Abstract
We addressed the limitations of subjective clinical tremor assessment by comparing routine neurological evaluation with a Tremor Occurrence Score derived from smartwatch sensor data, among 142 participants with Parkinson disease and 77 healthy controls. Our findings highlight the potential of smartwatches for automated tremor detection as a valuable addition to conventional assessments, applicable in both clinical and home settings.
Keywords: Parkinson disease, tremor, smart wearables, smartwatch, mobile apps, movement disorders, tremor documentation, tremor occurrence, tremor score
Introduction
Clinical assessment of tremor is limited by its subjectivity, making it challenging to detect subtle tremors (<0.05 g) [1]. The need for technology-based evaluations led to extensive research on sensor-based tremor assessments [2-6]. However, the benefits of smart consumer devices for clinical documentation remain unaddressed. We aimed to bridge this research gap by comparing tremor documentation from routine neurological assessments with a Tremor Occurrence Score (TOS) derived from smartwatch sensor recordings. We included 142 participants with Parkinson disease (PD) and 77 healthy controls. During clinical visits, routine neurological assessments were conducted and wrist-motion data were captured using a previously established Smart Device System (SDS) in a controlled setting [1,7]. The SDS is based on consumer smartwatches and smartphones. The smartwatches were validated in a geophysical experiment for tremor analysis with comparison to a gold-standard seismometer [1]. We investigated the potential of complementing routine tremor documentation with tremor capture using smartwatches [8].
Methods
Overview
The SDS parent study [1] recruited 450 participants, including patients with PD and other movement disorders and healthy controls. The SDS implemented data capture through electronic questionnaires and app-guided movement tasks with smartwatch sensing. Varghese et al [7] provide a detailed description of the study. Of the 450 participants, 101 were excluded due to diagnoses other than PD. Moreover, 130 patients with PD without explicit information regarding tremor in routine documentation were excluded, resulting in 219 participants. Table 1 summarizes the age, sex, and years from diagnosis onset distribution.
Table 1.
Overview of sex, age, and years to diagnosis onset for the 2 study cohorts.
| Characteristics | PDa cohort (n=142) | HCb cohort (n=77) | P value | ||||||
| Sex, n (%) | <.001 | ||||||||
|
|
Female | 42 (29.6) | 48 (62.3) |
|
|||||
|
|
Male | 100 (70.4) | 29 (37.7) |
|
|||||
| Age (y), mean (SD) | 64.9 (9.4) | 62.6 (12.4) | .08 | ||||||
| Years from diagnosis onset | N/Ac | ||||||||
|
|
Mean (SD) | 6.70 (5.36) | N/A |
|
|||||
|
|
Range, n (%) |
|
|||||||
|
|
|
0-4 | 60 (42.2) | N/A |
|
||||
|
|
|
5-9 | 39 (27.5) | N/A |
|
||||
|
|
|
10-14 | 31 (21.8) | N/A |
|
||||
|
|
|
15-19 | 6 (4.2) | N/A |
|
||||
|
|
|
≥20 | 6 (4.2) | N/A |
|
||||
aPD: Parkinson disease.
bHC: heathy control.
cN/A: not applicable.
Patients with PD were labeled as either (1) TremorDocumented: tremor was documented in routine documentation, regardless of type or amplitude (78/142, 54.9%), or (2) NoTremorDocumented: no information regarding tremor in routine documentation (64/142, 45.1%). We compared the routine tremor documentation to a Tremor Occurrence Score (TOS) from smartwatch recordings of a 20-second task, during which participants remained seated in an armchair. The TOS is normalized, from 0=no tremor occurrence to 1=tremor occurrence with a clear frequency peak, with 0.5 representing the average TOS of healthy controls. Details on calculations can be found in Multimedia Appendix 1 [9,10].
Ethical Considerations
The SDS parent study has been registered (ClinicalTrials.gov NCT03638479) and approved by the ethical board of the University of Münster and the physician’s chamber of Westphalia-Lippe (reference 2018-328-f-S). All participants gave written informed consent, and data were pseudonymized.
Results
Figure 1 shows boxplots of the TOSs according to participants’ routine tremor documentation. Overall, 16 (25%) out of 64 patients in the NoTremorDocumented group had a TOS ≥0.75 (the median TOS in the TremorDocumented group) and an average amplitude of 0.07 g. Particularly, 10 (16%) patients in the NoTremorDocumented group had a clear tremor (TOS >0.88, amplitude >0.10 g). The TremorDocumented group had a TOS ≥0.75 and an average amplitude of 0.11 g. Overall, patients with a clear but low-amplitude tremor (TOS ≥0.75, amplitude <0.05 g) were 1.59 times less likely to have a documented tremor (NoTremorDocumented) than those with a nonsubtle tremor (TOS ≥0.75, amplitude >0.05 g).
Figure 1.
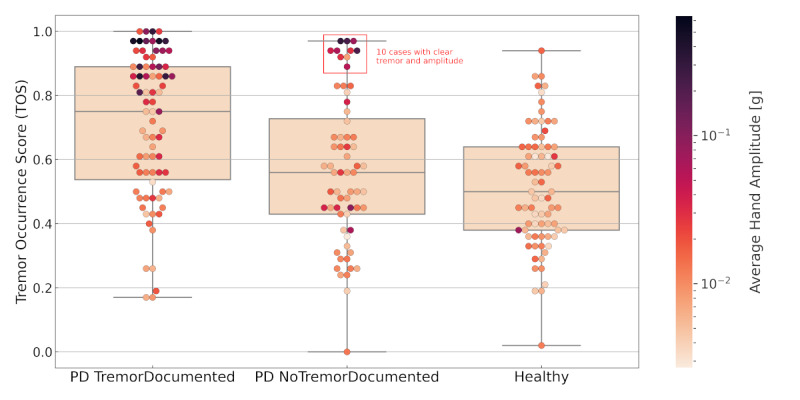
Boxplots of Tremor Occurrence Scores (TOSs) for all participants grouped by their routine tremor documentation. Each data point represents a participant of the respective group. Points are colored according to the average wrist amplitude in grams. The middle line is the median. The top and bottom sections are upper and lower quartiles, respectively. The box represents the middle 50% of TOSs for the group. The upper and lower whiskers represent scores outside the middle 50%. PD: Parkinson disease.
Discussion
Smartwatches can capture clear tremors in certain patients with PD, wherein the tremor was not documented during routine neurological assessments. Generally, patients with a clear but low-amplitude tremor (<0.05 g) were 1.59 times less likely to have a documented tremor than those with a strong tremor. We hypothesize that the tremor may not have been documented during the examination because of its small amplitude and difficulty of being seen. Moreover, a few patients (10/64, 16%) in the NoTremorDocumented group showed a clear tremor with visible amplitudes (>0.10 g), possibly due to the tremor being unnoticed or absent during routine examination. Likewise, tremors may not always manifest during smartwatch-based data capture, ultimately affecting the findings’ reproducibility.
Nevertheless, we suggest that integrating smartwatch-based data capture into routine neurological assessments enhances tremor documentation by providing objective numbers on frequency and amplitude for biomarker analysis. Smartwatch integration would further enable improved longitudinal assessment at home. The findings are not suitable for assessing the potential of diagnostic improvements, as routine manual documentation was only compared to smartwatch-based data capture. The parent study is currently evaluating a larger patient cohort, including differential diagnosis, to evaluate the diagnostic accuracy by using machine learning and more detailed tremor features, other movement characteristics, and nonmotor symptoms [1].
Acknowledgments
We acknowledge support from the Open Access Publication Fund of the University of Münster. We thank the Department of Neurology at the University Hospital Münster for integrating the study.
Abbreviations
- PD
Parkinson disease
- SDS
Smart Device System
- TOS
Tremor Occurrence Score
Tremor Occurrence Score (TOS) and tremor characteristic calculation.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Varghese J, Alen CMV, Fujarski M, Schlake GS, Sucker J, Warnecke T, Thomas C. Sensor validation and diagnostic potential of smartwatches in movement disorders. Sensors (Basel) 2021 Apr 30;21(9):3139. doi: 10.3390/s21093139. https://www.mdpi.com/resolver?pii=s21093139 .s21093139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espay AJ, Bonato P, Nahab FB, Maetzler W, Dean JM, Klucken J, Eskofier BM, Merola A, Horak F, Lang AE, Reilmann R, Giuffrida J, Nieuwboer A, Horne M, Little MA, Litvan I, Simuni T, Dorsey ER, Burack MA, Kubota K, Kamondi A, Godinho C, Daneault J, Mitsi G, Krinke L, Hausdorff JM, Bloem BR, Papapetropoulos S. Technology in Parkinson's disease: challenges and opportunities. Mov Disord. 2016 Dec;31(9):1272–1182. doi: 10.1002/mds.26642. http://europepmc.org/abstract/MED/27125836 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai H, Zhang P, Lueth T. Quantitative assessment of Parkinsonian tremor based on an inertial measurement unit. Sensors (Basel) 2015 Sep 29;15(10):25055–25071. doi: 10.3390/s151025055. https://www.mdpi.com/resolver?pii=s151025055 .s151025055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigcha L, Pavón I, Costa N, Costa S, Gago M, Arezes P, López JM, Arcas GD. Automatic resting tremor assessment in Parkinson's disease using smartwatches and multitask convolutional neural networks. Sensors (Basel) 2021 Jan 04;21(1):291. doi: 10.3390/s21010291. https://www.mdpi.com/resolver?pii=s21010291 .s21010291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin G, Halje P, Uzun S, Jakobsson A, Petersson P. Tremor evaluation using smartphone accelerometry in standardized settings. Front Neurosci. 2022 Aug 1;16:861668. doi: 10.3389/fnins.2022.861668. https://europepmc.org/abstract/MED/35979340 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ZhuParris A, de Goede AA, Yocarini IE, Kraaij W, Groeneveld GJ, Doll RJ. Machine learning techniques for developing remotely monitored central nervous system biomarkers using wearable sensors: a narrative literature review. Sensors (Basel) 2023 May 31;23(11):5243. doi: 10.3390/s23115243. https://www.mdpi.com/resolver?pii=s23115243 .s23115243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varghese J, Niewöhner S, Soto-Rey I, Schipmann-Miletić S, Warneke N, Warnecke T, Dugas M. A smart device system to identify new phenotypical characteristics in movement disorders. Front Neurol. 2019 Jan 30;10:48. doi: 10.3389/fneur.2019.00048. https://europepmc.org/abstract/MED/30761078 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reeder B, David A. Health at hand: a systematic review of smart watch uses for health and wellness. J Biomed Inform. 2016 Oct;63:269–276. doi: 10.1016/j.jbi.2016.09.001. https://linkinghub.elsevier.com/retrieve/pii/S1532-0464(16)30113-7 .S1532-0464(16)30113-7 [DOI] [PubMed] [Google Scholar]
- 9.Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, Raethjen J, Stamelou M, Testa CM, Deuschl G. Consensus statement on the classification of tremors. from the Task Force on Tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018 Jan 30;33(1):75–87. doi: 10.1002/mds.27121. https://europepmc.org/abstract/MED/29193359 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijayan V, Connolly JP, Condell J, McKelvey N, Gardiner P. Review of wearable devices and data collection considerations for connected health. Sensors (Basel) 2021 Aug 19;21(16):5589. doi: 10.3390/s21165589. https://www.mdpi.com/resolver?pii=s21165589 .s21165589 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tremor Occurrence Score (TOS) and tremor characteristic calculation.


