Abstract
Background
Because interventions are available to prevent further recurrence in patients with recurrent Clostridioides difficile infection (rCDI), we identified predictors of multiple rCDI (mrCDI) in adults at the time of presentation with initial CDI (iCDI).
Methods
iCDI was defined as a positive C difficile test in any clinical setting during January 2018–August 2019 in a person aged ≥18 years with no known prior positive test. rCDI was defined as a positive test ≥14 days from the previous positive test within 180 days after iCDI; mrCDI was defined as ≥2 rCDI. We performed multivariable logistic regression analysis.
Results
Of 18 829 patients with iCDI, 882 (4.7%) had mrCDI; 437 with mrCDI and 7484 without mrCDI had full chart reviews. A higher proportion of patients with mrCDI than without mrCDI were aged ≥65 years (57.2% vs 40.7%; P < .0001) and had healthcare (59.1% vs 46.9%; P < .0001) and antibiotic (77.3% vs 67.3%; P < .0001) exposures in the 12 weeks preceding iCDI. In multivariable analysis, age ≥65 years (adjusted odds ratio [aOR], 1.91; 95% confidence interval [CI], 1.55–2.35), chronic hemodialysis (aOR, 2.28; 95% CI, 1.48–3.51), hospitalization (aOR, 1.64; 95% CI, 1.33–2.01), and nitrofurantoin use (aOR, 1.95; 95% CI, 1.18–3.23) in the 12 weeks preceding iCDI were associated with mrCDI.
Conclusions
Patients with iCDI who are older, on hemodialysis, or had recent hospitalization or nitrofurantoin use had increased risk of mrCDI and may benefit from early use of adjunctive therapy to prevent mrCDI. If confirmed, these findings could aid in clinical decision making and interventional study designs.
Five percent of patients with initial Clostridioides difficile infection had up to 5 recurrences within 180 days. Significant risk factors for ≥2 recurrences among patients with initial Clostridioides difficile infection included older age, chronic hemodialysis, and recent hospitalization and nitrofurantoin use.
Clostridioides difficile infection (CDI) is a common healthcare-associated gastrointestinal infection, with an estimated >400 000 incident infections occurring annually in the United States [1]. Rates of recurrence vary, but generally, up to 25% of patients with an initial CDI (iCDI) may experience recurrent CDI (rCDI), with the majority of first recurrences occurring within 8 weeks of the initial episode [2]. rCDI is associated with significant morbidity and mortality, with 2.5 times higher hospital admission rate, 4 times longer hospital stay, and 33% higher all-cause mortality rate than iCDI [3, 4]. The economic burden of rCDI is also substantial; the attributable healthcare costs have been estimated to be $10 850 per recurrent episode [5].
Recent advancements in CDI treatment have focused on prevention of rCDI in adults, particularly multiple recurrences (≥2 rCDI). Although antibiotics alone, including extended or tapering courses, can be used to treat or prevent a first or subsequent recurrence, fecal microbiota transplantation (FMT) or live biotherapeutic products (REBYOTA or VOWST) following antibiotic therapy are currently recommended for the management of a second or subsequent recurrence [6–8]. Another adjunctive therapy for prevention of a first or subsequent recurrence includes bezlotoxumab, a human monoclonal antibody that binds to C difficile toxin B [9]. Despite the availability of these interventions to prevent multiple rCDI (mrCDI), little is known regarding which patients are at increased risk for mrCDI. We sought to describe the epidemiology of mrCDI across geographically diverse U.S. sites and identify predictors of mrCDI in adults at the time of presentation with iCDI.
METHODS
Surveillance Population and Case Definition
The Centers for Disease Control and Prevention's Emerging Infections Program (EIP) conducts population-based surveillance for CDI in 35 counties in 10 states (California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, New York, Oregon, and Tennessee) [10]. Laboratories serving the catchment areas reported all positive C difficile tests to EIP site staff. iCDI was defined as a positive C difficile molecular or toxin assay in any clinical setting during January 2018–August 2019 in a catchment-area resident aged ≥18 years with no prior positive test reported to EIP. rCDI was defined as a positive test ≥14 days from the previous positive test, whereas mrCDI was defined as a second or subsequent rCDI episode, within 180 days after iCDI.
Data Collection and Epidemiologic Classification
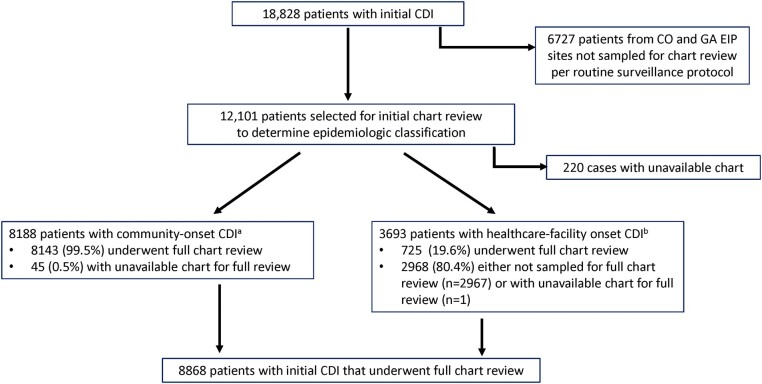
An initial chart review was performed by trained EIP site staff using a standardized case report form on all patients with iCDI from 8 EIP sites and on a random sample of patients from 2 sites with the largest surveillance population (Colorado and Georgia). Based on chart review, iCDI was epidemiologically classified as community onset if the C difficile–positive stool was collected as an outpatient or within 3 days of hospital admission; hospital onset if the positive stool was collected >3 days after hospital admission; or long-term care facility (LTCF) onset if the positive stool was collected in a LTCF or from a LTCF resident. Per routine surveillance protocol, all community-onset patients and a random 10% to 20% of healthcare facility-onset patients (ie, hospital onset and LTCF onset) underwent a subsequent full chart review to collect additional healthcare and medication exposures, comorbidities, and clinical course (Figure 1). All medication exposures (eg, antibiotics) were limited to the 12 weeks before iCDI; no information about subsequent antibiotics, other than those used in the treatment of iCDI, was collected. State death registries were used to obtain mortality within 180 days after iCDI.
Figure 1.
Flow diagram depicting the selection of patients with initial Clostridioides difficile infection for chart review and inclusion in the analysis. Abbreviations: CDI, Clostridioides difficile infection; EIP, Emerging Infections Program. aPatients with community-onset CDI who underwent a full chart review were less likely than those without a chart review to be community-associated (71.0% vs 93.3%; P = .004), but there were no differences in the proportion who were female (P = .10), aged ≥65 y (P = .93), or who had multiple recurrent CDI (P = 1.00) or died within 180 d of initial CDI (P = .69). bPatients with healthcare facility-onset CDI (ie, hospital-onset or long-term care facility onset CDI) who had a full chart review were less likely than those without a chart review to be long-term care facility-onset (32.7% vs 38.5%; P = .004), but there were no differences in the proportion who were female (P = .19), aged ≥65 y (P = .32), or who had multiple recurrent CDI (P = .51), or died within 180 d of initial CDI (P = .48).
Community-onset iCDI was further classified as community-associated if there was no documentation of an overnight stay in a healthcare facility in the preceding 12 weeks. All other community-onset iCDI that did not meet this criterion were classified as community-onset healthcare facility-associated (COHCFA), and, along with hospital-onset and LTCF-onset iCDI, were considered healthcare-associated iCDI.
Statistical Analysis
We used descriptive statistics to summarize rCDI data among all patients. To identify predictors of mrCDI, we restricted the analysis to patients who had a full chart review. Chi-square and Fisher exact tests (where applicable) were used to assess the associations between risk factors and patient groups with or without mrCDI (ie, ≤ 1 rCDI). We also calculated mrCDI attack rates and unadjusted relative risks for selected risk factors. Multiple imputation was performed for the race variable (18.9% of cases missing) and ethnicity variable (23.6% of cases missing) using the fully conditional specification method based on age, sex, epidemiologic classification, EIP site, and year [11]. Because our objective was to identify risk factors of patients who were at risk of mrCDI, regardless of when the recurrences occurred during the follow-up period, we used a logistic regression model instead of survival analysis. The following candidate variables were determined a priori to be potentially associated with mrCDI and were entered into an initial multivariable logistic regression model: age, sex, race/ethnicity, selected comorbidities and healthcare and medication exposures, hospitalization, intensive care unit (ICU) admission, and iCDI treatment. We also adjusted for healthcare facility onset status to account for the sampling of cases for full chart review. To reduce the number of variables in the model, we selected candidate variables with a P value <.1 in the initial model for inclusion in the final model. Patients without mrCDI who had died within 180 days of their iCDI were excluded from these models because they did not have a chance to develop mrCDI.
We performed a sensitivity analysis excluding patients with a single recurrence from the models (ie, comparing patients with mrCDI with patients without recurrence). We performed a second sensitivity analysis evaluating death as another outcome, where patients with mrCDI or those who had died within 180 days of iCDI were compared with patients without mrCDI who had survived.
Adjusted odds ratios (aOR) and 95% confidence intervals (CI) were calculated for the models. A 2-tailed P value <.05 was considered statistically significant. SAS statistical software version 9.4 (SAS Institute Inc, Cary, North Carolina) was used for all analyses. Datasets used for this analysis included 2018 surveillance data as of 17 November 2020 and 2019 surveillance data as of 12 October 2021.
Patient Consent Statement
This study was reviewed and approved by the Centers for Disease Control and Prevention institutional review board (see 45 C.F.R. part 46; 21 C.F.R. part 56.) and was deemed either nonresearch or received institutional review board approval with a waiver of informed consent in EIP sites. This activity did not include factors necessitating patient consent.
RESULTS
Description of mrCDI
Of 18 828 patients with iCDI, 15 367 (81.6%) had no rCDI, 2579 (13.7%) had a single rCDI, and 882 (4.7%) had mrCDI, ranging from 2 to 5 rCDI per patient within 180 days following iCDI (Table 1). Among patients with rCDI, 32.9% had their recurrent CDI episodes diagnosed at different laboratories. Of the 882 patients with mrCDI, 702 (79.6%) had 2 recurrences, 146 (16.6%) had 3 recurrences, 31 (3.5%) had 4 recurrences, and 3 (0.3%) had 5 recurrences. The median time between each subsequent recurrence varied slightly (Table 1), with an overall median of 43 days (interquartile range, 27–65 days).
Table 1.
Timing of Recurrent CDI From the Initial and Previous CDI Episodes
| All Patients With Initial CDI | |||
|---|---|---|---|
| N = 18 828 | Days From Initial CDI | Days From Previous CDI | |
| No. (%) | Median (IQR) | Median (IQR) | |
| First recurrence | 3461 (18.4) | 37 (25–65) | 37 (25–65) |
| Second recurrence | 882 (4.7) | 84 (60–117) | 44 (27–67) |
| Third recurrence | 180 (1.0) | 118.5 (97–148) | 43 (27–61) |
| Fourth recurrence | 34 (.2) | 140 (118–160) | 39 (26–51) |
| Fifth recurrence | 3 (.02) | 152 (150–174) | 37 (36–41) |
Abbreviations: CDI, Clostridioides difficile infection; IQR, interquartile range.
Table 2 shows the proportion of patients with subsequent recurrences by their baseline characteristics. Patients aged ≥65 years at the time of their iCDI, compared with other age groups (P ≤ .01), and patients with COHCFA or LTCF-onset iCDI, compared with community-associated or hospital-onset iCDI (P < .01), were more likely to have any number of rCDI during the 180-day follow-up period. Hispanic patients of any race and non-Hispanic White patients were more likely than other racial/ethnic groups to have up to 2 or more rCDI (P < .01). Patients with a toxin-positive iCDI were also more likely than those with a toxin-negative iCDI (ie, nucleic acid amplification test [NAAT] positive only) to have up to 2 or more rCDI (P < .01).
Table 2.
Frequency of Recurrent CDI Among Patients With Initial CDI by Their Baseline Characteristics
| Patient Characteristics at Time of Initial CDI | No. (%)a | Percent of Subgroupb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ≥1 rCDI | P Value | ≥2 rCDI | P Value | ≥3 rCDI | P Value | ≥4 rCDI | P Value | ||
| Patients with initial CDI | 18 828 (100.0) | 18.4 | 4.7 | … | 1.0 | … | .2 | … | |
| Age group, y (n = 18 828) | … | … | <.01 | … | <.01 | … | <.01 | … | .01 |
| 18–44 | 3771 (20.0) | 13.8 | 2.9 | … | .5 | … | .1 | … | |
| 45–64 | 5952 (31.6) | 17.7 | 4.1 | … | .7 | … | .1 | … | |
| ≥65 | 9105 (48.4) | 20.7 | 5.8 | … | 1.3 | … | .3 | … | |
| Sex (n = 18 828) | … | … | .09 | … | .27 | … | .15 | … | .26 |
| Male | 7895 (41.9) | 17.8 | 4.5 | … | .8 | … | .1 | … | |
| Female | 10 933 (58.1) | 18.8 | 4.8 | … | 1.0 | … | .2 | … | |
| Epidemiologic classification of initial CDI (n = 11 881)c | … | … | <.01 | … | <.01 | … | <.01 | … | <.01 |
| CA | 5825 (49.0) | 17.9 | 4.3 | … | .9 | … | .1 | … | |
| COHCFA | 2363 (19.9) | 24.2 | 6.7 | … | 1.4 | … | .3 | … | |
| HO | 2312 (19.5) | 14.0 | 3.2 | … | .7 | … | .1 | … | |
| LTCFO | 1381 (11.6) | 23.2 | 6.2 | … | 1.7 | … | .7 | … | |
| Race/ethnicity (n = 11 586)d | … | … | <.01 | … | <.01 | … | .10 | … | .53 |
| Hispanic, any race | 1105 (9.5) | 20.1 | 5.2 | … | 1.1 | … | .4 | … | |
| Non-Hispanic, White race | 7583 (65.5) | 21.0 | 5.4 | … | 1.3 | … | .2 | … | |
| Non-Hispanic, other racee | 2898 (25.0) | 17.4 | 3.9 | … | .8 | … | .2 | … | |
| Diagnostic test result for initial CDI (n = 10 153)f | … | … | <.01 | … | <.01 | … | .17 | … | .44 |
| Toxin-positive | 4970 (49.0) | 21.0 | 5.9 | … | 1.2 | … | .2 | … | |
| NAAT positive/toxin negative | 5183 (51.1) | 17.1 | 4.0 | … | 1.0 | … | .2 | … | |
Abbreviations: CA, community-associated; COHCFA, community-onset healthcare facility-associated; EIP, Emerging Infections Program; HO, hospital-onset; LTCFO, long-term care facility onset; NAAT, nucleic acid amplification test; rCDI, recurrent Clostridioides difficile infection.
aDisplays column percentages.
bDisplays row percentages.
cExcludes 6947 patients, of whom 220 (3.2%) had incomplete documentation of healthcare exposures in their medical records and 6727 (96.8%) were not selected by Georgia and Colorado EIP sites for chart review.
dExcludes 7242 patients, of whom 3052 (42.1%) were missing race/ethnicity data from the laboratory line list or medical records and 4190 (57.9%) were not selected by Georgia and Colorado EIP sites for chart review.
eAll non-Hispanic, non-White patients were grouped into a single category because of small numbers. These included patients of Black, Asian, American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, or ≥2 races.
fExcludes 8675 patients diagnosed by NAAT alone or as part of a multistep algorithm where the toxin result was not available.
Predictors of mrCDI
A full chart review was performed on 8868 patients with iCDI (Figure 1). Among those with data available, 97.7% (8063/8257) had documentation of diarrhea. Of the 8868 patients, 437 (4.9%) subsequently had mrCDI and 8431 (95.1%) did not have mrCDI. Of the 8431 patients without mrCDI, 947 (11.2%) had died within 180 days after iCDI. A comparison of the patients who had died and those who had survived is shown in Supplementary Table 1.
Table 3 compares the 437 patients with subsequent mrCDI to the 7484 patients without mrCDI (6326 patients without recurrence and 1158 with a single recurrence) who had survived the 180-day period after iCDI. A higher percentage of patients with mrCDI than those without mrCDI were aged ≥65 years at the time of their iCDI (57.2% vs 40.7%; P < .0001) and had healthcare-associated (42.6% vs 30.4%; P < .0001) and toxin-positive (33.6% vs 26.6%; P = .003) iCDI, although toxin-positivity status was known for <60% of patients. They were also more likely than those without mrCDI to have underlying conditions, including chronic kidney disease (22.0% vs 14.4%; P < .0001), diverticular disease (15.6% vs 11.2%; P = .006), and hematologic or solid tumor malignancy (17.9% vs 14.1%; P = .03). Healthcare and medication exposures in the 12 weeks preceding iCDI were also more common in patients with than without mrCDI, including prior hospitalization (39.6% vs 26.9%; P < .0001), long-term care facility stay (9.0% vs 4.9%; P = .0002), chronic hemodialysis (6.3% vs 3.0%; P = .0001), surgery (16.0% vs 12.1%; P = .02), antibiotic use (77.3% vs 67.3%; P < .0001), proton-pump inhibitor use (38.7% vs 33.0%; P = .02), and immunosuppressant use (30.6% vs 25.5%; P = .02). Patients with mrCDI were also more likely than those without mrCDI to have received treatment for their iCDI (97.8% vs 95.4%; P = .02). A separate comparison of patients with mrCDI to those without recurrence and to those with a single recurrence is shown in Supplementary Table 2.
Table 3.
Comparison of Characteristics Between Patients With and Without Multiple Recurrent CDI
| Patient Characteristics at Time of Initial CDI | Patients With mrCDI N = 437 No. (%) |
Patients Without mrCDI N = 7484a No. (%) |
P Value |
|---|---|---|---|
| Age group, y | … | … | <.0001 |
| 18–44 | 53 (12.1) | 1896 (25.3) | |
| 45–64 | 134 (30.7) | 2546 (34.0) | |
| ≥65 | 250 (57.2) | 3042 (40.7) | |
| Sex | … | … | .62 |
| Male | 164 (37.5) | 2898 (38.7) | |
| Female | 273 (62.5) | 4586 (61.3) | |
| Race/ethnicity | … | … | .004 |
| Hispanic, any race | 32 (7.3) | 611 (8.2) | |
| Non-Hispanic, White race | 271 (62.0) | 3988 (53.3) | |
| Non-Hispanic, other raceb | 49 (11.2) | 968 (12.9) | |
| Unknown race or ethnicity | 85 (19.5) | 1917 (25.6) | |
| Epidemiologic classification of initial CDI | … | … | <.0001 |
| Community-associated | 251 (57.4) | 5209 (69.6) | |
| Healthcare-associated | 186 (42.6) | 2275 (30.4) | |
| Community-onset healthcare-facility associated | 158 (36.2) | 1782 (23.8) | |
| Hospital-onset | 17 (3.9) | 348 (4.7) | |
| Long-term care facility-onset | 11 (2.5) | 145 (1.9) | |
| Diagnostic test result for initial CDI | … | … | .003 |
| Toxin-positive | 147 (33.6) | 1993 (26.6) | |
| NAAT-positive/toxin-negative | 106 (24.3) | 2225 (29.7) | |
| Unknown toxin status | 184 (42.1) | 3266 (43.6) | |
| Select underlying conditions | … | … | |
| Charlson comorbidity index ≥1 | 289/431 (67.1) | 4170/7400 (56.4) | <.0001 |
| Cardiac disease | 62/431 (14.4) | 862/7400 (11.7) | .09 |
| Chronic kidney disease | 95/431 (22.0) | 1068/7400 (14.4) | <.0001 |
| Chronic liver disease | 20/431 (4.6) | 365/7400 (4.9) | .79 |
| Chronic pulmonary disease | 82/431 (19.0) | 1286/7400 (17.4) | .38 |
| Diabetes mellitus | 102/431 (23.7) | 1602/7400 (21.7) | .32 |
| Diverticular disease | 67/431 (15.6) | 827/7400 (11.2) | .006 |
| Hematologic or solid tumor malignancy | 77/431 (17.9) | 1043/7400 (14.1) | .03 |
| Hematopoietic stem cell or solid organ transplant | 13/431 (3.0) | 194/7400 (2.6) | .62 |
| Inflammatory bowel disease | 33/431 (7.7) | 471/7400 (6.4) | .29 |
| Prior healthcare exposuresc | … | … | |
| Hospitalization | 172/434 (39.6) | 1995/7413 (26.9) | <.0001 |
| Long-term acute care hospital stay | 3/434 (.7) | 16/7412 (.2) | .08 |
| Long-term care facility stay | 39/434 (9.0) | 361/7410 (4.9) | .0002 |
| Emergency room visit | 130/434 (30.0) | 1944/7412 (26.2) | .09 |
| Observational unit stay | 11/431 (2.6) | 152/7407 (2.1) | .48 |
| Chronic hemodialysis | 27/432 (6.3) | 219/7411 (3.0) | .0001 |
| Surgery | 69/432 (16.0) | 895/7412 (12.1) | .02 |
| Any of the above | 256/433 (59.1) | 3474/7413 (46.9) | <.0001 |
| Prior medication exposuresc | … | … | |
| Any antibiotic | 327/423 (77.3) | 4883/7260 (67.3) | <.0001 |
| Select antibiotic classes: | … | … | |
| Beta-lactam/beta-lactamase inhibitor combinations | 136/423 (32.2) | 1815/7260 (25.0) | .001 |
| Carbapenems | 18/423 (4.3) | 192/7260 (2.6) | .05 |
| Cephalosporins | 156/423 (36.9) | 2082/7260 (28.7) | .0003 |
| Clindamycin | 50/423 (11.8) | 719/7260 (9.9) | .20 |
| Fluoroquinolones | 83/423 (19.6) | 1174/7260 (16.2) | .06 |
| Macrolides | 20/423 (4.7) | 335/7260 (4.6) | .91 |
| Nitrofurantoin | 18/423 (4.3) | 157/7260 (2.2) | .005 |
| Penicillins | 22/423 (5.2) | 470/7260 (6.5) | .30 |
| Proton-pump inhibitor | 164/424 (38.7) | 2384/7221 (33.0) | .02 |
| Immunosuppressant | 129/421 (30.6) | 1837/7215 (25.5) | .02 |
| Clinical course | … | … | |
| Hospital admissiond | 194 (44.4) | 3039/7471 (40.7) | .12 |
| Intensive-care unit staye | 8/434 (1.8) | 174/7467 (2.3) | .51 |
| Receipt of treatment for initial CDI | 405/414 (97.8) | 6603/6921 (95.4) | .02 |
| Metronidazole only | 91/414 (22.0) | 1459/6915 (21.1) | .67 |
| Oral/rectal vancomycin | 312/414 (75.4) | 5092/6915 (73.6) | .44 |
| Fidaxomicin | 8/414 (1.9) | 117/6915 (1.7) | .71 |
Any missing response to a variable is excluded from the denominator.
Abbreviations: CDI, Clostridioides difficile infection; mrCDI, multiple recurrent Clostridioides difficile infection; NAAT, nucleic acid amplification test.
aExcludes 947 patients who had died within 180 d of initial CDI diagnosis.
bAll non-Hispanic, non-White patients were grouped into a single category because of small numbers. These included patients of Black, Asian, American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, or ≥2 races.
cDuring the 12 wks prior to initial CDI diagnosis.
dHospitalized at the time of or within 6 d following initial CDI diagnosis.
eAdmitted to the intensive care unit on the day of or within 6 d following initial CDI diagnosis.
The initial multivariable model to identify predictors of mrCDI is shown in Supplementary Table 3. In the final multivariable analysis, age ≥65 years at the time of iCDI (aOR, 1.91; 95% CI, 1.55–2.35), chronic hemodialysis (aOR, 2.28; 95% CI, 1.48–3.51), hospitalization (aOR, 1.64; 95% CI, 1.33–2.01), and nitrofurantoin use (aOR, 1.95; 95% CI, 1.18–3.23) in the 12 weeks preceding iCDI were significantly associated with experiencing mrCDI within 180 days following iCDI (Table 4).
Table 4.
Final Multivariable Model: Characteristics of Initial CDI Associated With Developing Multiple Recurrent CDI
| Patient Characteristics at Time of Initial CDI | Adjusted Odds Ratio (95% CI) | P Value |
|---|---|---|
| ≥65 y of age | 1.91 (1.55–2.35) | <.0001 |
| Race/ethnicity | … | |
| Hispanic, any race | .89 (.60–1.31) | .55 |
| Non-Hispanic, White race | Referent | - |
| Non-Hispanic, Other racea | .75 (.55–1.02) | .07 |
| Healthcare-facility onset initial CDI | .73 (.49–1.09) | .13 |
| Inflammatory bowel disease | 1.46 (.99–2.15) | .05 |
| Chronic hemodialysis | 2.28 (1.48–3.51) | .0002 |
| Prior hospitalizationb | 1.64 (1.33–2.01) | <.0001 |
| Prior LTACH stayb | 2.96 (.82–10.67) | .10 |
| Prior nitrofurantoin useb | 1.95 (1.18–3.23) | .009 |
Abbreviation: CDI, Clostridioides difficile infection; CI, confidence interval; LTACH, long-term acute-care hospital.
aAll non-Hispanic, non-White patients were grouped into a single category because of small numbers. These included patients of Black, Asian, American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, or ≥2 races.
bDuring the 12 wks before initial CDI diagnosis.
Attack rates of mrCDI among patients with iCDI by selected risk factors are shown in Supplementary Table 4. The mrCDI attack rate among patients with iCDI who were aged ≥65 years, receiving chronic hemodialysis, or had prior hospitalization or nitrofurantoin use ranged from 7.6% to 11.0%. Among 1300 patients with 2 or more of these risk factors, the mrCDI attack rate was 10.2%.
In the first sensitivity analysis, we excluded patients from the comparator group that had a single recurrence and found the same variables remained significantly associated with mrCDI (Supplementary Tables 5 and 6). In the second sensitivity analysis that included death as an outcome, the following were significant predictors of mrCDI or death within 180 days after iCDI: aged ≥65 years at the time of iCDI; healthcare-facility onset iCDI; cardiac disease; chronic liver disease; chronic kidney disease; hematologic or solid tumor malignancy; hematopoietic stem cell or solid organ transplant; chronic hemodialysis; hospitalization, LTCF stay, surgery, immunosuppressant use, and beta-lactam/beta-lactamase inhibitor combination use in the 12 weeks preceding iCDI; and hospitalization and ICU admission at the time of or within 6 days following iCDI (Supplementary Tables 7 and 8).
DISCUSSION
In our large multisite analysis, 5% of patients with iCDI experienced mrCDI (2 or more recurrences) in the subsequent 180 days. By using population-based surveillance, which encompassed inpatient and outpatient settings, we were able to identify recurrent episodes diagnosed by different laboratories in a third of the patients who experienced rCDI. We found the frequency of recurrences was highest among patients aged ≥65 years or who had an initial COHCFA or LTCF-onset CDI. We also found that older age, receipt of chronic hemodialysis, recent hospitalization, and nitrofurantoin use were independent risk factors for mrCDI. The presence of 2 or more of these risk factors resulted in an mrCDI attack rate of 10.2%.
Several factors associated with advanced age might increase the risk of mrCDI, including impairment of the immune system from a decline in the quantity or function of antibody-producing and innate immune cells [12]. The inability to mount an adequate immune response to an initial episode of CDI increases the risk for recurrence [13, 14]. Intestinal microbiota can also change with age, resulting in reduced species diversity and smaller inhibitory effect on C difficile growth [15, 16]. Additionally, older patients are more likely to have underlying comorbidities and frequent antibiotic use that can contribute to the risk of mrCDI. Furthermore, they are also more likely to have frequent and prolonged hospitalizations and be admitted to LTCFs [17], all of which can lead to repeated exposures to C difficile.
We also found chronic hemodialysis to be a risk factor for mrCDI. A previous study found chronic kidney disease to be independently associated with mrCDI but did not specifically evaluate end-stage renal disease [18]. Data regarding recurrent CDI are limited among patients with end-stage renal disease, but 1 study reported a recurrence rate of 23.6% [19] and another study identified chronic dialysis as an independent risk factor for rCDI [20]. Similar to the older population, patients with end-stage renal disease have impairment of their immune system and frequent antibiotic use [21], which can contribute to the risk of mrCDI. Importantly, they also regularly encounter the healthcare environment for maintenance hemodialysis, which could lead to continued C difficile exposure. In fact, CDI outbreaks have been associated with outpatient dialysis facilities [22], where the physical layout, commonly an open floorplan with patients placed in close proximity to one another, and challenges with environmental cleaning and disinfection can lead to C difficile transmission [23].
Interestingly, we did not find an increased risk of mrCDI among patients with iCDI who had recent immunosuppressant use or another underlying condition that could result in an impaired immune system, such as hematologic or solid organ malignancy or transplant (Table 4). However, the immunological effect of immunosuppressive drugs is variable, and we did not collect information on duration of use that could impact risk of mrCDI.
We also assessed whether recent antibiotic exposure before iCDI might increase subsequent risk of mrCDI, given that gut microbiome disruption from antibiotic use can persist for several weeks to months. We included antibiotic classes with high or moderate CDI risk (Supplementary Table 3) [24, 25], as well as nitrofurantoin because of 1 study that found nitrofurantoin use before iCDI was a risk factor for a first recurrence [26]. Surprisingly, only nitrofurantoin use was associated with mrCDI in our study, even though it is not known to significantly affect bowel flora and has comparatively lower risk of CDI than other antibiotics [27]. The significant association with nitrofurantoin might reflect cumulative exposure to antibiotics for treatment or prevention of recurrent urinary tract infections (UTIs), including continued antibiotic use after iCDI. An increased risk of CDI has been shown in older patients receiving long-term antibiotic prophylaxis for UTIs [28]. Alternatively, it could reflect a propensity to mrCDI in patients who experience UTIs that is not related to antibiotic exposure. Separately, we also found that recent hospitalization among patients with iCDI was associated with mrCDI, which could be a marker for generally sicker patients with comorbid conditions who might be more likely to be readmitted and exposed to antibiotics.
We conducted a sensitivity analysis with either mrCDI or death as an outcome because emerging data suggest that FMT can reduce 90-day mortality among patients with rCDI [29]. Several significant risk factors for 180-day mrCDI or mortality were identified among patients with iCDI, with the strongest associations seen with older age, hospitalization, and ICU admission (≥2 times the odds of having either outcome), suggesting that investigations of FMT or live biotherapeutic products might prioritize studying these patients.
If confirmed, our findings could help guide patient selection in interventional trials of patients with iCDI to prevent rCDI. Although bezlotoxumab is indicated to reduce rCDI in patients receiving antibiotic treatment for iCDI who are at high risk of rCDI, both REBYOTA and VOWST are currently solely indicated for the prevention of mrCDI following rCDI. Potential settings in which to conduct these clinical trials would be places with longitudinal electronic medical records, including antibiotic exposures, such as the Veterans Health Administration, health maintenance organizations, or localities with highly functional health information exchanges. Such patients could be randomized to receive an intervention at the time of iCDI and followed electronically for extended outcomes.
Our study has several limitations. First, because the case definition was based on a laboratory diagnosis, it is possible recurrence could have been overestimated if some patients were colonized, had repeat testing without resolution of symptoms, or had a positive result for a test of cure rather than a true recurrence. Conversely, intensity of C difficile testing varies among institutions [30], and recurrence could have been underestimated if clinicians did not retest for C difficile when clinically indicated or patients sought care outside the surveillance catchment area. Second, >40% of iCDI cases were diagnosed with NAAT alone or as part of an algorithm where the toxin enzyme immunoassay result was not available, thus limiting our ability to evaluate toxin-positivity status as a predictor of mrCDI. Third, we could have underestimated risk factors for mrCDI if there was incomplete documentation of exposures or parts of the medical chart were unavailable for review. Although intervening antibiotics (ie, following iCDI or rCDI but before mrCDI) are an important risk factor for rCDI, we limited exposures to those before iCDI; future studies could assess the additional risk from intervening antibiotics on the outcome of mrCDI because those might be additional points to introduce interventions. Fourth, we did not adjust P values for multiple comparisons; however, the significant P values were several magnitudes smaller than .05 and would likely have remained significant had we used adjusted P values. Last, because of the sampling of healthcare-facility onset cases for full chart review, our data may not be representative of that patient population. However, an increasing proportion of CDI are now community onset [1].
In summary, we found 5% of patients with iCDI had up to 5 recurrences over the subsequent 180 days. Patients with iCDI who were older, on chronic hemodialysis, had recent hospitalization or nitrofurantoin use had increased risk of mrCDI and may benefit from early use of adjunctive therapy to prevent mrCDI. Confirmation of these findings could aid in clinical decision making and interventional study designs. Further efforts to improve the identification of patients at risk of mrCDI may require novel approaches, including exploring the use of biomarkers or gut metabolites to predict CDI recurrence.
Supplementary Material
Contributor Information
Alice Y Guh, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Rongxia Li, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Lauren Korhonen, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Lisa G Winston, School of Medicine, University of California, San Francisco, San Francisco, California, USA.
Erin Parker, California Emerging Infections Program, Oakland, California, USA.
Christopher A Czaja, Colorado Department of Public Health and Environment, Denver,Colorado, USA.
Helen Johnston, Colorado Department of Public Health and Environment, Denver,Colorado, USA.
Elizabeth Basiliere, Colorado Department of Public Health and Environment, Denver,Colorado, USA.
James Meek, Connecticut Emerging Infections Program, Yale School of Public Health, New Haven, Connecticut, USA.
Danyel Olson, Connecticut Emerging Infections Program, Yale School of Public Health, New Haven, Connecticut, USA.
Scott K Fridkin, Emory University School of Medicine, Atlanta, Georgia, USA.
Lucy E Wilson, University of Maryland Baltimore County, Baltimore, Maryland, USA.
Rebecca Perlmutter, Maryland Department of Health, Baltimore, Maryland, USA.
Stacy M Holzbauer, Minnesota Department of Health, St Paul, Minnesota, USA; Career Epidemiology Field Officer Program, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Paige D’Heilly, Minnesota Department of Health, St Paul, Minnesota, USA.
Erin C Phipps, New Mexico Emerging Infections Program, University of New Mexico, Albuquerque, New Mexico, USA.
Kristina G Flores, New Mexico Emerging Infections Program, University of New Mexico, Albuquerque, New Mexico, USA.
Ghinwa K Dumyati, New York Emerging Infections Program and University of Rochester Medical Center, Rochester, New York, USA.
Rebecca Pierce, Oregon Health Authority, Portland, Oregon, USA.
Valerie L S Ocampo, Oregon Health Authority, Portland, Oregon, USA.
Christopher D Wilson, Tennessee Department of Health, Nashville, Tennessee, USA.
Jasmine J Watkins, Tennessee Department of Health, Nashville, Tennessee, USA.
Dale N Gerding, Edward Hines, Jr. Veterans Affairs Hospital, Hines, Illinois, USA.
L Clifford McDonald, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments. We thank all the Emerging Infections Program (EIP) staff for their contribution, including Lena Tayo from California EIP and Melissa Christian from New Mexico EIP.
Financial support. This work was supported by the National Center for Emerging and Zoonotic Infectious Diseases at the U.S. Centers for Disease Control and Prevention through the Emerging Infections Program Cooperative Agreement.
Disclaimer. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
References
- 1. Guh AY, Mu Y, Winston LG, et al. . Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med 2020; 382:1320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsigrelis C. Recurrent Clostridioides difficile infection: recognition, management, prevention. Cleve Clin J Med 2020; 87:347–59. [DOI] [PubMed] [Google Scholar]
- 3. Olsen MA, Yan Y, Reske KA, Zilberberg MD, Dubberke ER. Recurrent Clostridium difficile infection is associated with increased mortality. Clin Microbiol Infect 2015; 21:164–70. [DOI] [PubMed] [Google Scholar]
- 4. Olsen MA, Yan Y, Reske KA, Zilberberg M, Dubberke ER. Impact of Clostridium difficile recurrence on hospital readmissions. Am J Infect Control 2015; 43:318–22. [DOI] [PubMed] [Google Scholar]
- 5. Zhang D, Prabhu VS, Marcella SW. Attributable healthcare resource utilization and costs for patients with primary and recurrent Clostridium difficile infection in the United States. Clin Infect Dis 2018; 66:1326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDonald LC, Gerding DN, Johnson S, et al. . Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. U.S. Food and Drug Administration . REBYOTA. Available at: https://www.fda.gov/vaccines-blood-biologics/vaccines/rebyota. Accessed 20 March 2024.
- 8. U.S. Food and Drug Administration . VOWST. Available at: https://www.fda.gov/vaccines-blood-biologics/vowst. Accessed 8 September 2023.
- 9. Lee Y, Lim WI, Bloom CI, Moore S, Chung E, Marzella N. Bezlotoxumab (Zinplava) for Clostridium difficile infection: the first monoclonal antibody approved to prevent the recurrence of a bacterial infection. P T 2017; 42:735–8. [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention . Clostridioides difficile infection (CDI) tracking. Available at: https://www.cdc.gov/hai/eip/cdiff-tracking.html. Accessed on 8 September 2023.
- 11. Van Buuren S, Brand JP, Groothuis-Oudshoorn CG, Rubin DB. Fully conditional specification in multivariate imputation. Stat Comput Sim 2006; 76:1049–64. [Google Scholar]
- 12. Shin JH, High KP, Warren CA. Older is not wiser, immunologically speaking: effect of aging on host response to Clostridium difficile infections. J Gerontol A Biol Sci Med Sci 2016; 71:916–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giannasca PJ, Warny M. Active and passive immunization against Clostridium difficile diarrhea and colitis. Vaccine 2004; 22:848–56. [DOI] [PubMed] [Google Scholar]
- 14. Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med 2000; 342:390–7. [DOI] [PubMed] [Google Scholar]
- 15. Claesson MJ, Cusack S, O’Sullivan O, et al. . Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A 2011; 108:4586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hopkins MJ, Macfarlane GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol 2002; 51:448–54. [DOI] [PubMed] [Google Scholar]
- 17. Kee VR. Clostridium difficile infection in older adults: a review and update on its management. Am J Geriatr Pharmacother 2012; 10:14–24. [DOI] [PubMed] [Google Scholar]
- 18. Ma GK, Brensinger CM, Wu Q, Lewis JD. Increasing incidence of multiply recurrent Clostridium difficile infection in the United States. Ann Intern Med 2017; 167:152–8. [DOI] [PubMed] [Google Scholar]
- 19. Tirath A, Tadros S, Coffin SL, et al. . Clostridium difficile infection in dialysis patients. J Investig Med 2017; 65:353–7. [DOI] [PubMed] [Google Scholar]
- 20. Bauer MP, Notermans DW, van Benthem BH, et al. . Clostridium difficile infection in Europe: a hospital-based survey. Lancet 2011; 377:63–73. [DOI] [PubMed] [Google Scholar]
- 21. Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol 2013; 9:255–65. [DOI] [PubMed] [Google Scholar]
- 22. See I, Bagchi S, Booth S, et al. . Outbreak of Clostridium difficile infections at an outpatient hemodialysis facility—Michigan, 2012–2013. Infect Control Hosp Epidemiol 2015; 36:972–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. D'Agata EMC, Apata IW, Booth S, et al. . Suggestions for the prevention of Clostridioides difficile spread within outpatient hemodialysis facilities. Kidney Int 2021; 99:1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother 2013; 57:2326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown KA, Langford B, Schwartz KL, Diong C, Garber G, Daneman N. Antibiotic prescribing choices and their comparative C. difficile infection risks: a longitudinal case-cohort study. Clin Infect Dis 2021; 72:836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okafor CM, Clogher P, Olson D, Niccolai L, Hadler J. Trends in and risk factors for recurrent Clostridioides difficile infection, New Haven County, Connecticut, USA, 2015–2020. Emerg Infect Dis 2023; 29:877–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huttner A, Verhaegh EM, Harbarth S, Muller AE, Theuretzbacher U, Mouton JW. Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother 2015; 70:2456–64. [DOI] [PubMed] [Google Scholar]
- 28. Langford BJ, Brown KA, Diong C, et al. . The benefits and harms of antibiotic prophylaxis for urinary tract infection in older adults. Clin Infect Dis 2021; 73:e782–91. [DOI] [PubMed] [Google Scholar]
- 29. Ianiro G, Murri R, Sciumè GD, et al. . Incidence of bloodstream infections, length of hospital stay, and survival in patients with recurrent Clostridioides difficile infection treated with fecal microbiota transplantation or antibiotics: a prospective cohort study. Ann Intern Med 2019; 171:695–702. [DOI] [PubMed] [Google Scholar]
- 30. Fridkin SK, Onwubiko UN, Dube W, et al. . Determinates of Clostridioides difficile infection (CDI) testing practices among inpatients with diarrhea at selected acute-care hospitals in Rochester, New York, and Atlanta, Georgia, 2020–2021. Infect Control Hosp Epidemiol 2022; 44:1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.