Abstract
An increased urinary albumin excretion rate is an important early risk factor for chronic kidney disease and other major outcomes and is usually measured using the urinary albumin-creatinine ratio (ACR). Obesity is highly prevalent in the general and chronic kidney disease populations and is an independent risk factor for moderately increased albuminuria (henceforth, moderate albuminuria). In this review, we describe how the ACR was developed and used to define moderate albuminuria. We then investigate how biases related to urinary creatinine excretion are introduced into the ACR measurement and how the use of the 30-mg/g threshold decreases the performance of the test in populations with higher muscle mass, with a primary focus on why and how this occurs in the obese population. The discussion then raises several strategies that can be used to mitigate such bias. This review provides a comprehensive overview of the medical literature on the uses and limitations of ACR in individuals with obesity and critically assesses related issues. It also raises into question the widely accepted 30-mg/g threshold as universally adequate for the diagnosis of moderate albuminuria. The implications of our review are relevant for clinicians, epidemiologists, and clinical trialists.
Introduction
Obesity is highly prevalent in the chronic kidney disease (CKD) population and is a major mediator for CKD.1,2 An increased urinary albumin excretion rate (AER) is an important early indicator of CKD and a major end point in kidney-oriented clinical trials.3 Obesity is an independent risk factor for moderately increased albuminuria (henceforth, moderate albuminuria),4,5 historically termed as microalbuminuria.6 Moderate albuminuria, defined as an AER ≥30 mg/d, predicts CKD stages 3 to 5, cardiovascular disease, and cardiovascular and overall mortality.7, 8, 9, 10, 11, 12, 13, 14 Early detection of an increased AER throughout the spectrum can help prompt interventions to improve outcomes. Thus, an accurate measurement of the AER is necessary for risk stratification, optimal clinical care, and advances in kidney-related research. This review will discuss the most common strategy used to measure moderate albuminuria—the urinary albumin-creatinine ratio (ACR) —and explain why its use in individuals with obesity is fraught with biases that reduce its performance.
Measuring and Defining Moderate Albuminuria
The gold standard method for measuring albuminuria involves adequately performed 24-hour urine collections. However, a timed urine collection is cumbersome to perform and prone to error from incomplete collections. Measurement of the ACR in a spot urine sample has therefore been widely adopted to estimate albuminuria as an alternative to 24-hour urine collection because of its ease and simplicity.15, 16, 17, 18, 19, 20, 21, 22, 23, 24
Most guidelines consider an ACR of ≥30 mg/g15, 16, 17,19,21,24 as the threshold for diagnosing moderate albuminuria. However, a lower ACR cutoff of >2 mg/mmol (ie, >18 mg/g) was recently recommended by Canadian guidelines,22 whereas a 2.5- to 3.5-mg/mmol (ie, 21-31 mg/g)20 cutoff was proposed by European guidelines. KDIGO guidelines15,16 recommend a 30-mg/g threshold for moderate albuminuria, which in the international system of units is equivalent to 3.4 mg/mmol. However, this international system of unit threshold was rounded out to 3 mg/mmol, which is equivalent to 26.5 mg/g, “for simplicity and to reflect that the chosen threshold is an approximation” (see Table 1 for definitions).15 Consequently, the recommended KDIGO threshold is lower when the international system of unit is used as in Europe or Canada than when the conventional unit system is used as in the United States. This difference in recommended thresholds is expected to affect the performance of ACR as a diagnostic test for moderate albuminuria.
Table 1.
Commonly Used Classifications for Obesity and Albuminuria
| Body mass index56 (kg/m2) | ||
| Underweight | <18.5 | |
| Healthy | ≥18.5 to 24.9 | |
| Overweight | ≥25.0 to 29.9 | |
| Obese | ≥30 | |
| Class I | 30.0-34.9 | |
| Class II | 35.0-39.9 | |
| Class III | ≥40 | |
| Albuminuria15 (mg/d) | ||
| A1: Normal to mildly increased | <30 | |
| A2: Moderately increased | 30-300 | |
| A3: Severely increased | >300 | |
| Albumin-to-creatinine ratio15 | mg/g | mg/mmol |
| A1: Normal to mildly increased | <30 | <3 |
| A2: Moderately increased | 30-300 | 3-30 |
| A3: Severely increased | >300 | >30 |
Development of the Urinary ACR
Fifty-three years ago, Barrat et al25 first reported in 57 children and adults that the spot urinary ACR concentration correlates well with albuminuria after adjusting for body weight (r = 0.96). The rationale for adjusting for the urinary creatinine concentration was that creatinine served “as a reference standard by virtue of its relatively constant rate of excretion.”26 That is, the advantage of ACR over measuring the urinary albumin concentration alone is that it corrects for effects of urine concentration and dilution. The ratio of albuminuria to creatininuria,
in which U stands for urinary, AER for the albumin excretion rate, and CER for the creatinine excretion rate. Because the ACR equals the ratio of albuminuria to creatininuria, ACR is an accurate predictor of albuminuria only if the inter- and intraindividual variability of its denominator, CER, is minimal.
Obesity and Other Factors That Influence the CER
Under steady-state conditions, creatinine excretion reflects creatinine generation. Most urinary creatinine originates from the conversion of creatine and phosphocreatine in muscle and its subsequent release into the extracellular compartment.27 Because about 98% of total body creatinine is found in muscles28 and because its conversion to creatinine occurs at a steady rate and is irreversible in vivo,27 the urinary excretion rate of creatinine mostly reflects muscle mass.28,29 Thus, expressing albumin excretion per unit of creatinine implies adjusting it per unit of muscle mass, as articulated by Fotheringham et al.30
Muscle mass markedly varies among adults and urine CERs follow this pattern. Table S1 and Fig S1 summarize 12 reports31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 analyzing data collected from 131,562 women and men who were enrolled in 18 large observational studies or clinical trials. The mean CER was 1351 ± 104 mg/d and the mean standard deviation was 428 mg ± 35 mg/d. The CER interval including approximately 95% of the participants (see detailed methods in Item S1) is broad, with the mean interval upper boundary being more than 4 times that of its lower limit. The consequence of this high interindividual CER variability on the ACR measurement derives from the mathematical relationship between the terms in the ratio—albumin and creatinine. For example, a given ACR of 20 mg/g obtained in 2 persons, one excreting 700 mg and the other 2,100 mg creatinine per day, will reflect an AER of 14 mg/d and 42 mg/d, respectively. A prime example of how muscle mass influences urinary CERs is the well-described difference between the sexes. Owing to greater muscle mass, men excrete 47% more creatinine than women per unit of time, as demonstrated in Table S2.31,34, 35, 36, 37, 38, 39,41, 42, 43, 44 As seen in Table S3 and Fig S2, CER distribution is about 40% wider in mixed male and female populations than in groups separated by sex.31,34, 35, 36, 37, 38, 39,41,42 Age is an important predictor of the CER, in which an older age is associated with reduced creatinine excretion owing to the sarcopenia of aging.35,45,46 Chronic illness may also lead to a loss of skeletal muscle mass and thus lower the CER.47,48
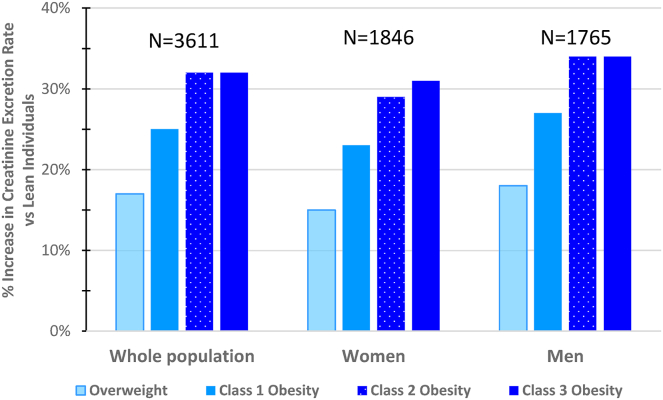
Although obesity’s effects on muscle mass and consequently the CER are large, it has drawn much less attention than the factors mentioned above. Body composition studies demonstrate that obesity is associated with increased lean body mass49, 50, 51, 52 and skeletal muscle mass.52 Studies using urinary creatinine as a marker of muscle mass37,39,53,54 have confirmed this phenomenon (Table S4 and Fig 1). Analyzing data from 3,611 participants of the Chronic Renal Insufficiency Cohort (CRIC) study, Fotheringham et al53 showed that the CER increases with body mass index (BMI) and waist circumference in both men and women. After adjustment for confounders, the CER was 17% and 28% higher in overweight and obese patients, respectively. A relationship of similar direction and amplitude was found between CER and more specific markers of obesity than BMI, namely waist circumference and fat mass. Abdelmalek et al37 confirmed that CER rises in conjunction with body weight in a population-based study that included 2,711 participants, with the middle and upper tertiles of body weight (mean BMI, 25.5 and 29.3 kg/m2) having 22% and 46% higher CERs, respectively, than the lower tertile (mean BMI, 22.8 kg/m2). Ogna et al54 found qualitatively similar results in a large cohort of healthy Swiss individuals. A fourth study by Johner et al39 confirmed the relationship between obesity and urinary creatinine, although with more modest differences. The aggregate mean increase in CER associated with obesity in the 9,124 women and men enrolled in these 4 studies was 32%.
Figure 1.
Increase in creatinine excretion rates in overweight and obese, compared with lean individuals. Studies: CRIC study53; PREVEND study37; VERA study39; Swiss Salt Survey54. Abbreviations: CRIC, Chronic Renal Insufficiency Cohort; PREVEND, Prevention of Renal and Vascular Endstage Disease; VERA, Verbundstudie Ernährungserhebung und Risikofaktoren-Analytik.
Markers of Obesity and Variability in CER
Of the 4 studies reviewed above, 339,53,54 used BMI to characterize body mass distribution. The fourth37 classified participants according to bodyweight tertiles while also providing data on BMI per tertile. BMI is the most commonly used first step in classifying people as underweight, healthy weight, overweight, or obese (Table 1).56 However, body weight (and consequently BMI) does not distinguish between muscle mass-related and adipose tissue-related body weight. Thus, individuals with high muscle mass and no excess adiposity may have an increased BMI and vice versa. In both situations, waist measurement will correct the diagnosis. Despite this limitation, BMI at the population level is an adequate measure of adiposity.55 Fotheringham et al53 demonstrated that the urinary CER increases with increasing adiposity levels, regardless of whether assessed based on BMI, waist circumference, or bioimpedance (Fig 2).
Figure 2.
Increase in creatinine excretion rates based on categories of BMI in women and men in the CRIC study, compared with lean individuals. BMI categories: lean, 18.5 to <25 kg/m2; overweight, 25 to <30 kg/m2; class 1 obesity, 30 to <35 kg/m2; class 2 obesity, 35 to <40 kg/m2; class 3 obesity, ≥40 kg/m2. Data were retrieved from the study by Fotheringham et al.53 Abbreviations: BMI, body mass index; CRIC, Chronic Renal Insufficiency Cohort.
Higher CERs and Bias in Obese Populations
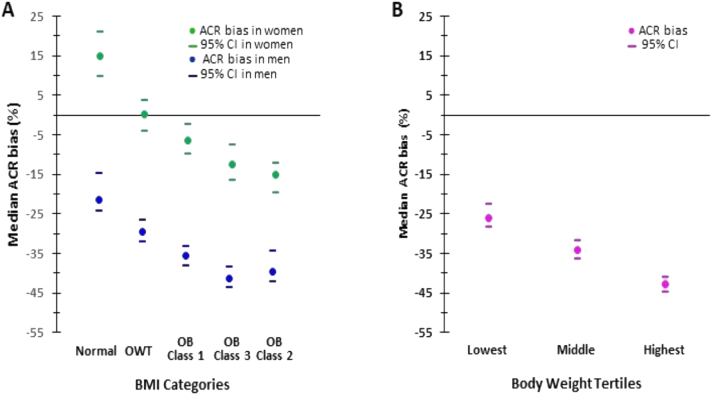
Any increase in the CER, including that owing to obesity, is expected to lead to an underestimation of albuminuria when evaluated with ACR.57 This effect was indeed observed in a secondary analysis of the Chronic Renal Insufficiency Cohort study,53 which showed that greater adiposity was associated with an increased CER and underestimation of albuminuria by ACR. An ACR bias that was positive in lean women became negative (+15% to -15%) as the BMI rose from the healthy range to class 3 obesity. An ACR bias, which was already negative in lean men, decreased further from -21% to ∼-40% as the BMI rose from the healthy range to class 2-3 obesity (Fig 3A). Abdelmalek et al37 observed a similar pattern, with an underestimation bias worsening from -26% to -43% with increasing body weight tertiles in the general population (Fig 3B).
Figure 3.
Bias in ACR based on body size and sex categories. Panel A was created from data from the study by Fotheringham et al53. In women, bias is positive at lean weight, nil at overweight, and negative in obesity, becoming more negative as obesity class escalates. In men, the underestimation bias, already apparent in lean men, worsens in magnitude as BMI increases from the lean range to class 2 obesity, in which it reaches a plateau. Panel B was created from data from the study by Abdelmalek et al37. An underestimation bias, apparent in the lower body weight tertile group, worsens with increasing body weight tertiles. Lowest tertile: <70.6 kg, BMI 22.8 ± 2.3; middle tertile: 70.6-82 kg, BMI 25.5 ± 2.5; upper tertile: >82 kg, BMI 29.3 ± 3.8. ACR bias was expressed as the percentage difference between ACR (mg/g) and measured 24-hour albuminuria (mg/24 h), with negative values indicating underestimation of albuminuria. Abbreviations: ACR, albumin-to-creatinine ratio; BMI, body mass index; CI, confidence interval; OB, obesity; OWT, overweight.
The bias in ACR introduced by obesity may affect clinical decision making and data interpretation in clinical and epidemiologic studies. AER13 and ACR14,58 both predict negative outcomes including all-cause mortality, with risks rising at albuminuria levels above 5-10 mg/d or mg/g. Obesity-related biases lead to the underestimation of risks though the clinical consequences may differ based on patient. For example, an obese man with an AER of 120 mg/d and a CER of 2,000 mg/d will have an ACR of 60 mg/g. This bias will not affect management because both AER and ACR are defined as moderately increased albuminuria. However, if this individual has an AER of 40 mg/d, the ACR will be 20 mg/g, which is below the moderately increased albuminuria range, as illustrated in Fig 4. According to guidelines, this ACR category does not require the same interventions as those used for moderately increased albuminuria, including mineralocorticoid or renin-aldosterone antagonists for reno- and/or cardiovascular protection59, 60, 61 (Box 1). Thus, this person will be undertreated despite having an AER in the higher-risk moderately increased range.13 Interestingly, when examined as a continuous variable, ACR has a less impactful bias than when viewed categorically.
Figure 4.
Expected misdiagnosis of moderate albuminuria based on sex according to various recommended cutoffs of urinary albumin and creatinine excretion.
Panels A and D show the expected misdiagnosis of moderate albuminuria for women and men, respectively, based on varying AERs and creatinine excretion rates using the 30-mg/g ACR cutoff. Panels B and E show these results using the 26.5-mg/g (3 mg/mmol) ACR cutoff, which is the international system of unit alternative threshold recommended by KDIGO (Kidney Disease: Improving Global Outcomes [KDIGO] CKD Work Group) for the diagnosis of moderate albuminuria.15 Panels C and F display misdiagnoses using the 25-mg/g ACR cutoff for women and 17-mg/g cutoff for men. Varying AERs are shown on the x-axis, whereas varying creatinine excretion rates are shown on the y-axis. The numbers in the areas defined by these axes represent ACR (mg/g) for different AER and urinary creatinine excretion rates. The proportion of the chart quadrant appearing in a pink background reflects the magnitude of moderate albuminuria underdiagnosis. It is highest when a 30-mg/g ACR cutoff is used and decreases as cutoffs lower, for both women and men. The extent of moderate albuminuria underdiagnosis is more frequent in men compared with women with all cutoffs. However, the difference between women and men is minimal when the lowest sex-specific cutoffs are used. The chart areas delimited by black dot lines include the ACR values for women and men with an AER between 30 and 70 mg/d. The proportion of false-negative ACR results (red cells) within this AER range is 17% in women and 48% in men when the 30-mg/g ACR cutoff is used. This proportion decreases in women to 11% and 8% with the 26.5-mg/g (3 mmol/g) and the 25-mg/g cutoffs, respectively. In men, the false-negative rate decreases to 38% and to 7% with the 26.5- (3 mmol/g) and the 17-mg/g cutoffs, respectively. It should be noted that these rates do not represent the proportion of false-negative tests in a given population because the relative prevalence of each ACR value is not known. The creatinine excretion rate range displayed in the charts is different for women and men because it reflects the average interval comprising 95% of the population as reported in 11 studies summarizing data from 131,845 participants enrolled in 17 cohorts (Table S2). The lower and upper limits of the intervals were rounded to the nearest hundreds. Abbreviations: ACR, albumin-to-creatinine ratio; AER, albumin excretion rate.
Box 1. Expected Benefits of Reclassifying Obese Individuals With CKD From the “Normal to Mildly increased” to the “Moderately Increased” Albuminuria Categories (A1-A2), According to KDIGO Guidelines16,62.
-
•
Pharmacologic treatment of hypertension in nondiabetic and diabetic patients with moderately increased albuminuria: ACEI/ARB treatment will be the first-line pharmacologic treatment at any eGFR level above 15 mL/min/1.73 m2 (grade 1B recommendation). In contrast, renin-angiotensin inhibition is not mandatory when AER is below the moderately increased level (A1 category).
-
•
Pharmacologic treatment of CKD in diabetic patients with an eGFR above 25 mL/min/1.73 m2 and moderately increased albuminuria: the presence of moderate albuminuria or above, despite treatment with ACEI/ARB and SGLT2i, is an indication for a nonsteroidal mineralocorticoid receptor antagonist (grade 2A recommendation).
-
•
Pharmacologic treatment of CKD in normotensive diabetic patients with moderately increased albuminuria: ACEI/ARB treatment may be considered (ungraded practice point).
-
•
Change in risk category: as compared with the “normal to mildly increased albuminuria” stage, moderately increased albuminuria is associated at any eGFR category with an increase in relative risk of kidney failure (need for dialysis or transplantation), cardiovascular disease, and mortality.
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AER, albumin excretion rate; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; SGLT-2i, sodium-glucose cotransporter 2 inhibitor.
Figure 4A-F illustrates how the 30-mg/g threshold for moderate albuminuria leads to a greater magnitude of misdiagnosis of this condition in men and women as CER increases, depending on CER and AER. As specified earlier, CER is higher in obese versus lean people.37,39,53,54 CER data collected from a general population cohort37 showed that the upper body weight tertile subgroup had a mean excretion rate of ∼1,650 mg/d and a mean + 2 SD value of ∼2,470 mg/d. Values for the corresponding measures in an obese male cohort39 were ∼1,920 and ∼2,950 mg/d. As shown in Fig 4, everyone with a CER of ≥1,200 mg/d (ie, most obese people) and an AER of 30-35 mg/d will be erroneously diagnosed as not having moderate albuminuria when the 30-mg/g ACR cutoff is used. This bias may lead to undertreatment of obese patients. Additionally, it may reduce their eligibility for clinical trials because enrollment of CKD patients often includes an ACR above a given threshold.3,63,64 The consequence of using an ACR threshold without considering the interindividual muscle mass variability is that a lean participant with an AER of 500 mg/d has a higher probability of being enrolled in a trial enrolling people with an ACR requirement of above 300 mg/g than an obese patient with a similar AER.
ACR has large (median 33%) day-to-day intraindividual variability, with lower levels seen in early morning.65 This biological variability occurs randomly and may thus be overcome by repeating measurements. In contrast, the 40%-43% obesity-related bias37,53 is a systematic error that will consistently lead to AER underestimation.
Consequences of Biases on the Performance of ACR in Diagnosing Moderate Albuminuria
Studies evaluating the validity of ACR30 mg/g (ACR with a 30-mg/g cutoff) in diagnosing moderate albuminuria in the general population and cohorts with diabetes mellitus and CKD showed that sensitivity ranged between 64% and 94%.30,36,66, 67, 68 The relatively low sensitivity of ACR using a 30-mg/g cutoff results from insufficient accuracy. Fotheringham et al,30 measuring accuracy in terms of the percentage of individuals with ACR measurements within 30% of the measured AER, showed that only 49% and 38% of participants in the CRIC and Diabetes Control and Complications Trial (DCCT) studies, respectively, had ACR values within this range. This study highlights that large discrepancies may exist in the performance of surrogate and gold standard kidney parameters between individuals and populations.
To our knowledge, no study has defined optimal cutoffs for the diagnosis of moderate albuminuria in obese individuals. Because the difference in CERs between obese and lean people is of similar magnitude to that between men and women, data on the sex-related sensitivity of ACR may shed light on adiposity-related differences in test sensitivity. Two studies evaluating the performance of ACR30 mg/g in detecting moderate albuminuria in 3 groups with diabetes mellitus,30 CKD,30 and CKD after transplantation36 showed 4%-11% lower sensitivity in men versus women. Studies evaluating optimal sex-related thresholds for ACR consistently revealed lower optimal ACR cutoffs for men than for women (17-26 mg/g for men vs 24-35 mg/g for women30,32,36,69, 70, 71), this sex-related difference being the consequence of difference in muscle mass. Three studies30,36,72 involving 2 large cohorts with CKD and diabetes,30 a population of renal transplant recipients,36 and an Indo-Asian group recruited from the general population72 showed that sex-associated thresholds (ie, 17 mg/g for men and 25 mg/g for women)69 improve ACR sensitivity in both sexes, as compared with the 30-mg/g cutoff. Figure 4 illustrates the improved ACR sensitivity conferred by sex-specific thresholds for given creatinine excretion and AER levels. The predictive value of albuminuria defined using sex-related ACR thresholds for cardiovascular disease58,73 and visceral obesity74 was demonstrated among the Framingham Offspring Study participants. Of note, although a low CER independently predicts adverse outcomes,34,75 there are conflicting data over whether ACR, which reflects both urinary albumin and creatinine excretion, has a more predictive power compared with creatinine excretion alone.76,77
Consequences of Intraindividual Variability of CER in Obesity
In contrast to the limitations of ACR as a screening test for moderate albuminuria in the obese population, its use is adequate for following changes in albuminuria over time and assessing responses to therapeutic interventions as long as body weight and muscle mass are relatively stable. However, following a significant weight loss, ACR results must be reinterpreted with the understanding that weight loss involves losing both fat and lean body mass.78, 79, 80, 81, 82, 83 The marked loss in muscle mass following bariatric/metabolic surgery leads to a drop in creatinine generation and excretion. Three studies reported on the change in CER during the first year following bariatric/metabolic surgery. CER decreased by 24%-26% at 6-10 months84,85,95 and 18%-23% at 12 months.84,85 The inevitable postoperative reduction in creatinine excretion leads to an overestimation of albuminuria based on ACR and an underestimation of the effect of the intervention on lowering albuminuria. Thus, an obese patient with 200-mg/d AER and 2,500-mg/d CER before bariatric/metabolic surgery may 6 months postsurgery have an AER of 100 mg/d and a CER of 2,000 mg/d. Although albuminuria has decreased by 50%, based on ACR, the decrease would only be 38% (ie, ∼80 mg/g and ∼50 mg/g before and after surgery, respectively). Similarly, an ACR that remains unchanged following bariatric/metabolic surgery obscures a reduction in albuminuria. This effect is also relevant to ongoing or future clinical trials of new and highly effective antiobesity drugs that are designed to evaluate for possible renoprotective effects.86,87 Any loss of muscle with weight loss could result in miscategorizing such treatments as having lesser or no benefit in reducing albuminuria.
Approaches to Mitigate ACR-Related Biases in Obese People
The appropriateness of the 30-mg/g ACR threshold for the diagnosis of moderate albuminuria has been controversial for almost 3 decades.57,69 Of note, the first study that evaluated the relationship between ACR and albuminuria enrolled children and adults,25 and the second, children88 only. In these studies, the authors adjusted AER for body size using body weight or body surface area. Because these factors are related to muscle mass, these initial studies indirectly adjusted for CER. The third such study,89 which included diabetic adults, showed that a 31-mg/g threshold for ACR performed well for the diagnosis of an AER of above 43 mg/d (30 μg/min) but not 30 mg/d. Later studies evaluating ACR performance in adults dropped body size adjustment for pragmatic reasons and used ACR to diagnose moderate albuminuria defined as albuminuria above 30 mg/d. ACR-related biases do not imply that this convenient test is intrinsically inadequate for the diagnosis of moderate albuminuria. Its performance may be improved by recognizing that the wide interindividual creatinine excretion variability implies that a given ACR value has different implications in people with different muscle mass.
Two approaches have been used to improve the suboptimal performance of ACR, both of which adjust for the interindividual variability of muscle mass and CER. The first approach adjusts cutoffs according to demographic parameters, including sex, that predict creatinine excretion. Sex-specific ACR cutoffs (17 mg/g for men and 25 mg/g for women) improve ACR performance, as shown earlier in this review. These sex-associated cutoffs represent a significant step forward in adjusting for interindividual variations in CER. Optimally adjusted cutoffs would also require adjustment for other predictors of muscle mass, such as age, adiposity, and comorbid conditions.
Another approach to improving ACR performance is to estimate AER for each individual, with estimated AER (eAER) being equal to ACR multiplied by an estimated CER. Three prediction equations for the estimation of CER30,35,90 based on demographic factors related to creatinine generation were developed using data collected from CKD30,35 and non-CKD populations.90 Fotheringham et al30 and Abdelmalek et al37 applied these 3 equations to a general population cohort37 and 2 populations with CKD or diabetes.30 These analyses demonstrated improved accuracy and decreased biases for eAER compared with ACR. eAER improved sensitivity and modestly lowered specificity compared with ACR30 mg/g. eAER and ACR17-25 mg/g (ACR with sex-associated cutoffs) had very similar sensitivity and specificity30 for the detection of moderate albuminuria. Inker91 has pointed out that the marked variability in accuracy among the 3 populations in these 2 studies suggests that these equations may not perform well for certain other populations. It should be noted that the eAER equations and the sex-associated cutoffs for ACR were developed in populations enrolled at least 3 decades ago. Since then, body weight has increased worldwide,92 and it is unclear how well the above formulas would perform in contemporary populations with a higher prevalence of obesity and more severe obesity. Aspirations for a universal and highly performing ACR cutoff or for an eAER equation that fits all patients may be unrealistic for a biochemical marker that is heavily dependent on muscle mass that itself is highly variable in demographic categories like sex and age.
Notably, most studies presented in this review were collected from populations living in North America or Europe. CERs in people from other geographic regions may markedly differ and our recommendations regarding optimal cutoffs and eAER may not be valid elsewhere. Inker91 proposed research to develop a prediction equation for eAER. However, different equations may be needed for diverse populations.
Considerations in the Clinical Assessment of Albuminuria
At the present time, how should clinicians best evaluate albuminuria? Available data do not provide a way to accurately estimate albuminuria in all populations using ACR with a 30-mg/g threshold for the diagnosis of moderate albuminuria. Lower 17- to 25-mg/g sex-specific cutoffs were proposed as an option by the 2003 National Kidney Foundation CKD guidelines93 but later abandoned by the 2012 KDIGO CKD guidelines.15 Some of the data presented in this study demonstrating that the 30-mg/g cutoff underestimates moderate albuminuria in the obese and a significant portion of the nonobese population were not available at the time the 2012 KDIGO guidelines were developed. Considering the evidence accumulated during the last decade, we suggest that guidelines committees re-evaluate the recommendation regarding ACR cutoffs and consider adopting lower thresholds for the diagnosis of moderate albuminuria. eAER equations30,35,90 may require further development for the following reasons: variability in accuracy between populations as pointed out by Inker91 and the absence of weight variables in one of the equations,30 which is expected to introduce biases in obese and underweight people. In addition, 2 of the equations use a race variable that requires refitting as recommended by the National Kidney Foundation–American Society of Nephrology task force for reassessment of the inclusion of race in GFR-estimating equations.94
Box 2 describes research goals to help optimize the use of ACR in obese individuals. Adequately performed, timed urine collections may be used for confirmatory purposes15 when a false-negative or false-positive result is suspected. This may occur in patients with an ACR level in the normal range and perceived high muscle mass or in people with an elevated ACR level and suspected sarcopenia. This initial 24-hour collection, if performed by compliant patients able to adequately collect urine, provides information on albuminuria and urinary CER. This initial CER will allow the calculation of eAER from ACR values obtained at follow-up (ACRf-u), as eAER at follow-up equals ACRf-u times the initial CER. This way of estimating albuminuria will adequately perform only as long as muscle mass is stable. An added value of performing an initial 24-hour urine collection is that it provides information regarding urinary sodium and urea excretion rates, hence on sodium and protein intake.
Box 2. Research Issues to Be Addressed in Optimizing the Performance of ACR in the Obese Population.
-
•
Estimate the medical and economic consequences of a missed diagnosis of moderate albuminuria in obese women and men based on age and comorbid conditions.
-
•
Estimate the medical and economic consequences of an overdiagnosis of moderate albuminuria in obese women and men according to age and comorbid conditions.
-
•
Answers to the above questions will provide tools to define the goals of ACR in terms of sensitivity and specificity when screening for moderate albuminuria and help define the desirable diagnostic thresholds.
-
•
These key issues should be evaluated as defined by Inker,91 starting with an accurate gold standard measurement, including the collection of urine under direct observation for 24 hours. This study should have a double aim, which is as follows: defining optimal cutoffs for the diagnosis of moderate albuminuria and developing an equation to estimate AER from ACR (eAER). The population studies should include multiple categories according to the following factors determining the creatinine excretion rate: sex, age, body mass and obesity markers (height, body weight, and waist circumference), and comorbid conditions. This large-scale study should include a sufficiently high number of patients from each class of obesity and should be geographically diverse.
Abbreviations: ACR, albumin-to-creatinine ratio; AER, albumin excretion rate; eAER, estimated AER.
How will the suggested solutions proposed in this review affect the performance of ACR30 mg/g to predict outcomes? As previously noted, ACR may be a better predictor of renal and cardiovascular events than AER,96,97 ACR being able to correct for incomplete urine collections and/or detect the 2 risk factors, albuminuria and low muscle mass, simultaneously. The proposed lowering of ACR cutoffs will not affect this improved performance. However, eAER may become less predictive compared with ACR in people with sarcopenia and in cases of incomplete urine collections.
It may be argued that reporting different reference limits for different populations complicates the interpretation of ACR results. Different reference limits for a given laboratory test are used to account for biological variability due to sex, age, diurnal and menstrual cycles, and body posture.98 Serum creatinine and creatininuria are examples of sex-specific reference values used in nephrology. In addition, clinical decision limits that vary according to health status (as diabetes status for cholesterol levels) are sometimes provided in lieu of conventional reference limits. Integrated electronic health records and laboratory systems facilitate the reporting of variable reference limits. The proposed changes are expected to benefit people with CKD, a CER above 1 g/d, and an AER in the lower range of moderate albuminuria, as shown in Fig 4, irrespective of obesity status. Considering the fact that most nonelderly obese people have a CER well above 1 g/d, and the increased prevalence of moderate albuminuria in obesity,4,5 the number of people misdiagnosed by the ACR test is likely to be substantial. Data from completed cohort studies and randomized controlled trials may provide more precise numbers.
In summary (see Box 3 for key points), we provide evidence that the mean CER is about a third higher in obese compared with normal-sized people of the same sex, with the mean CER in normal-sized adults being above 1,350 mg/d (a value notably well above the 1,000-mg/d creatinine-adjusting factor routinely accepted as the denominator in the ACR equation). The higher actual overaccepted 1-g reference CER leads to an underestimation bias in the majority of the population when albuminuria is estimated using ACR30 mg/g, and ultimately an underdiagnosis of moderate albuminuria. Moreover, the adiposity excess–related increase in CER further amplifies ACR-related biases occurring in normal-sized people, thereby leading to underestimation of the diagnosis of moderate albuminuria in obese more often than in normal-sized persons. Considering the high prevalence of moderate albuminuria among the large and growing obese population,4,5 this translates into an increased number of people at risk for cardiovascular disease and CKD in whom treatment decisions may be deferred. Additionally, these biases may hamper the recruitment of individuals with obesity and lead to false-negative outcomes in clinical trials investigating the effects of weight loss on kidney outcomes. Alternative strategies to assess albuminuria in such patients such as sex-related ACR cutoffs for the diagnosis of moderate albuminuria or eAER should be considered and gaps in our knowledge base addressed.
Box 3. Key Points of the Review.
-
•
Obesity is an independent risk factor for moderately increased albuminuria and predicts advanced CKD and cardiovascular outcomes.
-
•
Albuminuria is commonly estimated using ACR.
-
•
The recommended thresholds defining moderate albuminuria are 30 mg/d for AER and 30 mg/g for ACR.
-
•
Mean creatininuria in the general population is significantly higher than 1 g/d. As the creatinine excretion rate increases above 1 g/d, ACR decreases for any given albuminuria. This may lead to the underdiagnosis of moderate albuminuria when the 30-mg/g cutoff is used for ACR (ACR30 mg/g).
-
•
ACR30 mg/g underperformance is more pronounced as muscle mass and creatininuria increase, that is, more in men and young than in women and elderly.
-
•
Obese women and men have an approximately one-third higher creatininuria than lean people, amplifying the underestimation bias induced by ACR30 mg/g. This may lead to the underdiagnosis and undertreatment of people with obesity and moderate albuminuria, especially when albuminuria is only mildly elevated above 30 mg/g.
-
•
Decreasing ACR cutoffs from 30 to lower sex-adjusted cutoffs (25 and 17 mg/g for women and men, respectively) or estimating albuminuria using prediction equations for creatininuria improves ACR performance.
-
•
Further research is required to determine optimal cutoffs for people with obesity.
Abbreviations: ACR, albumin-to-creatinine ratio; AER, albumin excretion rate; CKD, chronic kidney disease.
Article Information
Authors’ Full Names and Academic Degrees
Avry Chagnac, MD, and Allon N. Friedman, MD.
Support
None.
Financial Disclosure
Dr Chagnac has no conflict of interest. Dr Friedman is an advisor to Gila Therapeutics and GI Dynamics, is on a steering committee of Eli Lilly, and owns stock in Eli Lilly.
Peer Review
Received August 7, 2023, as a submission to the expedited consideration track with 2 external peer reviews. Direct editorial input from the Editor-in-Chief. Accepted in revised form November 12, 2023.
Footnotes
Complete author and article information provided before references.
Figure S1: Creatinine excretion rate in the whole population.
Figure S2: Dispersion of the creatinine excretion rate in women, men, and mixed populations.
Item S1: Calculation of the dispersion of the creatinine excretion rate.
Table S1: Creatinine excretion rate and its variability in the whole population.
Table S2: Creatinine excretion rate and its variability in separate cohorts of women and men.
Table S3: Dispersion of the creatinine excretion rate in women, men, and mixed populations.
Table S4: Creatinine excretion rate in lean, overweight, and obese people.
Supplementary Materials
Figures S1-S2; Item S1; Tables S1-S4.
References
- 1.Friedman A.N., Ogden C.L., Hales C.M. Prevalence of obesity and CKD among adults in the United States, 2017-2020. Kidney Med. 2023;5(1) doi: 10.1016/j.xkme.2022.100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman A., Schauer P., Srinivasan B., et al. Obstacles and opportunities in managing coexisting obesity and CKD: report of a scientific workshop cosponsored by the National Kidney Foundation and The Obesity Society. Am J Kidney Dis. 2022;80(6):783–793. doi: 10.1053/j.ajkd.2022.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Levey A.S., Gansevoort R.T., Coresh J., et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75(1):84–104. doi: 10.1053/j.ajkd.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Chen J., Muntner P., Hamm L.L., et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140(3):167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 5.Garofalo C., Borrelli S., Minutolo R., Chiodini P., De Nicola L., Conte G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017;91(5):1224–1235. doi: 10.1016/j.kint.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Levey A.S., Eckardt K.U., Dorman N.M., et al. Nomenclature for kidney function and disease: report of a Kidney Disease: improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020;97(6):1117–1129. doi: 10.1016/j.kint.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Nelson R.G., Knowler W.C., Pettitt D.J., Saad M.F., Charles M.A., Bennett P.H. Assessment of risk of overt nephropathy in diabetic patients from albumin excretion in untimed urine specimens. Arch Intern Med. 1991;151(9):1761–1765. [PubMed] [Google Scholar]
- 8.Roest M., Banga J.D., Janssen W.M., et al. Excessive urinary albumin levels are associated with future cardiovascular mortality in postmenopausal women. Circulation. 2001;103(25):3057–3061. doi: 10.1161/hc2501.091353. [DOI] [PubMed] [Google Scholar]
- 9.Hillege H.L., Fidler V., Diercks G.F., et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106(14):1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 10.McCullough P.A., Jurkovitz C.T., Pergola P.E., et al. Independent components of chronic kidney disease as a cardiovascular risk state: results from the Kidney Early Evaluation Program (KEEP) Arch Intern Med. 2007;167(11):1122–1129. doi: 10.1001/archinte.167.11.1122. [DOI] [PubMed] [Google Scholar]
- 11.Levey A.S., de Jong P.E., Coresh J., et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 12.Fangel M.V., Nielsen P.B., Kristensen J.K., et al. Albuminuria and risk of cardiovascular events and mortality in a general population of patients with type 2 diabetes without cardiovascular disease: a Danish cohort study. Am J Med. 2020;133(6):e269–e279. doi: 10.1016/j.amjmed.2019.10.042. [DOI] [PubMed] [Google Scholar]
- 13.Smink P.A., Lambers Heerspink H.J., Gansevoort R.T., et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis. 2012;60(5):804–811. doi: 10.1053/j.ajkd.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Chronic Kidney Disease Prognosis Consortium. Matsushita K., van der Velde M., et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150. [Google Scholar]
- 16.Cheung A.K., Chang T.I., Cushman W.C., et al. Executive summary of the KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3):559–569. doi: 10.1016/j.kint.2020.10.026. [DOI] [PubMed] [Google Scholar]
- 17.National Kidney Foundation KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 18.James P.A., Oparil S., Carter B.L., et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 19.Inker L.A., Astor B.C., Fox C.H., et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 20.Piepoli M.F., Hoes A.W., Agewall S., et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnett D.K., Blumenthal R.S., Albert M.A., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;74(10):1376–1414. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabi D.M., McBrien K.A., Sapir-Pichhadze R., et al. Hypertension Canada’s 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Can J Cardiol. 2020;36(5):596–624. doi: 10.1016/j.cjca.2020.02.086. [DOI] [PubMed] [Google Scholar]
- 23.Unger T., Borghi C., Charchar F., et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association Professional Practice Committee. Draznin B., Aroda V.R., et al. 11. Chronic kidney disease and risk management: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(suppl 1):S175–S184. doi: 10.2337/dc22-S011. [DOI] [PubMed] [Google Scholar]
- 25.Barratt T.M., McLaine P.N., Soothill J.F. Albumin excretion as a measure of glomerular dysfunction in children. Arch Dis Child. 1970;45(242):496–501. doi: 10.1136/adc.45.242.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordin B.E. Assessment of calcium excretion from the urinary calcium/creatinine ratio. Lancet. 1959;2(7099):368–371. doi: 10.1016/s0140-6736(59)91635-6. [DOI] [PubMed] [Google Scholar]
- 27.Wyss M., Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 28.Heymsfield S.B., Arteaga C., McManus C., Smith J., Moffitt S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr. 1983;37(3):478–494. doi: 10.1093/ajcn/37.3.478. [DOI] [PubMed] [Google Scholar]
- 29.Rule A.D., Bailey K.R., Schwartz G.L., Khosla S., Lieske J.C., Melton L.J., 3rd For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int. 2009;75(10):1071–1078. doi: 10.1038/ki.2008.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fotheringham J., Campbell M.J., Fogarty D.G., El Nahas M., Ellam T. Estimated albumin excretion rate versus urine albumin-creatinine ratio for the estimation of measured albumin excretion rate: derivation and validation of an estimated albumin excretion rate equation. Am J Kidney Dis. 2014;63(3):405–414. doi: 10.1053/j.ajkd.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Connell S.J., Hollis S., Tieszen K.L., McMurray J.R., Dornan T.L. Gender and the clinical usefulness of the albumin: creatinine ratio. Diabet Med. 1994;11(1):32–36. doi: 10.1111/j.1464-5491.1994.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 32.Incerti J., Zelmanovitz T., Camargo J.L., Gross J.L., de Azevedo M.J. Evaluation of tests for microalbuminuria screening in patients with diabetes. Nephrol Dial Transplant. 2005;20(11):2402–2407. doi: 10.1093/ndt/gfi074. [DOI] [PubMed] [Google Scholar]
- 33.Remer T., Berkemeyer S., Rylander R., Vormann J. Muscularity and adiposity in addition to net acid excretion as predictors of 24-h urinary pH in young adults and elderly. Eur J Clin Nutr. 2007;61(5):605–609. doi: 10.1038/sj.ejcn.1602560. [DOI] [PubMed] [Google Scholar]
- 34.Oterdoom L.H., van Ree R.M., de Vries A.P., et al. Urinary creatinine excretion reflecting muscle mass is a predictor of mortality and graft loss in renal transplant recipients. Transplantation. 2008;86(3):391–398. doi: 10.1097/TP.0b013e3181788aea. [DOI] [PubMed] [Google Scholar]
- 35.Ix J.H., Wassel C.L., Stevens L.A., et al. Equations to estimate creatinine excretion rate: the CKD epidemiology collaboration. Clin J Am Soc Nephrol. 2011;6(1):184–191. doi: 10.2215/CJN.05030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erman A., Rahamimov R., Mashraki T., et al. The urine albumin-to-creatinine ratio: assessment of its performance in the renal transplant recipient population. Clin J Am Soc Nephrol. 2011;6(4):892–897. doi: 10.2215/CJN.05280610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdelmalek J.A., Gansevoort R.T., Lambers Heerspink H.J., Ix J.H., Rifkin D.E. Estimated albumin excretion rate versus urine albumin-creatinine ratio for the assessment of albuminuria: a diagnostic test study from the Prevention of Renal and Vascular Endstage Disease (PREVEND) Study. Am J Kidney Dis. 2014;63(3):415–421. doi: 10.1053/j.ajkd.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forni Ogna V., Ogna A., Vuistiner P., et al. New anthropometry-based age- and sex-specific reference values for urinary 24-hour creatinine excretion based on the adult Swiss population. BMC Med. 2015;13:40. doi: 10.1186/s12916-015-0275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johner S.A., Boeing H., Thamm M., Remer T. Urinary 24-h creatinine excretion in adults and its use as a simple tool for the estimation of daily urinary analyte excretion from analyte/creatinine ratios in populations. Eur J Clin Nutr. 2015;69(12):1336–1343. doi: 10.1038/ejcn.2015.121. [DOI] [PubMed] [Google Scholar]
- 40.Marco Mayayo M.P., Martinez Alonso M., Valdivielso Revilla J.M., Fernandez-Giraldez E. A new gender-specific formula to estimate 24-hour urine protein from protein to creatinine ratio. Nephron. 2016;133(4):232–238. doi: 10.1159/000447604. [DOI] [PubMed] [Google Scholar]
- 41.Stam S.P., Oste M.C.J., Eisenga M.F., et al. Posttransplant muscle mass measured by urinary creatinine excretion rate predicts long-term outcomes after liver transplantation. Am J Transplant. 2019;19(2):540–550. doi: 10.1111/ajt.14926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagenaar C.A., Dekker L.H., Navis G.J. Prevalence of sarcopenic obesity and sarcopenic overweight in the general population: the lifelines cohort study. Clin Nutr. 2021;40(6):4422–4429. doi: 10.1016/j.clnu.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 43.James G.D., Sealey J.E., Alderman M., et al. A longitudinal study of urinary creatinine and creatinine clearance in normal subjects. Race, sex, and age differences. Am J Hypertens. 1988;1(2):124–131. doi: 10.1093/ajh/1.2.124. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs D.R., Jr., Murtaugh M.A., Steffes M., Yu X., Roseman J., Goetz F.C. Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2002;155(12):1114–1119. doi: 10.1093/aje/155.12.1114. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson D.J., Piasecki M., Atherton P.J. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. 2018;47:123–132. doi: 10.1016/j.arr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metter E.J., Conwit R., Tobin J., Fozard J.L. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci. 1997;52(5):B267–B276. doi: 10.1093/gerona/52a.5.b267. [DOI] [PubMed] [Google Scholar]
- 47.Ebner N., Springer J., Kalantar-Zadeh K., et al. Mechanism and novel therapeutic approaches to wasting in chronic disease. Maturitas. 2013;75(3):199–206. doi: 10.1016/j.maturitas.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Cao R.Y., Li J., Dai Q., Li Q., Yang J. Muscle atrophy: present and future. Adv Exp Med Biol. 2018;1088:605–624. doi: 10.1007/978-981-13-1435-3_29. [DOI] [PubMed] [Google Scholar]
- 49.Forbes G.B., Welle S.L. Lean body mass in obesity. Int J Obes. 1983;7(2):99–107. [PubMed] [Google Scholar]
- 50.Forbes G.B. Lean body mass-body fat interrelationships in humans. Nutr Rev. 1987;45(8):225–231. doi: 10.1111/j.1753-4887.1987.tb02684.x. [DOI] [PubMed] [Google Scholar]
- 51.Baumgartner R.N., Heymsfield S.B., Roche A.F. Human body composition and the epidemiology of chronic disease. Obes Res. 1995;3(1):73–95. doi: 10.1002/j.1550-8528.1995.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 52.Walowski C.O., Braun W., Maisch M.J., et al. Reference values for skeletal muscle mass—current concepts and methodological considerations. Nutrients. 2020;12(3):755. doi: 10.3390/nu12030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fotheringham J., Weatherley N., Kawar B., Fogarty D.G., Ellam T. The body composition and excretory burden of lean, obese, and severely obese individuals has implications for the assessment of chronic kidney disease. Kidney Int. 2014;86(6):1221–1228. doi: 10.1038/ki.2014.112. [DOI] [PubMed] [Google Scholar]
- 54.Ogna A., Forni Ogna V., Bochud M., et al. Association between obesity and glomerular hyperfiltration: the confounding effect of smoking and sodium and protein intakes. Eur J Nutr. 2016;55(3):1089–1097. doi: 10.1007/s00394-015-0923-0. [DOI] [PubMed] [Google Scholar]
- 55.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894(i-xii):1–253. [PubMed] [Google Scholar]
- 56.NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US) National Heart, Lung, and Blood Institute; Bethesda, MD: 1998. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. [Google Scholar]
- 57.Ellam T.J. Albumin:creatinine ratio—a flawed measure? The merits of estimated albuminuria reporting. Nephron Clin Pract. 2011;118(4):c324–c330. doi: 10.1159/000323670. [DOI] [PubMed] [Google Scholar]
- 58.Ärnlöv J., Evans J.C., Meigs J.B., et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals : the Framingham Heart Study. Circulation. 2005;112(7):969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 59.Rossing P., Anker S.D., Filippatos G., et al. Finerenone in patients with chronic kidney disease and type 2 diabetes by sodium-glucose cotransporter 2 inhibitor treatment: the FIDELITY analysis. Diabetes Care. 2022;45(12):2991–2998. doi: 10.2337/dc22-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parving H.H., Lehnert H., Brochner-Mortensen J., et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 61.Makino H., Haneda M., Babazono T., et al. Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care. 2007;30(6):1577–1578. doi: 10.2337/dc06-1998. [DOI] [PubMed] [Google Scholar]
- 62.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102:S1–S127. doi: 10.1016/j.kint.2022.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Heerspink H.J.L., Stefansson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 64.Bakris G.L., Agarwal R., Anker S.D., et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 65.Lamb E., Jones G. In: Tietz Textbook of Laboratory Medicine. 7th ed. Rifai N., editor. Elsevier; 2022. Kidney function tests; pp. 352.e351–352.e360. [Google Scholar]
- 66.Wiegmann T.B., Chonko A.M., Barnard M.J., et al. Comparison of albumin excretion rate obtained with different times of collection. Diabetes Care. 1990;13(8):864–871. doi: 10.2337/diacare.13.8.864. [DOI] [PubMed] [Google Scholar]
- 67.Chaiken R.L., Khawaja R., Bard M., Eckert-Norton M., Banerji M.A., Lebovitz H.E. Utility of untimed urinary albumin measurements in assessing albuminuria in black NIDDM subjects. Diabetes Care. 1997;20(5):709–713. doi: 10.2337/diacare.20.5.709. [DOI] [PubMed] [Google Scholar]
- 68.Lopez-Pelayo I., Mazuecos Blanca M.A., Garcia Palacios M.V., Galan Sanchez F., Lopez Rodriguez F., Bailen Garcia M.A. How to manage kidney transplant recipients: deciding between glomerular filtration rate-estimating equations, creatinine clearance and albumin-creatinine ratio, or albumin excretion. Exp Clin Transplant. 2019;17(4):450–456. doi: 10.6002/ect.2017.0335. [DOI] [PubMed] [Google Scholar]
- 69.Warram J.H., Gearin G., Laffel L., Krolewski A.S. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol. 1996;7(6):930–937. doi: 10.1681/ASN.V76930. [DOI] [PubMed] [Google Scholar]
- 70.Harvey J.N., Hood K., Platts J.K., Devarajoo S., Meadows P.A. Prediction of albumin excretion rate from albumin-to-creatinine ratio. Diabetes Care. 1999;22(9):1597–1598. doi: 10.2337/diacare.22.9.1597. [DOI] [PubMed] [Google Scholar]
- 71.Houlihan C.A., Tsalamandris C., Akdeniz A., Jerums G. Albumin to creatinine ratio: a screening test with limitations. Am J Kidney Dis. 2002;39(6):1183–1189. doi: 10.1053/ajkd.2002.33388. [DOI] [PubMed] [Google Scholar]
- 72.Jafar T.H., Chaturvedi N., Hatcher J., Levey A.S. Use of albumin creatinine ratio and urine albumin concentration as a screening test for albuminuria in an Indo-Asian population. Nephrol Dial Transplant. 2007;22(8):2194–2200. doi: 10.1093/ndt/gfm114. [DOI] [PubMed] [Google Scholar]
- 73.Fox C.S., Gona P., Larson M.G., et al. A multi-marker approach to predict incident CKD and microalbuminuria. J Am Soc Nephrol. 2010;21(12):2143–2149. doi: 10.1681/ASN.2010010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foster M.C., Hwang S.J., Massaro J.M., et al. Association of subcutaneous and visceral adiposity with albuminuria: the Framingham Heart Study. Obesity (Silver Spring) 2011;19(6):1284–1289. doi: 10.1038/oby.2010.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tynkevich E., Flamant M., Haymann J.P., et al. Urinary creatinine excretion, measured glomerular filtration rate and CKD outcomes. Nephrol Dial Transplant. 2015;30(8):1386–1394. doi: 10.1093/ndt/gfv047. [DOI] [PubMed] [Google Scholar]
- 76.Koopman J.J.E., Scherzer R., Ix J.H., Shlipak M.G., Waikar S.S. A comparison of different estimates of albuminuria in association with mortality in epidemiologic research. Clin J Am Soc Nephrol. 2020;15(12):1814–1816. doi: 10.2215/CJN.07290520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wettersten N., Katz R., Shlipak M.G., et al. Urinary biomarkers and kidney outcomes: impact of indexing versus adjusting for urinary creatinine. Kidney Med. 2021;3(4):546–554.e1. doi: 10.1016/j.xkme.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carey D.G., Pliego G.J., Raymond R.L. Body composition and metabolic changes following bariatric surgery: effects on fat mass, lean mass and basal metabolic rate: six months to one-year follow-up. Obes Surg. 2006;16(12):1602–1608. doi: 10.1381/096089206779319347. [DOI] [PubMed] [Google Scholar]
- 79.Zalesin K.C., Franklin B.A., Lillystone M.A., et al. Differential loss of fat and lean mass in the morbidly obese after bariatric surgery. Metab Syndr Relat Disord. 2010;8(1):15–20. doi: 10.1089/met.2009.0012. [DOI] [PubMed] [Google Scholar]
- 80.Heymsfield S.B., Gonzalez M.C., Shen W., Redman L., Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev. 2014;15(4):310–321. doi: 10.1111/obr.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dixon J.B., Lambert E.A., Grima M., Rice T., Lambert G.W., Straznicky N.E. Fat-free mass loss generated with weight loss in overweight and obese adults: what may we expect? Diabetes Obes Metab. 2015;17(1):91–93. doi: 10.1111/dom.12389. [DOI] [PubMed] [Google Scholar]
- 82.Martinez M.C., Meli E.F., Candia F.P., et al. The impact of bariatric surgery on the muscle mass in patients with obesity: 2-year follow-up. Obes Surg. 2022;32(3):625–633. doi: 10.1007/s11695-021-05815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nuijten M.A.H., Eijsvogels T.M.H., Monpellier V.M., Janssen I.M.C., Hazebroek E.J., Hopman M.T.E. The magnitude and progress of lean body mass, fat-free mass, and skeletal muscle mass loss following bariatric surgery: a systematic review and meta-analysis. Obes Rev. 2022;23(1) doi: 10.1111/obr.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saliba J., Kasim N.R., Tamboli R.A., et al. Roux-en-Y gastric bypass reverses renal glomerular but not tubular abnormalities in excessively obese diabetics. Surgery. 2010;147(2):282–287. doi: 10.1016/j.surg.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lieske J.C., Collazo-Clavell M.L., Sarr M.G., Rule A.D., Bergstralh E.J., Kumar R. Gastric bypass surgery and measured and estimated GFR in women. Am J Kidney Dis. 2014;64(4):663–665. doi: 10.1053/j.ajkd.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.US National Library of Medicine A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW) https://clinicaltrials.gov/ct2/show/NCT03819153
- 87.US National Library of Medicine A Study of Tirzepatide (LY3298176) in Participants With Overweight or Obesity and Chronic Kidney Disease With or Without Type 2 Diabetes (TREASURE-CKD) https://clinicaltrials.gov/ct2/show/NCT05536804?cond=tirzepatide+kidney&draw=2&rank=2
- 88.Davies A.G., Postlethwaite R.J., Price D.A., Burn J.L., Houlton C.A., Fielding B.A. Urinary albumin excretion in school children. Arch Dis Child. 1984;59(7):625–630. doi: 10.1136/adc.59.7.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gatling W., Knight C., Hill R.D. Screening for early diabetic nephropathy: which sample to detect microalbuminuria? Diabet Med. 1985;2(6):451–455. doi: 10.1111/j.1464-5491.1985.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 90.Walser M. Creatinine excretion as a measure of protein nutrition in adults of varying age. JPEN J Parenter Enteral Nutr. 1987;11(suppl 5):73S–78S. doi: 10.1177/014860718701100510. [DOI] [PubMed] [Google Scholar]
- 91.Inker L.A. Albuminuria: time to focus on accuracy. Am J Kidney Dis. 2014;63(3):378–381. doi: 10.1053/j.ajkd.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 92.Collaborators G.B.D.O., Afshin A., Forouzanfar M.H., et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levey A.S., Coresh J., Balk E., et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 94.Delgado C., Baweja M., Crews D.C., et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. J Am Soc Nephrol. 2021;32(12):2994–3015. doi: 10.1681/ASN.2021070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park A.M., Storm D.W., Fulmer B.R., Still C.D., Wood G.C., Hartle J.E., 2nd A prospective study of risk factors for nephrolithiasis after Roux-en-Y gastric bypass surgery. J Urol. 2009;182(5):2334–2339. doi: 10.1016/j.juro.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 96.Lambers Heerspink H.J., Gansevoort R.T., Brenner B.M., et al. Comparison of different measures of urinary protein excretion for prediction of renal events. J Am Soc Nephrol. 2010;21(8):1355–1360. doi: 10.1681/ASN.2010010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vart P., Scheven L., Heerspink H.J.L., et al. Urine albumin-creatinine ratio versus albumin excretion for albuminuria staging: a prospective longitudinal cohort study. Am J Kidney Dis. 2016;67(1):70–78. doi: 10.1053/j.ajkd.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 98.American Board of Internal Medicine ABIM Laboratory Test Reference Ranges – July 2023. https://www.abim.org/Media/bfijryql/laboratory-reference-ranges.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S2; Item S1; Tables S1-S4.