Abstract
The role of phosphorylation in the dissociation of structural components of the herpes simplex virus type 1 (HSV-1) tegument was investigated, using an in vitro assay. Addition of physiological concentrations of ATP and magnesium to wild-type virions in the presence of detergent promoted the release of VP13/14 and VP22. VP1/2 and the UL13 protein kinase were not significantly solubilized. However, using a virus with an inactivated UL13 protein, we found that the release of VP22 was severely impaired. Addition of casein kinase II (CKII) to UL13 mutant virions promoted VP22 release. Heat inactivation of virions or addition of phosphatase inhibited the release of both proteins. Incorporation of radiolabeled ATP into the assay demonstrated the phosphorylation of VP1/2, VP13/14, VP16, and VP22. Incubation of detergent-purified, heat-inactivated capsid-tegument with recombinant kinases showed VP1/2 phosphorylation by CKII, VP13/14 phosphorylation by CKII, protein kinase A (PKA), and PKC, VP16 phosphorylation by PKA, and VP22 phosphorylation by CKII and PKC. Proteolytic mapping and phosphoamino acid analysis of phosphorylated VP22 correlated with previously published work. The phosphorylation of virion-associated VP13/14, VP16, and VP22 was demonstrated in cells infected in the presence of cycloheximide. Use of equine herpesvirus 1 in the in vitro release assay resulted in the enhanced release of VP10, the homolog of HSV-1 VP13/14. These results suggest that the dissociation of major tegument proteins from alphaherpesvirus virions in infected cells may be initiated by phosphorylation events mediated by both virion-associated and cellular kinases.
The herpesvirus tegument is a stable macromolecular structure formed by virion structural proteins. It is located between the capsid and the virus envelope (23). The proteins comprising this component of the virion are the first to be exposed to the intracellular environment of an infected cell and provide critical viral functions in the time between viral penetration of the cell and the synthesis of virus immediate-early proteins. The herpes simplex virus type 1 (HSV-1) tegument contains four major structural proteins: VP1/2, VP13/14, VP16, and VP22 (10, 23), the products of the UL36, UL47, UL48, and UL49 genes (2, 7, 13, 14, 25). These proteins constitute a major part of the mass of the virus particle (9). Another protein found in the tegument is the product of the UL13 gene, a putative protein kinase which may contribute to the phosphorylation of VP22, although the reason for its packaging in mature virions remains unclear (3, 4). Interestingly, it has been argued that this protein is required for the host shutoff function mediated by the virion-associated Vhs protein, also a tegument component (18). Tegument proteins have been assigned a variety of functions aside from the shutoff of host cell protein synthesis (8, 20), such as immediate-early gene transactivation (2). These tasks presumably require the dissociation of much of the tegument and the release of soluble proteins into the cytoplasm of the infected cell, but the mechanism of this is unknown. The tegument is stable at physiological salt concentrations, it does not require the presence of either envelope or capsid to maintain its structural integrity (12), and the interaction between tegument proteins in purified virions is likely to be ionic, not hydrophobic, in nature (16). In addition, tegument structures appear capable of self-assembly in the absence of virion maturation, giving rise to noninfectious virion-like L particles composed essentially of envelope and tegument (19, 24), and specific associations between individual tegument proteins are well documented (5, 21). Furthermore, at a later stage of infection in the cell, the tegument proteins which dissociated upon virion entry must associate to create the tegument of new virions. The apparently paradoxical nature of these observations suggests the involvement of a reversible cellular process in tegument association and dissociation. The phosphorylation of VP1/2, VP13/14, VP16, and VP22 has been demonstrated in vitro, in transfected cells and in infected cells later in infection (6, 11, 15–17). However, tegument proteins in purified virions are not phosphorylated (6, 7, 16). Phosphorylation and dephosphorylation therefore represent a candidate mechanism for the regulated dissociation and assembly of the HSV-1 tegument. In the work presented here, we studied the effect of phosphorylation on the release of soluble tegument proteins from purified virions, using a simple, reproducible, and robust in vitro assay system, and investigated the role that the UL13 virion protein kinase and cellular kinases may play in this process.
MATERIALS AND METHODS
Antibodies.
R218 is specific for VP1/2 and was prepared by inoculation of rabbits with VP1/2 purified by preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). R220 and R230, prepared in a similar manner, recognize VP13/14 (25) and VP16, respectively. R323 is specific for the UL13 protein kinase and was prepared by inoculation of rabbits with UL13 expressed as a fusion protein in bacteria. R205 is a rabbit polyclonal antiserum raised against detergent-purified capsid-tegument samples which recognizes VP13/14, VP16, and VP22. P43 is a mouse monoclonal immunoglobulin M which was raised against VP22 (5, 7). 13A9 is a monoclonal antibody which recognizes VP10 of equine herpesvirus 1 (EHV-1) (25).
Viruses.
The wild-type (wt) virus used in this work was HSV-1 strain 17+. The mutant virus UL13 lacZ, a kind gift from D. J. McGeoch, contains the lacZ gene of Escherichia coli inserted into the XhoI restriction site at sequence coordinate 28058 to interrupt the UL13 protein (3). The EHV-1 strain used in this study was AB1. Virions were purified from culture supernatants of infected cells by standard techniques.
Immunoprecipitations.
Infected cell monolayers were washed in phosphate-buffered saline and solubilized in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris HCl [pH 7.4], 150 mM NaCl, 0.1% SDS, 0.1% sodium deoxycholate, 0.5% Nonidet P-40 [NP-40]) containing a cocktail of protease inhibitors. The lysate was clarified by centrifugation at 12,000 × g for 30 min before incubation with a previously prepared antibody-protein A-Sepharose complex at 4°C overnight with mixing. After extensive washing in RIPA buffer, bound material was eluted with boiling SDS-PAGE sample buffer and analyzed by SDS-PAGE.
SDS-PAGE and Western blotting.
SDS-PAGE and Western blotting were carried out essentially as described previously (25). Samples were solubilized under reducing conditions in the presence of SDS. Gels contained 5 to 18% acrylamide cross-linked with N,N"-diallyltartardiamide (9). After a run, gels were fixed, dried, and exposed to autoradiographic film or stained with Coomassie blue to directly visualize protein. Alternatively, proteins were electrophoretically transferred to nitrocellulose or polyvinylidene difluoride (PVDF) membranes. Western blots were developed using horseradish peroxidase-conjugated secondary antisera (Sigma) and the Pierce SuperSignal enhanced chemiluminescence system according to the manufacturers’ instructions. Nonspecific binding was minimized by the addition of 1% nonfat dried skimmed milk to antibody preparations and 0.1% Tween 20 to wash buffers.
Production of HSV-1 capsid-tegument preparations.
Capsid-tegument structures were prepared by incubation of purified virions in 50 mM Tris (pH 7.4)–1% NP-40 for 5 min on ice followed by centrifugation at full speed in a microcentrifuge for 10 min. The resulting pellet was resuspended in 50 mM Tris (pH 7.4)–0.1 M NaCl. Endogenous kinase activity in such preparations was heat inactivated by incubation at 60°C for 10 min.
Tegument release assay.
Purified virion preparations (representing approximately 107 PFU) were incubated in 200 μl of 50 mM HEPES buffer (pH 7.4)–100 mM NaCl (HEPES-buffered saline [HBS]) and 1% Triton X-100 for 5 to 30 min at 37°C in the presence or absence of 1 mM MgCl2 and 1 mM ATP. The mixture was then layered onto 0.5 ml of 35% (wt/vol) sucrose in HBS in a microcentrifuge tube and centrifuged at 14,000 rpm for 30 min. The 200 μl of supernatant above the interface with the sucrose cushion was carefully removed and frozen as released, fully soluble protein. After aspiration of the sucrose cushion, the pelleted material in the bottom of the tube was recovered and frozen as insoluble capsid-tegument proteins. Detectable tegument proteins were found within the sucrose gradient (results not shown). These may represent partially dissociated tegument structures, but for the purposes of this study only fully soluble material was used. In some experiments, 150 U of calf intestinal phosphatase (CIP; Sigma) was incorporated into the incubation mixture.
In vitro phosphorylation reactions.
Protein kinase A (PKA) catalytic subunit and casein kinase II (CKII), along with their corresponding 10-fold-concentrated reaction buffers, were obtained from New England Biolabs. Protein kinase C (PKC) was obtained from Promega. In vitro phosphorylation reactions were carried out in the following conditions. For PKA and CKII, reactions were carried out with approximately 5 μg of heat-inactivated capsid-tegument preparation, 10× kinase reaction buffer, 5 U of PKA or CKII, 10 μM ATP, and 10 μCi of [γ-32P]ATP in a reaction volume of 50 μl. PKC incubations were typically carried out with 5 μg of heat-inactivated capsid-tegument, 5 μl of 10× reaction buffer (200 mM HEPES [pH 7.4], 100 mM MgCl2, 50 mM CaCl2, 2 mg of phosphatidylserine per ml, 100 μg of diolein per ml), 0.2 U of PKC, and 10 μCi of [γ-32P]ATP in a reaction volume of 50 μl. In vitro labeling of purified HSV-1 virions was carried out by adding purified virions to an equal volume of assay buffer (50 mM Tris [pH 8], 1 mM MgCl2, 0.5 M NaCl, 1% NP-40) in the presence of 1 μCi of [γ-32P]ATP. All reactions were typically carried out at 37°C for 30 min and were stopped by the addition of an equivalent volume of SDS-PAGE loading buffer and boiling.
Phosphopeptide mapping.
Phosphorylated virion samples were subjected to SDS-PAGE and transferred to a PVDF membrane. Phosphorylated proteins were visualized by exposing the membrane to autoradiographic film. The band representing phosphorylated VP22 was excised by using the film as a template as well as by staining the membrane with a reversible protein stain (Sigma) to directly visualize blotted proteins. After destaining according to the manufacturer’s instructions, the membrane fragments were divided into two pieces and resuspended in 50 mM Tris (pH 8)–0.1% SDS–0.1% NP-40 in the presence or absence of 1 μg of endoprotease LysC (endoC). The samples were incubated for 4 h at 37°C, after which an equal volume of SDS-PAGE loading buffer containing 5 mM dithiothreitol was added to each tube and the samples were boiled. After removal of the PVDF fragment, the samples were loaded into the well of an SDS-polyacrylamide gel along with a further 0.1 μg of protease. The gels were run, fixed, and dried, and phosphopeptide profiles were visualized by exposure to autoradiographic film.
Phosphoamino acid analysis.
Phosphorylated samples were blotted to a PVDF membrane, and phosphorylated VP22 was excised. Strips were rewetted by incubation in methanol for 30 s and in water for a further 30 s. Strips were then incubated in 6 N HCl for 1 h at 110°C, after which the membrane was removed and the sample was lyophilized. The sample was then resuspended in water containing 10 μg each of O-phospho-l-serine, O-phospho-l-threonine, and O-phospho-l-tyrosine (Sigma) and analyzed by thin-layer chromatography in a solvent consisting of 5 volumes of isobutyric acid and 3 volumes of 0.5 M NH4OH. Phosphoamino acids were visualized by autoradiography, and positions of markers were visualized by ninhydrin staining.
RESULTS
Phosphorylation upregulates HSV-1 tegument dissociation in vitro.
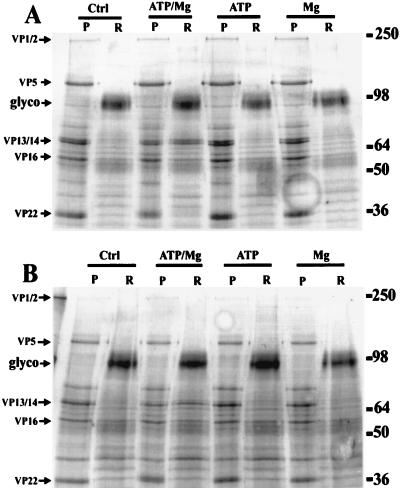
A simple in vitro assay was devised to study the effects of various conditions on the solubilization of herpesvirus tegument proteins from purified virions. Equal aliquots of wt and UL13 mutant virions were incubated at 37°C for 30 min in a simple HBS-detergent solution in the presence or absence of 1 mM ATP and/or 1 mM magnesium chloride. These samples were then fractionated into soluble and insoluble extracts by centrifugation through a sucrose cushion. The protein content of each fraction was visualized by SDS-PAGE and Coomassie blue staining. As shown in Fig. 1, the addition of ATP and magnesium chloride in combination but not individually to incubations of both wt and UL13 mutant virions resulted in the enhanced release of VP13/14 but not VP1/2. The visualization of another protein of interest, VP22, was hindered by the presence of the capsid protein VP21, which closely migrated with VP22 in the pelleted fraction. The partitioning of capsid (i.e., VP5 and VP21) and membrane glycoproteins in different samples confirmed the efficiency of the simple fractionation system used here.
FIG. 1.
SDS-PAGE analysis of HSV-1 in vitro tegument release assay. wt (A) and UL13 mutant (B) viruses were detergent treated and incubated at 37°C for 30 min in the presence or absence of 1 mM ATP and/or 1 mM magnesium chloride. They were then fractionated via sucrose gradient centrifugation into pelleted (P) or released (R) material. These samples were analyzed by SDS-PAGE, and the gels were stained with Coomassie blue to visualize total protein. Note in both viruses the partitioning of the major capsid protein VP5 into the pelleted fraction and of the major glycoproteins (glyco) into the soluble fraction, demonstrating effective fractionation. The major tegument proteins were identified by MW, indicated on the right in kilodaltons. VP1/2 was detected only in the pelleted material. The amount of VP13/14 in the released fraction of both viruses was increased by incubation with ATP and Mg in combination but not individually.
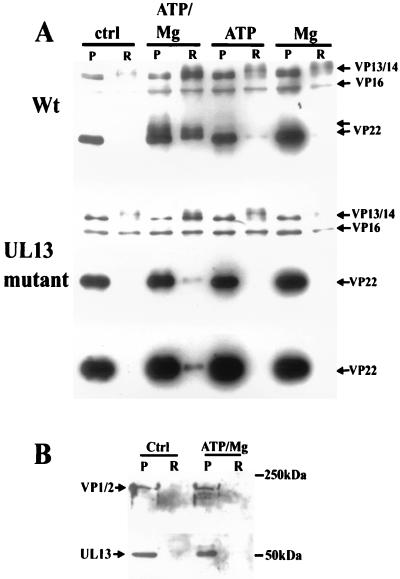
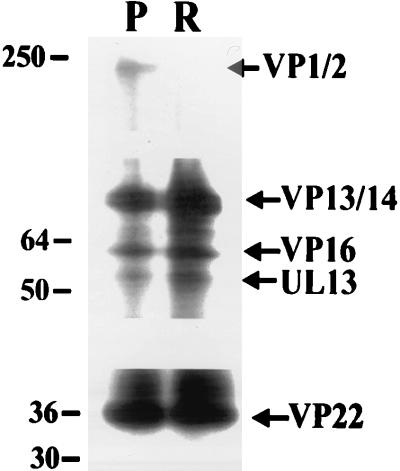
Since the visualization of VP22 was unclear with this staining technique, and to confirm the specificity of our observations, Western blotting of a portion of these samples was carried out with the polyclonal antibody R205, which recognizes VP13/14, VP16, and VP22 (Fig. 2A). The results showed conclusively that the release of VP13/14 and VP22 from the wt virion tegument in vitro was significantly enhanced by the combined presence of Mg and ATP. The release of VP16 was not detectably affected. The presence of higher-molecular-weight (MW) VP22 in both the pellet and the soluble fraction of samples incubated with ATP and Mg indicated that VP22 was being modified in some way, most likely by phosphorylation (6). With the UL13 mutant virus, the release of VP22 was significantly impaired. In addition, higher-MW forms of the protein were absent, indicating that the VP22 modification was also impaired. This observation is in agreement with previous work on this mutant virus (3) suggesting that UL13 contributes to the phosphorylation of VP22. The observation that VP1/2 was partitioned into the insoluble fraction in this assay system regardless of the experimental conditions was confirmed by Western blotting with the polyclonal antibody R218 (Fig. 2B). We therefore conclude that this large protein remains associated with the capsid in this assay. Surprisingly perhaps, Western blotting with R323 demonstrated that the great majority of the UL13 protein kinase also remained associated with the pelleted material (Fig. 2B). However, the presence of a higher-MW band in samples incubated with ATP and Mg suggested that the protein was modified in some way.
FIG. 2.
Western blotting analysis of in vitro tegument release assay. (A) The samples (pelleted [P] and released [R] material) shown in Fig. 1 were blotted to nitrocellulose and probed with antibody R205. The increased release of VP13/14 and VP22 from wt virus in the presence of ATP-Mg was confirmed. Note the higher-MW immunoreactive VP22 bands indicative of phosphorylation. In the UL13 mutant, VP22 release is retarded compared to wt, but VP13/14 release is unaffected. Note the lack of higher-MW VP22 bands with this mutant, even after blot overexposure. (B) The partitioning of VP1/2 into the pelleted fraction in the presence of ATP-Mg was confirmed by blotting with R218. The UL13 protein kinase was also shown to be primarily associated with the pelleted material under these conditions by blotting with R323; note, however, the higher-MW band seen in the presence of ATP-Mg.
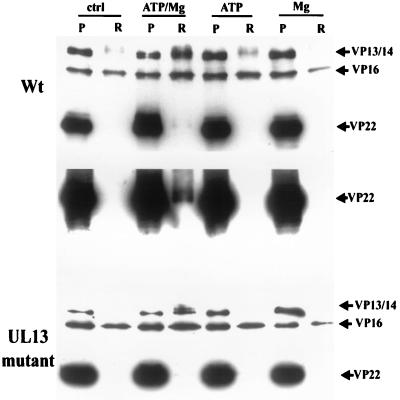
The kinetics of the release of VP13/14 and VP22 were investigated by repeating the above experiment with a shorter incubation time of 5 min. This analysis showed that release of VP13/14 occurred much more rapidly than that of VP22 (Fig. 3). For the UL13 mutant virus, similar results were obtained; VP13/14 was rapidly released, whereas VP22 was barely detectable in the soluble fraction even after extended exposures (Fig. 3).
FIG. 3.
Release of VP13/14 and release of VP22 have different characteristics. The experiment shown in Fig. 1 and 2 was repeated with incubation for 5 instead of 30 min. Blotting with R205 showed that VP13/14 release had reached significant levels by this time, whereas VP22 release appeared much slower; detection of VP22 in the soluble sample required blot overexposure. VP22 in the soluble fraction of the UL13 mutant virus under the same conditions was essentially undetectable. Abbreviations are as defined in the legend to Fig. 1.
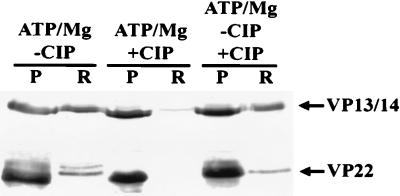
Our results suggested that the enhanced release of VP13/14 and VP22 was mediated by the phosphorylation of these proteins by different kinases in the purified virion preparations. This was confirmed in several ways. First, the presence of CIP in the assay severely inhibited the release of both VP13/14 and VP22 (Fig. 4). Interestingly, the addition of CIP to samples preincubated with ATP-Mg did not cause the reassociation of VP13/14 and VP22 with the virion despite the efficient dephosphorylation of the soluble VP22. The dissociation process therefore appears irreversible in this simple system. We next demonstrated the direct phosphorylation of a number of virion proteins by incorporating [γ-32P]ATP into the release assay (Fig. 5). VP1/2, VP13/14, VP16, VP22, and UL13 were all present in the insoluble fraction as phosphoproteins, while all but VP1/2 were present in the soluble fraction. A greater amount of phosphorylated material was present in the soluble rather than the insoluble fraction; the loading in the soluble lane of the gel represents one-third of that normally used to allow a side-by-side comparison of the two fractions without overexposure of the lane containing soluble protein. We therefore observed that although Western blotting analysis showed little difference in the amount of VP16 in each fraction (Fig. 1 to 3), the VP16 in the soluble fraction was more extensively phosphorylated. Furthermore, the increased sensitivity of this technique compared to Western blotting (Fig. 2B) allowed the observation that phosphorylated UL13 was also present in the soluble fraction. As expected, a greater proportion of phosphorylated VP13/14 and VP22 were found in the soluble fraction.
FIG. 4.
ATP-Mg-dependent tegument release in vitro is inhibited by the presence of phosphatase. Purified wt virions were incubated with ATP-Mg in the presence or absence of CIP for 30 min; addition of phosphatase inhibited VP13/14 and VP22 release. Note the absence of higher-MW forms of VP22 in the phosphatase-treated sample. A third sample was incubated without phosphatase for 30 min and then incubated in the presence of phosphatase for 30 min; the levels of VP13/14 and VP22 in the released (R) fraction were unaffected, although the presence of higher-MW VP22 forms was abolished. P, pelleted material.
FIG. 5.
Tegument proteins released in vitro in response to ATP-Mg are phosphorylated. [γ-32P]ATP was added to the release assay to reveal virion proteins which had undergone phosphorylation. The majority of radiolabeled material was in the released fraction. To allow side-by-side visualization of pelleted (P) and released (R) protein, the loading of the released fraction represents one-third of that used for the Western blots. In the pelleted fraction, the major tegument structural proteins VP1/2, VP13/14, VP16, and VP22 were labeled. The UL13 protein kinase was also labeled. In the released fraction, all of these proteins except VP1/2 were present. Sizes are indicated in kilodaltons.
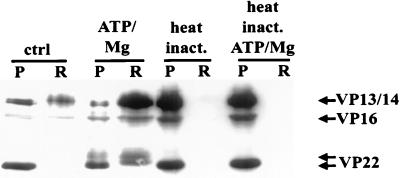
If kinases in the purified virus preparations were responsible for the phosphorylation and subsequent solubilization of VP22 and VP13/14, then this activity would be expected to be heat sensitive. Preincubation of virus preparations at 60°C for 10 min was sufficient to abolish the release of all proteins in the presence of ATP-Mg (Fig. 6). This treatment was also sufficient to abolish labeling of virion components with radiolabeled ATP (see Fig. 8).
FIG. 6.
Effects of heat inactivation (inact.) on ATP-Mg-dependent tegument release in vitro. The R205 Western blot demonstrates that preincubation of purified virions for 10 min at 60°C is sufficient to completely abolish ATP-Mg-dependent tegument release. Lanes are designated as for Fig. 1.
FIG. 8.
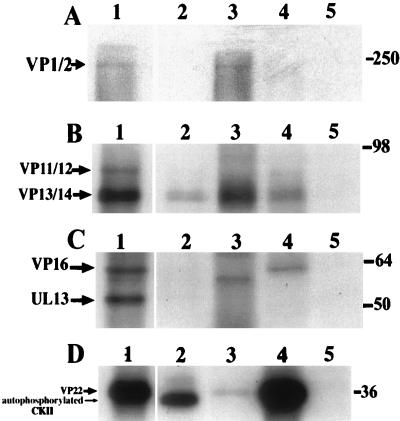
Substrate specificity of cellular kinases incubated with detergent-purified, heat-inactivated capsid tegument preparations in vitro. Purified wt virus preparations were detergent purified and heat treated at 60°C for 10 min. Samples were incubated with cellular kinases in the presence of [γ-32P]ATP and analyzed by SDS-PAGE followed by autoradiography. Lane 1 in each panel shows detergent-purified virions without heat treatment to demonstrate labeling by the endogenous virion kinase activity. Lanes 5 in panels A to C show heat-inactivated samples incubated with [γ-32P]ATP without added kinase; no labeling was observed. (A) Lanes 2 to 4 represent VP1/2 from heat-inactivated virions radiolabeled by PKC, CKII, and PKA, respectively. Note the weak labeling in the CKII incubation only. (B) Lanes 2 to 4 show VP13/14 labeling by PKC, CKII, and PKA, respectively. (C) Lanes 2 to 4 show incubations with PKC, CKII and PKA, respectively. VP16 is labeled only by PKA. The identity of the lower-MW band in the CKII incubation is unknown. UL13 was not labeled by any added cellular kinases. (D) Lane 2 shows the position of autophosphorylated CKII; lanes 3 and 4 demonstrate VP22 labeling by PKC and CKII, respectively; lane 5 shows the PKA incubation in which no VP22 labeling was seen.
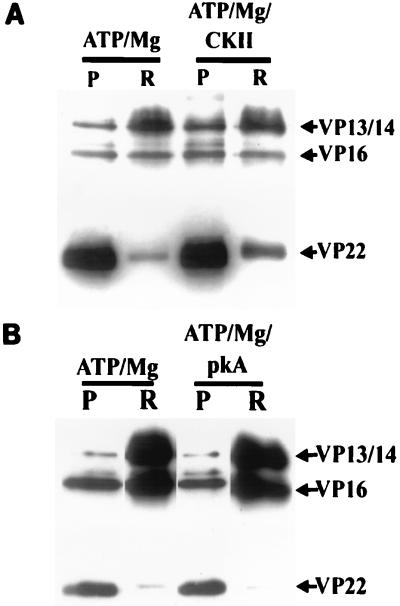
VP22 has previously been shown to be an excellent potential substrate for CKII but a very poor one for pkA (see reference 7 and Fig. 8). Since VP22 phosphorylation and release from capsid-tegument structures appear to be almost entirely dependent on the presence of UL13 (Fig. 2), we wished to test whether the addition of exogenous CKII to preparations of UL13 mutant virions could compensate for the absence of this protein (Fig. 7). Figure 7A demonstrates that the addition of CKII to such a preparation could indeed upregulate the release of VP22. In contrast, the addition of exogenous PKA had no effect on VP22 release (Fig. 7B). Interestingly, the addition of either enzyme also had little effect on the release of VP13/14, suggesting that the phosphorylation events which mediate the dissociation of this protein are not performed by these enzymes (although VP13/14 appears to be a competent substrate for both [see Fig. 8]).
FIG. 7.
CKII but not PKA can promote VP22 release in UL13 mutant virions in vitro. ATP-Mg-dependent VP22 release in vitro was severely impaired in purified UL13 mutant virions. The effect of adding exogenous CKII (A) or PKA (B) in conjunction with ATP-Mg to incubations of UL13 mutant virions was therefore studied. The samples were fractionated as before and analyzed by SDS-PAGE followed by Western blotting with R205. The amount of soluble VP22 was greater in the presence than on the absence of CKII. VP13/14 release appeared unaffected. Addition of PKA had no visible effect on VP22 or VP13/14 release compared to a mixture lacking this enzyme. P and R, pelleted and released material, respectively.
Both cellular and virion-associated kinases can phosphorylate tegument proteins in purified capsid-tegument structures.
The identities of the potent kinase activities responsible for the phosphorylation of the tegument proteins in the above assays were largely obscure, although the tegument-associated kinase UL13 appeared to contribute significantly to the phosphorylation of VP22. This finding implied that either an unidentified viral kinase or cellular kinases acquired during virion assembly mediated this phosphorylation. In vivo, exposure of the tegument proteins to cytoplasmic kinases after virion entry to an infected cell could also contribute to phosphorylation and subsequent dissociation. To examine this, we tested the ability of cellular kinases to phosphorylate tegument proteins in detergent-purified, heat-inactivated capsid-tegument structures. We found the inactivation of the virion-associated kinase activity by heat treatment to be essential for the acquisition of meaningful data free from interference from the potent endogenous virion-associated kinase activities. In Fig. 8, each panel represents a different autoradiographic exposure of the same experiment. Our results showed that VP1/2 was phosphorylated in both wt (Fig. 8A, lane 1) and UL13 mutant (not shown) capsid-tegument preparations and was weakly phosphorylated in the heat-inactivated samples by CKII (Fig. 8A, lane 3). VP13/14 was also phosphorylated in the untreated capsid-tegument samples and in addition was phosphorylated by all three kinases, PKA, PKC, and CKII (Fig. 8B, lanes 2 to 4). VP16 was phosphorylated in both capsid-tegument preparations but only by PKA in the heat-inactivated samples (Fig. 8C). A band of slightly lower MW than VP16 was observed in the CKII-phosphorylated sample (Fig. 8C, lane 3); phosphopeptide mapping demonstrated that this was not a different form of phosphorylated VP16 (data not shown). VP22 was phosphorylated in both wt and UL13 mutant capsid-tegument preparations. As observed previously (3), this phosphorylation was much reduced in the UL13 mutant sample (not shown). In the heat-inactivated sample, VP22 was phosphorylated by PKC and very efficiently by CKII, but not by PKA. It therefore appears that the major structural proteins in HSV-1 tegument preparations are accessible substrates for a variety of cellular kinases.
The possibility existed that the UL13-dependent, virion-associated kinase activity utilized phosphorylation sites different from those previously identified for cellular kinases in VP22. Since proteolytic digestion data have been previously presented to map the phosphorylation sites utilized by these kinases in VP22 (6), we used a similar approach to investigate if the same region was phosphorylated in our preparations.
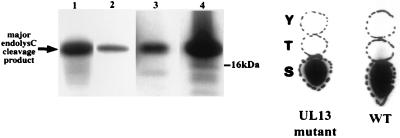
EndoC digestion of phosphorylated VP22 has previously localized all phosphorylation sites in the protein to a 20-kDa N-terminal region (6). EndoC digestion of phosphorylated VP22 from wt and UL13 mutant capsid-tegument structures yielded a similar result (Fig. 9). This finding implies that the UL13-dependent phosphorylation sites in VP22 are also located in this N-terminal region. Identical results were obtained from VP22 phosphorylated by PKC and CKII in heat-inactivated samples (Fig. 9), implying that the N-terminal sites in VP22 in these samples were accessible to these kinases.
FIG. 9.
EndoC mapping and phosphoamino acid analysis of in vitro-phosphorylated VP22. The phosphorylated VP22 species in Fig. 8 were analyzed by proteolytic mapping using endoC (endolysC). VP22 labeled by endogenous wt and UL13 mutant virion kinase activities was also subjected to phosphoamino acid analysis. Lanes 1 to 4, protein labeled by wt virions, UL13 mutant virions, PKC, and CKII, respectively. All phosphorylated VP22 forms produced a peptide of approximately 20 kDa. VP22 from wt and mutant UL13 virions was phosphorylated solely on serine.
Previous work has demonstrated that VP22 is phosphorylated on serines. In purified wt virions in this study, the endogenous virion kinase activities also phosphorylated VP22 solely on serine (Fig. 9).
Virion-associated tegument proteins are phosphorylated in infected cells in the absence of viral protein synthesis.
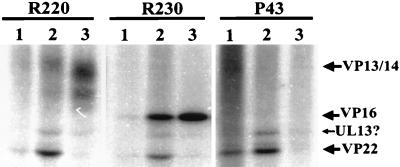
The in vitro data presented above predict that upon virion penetration of an infected cell, a proportion of VP13/14, VP16, and VP22 within the virion tegument will be phosphorylated. To investigate this, Vero cells were incubated with HSV-1 at a multiplicity of infection (MOI) of 50 in the presence of both 32P and cycloheximide for times ranging between 0 and 60 min. The cells were extracted in RIPA buffer, and immunoprecipitations were carried out with the antibodies R220, R230, and P43, which recognize VP13/14, VP16, and VP22, respectively. Precipitates were analyzed by SDS-PAGE and autoradiography. As shown in Fig. 10, VP13/14, VP16, and VP22 were all significantly radiolabeled under these conditions after 30 min of incubation. As predicted, therefore, virion-associated VP13/14, VP16, and VP22 were all phosphorylated during infection before the onset of viral protein synthesis. Interestingly, immunoprecipitations carried out with extracts prepared after 30 min of incubation resulted in the coimmunoprecipitation of other radiolabeled proteins. As the alignment of the three immunoprecipitations makes clear, these proteins likely represent other tegument components. Specifically, immunoprecipitation with both VP13/14- and VP16-specific antibodies results in the coimmunoprecipitation of phosphoproteins of MWs consistent with those of VP22 and, to a lesser extent, UL13. P43 immunoprecipitation resulted in the coimmunoprecipitation of a phosphoprotein with an MW consistent with that of UL13. An association between the transactivating domain of VP16 and VP22 in infected cells has been previously suggested (5). This experiment implies that such an association is broken relatively quickly in infected cells after virion penetration, allowing unmasking of the VP16 transactivation activity. By 60 min of incubation, all of these putative associations had been apparently abolished. These results are consistent with the data obtained in the in vitro assays, since it could be argued that as the phosphorylation of the tegument proteins within the cell is seen to increase, the association between them, as detected by coimmunoprecipitation, is seen to decrease.
FIG. 10.
Input virion-associated tegument proteins are phosphorylated in infected cells. Vero cells were labeled with 32P during infection with HSV-1 at an MOI of 50 in the presence of cycloheximide for 0, 30, and 60 min. Cell extracts from these time points (lanes 1 to 3, respectively, for each antibody) were subjected to immunoprecipitation with R220, R230, and P43 to capture VP13/14, VP16, and VP22 respectively. Precipitates were then subjected to SDS-PAGE and autoradiography. The positions of immunoprecipitated phosphoproteins are indicated with arrows. Phosphorylated VP13/14 and VP16 were first detected after 30 min of incubation and were more heavily labeled by 60 min. Phosphoproteins with MWs consistent with those of VP22 and UL13 were coimmunoprecipitated with VP13/14 and VP16 after 30 but not after 60 min of incubation. Phosphorylated VP22 could be immunoprecipitated almost immediately by P43, and UL13 appeared to be coimmunoprecipitated with VP22, after 30 min of incubation. Little VP22 was immunoprecipitated by P43 after 60 min of incubation (see text).
The lack of radiolabeled VP22 observed in the 60-min P43 immunoprecipitation does not imply that none is present in the cell at this time, since evidence suggesting that P43 recognizes a phosphorylation-sensitive epitope in VP22 has been obtained (16a). Since VP22 contains multiple phosphorylation sites (6), this antibody can still immunoprecipitate a proportion of radiolabeled VP22 at earlier incubation times, but by 60 min of incubation it is unable to immunoprecipitate significant amounts of protein because the progressive increase in VP22 phosphorylation has masked the P43 epitope. Note that this observation implies that more VP22 is phosphorylated by 30 min of incubation in this system than can be demonstrated by this antibody. The unusual properties of P43 may also help to explain the lack of coimmunoprecipitated VP16 and VP13/14 in the P43 incubations.
Tegument protein dissociation in EHV-1 is upregulated by ATP-Mg.
Since the possession of a tegument is a defining morphological feature of herpesviruses, it seemed possible that a common mechanism of tegument dissociation was shared between different viruses. We therefore investigated the effects of ATP-Mg on the dissociation of the EHV-1 tegument in our in vitro assay. Coomassie blue staining of soluble and insoluble fractions suggested that ATP-Mg enhanced the release of several structural proteins (results not shown), among them the major tegument protein VP10. The enhanced release of VP10 was confirmed by immunoblotting with the monoclonal antibody 13A9 (Fig. 11). This preliminary result was obtained in an assay system optimized for HSV-1; modification of this system may result in the identification of other proteins in the EHV-1 tegument whose release is upregulated by phosphorylation.
FIG. 11.
ATP-Mg upregulates the release of the EHV-1 tegument protein VP10 in vitro. SDS-PAGE and Western blot analysis of EHV-1 was used in the in vitro tegument release assay. The monoclonal anti-VP10 antibody 13A9 was used. The increased release of this protein in ATP-Mg-treated samples is clearly demonstrated. Lanes are labeled as for Fig. 1.
DISCUSSION
The major findings in this report relate directly to two of the less well understood areas of HSV-1 biology. The first of these are the immediate events in HSV-1 infection, which occur between virion penetration of the cell and the onset of viral protein synthesis. Progress in this area requires technically demanding experiments since, unlike later in infection, the only material the investigator can work with is that which constitutes the input virion. In this study, we addressed this problem both by using an in vitro system to mimic conditions during virion penetration and by artificially increasing the quantity of input viral material in infected cells by using high MOIs and utilizing cycloheximide to block the progression of infection. In the latter case, a similar experimental concept has recently been used to demonstrate the association of infecting virions with microtubules (22). We have used these techniques to demonstrate that major structural components of the HSV-1 tegument are phosphorylated upon entry to an infected cell (Fig. 10) and that in the case of VP13/14 and VP22, this phosphorylation mediates their dissociation from the virion in vitro (Fig. 1 to 7). Furthermore, it appears that the UL13 kinase mediates the dissociation of VP22 from wt purified virions, although CKII can mimic this effect (Fig. 7). We would speculate that in vivo, the major contributor to VP22 dissociation, by virtue of proximity and a higher local concentration, is UL13 rather than the cellular kinases previously shown to phosphorylate this protein (6). The coimmunoprecipitation of a phosphoprotein with an MW consistent with that of UL13 in cells infected in the presence of cycloheximide supports this view (Fig. 10). The potential delay in VP22 dissociation in the absence of UL13 may help to explain to the observed early lag in the growth curve of mutant UL13 viruses in cultured cells (3). The lack of UL13 could eventually be compensated for by phosphorylation of VP22 by cellular kinases. VP22 has been shown to be phosphorylated in transfected cells, demonstrating that a cellular kinase is capable of this function (6). Also of interest is the observation that VP1/2 does not dissociate from the capsid. This protein has been postulated to play a role in the insertion of the HSV-1 genome into the nucleus early in infection (1), and an affinity for the capsid is consistent with this function. It has previously been shown, however, that VP1/2 is a component of HSV-1 L particles, which lack capsids (24). Therefore, it is possible that VP1/2 interaction with the rest of the tegument is phosphorylation dependent but interaction with the capsid is phosphorylation independent.
The source of the virion-associated kinase activity responsible for the phosphorylation and release of VP13/14 is unclear; aside from UL13, no virion structural components with a proven kinase activity have been described, and UL13 deletion had no effect on VP13/14 release in our in vitro assay. It remains possible that a significant amount of a contaminating cell-derived kinase was present in our purified virion preparations. The way in which these preparations were made renders this unlikely unless the contaminating kinase was present within the envelope of the virions. A reasonable hypothesis is that the HSV-1 virion captures a cellular kinase activity during virion biogenesis, most likely as a component of the tegument or associated with the envelope. This possibility is under further investigation. In our solubility assays, a slight increase in VP13/14 release was sometimes apparent in the presence of ATP or Mg alone, although this never approached the levels observed when these two components were present in combination (Fig. 2A). This finding may be explained by the presence of nominal amounts of Mg and ATP in the purified virion preparation. Since phosphorylation is an enzyme-mediated event, the presence of minor amounts of Mg or ATP could easily effect such a release. We note that heat treatment completely abolishes tegument protein release (Fig. 6).
Our data are also of relevance in a second area, that of tegument acquisition by the virion. The site of tegument assembly and the mechanisms driving it are unknown. It seems logical to propose that tegument association will reflect its dissociation. A scenario for this association involving the local dephosphorylation of tegument proteins resulting in condensation around a maturing nucleocapsid can therefore be postulated. Such local dephosphorylation or the association of unphosphorylated newly synthesized tegument proteins could also result in the production of the noninfectious L particles observed with HSV-1 and other alphaherpesviruses (12, 24). In support of this view, preliminary data suggest that HSV-1 L particles show the same sensitivity to ATP-Mg in the in vitro release assay as that seen with purified virions. This implies that the effects of phosphorylation on tegument dissociation can occur independently of the presence of a capsid. These hypotheses will require testing by further experimental work.
The enhanced solubility of EHV-1 VP10 in the presence of ATP-Mg was of interest since VP10 is the EHV-1 homolog of HSV-1 VP13/14 (25). Several other viruses contain homologs of this protein, and this sensitivity to phosphorylation may represent a common feature of this family of virion structural protein. These preliminary data could therefore imply that phosphorylation of major structural components represents a general mechanism for the dissociation of alphaherpesvirus tegument structures.
ACKNOWLEDGMENTS
We acknowledge the financial support of The Wellcome Trust for this work.
We are grateful for the support of our colleagues in the Molecular Medicine Unit at St. James University Hospital, Leeds, United Kingdom.
REFERENCES
- 1.Batterson W, Furlong D, Roizman B. Molecular genetics of herpes simplex virus. VIII. Further characterisation of a temperature-sensitive mutant defective in the release of viral DNA and in other stages of the viral reproductive cycle. J Virol. 1983;45:397–407. doi: 10.1128/jvi.45.1.397-407.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell M E M, Palfreyman J W, Preston C M. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate-early transcription. J Mol Biol. 1984;180:1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- 3.Coulter L J, Moss H M W, Lang J, McGeoch D J. A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupted. J Gen Virol. 1993;74:387–395. doi: 10.1099/0022-1317-74-3-387. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham C, Davison A J, Dolan A, Frame M C, McGeoch D J, Meredith D M, Moss H W M, Orr A C. The UL13 virion protein of herpes simplex type 1 is phosphorylated by a novel virus-induced protein kinase. J Gen Virol. 1992;73:303–311. doi: 10.1099/0022-1317-73-2-303. [DOI] [PubMed] [Google Scholar]
- 5.Elliot G, Mouzakitis G, O’Hare P. VP16 interacts via its activation domain with VP22, a tegument protein of herpes simplex virus, and is relocated to a novel macromolecular assembly in coexpressing cells. J Virol. 1995;69:7932–7941. doi: 10.1128/jvi.69.12.7932-7941.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott G, O’Reilly D, O’Hare P. Phosphorylation of the herpes simplex virus type 1 tegument protein VP22. Virology. 1996;226:140–145. doi: 10.1006/viro.1996.0638. [DOI] [PubMed] [Google Scholar]
- 7.Elliott G D, Meredith D M. The herpes simplex virus type 1 tegument protein VP22 is encoded by gene UL49. J Gen Virol. 1992;73:723–726. doi: 10.1099/0022-1317-73-3-723. [DOI] [PubMed] [Google Scholar]
- 8.Fenwick M L, Walker M J. Suppression of the synthesis of cellular macromolecules by herpes simplex virus. J Gen Virol. 1978;41:37–51. doi: 10.1099/0022-1317-41-1-37. [DOI] [PubMed] [Google Scholar]
- 9.Heine J W, Honess R W, Cassai E, Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974;14:640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honess R W, Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpesvirus polypeptides in the infected cell. J Virol. 1973;12:640–651. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemaster S, Roizman B. Herpes simplex virus phosphoproteins. II. Characterisation of the virion protein kinase and of the polypeptides phosphorylated in the virion. J Virol. 1980;35:798–811. doi: 10.1128/jvi.35.3.798-811.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLauchlan J, Rixon F J. Characterisation of enveloped tegument structures (L particles) produced by alphaherpesviruses: integrity of the tegument does not depend upon the presence of capsid or envelope. J Gen Virol. 1992;73:269–276. doi: 10.1099/0022-1317-73-2-269. [DOI] [PubMed] [Google Scholar]
- 13.McLean G, Rixon F, Langeland N, Haarr L, Marsden H. Identification and characterisation of the virion protein products of herpes simplex virus type 1 gene UL47. J Gen Virol. 1990;71:2953–2960. doi: 10.1099/0022-1317-71-12-2953. [DOI] [PubMed] [Google Scholar]
- 14.McNabb D S, Courtney R J. Analysis of the UL36 open reading frame encoding the large tegument protein (ICP1/2) of herpes simplex virus type 1. J Virol. 1992;66:7581–7584. doi: 10.1128/jvi.66.12.7581-7584.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNabb D S, Courtney R J. Characterisation of the large tegument protein (ICP1/2) of herpes simplex virus type 1. Virology. 1992;190:221–232. doi: 10.1016/0042-6822(92)91208-c. [DOI] [PubMed] [Google Scholar]
- 16.Meredith D M, Lindsay J A, Halliburton I W, Whittaker G R. Post-translational modification of the tegument proteins (VP13 and VP14) of herpes simplex virus type 1 by glycosylation and phosphorylation. J Gen Virol. 1991;72:2771–2775. doi: 10.1099/0022-1317-72-11-2771. [DOI] [PubMed] [Google Scholar]
- 16a.Morrison, E. Unpublished data.
- 17.O’Reilly D, Hanscombe O, O’Hare P. A single serine residue at position 375 of VP16 is critical for complex assembly with Oct-1 and HCF and is a target of phosphorylation by casein kinase II. EMBO J. 1997;16:2420–2430. doi: 10.1093/emboj/16.9.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Overton H, McMillan D, Hope L, Wong-Kai-In P. Production of host shutoff-defective mutants of herpes simplex virus type 1 by inactivation of the UL13 gene. Virology. 1994;202:97–106. doi: 10.1006/viro.1994.1326. [DOI] [PubMed] [Google Scholar]
- 19.Rixon F J, Addison C, McLauchlan J. Assembly of enveloped tegument structures (L particles) can occur independently of virion maturation in HSV-1 infected cells. J Gen Virol. 1992;73:277–284. doi: 10.1099/0022-1317-73-2-277. [DOI] [PubMed] [Google Scholar]
- 20.Smibert C A, Johnson D J, Smiley J R. Identification and characterisation of the virion-induced host shutoff product of herpes simplex virus gene UL41. J Gen Virol. 1992;73:467–470. doi: 10.1099/0022-1317-73-2-467. [DOI] [PubMed] [Google Scholar]
- 21.Smibert C A, Popova B, Xiao P, Capone J P, Smiley J R. Herpes simplex virus VP16 forms a complex with the virion host shutoff protein vhs. J Virol. 1994;68:2339–2346. doi: 10.1128/jvi.68.4.2339-2346.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sodeik B, Ebersold M W, Helenius A. Microtubule-mediated transport of incoming herpes simplex virus type 1 capsids to the nucleus. J Cell Biol. 1997;130:1007–1022. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spear P G, Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpes virion. J Virol. 1972;9:143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szilagyi J F, Cunningham C. Identification and characterisation of a novel non-infectious herpes simplex virus-related particle. J Gen Virol. 1991;72:661–668. doi: 10.1099/0022-1317-72-3-661. [DOI] [PubMed] [Google Scholar]
- 25.Whittaker G R, Riggio M P, Halliburton I W, Killington R A, Allen G P, Meredith D M. Antigenic and protein sequence homology between VP13/14, a herpes simplex type 1 tegument protein, and gp10, a glycoprotein of equine herpesvirus 1 and 4. J Virol. 1991;65:2320–2326. doi: 10.1128/jvi.65.5.2320-2326.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]