Abstract
Salmonella-based cancer therapies show great potential in preclinical models, but for most cases the observed antitumor effect is transient. Understanding the basis of the antitumor efficacy might guide the design of improved strains that elicit long-lasting effects, paving the wave for clinical use. Here, we deepened into the role of macrophages and inflammasome activation in the context of Salmonella anti-melanoma effect. We showed inflammasome activation in melanoma cells upon infection, which correlated with cell surface exposure of gasdermin-D (GSDM-D) and calreticulin (CRT) and High mobility group box 1 protein (HMGB-1) release, suggesting immunogenic cell death, particularly pyroptosis. Salmonella infection upregulated levels of Caspase-11 (Casp11) mRNA, but not Nlrp3 or Nlrc4 mRNA, the only described inflammasome receptors engaged by Salmonella, suggesting that non-canonical inflammasome activation could be occurring in melanoma cells. Intratumoral administration of Salmonella to melanoma-bearing mice elicited local inflammasome activation and interleukin-1β (IL-1β) production together with tumor growth retardation and extended survival in wild type but not Caspase-1/11 (Casp1/11) knockout mice despite similar levels of intratumoral IL-1β in the later. Salmonella antitumor activity was also suppressed in melanoma bearing Nlrp3 knockout mice. Salmonella induced macrophage recruitment to the tumor site and infiltrating cells exhibited inflammasome activation. Depletion experiments confirmed that macrophages are also essential for Salmonella anti-melanoma effect. Intratumoral macrophages showed a marked M2/M1 shift soon after treatment but this inflammatory profile is then lost, which could explain the transient effect of therapy. All in all, our results highlight CASP-1/11 axis and macrophages as essential players in Salmonella-based cancer immunotherapy and suggest a possible target for future interventions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03148-x.
Keywords: Salmonella immunotherapy, Melanoma, Inflammasome, Macrophage recruitment, M2/M1 shift
Introduction
For many years, facultative anaerobic bacteria have been evaluated for the treatment of different types of cancer, showing highly effective in delaying tumor growth and prolonging survival in tumor-bearing mice [1–3]. Particularly, Salmonella has several features that make it an excellent candidate for immunotherapies. These include ability to grow easily under lab conditions, short replication cycles, ability to reach and replicate preferentially in tumor sites, and being facultative anaerobic, which enables them to reach both small oxic tumors or big hypoxic ones; amongst others ([4, 5], also reviewed in [6]). We have previously demonstrated that Salmonella Typhimurium LVR01 treatment induces the infiltration of immune cells as neutrophils and CD8 + T lymphocytes, correlating with a better outcome in both lymphoma and melanoma [7, 8]. Nevertheless, Salmonella antitumor effect is modest yet significant, and since the underlying mechanisms are not fully elucidated, additional evidence in this regard is imperative.
Inflammasome activation has emerged as an interesting topic since its discovery almost two decades ago. Salmonella can induce inflammasome assembly and activation in myeloid cells by at least two independent -but redundant- pathways. Direct recognition of intracellular flagellin by NLRC4/NAIP5, or NLRP3 indirect activation by the detection of common signals related to cell damage induced by infection such as changes in potassium levels or membrane disruption [9, 10]. In addition, the NLRP3 inflammasome may also be engaged by cytosolic LPS through direct binding to CASP-11 [11]. These events lead to the secretion of active IL-1β and IL-18 and may also promote a lytic type of caspase-dependent cell death named pyroptosis [12], which amplifies the inflammatory response through the release of DAMPs to the extracellular environment [13]. Gasdermin D (GSDM-D), the pore-forming effector protein of pyroptosis has also shown to be crucial in the release of IL-1β and IL-18 since they do not have a secretion signal [14]. The relationship between pyroptosis and cancer is controversial: on one hand, GSDM-D has been described as a tumor-suppressing molecule and on the other hand, a proinflammatory microenvironment can be tumor-promoting (reviewed in [15]).
Salmonella upregulates transcript levels of core molecules of inflammasome signaling when used as immunotherapy in colon carcinoma models [16] as well as triggers the production of dendritic cells-derived IL-1β, which leads to tumor growth suppression [17]. In this work, we pursued to establish the mechanisms behind Salmonella antitumor activity in a murine melanoma model deepening into the role of Salmonella-induced inflammasome activation. Our results show that upon infection, macrophages are recruited to the tumor site and both tumor and immune cells show rapid inflammasome activation. We also demonstrate that these events are indispensable for Salmonella to exert its antitumor effect. Loss over time of this acute inflammation profile could be a plausible explanation for the transient effect of Salmonella-based therapy.
Materials and methods
Bacterial strains
Salmonella enterica serovar Typhimurium LVR01, an avirulent aroC-mutant [18], was used in the present study. A LVR01 flagellin double knockout mutant (LVR01flag-) was constructed by phage P22 transduction of a fliC::KanR cassette from S. Enteritidis SEFK32 [19] and a fljB::CmR cassette from S. Typhimurium TH2795 [20], both strains kindly provided by Dr. Jean-Claude Sirard (Institut Pasteur of Lille, France). Correct insertion of each cassette was confirmed by PCR (primers available under request). Bacteria were routinely grown aerobically in an orbital shaking incubator (200 rpm) at 37 °C in LB broth (Difco, Detroit, MI), supplemented with kanamycin (50 μg/ml) and chloramphenicol (25 μg/ml) (both from Sigma-Aldrich, St. Louis, MO) when needed. Absence of flagellin expression was confirmed using agglutination tests for flagellar antigens with commercially available flagellar polyvalent sera (polyHA and polyHB, Difco, Detroit, MI). Additionally, phase-contrast microscope visualization of mid-log-phase bacterial cultures and motility tests were also performed. The latter consisted of plating bacteria in LB soft agar (0.3%) and measuring halo after incubating for 6 h at 37 °C.
Cell lines
B16F1, a C57BL/6 skin melanoma cell line, was obtained from American Type Culture Collection (ATCC, Manassas, VA). Tumor cells were cultured in DMEM medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum and 2 mM L-glutamine (DMEMsup) at 37 °C in 5% CO2.
Infection assays, caspase-1 activation and cell death assessment
B16F1 tumor cells were seeded in 24-well plates (Greiner bio-one, Germany) to a density of 5 × 105 cells per well. Salmonella LVR01 and LVR01flag- were diluted in the appropriate culture medium and added to the tumor cells at a multiplicity of infection (MOI) 100:1, and then incubated at 37 °C in 5% CO2 atmosphere. After 1 h, cell cultures were rinsed and incubated in medium containing gentamicin sulfate (100 μg/ml) (Sigma-Aldrich, St. Louis, MO) to kill extracellular, but not intracellular, bacteria. After 20 h cells were collected, washed twice with cold PBS, and then resuspended in 1X Binding Buffer (BD Pharmingen) at a final concentration of 1 × 106 cells/ml prior to staining with 5 µl of FITC-Annexin V (BD Pharmingen). Cells were then incubated for 15 min at room temperature (RT) in the dark. Ten minutes before flow cytometry analysis (FACS Canto II, BD Pharmingen), 5 µl of Propidium Iodide (300 ng/ml) (Sigma-Aldrich, St. Louis, MO) was added to the cells.
Inflammasome activation was measured using FAM-FLICA® Caspase-1 Assay Kit (ImmunoChemistry Technologies, MN) following manufacturer’s instructions.
For cell death assessment, 20 h after gentamicin addition cells were stained with either anti-GSDM-D antibody (ab209845, abcam) followed by secondary antibody (anti-rabbit IgG-FITC, Thermo Fisher Scientific); or anti-CRT antibody—Alexa Fluor® 647 (ab196159, abcam). Cell staining was analyzed by flow cytometry using FACS Canto II Flow cytometer equipped with six lasers (Becton–Dickinson, Oxford, UK). For data acquisition and analysis FACS Diva software (Becton–Dickinson) was used. Cell culture supernatants were assessed for HMGB-1 release, using HMGB1 ELISA Kit (IBL International, Germany) following manufacturer's instructions.
Quantitative RT-PCR
B16F1 cells were seeded and infected as described above. After 20 h of incubation, cells were resuspended in TRIzol (Invitrogen) and frozen at -80 °C to preserve RNA until extraction. In case of tumor homogenates, tumors were excised at different time points and preserved the same way in 500 μl TRIzol. RNA quality and quantity was assessed by spectrophotometric measurements at 260/280 nm using NanoDrop 2000 (Thermo Scientific). Prior to cDNA synthesis, 1 µg of total RNA was treated with DNAse-I (Invitrogen) according to manufacturer’s instructions. Retrotranscription was performed in a final volume of 20 µl in the presence of Random primers (200 ng), dNTPs (0.5 mM), DTT (0.01 M), RNaseOUT (40 U), and M-MLV Reverse Transcriptase (Invitrogen). Quantitative RT-PCRs for Nlrp3, Nlrc4, Asc, Casp1, Casp11, Il1b, Il18, Cxcl9, Cxcl10, Nos2, and Il12 mRNA was conducted using QuantiTect® SYBR® Green PCR Kit (Qiagen) in a Rotor-Gene 6000 (Corbett), primer sequences are available under request. Beta-Actin encoding gene Actb was used as house-keeping gene. The relative mRNA amount in each sample was calculated using the 2−ΔΔCt method as previously described [21] where ΔCt = Ctgene of interest—CtActb, and expressed as relative mRNA levels in the test groups compared to the control group.
Animals
Female C57BL/6 (DILAVE, Uruguay), and Casp1/11−/− or Nlrp3−/− mice (The Jackson Laboratories, ME, USA and bred at the Institut Pasteur Montevideo, Uruguay), 6–8 weeks old were used for in vivo experiments. All protocols for animal experimentation were carried out in accordance with procedures authorized by the University’s Ethical Committee for Animal Experimentation, Uruguay, to whom this project was previously submitted (CNEA No 0011/11).
Tumor model and immunization schedule
Tumor cells were grown in culture and harvested in log phase, washed twice with PBS, and resuspended in saline. Mice were injected into the right flank with 2.5 × 105 B16F1 cells. When tumors became palpable (average size 100 mm3), all mice were inoculated the same day directly into the tumor central area (intratumoral (i.t.) injections) with 1 × 106 cfu of Salmonella diluted in 100 μl of saline and then monitored for tumor growth every other day. Tumors were measured and volumes were calculated as length x width x depth x π/6. Mice were euthanized when tumors reached 4,000 mm3 or before if they showed any sign of distress. These time points were defined as survival time.
Tumor-Infiltrating cells analysis
In a different set of experiments, animals were sacrificed at different time points and each tumor was removed for further analysis. Analysis of tumor-infiltrating cells was conducted by flow cytometry on tumor homogenates by mechanical disruption. Cells were stained with FAM-FLICA® Caspase-1 Assay Kit (ImmunoChemistry Technologies, MN) for 30 min at 37 °C and after that they were immunostained for additional 30 min, 4 °C, with the following panel of antibodies: PE-conjugated anti-Ly6G, PercP/Cy5.5-conjugated anti-CD11b and APC-conjugated anti-F4/80 antibodies. For macrophage phenotyping, Fc block step was performed for 10 min and then the following panel of antibodies was used: PE/Cy7-conjugated anti-CD11c, APC-conjugated anti-CD206, APC/Cy7-conjugated CD11b, and Pacific Blue-conjugated anti-F4/80 (all reagents from BD Pharmingen or Biolegend, CA, USA). Cells were finally washed and analyzed by FACS as stated above.
Cytokine production assessment
IL-1β and IL-18 levels were quantified in tumor homogenates using ELISA MAX Deluxe Set Mouse IL-1β (Biolegend, CA, USA) and Platinum ELISA Mouse IL-18, Ready-To-Use Sandwich ELISA kit (eBioscience, CA, USA) according to manufacturer's instructions.
Macrophage depletion
Systemic macrophage depletion was carried out with Clophosome® clodronate liposomes (Neutral) (FormuMax Scientific, CA, USA). The regime consisted of 3 intraperitoneal doses of 700 μg/ml each separated by 5 days as previously described [22] and adapted to our model. PBMC was assessed for macrophage depletion over time. We obtained more than 70% of depletion 3 days after the third clodronate dose.
Statistical analysis
Differences in survival times were determined using Kaplan–Meier and log-rank test using GraphPad Prism 5 software. For the in vitro assays, the statistical significance of differences between study groups was analyzed using two ways ANOVA or Student T-test. In every case, a value of p < 0.05 was considered statistically significant.
Results
Salmonella induces inflammasome activation and cell death in melanoma cells
We have previously shown that Salmonella LVR01 infection resulted in decreased viability of melanoma B16F1-infected cells [8]. Thus, we assessed for different hallmarks of cell death. Twenty hours after Salmonella infection, B16F1 cells showed increased Annexin V expression in cell membranes (Fig. 1a). Besides, melanoma cells displayed GSDM-D expression which could be indicative of pyroptosis (Fig. 1b). Further, we found increased CRT membrane exposure as well as HMGB-1 release to the extracellular milieu both of which suggest immunogenic cell death (Fig. 1c, d).
Fig. 1.
B161 cells infected for 1 h with Salmonella resulted positive after 20 h for a Annexin V, b gasdermin-D, and c calreticulin expression. d HMGB-1 concentration in cell supernatants. e Relative Casp11 mRNA expression in B16F1 cells; f FLICA positive B16F1 cells. Results are shown as mean ± SEM. *p < 0.05, ***p ≤ 0.001. Representative dot plots for A-C and F are available in Suppl. Figure 1
We then evaluated inflammasome activation in Salmonella-infected melanoma cells. We found a marked increase in Casp11, but not Casp1, transcripts 20 h after Salmonella infection (Fig. 1e), while Nlrp3 and Nlrc4 transcripts were not detected (data not shown). Besides, FLICA staining showed that Salmonella infection induces CASP-1 activation in melanoma cells (Fig. 1f).
Salmonella induces inflammasome activation in the tumor microenvironment in melanoma-bearing mice
B16F1 melanoma-bearing mice received a single intratumoral injection of LVR01 and 72 h later tumors were assessed for expression of inflammasome-associated molecules. Figure 2 shows that Salmonella treatment induces increased levels of IL-1β, but not IL-18 protein in tumor homogenates (Fig. 2a). In addition, we observed increased levels of mRNA of inflammasome-related genes, such as Nlrp3 and Nlrc4 (Fig. 2b), both probably expressed by immune cells as we did not find these transcripts on melanoma infected cells. At later time points, this inflammatory expression profile decreases until most of the genes are not detected anymore, except for IL-1β that remains high (yet non-significant).
Fig. 2.
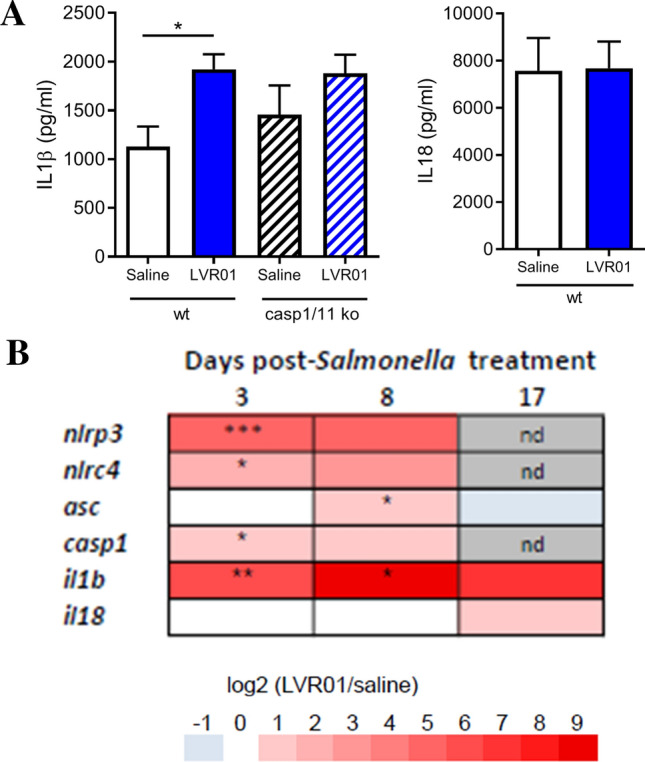
a IL1β and IL18 concentration in tumor homogenates 72 h post-Salmonella intratumoral injection. b Heatmap representing relative fold increase of inflammasome associated molecules on days 3, 7, and 18 post Salmonella intratumoral injection, nd: not detected. Data representative of two independent experiments. Results are shown as mean ± SEM. n = 12–13/group. *p < 0.05, **p < 0.01, ***p < 0.001
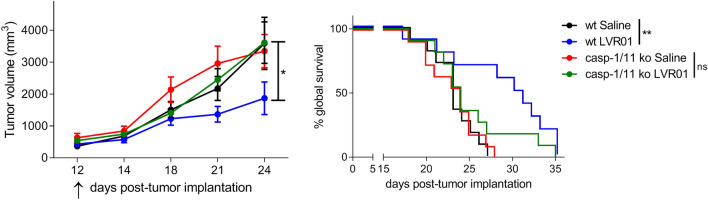
Salmonella antitumor effect depends on Caspase-1/11 activity
We have previously shown that intratumorally delivered LVR01 slows tumor growth and improves survival in melanoma-bearing mice [8]. We now demonstrate that this antitumor effect is dependent on the CASP-1/11 axis, inasmuch Salmonella antitumoral activity is lost in melanoma-bearing Casp1/11 ko mice (overall survival Control vs LVR01-treated p = 0.0038 in wt mice, p = 0.3489 in ko mice) (Fig. 3). Of note, intratumoral levels of IL-1β (around 2000 pg/ml) were similar in Salmonella-treated tumors in both wt and Casp1/11 ko mice (Fig. 2a).
Fig. 3.
Tumor growth (left) and survival (right) of LVR01 treated melanoma-bearing wt or Casp1/11 ko mice. Data representative of three independent experiments. Results are shown as mean ± SEM. n = 12–13/group. *p < 0.05, **p < 0.01. Arrow indicates Salmonella i.t. administration
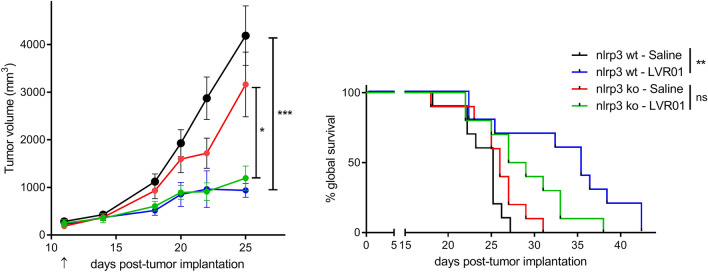
NLRP3 inflammasome activation is essential for antitumor effect while NLRC4 is dispensable
We found that Salmonella induces overexpression of NLRP3 at the tumor site (Fig. 2b), indicating local activation of NLRP3 inflammasome. Thus, we assessed whether this is involved in the antitumor activity. LVR01 treatment to melanoma-bearing Nlrp3 ko mice still induces tumor growth retardation although to a lesser extent than in wt mice and it does not extend overall survival (Fig. 4), suggesting that NLRP3 inflammasome engagement is central in Salmonella mediated antitumor activity.
Fig. 4.
Tumor growth (left) and survival (right) of LVR01-treated melanoma-bearing wt or nlrp3 ko mice, n = 12–13/group. Data representative of two independent experiments. Results are shown as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. Arrow indicates Salmonella i.t. administration
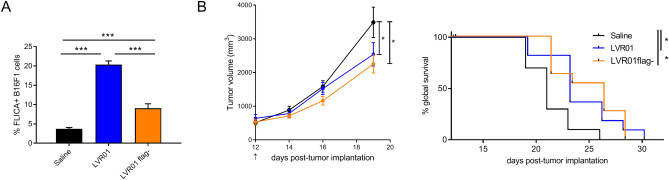
Flagellin, the main building block of Salmonella flagella, is an NLRC4 agonist and it has been described as the main effector for CASP-1 activation upon Salmonella infection [23]. Thus, we tested whether aflagellated Salmonella still retains anti-melanoma effect. For that, we constructed a Salmonella mutant lacking FliC and FljB (LVR01flag-). Melanoma cells infected with LVR01flag- still activate CASP-1, though to a much less extent than flagellin expressing LVR01 (Fig. 5a, Control vs LVR01 p = 4.33 × 10–10, Control vs LVR01flag- p = 9.20 × 10–6, LVR01 vs LVR01flag- p = 1.78 × 10–7). However, in the absence of flagellin Salmonella still retains antitumor effect as shown in Fig. 5b (overall survival Control vs LVR01flag- p = 0.0121), with no evident difference between strains (overall survival LVR01 vs LVR01flag- p = 0.8999).
Fig. 5.
a Percentage of FLICA positive B16F1 cells 20 h after infection with Salmonella, n = 3/condition. ***p < 0.001. b Tumor growth (left) and survival (right) of B16F1 tumor-bearing mice treated with different Salmonella. Results are shown as mean ± SEM. n = 12 per group. *p < 0.05. Arrow indicates Salmonella i.t. administration
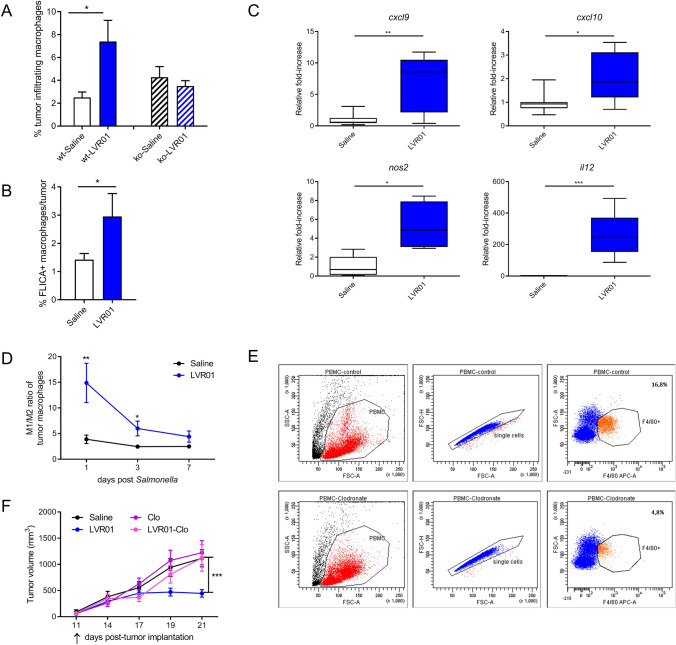
Salmonella treatment recruits macrophages to the tumor site and activates their inflammasome
We found that Salmonella recruits macrophages to the melanoma tumor site and also induces CASP-1 activation in a percentage of those tumor-infiltrating cells, but this recruitment is lost in Casp1/11 knockout mice (Fig. 6a and b). We also found Salmonella-induced upregulation of molecules that have been linked to the M1 phenotype, particularly Cxcl9, Cxcl10, Nos2, and Il12, in tumor microenvironment (Fig. 6c), suggesting a shift of intratumoral macrophages towards pro-inflammatory antitumoral phenotype. Indeed, we confirmed this phenomenon by determining the percentage of M2 (defined as CD206 + macrophages) and calculating the ratio M1/M2 within tumor-infiltrating macrophages. As seen in Fig. 6d, soon after Salmonella administration, we observed on average a 15/1 ratio of M1/M2 macrophages in Salmonella-treated mice, whereas the ratio was 4/1 in control mice. Increased M1/M2 ratio was maintained for a few days but significantly diminishes by day 7 post Salmonella until it becomes similar to that of control mice.
Fig. 6.
a Salmonella induced percentage of tumor-infiltrating macrophages and b FLICA positive macrophages, n = 3–5/group. c Relative Cxcl9, Cxcl10, Nos2, and Il12 mRNA expression in tumor homogenates 3 days post LVR01 i.t. treatment, n = 5–7/group. d M1/M2 ratio of tumor macrophages over time, n = 4–5/group. e Dot plots showing macrophage depletion in PBMC 3 days after finishing clodronate treatment. f Tumor growth of LVR01 treated melanoma-bearing mice with or without macrophage depletion, Clo: clodronate, n = 12–13/group. In every case, results are shown as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. Arrow indicates Salmonella i.t. administration
Macrophages are essential for Salmonella mediated anti-tumor activity
Since we found that Salmonella i.t. treatment results in CASP-1 activation in tumor-infiltrating macrophages (Fig. 6b) and that Salmonella antitumor activity is dependent on the CASP-1/11 axis (Fig. 3), we then wanted to assess whether macrophages are involved in Salmonella anticancer effectiveness. Melanoma-bearing mice in a macrophage depletion regime with clodronate liposomes (Fig. 6e) received i.t. Salmonella as previously. As shown in Fig. 6f, depletion of macrophages completely abrogates Salmonella antitumor activity.
Discussion
Salmonella has shown great potential for the development of new immunotherapies for cancer but the number of clinical trials is scarce and so far, they have not reproduced the effects observed in the preclinical models. Deciphering the molecular and cellular basis of the antitumor effectiveness of Salmonella might allow the designing of new strains with improved therapeutic efficacy and help to predict clinical responses.
In this work, we investigated Salmonella LVR01 antitumor activity in a melanoma preclinical model. We found that LVR01 induces GSDM-D expression, CRT membrane exposure as well as HMGB-1 release to the extracellular milieu all of which suggest immunogenic cell death [24], particularly pyroptosis [25]. Immunogenic cell death (ICD) drives adaptive immunity and the establishment of long-term immunological memory, influencing the cancer-immunity balance by skewing it towards antitumor immunity. Indeed, many agents used in cancer immunotherapies induce ICD [26]. Therefore, therapeutic engagement of ICD through Salmonella may represent an interesting approach to elicit more effective antitumor immunity [27]. Cleavage of GSDM-D protein is an important mediator of pyroptosis, a process that was originally described as a form of cell death mediated by CASP-1 cleavage [12] that ended up in the release of the inflammatory cytokine IL-1β [28]. We found that LVR01 infection significantly upregulated Casp11 transcripts in melanoma cells and induced CASP-1 activation as well as IL-1β release to the extracellular milieu in melanoma-bearing mice, suggesting that Salmonella-induced cell death at the tumor site is, at least in part, responsible for the antitumor activity. However, cleavage of inflammatory caspases as CASP-1 or CASP-11 does not always end up in cell death. Inflammatory caspases activation and GSDM-D cleavage have proven to have other physiological roles, inasmuch they are required to release IL-1β from living “hyperactive” cells [14]. We cannot exclude the possibility that this effect is taking place.
It has been previously reported that intravenous Salmonella administration upregulates transcript levels of core molecules of inflammasome signaling and triggers production of dendritic cells-derived IL-1β in tumors [16, 17]. It was also suggested that Salmonella antitumor activity is mediated by IL-1β since administration of this pro-inflammatory cytokine partially restored intrinsic antitumoral activity [17]. Here, we report that LVR01-induced CASP-1/11 activation is essential for Salmonella antitumor activity in melanoma-bearing mice since antitumor effect is lost in Casp1/11 deficient mice. However, we also found that high intratumoral production of IL-1β could still be found in melanoma bearing Casp1/11 ko mice (Fig. 2a), most likely due to inflammasome activation of B16F1 implanted cells, suggesting that this cytokine may contribute but not be essential for Salmonella antitumoral activity. Inflammasomes have been proposed as a target for cancer immunotherapies, particularly for melanoma which is highly plastic and therefore rapidly displays mechanisms for immunotherapy resistance [29]. Still, the role of inflammasome activation in tumor progression is controversial, and several reports have attributed it a double-edged sword effect [30, 31]. On one hand, increased expression of NLRP3 has been associated with multiple cancer types, melanoma included [32]. Conversely, IL-18 secretion induced by NLRP3 inflammasome activation in colorectal cancer has demonstrated to be protective, through IFN-γ production [33]. It is important to remark that despite IL-18 is considered an inflammasome-regulated cytokine, its modulation has proven to be different from that of IL-1β [34], and it has even been suggested that they may have opposite effects [35]. This could explain our results that showed no differences between Il18 mRNA expression and secretion after infection with Salmonella, while Il1β mRNA expression and protein production does increase upon treatment (Fig. 2). Another explanation for the IL-18 unchanged levels after therapy is that cancers, including melanoma, exhibit high basal amounts of IL-18 secretion [36], possibly masking a discrete effect induced by Salmonella.
The key defining event to predict the results from inflammasome activation in cancer could be the nature of the inflammation (acute vs chronic) since it is required to fight cancer cells but also represents a hallmark of cancer [37]. Our results show that Salmonella-induced inflammation in the tumor microenvironment is acute since inflammasome-related gene expression upon treatment dissipates over time (Fig. 2b). However other aspects like cancer type, stage, experimental conditions, microbiota, etc. are all additional factors that need to be taken into account when considering different outcomes. In line with this, it has been proposed that different subpopulations of macrophages (M1 or M2 phenotypes) exhibit differential inflammasome activation in response to stimuli [34], pinpointing that polarization is fundamental for shaping tumor microenvironment, which in turn is essential for the treatment outcome. Here, we found that Salmonella intratumoral administration induces a rapid phenotype shift of tumor-infiltrating macrophages towards classic M1, which together with the demonstration that macrophages are essential for the antitumor activity, suggests that this event is responsible for the antitumor activity of Salmonella. Nevertheless, by day 7 after treatment the phenotype shift is lost, coinciding with a progressive decrease in inflammasome-related gene expression upon treatment, being tempting to speculate that all these findings explain why Salmonella antitumor effect is only transient. In turn, this reinforces the requirement for strategies that can boost and/or maintain this antitumor phenotype. In this regard, it has been described that forced activation of Notch signaling increases M1 macrophages enhancing their antitumor capacity [38], suggesting that a mixed strategy including Salmonella to induce M1 profile and Notch signaling agent to maintain phenotype could be more beneficial in the long term.
We also found that Salmonella activates inflammasomes in tumor-infiltrating macrophages and that macrophages, Salmonella-mediated CASP-1 activation, and NLRP3 engagement are all essential for antitumor activity. The expression of inflammasome-related CASP-1 is up-regulated in M1 but not in M2 cells particularly in NLRP3 inflammasome engagement [34], suggesting that Salmonella mediated NLRP3 assembly resulted in M1 polarization of tumor-infiltrating macrophages and that this cell is at the center of its antitumor activity. This bacteria-induced phenotype shift has already been reported in human macrophages infected in vitro with an obligatory anaerobic Salmonella Typhimurium strain [39]. In addition, an engineered FlaB-secreting Salmonella strain also proved to be effective in reducing tumor size and prolonging survival in a colon cancer model, inducing a reduction in the percentage of M2-type macrophages as well as an increase in M1-type macrophages [40], pinpointing the antitumoral role of the latter.
In macrophages, the activation of CASP-1 induced by Salmonella is mainly mediated by the NLR family member NLRC4 that senses cytosolic flagellin. It has been proposed that Salmonella first engages NLRC4 and subsequently NLRP3, maximizing IL-1β and IL-18 production [9]. Nevertheless, the group of Tourlomousis recently showed that in absence of NLRC4, macrophages produce IL-1β via NLRP3 and because these cells do not rapidly die, they secrete large amounts of IL-1β between 6 and 24 h [41]. In this scenario NLRC4 restricts NLRP3 dependent production of inflammasome related proteins as IL-18 and IFN-γ [41], so not engaging NLRC4 would be an advantage. In line with this is the compelling recent finding that NLRC4 recognition suppresses, rather than facilitates, CD4 + T cell responses against Salmonella [41]. The authors suggest that flagellin modification could lead to stronger protective immunity to Salmonella, but in the context of our studies the lack of protective immunity could be a positive aspect to be further investigated. Our results here point out that regardless of the presence of flagellin, LVR01 is able to activate CASP-1 in melanoma cells (Fig. 5A) and to exert its antitumor effect (Fig. 5B) so in this case, NLRC4 engagement would be neither beneficial nor detrimental for the immunotherapy outcome.
In summary, our results demonstrate that Salmonella antitumor activity in melanoma is dependent on tumor-infiltrating M1 macrophages and CASP-1/11 inflammasome activation. This data may inform the development of new strains with improved antitumor effectiveness and will help to move forward the application of Salmonella-based immunotherapies in clinical setup.
Supplementary Information
Below is the link to the electronic supplementary material.
Footnotes
Précis: Salmonella antitumor activity requires functional CASP-1/11 activation, NLRP3 engagement, and recruitment of M1 macrophages to the tumor site. Absence of CASP-1/11 prevents macrophage intratumoral recruitment and hence the antitumor activity.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alejandro Chabalgoity and María Moreno share senior authorship.
Contributor Information
Jose Alejandro Chabalgoity, Email: chabalgoity.jose@gmail.com.
María Moreno, Email: mariamorenojauge@gmail.com.
References
- 1.Sznol M, et al. Use of preferentially replicating bacteria for the treatment of cancer. J Clin Invest. 2000;105(8):1027–1030. doi: 10.1172/JCI9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo X, et al. Antitumor effect of VNP20009, an attenuated Salmonella, in murine tumor models. Oncol Res. 2001;12(11–12):501–508. doi: 10.3727/096504001108747512. [DOI] [PubMed] [Google Scholar]
- 3.Avogadri F, et al. Cancer immunotherapy based on killing of Salmonella-infected tumor cells. Cancer Res. 2005;65(9):3920–3927. doi: 10.1158/0008-5472.CAN-04-3002. [DOI] [PubMed] [Google Scholar]
- 4.Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57(20):4537–4544. [PubMed] [Google Scholar]
- 5.Leschner S, et al. Tumor invasion of Salmonella enterica serovar Typhimurium is accompanied by strong hemorrhage promoted by TNF-alpha. PLoS One. 2009;4(8):e6692. doi: 10.1371/journal.pone.0006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno M, et al. Salmonella as live trojan horse for vaccine development and cancer gene therapy. Curr Gene Ther. 2010;10(1):56–76. doi: 10.2174/156652310790945566. [DOI] [PubMed] [Google Scholar]
- 7.Grille S, et al. Salmonella Enterica serovar Typhimurium immunotherapy for B-Cell Lymphoma induces broad antitumor immunity with therapeutic effect. Immunology. 2014;143(3):428–437. doi: 10.1111/imm.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vola M, et al. TLR7 agonist in combination with Salmonella as an effective antimelanoma immunotherapy. Immunotherapy. 2018;10(8):665–679. doi: 10.2217/imt-2017-0188. [DOI] [PubMed] [Google Scholar]
- 9.Broz P, et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010;207(8):1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong T, et al. Orchestration of NLRP3 Inflammasome Activation by Ion Fluxes. Trends Immunol. 2018;39(5):393–406. doi: 10.1016/j.it.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Shi J, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 12.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9(3):113–114. doi: 10.1016/S0966-842X(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 13.Lamkanfi M, et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol. 2010;185(7):4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evavold CL, et al. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity. 2018;48(1):35–44.e6. doi: 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia X, et al. The role of pyroptosis in cancer: pro-cancer or pro-"host"? Cell Death Dis. 2019;10(9):650. doi: 10.1038/s41419-019-1883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phan TX, et al. Activation of inflammasome by attenuated Salmonella typhimurium in bacteria-mediated cancer therapy. Microbiol Immunol. 2015;59(11):664–675. doi: 10.1111/1348-0421.12333. [DOI] [PubMed] [Google Scholar]
- 17.Kim JE, et al. Salmonella typhimurium Suppresses Tumor Growth via the Pro-Inflammatory Cytokine Interleukin-1beta. Theranostics. 2015;5(12):1328–1342. doi: 10.7150/thno.11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chabalgoity JA, et al. Salmonella typhimurium as a basis for a live oral Echinococcus granulosus vaccine. Vaccine. 2000;19(4–5):460–469. doi: 10.1016/S0264-410X(00)00197-3. [DOI] [PubMed] [Google Scholar]
- 19.Van Asten FJ, et al. Inactivation of the flagellin gene of Salmonella enterica serotype enteritidis strongly reduces invasion into differentiated Caco-2 cells. FEMS Microbiol Lett. 2000;185(2):175–179. doi: 10.1111/j.1574-6968.2000.tb09058.x. [DOI] [PubMed] [Google Scholar]
- 20.Didierlaurent A, et al. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J Immunol. 2004;172(11):6922–6930. doi: 10.4049/jimmunol.172.11.6922. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Miller MA, et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat Commun. 2015;6:8692. doi: 10.1038/ncomms9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franchi L. Role of inflammasomes in salmonella infection. Front Microbiol. 2011;2:8. doi: 10.3389/fmicb.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kepp O, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2015;3(9):e955691. doi: 10.4161/21624011.2014.955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sborgi L, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35(16):1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galluzzi L, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020 doi: 10.1136/jitc-2019-000337corr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed A, Tait SWG. Targeting immunogenic cell death in cancer. Mol Oncol. 2020;14(12):2994–3006. doi: 10.1002/1878-0261.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci. 2007;120(Pt 5):772–781. doi: 10.1242/jcs.03377. [DOI] [PubMed] [Google Scholar]
- 29.Emran AA, et al. Do innate killing mechanisms activated by inflammasomes have a role in treating melanoma? Pigment Cell Melanoma Res. 2020;33(5):660–670. doi: 10.1111/pcmr.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karan D. Inflammasomes: emerging central players in cancer immunology and immunotherapy. Front Immunol. 2018;9:3028. doi: 10.3389/fimmu.2018.03028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kantono M, Guo B. Inflammasomes and cancer: the dynamic role of the inflammasome in tumor development. Front Immunol. 2017;8:1132. doi: 10.3389/fimmu.2017.01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamoto M, et al. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta. J Biol Chem. 2010;285(9):6477–6488. doi: 10.1074/jbc.M109.064907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaki MH, et al. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol. 2010;185(8):4912–4920. doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Awad F, et al. Impact of human monocyte and macrophage polarization on NLR expression and NLRP3 inflammasome activation. PLoS One. 2017;12(4):e0175336. doi: 10.1371/journal.pone.0175336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Q, et al. New mechanisms of tumor-associated macrophages on promoting tumor progression: recent research advances and potential targets for tumor immunotherapy. J Immunol Res. 2016;2016:9720912. doi: 10.1155/2016/9720912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park H, et al. Enhanced IL-18 expression in common skin tumors. Immunol Lett. 2001;79(3):215–219. doi: 10.1016/S0165-2478(01)00278-4. [DOI] [PubMed] [Google Scholar]
- 37.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Wang YC, et al. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010;70(12):4840–4849. doi: 10.1158/0008-5472.CAN-10-0269. [DOI] [PubMed] [Google Scholar]
- 39.Yang M, et al. An obligatory anaerobic Salmonella typhimurium strain redirects M2 macrophages to the M1 phenotype. Oncol Lett. 2018;15(3):3918–3922. doi: 10.3892/ol.2018.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng JH, et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aak9537. [DOI] [PubMed] [Google Scholar]
- 41.Tourlomousis P, et al. Modifying bacterial flagellin to evade Nod-like Receptor CARD 4 recognition enhances protective immunity against Salmonella. Nat Microbiol. 2020;5(12):1588–1597. doi: 10.1038/s41564-020-00801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.