Abstract
Objectives
Programmed cell death-ligand 1 inhibitors plus chemotherapy (PD-L1 + Chemo) have achieved substantial progress in extensive-stage small-cell lung cancer (ES-SCLC). However, evidence about programmed cell death 1 inhibitors plus chemotherapy (PD-1 + Chemo) in SCLC is relatively lacking. Whether PD-1 inhibitors differ from PD-L1 inhibitors in their clinical outcomes remains controversial.
Materials and methods
We performed a meta-analysis to compare efficacy and safety of PD-L1 + Chemo vs PD-1 + Chemo in ES-SCLC by searching PubMed, Embase, the Cochrane Library, and major oncology conferences. We examined overall survival (OS) as the primary outcome. Secondary outcomes included progression-free survival (PFS), objective response rate (ORR), and treatment-related adverse events (AEs).
Results
We included four randomized trials (IMpower133, CASPIAN, KEYNOTE-604, and EA5161) with a total of 1553 patients. Direct comparison showed that PD-L1 + Chemo (PFS: hazard ratio [HR] 0.79; OS: HR 0.75) and PD-1 + Chemo (PFS: HR 0.72; OS: HR 0.77) significantly prolonged survival time compared with chemotherapy alone. But PD-L1 + Chemo (relative risk [RR]: 1.07) and PD-1 + Chemo (RR: 1.13) were not superior to chemotherapy alone in terms of ORR. Indirect comparison showed no significant difference in clinical efficacy between PD-L1 + Chemo and PD-1 + Chemo (OS: HR 0.99; PFS: HR 1.10; ORR: RR 0.95). We further stratified patients according to subgroups in terms of OS. In the subgroup of patients with brain metastasis, PD-L1 + Chemo tended to prolong OS (HR: 0.61, 0.28 to 1.32). There were no significant differences between PD-L1 + Chemo and PD-1 + Chemo regarding safety analyses. However, PD-L1 + Chemo exhibited a better safety profile in reducing the risk of treatment discontinuation due to AEs (RR: 0.43, 0.19 to 0.95) and pneumonia (pneumonia of any grade, RR: 0.59, 0.24 to 1.42; pneumonia of grade ≥ 3, RR: 0.37, 0.10 to 1.39).
Conclusions
PD-L1 + Chemo and PD-1 + Chemo provided a significant survival benefit relative to chemotherapy alone for ES-SCLC. The efficacy and safety of PD-L1 + Chemo and PD-1 + Chemo were similar based on current evidence.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03017-z.
Keywords: PD-L1 inhibitors, PD-1 inhibitors, Small-cell lung cancer, First-line therapy, Efficacy
Background
Small-cell lung cancer (SCLC), a devastating carcinoma with rapid proliferation and early widespread metastases, accounts for 13–15% of all lung cancers [1]. The five-year survival rate for patients with extensive-stage SCLC (ES-SCLC) is as low as 1–2% treated with palliative chemotherapy [2]. For the past two decades, etoposide plus either cisplatin or carboplatin has been the standard first-line treatment for patients with ES-SCLC [3–5]. However, most patients rapidly develop resistance to chemotherapy [6], so more efficacious treatments are urgently needed.
The advent of the immunotherapy era provides us with new treatment options. The IMpower133 study shows that atezolizumab plus chemotherapy significantly prolonged overall survival (OS) (HR: 0.76; 95% CI: 0.60 to 0.95) and progression-free survival (PFS) (HR: 0.77; 0.63 to 0.95) compared to chemotherapy alone [7]. The CASPIAN study also demonstrates a synergetic antitumor effect and survival benefits with durvalumab plus chemotherapy [8]. Based on the results of these two studies, programmed cell death-ligand 1 inhibitor plus chemotherapy (PD-L1 + Chemo) has been approved in the USA and recommended by the National Comprehensive Cancer Network clinical practice guidelines as first-line treatment for patients with ES-SCLC (NCCN guidelines, http://www.nccn.org/professionals/). Prior to that, programmed cell death 1 inhibitors plus chemotherapy (PD-1 + Chemo) have shown promising antitumor activity for non-small-cell lung cancer (NSCLC) in several randomized controlled trials (RCTs) [9, 10], but data on PD-1 + Chemo in SCLC are relatively scarce.
The KEYNOTE-604 study has recently reported that pembrolizumab plus chemotherapy statistically improves PFS [11]. Meanwhile, a phase 2 RCT shows that nivolumab plus chemotherapy significantly prolongs both PFS and OS [12]. However, whether PD-L1 inhibitors and PD-1 inhibitors deliver similar efficacy and safety in combination with chemotherapy in ES-SCLC remains controversial.
In this meta-analysis, we aimed to assess the differences between PD-L1 + Chemo and PD-1 + Chemo in first-line treatment for ES-SCLC.
Method
Study eligibility
The PubMed, Embase, Cochrane Library, and major oncology conferences were searched for relevant studies, with main subject terms of “pembrolizumab” or “nivolumab” or “atezolizumab” or “durvalumab” or “tremelimumab” or “avelumab” or “immune checkpoint inhibitor” or “immune therapy” or “immunotherapy” or “programmed cell death protein-1” or “programmed cell death-ligand 1” or “PD-1” or “PD-L1,” and “small-cell lung cancer” and “randomized controlled trial.” The specific search strategy is shown in Supplemental Method. The major oncology conferences included the American Society of Clinical Oncology, the European Society of Medical Oncology, the American Association for Cancer Research, and the World Conference on Lung Cancer. Study selection was independently conducted by two investigators, and the decision was to be judged by the third investigators if there were any disagreements during the process.
Quality assessment
We assessed the risk of bias for the included RCTs (Supplementary Table 1). The specific criteria were as follows: (1) sequence generation, (2) allocation concealment, (3) blinding, (4) incomplete outcome data, (5) selective reporting, and (6) other source of bias.
Data extraction
The following information was extracted from each study to a standard sheet: (1) trial details (study ID, patient characteristics, treatment regimen, etc.); (2) the outcomes, namely OS, PFS, objective response rate (ORR), and adverse events (AEs). We extracted the hazard ratio (HR) and its 95% confidence intervals (CIs) for OS and PFS, and the relative risk (RR) for ORR and AEs.
Statistical analysis
We conducted direct comparison between immune checkpoint inhibitors (ICIs) plus chemotherapy and chemotherapy alone, and indirect comparisons between PD-L1 + Chemo and PD-1 + Chemo. In direct comparisons, the HRs of PFS and OS were pooled using the inverse-variance weighted method, while dichotomous data regarding ORR and AEs were pooled with the RR, 95% CIs and P values using the MantelHaenszel method. Statistical heterogeneity was determined by the χ2 test and I2 statistic: If I 2 < 50% or P > 0.10 in the χ2 test indicated that significant heterogeneity did not exist, we used the fixed-effects model; otherwise, we used the random-effects model. In indirect comparisons, we used frequentist methods with the following formula [13]: log HRAB = log HRAC˗log HRBC, and its standard error (SE) for the log HR was . RR is calculated in a similar way. All statistical analyses were conducted using STATA (version 16.0).
Result
Characteristics of the eligible studies
This study included a total of four RCTs: Two explored the efficacy of anti-PD-L1 (atezolizumab or durvalumab) plus chemotherapy versus chemotherapy alone, and the other two explored the efficacy of anti-PD-1 (pembrolizumab or nivolumab) plus chemotherapy versus chemotherapy alone. The process of trial selection is shown in Supplementary Fig. 1. The main characteristics of the included RCTs and their outcomes are summarized in Table 1.
Table 1.
Characteristics of patients and outcomes of included trials
| Trial name | Arm | N | Median age | Males (%) | Smoke (%) | ECOG1 (%) | Brain metastases (%) | ORR (%) | Follow-up (m) | OS (m) | PFS (m) | HR for PFS 95%CI | HR for OS 95%CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMpower133 | Atezolizumab + chemotherapy | 201 | 64 | 64.2 | 95.5 | 63.7 | 8.5 | 60 | 22.9 | 12.3 | 5.2 | 0.77 (0.63–0.95) | 0.76 (0.60–0.95) |
| chemotherapy | 202 | 64 | 65.3 | 98.5 | 66.8 | 8.9 | 64 | 22.9 | 10.3 | 4.3 | Ref | Ref | |
| CASPIAN | Durvalumab + chemotherapy | 268 | 62 | 71 | 92 | 63 | 10 | 68 | 25.1 | 12.9 | 5.1 | 0.80 (0.66–0.96) | 0.75 (0.62–0.91) |
| chemotherapy | 269 | 63 | 68 | 94 | 67 | 10 | 58 | 25.1 | 10.5 | 5.4 | Ref | Ref | |
| KEYNOTE-604 | Pembrolizumab + chemotherapy | 228 | 64 | 66.7 | 96.5 | 73.7 | 14.5 | 71 | 10.8 | 4.5 | 0.75 (0.61–0.91) | 0.80 (0.64–0.98) | |
| chemotherapy | 225 | 65 | 63.1 | 96.4 | 75.1 | 9.8 | 62 | 9.7 | 4.3 | Ref | Ref | ||
| EA5161 | Nivolumab + chemotherapy | 80 | 52 | 11.3 | 5.5 | 0.65 (0.46–0.91) | 0.67 (0.46–0.98) | ||||||
| chemotherapy | 80 | 48 | 8.5 | 4.6 | Ref | Ref |
N Number; ECOG Eastern Cooperative Oncology Group; ORR Objective response rate; HR Hazard ratio; PFS Progression-free survival; OS Overall survival; Ref: Reference
Direct comparisons of PD-L1/PD-1 inhibitors plus chemotherapy versus chemotherapy alone
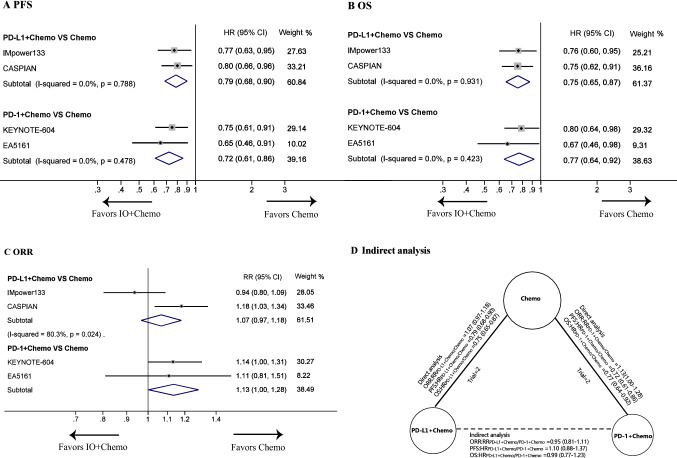
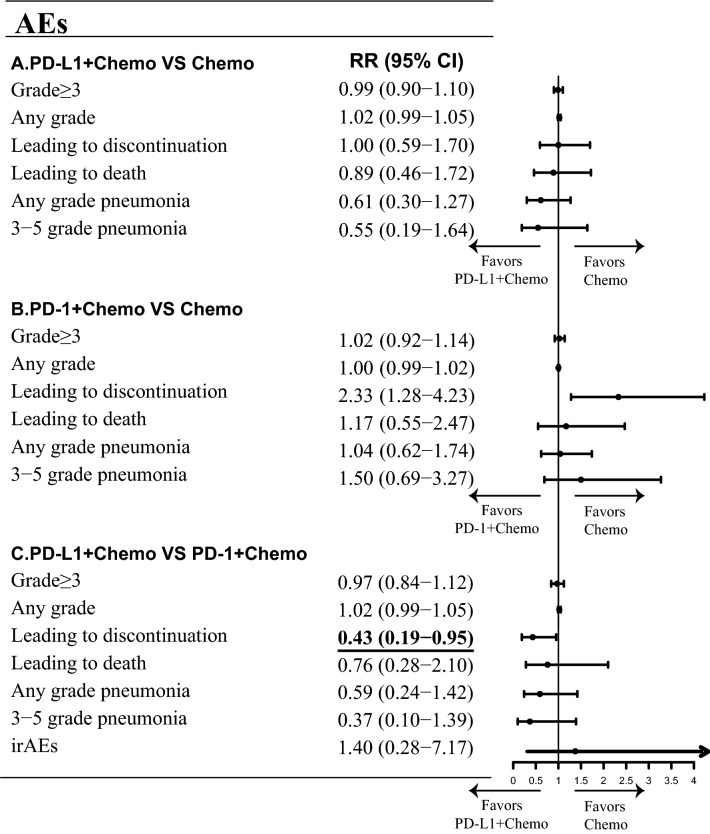
In direct comparisons, patients receiving PD-L1 + Chemo exhibited significantly longer PFS (HRPD-L1+Chemo/Chemo: 0.79, 95% CI: 0.68 to 0.90) and OS (HRPD-L1+Chemo/Chemo: 0.75, 0.65 to 0.87) than patients receiving chemotherapy alone. Similar improvements of PFS (HRPD-1+Chemo/Chemo: 0.72, 0.61 to 0.86) and OS (HRPD-1+Chemo/Chemo: 0.77, 0.64 to 0.92) were observed with PD-1 + Chemo relative to chemotherapy alone (Figs. 1a and 1b). However, no significant difference in ORR was found when a PD-L1 inhibitor (RRPD-L1+Chemo/Chemo: 1.07, 0.97 to 1.18) or a PD-1 inhibitor (RRPD-1+Chemo/Chemo: 1.13, 1.00 to 1.28) was added to chemotherapy (Fig. 1c). In safety analyses, the frequency of treatment discontinuation due to adverse reactions was increased by adding a PD-1 inhibitor to chemotherapy (RRPD-1+Chemo/Chemo: 2.33, 1.28 to 4.23) but not by adding a PD-L1 inhibitor (RRPD-L1+Chemo/Chemo: 1.00, 0.59 to 1.70). However, relative to chemotherapy, neither PD-1 + Chemo nor PD-L1 + Chemo was associated with significant differences in the frequency of AEs of any grade, AEs of grade ≥ 3, and treatment-related death (Figs. 2a and 2b).
Fig. 1.
Direct and indirect comparisons between PD-L1 inhibitors plus chemotherapy (PD-L1 + Chemo) or PD-1 inhibitors plus chemotherapy (PD-1 + Chemo) with chemotherapy alone. Figure a, b, and c showed the forest plot of hazard ratios (HRs) and risk ratios (RRs) directly comparing progression-free survival (PFS), overall survival (OS), and objective response rate (ORR) between PD-L1 + Chemo or PD-1 + Chemo with chemotherapy. The solid lines of Figure d represent the existence of direct comparisons between the treatments, whereas the dashed line represents the indirect comparison between PD-L1 + Chemo versus PD-1 + Chemo. The size of the circle corresponds to the number of enrolled patients. The horizontal line crossing the square represents the 95% CI. The diamonds represent the estimated overall effect, based on the meta-analysis. All statistical tests were two-sided. Abbreviations: IO, immuno-oncology; PD-L1, programmed cell death-ligand 1; PD-1, programmed cell death 1; Chemo, chemotherapy; HR, hazard ratio; RRs, risk ratios; CI, confidence interval
Fig. 2.
Direct and indirect comparisons of safety among PD-L1 + Chemo, PD-1 + Chemo, and chemotherapy. The forest plots of risk ratios (RRs) for safety comparing PD-L1 + Chemo with chemotherapy, PD-1 + Chemo with chemotherapy, and PD-L1 + Chemo with PD-1 + Chemo were shown in Figure a, b, and c, individually. The horizontal line crossing the dot represents the 95% CI. All statistical tests were two-sided. Abbreviations: PD-L1, programmed cell death-ligand 1; PD-1, programmed cell death 1; Chemo, chemotherapy; AEs, adverse events; irAEs, immune-related adverse events; RR, risk ratio
Indirect comparisons between PD-L1 + chemo versus PD-1 + chemo
Indirect comparisons showed that PD-L1 + Chemo was comparable with PD-1 + Chemo in terms of OS (HRPD-L1+Chemo/ PD-1+Chemo: 0.99, 0.77 to 1.23), PFS (HRPD-L1+Chemo/ PD-1+Chemo: 1.10, 0.88 to 1.37), and ORR (RRPD-L1+Chemo/ PD-1+Chemo: 0.95, 0.81 to 1.11) (Fig. 1d). Analyses of AEs suggested that the overall incidence of toxicity was similar between PD-L1 + Chemo and PD-1 + Chemo (Fig. 2c). However, the rate of drug discontinuation due to AEs was lower in patients receiving PD-L1 + Chemo (RRPD-L1+Chemo/ PD-1+Chemo: 0.43, 0.19 to 0.95). A trend toward reduced risk of pneumonia was also observed in the PD-L1 + Chemo group (pneumonia of any grade, RRPD-L1+Chemo/ PD-1+Chemo: 0.59, 0.24 to 1.42; pneumonia of grade ≥ 3, RRPD-L1+Chemo/ PD-1+Chemo: 0.37, 0.10 to 1.39).
Subgroup analysis for OS
Subgroup data were available in three studies: IMpower133, CASPIAN, and KEYNOTE-604. Analyses by gender, age, performance status, and brain metastasis status are shown in Fig. 3.
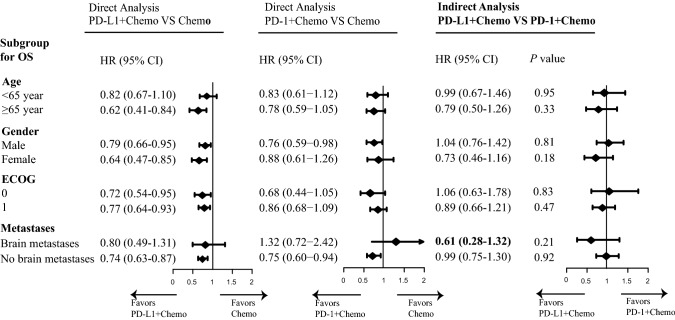
Fig. 3.
Subgroup analyses for overall survival in treatment of extensive-stage small-cell lung cancer. Subgroups including gender, age, performance status, and brain metastasis status were analyzed. The forest plots of hazard ratios (HRs) for OS comparing PD-L1 + Chemo with chemotherapy, PD-1 + Chemo with chemotherapy, and PD-L1 + Chemo with PD-1 + Chemo were shown in the left, middle, and right column, individually. The horizontal line crossing the dot represents the 95% CI. All statistical tests were two-sided. Abbreviations: PD-L1, programmed cell death-ligand 1; PD-1, programmed cell death 1; Chemo, chemotherapy; HR, hazard ratio
When compared PD-L1 + Chemo or PD-1 + Chemo with chemotherapy, a consistent OS benefit was seen across most of the specified subgroups except for the subgroup based on brain metastasis status at baseline. For patients without brain metastases, both PD-L1 + Chemo (HRPD-L1+Chemo/ Chemo: 0.74, 0.63 to 0.87) and PD-1 + Chemo (HRPD-1+Chemo/ Chemo: 0.75, 0.60 to 0.94) were superior to chemotherapy. For patients with brain metastases, PD-L1 + Chemo revealed a superior trend (HRPD-L1+Chemo/ Chemo: 0.80, 0.49 to 1.31) while PD-1 + Chemo revealed an inferior trend (HRPD-1+Chemo/ Chemo: 1.32, 0.72 to 2.42) relative to chemotherapy alone. Thus, PD-L1 + Chemo tended to reduce the risk of death relative to PD-1 + Chemo (HRPD-L1+Chemo/ PD-1+Chemo: 0.61; 0.28 to 1.32).
Discussion
To our knowledge, this is the first meta-analysis to specifically investigate the differences between PD-L1 + Chemo and PD-1 + Chemo in patients with ES-SCLC. The study demonstrated that PD-L1 + Chemo is comparable to PD-1 + Chemo in terms of OS, PFS, and ORR as first-line treatment for ES-SCLC. However, PD-L1 + Chemo revealed an advantage in the safety profile, with a significantly lower risk of treatment discontinuation caused by AEs, as well as a numerically lower risk of pneumonia compared with PD-1 + Chemo.
Differences between the clinical performance of PD-L1 inhibitors and PD-1 inhibitors have been reported in several studies. A published meta-analysis has shown that the risk of death is significantly reduced for patients treated with PD-1 + Chemo compared with PD-L1 + Chemo in NSCLC (HRPD-1+Chemo/ PD-L1+Chemo: 0.66, 0.48 to 0.90) [14]. The underlying mechanism remains to be fully elucidated but one possible reason may be that the interaction of PD-1 and PD-L2 may also inhibit the activation of T cells. And as we know, the PD-1 inhibitor could block the binding of PD-1 to both PD-L1 and PD-L2, while the PD-L1 inhibitor could inhibit only the binding of PD-1 to PD-L1. Thus, the tumor might escape antitumor immune response through the PD-1/PD-L2 axis when being treated with PD-L1 inhibitor [15]. However, for SCLC, our results showed no significant difference between PD-L1 + Chemo and PD-1 + Chemo in terms of OS (HRPD-L1+Chemo/ PD-1+Chemo: 0.99, 0.77 to 1.23) and PFS (HRPD-L1+Chemo/ PD-1+Chemo: 1.10, 0.88 to 1.37). And the results were consistent with another analysis performed by other method (Bayesian approach) [16]. The potential explanation could be that SCLC and NSCLC differ in their immune microenvironment, e.g., PD-L1 expression is typically low or absent in SCLC [17]. Another possible explanation is that PD-L1 binds two receptors, PD-1 and B7.1 (CD80), and B7.1 on tumor-associated dendritic cells (DCs) is a key costimulatory molecule to enhance T cell priming by B7.1/CD28 interaction. Thus, PD-L1 inhibitors play more roles than PD-1 inhibitors to relieve B7.1 by blocking PD-L1 on DCs, which further reinvigorates DCs function and help initiate anticancer T cell immunity [18].
However, the underlying mechanisms of differences between PD-L1 inhibitors and PD-1 inhibitors in SCLC remain to be investigated with well-defined animal models. Currently, it is clinically relevant to assess predictive biomarkers for PD-L1 inhibitors versus PD-1 inhibitors when combined with chemotherapy in SCLC. Although expression of PD-L1 has been considered as a potential predictive biomarker of response to ICIs in various cancer types, the reliability of PD-L1 expression in predicting efficacy of PD-L1/PD-1 + Chemo in SCLC is poor [7, 11]. For one reason, the biopsy samples from SCLC are often small and dominated by necrotic area. In the data of PD-L1 expression form study IMpower133, only 137 had evaluable tissue material in the all 403 patients enrolled, which reflected the difficulties in obtaining biopsy material [7]. For another reason, PD-L1 in SCLC is mainly expressed on tumor-infiltrating immune cell (IC), but not tumor cell (TC), which is different from NSCLC [19]. These suggest that PD-L1 expression may not be a perfect predictive biomarker for SCLC patients receiving PD-L1 + Chemo or PD-1 + Chemo. Tumor mutational burden (TMB) is another characteristic of the immune microenvironment for SCLC. However, among the four trials enrolled, the predictive value of TMB was only analyzed in IMpower133 and could not be pooled. More data are warranted to unveil the value of blood TMB or tissue TMB in SCLC. Other biomarkers, including but not limited to tumor microenvironment, gut microbiomics, and host immune status should be also studied in the future.
In subgroup analysis, there was a tendency to reduce the risk of death in patients with brain metastases for receiving PD-L1 + Chemo than PD-1 + Chemo (HRPD-L1+Chemo/ PD-1+Chemo: 0.61; 0.28 to 1.32). However, the result should be interpreted with caution because of the small population size for this subgroup in KEYNOTE-604. While the mechanisms behind the difference performance of PD-1 inhibitors versus PD-L1 inhibitors in patients with brain metastasis are unknown. Essential steps are urgently needed to explore the mechanism from the following aspects, including a prospective study to confirm this finding; more knowledge about the tumor microenvironment of brain metastases in SCLC; the difference of the ability to penetrate the bloodbrain barrier (BBB) between PD-L1 and PD-1 inhibitors.
As for safety, although there were no significant differences between PD-L1 + Chemo and PD-1 + Chemo for AEs of any grade, receiving PD-L1 + Chemo was associated with lower risk of treatment-related drug discontinuation. This might be related to the ability PD-L1 inhibitors of preserving normal immunological homoeostasis by retaining its programmed cell death-ligand 2. A previous study also revealed the best safety profile of atezolizumab in various cancers [20]. And the result suggests that PD-L1 + Chemo might have an advantage in reducing specific AEs such as pneumonia. Our results revealed that PD-L1 + Chemo tended to reduce incidence of pneumonia, compared with PD-1 + Chemo (pneumonia of any grade, RRPD-L1+Chemo/ PD-1+Chemo: 0.59, 0.24 to 1.42; pneumonia of grade ≥ 3, RRPD-L1+Chemo/ PD-1+Chemo: 0.37, 0.10 to 1.39). This was consistent with the previous finding that PD-1 inhibitors may increase the rate of pneumonia with NSCLC patients compared with PD-L1 inhibitors [21].
Although our study demonstrated that the clinical effects of PD-1 + Chemo and PD-L1 + Chemo were similar, the use of PD-1 + Chemo in the first-line treatment for ES-SCLC should be undertaken with caution for two reasons: In the EA5161 study, OS was a secondary endpoint, which does not meet the standard approved by the US Food and Drug Administration, and there has been no prospective head-to-head comparison between PD-L1 + Chemo and PD-1 + Chemo. Unfortunately, such a prospective study will probably not be done, which makes this indirect comparison important to meet current clinical requirements. The results of our study provide evidence to support the treatment choice for the application of PD-1 + Chemo in SCLC.
In conclusion, based on the present limited data, this study indicated that PD-L1 + Chemo and PD-1 + Chemo had no statistically significant differences in OS, PFS, and ORR for ES-SCLC. However, PD-L1 + Chemo exhibited a statistically better safety profile in reducing the risk of treatment discontinuation due to AEs and pneumonia.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- AEs
Adverse events
- BBB
Blood-brain barrier
- Chemo
Chemotherapy
- CI
Confidence interval
- DCs
Dendritic cells
- ECOG
Eastern Cooperative Oncology Group
- ESMO
European Society of Medical Oncology
- HRs
Hazard ratios
- IC
Immune cell
- ICIs
Immune checkpoint inhibitors
- irAEs
Immune-related adverse events
- NCCN
National Comprehensive Cancer Network
- NSCLC
Non-small-cell lung carcinoma
- ORR
Objective response rate
- OS
Overall survival
- PD-1
Programmed cell death 1
- PD-L1
Programmed cell death-ligand 1
- PFS
Progression-free survival
- RR
Relative risk
- SE
Standard error
- SCLC
Small-cell lung cancer
- TC
Tumor cell
- TMB
Tumor mutational burden
Author contributions
H.Y., P.C., and XY.C. contributed to data acquisition, data interpretation, and statistical analysis and drafting of the manuscript. C.C., XY.Z., and LN.H. contributed to data acquisition, data interpretation, and statistical analysis. YX.Z., SD.H., and B.Z. contributed to the study design, data acquisition, data interpretation, and statistical analysis. All the authors contributed to critical revision of the manuscript.
Funding
This study was funded by grants 81903176 from the National Natural Science Funds of China; 2019A1515011596 from the Science and Technology Program of Guangdong Province. C2019110 from Medical Scientific Research Foundation of Guangdong Province. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data availability
All data generated or analyzed during this study are included in the published article.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Précis: Our work confirmed that PD-L1+Chemo was not superior to PD-1+Chemo in terms of OS, PFS and ORR. However, PD-L1+Chemo tended to prolong OS for patients with brain metastases and exhibited a better safety profile relative to PD-1+Chemo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hui Yu, Ping Chen and Xiuyu Cai have contributed equally.
Contributor Information
Yixin Zhou, Email: zhouyx@sysucc.org.cn.
Shaodong Hong, Email: hongshd@sysucc.org.cn.
Bei Zhang, Email: zhangbei@sysucc.org.cn.
References
- 1.Oronsky B, Reid T, Oronsky A, Carter C. What's new in SCLC? a review. Neoplasia (New York, NY) 2017;19(10):842–847. doi: 10.1016/j.neo.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lally B, Urbanic J, Blackstock A, Miller A, Perry M. Small cell lung cancer: have we made any progress over the last 25 years? Oncologist. 2007;12(9):1096–1104. doi: 10.1634/theoncologist.12-9-1096. [DOI] [PubMed] [Google Scholar]
- 3.Stahel R, Thatcher N, Früh M, Le Péchoux C, Postmus P, Sorensen J, Felip E. 1st ESMO consensus conference in lung cancer; lugano 2010: small-cell lung cancer. Ann Oncol: Off J Eur Soc Med Oncol. 2011;22(9):1973–1980. doi: 10.1093/annonc/mdr313. [DOI] [PubMed] [Google Scholar]
- 4.Früh M, De Ruysscher D, Popat S, Crinò L, Peters S, Felip E. Small-cell lung cancer (SCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol : Off J Eur Soc Med Oncol. 2013;24:vi99–vi105. doi: 10.1093/annonc/mdt178. [DOI] [PubMed] [Google Scholar]
- 5.Kalemkerian G, Loo B, Akerley W, Attia A, Bassetti M, Boumber Y, Decker R, Dobelbower M, Dowlati A, Downey R, et al. NCCN guidelines insights: small cell lung cancer, version 2.2018. J Natl Compr Cancer Netw : JNCCN. 2018;16(10):1171–1182. doi: 10.6004/jnccn.2018.0079. [DOI] [PubMed] [Google Scholar]
- 6.Farago A, Keane F. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. 2018;7(1):69–79. doi: 10.21037/tlcr.2018.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S, Reck M, Mansfield A, Mok T, Scherpereel A, Reinmuth N, Garassino M, De Castro CJ, Califano R, Nishio M, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133) J Clin Oncol : Off J Am Soc Clin Oncol. 2021;39(6):619–630. doi: 10.1200/JCO.20.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair M, Özgüroğlu M, Ji J, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet (London, England) 2019;394(10212):1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair M, Powell S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 10.Langer C, Gadgeel S, Borghaei H, Papadimitrakopoulou V, Patnaik A, Powell S, Gentzler R, Martins R, Stevenson J, Jalal S, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudin C, Awad M, Navarro A, Gottfried M, Peters S, Csőszi T, Cheema P, Rodriguez-Abreu D, Wollner M, Yang J, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol : Off J Am Soc Clin Oncol. 2020;38(21):2369–2379. doi: 10.1200/JCO.20.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randomized phase II clinical trial of cisplatin/carboplatin and etoposide (CE) alone or in combination with nivolumab as frontline therapy for extensive-stage small cell lung cancer (ES-SCLC): ECOG-ACRIN EA5161 [https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.9000]
- 13.Bucher H, Guyatt G, Griffith L, Walter S. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691. doi: 10.1016/S0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 14.Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, Zhao Z, Zhao J, Chen S, Song J, et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: a systematic review and meta-analysis. JAMA Oncol. 2020;6(3):375–384. doi: 10.1001/jamaoncol.2019.5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Investig. 2015;125(9):3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ando K, Manabe R, Kishino Y, Kusumoto S, Yamaoka T, Tanaka A, Ohmori T, Ohnishi T, Sagara H. Comparative efficacy and safety of immunotherapeutic regimens with PD-1/PD-L1 inhibitors for previously untreated extensive-stage small cell lung cancer: a systematic review and network meta-analysis. Curr Oncol (Toronto, Ont) 2021;28(2):1094–1113. doi: 10.3390/curroncol28020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iams W, Porter J, Horn L. Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol. 2020;17(5):300–312. doi: 10.1038/s41571-019-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayoux M, Roller A, Pulko V, Sammicheli S, Chen S, Sum E, Jost C, Fransen M, Buser R, Kowanetz M, et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci Transl Med. 2020;12(534):eaav7431. doi: 10.1126/scitranslmed.aav7431. [DOI] [PubMed] [Google Scholar]
- 19.Kowanetz M, Zou W, Gettinger S, Koeppen H, Kockx M, Schmid P, Kadel E, Wistuba I, Chaft J, Rizvi N, et al. Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1) Proc Natl Acad Sci USA. 2018;115(43):E10119–E10126. doi: 10.1073/pnas.1802166115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu C, Chen Y, Du X, Liu J, Huang C, Chen L, Zhou G, Li W, Mao Y, Hsu C, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ (Clinical research ed) 2018;363:k4226. doi: 10.1136/bmj.k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khunger M, Rakshit S, Pasupuleti V, Hernandez A, Mazzone P, Stevenson J, Pennell N, Velcheti V. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152(2):271–281. doi: 10.1016/j.chest.2017.04.177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in the published article.