Summary
Background
No study has compared the virological and immunological status of young people with perinatally-acquired HIV infection (P-HIV) with that of people with HIV adulthood (A-HIV) having a similar duration of infection.
Methods
5 French cohorts of P-HIV and A-HIV patients with a known date of HIV-infection and receiving antiretroviral treatment (ART), were used to compare the following proportions of: virological failure (VF) defined as plasma HIV RNA ≥ 50 copies/mL, CD4 cell percentages and CD4:CD8 ratios, at the time of the most recent visit since 2012. The analysis was stratified on time since infection, and multivariate models were adjusted for demographics and treatment history.
Findings
310 P-HIV were compared to 1515 A-HIV (median current ages 20.9 [IQR:14.4–25.5] and 45.9 [IQR:37.9–53.5] respectively). VF at the time of the most recent evaluation was significantly higher among P-HIV (22.6%, 69/306) than A-HIV (3.3%, 50/1514); p ≤ 0.0001. The risk of VF was particularly high among the youngest children (2–5 years), adolescents (13–17 years) and young adults (18–24 years), compared to A-HIV with a similar duration of infection: adjusted Odds-Ratio (aOR) 7.0 [95% CI: 1.7; 30.0], 11.4 [4.2; 31.2] and 3.3 [1.0; 10.8] respectively. The level of CD4 cell percentages did not differ between P-HIV and A-HIV. P-HIV aged 6–12 and 13–17 were more likely than A-HIV to have a CD4:CD8 ratio ≥ 1: 84.1% vs. 58.8% (aOR = 3.5 [1.5; 8.3]), and 60.9% vs. 54.7% (aOR = 1.9 [0.9; 4.2]) respectively.
Interpretation
P-HIV were at a higher risk of VF than A-HIV with a similar duration of infection, even after adjusting for treatment history, whereas they were not at a higher risk of immunological impairment. Exposure to viral replication among young patients living with HIV since birth or a very early age, probably because of lower adherence, could have an impact on health, raising major concerns about the selection of resistance mutations and the risk of HIV transmission.
Funding
Inserm - ANRS MIE.
Keywords: Perinatal HIV infection, Cohort, Viral failure, Immunological outcome, Epidemiology
Research in context.
Evidence before this study
In 2024 we searched Pubmed using the terms “perinatally acquired HIV” “perinatal HIV infection” “viral suppression” “viral failure” “response to therapy” “virological outcome” “immunological outcome” “CD4” “CD4:CD8 ratio”.
Several cohort studies have underlined particular difficulties in the follow-up and treatment of people living with HIV after perinatal infection, particularly when they reach adolescence. Two studies compared the viral load according age: in the first one, the 13–17 year-old group was a mix of young adults with perinatally-acquired HIV and young people having acquired HIV through sexual intercourse, despite the fact that these two populations have very different medical histories. In the second one, the viral load was compared between perinatally and non-perinatally infected adolescents/young adults by age classes, which cannot take into account differences in HIV duration and medical histories. Two other studies compared patients with perinatal and non-perinatal HIV for age at the first treatment, but not for the time since the HIV acquisition. We did not find any studies comparing both immunological and virological status between patients living with HIV acquired perinatally and HIV acquired in adulthood taking into account the duration of the infection.
Added value of this study
To our knowledge, this is the first study to compare the immunovirological status of individuals aged 2–32 years, having acquired HIV perinatally (P-HIV) with individuals having acquired HIV in adulthood (A-HIV) with similar durations of infection. In addition, our study enables to consider five age categories of P-HIV, from childhood to adulthood, in relation to physiological, psychological, and behavioural changes, and changes in autonomy, with a possible impact on adherence to treatment, and to take into account the duration of infection and treatment history to compare P-HIV and A-HIV.
Immuno-virological data and treatment history were analysed using large national prospective paediatric and adult cohorts of patients recruited in hospital setting, including a large number of P-HIV, with follow-up durations of up to 30 years, thus corresponding to the first generation of adults living with HIV since birth or a very early age.
We found a large proportion of viral failure at the latest assessment among P-HIV, among the youngest children (2–5 years), adolescents (13–17 years) and young adults (18–24 years). We highlighted a higher risk of viral failure, than among people having acquired HIV in adulthood with a similar duration of infection, even after adjusting for treatment history. This larger proportion of viral load failure was not associated with more CD4+ T-cell depletion.
Implications of all the available evidence
In a country that offers free access to care and to a large panel of ART, virological control nevertheless remained more difficult to obtain in people living with HIV acquired perinatally than for those who acquired it during adulthood. Maintaining long-term viral suppression is crucial for all patients living with HIV, but it meets specific difficulties for people having acquired HIV perinatally, particularly for teenagers and young adults, compared to people who acquired HIV infection in adulthood. In addition, exposure to viral replication among these young patients living with perinatally-acquired HIV raises major concerns about the selection of resistant mutations and the risk of HIV transmission, and this could impact their future health.
Introduction
Perinatal HIV infection is classically defined as an infection transmitted during pregnancy, childbirth or breastfeeding, also called mother-to-child transmission. Antiretroviral treatment (ART) has dramatically decreased mortality among children and adolescents living with perinatal HIV (P-HIV) in high-income countries.1, 2, 3, 4 A European study reported a cumulative incidence estimate for mortality at the age of 15 of 0.8%.5 Studies describing current virological and immunological outcomes among P-HIV, many of whom are now reaching adulthood, are still few, and there is no study comparing outcomes between people having acquired HIV perinatally and those having acquired HIV in adulthood (A-HIV) with a similar duration of HIV infection.
Maintaining long-term viral suppression is crucial for all patients, but supporting P-HIV patients entails particular difficulties: treatment initiation at a time of physiological immaturity and its maintenance through physiological, psychological, and behavioural changes, exacerbated during adolescence, and the need to rely on caregiver for adherence. Several studies on paediatric cohorts have reported high virological failure (VF) rates, particularly during adolescence.6, 7, 8, 9
A collaborative study of European cohorts highlighted poorer virological responses at 12 months after ART initiation among children and adolescents than among adults.10 However, this study comparing outcomes only across age groups did not take the time since acquisition of HIV infection into account, and the 13–17 year-old group was a mix of patients having perinatally acquired HIV and patients having acquired it through sexual relation. Recent studies, with up to 3 years’ follow-up, have confirmed that the risk of VF is higher among P-HIV than A-HIV.11,12 In these studies, age at initiation of ART and treatment duration were considered, but the possibly different durations of HIV infection between the two populations were not.
Data on immunological recovery among P-HIV is also limited: there are concerns about their long-term immunological status as a consequence of poorer virological control, while greater thymic activity in children13,14 could provide greater potential for immune recovery.15
Our study aimed to compare the proportion of virological failure at latest assessment among P-HIV under treatment, according to different age strata corresponding to life developmental stages, with that observed among A-HIV after the same duration of HIV infection. We also compared immunological parameters between the two groups. This study used data from five large French national paediatric and adult cohorts, thus affording access to comprehensive immunological, virological and treatment histories.
Methods
Data sources
We used data from five ANRS French national cohorts, which included patients with HIV-1 infection acquired before the age of 13 (CO10-EPF, CO19-COVERTE), or in adulthood ≥15 years (CO9-COPANA, CO6-PRIMO, SEROPRI).
Eligible individuals with perinatally-acquired HIV infection (P-HIV)
The CO10-EPF cohort followed children living with HIV, up to the age of 17, born to more than 20,000 women living with HIV and enrolled since 1985 in around 100 hospital maternity facilities (N = 543) (Supplementary Fig. S0). From 2005, extended criteria enabled the inclusion of other children diagnosed with HIV at ≤13 years and antiretroviral therapy-naïve at the time of their first medical care in the participating paediatric hospital facilities (N = 174). For all children included in EPF-CO10, data was collected semestrially up to the last visit before the age of 18. In 2010–2015, CO19-COVERTE was set up to enrol patients, aged 18–25, diagnosed with HIV infection before the age of 13, previously (N = 298) or not (N = 179) included in the CO10-EPF cohort, with annual follow-up to 2018.
Patients were considered to have established or very probable perinatal HIV acquisition if they had been included since birth in the EPF cohort (HIV + mothers were enrolled in the cohort during pregnancy), or, for those included later after birth, if the perinatal HIV infection was documented before 13 years of age with no other route than mother-to-child transmission noted in the medical records (Supplementary Fig. S0). According to the age and calendar time, the diagnosis was based on by a first positive, RNA, DNA assay, p24 antigen or culture in the early months and/or an ELISA test after 18 months of age. For 48 of the 161 Coverte patients that we considered as P-HIV, only age or date of diagnosis and/or age at first antiretroviral treatment were available in medical record with no information on the initial method of diagnosis.
Eligible individuals with HIV infection acquired after the age of 15 (A-HIV)
Between 2004 and 2008, the ANRS CO9-COPANA Cohort included 800 antiretroviral-naive patients with a recent HIV-1 diagnosis (<1 year), with a follow-up until June 2016. Since 1996, the on-going ANRS CO6-PRIMO has enrolled > 2000 subjects diagnosed at the time of their primary HIV-1-infection. The ANRS SEROPRI cohort is a subset of 81 patients living with HIV-1 from the historical ANRS SEROCO cohort (enrolment from 1988 to 2001), for whom the date of HIV infection could be determined; they were still followed in 2008 and gave consent for the continuation of follow-up after 2008.
The date of HIV acquisition was estimated as follows: the date of onset of primary HIV infection symptoms minus 15 days, or the date of the incomplete Western blot minus one month, or the midpoint between negative and positive ELISA results within an interval of less than 24 months.
All patients in the P-HIV and A-HIV cohorts were followed by clinicians according to standard care, based on regularly updated national guidelines since 1990, with free access to care and treatment, viral load measurement at least twice a year, routinely available since 1995’s.
Study population
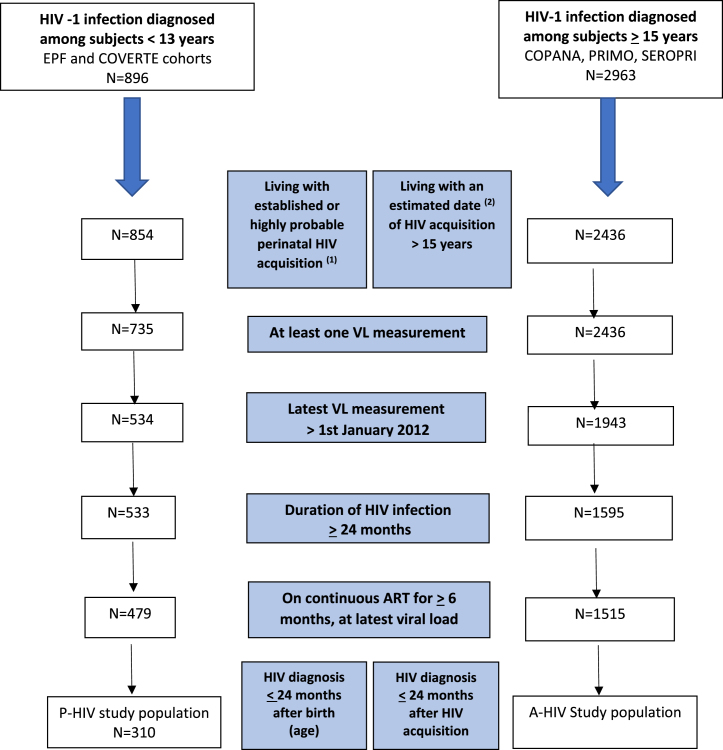
We included participants who had had an estimated date of HIV infection within a 24-month interval, who were still in follow-up after January 1st 2012, and who had undergone their latest plasma viral load (VL) measurement after 1st January 2012 while on prescribed continuous antiretroviral therapy for at least 6 months, without any reported interruption > 15 days. To enhance comparability, for both groups, we selected participants who had been diagnosed within 24 months following HIV acquisition and living with HIV for ≥2 years at the time of the latest VL evaluation (Fig. 1).
Fig. 1.
Flow chart from eligible P-HIV or A-HIV to the included study population. (1) Criteria described in the supplementary Supplementary Fig. S0. (2) Estimated as follows: the date of symptom onset of the primary HIV infection minus 15 days, or the date of the incomplete Western blot minus one month, or the midpoint between negative and positive ELISA results within an interval of less than 24 months.
Outcomes
The primary outcome was virological failure (VF), defined as the last VL ≥ 50 RNA copies/mL. We also considered CD4 percentages levels and CD4:CD8 ratios measured at the time of the latest VL assessment.
Main exposure
The mode of HIV acquisition was the main exposure: we compared patients with perinatal HIV (P-HIV), included in the EPF and COVERTE cohorts, with patients who acquired HIV during adulthood (A-HIV) in the SEROPRI, PRIMO and COPANA cohorts.
Five P-HIV age strata at the time of last VL evaluation were considered a priori, in relation to physiological, psychological, and behavioural changes, and changes in autonomy, that could have an impact on the adherence to treatment: two periods in childhood (preschool ages 2–5 years and compulsory schooling ages 6–12 years), two periods of transition to adulthood before and after the legal age of majority (teenagers aged 13–17 years and young adults aged 18–24 years) and one adult stratum of individuals aged 25–32 years.
As the ages also corresponded to the time elapsed since HIV infection for P-HIV, we defined, for A-HIV, same five strata of duration since their estimated date of infection.
Other risk factors
For all patients, gender, country of birth, and factors potentially associated with virological failure and immunological level were analysed: time from HIV acquisition to first ART, % CD4 and VL at first ART, calendar time and regimen type of first ART, number of different ART regimens, CD4% nadir, calendar time at the start and type of most recent ART regimen.
Statistical analysis
P-HIV and A-HIV were first compared according demographics and HIV history in each of the 5 five age/infection duration strata.
Univariate analysis was first performed to study the association between the main outcome (virological failure) and the main exposure (P-HIV vs. A-HIV) in each age/infection duration stratum, and with gender, country of birth, and ART history factors, separately for P-HIV and A-HIV (showed in Supplementary Table S1). Continuous variables are presented as medians (interquartile ranges), categorical variables as % (n). Comparisons between groups were made using the Kruskal–Wallis test for continuous variables, and the Chi-square test or Fisher’s test, as appropriate, for categorical variables.
Multivariate analysis was performed separately within each age/infection duration stratum to study the association of virological failure (VF) with P-HIV/A-HIV exposure. The initial model adjusted systematically for gender, country of birth, and ART history factors found in univariate analysis to be associated with the VF in the A-HIV or P-HIV group or both, at a p-value threshold < 0.2 to avoid over-adjustment we retained the overall number of ART regimens received, a switch being defined as any change in the number or type of ART, but without considering difference in formulation or dosage, as this partly reflects history of viral load control and possible past changes because of ART resistance, rather the calendar time at first ART, both being highly correlated. No subsequent variable selection procedure was needed as the adjusted ORs measuring the association of VF with P- vs. A-HIV acquisition were quite similar to crude ORs in the initial largest model, within each age/duration of infection stratum.
Logistic regressions were used to estimate crude and adjusted odds ratios. For each co-factor, the reference category was chosen among categories well represented in both the P-HIV and A-HIV groups.
Similar analyses were performed for the secondary outcomes including CD4 percentage ≥25%, and CD4:CD8 ratio ≥1.
The analyses were performed using SAS software, version 9.4 (SAS Inc., Cary, NC).
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the paper for publication.
Results
Study population
Of the 896 individuals enrolled in the paediatric cohorts, 854 had an established or highly probable perinatal HIV-1 infection (Supplementary Fig. S0), 479 had been living with HIV for ≥2 years, had been on continuous ART for ≥6 months, with the latest VL measurement after 1st January 2012 (Fig. 1). We excluded 169 individuals with a late HIV diagnosis (>24 months after infection). Thus 310 P-HIV were retained in the main analysis: 55.2% were female, and 5.8% were from sub-Saharan Africa. Of the 2963 individuals enrolled in the adult cohorts, 2436 had an estimated date of HIV acquisition. Among them, 1515 met the other criteria and were retained for the main analysis: 13.8% were female, 6.3% were from sub-Saharan Africa (Table 1).
Table 1.
Characteristics of the study population at latest viral load evaluation (2012–2018), according to the period of life of HIV-1 acquisition: perinatal or during adulthood–the French ANRS Coverte, CO10-EPF, Primo, Seropri, Copana cohorts.
| HIV acquired perinatally (P-HIV) N = 310 | HIV acquired in adulthood (A-HIV) N = 1515 | |
|---|---|---|
| Cohort | ||
| Only EPF | 147 (47.4) | |
| EPF and then COVERTE | 100 (32.3) | |
| Only COVERTE | 63 (20.3) | |
| COPANA | 162 (10.7) | |
| PRIMO | 1281 (84.6) | |
| SEROPRI | 72 (4.8) | |
| HIV transmission mode | ||
| Perinatal | 310 (100.0) | |
| Men having sex with men | 1061 (70.0) | |
| Heterosexual sex | 349 (23.0) | |
| Transfusion/occupational exposure | 16 (1.1) | |
| Injecting drug use | 8 (0.5) | |
| Unknown | 81 (5.4) | |
| Gender | ||
| Female | 171 (55.2) | 209 (13.8) |
| Country of birth | ||
| France | 276 (89.0) | 1274 (84.5) |
| Sub-Saharan Africa | 18 (5.8) | 95 (6.3) |
| Other | 16 (5.2) | 139 (9.2) |
| (Missing) | (7) | |
| Duration of HIV infection at latest VL evaluation, years | ||
| 2–5 years | 17 (5.5) | 472 (31.2) |
| 6–12 years | 49 (15.8) | 703 (46.4) |
| 13–17 years | 81 (26.1) | 210 (13.9) |
| 18–24 years | 73 (23.6) | 82 (5.4) |
| 25–32 years | 90 (29.0) | 48 (3.2) |
| Calendar period of latest VL measurement | ||
| 2012–2013 | 38 (12.3) | 100 (6.6) |
| 2014–2015 | 57 (18.4) | 175 (11.6) |
| 2016–2018 | 215 (69.4) | 1240 (81.9) |
| Median (IQR) date of most recent evaluation | 2016 [IQR:2015–2017] | 2016 [IQR:2016–2017] |
Data is presented as n (%) for patients with available data for the given variable.
The median age at the most recent evaluation of viral load on ART was 20.9 years [IQR:14.4–25.5] for P-HIV and 45.9 [IQR:37.9–53.5] years for A-HIV. The median dates were respectively 2016 [IQR:2015–2017] for P-HIV and 2016 [IQR:2016–2017] for A-HIV, p = 0.07. Among the 310 P-HIV, 17 were aged 2 to 5, 49 were 6–12, 81 were 13–17, 73 were 18–24, and 90 were 25–32. Among the 1515 A-HIV, 472, 703, 210, 82 and 48 respectively had reached corresponding durations of HIV infection.
Treatment history differed between the P-HIV and A-HIV groups, and some differences persisted after stratification by duration of HIV infection (Table 2). Whatever the duration of the HIV infection, P-HIV had initiated their first ART in earlier calendar times, at a higher CD4%, and they had longer durations of treatment than A-HIV. Young P-HIV aged under 18 were less likely to have had their last assessment within the most recent calendar time (2016–2018), or to be on a current regimen containing an integrase inhibitor and/or an entry inhibitor. In contrast, older P-HIV aged 18 or over were more often on integrase strand transfer inhibitors (INSTI)/or entry inhibitors than in their A-HIV counterparts: 37.0% vs. 30.5% (p < 0.05) in the [18–24 years] group, and 65.6% vs. 33.3% (p < 0.0001) in the [25–32 years] group respectively. Overall, 6 P-HIV and 12 A-HIV were on entry inhibitor; 5 P-HIV and 11 A-HIV were on maraviroc and 1 P-HIV vs. 2 A-HIV on T20.
Table 2.
Therapeutic history, according to the period of life of HIV-1 acquisition: in the perinatal period (P) or in adulthood (A), stratified on duration of HIV infection at the time of the latest viral load measurement (between 2012 and 2018)–the French ANRS Coverte, CO10-EPF, Primo Seropri, Copana cohorts.
| Duration of HIV infection | 2–5 years |
6–12 years |
13–17 years |
18–24 years |
25–32 years |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-HIV N = 17 | A N = 472 | p-value | P N = 49 | A N = 703 | p-value | P N = 81 | A N = 210 | p-value | P N = 73 | A N = 82 | p-value | P N = 90 | A N = 48 | p-value | |
| FEATURES AT FIRST ART | |||||||||||||||
| Time since HIV infection, months | |||||||||||||||
| Median | 4.6 | 1.4 | e | 4.2 | 15.5 | f | 2.8 | 1.9 | 8.2 | 1.6 | f | 17.0 | 80.6 | f | |
| IQR | (3.4–14.4) | (1.1–2.4) | (1.4–13.9) | (1.9–36.9) | (1.4–6.8) | (1.2–24.7) | (2.7–20.4) | (1.2–3.8) | (6.0–42.0) | (47.6–98.5) | |||||
| Year of initiation | |||||||||||||||
| Median | 2013 | 2013 | 2007 | 2009 | f | 1999 | 2002 | f | 1994 | 1997 | f | 1991 | 1995 | f | |
| IQR | (10–13) | (12–14) | (05–08) | (07–10) | (97–2002) | (00–04) | (93–95) | (96–98) | (89–92) | (92–97) | |||||
| 1st ART Regimen | |||||||||||||||
| INSTI, or entry inhibitor (±NRTI) | 5.9 (1) | 25.2 (119) | 0.0 (0) | 13.4 (94) | f | 0.0 (0) | 1.4 (3) | f | 0.0 (0) | 1.2 (1) | f | 0.0 (0) | 0.0 (0) | ||
| PI and NNRTI (±NRTI) | 0.0 (0) | 0.0 (0) | 4.1 (2) | 0.4 (3) | 6.2 (5) | 3.3 (7) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | |||||
| PI (±NRTI) | 76.5 (13) | 61 (288) | 69.4 (34) | 58.2 (409) | 39.5 (32) | 67.1 (141) | 2.7 (2) | 73.2 (60) | 6.7 (6) | 14.6 (7) | |||||
| NNRTI (±NRTI) | 17.6 (3) | 13.6 (64) | 18.4 (9) | 27.2 (191) | 7.4 (6) | 21.9 (46) | 2.7 (2) | 3.7 (3) | 1.1 (1) | 2.1 (1) | |||||
| Only NRTIs | 0.0 (0) | 0.2 (1) | 8.2 (4) | 0.9 (6) | 46.9 (38) | 6.2 (13) | 94.5 (69) | 22.0 (18) | 92.2 (83) | 83.3 (40) | |||||
| CD4% | |||||||||||||||
| Median | 45.0 | 27.0 | c | 32.0 | 22.0 | d | 36.0 | 22.0 | f | 34.0 | 26.0 | d | 28.0 | 18.0 | e |
| IQR | (22.0–56.5) | (20.0–34.0) | (16.0–49.0) | (17.0–27.0) | (23.0–49.0) | (16.0–29.0) | (20.0–43.0) | (18.0–30.0) | (18.0–39.0) | (12.0–24.0) | |||||
| VL at ART initiation (log10 copies/mL) | |||||||||||||||
| Median | 5.8 | 5.3 | 5.4 | 4.9 | 5.2 | 5.1 | 4.8 | 5.0 | 4.8 | 4.3 | |||||
| IQR | (5.3–6.6) | (4.6–6.1) | (3.9–6.1) | (4.3–5.4) | (4.0–5.8) | (4.5–5.7) | (4.4–5.2) | (4.3–5.7) | (4.2–5.0) | (3.9–4.8) | |||||
| FEATURES AT LATEST EVALUATION | |||||||||||||||
| Time since 1st ART (years) | |||||||||||||||
| Median | 4 | 3.5 | c | 9.4 | 7.0 | f | 16.2 | 14.0 | f | 22.4 | 19.1 | f | 25.6 | 20.9 | f |
| IQR | (3.6–5.0) | (2.5–4.4) | (8.0–10.6) | (5.8–9.0) | (14.3–17.1) | (12.9–15.4) | (20.2–23.3) | (18.4–20.0) | f | (24.0–27.1) | (19.1–24.2) | ||||
| Time since first combined therapya(years) | |||||||||||||||
| Median | 4.0 | 3.5 | c | 9.3 | 7.0 | f | 14.5 | 13.8 | c | 16.7 | 18.8 | f | 18.4 | 18.7 | |
| IQR | (3.6–5.0) | (2.5–4.4) | (7.9–10.6) | (5.7–8.9) | (12.8–16.0) | (11.8–15.2) | (14.1–18.5) | (17.9–19.5) | (15.4–19.8) | (17.3–19.9) | |||||
| Number of different ART regimens | |||||||||||||||
| Median | 3 | 2 | 3 | 3 | 5 | 5 | 9 | 5 | f | 12 | 9 | ||||
| IQR | (1–3) | (1–3) | (2–4) | (2–4) | (4–7) | (3–7) | (8–12) | (4–8) | (8–14) | (6–15) | |||||
| Nadir CD4%b | |||||||||||||||
| Median | 25.0 | 32.0 | d | 22.0 | 25.0 | 17.0 | 19.0 | d | 14.6 | 20.5 | d | 11.0 | 13.5 | ||
| IQR | (22.0–31.0) | (27.0–37.0) | (17.0–29.0) | (20.0–30.0) | (11.0–23.0) | (15.0–25.0) | (6.0–22.4) | (14.0–26.0) | (4.4–21.0) | (7.0–20.5) | |||||
| Year of initiation of last ART regimen, % (n) | |||||||||||||||
| ≤2006 | 0.0 (0) | 0.0 (0) | 12.2 (6) | 2.7 (19) | e | 8.6 (7) | 8.1 (17) | 0.0 (0) | 17.1 (14) | e | 2.2 (2) | 14.6 (7) | e | ||
| 2007–2013 | 35.3 (6) | 34.1 (161) | 55.1 (27) | 53.9 (379) | 53.1 (43) | 54.8 (115) | 50.7 (37) | 36.6 (30) | 32.2 (29) | 47.9 (23) | |||||
| 2014–2017 | 64.7 (11) | 65.9 (311) | 32.7 (16) | 43.4 (305) | 38.3 (31) | 37.1 (78) | 49.3 (36) | 46.3 (38) | 65.6 (59) | 37.5 (18) | |||||
| Last ART regimen, % (n) | |||||||||||||||
| INSTI, or entry inhibitor (+NRTI) | 11.8 (2) | 41.9 (198) | d | 12.2 (6) | 32.7 (230) | f | 14.8 (12) | 29.0 (61) | f | 37.0 (27) | 30.5 (25) | d | 62.6 (59) | 33.3 (16) | f |
| PI and NNRTI (±NRTI) | 0.0 (0) | 0.2 (1) | 2.0 (1) | 1.3 (9) | 3.7 (3) | 1.4 (3) | 1.4 (1) | 2.4 (2) | 2.2 (2) | 0.0 (0) | |||||
| PI (±NRTI) | 58.8 (10) | 20.6 (97) | 59.2 (29) | 18.1 (127) | 51.9 (42) | 14.3 (30) | 35.6 (36) | 18.3 (15) | 18.9 (17) | 18.8 (9) | |||||
| NNRTI (±NRTI) | 29.4 (5) | 36.4 (172) | 26.5 (13) | 46.9 (330) | 27.2 (22) | 54.3 (114) | 26.0 (19) | 43.9 (36) | 13.3 (12) | 27.1 (13) | |||||
| Only NRTIs | 0.0 (0) | 0.8 (4) | 0.0 (0) | 1.0 (7) | 2.5 (2) | 1.0 (2) | 0.0 (0) | 4.9 (4) | 0.0 (0) | 20.8 (10) | |||||
| Calendar period of latest VLmeasurement, % (n) | |||||||||||||||
| 2012–2013 | 5.9 (1) | 5.7 (27) | 14.3 (7) | 7.3 (51) | c | 29.6 (24) | 5.2 (11) | f | 6.9 (5) | 8.5 (7) | 1.1 (1) | 8.3 (4) | c | ||
| 2014–2015 | 17.7 (3) | 10.6 (50) | 20.4 (10) | 11.4 (80) | 27.2 (22) | 13.3 (28) | 17.8 (13) | 8.5 (7) | 10.0 (9) | 20.8 (10) | |||||
| 2016–2018 | 76.5 (13) | 83.7 (395) | 65.3 (32) | 81.4 (572) | 43.2 (35) | 81.4 (171) | 75.3 (55) | 82.9 (68) | 88.9 (80) | 70.8 (34) | |||||
Values in bold are median or percentages.
ART: antiretroviral therapy, VL: plasma viral load, INSTI: Integrase Strand Transfer Inhibitor, PI: Protease Inhibitor, NNRTI: Non-Nucleoside Reverse Transcriptase Inhibitor, NRTI: Nucleoside Reverse Transcriptase Inhibitor.
Defined as the concomitant use of ≥3 drugs from ≥2 classes of antiretroviral drugs (for 5 patients, the date of initiation of first combined therapy was unknown).
After initiation of ART.
p ≤ 0.05.
p ≤ 0.01.
p ≤ 0.001.
p ≤ 0.0001.
Virological control at the latest assessment
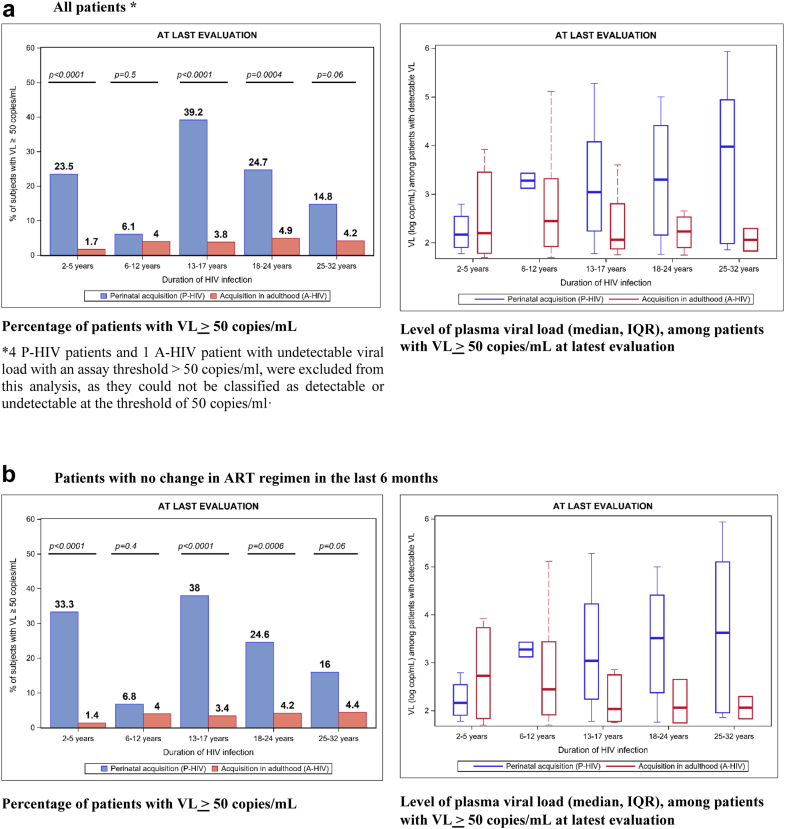
At the latest assessment, the percentages of VF (VL ≥ 50 cop/mL) among P-HIV ranged from 6.1% to 39.2% according to age stratum (Fig. 2). The proportion of VF was higher in the [13–17] age group (39.2%), followed by the [18–24] and [2–5] age groups (24.7% and 23.5% respectively). In contrast, the proportion of VF was below 5% among A-HIV across all strata of infection durations. Among the participants with VF, median VL was higher among P-HIV than among A-HIV: 1300 copies/mL (IQR: 145; 17,000) vs. 213 copies/mL (IQR: 78; 1219) (p = 0.004). Similar patterns were seen in an analysis restricted to participants with no change in ART regimen in the last 6 months.
Fig. 2.
Virological status at latest evaluation (2012–2018), according to the period of HIV acquisition, stratified by duration of HIV infection–the French ANRS Coverte, CO10-EPF, Primo Seropri, Copana cohorts.
In both P-HIV and A-HIV groups, the risk of experiencing VF was higher when the latest measurement dated from earlier calendar times, and it differed with the type of current regimen (Supplementary Table S1). The percentages of VF decreased from 36.8% in 2012–2013 to 17.4% in 2016–2018 among P-HIV, and from 8.1% to 2.6% among A-HIV. Cases where the last ART regimen contained NNRTI + PI together were few among both P-HIV and A-HIV. Among the others, the risk of VF was lower with a last regimen containing NNRTI than when it contained PI ( ± NRTI) in both the P-HIV and A-HIV groups. The risk of VF was also lower, among A-HIV only, when the last regimen contained INSTI or entry inhibitors than when it contained PI ( ± NRTI). Other factors were also specifically associated with VF in the A-HIV group: longer time-lapse before initiation of ART, lower CD4% at ART initiation, and a larger number of different regimens received.
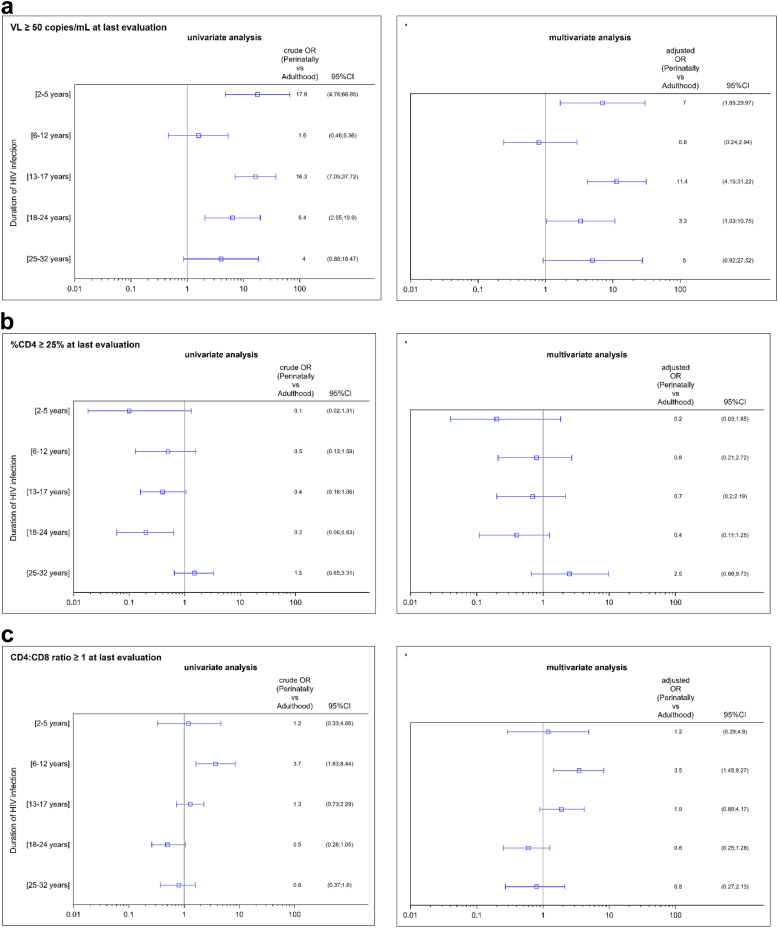
Overall, at the latest assessment, the proportion of participants with VF was significantly higher in the P-HIV group than in the A-HIV group, and in all strata of infection duration except the (6–12 years) group, where the percentage of VF was low, and the (25–32 years) group where the difference was of borderline significance (p = 0.06) (Fig. 2). The greatest differences were observed among the youngest children (2–5 years), adolescents (13–17 years) and young adults (18–24 years), and they remained significant in multivariate analysis, after adjustment for gender, country of birth, time lapse to first ART, number of different ART regimens, type of last regimen, and calendar time of latest VL measurement, with adjusted ORs of 7.0 [95% CI: 1.7; 30.0], 11.4 [95% CI: 4.2; 31.2] and 3.3 [95% CI: 1.03; 10.7] respectively for the [2–5 years], [13–17 years] and [18–24 years] strata (Fig. 3). Similar results were found in the sensitivity analysis restricted to participants with no regimen change in the last 6 months, with adjusted ORs of 12.7 [95% CI: 2.7; 60.1], 13.4 [95% CI: 4.3; 41.2] and 4.3 [95% CI: 1.1; 15.9] respectively.
Fig. 3.
Risk of presenting (a) a VL > 50 copies/mL, (b) a %CD4 > 25%, (c) a CD4:CD8 ratio >1, at the latest assessment, according to the period of HIV acquisition: perinatally (P-HIV) or during adulthood (A-HIV), stratified by duration of HIV-1 infection, in univariate and multivariate analyses∗–the French ANRS Coverte, CO10-EPF, Primo Seropri, Copana cohorts. ∗ Adjusted for: gender, country of birth, time-lapse between HIV acquisition and ART initiation, number of previous ART regimens, type of last regimen, and calendar period of last VL measurement).
Immunological status at the time of the latest VL measurement
Among P-HIV, 83.5% (218 of 261 with available measurements) had a CD4% ≥ 25% at the latest assessment (Supplementary Fig. S1). It was found for 91.7% and 93.2% of those aged [2–5] and [6–12], but dropped to 78.0%, and 77.5% in the [18–24] and [25–32] age groups respectively (chi-2 for trend, p = 0.11). After adjustment for demographic factors and therapeutic history, there was no statistically significant difference between P-HIV and A-HIV, whatever the duration of infection.
Compared to their A-HIV counterparts, P-HIV aged [6–12] and [13–17] were more likely to reach a CD4:CD8 ratio ≥1: 84.1% vs. 58.8% in the [6–12] year stratum (adjusted OR = 3.5 [1.5; 8.3]), and 60.9% vs. 54.7% in the [13–17] year stratum (adjusted OR = 1.9 [0.9; 4.2]) (Fig. 3). Compared to the youngest P-HIV, the proportion of participants with a CD4:CD8 ratio ≥1 was lower among P-HIV aged [18–24] and [25–32], but it was similar to that found among A-HIV with the same duration of infection: 32.8% vs. 48.1% (adjusted OR = 0.6 [0.3; 1.3]) and 39.2% vs. 45.7% (adjusted OR = 0.8 [0.3; 2.1]) respectively (Fig. 3).
Discussion
To our knowledge, this is the first study comparing the virological and immunological status of people living with perinatal HIV (P-HIV), at different key-periods in their physical and psychological development, to patients who acquired HIV during adulthood (A-HIV) with the same duration of infection, taking into account their therapeutic history, and using data from large paediatric and adult cohorts of 310 P-HIV and 1515 A-HIV.
The risk of virological failure (VF) at the latest visit was significantly higher among adolescents (13–17 years), young adults (18–24 years), and the youngest children (2–5 years) among P-HIV than among A-HIV with a similar duration of infection. This higher risk remained after adjustment for potential confounding factors, including therapeutic history and calendar time. Using the CDC Undetectable = Untransmittable (U U) threshold of 200 cp/mL, P-HIV adolescents and young adults remained at higher risk of VF than A-HIV, which raises concerns on the risk of onward transmission to sexual partners, and on their own immune deterioration (Supplementary Fig. S2).
We studied a large number of P-HIV individuals, with follow-up durations of up to 30 years, which corresponds to the first generation of adults living with HIV since birth or a very early age. We highlighted poor viral outcomes among both adolescents and young adults, with proportions of VF reaching 38.3% and 22.6% respectively. This is consistent with other studies reporting a greater risk of VF among P-HIV, particularly when they reach adolescence and adulthood.16,17 A VL ≥ 400 copies/mL was observed for 26% of adolescents on cART, transferred to adult care at a median age of 17.4 years.18 Older P-HIV (13–17 and 18–30-year-olds) were found to have spent more time with a VL ≥ 400 copies/mL and to be more exposed to non-suppressive cART, compared to 7–12-year-olds.19
The higher risk of VF among P-HIV than among A-HIV with the same duration of HIV infection could have several explanations. Therapeutic history differed between the groups, but the higher risk of VF in the P-HIV group remained significant after adjustment for the type of the last regimen, for the calendar time at last VL measurement under ART and the time-lapse between HIV acquisition and ART initiation, which together indirectly reflect the overall duration of the ART in each of the 5 age/infection duration strata. The difference in VF is thus not likely to be mainly explained by differences in effectiveness of the therapy received over time, or in the time-lapse between infection and first treatment. Moreover, in a context of free access to care and treatment, with a broad panel of antiretroviral drugs available, good viral control could be expected, whatever the treatment history. This was observed for A-HIV: more than 90% had a suppressed viral load, including those who had been living with HIV for the longest durations (25–32 years). We observed a decrease in VF prevalence in the more recent calendar times for both P-HIV and A-HIV participants, which is likely to be related to the availability of more potent, better tolerated drugs, including INSTI.20 However, the percentage of P-HIV with VF at the latest visit still reached 18.2% in the most recent calendar time (2016–2018), contrasting with the very low proportion (2.6%) among A-HIV subjects over the same period. Similar results for VF (at a threshold of 200 copies/ml) were found in the UK P-HIV cohort (median age: 23 years).21
We observed a larger proportion of VF in the youngest P-HIV group (2–5 years) than in the A-HIV group with the same duration of infection. A higher risk of VF among children aged under 3 years has already been reported.6 At this age, dependence on the caregiver (often the mother or other family members) and the social environment, difficulties related to drug administration, the palatability of formulations and the variability of ARV pharmacokinetics could explain these findings.
Teenagers and young adults in the P-HIV group also had poorer virological outcomes, while they had access to the same therapeutic options as the A-HIV group without the limitations of drug formulations. This is probably linked to complex issues in coping with HIV infection and its treatment, which car have a strong impact on treatment adherence.22 Adolescents and young adults are more likely to be less compliant.23 In HIV infection, specific factors also make adherence to treatment even more difficult than in other chronic conditions: reluctance to disclose HIV status, stigmatization, and disease denial.24 The transition from paediatric to adult healthcare provision could also be a destabilizing factor, because of the need to adapt to a new medical team and to changes in the physician-patient relationship. Adolescents and young adults living with HIV and moving from paediatric to adult care have been shown to be prone to VF.7
Among the P-HIV aged 25 years or over, which is a group that has been less widely studied in paediatric studies and has often included young people having acquired HIV through sexual relation, VF was less frequent than among adolescents and young adults having acquired HIV perinatally, but remained around 4- times higher than among A-HIV (p = 0.06).
The 6–12 year-old P-HIV group was the only age group with a fairly similar level of virological failure to the A-HIV group. Compared to the youngest children, their cognitive and emotional development could encourage medical teams and family to partially inform them about their virus to help coping with treatment, as recommended by French national guidelines in accordance with WHO for school-age children (6–12 years), before they enter the challenging period of adolescence. Partial diagnosis disclosure to children is reported to be associated with better adherence to treatment.25
Although each P-HIV age stratum size is fairly small, the statistical power was sufficient to reach statistical significance for such a large differences in viral failure proportion between P-HIV and A-HIV, in both univariate and multivariate analysis, in all strata, except in the 6–12 years stratum. For this school age children, odds ratios were much lower than in the other strata, which is consistent with a period that is possibly more favourable to a better adherence to treatment.
The poor virological control observed for P-HIV raises concerns about immune function. We found no significant difference in CD4% between the P-HIV and A-HIV groups, whatever the time lapse since HIV infection. In fact, P-HIV in the 6–12 and 13–17 age groups were more likely to exhibit a CD4:CD8 ratio ≥1 than their A-HIV counterparts. This difference between A-HIV and P-HIV was not observed in the [18–24] and [25–32] years groups, which could reflect a lesser potential for lymphopoiesis. This supports early ART initiation, as older P-HIV in our study started ART later. These results are consistent with the impact of greater thymic activity among children than among adults: among young P-HIV, the thymus produces new naïve T-cells which compensate for the destruction caused by HIV infection.15,26,27 While T-cell recovery after ART initiation is biphasic among adults, with a progressive repopulation phase of the naive cell pool following a fast phase of redistribution,28 it follows a more monophasic and uniform pattern among children throughout recovery.29 Higher and more lasting CD4+ cell counts and percentages have been reported among younger than older children at the time of initiation of cART.26,30,31
One study suggested that children starting ART at a younger age were more likely to recover a normal CD4:CD8 ratio, but the proportion of P-HIV in this study with a normal CD4:C8 ratio (62%) on ART (median age: 15.4 years) was compared to a global proportion reported in published studies on adults starting ART at the stage of chronic HIV infection.32 Here, using both paediatric and adult French cohorts coordinated by the same research unit, we were able to compare populations with similar durations of HIV infection and to adjust for known risk factors. We showed that P-HIV aged 6–12 and 13–17 were more likely to reach a normal CD4:CD8 ratio than A-HIV. It is worth noting that the proportion of A-HIV with a normal CD4:CD8 ratio was high in our study (62% of those with viral suppression), which is likely to be related to the large proportion of patients diagnosed and treated at the time of primary infection.33
The strength of our study lies in the large numbers of participants in the P-HIV and A-HIV groups, enrolled from several public hospital units across France, with prospective data collection over the long term, reaching nearly 30 years following HIV infection. All patients were in care through a network of hospital facilities and laboratories following national guidelines, and data was collected using standardised questionnaires in a similar way across the 5 cohorts, providing access to therapeutic, immunological and virological histories. We retained only patients with a short time to diagnosis, ≤24 months after HIV acquisition, which limited the potential selection bias of differential early access to treatment.
We acknowledge several limitations. Firstly, adherence and treatment resistance data, collected in a similar way, was not available to compare P-HIV and A-HIV. The national multicentre CO10-EPF cohort only collected routine annual medical follow-up of children up to the age of 17, to limit selection bias due to time consuming parental and clinician participation. Further qualitative or quantitative studies could focus more specifically on adherence data with ART drug blood level monitoring, global pill burden, or ART pharmaceutical formulation. Multivariate analyses were adjusted for the number of different previous ART regimens, more frequent among P-HIV, which could partly reflect past switches due to resistance.
Secondly, quantification thresholds for HIV plasma viral load assays have lowered over time; however, we analysed patients with the latest follow-up in 2012 or thereafter, thus limiting the problem of higher thresholds of quantification in earlier periods. Finally, the small number of P-HIV treated with INSTI as their first ART prevented us from assessing the impact of this potent class of ARV on the long-term incidence of VF. Further studies are needed to evaluate whether the expanded use of dolutegravir and bictegravir, various formulations (solid vs. liquid, single vs. multiple tablets) and new formulation (e.g., injectables) in the pediatric population could improve viral suppression among P-HIV.
In conclusion, maintaining virological suppression remains a challenge for patients having acquired HIV perinatally. We found they were at higher risk of VF than A-HIV with a similar duration of infection, even after adjusting for treatment history. This difference, which was the most marked for teenagers and young adults, is likely to result from problems of adherence specific to these periods in life. In a country which offers free access to care and to a large panel of ART, virologic control nevertheless remained more difficult to obtain among people living with HIV acquired perinatally than those living, with the same duration, with HIV acquired during adulthood. Although this did not translate into poorer immunological status, exposure to viral replication in these particular periods of life could have an impact on chronic inflammation, the development of comorbidities and the replenishment of the HIV reservoir, which are issues that warrant further studies. Our results also raise major concerns about the selection of resistance mutations and the risk of HIV transmission at critical times in life. Further studies are needed to assess the long-term impact of more potent and easier-to-take cART on VF among P-HIV subjects, with paediatric single-tablet regimens including INSTI.
Contributors
JW, CD, AF and JPV conceived and designed the paediatric cohorts, LM and CG conceived and designed the adult cohorts. RS, JW and JPV interpreted the findings and drafted the manuscript. RS and JJB carried out the current analysis. EA, JLC, FB and AE assessed and supervised the verification of the data in the paediatric and adult cohorts. PF, AF, CD, JLC, JJB, SB, CG, LM, TG, VAF were involved in revising the manuscript for important intellectual content. All authors had full access to all the data in the study, read and approved the final manuscript, and had final responsibility for the decision to submit for publication.
Data sharing statement
Data requests may be submitted to the scientific committee of the ANRS CO10-COverte-Primo studies (by email to josiane.warszawski@inserm.fr) and must be approved by the French data protection authority, la Commission Nationale de l’Informatique et des Libertes (CNIL). French law requires that everyone who wishes to access cohort data or clinical study data on humans must ask the French data protection authority (the CNIL) for permission. For further information, please see https://www.cnil.fr/. The ANRS CO10/Coverte/Primo scientific committees will evaluate each proposal for compatibility with general objectives, ethical approval, and informed consent forms of the cohort concerned, and for potential overlap with ongoing work.
Declaration of interests
LM has received grants from ANRS-MIE, SIDACTION, all of which were paid to her institution. PF has received grants from ANRS-MIE which were paid to his institution, personal fees from MSD France, ViiV Healthcare, Janssen Cilag Gilead Sciences, support for attending meetings/and or travel from MSD France, Gilead Sciences, ViiV Healthcare. PF reports to be EACS Panel member. RS has received grants from ANRS-MIE which were paid to her institution. JW has received grants from ANRS-MIE and Region Ile de France, all of which were paid to her institution.
All other authors declare no competing interests.
Acknowledgements
This study was supported by Inserm - ANRS MIE.
We thank also for their valuable contribution Nelly Briand, Laura Nailler, Mélanie Thoumine, Maud Brossard Alexandre Hoctin, Mathilde Ghislain, Camille Legeai, Olivia Dialla, Leila Ouzrout, Sandra Beramice, Geneviève Vaudre, Anne Persoz, Laurent Tran.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.100885.
Contributor Information
Josiane Warszawski, Email: josiane.warszawski@universite-paris-saclay.fr.
ANRS EPF:
H. Aumaitre, E. Froguel, F. Caby, S. Dellion, L. Gerard, F. Lucht, C. Chirouze, M. Dupon, Jl Schmit, C. Goujard, T. Allegre, B. Cazenave, G. Hittinger, P. De Truchis, J. Cailhol, C. Duvivier, A. Canestri, O. Bouchaud, M. Karmochkine, D. Salmon-Ceron, D. Zucman, E. Mortier, R. Tubiana, P.M. Girard, C. Pintado, A. Cabie, V. Rabier, P. Morlat, D. Neau, C. Genet, D. Makhloufi, S Bregigeon Ronot, J. Ghosn, V. Reliquet, P. Perré, Jl Pellegrin, C. Arvieux, C. Cheneau, L. Bernard, P. Delobel, R. Verdon, C. Jacomet, L. Piroth, F. Ajana, S. Bevilacqua, Y. Debab, A.L. Lecapitaine, L. Cotte, S. Mokhtari, P. Mercie, P. Poubeau, V. Garrait, Ma Khuong, G. Beck-Wirth, L. Blum, S. Blanche, F. Boccara, T. Prazuck, C. Barbuat, J.P. Viard, S. Stegmann-Planchard, B. Martha, J.M. Treluyer, E. Dore, C. Gaud, M. Niault, E. Fernandes, H. Hitoto, A. Compagnucci, N. Elenga, A. Faye, C. Dollfus, A. Chace, M. Levine, S.A. Martha, C. Floch-Tudal, K. Kebaïli, N. Entz-Werle, J. Tricoire, F. Mazingue, P. Bolot, P. Brazille, T. Goetghebuer, A.F. Gennotte, D. Van Der Linden, V. Schmitz, M. Moutschen, C. Crenn-Hebert, F. Habibi, A. Coursol, E. Guesdon, P.F. Ceccaldi, M. Dehlinger – Paul, E. Pannier, V. Marcou, J. Ghosn, V. Garrait, C. Elleau, M. Achkar, P. Delobel, M.O. Vareil, A. Chace, S. Couderc, C. Routier, M.A. Bouldouyre, F. Caby, L. Selleret, P. Bolot, A. Chabrol, C. Bellahcene, C. Pluchart, R. Tubiana, A. Yangui, D. Vignes, A. Alissa, A. Johnson, E. Lachassinne, A. Benbara, L. Karaoui, A. Bongain, B. Yakeu, J.L. Schmit, L. Cravello, C. Hubert, C. Dollfus, P. Faucher, D. Pinquier, C. Borie, D. Rocchi, C. Chirouze, C. Brunet-Cartier, C. Briandet, J. Brouard, A. Chalvon-Demersay, M. Rajguru, L. Bernard, K. Billiemaz, A. Fresard, A. Moulin, P. Fialaire, L. Mesnard, E. Werner, E. Vintejoux, J. Marian, S. Ranaivojaona, F. Bissuel, M. Abdelhadi, Y. Hammou, C. Genet-Villeger, Y. Hatchuel, N. Elenga, G. Hittinger, G. Bachelard, M. Medus, J. Dendale – Nguyen, T.S. Guimard, A. Martha, M. Rouha, P. Perfezou, L. De Saint Martin, S. Jaffuel, R. Buzele, C. Arvieux, M. Gousseff, C. Cudeville, M. Niault, V. Vitrat, C. Michau, G. Palenzuela, M. Driessen, B. Heller-Roussin, J.M. Labaune, B. Muanza, G. Hittinger, D. Makhloufi, J. Massardier, M. Partisani, C. Floch-Tudal, V. Marcou, I. Hau, C. Runel-Belliard, C. Brehin, A. Chace, K. Kebaili, M. Lalande, M. Lagree, N. Entz-Werle, K. Lacombe, J.-M. Molina, J. Ghosn, J. Reynes, O. Robineau, F. Raffi, P. Morlat, P. Delobel, A. Becker, C. Goujard, L. Weiss, T. Allègre, G. Pialoux, F. Souala, A. Rami, C. Katlama, A. Cabié, D. Makhloufi, J.-P. Viard, C. Cheneau, F. Bastides, D. Neau, H. Aumaitre, C. Duvivier, O. Bouchaud, P. Fialaire, L. Piroth, C. Michel, D. Salmon, J-D Le Lièvre, G. Hittinger, P. De Truchis, A. Sotto, C. Jacomet, E. Rouveix, A. Naqvi, D. Zucman, S. Brégigeon, R. Rodet, C. Chirouze, A. Simon-Coutelier, V. Garrait, J.-L. Esnault, E. Mortier, R. Buzelé, S. Bevilacqua, R. Verdon, A. Stein, C. Godin-Colet, G. Pichancourt, A. Chabrol, P. Caraux-Paz, M Mohseni Zadeh, L. Gérard, C. Lascaux-Cametz, L. Bodard, J.-L. Pellegrin, C. Genet, N. Ettahar, A. Uludag, F. Caby, E. Rosenthal, F. Prevoteau du Clary, A. Fresard, S. Jaureguiberry, L. Blum, P. Philibert, A.-L. Lecapitaine, Y. Debab, E. Chakvetadze, H. Champagne, M. Gousseff, E. Froguel, V. Daneluzzi, J. Goupil de Bouillé, A. Leprêtre, I. Lamaury, I. Darasteanu, B. Abraham, D. Garipuy, T. Prazuck, J.-L. Berger, J.-L. Schmit, K. Diallo, F. Gourdon, O. Vaillant, V. Gaborieau, B. Martha, J. Doll, D. Quinsat, L. Geffray, J.-J. Girard, D. Houlbert, C. Michau, B. Cazenave, V. Perronne, E. Klement, O. Antioniotti, C. Rouzioux, V. Avettand-Fenoel, O. Lortholary, J.P. Viard, S. Boucly, A. Maignan, C. Duvivier, R. Thiebaut, L. Meyer, F. Boufassa, M.A. Charles, R. Dray-Spira, C. Legeai, V. Amon, N. Benammar, R. Seng, G. Pialoux, L. Slama, P. Bonnard, C. Chakvetadze, T. L’Yavanc, J. Capeau, C. Vigouroux, S. Fellahi, J.P. Bastard, E. Oksenhendler, L. Gerard, J.F. Bourge, V. Bajzik, D. Sereni, C. Lascoux-Combe, C. Pintado, O. Taulera, L.V. Dien, J. Delgado, J.M. Molina, T. Saint-Marc, S. Ferret, J. Pavie, J.F. Bergmann, A. Rami, M. Parrinello, P.M. Girard, BLefebvre, C. Boudraa, B. Diallo, C. Lupin, S. Herson, A. Simon, N. Edeb, D. Salmon-Ceron, L. Guillevin, T. Tahi, M.P. Pietri, L. Weiss, D. Tisne-Dessus, C. Jalbert, P. Yeni, S. Matheron, G. Pahlavan, B. Phung, N. El-Alami Talbi, Z. Ramani, G. Catalano, C. Godard, F. Boue, V. Chambrin, D. Bornarel, H. Schoen, R. Carlier, B. Fantin, A. Uludag, C. Poder, R. Dhote, M. Bentata, P. Honore, O. Bouchaud, Xuan Tuyet, J.F. Delfraissy, C. Goujard, F. Chaix, M.T. Rannou, Y. Levy, A. Sobel, C. Dumont, A. Cabie, S. Abel, S. Pierre-François, V. Beaujolais, I. Poizot-Martin, O. Zaegel-Faucher, C. Debreux, J. Moreau, S. Mokhtari, E. Van Der Gheynst, M.C. Thiebaut-Drobacheff, A. Foltzer, B. Hoen, J.F. Faucher, H. Gil, M. Dupon, J.M. Ragnaud, I. Raymond, P. Morlat, I. Louis, M. Hessamfar, J. Reynes, V. Baillat, C Merle De Boever, C. Tramoni, A. Soufflet, P. Guadagnin, F. Bastides, P. Choutet, L. Bernard, F. Raffi, O. Mounoury, V. Reliquet, D. Brosseau, H. Hue, T. May, S. Wassoumbou, M. Stenzel, M.P. Bouillon, Y. Yazdanpanah, T. Huleux, E. Aissi, S. Pavel, D. Rey, C. Cheneau, P. Fischer, M. Partisani, G. Blaison, M Mohseni Zadeh, M. Martinot, A. Pachart, F. Jeanblanc, J.L. Touraine, C. Trepo, P. Miailhes, K. Kouadjo, V. Thoirain, C. Brochier, P. Perre, S. Leautez, J.L. Esnault, and I. Suaud
Appendix A. Supplementary data
References
- 1.de Martino M., Tovo P.A., Balducci M., et al. Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection. Italian Register for HIV Infection in Children and the Italian National AIDS Registry. JAMA. 2000;284:190–197. doi: 10.1001/jama.284.2.190. [DOI] [PubMed] [Google Scholar]
- 2.Gortmaker S.L., Hughes M., Cervia J., et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345:1522–1528. doi: 10.1056/NEJMoa011157. [DOI] [PubMed] [Google Scholar]
- 3.Gibb D.M., Duong T., Tookey P.A., et al. Decline in mortality, AIDS, and hospital admissions in perinatally HIV-1 infected children in the United Kingdom and Ireland. BMJ. 2003;327:1019. doi: 10.1136/bmj.327.7422.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sánchez J.M., Ramos Amador J.T., Fernández de Miguel S., et al. Impact of highly active antiretroviral therapy on the morbidity and mortality in Spanish human immunodeficiency virus infected children. Pediatr Infect Dis J. 2003;22:863–867. doi: 10.1097/01.inf.0000091282.70253.5f. [DOI] [PubMed] [Google Scholar]
- 5.Slogrove A.L., Schomaker M., Davies M.A., et al. The epidemiology of adolescents living with perinatally acquired HIV: a cross-region global cohort analysis. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunupuradah T., Sricharoenchai S., Hansudewechakul R., et al. Risk of first-line antiretroviral therapy failure in HIV-infected Thai children and adolescents. Pediatr Infect Dis J. 2015;34:e58–e62. doi: 10.1097/INF.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 7.Weijsenfeld A.M., Smit C., Cohen S., et al. Virological and social outcomes of HIV-infected adolescents and young adults in the Netherlands before and after transition to adult care. Clin Infect Dis. 2016;63:1105–1112. doi: 10.1093/cid/ciw487. [DOI] [PubMed] [Google Scholar]
- 8.Suaysod R., Ngo-Giang-Huong N., Salvadori N., et al. Treatment failure in HIV-infected children on second-line protease inhibitor-based antiretroviral therapy. Clin Infect Dis. 2015;61:95–101. doi: 10.1093/cid/civ271. [DOI] [PubMed] [Google Scholar]
- 9.Mu W., Bartlett A.W., Bunupuradah T., et al. Early and late virologic failure after virologic suppression in HIV-infected Asian children and adolescents. J Acquir Immune Defic Syndr. 2019;80:308–315. doi: 10.1097/QAI.0000000000001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabin C.A., Smith C.J., d’Arminio Montforte A., et al. Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group Response to combination antiretroviral therapy: variation by age. AIDS. 2008;22:1463–1473. doi: 10.1097/QAD.0b013e3282f88d02. [DOI] [PubMed] [Google Scholar]
- 11.Judd A., Lodwick R., Noguera-Julian A., et al. Higher rates of triple-class virological failure in perinatally HIV-infected teenagers compared with heterosexually infected young adults in Europe. HIV Med. 2017;18:171–180. doi: 10.1111/hiv.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han M., Law M.G., Egger, et al. Global estimates of viral suppression in children and adults on antiretroviral therapy adjusted for missing viral load measurements: a multiregional, retrospective cohort study in 31 countries. Lancet HIV. 2021;8:e766–e775. doi: 10.1016/S2352-3018(21)00265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinmann G.G., Klaus B., Müller-Hermelink H.K. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985;22:563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 14.Bains I., Antia R., Callard R., Yates A.J. Quantifying the development of the peripheral naive CD4+T cell pool in humans. Blood. 2009;113:5480–5487. doi: 10.1182/blood-2008-10-184184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanche S., Scott-Algara D., Le Chenadec J., et al. Naive T lymphocytes and recent thymic emigrants are associated with HIV-1 disease history in French adolescents and young adults infected in the perinatal period: the ANRS-EP38-IMMIP study. Clin Infect Dis. 2014;58:573–587. doi: 10.1093/cid/cit729. [DOI] [PubMed] [Google Scholar]
- 16.Dollfus C., Le Chenadec J., Faye A., et al. Long-term outcomes in adolescents perinatally infected with HIV-1 and followed up since birth in the French perinatal cohort EPF/ANRS CO10) Clin Infect Dis. 2010;51:214–224. doi: 10.1086/653674. [DOI] [PubMed] [Google Scholar]
- 17.Nachega J.B., Hislop M., Nguyen H., et al. Antiretoviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in Southern Africa. J Acquir Immune Defic Syndr. 2009;51(1):65–71. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins I.J., Foster C., Tostevin A., et al. Clinical status of adolescents with perinatal HIV at transfer to adult care in the UK/Ireland. Clin Infect Dis. 2017;64:1105–1112. doi: 10.1093/cid/cix063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neilan A.M., Karalius B., Patel K., et al. Association of risk of viremia, immunosuppression, serious clinical events, and mortality with increasing age in perinatally Human Immunodeficiency Virus infected youth. JAMA Pediatr. 2017;171:450–460. doi: 10.1001/jamapediatrics.2017.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nance R.M., Delaney J.A.C., Simoni J.M., et al. HIV viral suppression trends over time Among HIV Infected patients receiving care in the United States, 1997 to 2015: a Cohort Study. Ann Intern Med. 2018;169:376–384. doi: 10.7326/M17-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster C., Ayers S., Mcdonald S., et al. Clinical outcomes post transition to adult services in young adults with perinatally acquired HIV infection: mortality, retention in care and viral suppression. AIDS. 2020;34(2):261–266. doi: 10.1097/QAD.0000000000002410. [DOI] [PubMed] [Google Scholar]
- 22.Kim S.H., Gerver S.M., Fidler S., Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS. 2014;28:1945–1956. doi: 10.1097/QAD.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taddeo D., Egedy M., Frappier J.Y. Adherence to treatment in adolescents. Paediatr Child Health. 2008;13:19–24. doi: 10.1093/pch/13.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L., Munir K., Kanabkaew C., Le Coeur S. Factors influencing antiretroviral treatment suboptimal adherence among perinatally HIV-infected adolescents in Thailand. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odiachi A. The impact of disclosure on health and related outomes in human immunodeficiency virus-infect children a literature review. Front Public Health. 2017;5:231. doi: 10.3389/fpubh.2017.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibb D.M., Newberry A., Klein N., de Rossi A., Grosch-Woerner I., Babiker A. Immune repopulation after HAART in previously untreated HIV-1-infected children. Paediatric European network for treatment of AIDS (PENTA) steering committee. Lancet. 2000;355:1331–1332. doi: 10.1016/s0140-6736(00)02117-6. [DOI] [PubMed] [Google Scholar]
- 27.De Rossi A., Walker A.S., Klein N., De Forni D., King D., Gibb D.M. Increased thymic output after initiation of antiretroviral therapy in human immunodeficiency virus type 1-infected children in the Paediatric European Network for Treatment of AIDS (PENTA) 5 Trial. J Infect Dis. 2002;186:312–320. doi: 10.1086/341657. [DOI] [PubMed] [Google Scholar]
- 28.Pakker N.G., Notermans D.W., de Boer R.J., et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 29.Picat M.Q., Lewis J., Musiime V., et al. Predicting patterns sof long-term CD4 reconstitution in HIV Infected children starting antiretroviral therapy in Sub-Saharan Africa: a cohort-based modelling study. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simms V., Rylance S., Bandason T., et al. CD4+ cell count recovery following initiation of HIV antiretroviral therapy in older childhood and adolescence. AIDS. 2018;32:1977–1982. doi: 10.1097/QAD.0000000000001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker A.S., Doerholt K., Sharland M., Gibb D.M. Collaborative HIV paediatric study (CHIPS) steering committee. Response to highly active antiretroviral therapy varies with age: the UK and Ireland collaborative HIV paediatric study. AIDS. 2004;18:1915–1924. doi: 10.1097/00002030-200409240-00007. [DOI] [PubMed] [Google Scholar]
- 32.Seers T., Vassallo P., Pollock K., Thornhill J.P., Fidler S., Foster C. CD4:CD8 ratio in children with perinatally acquired HIV-1 infection. HIV Med. 2018;19:668–672. doi: 10.1111/hiv.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thornhill J., Inshaw J., Kaleebu P., et al. Brief Report: enhanced normalization of CD4/CD8 ratio with earlier antiretroviral therapy at primary HIV infection. J Acquir Immune Defic Syndr. 2016;73:69–73. doi: 10.1097/QAI.0000000000001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.