Abstract
Objectives:
To compare the performance of arterial spin labelling (ASL) in evaluating arteriovenous malformations (AVMs) against the current gold standard of catheter angiography.
Methods:
We systematically reviewed the published literature using EMBASE and Medline. We included studies that compared ASL to catheter angiography in the assessment of AVMs in three outcome domains: detection, angioarchitectural and haemodynamic features.
Results:
From 314 unique citations, 19 studies representing 289 patients with intracranial AVMs met our inclusion criteria. We did not pool data due to marked heterogeneity in study outcome measures. Seven studies showed high diagnostic performance of ASL in identifying arterial feeders, with sensitivity ranging from 84.6 to 100% and specificity ranging from 93.3 to 100%. Six studies showed strong ability in detecting arteriovenous shunting, with sensitivity ranging from 91.7 to 100% and specificity ranging from 90 to 100%. Seven studies demonstrated that ASL could identify nidal location and size as well as catheter angiography, while five studies showed relatively poorer performance in delineating venous drainage. Two studies showed 100% sensitivity of ASL in the identification of residual or obliterated AVMs following stereotactic radiosurgery.
Conclusions:
Despite limitations in the current evidence base and technical challenges, this review suggests that ASL has a promising role in the work-up and post-treatment follow-up of AVMs. Larger scale prospective studies assessing the diagnostic performance of ASL are warranted.
Advances in knowledge:
ASL demonstrates overall validity in the evaluation of intracranial AVMs.
Introduction
According to the International Society for the Study of Vascular Anomalies classification, arteriovenous malformations (AVMs) are high-flow, typically congenital lesions where there is an abnormal connection between feeding arteries and draining veins without an intervening capillary bed. 1 The result is that blood is shunted through a collection of dysmorphic vessels referred to as the nidus. 1,2 AVMs are rare and occur most frequently within the central nervous system, with an estimated incidence of approximately 1 per 100,000 per year in unselected populations. 3,4 Other sites include the trunk and the peripheries.
There are four primary treatment options for AVMs: conservative management, surgery, endovascular embolisation or radiosurgery. 5 Each has its own advantages and disadvantages, and the decision regarding which option to pursue is often decided based on patient characteristics and features of the AVM. For example, the Spetzler-Martin classification has been developed to predict risk of open neurosurgery for patients with AVMs, and accounts for size, location and venous drainage of the AVM. 6 Furthermore, advanced knowledge of the architectural features of the AVM, including the size and location of the nidus and information on feeding arteries, is essential for operative or endovascular planning. Moreover, some features such as deep location, single draining vein and deep venous drainage are associated with an increased risk of haemorrhage from intracranial AVMs, 4,7,8 allowing clinicians to identify high-risk patients. As such, imaging plays a pivotal role in the workup of AVMs. While CT angiography and magnetic resonance angiography (MRA) are important in the workup, catheter angiography is considered the gold standard. 5
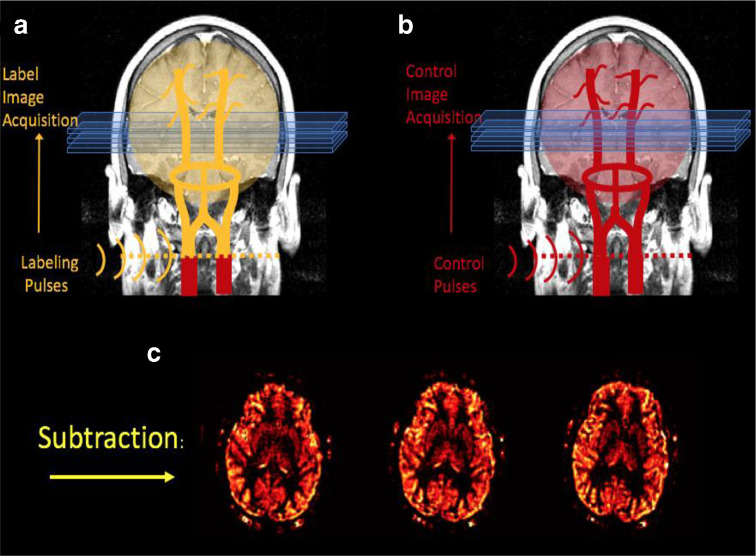
Arterial spin labelling (ASL) is a magnetic resonance (MR) perfusion technique where water in arterial blood is used as an endogenous freely diffusible tracer. ASL involves labelling inflowing arterial blood by applying an inversion radiofrequency pulse proximal to the imaging plane, the nature of which varies according to the specific method employed. 9 Subsequently, signal from the arterial blood flow in the imaging plane is subtracted from unlabelled control images to provide a subtracted image. As a result, the signal intensity in the resultant image is proportional to blood flow 10,11 (Figure 1). ASL has emerged as a useful tool in the assessment of a range of neurological conditions where cerebral perfusion is altered, ranging from acute strokes and tumours to vascular malformations and chronic cerebrovascular disease. 12–15
Figure 1.
A diagram outlining the principle of pseudocontinuous ASL imaging. Inflowing arterial water is labelled by applying an inversion radiofrequency pulse at the neck. A “labelled” image is then acquired. Subsequently, a “control” image is acquired without labelling. The final image is produced by subtracting the “labelled” image from the “control” image. Diagram reproduced with permission from the Functional MRI Laboratory, University of Michigan.
To our knowledge, there have been no systematic reviews focused on the utility of ASL in the imaging of AVMs. This systematic review therefore sought to compare the diagnostic performance of ASL in the assessment and characterisation of AVMs against the current gold standard of catheter angiography.
Methods
Following a predefined protocol (available from the authors on request), we systematically reviewed the peer-reviewed, published literature according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. 16 We searched two electronic databases using the search terms detailed in the Supplementary Material 1: EMBASE and Medline for articles with no time or language restrictions. Reference lists of included studies were screened to identify further data.
After screening titles and abstracts, we performed a full-text review of remaining articles. The screening and full-text review were conducted by two independent reviewers (SR and DM) with arbitration in case of disagreement (NK). The reviewers were blinded from each other in the selection process. Inclusion criteria included any primary study that: 1) involved any form of ASL imaging; 2) had adult or paediatric patients with AVMs; and 3) provided a direct comparison with catheter angiography.
During screening and full-text review, a study was excluded once it met any exclusion criterion. The exclusion criteria were always applied in the following order: 1) ASL imaging was not used; 2) no patients with intracranial AVMs were included in study cohort; 3) catheter angiography was not performed for comparison; 4) no appropriate outcome measures reported; and 5) individual case reports or conference abstracts.
Outcomes were categorised according to three domains: overall detection (including accuracy of presence, location and size), architectural features (including nidus, feeding arteries and draining veins) and haemodynamic features (including flow and arteriovenous shunting). We extracted any data that allow direct comparison of ASL performance with catheter angiography in these domains. We recorded information in a standardised database on the following: study design, patient characteristics, sample size, outcome measures and associated statistical significance, ASL protocols (including labelling method, MR field strength and sequence parameters) and information pertaining to the quality assessment outlined below.
We gave a descriptive analysis of the outcomes and provided a qualitative analysis of the data. We did not pool outcomes due to the marked heterogeneity in study outcome measures and populations.
Using an adapted QUADAS-2 tool, 17 we evaluated the quality of studies according to five categories, each reflecting a key domain of QUADAS-2:
Study design (under the domain “patient selection”)
Patient enrolment method (under “patient selection”)
Risk of individual interpreter subjectivity (under “index test” and “reference standard”)
Risk of recall bias (under “index test” and “reference standard”)
Time elapsed between ASL imaging and catheter angiography (“flow and timing”)
Details of how each study was assessed against each criterion is outlined in the Supplementary Material 1.
Results
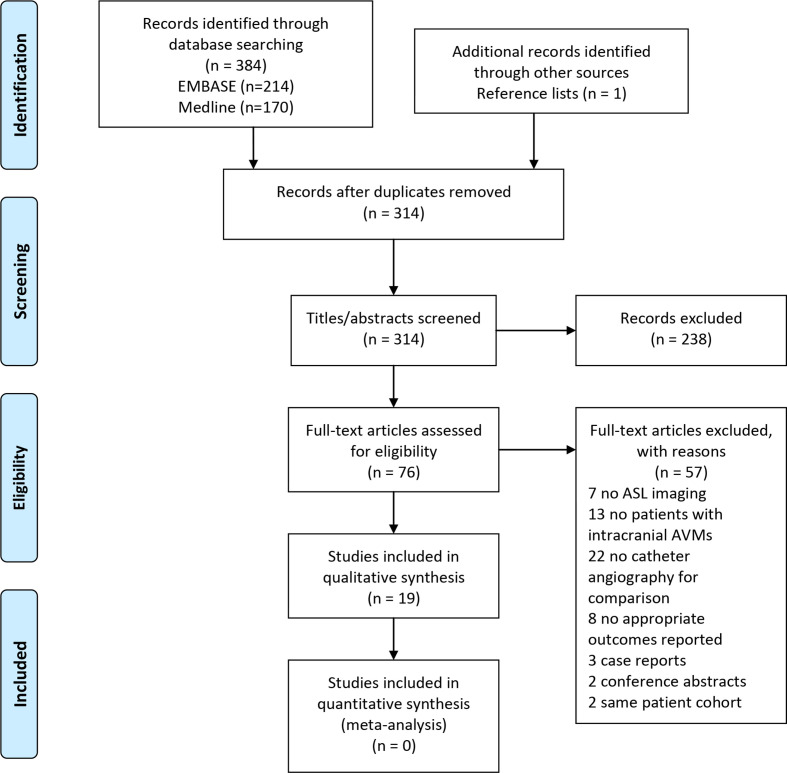
Of 314 unique records, we excluded 238 by abstract/title screening and 57 by full-text review (Figure 2). Nineteen studies met the inclusion criteria and provided outcomes on 289 patients with intracranial AVMs. There were no patients with AVMs located in other anatomical sites which met the inclusion criteria. A summary of patient characteristics and outcome measures by study is provided in Table 1.
Figure 2.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram outlining the selection process for studies
Table 1.
Summary of results for studies comparing the performance of ASL imaging in the assessment of intracranial AVMs with catheter angiography
| Study | Study design | Labelling method/MR field strength | Patient characteristics | Sample size | Outcome measure | Outcome value | Statistical significance |
| Kuluk 2010 | Prospective | Selective PASL / 3T | Symptomatic AVMs | 16 | Sensitivity in identification of feeding arteries in combination with MRA additional functional/anatomical information | 96–25% | |
| Amponsah 2012 | Retrospective | PASL / NR | γ knife radiosurgery follow up | 9 | Sensitivity to incomplete obliteration sensitivity to complete obliteration* | 100100% | 95% CI: 0.66–1 |
| Blauwblomme 2015 | Retrospective | pCASL / 1.5T | Paediatric (untreated or partially treated AVMs). Mean age: 10.8 (range 3.6–17) | 21 | Accuracy of nidus location | 100% | |
| Wu 2014 | Prospective | pCASL / 3T | Previously diagnosed AVMs | 3 | Sensitivity to arterial pedicles Accuracy of nidus location | 87.5100% | |
| Jensen-Kondering 2015 | Prospective | Superselective pCASL / 1.5T | Clinical cases. Age range 20–65, 1F | 2 | Sensitivity in identification of feeding arteries | 100% | |
| Sunwoo 2015 | Retrospective | pCASL / 3T | Treated & untreated AVMs. Mean age: 37.4 (range 14–72), 17F | 40 | Correlation between AVM signal intensity on ASL and degree of early vein opacification on DSA Correlation between AVM signal intensity and size on DSA | 0.638 0.561 | p < 0.001 p<0.001 |
| Schubert 2018 | Retrospective | pCASL / mixed 1.5 & 3T | Demonstrating AV shunting. Mean age: 58.5 (41–75), 5F | 8 | Agreement with DSA Spetzler-Martin classification (Cohen’s unweighted κ)Sensitivity to AV shunting (reader 1/2)Specificity to AV shunting (reader 1/2) | Reader 1: 0.43Reader 2: 0.6 100 % / 91.7100% / 100% | |
| Kodera 2017 | Retrospective | pCASL / 3T | Stereotactic radiosurgery follow up. Mean age: 48.7 (range 28–80), 3F | 7 | Sensitivity to disappearance of AV shunting | 100% | |
| Le 2012 | Retrospective | pCASL / 1.5T | Small AVMs (<2 cm) combined with 8 AVFs. Combined mean age: 58 (range 11–69), 6F | 15 (7 with AVMs) | Sensitivity of venous ASL signal for presence of AVM/AVF on DSA**Specificity of above**Positive predictive value of above**Negative predictive value of above** | 78–85%8874% | 95% CI: 63–89%68–95%73–96%57–87% |
| Suazo 2012 | Prospective | PASL / 3T | Post-embolisation. Mean age: 40 (range 15–51), 3F | 8 | Reduction in signal of AVM on ASL for 10–20% shunt reduction estimated on DSA (four patients) Reduction in signal for 30–50% shunt reduction (two patients) Reduction in signal for 80–100% shunt reduction (two patients) | −6%† to 14–25 to 60%–69 to 76% | |
| Yu 2014 | Prospective | Vessel-encoded pCASL / 3T | Consecutive patients with AVMs. Mean age: 32.8 (range 13–47), 8F | 18 | Standard labelling efficiency‡:Sensitivity in identification of feeding arteriesSpecificity of above Area under the curve (AUC) Custom labelling efficiency‡:Sensitivity in identification of feeding arteries Specificity of aboveArea under the curve (AUC) | 84.62–93.33%0.935 89.74–93.33%0.957 | p < 0.01 P<0.01 |
| Nabavizadeh 2014 | Retrospective | PASL / 3T | Paediatric patients with angiographically confirmed AV shunting. Mean age: 9.2 (1 day – 17.5), 8F | 19 | Median number of draining veins on DSA/median number on ASL Sensitivity in detecting AV shunting | 3.63/1.76 100% | p < 0.05 |
| Heit 2019 | Retrospective | pCASL / mixed 1.5 & 3T | Stereotactic surgery follow up. Mean age: 29 (range 14–55), 12F | 15 | Sensitivity of venous signal for residual AVM Specificity of above Positive predictive value of above Negative predictive value of above | 100–95%98100% | 95% CI: 90–100%70–100%90–100%80–100% |
| Fujima 2016 | Retrospective | PASL / 3T | Newly diagnosed AVMs. Mean age: 32.3 (range 5–69), 4F | 12 | Mean sensitivity for identification of feeding arteries (vessel selective/non-vessel selective)§Mean specificity of above Mean positive predictive value Mean negative predictive value Mean sensitivity for identification of draining veins (vessel selective/non-vessel selective)§Mean specificity of above Mean positive predictive valueMean negative predictive value Nidus size on DSA / vessel selective / non-vessel detective (mean ± SD, mm) Error values of assessment of % contribution of feeding arteries to overall blood flow of AVM, DSA / vessel-selective (mean ± SD, %) | 98 % / 91–100% / 95.5100% / 84.599.5% / 97.5% 62 % / 79100% / 93100% / 96.552% / 65% 36.8 ± 13.1/34.4±11.8/34.7±11.3 27.1±5.7/7.1±3.9 | p = 0.82 p<0.05 |
| Hodel 2017 | Prospective | pCASL / 3T | Suspected AV shunting. Mean age: 47.4 (range 11–81, 40F | 53 | Sensitivity for presence of AVMSensitivity in identification of AV shuntingSpecificity of abovePositive predictive value of aboveNegative predictive value of aboveArea under the curve | 9895% 9095%90%0.92 | 95% CI: 90–100%78–100%87–99%73–98%0.84–0.97 |
| Iryo 2016 | Retrospective | pCASL / 3T | Untreated AVMs. Mean age: 39.5 (range 7–65), 4F | 6 | Intermodality agreement for nidus size (Cohen’s κ)Intermodality agreement for arterial feedersIntermodality agreement for venous drainage | 1.00 0.88 0.80 | 95% CI: 1.00–1.000.671-1.00 0.449–1.00 |
| Cong 2018 | Prospective | PASL / 3 & 7T | Mean age: 25.1 ± 13.2, 6F | 8 | Agreement with DSA Spetzler-Martin classification at 3 T / 7TAgreement with venous drainage pattern on DSA at 3 T / 7T | 50 % / 87.5–50% / 87.5% | |
| Togao 2019 | Retrospective | PASL / 3T | Consecutive patients with AVMs. Mean age: 31.1 (range 9–63), 10F | 21 | Sensitivity for presence of AVM§Mean intermodality agreement for Spetzler-Martin classification (Cohen’s κ)§Mean intermodality agreement for venous drainage pattern§Nidus size on DSA / ASL observer 1/ASL observer 2 (mean ± SD, mm) | 95.1%0.748 0.718 32.1 ± 23.3/27.2±23.3/29.9±20.4 | Not significant |
| Raoult 2014 | Prospective | PASL / 3T | Mean age: 42.0 ± 15.6, 9F | 16 | Intermodality agreement for nidus size (Cohen’s κ) over two cardiac cyclesIntermodality agreement for arterial feedersIntermodality agreement for venous drainageIntermodality agreement for Spetzler-Martin classification | 1.00 0.85 0.82 0.89 | 95% CI: 1.00–1.000.65-1.00 0.59–1.00 0.71–1.00 |
AUC, Area Under the Curve; AVF, arteriovenous fistula; CI, confidence interval; DSA, digital subtraction angiogram; MRA, magnetic resonance angiography; NR, not reported; PASL, pulsed arterial spin labelling; pCASL, pseudo continuous arterial spin labelling; SD, standard deviation; T, Tesla.
Defined as complete absence of nidus and draining veins.
Outcomes reported are a combination of AVMs and AVFs as data were not stratified according by type of malformation
-6%* denotes a slight increase in AVM ASL signal observed in one patient in this cohort
Custom labelling method involved altering ASL imaging parameters according to AVM characteristics in individual patients. Standard labelling method was a standardised ASL imaging protocol applied uniformly for each patient, and is outlined in the supplementary appendix. Sensitivities and specificities reported are calculated at the optimal cut-off of supply fraction to achieve a targeted balance between sensitivity and specificity.
Outcome measures were reported as a mean of two independent readers.
Description of studies
The majority of studies were retrospective in study design (n = 11, 57.9%), 18–28 and the remaining were prospective comparative studies (n = 8, 42.1%). 29–36 Sample sizes in studies were relatively small, with a mean of 15.2 patients per study (range: 2–53). Labelling methods and magnetic field strengths used in each study are outlined in Table 1. Other details of MR parameters used were not as consistently reported and are reported in the Supplementary Material 1.
Most studies did not employ restrictions on patient or AVM characteristics, but tended to primarily enrol a large adult population (n = 10, 52.6%). Only two studies (10.5%) focused exclusively on paediatric patients, with a combined sample size of 40 patients. 19,24 Some had focused on the follow-up for procedures to treat the AVM (n = 4, 21.1%), namely poststereotactic radiosurgery 18,22,25 and postembolisation 32 with a total of 39 patients. Two studies only included patients with untreated AVMs, 26,27 one of which recruited newly diagnosed AVMs. 26 One study only recruited patients with small AVMs (defined as <2 cm) and was combined with patients with arteriovenous fistulas (AVFs). 23
Study quality
The majority of studies were retrospective and were therefore deemed relatively low quality in this category (n = 11, 57.9%). Only two studies (10.5%) described recruitment methods intended to minimise selection bias, by using consecutive patients. 28,33 The vast majority of studies used at least two interpreters to assess the ASL imaging and were therefore deemed high quality in this category (n = 17, 89.5%). Most studies had described sufficient blinding to be assessed as high quality (n = 11, 57.9%). ASL imaging and catheter angiography were performed within 6 months of one another in most studies (n = 14, 73.7%), whereas timing was not reported in the remaining five studies. A full list of study quality assessments is provided in Table 2.
Table 2.
A summary of quality assessment of studies included in qualitative synthesis
| Study | Study design | Patient enrolment | Interpreter subjectivity | Confirmation bias (blinding) | Delay between modalities |
|---|---|---|---|---|---|
| Kukuk 2010 | High | Low | High | High | High |
| Amponsah 2012 | Low | Low | Low | Low | Unclear |
| Blauwblomme 2015 | Low | Low | High | Unclear | High |
| Wu 2014 | High | Low | High | Unclear | High |
| Jensen-Kondering 2015 | High | Low | High | Unclear | Unclear |
| Sunwoo 2015 | Low | Low | High | High | High |
| Schubert 2018 | Low | Low | High | High | Unclear |
| Kodera 2017 | Low | Low | High | Unclear | Unclear |
| Le 2012 | Low | Low | High | High | High |
| Suazo 2012 | High | Low | Unclear | Unclear | High |
| Yu 2014 | High | High | High | High | High |
| Nabavizadeh 2014 | Low | Low | High | High | High |
| Heit 2019 | Low | Low | High | High | High |
| Fujima 2016 | Low | Low | High | High | High |
| Hodel 2017 | High | Low | High | High | High |
| Iryo 2016 | Low | Low | High | High | High |
| Cong 2018 | High | Low | High | Unclear | Unclear |
| Togao 2019 | Low | High | High | Low | High |
| Raoult 2014 | High | Low | High | High | High |
Quality assessment was carried out using an adapted QUADAS-2 tool. A colour-coded system is used to represent the quality of the study under each domain, as opposed to risk of bias. Green is used if the study quality was high, red if the study quality was low and grey if there was insufficient information reported to formulate a judgement.
Detection of AVMs
Three studies demonstrated a sensitivity of 78%, 23 95.1% 28 and 98%, 34 respectively, in detecting the overall presence of an AVM, with the limitation that the former reflected a mixed cohort of patients with small AVMs and AVFs. 23 Moreover, two studies investigating the identification of AVMs following stereotactic radiosurgery demonstrated 100% sensitivity in detecting residual AVMs, 18,25 and high specificities of 95% 25 and 100%. 18
Four studies evaluated concordance in Spetzler-Martin classifications between ASL and catheter angiography. 21,28,35,36 There was an overall moderate intermodality agreement among three studies, 21,28,36 with Cohen’s κ coefficients ranging from 0.43 21 to 0.89. 36 The fourth showed high concordance at 87.5% at 7T, reducing to 50% at 3T. The discrepancies at both field strengths were solely due to inaccurate or inadequate delineation of venous drainage. 35 Finally, one study found a statistically significant, moderately positive correlation between overall AVM signal intensity of the AVM and size of the AVM measured during catheter angiography (r = 0.561, p < 0.001), 20 irrespective of whether the AVM had been previously treated or not.
Angioarchitectural features of AVMs
A number of studies assessed performance in identifying arterial feeders (n = 7, 36.8%). 26,27,29–31,33,36 Across five studies, 26,29–31,33 sensitivity in this domain ranged from 84.6% 33 to 100% 31 (median: 91%). Across two studies, specificity ranged from 93.33% 33 to 100%. 26 Good diagnostic performance was demonstrated for both standard and custom labelling efficiencies, with an area under the curve value of 0.94 and 0.96, respectively, (p < 0.01). 33 Interestingly, one study identified an arterial feeder on ASL which was not accessible on catheter angiography due to altered vasculature. 31 The final two studies revealed high intermodality agreement for delineating arterial feeders at 0.85 36 and 0.88, 27 respectively.
Fewer studies assessed venous drainage (n = 5, 26.3%) 24,26–28,36 and showed relatively lower performance in this domain. One study found the mean sensitivity and specificity for the identification of draining veins by vessel selective ASL to be 62 and 100%, respectively. Meanwhile, non-vessel selective ASL had slightly higher sensitivity at 79% but lower specificity at 93%. 26 Moreover, in a study of paediatric patients, the median number of draining veins detected on DSA was approximately twice as many as on ASL (3.63 on DSA versus 1.76 on ASL, p < 0.05). 24 Three studies showed similar intermodality agreement for venous drainage patterns at 0.72, 28 0.82 36 and 0.80 (95% CI: 0.45–1.00), 27 respectively, with the latter marginally lower compared to both arterial drainage and nidus size. 27 One of these studies demonstrated markedly superior intermodality agreement an acquisition window over two cardiac cycles. 36
AVM nidus
Some studies reported outcome measures on the AVM nidus (n = 6, 31.6%). 19,26–28,30,36 Two studies demonstrated 100% accuracy of ASL in determining nidus location from a combined cohort of 24 patients, 19,30 21 of whom were paediatric patients. Two studies showed no significant differences in nidus size across DSA and ASL. 26,28 Similarly, two studies found excellent intermodality agreement between ASL and catheter angiography for nidus size, with an overall Cohen’s κ value of 1.00 from a total of 22 patients. 27,36
Haemodynamic features of AVMs
Some studies reported haemodynamic outcome measures of AVMs, specifically of AV shunting (n = 6, 31.6%). 20,21,24,25,32,34 Sensitivity in detecting AV shunting ranged from 91.7% 21 to 100%, 21,24 including 100% sensitivity in an exclusive cohort of 19 paediatric patients. 24 Specificity was measured by two studies and ranged from 90% 34 to 100%. 21 Importantly, the study with the largest sample size in this review (53 patients) showed robust diagnostic performance of ASL in detecting AV shunting. 34 From a sample size of eight patients, one study demonstrated good agreement in quantifying AV shunt reduction postembolisation with operator-based estimates during catheter angiography. 32 Similarly, a further study showed 100% sensitivity in detecting the disappearance of AV shunting from a cohort of seven patients following on from stereotactic radiosurgery. 22
Discussion
Nineteen studies evaluated the diagnostic performance of ASL in the characterisation of intracranial AVMs in comparison with catheter angiography. Findings suggest that ASL has promising diagnostic potential, particularly in ascertaining the location of the AVM and the nidus, determining the presence of AV shunting and identifying arterial feeders. Of all outcome measures, the evidence for arterial feeders 26,27,29–31,33,36 and AV shunting 20,21,24,25,32,34 are the strongest, with several studies showing consistently high diagnostic performance in these domains. Furthermore, there is some evidence that ASL may provide a robust non-invasive imaging alternative to catheter angiography in assessing the response to treatment following stereotactic radiosurgery and embolisation. However, there is evidence from five studies that suggests inferior performance in the characterisation of venous drainage. In addition, ASL appears to be a usable imaging tool, with high interobserver agreement, 20,23,25–27,33–35 adequate diagnostic quality 27,33,35 and acceptable scan times (with a mean reported time of 5 min). However, the strength of the evidence base is limited by small sample sizes and relatively low quality in some domains (particularly study design and patient recruitment).
Although heterogeneity in ASL labelling methods prevented analysis of its effects on the imaging of AVMs, there is preliminary evidence that increased field strength to 7T 35 and electrocardiogram gating over two cardiac cycles as opposed to one 36 can improve the diagnostic performance of ASL. Furthermore, although vessel-selective ASL confers the theoretical advantage of imaging AVMs according to their specific vascular territory, evidence from three studies demonstrates no significant difference in performance to non-selective methods. 29,31,33
Two further conference abstracts also addressed our research question. 37,38 The first study showed that ASL was able to successfully identify all three AVMs in patients with hereditary haemorrhagic telangiectasia, two of which were smaller than 1 cm. 37 Conversely, the second contrastingly showed a lower sensitivity of 72% and intermodality agreement with DSA of only 0.39 for ASL among paediatric patients in post-treatment AVM surveillance. However, specificity was 100% and sensitivity rose to 92% when using additional techniques that combined ASL with spoiled gradient recalled echo. 38 Moreover, ASL imaging has been shown to be useful in diagnosing paediatric cervicofacial AVMs by demonstrating increased lesional flow. 39
ASL may confer several advantages over both catheter angiography and conventional MRA. First, the use of intravenous gadolinium in some conventional MRA techniques may be avoided as ASL uses water as an endogenous tracer. 13 This is of clinical importance as ASL may offer an alternative option for patients with renal impairment who are at risk of nephrogenic systemic fibrosis, 40 and in other circumstances where gadolinium poses risks, such as in paediatric patients in the first year of life and during pregnancy. 41,42 Second, ASL may offer a safer, non-invasive alternative to catheter angiography in select cases, obviating the risks posed by the latter. 43,44 Third, ASL provides a functional AVM assessment that provides an objective, quantitative measure of flow. Fourth, ASL may identify some arterial feeders not detected on catheter angiography 31 and may offer better characterisation of low-flow segments of the nidus. 32 Fifth, strong pressure injection of contrast agent during catheter angiography may result in an overestimation in the proportion of blood flow in the nidus, due to reflux from other feeding arteries. This phenomenon may have been reflected in some studies in our review. 26,32 ASL may mitigate for this by providing a more physiological indication of cerebral blood flow. Sixth, ASL may occasionally be useful in identifying AVMs that may otherwise be obscured on DSA by mass effect secondary to intracerebral haemorrhage. 23
In contrast, there are a number of disadvantages in using ASL. The reduced reliability of ASL for venous drainage may be accounted for by the recovery of spin inversion in arterial water during its transit, such that the signal reduces by the time it enters venous drainage. 45 Furthermore, there was interstudy variation in the MR parameters employed in this review. Although no general trend was identified in whether the variation in these parameters significantly affected outcome measures, there were too few studies to formally assess this. Indeed, it has been shown that altering parameters such as postlabelling delay time can change nidal, venous and gray matter perfusion. 46 The sensitivity of ASL in evaluating small AVMs, low flow AVMs, small calibre arterial feeders and draining veins is also not clear from current evidence, and may represent another potential issue.
Moreover, AVMs in particular locations such as the apical cranial convexity may be more difficult to detect on ASL due to greater loss of signal as the labelled blood travels further from the labelling plane. 21 There may also be an underestimation of total blood flow in AVMs on ASL, as regions of interests may include not only the high-flow nidus but also the arterial feeders and draining veins, resulting in an average value for flow. 32 Another caution in the immediate postintervention period is that ASL may underestimate angiographically confirmed complete AV shunt occlusion, which may be due to dilatation of proximal segments of previous feeding arteries 32 or changes in autoregulation. 47 However, this can be mitigated by comparative use of MRA. Furthermore, ASL interpretation can be hindered by inhomogeneities in certain vessel territories 31 ; hyperintensity secondary to conditions such as seizures and luxury perfusion 48 ; and large changes in cerebral blood flow that occur with age. 49
Review limitations
First, the evidence we present is limited by the small sample sizes of many studies. Another consequence of this is that statistical significance was inconsistently reported, rendering interpretation of some data difficult. Second, the majority of studies were retrospective and of the remaining prospective studies, only one reported a robust recruitment strategy. This also limits the strength of the evidence base. Third, we could not evaluate the effect of interstudy variation in labelling methods and MR sequence parameters. Fourth, although a few studies did directly compare ASL with other MR imaging modalities, this was not the focus of our review. Finally, the study populations and the outcome measures varied widely, precluding our ability to pool data for meta-analysis.
Conclusions
Taken together, this review provides preliminary evidence on the reliability of ASL in evaluating intracranial AVMs. Although the quality of current evidence and technical limitations of ASL suggest that it will not replace conventional catheter angiography, it shows a promising role of ASL imaging in the workup of AVMs and their follow-up after treatment. There is sufficient evidence to justify larger scale prospective studies dedicated to assessing the diagnostic performance of ASL. Furthermore, there was no literature on ASL imaging of truncal or peripheral AVMs, highlighting another area for future research.
Supplementary Material
Footnotes
Acknowledgment: We would like to thank Keith Nockles, academic librarian at the University of Leicester, in supporting the generation of search terms.
Conflicts of interest: None to declare. This study was supported by the National Institute for Health Research (Academic Clinical Fellowship, SR). The funding organisation had no role in the design or conduct of this research.
Contributor Information
Sanjeev Ramachandran, Email: sanjeevramachandran.sr@gmail.com, University Hospitals of Leicester NHS Trust, Leicester, United Kingdom ; University of Leicester, Leicester, United Kingdom .
Deyashini Mukherjee, Email: deyashini93@hotmail.com, University Hospitals of Leicester NHS Trust, Leicester, United Kingdom ; University of Leicester, Leicester, United Kingdom .
Jonathan Delf, Email: jjw.delf@gmail.com, University Hospitals of Leicester NHS Trust, Leicester, United Kingdom .
Matthew James Bown, Email: mjb42@leicester.ac.uk, University Hospitals of Leicester NHS Trust, Leicester, United Kingdom ; University of Leicester, Leicester, United Kingdom .
Neghal Kandiyil, Email: neghal@gmail.com, University Hospitals of Leicester NHS Trust, Leicester, United Kingdom ; University of Leicester, Leicester, United Kingdom .
REFERENCES
- 1. Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, et al. . Vascular anomalies classification: recommendations from the International Society for the study of vascular anomalies . Pediatrics 2015. ; 136: e203 – 14 . doi: 10.1542/peds.2014-3673 [DOI] [PubMed] [Google Scholar]
- 2. Donnelly LF, Adams DM, Bisset GS . Vascular malformations and hemangiomas: a practical approach in a multidisciplinary clinic . AJR Am J Roentgenol 2000. ; 174: 597 – 608 . doi: 10.2214/ajr.174.3.1740597 [DOI] [PubMed] [Google Scholar]
- 3. Berman MF, Sciacca RR, Pile-Spellman J, Stapf C, Connolly ES, Mohr JP, et al. . The epidemiology of brain arteriovenous malformations . Neurosurgery 2000. ; 47: 389 – 97 . doi: 10.1097/00006123-200008000-00023 [DOI] [PubMed] [Google Scholar]
- 4. Al-Shahi R, Warlow C . A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults . Brain 2001. ; 124( Pt 10 ): 1900 – 26 . doi: 10.1093/brain/124.10.1900 [DOI] [PubMed] [Google Scholar]
- 5. Tranvinh E, Heit JJ, Hacein-Bey L, Provenzale J, Wintermark M . Contemporary imaging of cerebral arteriovenous malformations . AJR Am J Roentgenol 2017. ; 208: 1320 – 30 . doi: 10.2214/AJR.16.17306 [DOI] [PubMed] [Google Scholar]
- 6. Spetzler RF, Martin NA . A proposed grading system for arteriovenous malformations . J Neurosurg 1986. ; 65: 476 – 83 . doi: 10.3171/jns.1986.65.4.0476 [DOI] [PubMed] [Google Scholar]
- 7. Stapf C, Mast H, Sciacca RR, Choi JH, Khaw AV, Connolly ES, et al. . Predictors of hemorrhage in patients with untreated brain arteriovenous malformation . Neurology 2006. ; 66: 1350 – 5 . doi: 10.1212/01.wnl.0000210524.68507.87 [DOI] [PubMed] [Google Scholar]
- 8. Hademenos GJ, Massoud TF . Risk of intracranial arteriovenous malformation rupture due to venous drainage impairment. A theoretical analysis . Stroke 1996. ; 27: 1072 – 83 . doi: 10.1161/01.STR.27.6.1072 [DOI] [PubMed] [Google Scholar]
- 9. Petcharunpaisan S, Ramalho J, Castillo M . Arterial spin labeling in neuroimaging . World J Radiol 2010. ; 2: 384 – 98 . doi: 10.4329/wjr.v2.i10.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Detre JA, Alsop DC, Vives LR, Maccotta L, Teener JW, Raps EC . Noninvasive MRI evaluation of cerebral blood flow in cerebrovascular disease . Neurology 1998. ; 50: 633 – 41 . doi: 10.1212/WNL.50.3.633 [DOI] [PubMed] [Google Scholar]
- 11. Jezzard P, Chappell MA, Okell TW . Arterial spin labeling for the measurement of cerebral perfusion and angiography . J Cereb Blood Flow Metab 2018. ; 38: 603 – 26 . doi: 10.1177/0271678X17743240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Proisy M, Bruneau B, Rozel C, Tréguier C, Chouklati K, Riffaud L, et al. . Arterial spin labeling in clinical pediatric imaging . Diagn Interv Imaging 2016. ; 97: 151 – 8 . doi: 10.1016/j.diii.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 13. Ho M-L . Arterial spin labeling: clinical applications . J Neuroradiol 2018. ; 45: 276 – 89 . doi: 10.1016/j.neurad.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 14. Hendrikse J, Petersen ET, Golay X . Vascular disorders: insights from arterial spin labeling . Neuroimaging Clin N Am 2012. ; 22: 259 – 69 . doi: 10.1016/j.nic.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 15. Grade M, Hernandez Tamames JA, Pizzini FB, Achten E, Golay X, Smits M . A neuroradiologist's guide to arterial spin labeling MRI in clinical practice . Neuroradiology 2015. ; 57: 1181 – 202 . doi: 10.1007/s00234-015-1571-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher DLiberati ATetzlaff JAltman DG , . PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement . PLoS Med 2009. ; 6: e1000097 . doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies . Ann Intern Med 2011. ; 155: 529 – 36 . doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 18. Amponsah K, Ellis TL, Chan MD, Lovato JF, Bourland JD, deGuzman AF, et al. . Retrospective analysis of imaging techniques for treatment planning and monitoring of obliteration for gamma knife treatment of cerebral arteriovenous malformation . Neurosurgery 2012. ; 71: 893 – 900 . doi: 10.1227/NEU.0b013e3182672a83 [DOI] [PubMed] [Google Scholar]
- 19. Blauwblomme T, Naggara O, Brunelle F, Grévent D, Puget S, Di Rocco F, et al. . Arterial spin labeling magnetic resonance imaging: toward noninvasive diagnosis and follow-up of pediatric brain arteriovenous malformations . J Neurosurg Pediatr 2015. ; 15: 451 – 8 . doi: 10.3171/2014.9.PEDS14194 [DOI] [PubMed] [Google Scholar]
- 20. Sunwoo L, Sohn C-H, Lee JY, Yi KS, Yun TJ, Choi SH, et al. . Evaluation of the degree of arteriovenous shunting in intracranial arteriovenous malformations using pseudo-continuous arterial spin labeling magnetic resonance imaging . Neuroradiology 2015. ; 57: 775 – 82 . doi: 10.1007/s00234-015-1533-5 [DOI] [PubMed] [Google Scholar]
- 21. Schubert T, Clark Z, Sandoval-Garcia C, Zea R, Wieben O, Wu H, Wieben O, Turski PA, Johnson KM, et al. . Non contrast, pseudo-continuous arterial spin labeling and accelerated 3-dimensional radial acquisition intracranial 3-dimensional magnetic resonance angiography for the detection and classification of intracranial arteriovenous shunts . Invest Radiol 2018. ; 53: 80 – 6 . doi: 10.1097/RLI.0000000000000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kodera T, Arai Y, Arishima H, Higashino Y, Isozaki M, Tsunetoshi K, et al. . Evaluation of obliteration of arteriovenous malformations after stereotactic radiosurgery with arterial spin labeling MR imaging . Br J Neurosurg 2017. ; 31: 641 – 7 . doi: 10.1080/02688697.2017.1365818 [DOI] [PubMed] [Google Scholar]
- 23. Le TT, Fischbein NJ, André JB, Wijman C, Rosenberg J, Zaharchuk G . Identification of venous signal on arterial spin labeling improves diagnosis of dural arteriovenous fistulas and small arteriovenous malformations . AJNR Am J Neuroradiol 2012. ; 33: 61 – 8 . doi: 10.3174/ajnr.A2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nabavizadeh SA, Edgar JC, Vossough A . Utility of susceptibility-weighted imaging and arterial spin perfusion imaging in pediatric brain arteriovenous shunting . Neuroradiology 2014. ; 56: 877 – 84 . doi: 10.1007/s00234-014-1408-1 [DOI] [PubMed] [Google Scholar]
- 25. Heit JJ, Thakur NH, Iv M, Fischbein NJ, Wintermark M, Dodd RL, et al. . Arterial-spin labeling MRI identifies residual cerebral arteriovenous malformation following stereotactic radiosurgery treatment . J Neuroradiol 2020. ; 47: 13 – 19 . doi: 10.1016/j.neurad.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 26. Fujima N, Osanai T, Shimizu Y, Yoshida A, Harada T, Nakayama N, et al. . Utility of noncontrast-enhanced time-resolved four-dimensional Mr angiography with a vessel-selective technique for intracranial arteriovenous malformations . J Magn Reson Imaging 2016. ; 44: 834 – 45 . doi: 10.1002/jmri.25222 [DOI] [PubMed] [Google Scholar]
- 27. Iryo Y, Hirai T, Nakamura M, Kawano T, Kaku Y, Ohmori Y, et al. . Evaluation of intracranial arteriovenous malformations with four-dimensional Arterial-Spin labeling-based 3-T magnetic resonance angiography . J Comput Assist Tomogr 2016. ; 40: 290 – 6 . doi: 10.1097/RCT.0000000000000346 [DOI] [PubMed] [Google Scholar]
- 28. Togao O, Hiwatashi A, Yamashita K, Momosaka D, Obara M, Nishimura A, et al. . Acceleration-selective arterial spin labeling Mr angiography for visualization of brain arteriovenous malformations . Neuroradiology 2019. ; 61: 979 – 89 . doi: 10.1007/s00234-019-02217-w [DOI] [PubMed] [Google Scholar]
- 29. Kukuk GM, Hadizadeh DR, Boström A, Gieseke J, Bergener J, Nelles M, et al. . Cerebral arteriovenous malformations at 3.0 T: Intraindividual comparative study of 4D-MRA in combination with selective arterial spin labeling and digital subtraction angiography . Invest Radiol 2010. ; 45: 126 – 32 . doi: 10.1097/RLI.0b013e3181c7bcfe [DOI] [PubMed] [Google Scholar]
- 30. Wu H, Block WF, Turski PA, Mistretta CA, Rusinak DJ, Wu Y, et al. . Noncontrast dynamic 3D intracranial Mr angiography using pseudo-continuous arterial spin labeling (PCASL) and accelerated 3D radial acquisition . J Magn Reson Imaging 2014. ; 39: 1320 – 6 . doi: 10.1002/jmri.24279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jensen-Kondering U, Lindner T, van Osch MJP, Rohr A, Jansen O, Helle M, Van OM . Superselective pseudo-continuous arterial spin labeling angiography . Eur J Radiol 2015. ; 84: 1758 – 67 . doi: 10.1016/j.ejrad.2015.05.034 [DOI] [PubMed] [Google Scholar]
- 32. Suazo L, Foerster B, Fermin R, Speckter H, Vilchez C, Oviedo J, et al. . Measurement of blood flow in arteriovenous malformations before and after embolization using arterial spin labeling . Interv Neuroradiol 2012. ; 18: 42 – 8 . doi: 10.1177/159101991201800106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu SL, Wang R, Wang R, Wang S, Yao YQ, Zhang D, et al. . Accuracy of vessel-encoded pseudocontinuous arterial spin-labeling in identification of feeding arteries in patients with intracranial arteriovenous malformations . AJNR Am J Neuroradiol 2014. ; 35: 65 – 71 . doi: 10.3174/ajnr.A3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hodel J, Leclerc X, Kalsoum E, Zuber M, Tamazyan R, Benadjaoud MA, et al. . Intracranial arteriovenous shunting: detection with arterial spin-labeling and susceptibility-weighted imaging combined . AJNR Am J Neuroradiol 2017. ; 38: 71 – 6 . doi: 10.3174/ajnr.A4961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cong F, Zhuo Y, Yu S, Zhang X, Miao X, An J, et al. . Noncontrast-enhanced time-resolved 4D dynamic intracranial Mr angiography at 7T: a feasibility study . J Magn Reson Imaging 2018. ; 48: 111 – 20 . doi: 10.1002/jmri.25923 [DOI] [PubMed] [Google Scholar]
- 36. Raoult H, Bannier E, Robert B, Barillot C, Schmitt P, Gauvrit J-Y . Time-Resolved spin-labeled Mr angiography for the depiction of cerebral arteriovenous malformations: a comparison of techniques . Radiology 2014. ; 271: 524 – 33 . doi: 10.1148/radiol.13131252 [DOI] [PubMed] [Google Scholar]
- 37. Takenori A, Hirokazu F, Masahiro J, Kazunari Y . High detectability of non-contrast-enhanced Mr angiography using a silent scan for screening of cerebral arteriovenous malformations in HHT patients . Angiogenesis 2018. ; 21: 138 . [Google Scholar]
- 38. Chukus A, Iv M, Yeom K . Arterial spin labeling and ferumoxytol-based spoiled gradient recalled acquisition magnetic resonance imaging versus digital subtraction angiography for surveillance of residual brain arteriovenous malformations in children: a single-institution analysis of inter-modality reliability . Pediatr Radiol 2018. ; 48. [Google Scholar]
- 39. Boulouis G, Dangouloff-Ros V, Boccara O, Garabedian N, Soupre V, Picard A, et al. . Arterial spin-labeling to discriminate pediatric cervicofacial soft-tissue vascular anomalies . AJNR Am J Neuroradiol 2017. ; 38: 633 – 8 . doi: 10.3174/ajnr.A5065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramalho J, Semelka RC, Ramalho M, Nunes RH, AlObaidy M, Castillo M . Gadolinium-Based contrast agent accumulation and toxicity: an update . AJNR Am J Neuroradiol 2016. ; 37: 1192 – 8 . doi: 10.3174/ajnr.A4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adin ME, Kleinberg L, Vaidya D, Zan E, Mirbagheri S, Yousem DM . Hyperintense dentate nuclei on T1-weighted MRI: relation to repeat gadolinium administration . AJNR Am J Neuroradiol 2015. ; 36: 1859 – 65 . doi: 10.3174/ajnr.A4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D . High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted Mr images: relationship with increasing cumulative dose of a gadolinium-based contrast material . Radiology 2014. ; 270: 834 – 41 . doi: 10.1148/radiol.13131669 [DOI] [PubMed] [Google Scholar]
- 43. Kaufmann TJ . Huston J,3rd, Mandrekar JN, Schleck CD, Thielen Kr, Kallmes DF. complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients . Radiology 2007. ; 243: 812 – 9 . [DOI] [PubMed] [Google Scholar]
- 44. Alakbarzade V, Pereira AC . Cerebral catheter angiography and its complications . Pract Neurol 2018. ; 18: 393 – 8 . doi: 10.1136/practneurol-2018-001986 [DOI] [PubMed] [Google Scholar]
- 45. Lu H, Clingman C, Golay X, van Zijl PCM . Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla . Magn Reson Med 2004. ; 52: 679 – 82 . doi: 10.1002/mrm.20178 [DOI] [PubMed] [Google Scholar]
- 46. Lüdemann L, Jedrzejewski G, Heidenreich J, Han ET, Bruhn H . Perfusion imaging of cerebral arteriovenous malformations: a study comparing quantitative continuous arterial spin labeling and dynamic contrast-enhanced magnetic resonance imaging at 3 T . Magn Reson Imaging 2011. ; 29: 1157 – 64 . doi: 10.1016/j.mri.2011.07.026 [DOI] [PubMed] [Google Scholar]
- 47. Noguchi T, Irie H, Takase Y, Kawashima M, Ootsuka T, Nishihara M, et al. . Hemodynamic studies of intracranial dural arteriovenous fistulas using arterial spin-labeling MR imaging . Interv Neuroradiol 2010. ; 16: 409 – 19 . doi: 10.1177/159101991001600407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA . Arterial spin-labeling in routine clinical practice, part 3: hyperperfusion patterns . AJNR Am J Neuroradiol 2008. ; 29: 1428 – 35 . doi: 10.3174/ajnr.A1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu C, Honarmand AR, Schnell S, Kuhn R, Schoeneman SE, Ansari SA, et al. . Age-Related changes of normal cerebral and cardiac blood flow in children and adults aged 7 months to 61 years . J Am Heart Assoc 2016. ; 5: e002657 . doi: 10.1161/JAHA.115.002657 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.