Abstract
Multiparametric ultrasound (MPUS), combining conventional techniques (greyscale and colour Doppler ultrasound), ultrasound strain elastography, and contrast-enhanced ultrasound (CEUS), has been successfully used in the assessment of adult scrotal pathology. Contrast-enhanced ultrasound can confidently establish testicular tissue vascularity even in the small-volume paediatric testis. Elastography provides further assessment of tissue stiffness, potentially adding useful diagnostic information. In children, ultrasonography is particularly advantageous, being safe, radiation-free and negating the need for sedation or general anaesthesia during the imaging evaluation. In this review article, we aim to familiarise readers with the MPUS scanning protocol used for paediatric scrotal examination and provide an overview of scrotal MPUS features, with particular focus to clinical indications where MPUS may be advantageous over conventional ultrasonography.
Introduction
Scrotal pathology is common in the paediatric population, and a timely and correct diagnosis is necessary to avoid possible future infertility. Conventional ultrasonography techniques, including greyscale, colour and power Doppler ultrasonography, are widely employed for the evaluation of the morphology and vascularity of paediatric scrotal pathology, 1 being widely accessible and radiation-free. 2 Beyond conventional techniques, new advanced ultrasonography techniques have been developed, including contrast-enhanced ultrasonography (CEUS) and tissue elastography.
CEUS, with the currently commercially available second-generation ultrasound contrast agents (UCAs), has become a standard imaging modality in adult clinical practice with an excellent safety profile. 3 The use of CEUS in children is supported by clinical experience and growing evidence regarding safety and usefulness in many applications and is primarily driven by the need to avert radiation exposure and nephrotoxic contrast agents in this age group, 4–10 and the Food and Drug Administration in the United States recently approved the use of SonoVueTM under the commercial name LumasonTM for paediatric liver applications. 11 Scrotal CEUS is still performed in an off-label pattern in children, but using medications “off-label” is not rare in paediatric practice, 12–14 and CEUS may be considered appropriate in cases where its benefits is considered to outweigh the potential risks associated with alternative imaging investigations, such as the need to subject the child to conscious sedation or general anaesthesia and the potential long-term adverse effects of gadolinium agents for MRI, for paediatric patients. 15,16
Tissue elastography, another advanced ultrasonography technique, can be broadly classified into strain and shear-wave elastography. 17 In strain elastography (SE), a stress is applied by the transducer through manual compression, and the amount of target tissue deformation relative to the surrounding tissue is evaluated. In shear-wave elastography (SWE), an acoustic radiation force pulse is utilised to produce a longitudinal strain, and the speed of shear waves propagating perpendicular to the longitudinal strain is measured to obtain a quantitative estimation of tissue stiffness. 18 While only few studies deal with use of SWE for scrotal pathologies, 19,20 several investigations explored the use of SE in imaging of the testes. 21,22
It has been proposed that simultaneous use of conventional modes with the newer techniques such as CEUS, and tissue elastography, termed multiparametric ultrasound (MPUS) 23,24 could increase the diagnostic yield for appropriate clinical indications. The utilisation of MPUS for scrotal imaging in adults is supported in the literature 25–27 with increasing evidence that MPUS may at least be a problem solving tool in cases equivocal on conventional ultrasound. As a general statement, MPUS provides added value over conventional ultrasound in offering ability to definitively assess presence (or absence) of vascularity within the testicular parenchyma or focal lesions, and additional information regarding tissue stiffness for the differentiation between an abnormality and normal parenchyma. The literature regarding the developing use of MPUS for the scrotum in the paediatric population is scarce. 28–30 This could be partly explained by the fact that conventional ultrasound techniques already achieve adequate diagnostic accuracy in a range of common scrotal conditions in children. However, familiarity with the added value of MPUS scrotal imaging would allow practitioners to make use of these newer techniques appropriately when conventional ultrasound findings are equivocal and avert the need for CT or MRI along with their inherent disadvantages for children. In this review article, we aim to familiarise the readers with the MPUS scanning protocol used for paediatric scrotal examination, and provide an overview of scrotal MPUS features in a range of scrotal pathologies pertinent to the paediatric population, with particular focus to clinical indications where MPUS may be advantageous over conventional ultrasound.
Technical basis for MPUS
From birth to early childhood, the access to the scrotum is usually unobstructed. Occasionally, it may be necessary for the ultrasound operator to place a fingertip just above the scrotum on the inguinal canal to prevent movement of the testis. In the adolescents, scrotal ultrasound is performed with the patient in the supine position, holding the penis lifted up onto the abdomen, and the scrotum may be supported by a towel placed between the thighs. 1 Optimal visualisation of the paediatric scrotal contents is best achieved with high-resolution imaging using high-frequency linear transducers with a frequency ranging from 9 to 18 MHz. 31 In infants, a hockey-stick transducer with frequencies of 15–18 MHz may be used. Conventionally, an unenhanced greyscale ultrasound examination is performed complemented with colour and power Doppler ultrasound (CDUS). The transverse bilateral plane is important, so that the asymptomatic testis serves as a reference for size, echogenicity and Doppler signal.
To perform ultrasound strain elastography, a stress is applied through repeated manual compression of the ultrasound-transducer adjusted according to a visual indicator, and the amount of the target lesion deformation relative to the surrounding healthy tissue is evaluated and displayed by colour coding. 17 Qualitative or semi-quantitative measurement of the elasticity in relation to healthy testicular tissue can be obtained. For shear-wave elastography, measurements are generated without compression in the transverse plane obtained in the centre of the testis and the average of at least three readings is taken as the final result to reduce measurement variation. 32 With regards to safety, when the impulse used to produce the tissue displacement is mechanical in origin such as in strain elastography, there is no reason for more concern about the safety of elastography than for B-mode imaging. However, when acoustic radiation force impulses are used such as SWE, significant temperature rises may theoretically occur. It is therefore recommended that the ”as low as reasonably achievable” principle should be applied when setting the output for acoustically induced elastography methods and the scanning time should be kept short. 33
CEUS should be performed with the joint approval of the clinician caring for the child and radiologist after evaluation with greyscale and CDUS. In most cases, an informed verbal consent obtained from parents or legal guardians is all that may be required, but local policies may vary and a written consent for UCA administration may be a hospital requirement. There are no standardised dosage schemes of the administrated ultrasound contrast agent for paediatric scrotal CEUS. In principle, the amount of UCA required is higher compared to the amount required in examination for abdominal organs, as a relatively small number of microbubbles within the UCA resonate at the high frequency used for imaging superficial structures such as the scrotum. For adult scrotal CEUS examination, a bolus of 4.8 ml of SonoVueTM/LumasonTM is used. It is accepted that the dose of the administrated UCA may be reduced in children adjusted according to child’s age, body surface or body weight. A suggested dosage scheme is 0.6 mL in children <6 years old, 1.2 mL for those between 6 and 12 years old and 2.4 mL in those >12 years old. Catheters measuring 12–24 gauge can be used for the venous access for the administration of UCA. When performing the CEUS examination, the mechanical index (MI) should be kept as low as possible to avoid microbubble destruction, with current machines being able to achieve MI values as low as 0.08. After a bolus UCA injection, serial static images and multiframe cine-clips are acquired, and continuous observation is usually performed from the time of arrival of the microbubbles and until they disappear. Images are stored digitally. Quantitative analysis could be performed for comparison of the enhancement dynamics over a region of interest (ROI) during the post-processing step if required, with evaluation of various aspects of the wash-in and wash-out parameters in time–intensity curves.
Contraindications for CEUS include a history of known hypersensitivity to the active substance or excipients, children with right-to-left shunts, severe pulmonary hypertension and uncontrolled systemic hypertension. Although it has been suggested that theoretically microbubbles in may lower the threshold of tissue cavitation, this theoretical risk is balanced against the risk of alternative imaging or misdiagnosis. 30 The rate of anaphylactoid reactions to UCA in children is very low, 34–36 ready access to resuscitation equipment is mandatory and should be in close proximity to the room where CEUS examinations are conducted. All personnel involved with UCA administration in children should have the appropriate skills in identifying and treating a contrast reaction in a child.
Normal MPUS Anatomy (Figure 1)
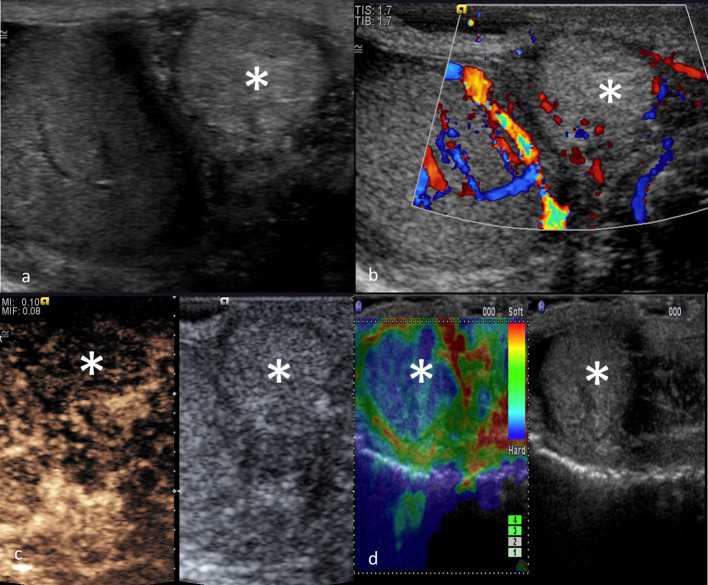
Figure 1.
MPUS of a normal testis. Greyscale (a) showing homogeneous echogenicity of the testis and a normal appearance of epididymis. CDUS (b) showing uniform distribution of blood flow signals. CEUS (c) showing uniform enhancement of the testicular parenchyma. On SE (d), the normal testis is relatively soft at elastography, with a stiffer sub albuginea ring which appears stiffer (blue). CDUS, colour-Doppler ultrasound; CEUS, contrast-enhanced ultrasound; MPUS, multiparametric ultrasound;SE, strain elastography.
On greyscale ultrasound, the normal testes are symmetrical, oval-shape organs characterised by a homogeneous pattern of medium-amplitude echoes. 2 After microbubble administration, arteries enhance first, followed within a few seconds by complete fill-in of the parenchyma. The testis and epididymis enhance quickly and intensively while scrotal wall enhancement is less pronounced. Unlike the liver, the testis has a single arterial supply and thus no clear cut-offs exist to differentiate enhancement phases. In general, an early phase lasts up to 40 s and a late phase to 90 s can be considered. Enhancement typically fades within 2–3 min. 37,38 On the SE, a normal testis is relatively “soft“ with a subalbugineal ring, which appears stiffer due to a higher number of connective septa arising from the tunica albuginea.
MPUS for paediatric scrotal pathology
Paediatric scrotal pathologies could be broadly grouped into the following categories: Torsion, Trauma, epididymoorchitis and tumours (and tumour-mimics). 2,39 Pain, swelling, redness, and a palpable mass are the typical manifestations of many paediatric scrotal pathologies, with pitfalls in their clinical diagnosis. The potential added value of scrotal MPUS for a range of clinical indication is summarised in Table 1.
Table 1.
Summary of the added value of MPUS in paediatric scrotal pathology by clinical indications
| Clinical indication | Added value of MPUS |
|---|---|
| Testicular torsion |
|
| Testicular infarction | Improved detection and depiction of the evolution of a segmental infarction with CEUS. |
| Trauma |
|
| Inflammation |
|
| Cyst |
|
| Solid tumour |
|
CDUS, colour-Doppler ultrasound;CEUS, contrast-enhanced ultrasound; SE, strain elastography.
Torsion
Testicular torsion
Testicular torsion is the rotation of the testis with torsion of the spermatic cord and is the most common cause of acute scrotum (86%) in adolescents (aged 13–21 years) and the second most common cause (34%) in younger children (0–12 years). 31,40 Testicular torsion should always be considered in the differential diagnosis in paediatric patients with acute onset of scrotal pain, nausea, abnormal lie, and loss of the ipsilateral cremasteric reflex, especially in adolescents. 31 Testicular torsion could lead to testicular ischemia and necrosis, and prompt and accurate diagnosis is crucial for testis salvage. 39 In suspected testicular torsion, immediate surgical exploration is advocated when the clinical findings are highly suspicious. Practically speaking, immediate ultrasound should only be performed either to explicate sonographic features supportive of an alternative diagnosis such as an epididymoorchitis when the clinical likelihood of torsion is low, or to establish the diagnosis of global testicular infarct in a potential missed torsion, without delaying clinical management. 41
The ultrasound examination should be performed by an experienced investigator as torsion is not an all-or-none phenomenon, but a complex condition with a spectrum of sonographic presentations. 26 Greyscale ultrasound findings in testicular torsion are variable: the testis may appear enlarged or demonstrate a heterogeneous echo pattern. Usually, the testicular echotexture shows a low echoic signal 4–6 h after the torsion due to oedema and swelling. After 24 h, a torted testis becomes more heterogeneous, reflecting congestion, ischemia, and/or haemorrhage. The most direct finding with respect to testicular torsion is a twisted spermatic cord, referred to as the “whirlpool sign.” 31 Colour Doppler studies with duplex spectral analysis of the testicular parenchyma and vessels are mandatory. Decreased or absent affected testicular flow has a sensitivity of 86–100%, and a specificity of 97.9–100%. 42 However, false-negative CDUS evaluations can occur in the setting of spontaneous de-torsion or intermittent torsion, which could exhibit hyperaemia and may be misdiagnosed as orchitis.
To date, no advantage of CEUS over CDUS has been found for acute testicular torsions in several case reports and investigations. 38,43–46 However, CEUS as an adjunct may be helpful in two specific situations in the paediatric population. Firstly, CEUS has the potential to provide improved sensitivity in detecting vascularity in the paediatric testis in pre-pubertal boys, in whom the testes are small and associated with relatively low flow and it is often difficult to demonstrate perfusion within a normal testis with CDUS (Figure 2). 28 Secondly, CEUS may be used for problem-solving to conclusively confirm the lack of perfusion in children with small testes in clinically suspected missed torsion with global infarct (Figure 3), where conventional ultrasound and CDUS imaging may result in diagnostic uncertainty due to apprehension of suboptimal assessment of flow in a child with testicular torsion. Strain elastography may show stiffer appearance as the parenchyma becomes oedematous. Although it does not help with the diagnosis, shear-wave elastography may have a prognostic value, as higher stiffness values caused by testicular torsion have been associated with qualitatively and quantitatively decreased spermatogenesis in an animal model. 47 These experimental findings, however, have not been validated in humans.
Figure 2.
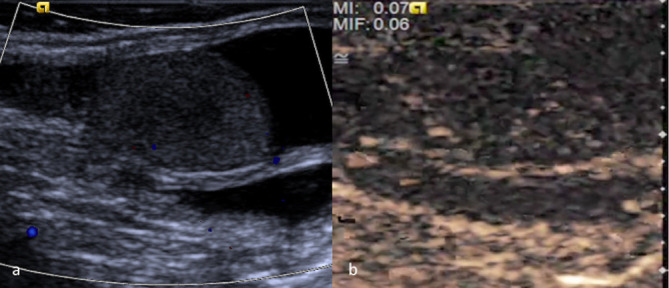
(a) CDUS of a testis in a pre-pubertal boy in whom the testes are small and demonstrate relatively low blood flow. It is difficult to demonstrate perfusion within the normal testis with CDUS. (b) CEUS of the same testis which confirms conclusively tissue perfusion by demonstrating movement of microbubbles within the vasculature. CDUS, colour-Doppler ultrasound; CEUS, contrast-enhanced ultrasound.
Figure 3.
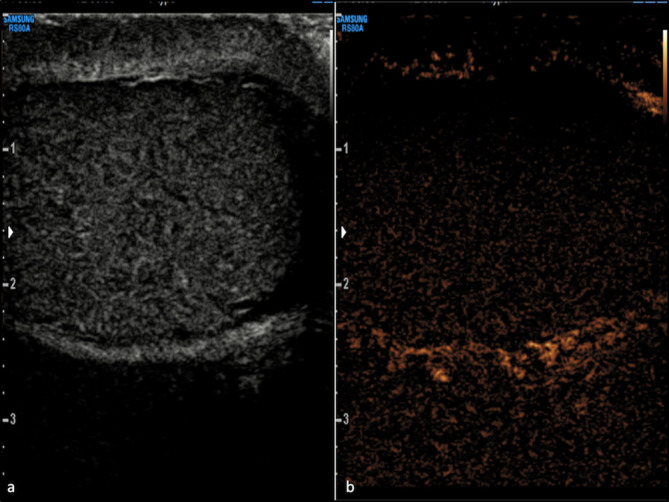
Global testicular infarct following a missed testicular torsion. Greyscale (a) showing diffuse heterogeneity of the testicular parenchyma. CEUS (b) conclusively demonstrates the absence of perfusion within a testis. CEUS, contrast-enhancedultrasound.
With regards to intermittent testicular torsion, MPUS could add useful information for evaluating a segmental infarction, which may be secondary to recurrent episodes of intermittent testicular torsion. When an intermittent torsion causes a segmental infarction, it may be barely detectable on the greyscale ultrasound in the early phase. Segmental infarcts may subsequently appear hypoechoic, with some hyperechoic areas if haemorrhage is present. Regardless of greyscale appearance, a segmental testicular infarction is invariably hypovascular or avascular on CDUS. 48 The differention of a segmental infarction from a hypovascular tumour such as a mixed germ cell tumour in paediatric patients may be problematic in rounded lesions when vascularity is not completely absent. 49 CEUS is able to demonstrate features suggestive of a segmental infarct, with distinct non-enhancing parenchymal lobules (Figure 4) with a perilesional rim enhancement in subacute segmental infarction which progressively decreases in size and eventually disappears. The evolution and size reduction of the abnormality confirms the diagnosis. 50–52 On SE, early after the onset of pain segmental testicular infarction can be stiff at the periphery and soft in the centre, due to the oedema and haemorrhagic changes. Later on, the lesion is usually soft. 27
Figure 4.
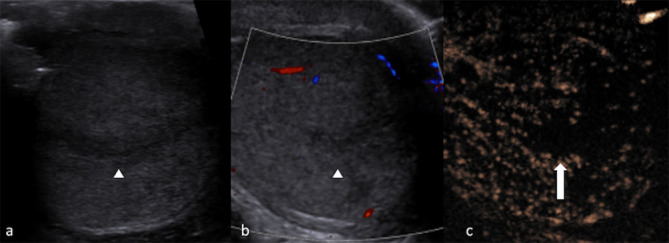
Testicular segmental infarct. Greyscale image (a) showing a focal area outlined by a hypoechoic halo. CDUS (b) demonstrates scarce blood flow signals. Arrowheads outline the abnormality in (a, b). Confirmation of lack of enhancement on CEUS (c) differentiates a segmental infarction (block arrow) from a vascular tumour. CDUS, colour-Doppler ultrasound; CEUS, contrast-enhanced ultrasound.
Trauma
Testicular trauma is common in children, usually due to a sporting activity. 53 Testicular trauma causes haemorrhage and infarction of the parenchyma which could ultimately lead to necrosis. In serious cases, disruption of the tunica albuginea accompanied by parenchymal protrusion can occur and is an indication for urgent surgery. 53 The essential remit of ultrasound in the setting of blunt scrotal injury is to confirm testicular viability and to establish the need for urgent operative intervention to salvage the testis. 54
Greyscale ultrasound findings of a traumatised testis include changes in parenchymal echogenicity due to haemorrhage and ischaemia, and irregular testicular contours or discontinuity of the tunica albuginea suggesting testicular rupture. However, the extent of haematoma and haemorrhagic areas may be underestimated on greyscale ultrasound, as the echogenicity of the haematoma can be similar to the normal testis. It is also challenging to clearly establish testicular tissue viability on CDUS, as the testicular vascular signal could be globally reduced on CDUS in the injured testis due to oedema. 53
CEUS is highly sensitive in demonstrating parenchymal vascularity and its changes in the traumatised testis. 37 CEUS has been found to show weak, inhomogeneous, patchy enhancement or complete loss of enhancement in a severely fractured testis. CEUS can conclusively delineate the fracture line and confirm the lack of enhancement in the non-vascularised parenchyma (Figure 5). It has been shown that CEUS adds to conventional ultrasound assessment in planning clinical and surgical management of the patient by establishing a clear boundary between viable and non-viable testicular tissue, allowing accurate surgical debridement. 54
Figure 5.
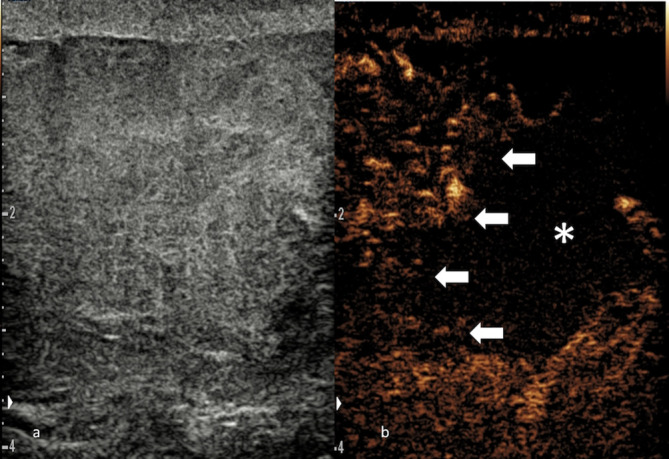
Testicular fracture. Greyscale mode (a) showing a diffusely heterogenous testis, where the viable part of the parenchyma cannot be differentiated form the non-viable. CEUS (b) conclusively delineates the fracture line (blocked arrows) and confirm the lack of enhancement in the traumatized, non-vascularized section of testicular parenchyma (*), thus guiding surgical treatment. CEUS, contrast-enhancedultrasound.
Following trauma, a haematoma may be sonographically complex, developing septations in the chronic phase or demonstrating penetrating vessels, features mimicking a small hypoechoic lesion. It is important to be confident that a focal intratesticular abnormality incidentally found after trauma is a hematoma rather than an incidental vascularised focal intratesticular lesion. MPUS could add diagnostic confidence if the haematoma demonstrates minimal internal enhancement on CEUS, and does not appear “hard“ on SE, with resolution on follow-up ultrasound examinations (Figure 6). 55
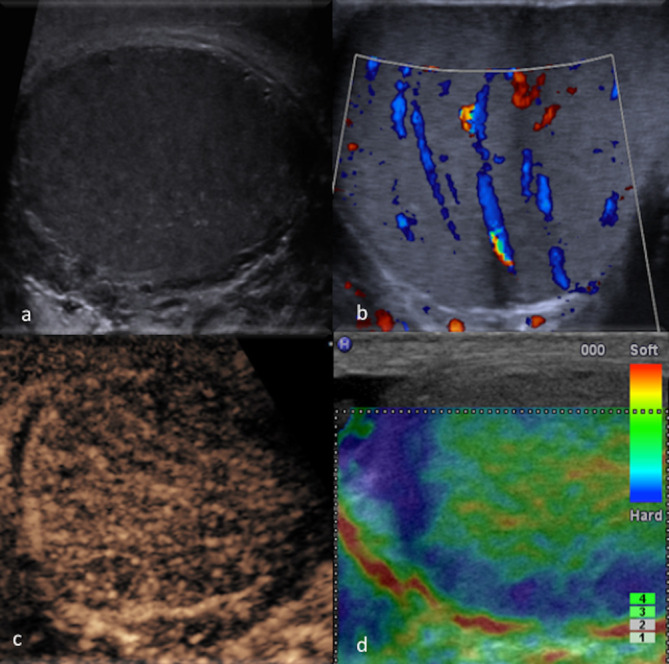
Figure 6.
MPUS of a testicular hematoma. The haematoma appears hypoechoic on greyscale (a) and avascular on CDUS (b). CEUS (c) can conclusively confirm the lack of enhancement in the non-vascularised haematomas (*). On SE (d), focal post-traumatic haematoma can show a variable pattern, but may not appear as hard as a focal malignancy. CDUS, colour-Doppler ultrasound; CEUS, contrast-enhanced ultrasound; SE, strainelastography
Inflammation
Inflammatory disease of the scrotum includes epididymoorchitis and is mainly idiopathic when affecting younger children or associated with sexually transmitted disease in adolescents. Inflammation of the epididymis (epididymitis) appears with enlargement of the epididymis with increased echogenicity and CDUS signal. The head of the epididymis is the most frequently affected part. Simultaneous disease of epididymis and testis can be found in up to 40% of cases. The findings are similar when the inflammation primarily affects the testis. Complications of severe inflammatory disease include abscess formation and venous infarction, although such entities may be less frequent in the paediatric population. 1 In the setting of inflammation, CEUS is virtually unnecessary in uncomplicated patients. Nonetheless, CEUS is valuable in the evaluation of complicated epididymoorchitis. Namely, it has been found to better delineate and help establish the diagnosis of abscesses and venous infarction in adult patients. 56 An abscess should typically exhibit a rim of increased enhancement but absolute lack of internal enhancement. The absence of internal vascularity as documented with CEUS was also reported for extratesticular abscesses. 56,57 CEUS is excellent in establishing the absence of vascularity (Figure 7). This is particularly useful in cases of abscesses containing echogenic material or extending across a large part of the hemiscrotum.
Figure 7.
MPUS of an epididymal abscess (*). Greyscale (a) showing a mixed echogenicity paratesticular lesion (asterisk) which shows intense peripheral vascularity on CDUS (b) but no internal blood flow signals. The lack of internal enhancement is better appreciated on CEUS (c), where the lesion is better demarcated. On SE (d) the abscess appears as “soft“ elasticity pattern (represented by the predominantly green colour pattern in this case). CDUS, colour-Doppler ultrasound; CEUS, contrast-enhanced ultrasound; MPUS, multiparametric ultrasound;SE, strain elastography
Tumours and Tumour-mimics
Cystic lesions
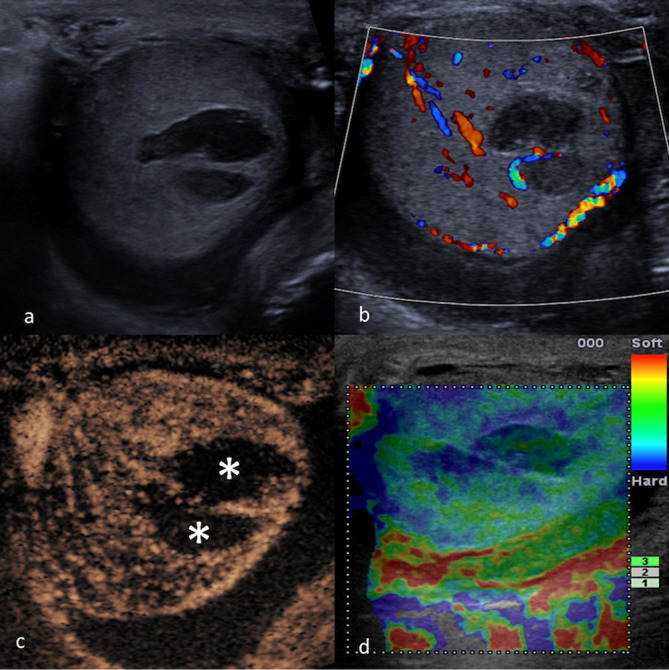
Simple cysts are only rarely found in the paediatric testis and either associated with rete testis or tunica albuginea. A complicated cyst containing proteinaceous content, blood or other form of echogenic debris may mimic a solid focal lesion. In this setting, it is of great importance to establish the absence of vascularity within the echogenic material. CEUS could be used to conclusively demonstrate the absence of internal vascularity with a complicated cyst and exclude the diagnosis of a neoplasm. Similarly, epidermoid cysts, which are well-defined lesions with a lamellated echogenicity consisting of multiple concentric layers of alternating echogenicity and an outer hyperechoic rim, could also mimic testicular tumours. The key to differentiate an epidermoid cyst from a vascular tumour is the confirmation of absence of internal vascularity. CEUS could be used in this context to conclusively demonstrate the absence of internal vascularity within an epidermoid cyst (Figure 8) to confirm benignity with increased confidence. 58
Figure 8.
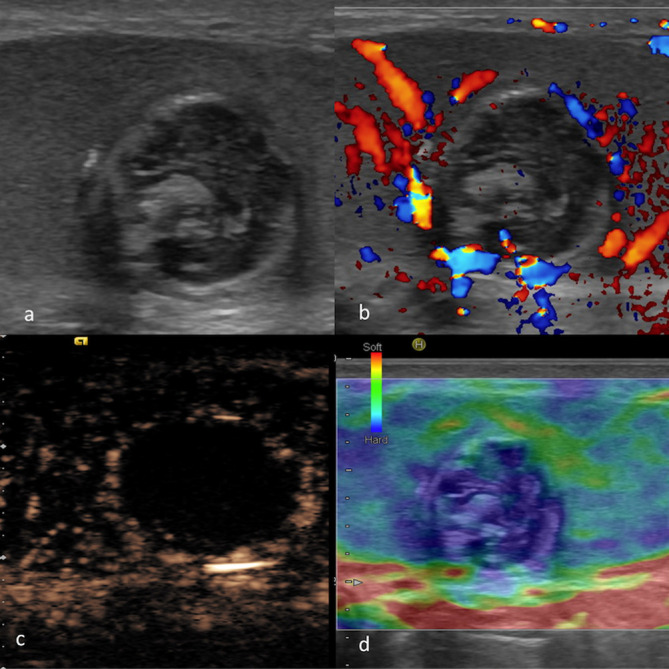
MPUS of an epidermoid cyst. Greyscale ultrasound (a) showing a rounded lesion of mixed echogenicity with partially calcified wall, which was avascular on CDUS (b). On MPUS, an epidermoid cyst typically appears as a sharply defined mass, completely unenhancing on CEUS (c) and “hard“ on SE (d) (indicated by the homogeneously “blue“ colour pattern). CDUS, colour-Doppler ultrasound; CEUS, contrast-enhanced ultrasound; MPUS, multiparametric ultrasound;SE, strain elastography.
Solid lesions
Testicular tumours in paediatric population are uncommon (1–2% of all paediatric solid neoplasms) with an incidence of 0.5–2/100,000 boys. 30,59 Unlike pre-pubertal lesions, most of post-pubertal intra testicular masses have similar pathology as adults, being often malignant and require orchiectomy. 59 Testicular tumours are categorised as non-germ cell tumours and germ cell tumours. The latter are further classified as seminomas and non-seminomatous tumours, which are the most common testicular tumour in boys. Yolk sac tumours are the most frequent type of germ cell tumour affecting prepubertal children. These tumours appear as heterogeneous, well-defined solid focal masses, although they may occasionally present with diffuse testicular enlargement. Other cellular types giving rise to tumours include cells of the gonadal stroma (Leydig cell tumour), sex cord cells (Sertoli cell tumour), and this group of tumours is referred to as non-germ cell tumours. Leydig cell tumours usually affect children between 3 and 6 years of age and may be a cause of precocious puberty. As in adults, Leydig cell are benign entities in over 90% of cases in the post-pubertal population.
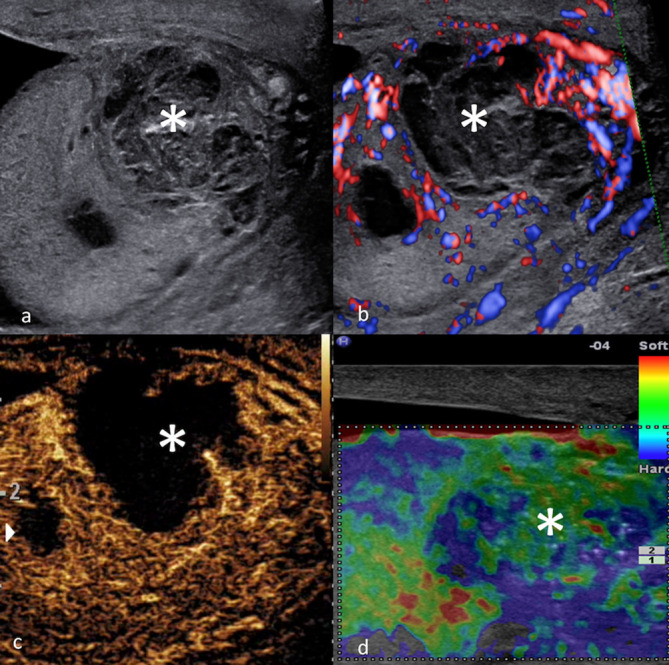
The finding of a vascular focal intratesticular lesion nearly always implies the presence of a malignant lesion, and orchidectomy is the standard surgical management. A testicular malignant tumour, broadly speaking, appears as a solid intratesticular mass, vascularised on CDUS and CEUS, and “hard“ on elastography (Figure 9). 25 Although the evaluation of vascularity is not a pre-rogative of CEUS alone, CEUS enables a substantial improvement in accuracy in detecting vascularity. SE on its own is not able to accurately differentiate malignant from benign testicular lesions, but current evidence suggests a “soft“ abnormality independent of vascularity, is probably benign. 21,22 It has been suggested that Leydig cell tumours display an enhancement pattern on CEUS of early avid hyperenhancement with delayed washout (Figure 10). 25,60 Although currently a research tool, there have been promising investigations on using CEUS time–intensity curves analysis to differentiate tumour types in the adult population. 61–63 It should however be clarified that differentiation between benign and malignant paediatric scrotal tumours with MPUS is an issue of current ongoing research and definite conclusions on this application may be drawn in the future.
Figure 9.
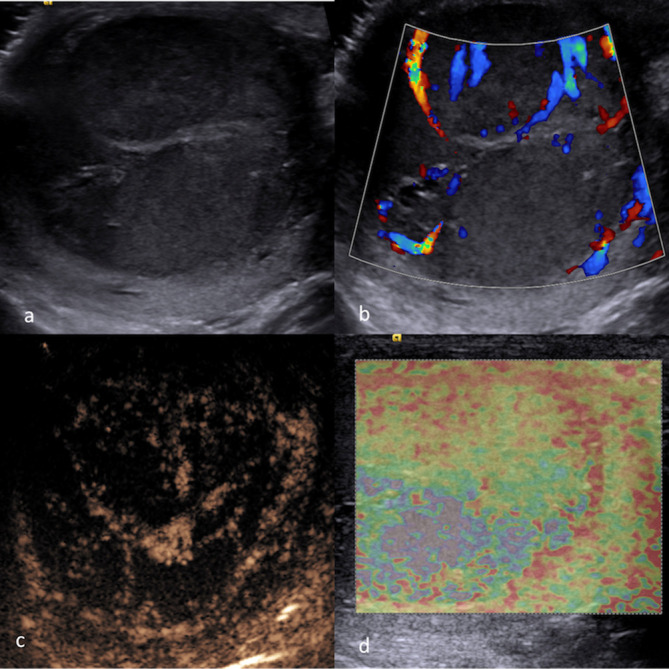
MPUS of a malignant testicular mixed germ cell tumor. Greyscale ultrasound (a) identifying the focal hypoechoic lesion. CDUS (b) shows blood flow signals within the lesion. On MPUS, the mixed germ cell tumour appeared as a solid intratesticular mass with enhancement on CEUS (c), and areas of “hardness“ (indicated by the presence of ill-demarcated “blue“ colour pattern) on SE (d). CDUS, colour-Doppler ultrasound; CEUS, contrast-enhanced ultrasound; MPUS, multiparametric ultrasound;SE, strain elastography.
Figure 10.
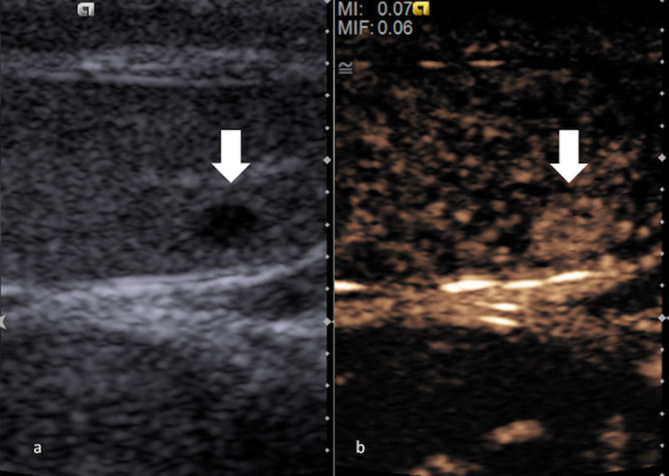
Greyscale ultrasound (a) showing a small hypoechoic Leydig cell tumour (block arrow). On CEUS (b), the Leydig cell tumour (block arrow) can demonstrate a strong, rapid and prolonged hyperenhancement. CEUS, contrast-enhancedultrasound.
Extratesticular masses
Solid or complex extratesticular scrotal masses, which can mimic intratesticular tumours, represent a range of tissues commonly encountered in paediatrics. These include congenital conditions such as supernumerary testes, benign lesions, such as an adenomatoid lesion (Figure 11) or a spermatic cord lipoma (Figure 12), but also life-threatening malignancies, such as a rhabdomyosarcoma. Unfortunately, on MPUS there is a considerable overlap in the appearances of many solid extratesticular masses, precluding a specific diagnosis in most cases. 57
Figure 11.
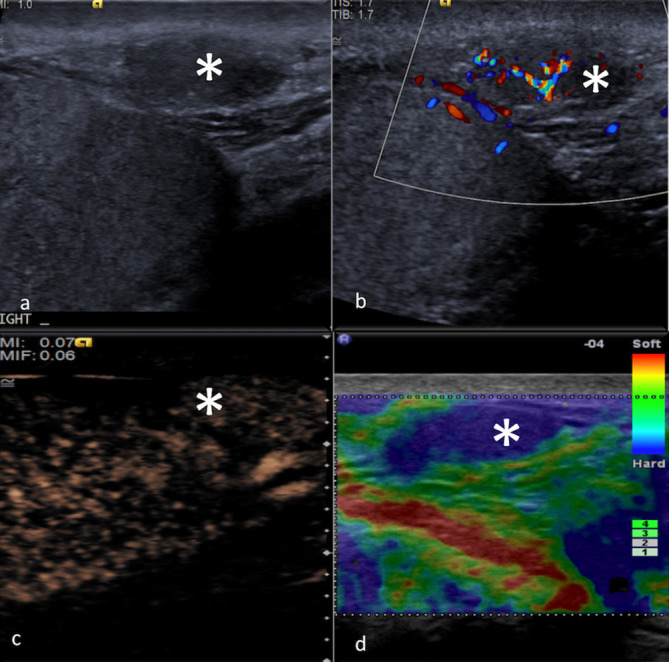
MPUS of an extratesticular adenomatoid lesion (*). On greyscale ultrasound (a) the lesion appears as a hypoechoic lesion in close proximity with the testis. CDUS (b) shows some degree of internal blood flow signals. On CEUS (c), the lesion is hypoenhancing relative to testicular parenchyma but this CEUS pattern is non-specific and the CEUS appearances of an extratesticular adenomatoid lesion can be variable. On SE (d), this lesion appears as “hard“ (represented by the homogeneously blue colour pattern), but the appearances on SE can be variable. CDUS, colour-Doppler ultrasound; CEUS, contrast-enhanced ultrasound; MPUS, multiparametric ultrasound;SE, strain elastography.
Figure 12.
MPUS of a spermatic cord lipoma (*). Greyscale (a) shows a hyperechoic extra testicular mass, with no internal vascularity noted on CDUS (b). CEUS (c) (split screen: CEUS on the left and the corresponding greyscale mode on the right) shows hypoenhancement relative to testicular parenchyma. The CEUS enhancement pattern of a spermatic cord lipoma however is variable. On SE (d) (split screen: SE on the left and the corresponding greyscale mode on the right) a “harder“ pattern is noted (represented by the predominantly blue colour pattern in this case). CDUS, colour-Doppler ultrasound; CEUS, contrast-enhanced ultrasound; MPUS, multiparametric ultrasound;SE, strain elastography.
Conclusion
Ultrasonography is well established as the first-line imaging method for children with scrotal pathology and often adequately identifies abnormalities to allow for appropriate clinical management. In the appropriate clinical indications, MPUS, in particular with CEUS, could increase operator diagnostic confidence and has the potential to influence, or even change, the diagnostic and therapeutic decisions.
Contributor Information
Dean Y. Huang, Email: dean.huang@nhs.net, Department of Radiology, King’s College Hospital, London. Denmark Hill, London SE5 9RS U.K, United Kingdom .
Filippo Pesapane, Email: filippopesapane@gmail.com, Breast Imaging Division, IEO European Institute of Oncology IRCCS, Via Giuseppe Ripamonti 435, 20141, Milan, Italy .
Vasileios Rafailidis, Email: v.rafailidis@nhs.net, Department of Radiology, King’s College Hospital, London. Denmark Hill, London SE5 9RS U.K, United Kingdom .
Annamaria Deganello, Email: adeganello@nhs.net, Department of Radiology, King’s College Hospital, London. Denmark Hill, London SE5 9RS U.K, United Kingdom .
Maria E. Sellars, Email: maria.sellars@nhs.net, Department of Radiology, King’s College Hospital, London. Denmark Hill, London SE5 9RS U.K, United Kingdom .
Paul S Sidhu, Email: paulsidhu@nhs.net, Department of Radiology, King’s College Hospital, London. Denmark Hill, London SE5 9RS U.K, United Kingdom .
REFERENCES
- 1. Delaney LR, Karmazyn B . Ultrasound of the pediatric scrotum . Semin Ultrasound CT MR 2013. ; 34: 248 – 56 . doi: 10.1053/j.sult.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 2. Sung EK, Setty BN, Castro-Aragon I . Sonography of the pediatric scrotum: emphasis on the Ts--torsion, trauma, and tumors . AJR Am J Roentgenol 2012. ; 198: 996 – 1003 . doi: 10.2214/AJR.11.8034 [DOI] [PubMed] [Google Scholar]
- 3. Piscaglia F, Bolondi L . Italian Society for ultrasound in M, biology Study Group on ultrasound contrast A. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations . Ultrasound Med Biol 2006. ; 32: 1369 – 75 . [DOI] [PubMed] [Google Scholar]
- 4. Darge K, Papadopoulou F, Ntoulia A, Bulas DI, Coley BD, Fordham LA, et al. . Safety of contrast-enhanced ultrasound in children for non-cardiac applications: a review by the Society for pediatric radiology (SPR) and the International contrast ultrasound Society (ICUs . Pediatr Radiol 2013. ; 43: 1063 – 73 . doi: 10.1007/s00247-013-2746-6 [DOI] [PubMed] [Google Scholar]
- 5. Coleman JL, Navid F, Furman WL, McCarville MB . Safety of ultrasound contrast agents in the pediatric oncologic population: a single-institution experience . AJR Am J Roentgenol 2014. ; 202: 966 – 70 . doi: 10.2214/AJR.13.12010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piskunowicz M, Kosiak W, Batko T, Piankowski A, Połczyńska K, Adamkiewicz-Drożyńska E . Safety of intravenous application of second-generation ultrasound contrast agent in children: prospective analysis . Ultrasound Med Biol 2015. ; 41: 1095 – 9 . doi: 10.1016/j.ultrasmedbio.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 7. Rosado E, Riccabona M . Off-Label use of ultrasound contrast agents for intravenous applications in children: analysis of the existing literature . J Ultrasound Med 2016. ; 35: e21 – 30 . doi: 10.7863/ultra.15.02030 [DOI] [PubMed] [Google Scholar]
- 8. Valentino M, Serra C, Zironi G, De Luca C, Pavlica P, Barozzi L . Blunt abdominal trauma: emergency contrast-enhanced sonography for detection of solid organ injuries . AJR Am J Roentgenol 2006. ; 186: 1361 – 7 . doi: 10.2214/AJR.05.0027 [DOI] [PubMed] [Google Scholar]
- 9. Menichini G, Sessa B, Trinci M, Galluzzo M, Miele V . Accuracy of contrast-enhanced ultrasound (CEUS) in the identification and characterization of traumatic solid organ lesions in children: a retrospective comparison with baseline US and CE-MDCT . Radiol Med 2015. ; 120: 989 – 1001 . doi: 10.1007/s11547-015-0535-z [DOI] [PubMed] [Google Scholar]
- 10. McCarville MB, Kaste SC, Hoffer FA, Khan RB, Walton RC, Alpert BS, et al. . Contrast-Enhanced sonography of malignant pediatric abdominal and pelvic solid tumors: preliminary safety and feasibility data . Pediatr Radiol 2012. ; 42: 824 – 33 . doi: 10.1007/s00247-011-2338-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Food & Drug Administration . APPROVED DRUG PRODUCT LIST . 2016. . Available from: http://www.fda.gov/downloads/drugs/developmentap- provalprocess/ucm071120.pdf .
- 12. Prandstetter C, Tamesberger M, Wagner O, Weissensteiner M, Wiesinger-Eidenberger G, Weidinger I, et al. . Medical prescriptions to premature and newborn infants in an Austrian neonatal intensive care unit . Klin Padiatr 2009. ; 221: 312 – 7 . doi: 10.1055/s-0029-1220903 [DOI] [PubMed] [Google Scholar]
- 13. Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane A, et al. . Survey of unlicensed and off label drug use in paediatric wards in European countries. European network for drug investigation in children . BMJ 2000. ; 320: 79 – 82 . doi: 10.1136/bmj.320.7227.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pandolfini C, Bonati M . A literature review on off-label drug use in children . Eur J Pediatr 2005. ; 164: 552 – 8 . doi: 10.1007/s00431-005-1698-8 [DOI] [PubMed] [Google Scholar]
- 15. Sellars ME, Deganello A, Sidhu PS . Paediatric contrast-enhanced ultrasound (CEUS): a technique that requires co-operation for rapid implementation into clinical practice . Ultraschall Med 2014. ; 35: 203 – 6 . doi: 10.1055/s-0034-1366567 [DOI] [PubMed] [Google Scholar]
- 16. Sidhu PS, Cantisani V, Deganello A, Dietrich CF, Duran C, Franke D, et al. . Role of contrast-enhanced ultrasound (CEUS) in paediatric practice: an EFSUMB position statement . Ultraschall Med 2017. ; 38: 33 – 43 . doi: 10.1055/s-0042-110394 [DOI] [PubMed] [Google Scholar]
- 17. Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, et al. . EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: basic principles and technology . Ultraschall Med 2013. ; 34: 169 – 84 . doi: 10.1055/s-0033-1335205 [DOI] [PubMed] [Google Scholar]
- 18. Kantarci F, Cebi Olgun D, Mihmanli I . Shear-Wave elastography of segmental infarction of the testis . Korean J Radiol 2012. ; 13: 820 – 2 . doi: 10.3348/kjr.2012.13.6.820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rocher L, Criton A, Gennisson J-L, Izard V, Ferlicot S, Tanter M, et al. . Testicular shear wave elastography in normal and infertile men: a prospective study on 601 patients . Ultrasound Med Biol 2017. ; 43: 782 – 9 . doi: 10.1016/j.ultrasmedbio.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 20. Camoglio FS, Bruno C, Peretti M, Bianchi F, Bucci A, Scirè G, et al. . The role of sonoelastography in the evaluation of testes with varicocele . Urology 2017. ; 100: 203 – 6 . doi: 10.1016/j.urology.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 21. Aigner F, De Zordo T, Pallwein-Prettner L, Junker D, Schäfer G, Pichler R, et al. . Real-Time sonoelastography for the evaluation of testicular lesions . Radiology 2012. ; 263: 584 – 9 . doi: 10.1148/radiol.12111732 [DOI] [PubMed] [Google Scholar]
- 22. Goddi A, Sacchi A, Magistretti G, Almolla J, Salvadore M . Real-Time tissue elastography for testicular lesion assessment . Eur Radiol 2012. ; 22: 721 – 30 . doi: 10.1007/s00330-011-2312-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sidhu PS, Ultrasound M . MPUS) imaging: terminology describing the many aspects of ultrasonography . Ultraschall Med 2015. ; 36: 315 – 7 . [DOI] [PubMed] [Google Scholar]
- 24. Auer T, De Zordo T, Dejaco C, Gruber L, Pichler R, Jaschke W, et al. . Value of multiparametric us in the assessment of intratesticular lesions . Radiology 2017. ; 285: 640 – 9 . doi: 10.1148/radiol.2017161373 [DOI] [PubMed] [Google Scholar]
- 25. Huang DY, Sidhu PS . Focal testicular lesions: colour Doppler ultrasound, contrast-enhanced ultrasound and tissue elastography as adjuvants to the diagnosis . Br J Radiol 2012. ; 85( special_issue_1 );:: S41 – 53 85 Spec No . doi: 10.1259/bjr/30029741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, et al. . The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (long version . Ultraschall Med 2018. ; 39: e2 – 44 . [DOI] [PubMed] [Google Scholar]
- 27. Bertolotto M, Muça M, Currò F, Bucci S, Rocher L, Cova MA . Multiparametric us for scrotal diseases . Abdom Radiol 2018. ; 43: 899 – 917 . doi: 10.1007/s00261-018-1510-7 [DOI] [PubMed] [Google Scholar]
- 28. Kitami M . Ultrasonography of pediatric urogenital emergencies: review of classic and new techniques . Ultrasonography 2017. ; 36: 222 – 38 . doi: 10.14366/usg.17011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rafailidis V, Arvaniti M, Rafailidis D, Sfoungaris D . Multiparametric ultrasound findings in a patient with polyorchidism . Ultrasound 2017. ; 25: 177 – 81 . doi: 10.1177/1742271X16689808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riccabona M, Lobo ML, Augdal TA, Avni F, Blickman J, Bruno C, et al. . European Society of paediatric radiology abdominal imaging Task force recommendations in paediatric uroradiology, part X: how to perform paediatric gastrointestinal ultrasonography, use gadolinium as a contrast agent in children, follow up paediatric testicular microlithiasis, and an update on paediatric contrast-enhanced ultrasound . Pediatr Radiol 2018. ; 48: 1528 – 36 . doi: 10.1007/s00247-018-4147-3 [DOI] [PubMed] [Google Scholar]
- 31. Aso C, Enríquez G, Fité M, Torán N, Piró C, Piqueras J, et al. . Gray-scale and color Doppler sonography of scrotal disorders in children: an update . RadioGraphics 2005. ; 25: 1197 – 214 . doi: 10.1148/rg.255045109 [DOI] [PubMed] [Google Scholar]
- 32. Trottmann M, Marcon J, D’Anastasi M, Bruce MF, Stief CG, Reiser MF, et al. . Shear-Wave elastography of the testis in the healthy man – determination of standard values . Clin Hemorheol Microcirc 2016. ; 62: 273 – 81 . doi: 10.3233/CH-162046 [DOI] [PubMed] [Google Scholar]
- 33. EFSUMB safety guidelines .. Available from: http://www.efsumb-portal.org/ep/article.php?id=271 .
- 34. Coleman JL, Navid F, Furman WL, McCarville MB . Safety of ultrasound contrast agents in the pediatric oncologic population: a single-institution experience . American Journal of Roentgenology 2014. ; 202: 966 – 70 . doi: 10.2214/AJR.13.12010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McMahon CJ, Ayres NA, Bezold LI, Lewin MB, Alonzo M, Altman CA, et al. . Safety and efficacy of intravenous contrast imaging in pediatric echocardiography . Pediatr Cardiol 2005. ; 26: 413 – 7 . doi: 10.1007/s00246-004-0795-1 [DOI] [PubMed] [Google Scholar]
- 36. Darge K, Papadopoulou F, Ntoulia A, Bulas DI, Coley BD, Fordham LA, et al. . Safety of contrast-enhanced ultrasound in children for non-cardiac applications: a review by the Society for pediatric radiology (SPR) and the International contrast ultrasound Society (ICUs . Pediatr Radiol 2013. ; 43: 1063 – 73 . doi: 10.1007/s00247-013-2746-6 [DOI] [PubMed] [Google Scholar]
- 37. Badea R, Lucan C, Suciu M, Vasile T, Gersak M . Contrast enhanced harmonic ultrasonography for the evaluation of acute scrotal pathology. A pictorial essay . Med Ultrason 2016. ; 18: 110 – 5 . doi: 10.11152/mu.2013.2066.181.esy [DOI] [PubMed] [Google Scholar]
- 38. Valentino M, Bertolotto M, Derchi L, Bertaccini A, Pavlica P, Martorana G, et al. . Role of contrast enhanced ultrasound in acute scrotal diseases . Eur Radiol 2011. ; 21: 1831 – 40 . doi: 10.1007/s00330-010-2039-5 [DOI] [PubMed] [Google Scholar]
- 39. Ben-Chaim J, Leibovitch I, Ramon J, Winberg D, Goldwasser B . Etiology of acute scrotum at surgical exploration in children, adolescents and adults . Eur Urol 1992. ; 21: 45 – 7 . [DOI] [PubMed] [Google Scholar]
- 40. Sidhu PS . Clinical and imaging features of testicular torsion: role of ultrasound . Clin Radiol 1999. ; 54: 343 – 52 . doi: 10.1053/crad.1999.0178 [DOI] [PubMed] [Google Scholar]
- 41. Zini L, Mouton D, Leroy X, Valtille P, Villers A, Lemaitre L, et al. . Should scrotal ultrasound be discouraged in cases of suspected spermatic cord torsion? Prog Urol 2003. ; 13: 440 – 4 . [PubMed] [Google Scholar]
- 42. Dogra VS, Gottlieb RH, Oka M, Rubens DJ . Sonography of the scrotum . Radiology 2003. ; 227: 18 – 36 . doi: 10.1148/radiol.2271001744 [DOI] [PubMed] [Google Scholar]
- 43. Cosgrove DO, Kiely P, Williamson R, Blomley MJK, Eckersley RJ . Ultrasonographic contrast media in the urinary tract . BJU Int 2000. ; 86( Suppl 1 ): 11 – 17 . doi: 10.1046/j.1464-410X.2000.00580.x [DOI] [PubMed] [Google Scholar]
- 44. Catalano O, Lobianco R, Sandomenico F, Mattace Raso M, Siani A . Real-Time, contrast-enhanced sonographic imaging in emergency radiology . Radiol Med 2004. ; 108( 5–6 ): 454 – 69 . [PubMed] [Google Scholar]
- 45. Yusuf GT, Sidhu PS . A review of ultrasound imaging in scrotal emergencies . J Ultrasound 2013. ; 16: 171 – 8 . doi: 10.1007/s40477-013-0033-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moschouris H, Stamatiou K, Lampropoulou E, Kalikis D, Matsaidonis D . Imaging of the acute scrotum: is there a place for contrast-enhanced ultrasonography? Int. braz j urol. 2009. ; 35: 692 – 705 discussion 702-695 . doi: 10.1590/S1677-55382009000600008 [DOI] [PubMed] [Google Scholar]
- 47. Zhang X, Lv F, Tang J, elastography Swave . Swe) is reliable method for testicular spermatogenesis evaluation after torsion . Int J Clin Exp Med 2015. ; 8: 7089 – 97 . [PMC free article] [PubMed] [Google Scholar]
- 48. Sriprasad S, Kooiman GG, Muir GH, Sidhu PS . Acute segmental testicular infarction: differentiation from tumour using high frequency colour Doppler ultrasound . Br J Radiol 2001. ; 74: 965 – 7 . doi: 10.1259/bjr.74.886.740965 [DOI] [PubMed] [Google Scholar]
- 49. Bertolotto M, Cantisani V, Valentino M, Pavlica P, Derchi LE . Pitfalls in imaging for acute scrotal pathology . Semin Roentgenol 2016. ; 51: 60 – 9 . doi: 10.1053/j.ro.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 50. Patel KV, Huang DY, Sidhu PS . Metachronous bilateral segmental testicular infarction: multi-parametric ultrasound imaging with grey-scale ultrasound, Doppler ultrasound, contrast-enhanced ultrasound (CEUS) and real-time tissue elastography (RTE . J Ultrasound 2014. ; 17: 233 – 8 . doi: 10.1007/s40477-014-0098-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zebari S, Huang DY, Wilkins CJ, Sidhu PS . Acute testicular segmental infarct following endovascular repair of a Juxta-renal abdominal aortic aneurysm: case report and literature review . Urology 2018. ;. [DOI] [PubMed] [Google Scholar]
- 52. Bertolotto M, Derchi LE, Sidhu PS, Serafini G, Valentino M, Grenier N, et al. . Acute segmental testicular infarction at contrast-enhanced ultrasound: early features and changes during follow-up . American Journal of Roentgenology 2011. ; 196: 834 – 41 . doi: 10.2214/AJR.10.4821 [DOI] [PubMed] [Google Scholar]
- 53. Bhatt S, Dogra VS . Role of US in testicular and scrotal trauma . RadioGraphics 2008. ; 28: 1617 – 29 . doi: 10.1148/rg.286085507 [DOI] [PubMed] [Google Scholar]
- 54. Hedayati V, Sellars ME, Sharma DM, Sidhu PS . Contrast-Enhanced ultrasound in testicular trauma: role in directing exploration, debridement and organ salvage . Br J Radiol 2012. ; 85: e65 – 8 . doi: 10.1259/bjr/95600238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yusuf G, Konstantatou E, Sellars ME, Huang DY, Sidhu PS . Multiparametric sonography of testicular hematomas: features on Grayscale, color Doppler, and contrast-enhanced sonography and strain elastography . J Ultrasound Med 2015. ; 34: 1319 – 28 . [DOI] [PubMed] [Google Scholar]
- 56. Lung PFC, Jaffer OS, Sellars ME, Sriprasad S, Kooiman GG, Sidhu PS . Contrast-Enhanced ultrasound in the evaluation of focal testicular complications secondary to epididymitis . AJR Am J Roentgenol 2012. ; 199: W345 – 54 . doi: 10.2214/AJR.11.7997 [DOI] [PubMed] [Google Scholar]
- 57. Rafailidis V, Robbie H, Konstantatou E, Huang DY, Deganello A, Sellars ME, et al. . Sonographic imaging of extra-testicular focal lesions: comparison of grey-scale, colour Doppler and contrast-enhanced ultrasound . Ultrasound 2016. ; 24: 23 – 33 . doi: 10.1177/1742271X15626195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patel K, Sellars ME, Clarke JL, Sidhu PS . Features of testicular epidermoid cysts on contrast-enhanced sonography and real-time tissue elastography . J Ultrasound Med 2012. ; 31: 115 – 22 . doi: 10.7863/jum.2012.31.1.115 [DOI] [PubMed] [Google Scholar]
- 59. Kleigman R. M, Behrman R. E, Jenson H. B . eds. Elder JS: Testicular tumor . In : Nelson Textbook of Pediatrics, ed 18 . Philadelphia: : PA, Saunders Elsevier; ; 2007. . pp. 2264 – 2265 . [Google Scholar]
- 60. Lock G, Schröder C, Schmidt C, Anheuser P, Loening T, Dieckmann K . Contrast-Enhanced ultrasound and real-time elastography for the diagnosis of benign Leydig cell tumors of the testis – a single center report on 13 cases . Ultraschall in Med 2014. ; 35: 534 – 9 . doi: 10.1055/s-0034-1385038 [DOI] [PubMed] [Google Scholar]
- 61. Luzurier A, Maxwell F, Correas JM, Benoit G, Izard V, Ferlicot S, et al. . Qualitative and quantitative contrast-enhanced ultrasonography for the characterisation of non-palpable testicular tumours . Clin Radiol 2018. ; 73: 322.e1 – 322.e9 . doi: 10.1016/j.crad.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 62. Isidori AM, Pozza C, Gianfrilli D, Giannetta E, Lemma A, Pofi R, et al. . Differential diagnosis of nonpalpable testicular lesions: qualitative and quantitative contrast-enhanced us of benign and malignant testicular tumors . Radiology 2014. ; 273: 606 – 18 . doi: 10.1148/radiol.14132718 [DOI] [PubMed] [Google Scholar]
- 63. Drudi FM, Valentino M, Bertolotto M, Malpassini F, Maghella F, Cantisani V, et al. . CEUS time intensity curves in the differentiation between Leydig cell carcinoma and seminoma: a multicenter study . Ultraschall Med 2016. ; 37: 201 – 5 . doi: 10.1055/s-0034-1398841 [DOI] [PubMed] [Google Scholar]