Abstract
Cancer immunotherapies may be limited by their failure to target cancer stem cells (CSCs). We previously described an approach to target these cells using a dendritic cell (DC) vaccine primed with lysates of CSCs identified by aldehyde dehydrogenase (ALDH). However, its clinical application is limited by the difficulty of obtaining adequate amounts of tumor from patient to make CSC lysate for vaccine preparation. To address this issue, we evaluated targeting ALDHhigh CSCs using two antigenic peptides derived from ALDH in D5 melanoma model in both protection and therapeutic settings. ALDH 1A1 or 1A3 peptide-DC vaccines primed cytotoxic T lymphocytes (CTLs) that specifically killed ALDHhigh D5 CSCs, with ALDH 1A1 + 1A3 dual peptides-DC vaccine mediating an additive CTL effect compared to single peptide-DC vaccines. In a tumor challenge model, ALDH peptide-DC vaccines induced significant protective immunity suppressing D5 tumor growth with the dual peptides-DC vaccine being superior to each peptide individually. In a therapeutic model, dual peptide-DC vaccine resulted in significant tumor growth suppression with anti-PD-L1 administration significantly augmenting this effect. Immune monitoring studies revealed that ALDH dual peptides-DC vaccination elicited strong T cell (CTL & IFNγ Elispot) and antibody immunity targeting ALDHhigh CSCs, resulting in significant reduction of ALDHhigh D5 CSCs. ALDH dual peptides-DC vaccination plus anti-PD-L1 administration resulted in increased recruitment of CD3+ TILs in the residual tumors and further reduction of ALDHhigh D5 CSCs. ALDH peptide(s)-based vaccine may allow for clinical translation via immunological targeting of ALDHhigh CSCs. Furthermore, this vaccine augments the efficacy of immune checkpoint blockade.
Keywords: Aldehyde dehydrogenase, Peptide, Dendritic cells, Vaccine, Cancer stem cells, Immunotherapy
Introduction
Cancer stem cells (CSCs) are intimately involved in tumor initiation and metastasis as well as in mediating resistance to chemotherapy/radiotherapy and evasion of immune surveillance [1]. Eliminating CSCs represents a significant challenge in cancer therapeutics. In previous studies, we reported that CSC lysate-pulsed dendritic cells (DC) vaccines were effective at preventing lung metastasis of murine melanoma D5 and subcutaneous tumor growth of murine head and neck squamous cell cancer (HNSCC) SCC7 by eliciting strong and specific anti-CSC immunity [2]. We demonstrated that this approach was particularly advantageous when deployed in the adjuvant setting [3, 4]. However, our previously reported methodology for preparing CSC vaccines involved isolating CSCs from bulk tumor and generating lysates utilized for priming DCs. This limits the clinical applicability of this approach due to the variable availability of tumor tissues obtainable from each patient. Targeting of shared CSC antigens instead of CSC cell lysates provides an approach amenable to development of an “off the shelf” therapeutic vaccine.
Aldehyde dehydrogenase (ALDH) is a detoxifying enzyme responsible for the oxidation of intracellular aldehydes [5]. High levels of ALDEFLUOR/ALDH activity have been successfully used as a marker to isolate CSC-enriched populations in a variety of solid tumors [2, 6, 7], including breast cancer, lung cancer, colon cancer, sarcoma and HNSCC. Although there are 19 members in the human ALDH family, the isoforms expressed in CSCs are primarily ALDH1A1 and ALDH1A3 [8]. In addition to serving as CSC markers, ALDH1A1 and ALDH1A3 also play important functional roles in these cells and thus have served as potential therapeutic targets [8, 9]. Disulfiram, an irreversible pan-ALDH inhibitor, blocked in vitro and in vivo irradiation-induced conversion of non-stem breast cancer cells into breast CSCs [10]. Moreover, ALDHhigh CSCs were recognized and eliminated in vitro by HLA-A2-restricted, ALDH1A188–96 peptide-specific CD8+ T cells; and adoptive transfer of these ALDH1A1 peptide-specific CD8+ T cells inhibited tumor growth and reduced lung metastases in vivo [11]. This demonstrated the feasibility of targeting CSC peptide via passive (T cell) immunotherapy vs. active (vaccine-based) immunotherapy as we report in this study. ALDH1A1 and ALDH1A3 are the predominant ALDH isozymes expressed in ALDHhigh CSCs of breast cancer, lung cancer, melanoma, and head and neck cancer [12]. Microarray analysis of ALDHhigh and ALDHlow cells from melanoma tumor cells revealed that seventeen ALDH genes were up-regulated and 2 genes were down-regulated in ALDHhigh cells [12]. Specifically, ALDHhigh cells expressed over 20-fold more ALDH1A1 and ALDH1A3 than ALDHlow cells. This study suggested that ALDH 1A1 and ALDH1A3 represent two ideal targets for immunological targeting of ALDHhigh CSCs. However, up to date, ALDH1A1 and ALDH1A3 inhibitors to target these two isoforms have shown significant limitations for clinical application. For example, CM037, a benzothienopyrimidine-based competitive ALDH1A1 inhibitor representing a potential therapeutic drug to target ALDH1A1 in CSCs in ovarian cancer and endometrial cancer, is ineffective in vivo likely due to its low solubility in aqueous solutions [13]. ALDH1A3 inhibitor, such as free citral, has also been found ineffective in vivo [14], while citral encapsulated by nanoparticle reduced the tumor growth [14]. In addition, while pan-ALDH inhibitor disulfiram was toxic to CSCs in a copper-dependent manner, disulfiram alone did not show inhibitory effect on the ALDH activity [15, 16]. We hypothesize that ALDH peptides can serve as CSC associated antigen(s) to prime dendritic cells to induce specific immunity against ALDHhigh CSCs. In the present study, we developed ALDH1A1 and 1A3 peptide(s)-DC vaccines and examined their efficacy and mechanism of action utilizing an immunocompetent murine model.
Cancer immunotherapy utilizing immune checkpoint blockade, including PD-1/PD-L1 inhibitors, represents a major advance in cancer therapeutics [17, 18]. However, the overall response rate for monoclonal antibodies targeting PD-1/PD-L1 is generally less than 30% in many solid malignancies, suggesting that many patients show primary resistance to checkpoint inhibitors. In addition, many of these responses are transitory. In that regard, our previous studies demonstrated that the efficacy of CSC lysate-DC vaccine was augmented significantly by the simultaneous administration of anti-PD-L1 monoclonal antibody [4]. We therefore examined the efficacy of adding PD-1/PD-L1 blockade to ALDH peptide DC vaccines.
Methods
Mice
Female C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were maintained in a pathogen-free environment and used at age 7–8 weeks or older. Principles of laboratory animal care (National Institutes of Health) were followed, and the University of Michigan Laboratory of Animal Medicine approved all animal protocols.
Murine cancer cells
The D5 cell line is a sub-clone of murine melanoma cell line B16 that is syngeneic to C57BL/6 mice. Subcutaneous inoculation of D5 cells into the flank of C57BL/6 mice was performed to establish both protection and established tumor therapy models. D5 cell lines are cultured in complete medium (CM) consisting of RPMI1640 (GIBCO, Gaithersburg, MD) and supplements in 5% CO2 atmosphere.
ALDEFLUOR assay
The ALDEFLUOR™ Kit (StemCell Technologies, British Columbia, Canada) was used to isolate ALDEFLUOR+/ALDHhigh CSCs from D5 cells according to the manufacturer’s instruction.
Preparation of ALDHhigh CSC-targeted DC vaccines
DCs were prepared as we described previously [4]. Briefly, murine bone marrow-derived cells were cultured in 10 ml complete medium supplemented with 20 ng/ml GM-CSF (GenScript, NJ) at a concentration of (2–4) × 105 cells/ml in non-tissue culture petri dishes (Corning Incorporated, Corning, NY). Half of the CM with GM-CSF was refreshed on day 3, 6 and 8. On day 10, DCs were harvested and loaded with ALDHhigh CSC lysate (control) or ALDH peptide(s). ALDHhigh D5 CSCs were frozen and thawed 3 times to make CSC lysate. ALDHhigh D5 CSC lysate was added to DCs at a 1:3 cell equivalent ratio. The DCs were then incubated at 37 °C for 24 h with 5% CO2. After incubation, ALDHhigh D5 lysate-pulsed DCs were used as vaccine (control) in the subsequent experiments.
The sequence of ALDH 1A1 peptide (LLYKLADLI) and 1A3 peptide (LLHQLADLV) was based on the study of Visus C et al. [8] as well as the information from GenBank accession no. NM_000689 and NM_053080. The two peptides were synthesized by Genscript (Piscataway, NJ). The ALDH 1A1 peptide and 1A3 peptide that exceeded 90% purity were analyzed by high-performance liquid chromatography and validated for identity by mass spectrometry. Lyophilized peptides were dissolved in ultrapure water at a concentration of 10 mg/ml and stored at -80 °C. ALDH 1A1 or/and 1A3 peptide(s) were added to DCs at a ratio of 0.8 mg single peptide or 1.6 mg dual peptides (0.8 mg each peptide)/2 million DCs, which were then incubated at 37 °C for 24 h with 5% CO2 and used as ALDH peptide(s)-DC vaccines in the following experiments.
Tumor models
To test the protective effect of ALDH peptide(s)-DC vaccine, 2 × 106 DCs as vaccine per C57BL/6 mouse were administered (s.c.) once a week for two weeks, starting 14 days before D5 tumor cell challenge (5 × 105 D5 cells/mouse). For the D5 tumor treatment model, 5 × 104 D5 cells were injected (s.c.) into the flank of each C57BL/6 mouse. One day after tumor inoculation, 2 × 106 DCs were administered (s.c.) once a week for two weeks, and anti-PD-L1 monoclonal antibody (Clone number 80, provided by Dr. Elaine Hurt, MedImmune, Gaithersburg, MD) was administered (i.p.) at 0.05 mg/mouse, 3 times/week every other day starting from the next day of each vaccination for two weeks. The long and short diameters of tumor were measured twice a week. Tumor volumes were calculated as (width2 × length)/2. At the endpoint (when tumor was > 20 mm in diameter), mice were sacrificed, and various samples such as spleen, peripheral blood, and residual tumor were harvested for immune function assays.
Cytotoxic T lymphocytes (CTLs) cytotoxicity
Spleens were harvested from normal healthy or tumor-bearing treated B6 mice and made into splenocyte cell suspensions. Splenic T cells were isolated by MACS separator kits (MiltenyiBiotec. Inc. Auburn, CA) with anti-CD3-coupled microbeads, followed by anti-CD3/anti-CD28 (BD Pharmingen, San Diego, CA) activation and IL-2 (Prometheus Laboratories, San Diego, CA) expansion, which consistently resulted in > 90% of CD3+ T cells[2]. We then co-cultured the ALDHhigh CSCs and ALDHlow non-CSCs as target cells with the CTLs as generated above for 6 h. After that, we detected the cytotoxicity of CTLs by a lactate dehydrogenase (LDH) release assay (CytoTox 96 Non-Radioactive Cytotoxicity Assay, Promega, Madison, WI) according to the manufacturer's protocol.
T cell proliferation
T cell proliferation was determined using a carboxyfluorescein succinimidyl ester (CFSE)-based assay as previously described [19]. In brief, the isolated splenic CD3+ T cells from C57BL/6 mice were labeled with 5 μM CFSE (eBioscience, San Diego, CA) and stimulated by ALDH 1A1, 1A3 single or dual peptide(s)-DC vaccine or ALDHhigh CSC lysate-DC vaccine (as positive control) at a 1:1 cell ratio of DCs to T cells. Cell proliferation was detected by monitoring the changes in fluorescence intensity of the labeled cells after 7 days of co-culture.
Detection of CD3+ tumor infiltrating lymphocytes (TILs)
At the end of treatment, residual tumors were resected and fixed in formalin and embedded in paraffin. Sections of 4 μm thickness were deparaffinized in xylene and rehydrated through graded alcohols. These deparaffinized slides were then processed in microwave heating in 10 mmol/L citrate buffer (pH 6.0) for 15 min for antigen retrieval, in the presence of 3% H2O2 for endogenous peroxidase inactivation, followed by incubation with anti‐CD3 primary antibody (Abcam, Cambridge, MA) at 1:100 dilution, at 4 °C overnight. After washing three times in PBS, the slides were incubated for 30 min in biotinylated secondary goat anti-rabbit IgG antibody (1:2000) (Sigma, Indianapolis, IN). Immunostaining was performed using a DAB kit (3, 3′ diaminobenzidine, DAKO) and manufacturer's instructions were followed (Abcam, Cambridge, MA).
Intracellular IFN-γ staining and enzyme-linked immunospot (Elispot) assay
To determine IFN-γ intracellular secretion, the stimulated spleen T cells with ALDH peptide(s)-DCs as described above were permeabilized with pre-chilled perm buffer III (BD Bioscience, San Jose, CA) at 4 °C for 30 min. After washing once with PBS, the cells were stained with fluorescein isothiocyanate (FITC)-labeled anti-mouse IFN-γ at 4 °C for 30 min, followed by flow cytometry.
Extracellular IFN-γ secretion of splenic T cells primed with ALDH peptide(s)-DC vaccine in response to D5 ALDHhigh CSCs versus ALDHlow non-CSCs was determined using a Mouse IFN-γ Elispot set (BD Biosciences, San Jose, CA) according to the manufacturer's protocol. In brief, we collected splenic cells from ALDH peptide(s)-DC vaccinated mice and used MACS separator kits (MiltenyiBiotec. Inc. Auburn, CA) with anti-CD3-coupled microbeads to isolate CD3+ T cells from the splenic cells. Then, 0.2 × 106 isolated CD3+ T cells were added to the anti-IFN-γ-coated 96-well polyvinylidene fluoride (PVDF) ELISPOT plate. The CD3+ T cells were re-stimulated with PBS (negative control), ALDHhigh CSCs, ALDHlow non-CSCs, or phytohemagglutinin (PHA) (positive control) at 37 °C for 18 h. After cell removal, plates were developed at room temperature for 2 h in the presence of 0.4 U/ml IFN-γ-specific alkaline phosphatase-coupled mAb. Spot detection was performed following incubation for 6 min in the dark with a 1-step nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate substrate (Thermo Fisher Scientific, Waltham, USA). IFN-γ-specific spot-forming cells (SFC) were counted using a Bioreader® 5000 Eα (BIO-SYS GmbH, Karbon, Germany).
IgG binding assay and antibody/complement–dependent cytotoxicity (CDC)
Plasma IgG was detected by mouse IgG ELISA quantitation set (Bethyl Laboratories, Montgomery, TX). ALDHhigh or ALDHlow D5 cells were incubated with the equal quantity of IgG, followed by incubation with FITC-conjugated anti-mouse IgG antibody (BD Bioscience, San Jose, CA). Binding of plasma IgG to ALDHhigh D5 cells was assessed by flow cytometry. CDC against ALDHhigh CSCs versus ALDHlow non-CSCs was measured as described previously [2]. Briefly, 105 viable ALDHhigh D5 CSCs were incubated with plasma harvested from animals subjected to various treatments for 1 h followed by cell culture in the presence of rabbit complement for another hour. Viable cells were then counted under a microscope after trypan blue staining to calculate cell lysis. % of viable cells = viable ALDHhigh CSCs after plasma and complement incubation/105. Lower % of.
viable ALDHhigh CSCs at the end of incubation indicates more cell lysis.
Detection of ALDHhigh CSCs and CD3+ T cells in residual tumor
Residual tumors were harvested from all mice at the end of experiments and cut into small piece (1–8 mm3) for digestion by 1 × collagenase/hyaluronidase (Stem Cell Technologies, Vancouver, BC, Canada) for 30–40 min to make single cell suspensions. These single cell suspensions were then used to detect the percentage of ALDHhigh D5 CSCs or CD3+ TILs utilizing the ALDEFLUOR™ Kit and APC-conjunct anti-CD3 (BD Biosciences, San Jose, CA), respectively, followed by flow cytometry.
Flow cytometry analysis
Cell surface expression (CD3, PD-1, PD-L1) and intracellular expression (IFN-γ, ALDH) were analyzed by flow cytometry. All FITC- or phycoerythrin- or peridinin chlorophyll protein-conjugated antibodies and matched isotype controls were purchased from BD Biosciences. Flow cytometry was performed on a LSRII flow cytometer (BD Biosciences) and analyzed by FlowJo™ version 10 (Tree Star, Inc., Ashland, OR).
Statistical analysis
Statistical analyses of CFSE, FACS, Elispot, ELISA, immunohistochemistry and CTL cytotoxicity were performed using Student’s t-test when comparing only 2 groups and using one-way ANOVA with Tukey's multiple comparisons when comparing > 2 groups. The growth curves were expressed as mean ± SE and compared by two-way ANOVA. P < 0.05 was considered statistically significant. Statistical analyses were performed with GraphPad Prism 9.0.
Results
ALDH 1A1 and/or 1A3 peptide(s)-DC vaccines induced T cell proliferation and anti-ALDHhigh CSC activity in vitro
As described above, high levels of ALDEFLUOR/ALDH activity have been successfully used as a single marker to isolate cancer stem cell-enriched populations in a variety of tumors. Functional activity in CSCs is primarily mediated by two isoforms: ALDH 1A1 and 1A3. We examined the effect of two peptides derived from ALDH 1A1 and 1A3, respectively, as described in the Materials and Methods (ALDH 1A1 and 1A3 peptides)-DC vaccine on T cell proliferation and function in vitro. Splenic CD3+ T cells from the normal B6 mice were labeled with CFSE followed by stimulation with unloaded-DC (as negative control), ALDH 1A1 peptide-DC, ALDH1A3 peptide-DC, ALDH 1A1 + 1A3 peptides-DC. D5 ALDHhigh CSC lysate-DC was used as a positive control. As shown in Fig. 1a, the T cell proliferation rate was 20.2%, 26.1% and 31.5% after ALDH 1A1, 1A3 and 1A1 plus 1A3 peptides-DC in vitro stimulation, respectively, demonstrating significant increases compared with the negative control (9.51% after unloaded-DC stimulation). The T cell proliferation rate after stimulation with ALDH 1A1 + 1A3 peptides-DC increased to 31.5%, which was comparable to 35.5% generated by the positive control (ALDHhigh D5 CSC lysate-DC). Figure 1b indicates that significantly stronger CD3+ T cell responses to the ALDH 1A1 and/or 1A3 peptide antigens could be elicited by the DCs presenting these peptide antigens than unloaded DCs.
Fig. 1.
ALDH peptide(s)-DC vaccine promoted the in vitro proliferation and anti-ALDHhigh CSC function of T cells. a: Flow cytometry indicated proliferation of CFSE labeled CD3+ T cells from the spleen of normal C57BL/6 mice stimulated by DCs loaded with ALDH 1A1 and/or 1A3 peptide(s). Representative results of the CFSE assay after 7-day co-culture of peptide(s)-DC: T cells at a 1:1 ratio are shown. b: The CFSE assays were performed three times. Statistical analyses are shown for T cell expansion in response to different ALDH peptide(s)-DC vaccines as indicated. c: The CD3+ T cells isolated from the spleen of normal B6 mice and stimulated by DCs loaded with ALDH 1A1 and/or 1A3 peptide(s) were co-incubated with D5 ALDHhigh versus ALDHlow target cells at a 10:1 E/T ratio. Data were representative of three experiments performed
We used these ALDH 1A1 and/or 1A3 peptides DC-stimulated T cells as CTLs and assessed their cytotoxicity to ALDHhigh CSCs vs. ALDHlow non-CSCs as measured by an LDH release assay. As shown in Fig. 1c, CTLs primed with ALDH 1A1 and/or 1A3 peptide(s)-DCs exhibited significantly higher killing of ALDHhigh D5 cells compared to the negative control (unloaded-DC primed T cells).
at a 10:1 effector (E): target cell (T) ratio (all p values < 0.05). Importantly, the dual (ALDH 1A1 + 1A3) peptides-DC-activated T cells killed a higher proportion of ALDHhigh CSCs than did single peptide-DC activated T cells (p = 0.0681 and p = 0.0206, respectively). However, the cytotoxicity elicited by ALDH peptide(s)-DC primed T cells was not observed when ALDHlow non-CSCs were used as a negative target control. These experiments demonstrated that ALDH 1A1 and/or 1A3 peptide(s)-DC vaccines could induce T cell anti-ALDHhigh CSC activity as well as T cell proliferation in vitro.
ALDH 1A1 plus 1A3 peptides-DC vaccines demonstrated additive effect against D5 tumor challenge in vivo
Based on the in vitro data as described above, we proceeded to test the effect of combined dual ALDH peptides-DC vaccine in a protective D5 tumor model. Two vaccinations were administered 14 and 7 days before tumor cell injection as shown in Fig. 2a. Treatment consisted of PBS control, single ALDH peptide-DC vaccine or dual peptides-DC vaccine. The ALDH 1A1 or 1A3 peptide-DC vaccine significantly inhibited subcutaneous tumor growth (p < 0.0001) compared to PBS control. Importantly, the ALDH 1A1 plus 1A3 dual peptides-DC vaccine inhibited tumor growth more than vaccination with single peptide vaccines (p = 0.018 and p = 0.082, respectively).
Fig. 2.
ALDH dual peptides-DC vaccine significantly inhibited D5 tumor growth in the protection model. a: D5 growth curves in mice treated with PBS, single ALDH peptide-DC vaccines and the dual peptides-DC vaccine. This experiment was repeated twice. The growth curves were expressed as mean ± SE and compared by two-way ANOVA. b: The resected residual tumors from hosts treated with the ALDH peptide(s)-DC vaccines
The representative pictures of residual subcutaneous tumors at the end of the experiment after resection are displayed (Fig. 2b), indicating that the dual ALDH peptides-DC vaccine could induce more potent suppression of tumor growth than single ALDH peptide-DC vaccines.
ALDH peptide(s)-DC vaccine elicited T cell anti-CSC immune responses
We collected the spleens, peripheral blood and residual tumors at the end of the experiments in Fig. 2 and used them to analyze the anti-CSC immune function in vitro. The primed splenic T cells isolated from ALDH 1A1, 1A3 or dual peptide-DC vaccinated mice exhibited increased cytotoxicity of ALDHhigh D5 CSCs than splenic T cells from PBS treated mice at a E/T = 10:1 ratio (p = 0.0449, p = 0.0063 and p = 0.0147, respectively, Fig. 3a). Of note, dual ALDH peptides-DC vaccine induced higher (p = 0.0111) cytotoxicity to ALDHhigh CSCs than ALDH1A1 peptide-DC vaccine. These elevated cytotoxicities were not observed against ALDHlow D5 non-CSC target cells. Importantly, dual ALDH peptides-DC vaccine induced significantly (p = 0.0302) higher cytotoxicity to ALDHhigh CSCs than to ALDHlow non-CSCs (Fig. 3a). These data indicate that ALDH peptide(s)-DC vaccine elicits specific anti-ALDHhigh CSC CTL activity. Using the same CTLs, we performed intracellular staining of IFN-γ to evaluate the cytokine response against ALDHhigh CSCs versus ALDHlow non-CSCs by flow cytometry. Compared with the 1.79% intracellular IFN-γ producing splenic T cells from PBS treated mice, increased intracellular IFN-γ secreting splenic T cells were conferred by ALDH peptide 1A1 (2.76%), 1A3 (3.83%) and dual 1A1 + 1A3 (7.18%)-DC vaccines in response to ALDHhigh CSCs (Fig. 3b). In contrast, augmented T cell responses were not elicited to ALDHlow non-CSCs (Fig. 3c), confirming ALDH peptide (s)-DC vaccines induced specific T cell immune responses to ALDHhigh CSCs. As shown in Fig. 3d, compared with PBS treated CTLs, CTLs primed by ALDH 1A1, 1A3 or dual peptide-DC vaccine secreted significantly more intracellular IFN-γ (p = 0.0255, p = 0.0330, and p = 0.0359, respectively) in response to ALDHhigh CSCs, but not ALDHlow non-CSCs. Of note, CTLs activated by dual ALDH peptides-DC vaccine secreted more intracellular IFN-γ (p = 0.0334) in response to ALDHhigh CSCs than to ALDHlow non-CSCs.
Fig. 3.
ALDH peptide(s)-DC vaccine elicited T cell immune responses against ALDHhigh CSCs. a: Cytotoxicity of spleen T cells isolated from D5-bearing mice treated with ALDH 1A1 and/or 1A3 peptides-DC vaccines. This experiment was repeated twice. b: Flow cytometry showed intracellular staining of IFN-γ secreting T cells induced by ALDH 1A1 and/or 1A3 peptide(s)-DC vaccine in response to ALDHhigh D5 CSCs. The top row shows flow cytometry scatter plots using the isotype control for anti-IFN-γ mAb. c: Flow cytometry showing the intracellular staining of IFN-γ secreting T cells primed by ALDH 1A1 and/or 1A3 peptide(s)-DC vaccine in response to ALDHlow D5 non-CSCs. d: The flow cytometry experiments for the detection of intracellular IFN-γ secreting T cells were repeated twice. Bar graph showing the statistical difference of IFN-γ secretion in response to ALDHhigh cells versus ALDHlow cells by T cells collected from animals subjected to various ALDH peptide(s)-DC vaccine treatment as indicated
Furthermore, Elispot assays demonstrated that the single peptide-DC vaccine and dual peptides-DC vaccine increased extracellular IFN-γ-producing splenic T cells in response to ALDHhigh D5 CSCs vs. ALDHlow D5 non-CSCs (Fig. 4a). As shown in Fig. 4b, the number of IFNγ-producing T cells from ALDH 1A3 peptide-DC and dual peptides-DC treated mice was significantly greater in response to D5 ALDHhigh CSCs than that in response to ALDHlow D5 non-CSCs (p = 0.0374 and p = 0.0116, respectively). In addition, ALDH 1A1, 1A3 and dual peptide(s)-DC elicited significantly more IFN-γ-producing T cells in response to ALDHhigh CSCs (p = 0.0250, p = 0.0241 and p = 0.0212, respectively) compared to PBS. These results verified that ALDH peptide(s)-DC vaccine stimulates specific T cell immune responses against ALDHhigh CSCs.
Fig. 4.
ALDH peptide(s)-DC vaccine increased extracellular IFN-γ secretion by T cells against ALDHhigh CSCs. a: Representative Elispot results showing the by splenic T cells primed with ALDH peptide(s)-DC vaccination in response to D5 ALDHhigh CSCs versus ALDHlow non-CSCs. b: The histogram graph showing the statistics of the Elispot data in a. The experiment was repeated twice
ALDH peptide(s)-DC vaccine elicited humoral anti-CSC immune responses
To explore whether ALDH peptide(s)-DC vaccination generates B cell immune responses against ALDHhigh CSCs, we evaluated the binding to ALDHhigh CSCs by immune sera collected from the animals subjected to ALDH peptide(s)-DC treatment. As shown in Fig. 5a, plasma IgG isolated from ALDH 1A1, 1A3 or dual peptide-DC vaccine-treated mice bound to ALDHhigh D5 CSCs significantly higher than plasma IgG collected from PBS-treated mice (p = 0.0028, p < 0.0001, p < 0.0001, respectively). The representative binding rates as revealed by flow cytometry were 5.3% for PBS control, and 12.2%, 15.9%, 16.2% for ALDH 1A1, 1A3 and dual peptide-DC vaccine treatment (Fig. 5a). To assess the potential immunological consequence of such binding, we conducted complement-dependent cytotoxicity(CDC)assays. The CDC assay showed that ALDH 1A1 or 1A3 peptide-DC vaccine-primed immune plasma killed ALDHhigh D5 CSCs significantly more than the control (p = 0.0007 and p < 0.0001, respectively). Importantly, dual ALDH peptide-DC vaccine-primed immune plasma demonstrated a significantly increased ALDHhigh CSCs lysis compared with plasma primed by each single peptide-DC vaccine (p < 0.0001, p = 0.0086, respectively) (Fig. 5b). In contrast, such lysis was not observed when ALDHlow non-CSCs were used as control targets. These data indicate that ALDH peptide(s)-DC vaccine can confer host humoral anti-ALDHhigh CSC specific immunity.
Fig. 5.
ALDH peptide(s)-DC vaccine induced humoral immune responses and CD3+ TILs a: ALDH peptide(s)-DC vaccine primed mice to produce plasma antibody which bound to ALDHhigh D5 CSCs. Flow cytometry scatter plots demonstrated the representative binding rate of plasma IgG to ALDHhigh D5 CSCs, and the histogram graph showed the mean ± standard error of the plasma binding rate to ALDHhigh D5 CSCs. This experiment was repeated three times. b: ALDH peptide(s)-DC vaccine-primed plasma antibody lysed ALDHhigh D5 CSCs via CDC. ALDHlow D5 non-CSCs were used as target control. This experiment was repeated twice. c: Flow cytometry showing the percentage of ALDHhigh CSCs in residual D5 tumors post-treatment as indicated. d: ALDH peptide-DC vaccination treatments induced CD3+ TILs. Representative IHC results showing CD3+ TILs in residual tumors harvested from the treated hosts. Tumor cells and background were stained with red and amaranth, respectively. The experiment was repeated twice. e: Bar graph comparing the CD3+ TILs induced by different treatments as indicated
To confirm the in vivo targeting of ALDHhigh CSCs by the ALDH peptide(s)-DC vaccine, we harvested residual tumors from the treated animals. The tumors were made into single cell suspensions and were stained by the ALDEFLUOR assay to quantify residual ALDHhigh CSCs. The proportion of ALDHhigh D5 cells in residual tumors was decreased by ALDH 1A1 (4.18%), 1A3 (3.42%) and dual peptide-DC 1A1/1A3-DC (1.96%) vaccines, compared with the PBS control (14.1%) (Fig. 5c), indicating that dual peptide DC vaccination results in effective reduction of the ALDHhigh CSCs from nearly 15% to less than 2%.
In addition, immunohistochemistry assays on residual tumors showed that both ALDH 1A1, ALDH 1A3 and ALDH 1A1 + 1A3 peptide(s)-DC vaccines induced more CD3+ tumor-infiltrating lymphocytes (TILs) than PBS (Fig. 5d). Moreover, dual 1A1 + 1A3 peptide-DC vaccines recruited more CD3+ T cells to infiltrate D5 tumor than each single peptide-DC vaccine (p = 0.0122, p = 0.0071, respectively) (Fig. 5e).
Anti-PD-L1 treatment significantly enhanced the anti-tumor effect of ALDH peptides-DC vaccine
We detected the expression of PD-L1 on D5 ALDHhigh CSCs, ALDHlow non-CSCs and unsorted D5 cells by flow cytometry. ALDHhigh D5 CSCs showed higher PD-L1 expression than ALDHlow D5 non- CSCs or unsorted D5 cells (P < 0.0001, P = 0.0032, respectively) (Fig. 6a and b). We therefore hypothesized that blockade of PD-1/PD-L1 pathway may enhance the efficacy of ALDH peptides-DC vaccines. To test this hypothesis, we examined the potential synergism of anti-PD-L1 treatment and dual ALDH peptides-DC vaccine in the D5 therapeutic model. In this D5 therapy model, therapy was not begun until after tumor implantation. Dual peptide-DC vaccine was administered (s.c.) once a week for two weeks starting 24 h after tumor inoculation with PBS as a control (n = 5), and anti-PD-L1 mAb was administered (i.p.) 3 times following each vaccination for a total of 6 times in 2 weeks with an anti-PD-L1 isotype (iso) control. As shown in Fig. 6c, dual peptide-DC vaccine significantly inhibited the growth of D5 tumor compared with PBS control (p < 0.0001) as we observed in our previous experiments. The addition of anti-PD-L1 resulted in a significantly greater inhibition of D5 tumor growth compared with dual peptide-DC vaccine alone (p = 0.0235) or anti-PD-L1 alone (p < 0.0001).
Fig. 6.
Anti-PD-L1 significantly enhanced the anti-tumor effect of ALDH dual peptide-DC vaccine in the D5 therapy model. a: PD-L1 expression of unsorted D5, ALDHhigh D5 CSCs and ALDHlow D5 non-CSCs as determined by flow cytometry. b: The expression of PD-L1 on unsorted D5 cells, ALDHhigh CSCs and ALDHlow non-CSCs. Data graph was generated using the flow plot data in three independent experiments. c: Tumor growth in the day 1 D5 therapy model in mice (n = 5 for each group) treated with PBS, dual peptide-DC with or without anti-PD-1 or anti-PD-L1 iso. Data are representative of three experiments. d: Cytotoxicity of splenic T cells isolated from D5-bearing mice treated in C (E/T = 10:1 ratio) against D5 ALDHhigh CSCs vs. ALDHlow non-CSCs. This experiment was repeated twice. e: IFNγ-producing splenic T cells from animals in C were assayed by Elispot in responses to D5 ALDHhigh CSCs versus ALDHlow non-CSCs. Data are presented as a histogram graph. This experiment was repeated twice
We tested the potential immune mechanism which may be involved in this effect. CTLs from the mice vaccinated with dual peptides-DC with or without anti-PD-L1 exerted greater cytotoxicity of ALDHhigh CSCs than CTLs from the mice treated with PBS (p = 0.0402 and p = 0.0454, respectively) (Fig. 6d). Interestingly, although anti-PD-L1 alone did not increase the cytotoxicity of CTLs against ALDHhigh CSCs compared with the CTLs from the PBS group, dual peptides-DC vaccine plus anti-PD-L1 significantly enhanced the cytotoxic effect of CTLs to ALDHhigh CSCs compared with the dual peptide-DC vaccine alone (p = 0.0362) or anti-PD-L1 administration alone (p = 0.0091). However, these effects were not observed when ALDHlow non-CSCs were used as target controls in the same CTL assays (Fig. 6d), confirming the specificity of ALDH dual peptides-DC vaccine induced immunity against ALDHhigh CSCs..
In addition, we measured IFN-γ secretion via Elispot (Fig. 6e). We found that the number of IFN-γ-producing T cells per 105 splenic T cells was significantly increased in mice subjected to dual peptides-DC vaccine in response to ALDHhigh CSCs (p = 0.0491) compared with PBS controls. Moreover, T cells from mice treated with dual peptide-DC vaccine plus anti-PD-L1 induced significantly stronger IFN-γ secretion in response to ALDHhigh CSCs than T cells from mice treated with dual peptide-DC vaccine (p = 0.0470) or anti-PD-L1 alone (p = 0.0211). Of note, we detected significantly fewer numbers of the IFN-γ-producing T cells in response to ALDHlow non-CSCs than to ALDHhigh CSCs by T cells obtained from mice treated with dual peptide-DC vaccine (p = 0.0369) or dual peptide-DC plus anti-PD-L1 (p = 0.0382), demonstrating ALDH peptides-DC vaccine elicited anti-ALDHhigh CSC specificity. Additionally, flow cytometry showed that the proportion of intracellular IFN-γ-producing T cells from the spleen of mice treated with PBS, dual peptide-DC and dual peptide-DC plus anti-PD-L1 was 0.9%, 4.3% and 9.9%, respectively (data not shown). Collectively, this group of data suggest that co-administration of anti-PD-L1 mAb significantly augmented the immune effect of ALDH peptides-DC vaccination by eliciting T cell immune responses against ALDHhigh CSCs.
Accumulating evidence suggests that TILs may serve as a biomarker to assess the efficacy of cancer immunotherapy [20, 21]. We found that ALDH 1A1 and/or 1A3 peptide(s)-DC vaccination increased CD3+ TILs in residual tumors harvested from the vaccine-treated hosts (Fig. 5d and e). To evaluate the immune response in tumors after the combined use of anti-PD-L1 with the vaccine, we examined CD3+ TILs by flow cytometry and histology from mice in the D5 therapy experiments in Fig. 6c. Representative immunohistochemistry (IHC) of the CD3+ TILs is shown in Fig. 7a. Both dual peptides-DC vaccine and anti-PD-L1 alone induced CD3+ TILs in residual tumors. Importantly, the combination of these two treatments recruited more CD3+ T cells to infiltrate the D5 tumor than each single treatment (p < 0.0001, p = 0.0041) (Fig. 7b). These data suggest that blockade of the PD-1/PD-L1 pathway enhanced the efficacy of ALDH dual peptides-DC vaccine (Fig. 6c) by increasing local CD3+ TILs in addition to enhancing systemic T cell and antibody immune responses to ALDHhigh CSCs as demonstrated above.
Fig. 7.
a: The representative IHC results confirming increased CD3+ TILs in residual tumors. Tumor cells and background were stained with green color, and the purple-stained cells indicate the CD3+ TILs. b: Bar graph showing the statistical difference of CD3+ TILs post-treatments as indicated. The experiment was repeated twice
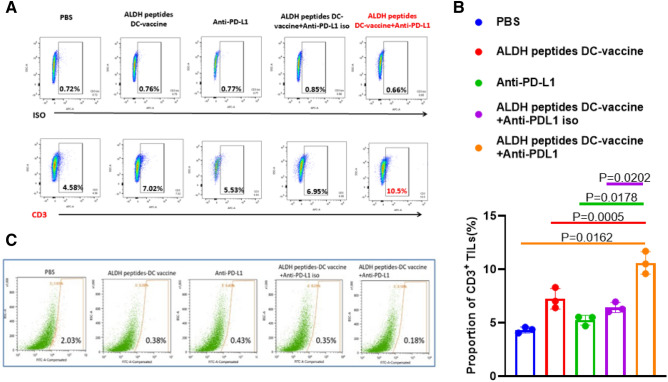
By flow cytometry, CD3+ TILs from mice after treatment were: PBS (4.58%), ALDH dual peptide-DC (7.02%) and dual peptide-DC plus anti-PD-L1 (10.5%) (Fig. 8a). Statistical analysis of three flow cytometry experiments showed that dual peptide-DC and anti-PD-L1 combined treatment group induced more TILs than monotherapy group (p = 0.0005 and p = 0.0178) (Fig. 8b). In addition, we examined the amount of residual CSCs at the end of the therapy experiments from Fig. 6c. By flow cytometry, both ALDH dual peptide-DC vaccine and anti-PD-L1 administration reduced the proportion of the ALDHhigh D5 cells in residual tumors (0.38% and 0.43%, respectively) compared with the PBS control (2.03%) (Fig. 8c). Of note, combined therapy of the two approaches reduced CSCs (0.18%) with the highest efficacy, indicating their interactive roles as evident by the reduction of ALDHhigh D5 cells by more than 90% compared to the PBS control.
Fig. 8.
Anti-PD-L1 enhanced the effect of ALDH peptides-DC vaccine via recruiting more CD3+ TILs and reducing the ALDHhigh CSCs. a: Anti-PD-L1 administration in addition to ALDH dual peptides-DC vaccination increased the CD3+ TILs as shown by flow cytometry. The single tumor cell suspension was made from residual D5 tumors harvested from the mice subjected to treatment as indicated. b: Bar graph showing the statistical difference of CD3+ TILs post-treatment as indicated. The experiment was repeated twice. c: ALDH dual peptides-DC vaccine with anti-PD-L1 decreased the ALDHhigh D5 cells in residual tumors. Flow cytometry showed the representative percentages of ALDHhigh D5 cells in residual tumors harvested from the mice post-treatment as indicated. Five mice were used each group
Discussion
Cancer stem cells promote the growth, metastasis and contribute to relapse of tumors. Furthermore, CSCs are relatively resistant to chemotherapy and radiotherapy [6]. Although immunotherapy represents an important breakthrough in cancer therapy, the limitation of these therapies may relate to their inability to effectively target the CSC population. The development of CSC targeting cancer vaccines has the potential to overcome this limitation. Over the past 50 years, many attempts have been made to generate cancer vaccines with limited success. However, it is important to note that the vast majority of cancer vaccines have focused on targeting differentiation associated antigens. These antigens are not expressed on CSCs, potentially limiting their clinical efficacy. Ex vivo-generated DCs loaded with tumor cell lysate or molecular antigens have been tested in the clinic and proven to be effective [22]. However, the clinical responses have been confined to a limited number of patients. In a phase I trial, Rudnick et al. evaluated the safety and potential synergy of surgical resection with Gliadel Wafer implantation, followed by autologous tumor lysate-pulsed DC vaccine in patients with malignant glioma. While the adjuvant autologous DC vaccine was safe, elicited modest immunogenicity, comparisons between vaccine responders and non-vaccine responders were not statistically significant [23]. Based on 173 published trials, Neller et al. described that 138 of 1711 patients treated with tumor cell lysate-based DC vaccines exhibited an objective response rate of 8.1%, and 63 of 1733 patients who were treated with molecularly defined antigen-based DC vaccines showed a response rate of 3.6% [24]. One of the major reasons responsible for these low response rates was the inability of these vaccines to induce anti-CSC immunity.
CSC-targeted immunotherapies represent promising advances in cancer treatment. We previously developed a vaccination strategy utilizing cell lysates of ALDHhigh CSCs to pulse DCs and found that vaccination of animals with these ALDHhigh CSC-targeted DCs resulted in significant anti-tumor immunity in prevention models [2]. Furthermore, we demonstrated in the adjuvant setting that ALDHhigh CSC lysate-DC vaccine significantly inhibited local recurrence after surgical resection of established tumor, compared with either ALDHlow or unsorted tumor cells lysate-DC vaccine [4]. In addition, using a radiation therapy model for established tumors, we reported that ALDHhigh CSC lysate-DC vaccination significantly inhibited tumor growth, reduced development of metastases and prolonged mice survival [3]. These results were consistent with two other studies in which pancreas and breast CSC lysate-pulsed DC vaccines, respectively, exhibited cytotoxic effect to CSCs [25, 26].
Although we have shown significant efficacy of vaccination with DCs pulsed with cell lysate of ALDHhigh CSCs (CSC-DC), obtaining adequate amounts of tumor from patients to isolate and make CSC lysate for vaccine production is labor intensive and often not feasible, thus representing a significant limitation for the clinical translation of this approach. Shared CSC antigens could serve as specific targets for cancer immunotherapy. For example, cytokine-induced killer cells armed with anti-CD3/anti-CD133 bispecific antibody significantly killed pancreatic and hepatic CD133high CSCs [27]. In addition, a specific chimeric antigen receptor (CAR) was designed to target CSC marker EpCAM, and the CAR therapy exerted significant anti-tumor activity against prostate cancer [28]. Studies have found that several isoforms of ALDH such as 1A1 and 1A3 not only regulate CSC function but also serve as markers for CSCs [29]. Visus et al. identified ALDH 1A1 as a novel CD8+ T cell-defined tumor antigen in head and neck squamous cell carcinoma [8], and HLA-A2-restricted, ALDH1A188–96 peptide-specific CD8+ T cells could recognize and eliminate ALDHhigh CSCs in vitro. The adoptive transfer of this ALDH1A1 peptide-specific CD8+ T cells inhibited tumor growth and reduced lung metastases in HNSCC and breast cancer in vivo [11]. However, Marcato found that CSC activity in patient with breast cancer correlated with the expression of isoform ALDH1A3 [30]. In other studies, both ALDH1A1 and ALDH1A3 have been identified as important contributors to CSC function [31, 32].
Our previous studies (2–4) demonstrated that ALDH may represent an ideal marker for CSC targeting. To eliminate the requirement to obtain intact CSCs as a source of antigen for CSC-targeted DC vaccine, we tested two peptides derived from ALDH1A1 (LLYKLADLI) and 1A3 (LLHQLADLV) as antigens to generate ALDH peptide-DC vaccines in this study. We found that ALDH 1A1 and 1A3 peptide-DC vaccines, respectively, induced T cell proliferation and anti-ALDHhigh CSC activity in vitro. CTL primed by these vaccines specific killed the ALDHhigh D5 CSCs. Importantly, we observed that an ALDH 1A1 + 1A3 dual peptides-DC vaccine demonstrated additive effect vs. each single peptide-DC vaccine. We further examined the efficacy of the ALDH peptide-DC vaccine in vivo in both the prevention and minimal disease therapy settings. ALDH1A1 and/or 1A3 peptide(s)-DC vaccine demonstrated significant protective immunity by inhibiting D5 tumor growth after challenge. In the minimal disease setting (day 1 tumor), ALDH peptide(s)-DC vaccination was efficacious in suppressing tumor growth that was enhanced by the co-administration of anti-PD-L1. In a double-blinded, randomized phase II trial in newly-diagnosed glioblastoma (GBM) patients, Wen et al. have recently evaluated an autologous DC vaccine pulsed with six synthetic peptide epitopes targeting GBM tumor/stem cell-associated antigens MAGE-1, HER-2, AIM-2, TRP-2, gp100 and IL-13Rα2 (ICT-107). The vaccine was well tolerated and increased progression-free survival by 2.2 months [33]. However, the primary endpoint of overall survival was not statistically increased. This study indicates that autologous DC vaccine targeting tumor/stem cell-associated antigens may not be sufficient, and the addition of anti-PD-L1 may increase its efficacy.
Immune monitoring in our study revealed that ALDH dual peptides-DC vaccination elicited strong T cell and antibody immunity targeting ALDHhigh CSCs, and this targeting was significantly elevated by co-administration of anti-PD-L1. As a result, the percentage of ALDHhigh D5 CSCs was significantly reduced by 90% after ALDH dual peptides-DC vaccination with anti-PD-L1 administration. Furthermore, we found that ALDH dual peptides-DC vaccination resulted in the recruitment of CD3+ TILs, which was also enhanced by anti-PD-L1 administration. Identification of the subsets of these CD3+ TILs and characterization of their function, e.g., T effectors versus Treg, warrant further investigation.
The study of interaction between CSCs and the immune system is of great interest, as CSCs may not only evade the immune system’s surveillance, but also use the immune system to promote their expansion and tumorigenesis [34]. The innate immune responses mediated by natural killer cells and macrophages have been reported to regulate the stemness of CSCs [35, 36]. Most notably, PD-L1 expression on CSCs was significantly elevated in various cancers, which has been suggested to facilitate CSC immune evasion [37, 38]. In a previous publication, we have demonstrated that a reduction in PD-L1 expression on tumor cells was strongly associated with ALDHhigh CSC-DC vaccine treatment [4]. On the other hand, PD-1/PD-L1 blockade together with vaccine therapy facilitated effector T cell infiltration into pancreatic tumors [39]. Another study confirmed that PD-1/PD-L1 blockade enhanced the efficacy of SA-GM-CSF surface-modified tumor vaccine in prostate cancer [40]. More recently, we have reported that anti-PD-L1 antibody significantly strengthened the therapeutic efficacy of an integrin β4 (ITGB4)-DC vaccine in both the 4T1 and SCC7 models [41]. ITGB4 has been shown to play an important role in the regulation of CSCs.
This work represents an addition to the initial work targeting ALDH peptide via passive (T cell) immunotherapy [8, 11], but with an alternative approach, e.g., active (vaccine-based) immunotherapy. The potential molecular and immune mechanisms underlining the anti-CSC effect of the vaccine need to be further clarified. Exploring the potential effect of the ALDH peptide(s)-DC vaccine combined with traditional cancer therapies, e.g., surgery, chemotherapy and radiation therapy, needs to be further explored to optimize the potential of this vaccine approach. Technically, ALDH peptide(s)-based vaccines may allow for the development of an “off-the-shelf” product as an immunologic approach for more broadly applicable clinical translation. Another important issue concerns the selectivity of this approach at generating anti-CSC responses while sparing normal tissue stem cells that also express ALDH. In our immunocompetent mouse models, we did not observe any systemic toxicity associated with vaccination. Although the mechanism of selective toxicity of CSCs vs normal tissue stem cells remains unknown, we have preliminary evidence for differential distribution of ALDH isoforms in these cells. Additional work will be required to further explore the mechanism(s) of this selective toxicity of CSCs vs normal tissue stem cells to understand the potential toxicity of these ALDH peptide(s)-based vaccines in clinical translation. It is critical to show the ALDH isoform expression on human melanoma tumors and identify HLA-A2 epitopes of these regions for human translation. This warranties further investigation.
Another area to explore is the identification of other CSC-associated antigens that could be utilized in combination with ALDH peptides. To identify other potential CSC antigens, we have begun to examine genes involved in reprogramming adult cells toward a more undifferentiated, pluripotent state including Sox2, Oct4 and Nanog, transcription factors capable of reprograming normal fibroblasts into induced pluripotent stem (iPS) cells [42]. The majority of CSCs express one or more of these reprogramming transcription factors. This concept is supported by recent evidence that a DC vaccine generated against iPS cells generates anti-tumor immunity across a spectrum of tumor types [43, 44] with no apparent systemic toxicity. Another recent report has demonstrated that pharmacologically modified pluripotent stem cells administered as a vaccine have anti-tumor effects in a murine breast cancer model [45]. Since these antigens are widely expressed in CSCs across a variety of tumor types, this approach may have wide applicability.
Author contributions
FL, ZJ and YYH performed the experiments, analyzed the data, prepared the figures and drafted the manuscript. QL and AEC designed the project and finalized the manuscript. Other authors provided assistance with partial experiments and data analysis. All authors read and approved the final manuscript.
Funding
This work was partially supported by Medimmune Inc. (MW & QL, No.16-PAF05892) and Gillson Longenbaugh Foundation (AEC & QL, No.C304136). F.L., J.Z. and Y.H. were supported in part by the China Scholarship Council, Hubei Cancer Hospital, and Wuhan Union Hospital, respectively.
Declarations
Conflict of interest
The author(s) declare that they have no conflict of interest.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Ethical approval
All the procedures used in the animal studies were approved by the Animal Care and Use Committee of the University of Michigan.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fei Liao, Jing Zhang and Yangyang Hu have contributed equally to this work.
Contributor Information
Alfred E. Chang, Email: aechang@umich.edu
Qiao Li, Email: qiaoli@umich.edu.
References
- 1.Marquardt S, Solanki M, Spitschak A, Vera J, Putzer BM. Emerging functional markers for cancer stem cell-based therapies: understanding signaling networks for targeting metastasis. Semin Cancer Biol. 2018;53:90–109. doi: 10.1016/j.semcancer.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Ning N, Pan Q, Zheng F, et al. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res. 2012;72:1853–1864. doi: 10.1158/0008-5472.CAN-11-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu L, Tao H, Chang AE, et al. Cancer stem cell vaccine inhibits metastases of primary tumors and induces humoral immune responses against cancer stem cells. Oncoimmunology. 2015;4:e990767. doi: 10.4161/2162402X.2014.990767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Y, Lu L, Xia Y, et al. Therapeutic efficacy of cancer stem cell vaccines in the adjuvant setting. Cancer Res. 2016;76:4661–4672. doi: 10.1158/0008-5472.CAN-15-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black WJ, Stagos D, Marchitti SA, Nebert DW, Tipton KF, Bairoch A, Vasiliou V. Human aldehyde dehydrogenase genes: alternatively spliced transcriptional variants and their suggested nomenclature. Pharmacogenet Genomics. 2009;19:893–902. doi: 10.1097/FPC.0b013e3283329023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shenoy A, Butterworth E, Huang EH. ALDH as a marker for enriching tumorigenic human colonic stem cells. Methods Mol Biol. 2012;916:373–385. doi: 10.1007/978-1-61779-980-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prince MEP, Zhou L, Moyer JS, et al. Evaluation of the immunogenicity of ALDH(high) human head and neck squamous cell carcinoma cancer stem cells in vitro. Oral Oncol. 2016;59:30–42. doi: 10.1016/j.oraloncology.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Visus C, Ito D, Amoscato A, et al. Identification of human aldehyde dehydrogenase 1 family member A1 as a novel CD8+ T-cell-defined tumor antigen in squamous cell carcinoma of the head and neck. Cancer Res. 2007;67:10538–10545. doi: 10.1158/0008-5472.CAN-07-1346. [DOI] [PubMed] [Google Scholar]
- 9.Duan JJ, Cai J, Guo YF, Bian XW, Yu SC. ALDH1A3, a metabolic target for cancer diagnosis and therapy. Int J Cancer. 2016;139:965–975. doi: 10.1002/ijc.30091. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Li W, Patel SS, et al. Blocking the formation of radiation-induced breast cancer stem cells. Oncotarget. 2014;5:3743–3755. doi: 10.18632/oncotarget.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visus C, Wang Y, Lozano-Leon A, et al. Targeting ALDH(bright) human carcinoma-initiating cells with ALDH1A1-specific CD8(+) T cells. Clin Cancer Res. 2011;17:6174–6184. doi: 10.1158/1078-0432.CCR-11-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Y, Dallaglio K, Chen Y, et al. ALDH1A isozymes are markers of human melanoma stem cells and potential therapeutic targets. Stem Cells. 2012;30:2100–2113. doi: 10.1002/stem.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muralikrishnan V, Hurley TD, Nephew KP. Targeting aldehyde dehydrogenases to eliminate cancer stem cells in gynecologic malignancies. Cancers (Basel) 2020 doi: 10.3390/cancers12040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas ML, de Antueno R, Coyle KM, Sultan M, Cruickshank BM, Giacomantonio MA, Giacomantonio CA, Duncan R, Marcato P. Citral reduces breast tumor growth by inhibiting the cancer stem cell marker ALDH1A3. Mol Oncol. 2016;10:1485–1496. doi: 10.1016/j.molonc.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu P, Brown S, Goktug T, et al. Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. Br J Cancer. 2012;107:1488–1497. doi: 10.1038/bjc.2012.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yip NC, Fombon IS, Liu P, et al. Disulfiram modulated ROS-MAPK and NFkappaB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br J Cancer. 2011;104:1564–1574. doi: 10.1038/bjc.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coukos G. Neoadjuvant immune-checkpoint blockade in resectable colon cancer. Nat Med. 2020;26:473–474. doi: 10.1038/s41591-020-0826-3. [DOI] [PubMed] [Google Scholar]
- 18.Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020 doi: 10.1126/science.aax0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu F, Hu Y, Zhou Y, Guo M, Lu J, Zheng W, Xu H, Zhao J, Xu L. MicroRNA-126 deficiency enhanced the activation and function of CD4(+) T cells by elevating IRS-1 pathway. Clin Exp Immunol. 2018;191:166–179. doi: 10.1111/cei.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orhan A, Vogelsang RP, Andersen MB, Madsen MT, Holmich ER, Raskov H, Gogenur I. The prognostic value of tumour-infiltrating lymphocytes in pancreatic cancer: a systematic review and meta-analysis. Eur J Cancer. 2020;132:71–84. doi: 10.1016/j.ejca.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Marin R, Reda S, Riobello C, Cabal VN, Suarez-Fernandez L, Vivanco B, Lopez F, Llorente JL, Hermsen MA. CD8(+) tumour-infiltrating lymphocytes and tumour microenvironment immune types as biomarkers for immunotherapy in sinonasal intestinal-type adenocarcinoma. Vaccines (Basel) 2020 doi: 10.3390/vaccines8020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol. 2017;38:577–593. doi: 10.1016/j.it.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Rudnick JD, Sarmiento JM, Uy B, et al. A phase I trial of surgical resection with Gliadel Wafer placement followed by vaccination with dendritic cells pulsed with tumor lysate for patients with malignant glioma. J Clin Neurosci. 2020;74:187–193. doi: 10.1016/j.jocn.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Neller MA, Lopez JA, Schmidt CW. Antigens for cancer immunotherapy. Semin Immunol. 2008;20:286–295. doi: 10.1016/j.smim.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Yin T, Shi P, Gou S, Shen Q, Wang C. Dendritic cells loaded with pancreatic Cancer Stem Cells (CSCs) lysates induce antitumor immune killing effect in vitro. PLoS ONE. 2014;9:e114581. doi: 10.1371/journal.pone.0114581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pham PV, Le HT, Vu BT, et al. Targeting breast cancer stem cells by dendritic cell vaccination in humanized mice with breast tumor: preliminary results. Onco Targets Ther. 2016;9:4441–4451. doi: 10.2147/OTT.S105239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang J, Li C, Wang Y, Lv H, Guo Y, Dai H, Wicha MS, Chang AE, Li Q. Cytokine-induced killer (CIK) cells bound with anti-CD3/anti-CD133 bispecific antibodies target CD133(high) cancer stem cells in vitro and in vivo. Clin Immunol. 2013;149:156–168. doi: 10.1016/j.clim.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Deng Z, Wu Y, Ma W, Zhang S, Zhang YQ. Adoptive T-cell therapy of prostate cancer targeting the cancer stem cell antigen EpCAM. BMC Immunol. 2015;16:1. doi: 10.1186/s12865-014-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcato P, Dean CA, Pan D, et al. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells. 2011;29:32–45. doi: 10.1002/stem.563. [DOI] [PubMed] [Google Scholar]
- 30.Vassalli G. Aldehyde dehydrogenases: not just markers, but functional regulators of stem cells. Stem Cells Int. 2019;2019:3904645. doi: 10.1155/2019/3904645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Alea M, McGrail K, Sanchez-Redondo S, et al. ALDH1A3 is epigenetically regulated during melanocyte transformation and is a target for melanoma treatment. Oncogene. 2017;36:5695–5708. doi: 10.1038/onc.2017.160. [DOI] [PubMed] [Google Scholar]
- 32.Tomita H, Tanaka K, Tanaka T, Hara A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget. 2016;7:11018–11032. doi: 10.18632/oncotarget.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen PY, Reardon DA, Armstrong TS, et al. A randomized double-blind placebo-controlled Phase II trial of dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin Cancer Res. 2019;25:5799–5807. doi: 10.1158/1078-0432.CCR-19-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sultan M, Coyle KM, Vidovic D, Thomas ML, Gujar S, Marcato P. Hide-and-seek: the interplay between cancer stem cells and the immune system. Carcinogenesis. 2017;38:107–118. doi: 10.1093/carcin/bgw115. [DOI] [PubMed] [Google Scholar]
- 35.Tallerico R, Todaro M, Di Franco S, et al. Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol. 2013;190:2381–2390. doi: 10.4049/jimmunol.1201542. [DOI] [PubMed] [Google Scholar]
- 36.Okuda H, Kobayashi A, Xia B, et al. Hyaluronan synthase HAS2 promotes tumor progression in bone by stimulating the interaction of breast cancer stem-like cells with macrophages and stromal cells. Cancer Res. 2012;72:537–547. doi: 10.1158/0008-5472.CAN-11-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta HB, Clark CA, Yuan B, et al. Tumor cell-intrinsic PD-L1 promotes tumor-initiating cell generation and functions in melanoma and ovarian cancer. Signal Transduct Target Ther. 2016 doi: 10.1038/sigtrans.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu JM, Xia W, Hsu YH, et al. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat Commun. 2018;9:1908. doi: 10.1038/s41467-018-04313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soares KC, Rucki AA, Wu AA, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. 2015;38:1–11. doi: 10.1097/CJI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi X, Zhang X, Li J, Zhao H, Mo L, Shi X, Hu Z, Gao J, Tan W. PD-1/PD-L1 blockade enhances the efficacy of SA-GM-CSF surface-modified tumor vaccine in prostate cancer. Cancer Lett. 2017;406:27–35. doi: 10.1016/j.canlet.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 41.Ruan S, Lin M, Zhu Y, et al. Integrin beta4-targeted cancer immunotherapies inhibit tumor growth and decrease metastasis. Cancer Res. 2020;80:771–783. doi: 10.1158/0008-5472.CAN-19-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 43.Cai S, Hou J, Fujino M, et al. iPSC-derived regulatory dendritic cells inhibit allograft rejection by generating alloantigen-specific regulatory T cells. Stem Cell Reports. 2017;8:1174–1189. doi: 10.1016/j.stemcr.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kooreman NG, Kim Y, de Almeida PE, et al. Autologous iPSC-based vaccines elicit anti-tumor responses in vivo. Cell Stem Cell. 2018;22(501–13):e7. doi: 10.1016/j.stem.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heront-Kishi MAA, Desterke C, Chaker D, de Goër de Herve MG, Turhan AG, Bennaceur-Griscelli A, Griscelli F (2020) Pharmacologically modified pluripotent stem cell-based cancer vaccines with anti-metastatic potential. BioRxiv. Doi: 10.1101/2020.05.27.118471
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.