Abstract
While natural killer (NK) cells are essential players in detection and elimination of malignant cells, these surveillance properties can be compromised by cancer cells. Since NK cell education primarily occurs in the bone marrow and lymphoid tissue, this process might be particularly affected by their infiltration with lymphoma cells. This study aimed to explore functional properties of diffuse large B-cell lymphoma (DLBCL) patient NK cells, which could potentially promote tumour immune evasion and disease propagation.
NK cells isolated from the peripheral blood (PB) of 26 DLBCL patients and 13 age-matched healthy controls (HC) were analysed.
The cytotoxic CD56dim subtype was the only one identified in patients. Compared to HC, patient cells demonstrated low levels of inhibitory CD158a/b along with decreased expression of activating NKG2D and CD161 and increased inhibitory NKG2A levels.
Patient NK cell cytotoxic activity was impaired, as were their degranulation and inflammatory cytokine production, which partially recovered following non-receptor-dependant stimulation.
The phenotypically skewed and restricted population of patient NK cells, along with their blunted cytotoxic and immune-regulatory activity, appear to be driven by exposure to lymphoma environment. These NK cell functional aberrations could support lymphoma immune evasion and should be considered in the era of cellular therapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03284-4.
Keyword: Natural killer cells, Diffuse large B-cell lymphoma, Immune evasion, Cytotoxicity, Inflammatory cytokines
Introduction
Natural killer (NK) cells, characterized by strong cytolytic responses and significant ability to secrete cytokines and chemokines, are key constituents of the innate immune surveillance system against cancer. The quality of NK cell responses depends on the cytokine microenvironment, as well as interactions with other cells of the immune system [1], and their effector function is regulated by a wide repertoire of activating and inhibitory receptors. Killer cell immunoglobulin-like receptors (KIRs), C-type lectin receptors (CLRs) and natural cytotoxicity receptors (NCRs) constitute the three major receptor families which, along with supporting co-receptors (Suppl. Table 1), are critical for NK activation [2].
The CD56bright mature NK cell subset is characterized by stronger inflammatory cytokine production and attenuated natural cytotoxicity potential, whereas the CD56dim subset represents terminally mature NK cells, that are more cytotoxic, express high surface density of KIR and have a strong antibody-dependent cellular cytotoxicity (ADCC) potential, which is related to their high expression of the FcγRIII (CD16) [3–5]. CD56bright cells are shown to comprise 25% of bone marrow (BM) mature NK cells, decreasing to approximately 10% in the peripheral blood (PB) [6]. NK cells are known to be capable of identifying changes occurring in transformed malignant cells [7], and their active involvement in the control of tumour propagation has been reported [8–10]. In a population-based cohort study, high cytotoxic activity of PB-NK cells has been found to be associated with reduced cancer risk [11].
Tumour escape from NK immune surveillance nonetheless occurs, through either the loss of NK activation pathways, or the acquisition of inhibitory signals [12]. Studies in solid tumours have demonstrated decreased NK activity, impaired perforin-dependent cytotoxicity, and aberrant expression of activating and inhibitory receptors on the NK cell surface [13, 14]. In acute myeloid leukaemia (AML), patient NK cells display low NCR surface density [15] and weak cytolytic activity in vitro [16, 17]. Moreover, over-expression of the inhibitory receptor NKG2A and reduced production of tumour necrosis factor α (TNFα) are associated with remission failure [17, 18]. Impaired NK cell function and low expression of activating receptors are also reported in myelodysplastic syndrome and multiple myeloma [19–21]. Lymphoproliferative disorders (LPDs) are of special interest in terms of malignancy-induced NK-cell modifications, since the major site of NK cell education, the lymphoid tissue, is infiltrated with lymphoma cells. While PB-NK cell properties could provide accurate information on the tumour-infiltrating counterpart, data regarding NK cell composition and functions in lymphoma are somewhat limited. In a key study by Danielou-Lazareth et al., PB-NK cells of DLBCL patients have demonstrated impaired ADCC as well as differences in their composition and surface receptor expression compared to those observed in healthy controls [22]. Available evidence in LPDs, particularly in diffuse large B-cell lymphoma (DLBCL), points to a correlation between a low percentage of PB-NK cells and a poor disease prognosis [23–25].
Given that major advances in the modern therapy of DLBCL are of immune-based nature [e.g. chimeric antigen receptor T cells (CAR-T cells); bispecific T-cell engagers, etc.], better understanding of lymphoma-driven mechanisms regulating the escape from immune surveillance is of particular importance. Accordingly, the objective of this study has been to explore potential NK cell aberrations in DLBCL patient PB that could compromise their role in immune surveillance, thus contributing to lymphoma cell escape.
Materials and methods
Samples
PB samples were obtained from newly diagnosed DLBCL patients at staging and from healthy controls (HC). Additionally, patient BM staging samples were included in the evaluation. The study was approved by the Rambam Institutional Review Board (approval #0114-17RMB). All participants signed the informed consent form. DLBCL patients whose disease was suspected to be transformed from follicular lymphoma (FL) or chronic lymphocytic leukaemia were excluded from the analysis. Patients with active infectious or autoimmune inflammatory diseases were also excluded.
NK cell isolation
NK cells were isolated from whole blood samples of HC and DLBCL patients by immunomagnetic negative selection using the EasySep™ Direct Human NK Cell Isolation kit according to the manufacturer’s protocol. The number and viability of cells were determined using a Neaubauer haemocytometer in conjunction with Trypan Blue exclusion assay. NK cell purity (CD3-/CD19-/CD56 + cells) was determined by flow cytometry in the separated cell pellet.
K562 cell line
K652, a human chronic myelogenous leukaemia human leukocyte antigen (HLA) null cell line [American Type Culture Collection (ATCC) reference CCL-243], served as an NK cell stimulus in the experiments. The line expresses ligands for activating NK receptors and is commonly used in co-cultures to activate NK cells [26–28].
FACS analysis
Data acquisition was performed in a BD FACSCanto™ II flow cytometer using FACSDiva™ software version 9.0 program (BD). Multi-parameter flow cytometry analysis of the expression pattern of NK cell receptors was performed with the Infinicyt™ software.
Antibodies
CD2-V450 BD, CD3-APC BD, CD3-PerCP-Cy5.5 BD, CD7-FITC BD, CD16-V450 BD, CD19-APC-H7 BD, CD45-V500 BD, CD56-Pe-Cy7 BD, CD57-FITC BD, CD94-APC BD, CD107a-FITC BioLegend, CD158a-APC BioLegend, CD158b-PE BioLegend, CD159a-PE BioLegend, CD161-FITC BioLegend, CD226-APC BioLegend, CD244-PE BD, CD314-FITC BioLegend, CD335-APC BioLegend, CD336-PE BioLegend, CD337-APC BioLegend, Granzyme b-FITC Fitzgerald, Perforin-FITC BD, TNFα-PE Beckman Coulter, INFγ-APC Invitrogen.
Staining protocols
Immunofluorescence surface staining of whole blood samples was performed using a lyse wash procedure according to the manufacturer's protocol. Combined staining for surface and intracellular antigens was performed using the Fix & Perm reagent kit according to the manufacturer’s instructions. A minimum of 2 × 105 events was acquired for each staining tube.
NK cell receptor phenotype
Immunophenotypic characterization of PB-NK cell receptors was performed using a panel of 8-color combinations of fluorochrome-conjugated monoclonal antibodies (MoAbs). For each sample, the percentage and mean fluorescent intensity (MFI) of total CD56 positive cells, CD56bright cells and CD56dim cells were analysed. The expression of each receptor was assessed on both dim and bright CD56-positive NK populations. MFI was expressed in arbitrary relative linear units scaled from 0 to 104.
Functional NK cell assays
Degranulation capacity and inflammatory cytokine production were utilized as markers of NK cell functionality. Both were assessed on patient and HC cells following activation by either the K562 cell line or a cocktail of phorbol 12-myristate 13-acetate (PMA) and ionomycin.
Degranulation assay
CD107a expression was measured as a marker for NK cell degranulation. Surface CD107a expression reflects merging of NK cell lytic granules, coated with this protein, with the cell membrane [27, 28].
Isolated PB-NK cells of patients and HC were plated in 96-well U-bottom plates at a concentration of 1.5–7 × 105 cells/ml in the presence of anti-CD107a FITC conjugated MoAbs. K562 target cells (0.3–1.2 × 105 cell/ml) alone, or in combination with IL-2 (final concentration of 100 U/ml; Invitrogen) were added to the plated NK cells (effector cells) and incubated at an effector/target (E:T) ratio 5:1 for 1 h at 37 °C in 5% CO2. PMA (50 nM) and ionomycin (1 µM) stimulated NK cells served as a positive control, whereas NK cells in conditioned medium [RPMI 1640 (Sigma), containing 10% FBS (Biological Industries), 2 mM l-glutamine (Biological Industries) and 1 µg/ml penicillin–streptomycin (Biological Industries), served as negative control. To prevent cytokine-containing vesicle exocytosis and avoid CD107a cellular internalization, following a 1-h incubation, brefeldin A (0.5-1 µM) and monensin (2-3 µM) were added to the aforementioned cell cultures and incubated under the same conditions for additional 4 h. At the end of incubation, NK cells were harvested, washed, and analysed by FACS.
Cytokine generation assay
Levels of interferon γ (IFNγ) and TNFα were measured intracellularly by FACS following the described-above incubation protocol. Upon 5-h incubation, NK cells were washed and stained with surface CD56 and CD45 antibodies, as well as with intracellular INFγ and TNFα antibodies according to the manufacturer's protocol.
NK cell cytotoxicity assay
A co-culture of NK cells with susceptible K652 cells at various E:T ratios was applied to assess cell-mediated cytotoxicity. Carboxyfluorescein succinimidyl ester (CFSE) was used to track K562 cells, with labelling performed according to the manufacturer's instructions. NK cells were co-incubated with K562 cells in 96-well U-bottom plates, at 5:1 through 0.625:1 E:T ratios. IL-2 (100 U/ml) was added to the 5:1 ratio co-culture to enhance NK activity. Target cells in the absence of effectors were used as negative controls, whereas 0.1% of Tween-20 detergent (Sigma) served as a positive control.
Following co-culture, K652 cells were stained with 7-amino-actinomycin D (7-AAD) to quantify the cells that had undergone lysis.
Cytotoxic activity was expressed as % specific lysis calculated with the following formula: %Spesific Lysis = 100 × (%Sample lysis − %Basal lysis)/ (100 − %Basal lysis). The term “sample lysis” refers to the percentage of cell lysis in the presence of NK cells at a given E:T ratio.
Statistical analysis
Descriptive statistics in terms of mean, standard deviation, median, and percentiles were used to evaluate the study parameters. The normal distribution of the quantitative parameters was assessed by the Kolmogorov–Smirnov test. Mann–Whitney U test with multiple comparisons was used for analysing differences between mature NK cells from DLBCL patients and HC. Two-tailed P < 0.05 was considered statistically significant.
Results
NK cells, derived from the PB of 26 newly-diagnosed DLBCL patients and 13 age-matched HCs, were analysed. Patient characteristics, including demographics, disease stage, International Prognostic Index (IPI), cell of origin [germinal centre B-cell like (GCB) versus non-GCB], BM involvement, LDH, β2-microglobulin, response to therapy and post-remission relapse rate, are detailed in Table 1.
Table 1.
Demographic and clinical characteristics of 26 DLBCL patients and 13 healthy controls
| DLBCL | HC | P-value | |
|---|---|---|---|
| N. patients | 26 | 13 | |
| Age, median (range) | 61 (30–80) | 50 (25–72) | 0.23 |
| Gender | 0.82 | ||
| Female | 14 (54%) | 8 (62%) | |
| Male | 12 (46%) | 5 (38%) | |
| Stage | |||
| 1 + 2 | 8 (31%) | ||
| 3 + 4 | 18 (69%) | ||
| IPI | |||
| I + II | 10 (39%) | ||
| III | 5 (19%) | ||
| IV + V | 11 (42%) | ||
| GCB | 14 (54%) | ||
| Non-GCB | 12 (46%) | ||
| BM involvement (biopsy) | |||
| Yes | 0 | ||
| No | 26 (100%) | ||
| BM involvement (flow cytometry) | |||
| Yes | 2 (8%) | ||
| No | 24 (92%) | ||
| LDH | |||
| Normal | 9 (35%) | ||
| High, > 225 U/L | 17 (65%) | ||
| β2-microglobulin | |||
| Normal | 11 (42%) | ||
| High, > 2530 ng/mL | 15 (58%) | ||
| Treatment response | |||
| CR | 25 (96%) | ||
| PR | 1 (4%) | ||
| PD | 0 | ||
| Post-remission relapse | 5 (20%) |
BM Bone marrow, CR Complete remission, GCB Germinal centre B-cell like, HC Healthy controls, IPI International prognostic index,LDH Lactate dehydrogenase, PR Partial remission, PD Progressive disease
NK cell subsets
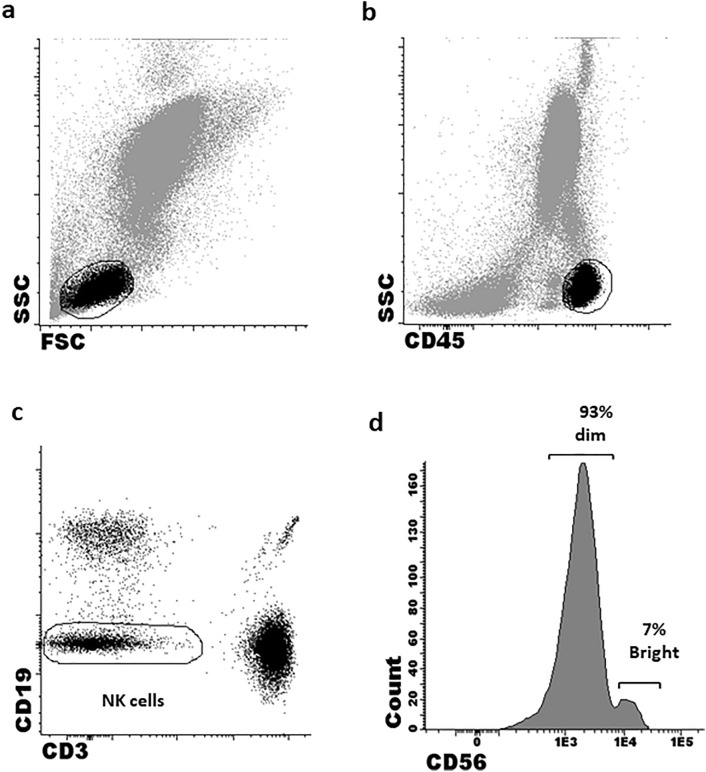
The purity of isolated PB-NK cells was 92.3 ± 2.1% and 93.2 ± 2.2% in DLBCL and HC samples, respectively. The total percentage of CD56 NK cells in patients and HCs was comparable. However, the distribution of mature NK subsets according to their CD56 intensity was found to be abnormal in patients. The percentage of CD56bright cells was significantly lower in patient compared to HC samples (3.5% vs. mean 5.7 ± 1.3%, respectively; p < 0.01) (Table 2). Similar to PB, patient BM-NK cells exhibited an abnormal maturation phenotype, with only 2/26 (7.7%) patients harbouring both CD56bright and CD56dim subsets. Moreover, even in these two patients, the percentage of CD56bright cells was remarkably low (2.5 ± 1.4%). Notably, none of the BM biopsies demonstrated lymphoma infiltration. Based on these findings, all further experiments and analyses of both patient and HC-NK cells were performed on the CD56dim subset only. Figure 1a-d depicts sequential gating of CD56dim NK cells. CD16, while uniformly expressed on CD56dim NK cells, demonstrated lower MFI on patient versus HC cells (p = 0.04; Table 2). Notably, NK cells obtained from patients at advanced disease stages displayed significantly decreased CD16 MFI compared to those presenting with early disease stages (p = 0.04; Suppl. Table 2).
Table 2.
Immunophenotypic characteristics of peripheral blood lymphocytes and NK subpopulations
| DLBCL N = 26 | HC N = 13 | P-value | |
|---|---|---|---|
| Lymphocytes median % [range] | 10.6 [5–19] | 12.1 [8–21] | 0.3 |
| NK subpopulation median % [range] | 10.4 [4–19] | 11 [6–15] | 0.4 |
| CD56 subsets | |||
| No | 25 (96%) | 3 (23.1%) | |
| Yes | 1 (4%) | 10 (76.9%) | |
| CD56bright (mean + SD) | 3.5% | 5.7% ± 1.3 | < 0.01 |
| CD16 MFI (on CD56dim population) | 2368 [1523–8390] | 3709 [2963–10426] | 0.04 |
HC Healthy controls, MFI Mean fluorescence intensity, NK Natural killer, SD Standard deviation
Fig. 1.
Sequential gating analysis of CD56 NK cell subsets. Lymphocytes were gated based on: a Their forward scatter/side scatter (FSC/SSC). b CD45 expression. c NK cells were identified as double-negative CD3 and CD19 lymphocytes. D NK cells were further divided into subsets according to the mean fluorescent intensity of CD56 expression into CD56dim and CD56bright cells
NK cell receptors
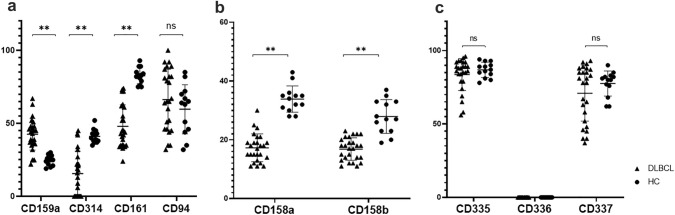
The expression of NK cell receptors, including CLRs, KIRs, NCRs, and other co-receptors, was assessed on patient and HC CD56dim PB-NK cells. The expression of the inhibitory CLR NKG2A (CD159a) was significantly higher in patient samples compared to HC (mean 42 ± 5.1% vs. 27 ± 2.3%; p < 0.01). Conversely, the levels of activating CLRs NKG2D (CD314) and CD161 were significantly lower in DLBCL than in HC samples (mean 15 ± 8% vs. 40.2 ± 4%; p < 0.01 and 47.8 ± 7% vs. 82.7 ± 5%, respectively; p < 0.01). The expression of CD94, a transmembrane-anchored glycoprotein, essential for NKG2A and NKG2D activation did not differ between patient and HC-NK cells (Fig. 2a). Additionally, patient PB-NK cells expressed significantly decreased levels of KIRs (CD158a and CD158b) compared to those of HC (16.2 ± 5% vs. 33.8 ± 2.1%; p < 0.01 and 17.6 ± 6% vs. 32.4 ± 3.1%, respectively; p < 0.01) (Fig. 2b). Unlike the aforementioned CLRs and KIRs, the expression of NCRs NKp46 (CD335), NKp44 (CD336), and NKp30 (CD337) did not significantly differ in patient and HC samples (Fig. 2c).
Fig. 2.
Phenotypic profile of NK cell receptors on the PB CD56dim subset. The percentage of PB-NK cells expressing various NK-specific receptors was assessed. a CLRs: CD159a, CD314, CD161 and CD94. b KIRs: CD158a and CD158b. c NCRs: CD335, CD336 and CD337. Patient and HC values are presented by triangles and circles, respectively. *P < 0.05; **P < 0.01; ns, not significant
Intracellular GanzymB and Perforin, as well as costimulatory molecules CD226 and CD244, showed no differences in their expression on patient- and HC-derived NK cells (data not shown).
Factors such as disease stage, IPI, LDH or cell of origin did not significantly impact receptor expression levels (Suppl. Table 2).
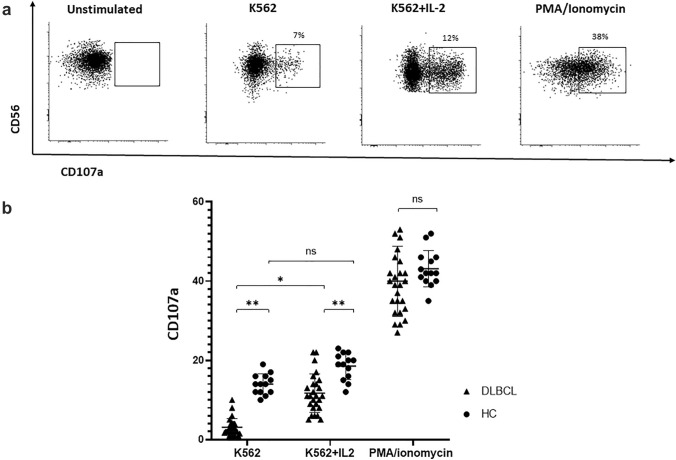
NK cell degranulation
CD107a expression on patient and HC-NK cells was assessed as an indicator of degranulation following activation by either K562 or PMA/ionomycin cocktail. The two sets of NK cells responded differently to K562 stimulation, with patient cells demonstrating significantly lower degranulation capacity than HC cells (2.8 ± 2.1% vs. 14.6 ± 1.5%, respectively; p < 0.01). IL-2 supplementation enhanced degranulation of both patient and HC-NK cells; however, the enhancement was significant only in patients (patients: 2.8 ± 2.1% vs. 11.8 ± 2.5%; p < 0.05; HCs: 14.6 ± 1.5% vs. 20.1 ± 2.7%; p = 0.06). Remarkably, the augmented degranulation following the IL-2 addition to patient cells was still significantly lower than that of K562 + IL-2 stimulated HC cells (11.8 ± 2.5% vs. 20.1 ± 2.7%, respectively; p < 0.01). Under the latter conditions, degranulation of NK cells derived from patients with advanced-stage disease (3–4) was significantly diminished compared to that observed in cells obtained from patients with limited-stage disease (1–2). LDH above the upper limit of normal as well as high IPI (IV-V versus 0-III) also negatively impacted the degranulation capacity of NK cells (p = 0.005 and p = 0.002, respectively; Suppl. Table 2).
PMA/ionomycin stimulation resulted in equally enhanced degranulation in NK cells from both DLBCL patients and HC (39.3 ± 8.2% vs. 42.6 ± 4.3%; p = 0.82) (Fig. 3a, b).
Fig. 3.
Degranulation of NK cells assessed by CD107a expression under various stimulation conditions CD107a expression was assessed on patient and HC PB-NK cells following activation with K562 cells alone (E:T ratio of 5:1), in the presence of IL-2, or PMA/ionomycin. a Representative FACS dot plot analysis of CD107a expression on CD56dim NK cell population under various stimulation conditions. b Median percentage of CD107a-positive CD56dim NK cells under various stimulation conditions. Patient and HC values are presented by triangles and circles, respectively. *P < 0.05; **P < 0.01; ns, not significant
Cytokine generation by NK cells
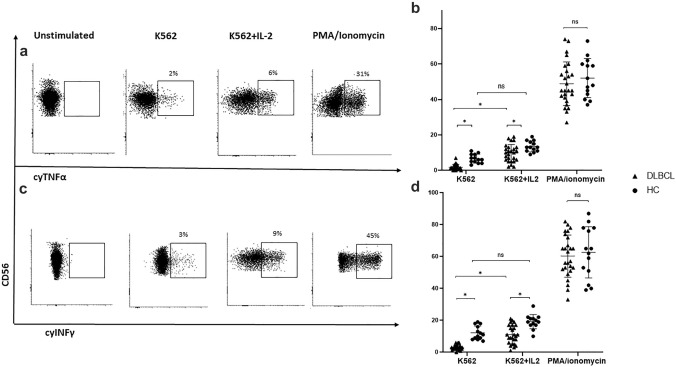
The production of intracellular TNFα and INFγ was evaluated as another indicator of NK cell activity. Similar to the findings regarding degranulation, K562-stimulated patient cells produced significantly lower levels of both cytokines relative to HC cells, equating to 2.4 ± 1.3% vs. 6.9 ± 2.3%, respectively, for TNFα (p < 0.05) and 3.2 ± 1.6% vs. 11.3 ± 2.6%, respectively, for IFNγ (p < 0.05). Among patient samples, cytokine production was significantly attenuated in NK cells obtained from patients with advanced-stage disease compared to those with limited-stage disease (p = 0.04 for TNFα and p = 0.02 for IFNγ; Suppl. Table 2).
The addition of IL-2 to K562 significantly enhanced the production of TNFα (2.4 ± 1.3% vs. 9.3 ± 3.3%, p < 0.05) and IFN γ (3.2 ± 1.6% vs.11.7 ± 3.3; p < 0.05) by patient NK cells. In HC-NK cells, the IL-2 addition resulted in a similar trend (TNFα: 6.9 ± 2.3% vs 12.8 ± 3.6%; p = 0.05; IFNγ: 11.3 ± 2.6% vs 18.4 ± 3.4%; p = 0.05). Despite the IL-2-related enhancement in the cytokine generation by patient NK cells, it remained lower than that produced by HC cells (TNFα: 9.3 ± 3.3% vs. 12.8 ± 3.6%; p < 0.05; IFNγ: 11.7% vs. 18.4 ± 3.4%; p < 0.05).
Following stimulation with PMA/ionomycin, generation of both cytokines was found to be comparably high in patient and HC-NK cells [TNFα: 47.8 ± 6.3% vs. 52.3 ± 5.7%, respectively; p = 0.79; IFNγ: 60.3 ± 5.3% vs. 64.1 ± 6.2%, respectively; p = 0.79] (Fig. 4a-d).
Fig. 4.
Generation of TNFα and INFγ by NK cells under various stimulation conditions Intracellular TNFα and INFγ expression was evaluated on patient and HC PB-NK cells following activation with K562 cells alone (E:T ratio of 5:1), in the presence of IL-2, or with PMA/ionomycin. a Representative FACS dot plot analysis of TNFα production under various stimulation conditions. b Median percentage of the TNFα-positive CD56dim NK cells under various stimulation conditions. c Representative FACS dot plot analysis of INFγ production under various stimulation conditions. d Median percentages of the INFγ-positive CD56dim NK cells under various stimulation conditions. Patient and HC values are presented by triangles and circles, respectively. *P < 0.05; **P < 0.01; ns, not significant
NK cell cytotoxicity
CFSE-labelled K562 cells were assessed for the percentage of apoptosis and necrosis following co-culture with purified NK cells. Dose-dependent lysis of K562 target cells was observed in the co-cultures derived from both patient and HC samples. However, patient NK cells demonstrated significantly lower cytotoxic activity. For instance, at the highest E:T ratio of 5:1, patient cells induced lysis of only 16.3% of target cells, whereas 24.2% of these cells were lysed following exposure to HC-NK cells (p < 0.01).
The addition of IL-2 to the 5:1 ratio co-culture increased cytotoxic activity of patient NK cells from 16.3% to 20.7% (p < 0.01) and of HC-NK cells from 24.2% to 28.1% (p = 0.05); however, patient cell cytotoxicity remained lower relative to that of HC cells (20.7% vs. 28.1%, respectively; p < 0.05) (Fig. 5). Across all the evaluated DLBCL co-culture conditions, the impairment in NK cell cytotoxic activity was more pronounced in samples derived from patients with a more advanced stage of disease (3–4 versus 1–2) and higher IPI (IV-V versus 0-III) (Suppl. Table 2).
Fig. 5.
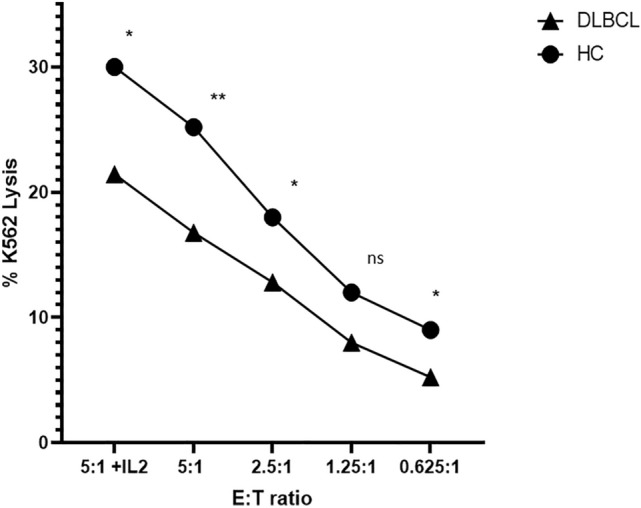
NK cell-induced cytolysis of target cells CFSE-labelled K562 cells were co-cultured with patient and HC PB-NK cells, and assessed for percentage of target necrotic and apoptotic cells. Line graph shows the median percentage of target cells that have undergone lysis at different effector to target concentrations (5:1 through 0.625:1). Patient and HC co-culture values are presented by triangles and circles, respectively. *P < 0.05; **P < 0.01; ns, not significant
Discussion
The current study provided evidence on phenotypic and functional aberrance of PB-NK cells derived from DLBCL patients. The observed phenotypic abnormalities included irregular distribution of mature subsets and an altered NK receptor repertoire, the latter being both discordant with the terminal differentiation state of the cell, and skewed towards an inhibitory phenotype. While virtually all mature NK cells in DLBCL were CD56dim, they unexpectedly expressed low levels of KIRs and aberrantly displayed several CLRs.
Malignancy-associated abnormal distribution of mature NK cell subsets was previously reported in the PB and tumour tissues. Tumour infiltrating lymphocytes in biopsies obtained from patients with various types of solid tumours were shown to be enriched with CD56bright cells, possibly chemo-attracted to the tumour sites to support immune evasion [29]. Tumour growth was found to interrupt normal maturation of NK cells in the BM. Notably, this tumour effect was reported to be independent of BM involvement [30], which was in accordance with our data demonstrating abnormal BM-NK cell distribution, despite the lack of BM infiltration with lymphoma cells. Evidence of tumour-induced effects on the disposition of PB-NK cells varies. Reports in-line with our findings demonstrating domination of the CD56dim subset in patient blood come from the chronic myeloid leukaemia setting [31], as well as from that of certain solid tumours. Indeed, in prostate cancer, a disease stage-dependent reduction in CD56bright cells was documented [32] with similar observations reported in patients with head and neck cancer. These data suggest that the paucity of the CD56bright population negatively impacts anti-cancer immune responses through diminished IFNγ secretion [33, 34]. Furthermore, in prostate cancer, a high CD56dim/CD56bright ratio, found in metastatic lymph nodes, is suspected to be the result of CD56bright cell recruitment from the PB to the pathologic lymph nodes to acquire cytotoxic abilities. This process could also account for the near-absence of CD56bright cells in patient PB found in the current study.
Somewhat distinct findings were reported in another study in DLBCL, where no statistically significant differences in the CD56dim/CD56bright subset ratio were observed between patients and controls [22], and in a study in hepatocellular carcinoma (HCC) where a reduction in CD56 + CD16 + NK-cells was observed [35]. The main reduction in this NK population in the latter disease originated from a reduction of the CD56dim population without a corresponding rise in the CD56bright subset. The differences in the findings at least in lymphoma patients might result from the variability in FACS data interpretation (e.g. the number of events counted, the cut-off between positive and negative cell populations) as well as from the composition of the patient population. Indeed, relatively high rates of CR were observed in the present study, however the documented 2-year PFS of 80% which is in-line with that published in the literature [36] along with the standard clinical and demographic characteristics of our patient cohort support its representativeness.
Abnormal signalling through both activating and inhibitory receptor pathways is likely to attenuate anti-cancer immune surveillance capacity of NK cells. In the current study, inhibitory KIRs CD158a and CD158b were under-expressed on patient NK cells. Since normal KIR expression is crucial for NK cell education, a process ensuring the formation of responsive, yet self-tolerant, effector cells [37, 38], the low expression of these KIRs on patient cells could abrogate NK cytotoxicity. In lung cancer, inhibitory KIR under-representation induced by tumour cells was shown to lead to NK cell functional defects [39]. At the same time, enhanced activating KIR expression was reported to be associated with the development of certain malignancies [40, 41], possibly through the induction of non-specific inflammatory responses [42]. Similar to KIRs, the pattern of CLR expression on patient NK cells was found to be abnormal in the current study and consistent with an inhibitory phenotype. Specifically, NKG2D, promoting cytotoxicity in response to malignant transformation [7], was under-represented in patients, whereas NKG2A, suppressing tumour-targeted NK-cell activity [43], was overexpressed. This unique CLR expression pattern, previously reported in other malignancy settings, such as acute leukaemia [44, 45] and breast cancer[46], is most likely to contribute to the suppression of anti-cancer responses in DLBCL. CD16, the FCγRIII crucial for NK ADCC, was found to be under-expressed on the CD56dim population in this study as well as in other reports [22, 35] and as it mediates the efficacy of anti-CD20 and anti-CD19 monoclonal antibody therapy in lymphoma, its under-expression may represent another mechanism compromising NK cell responses against lymphoma cells.
In the current study, patient NK cell activity was also found to be inferior to that of HC cells, as reflected by impaired direct lysis of malignant target cells. In our experiments, target cancer cells underwent significantly attenuated cytolysis when exposed to lymphoma patient NK cells, showing that the latter cells were dysfunctional and not only phenotypically aberrant. Further functional impairments such as decreased generation of intracellular IFNγ and TNFα as well as reduced degranulation observed in patient NK cells, corresponded to the aforementioned difference in cytolytic activity between patient and HC-NK cells. Similar NK-cell functional alterations were reported in other studies in cancer, including those in lymphoma. Pancreatic carcinoma cells co-cultured with NK cells were shown to attenuate NK cell cytotoxicity [47]. In patients with melanoma, disease metastasis was suggested to be linked to impaired perforin-dependent cytotoxicity and an exhausted phenotype of NK cells [48, 49] and in HCC, NK cells were shown to produce reduced IFN-γ and exhibit impaired K562 target cell lysis [35]. As to the lymphoma setting, NK-cell degranulation was reported to be impaired following CD16 ligation [22], as was the ability to produce IFN-γ, a process found to be related to fatty acid excess in the lymphoma environment which suppressed mitochondrial metabolism [50].
Importantly, the effects of the disease stage and IPI on the activity of patient-derived NK cells were not uniform, with the aberrant direct cell lysis being the most prominent and consistent defect in advanced-stage disease. Notably, the disease stage was reported to negatively impact the NK function in solid malignancies such as HCC [35] and breast cancer [51], as well as in other haematological malignancies, such as multiple myeloma (MM) [52]. While the cause-and-effect relationship between the disease stage and NK cell dysfunction is not completely delineated, the above-mentioned findings underscore the fact that NK cell modification is a dynamic process in the course of malignancy propagation. Recent evidence points to the involvement of checkpoint molecules such as PD-1, CTLA-4 and LAG-3 in NK-cell inhibition [53, 54], positioning checkpoint upregulation as a potential cancer-driven stage-dependent factor contributing to NK-cell exhaustion. This actually resembles tumour-induced modulation reported in T-cells [55, 56]. On the other hand, a decrease in NK cell cytotoxic activity was reported in individuals at risk for hereditary colorectal adenocarcinoma [13, 57] prior to the disease appearance. In such individuals, NK cell dysfunction was suggested to be responsible for insufficient immune-targeting of cancer-initiating cells [58].While these data on hereditary cancer syndromes imply that NK-cell inherent abnormalities might be risk factors for cancer initiation and propagation, our findings suggest that at least in DLBCL progression, NK cell functional aberration is an outcome of exposure to lymphoma environment, modifying their receptor expression, rather than a primary event.
Conclusion
In the current study, DLBCL patient NK cells demonstrated restricted immuno-modulatory and cytotoxic functions along with an aberrant surface receptor phenotype. This abnormal NK cell receptor repertoire most likely resulted from exposure to the lymphoma environment, which left intracellular processes intact. Such cellular dysfunction could promote lymphoma evasion from NK immune surveillance and disease propagation. The mechanisms underlying lymphoma-induced suppression of NK cell cytotoxic activity should be considered in the development of NK-based cellular therapies, anticipated to become an essential part of advanced treatment strategies in DLBCL.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to acknowledge with thanks the assistance of Sonia Kamenetsky in the preparation of the manuscript.
Abbreviations
- ADCC
Antibody-dependent cellular cytotoxicity
- AML
Acute myeloid leukaemia
- ATCC
American Type Culture Collection
- BM
Bone marrow
- CAR-T cell
Chimeric antigen receptor T-cell
- CFSE
Carboxyfluorescein succinimidyl ester
- CLRs
C-type lectin receptors
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- DLBCL
Diffuse large B-cell lymphoma
- E:T
Effector/target
- FACS
Fluorescence-activated cell sorting
- FL
Follicular lymphoma
- HC
Healthy controls
- HCC
Hepatocellular carcinoma
- HLA
Human leukocyte antigen
- IFNγ
Interferon γ
- KIRs
Killer cell immunoglobulin-like receptors
- LAG-3
Lymphocyte-activation gene 3
- LPDs
Lymphoproliferative disorders
- MFI
Mean fluorescent intensity
- MoAbs
Monoclonal antibodies
- NCRs
Natural cytotoxicity receptors
- NK
Natural killer
- PB
Peripheral blood
- PD-1
Programmed cell death protein 1
- PMA
Phorbol 12-myristate 13-acetate
- TNFα
Tumour necrosis factor α
- 7-AAD
7-Amino-actinomycin D
Author contribution
T.A.: designed and performed research, interpreted the data, wrote the paper, approved the final version of the paper. I.S.: performed research, approved the final version of the paper. M.K.: performed research, approved the final version of the paper. M.F.: performed research, approved the final version of the paper. M.H.: performed research, approved the final version of the paper. Y.O.: performed research, interpreted the data, approved the final version of the paper. G.S.: designed research, interpreted the data, approved the final version of the paper. S.R.-H.: designed and performed research, interpreted the data, wrote the paper, approved the final version of the paper.
Funding
This study was not supported by any funding.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors have no conflicts to declare.
Ethical approval
All the procedures involved in this study were in accordance with the ethical standards of the Institutional Review Board of the Rambam Health Care Campus (Approval #0114–17RMB) and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
All the participants signed the Informed Consent Form.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Galit Sarig, Email: g_sarig@rmc.gov.il.
Shimrit Ringelstein-Harlev, Email: s_ringelstein@rambam.health.gov.il.
References
- 1.Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7(9):703–714. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 2.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 3.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97(10):3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 4.Penack O, Gentilini C, Fischer L, Asemissen AM, Scheibenbogen C, Thiel E, Uharek L. CD56dimCD16neg cells are responsible for natural cytotoxicity against tumor targets. Leukemia. 2005;19(5):835–840. doi: 10.1038/sj.leu.2403704. [DOI] [PubMed] [Google Scholar]
- 5.Chan A, Hong DL, Atzberger A, Kollnberger S, Filer AD, Buckley CD, McMichael A, Enver T, Bowness P. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J Immunol. 2007;179(1):89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 6.Stabile H, Nisti P, Morrone S, Pagliara D, Bertaina A, Locatelli F, Santoni A, Gismondi A. Multifunctional human CD56 low CD16 low natural killer cells are the prominent subset in bone marrow of both healthy pediatric donors and leukemic patients. Haematologica. 2015;100(4):489–498. doi: 10.3324/haematol.2014.116053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27(45):5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 8.Harning R, Koo GC, Szalay J. Regulation of the metastasis of murine ocular melanoma by natural killer cells. Invest Ophthalmol Vis Sci. 1989;30(9):1909–1915. [PubMed] [Google Scholar]
- 9.Kozlowski JM, Fidler IJ, Campbell D, Xu ZL, Kaighn ME, Hart IR. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 1984;44(8):3522–3529. [PubMed] [Google Scholar]
- 10.Smyth MJ. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2008;20(4):631. doi: 10.1093/intimm/dxn028. [DOI] [PubMed] [Google Scholar]
- 11.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356(9244):1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 12.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121(1):1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markowitz JF, Aiges HW, Cunningham-Rundles S, Kahn E, Teichberg S, Fisher SE, Daum F. Cancer family syndrome: marker studies. Gastroenterology. 1986;91(3):581–589. doi: 10.1016/0016-5085(86)90626-8. [DOI] [PubMed] [Google Scholar]
- 14.Warren RP, Stembridge AM, Gardner EJ. Deficient immune function of peripheral blood mononuclear cells from patients with Gardner syndrome. Clin Exp Immunol. 1985;60(3):525–531. [PMC free article] [PubMed] [Google Scholar]
- 15.Sivori S, Pende D, Bottino C, Marcenaro E, Pessino A, Biassoni R, Moretta L, Moretta A. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur J Immunol. 1999;29(5):1656–1666. doi: 10.1002/(SICI)1521-4141(199905)29:05<1656::AID-IMMU1656>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, Gastaut JA, Pende D, Olive D, Moretta A. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99(10):3661–3667. doi: 10.1182/blood.v99.10.3661. [DOI] [PubMed] [Google Scholar]
- 17.Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R, Pavlu J, Brisley G, de Lavallade H, Sarvaria A, Marin D, Mielke S, Apperley JF, Shpall EJ, Barrett AJ, Rezvani K. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica. 2014;99(5):836–847. doi: 10.3324/haematol.2013.087536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss-Steider B, Soto-Cruz I, Martinez-Campos CA, Mendoza-Rincon JF. Expression of MICA, MICB and NKG2D in human leukemic myelomonocytic and cervical cancer cells. J Exp Clin Cancer Res. 2011;30:37. doi: 10.1186/1756-9966-30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epling-Burnette PK, Bai F, Painter JS, Rollison DE, Salih HR, Krusch M, Zou J, Ku E, Zhong B, Boulware D, Moscinski L, Wei S, Djeu JY, List AF. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 2007;109(11):4816–4824. doi: 10.1182/blood-2006-07-035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Correa B, Gayoso I, Bergua JM, Casado JG, Morgado S, Solana R, Tarazona R. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol. 2012;90(1):109–115. doi: 10.1038/icb.2011.15. [DOI] [PubMed] [Google Scholar]
- 21.Costello RT, Boehrer A, Sanchez C, Mercier D, Baier C, Le Treut T, Sebahoun G. Differential expression of natural killer cell activating receptors in blood versus bone marrow in patients with monoclonal gammopathy. Immunology. 2013;139(3):338–341. doi: 10.1111/imm.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danielou-Lazareth A, Henry G, Geromin D, Khaznadar Z, Briere J, Tamouza R, Cayuela JM, Thieblemont C, Toubert A, Dulphy N. At diagnosis, diffuse large B-cell lymphoma patients show impaired rituximab-mediated NK-cell cytotoxicity. Eur J Immunol. 2013;43(5):1383–1388. doi: 10.1002/eji.201242733. [DOI] [PubMed] [Google Scholar]
- 23.Plonquet A, Haioun C, Jais JP, Debard AL, Salles G, Bene MC, Feugier P, Rabian C, Casasnovas O, Labalette M, Kuhlein E, Farcet JP, Emile JF, Gisselbrecht C, Delfau-Larue MH, Groupe d'etude des lymphomes de la Peripheral blood natural killer cell count is associated with clinical outcome in patients with aaIPI 2–3 diffuse large B-cell lymphoma. Ann Oncol. 2007;18(7):1209–1215. doi: 10.1093/annonc/mdm110. [DOI] [PubMed] [Google Scholar]
- 24.He L, Zhu HY, Qin SC, Li Y, Miao Y, Liang JH, Xia Y, Wang Y, Wu YJ, Wang L, Fan L, Li JY, Xu W. Low natural killer (NK) cell counts in peripheral blood adversely affect clinical outcome of patients with follicular lymphoma. Blood Cancer J. 2016;6(8):e457. doi: 10.1038/bcj.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klanova M, Oestergaard MZ, Trneny M, Hiddemann W, Marcus R, Sehn LH, Vitolo U, Bazeos A, Goede V, Zeuner H, Knapp A, Sahin D, Spielewoy N, Bolen CR, Cardona A, Klein C, Venstrom JM, Nielsen T, Fingerle-Rowson G. Prognostic impact of natural killer cell count in follicular lymphoma and diffuse large B-cell lymphoma patients treated with immunochemotherapy. Clin Cancer Res. 2019;25(15):4634–4643. doi: 10.1158/1078-0432.CCR-18-3270. [DOI] [PubMed] [Google Scholar]
- 26.Andersson LC, Nilsson K, Gahmberg CG. K562–a human erythroleukemic cell line. Int J Cancer. 1979;23(2):143–147. doi: 10.1002/ijc.2910230202. [DOI] [PubMed] [Google Scholar]
- 27.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294(1–2):15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Krzewski K, Gil-Krzewska A, Nguyen V, Peruzzi G, Coligan JE. LAMP1/CD107a is required for efficient perforin delivery to lytic granules and NK-cell cytotoxicity. Blood. 2013;121(23):4672–4683. doi: 10.1182/blood-2012-08-453738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levi I, Amsalem H, Nissan A, Darash-Yahana M, Peretz T, Mandelboim O, Rachmilewitz J. Characterization of tumor infiltrating natural killer cell subset. Oncotarget. 2015;6(15):13835–13843. doi: 10.18632/oncotarget.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards JO, Chang X, Blaser BW, Caligiuri MA, Zheng P, Liu Y. Tumor growth impedes natural-killer-cell maturation in the bone marrow. Blood. 2006;108(1):246–252. doi: 10.1182/blood-2005-11-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierson BA, Miller JS. CD56+bright and CD56+dim natural killer cells in patients with chronic myelogenous leukemia progressively decrease in number, respond less to stimuli that recruit clonogenic natural killer cells, and exhibit decreased proliferation on a per cell basis. Blood. 1996;88(6):2279–2287. doi: 10.1182/blood.V88.6.2279.bloodjournal8862279. [DOI] [PubMed] [Google Scholar]
- 32.Koo KC, Shim DH, Yang CM, Lee SB, Kim SM, Shin TY, Kim KH, Yoon HG, Rha KH, Lee JM, Hong SJ. Reduction of the CD16(-)CD56bright NK cell subset precedes NK cell dysfunction in prostate cancer. PLoS ONE. 2013;8(11):e78049. doi: 10.1371/journal.pone.0078049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wulff S, Pries R, Borngen K, Trenkle T, Wollenberg B. Decreased levels of circulating regulatory NK cells in patients with head and neck cancer throughout all tumor stages. Anticancer Res. 2009;29(8):3053–3057. [PubMed] [Google Scholar]
- 34.de Jonge K, Ebering A, Nassiri S, Maby-El Hajjami H, Ouertatani-Sakouhi H, Baumgaertner P, Speiser DE. Circulating CD56(bright) NK cells inversely correlate with survival of melanoma patients. Sci Rep. 2019;9(1):4487. doi: 10.1038/s41598-019-40933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H, Tien P, Wang FS. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129(3):428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Tilly H, Morschhauser F, Sehn LH, Friedberg JW, Trneny M, Sharman JP, Herbaux C, Burke JM, Matasar M, Rai S, Izutsu K, Mehta-Shah N, Oberic L, Chauchet A, Jurczak W, Song Y, Greil R, Mykhalska L, Bergua-Burgues JM, Cheung MC, Pinto A, Shin HJ, Hapgood G, Munhoz E, Abrisqueta P, Gau JP, Hirata J, Jiang Y, Yan M, Lee C, Flowers CR, Salles G. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med. 2022;386(4):351–363. doi: 10.1056/NEJMoa2115304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell. 2010;142(6):847–856. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brodin P, Karre K, Hoglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30(4):143–149. doi: 10.1016/j.it.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Al Omar SY, Marshall E, Middleton D, Christmas SE. Increased killer immunoglobulin-like receptor expression and functional defects in natural killer cells in lung cancer. Immunology. 2011;133(1):94–104. doi: 10.1111/j.1365-2567.2011.03415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barani S, Hosseini SV, Ghaderi A. Activating and inhibitory killer cell immunoglobulin like receptors (KIR) genes are involved in an increased susceptibility to colorectal adenocarcinoma and protection against invasion and metastasis. Immunobiology. 2019;224(5):681–686. doi: 10.1016/j.imbio.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Butsch Kovacic M, Martin M, Gao X, Fuksenko T, Chen CJ, Cheng YJ, Chen JY, Apple R, Hildesheim A, Carrington M. Variation of the killer cell immunoglobulin-like receptors and HLA-C genes in nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2005;14(11 Pt 1):2673–2677. doi: 10.1158/1055-9965.EPI-05-0229. [DOI] [PubMed] [Google Scholar]
- 42.Muriuki BM, Forconi CS, Oluoch PO, Bailey JA, Ghansah A, Moormann AM, Ong'echa JM. Association of killer cell immunoglobulin-like receptors with endemic burkitt lymphoma in Kenyan children. Sci Rep. 2021;11(1):11343. doi: 10.1038/s41598-021-90596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamiya T, Seow SV, Wong D, Robinson M, Campana D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J Clin Invest. 2019;129(5):2094–2106. doi: 10.1172/JCI123955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez-Correa B, Morgado S, Gayoso I, Bergua JM, Casado JG, Arcos MJ, Bengochea ML, Duran E, Solana R, Tarazona R. Human NK cells in acute myeloid leukaemia patients: analysis of NK cell-activating receptors and their ligands. Cancer Immunol Immunother. 2011;60(8):1195–1205. doi: 10.1007/s00262-011-1050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419(6908):734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 46.Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Goncalves A, Andre P, Romagne F, Thibault G, Viens P, Birnbaum D, Bertucci F, Moretta A, Olive D. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121(9):3609–3622. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng YP, Zhang JJ, Liang WB, Tu M, Lu ZP, Wei JS, Jiang KR, Gao WT, Wu JL, Xu ZK, Miao Y, Zhu Y. Elevation of MMP-9 and IDO induced by pancreatic cancer cells mediates natural killer cell dysfunction. BMC Cancer. 2014;14:738. doi: 10.1186/1471-2407-14-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, Osman I, Bhardwaj N. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res. 2014;2(5):410–422. doi: 10.1158/2326-6066.CIR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jovic V, Konjevic G, Radulovic S, Jelic S, Spuzic I. Impaired perforin-dependent NK cell cytotoxicity and proliferative activity of peripheral blood T cells is associated with metastatic melanoma. Tumori. 2001;87(5):324–329. doi: 10.1177/030089160108700509. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi T, Lam PY, Jiang H, Bednarska K, Gloury R, Murigneux V, Tay J, Jacquelot N, Li R, Tuong ZK, Leggatt GR, Gandhi MK, Hill MM, Belz GT, Ngo S, Kallies A, Mattarollo SR. Increased lipid metabolism impairs NK cell function and mediates adaptation to the lymphoma environment. Blood. 2020;136(26):3004–3017. doi: 10.1182/blood.2020005602. [DOI] [PubMed] [Google Scholar]
- 51.Konjevic G, Jurisic V, Spuzic I. Association of NK cell dysfunction with changes in LDH characteristics of peripheral blood lymphocytes (PBL) in breast cancer patients. Breast Cancer Res Treat. 2001;66(3):255–263. doi: 10.1023/a:1010602822483. [DOI] [PubMed] [Google Scholar]
- 52.Jurisic V, Srdic T, Konjevic G, Markovic O, Colovic M. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med Oncol. 2007;24(3):312–317. doi: 10.1007/s12032-007-0007-y. [DOI] [PubMed] [Google Scholar]
- 53.Esen F, Deniz G, Aktas EC. PD-1, CTLA-4, LAG-3, and TIGIT: The roles of immune checkpoint receptors on the regulation of human NK cell phenotype and functions. Immunol Lett. 2021;240:15–23. doi: 10.1016/j.imlet.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 54.Quatrini L, Mariotti FR, Munari E, Tumino N, Vacca P, Moretta L. The Immune checkpoint PD-1 in natural killer cells: expression function and targeting in tumour immunotherapy. Cancers (Basel) 2020;12(11):3285. doi: 10.3390/cancers12113285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warren RP, Lum LG, Storb R. Is the leukocyte group-5a antigen associated with reduced NK cell function? Tissue Antigens. 1985;25(2):107–110. doi: 10.1111/j.1399-0039.1985.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 58.Tallerico R, Todaro M, Di Franco S, Maccalli C, Garofalo C, Sottile R, Palmieri C, Tirinato L, Pangigadde PN, La Rocca R, Mandelboim O, Stassi G, Di Fabrizio E, Parmiani G, Moretta A, Dieli F, Karre K, Carbone E. Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol. 2013;190(5):2381–2390. doi: 10.4049/jimmunol.1201542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.