Abstract
Introduction
The incidence of cancer during pregnancy and within first year post‐delivery, ie pregnancy‐associated cancer (PAC), is increasing in many countries, but little is known about risk factors for these trends. This study quantified incidence of PAC by trimesters and post‐delivery periods, and assessed the role of maternal age, parity, immigrant status, education, smoking and body mass index for the risk and incidence trends of PAC.
Material and methods
We used data from the national birth and cancer registers in Sweden during 1973–2017 to define a register‐based cohort of women aged 15–44 years. Incidence rates of PAC during pregnancy and up to 1 year post‐delivery were calculated per 100 000 deliveries per year. Poisson regression with multiple imputation estimated incidence rate ratios with 95% confidence intervals adjusted by year, age, previous parity, immigrant status, education, smoking and BMI during 1990–2017, when information on risk factors was available.
Results
Among 4 557 284 deliveries, a total of 1274 (during pregnancy) and 3355 (within 1 year post‐delivery) cases of PAC were diagnosed, with around 50 cases/year diagnosed during pregnancy and 110 cases/year during the first year post‐delivery in the latest period 2015–2017. The most common cancer types during pregnancy were malignant melanoma, breast and cervical cancer, together accounting for 57% of cases during pregnancy and 53% during the first year post‐delivery. The numbers of PAC were lower during pregnancy than during post‐delivery for all tumor types with lowest numbers during first trimester. The PAC incidence rates increased over calendar time. High maternal age at diagnosis, smoking, nulliparity and non‐immigrant background were associated with significantly higher risks of PAC. The increasing PAC incidence was in part explained by higher maternal age over time, but not by the other factors.
Conclusions
High maternal age is the strongest risk factor for PAC. We show for the first time that smoking, nulliparity and non‐immigrant background are also contributing risk factors for PAC. However, only high maternal age contributed significantly to the increasing incidence. Further studies on other potential risk factors for PAC are warranted, since our results indicate that age on its own does not fully explain the increase.
Keywords: breast cancer, cervical cancer, incidence, malignant melanoma, pregnancy, pregnancy‐associated cancer, risk factors
The incidence of cancer during pregnancy and first year post‐delivery is increasing over time, with breast cancer, malignant melanoma and cervical cancer accounting for over 50% of cases. Although maternal age, smoking, nulliparity and non‐immigrant background were identified as risk factors, only maternal age contributed, in part, to the increasing incidence over time.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- HER2
Human epidermal growth factor receptor 2
- ICD
International Classification of Diseases
- IRR
incidence rate ratio
- LISA
Longitudinal Integrated Database for Health Insurance and Labor Market Studies
- MBR
Medical Birth Register
- MICE
multiple imputation with chained equations
- PAC
pregnancy‐associated cancer
Key message.
The incidence of cancer during pregnancy and within 1 year post‐delivery is increasing. In this study, high maternal age, smoking, non‐immigrant background and nulliparity were identified as risk factors. However, only maternal age contributed, in part, to the increasing trends.
1. INTRODUCTION
Pregnancy‐associated cancer (PAC) is commonly defined as cancer occurring during pregnancy and the first year post‐delivery and has an estimated incidence of around one per 1000 deliveries. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 Cancer diagnosed during pregnancy is particularly challenging for the patient and attending physicians, as, depending on the trimester of diagnosis, certain diagnostic procedures and treatments must be avoided due to maternal and fetal risks. For this reason, it has been argued that cancer diagnosed during pregnancy should be studied separately from post‐delivery cases, which are also affected by hormonal and physiological changes but not complicated by treatment restrictions. 12 In addition, symptoms of cancer may be misinterpreted during pregnancy, which may delay the diagnosis until after delivery. Therefore, it is reasonable to assess also the incidence of cancer shortly after delivery in the same context as pregnant cases, as these cancers may have been detected earlier if not masked by pregnancy. 13
Only a few studies exist on PAC incidence and risk factors of PAC, mainly due to the scarcity of linkable population‐based data on cancer and pregnancies. An increasing PAC incidence over time has been reported from the Nordic countries, 5 , 6 , 11 the USA 3 , 9 , 10 and Australia‐Asia, 7 although not from all countries. 8 Increasing maternal age over time may in part explain the increasing incidence of PAC in some countries. 2 , 5 , 7 , 14 Apart from maternal age, it remains unknown which other risk factors contribute to the increasing incidence.
Trends in PAC incidence are affected both by the changing patterns of birth rates as well as cancer incidence trends, and could depend on risk factors related to both, such as age, number of children, socioeconomic factors and immigrant background. 15 To assess PAC incidence, complete population‐based birth and cancer data measured over time are required to obtain sufficient power and validity. Such individual‐level data are available from the Nordic population registries, which have recorded births and cancer cases since the 1960s and 1950s, respectively. These registers offer unique possibilities to estimate PAC incidence and determine potential risk factors and are thus essential to understand the epidemiology of PAC.
The aim of this study was to quantify the PAC incidence trends in Sweden by pregnancy trimesters and post‐delivery intervals, as well as by cancer type (all tumor types, malignant melanoma, breast and cervical cancer) and across calendar periods. A second aim was to assess potential risk factors of PAC, including maternal age, previous parity, immigrant background, educational level, smoking and pre‐pregnancy body mass index (BMI), as well as to what extent these risk factors were associated with the incidence trend.
2. MATERIAL AND METHODS
2.1. Study population
In this population‐based cohort study using registry data, we included all cases of PAC in Sweden 1973–2017 in women aged 15–44 years. The Swedish population and health registers record information on all Swedish residents, and include a unique personal identification number (PIN) that enables individual‐level deterministic cross‐linkages between registers. The study cohort was defined in the Swedish Multigeneration Register, which is based on the Swedish Total Population Register and encompasses residents born in 1932 or later who were alive in 1961. We included female residents who were aged 15–44 years at any time during 1973 and 2017. By individual linkage to the Medical Birth Register (MBR), which was established in 1973, we identified delivery dates of all births, including live births and stillbirths (from week 28 until 2007, from week 22 after 2008) from 1973 to 2017. From the Swedish National Cancer Register, which includes compulsory information on reported new cases of cancer since 1958, we included all cancers occurring at ages 15–44 years during the same period. Information on date of diagnosis and type of cancer was included. The cancer registry uses the International Classification of Diseases Oncology version 3 (ICD‐O‐3), as well as back‐translated diagnoses to ICD version 7 to enable comparisons over calendar time. We assessed all tumor types combined (ICD‐7140–207) and site‐specific diagnoses (Table S1). We included the first occurrence of cancer in each woman, thus women with a history of cancer prior to the PAC index pregnancy were excluded. Similarly, in the group of women without cancer we only counted deliveries that were free of maternal history of cancer.
2.2. Definition of PAC
By linking the cancer data to the birth information we were able to identify cancer diagnoses which occurred near a pregnancy. Pregnancy‐associated cancer was defined based on the date of cancer diagnosis in relation to estimated conception date and delivery date. If conception date was available (defined by last menstrual period, ultrasound or in vitro fertilization), timing of diagnosis was defined by trimesters (1st: 0–97 days; 2nd: 98–188 days; 3rd: >188 days) or during post‐delivery intervals (1–92, 93–183, 184–274, 275–365 days post‐delivery). If conception date was not available (0.2%), trimesters were defined using delivery date (1st: 280–183 days, 2nd: 182–92 days; 3rd: 91–0 days prior to delivery date).
2.3. Risk factors
Maternal age was measured at cancer diagnosis and categorized in 5‐year groups from 15 to 44 years, and calendar period at cancer diagnosis was categorized as 1973–1979, 1980–1984, 1985–1989, etc., to 2015–2017. Previous parity was calculated as number of deliveries in the birth register at the time of cancer diagnosis, excluding the delivery associated with the PAC. Information on education was available from 1990 in the Longitudinal Integrated Database for Health Insurance and Labor Market Studies (LISA) held at Statistics Sweden, and we included the highest attained education level at the date of cancer diagnosis (categorized as <10 years (primary education), 10–13 years (secondary education), undergraduate (<3 years of tertiary education) and postgraduate (≥3 years of tertiary education)). From the MBR, we included information on maternal smoking and pre‐pregnancy BMI measured at first antenatal visit from 1990 and onwards. Immigrant background was categorized as non‐immigrant and immigrant based on birth country information from Statistics Sweden. Similarly, we categorized deliveries among women without cancer based on age, year, parity, immigrant background, education, smoking and BMI at the delivery date.
2.4. Statistical analyses
We calculated descriptive frequencies of PAC by cancer site and by trimesters and post‐delivery intervals. To account for the fact that the number of deliveries at risk may vary over time, we estimated incidence rates of PAC per 100 000 deliveries with the number of PAC cases divided by the number of deliveries in each calendar year. Number of deliveries is an approximation for the pregnant population at risk for PAC in a given age and year, and a standard method for estimating PAC incidence. 15 , 16 For the PAC cases, the age and year of cancer diagnosis was used, rather than age and year of delivery.
The PAC incidence rates in 1990–2017 were modeled using Poisson regression for count data with a log link function and the number of deliveries at risk as offset. For all tumor types, malignant melanoma, breast and cervical cancer, we estimated incidence rate ratios with 95% confidence intervals (CI) for associations with risk factors in a multivariable model adjusted for year, age, parity, immigrant status, education, smoking and BMI. To assess the impact of risk factors on the time trends, a model with calendar period as main exposure was stepwise adjusted for risk factors. Due to missing information on education, smoking and BMI, we used multiple imputation with chained equations (MICE) producing 30 imputed datasets to which the Poisson models were applied. 17 The associations with risk factors were assessed with two‐sided Wald tests and a significance level at 0.05.
2.5. Ethics statement
Ethical approval for the study was granted by the Swedish Ethical Review Authority (Dnr 2010–1950‐31/4 on January 19, 2011, with amendments (2011–599‐32) on April 13, 2011, (2018/1293–32) on July 4, 2018 and (2022/02992–02) on June 15, 2022. The data were analyzed after pseudo‐anonymization, i.e. including no directly identifying information such as name or personal identification number. No informed consent was required according to Swedish legislation.
3. RESULTS
3.1. Descriptive numbers of PAC
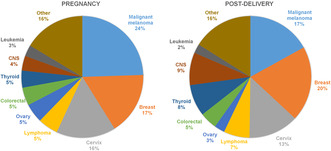
Among 4 557 284 deliveries between 1973 and 2017, 1274 cases of PAC during pregnancy and 3355 cases within 1 year post‐delivery were diagnosed (Table 1). Malignant melanoma, breast cancer and cervical cancer were the three most common cancer types during pregnancy, comprising 57% of all cases during pregnancy and 53% of cases post‐delivery. Malignant melanoma (24.5%) was the most common cancer type during pregnancy, and breast cancer (20.0%) was the most common cancer type post‐delivery (Figure 1).
TABLE 1.
Numbers of pregnancy‐associated cancers in Sweden 1973–2017 by tumor type and by background variables.
| All deliveries | PAC during pregnancy | PAC during 0–12 months postdelivery | ||||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | Rate/100 000 | n | % | Rate/100 000 | |
| Total (row %) | 4 557 284 | 100.0 | 1274 | 0.03 | 28.0 | 3355 | 0.07 | 73.6 |
| Tumor type | ||||||||
| All tumor types | 4629 | 100.0 | 1274 | 100.0 | 28.0 | 3355 | 100.0 | 73.6 |
| Breast | 882 | 19.1 | 212 | 16.6 | 4.7 | 670 | 20.0 | 14.7 |
| Malignant melanoma | 881 | 19.0 | 312 | 24.5 | 6.9 | 569 | 17.0 | 12.5 |
| Cervix | 647 | 14.0 | 201 | 15.8 | 4.4 | 446 | 13.3 | 9.8 |
| Ovary | 157 | 3.4 | 65 | 5.1 | 1.4 | 92 | 2.7 | 2.0 |
| Central nervous system | 341 | 7.4 | 50 | 3.9 | 1.1 | 291 | 8.7 | 6.4 |
| Colon/rectum | 214 | 4.6 | 61 | 4.8 | 1.3 | 153 | 4.6 | 3.4 |
| Thyroid | 340 | 7.3 | 58 | 4.6 | 1.3 | 282 | 8.4 | 6.2 |
| Lymphoma | 299 | 6.5 | 68 | 5.3 | 1.5 | 231 | 6.9 | 5.1 |
| Leukemia | 119 | 2.6 | 38 | 3.0 | 0.8 | 81 | 2.4 | 1.8 |
| Other | 749 | 16.2 | 209 | 16.4 | 4.6 | 540 | 16.1 | 11.8 |
| Timing of cancer diagnosis | ||||||||
| First trimester | 307 | 6.6 | 307 | 24.1 | 6.7 | |||
| Second trimester | 464 | 10.0 | 464 | 36.4 | 10.2 | |||
| Third trimester | 503 | 10.9 | 503 | 39.5 | 11.0 | |||
| 0–3 months post‐delivery | 839 | 18.1 | 839 | 25.0 | 18.4 | |||
| 3–6 months post‐delivery | 820 | 17.7 | 820 | 24.4 | 18.0 | |||
| 6–9 months post‐delivery | 822 | 17.8 | 822 | 24.5 | 18.0 | |||
| 9–12 months post‐delivery | 874 | 18.9 | 874 | 26.1 | 19.2 | |||
| Year at delivery/diagnosis | ||||||||
| 1973–1979 | 696 286 | 15.3 | 115 | 9.0 | 16.5 | 342 | 10.2 | 49.1 |
| 1980–1984 | 459 761 | 10.1 | 98 | 7.7 | 21.3 | 267 | 8.0 | 58.1 |
| 1985–1989 | 520 782 | 11.4 | 115 | 9.0 | 22.1 | 309 | 9.2 | 59.3 |
| 1990–1994 | 582 673 | 12.8 | 129 | 10.1 | 22.1 | 369 | 11.0 | 63.3 |
| 1995–1999 | 446 003 | 9.8 | 122 | 9.6 | 27.4 | 307 | 9.2 | 68.8 |
| 2000–2004 | 458 624 | 10.1 | 140 | 11.0 | 30.5 | 352 | 10.5 | 76.8 |
| 2005–2009 | 511 585 | 11.2 | 191 | 15.0 | 37.3 | 468 | 13.9 | 91.5 |
| 2010–2014 | 546 984 | 12.0 | 209 | 16.4 | 38.2 | 600 | 17.9 | 109.7 |
| 2015–2017 | 334 586 | 7.3 | 155 | 12.2 | 46.3 | 341 | 10.2 | 101.9 |
| Age at delivery/diagnosis | ||||||||
| 15–19 | 131 188 | 2.9 | 7 | 0.5 | 5.3 | 28 | 0.8 | 21.3 |
| 20–24 | 883 077 | 19.4 | 100 | 7.8 | 11.3 | 261 | 7.8 | 29.6 |
| 25–29 | 1 570 294 | 34.5 | 382 | 30.0 | 24.3 | 816 | 24.3 | 52.0 |
| 30–34 | 1 306 059 | 28.7 | 448 | 35.2 | 34.3 | 1225 | 36.5 | 93.8 |
| 35–39 | 561 175 | 12.3 | 270 | 21.2 | 48.1 | 784 | 23.4 | 139.7 |
| 40–44 | 105 491 | 2.3 | 67 | 5.3 | 63.5 | 241 | 7.2 | 228.5 |
| Live birth, gestational week | ||||||||
| 22–27 | 10 138 | 0.2 | 16 | 1.3 | 157.8 | 17 | 0.5 | 167.7 |
| 28–31 | 23 086 | 0.5 | 77 | 6.1 | 333.5 | 31 | 0.9 | 134.3 |
| 32–36 | 209 436 | 4.6 | 293 | 23.2 | 139.9 | 225 | 6.7 | 107.4 |
| 37–41 (term) | 3 922 539 | 86.4 | 821 | 65.0 | 20.9 | 2846 | 85.1 | 72.6 |
| 42+ | 366 726 | 8.1 | 53 | 4.2 | 14.5 | 219 | 6.5 | 59.7 |
| Missing gestational age | 8429 | 0.2 | 4 | 0.3 | 47.5 | 7 | 0.2 | 83.0 |
| Stillbirth, gestational week | ||||||||
| 22–27 | 1138 | 6.7 | 1 | 10.0 | 87.9 | 0 | 0.0 | 0.0 |
| 28–31 | 2610 | 15.4 | 3 | 30.0 | 114.9 | 2 | 20.0 | 76.6 |
| 32–36 | 4949 | 29.2 | 4 | 40.0 | 80.8 | 3 | 30.0 | 60.6 |
| 37–41 (term) | 7327 | 43.3 | 2 | 20.0 | 27.3 | 5 | 50.0 | 68.2 |
| 42+ | 752 | 4.4 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 |
| Missing gestational age | 154 | 0.9 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 |
| Parity prior to current delivery | ||||||||
| 0 | 1 948 329 | 42.8 | 527 | 41.4 | 27.0 | 1225 | 36.5 | 62.9 |
| 1 | 1 664 925 | 36.5 | 449 | 35.2 | 27.0 | 1263 | 37.6 | 75.9 |
| 2+ | 944 030 | 20.7 | 298 | 23.4 | 31.6 | 867 | 25.8 | 91.8 |
| Immigrant status | ||||||||
| Non‐immigrant | 3 778 450 | 82.9 | 1083 | 85.0 | 28.7 | 2815 | 83.9 | 74.5 |
| Immigrant | 778 544 | 17.1 | 191 | 15.0 | 24.5 | 540 | 16.1 | 69.4 |
| Missing | 290 | 0.0 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 |
| Education level (1990–2017) | ||||||||
| <10 years | 365 994 | 12.7 | 91 | 9.5 | 24.9 | 239 | 9.9 | 65.3 |
| 10–13 years | 1 286 121 | 44.6 | 385 | 40.4 | 29.9 | 995 | 41.1 | 77.4 |
| Undergraduate | 403 519 | 14.0 | 131 | 13.7 | 32.5 | 361 | 14.9 | 89.5 |
| Postgraduate | 755 377 | 26.2 | 337 | 35.3 | 44.6 | 788 | 32.6 | 104.3 |
| Missing | 69 444 | 2.4 | 10 | 1.0 | 14.4 | 35 | 1.4 | 50.4 |
| Smoking (1990–2017) | ||||||||
| No | 2 250 613 | 78.1 | 707 | 74.1 | 31.4 | 1930 | 79.8 | 85.8 |
| Yes | 482 938 | 16.8 | 157 | 16.5 | 32.5 | 350 | 14.5 | 72.5 |
| Missing | 146 904 | 5.1 | 90 | 9.4 | 61.3 | 138 | 5.7 | 93.9 |
| Pre‐pregnancy BMI (1990–2017) | ||||||||
| <18.5 kg/m2 | 61 526 | 2.1 | 19 | 2.0 | 30.9 | 47 | 1.9 | 76.4 |
| 18.5–24.9 kg/m2 | 1 449 117 | 50.3 | 467 | 49.0 | 32.2 | 1243 | 51.4 | 85.8 |
| 25.0–29.9 kg/m2 | 567 575 | 19.7 | 202 | 21.2 | 35.6 | 505 | 20.9 | 89.0 |
| ≥30.0 kg/m2 | 255 824 | 8.9 | 77 | 8.1 | 30.1 | 205 | 8.5 | 80.1 |
| Missing | 546 413 | 19.0 | 189 | 19.8 | 34.6 | 418 | 17.3 | 76.5 |
FIGURE 1.
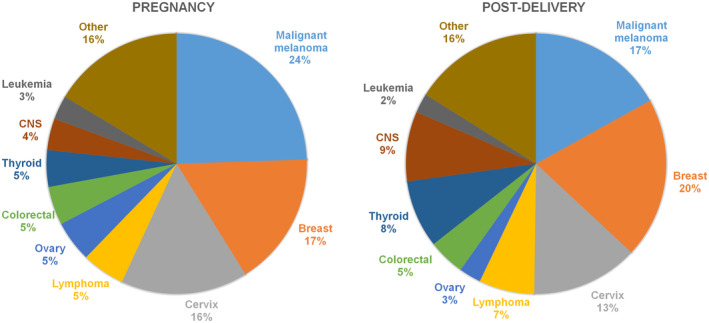
Distribution of tumor types during pregnancy and within 1 year post‐delivery.
The numbers of PAC increased over calendar years, with numbers depending not only on cancer but also on changes in birth rates per year (Table 1). Cancer during pregnancy, as well as cancer during the first year post‐delivery, were both most common at ages 30–34 (35.2% and 36.5%, respectively). The rates of PAC increased by age group, with the highest rates in women aged 40–44 (63.5/100000 during pregnancy and 228.5/100000 during the first year post‐delivery). Preterm delivery was more common for PAC during pregnancy (30.6%) compared with all deliveries (5.3%) and PAC within 1 year post‐delivery (8.1%). Stillbirth rates were low in all groups. Parity, education, immigration background, educational level, smoking status and BMI only differed marginally in women with PAC compared with the population.
The number of PAC was lower during pregnancy compared with 3‐month post‐delivery intervals, with some differences for the three major cancer types (Figure 2, Figure S1). The numbers of malignant melanoma were similar pre‐ and post‐delivery, whereas the numbers of breast cancer increased from first to third trimester and across post‐delivery intervals. For cervical cancer, the highest number of cases were diagnosed 3–6 months post‐delivery.
FIGURE 2.
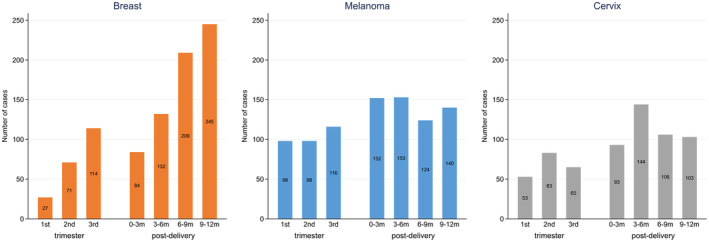
Diagnosis of cancer in relation to time of pregnancy and post‐delivery period, for breast cancer, malignant melanoma and cervical cancer.
3.2. Incidence trends of PAC by year and age
Crude incidence rates of PAC increased significantly over calendar time from 21.3 per 100 000 in 1980–1984 to 38.2 per 100 000 in 2010–2014, with about half the incidence during pregnancy compared with during first year post‐delivery (Figure 3, top left; Table 1). The crude incidence increased over time in all trimesters and post‐delivery intervals (Figure 3, bottom left). The crude incidence rates of PAC were also strongly and significantly associated with increasing maternal age (Figure 3, top right) and by trimester (Figure 3, bottom right).
FIGURE 3.
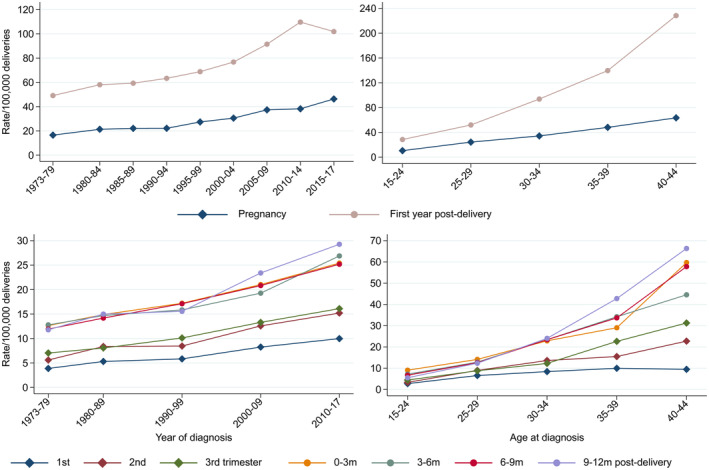
Crude incidence rates of pregnancy‐associated cancer (diagnosis during pregnancy and within 1 year post‐delivery) (top panel) and for trimesters and 3‐month intervals post‐delivery (bottom panel), all tumor types combined.
3.3. Risk factors for PAC
During pregnancy, the PAC incidence rates of all tumor types, breast cancer, malignant melanoma and cervical cancer, increased across calendar periods in Sweden, after adjustment for age, parity, immigration status, education, smoking and BMI (Table 2). Increasing maternal age was strongly and significantly associated with higher PAC incidence of all tumor types, breast cancer, malignant melanoma and cervical cancer, hence the highest incidence rates were found in the oldest age group (40–44 years) and the lowest at ages below 30. Nulliparity was significantly associated with higher rates of PAC (all tumor types), whereas parity was not significantly associated with PAC incidence for breast cancer, malignant melanoma or cervical cancer separately. Immigrant background was significantly associated with a lower PAC rate for all tumor types and malignant melanoma; it was borderline significant for cervical cancer, but not significantly associated with breast cancer. Education and BMI were not significantly associated with PAC rates. Smoking was significantly associated with a higher risk of PAC during pregnancy (incidence rate ratio = 1.28, 95% CI 1.07–1.54), especially cervical cancer (incidence rate ratio = 2.32, 95% CI 1.52–3.54).
TABLE 2.
Associations between incidence of cancer during pregnancy and calendar year, age, parity, immigrant status, education, smoking and BMI by tumor type in Sweden 1990–2017.
| All tumor types | Malignant melanoma | Breast | Cervix | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | IRR a | 95% CI | n | IRR a | 95% CI | n | IRR a | 95% CI | n | IRR a | 95% CI | |
| Year at delivery/diagnosis | ||||||||||||
| 1990–1994 | 129 | 1.00 | Ref | 31 | 1.00 | Ref | 19 | 1.00 | Ref | 23 | 1.00 | Ref |
| 1995–1999 | 122 | 1.18 | 0.92–1.51 | 42 | 1.69 | 1.06–2.69 | 21 | 1.27 | 0.68–2.37 | 16 | 0.92 | 0.48–1.74 |
| 2000–2004 | 140 | 1.19 | 0.94–1.52 | 31 | 1.10 | 0.67–1.82 | 26 | 1.31 | 0.72–2.39 | 17 | 0.84 | 0.45–1.59 |
| 2005–2009 | 191 | 1.43 | 1.13–1.79 | 45 | 1.42 | 0.89–2.28 | 38 | 1.57 | 0.90–2.76 | 24 | 1.11 | 0.62–2.00 |
| 2010–2014 | 209 | 1.48 | 1.18–1.86 | 53 | 1.65 | 1.04–2.61 | 40 | 1.52 | 0.87–2.67 | 29 | 1.32 | 0.75–2.33 |
| 2015–2017 | 155 | 1.81 | 1.42–2.31 | 33 | 1.73 | 1.05–2.88 | 31 | 1.92 | 1.07–3.47 | 33 | 2.51 | 1.44–4.36 |
| P < 0.0001 | P = 0.1035 | P = 0.3590 | P = 0.0011 | |||||||||
| Age at delivery/diagnosis | ||||||||||||
| 15–24 | 58 | 0.32 | 0.24–0.43 | 9 | 0.20 | 0.10–0.40 | 1 | 0.03 | 0.00–0.22 | 9 | 0.27 | 0.13–0.57 |
| 25–29 | 263 | 0.74 | 0.63–0.87 | 68 | 0.66 | 0.48–0.91 | 30 | 0.45 | 0.29–0.69 | 47 | 0.81 | 0.54–1.21 |
| 30–34 | 351 | 1.00 | Ref | 105 | 1.00 | Ref | 70 | 1.00 | Ref | 52 | 1.00 | Ref |
| 35–39 | 219 | 1.36 | 1.14–1.61 | 41 | 0.87 | 0.60–1.25 | 56 | 1.70 | 1.19–2.43 | 28 | 1.21 | 0.76–1.93 |
| 40–44 | 55 | 1.74 | 1.31–2.32 | 12 | 1.35 | 0.74–2.47 | 18 | 2.78 | 1.64–4.70 | 6 | 1.33 | 0.57–3.12 |
| P < 0.0001 | P = 0.0001 | P < 0.0001 | P = 0.0053 | |||||||||
| Parity prior to current delivery | ||||||||||||
| 0 | 402 | 1.00 | Ref | 97 | 1.00 | Ref | 64 | 1.00 | Ref | 67 | 1.00 | Ref |
| 1 | 337 | 0.85 | 0.73–0.98 | 90 | 0.93 | 0.70–1.25 | 65 | 0.89 | 0.62–1.26 | 49 | 0.77 | 0.53–1.12 |
| 2+ | 207 | 0.81 | 0.67–0.97 | 48 | 0.91 | 0.62–1.32 | 46 | 0.86 | 0.57–1.29 | 26 | 0.65 | 0.40–1.06 |
| Not counting index birth | P = 0.0294 | P = 0.8456 | P = 0.7171 | P = 0.1634 | ||||||||
| Immigrant status | ||||||||||||
| Non‐immigrant | 790 | 1.00 | Ref | 223 | 1.00 | Ref | 141 | 1.00 | Ref | 122 | 1.00 | Ref |
| Immigrant | 156 | 0.76 | 0.64–0.91 | 12 | 0.23 | 0.13–0.41 | 34 | 0.88 | 0.60–1.29 | 20 | 0.64 | 0.40–1.04 |
| Imputed | 0 | 0 | 0 | 0 | ||||||||
| P = 0.0025 | P < 0.0001 | P = 0.5071 | P = 0.0728 | |||||||||
| Education | ||||||||||||
| <10 years | 88 | 0.93 | 0.74–1.19 | 12 | 0.63 | 0.34–1.17 | 16 | 1.32 | 0.74–2.35 | 12 | 0.73 | 0.39–1.37 |
| 10–13 years | 382 | 1.00 | Ref | 99 | 1.00 | Ref | 52 | 1.00 | Ref | 63 | 1.00 | Ref |
| Undergraduate | 130 | 0.94 | 0.77–1.16 | 34 | 0.94 | 0.63–1.40 | 29 | 1.34 | 0.85–2.13 | 22 | 1.04 | 0.63–1.70 |
| Postgraduate | 336 | 1.11 | 0.95–1.31 | 90 | 1.15 | 0.84–1.58 | 76 | 1.53 | 1.04–2.23 | 43 | 0.93 | 0.60–1.44 |
| Imputed | 10 | 0 | 2 | 2 | ||||||||
| P = 0.3224 | P = 0.3077 | P = 0.1788 | P = 0.7653 | |||||||||
| Cigarette smoking | ||||||||||||
| No | 702 | 1.00 | Ref | 186 | 1.00 | Ref | 140 | 1.00 | Ref | 83 | 1.00 | Ref |
| Yes | 154 | 1.28 | 1.07–1.54 | 34 | 1.16 | 0.79–1.70 | 21 | 1.06 | 0.65–1.71 | 34 | 2.32 | 1.52–3.54 |
| Imputed | 90 | 15 | 14 | 25 | ||||||||
| P = 0.0069 | P = 0.4388 | P = 0.8267 | P = 0.0001 | |||||||||
| Pre‐pregnancy BMI | ||||||||||||
| <18.5 | 19 | 1.18 | 0.74–1.86 | 2 | 0.45 | 0.11–1.81 | 5 | 2.07 | 0.82–5.26 | 3 | 1.32 | 0.44–3.95 |
| 18.5–24.9 | 467 | 1.00 | Ref | 130 | 1.00 | Ref | 82 | 1.00 | Ref | 63 | 1.00 | Ref |
| 25.0–29.9 | 202 | 1.09 | 0.92–1.28 | 45 | 0.91 | 0.65–1.28 | 41 | 1.29 | 0.87–1.91 | 23 | 0.88 | 0.53–1.43 |
| 30.0+ | 77 | 0.94 | 0.72–1.23 | 15 | 0.70 | 0.40–1.22 | 12 | 0.93 | 0.49–1.77 | 13 | 1.06 | 0.55–2.04 |
| Imputed | 181 | 43 | 35 | 40 | ||||||||
| P = 0.6651 | P = 0.4087 | P = 0.3321 | P = 0.8897 | |||||||||
| Cases with information imputed, total | 202 | 45 | 39 | 43 | ||||||||
Model included year, age, parity, immigration status, education, smoking and body mass index (BMI). Education, smoking and BMI imputed using MICE, 10 cycles of chained equations and 30 sets.
Within 1 year post‐delivery, the PAC incidences of all tumor types, breast cancer, malignant melanoma and cervical cancer, were higher in the recent calendar years (Table 3). Increasing maternal age was strongly and significantly associated with PAC incidence for all tumor types, breast cancer and malignant melanoma, while the effect was less marked for cervical cancer. Previous parity was not significantly associated with PAC incidence. Immigrant background was significantly associated with a lower PAC rate within 1 year post‐delivery for all tumor types, malignant melanoma and cervical cancer, but not for breast cancer. Educational level was not significantly associated with higher rates of PAC, except for cervical cancer, where higher education (undergraduate and postgraduate) was significantly associated with lower rates (P = 0.0159). Smoking was not significantly associated with PAC incidence rates, nor was BMI.
TABLE 3.
Associations between incidence of cancer during first year post‐delivery and calendar year, age, parity, immigrant status, education, smoking and BMI by cancer type in Sweden 1990–2017.
| All tumor types | Malignant melanoma | Breast | Cervix | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | IRR a | 95% CI | n | IRR a | 95% CI | n | IRR a | 95% CI | n | IRR a | 95% CI | |
| Year at delivery/diagnosis | ||||||||||||
| 1990–1994 | 369 | 1.00 | Ref | 62 | 1.00 | Ref | 74 | 1.00 | Ref | 39 | 1.00 | Ref |
| 1995–1999 | 307 | 1.01 | 0.87–1.18 | 49 | 0.95 | 0.65–1.38 | 58 | 0.88 | 0.62–1.24 | 32 | 1.02 | 0.64–1.63 |
| 2000–2004 | 352 | 1.05 | 0.91–1.22 | 63 | 1.10 | 0.77–1.56 | 60 | 0.77 | 0.55–1.08 | 54 | 1.61 | 1.06–2.44 |
| 2005–2009 | 468 | 1.22 | 1.06–1.40 | 78 | 1.20 | 0.85–1.68 | 113 | 1.20 | 0.89–1.62 | 54 | 1.48 | 0.97–2.26 |
| 2010–2014 | 600 | 1.49 | 1.30–1.70 | 127 | 1.89 | 1.38–2.59 | 130 | 1.28 | 0.96–1.73 | 75 | 2.03 | 1.36–3.03 |
| 2015–2017 | 341 | 1.39 | 1.20–1.62 | 75 | 1.88 | 1.33–2.66 | 65 | 1.05 | 0.74–1.48 | 47 | 2.13 | 1.38–3.30 |
| P < 0.0001 | P < 0.0001 | P = 0.0136 | P = 0.0006 | |||||||||
| Age at delivery/diagnosis | ||||||||||||
| 15–24 | 161 | 0.33 | 0.27–0.39 | 22 | 0.28 | 0.18–0.45 | 4 | 0.04 | 0.02–0.11 | 18 | 0.22 | 0.13–0.36 |
| 25–29 | 525 | 0.55 | 0.49–0.62 | 110 | 0.64 | 0.50–0.82 | 58 | 0.30 | 0.22–0.40 | 71 | 0.48 | 0.36–0.64 |
| 30–34 | 929 | 1.00 | Ref | 171 | 1.00 | Ref | 193 | 1.00 | Ref | 139 | 1.00 | Ref |
| 35–39 | 617 | 1.44 | 1.29–1.59 | 117 | 1.52 | 1.20–1.93 | 181 | 2.00 | 1.63–2.46 | 63 | 0.98 | 0.73–1.33 |
| 40–44 | 205 | 2.43 | 2.09–2.84 | 34 | 2.34 | 1.61–3.40 | 64 | 3.58 | 2.68–4.77 | 10 | 0.79 | 0.42–1.51 |
| P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | |||||||||
| Parity prior to current delivery | ||||||||||||
| 0 | 921 | 1.00 | Ref | 186 | 1.00 | Ref | 155 | 1.00 | Ref | 115 | 1.00 | Ref |
| 1 | 922 | 0.96 | 0.88–1.06 | 181 | 0.93 | 0.76–1.15 | 198 | 1.04 | 0.84–1.28 | 117 | 0.98 | 0.75–1.27 |
| 2+ | 594 | 0.90 | 0.80–1.00 | 87 | 0.72 | 0.55–0.95 | 147 | 0.95 | 0.75–1.21 | 69 | 0.89 | 0.64–1.23 |
| Not counting index birth | P = 0.1555 | P = 0.0607 | P = 0.7523 | P = 0.7609 | ||||||||
| Immigrant status | ||||||||||||
| Non‐immigrant | 1998 | 1.00 | Ref | 424 | 1.00 | Ref | 391 | 1.00 | Ref | 262 | 1.00 | Ref |
| Immigrant | 439 | 0.83 | 0.75–0.92 | 30 | 0.27 | 0.19–0.39 | 109 | 1.07 | 0.86–1.33 | 39 | 0.53 | 0.38–0.75 |
| Imputed | 0 | 0 | 0 | 0 | ||||||||
| P = 0.0006 | P < 0.0001 | P = 0.5704 | P = 0.0004 | |||||||||
| Education | ||||||||||||
| <10 years | 239 | 0.98 | 0.84–1.13 | 27 | 0.85 | 0.56–1.29 | 38 | 0.82 | 0.57–1.18 | 36 | 1.20 | 0.81–1.75 |
| 10–13 years | 995 | 1.00 | Ref | 172 | 1.00 | Ref | 189 | 1.00 | Ref | 140 | 1.00 | Ref |
| Undergraduate | 361 | 0.95 | 0.84–1.08 | 84 | 1.25 | 0.96–1.63 | 81 | 0.98 | 0.75–1.27 | 35 | 0.67 | 0.46–0.97 |
| Postgraduate | 788 | 0.92 | 0.83–1.02 | 167 | 1.03 | 0.81–1.30 | 182 | 0.92 | 0.74–1.15 | 87 | 0.70 | 0.52–0.93 |
| Imputed | 54 | 4 | 10 | 3 | ||||||||
| P = 0.4208 | P = 0.2622 | P = 0.6897 | P = 0.0159 | |||||||||
| Cigarette smoking | ||||||||||||
| No | 1930 | 1.00 | Ref | 377 | 1.00 | Ref | 414 | 1.00 | Ref | 237 | 1.00 | Ref |
| Yes | 350 | 1.02 | 0.91–1.14 | 50 | 0.84 | 0.62–1.14 | 51 | 0.78 | 0.58–1.05 | 52 | 1.08 | 0.79–1.48 |
| Imputed | 157 | 27 | 35 | 12 | ||||||||
| P = 0.7710 | P = 0.2583 | P = 0.1007 | P = 0.6298 | |||||||||
| Pre‐pregnancy BMI | ||||||||||||
| <18.5 | 47 | 1.11 | 0.82–1.48 | 6 | 0.76 | 0.33–1.71 | 8 | 1.09 | 0.52–2.27 | 7 | 1.30 | 0.61–2.77 |
| 18.5–24.9 | 1243 | 1.00 | Ref | 244 | 1.00 | Ref | 244 | 1.00 | Ref | 158 | 1.00 | Ref |
| 25.0–29.9 | 505 | 0.98 | 0.88–1.09 | 96 | 1.01 | 0.78–1.30 | 103 | 0.99 | 0.78–1.25 | 71 | 1.09 | 0.83–1.44 |
| 30.0+ | 205 | 0.86 | 0.74–1.00 | 36 | 0.84 | 0.58–1.20 | 46 | 0.97 | 0.71–1.32 | 28 | 0.90 | 0.60–1.35 |
| Imputed | 437 | 72 | 99 | 37 | ||||||||
| P = 0.2118 | P = 0.6963 | P = 0.9908 | P = 0.7497 | |||||||||
| Cases with information imputed, total | 485 | 81 | 108 | 43 | ||||||||
Model included year, age, parity, immigration status, education, smoking and body mass index (BMI). Education, smoking and BMI imputed using MICE, 10 cycles of chained equations and 30 sets.
3.4. Impact of risk factors on incidence trends
Presenting the incidence trends as incidence rate ratios with period 1990–1994 as reference point, enabled stepwise adjustments by risk factors (Figure 4; Tables S2 and S3). During pregnancy, adjustment for age reduced the incidence rate ratio (all tumor types) over calendar time (Figure 4, top panel). Additional adjustments for previous parity, immigrant background, educational level, smoking or BMI did not impact the trends of all tumor types. All estimates from the fully adjusted models in Figure 4 are presented in Tables 2 and 3. The PAC incidence increased the most for breast cancer over calendar time, and adjustment for age reduced the calendar time effect substantially. Further adjustment for potential risk factors did not change the pattern, except for smoking, which lowered the trend slightly. For malignant melanoma and cervical cancer, the adjustment for age led to slight reductions in the trends, while adjustment for the remaining factors only had minor impact on the trends. Within 1 year post‐delivery, the incidence trend (all tumor types) increased over time with the most pronounced trend for malignant melanoma (Figure 4, bottom panel). Adjustment for age lowered the incidence substantially, while additional adjustments did not change the incidence patterns. For cervical cancer, the impact of adjustments was less consistent.
FIGURE 4.
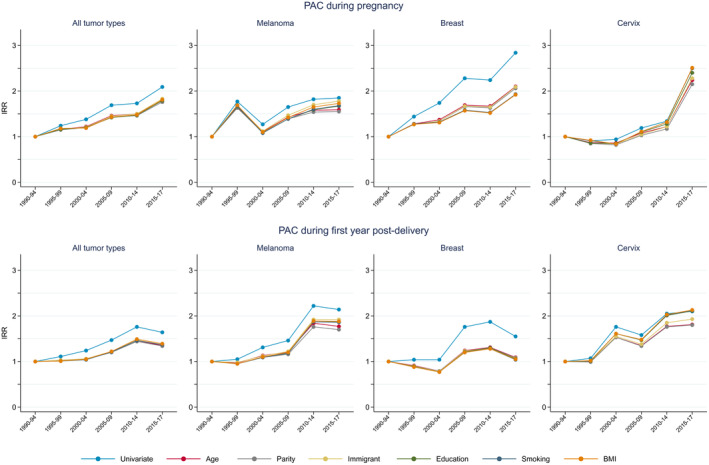
Adjusted incidence rate ratios of pregnancy‐associated cancer during pregnancy and 1 year post‐delivery by calendar period of diagnosis, stepwise adjusted for age, parity, immigrant status, education, smoking and BMI 1990–2017 in Sweden.
4. DISCUSSION
In this population‐based study, we found that malignant melanoma, breast and cervical cancer accounted for over 50% of the cases of PAC. In particular, the incidence rate of malignant melanoma during pregnancy was higher than in previous studies, indicating that hospital‐based studies may miss a substantial proportion of cases. 18 The total numbers of PAC were lower during pregnancy, particularly during first and second trimesters, compared with the first year post‐delivery. The incidence during the first trimester remained low throughout the study period. This suggests a true lower risk during pregnancy, delay in diagnosis or an under‐ascertainment of cases due to miscarriages and legal abortions which we were not able to account for. The incidence of PAC during pregnancy and within 1 year post‐delivery increased over time, which was only in part explained by increasing age at birth. Higher maternal age was the strongest risk factor for PAC, in particular for breast cancer, but also for malignant melanoma and cervical cancer. Nulliparity and non‐immigrant background were associated overall with higher risk of PAC, whereas smoking was only associated with increased rates of cancer during pregnancy, and in particular for cervical cancer. However, parity, immigrant background, education, smoking and BMI did not contribute to the increasing trend.
The incidence estimates in this study are in line with previous studies. 4 , 15 An increasing incidence over time has been reported from most 3 , 5 , 6 , 7 , 9 , 10 , 11 but not all 8 countries. Our consistent finding of increasing incidences across several cancer types suggests that common factors, such as maternal age rather than cancer‐specific risk factors are involved. Since the PAC incidence rates depend both on the underlying cancer incidence and on the birth rates in the population, changes in one or both will influence the PAC incidence trends. Cancer incidence rates in women under 40 years of age have increased over time in Sweden and elsewhere. 19 , 20 Additionally, the incidence of malignant melanoma and breast cancer continuously increases with age in premenopausal women, while the highest incidence of cervical cancer is at ages 30–39 years. 19 For the incidence trend of PAC, it is important to disentangle the magnitude of the contributions of underlying cancer trends, from the contributions of increasing maternal age and other factors associated with birth rates.
Childbearing patterns change over long periods of time, and birth rates exhibit large cyclic differences across years. For estimation of PAC incidence it is crucial to adjust for the number of deliveries at risk per year. In Sweden, there has been a substantial and continuous increase in maternal age over the last decades (Figure S2). However, temporary changes also occur, eg a recent “third child trend” during the early 2000s, with more women giving birth to three children rather than two. 21 These trends in childbearing should impact on PAC incidence trends.
It is important to include cancer cases in early pregnancy when estimating the PAC incidence to provide a full picture of the burden of disease, including terminations of pregnancy due to cancer diagnosis. The true number of PAC cases for the first two trimesters was difficult to estimate in our study, since data on legal terminations and spontaneous abortions are not available in MBR. This underestimated the incidence of PAC during pregnancy. The legislation of legal pregnancy terminations varies worldwide (in Sweden until 19 gestational weeks) and will be reflected in the number of PAC cases for the first two trimesters. Results from a Danish study, where information on legal and spontaneous abortion is available in registers, indicate that the incidence in the first trimester is likely higher if abortions are accounted for, yet abortions cannot explain the overall decline in first two trimesters. 5
Other reasons behind the lower risk during pregnancy compared with post‐delivery could be a delay in detection due to masked or misinterpreted symptoms during pregnancy. 13 , 22 There may also be a lower risk due to pregnancy‐induced hormonal and immunological changes that could suppress tumor development. 1
We found increased rates of cervical cancer 3–6 months after delivery compared with the other post‐delivery intervals. In Sweden, national guidelines recommend that pregnant women be screened for cervical cancer at the first prenatal visit (if no previous screening test was done within 2.5 years), while diagnostic examinations are generally avoided during the puerperium due to lower reliability. The first post‐delivery return visit usually occurs at 6–8 weeks after delivery, and includes contraceptive counseling but no recommendation for additional screening.
Similar to previous studies, we found that maternal age is the strongest risk factor for PAC. 2 , 5 , 7 Although absolute numbers of PAC are highest at ages 30–34 years, the cancer risk among pregnant women is highest in women aged 35–44. The number of pregnant women is highest below age 30, whereas the cancer risk in general increases with age. Hence, a shift to higher maternal age over calendar time will lead to more women being diagnosed with PAC because the underlying cancer risk is higher. We and others have found that higher maternal age is a risk factor for PAC across all cancer types and with the strongest association for breast cancer. 2 , 5 , 7 , 14 In contrast to the increasing incidence of PAC across age, the proportion of cancer cases that have a pregnancy‐associated cancer decreases with age. In our previous publication based on the same data, we found that only 1% of all cancer cases diagnosed in women aged 40–44 were pregnancy‐associated. 6 In women aged 25–29 and 30–34 years, where the background cancer risk is much lower, 16% and 14% of all cancer cases, respectively, were pregnancy‐associated.
Although nulliparity was associated with a higher risk for PAC during pregnancy (all tumor types), we found no significant association with parity for the three most common cancer types: malignant melanoma, breast cancer and cervical cancer. Immigrant background was associated with a significantly lower risk for PAC, which was pronounced for malignant melanoma. This is likely an effect of the higher incidence of malignant melanoma in general among Swedish‐born women. 23 There was a borderline association between immigrant background and lower risk of pregnancy‐associated cervical cancer both during and after pregnancy, which is likely due to a lower screening attendance among foreign‐born women. 24 , 25 Our finding of an increased risk of cervical cancer during pregnancy among smokers was expected, considering that smoking is a well‐established risk factor for cervical cancer. 26 However, this has not previously been reported for PAC and was in contrast to the null finding for smoking and cervical cancer within 1 year post‐delivery.
The incidence of PAC is influenced by risk factors, such as skin type and lifestyle factors, but also by childbearing patterns, legislation of pregnancy termination and screening routines, which may vary across countries and populations. However, the increasing incidence pattern of PAC in our study appears to be a general trend in line with other studies. 4 , 15
This study represents one of the largest studies to date on several previously not investigated risk factors for PAC incidence trends. The most important strength was the population‐based data from the cancer and birth registers, which provided essentially complete, unbiased ascertainment of cancer cases and births over a study period of 50 years. In comparison with studies based on single‐ or multicenter materials, the population‐based registers provide data on all cases in the population, regardless of severity or type of clinic. The medical birth register in Sweden is essentially complete, with <1% of births missing. 27
A limitation was the lack of information on miscarriages in MBR before week 28 (1973–2007) and week 22 (since 2008), which likely underestimated the incidence of PAC in the first and second trimesters. Furthermore, no information on terminated pregnancies was available, which may have influenced estimates for the earlier period, when treatment options were limited. The lack of information on terminated pregnancies may also cause a larger underestimation of PAC incidence for less favorable cancers, where treatment cannot be given during pregnancy or has to be postponed until after delivery. The available evidence of safety regarding systemic treatments during pregnancy has gradually increased over time, which has likely reduced this underestimation. For instance, several types of chemotherapy have been considered safe during the second and third trimesters since the early 2000s. In addition, we had no information on the proportion of breast cancers detected by screening (Swedish women have been invited to screening from age 40 since the mid‐1990s). However, since only 1% of PACs are diagnosed in women 40–44 years, the impact of screening on the incidence estimates must be limited. Lastly, for the PAC cases, the year of cancer diagnosis (rather than delivery year) was used to calculate the incidence rate, and thus there is a minor discrepancy between year of case and year at risk.
5. CONCLUSION
In this large population‐based study, malignant melanoma, breast and cervical cancer were the three most common cancer types of PAC, where malignant melanoma has been largely under‐reported in previous studies. We found, in line with earlier studies, that high maternal age was a strong risk factor for PAC. Furthermore, nulliparity, non‐immigrant background and smoking were for the first time shown to constitute risk factors for PAC, whereas education and BMI were not. However, only maternal age contributed significantly to the increasing incidence. Further studies on other potential risk factors for PAC are warranted, since our results indicate that age on its own does not fully explain the higher incidence. Epidemiological studies of PAC are important to guide healthcare professionals to plan and evaluate strategies to prevent PAC and to provide population‐based evidence for the management of patients with PAC.
AUTHOR CONTRIBUTIONS
FEL, HS and ALVJ conceived and designed the study. FEL analyzed the data. FL, HS and ALVJ interpreted the data and wrote the first version of the paper. All authors revised the paper critically and finally approved it.
FUNDING INFORMATION
This work was supported by the Radiumhemmet Research Foundations (grant number 191132/2019, 221153/2022 Anna Johansson), The Swedish Breast Cancer Association (grant number 23/2022 Anna Johansson), Karolinska Institutet Foundations (grant number: FS‐2020‐01400 Frida Lundberg, FS‐2020‐01405 Anna Johansson) and the Swedish Research Council (grant number: 2021–01657 Anna Johansson).
CONFLICT OF INTEREST STATEMENT
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Supporting information
Figure S1: Distribution of cancer during trimesters and 3‐month post‐delivery intervals, by tumor type.
Figure S2: Temporal trends in age at births in Sweden 1973–2020.
Table S1: Included tumor types and corresponding ICD version 7 codes used in Sweden.
Table S2: Associations between cancer incidence during pregnancy and calendar year with stepwise adjustment for age, parity, immigrant status, education, smoking and BMI by tumor type. Estimates in Figure 4.
Table S3: Associations between cancer incidence first year post‐delivery and calendar year with stepwise adjustment for age, parity, immigrant status, education, smoking and BMI by tumor type. Estimates in Figure 4.
Lundberg FE, Stensheim H, Ullenhag GJ, et al. Risk factors for the increasing incidence of pregnancy‐associated cancer in Sweden – a population‐based study. Acta Obstet Gynecol Scand. 2024;103:669‐683. doi: 10.1111/aogs.14677
Frida E. Lundberg and Hanne Stensheim contributed equally.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from register holders (Statistics Sweden and the Swedish National Board of Health and Welfare). The data are not publicly available due to restrictions by Swedish and European law, in order to protect patient privacy. Data are available from the register holders for researchers with relevant ethical approvals and who meet the criteria for access to confidential data.
REFERENCES
- 1. Andersson TM, Johansson AL, Fredriksson I, Lambe M. Cancer during pregnancy and the postpartum period: a population‐based study. Cancer. 2015;121:2072‐2077. [DOI] [PubMed] [Google Scholar]
- 2. Bannister‐Tyrrell M, Roberts CL, Hasovits C, Nippita T, Ford JB. Incidence and outcomes of pregnancy‐associated melanoma in New South Wales 1994‐2008. Aust N Z J Obstet Gynaecol. 2015;55:116‐122. [DOI] [PubMed] [Google Scholar]
- 3. Cottreau CM, Dashevsky I, Andrade SE, et al. Pregnancy‐associated cancer: a U.S. population‐based study. J Womens Health. 2018;28:250‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dalmartello M, Negri E, La Vecchia C, et al. Frequency of pregnancy‐associated cancer: a systematic review of population‐based studies. Cancer. 2020;12:1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eibye S, Kjaer SK, Mellemkjaer L. Incidence of pregnancy‐associated cancer in Denmark, 1977‐2006. Obstet Gynecol. 2013;122:608‐617. [DOI] [PubMed] [Google Scholar]
- 6. Johansson ALV, Fredriksson I, Mellemkjaer L, et al. Cancer survival in women diagnosed with pregnancy‐associated cancer: an overview using nationwide registry data in Sweden 1970‐2018. Eur J Cancer. 2021;155:106‐115. [DOI] [PubMed] [Google Scholar]
- 7. Lee YY, Roberts CL, Dobbins T, et al. Incidence and outcomes of pregnancy‐associated cancer in Australia, 1994‐2008: a population‐based linkage study. BJOG. 2012;119:1572‐1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parazzini F, Franchi M, Tavani A, Negri E, Peccatori FA. Frequency of pregnancy related cancer: a population based linkage study in Lombardy. Italy Int J Gynecol Cancer. 2017;27:613‐619. [DOI] [PubMed] [Google Scholar]
- 9. Rodriguez AO, Chew H, Cress R, et al. Evidence of poorer survival in pregnancy‐associated breast cancer. Obstet Gynecol. 2008;112:71‐78. [DOI] [PubMed] [Google Scholar]
- 10. Smith LH, Danielsen B, Allen ME, Cress R. Cancer associated with obstetric delivery: results of linkage with the California cancer registry. Am J Obstet Gynecol. 2003;189:1128‐1135. [DOI] [PubMed] [Google Scholar]
- 11. Stensheim H, Møller B, van Dijk T, Fossa SD. Cause‐specific survival for women diagnosed with cancer during pregnancy or lactation: a registry‐based cohort study. J Clin Oncol. 2009;27:45‐51. [DOI] [PubMed] [Google Scholar]
- 12. Amant F, Lefrere H, Borges VF, et al. The definition of pregnancy‐associated breast cancer is outdated and should no longer be used. Lancet Oncol. 2021;22:753‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johansson ALV, Weibull CE, Fredriksson I, Lambe M. Diagnostic pathways and management in women with pregnancy‐associated breast cancer (PABC): no evidence of treatment delays following a first healthcare contact. Breast Cancer Res Treat. 2019;174:489‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andersson TM, Johansson AL, Hsieh CC, Cnattingius S, Lambe M. Increasing incidence of pregnancy‐associated breast cancer in Sweden. Obstet Gynecol. 2009;114:568‐572. [DOI] [PubMed] [Google Scholar]
- 15. Stensheim H, Johansson ALV. Epidemiology. In: Amant F, ed. Textbook of Cancer in Pregnancy. International Network for Cancer, Infertility and Pregnancy (INCIP)/European Society for Gynaecological Oncology (ESGO); 2017. [Google Scholar]
- 16. Johansson ALV, Stensheim H. Epidemiology of pregnancy‐associated breast cancer. In: Alipour S, Omranipour R, eds. Diseases of the Breast during Pregnancy and Lactation. Springer Nature Switzerland AG; 2020:75‐79. [DOI] [PubMed] [Google Scholar]
- 17. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377‐399. [DOI] [PubMed] [Google Scholar]
- 18. de Haan J, Verheecke M, Van Calsteren K, et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20‐year international cohort study of 1170 patients. Lancet Oncol. 2018;19:337‐346. [DOI] [PubMed] [Google Scholar]
- 19. Larønningen SFJ, Beydogan H, Bray F, et al. NORDCAN: cancer incidence, mortality, prevalence and survival in the Nordic countries, version 9.2 (23.06.2022). Association of the Nordic Cancer Registries. Cancer Registry of Norway. Accessed January 5, 2023 2022. https://nordcan.iarc.fr/
- 20. Gupta S, Harper A, Ruan Y, et al. International trends in the incidence of cancer among adolescents and Young adults. J Natl Cancer Inst. 2020;112:1105‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Statistics Sweden Demographic Reports . A third child ‐ a new trend? 2011;1:15‐20. [Google Scholar]
- 22. Langer A, Mohallem M, Stevens D, Rouzier R, Lerebours F, Chérel P. A single‐institution study of 117 pregnancy‐associated breast cancers (PABC): presentation, imaging, clinicopathological data and outcome. Diagn Interv Imaging. 2014;95:435‐441. [DOI] [PubMed] [Google Scholar]
- 23. Ljung R, Talbäck M, Feychting M. Incident malignant melanoma in Sweden: the importance of accounting for skin complexion in the population. Epidemiology. 2022;33:e11‐e12. [DOI] [PubMed] [Google Scholar]
- 24. Azerkan F, Sparen P, Sandin S, Tillgren P, Faxelid E, Zendehdel K. Cervical screening participation and risk among Swedish‐born and immigrant women in Sweden. Int J Cancer. 2012;130:937‐947. [DOI] [PubMed] [Google Scholar]
- 25. Broberg G, Wang J, Ostberg AL, et al. Socio‐economic and demographic determinants affecting participation in the Swedish cervical screening program: a population‐based case‐control study. PloS One. 2018;13:e0190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malevolti MC, Lugo A, Scala M, et al. Dose‐risk relationships between cigarette smoking and cervical cancer: a systematic review and meta‐analysis. Eur J Cancer Prev. 2023;32:171‐183. [DOI] [PubMed] [Google Scholar]
- 27. Laugesen K, Ludvigsson JF, Schmidt M, et al. Nordic health registry‐based research: a review of health care systems and key registries. Clin Epidemiol. 2021;13:533‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Distribution of cancer during trimesters and 3‐month post‐delivery intervals, by tumor type.
Figure S2: Temporal trends in age at births in Sweden 1973–2020.
Table S1: Included tumor types and corresponding ICD version 7 codes used in Sweden.
Table S2: Associations between cancer incidence during pregnancy and calendar year with stepwise adjustment for age, parity, immigrant status, education, smoking and BMI by tumor type. Estimates in Figure 4.
Table S3: Associations between cancer incidence first year post‐delivery and calendar year with stepwise adjustment for age, parity, immigrant status, education, smoking and BMI by tumor type. Estimates in Figure 4.
Data Availability Statement
The data that support the findings of this study are available from register holders (Statistics Sweden and the Swedish National Board of Health and Welfare). The data are not publicly available due to restrictions by Swedish and European law, in order to protect patient privacy. Data are available from the register holders for researchers with relevant ethical approvals and who meet the criteria for access to confidential data.


