Key Points
Question
Among people receiving care in opioid treatment programs, does facilitated telemedicine for hepatitis C treatment increase cure rates compared with standard-of-care referral to hepatitis specialists?
Findings
Cure percentages were 90.2% in telemedicine and 39.4% in referral, with an estimated logarithmic odds ratio of the study group effect of 2.9. Among cured participants, illicit drug use decreased significantly. We observed minimal reinfections during 2 years of follow-up.
Meaning
Facilitated telemedicine integrated into opioid treatment programs resulted in significantly higher cure rates, with significant reductions in illicit drug use and minimal reinfections; facilitated telemedicine increases hepatitis C treatment access for underserved populations.
Abstract
Importance
Facilitated telemedicine may promote hepatitis C virus elimination by mitigating geographic and temporal barriers.
Objective
To compare sustained virologic responses for hepatitis C virus among persons with opioid use disorder treated through facilitated telemedicine integrated into opioid treatment programs compared with off-site hepatitis specialist referral.
Design, Setting, and Participants
Prospective, cluster randomized clinical trial using a stepped wedge design. Twelve programs throughout New York State included hepatitis C–infected participants (n = 602) enrolled between March 1, 2017, and February 29, 2020. Data were analyzed from December 1, 2022, through September 1, 2023.
Intervention
Hepatitis C treatment with direct-acting antivirals through comanagement with a hepatitis specialist either through facilitated telemedicine integrated into opioid treatment programs (n = 290) or standard-of-care off-site referral (n = 312).
Main Outcomes and Measures
The primary outcome was hepatitis C virus cure. Twelve programs began with off-site referral, and every 9 months, 4 randomly selected sites transitioned to facilitated telemedicine during 3 steps without participant crossover. Participants completed 2-year follow-up for reinfection assessment. Inclusion criteria required 6-month enrollment in opioid treatment and insurance coverage of hepatitis C medications. Generalized linear mixed-effects models were used to test for the intervention effect, adjusted for time, clustering, and effect modification in individual-based intention-to-treat analysis.
Results
Among 602 participants, 369 were male (61.3%); 296 (49.2%) were American Indian or Alaska Native, Asian, Black or African American, multiracial, or other (ie, no race category was selected, with race data collected according to the 5 standard National Institutes of Health categories); and 306 (50.8%) were White. The mean (SD) age of the enrolled participants in the telemedicine group was 47.1 (13.1) years; that of the referral group was 48.9 (12.8) years. In telemedicine, 268 of 290 participants (92.4%) initiated treatment compared with 126 of 312 participants (40.4%) in referral. Intention-to-treat cure percentages were 90.3% (262 of 290) in telemedicine and 39.4% (123 of 312) in referral, with an estimated logarithmic odds ratio of the study group effect of 2.9 (95% CI, 2.0-3.5; P < .001) with no effect modification. Observed cure percentages were 246 of 290 participants (84.8%) in telemedicine vs 106 of 312 participants (34.0%) in referral. Subgroup effects were not significant, including fibrosis stage, urban or rural participant residence location, or mental health (anxiety or depression) comorbid conditions. Illicit drug use decreased significantly (referral: 95% CI, 1.2-4.8; P = .001; telemedicine: 95% CI, 0.3-1.0; P < .001) among cured participants. Minimal reinfections (n = 13) occurred, with hepatitis C virus reinfection incidence of 2.5 per 100 person-years. Participants in both groups rated health care delivery satisfaction as high or very high.
Conclusions and Relevance
Opioid treatment program–integrated facilitated telemedicine resulted in significantly higher hepatitis C virus cure rates compared with off-site referral, with high participant satisfaction. Illicit drug use declined significantly among cured participants with minimal reinfections.
Trial Registration
ClinicalTrials.gov Identifier: NCT02933970
This study discusses whether facilitated telemedicine for hepatitis C treatment increases cure rates compared with standard-of-care referral to hepatitis specialists.
Introduction
Access to high-quality, convenient health care is a limited resource in the United States, especially for underserved populations.1,2 Because of telemedicine’s ability to transcend geographic and temporal boundaries for health care delivery, it may increase health care access.3,4 For underserved populations, however, augmenting health care access through telemedicine poses technical and social challenges, such as limited access to digital technology, adequate broadband strength, and trust in technology.5 Thus, novel approaches, such as facilitated telemedicine, in which a health care staff member facilitates in-person connectivity between a patient and an off-site clinician, are required to increase telemedicine entry points, especially for underserved populations.6
People with opioid use disorder are an underserved population largely because of societal stigma. Stigma and shunning frequently encountered in conventional medical settings result in restricted health care access, including for hepatitis C virus (HCV) infection.7 Pooled HCV incidence is 12.1 per 100 person-years among people who inject drugs,8 and restricted access to direct-acting antivirals (DAAs) is a leading public health issue. Because of highly efficacious DAAs, many jurisdictions seek HCV elimination by 2030,9,10 which requires improving DAA access by people with opioid use disorder.11 Opioid treatment programs (OTPs) are convenient, comfortable, and nonstigmatizing health care delivery sites that successfully integrate medical and behavioral treatment for opioid use disorder.12,13,14 In a single-group, single-site study, HCV care through OTP-integrated facilitated telemedicine encounters integrated into OTPs resulted in high cure rates with high patient satisfaction.15,16
To assess OTP-integrated facilitated telemedicine’s ability to increase HCV access to underserved populations, we evaluated the OTP-integrated facilitated telemedicine model among people with opioid use disorder. We designed a pragmatic clinical trial using the stepped wedge design to compare OTP-integrated facilitated telemedicine with usual care, off-site referral to hepatitis specialists.
Methods
Study Description
We conducted a multisite, nonblinded, pragmatic clinical trial at 12 OTPs throughout New York State (study details, including stepped wedge design rationale, are presented elsewhere17; Supplements 2, 3, and 4). Study recruitment commenced on March 1, 2017, and concluded on February 29, 2020. Participants with sustained virologic response (SVR) received 2-year follow-up for HCV reinfection assessment.
Study Sites, Site Recruitment, and Regulatory Approval
The New York State Office of Addiction Services and Supports oversees opioid use disorder treatment in New York. The office’s collaboration was instrumental in site recruitment and providing cluster-level demographic data for the randomization. For study participation, we required a minimum of 50 HCV-seropositive patients per site. We obtained coordinating and subsite institutional review board approval. Participants provided written informed consent to study case managers before enrollment. This study followed the CONSORT guidelines for reporting stepped wedge cluster randomized trials.
Study Design, Randomization, and Sample Size
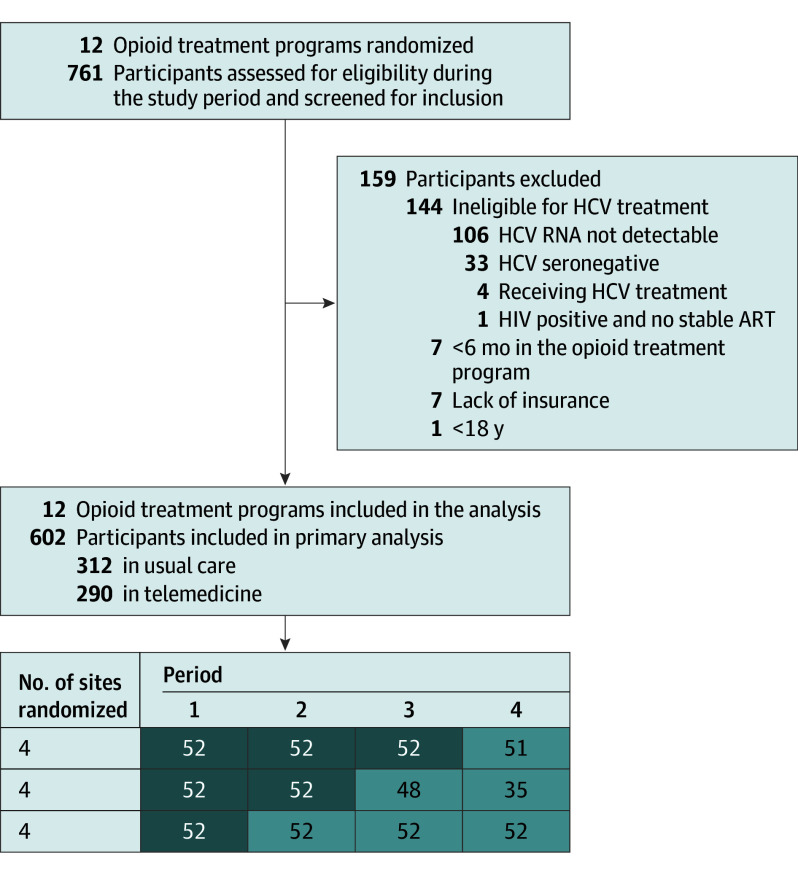
In the cross-sectional stepped wedge design, study group assignment was conducted at the cluster level, and we used covariate-constrained randomization (eAppendix 1 in Supplement 1).17,18,19,20 The final randomization was kept confidential, and we notified sites 30 days before commencing OTP-integrated facilitated telemedicine. We conducted the study during 3 separate steps with 4 periods consisting of 9 months each, and each step consisted of 4 clusters (Figure 1). Thirteen participants were enrolled per site per period without participant crossover, and we performed the analysis according to the allocated schedule. The projected sample was 624 participants, 312 per group, resulting in 12 clusters.17 We recruited 602 participants (recruitment rate = 96.5%), 312 in referral and 290 in OTP-integrated facilitated telemedicine. Selection bias is discussed in eAppendix 5 in Supplement 1.
Figure 1. Recruitment, Randomization, and Patient Follow-Up in the Stepped Wedge Cluster Randomized Opioid Treatment Program–Integrated Facilitated Telemedicine Trial.
The number of sites randomized and individuals analyzed per period is illustrated at the bottom of the figure. Usual care is shown in dark shading and opioid treatment program–integrated facilitated telemedicine is shown in light shading. ART indicates antiretroviral therapy; HCV, hepatitis C virus.
Study Conduct
Consistent with the Office of Addiction Services and Supports recommendations, each site measured HCV antibodies annually. Each site provided lists of HCV-seropositive and, in some cases, HCV RNA–positive individuals. Study case managers had experience working in the health care sector, particularly with people with opioid use disorder. They worked closely with OTP staff, especially counselors, to identify eligible participants and to address potential retention obstacles.21 Study inclusion criteria required 6-month active OTP enrollment, detectable HCV RNA, aged 18 years or older, and insurance coverage of DAAs. Exclusion criteria were HCV seronegativity, DAA treatment at screening, and HIV-seropositive individuals not receiving stable antiretroviral therapy because of adherence concerns. Decompensated individuals with cirrhosis could participate. During the screening visit, we assessed HCV RNA, HCV genotype, hepatitis panel, complete blood cell count, complete metabolic panel, prothrombin time, and HIV status.
Usual Care and OTP-Integrated Facilitated Telemedicine
Participants in usual care received an off-site hepatitis specialist (ie, hepatologist, infectious diseases physician, or primary care physician) referral following the standard of care at each site. Participants provided permission for study case managers to obtain medical records from the hepatitis specialist to determine referral outcomes, including whether (and when) HCV treatment was initiated, when it was completed, and treatment outcome. After referral, case managers inquired monthly with participants and the referring clinicians’ offices to assess referral outcomes. In OTP-integrated facilitated telemedicine, participants had an initial telemedicine encounter facilitated by study case managers on-site in the OTP. Blood for testing was obtained by venipuncture according to local procedures. The telemedicine clinician subsequently evaluated participants, ordering DAAs electronically that were delivered to the OTP monthly (as refills required) and dispensing them with methadone. The OTPs dispensed take-home DAA doses for participant self-medication on days when the participant did not appear in person in the OTP. Telemedicine clinicians consisted of 2 gastroenterologist-hepatologists (A.H.T. and A.M.D.) and a nurse practitioner who had HCV treatment experience. These clinicians individualized treatment of patients with cirrhosis. In both groups, HCV RNA levels were assessed at treatment completion and at weeks 4 and 12 (ie, SVR assessment) posttreatment.
Study Outcomes
The primary outcome was SVR (ie, undetectable HCV RNA 12 weeks after treatment cessation). Predefined secondary outcomes included a comparison of treatment initiation and completion rates, participant satisfaction with health care delivery, and treatment adherence rates between groups. Hepatitis C virus reinfection was an exploratory outcome.
After extensive stakeholder (ie, patients, sponsor, study patient advisory committee, and frontline OTP staff) discussion, we prespecified that participants without an initial hepatitis specialist visit within 5 months of enrollment would cease trial participation.22,23,24 A recent study reported that 75% of Medicaid-insured individuals who initiated DAAs did so within 6 months of an initial HCV diagnosis.25 Furthermore, in chronic HCV infection, spontaneous resolution occurs at 0.36% per person-year of follow-up, an extremely rare event.26 All other participants initiated treatment.
Missing Data
In designing this study, we implemented strategies17 to minimize missing data.27 Despite these strategies, missing data still occurred (eAppendix 2 in Supplement 1). The trial dropout rate was 243 of 602 participants (40.4% overall), 204 of 312 (65.4%) in referral and 39 of 290 (13.4%) in OTP-integrated facilitated telemedicine, calculated with the number of participants without SVR assessments. We illustrate reasons for premature participant discontinuation in eTable 1 in Supplement 1. We assumed that the missing mechanism was missing at random; to handle missing data, we used multivariate imputation by chained equations,28 which was permitted by a sufficiently low estimated intraclass correlation coefficient of 0.099 obtained through ICCbin (Monte Carlo method) in R version 4.1.1 (R Foundation for Statistical Computing).29
We illustrate details and variable justification for construction of the imputation model and variables with missing data in eAppendix 2.1 in Supplement 1. We performed analysis with 20 imputed data sets and summarized the results using Rubin rules for combining estimates and SEs (eTables 2 and 3 and eFigure 2 in Supplement 1). We enumerate participant deaths in eTable 4 in Supplement 1 and associated analytic issues in eAppendix 2.2 in Supplement 1. Deaths were treated as missing at random.30,31,32,33
Statistical Analysis
Analyses were individual-based intention to treat using generalized linear mixed models.34 They were also cluster level, within period, robust, and nonparametric.35 Data were analyzed from December 1, 2022, through September 1, 2023.
Factors Associated With SVR
We used covariates such as age, race, sex, and ethnicity in the covariate-constrained randomization.17 These covariates are no longer SVR predictors because DAAs are highly efficacious.36 Therefore, although we are expected to adjust the generalized linear mixed models for randomization covariates, given that the covariates themselves are not SVR predictors and may create very small strata, their inclusion in the model is not recommended.37
Modeling
The primary outcome was binary; SVR rates between the 2 groups in which SVR = 1 indicated HCV cure and SVR = 0 indicated treatment nonresponse.
We used generalized linear mixed models,34 adjusted for confounding by calendar time incorporated as a categorical variable. The model accounted for clustering by incorporating a random site effect. This model assumed that the effects of time were common to all clusters, and the correlation between any 2 observations in the same cluster was the same and independent of the time step.
To account for effect modification, we incorporated a time by intervention interaction effect (eAppendix 4 in Supplement 1). eAppendix 3.1 in Supplement 1 presents a nonparametric, cluster-level, robust, within-period analysis to estimate the intervention effect that avoids the generalized linear mixed models assumptions.35
Subgroup Analysis
A priori subgroups of interest included comorbid medical and mental health conditions (specifically depression or anxiety), fibrosis stage (binary [F3, F3-F4, or F4] vs all other stages),38 and participants’ residence location (specifically urban or rural classification: US Department of Agriculture Economic Research Service rural-urban commuting area codes). Furthermore, we examined subgroups defined by sex, ethnicity, location, and race.39,40 These analyses were exploratory, and we used generalized linear mixed models with unadjusted 2-sided P = .05.
Adherence Analysis
We defined adherence as the percentage of participants who took greater than or equal to 90% (high) vs less than 90% (low) of prescribed DAAs.41 We assessed HCV medication adherence through participant self-report of missed DAA doses in the preceding 2 weeks.
Effects of COVID-19
We followed the CONSERVE statement for reporting the impact of COVID-19.42 COVID-19 restrictions had minimal effects on the study because recruitment had concluded and all sites had already transferred to the OTP-integrated facilitated telemedicine group. The cessation of in-person visits, however, necessitated shifts in data collection methods (ie, through telephone) and intervention delivery. Protocol modifications were approved by the study sponsor. Therefore, no model adjustments were needed, as explained elsewhere.17
Exploratory Analysis
We evaluated variable distribution by using graphic analysis and descriptive statistics. Continuous variables are presented by either their means and SDs or medians and IQRs. We present categorical variables as counts and percentages. We performed statistical analyses with SAS 9.4 with add-on analytic products of SAS/STAT 15.2 (SAS Institute) and R version 4.1.1 (R Packages, RStudio; R Foundation for Statistical Computing) as appropriate.
Computing Incidence Density
We followed up cured participants for up to 2 years with HCV RNA determinations every 6 months to assess for reinfection, defined as recurrent viremia after obtaining an SVR. We computed the incidence density (ie, number of reinfections during person-years of follow-up) among SVR participants.
Results
Cluster Recruitment and Randomization
We approached and recruited 12 OTPs. All 12 OTPs began and completed the trial. Further details are provided elsewhere17 and in eAppendix 5 in Supplement 1.
Baseline Characteristics
We screened 761 individuals for study eligibility, and 159 (20.9%) were excluded (Figure 1) because of lacking insurance, participating fewer than 6 months in the OTP, being younger than 18 years, and having HCV treatment ineligibility. We enrolled 602 individuals, 312 (51.8%) in referral and 290 (48.2%) in OTP-integrated facilitated telemedicine. Baseline characteristics were well balanced between both groups (Table 1; eFigures 1, 3, and 4 in Supplement 1). The mean (SD) age of the enrolled participants in the telemedicine group was 47.1 (13.1) years; that of the referral group was 48.9 (12.8) years. A total of 369 participants were male (61.3%) and 233 were female (38.7%), approximately equally balanced between White (306 [50.8%]) and non-White (296 [49.2%]) races. A total of 164 participants (27.2%) were American Indian or Alaska Native, Asian, multiracial, or other (ie, no race category was selected); 132 (21.9%) were Black or African American; and 185 were Hispanic or Latino/a. Race data were collected according to the 5 standard National Institutes of Health categories. A total of 138 participants (22.9%) had cirrhosis.
Table 1. Baseline Characteristics of Study Participants Comparing Opioid Treatment Program–Integrated Facilitated Telemedicine With Off-Site Referral.
| Demographics | No. (%) | |
|---|---|---|
| Telemedicine (n = 290) | Referral (n = 312) | |
| Age at consent, y | ||
| Mean (SD) | 47.1 (13.1) | 48.9 (12.8) |
| Median (IQR) | 46.0 (36.3-58.0) | 50.0 (37.8-60.0) |
| Sex | ||
| Female | 115 (39.7) | 118 (37.8) |
| Male | 175 (60.3) | 194 (62.2) |
| Hispanic or Latino/a | 89 (30.7) | 96 (30.8) |
| Racea | ||
| Black or African American | 49 (16.9) | 83 (26.6) |
| White | 155 (53.4) | 151 (48.4) |
| Other | 86 (29.7) | 78 (25.0) |
| Geographic location | ||
| Urban | 245 (84.5) | 267 (85.6) |
| Comorbid condition | ||
| Anxiety or depression | 90 (31.0) | 79 (25.3) |
| Other comorbid conditions besides anxiety or depressionb | 88 (30.3) | 91 (29.2) |
| No comorbid condition or unsure | 112 (38.6) | 142 (45.5) |
| HIV | 6 (2.1) | 18 (5.8) |
| DAST-10 score at screening visitc | ||
| Mean (SD) | 4.8 (3.1) | 4.5 (3.2) |
| Median (IQR) | 5 (2-8) | 4 (1-7) |
| Virology and fibrosis variables | ||
| HCV RNA (10 log IU/mL) | ||
| Mean (SD) | 5.9 (1.0) | 5.9 (0.9) |
| Median (IQR) | 6.0 (5.5-6.6) | 6.1 (5.4-6.6) |
| HCV genotyped | ||
| 1 | 2 (0.7) | 4 (1.3) |
| 1a | 172 (59.3) | 193 (61.9) |
| 1b | 28 (9.7) | 38 (12.2) |
| 2 | 4 (1.4) | 0 |
| 2a | 2 (0.7) | 0 |
| 2b | 18 (6.2) | 18 (5.8) |
| 3 | 27 (9.3) | 22 (7.1) |
| 3a | 22 (7.6) | 17 (5.4) |
| 4 | 5 (1.7) | 1 (0.3) |
| 4a | 0 | 4 (1.3) |
| HIV | 6 (2.1) | 18 (5.8) |
| Fibrosis (APRI category)e | ||
| 0-1, No fibrosis or mild fibrosis | 155 (53.4) | 145 (46.5) |
| 2, Moderate fibrosis | 44 (15.2) | 60 (19.2) |
| 3, Advanced fibrosis | 29 (10.0) | 31 (9.9) |
| 4, Cirrhosis | 62 (21.4) | 76 (24.4) |
| Adherence variables | ||
| No. of months in methadone programf | ||
| Mean (SD) | 52.2 (72.4) | 57.6 (62.1) |
| Median (IQR) | 20 (11-65) | 32 (13-83) |
| NIDA Quick Screen | ||
| Answers per question | ||
| In the past year, how often have you used prescription drugs for nonmedical reasons? | ||
| Daily or almost daily | 15 (5.2) | 12 (3.8) |
| Weekly | 12 (4.1) | 9 (2.9) |
| Monthly | 10 (3.4) | 17 (5.4) |
| Once or twice | 53 (18.3) | 40 (12.8) |
| Never | 193 (66.6) | 220 (70.5) |
| In the past year, how often have you used illicit drugs?g | ||
| Daily or almost daily | 46 (15.9) | 37 (11.9) |
| Weekly | 45 (15.5) | 42 (13.5) |
| Monthly | 51 (17.6) | 39 (12.5) |
| Once or twice | 38 (13.1) | 57 (18.3) |
| Never | 103 (35.5) | 123 (39.4) |
Abbreviations: APRI, AST to platelet ratio index; DAST-10, Drug Abuse Screening Test; HCV, hepatitis C virus; NIDA, National Institute on Drug Abuse.
Race data were collected according to the 5 standard National Institutes of Health categories.43 Other races include American Indian or Alaska Native, Asian, multiracial, and other (ie, no race category was selected).
Other comorbid conditions besides anxiety and depression include cardiac, gastrointestinal and liver, pulmonary, rheumatologic, diabetes and endocrine, kidney, cancer, and psychiatric disorders. Comorbid conditions were assessed by case manager review of the medical record.
The score variable for DAST-10 is calculated as the total number of yes responses (which receive 1 point each), except for 1 question for which no receives 1 point. The DAST-10 score ranges from 0 to 10, and a score from 3 to 5 represents a moderate degree of problems related to drug abuse. The DAST-10 questionnaire covers the use of prescribed or over-the-counter medications and drugs in excess of the directions and any nonmedical use of drugs, including cannabis, solvents, tranquilizers, barbiturates, cocaine, stimulants, hallucinogens, and narcotics.44
Hepatitis C virus genotype was assessed with reverse transcriptase–polymerase chain reaction and the INNO-LiPA HCV genotype 2.0 DNA line probe assay (Siemens). Hepatitis C virus genotype analysis is based on the DNA sequence of the core and the 5′ UTR of the HCV genome and categorizes the virus into distinct types (eg, 1-6) and subtypes (ie, a, b, c).
The APRI was assessed as follows according to Raab et al38: APRI value stage interpretation: less than 0.5, F0 to F1 indicates no or mild fibrosis; 0.5 to less than 0.7, F2 indicates moderate fibrosis; 0.7 to 1.0, F3 indicates advanced fibrosis; and greater than 1.0, F4 indicates cirrhosis.
A total of 292 of 312 participants in referral (93.6%) and 279 of 290 participants in telemedicine (96.2%) appeared daily in person for methadone dispensing. The remainder of participants adhered to a schedule requiring weekly or monthly in-person appearance in the opioid treatment program to receive methadone.
Illicit drug use was assessed by a question on the NIDA Quick Screen questionnaire.45
HCV Treatment Cascade
Of 312 referral participants, 297 (95.2%) obtained an initial visit with the study case manager, and 126 (40.4%) initiated DAAs (Table 2). Direct-acting antivirals, as prescribed, are shown in eTable 5 in Supplement 1. Of these participants, 116 completed treatment and 108 had an SVR assessment, of whom 106 (30.4%) achieved an SVR and 2 had detectable virus. Of 290 participants in the OTP-integrated facilitated telemedicine group, 280 (96.6%) completed an initial visit and 268 (92.4%) initiated HCV treatment. Of these participants, 261 completed treatment and 251 had an SVR assessment, 246 (84.8%) with undetectable virus and 5 with detectable virus. The SVR assessments were performed at the appropriate visit in 249 of 251 (99.2%) OTP-integrated facilitated telemedicine visits compared with 66 of 108 (61.1%) referral visits.
Table 2. Hepatitis C Virus Care Cascade.
| No. (%) | Log odds estimate (95% CI) | ||
|---|---|---|---|
| OTP-integrated facilitated telemedicine (n = 290) | Referral (n = 312) | ||
| Visit 1a | 280 (96.6) | 297 (95.2) | 0.1 (−0.8 to 1.0) |
| Treatment initiation | 268 (92.4) | 126 (40.4) | 2.8 (2.3 to 3.3) |
| Treatment completion | 261 (90.0) | 116 (37.2) | 2.7 (2.2 to 3.1) |
| Sustained virologic response assessed | 251 (86.6) | 108 (34.6) | 2.4 (2.0 to 2.9) |
| Observed sustained virologic response | 246 (84.8) | 106 (34.0) | 2.3 (1.9 to 2.7) |
Abbreviation: OTP, opioid treatment program.
The percentage of study participants in both groups who attended the initial visit with the case manager to provide blood for testing for the initial telemedicine encounter or to obtain a referral to an off-site hepatitis C virus clinician. The log odds estimates and associated 95% CIs for comparing the proportions in the 2 groups were obtained by fitting a linear mixed model incorporating the study group effect and a random effect to account for clustering.
Among participants who initiated therapy, the observed SVR rate was similar between the groups (246 of 268 [91.8%] in OTP-integrated facilitated telemedicine vs 106 of 126 [84.1%] in referral). Among participants with SVR determination, detectable HCV RNA occurred at a comparable frequency between the 2 groups (5 of 251 [2.0%] in OTP-integrated facilitated telemedicine vs 2 of 108 [1.9%] in referral).
Models Adjusted for Time, Clustering Effect, and Effect Modification
We analyzed the data according to the intended randomization schedule. The estimated intraclass correlation coefficient was 0.099 (95% CI, 0-0.2). The intention-to-treat analysis used data from 602 participants, with missing values imputed as described. The overall SVR percentages were 262 of 290 (90.3%) in the OTP-integrated facilitated telemedicine group compared with 123 of 312 (39.4%) in the referral group. The estimate of the logarithmic odds ratio of the time-averaged intervention effect, obtained from combining the results of the 20 imputed data sets, was 2.9 (95% CI, 2.0-3.5; P < .001) using generalized linear mixed models as described in the Modeling section.
When the model accounted for effect modification using time as a continuous variable, the intervention effect estimate was still significant (2.8; 95% CI, 0.8-4.8; P = .004). The interaction coefficient was −0.002 (95% CI, –0.64 to 0.64; P = 0.5), indicating no effect modification.
Timing of Treatment Uptake
The time between screening and initial appointments was significantly shorter in OTP-integrated facilitated telemedicine (referral median, 18 days [IQR, 7-35 days]; OTP-integrated facilitated telemedicine median, 14 days [IQR, 7-26 days]; test statistic = 2.1; P = .04).46 Similarly, the duration between the initial visit and DAA initiation was significantly shorter in OTP-integrated facilitated telemedicine (referral mean [SD], 123.5 [92.4] days; OTP-integrated facilitated telemedicine mean [SD], 49.9 [48.1] days; test statistic = 3.85; P < .001).47
Substance Use
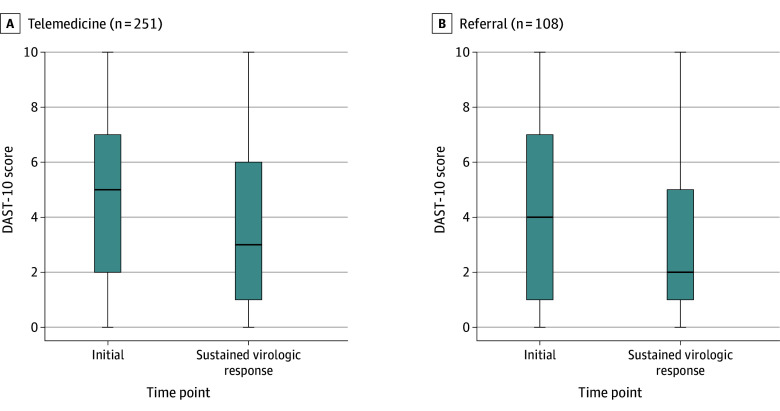
At baseline, the mean (SD) response score for the Drug Abuse Screening Test was 4.45 (3.23) and 4.82 (3.10) in the referral and OTP-integrated facilitated telemedicine groups, respectively, in which a score between 3 and 5 represents moderate problems with drug abuse.44 At the SVR time point, the Drug Abuse Screening Test score decreased significantly among HCV-cured participants in referral, with a median at the initial time point of 4 (IQR, 1-7) and 2 (IQR, 1-5; P = .001 for both) and a median of 5 (IQR, 2-7) and 3 (IQR, 1-6; P < .001 for both) in OTP-integrated facilitated telemedicine, respectively (Figure 2).47
Figure 2. Distributions of Scores Obtained From the Drug Abuse Screening Test (DAST-10) at the Initial and Sustained Virologic Response Visits.
The Tukey boxplot illustrates a significant decline in DAST-10 scores in individuals cured of hepatitis C virus infection in facilitated telemedicine (P < .001) and referral (P = .001). The box extends from the 25th to the 75th percentile, with the line in the middle of the box depicting the median. The lines extending from the top and bottom of the box depict the upper and lower values. For information on the components and scoring of the DAST-10, see the footnote in Table 1.
HCV Adherence
We observed very high DAA adherence. Among participants with non-SVR in the OTP-integrated facilitated telemedicine group, 4 of 5 (80%) had 90% adherence at treatment weeks 6 and 12 (eTable 6 in Supplement 1). We observed no differences in methadone doses between study groups (eFigure 5 in Supplement 1) or when stratified by participants with a treatment start date, treatment end date, or SVR (eFigure 6 in Supplement 1).
Subgroup Analysis
The effects of the intervention on the primary outcome were examined in prespecified subgroups (fibrosis stage, comorbid medical conditions, residence, sex, race, and ethnicity) (Figure 3). We present the time-adjusted effects (log odds) for the subgroups of interest and their 95% CIs. All results favored the intervention. We consider these analyses exploratory owing to lack of adjustment for multiplicity of testing. However, only 2 of 28 Hispanic female participants in referral achieved an SVR compared with 23 of 26 participants in OTP-integrated facilitated telemedicine (eAppendix 6 and eTable 7 in Supplement 1). These findings do not appear to be due to English proficiency (eTable 8 in Supplement 1). Similar results (ie, favoring OTP-integrated facilitated telemedicine) were observed among rural participants (eFigure 7 in Supplement 1).
Figure 3. Hepatitis C Virus Cure Subgroup Analysis.
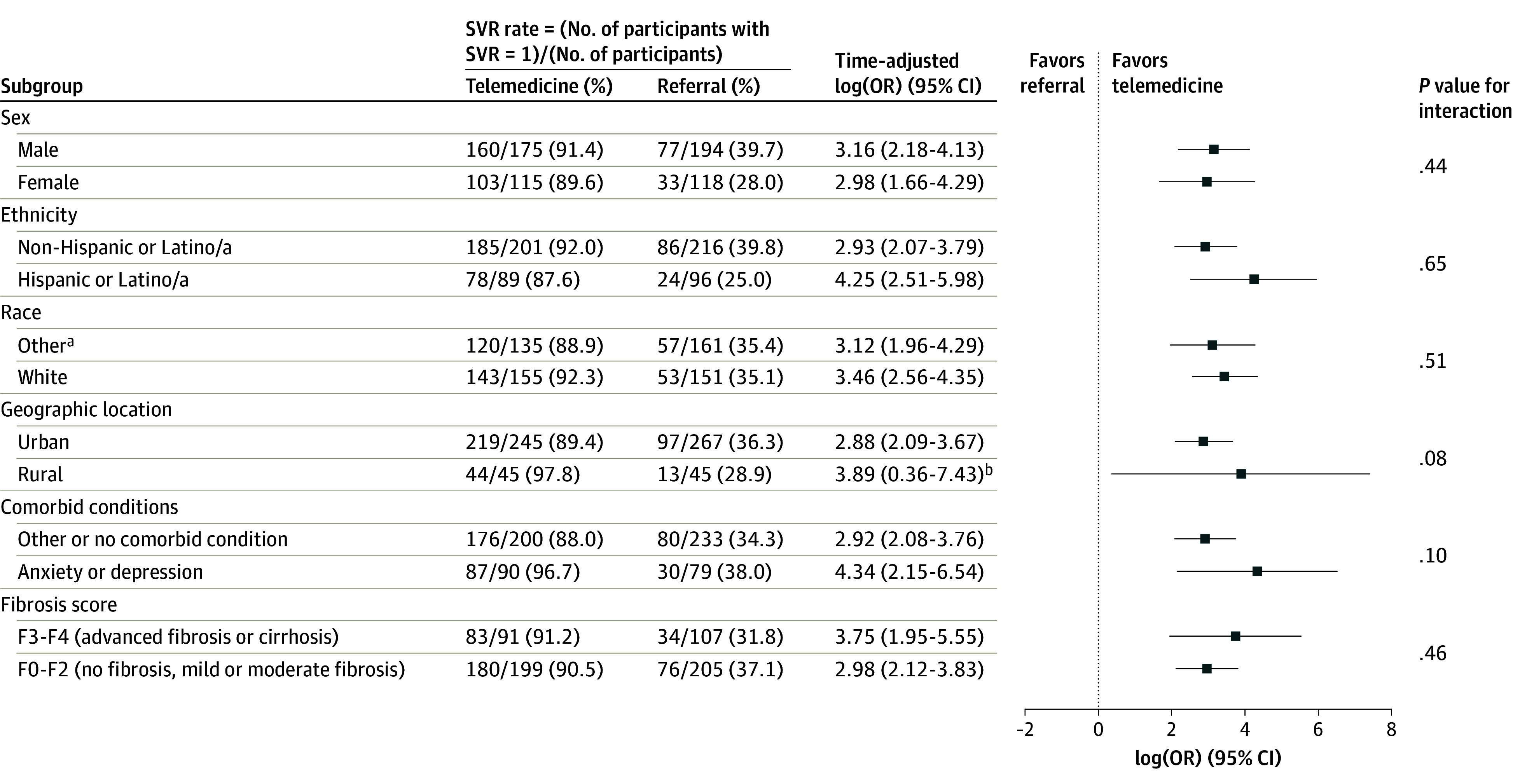
Dot and whisker plot of time-adjusted log(OR) with associated 95% CIs for various subgroups of interest. The level of α = .05 (2-tailed), and no adjustments for multiplicity were made. OR indicates odds ratio; SVR, sustained virologic response.
aRace data were collected according to the 5 standard National Institutes of Health categories.43 Other races include American Indian or Alaska Native, Asian, multiracial, and other (ie, no race category was selected).
bA normal distribution prior was used and the results were obtained with the blme R package, which encodes bayesian methods for fitting linear mixed models; priors were used for the model parameters.
Follow-Up Data
We observed 3 reinfections in referral, with a total follow-up of 162.0 person-years. In OTP-integrated facilitated telemedicine, we noted 10 reinfections, with a total follow-up of 365.2 person-years. The overall incidence density rate was 2.5 per 100 person-years of follow-up, with rates of 2.7 reinfections per 100 person-years of follow-up in OTP-integrated facilitated telemedicine and 1.9 in referral. No significant differences existed between the 2 study groups in the number of reinfections (eAppendix 7 in Supplement 1).
COVID-19 Effects on the Study
COVID-19 pandemic lockdowns resulted in an increase in methadone and DAA take-home doses. Although no significant DAA interruptions or discontinuations occurred among participants who had initiated treatment before the COVID-19 lockdowns, treatment initiation was delayed for 13 participants, and SVR assessment was also delayed.
Discussion
In this pragmatic trial, 262 of 290 participants (90.3%) in the OTP-integrated facilitated telemedicine group achieved an SVR compared with 123 of 312 participants (39.4%) in referral. These participants also initiated DAAs significantly more expeditiously. Our patient population consisted of similar numbers of White and non-White races, one-third were Hispanic, one-third had anxiety or depression, and a quarter had cirrhosis. Illicit drug use decreased significantly for cured participants in both groups. An SVR was durable, with minimal reinfections occurring during the 2-year follow-up period. No significant differences between groups were identified in terms of anxiety or depression, fibrosis score, urban or rural residence, or demographics.
Our study has several desirous attributes for well-designed pragmatic clinical trials,48 including intervention implementation in routine clinical settings with usual staff and workflows. Our study sites routinely provide opioid use disorder treatment to a population with high HCV prevalence and incidence. Study case managers were fully integrated into OTP workflows and OTP-integrated facilitated telemedicine encounters, and they educated and communicated with study participants.21,49 Furthermore, research conduct in comfortable and destigmatizing OTPs facilitated trial performance because people with opioid use disorder often encounter stigma and shunning in conventional health care settings.50,51,52 Additionally, our primary outcome, SVR, is meaningful to patients, clinicians, and payers.53
Participants were highly satisfied with health care delivery through OTP-integrated facilitated telemedicine, equivalent to in-person encounters, with clinician empathetic characteristics rated higher than logistic attributes such as accessibility and convenience.54 Our qualitative data suggest that an HCV cure fosters self-confidence, promoting overall well-being.52 Our observation of significant decreases in illicit drug use among HCV-cured participants is consistent with prior studies that showed improved HCV and opioid use disorder treatment outcomes with simultaneous treatment of both entities.55,56,57,58,59 Although desired, physical integration of medical and behavioral therapy has been difficult to achieve60,61; OTP-integrated facilitated telemedicine appears to integrate HCV and opioid use disorder care with improved convenience, accessibility, and flexibility. Situating telemedicine encounters in OTPs mitigated potential broadband access issues and introduced new telemedicine access points.3
Through OTP-integrated facilitated telemedicine, we observed that a high percentage of Hispanic women achieved an SVR. Hispanic individuals have lower DAA treatment initiation rates owing to mistrust of health care clinicians, incarceration, homelessness, lack of insurance, cultural and linguistic barriers, and fear of deportation.62,63,64 Stabilization of opioid use disorder through methadone treatment with HCV care via OTP-integrated facilitated telemedicine may have satisfactorily addressed obstacles encountered by Hispanic women in other health care settings. Similarly, we observed that rural inhabitants achieved a higher SVR percentage than urban individuals. These findings should be interpreted cautiously because of the small sample sizes and noncorrection for multiple comparisons (eg, urban or rural). Sufficiently powered clinical trials are warranted to address the effectiveness of OTP-integrated facilitated telemedicine in these populations.4,6 The COVID-19 pandemic delayed HCV treatment initiation and SVR assessments for a few participants, although it did not affect recruitment, which was completed 2 weeks before the lockdowns.
Although participating OTPs had minimal prior research experience, they successfully completed a pragmatic clinical trial using a rigorous design. To increase clinical transferability, we required insurance coverage of DAAs. We enrolled 602 of 624 projected participants (96.5%) and fully enrolled the usual care group. We sought to minimize bias by notifying sites only 1 month before telemedicine initiation.
Limitations
In terms of limitations, although our sites represent almost all New York State metropolitan areas, New York methadone reimbursement practices are more generous compared with those of other states, and we had a relatively low number of participating OTPs. Additionally, methadone treatment requires a more frequent in-person appearance than other substance use treatment approaches, including buprenorphine. Our inclusion criteria required 6 months of active OTP enrollment because prior data suggest that approximately 50% of patients admitted to an OTP will discontinue within the first 3 months.65 Future investigations should address transferability of OTP-integrated facilitated telemedicine, as well as the role of socioeconomic factors, and include more recent entrants to substance use treatment. Future study designs might also not require SVR assessments because DAAs are so efficacious.
Conclusions
In conclusion, OTP-integrated facilitated telemedicine resulted in substantially higher SVR rates than off-site referral. Our intervention successfully builds patient-clinician trust across the screen, and significant decreases in substance use were observed in cured participants with minimal HCV reinfections. Opioid treatment program–integrated facilitated telemedicine promotes increased access and integrates HCV treatment into venues that offer opioid use disorder treatment.6
eAppendix 1. Randomization
eFigure 1. Age Distributions at Baseline (N= 602) Between Referral and Opioid Treatment Program-Integrated Facilitated Telemedicine Arms
eAppendix 2. Treatment of Missing Data
eTable 1. Reasons for Early Termination of Study Participants (i.e., Dropout) by Study Arm
eAppendix 2.1. Justification of Variables Included in the Model
eTable 2. Descriptive Statistics of Sustained Virological Responses Associated With the 20 Imputed Datasets
eFigure 2. Distribution of Imputed and Observed Drug Abuse Screening Test (DAST10) Levels per Arm
eTable 3. Percentages of Observed and Imputed, per Arm, Illegal Drug Use per Category as Obtained by Drug Abuse Screening Test (DAST-10)
eAppendix 2.2. Deaths
eTable 4. Deceased Participants by Relevant Demographic and Residence Location Variables
eAppendix 3. Nonparametric Analysis
eAppendix 3.1. Cluster-Level Analysis
eAppendix 4. Effect Modification
eAppendix 5. Selection Bias
eFigure 3. Distribution of Drug Abuse Screening Test (DAST10) Scores in the Referral and Opioid Treatment Program-Integrated Telemedicine Arms
eFigure 4. Distribution of the Number of Months Participants Are in the Methadone Program for the Referral and Opioid Treatment Program-Integrated Telemedicine Arms
eTable 5. Direct Acting Antiviral Medications Prescribed to Study Participants
eTable 6. Adherence to Direct Acting Antiviral Medication by Treatment Week
eFigure 5. Boxplots Comparing the Distribution of Methadone Dose in the Referral (Usual Care) and Opioid Treatment Program-Integrated Telemedicine Arms
eFigure 6. Boxplots Comparing Methadone Doses in Opioid Treatment Program-Integrated Telemedicine and Referral Arms
eAppendix 6. Observations Related to Subgroups
eTable 7. Sustained Virological Response Rates by Period for Hispanic Females in Referral and Opioid Treatment Program-Integrated Telemedicine Arms
eTable 8. English Language Ability for Hispanic Females and Males
eFigure 7. Number of Rural Participants Achieving a Sustained Virologic Response (SVR) Stratified by Study Arm
eAppendix 7. Analysis of Incidence Rates
eReferences.
Trial Protocol: Statistical Analysis and Final Results Overview Including Heterogeneity of Treatment Effects
Trial Protocol: Supplemental Material Supporting Reference 17: Patient-Centered HCV Care via Telemedicine for Individuals on Medication for Opioid Use Disorder
Trial Protocol
Data Sharing Statement
References
- 1.Daniel H, Bornstein SS, Kane GC, et al. ; Health and Public Policy Committee of the American College of Physicians . Addressing social determinants to improve patient care and promote health equity: an American College of Physicians position paper. Ann Intern Med. 2018;168(8):577-578. doi: 10.7326/M17-2441 [DOI] [PubMed] [Google Scholar]
- 2.Outland BE, Erickson S, Doherty R, Fox W, Ward L; Medical Practice and Quality Committee of the American College of Physicians . Reforming physician payments to achieve greater equity and value in health care: a position paper of the American College of Physicians. Ann Intern Med. 2022;175(7):1019-1021. doi: 10.7326/M21-4484 [DOI] [PubMed] [Google Scholar]
- 3.Mehrotra A, Bhatia RS, Snoswell CL. Paying for telemedicine after the pandemic. JAMA. 2021;325(5):431-432. doi: 10.1001/jama.2020.25706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zulman DM, Verghese A. Virtual care, telemedicine visits, and real connection in the era of COVID-19: unforeseen opportunity in the face of adversity. JAMA. 2021;325(5):437-438. doi: 10.1001/jama.2020.27304 [DOI] [PubMed] [Google Scholar]
- 5.Talal AH, Sofikitou EM, Jaanimägi U, Zeremski M, Tobin JN, Markatou M. A framework for patient-centered telemedicine: application and lessons learned from vulnerable populations. J Biomed Inform. 2020;112:103622. doi: 10.1016/j.jbi.2020.103622 [DOI] [PubMed] [Google Scholar]
- 6.Herzer KR, Pronovost PJ. Ensuring quality in the era of virtual care. JAMA. 2021;325(5):429-430. doi: 10.1001/jama.2020.24955 [DOI] [PubMed] [Google Scholar]
- 7.Biancarelli DL, Biello KB, Childs E, et al. Strategies used by people who inject drugs to avoid stigma in healthcare settings. Drug Alcohol Depend. 2019;198:80-86. doi: 10.1016/j.drugalcdep.2019.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Artenie A, Stone J, Fraser H, et al. ; HIV and HCV Incidence Review Collaborative Group . Incidence of HIV and hepatitis C virus among people who inject drugs, and associations with age and sex or gender: a global systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(6):533-552. doi: 10.1016/S2468-1253(23)00018-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . Combating hepatitis B and C to reach elimination by 2030: advocacy brief. Accessed February 25, 2023. https://apps.who.int/iris/handle/10665/206453
- 10.US Department of Health and Human Services . Viral hepatitis: national strategic plan: a roadmap to elimination for the United States 2021-2025. Accessed January 19, 2021. https://www.hhs.gov/sites/default/files/Viral-Hepatitis-National-Strategic-Plan-2021-2025.pdf
- 11.Fleurence RL, Collins FS. A national hepatitis C elimination program in the United States: a historic opportunity. JAMA. 2023;329(15):1251-1252. doi: 10.1001/jama.2023.3692 [DOI] [PubMed] [Google Scholar]
- 12.Earnshaw V, Smith L, Copenhaver M. Drug addiction stigma in the context of methadone maintenance therapy: an investigation into understudied sources of stigma. Int J Ment Health Addict. 2013;11(1):110-122. doi: 10.1007/s11469-012-9402-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Islam MM. Missed opportunities for hepatitis C testing and other opportunistic health care. Am J Public Health. 2013;103(12):e6. doi: 10.2105/AJPH.2013.301611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.New York State Office of Addiction Services and Supports . Person-centered care guidance for OASAS certified programs. Accessed September 9, 2021. https://oasas.ny.gov/system/files/documents/2020/01/oasasperson-centeredcareguidance.pdf
- 15.Talal AH, Andrews P, McLeod A, et al. Integrated, co-located, telemedicine-based treatment approaches for hepatitis C virus management in opioid use disorder patients on methadone. Clin Infect Dis. 2019;69(2):323-331. doi: 10.1093/cid/ciy899 [DOI] [PubMed] [Google Scholar]
- 16.Talal AH, McLeod A, Andrews P, et al. Patient reaction to telemedicine for clinical management of hepatitis C virus integrated into an opioid treatment program. Telemed J E Health. 2019;25(9):791-801. doi: 10.1089/tmj.2018.0161 [DOI] [PubMed] [Google Scholar]
- 17.Talal AH, Markatou M, Sofikitou EM, et al. Patient-centered HCV care via telemedicine for individuals on medication for opioid use disorder: Telemedicine for Evaluation, Adherence and Medication for Hepatitis C (TEAM-C). Contemp Clin Trials. 2022;112:106632. doi: 10.1016/j.cct.2021.106632 [DOI] [PubMed] [Google Scholar]
- 18.Chaudhary MA, Moulton LH. A SAS macro for constrained randomization of group-randomized designs. Comput Methods Programs Biomed. 2006;83(3):205-210. doi: 10.1016/j.cmpb.2006.04.011 [DOI] [PubMed] [Google Scholar]
- 19.Moulton LH. Covariate-based constrained randomization of group-randomized trials. Clin Trials. 2004;1(3):297-305. doi: 10.1191/1740774504cn024oa [DOI] [PubMed] [Google Scholar]
- 20.Moulton LH, Golub JE, Durovni B, et al. Statistical design of THRio: a phased implementation clinic-randomized study of a tuberculosis preventive therapy intervention. Clin Trials. 2007;4(2):190-199. doi: 10.1177/1740774507076937 [DOI] [PubMed] [Google Scholar]
- 21.Talal AH, Jaanimägi U, Davis K, et al. Facilitating engagement of persons with opioid use disorder in treatment for hepatitis C virus infection via telemedicine: stories of onsite case managers. J Subst Abuse Treat. 2021;127:108421. doi: 10.1016/j.jsat.2021.108421 [DOI] [PubMed] [Google Scholar]
- 22.Schackman BR, Teixeira PA, Beeder AB. Offers of hepatitis C care do not lead to treatment. J Urban Health. 2007;84(3):455-458. doi: 10.1007/s11524-007-9180-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grebely J, Raffa JD, Lai C, et al. Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepat. 2009;16(5):352-358. doi: 10.1111/j.1365-2893.2009.01080.x [DOI] [PubMed] [Google Scholar]
- 24.Wester C, Osinubi A, Kaufman HW, et al. Hepatitis C virus clearance cascade—United States, 2013-2022. MMWR Morb Mortal Wkly Rep. 2023;72(26):716-720. doi: 10.15585/mmwr.mm7226a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson WW, Symum H, Sandul A, et al. Vital signs: hepatitis C treatment among insured adults—United States, 2019-2020. MMWR Morb Mortal Wkly Rep. 2022;71(32):1011-1017. doi: 10.15585/mmwr.mm7132e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bulteel N, Partha Sarathy P, Forrest E, et al. Factors associated with spontaneous clearance of chronic hepatitis C virus infection. J Hepatol. 2016;65(2):266-272. doi: 10.1016/j.jhep.2016.04.030 [DOI] [PubMed] [Google Scholar]
- 27.Li T, Hutfless S, Scharfstein DO, et al. Standards should be applied in the prevention and handling of missing data for patient-centered outcomes research: a systematic review and expert consensus. J Clin Epidemiol. 2014;67(1):15-32. doi: 10.1016/j.jclinepi.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1-67. doi:https://www.jstatsoft.org/article/view/v045i03
- 29.Chakraborty H, Hossain A. R package to estimate intracluster correlation coefficient with confidence interval for binary data. Comput Methods Programs Biomed. 2018;155:85-92. doi: 10.1016/j.cmpb.2017.10.023 [DOI] [PubMed] [Google Scholar]
- 30.Schafer JL, Olsen MK. Multiple imputation for multivariate missing-data problems: a data analyst’s perspective. Multivariate Behav Res. 1998;33(4):545-571. doi: 10.1207/s15327906mbr3304_5 [DOI] [PubMed] [Google Scholar]
- 31.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40-49. doi: 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y. Diagnostic checking of multiple imputation models. Adv Stat Anal. 2022;106(2):271-286. doi: 10.1007/s10182-021-00429-1 [DOI] [Google Scholar]
- 33.Schafer JL. Analysis of Incomplete Multivariate Data. Chapman & Hall/CRC; 1997. doi: 10.1201/9781439821862 [DOI] [Google Scholar]
- 34.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28(2):182-191. doi: 10.1016/j.cct.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 35.Thompson JA, Davey C, Fielding K, Hargreaves JR, Hayes RJ. Robust analysis of stepped wedge trials using cluster-level summaries within periods. Stat Med. 2018;37(16):2487-2500. doi: 10.1002/sim.7668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel . Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020;71(2):686-721. doi: 10.1002/hep.31060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raab GM, Day S, Sales J. How to select covariates to include in the analysis of a clinical trial. Control Clin Trials. 2000;21(4):330-342. doi: 10.1016/S0197-2456(00)00061-1 [DOI] [PubMed] [Google Scholar]
- 38.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518-526. doi: 10.1053/jhep.2003.50346 [DOI] [PubMed] [Google Scholar]
- 39.Varadhan R, Segal JB, Boyd CM, Wu AW, Weiss CO. A framework for the analysis of heterogeneity of treatment effect in patient-centered outcomes research. J Clin Epidemiol. 2013;66(8):818-825. doi: 10.1016/j.jclinepi.2013.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM. Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide. Agency for Healthcare Research & Quality; 2013. [PubMed] [Google Scholar]
- 41.Ward KM, Falade-Nwulia O, Moon J, et al. Nonadherence to ledipasvir/sofosbuvir did not predict sustained virologic response in a randomized controlled trial of human immunodeficiency virus/hepatitis C virus coinfected persons who use drugs. J Infect Dis. 2022;225(5):903-911. doi: 10.1093/infdis/jiab477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orkin AM, Gill PJ, Ghersi D, et al. ; CONSERVE Group . Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other extenuating circumstances: the CONSERVE 2021 statement. JAMA. 2021;326(3):257-265. doi: 10.1001/jama.2021.9941 [DOI] [PubMed] [Google Scholar]
- 43.National Institutes of Health . Racial and ethnic categories and definitions for NIH diversity programs and for other reporting purposes. Published April 8, 2015. Accessed September 23, 2016. https://grants.nih.gov/grants/guide/notice-files/not-od-15-089.html
- 44.Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363-371. doi: 10.1016/0306-4603(82)90005-3 [DOI] [PubMed] [Google Scholar]
- 45.NIDA Quick Screen V1.0. Accessed July 3, 2021. https://nida.nih.gov/sites/default/files/pdf/nmassist.pdf
- 46.Rosner B, Glynn RJ, Lee ML. Incorporation of clustering effects for the Wilcoxon rank sum test: a large-sample approach. Biometrics. 2003;59(4):1089-1098. doi: 10.1111/j.0006-341X.2003.00125.x [DOI] [PubMed] [Google Scholar]
- 47.Rosner B, Glynn RJ, Lee ML. The Wilcoxon signed rank test for paired comparisons of clustered data. Biometrics. 2006;62(1):185-192. doi: 10.1111/j.1541-0420.2005.00389.x [DOI] [PubMed] [Google Scholar]
- 48.Ford I, Norrie J. Pragmatic trials. N Engl J Med. 2016;375(5):454-463. doi: 10.1056/NEJMra1510059 [DOI] [PubMed] [Google Scholar]
- 49.Dickerson SS, George SJ, Ventuneac A, Dharia A, Talal AH. Integrating facilitated telemedicine for hepatitis C virus treatment within opioid treatment programs: staff experiences. Poster presented at: 2023 American Telemedicine Association Conference and Expo; March 4-6, 2023; San Antonio, TX. [Google Scholar]
- 50.Zeremski M, Zibbell JE, Martinez AD, Kritz S, Smith BD, Talal AH. Hepatitis C virus control among persons who inject drugs requires overcoming barriers to care. World J Gastroenterol. 2013;19(44):7846-7851. doi: 10.3748/wjg.v19.i44.7846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris M, Guy D, Picchio CA, White TM, Rhodes T, Lazarus JV. Conceptualising hepatitis C stigma: a thematic synthesis of qualitative research. Int J Drug Policy. 2021;96:103320. doi: 10.1016/j.drugpo.2021.103320 [DOI] [PubMed] [Google Scholar]
- 52.Talal AH, Jaanimägi U, Dharia A, Dickerson SS. Facilitated telemedicine for hepatitis C virus: addressing challenges for improving health and life for people with opioid use disorder. Health Expect. 2023;26(6):2594-2607. doi: 10.1111/hex.13854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reynolds A. The value of an HCV cure: curing HCV benefits the individual—and society. Posit Aware. 2015;27(4):20-23. [PubMed] [Google Scholar]
- 54.Talal AH, Sofikitou EM, Wang K, Dickerson S, Jaanimägi U, Markatou M. High satisfaction with patient-centered telemedicine for hepatitis C virus delivered to substance users: a mixed-methods study. Telemed J E Health. 2023;29(3):395-407. doi: 10.1089/tmj.2022.0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Artenie AA, Cunningham EB, Dore GJ, et al. Patterns of drug and alcohol use and injection equipment sharing among people with recent injecting drug use or receiving opioid agonist treatment during and following hepatitis C virus treatment with direct-acting antiviral therapies: an international study. Clin Infect Dis. 2020;70(11):2369-2376. doi: 10.1093/cid/ciz633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenthal ES, Silk R, Mathur P, et al. Concurrent initiation of hepatitis C and opioid use disorder treatment in people who inject drugs. Clin Infect Dis. 2020;71(7):1715-1722. doi: 10.1093/cid/ciaa105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Springer SA, Del Rio C. Co-located opioid use disorder and hepatitis C virus treatment is not only right, but it is also the smart thing to do as it improves outcomes! Clin Infect Dis. 2020;71(7):1723-1725. doi: 10.1093/cid/ciaa111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bartlett SR, Wong S, Yu A, et al. The impact of current opioid agonist therapy on hepatitis C virus treatment initiation among people who use drugs from the direct-acting antiviral (DAA) era: a population-based study. Clin Infect Dis. 2022;74(4):575-583. doi: 10.1093/cid/ciab546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cunningham EB, Wheeler A, Hajarizadeh B, et al. Interventions to enhance testing, linkage to care, and treatment initiation for hepatitis C virus infection: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(5):426-445. doi: 10.1016/S2468-1253(21)00471-4 [DOI] [PubMed] [Google Scholar]
- 60.Bornstein S. The challenges of behavioral health integration: the persistence of the mind-body problem. Ann Intern Med. 2020;173(2):151-152. doi: 10.7326/M20-2887 [DOI] [PubMed] [Google Scholar]
- 61.Heath B, Wise Romero P, Reynolds KA. Table 1: six levels of collaboration/integration (core descriptions). Accessed July 25, 2022. https://www.thenationalcouncil.org/wp-content/uploads/2020/01/CIHS_Framework_Final_charts.pdf?daf=375ateTbd56
- 62.Wong RJ, Jain MK, Therapondos G, et al. Race/ethnicity and insurance status disparities in access to direct acting antivirals for hepatitis C virus treatment. Am J Gastroenterol. 2018;113(9):1329-1338. doi: 10.1038/s41395-018-0033-8 [DOI] [PubMed] [Google Scholar]
- 63.Taylor BS, Hanson JT, Veerapaneni P, Villarreal R, Fiebelkorn K, Turner BJ. Hospital-based hepatitis C screening of baby boomers in a majority Hispanic south Texas cohort: successes and barriers to implementation. Public Health Rep. 2016;131(suppl 2):74-83. doi: 10.1177/00333549161310S212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mittal A, Kosinski KC, Stopka TJ. HCV treatment access among Latinxs who inject drugs: qualitative findings from Boston, Massachusetts, 2016. Harm Reduct J. 2019;16(1):44. doi: 10.1186/s12954-019-0314-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kasdan A, Marotta P, Hamburg A. Beyond methadone: improving health and empowering patients in opioid treatment programs. Accessed October 20, 2015. https://www.researchgate.net/publication/271645736_improving_health_and_empowering_patients_in_opioid_treatment_programs
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Randomization
eFigure 1. Age Distributions at Baseline (N= 602) Between Referral and Opioid Treatment Program-Integrated Facilitated Telemedicine Arms
eAppendix 2. Treatment of Missing Data
eTable 1. Reasons for Early Termination of Study Participants (i.e., Dropout) by Study Arm
eAppendix 2.1. Justification of Variables Included in the Model
eTable 2. Descriptive Statistics of Sustained Virological Responses Associated With the 20 Imputed Datasets
eFigure 2. Distribution of Imputed and Observed Drug Abuse Screening Test (DAST10) Levels per Arm
eTable 3. Percentages of Observed and Imputed, per Arm, Illegal Drug Use per Category as Obtained by Drug Abuse Screening Test (DAST-10)
eAppendix 2.2. Deaths
eTable 4. Deceased Participants by Relevant Demographic and Residence Location Variables
eAppendix 3. Nonparametric Analysis
eAppendix 3.1. Cluster-Level Analysis
eAppendix 4. Effect Modification
eAppendix 5. Selection Bias
eFigure 3. Distribution of Drug Abuse Screening Test (DAST10) Scores in the Referral and Opioid Treatment Program-Integrated Telemedicine Arms
eFigure 4. Distribution of the Number of Months Participants Are in the Methadone Program for the Referral and Opioid Treatment Program-Integrated Telemedicine Arms
eTable 5. Direct Acting Antiviral Medications Prescribed to Study Participants
eTable 6. Adherence to Direct Acting Antiviral Medication by Treatment Week
eFigure 5. Boxplots Comparing the Distribution of Methadone Dose in the Referral (Usual Care) and Opioid Treatment Program-Integrated Telemedicine Arms
eFigure 6. Boxplots Comparing Methadone Doses in Opioid Treatment Program-Integrated Telemedicine and Referral Arms
eAppendix 6. Observations Related to Subgroups
eTable 7. Sustained Virological Response Rates by Period for Hispanic Females in Referral and Opioid Treatment Program-Integrated Telemedicine Arms
eTable 8. English Language Ability for Hispanic Females and Males
eFigure 7. Number of Rural Participants Achieving a Sustained Virologic Response (SVR) Stratified by Study Arm
eAppendix 7. Analysis of Incidence Rates
eReferences.
Trial Protocol: Statistical Analysis and Final Results Overview Including Heterogeneity of Treatment Effects
Trial Protocol: Supplemental Material Supporting Reference 17: Patient-Centered HCV Care via Telemedicine for Individuals on Medication for Opioid Use Disorder
Trial Protocol
Data Sharing Statement