Abstract
Objective:
To investigate the specificity, clinical implication and prognostic value of MRI adipocytic maturation (MAM) in myxoid/round cells liposarcomas (MRC-LPS) treated with neoadjuvant chemotherapy (NAC).
Methods:
Of the 89 patients diagnosed with MRC-LPS at our sarcoma reference center between 2008 and 2018, 28 were included as they were treated with NAC, surgery and radiotherapy. All patients underwent contrast-enhanced MRIs at baseline and late evaluation. A control cohort of 13 high-grade pleomorphic and dedifferentiated LPS with same inclusion criteria was used to evaluate the specificity of MAM in MRC-LPS. Two radiologists analyzed the occurrence of MAM, changes in the tumor architecture, shape and surrounding tissues during NAC. Pathological features of tumor samples were reviewed and correlated with MRI. Metastatic relapse-free survival was estimated with Kaplan–Meier curves and Cox models. Associations between prognostic T1-based delta-radiomics features and MAM were investigated with Student t-test.
Results:
MAM was more frequent in MRC-LPS (p = 0.045) and not specific of any type of chemotherapy (p = 0.7). Regarding MRC-LPS, 14 out of 28 patients (50%) demonstrated MAM. Eight patients showed metastatic relapses. MAM was not associated with metastatic relapse-free survival (p = 0.9). MAM correlated strongly with the percentage of histological adipocytic differentiation on surgical specimen (p < 0.001), which still expressed the tumor marker NY-ESO-1. None of the prognostic T1-based delta-radiomics features was associated with MAM.
Conclusion:
MAM seems a neutral event during NAC.
Advances in knowledge:
MAM predominated in MRC-LPS and was not specific of a type of chemotherapy. Occurrence of MAM was not associated with better patients’ metastasis free survival.
Introduction
Myxoid/round cells liposarcoma (MRC-LPS) is the most frequent histological type of liposarcoma in children, young and middle-aged adults. MRC-LPS is characterized by translocations that fuse DDIT3 to FUS or EWSR1. 1,2 The presence of round cells over 5% of tumor volume correlates with the worse outcome together with age, depth, size and resection margins. 1,3–7 Due to their chemosensitivity, patients with locally advanced MRC-LPS are treated with neoadjuvant chemotherapy (NAC), curative surgery and radiotherapy in Sarcoma Reference Centers. 8–10 In soft-tissue sarcomas, response to chemotherapy is usually assessed with contrast-enhanced MRIs. Decreases in tumor enhancement, peritumoral edema, peritumoral enhancement or changes in tumor heterogeneity and shape (evaluated with delta-radiomics) have demonstrated significant correlations with chemotherapeutic responses. 11–15
Previous studies have highlighted original radiological patterns of responses to treatment in MRC-LPS. Besides dimensional response or change in contrast-enhancement, MRC-LPS can demonstrate an increase in its amount of mature fat component, also named ‘adipocytic maturation’, which can be captured with a conventional MRI through T1 weighted imaging (WI) or DIXON Fat image. This phenomenon was initially thought to be specific of trabectedin—a marine-derived alkaloid—but was later found with cytotoxic chemotherapy and radiotherapy. 16–19 However, the clinical meaning of adipocytic maturation remains unclear. This phenomenon can be accompanied with hyalinization and decreased vascularity, possibly indicating a good response to treatment. 17–19
Therefore, our aims were to investigate the clinical, histological and prognostic values of adipocytic maturation seen on MRI of MRC-LPS during NAC.
Methods and materials
Study design
This single-center study was IRB-approved. The requirement for informed consent was waived by its retrospective nature.
We included all consecutive adult patients with histologically proven, locally-advanced MRC-LPS in the pathological database of our sarcoma reference center, from January 2008 to January 2018 (n = 89). The inclusion criteria were: administration of NAC (4–6 cycles at most) followed by curative surgery, adjuvant radiotherapy (n = 44) and available contrast-enhanced MRI at baseline (MRI-0) and during chemotherapy (MRI-1, performed at least after four cycles - n = 28).
The following co-variables were retrieved from medical reports: age, gender, performance status, depth (relative to superficial fascia), location, initial biopsy modality (surgical biopsy, imaging-guided microbiopsy, clinical microbiopsy), round cells on microbiopsy, type of NAC (anthracycline-based vs trabectedin), number of cycles and surgical margins.
We reported the occurrence of local relapses, metastatic relapses and disease-related deaths. We defined local relapse-free survival (LRFS) as the time from surgery to local relapse in months; metastatic relapse-free survival (MFS) as the time from surgery to metastatic relapse in months; and overall survival as the time from surgery to death-related disease in months. Patients without events during the study period were censored. No patient missed follow-ups. Follow-ups consisted in clinical examinations and chest radiographs every 3 months for 2 years, then every 6 months for 5 years, and then annually, with supplementary local MRI, spine MRI and chest CT-scan in case of abnormal findings. Since 2015, patients undergo whole-body MRIs every year. All relapses were histologically proven.
The study consisted in five steps:
Assessment of the specificity of MAM to MRC-LPS. To do so, we built a control cohort with other liposarcomas that met the same inclusion criteria as the MRC-LPS cohort (n = 13).
Assessment of the specificity of MAM to a type of chemotherapy in MRC-LPS.
Prognostic value of MAM in MRC-LPS.
Radiopathological correlations in MRC-LPS.
Correlation between MAM and relevant T1-based delta-radiomics features (RFs) in MRC-LPS.
The flow-chart of the study can be found in Supplementary Material 1.
MRI acquisition
MRI examinations were carried out on 1.5 T MR systems with adjustment of coils, field of views and matrix depending on the sizes and locations of the tumors. Most examinations (34/56, 60.7%) were performed at our institution (MAGNETOM Aera, Siemens Healthineers).
All the MRI examinations included sequences that enabled the estimation of the proportion of fat: either two-dimensional (2D) turbo spin echo T1WI with and without fat suppression (n = 21, echo-time/repetition-time: 10–15/500–700 ms), or DIXON gradient echo T1WI with Fat image (n = 7). Moreover, the following sequences were systematically available: T2 WI with or without fat-suppression (echo-time/repetition-time: 70–130/2400–6800 ms) and fat–suppressed contrast-enhanced T1 WI (CE-T1WI) for which different sequences were used (different fat suppression techniques, gradient echo or spin echo, 2D or 3D, with different contrast agents and different delays from injection to acquisition). Section thickness ranged from 3 to 5 mm.
MRI analysis
Two radiologists (one senior radiologist and one fellow with 6 months of residency training in our sarcoma reference center) performed a double-blind review of the imaging dataset on a dedicated PACS workstation. Next, they performed a consensual reading on which the analysis was based. 1 month later, the senior radiologist performed a second reading blinded to the previous one. Intra- and interobserver agreements are given in Supplementary Material 1. The following radiological variables named ‘semantic’ were reported: longest diameter (LD, in mm) at baseline and during treatment with calculation of change in LD and response status according to RECIST 1.1 20 ; MAM (defined as appearance of fatty signal inside the tumor, categorized as: absent or present); change in fibrotic component (defined as area with low signal intensity (SI) on T2WI and T1 WI, categorized as: decrease, stable and increase); change in necrotic component (defined as area with fluid-like SI on T2WI and no contrast-enhancement, and categorized as: decrease, stable and increase); retraction (defined as retractile change in shape with borders becoming concave, categorized as: absent, limited and marked); change in peritumoral edema (defined as high SI at T2WI with infiltrative and feathery borders that are distinguishable from the apparent tumor borders and without mass effects, categorized as: decrease (or initially absent - remaining absent), stable and increase); and change in peritumoral enhancement (defined as change in contrast-enhancement at CE-T1WI beyond the apparent tumor borders without mass effects, categorized as: decrease (or initially absent - remaining absent), stable and increase).
Pathological analysis
A pathologist with expertise in sarcomas reviewed all surgical specimens for quantitative assessment of the relative percentages of viable cells, necrosis, fibrosis, fatty components on hematoxylin and eosin stained slices (HES). A good histological response was defined as <10% viable cells on the whole tumor volume. 21
The cases with marked adipocytic maturation on HES (n = 9) were complemented with immunohistochemistry in order to assess whether the mature adipocytic component would express the pathognomonic marker of MRC-LPS, named NY-ESO-1, or if this component would only correspond to mature, scar adipose tissue. 22 For immunohistochemistry, the slides were deparaffinized in xylene and hydrated in alcohol. NY-ESO1 expression was assessed with a mouse monoclonal antibody E978 (provider: Santa Cruz). Incubation and staining were performed on a Benchmark-ultra automate (Ventana) using diamino-benzidine as chromogen (Ultra-View Universal DAB, Ventana).
Delta-radiomics analysis
Since the best MR sequence to capture MAM is pre-contrast T1WI, we hypothesized that (i) a delta-radiomics approach based on this sequence could help to capture different spatial patterns of MAM during NAC and (ii) that some of these patterns could correlate with MFS.
To do so, we first extracted the RFs from MRI-0 and MRI-1 of the subset of 21 patients with pre-contrast 2D turbo spin echo T1WI. The radiomics pipeline consisted in the following post-processing pipeline: slices were resampled using bilinear interpolation to obtain a common isotropic in plane resolution of 1 × 1 mm2 and a thickness of 4 mm. SI on T1WI were normalized for non-uniform intensity using N4ITK bias field correction. 23 The intensity ranges were standardized using histogram-matching with the normalized acquisition of a healthy volunteer’s thigh as a reference. 24 A senior radiologist manually segmented the whole tumor volumes on T 1WI with the help of other sequences to adjust the tumor boundaries. The extraction of 45 3D-radiomics features was performed with LIFEx (v. 4.7, Inserm, Orsay, France). 25 Standardized SIs were discretized in 128 fixed bins of a bin size of 0.0315. 3 shape features, 10 histogram-based and 32 second-order texture features from gray-level co-occurrence matrix (GLCM, n = 7 with a distance of 4 voxels to neighbors), gray-level run length matrix (GLRLM, n = 11), neighborhood gray-level different matrix (NGLDM, n = 3) and gray-level zone length matrix (GLZLM, n = 11) were estimated (Supplementary Material 1—Details for the calculation of the features can be found at: https://www.lifexsoft.org/index.php/resources/19-texture/). Then, we calculated the relative change in each RF “X” from MRI-0 to MRI-1, as Δ-X = 100x(XMRI-1-X MRI-0)/X MRI-0, which was further standard-scaled.
Statistical analysis
Statistical analysis was performed using R v. 3.5.2. All tests were two-tailed. A p-value < 0.05 was deemed significant.
Associations between categorical/ordinal variables were tested with Fisher or χ2 tests.
Regarding semantic radiological features, Kaplan–Meier curves for MFS were drawn and differences in survival measures were assessed with log-rank tests.
Regarding radiopathological correlations, the hazard ratio [HR, with 95% confidence interval (95% CI)] of each continuous variable (i.e. the amount of each histological component on post-NAC surgical specimen) was estimated with univariate Cox model. These variables were dichotomized according to their median and we assessed their prognostic value with these cut-offs, as well as the prognostic value of the histological response, by using log-rank tests. Moreover, the correlation between the occurrence of MAM and the percentages of lipoblasts on post-NAC surgical specimen was assessed with the Spearman test.
Regarding radiomics, we performed univariate Cox regression between MFS and each delta-RF to estimate HR. We identified those that were significantly predictive of metastatic relapses (named “relevant RF”) and we tested their associations with MAM using Student's t-tests.
Results
Study population
The final number of patients was 28 (14 females, median age: 43.5 years old—Table 1). Tumors were all deep-seated and mostly located in lower limbs (25/28, 89.3%). Median size was 173.5 mm (range: 34–270). All patients underwent biopsies before treatment that showed round cells in 10/28 cases (64.3%). The median delay between baseline and late evaluations by MRI was 137 days (range: 89–207).
Table 1.
Characteristics of the study population
| Variables | Patients |
|---|---|
| Age | |
| Mean ± SD |
49.1 ± 13.3 |
| Median (range) |
43.5 (29–75) |
| Gender | |
| Men |
14/28 (50%) |
| Women |
14/28 (50%) |
| WHO-PS | |
| PS 0 |
26/28 (92.9%) |
| PS 1 |
2/28 (7.1%) |
| Location | |
| Upper limb | 1/28 (3.6%) |
| Pelvic girdle | 2/28 (7.1%) |
| Lower limb | 25/28 (89.3%) |
| Depth | |
| Deep | 28/28 (100%) |
| Deep and superficial | 0/28 (0%) |
| Superficial | 0/28 (0%) |
| Initial size (mm) | |
| Mean ± SD | 163.3 ± 59.6 |
| Median (range) | 173.5 (34–270) |
| Initial volume (cm3) | |
| Mean ± SD | 834.5 ± 757.3 |
| Median (range) | 468.6 (50.6–2541.9) |
| Chemotherapy type | |
| Anthracycline-based | 18/28 (64.3%) |
| Trabectedin | 10/28 (35.7%) |
| No. of chemotherapy cycles | |
| Four cycles | 6/28 (21.4%) |
| Six cycles | 22/28 (78.6%) |
| Initial sampling modality | |
| Imaging-guided microbiopsy | 16/28 (57.1%) |
| Clinical microbiopsy | 9/28 (32.1%) |
| Surgical biopsy | 3/28 (10.7%) |
| Presence of round cells on initial sampling | |
| Yes | 10/28 (35.7%) |
| No | 18/28 (64.3%) |
No, number; SD, standard deviation; WHO-PS, World Health Organization performance status.
Note: Data are number of patients with percentage in parentheses (except for age, longest diameter and volume, which are given as mean and standard deviation, as well as median with range in parentheses).
Specificity of MAM among liposarcomas
The control cohort included 13 patients who were all treated with anthracycline-based chemotherapy. Table 2 shows the clinical features of the control cohort. 8 patients (8/13, 61.5%) had pleomorphic LPS and 5 (5/13, 38.5%) had dedifferentiated LPS. MAM was seen in 2/13 patients with non-MRC-LPS (15.4% - Figure 1) and in 14/28 patients with MRC-LPS (50%) leading to a significant association between MAM and MRC-LPS (p = 0.045). Regarding patients from the two cohorts who were exclusively treated with anthracycline-based NAC, 10 our of 18 patients (55.6%) with MRC-LPS showed a MAM while 2 out of 13 patients (15.4%) from the control cohort did, which also led to a significant association between the subtype of LPS and occurrence of MAM (p = 0.033).
Table 2.
Characteristics of the ‘other liposarcomas’ cohort
| Variables | Patients |
| Age | |
| Mean ± sd | 60.6 ± 9.9 |
| Median (range) | 61 (43–73) |
| Gender | |
| Males | 7/13 (53.8) |
| Females | 6/13 (46.2) |
| WHO-PS | |
| PS 0 | 12/13 (92.3) |
| PS 1–2 | 1/13 (7.2) |
| Location | |
| Trunk wall | 2/13 (15.4) |
| Lower limb | 10/13 (84.6) |
| Depth | |
| Deep | 13/13 (100) |
| Initial size (mm) | |
| Mean ± sd | 134 ± 65.5 |
| Median (range) | 130.5 (60–260) |
| Histological type | |
| Pleomorphic LPS | 8/13 (61.5) |
| Dedifferentiated LPS | 5/13 (38.5) |
| Histological grade on microbiopsy | |
| Grade 1 | 0/13 (0) |
| Grade 2 | 1/13 (7.2) |
| Grade 3 | 12/13 (92.3) |
| Chemotherapy type | |
| Anthracycline-based | 13/13 (100) |
LPS, liposarcoma; SD, standard deviation; WHO-PS, World Health Orgnanization performance status.
NOTE. Data are number of patients with percentage in parentheses (except for age, longest diameter and volume, which are given as mean, standard deviation, median with range in parentheses).
Figure 1.
MRI adipocytic differentiation in pleomorphic liposarcoma during anthracycline-based chemotherapy. A 56-year-old female presented with a 14cm-long Grade III pleomorphic liposarcoma of the left lumbar muscles. On baseline MRI, the tumor displayed areas with fatty signal, i.e. areas with high SI on Fat image of Dixon gradient echo T 1 weighted imaging (‘Ax Dixon Fat T1’) and sagittal turbo spin echo T 1 weighted imaging (‘Sag T1’). During chemotherapy, MRI performed after 2 and 6 cycles (‘Post-C2’ and ‘Post-C6’. respectively) showed an increase in the amount of fatty areas (white arrows), especially at the lower and anterior sides of the tumor—without noticeable change in longest diameter (+0.5%). SI, signal intensity.
Specificity of MAM to the type of chemotherapy
10 out of the 18 MRC-LPS patients treated with anthracycline demonstrated MAM (10/18, 56%) while MAM was seen on 4/10 (40%) MRC-LPS patients treated with trabectedin, which was not statistically different (p = 0.7).
Prognostic value of MAM and other semantic radiological features
There were eight metastatic relapses, two local relapses and three deaths related to disease. The MFS probabilities at 1 year, 2 years and 5 years were 0.918 [95%CI=(0.816; 1.0)], 0.870 [95%CI=(0.742; 1.0)] and 0.609 [95%CI=(0.413; 0.897)], respectively.
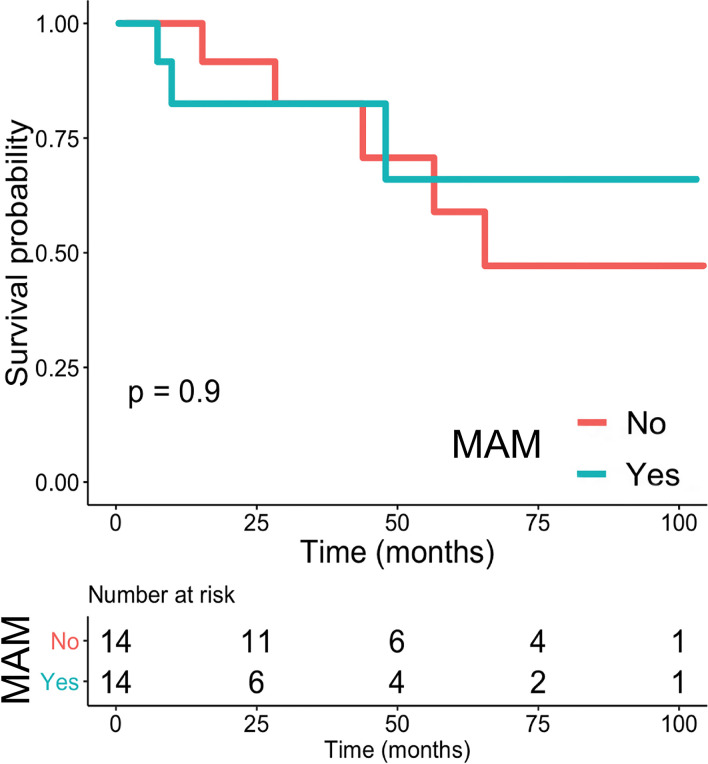
Table 3 shows the prognostic value of the radiological features. Of the 14 MRC-LPS patients with MAM, 3 had a metastatic relapse (3/14, 21.4%). MAM did not correlate with MFS (p = 0.9). Figure 2 shows two cases of MRC-LPS patients with MAM but different outcomes.
Table 3.
Assessment of the prognostic value of semantic radiological features for metastatic relapse after initial treatment (univariate analysis)
| Variables | No. of patients | No. of events | log-rank p-value | 2 years survival probability |
|---|---|---|---|---|
| Retraction | ||||
| No | 7 | 3 | 0.3 | 0.75 [0.43; 1.00] |
| Yes | 17 | 5 | 0.87 [72.7; 1.00] | |
| Yes, marked | 4 | 0 | 1.00 [1.00; 1.00] | |
| Retraction - dichotomized | ||||
| No | 7 | 3 | 0.2 | 0.75 [42.6; 1.00] |
| Yes | 21 | 5 | 0.90 [0.78; 1.00] | |
| MAM | ||||
| Absent | 14 | 5 | 0.9 | 0.92 [0.77; 1.00] |
| Present | 14 | 3 | 0.83 [0.63; 1.00] | |
| Change in fibrotic component | ||||
| Decrease or absent | 0 | 0 | 1.0 | - |
| Stable | 16 | 5 | 1.00 [1.00; 1.00] | |
| Increase | 12 | 3 | 0.67 [0.41; 1.00] | |
| Change in necrotic component | ||||
| Decrease or absent | 2 | 1 | 0.5 | 0.50 [0.12; 1.00] |
| Stable | 17 | 6 | 0.88 [0.73; 1.00] | |
| Increase | 8 | 0 | 1.00 [1.00; 1.00] | |
| Change in peritumoral edemaa | ||||
| Decrease or absent | 21 | 4 | <0.001*** | 0.88 [0.73; 1.00] |
| Stable | 2 | 1 | 1.00 [1.00; 1.00] | |
| Worsening | 1 | 1 | - | |
| Change in peritumoral edema - dichotomizeda | ||||
| Decrease or absent | 21 | 4 | <0.001*** | 0.88 [0.73; 1.00] |
| Stable or worsening | 3 | 2 | 0.67 [0.30; 1.00] | |
| Change in peritumoral enhancementb | ||||
| Decrease or absent | 21 | 4 | 0.09 | 0.87 [0.72; 1.00] |
| Stable | 2 | 1 | 1.00 [1.00; 1.00] | |
| Worsening | 2 | 2 | 0.50 [0.13; 1.00] | |
| Change in peritumoral enhancement - dichotomizedb | ||||
| Decrease or absent | 21 | 4 | 0.09 | 0.87 [0.72; 1.00] |
| Stable or worsening | 4 | 3 | 0.75 [0.43; 1.00] | |
| Reponse status (RECIST v1.1) | ||||
| Stable disease | 26 | 8 | 0.4 | 0.86 [0.73; 1.00] |
| Progressive disease | 2 | 0 | 1.00 [1.00; 1.00] | |
MAM, MRI adipocytic maturation.
*: p<0.05. **: p<0.005. ***: p<0.001
Four patients were not analysable for change in peritumoral edema.
Three patients were not analysable for change in peritumoral enhancement.
Figure 2.
Spectrum of MAM in MRC-LPS (with different outcomes). (A) A 41 years old female presented with deep-seated, 11 cm long MRC-LPS of the thigh. From baseline to early and late evaluation MRIs (Post-2 and 6 cycles of anthracycline-based regimen), there was a progressive increase of surface area displaying a fatty signal on MRI (MAM, white arrows) together with change in shape consistent with a retraction (from convex to concave borders). (B) The histogram of the whole tumor segmented on normalized T 1WI demonstrated a decay of the histogram towards higher normalized SIs from baseline to post-C6 evaluation. On surgical specimen, there were 70% viable cells (including 75% of mature lipoblasts) and 30% necrosis. Three and a half years later, the patient has not demonstrated any relapse. (C) A 65-year-old male presented with a deep-seated, locally advanced MRC-LPS of the left gluteal muscles. During anthracycline-based chemotherapy, there was a progressive increase in areas with fatty signal (white arrows), which also translated toward an increase decay of the histogram toward higher SIs on normalized T 1WI and compatible with a MAM. Surgical specimen showed 40% viable tumor cells including 50% of mature lipoblasts. A metastatic relapse involving the vertebral column and lung was diagnosed 10 months later. Post-Cx: Post ‘x’ cycles of chemotherapy. MAM, MRI adipocytic maturation; MRC-LPS, myxoid/round cells liposarcomas; SI, signal intensity; WI, weighted imaging.
Two patients (2/28, 7.1%) demonstrated a progressive disease according to RECIST but none of them demonstrated any relapse. RECIST did not correlate with MFS (p = 0.4). 8 patients (8/28, 28.6%) showed an increase in necrotic component and 12 in fibrotic component (12/28, 42.9%) but this was not associated with MFS (p = 0.5 and 1.0, respectively). Retraction during NAC was seen in 21 patients. It was moderate in 17 patients and marked in 4 patients. None of the patients with a marked retraction showed metastatic relapse, but this variable did not correlate with MFS (p = 0.3). Change in peritumoral edema was analyzable in 24 patients. Stability or worsening of a pre-existing surrounding edema was seen in 3 cases (3/24, 12.5%) and 2 of them (2/3, 33.3%) showed a metastatic relapse. It was significantly associated with MFS (p = 0.02). Change in peritumoral enhancement was analysable in 25 patients. Stability or worsening of a pre-existing surrounding edema was seen in 4 cases (4/25, 16%) and 3 of them (3/4, 75%) showed a metastatic relapse (p = 0.09). Figure 3 illustrates the other radiological pattern of response seen in MRC-LPS. Figure 4 summarizes the survival analysis for MAM.
Figure 3.
MRI response patterns in MRC-LPS during neo-adjuvant chemotherapy. A 66-year-old male presented with a deep-seated, locally advanced MRC-LPS of the left thigh. (A) On baseline MRI, the tumor showed a peritumoral oedema at its upper side (black arrows), as well as peritumoral enhancement along the superficial and deep fascia (white arrow). (B) The MRI performed after six-cycles of anthracycline-based chemotherapy demonstrated a disappearance of the peritumoral edema as well as the peritumoral enhancement. Areas with low SIs on T 2WI appeared (dashed arrows. compatible with fibrosis), surrounding a large area of necrosis with high SI on T 2WI and no contrast-enhancement (white arrow heads). ‘Ax T 2’, axial turbo spin echo T 2WI; ‘AxT1 FS +’, axial Fat Sat T 1WIafter intravenous injection of gadolinium chelates; MRC-LPS, myxoid/round cell liposarcoma;‘Sag T 2STIR’,sagittal short inversion time recovery turbo spin echo T 2WI; SI, signal intensity; ‘SubT 1 +’, subtraction ofpost-contrast and pre-contrast Fat Sat T 1WI;WI, weighted imaging..
Figure 4.
Kaplan–Meier Survival curve for metastatic relapse of MRC-LPS depending on presence or absence of MAM. MAM,MRI adipocytic maturation, MRC-LPS, myxoid/round cell liposarcoma.
Radiopathological correlations and prognostic value of post-NAC histological features
Radiopathological correlations were performed in 26/28 (92.9%) cases. HES slices were missing for two patients for whom histological response was obtained in pathological reports.
Qualitative assessment by the pathologist showed that lipoblasts in MAM often had a more mature aspect and larger size than lipoblasts on pre-therapeutic samples.
Regarding immunohistochemistry with NY-ESO1 antibodies, all cases displayed a strong and diffuse immunostaining of the nucleus of all lipoblasts consistent with their tumoral nature (Figure 5).
Figure 5.
Radiopathological correlations in a patient with MRI adipocytic maturation. (A) HES slices of the surgical specimen after neoadjuvant chemotherapy demonstrated large areas made of mature fat (black arrows) at the borders of the tumor and close to large strands of fibrosis (black asteroid), together with areas with persisting stainable tumor cells inside myxoid stroma (black circle). (B) Magnification showed several closely packed round and oval large mature lipoblasts of various sizes together with usual smaller lipoblasts (black dashed arrow). (C) Immunostaining with NY-ESO-1 antibody demonstrated a strong and diffuse nuclear staining of all tumor cells (adipocytic and non-adipocytic, white arrowheads) consistent with their tumoral nature (MRI of this patient can be seen in Figure 3A). HES,hematoxylin and eosin stained.
There were three good histological responders (3/28, 10.7%). None of the patients showed residual round cells component. The median percentage of lipoblasts was 5% (range: 0–74.2). A significant correlation was found between the percentage of lipoblasts on surgical specimen and MAM (ρ = 0.608, p < 0.001).
Regarding the prognostic value of the amount of lipoblasts on post-chemotherapy surgical specimens, there was no correlation with MFS [HR = 0.99, 95%CI (0.94; 1.05), p = 0.8]. Once dichotomized by its median value, there was no statistical difference between the MFS curves (p = 1.0). Table 4 provides the quantification of the other histological components on post-NAC surgical specimens with their corresponding predictive value for MFS. None of them was a predictor of MFS.
Table 4.
Assessment of the prognostic value of pathological features for metastatic relapse after initial treatment (univariate analysis)
| Variables | Mean ± SD | Median (range) | Modalitiesa | No. of patients | No. of events | 2 years survival probability | log rank p-value | HR (95% CI)b |
|---|---|---|---|---|---|---|---|---|
| Lipoblasts (%) | 14.3 ± 21.7 | 4 (0–74.2) | <5% | 14 | 4 | 0.91 [0.75; 1.0] | 1.0 | 0.99 [0.94; 1.05] |
| ≥5% | 12 | 3 | 0.78 [0.55; 1.0] | |||||
| Necrosis (%) | 25.5 ± 26.6 | 10 (0–90) | <10% | 14 | 2 | 1.0 [1.0; 1.0] | 0.1 | 1.02 [0.99; 1.04] |
| ≥10% | 12 | 4 | 0.62 [0.35; 1.0] | |||||
| Fibrosis (%) | 39.3 ± 27.7 | 40 (0–96) | <35% | 12 | 4 | 0.65 [0.39; 1.0] | 0.2 | 0.99 [0.97; 1.02] |
| ≥35% | 14 | 2 | 1.0 [1.0; 1.0] | |||||
| Viable cells (%) | 35.2 ± 22.4 | 30 (1–85) | <30% | 14 | 5 | 0.85 [0.70; 1.0] | 0.2 | 0.97 [0.93; 1.01] |
| ≥30% | 12 | 1 | 1.0 [1.0; 1.0] | |||||
| - | - | <50% | 15 | 5 | 0.86 [0.69; 1.0] | 0.8 | - | |
| ≥50% | 8 | 1 | 0.83 [0.58; 1.0] | |||||
| Histological response c | Good | 3 | 1 | 0.68 [0.30; 1.0] | 0.7 | - | ||
| Poor | 25 | 7 | 0.91 [0.79; 1.0] | |||||
95% CI, 95% confidence interval; HR, hazard ratio; No., number; SD, standard deviation.
Note. The percentages of lipoblasts, necrosis, fibrosis and viable cells were assessed on surgical specimen following neoadjuvant chemotherapy. None of the specimen demonstrated residual viable round cell component.
: ‘Modalities’ corresponds to the modalities of the histological variables after dichotomization according their median value.
: Hazard ratio corresponds to the hazard ratio of the numeric/continuous percentage in each histological component.
: Histological response was defined as good if less than 10% of viable cells were present.
Correlations between MAM and relevant delta-RF
Three delta-RFs, assessed on pre-contrast T 1WI from baseline and late MRIs, were correlated with MFS at univariate analysis: Δ-GLZLM_LZE [HR = 2.56, 95% CI (1.15; 5.70), p = 0.022], Δ-GLZLM_LZLGE [HR = 2.97, 95%CI (1.17; 7.51), p = 0.021] and Δ-GLZLM_LZHGE [HR = 2.40, 95%CI (1.13; 5.09), p = 0.023]. Results for the other δ-RFs are given in Table 5. These three relevant RFs were not significantly associated with MAM (p = 0.8, 1.0 and 0.7, respectively).
Table 5.
Summary of the δ radiomics features extracted form T1-weighted-imaging correlating with MFS) at univariate analysis
| Variables | HR | lower limit of 95% CI | Upper limit of 95% CI | p-value |
|---|---|---|---|---|
| Δ-CONVENTIONAL_min | 0.8661 | 0.3524 | 2.1290 | 0.7541 |
| Δ-CONVENTIONAL_mean | 1.2306 | 0.3965 | 3.8196 | 0.7196 |
| Δ-CONVENTIONAL_std | 1.5381 | 0.6250 | 3.7852 | 0.3488 |
| Δ-CONVENTIONAL_max | 2.4750 | 0.9563 | 6.4054 | 0.0618 |
| Δ-HISTO_Skewness | 1.2459 | 0.4860 | 3.1940 | 0.6471 |
| Δ-HISTO_Kurtosis | 1.0169 | 0.3130 | 3.3030 | 0.9778 |
| Δ-HISTO_ExcessKurtosis | 1.0169 | 0.3130 | 3.3030 | 0.9778 |
| Δ-HISTO_Entropy_log10 | 1.7105 | 0.5699 | 5.1338 | 0.3384 |
| Δ-HISTO_Entropy_log2 | 1.7105 | 0.5699 | 5.1338 | 0.3384 |
| Δ-HISTO_Energy | 0.4130 | 0.0884 | 1.9286 | 0.2607 |
| Δ-SHAPE_Volume | 1.3800 | 0.6998 | 2.7214 | 0.3525 |
| Δ-SHAPE_Sphericity | 0.5911 | 0.2016 | 1.7331 | 0.3381 |
| Δ-SHAPE_Compacity | 1.6085 | 0.7091 | 3.6484 | 0.2554 |
| Δ-GLCM_Homogeneity | 0.9754 | 0.3720 | 2.5577 | 0.9596 |
| Δ-GLCM_Energy | 0.3595 | 0.0428 | 3.0222 | 0.3463 |
| Δ-GLCM_Contrast | 1.1082 | 0.4141 | 2.9662 | 0.8379 |
| Δ-GLCM_Correlation | 5.5599 | 0.7001 | 44.1561 | 0.1047 |
| Δ-GLCM_Entropy_log10 | 1.6449 | 0.5420 | 4.9920 | 0.3796 |
| Δ-GLCM_Entropy_log2 | 1.6449 | 0.5420 | 4.9920 | 0.3796 |
| Δ-GLCM_Dissimilarity | 1.0379 | 0.3964 | 2.7174 | 0.9396 |
| Δ-GLRLM_SRE | 0.8767 | 0.3705 | 2.0744 | 0.7645 |
| Δ-GLRLM_LRE | 1.0363 | 0.3587 | 2.9939 | 0.9475 |
| Δ-GLRLM_LGRE | 0.8475 | 0.2316 | 3.1007 | 0.8025 |
| Δ-GLRLM_HGRE | 1.2436 | 0.4036 | 3.8316 | 0.7042 |
| Δ-GLRLM_SRLGE | 0.8684 | 0.2639 | 2.8573 | 0.8163 |
| Δ-GLRLM_SRHGE | 0.9396 | 0.3829 | 2.3055 | 0.8918 |
| Δ-GLRLM_LRLGE | 0.8973 | 0.2911 | 2.7662 | 0.8504 |
| Δ-GLRLM_LRHGE | 1.1634 | 0.4321 | 3.1328 | 0.7645 |
| Δ-GLRLM_GLNU | 1.4006 | 0.7484 | 2.6213 | 0.2920 |
| Δ-GLRLM_RLNU | 1.3052 | 0.7141 | 2.3856 | 0.3867 |
| Δ-GLRLM_RP | 0.9219 | 0.3691 | 2.3023 | 0.8617 |
| Δ-NGLDM_Coarseness | 0.0082 | 0.0000 | 48.3822 | 0.2784 |
| Δ-NGLDM_Contrast | 0.5811 | 0.1736 | 1.9453 | 0.3786 |
| Δ-NGLDM_Busyness | 1.1679 | 0.5536 | 2.4638 | 0.6837 |
| Δ-GLZLM_SZE | 2.2127 | 0.4926 | 9.9389 | 0.3001 |
| Δ-GLZLM_LZE | 2.5556 | 1.1460 | 5.6987 | 0.0218* |
| Δ-GLZLM_LGZE | 1.1877 | 0.4367 | 3.2306 | 0.7361 |
| Δ-GLZLM_HGZE | 2.2952 | 0.4634 | 11.3693 | 0.3088 |
| Δ-GLZLM_SZLGE | 1.3891 | 0.4954 | 3.8949 | 0.5321 |
| Δ-GLZLM_SZHGE | 2.4182 | 0.7490 | 7.8076 | 0.1398 |
| Δ-GLZLM_LZLGE | 2.9687 | 1.1741 | 7.5064 | 0.0215* |
| Δ-GLZLM_LZHGE | 2.3952 | 1.1280 | 5.0861 | 0.0230* |
| Δ-GLZLM_GLNU | 1.2711 | 0.8031 | 2.0118 | 0.3058 |
| Δ-GLZLM_ZLNU | 1.2809 | 0.8138 | 2.0162 | 0.2848 |
| Δ-GLZLM_ZP | 1.1313 | 0.5753 | 2.2247 | 0.7207 |
CI, confidence interval; HR, hazard ratio; MFS, metastatic-relapse free survival.
p < 0.05, **: p < 0.005, ***:p < 0.001
Discussion
Adipocytic maturation occurs during chemotherapy in MRC-LPS and its prognostic significance is still controversial. Overall, our results demonstrate that MAM is a common event, found in half of MRC-LPS patients treated with NAC. Nonetheless, it is not specific and can be seen in other high-grade liposarcomas, although at lower frequencies. Moreover, MAM did not correlate with patients’ outcome; neither did any histological marker on post-NAC surgical specimen—including the percentage of lipoblasts and viable cells.
Herein, the incidence of MAM in the MRC-LPS cohort is slightly higher than in previous studies. Its incidence ranged from 34.8 to 38% during radiotherapy alone. 17,26 MAM was identified in 6/22 (22.7%) tumors treated with a combination of radiotherapy and anthracycline in the study by Wang et al. 19 An explanation could be that MAM is favored by NAC rather than radiotherapy. In contrast with the study by Wortman et al, which did not find MAM in pleomorphic LPS undergoing radiotherapy, we found that MAM also occurred in two patients with pleomorphic liposarcomas. 17 In addition, their study did not include dedifferentiated LPS. Though not described in radiological study, it should be noted that clinicopathological studies previously reported MAM in pleomorphic LPS. 27 Herein, the incidence of MAM was not significantly different between trabectedin and anthracycline though it was initially suspected of being specific of trabectedin. 28 However, our study also highlights the significantly higher prevalence of MAM in MRC-LPS treated with anthracycline-based NAC compared with other LPS subtypes.
The underlying molecular pathways of adipocytic differentiation are likely to depend on chemotherapy and the subtype of liposarcoma. Regarding MRC-LPS, the recurrent translocations induced the creation of pro-oncogenic fusion transcripts FUS-DDIT3 or EWS-DDIT3. It has been shown that these transcripts inhibit key transcription factors involved in the adipogenesis. 29 Di Giandomenico et al have found that trabectedin was specifically able to release specific promoters’ regions from the fusion transcript FUS-DDIT3, hence enabling an adipocytic differentiation not seen with anthracycline. 28
Interestingly, we found that the adipocytic cells present in post treated MRC-LPS are still tumorigenic and still express the biomarker NY-ESO-1, which is a pathognomonic nuclear marker of MRC-LPS. 22 Consequently, this apparently mature fatty component should be labeled as a viable tumor component and not confused with a type of healing tissue—by analogy with fatty replacement that can be seen in some benign and malignant bone tumors in the healing process (for instance myeloma). Further studies should investigate if these lipoblasts in MAM have the ability to dedifferentiate again into classical tumoral lipoblasts found in naïve MRC-LPS, once the antitumoral effect of the chemotherapy has been removed. Other studies also support this assumption that the presence of fatty areas in MRC-LPS should not be interpreted as a favorable event. First, Kuyumcu et al have found that high fat content in MRC-LPS was not associated with better outcome. 30 Second, 2 out of the 6 (33.3%) patients with MAM in the study by Wang et al had a metastatic relapse. 19 Third, high PPARγ expression, which is involved in the adipocytic maturation of MRC-LPS, was an independent adverse prognostic factor of metastatic relapse in a recent study of 46 patients with MRC-LPS. 31
It should be noted that the distinction between lipoblasts in MAM from usual MRC-LPS lipoblasts (i.e. pre-therapeutic tumoral lipoblasts) could be challenging for pathologists. However, lipoblasts in MAM are characterized by their mature aspect and even their resemblance with non-neoplastic adult adipose tissue. They can exhibit large lipid-containing vacuole with differentiated nucleus. Moreover, the presence of surrounding hyalinization could help identifying MAM. Finally, at our institution, the surgical specimens are routinely oriented by the surgeon and the pathologist to facilitate correlations. Hence, we were able to select HES slices where MAM was marked on MRI.
Though occurrence of MAM was not associated with MFS in our study, we identified other imaging features that could help predict the prognosis of patients with locally advanced tumors. We found that the decreases in peritumoral edema and enhancement were associated with less metastatic relapses, in agreement with previous studies. 13 We hypothesized that this positive effect was due to the action of NAC on satellite tumor cells, and/or to a decrease in peritumoral inflammation, and/or to a decrease in the tumoral mass effect on vessels or nerves. The assessment of the changes in the surrounding tissue is specific to MRI and cannot be performed during the pathological analysis of the surgical specimen. We also found 3 T 1-based delta-RFs from GLZLM analysis that correlated well with MFS at univariate analysis. This concurs with previous radiomics studies based on soft-tissue sarcoma MRIs and CTs, highlighting their potential to improve prediction of the outcome of patients undergoing NAC or neoadjuvant radiotherapy. 14,32 Nevertheless, MAM did not correlate with these three relevant RFs. The explanations for this negative result are probably multifactorial. First, although we hypothesized that the most obvious reason for changes in tumor heterogeneity on pre-contrast T 1WI was the occurrence of MAM, these changes may also be due to hemorrhage—exhibiting high SI on T 1WI—and modifications in other tumor components, such as increased necrosis, cystic changes or fibrosis—all exhibiting very low SI on T 1WI. During their reading of the imaging data set, the radiologists were able to distinguish these phenomena from MAM because they analyzed all the MRI sequences at the same time. On the contrary, our univariate delta-radiomics approach mixed all the possible architectural alterations without distinction. Second, our exploratory delta-radiomics analysis may have lacked of statistical power because of the low number of patients. Third, the patterns of MAM that we have quantified with first and second orders textural RFs may simply not be associated with survivals, as is the case with our radiological assessment of MAM.
Finally, the results of our pathological analysis question the relevance of the current definition for a good histological response in patients with MRC-LPS. Only 3 patients out of 28 showed a good response, defined as less than 10% viable cells on surgical specimen, and one of them had a metastatic relapse. 21 It should be noted that the histological response for soft-tissue sarcomas has been defined on a large retrospective cohort of patients with various subtypes of sarcomas. Hence, this criterion may not be appropriate for MRC-LPS alone. The assessment of the histological response for this particular histological subtype remains challenging for pathologists because of the lack of data regarding the prognostic value of lipoblasts on surgical specimen and if their presence should be considered as a positive predictor. Since the histological response is just an intermediate endpoint of which the aim is to estimate the risk of relapse once the initial therapeutic management has been finished, we investigated the prognostic value of each histological component on post-NAC surgical specimens. Our study was not able to find any correlation. Thus, further larger multicentric prognostic studies combining radiologic, radiomics, histopathological and molecular features of MRC-LPS are required.
Our study has several limits. First, this is a retrospective single-center study with a limited number of patients. However, with 28 MRC-LPS and 13 other high-grade liposarcomas, this remains one of the largest radiological cohorts of liposarcomas undergoing NAC, surgery and radiotherapy with clinical follow-ups. This is also the largest cohort with radiopathological correlations of MRC-LPS after NAC. However, the subgroups of patients with pleomorphic LPS and dedifferentiated LPS were too small to draw negative or positive conclusions if the same survival analyses had been performed. Associations between prognosis and MAM in these subgroups should be investigated in dedicated studies. Furthermore, to the best of our knowledge, NY-ESO-1 immunostaining has never been performed in this setting. Second, the number of metastatic relapses was rather low (8/28, 28.6%), which is partly responsible for the lack of power in our statistical analyses. Third, there was a lack of standardization in the imaging protocol due to the retrospective nature of the study. Changes in the fatty areas were assessed using a semi-quantitative scale and further dichotomized as present versus absent, which may have also lead to a lack of statistical power in the analysis. However, we tried to limit the subjectivity bias by performing a consensual reading. Moreover, fatty signal variations were assessed either on turbo spin echo T 1WI, or on DIXON gradient echo T 1WI. To limit bias in the radiomics substudy, we purposely decided to perform the RF extraction on the same turbo spin echo T 1WI sequences, i.e. in only 21 patients. Due the small number of patients in this exploratory delta-radiomics analysis, we did not perform a multivariate prognostic modeling. However, larger studies should systematically adjust their results with potentially confounding variables including the type of chemotherapy because it may influence the spatial patterns of response on pre-contrast T 1WI. Moreover, we did not perform corrections for multiple comparisons in the univariate assessment of correlations between MFS and delta-RFs, which is at risk of false discovery. Indeed, since our radiomics approach was exploratory, without reference in the literature regarding the correlations between T 1-based delta-RFs and MFS, we chose to report the raw univariate p-values without doing any mathematical corrections. 33–35 In such context, the need for multiplicity adjustment is not consensual because it could obscure possible important findings. Nevertheless, our radiomics results will have to be validated in independent cohort. Finally, alternative techniques for quantifying fat could have been tried, such as proton magnetic resonance spectroscopy-derived density fat fraction used in liver imaging or with multipoints DIXON imaging. 18,36 Such techniques could have provided more robust estimation of MAM than simple qualitative assessment and enabled to investigate the predictive values of different cut-offs for the changes in intra tumoral fat signal.
To conclude, through a multilevel and integrative analysis of clinical, histopathological, radiological and radiomics features of MRC-LPS during NAC, our study brings original information regarding MAM. This phenomenon can be observed in any subtype of liposarcomas, with any type of chemotherapy, though it predominates in MRC-LPS, and is not an indicator of better responses or outcomes.
Supplementary Material
Footnotes
Acknowledgements: The authors would like to thank Mrs Camille Martinerie for medical writing service.
The authors Amandine Crombe and Maxime Sitbon contributed equally to the work.
Contributor Information
Amandine Crombe, Email: crombeamandine2@gmail.com, Department of Radiology, Institut Bergonie, F-33000, Bordeaux, France ; University of Bordeaux, F-33000, Bordeaux, France ; Modelisation in Oncology (MOnc) Team, INRIA Bordeaux-Sud-Ouest, CNRS UMR 5251 & Université de Bordeaux, F-33405, Talence, France .
Maxime Sitbon, Email: sitbon.maxime@gmail.com, Department of Radiology, Institut Bergonie, F-33000, Bordeaux, France .
Eberhard Stoeckle, Email: e.stoeckle@bordeaux.unicancer.fr, Department of Surgery, Institut Bergonie, F-33000, Bordeaux, France .
Antoine Italiano, Email: a.italiano@bordeaux.unicancer.fr, Department of Medical Oncology, Institut Bergonie, F-33000, Bordeaux, France .
Xavier Buy, Email: x.buy@bordeaux.unicancer.fr, Department of Radiology, Institut Bergonie, F-33000, Bordeaux, France .
François Le Loarer, Email: f.le-loarer@bordeaux.unicancer.fr, University of Bordeaux, F-33000, Bordeaux, France ; Department of Pathology, Institut Bergonie, F-33000, Bordeaux, France .
Michèle Kind, Email: m.kind@bordeaux.unicancer.fr, Department of Radiology, Institut Bergonie, F-33000, Bordeaux, France .
REFERENCES
- 1. Antonescu CR, Tschernyavsky SJ, Decuseara R, Leung DH, Woodruff JM, Brennan MF, et al. . Prognostic impact of p53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinicopathologic study of 82 cases . Clin Cancer Res 2001. ; 7: 3977 – 87 . [PubMed] [Google Scholar]
- 2. Turc-Carel C, Limon J, Dal Cin P, Rao U, Karakousis C, Sandberg AA, et al. . Cytogenetic studies of adipose tissue tumors. II. Recurrent reciprocal translocation t(12;16)(q13;p11) in myxoid liposarcomas . Cancer Genet Cytogenet 1986. ; 23: 291 – 9 . doi: 10.1016/0165-4608(86)90011-7 [DOI] [PubMed] [Google Scholar]
- 3. Haniball J, Sumathi VP, Kindblom L-G, Abudu A, Carter SR, Tillman RM, et al. . Prognostic factors and metastatic patterns in primary myxoid/round-cell liposarcoma . Sarcoma 2011. ; 2011: 538085 10.1155/2011/538085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engström K, Bergh P, Gustafson P, Hultborn R, Johansson H, Löfvenberg R, et al. . Liposarcoma: outcome based on the Scandinavian sarcoma group register . Cancer 2008. ; 113: 1649 – 56 . doi: 10.1002/cncr.23784 [DOI] [PubMed] [Google Scholar]
- 5. Asano N, Susa M, Hosaka S, Nakayama R, Kobayashi E, Takeuchi K, et al. . Metastatic patterns of myxoid/round cell liposarcoma: a review of a 25-year experience . Sarcoma 2012. ; 2012: 345161 10.1155/2012/345161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fuglø HM, Maretty-Nielsen K, Hovgaard D, Keller Johnny Ø, Safwat AA, Petersen MM, et al. . Metastatic pattern, local relapse, and survival of patients with myxoid liposarcoma: a retrospective study of 45 patients . Sarcoma 2013. ; 2013: 548628 10.1155/2013/548628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fiore M, Grosso F, Lo Vullo S, Pennacchioli E, Stacchiotti S, Ferrari A, et al. . Myxoid/round cell and pleomorphic liposarcomas: prognostic factors and survival in a series of patients treated at a single institution . Cancer 2007. ; 109: 2522 – 31 . doi: 10.1002/cncr.22720 [DOI] [PubMed] [Google Scholar]
- 8. Katz D, Boonsirikamchai P, Choi H, Lazar AJ, Wang W-L, Xiao L, et al. . Efficacy of first-line doxorubicin and ifosfamide in myxoid liposarcoma . Clin Sarcoma Res 2012. ; 2: 2 . doi: 10.1186/2045-3329-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones RL, Fisher C, Al-Muderis O, Judson IR . Differential sensitivity of liposarcoma subtypes to chemotherapy . Eur J Cancer 2005. ; 41: 2853 – 60 . doi: 10.1016/j.ejca.2005.07.023 [DOI] [PubMed] [Google Scholar]
- 10. Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, et al. . Soft tissue and visceral sarcomas: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up . Ann Oncol 2018. ; 29( Suppl 4 ): iv268 – 9 . doi: 10.1093/annonc/mdy321 [DOI] [PubMed] [Google Scholar]
- 11. Taieb S, Saada-Bouzid E, Tresch E, Ryckewaert T, Bompas E, Italiano A, et al. . Comparison of response evaluation criteria in solid tumours and Choi criteria for response evaluation in patients with advanced soft tissue sarcoma treated with trabectedin: a retrospective analysis . Eur J Cancer 2015. ; 51: 202 – 9 . doi: 10.1016/j.ejca.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 12. Soldatos T, Ahlawat S, Montgomery E, Chalian M, Jacobs MA, Fayad LM, et al. . Multiparametric assessment of treatment response in high-grade soft-tissue sarcomas with anatomic and functional MR imaging sequences . Radiology 2016. ; 278: 831 – 40 . doi: 10.1148/radiol.2015142463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crombé A, Le Loarer F, Stoeckle E, Cousin S, Michot A, Italiano A, et al. . Mri assessment of surrounding tissues in soft-tissue sarcoma during neoadjuvant chemotherapy can help predicting response and prognosis . Eur J Radiol 2018. ; 109: 178 – 87 . doi: 10.1016/j.ejrad.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 14. Crombé A, Périer C, Kind M, De Senneville BD, Le Loarer F, Italiano A, et al. . T2 -based MRI Delta-radiomics improve response prediction in soft-tissue sarcomas treated by neoadjuvant chemotherapy . J Magn Reson Imaging 2019. ; 50: 497 – 510 . doi: 10.1002/jmri.26589 [DOI] [PubMed] [Google Scholar]
- 15. Crombé A, Le Loarer F, Cornelis F, Stoeckle E, Buy X, Cousin S, et al. . High-Grade soft-tissue sarcoma: optimizing injection improves MRI evaluation of tumor response . Eur Radiol 2019. ; 29: 545 – 55 . doi: 10.1007/s00330-018-5635-4 [DOI] [PubMed] [Google Scholar]
- 16. Assi T, Kattan J, El Rassy E, Honore C, Dumont S, Mir O, et al. . A comprehensive review of the current evidence for trabectedin in advanced myxoid liposarcoma . Cancer Treat Rev 2019. ; 72: 37 – 44 . doi: 10.1016/j.ctrv.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 17. Wortman JR, Tirumani SH, Tirumani H, Shinagare AB, Jagannathan JP, Hornick JL, et al. . Neoadjuvant radiation in primary extremity liposarcoma: correlation of MRI features with histopathology . Eur Radiol 2016. ; 26: 1226 – 34 . doi: 10.1007/s00330-015-3953-3 [DOI] [PubMed] [Google Scholar]
- 18. Skorpil M, Rydén H, Wejde J, Lidbrink E, Brosjö O, Berglund J, et al. . The effect of radiotherapy on fat content and fatty acids in myxoid liposarcomas quantified by MRI . Magn Reson Imaging 2017. ; 43: 37 – 41 . doi: 10.1016/j.mri.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 19. Wang W-L, Katz D, Araujo DM, Ravi V, Ludwig JA, Trent JC, et al. . Extensive adipocytic maturation can be seen in myxoid liposarcomas treated with neoadjuvant doxorubicin and ifosfamide and pre-operative radiation therapy . Clin Sarcoma Res 2012. ; 2: 25 . doi: 10.1186/2045-3329-2-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1 . Eur J Cancer 2009. ; 45: 228 – 47 . doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 21. Cousin S, Crombe A, Stoeckle E, Brouste V, Le Loarer F, Lucchesi C, et al. . Clinical, radiological and genetic features, associated with the histopathologic response to neoadjuvant chemotherapy (NAc) and outcomes in locally advanced soft tissue sarcoma (STS) patients (PTS . JCO 2017. ; 35( 15_suppl ): 11014 10.1200/JCO.2017.35.15_suppl.11014 [DOI] [Google Scholar]
- 22. Hemminger JA, Iwenofu OH . Ny-Eso-1 is a sensitive and specific immunohistochemical marker for myxoid and round cell liposarcomas among related mesenchymal myxoid neoplasms . Mod Pathol 2013. ; 26: 1204 – 10 . doi: 10.1038/modpathol.2013.65 [DOI] [PubMed] [Google Scholar]
- 23. Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. . N4ITK: improved N3 bias correction . IEEE Trans Med Imaging 2010. ; 29: 1310 – 20 . doi: 10.1109/TMI.2010.2046908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nyúl LG, Udupa JK . On standardizing the Mr image intensity scale . Magn Reson Med 1999. ; 42: 1072 – 81 . doi: [DOI] [PubMed] [Google Scholar]
- 25. Nioche C, Orlhac F, Boughdad S, Reuzé S, Goya-Outi J, Robert C, et al. . LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity . Cancer Res 2018. ; 78: 4786 – 9 . doi: 10.1158/0008-5472.CAN-18-0125 [DOI] [PubMed] [Google Scholar]
- 26. Salduz A, Alpan B, Valiyev N, Özmen E, İribaş A, Ağaoğlu F, et al. . Neoadjuvant radiotherapy for myxoid liposarcomas: oncologic outcomes and histopathologic correlations . Acta Orthop Traumatol Turc 2017. ; 51: 355 – 61 . doi: 10.1016/j.aott.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Demetri GD, Fletcher CD, Mueller E, Sarraf P, Naujoks R, Campbell N, et al. . Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma . Proc Natl Acad Sci U S A 1999. ; 96: 3951 – 6 . doi: 10.1073/pnas.96.7.3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Di Giandomenico S, Frapolli R, Bello E, Uboldi S, Licandro SA, Marchini S, et al. . Mode of action of trabectedin in myxoid liposarcomas . Oncogene 2014. ; 33: 5201 – 10 . doi: 10.1038/onc.2013.462 [DOI] [PubMed] [Google Scholar]
- 29. Kuroda M, Ishida T, Takanashi M, Satoh M, Machinami R, Watanabe T, et al. . Oncogenic transformation and inhibition of adipocytic conversion of preadipocytes by TLS/FUS-CHOP type II chimeric protein . Am J Pathol 1997. ; 151: 735 – 44 . [PMC free article] [PubMed] [Google Scholar]
- 30. Kuyumcu G, Rubin BP, Bullen J, Ilaslan H . Quantification of fat content in lipid-rich myxoid liposarcomas with MRI: a single-center experience with survival analysis . Skeletal Radiol 2018. ; 47: 1411 – 7 . doi: 10.1007/s00256-018-2974-9 [DOI] [PubMed] [Google Scholar]
- 31. Takeuchi A, Yamamoto N, Shirai T, Hayashi K, Miwa S, Munesue S, et al. . Clinical relevance of peroxisome proliferator-activated receptor-gamma expression in myxoid liposarcoma . BMC Cancer 2016. ; 16: 442 . doi: 10.1186/s12885-016-2524-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peeken JC, Bernhofer M, Spraker MB, Pfeiffer D, Devecka M, Thamer A, et al. . Ct-Based radiomic features predict tumor grading and have prognostic value in patients with soft tissue sarcomas treated with neoadjuvant radiation therapy . Radiother Oncol 2019. ; 135: 187 – 96 . doi: 10.1016/j.radonc.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 33. Rothman KJ . No adjustments are needed for multiple comparisons . Epidemiology 1990. ; 1: 43 – 6 . doi: 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- 34. Perneger TV . What's wrong with Bonferroni adjustments . BMJ 1998. ; 316: 1236 – 8 . doi: 10.1136/bmj.316.7139.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ottenbacher KJ . Quantitative evaluation of multiplicity in epidemiology and public health research . Am J Epidemiol 1998. ; 147: 615 – 9 . doi: 10.1093/oxfordjournals.aje.a009501 [DOI] [PubMed] [Google Scholar]
- 36. Reeder SB, Cruite I, Hamilton G, Sirlin CB . Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy . J Magn Reson Imaging 2011. ; 34: 729 – 49 spcone . doi: 10.1002/jmri.22580 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.