Abstract
IMPORTANCE
Only modest attention has been paid to the contributions of social determinants of health to atrial fibrillation risk factors, diagnosis, symptoms, management, and outcomes. The diagnosis of atrial fibrillation provides unique challenges exacerbated by the arrhythmia’s often paroxysmal nature and individuals’ disparate access to healthcare and technologies that facilitate detection. Social determinants of health impact access to care and management decisions for atrial fibrillation, increasing the likelihood of adverse outcomes among individuals who experience systemic disadvantages. Developing effective approaches to address modifiable social determinants of health requires research to eliminate the substantive inequities in health care delivery and outcomes in atrial fibrillation.
OBSERVATIONS
The National Heart, Lung, and Blood Institute convened an expert panel to identify major knowledge gaps and research opportunities in the field of social determinants of atrial fibrillation. The workshop addressed the following social determinants: (1) socioeconomic status and access to care; (2) health literacy; (3) race, ethnicity, and racism; (4) sex and gender; (5) shared decision-making in systemically disadvantaged populations; and (6) place, including rurality, neighborhood, and community. Many individuals with AF have multiple adverse social determinants, which may cluster in the individual and in systemically disadvantaged places (e.g., rural locations, urban neighborhoods). Cumulative disadvantages may accumulate over the life course and contribute to inequities in the diagnosis, management, and outcomes in AF.
CONCLUSIONS AND RELEVANCE
Workshop participants identified multiple critical research questions and approaches to catalyze social determinants of health research that address the distinctive aspects of atrial fibrillation. The long-term aspiration of this work is to eradicate the substantive inequities in atrial fibrillation diagnosis, management, and outcomes across populations. Social determinants of health (SDOH) are the influence of circumstances and systems in which individuals are born, live, work, age, and access healthcare. An American Heart Association statement called for scientists to research and promote an evidence base to eliminate the deleterious cardiovascular consequences of SDOH, including education, income, race, ethnicity, social support, culture/language, access to care, and residential environments, but did not include atrial fibrillation (AF).1 The contributions of SDOH to cardiovascular health and various cardiovascular diseases have been well documented,2 in contrast to AF. As AF is a highly prevalent and morbid condition,2 investigating individual and systemic SDOH is essential for elucidating mechanisms and eliminating inequities in the diagnosis, management, and outcomes of AF.
Several features of AF render the condition susceptible to SDOH-related inequities. AF may be paroxysmal, and often is clinically unrecognized. Systemically disadvantaged patients have less access to screening and digital technology and hence are less likely to have their AF diagnosed. In addition, AF management may require expensive therapies that do not alter symptoms (e.g., anticoagulation), or that require specialty care access (e.g., ablation). Delayed or inadequate management may contribute to health inequities, particularly in patients who are uninsured, underinsured, or in rural communities.
The National Heart, Lung, and Blood Institute (NHLBI) sponsored a workshop series to prioritize substantive research opportunities to address gaps in AF knowledge and care.3–7 On May 13, 2021, the NHLBI Research Working Group brought together thought leaders on SDOH to provide the background, knowledge gaps, and research priorities to accelerate the evidence for interventions aimed at SDOH disadvantages and remediation in the context of AF. The workshop covered the following aspects of SDOH as pertinent across the life course of AF: (1) socioeconomic status (SES) and access to care; (2) health literacy; (3) race, ethnicity, and racism; (4) sex and gender; (5) shared decision-making (SDM) in systemically disadvantaged populations; and (6) place (Figure 1).
Socioeconomic Status and Access to Care
Background
Lower SES has been associated with higher risk of AF.8 Similarly, among individuals with AF, lower individual and area-level SES markers have been associated with higher risks of death and AF-related complications, including ischemic stroke and heart failure.9 The association between SES and outcomes in AF partially may be explained by lower awareness of an AF diagnosis among individuals with lower SES or who live in areas with lower SES.10 In addition, individuals with lower SES are less likely to have cardiologist involvement in their disease management, receive oral anticoagulants (OACs), and be treated with rhythm control approaches.11 Moreover, individuals with lower SES are more likely to receive warfarin than direct OACs12 and have worse INR control and medication adherence.13
Knowledge Gaps
A better understanding of the contributions that individual and community SES characteristics have to access to AF care and treatments is necessary to inform the design of interventions to improve treatment uptake and outcomes in AF (Figure 2). Individual social risk factors may impair access to health screening leading to less AF detection, underprescribing of guideline-recommended treatment for AF, lower adherence to prescribed therapy, and lower utilization of AF procedures such as catheter ablation.14 In addition, area-level SES is correlated with risk factors for AF and AF-related outcomes, including health behaviors, food insecurity, social connections,15 and health care access.16 Data are lacking on the contribution of SES to management of cardiovascular risk factors and diseases that contribute to AF onset and subsequent complications. Furthermore, patients who are uninsured or underinsured, as well as those with the lowest levels of SES have been disproportionately excluded from research studies, a key limitation undermining generalizability of extant studies. Approaches to routinely collect standardized information, as delineated in PhenX,17 on individual and area-level SES in electronic health records, other observational and clinical data, and clinical trials require continued development and implementation. The Table outlines research opportunities.
Figure 2. Socioeconomic status (SES) & access to care in atrial fibrillation (AF).
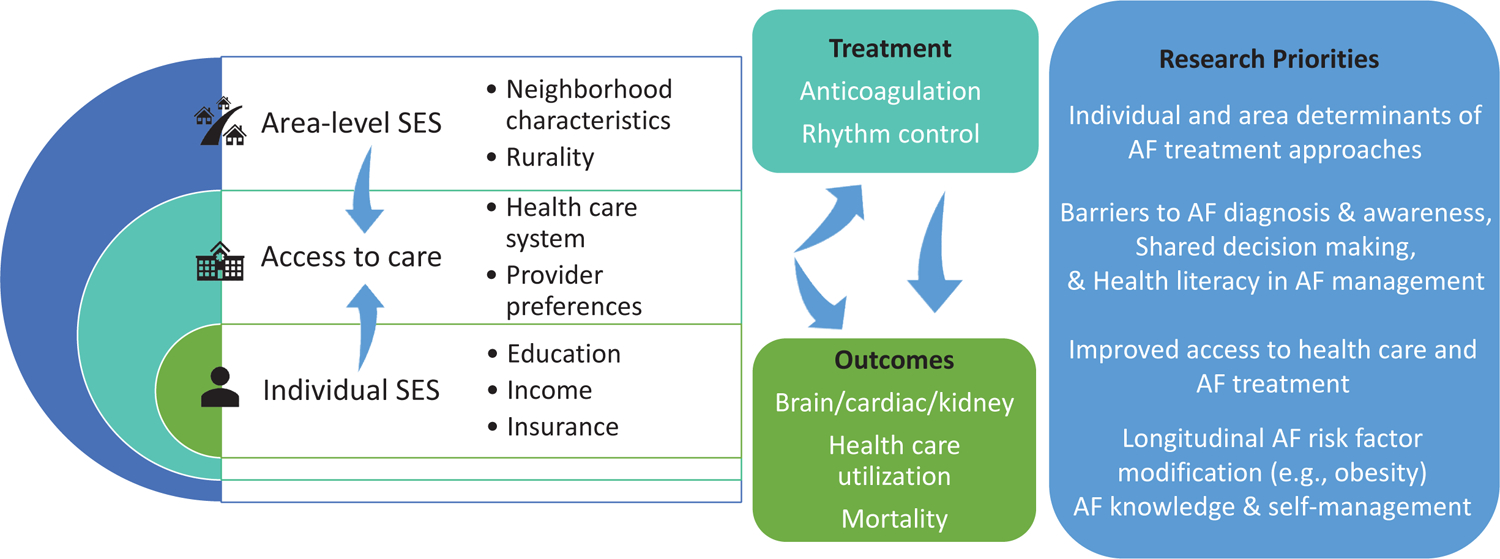
Socioeconomic factors influence access to treatment and outcomes. Research priorities include identifying individual and area determinants of AF treatment approaches, practice patterns, patient factors, and shared decision-making to develop effective ways to improve AF diagnosis, treatment, and outcomes. See also table in eSupplement.
Table.
Prioritized Research Opportunities for Atrial Fibrillation (AF) Social Determinants of Health (SDOH)
| Socioeconomic status and access to care | |
| 1. | Examine inequitable individual and neighborhood SES determinants of OAC initiation, adherence, and persistence and use of rhythm control therapies. |
| 2. | Conduct implementation studies and pragmatic randomized trials that modify clinician and healthcare system-factors implicated in SES inequities in AF treatment and outcomes. |
| Health literacy | |
| 1. | Using mixed-methods studies, identify barriers and facilitators to integration of standardized assessments of health literacy into clinical care and clinical trials in patients with AF. |
| 2. | Evaluate if health literacy is a risk factor, modifier, or mediator of adverse outcomes in patients with AF. |
| 3. | Using a pragmatic clinical trial design, test the hypothesis that accessible, patient-facing health literacy appropriate education improves metrics of patient-reported measures (e.g., decision-quality), guideline-based care, and clinical outcomes in AF. |
| Race, ethnicity, and racism | |
| 1. | Determine the association of systemic racism with AF incidence, treatment, and outcomes by examining health system markers of systemic racism such as clinician race and ethnicity, racially discordant encounters, availability of additional services to support social needs, and quality improvement initiatives targeting inequities and practice level bias. |
| 2. | Identify innovative multilevel interventions and novel technologies that specifically target reducing racial and ethnic inequities in guideline-based AF treatment and investigate their effect on outcomes in randomized pragmatic and setting-based cluster randomized trials. |
| Sex and gender | |
| 1. | Conduct observational cohort and pragmatic trials to evaluate potential mediation of sex differences in AF outcomes by individual, clinician, and systemic level SDOH to identify underlying contributors to sex differences in treatment of AF considering both biological and social determinants. Conduct pragmatic randomized trials testing educational and SDOH interventions designed to modify clinician and healthcare system-factors that may contribute to diminishing sex differences in AF treatment and outcomes. |
| 2. | Study the incidence, prevalence, risk factors, and outcomes of AF in sexual and gender diverse (particularly transgender) individuals, including assessment of treatment and outcomes in individuals receiving gender affirming therapies. |
| Shared decision-making (SDM) | |
| 1. | Perform mixed-methods studies to examine therapeutic options presented to patients and the decision-making outcome to study the influence of patient, clinician, and systemic factors in patients with AF in communities with low access to specialty and tertiary care. |
| 2. | Conduct comparative effectiveness trials to determine whether non-culturally tailored SDM aids achieve similar decision-outcomes compared with culturally tailored aids among UREG individuals with AF. |
| Place, including rurality, neighborhood, and community | |
| 1. | Assess relations of living in areas with limited resources with adverse AF outcomes. |
| 2. | Examine associations of place characteristics with AF incidence and prevalence in studies with more robust ascertainment of AF using various long-term ECG monitoring strategies, providing devices to individuals/groups that lack resources for purchase. |
Abbreviations: OAC, oral anticoagulation; shared decision-making, SDM; SES, socioeconomic status; UREG, underrepresented racial and ethnic groups. Please see supplementary table for full recommendations.
Health Literacy
Background
Health literacy is defined as the capacity to obtain, process, and understand health information.14 Limited health literacy is prevalent in an estimated one-third to one-half of US adults.18 Evaluation of health literacy is integral to SDOH, given the disproportionate prevalence of limited health literacy in systemically disadvantaged populations, as identified by race, ethnicity, income, health insurance status, and markers of social adversity.19 Compared with those possessing adequate health literacy, individuals with limited health literacy have higher hospitalization rates and poorer health status, as well as lower likelihood of medication adherence and less access to primary and specialty care.20
Limited health literacy is associated with the presence and poorer control of risk factors for AF (e.g., smoking, lower physical activity, higher body mass index), as well as with worse cardiovascular outcomes associated with AF.14 Individuals with limited health literacy may be less likely to seek care and experience challenges with the specialized terminology and symptom monitoring integral to AF evaluation and care, and likewise have decreased adherence to complex medications, such as anticoagulation.21 As such, health literacy may constitute (a) a mediator or moderator of associations between SDOH and health outcomes; (b) an individual-level social risk factor; and (c) a focal target for enhanced care delivery and promotion of health equity.
Knowledge Gaps
Three key unanswered questions have been identified in the study of health literacy and AF (Figure 3). First, how can health literacy be integrated into research on diagnosis, treatment, and outcomes in AF? Health literacy assessments are not routinely included in electronic health records, observational studies, registries, or clinical trials of AF, which limits generalizability of research and leaves critical questions unanswered. Successful strategies for incorporating health literacy in clinical trials to provide accessible, patient-facing content have been identified, including presenting multiple forms of information, involving patients to optimize information, and standardizing health literacy outcome measures.22
Figure 3. Knowledge gaps and research opportunities in health literacy (HL) and atrial fibrillation (AF).
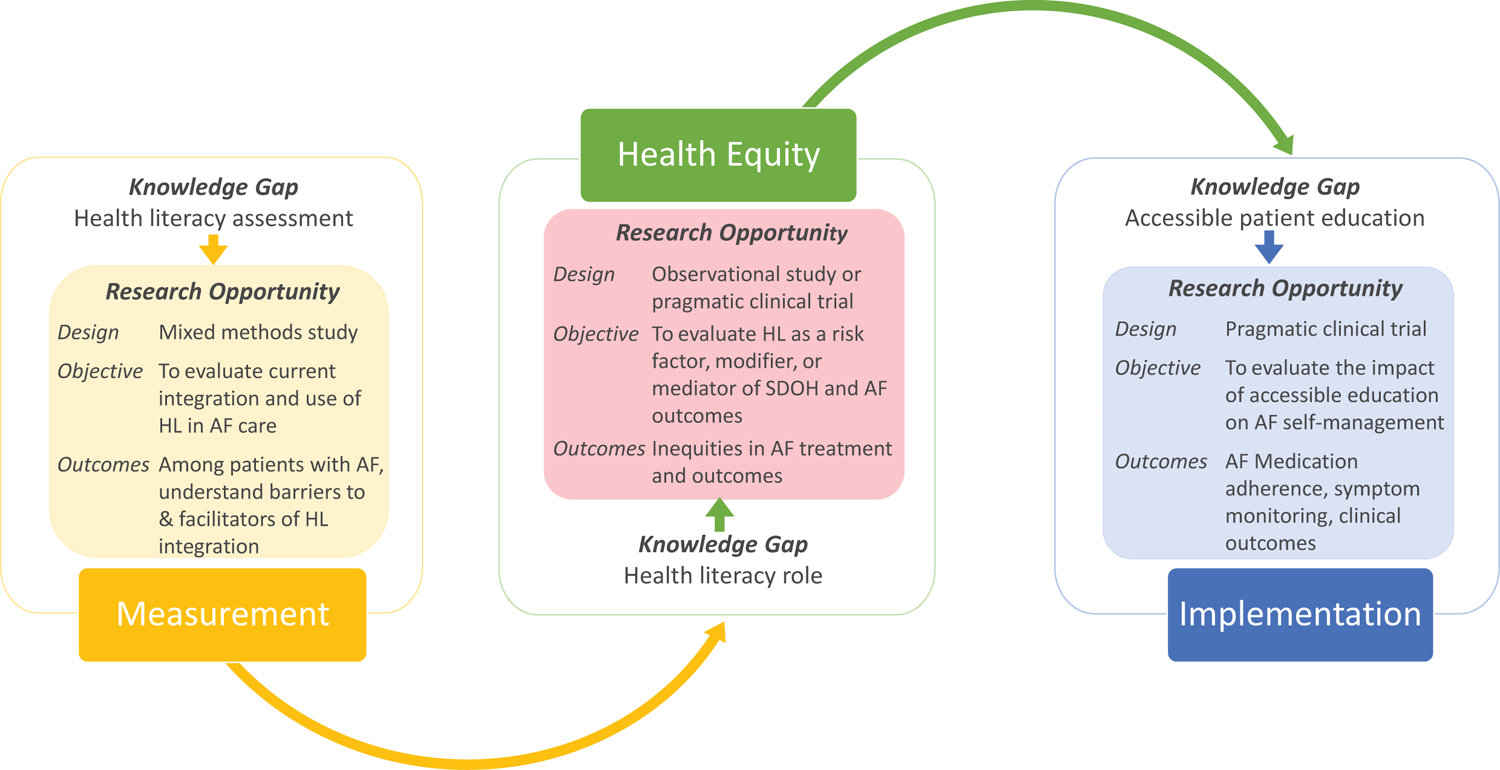
The figure presents the essential steps towards integration of HL into the framework of AF care, stemming from measurement, evaluation, and implementation. SDOH, social determinants of health. See also table in eSupplement.
Second, does health literacy constitute a risk factor, modifier, or mediator of health inequities in AF and can health literacy be considered in interventions to address inequities? As awareness of SDOH and their effect on AF grows, understanding how health literacy mediates inequities related to other forms of SDOH may identify critical targets to promote greater health equity.
Third, how does health literacy affect patients’ experience with AF, and whether clinical care processes address these associations? Health literacy has substantive, varied effects on self-management, symptom monitoring, engagement in care, and medication persistence. Addressing health literacy and its accompanying challenges can enrich interventions to improve treatment adherence. The Table outlines research opportunities.
Race, Ethnicity, Racism, and AF
Background
Racial inequities in health and health care have been extensively documented across cardiovascular conditions, including AF.23–25 The majority of adequately powered studies examining racial variation, including genetic studies, have documented that White individuals have higher risk of AF relative to other examined racial and ethnic groups.26 Despite Black adults being less likely to be diagnosed with AF,27 the likelihood of death and disability from AF is greater in individuals who are Black or from other underrepresented racial and ethnic groups (UREG) compared with White individuals.2,23 We define UREG as Black, Hispanic/Latino, Asian, American Indians/Alaskan Native, and Hawaiian/Pacific Islanders.
The determinants of racial inequities in AF are complex, broad, and partially rooted in systemic racism (structural, institutional, interpersonal, and internalized).26,28,29 First, the greater prevalence of AF clinical risk factors such as hypertension, diabetes, and obesity in UREG versus White individuals likely contributes to higher AF-related complications. Second, lower awareness and detection of AF among Black individuals has been reported, particularly in the absence of routine screening.2,30,31 Third, differential management of AF by race and ethnicity has been observed,26 with prior analyses describing lower rates of warfarin and direct OAC therapy among Black, Hispanic patients, and American Indian/Alaska Native individuals with AF.25 Additionally, racial and ethnic inequities in the use of antiarrhythmic drugs, cardioversion, and catheter ablation have been reported.32
Knowledge Gaps
Multiple gaps are highlighted in the area of race, ethnicity, and AF (eFigure 1). Despite global efforts to improve representation of UREG individuals in research, Black, Hispanic, American Indian/Alaska Native, Hawaiian/Pacific Islander, and Asian American individuals remain poorly represented in genetic studies,33 registries, and clinical trials of AF,34,35 particularly novel therapies and procedural interventions.36 Underrepresentation contributes to the tendency for individuals from UREG to be treated as monolithic entities, despite substantial subgroup variation in SDOH, management, and outcomes. With improved representation of UREG individuals in future studies, consideration should be given to collecting and reporting more granular and disaggregated race and ethnicity data for exploratory analyses. Furthermore, if possible, analyses of White vs. non-White individuals should be avoided because of the problematic nature of positing White individuals as the reference group and combining all other UREG individuals.
The reasons for racial and ethnic differences in AF incidence remain enigmatic. Future AF ascertainment studies should include individuals who identify as from UREG and might include measures of subclinical AF along with cardiac structure and function. The roles of digital health and remote patient monitoring to reduce AF health inequities across UREG communities need to be examined.
There remains limited understanding of the contributions of systemic racism, including neighborhood segregation, suboptimal patient-clinician relations/communication, and underinsurance experienced by UREG individuals to inequitable AF treatment and outcomes.37 How interventions that build patient trust will interact with race and ethnicity to affect AF care and outcomes is poorly understood. Investigative strategies might include workforce diversification and equity training, as well as enhancing community partnerships. There have been few multilevel interventions addressing patient, clinician, health system, and community-level factors to address unequal rates of control of AF risk factors and medical comorbidities among UREG communities.
Finally, there are significant opportunities to understand the relationships among race and ethnicity as a social construct, genetic ancestry, and biological traits38 to more precisely determine risk prediction of AF onset and outcomes and to potentially guide treatment. The Table outlines research opportunities.
Sex, Gender, and AF
Background
AF incidence is lower in women than men,2 until the prevalence in women surpasses that in men after age 75 years, when approximately 60% of individuals with AF are women;39 the overall prevalence of AF in women across the lifespan is equivalent to that in men.39 Once AF develops, women have more symptoms,40 worse quality of life,40 and are at higher risk for stroke40,41 and other adverse outcomes.41 Women with AF report significantly higher financial stress, traumatic life events, and neighborhood stress than women without AF.42 Not only are women with AF at twice the risk of stroke as compared with men,41 AF-related strokes are more severe and disabling in women.43
Within health systems there are also inequities in diagnosis and treatment of AF. Despite having more severe symptoms, women with AF have lower rates of OAC use, are less likely to see an electrophysiologist,44 and are less likely to undergo electrical cardioversion45 or catheter ablation for AF.32,44 These differences in AF incidence and treatment may have a biological component (e.g., sex differences in height, left atrial size, AF pattern on presentation) as well as a component based upon gender differences in socially constructed roles (i.e., primary caregiver, educational attainment, occupational roles) resulting in inequities in income, SES, and access to care.46 There are also risk factors that encompass both domains, such as the positive association between increasing number of pregnancies and AF among women,47 which may have both biological and social components.
Risk factors and outcomes of AF are poorly understood for sexual and gender diverse (e.g., lesbian, gay, bisexual, and transgender) individuals. While cardiovascular risk factors may vary across sexual and gender diverse groups, research in this area is limited without data convincingly demonstrating whether or not various groups are at higher risk of cardiovascular diseases than others or than cisgender individuals.48 Using the National Inpatient Sample, investigators reported that 2.9% of transgender patients undergoing gender-reassignment surgery had concomitant AF, which was associated with elevated risk of in-hospital mortality.49 However, there is a scarcity of data on the impact of gender-affirming therapies on outcomes, and available studies are limited by sample size, methodological issues, or study design.48
Knowledge Gaps
Reasons underlying the higher rates of AF-associated morbidity and mortality in women and sexual and gender diverse individuals require further investigation (eFigure 2). A better understanding of the effect of SDOH on AF incidence and outcomes in women and gender diverse individuals is a crucial first step toward designing population-based interventions to improve health outcomes in AF and in associated comorbid conditions such as heart failure, stroke, and dementia. There are also significant gaps in knowledge regarding other sex- and gender-specific risk factors that affect AF incidence and outcomes, such as pregnancy, contraception, and hormonal therapy.
With respect to AF therapy, it is unclear why women are less likely than men to see an electrophysiologist or to receive cardioversion or catheter ablation, and whether varying communication styles across sex and gender may contribute to these health care inequities. Given recent data regarding the benefits of AF ablation as an effective treatment strategy in reducing risk of recurrent AF and improving quality of life compared with drug therapy,50,51 it will be important to understand to what extent the lower rates of catheter ablation in women might be due to underlying biological sex differences versus social components inclusive of sex such as gender biases, differential access to care, patient preference, differences in health literacy or SDM, or delays in diagnosis resulting in higher risk profiles among women at the time of presentation. Comparative data regarding therapeutic approaches for AF in women and sexual and gender diverse individuals are lacking and will require adequate representation of such individuals in large, randomized trials to provide power for sex- and gender-specific analyses. Finally, relatively little is known about the incidence and outcomes of AF in sexual and gender diverse individuals, and the biological effects of gender-affirming therapies on AF. More research is needed to determine the risk of AF in transgender women and men, and the impact of hormonal therapy on AF and stroke risk. The Table outlines research opportunities.
AF Shared Decision-Making (SDM) in Systemically Disadvantaged Populations
Background
SDM is the process by which patients and clinicians work together to align medical decisions with the best available evidence and patients’ values and preferences.52 Decisions around treatment in AF are well-suited for SDM because stroke prevention and rate/rhythm control involve weighing risks and benefits of different strategies.53 Although OACs reduce stroke risk, they are associated with increased bleeding risk. Similarly, rhythm control strategies such as catheter ablation also involve trade-offs related to the possible neutral effect on survival, risk of procedural complications, and the salutary effect on quality of life.
Since 2014, professional societies have recommended the use of SDM in deciding antithrombotic care.54 However, research suggests that patients are rarely involved in AF decision-making.55 In a US-based registry, half of patients with AF reported that decisions around stroke prevention and rate/rhythm control were made entirely by their clinicians, with no patient input, and only one in four patients reported that their treatment strategy was the result of SDM.55 One strategy for improving SDM is the use of well-designed decision aids; one review for stroke prevention in AF identified 14 decision aids. The aids resulted in small improvements in patient knowledge and less decisional conflict, but whether they improve outcomes remains unknown.56
Knowledge Gaps
SDM in AF is particularly relevant in systemically disadvantaged populations, because SDOH influence patients’ ability to access and adhere to treatment strategies. However, there remain major evidence gaps on how best to support systemically disadvantaged populations (Figure 4). Evidence gaps have been organized around the Ottawa Decision Support Framework,57 which states that people have decision needs that can be addressed through decision support to improve outcomes. It is important to explicitly acknowledge that SDM outcomes can compete with clinical outcomes. For example, whereas a well-informed patient’s decision to forgo OAC may be a “quality” decision (informed, value-concordant), it is also associated with increased risk of stroke.
Figure 4. Knowledge gaps and research opportunities in shared decision-making (SDM) in systemically disadvantaged populations with atrial fibrillation (AF).
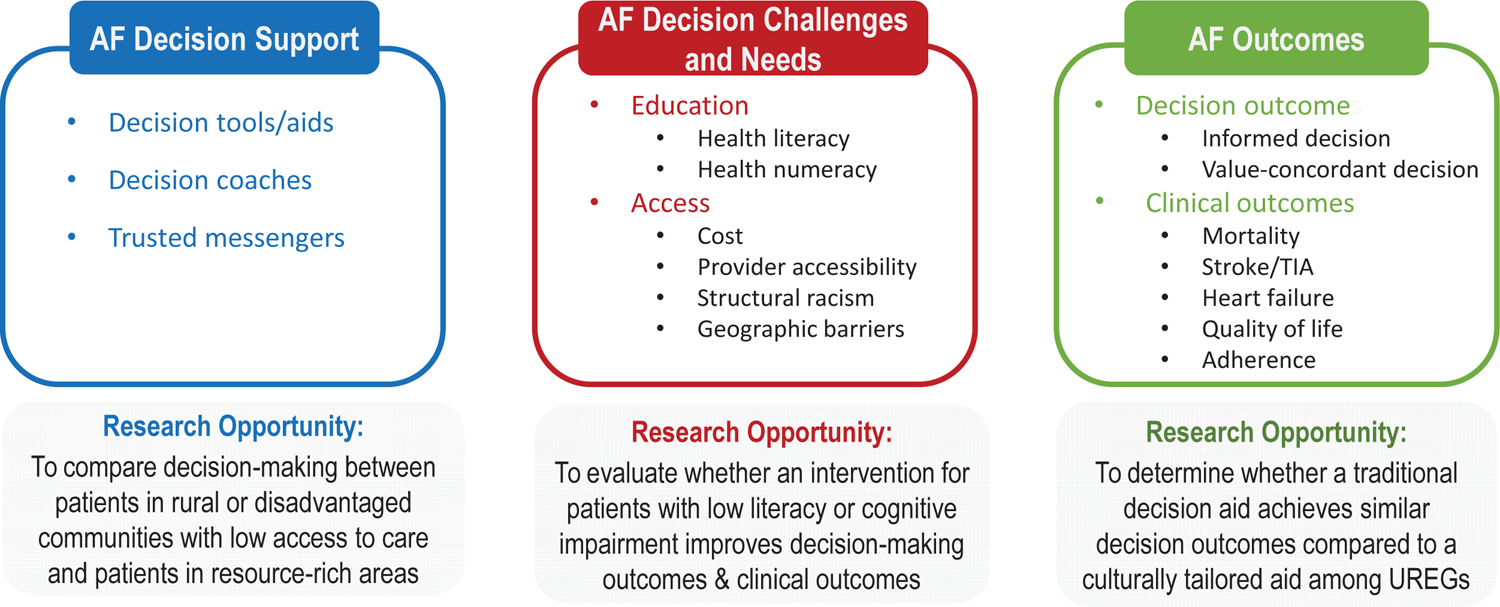
Knowledge gaps were organized around the Ottawa Decision Support Framework,57 which states that people have decision needs that can be addressed through decision support to improve decision outcomes. Knowledge gaps include 1) how do education level and cognitive impairment influence decision-making in AF, and what is their relationship to outcomes; 2) how does healthcare access influence decision-making in AF and outcomes. To determine the most effective decision aid to improve patient reported measures and clinical outcomes, it is necessary to address whether cultural tailoring improves the outcomes of decision interventions and the incorporation of trusted messengers in SDM in AF. UREG, underrepresented racial and ethnic group. See also table in eSupplement.
It remains unclear how low health literacy, numeracy, education, and cognition affect SDM in AF. Additionally, the optimal strategies to improve SDM outcomes in systemically disadvantaged populations need further development. It is unclear how low access to care due to geographic or cost barriers affect SDM.58 Finally, there is a paucity of theory-informed interventions including culturally tailored decision aids59 decision coaches, trusted messengers, and communication training to best support SDM in UREG individuals with AF. The Table outlines research opportunities.
Place Characteristics and AF
Background
Despite the higher prevalence of AF risk factors in systemically disadvantaged neighborhoods, findings from studies relating neighborhood conditions to AF incidence are mixed. A meta-analysis of 18 studies reported that short-term (PM2.5, SO2, NO2) and long-term (PM2.5, SO2, NO2, CO) exposures to air pollutants were associated with a higher risk of AF.60 A cohort study in a large health system found patients living in high and intermediate poverty neighborhoods were more likely to develop AF than those in low poverty neighborhoods.61 In another cohort, participants living in neighborhoods with greater healthy food availability were less likely to develop AF during followup.62 However, other neighborhood features, including walkability, physical resources, social cohesion, and safety were not associated with incident AF.62 Other studies have conflicting findings.63,64
There is stronger evidence relating place characteristics to poorer outcomes among patients with AF. Investigators reported increased mortality risk among Swedish patients with AF in low versus middle SES neighborhoods.65 The socioeconomic association was stronger in men than women, suggesting possible effect modification by sex/gender. A US study reported that patients with AF living in neighborhoods with the highest versus lowest quartile of PM2.5 exposure had the highest risk of stroke.66 Furthermore, in the US National Inpatient Sample of patients hospitalized with an AF diagnosis, those admitted to rural compared with urban hospitals had a higher risk of death.67
The exact reasons for poorer outcomes in patients with AF living in systemically disadvantaged neighborhoods and rural areas are unclear and likely multifactorial. Patients living in rural and systemically disadvantaged neighborhoods have a greater burden of traditional cardiovascular risk factors and comorbidities that could lead to poor AF outcomes.16,65 They may also have more limited, less convenient access to healthcare facilities, which is important for managing AF. In addition, patients with AF living in systemically disadvantaged neighborhoods and rural areas have lower OAC prescription rates.68,69
Knowledge Gaps
Several knowledge gaps exist on the effect of place on AF (eFigure 3). First, few studies, especially in the US, have examined the relations between place and AF-related outcomes, and most were limited to all-cause mortality. Relating place characteristics to more specific AF-related outcomes such as stroke, cognitive decline, heart failure, and quality of life would provide greater insights into the most salient intervention strategies.
Second, without increasing AF awareness and optimizing detection of AF using advanced ECG monitoring technology, an incomplete picture of the burden of AF may lead to differential detection by geographic, systemic, and neighborhood-related characteristics. Specifically, people living in more resource-deprived neighborhoods may have lower detection of AF. They also may be more likely to face geographic and systemic barriers to obtaining the regular health care needed to detect AF,16,70 and specialty care once detected.
Third, little is known about the influence of place characteristics on the occurrence and management of AF-related thromboembolism. Successful prevention of thromboembolic and other complications by appropriate use of stroke reduction therapies and risk factor treatment requires regular access to healthcare services, which are not readily accessible in certain areas. The Table outlines research opportunities.
Discussion
Clinicians, scientists, and the public are increasingly aware that complex individual and community level SDOH influence the risk of AF and its complications (Figure 1). The NHLBI Workshop participants identified multiple knowledge gaps and research opportunities to accelerate SDOH research related to AF (Table).
Figure 1. Social determinants of health (SDOH) in atrial fibrillation (AF), cross-cutting themes.
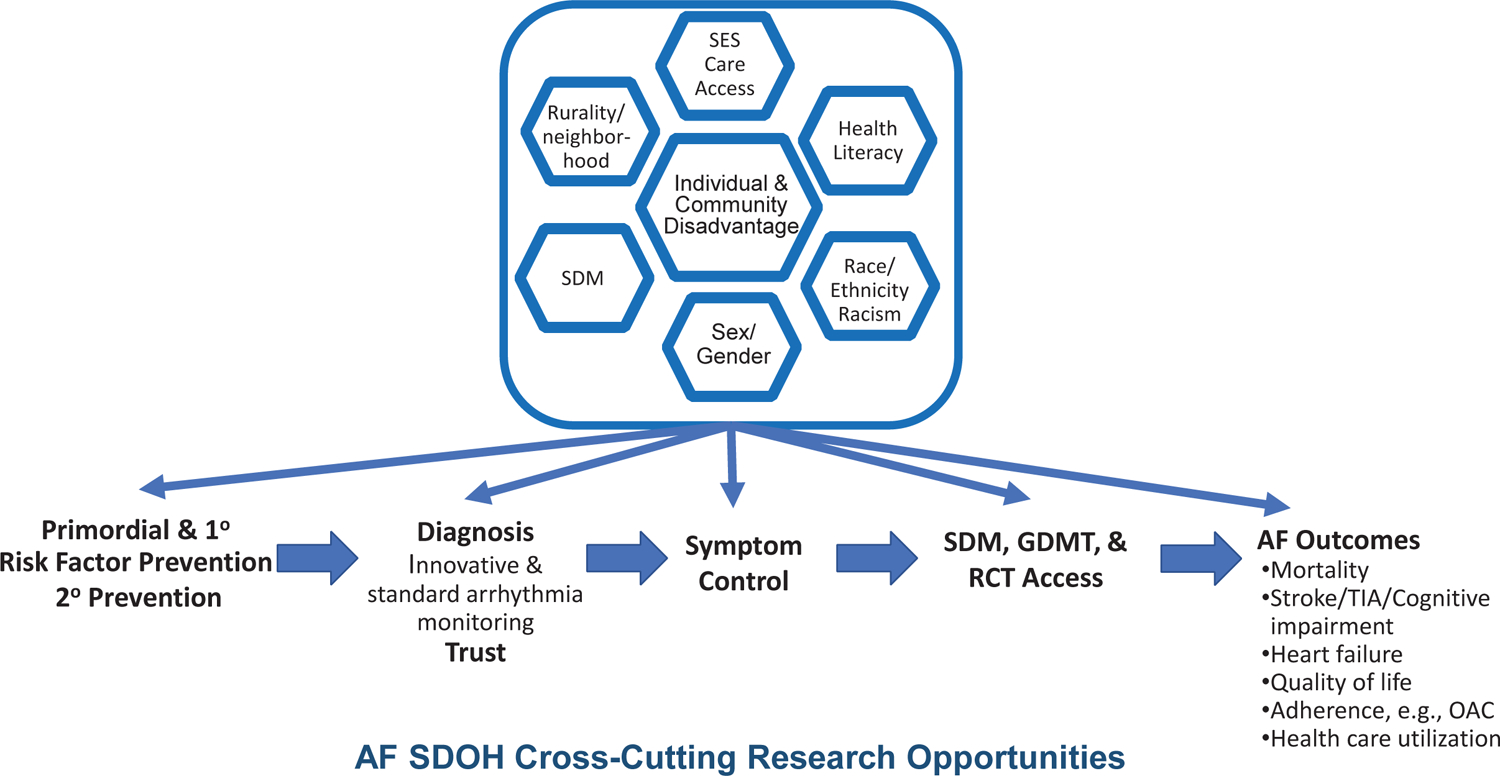
Individuals and communities accumulate disadvantages across the life course. Systemically disadvantaged SDOH are displayed in the top half of the figure, including: (1) socioeconomic status (SES) and access to care; (2) health literacy; (3) race, ethnicity, and racism; (4) sex and gender; (5) shared decision-making (SDM) in systemically disadvantaged populations; and (6) place (e.g., rurality, neighborhood, and community). The disadvantages accrue throughout the life course of individuals developing AF from the primordial emergence of risk factors, to primary prevention of risk factors, to secondary prevention of established heart failure and myocardial infarction. Disadvantages manifest from inequities in diagnosis, awareness, and symptoms. Inequities also emerge in receipt of guideline-directed medical therapies (GDMT) and access to randomized controlled trials (RCT), which lack diversity, yet inform GDMT. Each step along the lifecourse of AF contributes to inequities in AF outcomes, including stroke, cognitive decline, heart failure, myocardial infarction, chronic kidney disease, quality of life, health care utilization, and mortality. See also table in eSupplement.
Workshop participants noted a number of themes that cut across the 6 focus areas. Individuals with AF may have multiple intersectional identities (e.g., sex/gender, race/ethnicity) and may be subjected to complex systemic disadvantages. Similarly, SDOH often cluster in individuals and communities—a concept known as cumulative disadvantage71 (Figure 1). Cumulative disadvantages accumulate throughout the life course,71 and have been associated with cardiovascular mortality in women,72 but have rarely been studied in AF.73 The development of advanced statistical and machine learning approaches may allow for analyses of complex intersectional identities and multiple life course SDOH in cohort, electronic health record, and longitudinal clinical trial datasets. Ensuring equitable access to broadband and mobile health technologies will enhance research in technologically systemically disadvantaged populations. Such access may facilitate bringing research and AF management to where patients live, work, and age, thereby reducing health inequities.
A barrier to robust SDOH research has been the lack of standardized approaches to collecting data and definitions. The National Institute of Minority Health and Health Disparities funded the PhenX project to select high-quality SDOH measures and create an investigator toolkit. The resultant toolkit can be readily implemented in REDCap, and includes 16 core, 12 individual, and 10 structural SDOH tools.17 The ability to easily share and combine SDOH data from multiple studies using standardized PhenX SDOH tools has the potential to increase the scientific power of individual studies and clinical trials.
AF clinical practice guidelines are largely silent on the role of SDOH (e.g., SES, health literacy, racism, rurality, neighborhood).74,75 A contributing factor is that many of the groups disproportionately experiencing SDOH are underrepresented in existing clinical trials. Specifically, the underreporting of race and ethnicity and lack of representation in AF clinical trials of Black, Hispanic, Native American/Alaska Native, Native Hawaiian/Pacific Islander, Asian Americans, older individuals,34,35 and lower income individuals are well established. Similarly, most AF clinical trials do not report on participants’ educational attainment or health literacy level. Hence, there are substantive concerns about the generalizability of AF clinical trial findings. Workshop participants advocated that trialists must urgently develop and implement solutions to achieve the goal of representative enrollment in research studies. Clinical trials should report on the adequacy of such efforts to obtain FDA approval and publication of their trial findings. Pragmatic intervention and implementation science-based trials provide an opportunity to focus on systemically disadvantaged groups and address SDOH that contribute to underrepresentation and adverse AF-related outcomes.
Our workshop had limitations in scope. We did not cover all SDOH that may influence AF care, such as incarceration, food insecurity, housing insecurity, or prenatal and generational deprivation. Similarly, we did not address the potential biological underpinnings determining the influence of SDOH on individuals including allostatic load, chronic stress, and systemic inflammation.1 In addition, the vast majority of the literature we cited was from North America. The workshop participants note that future research on SDOH in AF also is needed from low- and middle-income countries, to represent global SDOH more broadly.
SDOH in AF operate throughout the life course of individuals and differentially across communities. The workshop participants hope that identifying major knowledge gaps and research opportunities in AF related to SDOH will markedly advance AF management and discovery and help reduce AF health inequities.
Supplementary Material
Key Points.
Question
What are key research priorities to eliminate inequities in atrial fibrillation (AF) diagnosis, management, and outcomes related to social determinants such as socioeconomic status, health literacy, race/ethnicity, sex/gender, shared decision-making access, and place?
Findings
The National Heart, Lung, and Blood Institute’s AF Research Working Group assembled experts who identified knowledge gaps and research opportunities essential to advancing health equity across populations from AF diagnosis to outcomes.
Meaning
Whereas the influence of social determinants of cardiovascular health have been described, eliminating health inequities in AF care requires intensive social determinants research across the continuum of care for patients with AF.
Tweet.
NHLBI’s expert consensus panel magnifies the importance of social determinants of health in atrial fibrillation’s (#AFIB) diagnosis, management & outcomes, & actionable research priorities to address inequities in the care & prognosis of patients with #AFIB! #SDOH #HealthEquity
Sources of Funding
Dr. Benjamin receives research funding from National Institutes of Health, National Heart, Lung, and Blood Institute grants R01HL128914, 2R01HL092577, R01HL141434 01A1, NIH National Institute of Aging grant R01AG066010 and American Heart Association: 18SFRN34110082.
Dr. Thomas reports funding from Patient Centered Outcomes Research Institute 1503-29746 and US National Institutes of Health 5UL1-TR001117-03, 1R01-MD013493-01, 1R01-MD011606-03, 5U54MD012530-03, 5R25HL135304-05
Dr. Go receives research funding from National Institutes of Health, National Heart, Lung, and Blood Institute R01HL142834 and National Institute of Diabetes and Digestive and Kidney Diseases R01DK103612.
Dr. Albert research funding from National Institutes of Health, National Heart, Lung, and Blood Institute R01HL116690
Dr. Essien receives research funding from the Veterans Health Administration IK2HX003176.
Dr. Hernandez receives research funding from National Institutes of Health, National Heart, Lung, and Blood Institute R01HL157051 and K01HL142847.
Dr. Levy receives research funding from US National Institutes of Health, National Heart, Lung, and Blood Institute R01 HL153607, R01 HL163377, R01 HL146059, R01 HL127215, T32 HL120822, and National Institute of Minority Health and Health Disparities P50 MD017351, as well as the American Heart Association Health Equity Research Network.
Dr. Magnani receives research funding from US National Institutes of Health, National Heart, Lung, and Blood Institute R01HL143010, R33HL144669, and K24HL160527.
Dr. Rodriguez receives funding from National Institutes of Health, National Heart, Lung, and Blood Institute and American Heart Association.
Dr. Alonso receives funding from National Institutes of Health, National Heart, Lung, and Blood Institute grants K24HL148521 and R01HL137338, and American Heart Association: 16EIA26410001.
Dr. Kershaw receives research funding from the National Institutes of Health, National Institute on Aging R01AG067557, R01AG062180, and R21AG069435.
Dr. Chamberlain receives funding from the National Institutes of Health, National Institute on Aging grants R21AG62580, R21AG64804, and the Patient Centered Outcomes Research Institute grant RICRN-2020-009.
Disclosures
No disclosures relevant to manuscript: Drs: Benjamin, Alonso, Chamberlain, Cooper, Desvigne-Nickens, Essien, Kershaw, Magnani, Matlock, Soliman; Ms. Hills
Dr Thomas reports serving as a consultant for Biosense Webster, Pfizer, Bristol Myers Squibb, Johnson & Johnson, Advisory Council for NHLBI, Steering Committee for REACT AF (Rhythm Evaluation for Anticoagulation with Continuous monitoring of Atrial Fibrillation).
Dr Go is a member of the Operations Committee and Steering Committee for the GUARD-AF Study (A Study to Determine if Identification of Undiagnosed Atrial Fibrillation in People at Least 70 Years of Age Reduces the Risk of Stroke; NCT04126486) sponsored by Bristol Meyers Squibb and Pfizer. Dr. Go has also received research funding through his institution from Bristol Meyers Squibb; iRhythm Technologies; and Janssen Research and Development.
Dr Albert is a member of the Data Safety and Monitoring Board for the Apple Watch Study and is on the Steering Committee for the CHANGE AF study.
Dr Hernandez reports serving as a Consultant to Pfizer, Bristol Myers Squibb
Dr. Levy is Immediate Past Chair, Accreditation Oversight Committee and current member of the National Cardiovascular Data Registry Oversight Committee, American College of Cardiology; President, Southeast Michigan American Heart Association.
Dr O’Brien reports research grants to her institution from Pfizer, Bristol Myers Squibb, and Novartis.
Dr. Russo has received research support (Boston Scientific, Medilynx); Consultant/Advisory Board (Biosense Webster, Boston Scientific, Medtronic); Research Steering Committee (Apple Heart Study, Boston Scientific, Medtronic)
Dr. Rodriguez has received honoraria from Merck and research support from and participated on an Advisory Board for Amgen.
Dr Al-Khatib has received research funding from Medtronic, Boston Scientific, and Abbott.
Abbreviations:
- AF
atrial fibrillation
- NHLBI
National Heart, Lung, and Blood Institute
- OAC
oral anticoagulant
- SDM
shared decision-making
- SDOH
social determinants of health
- SES
socioeconomic status
- UREG
underrepresented racial and ethnic groups
Footnotes
Disclaimer The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the United States Department of Health and Human Services
REFERENCES
- 1.Havranek EP, Mujahid MS, Barr DA, et al. Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2015;132(9):873–898. doi: 10.1161/CIR.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 2.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145(8):e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Chen PS, Bild DE, et al. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119(4):606–618. doi: 10.1161/CIRCULATIONAHA.108.825380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Khatib SM, Benjamin EJ, Albert CM, et al. Advancing Research on the Complex Interrelations Between Atrial Fibrillation and Heart Failure: A Report From a US National Heart, Lung, and Blood Institute Virtual Workshop. Circulation. 2020;141(23):1915–1926. doi: 10.1161/CIRCULATIONAHA.119.045204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Khatib SM, Benjamin EJ, Buxton AE, et al. Research Needs and Priorities for Catheter Ablation of Atrial Fibrillation: A Report From a National Heart, Lung, and Blood Institute Virtual Workshop. Circulation. 2020;141(6):482–492. doi: 10.1161/CIRCULATIONAHA.119.042706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Go AS, Desvigne-Nickens P, et al. Research Priorities in Atrial Fibrillation Screening: A Report From a National Heart, Lung, and Blood Institute Virtual Workshop. Circulation. 2021;143(4):372–388. doi: 10.1161/CIRCULATIONAHA.120.047633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Al-Khatib SM, Desvigne-Nickens P, et al. Research Priorities in the Secondary Prevention of Atrial Fibrillation: A National Heart, Lung, and Blood Institute Virtual Workshop Report. J Am Heart Assoc. 2021;10(16):e021566. doi: 10.1161/JAHA.121.021566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mou L, Norby FL, Chen LY, et al. Lifetime Risk of Atrial Fibrillation by Race and Socioeconomic Status: ARIC Study (Atherosclerosis Risk in Communities). Circ Arrhythm Electrophysiol. 2018;11(7):e006350. doi: 10.1161/CIRCEP.118.006350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lunde ED, Nielsen PB, Riahi S, et al. Associations between socioeconomic status, atrial fibrillation, and outcomes: a systematic review. Expert Rev Cardiovasc Ther. 2018;16(11):857–873. doi: 10.1080/14779072.2018.1533118 [DOI] [PubMed] [Google Scholar]
- 10.Frewen J, Finucane C, Cronin H, et al. Factors that influence awareness and treatment of atrial fibrillation in older adults. QJM. 2013;106(5):415–424. doi: 10.1093/qjmed/hct060 [DOI] [PubMed] [Google Scholar]
- 11.Eberly LA, Garg L, Yang L, et al. Racial/Ethnic and Socioeconomic Disparities in Management of Incident Paroxysmal Atrial Fibrillation. JAMA Netw Open. 2021;4(2):e210247. doi: 10.1001/jamanetworkopen.2021.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurusamy VK, Brobert G, Vora P, Friberg L. Sociodemographic factors and choice of oral anticoagulant in patients with non-valvular atrial fibrillation in Sweden: a population-based cross-sectional study using data from national registers. BMC Cardiovasc Disord. 2019;19(1):43. doi: 10.1186/s12872-019-1029-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N, Brooks MM, Hernandez I. Latent Classes of Adherence to Oral Anticoagulation Therapy Among Patients With a New Diagnosis of Atrial Fibrillation. JAMA Netw Open. 2020;3(2):e1921357. doi: 10.1001/jamanetworkopen.2019.21357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnani JW, Mujahid MS, Aronow HD, et al. Health Literacy and Cardiovascular Disease: Fundamental Relevance to Primary and Secondary Prevention: A Scientific Statement From the American Heart Association. Circulation. 2018;138(2):e48–e74. doi: 10.1161/CIR.0000000000000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–145. doi: 10.1111/j.1749-6632.2009.05333.x [DOI] [PubMed] [Google Scholar]
- 16.Harrington RA, Califf RM, Balamurugan A, et al. Call to Action: Rural Health: A Presidential Advisory From the American Heart Association and American Stroke Association. Circulation. 2020;141(10):e615–e644. doi: 10.1161/CIR.0000000000000753 [DOI] [PubMed] [Google Scholar]
- 17.Cox LA, Hwang S, Haines J, et al. Using the PhenX Toolkit to Select Standard Measurement Protocols for Your Research Study. Curr Protoc. 2021;1(5):e149. doi: 10.1002/cpz1.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy (NCES 2006–483). US Department of Education, Washington, DC: National Center for Education Statistics. 2006. doi:https://nces.ed.gov/pubs2006/2006483.pdf [Google Scholar]
- 19.Schillinger D. The Intersections Between Social Determinants of Health, Health Literacy, and Health Disparities. Stud Health Technol Inform. 2020;269:22–41. doi: 10.3233/SHTI200020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Conor R, Moore A, Wolf MS. Health Literacy and Its Impact on Health and Healthcare Outcomes. Stud Health Technol Inform. 2020;269:3–21. doi: 10.3233/SHTI200019 [DOI] [PubMed] [Google Scholar]
- 21.Cabellos-Garcia AC, Martinez-Sabater A, Castro-Sanchez E, Kangasniemi M, Juarez-Vela R, Gea-Caballero V. Relation between health literacy, self-care and adherence to treatment with oral anticoagulants in adults: a narrative systematic review. BMC Public Health. 2018;18(1):1157. doi: 10.1186/s12889-018-6070-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bader M, Zheng L, Rao D, et al. Towards a more patient-centered clinical trial process: A systematic review of interventions incorporating health literacy best practices. Contemp Clin Trials. 2022;116:106733. doi: 10.1016/j.cct.2022.106733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnani JW, Norby FL, Agarwal SK, et al. Racial Differences in Atrial Fibrillation-Related Cardiovascular Disease and Mortality: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA Cardiol. 2016;1(4):433–441. doi: 10.1001/jamacardio.2016.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golwala H, Jackson LR 2nd, Simon DN, et al. Racial/ethnic differences in atrial fibrillation symptoms, treatment patterns, and outcomes: Insights from Outcomes Registry for Better Informed Treatment for Atrial Fibrillation Registry. Am Heart J. 2016;174:29–36. doi: 10.1016/j.ahj.2015.10.028 [DOI] [PubMed] [Google Scholar]
- 25.Essien UR, Kim N, Hausmann LRM, et al. Disparities in Anticoagulant Therapy Initiation for Incident Atrial Fibrillation by Race/Ethnicity Among Patients in the Veterans Health Administration System. JAMA Netw Open. 2021;4(7):e2114234. doi: 10.1001/jamanetworkopen.2021.14234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamirisa KP, Al-Khatib SM, Mohanty S, et al. Racial and Ethnic Differences in the Management of Atrial Fibrillation. CJC Open. 2021;3(12 Suppl):S137–S148. doi: 10.1016/j.cjco.2021.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez CJ, Soliman EZ, Alonso A, et al. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol. 2015;25(2):71–76, 76 e71. doi: 10.1016/j.annepidem.2014.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Churchwell K, Elkind MSV, Benjamin RM, et al. Call to Action: Structural Racism as a Fundamental Driver of Health Disparities: A Presidential Advisory From the American Heart Association. Circulation. 2020;142(24):e454–e468. doi: 10.1161/CIR.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 29.Lett E, Asabor E, Beltran S, Cannon AM, Arah OA. Conceptualizing, Contextualizing, and Operationalizing Race in Quantitative Health Sciences Research. Ann Fam Med. 2022;20(2):157–163. doi: 10.1370/afm.2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meschia JF, Merrill P, Soliman EZ, et al. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010;41(4):581–587. doi: 10.1161/STROKEAHA.109.573907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heckbert SR, Austin TR, Jensen PN, et al. Differences by Race/Ethnicity in the Prevalence of Clinically Detected and Monitor-Detected Atrial Fibrillation: MESA. Circ Arrhythm Electrophysiol. 2020;13(1):e007698. doi: 10.1161/CIRCEP.119.007698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel N, Deshmukh A, Thakkar B, et al. Gender, Race, and Health Insurance Status in Patients Undergoing Catheter Ablation for Atrial Fibrillation. Am J Cardiol. 2016;117(7):1117–1126. doi: 10.1016/j.amjcard.2016.01.040 [DOI] [PubMed] [Google Scholar]
- 33.Roselli C, Chaffin MD, Weng LC, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018;50(9):1225–1233. doi: 10.1038/s41588-018-0133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarraju A, Maron DJ, Rodriguez F. Under-Reporting and Under-Representation of Racial/Ethnic Minorities in Major Atrial Fibrillation Clinical Trials. JACC Clin Electrophysiol. 2020;6(6):739–741. doi: 10.1016/j.jacep.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan MZ, Munir MB, Khan SU, et al. Representation of women, older patients, ethnic, and racial minorities in trials of atrial fibrillation. Pacing Clin Electrophysiol. 2021;44(3):423–431. doi: 10.1111/pace.14178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ethoan LN, Takata Y, Sakuma KK, Irvin VL. Trends in Clinical Research Including Asian American, Native Hawaiian, and Pacific Islander Participants Funded by the US National Institutes of Health, 1992 to 2018. JAMA Netw Open. 2019;2(7):e197432. doi: 10.1001/jamanetworkopen.2019.7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones CP. Toward the Science and Practice of Anti-Racism: Launching a National Campaign Against Racism. Ethn Dis. 2018;28(Suppl 1):231–234. doi: 10.18865/ed.28.S1.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo G, Fu Y, Lee H, Cai T, Mullan Harris K, Li Y. Genetic bio-ancestry and social construction of racial classification in social surveys in the contemporary United States. Demography. 2014;51(1):141–172. doi: 10.1007/s13524-013-0242-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155(5):469–473. doi: 10.1001/archinte.1995.00430050045005 [DOI] [PubMed] [Google Scholar]
- 40.Piccini JP, Simon DN, Steinberg BA, et al. Differences in Clinical and Functional Outcomes of Atrial Fibrillation in Women and Men: Two-Year Results From the ORBIT-AF Registry. JAMA Cardiol. 2016;1(3):282–291. doi: 10.1001/jamacardio.2016.0529 [DOI] [PubMed] [Google Scholar]
- 41.Emdin CA, Wong CX, Hsiao AJ, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ. 2016;532:h7013. doi: 10.1136/bmj.h7013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westcott SK, Beach LY, Matsushita F, et al. Relationship Between Psychosocial Stressors and Atrial Fibrillation in Women >45 Years of Age. Am J Cardiol. 2018;122(10):1684–1687. doi: 10.1016/j.amjcard.2018.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomita H, Hagii J, Metoki N, et al. Impact of Sex Difference on Severity and Functional Outcome in Patients with Cardioembolic Stroke. J Stroke Cerebrovasc Dis. 2015;24(11):2613–2618. doi: 10.1016/j.jstrokecerebrovasdis.2015.07.016 [DOI] [PubMed] [Google Scholar]
- 44.Bhave PD, Lu X, Girotra S, Kamel H, Vaughan Sarrazin MS. Race- and sex-related differences in care for patients newly diagnosed with atrial fibrillation. Heart Rhythm. 2015;12(7):1406–1412. doi: 10.1016/j.hrthm.2015.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lip GY, Laroche C, Boriani G, et al. Sex-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Observational Research Programme Pilot survey on Atrial Fibrillation. Europace. 2015;17(1):24–31. doi: 10.1093/europace/euu155 [DOI] [PubMed] [Google Scholar]
- 46.Lindley KJ, Aggarwal NR, Briller JE, et al. Socioeconomic Determinants of Health and Cardiovascular Outcomes in Women: JACC Review Topic of the Week. J Am Coll Cardiol. 2021;78(19):1919–1929. doi: 10.1016/j.jacc.2021.09.011 [DOI] [PubMed] [Google Scholar]
- 47.Wong JA, Rexrode KM, Sandhu RK, Conen D, Albert CM. Number of Pregnancies and Atrial Fibrillation Risk: The Women’s Health Study. Circulation. 2017;135(6):622–624. doi: 10.1161/CIRCULATIONAHA.116.026629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Streed CG Jr., Beach LB, Caceres BA, et al. Assessing and Addressing Cardiovascular Health in People Who Are Transgender and Gender Diverse: A Scientific Statement From the American Heart Association. Circulation. 2021;144(6):e136–e148. doi: 10.1161/CIR.0000000000001003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antwi-Amoabeng D, Doshi R, Adalja D, et al. Burden of arrythmias in transgender patients hospitalized for gender-affirming surgeries. J Arrhythm. 2020;36(4):797–800. doi: 10.1002/joa3.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russo AM, Zeitler EP, Giczewska A, et al. Association Between Sex and Treatment Outcomes of Atrial Fibrillation Ablation Versus Drug Therapy: Results From the CABANA Trial. Circulation. 2021;143(7):661–672. doi: 10.1161/CIRCULATIONAHA.120.051558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mark DB, Anstrom KJ, Sheng S, et al. Effect of Catheter Ablation vs Medical Therapy on Quality of Life Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321(13):1275–1285. doi: 10.1001/jama.2019.0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chung MK, Fagerlin A, Wang PJ, et al. Shared Decision Making in Cardiac Electrophysiology Procedures and Arrhythmia Management. Circ Arrhythm Electrophysiol. 2021;14(12):e007958. doi: 10.1161/CIRCEP.121.007958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brand-McCarthy SR, Delaney RK, Noseworthy PA. Can Shared Decision Making Improve Stroke Prevention in Atrial Fibrillation?: Implications of the Updated Guidelines. Circ Cardiovasc Qual Outcomes. 2020;13(3):e006080. doi: 10.1161/CIRCOUTCOMES.119.006080 [DOI] [PubMed] [Google Scholar]
- 54.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199–267. doi: 10.1161/CIR.0000000000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ali-Ahmed F, Pieper K, North R, et al. Shared decision-making in atrial fibrillation: patient-reported involvement in treatment decisions. Eur Heart J Qual Care Clin Outcomes. 2020;6(4):263–272. doi: 10.1093/ehjqcco/qcaa040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torres Roldan VD, Brand-McCarthy SR, Ponce OJ, et al. Shared Decision Making Tools for People Facing Stroke Prevention Strategies in Atrial Fibrillation: A Systematic Review and Environmental Scan. Med Decis Making. 2021;41(5):540–549. doi: 10.1177/0272989X211005655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stacey D, Legare F, Boland L, et al. 20th Anniversary Ottawa Decision Support Framework: Part 3 Overview of Systematic Reviews and Updated Framework. Med Decis Making. 2020;40(3):379–398. doi: 10.1177/0272989X20911870 [DOI] [PubMed] [Google Scholar]
- 58.Kunneman M, Branda ME, Hargraves IG, et al. Assessment of Shared Decision-making for Stroke Prevention in Patients With Atrial Fibrillation: A Randomized Clinical Trial. JAMA Intern Med. 2020;180(9):1215–1224. doi: 10.1001/jamainternmed.2020.2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alden DL, Friend J, Schapira M, Stiggelbout A. Cultural targeting and tailoring of shared decision making technology: a theoretical framework for improving the effectiveness of patient decision aids in culturally diverse groups. Soc Sci Med. 2014;105:1–8. doi: 10.1016/j.socscimed.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 60.Chen M, Zhao J, Zhuo C, Zheng L. The Association Between Ambient Air Pollution and Atrial Fibrillation. Int Heart J. 2021;62(2):290–297. doi: 10.1536/ihj.20-523 [DOI] [PubMed] [Google Scholar]
- 61.Essien UR, McCabe ME, Kershaw KN, et al. Association Between Neighborhood-Level Poverty and Incident Atrial Fibrillation: a Retrospective Cohort Study. J Gen Intern Med. 2022;37(6):1436–1443. doi: 10.1007/s11606-021-06976-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garg PK, Jorgensen N, Diez-Roux AV, et al. Neighborhood environments and risk of incident atrial fibrillation: The Multi-Ethnic Study of Atherosclerosis. Eur J Prev Cardiol. 2020;27(19):2116–2118. doi: 10.1177/2047487319866020 [DOI] [PubMed] [Google Scholar]
- 63.Zoller B, Li X, Sundquist J, Sundquist K. Neighbourhood deprivation and hospitalization for atrial fibrillation in Sweden. Europace. 2013;15(8):1119–1127. doi: 10.1093/europace/eut019 [DOI] [PubMed] [Google Scholar]
- 64.Murphy NF, Simpson CR, Jhund PS, et al. A national survey of the prevalence, incidence, primary care burden and treatment of atrial fibrillation in Scotland. Heart. 2007;93(5):606–612. doi: 10.1136/hrt.2006.107573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wandell P, Carlsson AC, Gasevic D, Sundquist J, Sundquist K. Neighbourhood socio-economic status and all-cause mortality in adults with atrial fibrillation: A cohort study of patients treated in primary care in Sweden. Int J Cardiol. 2016;202:776–781. doi: 10.1016/j.ijcard.2015.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rhinehart ZJ, Kinnee E, Essien UR, et al. Association of Fine Particulate Matter and Risk of Stroke in Patients With Atrial Fibrillation. JAMA Netw Open. 2020;3(9):e2011760. doi: 10.1001/jamanetworkopen.2020.11760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Neal WT, Sandesara PB, Kelli HM, Venkatesh S, Soliman EZ. Urban-rural differences in mortality for atrial fibrillation hospitalizations in the United States. Heart Rhythm. 2018;15(2):175–179. doi: 10.1016/j.hrthm.2017.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carlsson AC, Wandell P, Gasevic D, Sundquist J, Sundquist K. Neighborhood deprivation and warfarin, aspirin and statin prescription - A cohort study of men and women treated for atrial fibrillation in Swedish primary care. Int J Cardiol. 2015;187:547–552. doi: 10.1016/j.ijcard.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Norby FL, Lutsey PL, Shippee ND, et al. Direct Oral Anticoagulants and Warfarin for Atrial Fibrillation Treatment: Rural and Urban Trends in Medicare Beneficiaries. Am J Cardiovasc Drugs. 2022;22(2):207–217. doi: 10.1007/s40256-021-00502-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White K, Haas JS, Williams DR. Elucidating the role of place in health care disparities: the example of racial/ethnic residential segregation. Health Serv Res. 2012;47(3 Pt 2):1278–1299. doi: 10.1111/j.1475-6773.2012.01410.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dupre ME. Educational differences in health risks and illness over the life course: a test of cumulative disadvantage theory. Soc Sci Res. 2008;37(4):1253–1266. doi: 10.1016/j.ssresearch.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 72.Johnson-Lawrence V, Galea S, Kaplan G. Cumulative socioeconomic disadvantage and cardiovascular disease mortality in the Alameda County Study 1965 to 2000. Ann Epidemiol. 2015;25(2):65–70. doi: 10.1016/j.annepidem.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonaccio M, Di Castelnuovo A, Costanzo S, et al. Life Course Socioeconomic Status and Risk of Hospitalization for Heart Failure or Atrial Fibrillation in The Moli-Sani Study Cohort. Am J Epidemiol. 2021;190(8):1561–1571. doi: 10.1093/aje/kwab046 [DOI] [PubMed] [Google Scholar]
- 74.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 75.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


