Abstract
The strain-specific spectrum of liver disease following murine hepatitis virus type 3 (MHV-3) infection is dependent on inflammatory mediators released by macrophages. Production of nitric oxide (NO) by macrophages has been implicated in resistance to a number of viruses, including ectromelia virus, vaccinia virus, and herpes simplex virus type 1. This study was undertaken to define the role of NO in MHV-3 infection. Gamma interferon-induced production of NO inhibited growth of MHV-3 in a murine macrophage cell line (RAW 264.7). Viral inhibitory activity was reproduced by the NO donor S-nitroso-N-acetyl-dl-penicillamine (SNAP), whereas N-acetyl-dl-pencillamine (NAP), an inactive analog of SNAP, had no effect. Electron microscopy studies confirmed the inhibitory effects of NO on viral replication. Peritoneal macrophages isolated from A/J mice known to be resistant to MHV-3 produced a fivefold-higher level of NO and higher levels of mRNA transcripts of inducible NO synthase in response to gamma interferon than macrophages from susceptible BALB/cJ mice. SNAP inhibited growth of MHV-3 in macrophages from both strains of mice to similar degrees. In vivo inhibition of NO by N-monomethyl-l-arginine resulted in loss of resistance to MHV-3 in A/J mice. These results collectively demonstrate a defect in the production of NO in macrophages from susceptible BALB/cJ mice and define the importance of endogenous NO in resistance to MHV-3 infection in resistant A/J mice.
Nitric oxide (NO) has now been established to be an important endogenous messenger molecule in mammals and has been implicated in a wide range of biological processes, such as regulation of vasomotor tone, neurotransmission, and host defenses against intracellular pathogens (33). It is synthesized from l-arginine by a family of complex enzymes known as NO synthases (NOS), which includes at least three different isoforms: neuronal (nNOS, NOS1), inducible (iNOS, NOS2), and endothelial constitutive (ecNOS, NOS3), originally purified from neurons, cytokine-activated macrophages, and endothelium, respectively (43). The three NOS isoforms are expressed in a wide range of cell types and tissues, and a cell may even express two NOS isoforms. NOS are cytochrome P-450-like heme proteins which catalyze the NADPH-dependent, five-electron oxidation of l-arginine to generate NO and l-citrulline. The nNOS and ecNOS isoforms are constitutively expressed and are activated through stimulation of the Ca2+-calmodulin signaling pathway. iNOS is a Ca2+-independent, high-output enzyme whose expression can be stimulated by cytokines or lipopolysaccharide (LPS) (4) in almost every murine tissue and cell type over a period of hours. Although, for reasons as yet unclear, human cells express less iNOS mRNA in response to cytokines than rodent cells (9), sepsis and “septic-like” states in humans have been associated with increased urinary excretion of nitrite, suggesting that in vivo activation of the l-arginine–NO pathway does occur in humans (10, 15). Functional characterization of the murine iNOS promoter has demonstrated a proximal region which interacts with the NF-κβ trans-acting factor and which is critical for LPS-induced transcription of iNOS, as well as a more distal region involved in gamma interferon (IFN-γ)-stimulated changes in transcription (45). IFN-γ and LPS also exert their stimulatory effects on NO synthesis by stabilizing iNOS mRNA transcripts (44).
The induction of an antiviral state by interferons is an early response to viral infection that is essential for host survival. IFN-γ-induced nitric oxide production by macrophages has been implicated in resistance to intracellular pathogens, such as parasites (11, 23, 28, 30, 39), fungi (32), mycobacteria (5, 8), and more recently viruses, including ectromelia virus (18), vaccinia virus (14), and herpes simplex virus type 1 (2). In biological systems, NO reacts with oxygen (O2), superoxide (O2−), and transition metals, leading to the formation of reactive products that support additional nitrosative reactions at thiol groups (38). Iron-sulfur clusters and heme proteins which are now known to be regulated by NO include membrane, cytosolic, and nuclear proteins involved in signal transduction, cellular respiration and metabolism, DNA synthesis (19, 20), and initiation of transcription (20). Although the exact mechanism by which NO exerts its antiviral action remains unknown, the multiplicity of its host target enzymes makes it probable that multiple alterations in host cell proteins are involved. In vaccinia virus infection of RAW 264.7 cells, NO has been shown to inhibit viral DNA synthesis, late protein synthesis, and virus particle formation (14).
We wished to study the role of NO in a murine model of fulminant viral hepatitis. Murine hepatitis virus strain 3 (MHV-3), a single-stranded positive-sense RNA coronavirus, induces a strain-specific pattern of disease in inbred laboratory mice, which has served as an extremely useful experimental model for the study of host resistance/susceptibility to human fulminant viral hepatitis. Susceptible inbred mouse strains, such as BALB/c or C57BL/6, develop fulminant hepatitis and die within 3 to 5 days following parenteral inoculation of the virus. In contrast, A/J mice are resistant, develop no clinical signs of hepatitis, and clear the virus within 10 days of infection (3). As the virus grows in both susceptible and resistant mice (27), we have previously suggested that differences in the host inflammatory response account for resistance/susceptibility to MHV-3 (3, 21). A pivotal role for IFN-γ in murine viral hepatitis was suggested with the finding that treatment of A/J mice with anti-IFN-γ serum rendered them susceptible to MHV-3-induced disease (24). Furthermore, macrophages from resistant mice but not those from susceptible mice are able to restrict the growth of MHV-3 when activated with IFN-γ (29, 35).
The present study demonstrated that IFN-γ-induced production of NO inhibits the growth of MHV-3 in RAW 264.7 cells and that IFN-γ-activated macrophages from resistant A/J mice produce greater amounts of NO and are able to restrict viral replication to a greater degree than macrophages from IFN-γ-activated susceptible BALB/cJ mice. Furthermore, treatment of A/J mice with N-monomethyl-l-arginine (l-NMMA), an inhibitor of NO synthesis, increases mortality following MHV-3 infection, demonstrating the importance of NO production in resistance to MHV-3 in vivo.
MATERIALS AND METHODS
Mice.
Eight- to twelve-week-old female BALB/cJ and A/J mice were obtained from Jackson Laboratory (Bar Harbor, Maine). They were housed in microisolator cages and fed a standard chow and water ad libitum. Random mice were tested, and were all seronegative for routine viruses, including MHV.
Virus.
MHV-3 was plaque purified on DBT cells and grown to a titer of 107 PFU/ml in 17CL1 cells (22). Viral titers were tested in a standard plaque assay on monolayers of L2 cells as previously described (21). The origin and growth of 17CL1, DBT, and L2 cells have been described elsewhere (16, 40).
Cells.
RAW 264.7 cells (ATCC TIB71), a murine macrophage cell line transformed with the Abelson leukemia virus (37), were propagated in Dulbecco modified Eagle medium (ICN Biomedicals Inc., Costa Mesa, Calif.) containing 400 μM l-arginine, buffered with 16 mM MOPS [(N-morpholino)propane sulfonic acid] -HEPES-TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid] (Sigma Chemical Co., St. Louis, Mo.)–0.3% sodium bicarbonate and supplemented with 2 mM glutamine (Sigma), 100 U each of penicillin and streptomicin per ml, and 10% heat-inactivated fetal calf serum (Flow Laboratories, Mississauga, Ontario, Canada) (ADME-10). Peritoneal macrophages were obtained by lavage of the peritoneal cavity with 10 ml of RPMI 1640 (ICN Biomedicals) supplemented with 2 mM glutamine 4 days after intraperitoneal (i.p.) administration of 1.5 ml of 3% thioglycolate (Difco Laboratories, Detroit, Mich.) as previously described (21).
The cells were washed once and suspended in ADME-2 at 5 × 105/ml (2 ml/well) in six-well plates (Corning Glass Works, Corning, N.Y.). Two hours later, the nonadherent cells were removed and the adherent cells were infected with 1,000 PFU of MHV-3 (multiplicity of infection [MOI] of 0.001). After viral adsorption for 30 min at 22°C, 2 ml of ADME-2 with or without other reagents was added per well and the cells were incubated at 37°C; 24 to 48 h later, the cells were scraped off and stored at −70°C until viral titers and nitrite levels were measured.
Reagents.
S-Nitroso-N-acetyl-dl-penicillamine (SNAP; Alexis Corporation, San Diego, Calif.) was reconstituted in methanol at a concentration of 250 to 500 μM prior to use. At these concentrations, SNAP was not toxic to RAW cells, whereas at a concentration of 750 μM or greater, significant toxicity was seen. N-Acetyl-dl-penicillamine (NAP; Sigma) was reconstituted in methanol at a concentration of 250 to 500 μM prior to use. Recombinant murine IFN-γ (Pharmingen, San Diego, Calif.) was reconstituted in ADME-2. l-NMMA (Sigma) was dissolved in phosphate-buffered saline (PBS) and filter sterilized. The Griess reagent (11) was prepared fresh for each experiment by mixing equal amounts of 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride (Sigma) in water and 1% sulfanilamide (Sigma) in 5% phosphoric acid. The two constituents were stored at 4°C in dark bottles.
Nitrite assay.
Freeze-thawed samples were centrifuged at 300 × g for 10 min; 100-μl aliquots of the supernatants were then mixed with an equal volume of Griess reagent (see above) and incubated for 10 min at 37°C. The optical density at 540 nm was determined on an automated multiscan spectrophotometer (Flow Laboratories). The nitrite concentration was determined by using sodium nitrite (Sigma) as a standard for each experiment. The absorbance of medium alone was subtracted from the value obtained for each sample.
Northern blotting.
After appropriate treatment, peritoneal macrophages from A/J and BALB/cJ mice were washed with PBS, pelleted, and frozen in liquid nitrogen. Total RNA was isolated by 8 M acid-guanidinium hydrochloride extraction in a modified procedure described by Evans and Kamdar (6). The amount and purity of RNA were quantified by measuring the optical densities at 260 and 280 nm in a Spectronic 1001 spectrophotometer (Bausch & Lomb), and 15 μg of total RNA was added per lane. RNA was resolved on a 0.8% agarose gel containing MOPS-formaldehyde and transferred onto a nitrocellulose membrane (Schleicher & Schuell, Keene, N.H.). Forty nanograms of a cDNA probe for murine iNOS (26) was labeled by using a random priming DNA labeling system (Pharmacia Inc., Montreal, Quebec, Canada) with [α-32P]dCTP (specific activity, >3,000 Ci/mmol; Amersham, Mississauga, Ontario, Canada) to a specific activity of 4 × 108 cpm/μg. Membranes were prehybridized for 5 h at 42°C in a mixture of 50% formamide, 5× Denhardt’s solution, 0.2% sodium dodecyl sulfate, 100 μg of denatured salmon sperm DNA per ml, and 5× SSPE buffer (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]). Hybridization was carried out overnight in the same mixture at 42°C. The membranes were then washed under medium-stringency conditions and exposed to Kodak XAR-5 film with intensifying screens for 24 h at −70°C. To confirm the integrity and amount of RNA added to each lane, the membrane was reprobed with a human glyceraldehyde-3-phosphate dehydrogenase cDNA probe (42).
Electron microscopy.
After aspiration of the medium, RAW 264.7 cells which had been infected with MHV-3 at an MOI of 2.5 for 24 h, to ensure that 100% of cells were infected, in the presence or absence of IFN-γ or SNAP were fixed for 30 min with PBS containing 2.5% glutaraldehyde at 4°C and postfixed in 1% osmic acid in cacodylate buffer. Dehydration in acetone was followed by embedding in epoxy resin. Thin sections were stained with 2% aqueous uranyl acetate followed by lead citrate. The sections were examined in a Philips 400 electron microscope at 60 kV.
Statistical analysis.
Data are expressed as means ±1 standard deviation (SD) where applicable. Student’s t test for unpaired samples (two tailed) was used to analyze the data. The effect of l-NMMA treatment on survival in MHV-3-infected A/J mice was analyzed by survival analysis using the Kaplan-Meier method.
RESULTS
Effect of SNAP on growth of MHV-3 in macrophages.
The addition of SNAP, an NO donor (7), to RAW 264.7 cells inhibited the growth of MHV-3 (Fig. 1A), whereas the vehicle control had no effect. The inhibition was not due to toxic effects on RAW 264.7 cells, as demonstrated by trypan blue staining. In additional studies, using peritoneal macrophages from both A/J and BALB/cJ mice, SNAP inhibited MHV-3 replication to a similar degree. In contrast, NAP had no inhibitory effects on viral replication (Fig. 1B and C).
FIG. 1.
SNAP specifically inhibits MHV-3 replication in RAW 264.7 (A), A/J (B), and BALB/cJ (C) macrophages. We infected 106 macrophages with 1,000 PFU of MHV-3 (MOI of 0.001) in the presence or absence of 500 μM SNAP or 500 μM NAP for 24 h. SNAP, NAP, and the control vehicle, 0.5% methanol, were added every 4 h. Viral titers were measured by plaque assay. The control vehicle had no effect (not shown). Each bar represents the mean ± SD of the results from four independent experiments. Viral titers were compared by an unpaired t test (∗, two-tailed P value < 0.05).
To determine whether SNAP directly affected viral infectivity, MHV-3 was incubated with SNAP for 45 min on ice and then assayed on monolayers of L2 cells. Levels of replication of virus were equivalent in the presence and absence of SNAP (data not shown).
Effects of IFN-γ and l-NMMA on MHV-3 replication.
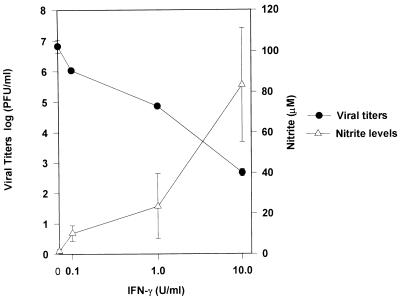
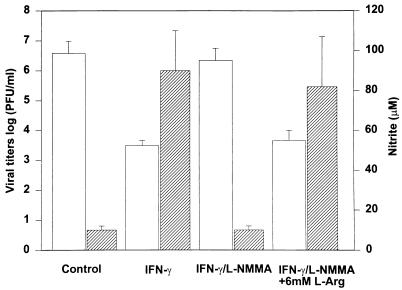
Recombinant murine IFN-γ induced NO production and restricted viral replication in RAW 264.7 cells in a concentration-dependent fashion over the range 0.1 to 10 U/ml (Fig. 2). The addition of l-NMMA at a concentration of 1,000 μM in combination with IFN-γ at 10 U/ml resulted in inhibition of nitrite production by 88% and a significant (1,000-fold) increase in peak viral titers compared to that with IFN-γ alone (two-tailed P value = 0.01). The addition of l-arginine (6 mM) completely reversed the inhibitory effect of l-NMMA (Fig. 3).
FIG. 2.
IFN-γ inhibits MHV-3 replication and induces NO production in RAW 264.7 cells. We infected 106 RAW 264.7 macrophages with 1,000 PFU of MHV-3 (MOI of 0.001) in the presence or absence of IFN-γ at doses ranging from 0.1 to 10 U/ml. Viral titers and nitrite levels were measured after 48 h. Values are means ± SDs of results from three separate experiments.
FIG. 3.
l-NMMA blocks IFN-γ-induced inhibition of viral replication and NO production in RAW 264.7 cells. We infected 106 RAW 264.7 macrophages with 1,000 PFU of MHV-3 (MOI of 0.001) in the presence or absence of IFN-γ (10 U/ml), with and without l-NMMA (1,000 μM) and l-arginine (l-Arg; 6 mM), as shown. Viral titers (□) and nitrite levels ( ) were measured after 48 h. Values are means ± SDs of results from three separate experiments.
Effects of IFN-γ and SNAP on viral particle formation in RAW 264.7 macrophages.
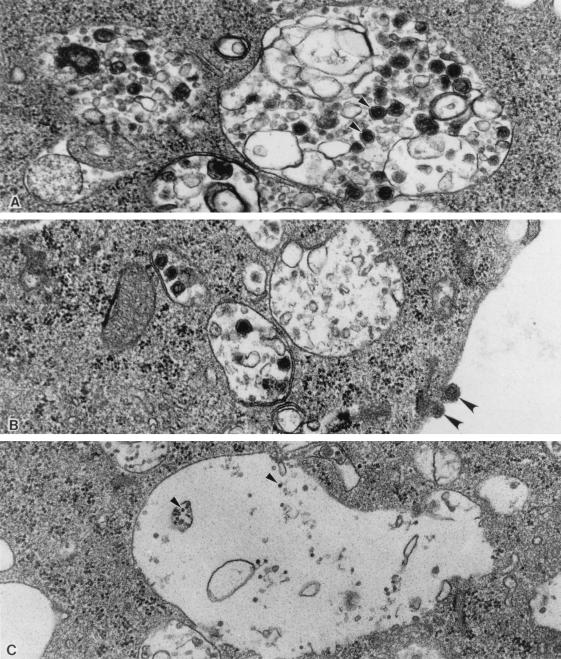
RAW 264.7 cells infected with MHV-3 for 24 h showed evidence of cell damage, particularly in the presence of numerous cytoplasmic vacuoles which occupied large areas of the cell cytoplasm (Fig. 4); few vacuoles only were seen in the noninfected controls (not shown). The clusters of small particles seen free in the cytoplasm outside the vacuoles are ribosomes. Numerous viral particles (virions) were seen within the cytoplasmic vacuoles of the infected cells. These were spheroidal and had an electron-dense core and the typical overall structure of coronaviruses. The sizes of viruses in this family vary from 80 to 160 nm (17); the ones illustrated in Fig. 4 range from 75 to 125 nm. They were very numerous in the MHV-3-infected nontreated group (Fig. 4A), less frequent in the MHV-3-infected interferon-treated cells (Fig. 4B), and undetectable in the MHV-3-infected, SNAP-treated group (Fig. 4C). In the MHV-infected, SNAP-treated cells, we found (Fig. 4C) very small, uniform, electron-dense particles (approximately 18 nm) which were not seen in the other groups; they may represent viral protein.
FIG. 4.
Electron micrographs of RAW 264.7 mouse macrophages infected with MHV-3 for 24 h. Cytoplasmic vacuoles in the periphery of cultured cells are shown. (A) MHV-3. Note spherical virions measuring 75 to 125 nm (arrowheads) within vacuoles. The electron-dense nucleocapsid is visible within many of the virions. These virions are typical of coronaviruses. (B) MHV-3 plus IFN-γ. The findings are similar to those in panel A, but there are fewer virions. Note also spherical virions adherent to the cell membrane (arrowheads). (C) MHV-3 plus SNAP. No virions are seen. The small dense particles (approximately 18 nm in diameter; arrowheads) may be viral protein. (Magnification, ×55,000).
Effects of IFN-γ and l-NMMA on NO production and MHV-3 replication in peritoneal macrophages.
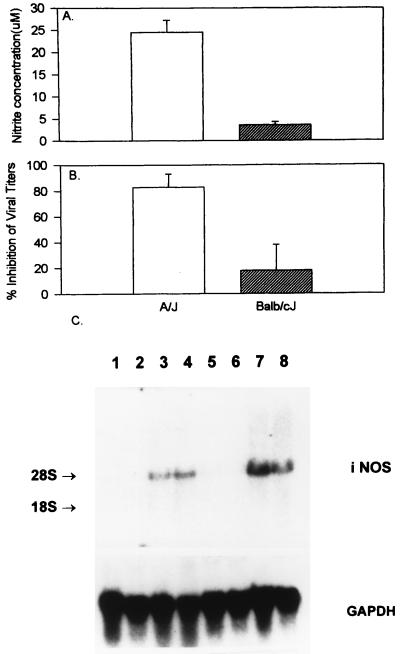
A/J macrophages produced significantly higher levels of nitrite than BALB/cJ macrophages in response to IFN-γ at all concentrations studied (10 to 1,000 U/ml) (Fig. 5A). Northern blot hybridization revealed higher steady-state iNOS mRNA levels following activation with IFN-γ in A/J than in BALB/cJ macrophages (Fig. 5C). No iNOS mRNA transcripts were detected in either A/J or BALB/cJ macrophages infected with MHV-3 for 8 h.
FIG. 5.
(A) IFN-γ induces greater NO production in A/J than in BALB/cJ macrophages. We treated 106 macrophages from susceptible BALB/cJ or resistant A/J mice with IFN-γ (100 U/ml) for 48 h. Nitrite levels were measured by a colorimetric assay using the Griess reagent. The experiment was done three times in triplicate wells. Values are means ± SDs of results from three separate experiments. (B) IFN-γ significantly inhibits MHV-3 replication in A/J macrophages only. We infected 106 macrophages from susceptible BALB/cJ or resistant A/J mice with 1,000 PFU of MHV-3 in the presence or absence of IFN-γ (100 U/ml). Viral titers were measured after 48 h, and percent inhibition of viral replication was calculated by using the following formula: (viral titer in control sample − viral titer in study sample)/(viral titer in control sample) × 100. Values are means ± SDs of results from three separate experiments. (C) IFN-γ induces greater iNOS mRNA expression in A/J than in BALB/cJ macrophages. We infected 106 macrophages from susceptible BALB/cJ mice (lanes 1 to 4) or resistant A/J mice (lanes 5 to 8) with 1,000 PFU of MHV-3 in the presence (lanes 3 and 7) or absence (lanes 2 and 6) of IFN-γ (100 U/ml) or treated them with IFN-γ (100 U/ml) alone (lanes 4 and 8). Lanes 1 and 5 contain unstimulated macrophages. Following incubation for 8 h, total RNA was extracted and 15 μg was added per lane. The RNA was separated on an agarose gel, transferred to a nitrocellulose membrane, and probed with an iNOS cDNA probe. A glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe was used to ensure equal loading of all lanes.
IFN-γ at doses of 10 to 1,000 U/ml, added immediately following viral adsorption, significantly inhibited peak viral growth in A/J macrophages only, not in BALB/cJ macrophages, with maximum inhibition at 100 U/ml and no further inhibition even at 1,000 U/ml (P = 0.01), as seen in Fig. 5B. The addition of l-NMMA at a concentration of 1,000 μM only partly reversed the antiviral action of IFN-γ in A/J macrophages, despite inhibiting NO production by 93% (not shown).
Although no iNOS mRNA transcripts were detected in peritoneal macrophages infected with MHV-3 for 8 h (Fig. 5C), low (<10 μM) levels of nitrite were measured after MHV-3 infection of RAW 264.7 cells and A/J peritoneal macrophages for 48 h (Fig. 5A).
Effect of l-NMMA on resistance of A/J mice to MHV-3.
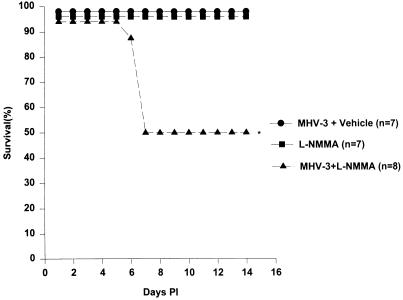
The importance of endogenous NO production in host resistance following MHV-3 infection in vivo was next examined. As shown in Fig. 6, treatment of A/J mice with l-NMMA (4 mg/day/mouse i.p.) for 2 weeks following infection with MHV-3 (10 PFU i.p.) resulted in 50% mortality. The liver pathology was consistent with fulminant hepatitis with widespread fibrin deposition and hepatocellular necrosis as is seen in susceptible BALB/cJ mice. None of the animals that received MHV-3 or l-NMMA alone died. The mean survival time in the group receiving both l-NMMA and MHV-3 was 10.38 days (95% confidence interval [CI], 7.85 to 12.90 days; determined by Kaplan-Meier survival analysis) and was thus significantly shorter than in either control group, where all animals survived for 14 days.
FIG. 6.
l-NMMA causes partial loss of resistance to MHV-3. Resistant A/J mice were infected with 10 PFU of MHV-3 i.p. and treated daily with l-NMMA (4 mg/day i.p.) for 14 days (n = 8). Control mice were either infected with 10 PFU of MHV-3 i.p. (n = 7) or treated with l-NMMA (4 mg/day i.p.) (n = 7) for 14 days. Mean survival times and 95% CI in each group were calculated by survival analysis using the Kaplan-Meier method. ∗, mean survival time in this group was 10.38 days, and the upper limit of the 95% CI was 12.9 days.
DISCUSSION
These studies demonstrate a linkage between NO production and genetic resistance to MHV-3 infection both in vivo and in vitro. Although NO production or lack of production has been implicated in the pathogenesis of a number of diseases (8, 12, 23, 28, 30, 32, 39, 41, 46), this is the first demonstration of a strain-dependent difference in the production of NO and resistance to a viral pathogen and explains the interferon nonresponsiveness of BALB/cJ mice.
In a previous study, we reported that the pattern of disease after MHV-3 infection correlated with macrophage activation and not viral replication (36). Macrophages from susceptible BALB/cJ mice following MHV-3 infection produced greater amounts of interleukin 1, tumor necrosis factor alpha, leukotriene B4, and a unique cellular procoagulant with prothrombinase activity than macrophages from resistant A/J mice. In the present study, we focus on the role of NO in resistance and susceptibility to MHV-3 infection.
The addition of SNAP, an NO donor, to RAW 264.7 cells and peritoneal macrophages from both resistant A/J and susceptible BALB/cJ mice inhibited the growth of MHV-3. This antiviral effect was specific, as demonstrated by the fact that NAP, an inactive analog of SNAP, had no inhibitory effects, and furthermore was not due to toxic effects on macrophages, as SNAP did not affect cell viability. Croen (2) also showed that SNAP, at a concentration of 500 μM added every 4 h, did not cause cell death, although it inhibited DNA and protein synthesis in RAW 264.7 cells to a degree similar to that effected by IFN-γ and LPS used in combination. Mammalian cells utilize several defense mechanisms against oxidant stress, including manganese-specific superoxide dismutase, glucose-6-phosphate dehydrogenase, and glutathione S-transferase, which are all activated following cytokine activation of macrophages (38). Thus, although NO does not appear to be directly toxic to MHV-3 virions or the host macrophages, it produces changes in host macrophages that interfere with viral replication, likely through effects on a multiplicity of target enzymes (18). The electron microscopy studies using MHV-3-infected RAW 264.7 cells demonstrate that NO is a potent inhibitor of viral particle formation and that it can reproduce the antiviral action of IFN-γ.
Furthermore, IFN-γ inhibited the growth of MHV-3 in RAW 264.7 cells in part through inducing NO production, as demonstrated by the increase in viral titers concomitant with inhibition of nitrite synthesis seen in the presence of l-NMMA. l-Arginine, the substrate of NOS, reversed the inhibitory of effect of l-NMMA. The fact that the inhibitory effect of IFN-γ was only partly reversed by l-NMMA indicates that at this dose (10 U/ml), IFN-γ must exert its antiviral action through other mechanisms as well, such as the induction of phosphatidylinositol kinase, 2′5′-oligoadenylate synthetase, indoleamine 2,3-dioxygenase, Mx proteins, 9-27 protein, and other, as yet unknown, mechanisms (18). In support of this, Harris et al. (14) also found that at high doses of IFN-γ, l-NMMA does not produce an increase in viral titers, suggesting that NO production is not the only pathway mediating the antiviral action of IFN-γ.
Macrophages from resistant A/J mice treated with IFN-γ (10 to 1,000 U/ml) produced higher levels of iNOS mRNA transcripts and higher levels of nitrite than macrophages from susceptible BALB/cJ mice. IFN-γ, at all concentrations studied, significantly inhibited the growth of MHV-3 in macrophages from resistant A/J mice, with maximum inhibition at a concentration of 100 U/ml. In contrast, no significant inhibition was seen in macrophages from susceptible BALB/cJ mice treated with IFN-γ. The relatively poor inhibition of the growth of MHV-3 in A/J macrophages compared to RAW 264.7 cells is consistent with the lower levels of nitrite induced by IFN-γ in A/J macrophages than in RAW 264.7 cells. It is also in agreement with other reports stating that multiple costimulatory signals are required for both effective synthesis of NO and induction of macrophage microbicidal activity (31). Our data are consistent with the observations of Pereira and colleagues (25, 29), who have demonstrated that macrophages from A/J but not BALB/cJ mice are able to restrict the growth of MHV-3 in response to IFN-γ. Recently, we have shown that A/J mice produce significantly higher concentrations of IFN-γ than BALB/cJ mice following MHV-3 infection in vivo (34). Thus, the data in this report provide an explanation, a difference in response to IFN-γ rather than production of IFN-γ.
We have recently shown that following infection with MHV-3, A/J mice generate a Th1 T-helper cell response, known to be associated with the production of IFN-γ (1, 34). Treatment of A/J mice with anti-IFN-γ serum has been reported to lead to loss of resistance to MHV-3 infection (24). Our studies extend these observations by the demonstration that blocking NO production in vivo by treating mice with l-NMMA, a nontoxic competitive inhibitor of l-arginine, also causes loss of resistance of A/J mice to MHV-3 infection, resulting in severe hepatocellular necrosis and fibrin deposition similar to the pathology of susceptible BALB/cJ mice. Thus, in vivo the production of NO is a critical mediator of the antiviral action of IFN-γ, although we have not excluded other possible protective effects of NO, such as vasomotor effects or modulation of T-helper cell differentiation. Treatment with l-NMMA has been shown to increase mortality in another model of fulminant hepatitis induced by corynebacterium-LPS, and it was suggested that endogenous NO production may exert a beneficial vasodilator effect on the hepatic microcirculation (13). Thus, although NO has a direct inhibitory effect on viral replication in macrophages in vitro, it will be important in future studies to determine whether this is the only mechanism by which it exerts its protective effect in vivo. In a model of virus-induced interstitial pneumonitis, production of cytokines such as tumor necrosis factor alpha also accounted for disease, whereas in contrast to the MHV-3 model of fulminant hepatic failure, production of NO provoked and aggravated the interstitial pneumonitis (41). Thus, it is apparent that production of NO is protective in some disease states but deleterious in others.
This study demonstrates the importance of NO production in resistance to fulminant viral hepatitis caused by MHV-3. The correlation between NO production in response to IFN-γ and host resistance to viral hepatitis is of potentially great interest for the elucidation of mechanisms of host resistance/susceptibility to viral pathogens. Furthermore, it should provide for the future development of effective antiviral therapy.
ACKNOWLEDGMENT
This work was supported by group grant PG 11810 from the Medical Research Council of Canada.
REFERENCES
- 1.Chung S, Gorczynski R, Cruz B, Fingerote R, Skamene E, Perlman S, Leibowitz J L, Fung L S, Flowers M, Levy G A. A TH1 helper cell line (3E9.1) from resistant A/J mice inhibits induction of macrophage procoagulant activity (PCA) in vitro and protects against MHV-3 mortality in vivo. Immunology. 1994;83:353–361. [PMC free article] [PubMed] [Google Scholar]
- 2.Croen K. Evidence for an antiviral effect of nitric oxide. Inhibition of herpes simplex type 1 replication. J Clin Invest. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dindzans V J, Skamene E, Levy G A. Susceptibility/resistance to mouse hepatitis virus strain 3 and macrophage procoagulant activity are genetically linked and controlled by two-non-H-2-linked genes. J Immunol. 1986;137:2355–2360. [PubMed] [Google Scholar]
- 4.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 5.Doi T, Ando M, Akaike T, Suga M, Sato K, Maeda H. Resistance to nitric oxide in Mycobacterium aviumcomplex and its implication in pathogenesis. Infect Immun. 1993;61:1980–1989. doi: 10.1128/iai.61.5.1980-1989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans R, Kamdar S J. Stability of RNA isolated from macrophages depends on the removal of an RNA degrading activity early in the extraction procedure. BioTechniques. 1990;8:357–360. [PubMed] [Google Scholar]
- 7.Field L, Dilts R V, Ravichandran R, Lenhert P G, Carnahan G E. An unusually stable thionitrite from N-acetyl-d,l-penicillamine; X-ray crystal and molecular structure of 2-(acetylamino)-2-carboxy-1,1-dimethylethyl thionitrite. J Chem Soc Chem Commun. 1978;1978:249–250. [Google Scholar]
- 8.Flesch I E A, Hess J H, Oswald I P, Kaufmann S H E. Growth inhibition of Mycobacterium bovis by IFN-gamma stimulated macrophages: regulation by endogenous tumor necrosis factor-alpha and by IL-10. Int Immunol. 1994;6:693–700. doi: 10.1093/intimm/6.5.693. [DOI] [PubMed] [Google Scholar]
- 9.Granger D L. Macrophage production of nitrogen oxides in host defence against microorganisms. Res Immunol. 1991;142:570–572. doi: 10.1016/0923-2494(91)90104-q. [DOI] [PubMed] [Google Scholar]
- 10.Green L C, DeLuzuriaga K R, Wagner D A, Rand W, Istfan N, Young V R, Tannenbaum S R. Nitrate biosynthesis in man. Proc Natl Acad Sci USA. 1981;78:7764–7768. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 12.Green S J, Meltzer M S, Hibbs J B, Nacy C A. Activated macrophages destroy Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J Immunol. 1990;144:278–283. [PubMed] [Google Scholar]
- 13.Harbrecht B G, Stadler J, Demetris A J, Simmons R L, Billiar T R. Nitric oxide and prostaglandins interact to prevent hepatic damage during murine endotoxemia. Am J Physiol. 1994;266(6 Pt. 1):G1004–G1010. doi: 10.1152/ajpgi.1994.266.6.G1004. [DOI] [PubMed] [Google Scholar]
- 14.Harris N R, Buller M L, Karupiah G. Gamma-interferon-induced, nitric oxide-mediated inhibition of vaccinia virus replication. J Virol. 1995;69:910–915. doi: 10.1128/jvi.69.2.910-915.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibbs J J, Westenfelder C, Taintor R, Vavrim Z, Kablitz C, Baranowski R L, Ward J H, Menlove R L, McMurry M P, Kushner J P. Evidence for cytokine-inducible nitric oxide synthase from l-arginine in patients receiving interleukin-2 (IL-2) therapy. J Clin Invest. 1992;89:867–877. doi: 10.1172/JCI115666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirano M, Fujiwara K, Hino S, Matumoto M. Replication and plaque formation of mouse hepatitis virus (MHV-2) in mouse cell line DBT culture. Arch Gesamte Virusforsch. 1974;44:298–302. doi: 10.1007/BF01240618. [DOI] [PubMed] [Google Scholar]
- 17.Holmes K V. Coronaviridae and their replication. In: Fields B N, Knipe D M, Chanock R M, Hirsch M S, Melnick J L, Monath T P, Roizman B, editors. Fields virology. New York, N.Y: Raven Press; 1990. pp. 841–856. [Google Scholar]
- 18.Karupiah G, Xie Q, Buller M L, Nathan C, Duarte C, MacMicking J D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 19.Kwon N S, Stuehr D J, Nathan C F. Inhibition of tumor cell ribonucleotide reductase by macrophage-derived nitric oxide. J Exp Med. 1991;174:761–767. doi: 10.1084/jem.174.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lepoivre M, Chenais B, Yapo A, Lemaire G, Thelander L, Tenu J P. Alterations of ribonucleotide reductase activity following induction of the nitrite-generating pathway in adenocarcinoma cells. J Biol Chem. 1990;265:14143–14149. [PubMed] [Google Scholar]
- 21.Levy G A, Leibowitz J L, Edgington T S. Induction of monocyte procoagulant activity by murine hepatitis virus type 3 parallels disease susceptibility in mice. J Exp Med. 1981;154:1150–1163. doi: 10.1084/jem.154.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Fung L S, Crow A, Myers-Mason N, Leibowitz J L, Cole E, Levy G A. Monoclonal anti-prothrombinase (3D4.3) prevents mortality from murine hepatitis virus infection (MHV-3) J Exp Med. 1992;1763:689–697. doi: 10.1084/jem.176.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liew F Y, Li Y, Moss D, Parkinson C, Rogers M V, Moncada S. Resistance to Leishmania major infection correlates with the induction of nitric oxide synthase in murine macrophages. Eur J Immunol. 1991;21:3009–3014. doi: 10.1002/eji.1830211216. [DOI] [PubMed] [Google Scholar]
- 24.Lucchiari M A, Modolell M, Eichmann K, Pereira C A. In vivo depletion of interferon-gamma leads to susceptibility of A/J mice to mouse hepatitis virus 3 infection. Immunobiology. 1992;185:475–482. doi: 10.1016/S0171-2985(11)80089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucchiari M A, Martin J P, Modolell M, Pereira C A. Acquired immunity of A/J mice to mouse hepatitis virus 3 infection: dependence of interferon-gamma synthesis and macrophage sensitivity to interferon-gamma. J Gen Virol. 1991;72:1317–1322. doi: 10.1099/0022-1317-72-6-1317. [DOI] [PubMed] [Google Scholar]
- 26.Lyons C R, Orloff G J, Cunningham J M. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992;267:6370–6374. [PubMed] [Google Scholar]
- 27.Macnaughton M R, Patterson S. Mouse hepatitis virus strain 3 infection of C57, A/Sn and A/J strain mice and their macrophages. Arch Virol. 1980;66:71–75. doi: 10.1007/BF01315046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manuel J, Corradin S B, Buchmuller Rouiller Y. Nitrogen and oxygen metabolites and the killing of Leishmania by activated murine macrophages. Res Immunol. 1991;142:577–579. doi: 10.1016/0923-2494(91)90106-s. [DOI] [PubMed] [Google Scholar]
- 29.Mello I G C, Vassao R C, Pereira C A. Virus specificity of the antiviral state induced by IFN gamma correlates with resistance to MHV-3. Arch Virol. 1993;132:281–289. doi: 10.1007/BF01309539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munoz-Fernandez M A, Fernandez M A, Fresno M. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-alpha and IFN-gamma through a nitric-oxide dependent mechanism. Immunol Lett. 1992;33:35–40. doi: 10.1016/0165-2478(92)90090-b. [DOI] [PubMed] [Google Scholar]
- 31.Nacy C A, Nelson B J, Meltzer M S, Green S J. Cytokines that regulate macrophage production of nitrogen oxides and expression of antileishmanial activities. Res Immunol. 1991;142:573–576. doi: 10.1016/0923-2494(91)90105-r. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura L T, Wu-Hsieh B A, Howard D H. Recombinant murine gamma interferon stimulates macrophages of the RAW cell line to inhibit intracellular growth of Histoplasma capsulatum. Infect Immun. 1994;62:680–684. doi: 10.1128/iai.62.2.680-684.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 34.Ning Q, Brown D, Parodo J, Cattral M, Gorczynski R, Cole E, Fung L S, Ding J W, Liu M F, Rotstein O, Phillips M J, Levy G A. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol. 1997;160:3487–3493. [PubMed] [Google Scholar]
- 35.Pereira C A, Lucchiari M A, Modolell M, Kuhn L, Lefkovits I. An attempt to identify gene products related to the induction of an antiviral state in macrophages resistant and sensitive to IFN-gamma. Res Virol. 1993;144:479–486. doi: 10.1016/s0923-2516(06)80063-4. [DOI] [PubMed] [Google Scholar]
- 36.Pope M, Rotstein O, Cole E, Sinclair S, Parr R, Cruz B, Fingerote R, Chung S, Gorczynski R, Fung L, Leibowitz J, Rao Y S, Levy G. Pattern of disease after murine hepatitis virus strain 3 infection correlates with macrophage activation and not viral replication. J Virol. 1995;69:5252–5260. doi: 10.1128/jvi.69.9.5252-5260.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raschke W C, Baird S, Ralph P, Nakooinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978;15:261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- 38.Stamler J S. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 39.Stenger S, Thuring H, Rollinghoff M, Bogdan C. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J Exp Med. 1994;180:783–793. doi: 10.1084/jem.180.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sturman L S, Takemoto K K. Enhanced growth of a murine coronavirus in transformed mouse cells. Infect Immun. 1972;6:501–507. doi: 10.1128/iai.6.4.501-507.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka K, Nakazawa H, Okada K, Umezawa K, Fukuyama N, Koga Y. Nitric oxide mediates murine cytomegalovirus-associated pneumonitis in lungs that are free of the virus. J Clin Invest. 1997;100:1822–1830. doi: 10.1172/JCI119710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tokunaga K, Nakamura Y, Sakata K, Fujimori K, Ohkubo M, Sawada K, Sakiyana S. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987;47:5616–5619. [PubMed] [Google Scholar]
- 43.Wang Y, Marsden P A. Nitric oxide synthases: biochemical and molecular regulation. Curr Opin Nephrol Hypertens. 1995;4:12–22. doi: 10.1097/00041552-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Weisz A, Oguchi S, Gratiello L, Esumi H. Dual mechanism for the control of inducible-type NO synthase gene expression in macrophages during activation by interferon-gamma and bacterial lipopolysaccharide. Transcriptional and post-transcriptional regulation. J Biol Chem. 1994;269:8324–8333. [PubMed] [Google Scholar]
- 45.Xie Q W, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon-gamma and bacterial lipopolysaccharide. J Exp Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaragoza C, Ocampo C J, Saura M, McMillan A, Lowenstein C J. Nitric oxide inhibition of coxsackievirus replication in vitro. J Clin Invest. 1997;100:1760–1767. doi: 10.1172/JCI119702. [DOI] [PMC free article] [PubMed] [Google Scholar]