Abstract
The escape of human immunodeficiency virus type 1 from effects of neutralizing antibodies was studied by using neutralization-resistant (NR) variants generated by growing the neutralization-sensitive (NS) wild-type MN virus in the presence of human serum with neutralizing antibodies, more than 99% of which were directed at the V3 region of gp120. The variants obtained had broad neutralization resistance to human sera, without limitation with respect to the V3 specificity of the sera. The molecular basis for the resistance was evaluated with molecularly cloned viruses, as well as with pseudoviruses expressing envelope glycoproteins of the NS and NR phenotypes. Nucleotide sequence analyses comparing NS and NR clones revealed a number of polymorphisms, including six in the V1/V2 region, two in C4/V5 of gp120, three in the leucine zipper (LZ) domain of gp41, and two in the second external putative α-helix region of gp41. A series of chimeras from NS and NR env genes was constructed, and each was presented on pseudoviruses to locate the domain(s) which conferred the phenotypic changes. The neutralization phenotypes of the chimeric clones were found to be dependent on mutations in both the C4/V5 region of gp120 and the LZ region of gp41. Additionally, interaction between mutations in gp120 and gp41 was demonstrated in that a chimeric env gene consisting of a gp120 coding sequence from an NS clone and a gp41 sequence from an NR clone yielded a pseudovirus with minimal infectivity. The possible significance of predicted amino acid changes in these domains is discussed. The results indicate that polyvalent antibodies predominantly directed against V3 can induce NR through selection for mutations that alter interactions of other domains in the envelope complex.
Lentivirus infections are characteristically persistent, with long periods of time elapsing between the onset of infection and severe or fatal consequences (30). In some cases, particularly visna virus and equine infectious anemia virus infections, periods of clinical latency during persistent infection are periodically interrupted by episodes of clinical illness (11, 35). Certain data support the hypothesis that these episodes are precipitated by the emergence of variant viruses that have mutated to become resistant to the previously developed immune response and are capable of unchecked replication until a new response develops (36, 55). Theoretically, mutations affecting susceptibility to any immune effector mechanism could result in such escape from immune surveillance. In the cases of human immunodeficiency virus (HIV) and simian immunodeficiency virus, epitopes that are targets for neutralizing antibodies (NA) and cytotoxic T cells reside in variable regions of the surface envelope glycoproteins, such that it is often posited that this variability is the result of mutants emerging in the face of the selective pressure of these immune responses (22, 29, 47, 56, 79). An important feature of these infections that relates to the potential for mutant strains to emerge is that the period of clinical latency is actually characterized by viral replication that occurs at a high rate (27, 57). Indeed, initial infection is associated with a very high level of replication, and sometimes resulting clinical manifestations, which is only partially suppressed by the development of immune responses (9, 37, 41, 62, 78). There is sufficient infidelity of the viral DNA polymerase that extensive genetic diversity accumulates during ongoing viral replication (20, 31). In the presence of selective immunological pressure, mutations that result in escape from immune surveillance are to be expected.
NA are critical for protective immunity against many viruses, and the capacity of candidate HIV vaccines to induce NA has been a major focus of vaccine development efforts (15, 40, 59, 78). Of the neutralization domains that have been defined on the envelope glycoproteins of HIV type 1 (HIV-1), two are in variable regions of gp120, including the immunodominant V3 region (4, 7, 17, 28, 29, 48, 49, 51, 69, 72). The variability in this region is reflected in the significant differences that exist among consensus sequences of different clades of HIV-1, raising concern that multivalent vaccines may be needed to induce protection against different subtypes of HIV-1 (40, 52). It is a reasonable hypothesis that immunological selection is a source of antigenic diversity of the V3 region, and it is even possible that neutralization is the principal source of global genetic variation in V3. However, a model that assumes that the antigenic diversity resulting from immunological selection occurs principally through changes in the primary structure of the variable neutralization determinants, V1/V2 and V3, must take into account the effects of NA directed at other neutralization determinants on the potential for immunological escape of new variants.
Despite the potential importance of studies of this issue, relatively little data are available regarding neutralization escape mutation of HIV-1 occurring in vivo, or in vitro as a result of selection in the presence of human sera. The extensive epitope mapping of the envelope that has been accomplished has been associated with the development and characterization of a large number of viruses with mutations at binding sites for monoclonal NA (4, 18, 26, 32, 44–46, 53, 64, 67, 70, 77). While these mutants may be considered escape mutants, they may not represent the mutations that are likely to be selected during infection when antibodies with multiple specificities may be present. Korber et al. demonstrated variability in the apical sequences of V3 within individual patients, but they did not present evidence that the variability reflected the selective pressure of immune responses (37). Studies of a neutralization-resistant (NR) variant recovered from an experimentally infected chimpanzee and of such a variant selected with human serum in vitro found that mutations conferring resistance to antibodies directed against gp120 epitopes were located in gp41, not in gp120 variable regions (2, 6, 60, 61, 76). Thus, although it seems intuitively evident that neutralization epitopes contained within regions of the envelope that are inherently variable would be subject to immunological selection, there is very little data to define the nature of the selection process in these regions that actually does occur in vivo.
In this report, we describe additional NR viruses selected in vitro by using human serum. The conditions used included the use of a serum for selection that neutralized the virus strain subjected to immunological selection in a manner that could be nearly completely inhibited by a short synthetic peptide homologous to the V3 neutralization determinant of the virus. In this way, we could test the hypothesis that such selective pressure would generate diversity in the neutralization determinant of the V3 region of the protein. Mutants were generated that had a surprising phenotype of broad, rather than narrow, V3-specific neutralization resistance. To further evaluate the envelope glycoproteins from the wild-type (wt) neutralization-sensitive (NS) virus and the NR escape mutant, the env genes were expressed on pseudoviruses, which were then used to characterize the phenotype, as well as study the basis for neutralization escape by constructing a series of chimeric envelope glycoproteins and sequencing the entire env genes. The data we present here establish that non-V3 mutations in gp120 and gp41 confer neutralization resistance to anti-V3 antibodies and indicate previously unrecognized interactions between regions of the two envelope components.
MATERIALS AND METHODS
Plasmids.
Plasmid pNL-Luc-E−R−, which contains an HIV-1 NL4-3 genome with a luciferase reporter gene and defective env and vpr genes, was provided by N. Landau (Aaron Diamond AIDS Research Center, The Rockefeller University, New York, N.Y.) (13, 23). Plasmid pSV7d was a gift from P. Luciw (University of California, Davis) and R. L. Burke (Chiron Corp., Emeryville, Calif.) (65).
Viruses and cell cultures.
The MN strain of HIV-1 was a gift from Robert Gallo, Bethesda, Md. (6). Virus pools were prepared in H9 cell cultures (also from R. Gallo) by methods previously described (73). Briefly, cultures were monitored for giant-cell formation; the cells were centrifuged at the optimum time; the medium was removed; the cells were resuspended in phosphate-buffered saline, shaken vigorously, and recentrifuged; and the supernatant was harvested for use as a virus pool. The Molt 3 cell line was obtained from the American Type Culture Collection (Manassas, Va.). The PM1 cell line was obtained from the National Institutes of Health (NIH) AIDS Reagent Program (provided by P. Lusso and M. Reitz) (43). The 293T cell line was obtained from the American Type Culture Collection with permission from the Rockefeller Institute (42). H9, Molt 3, and PM1 cell cultures were maintained in RPMI 1640 medium supplemented with fetal bovine serum (10%) and antibiotics. The 293T cells were maintained in Dulbecco’s minimal essential medium with similar supplements.
Isolation of NR variants.
NR mutants of the MN virus were obtained by growing the virus in Molt 3 cells in the presence of a selected human serum with an NA titer of 1:25,600 (data presented in Table 1 and below). Serial 10-fold dilutions of the virus were mixed with the serum at a 1:250 dilution, incubated for 1 h at 37°C, and then inoculated into microtiter wells with Polybrene-treated Molt 3 cells in a manner analogous to that used for cell inoculation in NA assays. The wells were observed approximately on a daily basis for giant-cell formation, wells in which evidence of virus growth appeared to begin with the formation of a single giant cell were identified, and the wells representing the most dilute virus inoculum at which such growth was observed were selected for further processing. The contents of each well were passed individually to wells of a 24-well plate and maintained in the same serum concentration until maximum giant-cell formation had passed. At that point, supernatant fluid from each well was harvested and passed to new microtiter cultures of Molt 3 cells by using the same selection procedure as before but a serum concentration of 1:100. The contents of single wells representing the most dilute virus concentration causing giant-cell formation as described above were again passed to wells of 24-well plates. The process was repeated a third time in the presence of a 1:50 serum dilution. The viruses harvested from single wells after the third passage were propagated further in H9 cells to form virus pools by using the same procedure as for the parent virus, except that the growth medium was routinely supplemented with the serum used for the selection process at a 1:50 dilution. In one case, the human serum was not added to allow testing of whether residual antibodies affected the phenotypes obtained.
TABLE 1.
Inhibitory effects of synthetic MN strain V3 peptide on NA in sera from patients
| Seruma | Reciprocal of NA titer at peptide conc (per ml) of:
|
||||||
|---|---|---|---|---|---|---|---|
| 0 ng | 0.1 ng | 1.0 ng | 10 ng | 0.1 μg | 1.0 μg | 10 μg | |
| NY-1 | 800 | 200 | 200 | 200 | 200 | 100 | 100 |
| NY-2 | 12,800 | 12,800 | 6,400 | 12,800 | 12,800 | 12,800 | 12,800 |
| NY-3 | 25,600 | 6,400 | 3,200 | 1,600 | 800 | 400 | 200 |
| NY-4 | 25,600 | 6,400 | 3,200 | 1,600 | 1,600 | 1,600 | 1,600 |
| NY-5 | 12,800 | 6,400 | 3,200 | 1,600 | 1,600 | 1,600 | 800 |
| NY-6 | 3,200 | 800 | 400 | 400 | 400 | 400 | 400 |
| NY-7 | 6,400 | 3,200 | 1,600 | 1,600 | 1,600 | 800 | 1,600 |
| NY-8 | 25,600 | 3,200 | 3,200 | 1,600 | 800 | 800 | 800 |
| NY-9 | 51,200 | 25,600 | 6,400 | 3,200 | 1,600 | 1,600 | 1,600 |
| NY-10 | 6,400 | 12,800 | 12,800 | 6,400 | 1,600 | 1,600 | 1,600 |
Sera were obtained from the NYBC.
Virus neutralization assays.
The sera used in this study were from studies conducted previously in our laboratory or were provided by A. Prince, New York Blood Center (NYBC). The former were obtained from individuals who participated in studies of AIDS pathogenesis at the NIH, and the latter were from individuals who were candidate blood donors and were collected and used in compliance with applicable requirements for the protection of human test subjects. All sera from HIV-1-infected donors were repeatedly reactive in an HIV-1 enzyme-linked immunosorbent assay and confirmed positive in Western blot assays. The synthetic peptides used in the neutralization assays were produced by solid-phase synthesis as previously described and were a gift from K. Seamon (Food and Drug Administration, Bethesda, Md.) (6). Neutralization assays were performed with Molt 3 cell cultures by using inhibition of giant-cell formation as the endpoint, as previously described (33, 73). Briefly, sera and viruses were incubated together at 37°C for 60 min prior to the addition of cells suspended in medium containing Polybrene. The virus was allowed to adsorb to the cells for an additional 60 min, and then the plates were centrifuged at low speed, additional medium was added, and the cultures were incubated for 5 or 6 days. Giant cells were counted microscopically, and the number of giant cells per field was determined for each well. Serial twofold dilutions of the sera were prepared by beginning at 1:8 or 1:10. The NA titer of each serum was considered to be the highest dilution at which greater than 50% inhibition of giant-cell formation occurred. Sera that did not neutralize at a 1:8 dilution were assigned a titer of 1:4. To test the inhibitory effects of synthetic peptides on the neutralizing activity of individual sera, the sera were incubated in specific peptides at concentrations ranging from 0.1 ng/ml to 100 μg/ml for 30 min at 37°C prior to addition to the virus suspension to be neutralized.
Preparation and sequencing of the V3 region.
DNA fragments corresponding to the V3 regions of the MN virus and NR variants derived from it were synthesized by PCR (50). Template DNA was prepared by extraction from acutely infected H9 cells (25). The sequence of the sense primer used was 5′AATGCTAAAACCATAATAGTACAGCTG3′, corresponding to nucleotides (nt) 853 to 879 in the MNCG sequence (21, 52). The sequence of the antisense primer was 5′TTACAATTTCTGGGTCCCCTCCTGAGGAT3′, corresponding to nt 1126 to 1098. The reaction was performed in the presence of 200 μM each deoxynucleoside triphosphate, 10 mM Tris hydrochloride (pH 8.3), 500 mM KCl, 15 mM MgCl2, 0.01% gelatin, and 2 U of Taq DNA polymerase (Perkin Elmer Corp., Norwalk, Conn.). Reaction mixtures were initially heated to 94°C for 5 min, and then 25 cycles were carried out involving denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min, followed by a terminal extension reaction at 72°C for 5 min. The PCRs with the MN and resistant strain DNA templates were all conducted at the same time, and this was the first time MN strain env DNA was amplified in our laboratory.
The PCR fragments were inserted by blunt-end ligation into the vector pGem3Zf-(Promega, Madison, Wis.) and sequenced on both strands with Sequenase (U.S. Biomedical, Cleveland, Ohio) by using the pUC/M13 universal sequencing primers (Promega). Analyses were performed by using the EditSeq and MegAlign programs (DNASTAR Inc., Madison, Wis.) (24). The cloning and sequencing were performed at Procons Laboratories, Inc., Amherst, N.Y.
Construction of molecular clones of full-length viruses.
Molecular clones of full-length genomes of infectious viruses were obtained from unintegrated circular viral DNA isolated from acutely infected H9 cells (25). The DNA was digested with EcoRI, and the circularly permuted viral DNA was cloned by using EcoRI-digested λgtWES and Escherichia coli LE 392 (1, 14, 38, 71). The cloned EcoRI inserts were then transferred into pBR322. The plasmid DNA was then digested with EcoRI, ligated to form concatemers which regenerated full-length viral DNA, and transfected into HeLa cells by the calcium phosphate technique. After 2 days, the transfected HeLa cells were cocultivated with H9 cells, and virus production was monitored by reverse transcriptase assay (1, 14). Virus was harvested from the cultures at the time of peak reverse transcriptase production. A single infectious molecular clone of MN was obtained, and an additional single infectious clone of one of the variant viruses, designated E4, was obtained. These molecular clones were named MNTQ and MNE4, respectively, and sequenced in full or in part by using Sequenase as previously described (14).
Envelope glycoprotein expression vector construction and sequence analysis of env clones.
The env genes were amplified by PCR either from a molecularly cloned virus or a wt MN virus pool. To clone the wt MN env gene, viral RNA was extracted by phenol-guanidium thiocyanate (RNA Stat-50; Tel-Test Inc., Friendswood, Tex.), and cDNA was synthesized by using avian myeloblastosis virus reverse transcriptase (cDNA Cycle Kit; Invitrogen, San Diego, Calif.). A set of primers was designed to amplify the env gene as follows: sense, 5′CGACGAAGGATCCCTCAAGACAGT3′ (nt 6007 to 6030 on MNCG); antisense, 5′GACCATTTGCCACTCGAGTTATAGCAAAGCCCTTTCC3′ (nt 8827 to 8791). Restriction enzyme recognition sequences for BamHI and XhoI (underlined) were incorporated into the primers. The antisense primer was specially designed to compensate for a deletion which was observed in the 3′ end of the env gene extending to the nef gene sequence in the two molecularly cloned MN viruses compared with the MNCG sequence. Amplifications were accomplished by using rTth, XL (Perkin Elmer), which contains Vent DNA polymerase and is predicted to introduce less than one mutation per env gene copy (3, 5). PCR was performed with 200 μM each deoxynucleoside triphosphate, 20 pmol of each primer, 1 mM magnesium acetate, and 2 U of rTth, XL for 30 cycles (94°C for 15 s, 68°C for 3 min) by using a Gene Amp PCR System 2400 thermal cycler (Perkin Elmer, Foster City, Calif.). The env gene was also amplified directly from molecularly cloned E4 DNA by using the same primers. The reaction product was purified with the Wizard prep PCR purification system (Promega), digested with BamHI and XhoI, and ligated into the BamHI and SalI sites of pSV7d. The DNA was transformed into competent HB101 cells (GIBCO-BRL, Gaithersburg, Md.), and clones were screened for env gene inserts. The resulting clone was named pSV-Vx or pSV-Ex, where x is the clone number and V or E indicates that the clone was obtained from a viral RNA or MNE4 DNA template, respectively.
Nucleotide sequence analysis of these env genes was performed in both direction by dye terminator cycle sequencing with AmpliTaq DNA polymerase FS (Perkin Elmer) for 25 cycles (96°C for 10 s, 50°C for 5 s, 60°C for 4 min). Sequencing gels were run and analyzed with an Applied Biosystems Prism 377 DNA sequencer.
Pseudovirus construction and assays for infectivity and neutralization.
Pseudoviruses were constructed and assayed by using methods similar to those described previously (10). The env plasmid DNA and pNL-Luc-E−R− were cotransfected into 50% confluent 293T cells by calcium phosphate transfection (Promega). The culture medium was replaced with fresh medium containing 1 μM sodium butyrate (66) at 18 h posttransfection. At 48 h after transfection, the pseudovirus-containing supernatants were harvested, filtered through a 45-μm-pore-size sterile filter (Millipore Corp., Bedford, Mass.), supplemented with additional fetal bovine serum to a final concentration of 20%, and stored at −80°C.
To measure the infectivity of pseudoviruses, a luminescence assay with PM1 cells was used as previously described (58). We have shown that similar results are obtained by neutralization assay of T- and M-tropic viruses with Molt 3 cells or PHA-blasts, respectively, and corresponding pseudoviruses with PM1 cells (58). PM1 cells (1.5 × 104) were inoculated with a serially diluted pseudovirus in 96-well plates with U-bottom wells. The cultures were incubated for 3 days at 37°C, after which the cells were washed with cold phosphate-buffered saline and lysed with 10 μl of cell lysis buffer (Promega). The amount of luciferase activity in each well was determined with 100 μl of substrate (Promega) in a Luminoscan luminometer (Labsystems, Inc., Needham, Mass.). Infectivity endpoints were determined by a modified Reed-Muench method; an individual well was considered positive if the luminescence was at least 10-fold greater than the mean of 12 to 18 negative control wells, and the endpoint was considered to be the highest dilution at which the calculated frequency of positivity was ≥50% (39). Luminescence resulting from infection with minimally diluted samples of nonchimeric pseudoviruses was generally at least 1,000-fold greater than the background.
The neutralization phenotype of each pseudovirus was tested in a manner similar to that of the infectivity assay, except that aliquots of 25 μl of serially diluted serum were mixed with equal volumes of the appropriately diluted pseudovirus and incubated for 1 h at 37°C, after which PM1 cell suspensions were added. The pseudovirus dilutions were selected to produce luminescence in the presence of nonneutralizing serum of about 100 times the background. The neutralization endpoints were calculated by a modified Reed-Muench method in which the endpoint was considered to be the highest serum dilution calculated to have a frequency of ≥50% for a reduction of luminescence by ≥90% compared to the nonneutralized control. Reference neutralizing sera 1 and 2 and the negative reference serum were used as controls (NIH AIDS Reagent Program, provided by L. Vujcic and G. Quinnan) (74).
Construction of envelope glycoprotein chimeras.
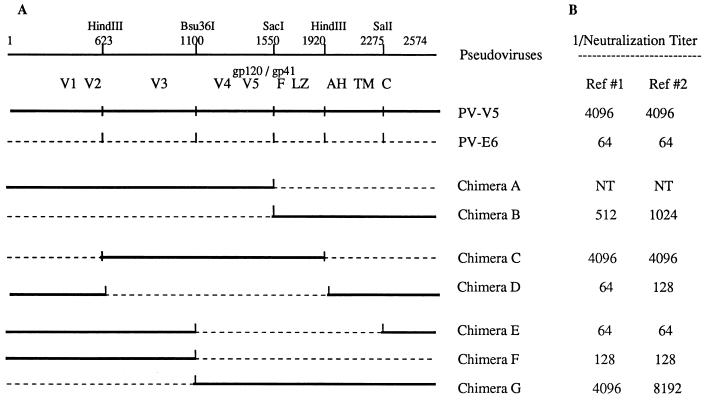
Several chimeric env clones were constructed by exchanging the fragments of NS (pSV-V5) and NR (pSV-E6) parental env clones after digesting the DNAs with restriction enzymes. For the specific restriction enzymes and the locations of their recognition sequences, see Fig. 2. The nucleotide positions are numbered based on MNCG sequences by counting from the start codon of the env gene (21). Chimeras A and B were constructed by digesting the plasmids with EcoRI (upstream of the env start codon in pSV7d) and SacI (nt 1550). Chimera A contains the entire sequence of gp120 from pSV-V5 with the rest of the region (gp41) from pSV-E6, since the SacI site is located 4 amino acids downstream from the cleavage site of gp120 and gp41. Chimeras C and D were constructed by exchanging the 1,297-bp fragments between the two HindIII sites at nt 623 and 1920. To construct chimera D, the third HindIII site located at nt 2004 of the env gene of pSV-V5 was removed by introducing a silent mutation (C to T at nt 2007) by using-site directed mutagenesis (Quick Change Mutagenesis Kit; Stratagene). The sequence of the mutated segment was verified, and it was transferred into the corresponding region of pSV-V5. The phenotype of the V5 envelope was the same before and after mutagenesis. Chimera E contains a fragment between the Bsu36I (nt 1100) and SalI (nt 2275) sites from pSV-E6 and the rest of the sequences from pSV-V5. Chimeras F and G were generated by exchanging the fragments between the EcoRI and Bsu36I sites. Pseudoviruses expressing chimeric env genes were prepared as described above. The infectivity and neutralization phenotypes of these pseudoviruses were measured in PM1 cells.
FIG. 2.
Map comparing the infectivity and neutralization sensitivity phenotypes of chimeras and parent env clones pSV-V5 and pSV-E6. (A) Series of chimeras constructed by digesting env genes with restriction endonucleases and exchanging fragments. (B) Neutralization phenotypes of chimeras. Assays were conducted with pseudoviruses against reference sera 1 and 2. The neutralization endpoint of chimera A was not determined due to its poor infectivity. F, fusion domain; TM, transmembrane domain; C, cytoplasmic sequence; NT, not tested.
Nucleotide sequence accession numbers.
The full-length genome of the MNTQ clone and the env genes of MNE4, pSV-E6, and pSV-V5 have been sequenced (GenBank accession no. AF075719 through AF075722).
RESULTS
Serum for immunological selection and neutralization resistance assay of selected variants.
Ten sera were screened for V3 specificity of NA activity. The synthetic peptide used in this assay as a blocking reagent consisted of the sequence RIHIGPGRAFYTTKNIIGTIRQA, corresponding to amino acid residues 311 to 333 of the MN strain envelope. Three patterns of V3 specificity were observed, as illustrated in Table 1. The NA activity of serum NY-3, and possibly serum NY-1, was nearly completely inhibited by the synthetic peptide, with progressive inhibition occurring at each incremental increase in concentration throughout the range of peptide concentrations tested; concentrations higher than 100 μg/ml were cytotoxic and could not be tested. Thus, serum NY-3 was inhibited more than 99% by the peptide. In contrast to serum NY-3, serum NY-2 was not inhibited by the synthetic peptide at all. The NA activity of the other seven sera tested was partially inhibited, and inhibitory concentrations were reached after which further peptide concentration increases caused no additional inhibition. These results are consistent with results we have obtained in other studies. In view of these data, it was considered that serum NY-3 may neutralize predominantly by binding to the principal neutralizing determinant on V3, and this serum was used for immunological selection.
Progeny viruses from four individual wells were picked and propagated under selection conditions as described above. These variants were designated C2, E4, E11, and F3. The results of initial NA assays with these variants are shown in Table 2. The four viral variants were highly resistant to neutralization by the serum used for selection (NY-3), as well as by the other sera shown. The variants were similarly resistant to neutralization by five sera in which the NA could be partially blocked by the synthetic peptide, as well as by two sera in which the NA could not be blocked. Similar neutralization resistance was observed with all of numerous additional sera tested, none of which had activity greater than that of NY-9 shown in Table 2 (data not shown). One virus pool of the E4 variant which was prepared in the absence of NY-3 serum displayed neutralization resistance similar to that of the pool shown in Table 2 (results not shown).
TABLE 2.
Neutralization of HIV-1 strain MN and four NR variant viruses obtained from it by human sera and lack of correlation between susceptibility to V3 peptide blocking of strain MN neutralization
| Human seruma | Reciprocal of NA titer against virus variant:
|
Fold inhibition of MN NA by V3 peptideb | ||||
|---|---|---|---|---|---|---|
| MN | C2 | E4 | E11 | F3 | ||
| NY-2 | 12,800 | 256 | 128 | 128 | 256 | 1 |
| NY-3 | 25,600 | 32 | 8 | 16 | 16 | 128 |
| NY-5 | 12,800 | 64 | 32 | 64 | 64 | 16 |
| NY-7 | 6,400 | 32 | 16 | NTc | NT | 4 |
| NY-9 | 51,200 | 1,024 | 256 | 512 | 256 | 32 |
| L-1 | 12,800 | 64 | 32 | 32 | 64 | NT |
| L-2 | 512 | 16 | NT | NT | NT | 1 |
| L-3 | 1,024 | 16 | 16 | NT | NT | 4 |
Sera were obtained from the NYBC (NY designations) and from patients who participated in studies of AIDS pathogenesis at the NIH (L designations).
Fold inhibition was determined at a peptide concentration of 100 μg/ml for NY-7, L-2, and L-3 and at 10 μg/ml for the rest of the sera.
NT, not tested.
V3 region sequences of MN and its NR variants.
To evaluate the V3 region sequences of the NR viruses, clones of PCR-derived DNA of the MN strain and the variants were sequenced in accordance with the following algorithm: if the sequences of two clones from one variant were identical, no additional clones were sequenced, while if they differed, three additional clones were sequenced. Thus, two clones from C2 and five clones from each of the other strains were sequenced. In comparison to the sequence of MNCG published in the database, all but one of the MN and all of the NR virus PCR clones had a 294N/K change before V3, all had a 327I/K mutation within V3, and all of them but one of the MN clones had a 336N/I substitution after V3. MNCG is a full-length, noninfectious genomic clone of the MN virus (51). There were also sporadic changes in five of the clones sequenced. None of these distinguished the MN clones from the variant clones. However, there was one distinguishing difference between the MN clones and the NR variant clones, in that 16 of the 17 NR clones had a 307N/I substitution. Only one of the five E11 clones lacked this mutation.
Tests for the effect of the 307N/I substitution on the specificity of human anti-V3 NA.
Because of the potential importance of the 307N/I substitution, additional studies were performed. Residue 307 is proximal to the region of V3 which has been mapped to the neutralization determinant (29). To test whether the amino acid change at this position alters the specificity of NA interaction with V3, we tested NA blocking by synthetic peptides homologous to the consensus sequences in our MN and escape virus pools from residues 306 to 329. The MN and variant peptides blocked MN virus neutralization by sera NY-1 and NY-3 equivalently and also blocked escape virus neutralization (variants F3 and E11) equivalently (data not shown). These reciprocal blocking experiments were intended to test whether the mutation altered the antigenic specificity of V3 recognition and not whether the mutation might have other significant conformational effects.
Neutralization phenotypes of viruses produced from molecular clones.
Virus pools were prepared from molecular clones for use in neutralization assays. The MN and E4 clones produced viruses that replicated with replication kinetics similar to those of the parent MN virus, and both produced giant cells in Molt 3 cell cultures (data not shown). These virus pools were compared with pools of uncloned viruses in neutralization assays, as shown in Table 3. The sera used in the assay whose results are shown had been previously tested in our laboratory and found to have high titers of NA against HIV-1 MN. All of these sera neutralized the uncloned MN pool at the highest serum dilution tested, 1:5,120. The virus pool derived from the MN molecular clone was relatively resistant to neutralization compared to the uncloned MN virus pool with an intermediate phenotype (NI). The individual sera had at least 4- to 64-fold greater neutralizing activity against the uncloned than the cloned MN virus. Both the cloned and uncloned E4 virus pools were highly resistant to neutralization, as expected. Both were consistently more resistant to neutralization than the cloned MN virus pool.
TABLE 3.
Neutralization of molecularly cloned and uncloned HIV-1 MN and NR variant E4
| Human serum | Reciprocal of NA titer against virus pool prepared froma:
|
|||
|---|---|---|---|---|
| MN
|
E4
|
|||
| Uncloned | Cloned | Uncloned | Cloned | |
| Reference 1 | >5,120 | 80 | 8 | 8 |
| Reference 2 | >5,120 | 320 | 32 | 128 |
| NY-3 | >5,120 | 80 | 8 | <8 |
| NY-4 | >5,120 | 160 | <8 | NTb |
| NY-5 | >5,120 | 80 | <8 | 16 |
| NY-9 | >5,120 | 1,280 | 256 | NT |
| L-1 | >5,120 | 80 | 8 | 32 |
| L-4 | 5,120 | 640 | 128 | 128 |
| L-5 | >5,120 | 640 | 64 | 64 |
In assays using the MN virus, sera were tested in serial twofold dilutions beginning at an initial dilution of 1:10, while in assays using the E4 virus, the starting dilution was 1:8.
NT, not tested.
Neutralization phenotype of pseudoviruses produced from various env clones.
env genes from viruses with different neutralization phenotypes were PCR amplified and cloned. Pseudoviruses were constructed by using several clones each from a wt MN virus pool and the molecular clone of MNE4, and these were tested for their biological phenotypes. Neutralization results obtained with representative clones derived from the MN virus and MNE4 compared to their parents are presented in Table 4. Pseudoviruses expressing envelope glycoproteins from MNE4 were variably NR, while those from the MN virus were consistently NS. Based on these neutralization results, the pSV-V5 clone was selected for further study since it was representative of the pSV-V clones, and the pSV-E6 clone was selected since it had the most resistant phenotype among those we tested. Pseudoviruses expressing these two clones were named PV-V5 and PV-E6. A flow chart of the MN virus and different virus clones derived from it is presented in Fig. 1.
TABLE 4.
Neutralization of pseudoviruses constructed from different env clones
| Serumb | Reciprocal of NA titer againsta:
|
||||||
|---|---|---|---|---|---|---|---|
| MN virus | MN virus-derived pseudovirus:
|
MNE4 | MNE4-derived pseudovirus:
|
||||
| PV-V5 | PV-V32 | PV-V36 | PV-E6 | PV-E14 | |||
| Reference 1 | >5,120 | 4,096 | 2,048 | 2,048 | 8 | 64 | 128 |
| Reference 2 | >5,120 | 4,096 | 4,096 | 2,048 | 128 | 64 | 256 |
| NY-2 | 12,800 | 16,384 | NTd | NT | NT | 512 | NT |
| NY-3c | 25,600 | 8,192 | NT | NT | <8 | 32 | NT |
| NY-4 | 25,600 | 8,192 | NT | NT | <8 | 256 | NT |
| NY-5 | 12,800 | 16,384 | NT | NT | 16 | 16 | NT |
| NY-7 | 6,400 | 8,192 | NT | NT | <8 | 512 | NT |
| NY-8 | 25,600 | 16,384 | NT | NT | NT | 256 | NT |
| NY-9 | 51,200 | ≥32,768 | NT | NT | NT | 512 | NT |
| NY-10 | 6,400 | 16,384 | NT | NT | NT | 512 | NT |
Neutralization of MN virus and MNE4 was assayed with Molt 3 cells, and the assays for the pseudoviruses were done with PM1 cells as described in Materials and Methods.
Human sera obtained from the NYBC (NY designations) and reference sera were used.
Serum that was used for selection of NR variants.
NT, not tested.
FIG. 1.
Schematic diagram of the production of the HIV-1 strain MN whole viral genome (MNTQ and MNE4) and env clones (V5 and E6).
Neutralization phenotype of chimeric envelope glycoproteins.
To evaluate the domains of the envelope responsible for the neutralization phenotype, a series of chimeric env clones were constructed from representative NS and NR env clones pSV-V5 and pSV-E6, respectively. The chimeric pseudoviruses were first screened for infectivity in PM1 cells. The pseudovirus constructed with chimeric env clone A was poorly infectious, while the other chimeras made were as infectious as their parents, although the relative titer was slightly variable after each transfection (data not shown). Neutralization phenotypes of chimeras were determined and compared with those of parental env clones. Chimera B derived its 5′ sequences, up to the SacI site located 4 amino acids carboxyl to the gp120-gp41 cleavage site, from pSV-V5 and its 3′ sequences from pSV-E6. It was consistently of intermediate neutralization sensitivity (Fig. 2). Neutralization endpoint titers could not be calculated for the converse construct, chimera A, because the virus titer was very low. However, it did appear to be consistently less sensitive to neutralization than PV-V5 (data not shown). These results indicate that both gp120 and gp41 apparently contribute to the neutralization phenotype. The phenotype of chimeras E and F was clearly NR, not different from that of PV-E6, while that of chimera G was as NS as PV-V5. These data indicate that the neutralization phenotype segregates with the C-terminal portion of gp120 plus gp41, not the N-terminal part of gp120. In addition, since no phenotypic difference was observed between chimeras E and F, the cytoplasmic tail stretching from the SalI site to the 3′ end of the envelope did not appear to be responsible for the phenotype. Since chimera C was NS and chimera D was NR, it appeared that the phenotype segregated within the N-terminal region of gp41, including the fusion domain and the leucine zipper (LZ) but ending before the membrane-proximal α-helix (AH) (12, 19). Considering the data for all of the phenotypes of the chimeric env clones, the region responsible for the neutralization phenotype difference falls between the Bsu36I site in the C terminus of gp120 and the second HindIII site in the N terminus of gp41.
Nucleotide sequences of the env genes with various neutralization sensitivities.
The published MNCG sequence differs from those of all four clones at a number of residues, including those residues indicated above which distinguished the MN virus V3 region PCR DNA from MNCG. Compared to MNCG, both the MNTQ and MNE4 clones have a deletion mutation that extends from the second-to-last nucleotide in the env gene about 250 nt into the nef gene, so that no nef gene product can be made. An in-frame stop codon occurs 4 amino acids downstream from the usual termination site of the env gene. Additional PCR studies we have conducted by using MN strain RNA as the template have demonstrated that the parent virus pool comprises a mixture of genomes with and without the nef deletion (data not shown).
The predicted amino acid sequences of the envelope glycoproteins corresponding to our four clones were compared, and polymorphisms that distinguish pSV-E6 and MNE4 from pSV-V5 are shown in Table 5. There were four additional sites at which MNTQ differed from pSV-V5, i.e., 281D/N, 307N/I, 407T/N, and 544L/P, at which pSV-V5 and MNE4 shared the same amino acids (not shown in Table 5). Unexpectedly, pSV-E6, which was derived from the MNE4 clone by PCR amplification, showed a 3-nt difference from the MNE4 sequence at nt 1631, 1703, and 1959, of which the first two are predicted to give rise to amino acid changes at residues 544 (L/P) and 568 (R/Q) and the third nucleotide substitution is silent. The finding of nucleotide changes at 3 of 2,574 bp in our synthetic PCR was surprising, considering the low predicted error rate of rTth, XL (3, 5). Comparison of pSV-V5 and pSV-E6 showed 16 predicted amino acid changes. The mutations which distinguish the two regions in pSV-V5 and pSV-E6 to which the neutralization phenotype mapped in the chimeras are at residues 420 and 460 in C4 and V5 of gp120 and at 562, 565, and 582 of the LZ region of gp41. Among these changes, the polymorphism at residue 460 was unique in that NI clone MNTQ differed from NS clone pSV-V5 (M versus E) and NR clones MNE4 and pSV-E6 (M versus I).
TABLE 5.
Amino acids in the envelope glycoproteins of NR variants compared with those of NS V5
| Position | Amino acid on envelope derived from:
|
Location (domain) | |||
|---|---|---|---|---|---|
| E6 | MNE4 | MNTQ | V5 | ||
| 141 | Asn | Asn | Asn | Asp | V1 |
| 144 | Asp | Asp | Asp | Ala | V1 |
| 172 | Asp | Asp | Asp | Asn | V2 |
| 174 | Val | Val | Met | Met | V2 |
| 188 | Ser | Ser | Pro | Pro | V2 |
| 196 | Tyr | Tyr | Tyr | His | V2 |
| 336 | Ile | Ile | Ile | Thr | C3 |
| 420 | Ile | Ile | Ile | Val | C4 |
| 460 | Ile | Ile | Met | Glu | V5 |
| 562 | Ser | Ser | Ser | Ala | LZa |
| 565 | His | His | His | Asn | LZ |
| 582 | Leu | Leu | Leu | Gln | LZ |
| 663 | Glu | Glu | Glu | Gly | AHb |
| 668 | Ala | Ala | Ala | Glu | AH |
| 819 | Ala | Ala | Ala | Thr | Cc |
| 828 | Asp | Asp | Asp | Asn | C |
LZ-like domain.
AH region located between LZ and transmembrane domain.
C, cytoplasmic.
DISCUSSION
In this study, we attempted to induce mutations in the MN strain of HIV-1 by subjecting the virus to the immunological selective pressure associated with growth in the presence of human serum with high NA activity directed predominately against the V3 region neutralization determinant. We hypothesized that this selective pressure would result in a mutation(s) in the V3 neutralization determinant itself and that the mutation(s) would result in selective resistance to neutralization by the serum used for the selection process and other sera that reacted selectively with the MN V3 neutralization determinant. The four different NR viruses so derived were found to be broadly resistant to neutralization by all of the human sera that we tested, including some that had NA activity that could not be demonstrated to be directed against the V3 determinant by peptide blocking experiments. Sequencing of the PCR DNA spanning the V3 regions of the four different NR viruses derived by this procedure did not reveal any mutations in the neutralization determinant itself. A mutation at residue 307(N/I) was found in 16 of 17 PCR clones from resistant viruses, but an effect of this mutation on antigen recognition specificity could not be demonstrated in peptide blocking studies, and cloned env genes from one of the escape viruses with the NR phenotype did not have this mutation. Studies conducted by using molecular virus clones and env gene clones expressed on pseudoviruses demonstrated that the NR phenotype mapped to mutations in the C-terminal region of gp120 and the N-terminal region of gp41. The data suggest an interaction between these two regions which affects sensitivity to NA directed at V3.
To demonstrate the molecular basis for the neutralization phenotype in the variants obtained by immune selection, two full-length molecular virus clones were prepared, one from our parent MN virus and the other from E4, one of the NR variants. It was hoped that an NS MN virus clone would be obtained into which it would be possible to introduce mutations found in the E4 virus clone to evaluate the role of each. Instead, MN virus clone MNTQ had an NI phenotype, while the E4 clone MNE4 was as resistant as the virus pool from which it was obtained. The finding that the MNTQ clone had intermediate neutralization sensitivity emphasizes the facts that the virus pool from which it was derived is a quasispecies mixture and that the immune selection may simply have facilitated the outgrowth of NR variants. Since the molecular virus clones could not be assessed directly to determine the basis for the NR phenotype, pseudoviruses were prepared which expressed envelope glycoproteins of interest. Neutralization phenotypes of pseudoviruses expressing MN virus envelope glycoproteins were consistently NS, while MNE4-derived clones tended to be NR, with some diversity. MN-derived clone pSV-V5 and the most NR E4-derived clone, pSV-E6, were selected for further study. PV-V5 was approximately as NS as the parent MN virus, while PV-E6 appeared to be slightly less NR than the E4 virus or the MNE4 molecular virus clone. Chimeras between these two env clones were constructed by using pre-existing restriction sites to further elucidate the bases for neutralization phenotype differences. The chimeras varied in both infectivity and neutralization phenotype. The infectivity phenotypes of different chimeras were interesting in that chimera A was poorly infectious while others were as infectious as their parents. The poor infectivity of chimera A apparently indicates that certain domains of the parental genes are incompatible when expressed together. More studies are needed to define the specific mutations and domains responsible for this phenotype.
The neutralization phenotype of the MN virus and variants appeared to depend on mutations in domains of gp120 and gp41, as determined by studies with chimeric genes. Sequence analyses revealed the amino acid changes that might be responsible for the phenotype changes within these domains to be residues 420(V/I) and 460(E/I) of gp120, which are located in C4 and V5, respectively, and three mutations in the LZ region of gp41 (63). These results are somewhat similar to those of a study of an NR variant of simian immunodeficiency virus that evolved during macaque infection in that the phenotype in that case was determined by the region of the env gene extending from V4 of gp120 through the 5′ portion of gp41 (34). However, the majority of the amino acid changes were clustered in V4 and resulted in the introduction of additional glycosylation sites, leading the investigators to suggest that the increased glycosylation was the mechanism for neutralization resistance. The mutation in C4/V5 of our NR clones did not affect glycosylation. In our study, mutations in both the C4/V5 region of gp120 and the LZ region of gp41 appeared to be responsible for the neutralization phenotype, but the role of each specific mutation needs to be further evaluated. Mutations in the LZ region of gp41 that affect the neutralization sensitivity of a virus have been previously reported (60, 68, 75). Reitz and colleagues found that the A/T mutation at residue 582 conferred the NR phenotype on the IIIB strain variant selected in vitro (60). Thali et al. have further shown that this mutation conferred resistance to neutralization by monoclonal antibodies directed against conformationally dependent epitopes in the CD4bs (68). There had been no report that any mutations in the C4/V5 region directly caused neutralization resistance, but interestingly, our mutation at residue 460 is very close to the conserved neutralization epitope in the CD4bs. Additionally, residue 460 is the only position at which we observed distinguishing amino acid substitutions in clones with the NS, NI, and NR phenotypes. Our data provide the first demonstration that mutations in the C terminus of gp120 can contribute to the NR phenotype and confirmatory demonstration that mutations in LZ residues do contribute to the NR phenotype.
The importance of interaction between gp120 and gp41 for determination of the neutralization phenotype was shown previously in a virus clone derived from a chimpanzee-passaged HIV-1 IIIB isolate (2). Back et al. found that mutations in and near the neutralization epitope of gp41 defined by the 2F5 monoclonal antibody affected the recognition of V3 by a monoclonal NA, as well as human sera. This 2F5 epitope may overlap the AH region that forms the molecular clasp with the LZ region (8). Our variants also had mutations in this region at residues 663(G/E) and 668(E/A), but these mutations did not appear to contribute to the neutralization phenotype. However, the AH mutations we observed could be second-site mutations compensatory for LZ or gp120 mutations. Interactions between regions of gp120 and gp41 apparently underlie the poor infectivity of chimera A, which could indicate that compensatory mutations stabilize the envelope with the NR phenotype mutations. The specific mutations affecting these interactions need further study. A mutation at the residue corresponding to amino acid 420 in MN, located in the C4 domain, was previously found to be responsible for a change in the infectivity of the NL4-3 strain (16). Freed and Martin found that a single amino acid change (I/M) at this position restored infectivity to a replication-defective virus, which demonstrated an interaction between the C4 and V1/V2 regions of gp120 required for proper envelope function. Some of the mutations present in our NR clones may be compensatory for the mutations conferring the NR phenotype. Further study of these mutants may provide insights into the nature of the interacting regions in gp120 and gp41, as well as the mechanism whereby gp41 mutations may influence the sensitivity of gp120 epitopes to effects of NA.
The results of this study provide another example of the association of resistance to NA directed toward the V3 neutralization determinant and other non-V3 neutralization domains with mutations in other regions of the envelope proteins. A mutation in the V3 region proximal to the neutralization epitope was found in the majority of the V3 sequences we examined from our uncloned NR virus DNA. We have not determined whether this mutation contributes to the NR phenotype by conformational effects, since it was not present in the NR clones we studied functionally. The NR viruses obtained by Nara et al. as a result of in vivo chimpanzee passage were selected by the immune response that occurred naturally in vivo (2, 54). Since the early NA response was V3 directed, the changes observed may well have been the result of selection by anti-V3 antibodies. In contrast, the in vitro immunological selection conducted by Robert-Guroff et al. probably involved antibodies mostly directed at non-V3 epitopes, since the IIIB strain NA in most sera from infected humans appear to neutralize predominantly through binding to non-V3 epitopes (60, 61, 68, 76). In the latter case, therefore, it is not surprising that the mechanisms of resistance involved mutations outside V3. The immunological selection procedure we performed was highly targeted toward V3, since a synthetic MN V3 peptide blocked neutralization of the MN virus by the serum used for selection 128-fold. Thus, our experiments were a more direct test than the previously published studies of the hypothesis that V3-directed immunological selection would result in the emergence of escape mutations in V3. Instead, the mutations responsible for this phenotype occurred in other domains of the envelope glycoprotein. Our findings and those of others are consistent, therefore, in suggesting that the human and chimpanzee immune responses exert more powerful selective pressure for neutralization escape to occur through mutations that work indirectly to limit the neutralizing effects of antibody binding to known gp120 neutralization epitopes.
Highly NS, T-tropic, cell culture-passaged virus strain MN was used in our studies because it has V3-dependent neutralization sensitivity, which made it possible to study the selective effects of human sera that contain NA directed predominately at V3. The uncloned virus and polyvalent human serum were used in our selections to mimic, in those respects, the selection that might occur in vivo. Since both laboratory passage and T tropism could affect neutralization sensitivity, it is not known whether viruses which are not T tropic or cell culture passaged would respond in the same way to similar selection pressure. However, the similarities between our observations and those of others discussed above, with respect to the locations of mutations in gp41 associated with resistance to neutralization directed at gp120 epitopes, support the interpretation that the mutations we observed may reflect mechanisms of immunological escape common to T-tropic viruses in vivo and in vitro. The combined effects of the C4-V5 and LZ mutations in our clones on their global neutralization resistance may thus be relevant to the mechanisms of neutralization resistance of HIV in general.
ACKNOWLEDGMENTS
This work was supported in part by Uniformed Services University of the Health Sciences grant RO87EZ and Public Health Service grant AI37438 from the National Institute of Allergy and Infectious Diseases.
We thank Kenneth Seamon for provision of the synthetic peptides used in this study and Yuxin Ran, Wendy Glass, Rekha Rapaka, and Sylvester Daniel for technical assistance. We are indebted to Alicia Buckler-White for gene sequencing and to Malcolm Martin for advice and support.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Back N K T, Smit L, Schutten M, Nara P L, Tersmette M, Goudsmit J. Mutations in human immunodeficiency virus type 1 gp41 affect sensitivity to neutralization by gp120 antibodies. J Virol. 1993;67:6897–6902. doi: 10.1128/jvi.67.11.6897-6902.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes W M. The fidelity of Taq polymerase catalyzing PCR is improved by an N-terminal deletion. Gene. 1992;112:29–35. doi: 10.1016/0378-1119(92)90299-5. [DOI] [PubMed] [Google Scholar]
- 4.Broliden P, von Gegerfelt A, Clapham P, Rosen J, Fenyo E, Wahren B, Broliden K. Identification of human neutralization-inducing regions of the human immunodeficiency virus type 1 envelope glycoproteins. Proc Natl Acad Sci USA. 1992;89:471–475. doi: 10.1073/pnas.89.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cariello N F, Swenberg J A, Skopek T R. Fidelity of Thermococcus litoralis DNA polymerase (Vent) in PCR determined by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1991;19:4193–4198. doi: 10.1093/nar/19.15.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrow E W, Vujcic L K, Glass W L, Seamon K B, Rastogi S C, Hendry R M, Boulos R, Bzila N, Quinnan G V. High prevalence of antibodies to the gp120 V3 region principal neutralizing determinant of HIV-1MN in sera from Africa and the Americas. AIDS Res Hum Retroviruses. 1991;7:831–838. doi: 10.1089/aid.1991.7.831. [DOI] [PubMed] [Google Scholar]
- 7.Chamat S, Nara P, Berquist L, Whalley A, Morrow W J M, Kohler H, Kang C-Y. Two major groups of neutralizing anti-gp120 antibodies exist in HIV infected individuals. J Immunol. 1992;149:645–654. [PubMed] [Google Scholar]
- 8.Chen C-H, Matthews T J, McDanal C B, Bolognesi D P, Greenberg M L. A molecular clasp in the human immunodeficiency virus (HIV) type 1 TM protein determines the anti-HIV fusion activity of gp41 derivatives: implications for viral fusion. J Virol. 1995;69:3771–3777. doi: 10.1128/jvi.69.6.3771-3777.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffin J. Genetic variation in AIDS viruses. Cell. 1986;46:1–4. doi: 10.1016/0092-8674(86)90851-2. [DOI] [PubMed] [Google Scholar]
- 10.Conner R I, Sheridan K E, Lai C, Zhang L, Ho D D. Characterization of the functional properties of env genes from long-term survivors of human immunodeficiency virus type 1 infection. J Virol. 1996;70:5306–5311. doi: 10.1128/jvi.70.8.5306-5311.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson M. Maedi/visna: a review. Vet Rec. 1980;106:212–216. doi: 10.1136/vr.106.10.212. [DOI] [PubMed] [Google Scholar]
- 12.Delwart E L, Mosialos G, Gilmore T. Retroviral envelope glycoproteins contain a leucine zipper-like repeat. AIDS Res Hum Retroviruses. 1990;6:703–706. doi: 10.1089/aid.1990.6.703. [DOI] [PubMed] [Google Scholar]
- 13.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major coreceptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 14.Englund G, Hoggan M, Theodore T, Martin M A. A novel HIV-1 isolate containing alterations affecting the NF-κB element. Virology. 1991;181:150–157. doi: 10.1016/0042-6822(91)90479-u. [DOI] [PubMed] [Google Scholar]
- 15.Fauci A S, Fishinger P J. The development of an AIDS vaccine: progress and promise. Public Health Rep. 1988;103:230–236. [PMC free article] [PubMed] [Google Scholar]
- 16.Freed E O, Martin M. Evidence for a functional interaction between the V1/V2 and C4 domains of human immunodeficiency virus type 1 envelope glycoprotein gp120. J Virol. 1994;68:2503–2512. doi: 10.1128/jvi.68.4.2503-2512.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fung M S C, Sun C R Y, Gordon W L, Liou R-S, Chang T W, Sun W N C, Daar E S, Ho D D. Identification and characterization of a neutralization site within the second variable region of human immunodeficiency virus type 1 gp120. J Virol. 1992;66:848–856. doi: 10.1128/jvi.66.2.848-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fung M S C, Sun C, Sun N C, Chang N Y, Chang T W. Monoclonal antibodies that neutralize HIV-1 virions and inhibit syncytium formation by infected cells. Bio/Technology. 1987;5:940–946. [Google Scholar]
- 19.Gallaher W R, Ball J M, Garry R F, Griffin M C, Montelaro R C. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res Hum Retriviruses. 1989;5:431–438. doi: 10.1089/aid.1989.5.431. [DOI] [PubMed] [Google Scholar]
- 20.Goodenow M, Huet T, Saurin W, Kwok S, Sninsky J, Wain-Hobson S. HIV-1 isolates are rapidly evolving quasispecies: evidence for viral mixtures and preferred nucleotide substitutions. J Acquired Immune Defic Syndr. 1989;2:344–352. [PubMed] [Google Scholar]
- 21.Gurgo C, Guo H G, Franchini G, Aldovini A, Collati E, Farrel K, Wong-Stahl F, Gallo R C, Reitz M S. Envelope sequences of two new United States HIV-1 isolates. Virology. 1988;164:531–536. doi: 10.1016/0042-6822(88)90568-5. [DOI] [PubMed] [Google Scholar]
- 22.Haigwood N L, Shuster J R, Moore G K, Lee H, Skiles P V, Higgins K W, Barr P J, George-Nascimento C, Steimer K S. Importance of hypervariable regions of HIV-1 gp120 in the generation of virus neutralizing antibodies. AIDS Res Hum Retroviruses. 1990;6:855–869. doi: 10.1089/aid.1990.6.855. [DOI] [PubMed] [Google Scholar]
- 23.He J, Choe S, Walker R, Di Marzio P, Morgan D O, Landau N R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins D G, Sharp P M. Fast and sensitive multiple sequence alignment on a microcomputer. CABIOS. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- 25.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 26.Ho D D, Fung M S C, Cao Y, Li X L, Sun C, Chang T W. Another discontinuous epitope on glycoprotein gp120 that is important in human immunodeficiency virus type 1 neutralization is identified by a monoclonal antibody. Proc Natl Acad Sci USA. 1991;88:8949–8952. doi: 10.1073/pnas.88.20.8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho D D, Moudgil T, Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989;321:1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- 28.Hwang S S, Boyle T J, Lyerly H K, Cullen B R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991;253:71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- 29.Javaherian K, Langois A J, McDanal C, Ross K L, Eckler L I, Jellis C L, Profy A T, Rusche J R, Bolognesi D P, Putney S D, Matthews T J. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci USA. 1989;86:6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joag S V, Stephens E B, Narayan O. Lentiviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1977–1996. [Google Scholar]
- 31.Kalams S A, Johnson R P, Dynan M J, Hartman K E, Harrer T, Harrer E, Trocha A K, Blattner W A, Buchbinder S P, Walker B D. T cell receptor usage and fine specificity of human immunodeficiency virus 1-specific cytotoxic T lymphocyte clones: analysis of quasispecies recognition reveals a dominant response directed against a minor in vivo variant. J Exp Med. 1996;183:1669–1679. doi: 10.1084/jem.183.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karwowska S, Gorny M K, Buchbinder A, Gianakakos V, Williams C, Fuerst T, Zolla-Pazner S. Production of human monoclonal antibodies specific for conformational and linear non-V3 epitopes of gp120. AIDS Res Hum Retroviruses. 1992;8:1099–1106. doi: 10.1089/aid.1992.8.1099. [DOI] [PubMed] [Google Scholar]
- 33.Katzenstein D A, Vujcic L K, Latif A, Boulos R, Halsey N A, Quinn T C, Rastogi S C, Quinnan G V. Human immunodeficiency virus neutralizing antibodies in sera from North America and Africa. J Acquired Immune Defic Syndr. 1990;3:810–816. [PubMed] [Google Scholar]
- 34.Kinsey N E, Anderson M G, Unangst T J, Joag S V, Narayan O, Zink M C, Clements J E. Antigenic variation of SIV: mutations in V4 alter the neutralization profile. Virology. 1996;221:14–21. doi: 10.1006/viro.1996.0348. [DOI] [PubMed] [Google Scholar]
- 35.Konno S, Yammamoto H. Pathology of equine infectious anemia. Cornell Vet. 1970;60:393–449. [PubMed] [Google Scholar]
- 36.Kono Y, Kobayashi K, Fukunaga Y. Antigenic drift of equine infectious anemia virus in chronically infected horses. Arch Gesamte Virusforsch. 1973;41:1–10. doi: 10.1007/BF01249923. [DOI] [PubMed] [Google Scholar]
- 37.Korber B, Wolinsky S, Haynes B, Kunstman K, Levy R, Furtado M, Otto P, Myers G. HIV-1 intrapatient sequence diversity in the immunogenic V3 region. AIDS Res Hum Retroviruses. 1992;8:1461–1465. doi: 10.1089/aid.1992.8.1461. [DOI] [PubMed] [Google Scholar]
- 38.Leder P, Tiemeier D, Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the Lambda gtWES system. Science. 1977;196:175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- 39.Lennette E H. General principles underlying laboratory diagnosis of viral and rickettsial infections. In: Lennette E H, Schmidt M J, editors. Diagnostic procedures of viral and rickettsial diseases. New York, N.Y: American Public Health Association; 1964. p. 45. [Google Scholar]
- 40.Letvin N. Vaccines against human immunodeficiency virus—progress and prospects. N Engl J Med. 1993;329:1400–1405. doi: 10.1056/NEJM199311043291908. [DOI] [PubMed] [Google Scholar]
- 41.Levy J A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liou H-C, Sha W C, Scott M L, Baltimore D. Sequential induction of NF-κB/Rel family proteins during B-cell terminal differentiation. Mol Cell Biol. 1994;14:5349–5359. doi: 10.1128/mcb.14.8.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lusso P, Cocchi F, Balotta C, Markham P D, Louie A, Farci P, Pal R, Gallo R C, Reitz M S., Jr Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1. J Virol. 1995;69:3712–3720. doi: 10.1128/jvi.69.6.3712-3720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsushita S, Robert-Guroff M, Rusche J, Koito A, Hattori T, Hoshino H, Javaherian K, Jellis C, Takatsuki K, Putney S. Characterization of a human immunodeficiency virus neutralizing monoclonal antibody and mapping of the neutralizing epitope. J Virol. 1988;62:2107–2114. doi: 10.1128/jvi.62.6.2107-2114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKeating J A, Gow J, Goudsmit J, Pearl L H, Mulder C, Weiss R A. Characterization of HIV-1 neutralization escape mutants. AIDS. 1989;3:777–784. doi: 10.1097/00002030-198912000-00001. [DOI] [PubMed] [Google Scholar]
- 46.McKeating J A, Schotton C, Cordell J, Graham S, Balfe P, Sullivan N, Charles M, Page M, Bolmstedt A, Olofsson S, Kayman S C, Wu Z, Pinter A, Dean C, Sodroski J, Weiss R A. Characterization of neutralizing monoclonal antibodies to linear and conformation-dependent epitopes within the first and second variable domains of human immunodeficiency virus type 1 gp120. J Virol. 1993;67:4932–4944. doi: 10.1128/jvi.67.8.4932-4944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Modrow S, Hahn B H, Shaw G M, Gallo R C, Wong-Staal F, Wolf H. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J Virol. 1987;61:570–578. doi: 10.1128/jvi.61.2.570-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore J P, Ho D D. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol. 1993;67:863–875. doi: 10.1128/jvi.67.2.863-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore J P, Sattentau Q J, Yoshiyama H, Thali M, Charles M, Sullivan N, Poon S-W, Fung M S, Traincard F, Pinkus M, Robey G, Robinson J E, Ho D D, Sodroski J. Probing the structure of the V2 domain of human immunodeficiency virus type 1 surface glycoprotein gp120 with a panel of eight monoclonal antibodies: human immune response to the V1 and V2 domains. J Virol. 1993;67:6136–6151. doi: 10.1128/jvi.67.10.6136-6151.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mullis K B, Faloona F A. Specific synthesis of DNA in vitro via a polymerase catalyzed chain reaction. Methods Enzymol. 1987;255:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 51.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralization epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Myers G, Berzofsky J A, Korber B, Smith R F, Pavlakis G N. Human retroviruses and AIDS 1992. Los Alamos, N. Mex: Los Alamos National Laboratory; 1993. [Google Scholar]
- 53.Nakamura G R, Byrn R, Wilkes D M, Fox J A, Hobbs M R, Hastings R, Wessling H C, Norcross M A, Fendly B M, Berman P W. Strain specificity and binding affinity requirements of neutralizing monoclonal antibodies to the C4 domain of gp120 from human immunodeficiency virus type 1. J Virol. 1993;67:6179–6191. doi: 10.1128/jvi.67.10.6179-6191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nara P L, Smit L, Dunlop N, Hatch W, Merges M, Waters D, Kelliher J, Gallo R C, Fischinger P J, Goudsmit J. Emergence of viruses resistant to neutralization by V3 specific antibodies in experimental human immunodeficiency virus type 1 IIIB infection of chimpanzees. J Virol. 1990;64:3779–3791. doi: 10.1128/jvi.64.8.3779-3791.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Narayan O, Griffin D E, Chase J. Antigenic drift of visna virus in persistently infected sheep. Science. 1977;197:376–378. doi: 10.1126/science.195339. [DOI] [PubMed] [Google Scholar]
- 56.Nixon D F, Broliden K, Ogg G, Broliden P A. Cellular and humoral antigenic epitopes in HIV and SIV. Immunology. 1992;76:515–534. [PMC free article] [PubMed] [Google Scholar]
- 57.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell lifespan, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 58.Quinnan, G. V., P. F. Zhang, D. W. Fu, M. Dong, and J. B. Margolick. Evolution of neutralizing antibody response against HIV type 1 virions and pseudovirions in Multicenter AIDS Cohort Study participants. AIDS Res. Hum. Retroviruses, in press. [DOI] [PubMed]
- 59.Quinnan G V., Jr . Immunization against viral diseases. In: Galasso G, Merigan T C, Whitley R, editors. Antiviral agents and viral diseases of man. Philadelphia, Pa: Raven Press; 1997. pp. 791–833. [Google Scholar]
- 60.Reitz M S, Wilson C, Naugle C, Gallo R C, Robert-Guroff M. Generation of a neutralization resistant variant of HIV-1 is due to selection for a point mutation in the envelope gene. Cell. 1988;54:57–63. doi: 10.1016/0092-8674(88)90179-1. [DOI] [PubMed] [Google Scholar]
- 61.Robert-Guroff M, Reitz M S, Robey W G, Gallo R C. In vitro generation of an HTLV-III variant by neutralizing antibody. J Immunol. 1986;137:3306–3309. [PubMed] [Google Scholar]
- 62.Sawyer L A, Katzenstein D A, Hendry R M, Boone E J, Vujcic L K, Williams C C, Zeger S L, Saah A J, Rinaldo C R, Jr, Phair J P, Giorgi J V, Quinnan G V., Jr Possible beneficial effects of neutralizing antibodies and antibody-dependent cell-mediated cytotoxicity in human immunodeficiency virus infection. AIDS Res Hum Retroviruses. 1990;6:341–356. doi: 10.1089/aid.1990.6.341. [DOI] [PubMed] [Google Scholar]
- 63.Shugars D C, Wild C T, Greenwell T, Matthews T J. Biophysical characterization of recombinant proteins expressing the leucine zipper like domain of the human immunodeficiency virus type 1 transmembrane protein gp41. J Virol. 1996;70:2982–2991. doi: 10.1128/jvi.70.5.2982-2991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skinner M A, Ting R, Langlois A J, Weinhold K J, Lyerly H K, Jahaverian K, Matthews T J. Characteristics of a neutralizing monoclonal antibody to the HIV envelope glycoprotein. AIDS Res Hum Retroviruses. 1988;4:187–197. doi: 10.1089/aid.1988.4.187. [DOI] [PubMed] [Google Scholar]
- 65.Stuve L L, Brown-Shimer S, Pachl C, Naharian R, Diaz D, Burke R L. Structure and expression of the herpes simplex virus type 2 glycoprotein gB gene. J Virol. 1987;61:326–335. doi: 10.1128/jvi.61.2.326-335.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sutton R E, Littman D R. Broad host range of human T-cell leukemia virus type 1 demonstrated with an improved pseudotyping system. J Virol. 1996;70:7322–7326. doi: 10.1128/jvi.70.10.7322-7326.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takeda A, Robinson J E, Ho D D, Debouck C, Haigwood N L, Ennis F A. Distinction of human immunodeficiency virus type 1 neutralization and infection enhancement by human monoclonal antibodies to glycoprotein 120. J Clin Invest. 1992;89:1952–1957. doi: 10.1172/JCI115802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thali M, Charles M, Furman C, Cavacini L, Posner M, Robinson J, Sodroski J. Resistance to neutralization by broadly reactive antibodies to the human immunodeficiency virus type 1 gp120 glycoprotein conferred by a gp41 amino acid change. J Virol. 1994;68:674–680. doi: 10.1128/jvi.68.2.674-680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thali M, Furman C, Ho D D, Robinson J, Tilley S, Pinter A, Sodroski J. Discontinuous, conserved neutralization epitopes overlapping the CD4-binding region of human immunodeficiency virus type 1 gp120 envelope glycoprotein. J Virol. 1992;66:5635–5641. doi: 10.1128/jvi.66.9.5635-5641.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thali M, Moore J P, Furman C, Charlis M, Ho D D, Robinson J, Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Theodore, T. S., G. Englund, A. Buckler-White, C. E. Buckler, M. A. Martin, and K. W. C. Peden. Construction and characterization of a stable full-length cacrophage-tropic HIV type 1 molecular clone that directs the production of high titers of progeny virions. AIDS Res. Hum. Retroviruses 12:191–194. [DOI] [PubMed]
- 72.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivassan K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vujcic L K, Shepp D H, Klutch M, Wells M A, Hendry R M, Wittek A E, Krilov L, Quinnan G V. Use of a sensitive neutralization assay to measure the prevalence of antibodies to the human immunodeficiency virus. J Infect Dis. 1988;157:1047–1050. doi: 10.1093/infdis/157.5.1047. [DOI] [PubMed] [Google Scholar]
- 74.Vujcic L K, Quinnan G V., Jr Preparation and characterization of human HIV type 1 neutralizing reference sera. AIDS Res Hum Retroviruses. 1995;11:783–787. doi: 10.1089/aid.1995.11.783. [DOI] [PubMed] [Google Scholar]
- 75.Watkins B A, Buge S, Aldrich K, Davis A E, Robinson J, Reitz M S, Jr, Robert-Guroff M. Resistance of human immunodeficiency virus type 1 to neutralization by natural antisera occurs through single amino acid substitutions that cause changes in antibody binding at multiple sites. J Virol. 1996;70:8431–8437. doi: 10.1128/jvi.70.12.8431-8437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson C, Reitz M S, Aldrich K, Klasse P J, Blomberg J, Gallo R C, Robert-Guroff M. The site of an immune-selected point mutation in the transmembrane protein of human immunodeficiency virus type 1 does not constitute the neutralization epitope. J Virol. 1990;64:3240–3248. doi: 10.1128/jvi.64.7.3240-3248.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wyatt R W, Sullivan N, Thali M, Repke H, Ho D, Robinson J, Posner M, Sodroski J. Functional and immunologic characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J Virol. 1993;67:4557–4565. doi: 10.1128/jvi.67.8.4557-4565.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 79.Zwart G, Langeduk H, Van der Hoek L, Jean-Jacques D J, Wolfs T F W, Ramautarsing C, Bakker M, De Rinde A, Goudsmit J. Immunodominance and antigenic variation of the principal neutralization domain of HIV-1. Virology. 1991;181:481–489. doi: 10.1016/0042-6822(91)90880-k. [DOI] [PubMed] [Google Scholar]