Abstract
Macrophages are important precursor cell types of the innate immune system and bridge adaptive immune responses through the antigen presentation system. Meanwhile, macrophages constitute substantial portion of the stromal cells in the tumor microenvironment (TME) (referred to as tumor-associated macrophages, or TAMs) and exhibit conflicting roles in the development, invasion, and metastasis of thyroid cancer (TC). Moreover, TAMs play a crucial role to the behavior of TC due to their high degree of infiltration and prognostic relevance. Generally, TAMs can be divided into two subgroups; M1-like TAMs are capable of directly kill tumor cells, and recruiting and activating other immune cells in the early stages of cancer. However, due to changes in the TME, M2-like TAMs gradually increase and promote tumor progression. This review aims to discuss the impact of TAMs on TC, including their role in tumor promotion, gene mutation, and other factors related to the polarization of TAMs. Finally, we will explore the M2-like TAM-centered therapeutic strategies, including chemotherapy, clinical trials, and combinatorial immunotherapy.
Keywords: Tumor-associated macrophages, Tumor microenvironment, Cancer metastasis, Thyroid cancer, Immunotherapy
Introduction
Thyroid cancer (TC) is one of the most common endocrine tumors in the world, with a high incidence rate, attracted more and more attention [1–3]. There are several subtypes of TC, with a variety of prognostic and therapeutic options. For example, follicular-cell-derived cancer accounts for the majority of TC and divided into follicular thyroid cancer (FTC), invasive-encapsulated follicular variant papillary cancer, papillary thyroid cancer (PTC), oncocytic carcinoma of the thyroid, poorly differentiated thyroid cancer, differentiated high-grade thyroid cancer, and anaplastic thyroid cancer (ATC) based on the cell of origin, pathologic features, biological behavior, and molecular classification [2, 4]. Among them, PTC and FTC are "mild" and have a good prognosis, while ATC is fierce, highly malignant and has a poor prognosis [2]. Currently, the conventional treatment for TC includes surgery, radiation therapy and thyroid hormone preparation, but satisfactory outcomes are sometimes difficult to achieve [5]. Tumors mainly through tumor-infiltrating immune cells, immunomodulatory molecules and soluble factors interact with the surrounding immune microenvironment during their development, to weaken the anti-tumor activity of the immune system, thereby mediating the immune tolerance and causing tumor escape [6, 7].
Macrophages constitute a substantial portion of the stromal cells in the tumor microenvironment (TME) (referred to as tumor-associated macrophages, or TAMs) and exhibit conflicting roles in the development, invasion, and metastasis of TC [8–10]. TAMs can be divided into two subgroups, M1-like TAMs and M2-like TAMs. M1-like TAMs are capable of directly killing tumor cells, and recruiting and activating other immune cells in the early stages of cancer. However, M2-like TAMs promote tumor progression. Moreover, TAMs play a crucial role in the behavior of TC due to their high degree of infiltration and prognostic relevance [7, 9, 11]. It has been found that TAMs correlate with extrathyroidal extension and capsular invasion in poorly DTC [12]. ATC also had the highest density of TAMs in TME resulting in decreased survival rates [7]. And in PTC, the density of TAM positively correlates with lymph node metastasis, larger tumors and poorer survival rates [9, 13]. In addition, TAMs were increased and correlated with tumor size, epithelial characteristics, lymph node metastases, and a reduced CD4/CD8 positive T cell ratio in the v-Raf murine sarcoma viral oncogene homolog B (BRAF) mouse model [14, 15]. The previous studies have also demonstrated that chemokine C-C motif ligand 2 (CCL2) levels are associated with TAM levels [16, 17], and the presence of large amounts of TAMs was more frequently associated with the poor prognosis of patients with TC [14]. This review aims to discuss the impact of TAMs on TC, including their role in tumor promotion, gene mutation, and other factors related to the polarization of TAMs.
Thyroid cancer and tumor microenvironment
TME is primarily made up of stromal cells, innate and adaptive immune cells, endothelial cells, cytokines, and chemokines [18, 19]. And it changes metabolic, secretory, immunological, and other factors that affect cancer development and promotes the growth and spread of tumors [20, 21]. It has been demonstrated that TME, infiltrating immune cells and immunotherapeutic effect were different for subtypes of TC [10, 14, 22, 23]. Furthermore, it has been confirmed that the disruption of the delicate balance of the original microenvironment encouraged the migration and proliferation of TC [8, 24]. Contrarily, tumor immune escape is another way for TC cells to survive and proliferate in vivo through TME [25]. Although tumor cells are often destroyed by immune cells in the early stages of cancer, TME can help tumor cells evade immune surveillance and even prevent immune cells from producing cytotoxic effects on the tumor cells [10, 20, 26, 27]. Macrophages occupy high percentage in the TME referred to as TAMs, play a crucial role in the regulation of the immune system and TC cells [28–30]. The different functions of TAMs have been classified into two opposing phenotypes, M1-like TAMs with a pro-inflammatory pathogen-killing capacity and M2-like TAMs that promote tissue remodeling, angiogenesis, and a key role in TC progression [8, 31–33]. In addition, as the degree of infiltration of M2-like TAM was strongly associated with the progression of TC suggested that the TAMs-targeting therapeutic approach could be the potential approach for TC [6, 9, 13, 34].
The classifications and functions of TAMs
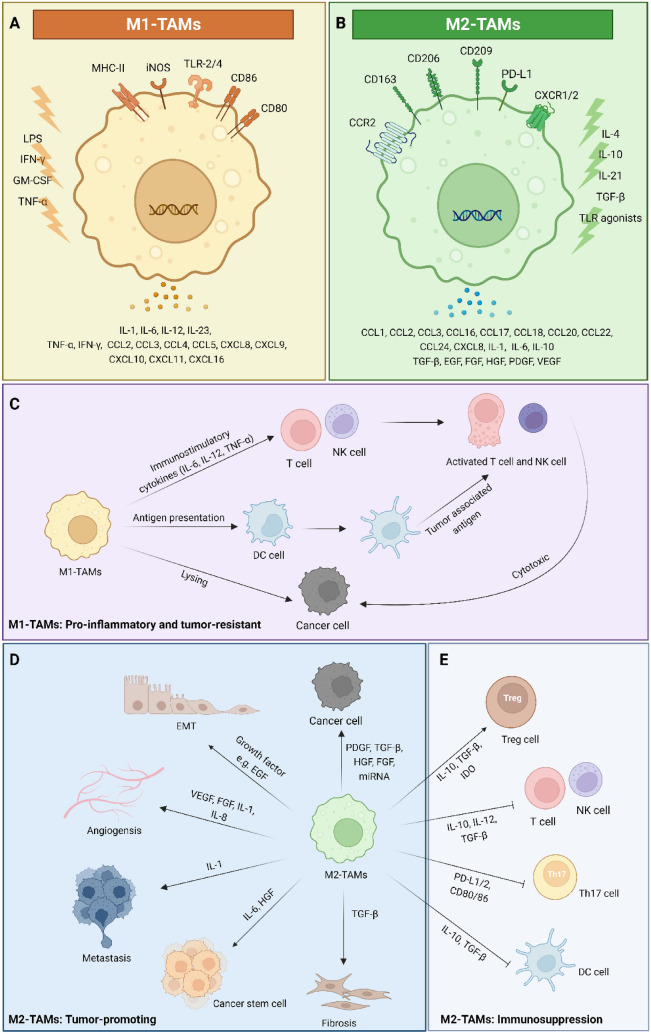
TAMs are generally divided into two phenotypes: M1-like TAMs, which contribute to the ability of the immune system to control malignancy, and M2-like TAMs, which accelerate tumor growth and reduce the anti-tumor effect of the immune system [35, 36]. Figure 1 A and B describe the subpopulations and functions of TAMs with identifiable markers and secretions. In detail, M1-like TAMs could activate innate or adaptive lymphocyte-mediated mechanisms of tumor resistance. The immunostimulatory cytokines such as interleukin (IL)-6, IL-12, and tumor necrosis factor-α (TNF-α) from M1 phenotype TAMs can enhance the anti-tumor ability of T cells and natural killer (NK) cells [37, 38]. Meanwhile, M1-like TAMs can promote can act as specialized antigen-presenting cells (APCs) when properly activated [39]. Also, the M1-like TAMs have the potential to kill tumor cells, relying mainly on antibody-dependent cellular cytotoxicity and autophagocytosis, which can cause vascular damage and tumor necrosis [40]. In contrast, TAMs are predominantly of the M2 phenotype in most solid tumors and promote the proliferation of cancer cells, angiogenesis in the TME, and suppression of innate and adaptive immune responses [27, 41, 42]. Several studies have shown that M2-like TAMs secretion of fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) are essential to supporting the progress of the tumor-associated vascular system, a process by which new blood vessels sprout from existing vessels or through the proliferation, motility, and accumulation of vascular endothelial cells [13, 43–45]. Moreover, M2-like TAMs cause immunosuppression by expressing inhibitory receptors or immune checkpoint ligands, such as programmed death-ligand1/2 (PD-L1/2), and CD80/CD86 [42, 46, 47] and the cytokines including IL-10 and transforming growth factor-β (TGF-β). In addition, M2-like TAMs promote metastasis of tumors by secreting growth factors that support tumor angiogenesis and neointimal formation as well as epithelial-to-mesenchymal transition (EMT) and tissue remodeling [30, 45, 48]. The extracellular vehicles (EVs) released by M2-like TAMs are also responsible for cancer metastasis by transferring certain microRNA (miRNA) in the colorectal cancer model [49]. TAMs from different polarization may perform different functions in the TME of cancer, which provides an opportunity to target immunotherapies more precisely (Fig. 1 C-E). Hence, the ideal strategy would involve selective targeting of M2-like TAMs and maintaining the functionality of M1-like TAMs without compromising the homeostatic immune system in vivo.
Fig. 1.
Surface markers and the functions with secretions of TAMs in tumor microenvironment. TAMs are classified as M1 and M2 polarizations, and plasticity is an important characteristic. TAMs can be characterized by the expression of different surface markers, explaining the variation of M1/M2-like TAMs in the TME for the same or different markers and receptors. In A, M1-like TAMs that could be induced by LPS + IFN-γ with anti-tumor functions can be stimulated by immunostimulatory cytokines, and MHC-II molecules are required for effective antigen presentation. In addition, some surface proteins of M1-like TAMs, CD80, and CD86, were also upregulated. M1-like TAMs also produce chemokines such as CXCL10 that promote T cell recruitment and activation. On the right, M2-like TAMs that could be induced by IL-4 with pro-tumorigenic functions are regulated by the hypoxic tumor microenvironment and immunosuppressive mediators (IL-10, TGF-β etc.). Similarly, some surface proteins of M2-like TAM were upregulated, including CD163, and CD206. The M1-like TAMs have the functions of phagocytosis and lysis of tumor cells and can promote inflammation and anti-tumor effect. Moreover, M1-like TAMs enhance the activity of antigen-presenting cells (DC cells) and promote the cytotoxic effects of other cancer killing leukocytes (T cells and NK cells) (C). However, M2-like TAMs are tumor-promoting activities, commonly through the secretion of growth factors (including EGF, FGF, HGF, PDGF, VEGF, and TGF-β, etc.) that support tumor angiogenesis and neointima formation as well as EMT and tissue remodeling (B). Meanwhile, M2-like TAMs promote and induce the proliferation and metastasis of cancer cells by creating an immunosuppressive TME (D and E). This figure was created using BioRender.com. Abbreviations: CCR2, C-C chemokine receptor type 2; CXCL8, chemokine C-X-C motif ligand 8; CXCR1/2, C-X-C chemokine receptor 1/2; DC cell, dendritic cell; EGF, epidermal growth factor; EMT, epithelial-to-mesenchymal transition; FGF, fibroblast growth factor; GM-CSF, granulocyte–macrophage colony-stimulating factor; HGF, hepatocyte growth factor; IDO, indoleamine 2,3-dioxygenase; IL-1, interleukin-1; IFN-γ, interferons-γ; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; MHC-II, major histocompatibility complex class II; NK cell, natural killer cell; PDGF, platelet-derived growth factor; PD-L1/2, programmed death-ligand 1/2; TGF-β, transforming growth factor-β; Th17 cell, T helper 17 cell; TLR, toll-like receptor, TNF-α, tumor necrosis factor-α; Treg cell, regulatory T cell; VEGF, vascular endothelial growth factor
The factors related to the polarization of TAMs and the treatment in TC
The gene mutations associated with polarization of TAMs in TC
Similar to other malignancies, TME of TC consists of immune cells (macrophages, mast cells, and lymphocytes) and soluble mediators (chemokines, cytokines, and growth factors) that are active in and around cancer cells [50, 51]. However, in contrast to other tumors, the prognosis of TC was often associated with complex genetic mutations [14, 52, 53]. For example, the BRAF mutations are most common in PTC or ATC [54]; RAS mutations are found predominantly in FTC [55]; and RET proto-oncogene mutations are thought to be the cause of the majority of medullary thyroid cancer (MTC) [56]. Also, current single-cell technologies, particularly single-cell RNA sequencing (scRNA-seq) demonstrated the impact of cellular subtypes on disease progression including TC, and improved the identification of biomarkers for stratification of patients. For example, the technology of scRNA-seq could quantify the cellular components and their interactions in the TME, and improve the understanding about the heterogeneity and gene mutation of TC [57]. At the same time, many gene mutations in different subtypes of TC have been proved to be associated with TAMs and the progression of the tumor [13, 15, 26, 43, 58–62]. A study has shown that protein levels of the CCL2, a major tumor-derived serum chemokine of monocytes, were regulated by RET/PTC in thyroid cells [58]. Simultaneously, previous studies have demonstrated that CCL2 levels are associated with TAMs levels [16, 17], and the presence of large amounts of TAMs was more frequently associated with the poor prognosis of patients with TC [14]. In the BRAFV600E mouse model, TAMs were increased and correlated with tumor size, epithelial characteristics, lymph node metastases, and a reduced CD4/CD8 positive T cell ratio, thus supporting a potential immunosuppressive effect of PTC [14, 15]. Additionally, the BRAF mutation was found to be higher in PTC than in benign thyroid tissues and was associated with poor prognostic factors such as M2-like TAMs, angiogenesis-related genes, elevated tumor, node, and metastasis (TNM) staging [43, 60]. The BRAF mutation also related with presence of myeloid-derived suppressor cells (MDSCs) [53], promoted the TAMs polarized toward an M2 phenotype, and can assist the TC cells in escaping from immune killing [18]. It has also been shown that the BRAF mutation has independent prognostic value as a recurrence of PTC and correlates with TAM polarization [15, 63]. Moreover, the TAMs account for a large proportion of tumor-infiltrating immune cells in TC compared with other tumors and are highly plastic [9, 13, 34, 64], and therapies targeting TAMs could be relevant in TC. Therefore, TAMs are the newly insightful therapeutic approach due to the polarization, pro-tumorigenic properties [6, 65, 66], gene mutation-related specificity[8, 9, 15, 64], and high density in the TC [6, 7, 67].
Cytokines associated with polarization of TAMs in the TC
M1/M2-like TAMs are highly plastic cells and their function can change significantly depending on microenvironmental signals from the TME of TC [52, 68–71]. For example, a previous study proved that the high expression of C-X-C chemokine receptor type (CXCR4) recruits more TAMs [72]. At the same time, CXCR4 is often overexpressed by TC cells because of RET rearrangements and the quantity of CXCR4 expressed by primary TC corresponds with the degree TAMs and lymph node metastasis [67, 73, 74]. Moreover, elevated chemokine C-X-C motif ligand (CXCL)16 expression in the medium mediated the invasion of PTC tumor cells when PTC cells and TAMs are co-cultured with the high percentage of M2-like TAMs has also been demonstrated [52]. Similarly, CSF-1 has also been studied in the TC as a major factor controlling the growth and differentiation of TAMs [14]. Previous studies had demonstrated that the coordination of autocrine and paracrine interactions between TC cells and TAMs is accomplished by chemokines and cytokines [6, 10, 14, 32, 60]. Also, the chemokines and cytokines in the TME of TC are core regulators and have been identified as one of the hallmark drivers of cancer [75]. The following types of factors have been shown to correlate with the development of TC and TAMs, including VEGF [28], CXCL1 [54], 7 [54], 8 [9, 18, 32], 12 [72, 76], 16 [43, 60], CXCR4 [67, 77–79], colony-stimulating factor 1(CSF-1) [14, 73], C-C chemokine receptor (CCR) type 2 [32, 75] and cytokines including IL-1 [18, 28], 6 [6], 8 [18, 34, 75, 80], 32 [80].
The treatment method associated with polarization of TAMs in TC
Currently, the main treatments for not-resectable cancerous diseases, radiotherapy, and chemotherapy, both cause tissue damage and cancer cell death due to local or systemic inflammation [18, 81–83]. Previous studies found that after ablative radiotherapy, the innate immune system was activated by inflammatory cytokines [84] and pro-fibrotic factors that recruited TAMs and promoted tumor recurrence and progression [69, 85]. Furthermore, the radiotherapy also induced senescence of TC cells, and the senescent cells triggered the polarization of M2-like TAMs accompanied by increased expression of CCL17, CCL18, IL-18, and TGF-β [69, 86]. In addition, differentiated M2-like TAMs promote the stemness and migration of tumor cells [30, 48, 68, 86, 87]. The study of TAMs should be more significant and necessary since radiotherapy is essential in the treatment of intractable TC. In addition to radiotherapy, chemotherapy for the TC, including systemic drugs targeting tumor angiogenesis [88], and targeted molecular drugs such as multikinase and rapamycin inhibitors, could also induce M2-differentiation of TAMs [18, 89, 90]. For example, in the treatment of ATC, Lenvatinib monotherapy and even in combination therapies with programmed cell death protein (PD-1)/PD-L1 inhibitor increased the density of M2-like TAMs [91]. Moreover, TAMs also modulate other immune cells in the TEM of TC. For example, TAMs express T cell immune checkpoint ligands and directly inhibit T cell functions, while also secreting cytokines such as IL-10 and TGF-β that contribute to the maintenance of a strong immunosuppressive TME [28, 45, 92, 93]. Since TAMs can promote tumor growth and metastasis by secreting cytokines or producing immunosuppressive TME, removal of TAMs or alteration of TME has been confirmed could reduce the progression of TC [14, 91, 94–96]. Considering the plasticity of TAMs, the concept that reprogramming the polarization may affect the function of TAMs, has also attracted significant attention in the treatment of TC [6, 45, 68, 70, 76, 77, 79, 97].
Other factors in TME that associated with polarization of TAMs in the TME of TC
As one of the most relevant intercellular communication mechanisms between cells in the TME, EVs could also reprogram the host cell and affect the polarization of TAMs [98, 99]. For example, colorectal cancer cell-derived EVs containing miR-934 induced CD163 positive M2 polarization of TAMs by activating the phosphoinositide-3 kinases (PI3K)/AKT signaling pathway and enhanced invasion and liver metastasis [100]. Similarly, a study reported that epithelial ovarian cancer-derived exosomal miRNA-222-3p induced polarization of TAMs to the M2 phenotype by activating the SOCS3/STAT3 signaling cascade [101]. Furthermore, it has also been demonstrated that colorectal tumor-secreted EVs with miRNA-145 promote the polarization of THP-1 to M2-like TAMs, leading to the downregulation of IL-12 and the upregulation of IL-10 [102]. In addition, it has been reported that exosomes derived from hypoxic epithelial ovarian cancer were enriched in miRNA-21-3p, miRNA-125b-5p, and miRNA-181-5p, and these miRNAs promoted M2 polarization of TAMs by activating the SOCS4/5/STAT3/hypoxia inducible factor-1α (HIF-1α) signaling cascade [103]. These studies demonstrated that miRNAs are primarily involved in EV-mediated M2-like TAM polarization and miRNAs are hallmarks of tumor-derived EVs a cargo consisting of multiple miRNAs (41.7% mature miRNAs of all RNAs in EVs) [104]. Additionally, the lactate in the TME also affect polarization of TAMs and has been shown to upregulate M2-like TAM by enhancing aerobic glycolysis in recent studied of TC [105, 106]. Therefore, an in-depth understanding of the changes in the complex relationship between tumor cells, TME and TAMs may provide new ideas for the treatment of TC.
TAMs-centered treatment strategy in TC
Therapeutic strategies aimed at targeting TAMs or modulating their activity are under development and are being applied in both clinical trials and animal experiments [27, 92, 107]. More than 90% of TC patients are PTC and FTC with relatively good prognoses, but there are still some patients with refractory TC. For example, the treatment and prognosis of patients with ATC were very limited, but the proportion of TAM is positively correlated with the aggressive tumor, so targeting TAM may provide new ideas for refractory TC [7, 10, 34, 61]. Additionally, another study revealed significant differences in TAM density between subtypes, leading the researchers to hypothesize that TAM density may be a prognostic factor for TC [65]. Several TAM targeting techniques have been investigated, including TAM depletion to decrease the pro-tumorigenic activity of TAMs [15, 58, 107, 108], inhibition of TAM[14, 94, 108, 109] recruitment from monocytes [14, 75], reprogramming of the TAMs to an M1-like phenotype [28, 32, 68, 70, 110, 111], and dissolving the immunosuppressive environment with the recovery of tumor-killing activities of cytotoxic T cells [6, 91, 112, 113]. TAMs, the primary cells that mediate the relationship between cancer and inflammation, undoubtedly play a significant role in opening the door for a novel approach to the treatment of TC. Figure 2 summarized the studies reporting TAMs targeting strategies and methods for the dichotomous behavior of TAMs and the existing approach applied for TC was marked with asterisks *.
Fig. 2.
Therapeutic strategies targeting and reprogramming TAMs: The strategies are divided into four main categories: (1) elimination of TAMs and inhibition of monocyte differentiation to TAMs; (2) reprogramming TAMs to the anti-tumor activity based on the polarization to the M1-like TAMs and expression of markers targeting M2-like TAMs; (3) reprogramming based on the phagocytosis function of TAMs; (4) inhibiting the immune suppression microenvironment and allow cytotoxic T cells activity. *Articles marked with asterisk are studies for thyroid cancer. This figure was created using BioRender.com. Abbreviations: CCR2, C-C chemokine receptor type 2; CCL2, chemokine C-C motif ligand 2; CSF-1, colony-stimulating factor-1; CSF-1R, colony-stimulating factor-1 receptor; CTLA-4, cytotoxic T-lymphocyte-associated protein-4; EVs, extracellular vesicles; HDAC, histone deacetylase; LILRB-2, leukocyte immunoglobulin-like receptor subfamily B member 2; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PI3K, phosphoinositide 3-kinases; SIRP-α, Signal regulatory protein α; STAT3, signal transducer and activator of transcription 3; TIM3, T cell immunoglobulin and mucin domain-containing protein 3; TLR, toll-like receptors; VEGF, vascular endothelial growth factor
TAM depletion and inhibition of recruitment
Targeting the Elimination of TAMs
A tyrosine kinase receptor named CSF-1 receptor (CSF-1R), expressed by macrophages, activated the recruitment of monocytes to TAMs, leading to the reprogramming of these TAMs to the M2 phenotype, which has been demonstrated in many animal tumor models including TC [14, 114], primary human macrophages[115] and clinical trial [94]. Therefore, blockade of the CSF-1R and CSF-1 axis may be a viable strategy for targeting tumor suppressor TAMs with an M2 phenotype [114–116]. In many clinical studies, including TC, various antibodies and small compounds predominantly targeting CSF-1R are being investigated [65, 92]. Small molecules such as PLX3397 [94, 117] and JNJ-40346527 [108, 109] have been examined in the clinical trials, while other small molecules such as ARRY-382 [118], PLX7486 [119], and BLZ945 [120] are currently under clinical trials investigation. Other monoclonal antibodies targeting CSF-1R or its ligand CSF-1, such as emactzumab [121], AMG820 [122, 123], cabiralizumab [124, 125], and MCS110 [126], are also currently being clinical trials investigated as monotherapy or in combination.
Clinical trials [94] and experimental animal studies [127] using CSF-1R antibodies or in combination with other checkpoints clinical trials [128, 129] have also been conducted for TC. The findings from phase I and phase II trials demonstrated that PLX3397 was well tolerated at a dosage of 1000 mg, and the extension study found that 12 of 23 patients (52%) had an anti-tumor response after treatment [130]. Furthermore, CSF-1/CSF-1R-targeted therapy is tolerable to date, suggesting the possibility of combination therapy with current immunotherapy options, including immune checkpoint inhibitors. Additionally, conditional activation of BRAFV600E increased the expression of the TAM chemoattractant CSF-1, and targeting CSF-1-expressing cells reduced TAMs had been proved in animal models [14, 65]. This strategy also induced smaller tumors, reduced PTC proliferation, and restored the thyroid follicular architecture. The CSF-1R inhibitor treatment also impaired the progression of PTC and TAM recruitment. This study primarily focused on PTC and FTC and demonstrated that TAMs are pro-tumorigenic in TC and can be used as targeting pharmacology. Moreover, it may be potentially useful for patients with advanced TC, such as ATC. The clinical immunotherapy strategies for TC are listed in Table 1.
Table 1.
Immunotherapy strategies and therapeutic targets for thyroid cancer
| Drug | Therapeutic targets | Patient population | Status | Trial registry number |
|---|---|---|---|---|
| LY3022855 | CSF-1R | Head and neck carcinoma | Completed | NCT01346358 |
| LY3022855 plus Tremelimumab and Durvalumab | CSF-1R plus PD-1 | Advanced solid tumors | Completed | NCT02718911 |
| Tremelimumab plus Durvalumab | PD-1 | Metastatic TC | Recruiting | NCT03753919 |
| Pembrolizumab | PD-1 | ATC | Completed | NCT02688608 |
| Pembrolizumab | PD-1 | ATC | Recruiting | NCT05119296 |
| Pembrolizumab | PD-1 | Advanced solid tumors (TC) | Recruiting | NCT02628067 |
| Pembrolizumab | PD-1 | FTC | Completed | NCT02054806 |
| Pembrolizumab plus Docetaxel | PD-1 | TC | Recruiting | NCT03360890 |
| Pembrolizumab plus SO-C101 | PD-1 | Advanced solid tumors (TC) | Recruiting | NCT04234113 |
| Pembrolizumab plus Lenvatinib | PD-1 plus VEGFR | ATC | Recruiting | NCT04171622 |
| Pembrolizumab plus Lenvatinib | PD-1 plus VEGFR | DTC | Active, not recruiting | NCT02973997 |
| Pembrolizumab plus Dabrafenib and Trametinib | PD-1 plus BRAF and MEK | ATC | Recruiting | NCT04675710 |
| PDR001 | PD-1 | ATC | Completed | NCT02404441 |
| PDR001 plus Dabrafenib and Trametinib | PD-1 plus BRAF and MEK | TC | Active, not recruiting | NCT04544111 |
| Vudalimab | PD-1 and CTLA-4 | ATC | Recruiting | NCT05453799 |
| Nivolumab plus Ipilimumab | PD-1 plus CTLA-4 | Rare tumor (TC) | Active, not recruiting | NCT02834013 |
| Nivolumab plus Ipilimumab and Cabozantinib | PD-1 plus CTLA-4 and VEGFR | Advanced DTC | Active, not recruiting | NCT03914300 |
| Nivolumab and Cabozantinib | PD-1 plus VEGFR | Advanced tumor (TC) | Recruiting | NCT04514484 |
| Nivolumab and Lenvatinib | PD-1 plus VEGFR | ATC | Recruiting | NCT05696548 |
| Nivolumab plus Encorafenib and Binimetinib | PD-1 plus BRAF and MEK | TC | Recruiting | NCT04061980 |
| CemIplimab plus Dabrafenib and Trametinib | PD-1 plus BRAF and MEK | ATC | Recruiting | NCT04238624 |
| Tislelizumab plus Surufatinib | PD-1 plus VEGFR | Advanced solid tumors (ATC) | Active, not recruiting | NCT04579757 |
| Tislelizumab plus Anlotinib and Radiotherapy | PD-1 plus VEGFR | ATC | Recruiting | NCT05659186 |
| AIC100 Chimeric Antigen Receptor (CAR) T cells | PD-1 and CTLA-4 | TC | Recruiting | NCT04420754 |
| Atezolizumab plus Cabozantinib | PD-L1 plus VEGFR | ATC | Active, not recruiting | NCT04400474 |
| Atezolizumab plus Cobimetinib | PD-L1 plus MET | TC | Completed | NCT01988896 |
| Durvalumab and Radiotherapy | PD-L1 | TC | Active, not recruiting | NCT03215095 |
| Vorinostat | HDAC | TC | Completed | NCT00134043 |
| PCI-24781 plus Pazopanib | HDAC plus VEFGR | Metastatic solid tumors (TC) | Recruiting | NCT01543763 |
Abbreviations: ATC, anaplastic thyroid cancer; BRAF, v-Raf murine sarcoma viral oncogene homolog B; CSF-1R, colony-stimulating factor 1 receptor; CTLA-4, cytotoxic T-lymphocyte-associated protein-4; DTC, differentiated thyroid cancer; FTC, follicular thyroid cancer; HDAC, histone deacetylase; MEK, mitogen-activated protein kinase; MET, receptor tyrosine kinase of MET proto-oncogene; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PI3K, phosphoinositide 3-kinases; TC, thyroid cancer; VEGFR, vascular endothelial growth factor receptor
In addition, VEGF can lead to massive infiltration of TAMs into the TC, and a previous study indicated upregulation of VEGF-A expression in ATC patients, which demonstrated that suppression of VEGF might also be a potential strategy for the depletion of TAMs in the TC [65, 131]. Previous studies in hepatocellular carcinoma cell lines [44] and patients of colorectal cancer [132] have observed that a VEGF-depleted environment attenuates the tumor-promoting function of TAMs by reducing cytokine secretion. However, the results also demonstrated that the inhibition of VEGF secretion by cancer cells did not alter the M2 polarization of macrophages in TME. The proangiogenic of TAMs and their assistance in tumor metastasis has been reported in several studies including experiment research and the tissue of patients [43, 44, 133]; however, there are limited studies targeting this modality in TC. Furthermore, another therapeutic strategy is the selective depletion of TAM, employing bisphosphonates including clodronate and zoledronic acid. The previous result of the animal model has shown that injection of clodronate with liposomes into the mice reduced the M2-like TAMs and inhibited the lung metastasis of ATC [95]. In another retrospective clinical study, zoledronic acid was effective in reducing new metastases and improving survival in patients with bone metastases with FTC [134]. Moreover, it was also indicated that the potential therapeutic mechanism may be due to zoledronic acid inhibiting the growth of TAMs, which often overexpress osteoclast-inducing factors to prompt bone resorption or osteolysis [135].
Targeting the inhibition of monocyte recruitment
TAM expansion in cancers is typically mediated by monocyte recruitment via the CCL2-CCR2 axis, including TC [14, 32, 64]. Several investigations have established the role of CCL2, a potent chemoattractant for monocytes, T cells, and NK cells, in the accumulation of TAMs in animal tumor models and the sample of patients [14, 16, 17, 32, 75]. Additionally, it has been demonstrated that patients with ATC have elevated serum CCL2 levels [75], indicating that CCL2 and its receptor, CCR2, may be potential therapeutic targets for TC patients. Another study revealed that vitamins can function as an independent factor to decrease the migration of TC cell lines by lowering the levels of CCL2 and CCL8 [136]. The relationship between vitamins and inflammation in tumors is currently the subject of one clinical trial; additional findings may be attained in the future [137]. In addition, the lack of experimental and clinical trials of TC and the findings from other animal models of cancer calls for careful consideration of anti-CCL2 drugs as monotherapy [138]. The researchers emphasized the TME as a key determinant of successful anti-metastatic therapy and indicated the need for additional biological knowledge to effectively inhibit TAMs. For example, CCL2 inhibition severely depletes monocytes while also increasing the risk of compensatory macrophage growth if recruitment is inhibited, and CCL2-CCR2 communication is essential for monocytes to enter the circulation from the bone [139]. However, prolonged systemic depletion of TAMs may lead to host immunosuppression and susceptibility to opportunistic infections, so targeting downstream mediators of TAMs may be an alternative strategy [92, 140].
Reprogramming of TAMs
TAMs are typically pro-tumorigenic but can be reprogrammed to suppress tumor development by triggering the immune system [27, 97, 141]. According to this scenario, it may be possible for TC to employ plasticity therapeutically to restore the anti-cancer capabilities of TAMs [6, 69, 70, 86, 142].
Targeting the specific marker of TAMs
Targeting immunosuppressive TAMs effectively is significantly hampered by the absence of specific protein markers expressed on M2-like TAMs. Several new ATC-specific immune checkpoint genes have been experimentally identified by using the tissue sample of patients, including the immunosuppressive molecule leukocyte immunoglobulin-like receptor subfamily B member-2 (LILRB-2) [10]. The combination of antibodies to LILRB-2 and anti-PD-L1 attenuates the inhibitory effect of TAMs on T cell proliferation and alters the TME to induce anti-tumor immunity in the animal model [143]. Furthermore, stabilin-1 has been shown to be expressed primarily by TAMs in human gastric cancer tissues, and a higher density of stabilin-1-positive cells was linked with a lower survival rate [144]. In addition, BRAFV600E expression in mice models of PTC showed the high recruitment of stabilin-1-positive TAMs and induced the immunosuppressive effect [54]. Moreover, increasing evidence suggests that targeting the mannose receptor (MR), CD206, which is highly expressed on M2-like TAMs, is a valuable alternative [145, 146]. In addition, a synthetic peptide, RP-182, can modulate the conformational switch of the MR expressed on M2-like TAMs, induce phagocytosis, phagosome-lysosome formation in macrophages, and transfer TAMs from the M2 to the M1 phenotype, increasing innate and adaptive anti-tumor immune responses and improving tumor treatment outcomes [147]. Both in vitro and in vivo experiments have demonstrated that TC-derived medium significantly increased MR expression in TAMs [6, 148]. Also, activated TAMs can establish a tumor-promoting environment and promote the progression of TC cells [6]. These findings indicate that targeting M2-like TAM markers may be a potential therapeutic approach for patients with TC.
Targeting the polarization signaling pathway-PI3K inhibitor
The PI3K signaling pathway is involved in almost all types of signaling and acts as a molecular switch that increases immunosuppressive to immunostimulatory activity in the TAMs [92]. A previous study confirmed that the genetic ablation of PI3K decreased hypoxic stabilization and the TAMs-related proangiogenic factors while inducing the secretion of proinflammatory cytokines [11]. Furthermore, it has been confirmed that M2-like TAMs could activate the PI3K pathway and promote TC stem cell proliferation and metastasis in the ATC in experiment research and the tissue of patients [30]. Moreover, a multicenter phase II pilot study showed that PI3K inhibitors can reduce tumor growth in rats; however, no survival benefit was obtained, which may be due to incomplete inhibition of oncogenic pathways and/or escape mechanisms [149]. These results suggest that the PI3K inhibitor is not sufficient alone, but it could reprogram TAMs and reduce the formation of TC stem cells. Therefore, PI3K could be a potential future therapeutic target for TC treatment.
Targeting the polarization signaling pathway-Histone deacetylase (HDAC) inhibitor
HDAC inhibitors are well-known epigenetic modulators with therapeutic potential for a variety of cancer by modifying the polarization of TAMs [27, 150, 151]. Recently, a study has linked HDAC inhibitors with immune-mediated anti-cancer effects, influenced the efficiency of immunotherapy, and reduced the M2-like TAMs in the animal model [150]. In other animal experiment, researchers found the release of inflammatory cytokines was increased as a result of MP195, a selective HDAC2 inhibitor with the highest concentration, which increased the proportion of M1-like TAMs [151]. Furthermore, celastrol, a novel HDAC inhibitor, was shown to modulate TAM polarization from M2 to M1 and inhibited colorectal cancer growth in the animal model [152]. However, in phase II clinical study designed to evaluate the objective response to HDAC inhibitor (vorinostat) in 19 patients with progressive TC, no patients achieved a partial or complete response [153]. Recently, the combination of TMP195 and PD-1 blockade may provide a therapeutic strategy for colorectal cancer-bearing mice [154], which may provide a novel combination therapy for TC as HDAC inhibitors have no significant effect as monotherapy against TC [153, 155, 156].
Targeting the polarization signaling pathway-signal transducer and activator of transcription 3 (STAT3) inhibitor
An experimental study demonstrated the regulating potential of the T cell immunoglobulin and mucin domain 3 (TIM3) pathway in TC where it was found that TIM3 blockage partially reversed TAM polarization [6]. Furthermore, one animal study indicated that TIM3 activated the STAT3 signaling, increased M2-like TAM polarization, promoted the epithelial-mesenchymal transition of the tumor cells, and finally induced the lung metastasis of osteosarcoma [87]. Clinical trial exploring the therapeutic effects is currently being conducted for solid cancers, and in vitro study showing the therapeutic potential of anti-TIM3 antibodies have shown encouraging results in ATC [6, 157]. Therefore, TIM3 blockers have tremendous potential for TC immunotherapy in conjunction with the reprogramming of TAMs. In addition, STAT3 has been shown to directly induce the expression of the marker protein CD163 in macrophages and induce the change in TAMs from the M1 to the M2 phenotype of human monocyte-derived macrophages and animal models [158, 159]. Moreover, another experimental study in TC found miRNA-324-5p could affect the polarization of TAMs through the STAT3 signaling pathway [142]. The findings also suggested that miRNA-324-5p induced the invasion or migration of endothelial cells and the polarization of M2-like TAMs via VEGF and IL-4 or IL-13, respectively. Several STAT3 inhibitors are being tested in clinical trials, including TTO101 [160–163], OPB-31121 [164–166], and Imx110 [167], but trials for TC patients are still not available. Although some methods were still not applied in TC, TAMs targeting strategies are classified in Table 2.
Table 2.
Therapeutic strategies aimed into TAMs
| Treatment strategies | Mechanisms | Targets | References |
|---|---|---|---|
| The depletion of TAMs | Metastasis and Angiogenesis | Anti-VEGF | [131]* |
| Apoptosis of the TAMs | Bisphosphonates | [95]* | |
| Monocyte recruitment | CSF-1 and CSF-1R axis | [14]* | |
| CCL-2 and CCR-2 axis | [75, 136]* | ||
| Phenotype-based reprogramming | Targeting polarization of TAMs | PI3K signaling pathway | [30, 105]* |
| HDAC signaling pathway | [156]* | ||
| STAT3 signaling pathway | [6]* | ||
| TLR signaling pathway | [169–171] | ||
| Reagents | [68, 70]* | ||
| miRNA inhibitor | [142]* | ||
| Viruses and bacteria | [32, 110, 187]* | ||
| EVs | [33]* | ||
| Nanoparticles | [219, 220]* | ||
| Targeting M2-like TAMs expression markers | Anti-Stabilin-1 | [54]* | |
| Anti-LILRB | [10]* | ||
| Anti-MARCO | [251] | ||
| Anti-MR | [147] | ||
| Function-based reprogramming | Targeting phagocytic activity of TAMs | PD-1 and PD-L1 axis | [230] |
| CD47 and SIRP-α axis | [10, 111]* | ||
| Macrophage engineering | Anti-HER2 CAR-TAMs | [256] | |
| Alternation of Immune suppression TME | Activation of T cells | PD-1 and PD-L1 axis | [91, 112, 231, 234]* |
| CTLA-4 and CD80/86 axis | [235, 264]* |
*Articles marked with asterisk are studies for thyroid cancer
Strategies to deplete or inactivate TAMs (by targeting cytokines, the CSF1-CSF1R axis, or using bisphosphonates) and strategies to inhibit monocyte recruitment to TAMs (targeting the CCL2-CCR2 axis) have been widely used in the treatment of tumors, including TC. Furthermore, changing the phenotype of TAMs from a protumor effect to an anti-tumor state may be another preferable therapeutic approach. It can primarily be divided into two types: (1) phenotype-based reprogramming and (2) function-based reprogramming. These recommendations are under development, and several reprogramming strategies have been tested, some of which are already applicable to TC. Additionally, TAMs also have protumor effects by suppressing immune activation. Therefore, TC therapy involves blocking immunosuppressive molecules in TAMs, which has been investigated in TC. The expression of chimeric antigen receptors (CARs) by TAMs which is currently in clinical trials for breast cancer but has not yet been studied in TC, is another provocative therapeutic strategy
Abbreviations: CAR-TAMs, chimeric antigen receptor-tumor associated macrophages; CCR2, C-C chemokine receptor type 2; CCL2, chemokine C-C motif ligand 2; CSF-1, colony-stimulating factor; CSF-1R, colony-stimulating factor receptor; CTLA-4, cytotoxic T-lymphocyte-associated protein-4; EVs, extracellular vesicles; HER2, receptor tyrosine-protein kinase erbB-2, HDAC, histone deacetylase; LILRB-2, leukocyte immunoglobulin-like receptor subfamily B member 2; MARCO, macrophage receptor with collagenous structure; MR, mannose receptor; PD-1, programmed cell protein 1; PD-L1, programmed death-ligand 1; PI3K, phosphoinositide 3-kinases; SIRP-α, signal regulatory protein-α; STAT3, signal transducer and activator of transcription 3; TC, thyroid cancer; TIM3, T cell immunoglobulin and mucin domain-containing protein 3; TLR, toll-like receptor; VEGF, vascular endothelial growth factor
Targeting the polarization signaling pathway with toll-like receptor (TLR) agonists
TAMs have been observed to become pro-inflammatory when exposed to TLRs in TME and restricted tumor progression in the animal tumor model [23, 168]. For example, intra-tumor administration of TLR-7 and TLR-9 increased monocyte infiltration and repolarized TAMs toward a proinflammatory phenotype in breast tumor mice models [169]. Furthermore, due to changes in the TAM phenotype, a similar effect was observed with TLR-7 and TLR-8 agonists that cause tumor regression in the mice models of melanoma [170, 171]. TLR-4 expression in TC is related to tumor metastasis, aggressiveness, and the BRAFV600E mutation, according to two studies using the tissue of patients and animal experiments [172, 173]. However, the therapeutic role of TLR agonists in TC with TAMs has not been studied. Currently, four TLR7 agonists (DSP-0509 [174], BNT411 [175], BDC-1001 [176], BDBD018 [177] and three TLR9 agonists (SD101 [178–180], CMP-001 [181–183], and tilsotolimod [184–186]) are being tested in clinical trials for their anti-tumor properties, and several clinical trials for solid tumor [175, 177, 186] and advanced cancer [184] may include patients with TC.
Targeting other factors related to the reprogramming of TAMs
Drugs could be used for the reprogramming of TAMs in addition to signaling pathway regulators in TC [68, 70]. For example, bleomycin, which primarily inhibits DNA synthesis, reverses the M2-like into M1-like TAMs and significantly decreases the cell proliferation, migration, and invasion of the TPC-1 cell line [70]. The M2-like TAM marker CD206 was suppressed by treatment with bleomycin, whereas the M1 phenotype marker CD80 and the major M1 secretagogues (TNF-α and IL-1β) were increased. Similarly, zoledronic acid treatment prevented the M2 polarization of the THP-1 monocytes cell line, thus inhibiting the stemness and metastasis of TC cells [68]. Furthermore, zoledronic acid with radioactive iodine has been proven effective in reducing new metastases and improving survival in patients with DTC bone metastases in one retrospective review conducted on 50 patients [134]. Zoledronic acid inhibits the growth of TAMs in TC cell lines, and TAMs often overexpress osteoclast-inducing factors, which contribute to bone resorption or osteolysis [135]. Therefore, targeting TAMs may be a potential therapeutic mechanism for the treatment of TC bone metastasis. Except for the reagents, studies on altering the differentiation of TAMs by the virus have also been conducted for the treatment of TC. For example, two experimental studies have confirmed that the oncolytic virus inhibited ATC growth [187] and switched M2-like TAMs toward an M1 phenotype [32]. In addition, the oncolytic activity of the virus (dl922-947) could be increased by a poly ADP ribose polymerase (PARP) inhibitor and inhibited the progression of ATC both in vitro and in vivo experiment [110]. Expect the virus, bacteria (e.g., Mycobacterium indicus pranii (Mw)) can also increase TAMs to repolarize to the M1 phenotype with the reduction of regulatory T cells in the animal models of melanoma [65, 188]. Additionally, Mw upregulated the expression of CD80/CD86 positive macrophages in a tuberculosis model by stimulating the nuclear factor-κB (NF-κB) signaling pathway [189]. Further research is required to determine whether these novel bacteria and the combination therapy could be used in TC.
EVs in TME have been demonstrated in many studies to influence TC development by mediating intercellular signaling and also affecting TAM polarization [33, 190, 191]. For example, research on the TC mice model showed that CXCR4 expression in PTC is restricted by EVs containing miRNA-655-3p, which prevents TAM growth, invasion, and M2 polarization [33]. By targeting various transcription factors and bridging proteins, miRNA-29a-3p [192], miRNA-103 [193], miRNA-145 [102], miRNA-203 [194], miRNA-222 [101], miRNA-934 [100], and miRNA-940 [195] induced M2 polarization. On the other hand, the miRNA-9 [196], miRNA-16 [197], miRNA-21 [198], miRNA-127 [199], miRNA-125b [200, 201], miRNA-155 [200] contained in the EVs related to M1 polarization have been reported by various experimental studies. In addition, the surface glycosylation profile of the EVs has been found to contain a significant amount of mannose, making it a suitable ligand for MR, which can target M2-like TAMs [202, 203]. Additionally, modification of the EVs by molecular engineering could help reduce the immunosuppression of TME [145]. Furthermore, a study has demonstrated that EV-mimics from M1 macrophages can directly repolarize M2 into M1-like TAMs that release proinflammatory cytokines, induce anti-tumor immune responses, and enhance the anti-cancer efficacy of PD-L1 [204]. This approach offers the possibility of programmed polarization of TAMs, and previous studies have already provided possible candidate strategies [145, 205]. In addition to the immunomodulation ability, several studies have shown that miRNAs in EVs are highly stable and protected by lipid bilayers, making them suitable and promising tumor markers for the clinical diagnosis of TC [206–209].
In addition to the EVs, nanoparticles alone or with chemotherapy can also be intelligently designed to promote M1-polarization and inhibited the progress in the mice model of melanoma [210–212]. Additionally, nanoparticles that mimic NK cell membranes can modulate TME, increase the percentage of M1-like TAMs, and polarize TAMs, improving both in vitro and in vivo immunotherapy for breast cancer [213]. Also, the effects of glycocalyx-mimetic nanoparticles on mouse primary peritoneal macrophage polarization have been investigated. The findings revealed that macrophage cells of mice were successfully repolarized to the M1 phenotype with increased expression of CD86 markers and elevated IL-12 levels [214]. Cytokines such as IL-12 was considered a typical candidate marker for promoting the reversal of M2-like TAMs to an M1 phenotype including TC both in clinical and animal study [13, 93, 215]. Previous research focusing on this characteristic has created pH-sensitive polymeric nanoparticles to encapsulate IL-12 for targeted immunotherapy. The nanoparticles loaded with IL-12 can passively accumulate and release IL-12 at tumor sites and exert therapeutic effects by promoting the polarization of the TAMs to the M1 phenotype in tumors by using the melanoma mice model [216]. Similarly, a plasmid DNA encoding the IL-12 gene is delivered into TAMs using a multifunctional fusion peptide-modified macrophage and tumor-targeted delivery system. This in vitro study indicated the nanoparticles enhanced IL-12 production, increased the release of proinflammatory cytokines, upregulated the M1 marker (CD80), and downregulated the M2 marker (CD206) [217]. Furthermore, to promote the repolarization of TAMs toward the M1 phenotype, clinical trials have respected for various transcriptional signaling drugs that can be flexibly loaded into nanoparticles, such as CD47-signaling regulatory protein-α (SIRP-α) antibodies [218] and TLR agonists [23]. In addition, it is reassuring to note that nanoparticles are typically decorated with specific targeting ligands, which can facilitate the successful transfer of signaling modulators in solid tumor mice models, including TC [219, 220]. The unmodified gold nanoparticles limited the growth of PTC cells (BCPAP and TPC-1), including cell proliferation, migration, and invasion, as demonstrated in a previous study [220]. The novel gold nanomedicine CYT-21625, which was developed for the targeted delivery of TNF-α with paclitaxel, has also shown significant inhibition of the progression of ATC in animal models [219].
Targeting phagocytic activity of TAMs
Tumor cells can evade clearance by macrophages by overexpressing anti-phagocytic surface proteins. The CD47 associated with macrophage SIRP-α is the source of anti-phagocytic signals that have been the subject of most research and documentation [221–223]. According to the study using the ATC mouse model, inhibiting CD47 increased phagocytosis and resulted in the overexpression of CD11B and CD80 on TAMs [111]. Moreover, in addition to the CD47 antibody, TTI-621, a fully human recombinant protein that blocks CD47-SIRP-α has been applied in-human phase I clinical trials [224]. One experiment study also confirmed the LILRB1 on TAMs is a novel signal to inhibit phagocytosis of cancer cells that evade SIRPα-CD47 blockers and the expression was increased in the ATC patients [10, 225]. While, the clinical trials for the TC are still being developed, and dozens of drugs targeting CD47 are currently being recruited; their names cannot be listed here in detail. Monoclonal antibodies that target the interaction between PD-1 and PD-L1 have shown clinical significance in combating a variety of cancers [226–228]. Recent experiment and clinical studies have demonstrated that TAMs also express PD-1 and are related to the progress of tumors [46, 229]. Moreover, a previous study confirmed that the PD-1 expression of TAMs was negatively correlated with phagocytic potency against tumor cells [230] and that blocking PD-1 reduced tumor growth in the model of orthotopic murine ATC [231].
Alteration of the immune suppression of TME
In the mice model of breast cancer and osteosarcoma, targeting PD-1/PD-L1 in TAMs, which converts its phenotype into an anti-tumor phenotype, directly leads to increase T cell-mediated immune surveillance [46, 232]. Another animal study of melanoma also demonstrated that anti-PD-L1 treatment increased M1-like TAM cell proliferation, survival, and activation, as well as upregulated proinflammatory-related pathways [47]. In another animal study of melanoma, targeted TAM depletion created a favorable environment to facilitate local and systemic delivery of antibodies against PD-1 antibody-adherent platelets [233]. The TME was further reprogrammed and promoted T cell infiltration into the tumor tissue by eliminating TAM. The antitumor of targeting PD-1/PD-L1 was also performed to enhance the efficacy of Lenvatinib by altering the TME in the mice of ATC [91]. In addition, another study showed that when a combination of BRAF inhibitors and anti-PD-1/PD-L1 antibodies was used, the reduction in MDSCs was usually accompanied by an increase in M1-like TAMs and reduced tumor volume in an orthotopic mouse model [231]. Except for the experimental studies, the clinical trials of immunotherapy against PD-1/PD-L1 have also been applied in TC [234], and the results show promise in patients with ATC [112]. In detail, two of 22 patients in a clinical trial of the anti-PD-1 antibody (pembrolizumab) in patients with advanced and PD-L1-positive TC confirmed a partial response [234]. In another phase II clinical trial for ATC, 42 patients had three complete responses and five partial responses to anti-PD-1 therapy [112]. Although it is well established that PD-1/PD-L1 blockade activates T cells, little is known about the role of a combination of targeting TAMs and TME in TC. The role of PD-1/PD-L1 blockers on TAMs in TC should not be neglected by the focus on T-cell signaling, as the effect on TAMs may inform the assessment of therapeutic efficacy and suggest alternative treatments.
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is another immune checkpoint inhibitor of TC that functions by unbreaking the T cells to turn on the anti-tumor immune response [113, 235]. In a phase II trial for TC, anti-CTLA-4 and anti-PD-1 combination therapy resulted in an objective response rate of 12%, with two partial responses in 17 patients [113]. In another phase 1 dose-escalation study, tumor reduction was demonstrated with anti-CTLA-4 and anti-PD-1 combination therapy, but the sample of patients with TC patients in this study was too small [235]. Despite the rapid advancement of immunotherapy technology, particularly with the development of immune checkpoint inhibitors, there are limited studies for TC [236, 237].
The advantages and prospects of TAM-targeted therapy in TC
As mentioned, the polarization of TAMs was strongly associated with TME of TC and correlated with aggressiveness of the cancer, which may provide a new platform for the treatment of TC that are refractory to conventional treatment strategies [13, 14, 18, 34, 58, 61, 62, 238]. First of all, the density of TAMs is increased in PTC tissues compared to benign thyroid disease [13]; and PTC with BRAF mutation significantly increases the expression of CSF-1 and CCL2 which attracted the TAMs [14]. Moreover, the TAMs correlated with lymph node metastasis [9, 13] and were used to assess the prognosis of TC [34]. Thus, developing TAM-targeted therapies requires a thorough understanding of the role of TAMs in the progression of TC as well as the effects of therapeutic strategies on TME. Also, it has been previously suggested that TAM-targeting therapeutics may offer a new prospect for EV-mediated tumor immunotherapy [205]. For example, EVs have been shown to act as effective immunomodulators of TAM depletion through the synthesis of paclitaxel-containing microvesicles and an ultrasound-mediated delivery method [239]. Moreover, a previous study found that nanovesicles from M1-like TAMs can directly regulate the transition of M2 to the M1 phenotype by modulating miRNA expression profiles [204], and the IL-4 receptor [240]. Therefore, the design of new therapeutic EVs, combined with the TAMs-targeted strategy, may herald a new era in cancer immunotherapy, including TC. Unfortunately, there is limited research on TAM in TC, particularly in patients who have recently undergone chemotherapy or radiotherapy. Due to the high plasticity of TAMs and the high density in TC, TAM-centered therapy, and the combinatorial treatment with chemotherapy, radiotherapy, or immunotherapy may become a new therapeutic opportunity for TC.
The limitation of the existing strategies
Appealing therapeutic strategies in TC include the depletion of TAMs and inhibition of monocyte recruitment to inhibit tumor development while preserving the cell types that support a protective immune response [27, 41, 66, 92, 97, 241]. However, the successful clinical application of these strategies requires careful investigations to overcome the current limitations. For instance, clodronate is a drug that can completely eliminate TAMs and hence is a simple and effective strategy. However, the drug may cause high toxicity if patients are treated for prolonged periods [92]. Moreover, osteonecrosis of the jaw (ONJ), a rare but severe side effect in TC patients, was previously linked to oral and systemic administration of bisphosphonates [242]. In addition, ONJ may worsen in patients using a combination of bisphosphonates and tyrosine kinase inhibitors (TKIs) for DTC and ATC [89, 243]. Furthermore, at the clinical level, this approach has not been inconsistently tested in clinical trials in different cancers [244–246]. These results suggest that different cancers or even various subtypes of the same cancer such as triple-negative breast cancer, and ATC may have side effects due to high doses application of the drugs. On the other hand, the depletion of TAM also affects other cells, e.g., M1-like TAM produces pro-inflammatory cytokines and up-regulates major histocompatibility complex class II (MHC-II) that promotes the anti-tumor activity of T cells [247, 248]. Research has shown that T cell-mediated immunity depends on monocytes and macrophages [247], also for anti-PD-1 [249] and anti-CTLA-4 therapies [250]. Complete TAM depletion makes it challenging to combine checkpoint inhibitors and immunotherapy [66]. In addition, the complex composition of the TME leads to dynamic interactions between TAMs and other immune cells that may vary with the progression of cancer, for which deletion of TAMs may disrupt the equilibrium structure and promote tumor progression. Another strategy is to target cancer-associated myeloid immature progenitors, which are now known to be highly heterogeneous and complex, posing a challenge to the development of myeloid cell-targeted immunotherapies [19, 248]. One of the greatest challenges is identifying specific markers [92], and the massive cell depletion in immature populations may result in multi-organ side effects. Hence, feasible alternatives to consider include temporary TAM ablation and targeting the recovery periods during which monocytes can be attracted before becoming pro-tumoral TAMs.
Therefore, a better strategy in the future would be to enhance the activity of anti-tumor TAMs or repolarize existing TAMs to produce anti-tumor activity, but the results for TC are rare. For example, the methods to repolarize M2 to M1-like TAMs for new characteristic gene markers have been used in melanoma and breast cancer, such as macrophage receptor with collagenous structure (MARCO) antibody therapy [27, 66, 92, 251], but no experimental or clinical trials have been conducted for TC. Additionally, the novel signature genes of TAMs have been identified, including scavenger receptor-triggering receptors expressed on myeloid cells 2 (TREM2) [252], apolipoprotein E (APOE) [169], secreted phosphoprotein 1 (SPP1) [10, 253], and V-set and immunoglobulin domain containing 4 (VSIG4) [10]. Additional single-cell RNA-seq studies also indicated that TAMs were frequently present with both pro-tumor and anti-tumor signatures [254]. This phenomenon suggests that macrophages in the TME may not have conventional M1/M2 polarization [253]. Understanding the role of novel TAMs subsets in TME may require new technologies like spatial transcriptomics and multiplex immunofluorescence [255]. Therefore, the identification and discovery of targets for reprogramming TAMs is critical for transforming TME from a pro-cancer to an anti-cancer function. An additional provocative therapeutic strategy of TAM manipulation has been introduced, including the engineering of macrophages to express chimeric antigen receptors (CAR) [27]. To date, CAR-TAMs studies are mainly at the preclinical stage, with data confirming their efficacy (growth-inhibiting tumor phagocytosis) in solid tumors [256–258]. However, whether it is the identification and discovery of targets for reprogrammed TAMs or clinical trials of CAR-TAMs, there is limited research in TC.
In addition to the issues of appeal and opportunities, many aspects need to be expanded upon. How can a balance be struck between TAMs and other immune cells to favor the acquired immune system over tumor development in the ever-changing state of TME? Does the composition of TME change before and after treatment? Does this change allow TAMs to develop chemotherapy resistance? How should subsequent treatment modalities and medications be organized for TAMs that have developed resistance? How can different subtypes of TC affect the polarization of TAMs, and what are the different therapeutic approaches that need to be proposed for the different subtypes? How could patients of different genders be applied in the clinical setting to develop treatment modalities for TAMs? Many studies have previously shown that sex hormones directly influence the characterization of TAMs, although this has not yet been explored in TC [259–263]. These are the major questions that need to be explored and answered subsequently.
Conclusions
To date, tremendous efforts have been made to promote immunotherapy to TC. TME plays an important role in TC genesis, metastasis, and stem cell proliferation, and TAM accounts for the largest proportion of TME cells. Although it is currently believed that high infiltration of M2-like TAMs supports TME and promotes TC growth, the study of TAM in TC is still a new field. Meanwhile, targeting therapy of TAMs in TC has the following advantages: 1. TAMs have a high degree of infiltration in TC and related with the prognosis of the cancer; 2. common genetic mutations in TC related with the polarization of TAMs, e.g., BRAF mutation has been shown to cause the TAMs polarization into M2 phenotype; 3. Since the gene mutation of BRAF correlated with high densities of M2-like TAM, targeted therapies can be better developed based on the subgroup of TC. Numerous studies have demonstrated the importance of innate immune cells in preventing the onset and progression of cancer, and TAMs may be a promising target in the future. Thus, targeting therapy of TAMs provides a platform to be considered for immunotherapy of TC. Despite the favorable prognosis of TC, more therapeutic strategies based on or in combination with TAMs need to be explored in the future.
Acknowledgements
Not applicable.
Abbreviations
- ATC
Anaplastic thyroid cancer
- APC
Antigen-presenting cells
- APOE
Apolipoprotein E
- BRAF
V-Raf murine sarcoma viral oncogene homolog B
- CAR-TAMs
Chimeric antigen receptor-tumor-associated macrophages
- CCL
Chemokine C-C motif ligand
- CCR
C-C chemokine receptor type
- CSF-1
Colony-stimulating factor
- CSF-1R
Colony-stimulating factor receptor
- CTLA-4
Cytotoxic T-lymphocyte-associated protein-4
- CXCL
Chemokine C-X-C motif ligand
- CXCR
C-X-C chemokine receptor
- DC cell
Dendritic cell
- DTC
Differentiated thyroid cancer
- EGF
Epidermal growth factor
- EMT
Epithelial-mesenchymal transition
- EVs
Extracellular vehicles
- FGF
Fibroblast growth factor
- FTC
Follicular thyroid cancer
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- HDAC
Histone deacetylase
- HER2
Receptor tyrosine-protein kinase erbB-2
- HGF
Hepatocyte growth factor
- HIF-1α
Hypoxia inducible factor-1α
- IDO
Indoleamine 2,3-dioxygenase
- IFN-γ
Interferons-γ
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- LILRB1/2
Leukocyte immunoglobulin-like receptor subfamily B1/2
- LPS
Lipopolysaccharide
- MARCO
Macrophage receptor with collagenous structure
- MDSC
Myeloid-derived suppressor cells
- MEK
Mitogen-activated protein kinase
- MET
Receptor tyrosine kinase of MET proto-oncogene
- MHC-II
Major histocompatibility complex class II
- MTC
Medullary thyroid cancer
- miRNA
MicroRNA
- MR
Mannose receptor
- NF-κB
Nuclear factor-κB
- NK cell
Natural killer cell
- ONJ
Osteonecrosis of the jaw
- PARP
Poly ADP ribose polymerase
- PD-1/2
Programmed cell death protein 1/2
- PDGF
Platelet-derived growth factor
- PI3K
Phosphoinositide 3-kinases
- PD-L1/2
Programmed death-ligand 1/2
- PTC
Papillary thyroid cancer
- scRNA-seq
Single-cell RNA sequencing
- SIRP-α
Signal regulatory protein-α
- SPP1
Secreted phosphoprotein 1
- STAT3
Signal transducer and activator of transcription 3
- TAMs
Tumor-associated macrophage
- TC
Thyroid cancer
- TGF-β
Transforming growth factor-β
- Th17 cell
T helper 17 cell
- TIM3
T cell immunoglobulin and mucin domain-containing protein 3
- TKIs
Tyrosine kinase inhibitors
- TLR
Toll-like receptor
- TME
Tumor microenvironment
- TNF-α
Tumor necrosis factor-α
- TNM
Tumor, node and metastasis
- Treg cell
Regulatory T cell
- TREM2
Scavenger receptor-triggering receptors expressed on myeloid cells 2
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
- VSIG4
V-set and immunoglobulin domain containing 4
Author contributions
In this paper, B-CA and XJL planned the project and the main conceptual ideas. LZ drafted the initial draft of the manuscript and designed the figures. PG and XJ contributed significantly to the modification and discussion of the manuscript. All authors have approved the final version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT), Grant/Award Number: NRF-2022R1A2C2005057.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiuli Jing, Email: jingxiuli84@163.com.
Byeong-Cheol Ahn, Email: abc2000@knu.ac.kr.
References
- 1.Wu J, Zhao X, Sun J, Cheng C, Yin C, Bai R. The epidemic of thyroid cancer in China: current trends and future prediction. Front Oncol. 2022;12:932729. doi: 10.3389/fonc.2022.932729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Megwalu UC, Moon PK. Thyroid cancer incidence and mortality trends in the United States: 2000–2018. Thyroid. 2022;32:560–570. doi: 10.1089/thy.2021.0662. [DOI] [PubMed] [Google Scholar]
- 3.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. 2017;317:1338–1348. doi: 10.1001/jama.2017.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baloch ZW, Asa SL, Barletta JA, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022;33:27–63. doi: 10.1007/s12022-022-09707-3. [DOI] [PubMed] [Google Scholar]
- 5.Oh JM, Ahn BC. Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS. Theranostics. 2021;11:6251–6277. doi: 10.7150/thno.57689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stempin CC, Geysels RC, Park S, et al. Secreted factors by anaplastic thyroid cancer cells induce tumor-promoting M2-like macrophage polarization through a TIM3-dependent mechanism. Cancers (Basel) 2021;13:1. doi: 10.3390/cancers13194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung KY, Cho SW, Kim YA, Kim D, Oh BC, Park DJ, Park YJ. Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. J Pathol Transl Med. 2015;49:318–324. doi: 10.4132/jptm.2015.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho SW, Kim YA, Sun HJ, Kim YA, Oh BC, Yi KH, Park DJ, Park YJ. CXCL16 signaling mediated macrophage effects on tumor invasion of papillary thyroid carcinoma. Endocr Relat Cancer. 2016;23:113–124. doi: 10.1530/ERC-15-0196. [DOI] [PubMed] [Google Scholar]
- 9.Fang W, Ye L, Shen L, et al. Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis. 2014;35:1780–1787. doi: 10.1093/carcin/bgu060. [DOI] [PubMed] [Google Scholar]
- 10.Pan Z, Bao L, Lu X, et al. IL2RA(+)VSIG4(+) tumor-associated macrophage is a key subpopulation of the immunosuppressive microenvironment in anaplastic thyroid cancer. Biochim Biophys Acta Mol Basis Dis. 2023;1869:166591. doi: 10.1016/j.bbadis.2022.166591. [DOI] [PubMed] [Google Scholar]
- 11.Joshi S, Singh AR, Zulcic M, Durden DL. A macrophage-dominant PI3K isoform controls hypoxia-induced HIF1alpha and HIF2alpha stability and tumor growth, angiogenesis, and metastasis. Mol Cancer Res. 2014;12:1520–1531. doi: 10.1158/1541-7786.MCR-13-0682. [DOI] [PubMed] [Google Scholar]
- 12.Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. 2008;15:1069–1074. doi: 10.1677/ERC-08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qing W, Fang WY, Ye L, et al. Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid. 2012;22:905–910. doi: 10.1089/thy.2011.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryder M, Gild M, Hohl TM, Pamer E, Knauf J, Ghossein R, Joyce JA, Fagin JA. Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid cancer progression. PLoS ONE. 2013;8:e54302. doi: 10.1371/journal.pone.0054302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho JW, Kim WW, Lee YM, et al. Impact of tumor-associated macrophages and BRAF(V600E) mutation on clinical outcomes in patients with various thyroid cancers. Head Neck. 2019;41:686–691. doi: 10.1002/hed.25469. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto T, Murakami R, Hamanishi J, et al. B7–H3 suppresses antitumor immunity via the CCL2-CCR2-M2 macrophage axis and contributes to ovarian cancer progression. Cancer Immunol Res. 2022;10:56–69. doi: 10.1158/2326-6066.CIR-21-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Yao W, Yuan Y, et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157–167. doi: 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari SM, Fallahi P, Galdiero MR, et al. Immune and Inflammatory Cells in Thyroid Cancer Microenvironment. Int J Mol Sci. 2019;20:1. doi: 10.3390/ijms20184413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin E, Koo JS. Cell component and function of tumor microenvironment in thyroid cancer. Int J Mol Sci. 2022;23:1. doi: 10.3390/ijms232012578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menicali E, Guzzetti M, Morelli S, Moretti S, Puxeddu E. Immune landscape of thyroid cancers: new insights. Front Endocrinol (Lausanne) 2020;11:637826. doi: 10.3389/fendo.2020.637826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan Z, Xu T, Bao L, et al. CREB3L1 promotes tumor growth and metastasis of anaplastic thyroid carcinoma by remodeling the tumor microenvironment. Mol Cancer. 2022;21:190. doi: 10.1186/s12943-022-01658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Peng W, Li C, Qin R, Zhong Z, Sun C. Identification of an immune-related signature indicating the dedifferentiation of thyroid cells. Cancer Cell Int. 2021;21:231. doi: 10.1186/s12935-021-01939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ, Weissleder R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng. 2018;2:578–588. doi: 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bao L, Xu T, Lu X, Huang P, Pan Z, Ge M. Metabolic reprogramming of thyroid cancer cells and crosstalk in their microenvironment. Front Oncol. 2021;11:773028. doi: 10.3389/fonc.2021.773028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galdiero MR, Varricchi G, Marone G. The immune network in thyroid cancer. Oncoimmunology. 2016;5:e1168556. doi: 10.1080/2162402X.2016.1168556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angell TE, Lechner MG, Jang JK, Correa AJ, LoPresti JS, Epstein AL. BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid. 2014;24:1385–1393. doi: 10.1089/thy.2014.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Yrigoyen M, Cassetta L, Pollard JW. Macrophage targeting in cancer. Ann N Y Acad Sci. 2021;1499:18–41. doi: 10.1111/nyas.14377. [DOI] [PubMed] [Google Scholar]
- 28.Liu Q, Sun W, Zhang H. Roles and new insights of macrophages in the tumor microenvironment of thyroid cancer. Front Pharmacol. 2022;13:875384. doi: 10.3389/fphar.2022.875384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li B, Liu S, Yang Q, Li Z, Li J, Wu J, Sun S, Xu Z, Wu Q. Macrophages in tumor-associated adipose microenvironment accelerate tumor progression. Adv Biol (Weinh) 2023;7:e2200161. doi: 10.1002/adbi.202200161. [DOI] [PubMed] [Google Scholar]
- 30.Lv J, Liu C, Chen FK, Feng ZP, Jia L, Liu PJ, Yang ZX, Hou F, Deng ZY. M2-like tumour-associated macrophage-secreted IGF promotes thyroid cancer stemness and metastasis by activating the PI3K/AKT/mTOR pathway. Mol Med Rep. 2021;24:1. doi: 10.3892/mmr.2021.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan Z, Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther. 2021;6:127. doi: 10.1038/s41392-021-00506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Passaro C, Borriello F, Vastolo V, Di Somma S, Scamardella E, Gigantino V, Franco R, Marone G, Portella G. The oncolytic virus dl922-947 reduces IL-8/CXCL8 and MCP-1/CCL2 expression and impairs angiogenesis and macrophage infiltration in anaplastic thyroid carcinoma. Oncotarget. 2016;7:1500–1515. doi: 10.18632/oncotarget.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiao L, Dong C, Jia W, Ma B. Exosomal miR-655-3p inhibits growth, and invasion and macrophage M2 polarization through targeting CXCR4 in papillary thyroid carcinoma. Acta Biochim Pol. 2022;69:773–779. doi: 10.18388/abp.2020_6027. [DOI] [PubMed] [Google Scholar]
- 34.Onuma AE, Schoenfield L, Shen C, Edwards C, Phay JE, Shirley LA, Tsung A. Prognosis of macrophage density in the absence of neutrophils in differentiated thyroid cancer. J Surg Res. 2020;256:458–467. doi: 10.1016/j.jss.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Yao Y, Xu XH, Jin L. Macrophage polarization in physiological and pathological pregnancy. Front Immunol. 2019;10:792. doi: 10.3389/fimmu.2019.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dungan LS, McGuinness NC, Boon L, Lynch MA, Mills KH. Innate IFN-γ promotes development of experimental autoimmune encephalomyelitis: a role for NK cells and M1 macrophages. Eur J Immunol. 2014;44:2903–2917. doi: 10.1002/eji.201444612. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Geng X, Hou J, Wu G. New insights into M1/M2 macrophages: key modulators in cancer progression. Cancer Cell Int. 2021;21:389. doi: 10.1186/s12935-021-02089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kranz LM, Diken M, Haas H, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–382. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21:799–820. doi: 10.1038/s41573-022-00520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim MJ, Sun HJ, Song YS, Yoo SK, Kim YA, Seo JS, Park YJ, Cho SW. CXCL16 positively correlated with M2-macrophage infiltration, enhanced angiogenesis, and poor prognosis in thyroid cancer. Sci Rep. 2019;9:13288. doi: 10.1038/s41598-019-49613-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okikawa S, Morine Y, Saito Y, et al. Inhibition of the VEGF signaling pathway attenuates tumor-associated macrophage activity in liver cancer. Oncol Rep. 2022;47:1. doi: 10.3892/or.2022.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kabasawa T, Ohe R, Aung NY, Urano Y, Kitaoka T, Tamazawa N, Utsunomiya A, Yamakawa M. Potential role of M2 TAMs around lymphatic vessels during lymphatic invasion in papillary thyroid carcinoma. Sci Rep. 2021;11:1150. doi: 10.1038/s41598-020-80694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jing W, Guo X, Wang G, Bi Y, Han L, Zhu Q, Qiu C, Tanaka M, Zhao Y. Breast cancer cells promote CD169(+) macrophage-associated immunosuppression through JAK2-mediated PD-L1 upregulation on macrophages. Int Immunopharmacol. 2020;78:106012. doi: 10.1016/j.intimp.2019.106012. [DOI] [PubMed] [Google Scholar]
- 47.Hartley GP, Chow L, Ammons DT, Wheat WH, Dow SW. Programmed cell death ligand 1 (PD-L1) signaling regulates macrophage proliferation and activation. Cancer Immunol Res. 2018;6:1260–1273. doi: 10.1158/2326-6066.CIR-17-0537. [DOI] [PubMed] [Google Scholar]
- 48.Lv J, Feng ZP, Chen FK, Liu C, Jia L, Liu PJ, Yang CZ, Hou F, Deng ZY. M2-like tumor-associated macrophages-secreted Wnt1 and Wnt3a promotes dedifferentiation and metastasis via activating β-catenin pathway in thyroid cancer. Mol Carcinog. 2021;60:25–37. doi: 10.1002/mc.23268. [DOI] [PubMed] [Google Scholar]
- 49.Lan J, Sun L, Xu F, et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019;79:146–158. doi: 10.1158/0008-5472.CAN-18-0014. [DOI] [PubMed] [Google Scholar]
- 50.Matsubayashi S, Kawai K, Matsumoto Y, et al. The correlation between papillary thyroid carcinoma and lymphocytic infiltration in the thyroid gland. J Clin Endocrinol Metab. 1995;80:3421–3424. doi: 10.1210/jcem.80.12.8530576. [DOI] [PubMed] [Google Scholar]
- 51.Cunha LL, Ward LS. Translating the immune microenvironment of thyroid cancer into clinical practice. Endocr Relat Cancer. 2022;29:R67–R83. doi: 10.1530/ERC-21-0414. [DOI] [PubMed] [Google Scholar]
- 52.Stempin CC, Geysels RC, Park S, et al. Secreted factors by anaplastic thyroid cancer cells induce tumor-promoting M2-like macrophage polarization through a TIM3-dependent mechanism. Cancers. 2021;13:4821. doi: 10.3390/cancers13194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang P, Guan H, Yuan S, et al. Targeting myeloid derived suppressor cells reverts immune suppression and sensitizes BRAF-mutant papillary thyroid cancer to MAPK inhibitors. Nat Commun. 2022;13:1588. doi: 10.1038/s41467-022-29000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spourquet C, Delcorte O, Lemoine P, et al. BRAF(V600E) expression in thyrocytes causes recruitment of immunosuppressive STABILIN-1 macrophages. Cancers (Basel) 2022;14:1. doi: 10.3390/cancers14194687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xing M. Clinical utility of RAS mutations in thyroid cancer: a blurred picture now emerging clearer. BMC Med. 2016;14:12. doi: 10.1186/s12916-016-0559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aydogan BI, Yuksel B, Tuna MM, et al. Distribution of RET mutations and evaluation of treatment approaches in hereditary medullary thyroid carcinoma in Turkey. J Clin Res Pediatr Endocrinol. 2016;8:13–20. doi: 10.4274/jcrpe.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pu W, Shi X, Yu P, et al. Single-cell transcriptomic analysis of the tumor ecosystems underlying initiation and progression of papillary thyroid carcinoma. Nat Commun. 2021;12:6058. doi: 10.1038/s41467-021-26343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borrello MG, Alberti L, Fischer A, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci USA. 2005;102:14825–14830. doi: 10.1073/pnas.0503039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho JW, Kim WW, Lee YM, et al. Impact of tumor-associated macrophages and BRAF. Head Neck. 2019;41:686–691. doi: 10.1002/hed.25469. [DOI] [PubMed] [Google Scholar]
- 60.Cho SW, Kim YA, Sun HJ, Oh BC, Yi KH, Park DJ, Park YJ. CXCL16 signaling mediated macrophage effects on tumor invasion of papillary thyroid carcinoma. Endocr Relat Cancer. 2016;23:113–124. doi: 10.1530/ERC-15-0196. [DOI] [PubMed] [Google Scholar]
- 61.Luo Y, Yang YC, Ma B, Xu WB, Liao T, Wang Y. Integrated analysis of novel macrophage related signature in anaplastic thyroid cancer. Endocrine. 2022;78:517–530. doi: 10.1007/s12020-022-03179-5. [DOI] [PubMed] [Google Scholar]
- 62.Melillo RM, Castellone MD, Guarino V, et al. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest. 2016;126:1603. doi: 10.1172/JCI87345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33:42–50. doi: 10.1200/JCO.2014.56.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferrari SM, Elia G, Piaggi S, Baldini E, Ulisse S, Miccoli M, Materazzi G, Antonelli A, Fallahi P. CCL2 is modulated by cytokines and PPAR-γ in anaplastic thyroid cancer. Anti-Cancer Agents Med Chem. 2018;18:458–466. doi: 10.2174/1871520617666170719152349. [DOI] [PubMed] [Google Scholar]
- 65.Ma M, Lin B, Wang M, Liang X, Su L, Okose O, Lv W, Li J. Immunotherapy in anaplastic thyroid cancer. Am J Transl Res. 2020;12:974–988. [PMC free article] [PubMed] [Google Scholar]
- 66.Cassetta L, Kitamura T. Targeting tumor-associated macrophages as a potential strategy to enhance the response to immune checkpoint inhibitors. Front Cell Dev Biol. 2018;6:38. doi: 10.3389/fcell.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim DI, Kim E, Kim YA, Cho SW, Lim JA, Park YJ. Macrophage densities correlated with CXC chemokine receptor 4 expression and related with poor survival in anaplastic thyroid cancer. Endocrinol Metab (Seoul) 2016;31:469–475. doi: 10.3803/EnM.2016.31.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lv J, Chen FK, Liu C, Liu PJ, Feng ZP, Jia L, Yang ZX, Hou F, Deng ZY. Zoledronic acid inhibits thyroid cancer stemness and metastasis by repressing M2-like tumor-associated macrophages induced Wnt/β-catenin pathway. Life Sci. 2020;256:117925. doi: 10.1016/j.lfs.2020.117925. [DOI] [PubMed] [Google Scholar]
- 69.Mazzoni M, Mauro G, Erreni M, et al. Senescent thyrocytes and thyroid tumor cells induce M2-like macrophage polarization of human monocytes via a PGE2-dependent mechanism. J Exp Clin Cancer Res. 2019;38:208. doi: 10.1186/s13046-019-1198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu H, Dong H, Jiang L, Li Z, Ma X. Bleomycin inhibits proliferation and induces apoptosis in TPC-1 cells through reversing M2-macrophages polarization. Oncol Lett. 2018;16:3858–3866. doi: 10.3892/ol.2018.9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo H, Xia X, Kim GD, et al. Characterizing dedifferentiation of thyroid cancer by integrated analysis. Sci Adv. 2021;7:1. doi: 10.1126/sciadv.abf3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu X, Wang D, Wang X, Sun S, Zhang Y, Wang S, Miao R, Xu X, Qu X. CXCL12/CXCR4 promotes inflammation-driven colorectal cancer progression through activation of RhoA signaling by sponging miR-133a-3p. J Exp Clin Cancer Res. 2019;38:32. doi: 10.1186/s13046-018-1014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aust G, Hofmann A, Laue S, Ode-Hakim S, Scherbaum WA. Differential regulation of granulocyte-macrophage colony-stimulating factor mRNA and protein expression in human thyrocytes and thyroid-derived fibroblasts by interleukin-1 alpha and tumor necrosis factor-alpha. J Endocrinol. 1996;151:277–285. doi: 10.1677/joe.0.1510277. [DOI] [PubMed] [Google Scholar]
- 74.Li W, Liu Z, Cen X, Xu J, Zhao S, Wang B, Zhang W, Qiu M. Integrated analysis of fibroblasts molecular features in papillary thyroid cancer combining single-cell and bulk RNA sequencing technology. Front Endocrinol (Lausanne) 2022;13:1019072. doi: 10.3389/fendo.2022.1019072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferrari SM, Elia G, Piaggi S, Baldini E, Ulisse S, Miccoli M, Materazzi G, Antonelli A, Fallahi P. CCL2 is Modulated by Cytokines and PPAR-γ in Anaplastic Thyroid Cancer. Anticancer Agents Med Chem. 2018;18:458–466. doi: 10.2174/1871520617666170719152349. [DOI] [PubMed] [Google Scholar]
- 76.Wang D, Wang X, Si M, Yang J, Sun S, Wu H, Cui S, Qu X, Yu X. Exosome-encapsulated miRNAs contribute to CXCL12/CXCR4-induced liver metastasis of colorectal cancer by enhancing M2 polarization of macrophages. Cancer Lett. 2020;474:36–52. doi: 10.1016/j.canlet.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Baek SH, Lee HW, Gangadaran P, Oh JM, Zhu L, Rajendran RL, Lee J, Ahn BC. Role of M2-like macrophages in the progression of ovarian cancer. Exp Cell Res. 2020;395:112211. doi: 10.1016/j.yexcr.2020.112211. [DOI] [PubMed] [Google Scholar]
- 78.de Azevedo RA, Shoshan E, Whang S, Markel G, Jaiswal AR, Liu A, Curran MA, Travassos LR, Bar-Eli M. MIF inhibition as a strategy for overcoming resistance to immune checkpoint blockade therapy in melanoma. Oncoimmunology. 2020;9:1846915. doi: 10.1080/2162402X.2020.1846915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghoochani A, Schwarz MA, Yakubov E, Engelhorn T, Doerfler A, Buchfelder M, Bucala R, Savaskan NE, Eyüpoglu IY. MIF-CD74 signaling impedes microglial M1 polarization and facilitates brain tumorigenesis. Oncogene. 2016;35:6246–6261. doi: 10.1038/onc.2016.160. [DOI] [PubMed] [Google Scholar]
- 80.Sloot YJE, Rabold K, Ulas T, et al. Interplay between thyroid cancer cells and macrophages: effects on IL-32 mediated cell death and thyroid cancer cell migration. Cell Oncol (Dordr) 2019;42:691–703. doi: 10.1007/s13402-019-00457-9. [DOI] [PubMed] [Google Scholar]
- 81.He L, Qing F, Li M, Lan D. Effects of laparoscopic and traditional open surgery on the levels of IL-6, TNF-α, and Gal-3 in patients with thyroid cancer. Gland Surg. 2021;10:1085–1092. doi: 10.21037/gs-21-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Madani I, De Neve W, Mareel M. Does ionizing radiation stimulate cancer invasion and metastasis? Bull Cancer. 2008;95:292–300. doi: 10.1684/bdc.2008.0598. [DOI] [PubMed] [Google Scholar]
- 83.Sherman EJ, Harris J, Bible KC, et al. Radiotherapy and paclitaxel plus pazopanib or placebo in anaplastic thyroid cancer (NRG/RTOG 0912): a randomised, double-blind, placebo-controlled, multicentre, phase 2 trial. Lancet Oncol. 2023;24:175–186. doi: 10.1016/s1470-2045(22)00763-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cui YH, Suh Y, Lee HJ, et al. Radiation promotes invasiveness of non-small-cell lung cancer cells through granulocyte-colony-stimulating factor. Oncogene. 2015;34:5372–5382. doi: 10.1038/onc.2014.466. [DOI] [PubMed] [Google Scholar]
- 85.Mathew B, Jacobson JR, Siegler JH, et al. Role of migratory inhibition factor in age-related susceptibility to radiation lung injury via NF-E2-related factor-2 and antioxidant regulation. Am J Respir Cell Mol Biol. 2013;49:269–278. doi: 10.1165/rcmb.2012-0291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang C, Gu X, Pan M, Yuan Q, Cheng H. Senescent thyroid tumor cells promote their migration by inducing the polarization of M2-like macrophages. Clin Transl Oncol. 2021;23:1253–1261. doi: 10.1007/s12094-020-02516-2. [DOI] [PubMed] [Google Scholar]
- 87.Cheng Z, Wang L, Wu C, Huang L, Ruan Y, Xue W. Tumor-derived exosomes induced M2 macrophage polarization and promoted the metastasis of osteosarcoma cells through Tim-3. Arch Med Res. 2021;52:200–210. doi: 10.1016/j.arcmed.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 88.Weitzman SP, Sherman SI. Novel drug treatments of progressive radioiodine-refractory differentiated thyroid cancer. Endocrinol Metab Clin North Am. 2019;48:253–268. doi: 10.1016/j.ecl.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 89.Kim DW, Jung YS, Park HS, Jung HD. Osteonecrosis of the jaw related to everolimus: a case report. Br J Oral Maxillofac Surg. 2013;51:e302–e304. doi: 10.1016/j.bjoms.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 90.Magaway C, Kim E, Jacinto E. Targeting mTOR and metabolism in cancer: lessons and innovations. Cells. 2019;8:1. doi: 10.3390/cells8121584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gunda V, Gigliotti B, Ashry T, et al. Anti-PD-1/PD-L1 therapy augments lenvatinib's efficacy by favorably altering the immune microenvironment of murine anaplastic thyroid cancer. Int J Cancer. 2019;144:2266–2278. doi: 10.1002/ijc.32041. [DOI] [PubMed] [Google Scholar]
- 92.Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17:887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- 93.Huang JK, Ma L, Song WH, Lu BY, Huang YB, Dong HM, Ma XK, Zhu ZZ, Zhou R. LncRNA-MALAT1 promotes angiogenesis of thyroid cancer by modulating tumor-associated macrophage FGF2 protein secretion. J Cell Biochem. 2017;118:4821–4830. doi: 10.1002/jcb.26153. [DOI] [PubMed] [Google Scholar]
- 94.Dowlati A, Harvey RD, Carvajal RD, et al. LY3022855, an anti-colony stimulating factor-1 receptor (CSF-1R) monoclonal antibody, in patients with advanced solid tumors refractory to standard therapy: phase 1 dose-escalation trial. Invest New Drugs. 2021;39:1057–1071. doi: 10.1007/s10637-021-01084-8. [DOI] [PubMed] [Google Scholar]
- 95.Li XJ, Gangadaran P, Kalimuthu S, Oh JM, Zhu L, Jeong SY, Lee SW, Lee J, Ahn BC. Role of pulmonary macrophages in initiation of lung metastasis in anaplastic thyroid cancer. Int J Cancer. 2016;139:2583–2592. doi: 10.1002/ijc.30387. [DOI] [PubMed] [Google Scholar]
- 96.Varricchi G, Loffredo S, Marone G, Modestino L, Fallahi P, Ferrari SM, de Paulis A, Antonelli A, Galdiero MR. The immune landscape of thyroid cancer in the context of immune checkpoint inhibition. Int J Mol Sci. 2019;20:1. doi: 10.3390/ijms20163934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen S, Morine Y, Tokuda K, Yamada S, Saito Y, Nishi M, Ikemoto T, Shimada M. Cancer-associated fibroblast-induced M2-polarized macrophages promote hepatocellular carcinoma progression via the plasminogen activator inhibitor-1 pathway. Int J Oncol. 2021;59:1. doi: 10.3892/ijo.2021.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rashid K, Ahmad A, Meerasa SS, Khan AQ, Wu X, Liang L, Cui Y, Liu T. Cancer stem cell-derived exosome-induced metastatic cancer: an orchestra within the tumor microenvironment. Biochimie. 2023 doi: 10.1016/j.biochi.2023.03.014. [DOI] [PubMed] [Google Scholar]
- 99.Gonzalez-Melero L, Hernandez RM, Santos-Vizcaino E, Igartua M. Tumour-derived extracellular vesicle based vaccines for melanoma treatment. Drug Deliv Transl Res. 2023 doi: 10.1007/s13346-023-01328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao S, Mi Y, Guan B, et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13:156. doi: 10.1186/s13045-020-00991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ying X, Wu Q, Wu X, Zhu Q, Wang X, Jiang L, Chen X. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget. 2016;7:43076–43087. doi: 10.18632/oncotarget.9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shinohara H, Kuranaga Y, Kumazaki M, Sugito N, Yoshikawa Y, Takai T, Taniguchi K, Ito Y, Akao Y. Regulated polarization of tumor-associated macrophages by miR-145 via colorectal cancer-derived extracellular vesicles. J Immunol. 2017;199:1505–1515. doi: 10.4049/jimmunol.1700167. [DOI] [PubMed] [Google Scholar]
- 103.Chen X, Zhou J, Li X, Wang X, Lin Y. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 2018;435:80–91. doi: 10.1016/j.canlet.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 104.Huang X, Yuan T, Liang M, et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol. 2015;67:33–41. doi: 10.1016/j.eururo.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang D, Qiu H, Miao L, Guo L, Zhang X, Lin M, Li Z, Li F. Cdc42 promotes thyroid cancer cell proliferation and migration and tumor-associated macrophage polarization through the PTEN/AKT pathway. J Biochem Mol Toxicol. 2022;36:e23115. doi: 10.1002/jbt.23115. [DOI] [PubMed] [Google Scholar]
- 106.Hou X, Shi X, Zhang W, et al. LDHA induces EMT gene transcription and regulates autophagy to promote the metastasis and tumorigenesis of papillary thyroid carcinoma. Cell Death Dis. 2021;12:347. doi: 10.1038/s41419-021-03641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cassetta L, Pollard JW. A timeline of tumour-associated macrophage biology. Nat Rev Cancer. 2023;23:238–257. doi: 10.1038/s41568-022-00547-1. [DOI] [PubMed] [Google Scholar]
- 108.von Tresckow B, Morschhauser F, Ribrag V, et al. An open-label, multicenter, phase I/II study of JNJ-40346527, a CSF-1R inhibitor, in patients with relapsed or refractory hodgkin lymphoma. Clin Cancer Res. 2015;21:1843–1850. doi: 10.1158/1078-0432.CCR-14-1845. [DOI] [PubMed] [Google Scholar]
- 109.Pass HI, Lavilla C, Canino C, Goparaju C, Preiss J, Noreen S, Blandino G, Cioce M. Inhibition of the colony-stimulating-factor-1 receptor affects the resistance of lung cancer cells to cisplatin. Oncotarget. 2016;7:56408–56421. doi: 10.18632/oncotarget.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Passaro C, Volpe M, Botta G, Scamardella E, Perruolo G, Gillespie D, Libertini S, Portella G. PARP inhibitor olaparib increases the oncolytic activity of dl922-947 in in vitro and in vivo model of anaplastic thyroid carcinoma. Mol Oncol. 2015;9:78–92. doi: 10.1016/j.molonc.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schürch CM, Roelli MA, Forster S, et al. Targeting CD47 in anaplastic thyroid carcinoma enhances tumor phagocytosis by macrophages and is a promising therapeutic strategy. Thyroid. 2019;29:979–992. doi: 10.1089/thy.2018.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Capdevila J, Wirth LJ, Ernst T, et al. PD-1 blockade in anaplastic thyroid carcinoma. J Clin Oncol. 2020;38:2620–2627. doi: 10.1200/JCO.19.02727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chae YK, Othus M, Patel S et al. (2020) 270 A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART) SWOG S1609: the thyroid tumor cohort. J ImmunoTherapy Cancer 8: A161-A. 10.1136/jitc-2020-SITC2020.0270
- 114.Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rodriguez-Perdigon M, Jimaja S, Haeni L, Bruns N, Rothen-Rutishauser B, Ruegg C. Polymersomes-mediated delivery of CSF1R inhibitor to tumor associated macrophages promotes M2 to M1-Like macrophage repolarization. Macromol Biosci. 2022;22:e2200168. doi: 10.1002/mabi.202200168. [DOI] [PubMed] [Google Scholar]
- 116.Wen J, Wang S, Guo R, Liu D. CSF1R inhibitors are emerging immunotherapeutic drugs for cancer treatment. Eur J Med Chem. 2023;245:114884. doi: 10.1016/j.ejmech.2022.114884. [DOI] [PubMed] [Google Scholar]
- 117.Roskoski R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2020 update. Pharmacol Res. 2020;152:104609. doi: 10.1016/j.phrs.2019.104609. [DOI] [PubMed] [Google Scholar]
- 118.A Study of ARRY-382 in Patients With Selected Advanced or Metastatic Cancers. https://ClinicalTrials.gov/show/NCT01316822
- 119.Phase 1 Study of PLX7486 as Single Agent in Patients With Advanced Solid Tumors. https://ClinicalTrials.gov/show/NCT01804530
- 120.A Study of BLZ945 Single Agent or BLZ945 in Combination With PDR001 in Advanced Solid Tumors. https://ClinicalTrials.gov/show/NCT02829723
- 121.Gomez-Roca CA, Italiano A, Le Tourneau C, et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann Oncol. 2019;30:1381–1392. doi: 10.1093/annonc/mdz163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Papadopoulos KP, Gluck L, Martin LP, et al. First-in-human study of AMG 820, a monoclonal anti-colony-stimulating factor 1 receptor antibody, in patients with advanced solid tumors. Clin Cancer Res. 2017;23:5703–5710. doi: 10.1158/1078-0432.CCR-16-3261. [DOI] [PubMed] [Google Scholar]
- 123.Razak AR, Cleary JM, Moreno V, et al. Safety and efficacy of AMG 820, an anti-colony-stimulating factor 1 receptor antibody, in combination with pembrolizumab in adults with advanced solid tumors. J Immunother Cancer. 2020;8:1. doi: 10.1136/jitc-2020-001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Study of Cabiralizumab in Combination With Nivolumab in Patients With Selected Advanced Cancers. https://ClinicalTrials.gov/show/NCT02526017
- 125.A Study of Cabiralzumab Given by Itself or With Nivolumab in Advanced Cancer or Cancer That Has Spread. https://ClinicalTrials.gov/show/NCT03158272
- 126.MCS110 With BRAF/MEK Inhibition in Patients With Melanoma. https://ClinicalTrials.gov/show/NCT03455764
- 127.Luo J, Wang Y, Zhao L, Wang C, Zhang Z. Anti-anaplastic thyroid cancer (ATC) effects and mechanisms of PLX3397 (Pexidartinib), a multi-targeted tyrosine kinase inhibitor (TKI) Cancers (Basel) 2022;15:1. doi: 10.3390/cancers15010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.A Combination Clinical Study of PLX3397 and Pembrolizumab To Treat Advanced Melanoma and Other Solid Tumors. https://ClinicalTrials.gov/show/NCT02452424
- 129.A Study of LY3022855 in Combination With Durvalumab or Tremelimumab in Participants With Advanced Solid Tumors. https://ClinicalTrials.gov/show/NCT02718911
- 130.Tap WD, Wainberg ZA, Anthony SP, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med. 2015;373:428–437. doi: 10.1056/NEJMoa1411366. [DOI] [PubMed] [Google Scholar]
- 131.Salajegheh A, Pakneshan S, Rahman A, et al. Co-regulatory potential of vascular endothelial growth factor-A and vascular endothelial growth factor-C in thyroid carcinoma. Hum Pathol. 2013;44:2204–2212. doi: 10.1016/j.humpath.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 132.Hamada Y, Tanoue K, Kita Y, et al. Vascular endothelial growth factor inhibitors promote antitumor responses via tumor microenvironment immunosuppression in advanced colorectal cancer. Scand J Gastroenterol. 2023;1:1–12. doi: 10.1080/00365521.2023.2194011. [DOI] [PubMed] [Google Scholar]
- 133.Dong F, Ruan S, Wang J, Xia Y, Le K, Xiao X, Hu T, Wang Q. M2 macrophage-induced lncRNA PCAT6 facilitates tumorigenesis and angiogenesis of triple-negative breast cancer through modulation of VEGFR2. Cell Death Dis. 2020;11:728. doi: 10.1038/s41419-020-02926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Andrade F, Probstner D, Decnop M, Bulzico D, Momesso D, Corbo R, Vaisman M, Vaisman F. The impact of zoledronic acid and radioactive iodine therapy on morbi-mortality of patients with bone metastases of thyroid cancer derived from follicular cells. Eur Thyroid J. 2019;8:46–55. doi: 10.1159/000493190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Iniguez-Ariza NM, Bible KC, Clarke BL. Bone metastases in thyroid cancer. J Bone Oncol. 2020;21:100282. doi: 10.1016/j.jbo.2020.100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Coperchini F, Greco A, Croce L, Petrosino E, Grillini B, Magri F, Chiovato L, Rotondi M. Vitamin D reduces thyroid cancer cells migration independently from the modulation of CCL2 and CXCL8 chemokines secretion. Front Endocrinol (Lausanne) 2022;13:876397. doi: 10.3389/fendo.2022.876397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.The Role of Vitamin D3 Supplementation in Advanced Cancer Patients With Pain. https://ClinicalTrials.gov/show/NCT05450419
- 138.Bonapace L, Coissieux M-M, Wyckoff J, Mertz KD, Varga Z, Junt T, Bentires-Alj M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–133. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- 139.Hitchcock JR, Watson CJ. Anti-CCL2: building a reservoir or opening the floodgates to metastasis? Breast Cancer Res. 2015;17:68. doi: 10.1186/s13058-015-0573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arwert EN, Harney AS, Entenberg D, Wang Y, Sahai E, Pollard JW, Condeelis JS. A Unidirectional Transition from Migratory to Perivascular Macrophage Is Required for Tumor Cell Intravasation. Cell Rep. 2018;23:1239–1248. doi: 10.1016/j.celrep.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Khan SU, Khan MU, Din AU, M, Khan IM, Khan MI, Bungau S, Hassan SSU, Reprogramming tumor-associated macrophages as a unique approach to target tumor immunotherapy. Front Immunol. 2023;14:1166487. doi: 10.3389/fimmu.2023.1166487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang Y, Xia S, Zhang L, Wang W, Chen L, Zhan W. MiR-324-5p/PTPRD/CEBPD axis promotes papillary thyroid carcinoma progression via microenvironment alteration. Cancer Biol Ther. 2020;21:522–532. doi: 10.1080/15384047.2020.1736465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen HM, van der Touw W, Wang YS, et al. Blocking immunoinhibitory receptor LILRB2 reprograms tumor-associated myeloid cells and promotes antitumor immunity. J Clin Invest. 2018;128:5647–5662. doi: 10.1172/JCI97570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yin SP, Gao Y, Xie XS, Xu DD, Riabov V, Du WD. Accumulation of stabilin-1 positive macrophages in the early stage of gastric cancer is associated with short cumulative survival. Oncol Lett. 2020;19:2404–2412. doi: 10.3892/ol.2020.11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fiani ML, Barreca V, Sargiacomo M, Ferrantelli F, Manfredi F, Federico M. Exploiting manipulated small extracellular vesicles to subvert immunosuppression at the tumor microenvironment through mannose receptor/CD206 targeting. Int J Mol Sci. 2020;21:1. doi: 10.3390/ijms21176318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhou Y, Do DC, Ishmael FT, et al. Mannose receptor modulates macrophage polarization and allergic inflammation through miR-511-3p. J Allergy Clin Immunol. 2018;141:350–64.e8. doi: 10.1016/j.jaci.2017.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jaynes JM, Sable R, Ronzetti M, et al. Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses. Sci Transl Med. 2020;12:1. doi: 10.1126/scitranslmed.aax6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mazzoni M, Mauro G, Minoli L, et al. Senescent thyrocytes, similarly to thyroid tumor cells, elicit M2-like macrophage polarization in vivo. Biology (Basel) 2021;10:1. doi: 10.3390/biology10100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Borson-Chazot F, Dantony E, Illouz F, et al. Effect of Buparlisib, a Pan-Class I PI3K inhibitor, in refractory follicular and poorly differentiated thyroid cancer. Thyroid. 2018;28:1174–1179. doi: 10.1089/thy.2017.0663. [DOI] [PubMed] [Google Scholar]
- 150.Kim YD, Park SM, Ha HC, Lee AR, Won H, Cha H, Cho S, Cho JM. HDAC inhibitor, CG-745, enhances the anti-cancer effect of anti-PD-1 immune checkpoint inhibitor by modulation of the immune microenvironment. J Cancer. 2020;11:4059–4072. doi: 10.7150/jca.44622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Han Y, Sun J, Yang Y, Liu Y, Lou J, Pan H, Yao J, Han W. TMP195 exerts antitumor effects on colorectal cancer by promoting M1 macrophages polarization. Int J Biol Sci. 2022;18:5653–5666. doi: 10.7150/ijbs.73264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang S, Hu G, Chen L, Ma K, Hu C, Zhu H, Xu N, Zhou C, Liu M. Celastrol acts as a new histone deacetylase inhibitor to inhibit colorectal cancer cell growth via regulating macrophage polarity. Cell Biol Int. 2023;47:492–501. doi: 10.1002/cbin.11952. [DOI] [PubMed] [Google Scholar]
- 153.Woyach JA, Kloos RT, Ringel MD, Arbogast D, Collamore M, Zwiebel JA, Grever M, Villalona-Calero M, Shah MH. Lack of therapeutic effect of the histone deacetylase inhibitor vorinostat in patients with metastatic radioiodine-refractory thyroid carcinoma. J Clin Endocrinol Metab. 2009;94:164–170. doi: 10.1210/jc.2008-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen JS, Hsieh YC, Chou CH, Wu YH, Yang MH, Chu SH, Chao YS, Chen CN. Chidamide plus tyrosine kinase inhibitor remodel the tumor immune microenvironment and reduce tumor progression when combined with immune checkpoint inhibitor in naïve and anti-PD-1 resistant CT26-bearing mice. Int J Mol Sci. 2022;23:1. doi: 10.3390/ijms231810677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Spartalis E, Kotrotsios K, Chrysikos D, et al. Histone deacetylase inhibitors and papillary thyroid cancer. Curr Pharm Des. 2021;27:2199–2208. doi: 10.2174/1381612826666201211112234. [DOI] [PubMed] [Google Scholar]
- 156.Lin SF, Lin JD, Chou TC, Huang YY, Wong RJ. Utility of a histone deacetylase inhibitor (PXD101) for thyroid cancer treatment. PLoS ONE. 2013;8:e77684. doi: 10.1371/journal.pone.0077684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sym023 (Anti-TIM-3) in Patients With Advanced Solid Tumor Malignancies or Lymphomas. https://ClinicalTrials.gov/show/NCT03489343
- 158.Solís-Martínez R, Cancino-Marentes M, Hernández-Flores G, et al. Regulation of immunophenotype modulation of monocytes-macrophages from M1 into M2 by prostate cancer cell-culture supernatant via transcription factor STAT3. Immunol Lett. 2018;196:140–148. doi: 10.1016/j.imlet.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 159.Kubala MH, Punj V, Placencio-Hickok VR, Fang H, Fernandez GE, Sposto R, DeClerck YA. Plasminogen activator inhibitor-1 promotes the recruitment and polarization of macrophages in cancer. Cell Rep. 2018;25:2177–91.e7. doi: 10.1016/j.celrep.2018.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Oral STAT3 Inhibitor, TTI-101, in Patients With Advanced Cancers. https://ClinicalTrials.gov/show/NCT03195699
- 161.Phase I/II Open Label Study Evaluating the Safety and Efficacy of Combining STAT3 Inhibition (TTI-101) With Anti-PD-1 Therapy (Pembrolizumab) in Patients With Recurrent or Metastatic (RM) Head and Neck Squamous Cell Carcinoma (HNSCC). https://ClinicalTrials.gov/show/NCT05668949
- 162.A Study of TTI-101 as Monotherapy and in Combination in Participants With Locally Advanced or Metastatic, and Unresectable Hepatocellular Carcinoma. https://ClinicalTrials.gov/show/NCT05440708
- 163.Study of TTI-101 in Combination for Participants With Metastatic Hormone Receptor-Positive and Human Epithelial Receptor 2 (HER2)-Negative Breast Cancer. https://ClinicalTrials.gov/show/NCT05384119
- 164.Phase 1 Study of OPB-31121 in Patients With Relapsed or Refractory Non-Hodgkin's Lymphoma or Multiple Myeloma. https://ClinicalTrials.gov/show/NCT00511082
- 165.Phase I/II Study of OPB-31121 in Patients With Progressive Hepatocellular Carcinoma. https://ClinicalTrials.gov/show/NCT01406574
- 166.Study to Assess OPB-31121 in Advanced Leukemias or Myelodysplastic Syndromes. https://ClinicalTrials.gov/show/NCT01029509
- 167.IMX-110 in Patients With Advanced Solid Tumors. https://ClinicalTrials.gov/show/NCT03382340
- 168.Vidyarthi A, Khan N, Agnihotri T, et al. TLR-3 stimulation skews M2 macrophages to M1 through IFN-alphabeta signaling and restricts tumor progression. Front Immunol. 2018;9:1650. doi: 10.3389/fimmu.2018.01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Le Mercier I, Poujol D, Sanlaville A, et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res. 2013;73:4629–4640. doi: 10.1158/0008-5472.CAN-12-3058. [DOI] [PubMed] [Google Scholar]
- 170.Mullins SR, Vasilakos JP, Deschler K, et al. Intratumoral immunotherapy with TLR7/8 agonist MEDI9197 modulates the tumor microenvironment leading to enhanced activity when combined with other immunotherapies. J Immunother Cancer. 2019;7:244. doi: 10.1186/s40425-019-0724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Singh M, Khong H, Dai Z, Huang XF, Wargo JA, Cooper ZA, Vasilakos JP, Hwu P, Overwijk WW. Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation. J Immunol. 2014;193:4722–4731. doi: 10.4049/jimmunol.1401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hagström J, Heikkilä A, Siironen P, Louhimo J, Heiskanen I, Mäenpää H, Arola J, Haglund C. TLR-4 expression and decrease in chronic inflammation: indicators of aggressive follicular thyroid carcinoma. J Clin Pathol. 2012;65:333–338. doi: 10.1136/jclinpath-2011-200402. [DOI] [PubMed] [Google Scholar]
- 173.Peyret V, Nazar M, Martín M, et al. Functional toll-like receptor 4 overexpression in papillary thyroid cancer by MAPK/ERK-induced ETS1 transcriptional activity. Mol Cancer Res. 2018;16:833–845. doi: 10.1158/1541-7786.MCR-17-0433. [DOI] [PubMed] [Google Scholar]
- 174.A Study of DSP-0509 in Patients With Advanced Solid Tumors to Determine the Safety and the Pharmacokinetic Profile. https://ClinicalTrials.gov/show/NCT03416335
- 175.Safety, Pharmacokinetics, Pharmacodynamics, and Preliminary Efficacy Trial of BNT411. https://ClinicalTrials.gov/show/NCT04101357
- 176.A First-in-human Study Using BDC-1001 as a Single Agent and in Combination With Nivolumab in Advanced HER2-Expressing Solid Tumors. https://ClinicalTrials.gov/show/NCT04278144
- 177.Clinical Study of BDB018: Monotherapy and in Combination With Pembrolizumab in Subjects With Advanced Solid Tumors. https://ClinicalTrials.gov/show/NCT04840394
- 178.Pressure Enabled Delivery of SD-101 With Checkpoint Blockade for Primary Liver Tumors. https://ClinicalTrials.gov/show/NCT05220722
- 179.Pressure Enabled Intrapancreatic Delivery of SD-101 With Checkpoint Blockade for Locally Advanced Pancreatic Adenocarcinoma. https://ClinicalTrials.gov/show/NCT05607953
- 180.TLR9 Agonist SD-101, Ibrutinib, and Radiation Therapy in Treating Patients With Relapsed or Refractory Grade 1-3A Follicular Lymphoma. https://ClinicalTrials.gov/show/NCT02927964
- 181.CMP-001 and Pre-operative Stereotactic Body Radiation Therapy (SBRT) in Early Stage Triple Negative Breast Cancer (TNBC). https://ClinicalTrials.gov/show/NCT04807192
- 182.CMP-001 for Relapsed and Refractory Lymphoma. https://ClinicalTrials.gov/show/NCT03983668
- 183.CMP-001 in Combination With IV PD-1-Blocking Antibody in Subjects With Certain Types of Advanced or Metastatic Cancer. https://ClinicalTrials.gov/show/NCT04916002
- 184.Intratumoral Tilsotolimod, a TLR-9 Agonist, Together With Intratumoral Ipilimumab and Intravenous Nivolumab in Patients With Advanced Cancers. https://ClinicalTrials.gov/show/NCT04270864
- 185.A Randomized Controlled Phase II Trial With Intradermal IMO-2125 in Pathological Tumor Stage (p) T3-4 cN0M0 Melanoma. https://ClinicalTrials.gov/show/NCT04126876
- 186.Study of Tilsotolimod in Combination With Nivolumab and Ipilimumab for the Treatment of Solid Tumors (ILLUMINATE-206). https://ClinicalTrials.gov/show/NCT03865082
- 187.Jiang K, Song C, Kong L, et al. Recombinant oncolytic Newcastle disease virus displays antitumor activities in anaplastic thyroid cancer cells. BMC Cancer. 2018;18:746. doi: 10.1186/s12885-018-4522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Banerjee S, Halder K, Ghosh S, Bose A, Majumdar S. The combination of a novel immunomodulator with a regulatory T cell suppressing antibody (DTA-1) regress advanced stage B16F10 solid tumor by repolarizing tumor associated macrophages in situ. Oncoimmunology. 2015;4:e995559. doi: 10.1080/2162402X.2014.995559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kumar P, Tyagi R, Das G, Bhaskar S. Mycobacterium indicus pranii and Mycobacterium bovis BCG lead to differential macrophage activation in Toll-like receptor-dependent manner. Immunology. 2014;143:258–268. doi: 10.1111/imm.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mardente S, Aventaggiato M, Splendiani E, Mari E, Zicari A, Catanzaro G, Po A, Coppola L, Tafani M. Extra-cellular vesicles derived from thyroid cancer cells promote the epithelial to mesenchymal transition (EMT) and the transfer of malignant phenotypes through immune mediated mechanisms. Int J Mol Sci. 2023;24:1. doi: 10.3390/ijms24032754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bravo-Miana RdC, Soler MF, Ceschin DG, et al. Extracellular vesicles from thyroid cancer harbor a functional machinery involved in extracellular matrix remodeling. Eur J Cell Biol. 2022;101:151254. doi: 10.1016/j.ejcb.2022.151254. [DOI] [PubMed] [Google Scholar]
- 192.Cai J, Qiao B, Gao N, Lin N, He W. Oral squamous cell carcinoma-derived exosomes promote M2 subtype macrophage polarization mediated by exosome-enclosed miR-29a-3p. Am J Physiol Cell Physiol. 2019;316:C731–C740. doi: 10.1152/ajpcell.00366.2018. [DOI] [PubMed] [Google Scholar]
- 193.Hsu YL, Hung JY, Chang WA, Jian SF, Lin YS, Pan YC, Wu CY, Kuo PL. Hypoxic lung-cancer-derived extracellular vesicle microRNA-103a increases the oncogenic effects of macrophages by targeting PTEN. Mol Ther. 2018;26:568–581. doi: 10.1016/j.ymthe.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Takano Y, Masuda T, Iinuma H, et al. Circulating exosomal microRNA-203 is associated with metastasis possibly via inducing tumor-associated macrophages in colorectal cancer. Oncotarget. 2017;8:78598–78613. doi: 10.18632/oncotarget.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chen X, Ying X, Wang X, Wu X, Zhu Q. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol Rep. 2017;38:522–528. doi: 10.3892/or.2017.5697. [DOI] [PubMed] [Google Scholar]
- 196.Thulin P, Wei T, Werngren O, Cheung L, Fisher RM, Grandér D, Corcoran M, Ehrenborg E. MicroRNA-9 regulates the expression of peroxisome proliferator-activated receptor δ in human monocytes during the inflammatory response. Int J Mol Med. 2013;31:1003–1010. doi: 10.3892/ijmm.2013.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jang JY, Lee JK, Jeon YK, Kim CW. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer. 2013;13:421. doi: 10.1186/1471-2407-13-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shao Y, Chen T, Zheng X, et al. Colorectal cancer-derived small extracellular vesicles establish an inflammatory premetastatic niche in liver metastasis. Carcinogenesis. 2018;39:1368–1379. doi: 10.1093/carcin/bgy115. [DOI] [PubMed] [Google Scholar]
- 199.Ying H, Kang Y, Zhang H, Zhao D, Xia J, Lu Z, Wang H, Xu F, Shi L. MiR-127 modulates macrophage polarization and promotes lung inflammation and injury by activating the JNK pathway. J Immunol. 2015;194:1239–1251. doi: 10.4049/jimmunol.1402088. [DOI] [PubMed] [Google Scholar]
- 200.Su MJ, Aldawsari H, Amiji M. Pancreatic cancer cell exosome-mediated macrophage reprogramming and the role of microRNAs 155 and 125b2 transfection using nanoparticle delivery systems. Sci Rep. 2016;6:30110. doi: 10.1038/srep30110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Trivedi M, Talekar M, Shah P, Ouyang Q, Amiji M. Modification of tumor cell exosome content by transfection with wt-p53 and microRNA-125b expressing plasmid DNA and its effect on macrophage polarization. Oncogenesis. 2016;5:e250. doi: 10.1038/oncsis.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Williams C, Royo F, Aizpurua-Olaizola O, Pazos R, Boons GJ, Reichardt NC, Falcon-Perez JM. Glycosylation of extracellular vesicles: current knowledge, tools and clinical perspectives. J Extracell Vesicles. 2018;7:1442985. doi: 10.1080/20013078.2018.1442985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gerlach JQ, Griffin MD. Getting to know the extracellular vesicle glycome. Mol Biosyst. 2016;12:1071–1081. doi: 10.1039/c5mb00835b. [DOI] [PubMed] [Google Scholar]
- 204.Choo YW, Kang M, Kim HY, et al. M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano. 2018;12:8977–8993. doi: 10.1021/acsnano.8b02446. [DOI] [PubMed] [Google Scholar]
- 205.Luo H, Zhang H, Mao J, et al. Exosome-based nanoimmunotherapy targeting TAMs, a promising strategy for glioma. Cell Death Dis. 2023;14:235. doi: 10.1038/s41419-023-05753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lee JC, Zhao JT, Gundara J, et al. Papillary thyroid cancer-derived exosomes contain miRNA-146b and miRNA-222. J Surg Res. 2015;196(1):39–48. doi: 10.1016/j.jss.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 207.Jiang K, Li G, Chen W, et al. Plasma exosomal miR-146b-5p and miR-222-3p are potential biomarkers for lymph node metastasis in papillary thyroid carcinomas. Oncol Targets Ther. 2020;13:1311–1319. doi: 10.2147/OTT.S231361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pan Q, Zhao J, Li M, Liu X, Xu Y, Li W, Wu S, Su Z. Exosomal miRNAs are potential diagnostic biomarkers between malignant and benign thyroid nodules based on next-generation sequencing. Carcinogenesis. 2020;41:18–24. doi: 10.1093/carcin/bgz160. [DOI] [PubMed] [Google Scholar]
- 209.Liang M, Yu S, Tang S, et al. A panel of plasma exosomal miRNAs as potential biomarkers for differential diagnosis of thyroid nodules. Front Genet. 2020;11:449. doi: 10.3389/fgene.2020.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Han S, Wang W, Wang S, et al. Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes. Theranostics. 2021;11:2892–2916. doi: 10.7150/thno.50928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Niu M, Naguib YW, Aldayel AM, Shi Y-c, Hursting SD, Hersh MA, Cui Z. Biodistribution and in vivo activities of tumor-associated macrophage-targeting nanoparticles incorporated with doxorubicin. Mol Pharm. 2014;11:4425–4436. doi: 10.1021/mp500565q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cao M, Yan H, Han X, et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer. 2019;7:326. doi: 10.1186/s40425-019-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Deng G, Sun Z, Li S, Peng X, Li W, Zhou L, Ma Y, Gong P, Cai L. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12:12096–12108. doi: 10.1021/acsnano.8b05292. [DOI] [PubMed] [Google Scholar]
- 214.Su L, Zhang W, Wu X, Zhang Y, Chen X, Liu G, Chen G, Jiang M. Glycocalyx-mimicking nanoparticles for stimulation and polarization of macrophages via specific interactions. Small. 2015;11:4191–4200. doi: 10.1002/smll.201403838. [DOI] [PubMed] [Google Scholar]
- 215.Habanjar O, Bingula R, Decombat C, Diab-Assaf M, Caldefie-Chezet F, Delort L. Crosstalk of inflammatory cytokines within the breast tumor microenvironment. Int J Mol Sci. 2023;24:1. doi: 10.3390/ijms24044002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang Y, Lin YX, Qiao SL, An HW, Ma Y, Qiao ZY, Rajapaksha RP, Wang H. Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumor microenvironment. Biomaterials. 2017;112:153–163. doi: 10.1016/j.biomaterials.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 217.He XY, Liu BY, Xu C, Zhuo RX, Cheng SX. A multi-functional macrophage and tumor targeting gene delivery system for the regulation of macrophage polarity and reversal of cancer immunoresistance. Nanoscale. 2018;10:15578–15587. doi: 10.1039/c8nr05294h. [DOI] [PubMed] [Google Scholar]
- 218.Kulkarni A, Chandrasekar V, Natarajan SK, et al. A designer self-assembled supramolecule amplifies macrophage immune responses against aggressive cancer. Nat Biomed Eng. 2018;2:589–599. doi: 10.1038/s41551-018-0254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nilubol N, Yuan Z, Paciotti GF, et al. Novel dual-action targeted nanomedicine in mice with metastatic thyroid cancer and pancreatic neuroendocrine tumors. J Natl Cancer Inst. 2018;110:1019–1029. doi: 10.1093/jnci/djy003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu F, Ma D, Chen W, et al. Gold nanoparticles suppressed proliferation, migration, and invasion in papillary thyroid carcinoma cells via downregulation of CCT3. J Nanomater. 2019;2012:1. doi: 10.1155/2019/1687340. [DOI] [Google Scholar]
- 221.Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Weiskopf K, Jahchan NS, Schnorr PJ, et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Investig. 2016;126:2610–2620. doi: 10.1172/JCI81603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu R, Wei H, Gao P, et al. CD47 promotes ovarian cancer progression by inhibiting macrophage phagocytosis. Oncotarget. 2017;8:39021. doi: 10.18632/oncotarget.16547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ansell SM, Maris MB, Lesokhin AM, et al. Phase I study of the CD47 blocker TTI-621 in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2021;27:2190–2199. doi: 10.1158/1078-0432.CCR-20-3706. [DOI] [PubMed] [Google Scholar]
- 225.Barkal AA, Weiskopf K, Kao KS, et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. 2018;19:76–84. doi: 10.1038/s41590-017-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Cercek A, Lumish M, Sinopoli J, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. 2022;386:2363–2376. doi: 10.1056/NEJMoa2201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Janjigian YY, Kawazoe A, Yanez P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727–730. doi: 10.1038/s41586-021-04161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cho BC, Abreu DR, Hussein M, et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022;23:781–792. doi: 10.1016/S1470-2045(22)00226-1. [DOI] [PubMed] [Google Scholar]
- 229.Liu Y, Zugazagoitia J, Ahmed FS, Henick BS, Gettinger SN, Herbst RS, Schalper KA, Rimm DL. Immune cell PD-L1 colocalizes with macrophages and is associated with outcome in PD-1 pathway blockade therapy. Clin Cancer Res. 2020;26:970–977. doi: 10.1158/1078-0432.CCR-19-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gunda V, Gigliotti B, Ndishabandi D, et al. Combinations of BRAF inhibitor and anti-PD-1/PD-L1 antibody improve survival and tumour immunity in an immunocompetent model of orthotopic murine anaplastic thyroid cancer. Br J Cancer. 2018;119:1223–1232. doi: 10.1038/s41416-018-0296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dhupkar P, Gordon N, Stewart J, Kleinerman ES. Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Med. 2018;7:2654–2664. doi: 10.1002/cam4.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Li Z, Ding Y, Liu J, et al. Depletion of tumor associated macrophages enhances local and systemic platelet-mediated anti-PD-1 delivery for post-surgery tumor recurrence treatment. Nat Commun. 2022;13:1845. doi: 10.1038/s41467-022-29388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mehnert JM, Varga A, Brose MS, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer. 2019;19:196. doi: 10.1186/s12885-019-5380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sakamuri D, Glitza IC, Betancourt Cuellar SL, et al. Phase I dose-escalation study of anti-CTLA-4 antibody ipilimumab and lenalidomide in patients with advanced cancers. Mol Cancer Ther. 2018;17:671–676. doi: 10.1158/1535-7163.MCT-17-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Vudalimab for the Treatment of Locally Advanced or Metastatic Anaplastic Thyroid Cancer or Hurthle Cell Thyroid Cancer. https://ClinicalTrials.gov/show/NCT05453799
- 237.Testing the Combination of Cabozantinib, Nivolumab, and Ipilimumab (CaboNivoIpi) for Advanced Differentiated Thyroid Cancer. https://ClinicalTrials.gov/show/NCT03914300
- 238.Cunha LL, Marcello MA, Ward LS. The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocr Relat Cancer. 2014;21:R85–R103. doi: 10.1530/ERC-13-0431. [DOI] [PubMed] [Google Scholar]
- 239.Luo T, Sun J, Zhu S, et al. Ultrasound-mediated destruction of oxygen and paclitaxel loaded dual-targeting microbubbles for intraperitoneal treatment of ovarian cancer xenografts. Cancer Lett. 2017;391:1–11. doi: 10.1016/j.canlet.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 240.Gunassekaran GR, Poongkavithai Vadevoo SM, Baek MC, Lee B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials. 2021;278:121137. doi: 10.1016/j.biomaterials.2021.121137. [DOI] [PubMed] [Google Scholar]
- 241.Zhang W, Zhu XD, Sun HC, et al. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420–3430. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 242.Lorusso L, Pieruzzi L, Gabriele M, Nisi M, Viola D, Molinaro E, Bottici V, Elisei R, Agate L. Osteonecrosis of the jaw: a rare but possible side effect in thyroid cancer patients treated with tyrosine-kinase inhibitors and bisphosphonates. J Endocrinol Invest. 2021;44:2557–2566. doi: 10.1007/s40618-021-01634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Beuselinck B, Wolter P, Karadimou A, et al. Concomitant oral tyrosine kinase inhibitors and bisphosphonates in advanced renal cell carcinoma with bone metastases. Br J Cancer. 2012;107:1665–1671. doi: 10.1038/bjc.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gralow JR, Barlow WE, Paterson AHG, et al. Phase III randomized trial of bisphosphonates as adjuvant therapy in breast cancer: S0307. J Natl Cancer Inst. 2020;112:698–707. doi: 10.1093/jnci/djz215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gainford MC, Dranitsaris G, Ooi W, Vanhuyse M, Clemons M. Comparing the results of bisphosphonate use in clinical trials with actual practice: a case of apples and oranges? Curr Oncol. 2006;13:187–190. doi: 10.3390/curroncol13050018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zylberberg HM, Rustgi SD, Yang A, Aronson A, Kessel E, Amin S, Lucas AL. Bisphosphonate use does not impact survival in patients with pancreatic cancer: a propensity score matching analysis. Gut Liver. 2021;15:782–790. doi: 10.5009/gnl20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Guerriero JL. Macrophages: their untold story in T cell activation and function. Int Rev Cell Mol Biol. 2019;342:73–93. doi: 10.1016/bs.ircmb.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 248.Wang Y, Johnson KCC, Gatti-Mays ME, Li Z. Emerging strategies in targeting tumor-resident myeloid cells for cancer immunotherapy. J Hematol Oncol. 2022;15:118. doi: 10.1186/s13045-022-01335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Krieg C, Nowicka M, Guglietta S, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. 2018;24:144–153. doi: 10.1038/nm.4466. [DOI] [PubMed] [Google Scholar]
- 250.Romano E, Kusio-Kobialka M, Foukas PG, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci USA. 2015;112:6140–6145. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.La Fleur L, Botling J, He F, et al. Targeting MARCO and IL37R on immunosuppressive macrophages in lung cancer blocks regulatory T cells and supports cytotoxic lymphocyte function. Cancer Res. 2021;81:956–967. doi: 10.1158/0008-5472.CAN-20-1885. [DOI] [PubMed] [Google Scholar]
- 252.Nakamura K, Smyth MJ. TREM2 marks tumor-associated macrophages. Signal Transduct Target Ther. 2020;5:233. doi: 10.1038/s41392-020-00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Li PH, Kong XY, He YZ, Liu Y, Peng X, Li ZH, Xu H, Luo H, Park J. Recent developments in application of single-cell RNA sequencing in the tumour immune microenvironment and cancer therapy. Mil Med Res. 2022;9:52. doi: 10.1186/s40779-022-00414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Azizi E, Carr AJ, Plitas G, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018;174:1293–308.e36. doi: 10.1016/j.cell.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hirz T, Mei S, Sarkar H, et al. Dissecting the immune suppressive human prostate tumor microenvironment via integrated single-cell and spatial transcriptomic analyses. Nat Commun. 2023;14:663. doi: 10.1038/s41467-023-36325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Schepisi G, Gianni C, Palleschi M, Bleve S, Casadei C, Lolli C, Ridolfi L, Martinelli G, De Giorgi U. The new frontier of immunotherapy: chimeric antigen receptor T (CAR-T) cell and macrophage (CAR-M) therapy against breast cancer. Cancers (Basel) 2023;15:1. doi: 10.3390/cancers15051597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.CAR-macrophages for the Treatment of HER2 Overexpressing Solid Tumors. https://ClinicalTrials.gov/show/NCT04660929
- 258.Cohort Study to Determine the Antitumor Activity of New CAR-macrophages in Breast Cancer Patients' Derived Organoids. https://ClinicalTrials.gov/show/NCT05007379
- 259.Reyes-García J, Montaño LM, Carbajal-García A, Wang YX. Sex hormones and lung inflammation. Adv Exp Med Biol. 2021;1304:259–321. doi: 10.1007/978-3-030-68748-9_15. [DOI] [PubMed] [Google Scholar]
- 260.Becerra-Díaz M, Lerner AD, Yu DH, Thiboutot JP, Liu MC, Yarmus LB, Bose S, Heller NM. Sex differences in M2 polarization, chemokine and IL-4 receptors in monocytes and macrophages from asthmatics. Cell Immunol. 2021;360:104252. doi: 10.1016/j.cellimm.2020.104252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Keselman A, Fang X, White PB, Heller NM. Estrogen signaling contributes to sex differences in macrophage polarization during asthma. J Immunol. 2017;199:1573–1583. doi: 10.4049/jimmunol.1601975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Adachi A, Honda T, Egawa G, et al. Estradiol suppresses psoriatic inflammation in mice by regulating neutrophil and macrophage functions. J Allergy Clin Immunol. 2022;150:909–19.e8. doi: 10.1016/j.jaci.2022.03.028. [DOI] [PubMed] [Google Scholar]
- 263.Becerra-Díaz M, Strickland AB, Keselman A, Heller NM. Androgen and androgen receptor as enhancers of M2 macrophage polarization in allergic lung inflammation. J Immunol. 2018;201:2923–2933. doi: 10.4049/jimmunol.1800352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Chae YK, Othus M, Patel S, et al. 270 A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART) SWOG S1609: the thyroid tumor cohort. J Immunother Cancer. 2020;8:A161. doi: 10.1136/jitc-2020-SITC2020.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]