Abstract
The highly enantioselective synthesis of (R)-sitagliptin has been achieved through a series of key steps, including the aza-Michael addition and Baeyer–Villiger oxidation. The enantioselective aza-Michael addition involved the reaction of tert-butyl β-naphthylmethoxycarbamate with (E)-1-(4-methoxyphenyl)-4-(2,4,5-trifluorophenyl)but-2-en-1-one, utilizing a quinine-derived C(9)-urea ammonium catalyst under phase-transfer catalytic conditions. The aza-Michael addition successfully introduced chirality to the amine in (R)-sitagliptin with 96% ee. The subsequent Baeyer–Villiger oxidation of the aza-Michael adduct led to the formation of 4-methoxyphenyl ester. Hydrolysis and amide coupling were then employed to construct the amide moiety. Further deprotections were performed to complete the synthesis of (R)-sitagliptin (7 steps, 41%, 96% ee).
Introduction
Type 2 diabetes stands out as one of the most prevalent diseases, with the quest for effective therapies being a central focus in the field of drug discovery.1 Dipeptidyl peptidase IV (DPP-4), a relatively recent target for therapeutic interventions in type 2 diabetes, has garnered significant attention.2 DPP-4 inhibitors serve as agents that elevate the levels of glucagon-like peptide-1 (GLP-1), thereby triggering insulin secretion and lowering blood glucose levels.3
Sitagliptin 1 is a widely prescribed oral antidiabetic medication that gained FDA approval in 2006 as the pioneering DPP-4 inhibitor (see Figure 1).4 Since Merck first disclosed the synthetic pathway for (R)-sitagliptin in 2005, numerous subsequent synthetic methods have been reported, featuring adjustments to key intermediates and reaction conditions. These developments have unfolded over the past few decades, driven by the immense commercial significance of sitagliptin. The reported synthetic approaches can be categorized based on the chiral induction strategy for β-amino acids, including the utilization of chiral starting materials,5 chiral auxiliaries,4,6 biocatalysis,7 organocatalysis,8 and transition-metal-based organometallic catalysis.9 While a considerable number of synthetic examples have emerged through transition-metal-based catalysis, instances of organocatalysis remain relatively scarce (Scheme 1). Herein, we present an efficient synthetic methodology for (R)-sitagliptin via enantioselective phase-transfer organocatalysis, which is a well-known economic and environmentally friendly green chemistry that is beneficial for industrial processes.
Figure 1.
Structure of (R)-sitagliptin 1.
Scheme 1. Enantioselective Synthesis of (R)-Sitagliptin by Organocatalysis.
Results and Discussion
In a previous study, we reported the highly enantioselective conjugate addition of tert-butyl-N-alkoxycarbamates to a series of cyclic α,β-unsaturated ketones, esters, and amides.10 The versatile methodology has proven to be highly effective in the synthesis of chiral cyclic 1,3-aminoalcohols, which serve as essential chiral building blocks in the field of medicinal chemistry. Our intention is to establish the synthesis of (R)-sitagliptin via the enantioselective conjugate addition in the acyclic system. As depicted in the synthetic strategy (Scheme 2), the key intermediate 4 can be synthesized by the enantioselective aza-Michael addition of phenylketone 2 followed by the Baeyer–Villiger oxidation. Subsequent amide coupling and deprotections lead to (R)-sitagliptin. Due to the low reactivities of the corresponding α,β-unsaturated ester and amide, α,β-unsaturated phenylketone (2) was chosen as a Michael acceptor.
Scheme 2. Retrosynthetic Strategy of (R)-Sitagliptin.
First, we synthesized substrate 7 for the aza-Michael addition (Scheme 3). Employing a Wittig coupling reaction between commercially available 2,4,5-trifluorophenylacetaldehyde (5) and benzoylmethylenetriphenylphosphoranes (6a–h) afforded Michael acceptors 7a–h (52–84%) (Table 1).11 Among the used solvents, dichloromethane showed the best chemical yield (entries 3 and 5–7) at 0 °C. No reaction proceeded at −20 °C, and the optimal temperature was 0 °C (entries 2–4). In the case of the functional group on the phenyl ring, the electron-donating 4-methoxy group showed the best result (entry 12). In our attempts to test the enantioselective Michael addition using representative substrate 7a, we initially employed previously established reaction conditions involving aqueous KOH (1.2 equiv) in toluene, along with catalyst 9 or 10(12) (10 mol %) at room temperature (Table 2).10 However, this resulted in the formation of a significant amount of the double bond migrated isomer of 7a, both at room temperature and 0 °C, leading to a low chemical yield and moderate enantioselectivity (entries 1 and 2). Subsequently, we explored lower temperatures, specifically −20 °C, which led to the formation of the corresponding addition adduct (11a) without isomerization of 7a, achieving a high chemical yield and moderate enantioselectivity (entry 3, 84%, 87% ee). We observed that reducing the reaction concentration provided benefits in terms of both chemical yield and enantioselectivity, with the optimal concentration being 0.05 M (entries 3–6). Recognizing that the ratio of the organic phase to the water phase could impact both chemical yield and enantioselectivity in phase-transfer catalysis, we experimented with varying the volume of toluene and water in reactions at −40 °C. However, no significant variation in enantioselectivity was observed (entries 7–9). In addition, temperatures lower than −20 °C did not show a benefit in enantioselectivity (entries 5 and 7). Notably, there was also no significant change in enantioselectivity with other various bases, although their chemical yields were lower compared to aq. KOH (entries 10–12). In the case of quinidine-based catalyst 10,13 the enantioselectivity was lower than that of quinine-based catalyst 9, with a significantly longer reaction time (entry 13, 85%, −81% ee). The slow reaction rate may be attributed to a less favorable binding conformation.
Scheme 3. Synthetic Route of (R)-Sitagliptin Precursors.

Table 1. Optimization of the Wittig Reaction for 7a.
| entry | 6 (equiv) | R | solvent | temp. (°C) | time (h) | yield (%)b |
|---|---|---|---|---|---|---|
| 1 | 6a (1.0) | H | DCM | 25 | 12 | 35 |
| 2 | 6a (1.5) | H | DCM | 25 | 12 | 42 |
| 3 | 6a (1.5) | H | DCM | 0 | 24 | 65 |
| 4 | 6a (1.5) | H | DCM | –20 | 48 | 0 |
| 5 | 6a (1.5) | H | Et2O | 0 | 24 | 62 |
| 6 | 6a (1.5) | H | THF | 0 | 24 | 58 |
| 7 | 6a (1.5) | H | DMF | 0 | 24 | 47 |
| 8 | 6b (1.5) | 4-F | DCM | 0 | 24 | 62 |
| 9 | 6c (1.5) | 3-F | DCM | 0 | 24 | 71 |
| 10 | 6d (1.5) | 4-Cl | DCM | 0 | 24 | 52 |
| 11 | 6e (1.5) | 4-Br | DCM | 0 | 24 | 58 |
| 12 | 6f (1.5) | 4-CH3O | DCM | 0 | 24 | 84 |
| 13 | 6g (1.5) | 3-CH3O | DCM | 0 | 24 | 72 |
| 14 | 6h (1.5) | 4-CH3 | DCM | 0 | 24 | 57 |
Reactions were performed with 1.0 or 1.5 equiv of Wittig reagents 6 under the given conditions.
Isolated yields.
Table 2. Optimization of Reaction Conditiona.

| entry | base | concn (M) | solvent (PhCH3:H2O) | temp. (°C) | time (h) | yield (%)b | ee (%)c |
|---|---|---|---|---|---|---|---|
| 1 | KOH | 0.15 | 8:1 | 25 | 1 | 50 | 83 |
| 2 | KOH | 0.15 | 8:1 | 0 | 1 | 71 | 85 |
| 3 | KOH | 0.15 | 8:1 | –20 | 1 | 84 | 87 |
| 4 | KOH | 0.10 | 8:1 | –20 | 2 | 96 | 92 |
| 5 | KOH | 0.05 | 8:1 | –20 | 2 | 92 | 93 |
| 6 | KOH | 0.025 | 8:1 | –20 | 5 | 85 | 92 |
| 7 | KOH | 0.05 | 8:1 | –40 | 4 | 92 | 93 |
| 8 | KOH | 0.05 | 16:1 | –40 | 4 | 92 | 93 |
| 9 | KOH | 0.05 | 32:1 | –40 | 4 | 92 | 93 |
| 10 | K2CO3 | 0.05 | 8:1 | –20 | 48 | 75 | 92 |
| 11 | Cs2CO3 | 0.05 | 8:1 | –20 | 24 | 70 | 92 |
| 12 | K3PO4 | 0.05 | 8:1 | –20 | 4 | 81 | 92 |
| 13d | KOH | 0.05 | 8:1 | –20 | 24 | 85 | –81 |
Reactions were performed with 2.0 equiv of tert-butyl carbamate 8 and 1.2 equiv of base under the given conditions.
Isolated yields.
Enantiopurity was determined by high-performance liquid chromatography (HPLC) analysis using a chiral column (DAICEL Chiralpak AD-H).
Reactions were performed with catalyst 10, and the enantiomer of 11a was obtained.
Next, we decided to explore variations in the functional groups attached to the benzoyl part of substrate 7a under the optimized PTC conditions (Table 2, entry 5). As illustrated in Scheme 4, among the various functional groups investigated, the p-methoxy group (7f, 3.26 mmol, 1 g) exhibited the highest level of enantioselectivity and chemical yield (11f, 94%, 96% ee). The presence of electron-withdrawing groups at the para-position may enhance the reactivity of the Michael acceptor, consequently leading to decreased enantioselectivity due to an increase in noncatalytically mediated reactions.
Scheme 4. Substrate Optimization of aza-Michael Reaction,,

Reactions were performed with 2.0 equiv of tert-butyl carbamates and 1.2 equiv of 50% KOH (aq.) under the given conditions.
Isolated yields.
Enantiopurity was determined by HPLC analysis using a chiral column (DAICEL Chiralpak AD-H) (see the Supporting Information).
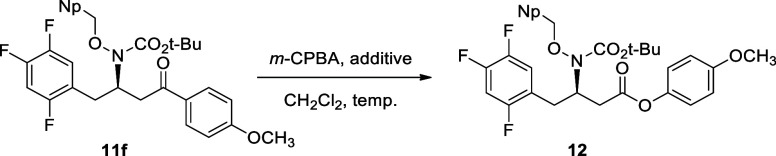
Subsequently, we proceeded with the optimization of the Baeyer–Villiger oxidation of 11f. In this regard, the Baeyer–Villiger oxidation was systematically explored across diverse reaction conditions, employing m-CPBA as the oxidizing agent (Table 3).14 Almost no reaction was observed under acidic conditions utilizing trifluoroacetic acid (TFA) and hexafluoroisopropanol (HFIP) (entries 1 and 2). Nevertheless, under basic conditions employing NaHCO3 and with the maintenance of neutrality through a phosphate buffer in the presence of HFIP, the desired product was obtained in low chemical yields (entries 3–5). Remarkably, the highest chemical yield was successfully achieved under anhydrous conditions utilizing 4 Å molecular sieves (entry 6, 90%).
Table 3. Optimization of the Baeyer–Villiger Oxidationa.
| entry | additive | temp. (°C) | time (h) | yield (%)b |
|---|---|---|---|---|
| 1 | TFA (1 equiv) | 25 | 24 | trace |
| 2 | HFIPc (40 equiv) | 25 | 24 | trace |
| 3 | HFIP, phosphate buffer (pH: 7.6) | 25 | 48 | 31 |
| 4 | HFIP, phosphate buffer (pH: 7.6) | 40 | 48 | 17 |
| 5 | NaHCO3 (2 equiv) | 25 | 24 | 24 |
| 6 | NaHCO3 (2 equiv), 4 Å molecular sieves | 25 | 24 | 90 |
Reactions were performed with 1.5 equiv of m-CPBA.
Isolated yields.
1,1,1,3,3,3-Hexafluoro-2-propanol.
We finalized the synthesis of (R)-sitagliptin from compound 12 (Scheme 5). The hydrolysis of 12 under alkaline conditions, followed by amide coupling with the commercially available triazole 13 in the presence of EDC, resulted in the formation of amide 14 (83% yield from 12).8d The hydrogenolysis of 14, utilizing Raney-Ni under atmospheric H2,15 produced carbamate 15 (75%) {[α]D23 = +22.0 (c 1.0, CHCl3); Lit.8bR-15, [α]D23 = +23.2 (c 1.0, CHCl3)}. Lastly, the deprotection of the N-Boc group under 0.5 N HCl/MeOH acidic conditions successfully furnished the target compound (R)-sitagliptin·HCl. The overall yield in 7 steps starting from compound 5 was 41%.
Scheme 5. Finalization of the Synthesis of (R)-Sitagliptin.

A plausible mechanism for the aza-Michael addition is depicted in Figure 2 based on insights from density functional theory (DFT) calculations.16 Initially, the carbamate anion (10) forms an ionic complex with the quaternary ammonium cation or hydrogen bonding with the α–C–H of the ammonium cation of catalyst 9. Two notable π–π stacking interactions occur: one between the two phenyl groups of (CF3)2Ph in catalyst 9 and the other between the quinoline group of catalyst 9 and the β-naphthyl group of carbamate 8. These interactions collectively contribute to a more rigid conformation. Simultaneously, the enone (7f) engages in hydrogen bond interactions with the C(9) urea group of catalyst 9. In accordance with the experimental findings, the up-face of enone 7f remains accessible for the nucleophilic carbamate N-anion (8) to approach, resulting in the formation of the R-enantiomer of 11f.
Figure 2.
Plausible mechanism of the aza-Michael addition of 7f by DFT calculations (gray: catalyst 9, yellow: carbamate 8, purple: substrate 7f; oxygen: red, nitrogen: blue, fluoro: green).
Conclusions
We have successfully established an efficient synthetic pathway for (R)-sitagliptin utilizing organocatalysis. The chirality of the primary amine was effectively introduced through the aza-Michael addition of tert-butyl β-naphthylmethoxycarbamate (8) to (E)-1-(4-p-methoxyphenyl)-4-(2,4,5-trifluorophenyl)but-2-en-1-one (7f), employing the quinine-derived bifunctional phase-transfer catalyst (9) (94% and 96% ee). Subsequent steps, including the Baeyer–Villiger oxidation, hydrolysis, and amide coupling, were instrumental in constructing the core structure of (R)-sitagliptin. The final stage of deprotections successfully completed the synthesis of (R)-sitagliptin·HCl, achieved in a total of 7 steps with an overall yield of 41% and an enantiomeric purity of 96%.
Experimental Section
General Information
All reagents purchased from commercial sources were used without further purification. The commercially available KOH pellet (99%) was ground to prepare solid KOH as a powder form. 50% w/v aqueous KOH was used as a stock solution. For the reactions that required heating, a sand bath was used as a heat source. Organic solvents were concentrated under reduced pressure using a Büchi rotary evaporator. Phase-transfer catalysts (910) were prepared according to the reported procedure. Thin layer chromatography (TLC) analyses were performed using precoated TLC plates (TLC Silica gel 60 F254). Flash column chromatography was carried out using E. Merck Silica gel 60 (0.040–0.063 mm). Hitachi (UV detector L-2130, Pump L-2130, and software LaChrome 890-8800-12) was used for HPLC. The values of enantiomeric excess (ee) of chiral products were determined by HPLC using 4.6 mm × 250 mm Daicel Chiralpak AD-H. Nuclear magnetic resonance (1H NMR and 13C NMR) spectra were measured on JEOL JNM-ECZ400s [400 MHz (1H), 101 MHz (13C)], Bruker AVANCE 500 [500 MHz (1H), 126 MHz (13C)], and 800 MHz Bruker Avance III HD spectrometer [800 MHz (1H), 201 MHz (13C)] using deuterated solvents and are reported in ppm relative to CDCl3 (δ 7.24), CD3OD (δ 3.31), and DMSO-d6 (δ 2.50) for 1H NMR and relative to the central CDCl3 (δ 77.23), CD3OD (δ 49.0), and DMSO-d6 (δ 39.5) resonance for 13C NMR. Coupling constants (J) in 1H NMR are reported in hertz. Low-resolution mass spectra (LRMS) and high-resolution mass spectra (HRMS) were measured on JEOL JMS-700 spectrometers (double-focusing mass analyzer). Melting points were measured on a Büchi B-540 melting point apparatus and are not corrected. Infrared (IR) spectra were recorded on JASCO FT/IR-4200 spectrometers. Optical rotations were measured on a JASCO P-2000 digital polarimeter and calibrated with pure solvent as the blank.
General Procedure for the Synthesis of α,β-Unsaturated Phenylketones (7a)
A mixture of 2-(2,4,5-trifluorophenyl)acetaldehyde (5) (0.90 g, 5.17 mmol) and 1-phenyl-2-(triphenylphosphoranylidene)ethanone (6a) (2.95 g, 7.76 mmol) in methylene chloride (10 mL) of a round-bottom flask (50 mL) was stirred at 0 °C. After the reaction was completed, the reaction mixture was diluted with ethyl acetate (200 mL), washed with water (40 mL × 2), dried over anhydrous magnesium sulfate, filtered, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, hexane/ether/dichloromethane = 12:1:1) to afford 7a (928 mg, 3.36 mmol, 65% yield) as a white solid.
General Procedure for the Enantioselective Phase-Transfer Catalytic aza-Michael Reaction (11a)
To a solution of tert-butyl β-naphthylmethoxycarbamate (8) (26.8 mg, 0.097 mmol, 2.0 equiv) and cinchona-derived chiral bifunctional tetraalkylammonium bromide (9) (4.33 mg, 0.005 mmol, 10 mol %) in toluene (950 μL, 0.05 M) of a round-bottom flask (5 mL) was added (E)-1-phenyl-4-(2,4,5-trifluorophenyl)but-2-en-1-one (7a) (15 mg, 0.049 mmol, 1.0 equiv) at −20 °C. Then, 50% w/v aqueous KOH (6.59 μL, 0.059 mmol, 1.2 equiv) and water (23.09 μL) were added to the reaction mixture and stirred until the starting material disappeared at −20 °C. After the reaction was completed, the reaction mixture was diluted with ethyl acetate (10 mL), washed with water (5 mL × 2), dried over anhydrous magnesium sulfate, filtered, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, hexane: EtOAc = 10:1) to afford 11a (18.3 mg, 0.033 mmol, 92% yield) as a white solid [α]D20 = +3.64 (c 1.0, CHCl3). The enantioselectivity was determined by chiral HPLC analysis (DAICEL Chiralpak AD-H, hexane: ethanol = 95:5, flow rate = 1.0 mL/min, 23 °C, λ = 250 nm), retention time: minor isomer 8.38 min, major isomer 11.79 min, 93% ee.
Procedure for the Preparation of Phase-Transfer Catalyst (10)
Under an argon atmosphere, urea10 (2 g, 3.46 mmol, 1.0 equiv) was dissolved in tetrahydrofuran (17 mL) of a round-bottom flask (100 mL); then, 2,4-bis(trifluoromethyl)benzyl bromide (778 μL, 4.15 mmol, 1.2 equiv) was added. The mixture was stirred at 60 °C for 24 h. After the reaction was finished, the mixture was concentrated under reduced pressure. After purification of the residue by column chromatography on silica gel with hexane-ethyl acetate (5:1 to EtOAc only) to ethyl acetate-methanol (EtOAc only to 8:1), molecule 10 was obtained as a white solid (mp 158–162 °C, 642.9 mg, 21% yield); 1H NMR (400 MHz, CD3OD) δ 8.78 (d, J = 4.1 Hz, 1H), 8.23 (d, J = 8.2 Hz, 1H), 8.17 (s, 1H), 8.13 (d, J = 8.2 Hz, 1H), 7.94–7.99 (m, 3H), 7.81 (d, J = 4.1 Hz, 1H), 7.71 (s, 1H), 7.48 (dd, J = 9.4, 2.5 Hz, 1H), 7.43 (s, 1H), 6.47 (d, J = 9.6 Hz, 1H), 5.77–5.85 (m, 1H), 5.24–5.36 (m, 4H), 5.10–5.12 (m, 1H), 4.05 (s, 5H), 3.78–3.84 (m, 1H), 3.49 (s, 1H), 2.75 (s, 1H), 2.09–2.16 (m, 1H), 1.95 (s, 2H), 1.83 (s, 1H), 1.39 (s, 1H), 1.21–1.28 (m, 1H) ppm; 13C NMR (101 MHz, CD3OD) δ 159.3, 154.6, 147.3, 144.2, 141.1, 137.9, 136.4, 133.1, 132.7, 131.9, 131.6, 130.7, 129.9, 129.3, 127.4, 124.8, 124.7, 124.7, 123.0, 122.0, 119.9, 118.0, 116.9, 114.9, 114.9, 101.0, 70.2, 57.3, 55.1, 54.1, 36.8, 31.4, 28.1, 25.4, 23.6, 22.4 ppm; IR (neat) 1542, 1508, 1280 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C38H34F12N4O2]+ ([M + H]+) 805.2406; found 805.2421. [α]D20 = −2.21 (c 1.0, CH3OH).
Analytical Data
(E)-1-Phenyl-4-(2,4,5-trifluorophenyl)but-2-en-1-one (7a)
Following the general procedure (2), molecule 7a was obtained as a white solid (mp 74 °C, 928 mg, 65% yield); 1H NMR (400 MHz, CDCl3) δ 7.89 (dd, J = 8.2, 1.4 Hz, 2H), 7.54–7.58 (m, 1H), 7.46 (t, J = 7.5 Hz, 2H), 6.84–7.11 (m, 4H), 3.59 (d, J = 6.9 Hz, 2H) ppm; 13C NMR (101 MHz, CDCl3) δ 190.4, 144.5, 137.6, 137.5, 133.1, 128.7, 128.6, 127.5, 118.5, 118.3, 105.8, 105.6, 31.5 ppm; IR (neat) 1673, 1623 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C16H12F3O]+ ([M + H]+) 277.0840; found 277.0836.
(E)-1-(4-Fluorophenyl)-4-(2,4,5-trifluorophenyl)but-2-en-1-one (7b)
Following the general procedure (2), molecule 7b was obtained as a white solid (mp 46 °C, 99.2 mg, 62% yield); 1H NMR (400 MHz, CDCl3) δ 7.92 (td, J = 5.9, 2.6 Hz, 2H), 6.91–7.15 (m, 5H), 6.82–6.85 (m, 1H), 3.59 (d, J = 6.6 Hz, 2H) ppm; 13C NMR (101 MHz, CDCl3) δ 188.6, 167.0, 164.5, 157.1, 154.7, 144.7, 133.9, 131.3, 131.2, 127.0, 118.5, 118.3, 116.0, 115.8, 106.1, 105.9, 105.8, 105.6, 31.5 ppm; IR (neat) 1673 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C16H11F4O]+ ([M + H]+) 295.0746; found 295.0740.
(E)-1-(3-Fluorophenyl)-4-(2,4,5-trifluorophenyl)but-2-en-1-one (7c)
Following the general procedure (2), molecule 7c was obtained as a brown solid (mp 45 °C, 120 mg, 71% yield); 1H NMR (400 MHz, CDCl3) δ 7.66 (d, J = 7.8 Hz, 1H), 7.58 (dt, J = 9.5, 2.1 Hz, 1H), 7.44 (td, J = 8.0, 5.5 Hz, 1H), 7.23–7.28 (m, 1H), 6.92–7.13 (m, 3H), 6.81 (d, J = 15.6 Hz, 1H), 3.60 (d, J = 6.9 Hz, 2H) ppm; 13C NMR (101 MHz, CDCl3) δ 189.0, 164.1, 161.7, 145.4, 139.7, 139.7, 130.4, 130.4, 127.0, 124.3, 120.2, 120.0, 118.5, 118.5, 118.3, 118.3, 115.4, 115.3, 106.1, 105.9, 105.8, 105.6, 31.6 ppm; IR (neat) 1674, 1626 cm–1; HRMS (EI) m/z: [M]+ calcd for [C16H10F4O]+ ([M]+) 294.0667; found 294.0662.
(E)-1-(4-Chlorophenyl)-4-(2,4,5-trifluorophenyl)but-2-en-1-one (7d)
Following the general procedure (2), molecule 7d was obtained as a brown solid (mp 46 °C 93 mg, 52% yield); 1H NMR (400 MHz, CDCl3) δ 7.83 (dt, J = 8.7, 2.2 Hz, 2H), 7.43 (dt, J = 8.8, 2.2 Hz, 2H), 6.91–7.11 (m, 3H), 6.82 (d, J = 15.6 Hz, 1H), 3.59 (d, J = 6.4 Hz, 2H) ppm; 13C NMR (101 MHz, CDCl3) δ 189.0, 157.2, 157.1, 154.7, 150.3, 148.2, 148.2, 148.1, 148.1, 147.9, 147.8, 145.1, 139.5, 135.9, 130.0, 129.1, 127.0, 121.2, 121.1, 121.0, 120.9, 118.5, 118.4, 118.3, 118.3, 106.1, 105.9, 105.8, 105.6, 31.5 ppm; IR (neat) 1672 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C16H11ClF3O]+ ([M + H]+) 311.0451; found 311.0441.
(E)-1-(4-Bromophenyl)-4-(2,4,5-trifluorophenyl)but-2-en-1-one (7e)
Following the general procedure (2), molecule 7e was obtained as a white solid (mp 44 °C, 118 mg, 58% yield); 1H NMR (400 MHz, CDCl3) δ 7.75 (dt, J = 9.0, 2.1 Hz, 2H), 7.60 (dt, J = 9.0, 2.1 Hz, 2H), 6.91–7.11 (m, 3H), 6.81 (d, J = 15.1 Hz, 1H), 3.59 (d, J = 6.4 Hz, 2H) ppm; 13C NMR (101 MHz, CDCl3) δ 189.2, 145.2, 136.3, 132.0, 130.1, 128.2, 126.9, 118.5, 118.4, 118.3, 118.2, 106.1, 105.9, 105.8, 105.6, 31.6 ppm; IR (neat) 1672, 1622 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C16H11BrF3O]+ ([M + H]+) 354.9945; found 354.9942.
(E)-1-(4-Methoxyphenyl)-4-(2,4,5-trifluorophenyl)but-2-en-1-one (7f)
Following the general procedure (2), molecule 7f was obtained as a white solid (mp 88 °C, 1.33 g, 84% yield); 1H NMR (400 MHz, CDCl3) δ 7.90 (dt, J = 9.5, 2.5 Hz, 2H), 6.85–7.08 (m, 6H), 3.87 (s, 3H), 3.58 (d, J = 6.9 Hz, 2H) ppm; 13C NMR (101 MHz, CDCl3) δ 188.6, 163.6, 143.5, 131.0, 130.5, 127.2, 118.5, 118.4, 118.3, 118.2, 113.9, 106.0, 105.8, 105.7, 105.5, 77.4, 77.1, 76.8, 55.6, 31.5 ppm; IR (neat) 1668, 1621 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C17H14F3O2]+ ([M + H]+) 307.0946; found 307.0951.
(E)-1-(3-Methoxyphenyl)-4-(2,4,5-trifluorophenyl)but-2-en-1-one (7g)
Following the general procedure (2), molecule 7g was obtained as a colorless oil (127 mg, 72% yield); 1H NMR (400 MHz, CDCl3) δ 7.42–7.46 (m, 2H), 7.36 (t, J = 7.8 Hz, 1H), 6.91–7.13 (m, 4H), 6.82–6.89 (m, 1H), 3.85 (s, 3H), 3.59 (d, J = 6.4 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ 190.1, 160.0, 144.5, 139.0, 129.7, 127.5, 121.2, 119.6, 118.5, 118.3, 112.9, 106.0, 105.8, 105.8, 105.6, 77.4, 77.1, 76.8, 55.5, 31.5 ppm; IR (neat) 1672, 1624 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C17H14F3O2]+ ([M + H]+) 307.0946; found 307.0942.
(E)-1-(p-Tolyl)-4-(2,4,5-trifluorophenyl)but-2-en-1-one (7h)
Following the general procedure (2), molecule 7h was obtained as a white solid (mp 85 °C, 95 mg, 57% yield); 1H NMR (400 MHz, CDCl3) δ 7.80 (d, J = 7.8 Hz, 2H), 7.26 (d, J = 6.9 Hz, 2H), 6.85–7.08 (m, 4H), 3.58 (d, J = 6.4 Hz, 2H), 2.40 (s, 3H) ppm; 13C NMR (101 MHz, CDCl3) δ 189.9, 144.0, 135.0, 129.4, 128.8, 127.5, 121.5, 121.4, 121.3, 121.2, 118.5, 118.4, 118.3, 118.2, 106.0, 105.8, 105.7, 105.5, 77.4, 77.1, 76.8, 31.5, 21.8 ppm; IR (neat) 1671, 1616 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C17H14F3O]+ ([M + H]+) 291.0997; found 291.1000.
(R)-tert-Butyl-(Naphthalen-2-ylmethoxy)(4-oxo-4-phenyl-1-(2,4,5-trifluorophenyl)butan-2-yl)carbamate (11a)
Following the general procedure (3), molecule 11a was obtained as a white solid (mp 85 °C, 27 mg, 92% yield); 1H NMR (400 MHz, CDCl3) δ 7.73–7.85 (m, 6H), 7.47–7.54 (m, 4H), 7.34–7.38 (m, 2H), 7.11–7.18 (m, 1H), 6.84 (td, J = 9.6, 6.6 Hz, 1H), 5.05 (d, J = 10.5 Hz, 1H), 4.86–4.94 (m, 2H), 3.25 (dd, J = 16.9, 5.9 Hz, 1H), 3.07 (dd, J = 17.2, 7.1 Hz, 1H), 2.86–2.99 (m, 2H), 1.39 (s, 9H) ppm; 13C NMR (101 MHz, CDCl3) δ 197.4, 156.5, 136.6, 133.4, 133.3, 133.2, 128.7, 128.6, 128.4, 128.1, 128.0, 127.8, 127.1, 126.5, 126.4, 121.9, 121.8, 121.7, 121.7, 119.5, 119.4, 119.3, 119.2, 105.5, 105.3, 105.2, 105.0, 81.9, 78.0, 56.6, 41.5, 30.6, 28.2 ppm; IR (neat) 1706, 1520 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C32H31F3NO4]+ ([M + H]+) 550.2205; found 550.2193. The enantioselectivity was determined by chiral HPLC analysis (DAICEL Chiralpak AD-H, hexane: ethanol = 95:5, flow rate = 1.0 mL/min, 23 °C, λ = 250 nm), retention time: minor isomer 6.06 min, major isomer 6.58 min, 93% ee, [α]D20 = +3.64 (c 1.0, CHCl3).
(R)-tert-Butyl-(4-(4-Fluorophenyl)-4-oxo-1-(2,4,5-trifluorophenyl)butan-2-yl)(naphthalen-2-ylmethoxy)carbamate (11b)
Following the general procedure (3), molecule 11b was obtained as a white solid (mp 95 °C, 27 mg, 92% yield); 1H NMR (400 MHz, CDCl3) δ 7.79–7.85 (m, 3H), 7.76 (s, 1H), 7.69 (dd, J = 8.7, 5.5 Hz, 2H), 7.47–7.51 (m, 3H), 7.14 (q, J = 8.7 Hz, 1H), 6.97 (t, J = 8.5 Hz, 2H), 6.84 (td, J = 9.6, 6.9 Hz, 1H), 5.03 (d, J = 11.0 Hz, 1H), 4.87 (d, J = 10.5 Hz, 1H), 4.83–4.90 (m, 1H), 3.15 (dd, J = 17.2, 6.2 Hz, 1H), 2.84–2.99 (m, 3H), 1.39 (s, 9H) ppm; 13C NMR (101 MHz, CDCl3) δ 195.7, 167.1, 164.5, 156.6, 133.5, 133.4, 133.2, 130.7, 130.6, 128.6, 128.4, 128.1, 127.8, 127.1, 126.5, 126.4, 121.8, 121.6, 119.5, 119.4, 119.3, 119.2, 115.8, 115.6, 105.5, 105.3, 105.2, 105.0, 82.0, 77.9, 56.4, 41.3, 30.6, 28.2, 27.7 ppm; IR (neat) 1689, 1598 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C32H30F4NO4]+ ([M + H]+) 568.2111; found 568.2133. The enantioselectivity was determined by chiral HPLC analysis (DAICEL Chiralpak AD-H, hexane: ethanol = 95:5, flow rate = 1.0 mL/min, 23 °C, λ = 250 nm), retention time: minor isomer 5.73 min, major isomer 6.74 min, 94% ee, [α]D20 = −39.10 (c 1.0, CHCl3).
(R)-tert-Butyl-(4-(3-Fluorophenyl)-4-oxo-1-(2,4,5-trifluorophenyl)butan-2-yl)(naphthalen-2-ylmethoxy)carbamate (11c)
Following the general procedure (3), molecule 11c was obtained as a white solid (mp 81 °C, 25 mg, 85% yield); 1H NMR (400 MHz, CDCl3) δ 7.78–7.85 (m, 3H), 7.76 (s, 1H), 7.43–7.52 (m, 5H), 7.29 (td, J = 7.9, 5.6 Hz, 1H), 7.10–7.22 (m, 2H), 6.84 (td, J = 9.6, 6.6 Hz, 1H), 5.03 (d, J = 10.5 Hz, 1H), 4.87 (d, J = 10.5 Hz, 1H), 4.84–4.91 (m, 1H), 3.20 (dd, J = 16.9, 6.4 Hz, 2H), 2.84–2.99 (m, 3H), 1.40 (s, 9H) ppm; 13C NMR (101 MHz, CDCl3) δ 196.1, 164.1, 161.6, 156.5, 138.6, 133.4, 133.3, 133.2, 130.4, 130.3, 128.6, 128.4, 128.1, 127.8, 127.0, 126.5, 126.4, 123.8, 121.8, 121.7, 121.6, 121.5, 120.4, 120.2, 119.5, 119.4, 119.3, 119.2, 114.9, 114.6, 105.5, 105.3, 105.2, 105.0, 82.1, 78.0, 56.5, 41.5, 30.7, 28.2 ppm; IR (neat) 1705, 1589 cm–1; HRMS (CI) m/z: [M + H]+ calcd for [C32H30F4NO4]+ ([M + H]+) 568.2015; found 568.2103. The enantioselectivity was determined by chiral HPLC analysis (DAICEL Chiralpak AD-H, hexane: ethanol = 95:5, flow rate = 1.0 mL/min, 23 °C, λ = 250 nm), retention time: minor isomer 5.71 min, major isomer 6.31 min, 95% ee, [α]D20 = +7.30 (c 1.0, CHCl3).
(R)-tert-Butyl-(4-(4-Chlorophenyl)-4-oxo-1-(2,4,5-trifluorophenyl)butan-2-yl)(naphthalen-2-ylmethoxy)carbamate (11d)
Following the general procedure (3), molecule 11d was obtained as a white solid (mp 92 °C, 25 mg, 88% yield); 1H NMR (400 MHz, CDCl3) δ 7.78–7.84 (m, 3H), 7.75 (s, 1H), 7.60 (d, J = 8.7 Hz, 2H), 7.47–7.53 (m, 3H), 7.27 (d, J = 8.7 Hz, 2H), 7.11–7.17 (m, 1H), 6.84 (td, J = 9.6, 6.7 Hz, 1H), 5.03 (d, J = 10.5 Hz, 1H), 4.87 (d, J = 11.0 Hz, 1H), 4.83–4.90 (m, 1H), 3.14 (dd, J = 17.4, 6.4 Hz, 1H), 2.84–2.97 (m, 3H), 1.40 (s, 9H) ppm; 13C NMR (101 MHz, CDCl3) δ 196.1, 156.6, 147.7, 145.5, 139.7, 134.8, 133.4, 133.3, 133.2, 129.4, 128.9, 128.6, 128.4, 128.1, 127.8, 127.1, 126.5, 126.4, 121.7, 121.6, 119.5, 119.4, 119.3, 119.2, 105.5, 105.3, 105.2, 105.0, 82.0, 77.9, 56.4, 41.3, 30.7, 28.2 ppm; IR (neat) 1689, 1589 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C32H30ClF3NO4]+ ([M + H]+) 584.1815; found 584.1823. The enantioselectivity was determined by chiral HPLC analysis (DAICEL Chiralpak AD-H, hexane: ethanol = 95:5, flow rate = 1.0 mL/min, 23 °C, λ = 250 nm), retention time: minor isomer 6.21 min, major isomer 7.04 min, 94% ee, [α]D20 = −3.12 (c 1.0, CHCl3).
(R)-tert-Butyl-(4-(4-Bromophenyl)-4-oxo-1-(2,4,5-trifluorophenyl)butan-2-yl)(naphthalen-2-ylmethoxy)carbamate (11e)
Following the general procedure (3), molecule 11e was obtained as a white solid (mp 95 °C, 25 mg, 90% yield); 1H NMR (400 MHz, CDCl3) δ 7.77–7.84 (m, 3H), 7.74 (s, 1H), 7.47–7.53 (m, 5H), 7.43 (dt, J = 8.8, 1.9 Hz, 2H), 7.11–7.17 (m, 1H), 6.84 (td, J = 9.6, 6.6 Hz, 1H), 5.03 (d, J = 10.5 Hz, 1H), 4.87 (d, J = 10.5 Hz, 1H), 4.83–4.90 (m, 1H), 3.14 (dd, J = 17.2, 6.2 Hz, 1H), 2.83–2.97 (m, 3H), 1.40 (s, 9H) ppm; 13C NMR (101 MHz, CDCl3) δ 196.2, 156.6, 150.2, 147.7, 135.2, 133.4, 133.3, 133.2, 131.9, 129.5, 128.6, 128.5, 128.4, 128.1, 127.8, 127.1, 126.5, 126.4, 121.7, 121.6, 119.5, 119.2, 105.5, 105.3, 105.2, 105.0, 82.0, 77.9, 56.4, 41.3, 30.7, 28.2 ppm; IR (neat) 1706, 1585 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C32H30BrF3NO4]+ ([M + H]+) 628.1310; found 628.1287. The enantioselectivity was determined by chiral HPLC analysis (DAICEL Chiralpak AD-H, hexane: ethanol = 95:5, flow rate = 1.0 mL/min, 23 °C, λ = 250 nm), retention time: minor isomer 6.85 min, major isomer 7.67 min, 89% ee, [α]D20 = −5.64 (c 1.0, CHCl3).
(R)-tert-Butyl-(4-(4-Methoxyphenyl)-4-oxo-1-(2,4,5-trifluorophenyl)butan-2-yl)(naphthalen-2-ylmethoxy)carbamate (11f)
Following the general procedure (3), molecule 11f was obtained as a white solid (mp 88 °C, 1.77 g, 94% yield); 1H NMR (400 MHz, CDCl3) δ 7.79–7.85 (m, 3H), 7.77 (s, 1H), 7.71–7.73 (m, 2H), 7.47–7.53 (m, 3H), 7.11–7.18 (m, 1H), 6.79–6.87 (m, 3H), 5.04 (d, J = 10.6 Hz, 1H), 4.87 (d, J = 10.6 Hz, 1H), 4.88–4.93 (m, 1H), 3.84 (s, 3H), 3.20 (dd, J = 16.9, 6.2 Hz, 1H), 3.01 (dd, J = 17.0, 7.4 Hz, 1H), 2.85–2.96 (m, 2H), 1.39 (s, 9H) ppm; 13C NMR (101 MHz, CDCl3) δ 195.8, 163.6, 156.5, 133.5, 133.4, 133.2, 130.4, 129.7, 128.5, 128.4, 128.2, 127.8, 127.1, 126.4, 126.3, 122.0, 121.9, 121.8, 121.8, 121.7, 119.5, 119.4, 119.3, 119.2, 113.8, 105.4, 105.2, 105.1, 105.0, 81.9, 78.0, 56.7, 55.6, 41.1, 30.6, 28.2 ppm; IR (neat) 1707, 1601 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C33H33F3NO5]+ ([M + H]+) 580.2311; found 580.2316. The enantioselectivity was determined by chiral HPLC analysis (DAICEL Chiralpak AD-H, hexane: ethanol = 95:5, flow rate = 1.0 mL/min, 23 °C, λ = 250 nm), retention time: minor isomer 10.44 min, major isomer 11.84 min, 96% ee, [α]D20 = −14.92 (c 1.0, CHCl3).
(R)-tert-Butyl-(4-(3-Methoxyphenyl)-4-oxo-1-(2,4,5-trifluorophenyl)butan-2-yl)(naphthalen-2-ylmethoxy)carbamate (11g)
Following the general procedure (3), molecule 11g was obtained as a colorless oil (27 mg, 94% yield); 1H NMR (400 MHz, CDCl3) δ 7.79–7.85 (m, 3H), 7.76 (s, 1H), 7.46–7.53 (m, 3H), 7.38 (s, 1H), 7.22–7.28 (m, 3H), 7.05–7.17 (m, 2H), 6.84 (td, J = 9.7, 6.4 Hz, 1H), 5.04 (d, J = 10.1 Hz, 1H), 4.88–4.94 (m, 1H), 3.80 (s, 3H), 3.27 (dd, J = 16.9, 6.4 Hz, 1H), 3.07 (dd, J = 16.9, 6.9 Hz, 1H), 2.85–3.10 (m, 2H), 1.39 (s, 9H) ppm; 13C NMR (101 MHz, CDCl3) δ 197.1, 159.8, 156.5, 138.1, 133.4, 133.3, 133.2, 129.7, 128.5, 128.4, 128.1, 127.8, 127.0, 126.4, 126.3, 120.7, 120.0, 112.1, 82.0, 78.0, 56.6, 55.5, 41.4, 30.7, 28.2 ppm; IR (neat) 1748, 1706 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C33H33F3NO5]+ ([M + H]+) 580.2311; found 580.2317. The enantioselectivity was determined by chiral HPLC analysis (DAICEL Chiralpak AD-H, hexane: ethanol = 95:5, flow rate = 1.0 mL/min, 23 °C, λ = 250 nm), retention time: minor isomer 5.12 min, major isomer 6.20 min, 91% ee, [α]D20 = −28.60 (c 1.0, CHCl3).
(R)-tert-Butyl-(naphthalen-2-ylmethoxy)(4-oxo-4-(p-tolyl)-1-(2,4,5-trifluorophenyl)butan-2-yl)carbamate (11h)
Following the general procedure (3), molecule 11h was obtained as a white solid (mp 73 °C, 26 mg, 89% yield); 1H NMR (400 MHz, CDCl3) δ 7.78–7.85 (m, 3H), 7.76 (s, 1H), 7.66 (d, J = 8.2 Hz, 2H), 7.47–7.53 (m, 3H), 7.11–7.17 (m, 3H), 6.83 (td, J = 9.7, 6.6 Hz, 1H), 5.04 (d, J = 10.5 Hz, 1H), 4.86 (d, J = 10.5 Hz, 1H), 3.25 (dd, J = 16.9, 5.9 Hz, 1H), 3.06 (dd, J = 16.9, 6.9 Hz, 1H), 2.85–2.98 (m, 2H), 2.38 (s, 3H), 1.39 (s, 9H) ppm; 13C NMR (101 MHz, CDCl3) δ 197.0, 156.5, 144.2, 134.2, 133.4, 133.2, 129.4, 128.5, 128.4, 128.2, 127.8, 127.0, 126.4, 126.3, 112.2, 112.1, 112.0, 81.9, 78.1, 56.6, 41.3, 30.6, 28.2, 21.7 ppm; IR (neat) 1706, 1607 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C33H33F3NO4]+ ([M + H]+) 564.2362; found 564.2345. The enantioselectivity was determined by chiral HPLC analysis (DAICEL Chiralpak AD-H, hexane: 2-propanol = 98:2, flow rate = 1.0 mL/min, 23 °C, λ = 254 nm), retention time: minor isomer 15.54 min, major isomer 28.92 min, 92% ee, [α]D20 = −24.43 (c 1.0, CHCl3).
(R)-4-Methoxyphenyl-3-((tert-butoxycarbonyl)(naphthalen-2-ylmethoxy)amino)-4-(2,4,5-trifluorophenyl)butanoate (12)
In a one-neck round-bottom flask, (R)-tert-butyl-(4-(4-methoxyphenyl)-4-oxo-1-(2,4,5-trifluorophenyl)butan-2-yl)(naphthalen-2-ylmethoxy)carbamate (11f) (238 mg, 0.41 mmol) and sodium bicarbonate (69 mg, 0.82 mmol, 2 equiv) were dissolved in dichloromethane (2 mL) under an argon atmosphere and stirred for 10 min. m-Chloroperoxybenzoic acid (138 mg, 0.62 mmol) in dichloromethane (2 mL) was dried with the excess amount of 4 Å molecular sieves and slowly added to the reaction mixture at 0 °C. The reaction was stirred for 24 h at room temperature until the TLC analysis showed that the reaction was complete. After completion of the reaction, the residue was diluted with dichloromethane (30 mL), quenched with sodium sulfite solution (10 mL), and extracted. The organic phase was washed with sodium bicarbonate solution (10 mL) and brine (5 mL), dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, hexane/ethyl acetate = 10:1) to afford compound 12 as a colorless oil (219 mg, 90% yield); 1H NMR (400 MHz, CDCl3) δ 7.87–7.82 (m, 4H), 7.59 (d, J = 8.7 Hz, 1H), 7.50 (td, J = 6.4, 3.4 Hz, 2H), 7.10 (q, J = 8.7 Hz, 1H), 6.96–6.81 (m, 5H), 5.15 (d, J = 10.1 Hz, 1H), 4.96 (d, J = 9.6 Hz, 1H), 4.89–4.82 (m, 1H), 3.76 (s, 3H), 3.02–2.89 (m, 3H), 2.80 (dd, J = 15.1, 5.9 Hz, 1H), 1.39 (s, 9H) ppm; 13C NMR (101 MHz, CDCl3) δ 170.0, 157.4, 156.5, 144.1, 133.4, 133.3, 133.0, 128.5, 128.4, 128.2, 127.8, 126.9, 126.4, 126.3, 122.3, 121.3, 121.2, 121.1, 121.0, 119.7, 119.6, 119.5, 119.4, 114.5, 105.6, 105.4, 105.3, 105.1, 82.1, 78.5, 57.4, 55.7, 37.9, 30.7, 28.2 ppm; IR (neat) 1647, 1622 cm–1; HRMS (FAB) m/z: [M-Boc + H]+ calcd for [C28H25F3NO4]+ ([M-Boc + H]+) 496.1736; found 496.1739 [α]D20 = +6.88 (c 1.0, CHCl3).
(R)-3-((tert-Butoxycarbonyl)(naphthalen-2-ylmethoxy)amino)-4-(2,4,5-trifluorophenyl)butanoic Acid (4)
(R)-4-Methoxyphenyl-3-((tert-butoxycarbonyl)(naphthalen-2-ylmethoxy)amino)-4-(2,4,5-trifluorophenyl)butanoate (12) (214 mg, 0.36 mmol) was dissolved in tetrahydrofuran (1.2 mL, 0.3 M), and KOH (1 M in water, 1.1 mL, 3 equiv) was added to the reaction mixture and stirred for room temperature. The reaction was stirred for 3 h until the TLC analysis showed that the reaction was complete. After completion of the reaction, the residue was diluted with ethyl acetate (30 mL), washed with ammonium chloride solution (15 mL), dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, hexane/ethyl acetate = 1:1 to ethyl acetate only) to afford compound 4 as a yellow sticky caramel (172 mg, 98% yield); 1H NMR (400 MHz, CDCl3) δ 7.82–7.86 (m, 4H), 7.53–7.56 (m, 1H), 7.47–7.51 (m, 2H), 6.81–7.08 (m, 2H), 5.10 (d, J = 9.6 Hz, 1H), 4.90 (d, J = 9.6 Hz, 1H), 4.70–4.77 (m, 1H), 2.79–2.91 (m, 3H), 2.55–2.60 (m, 1H), 1.40 (s, 9H) ppm; 13C NMR (101 MHz, CDCl3) δ 176.5, 157.6, 157.5, 156.6, 155.2, 155.1, 148.0, 147.8, 147.7, 145.4, 145.4, 145.3, 145.2, 133.4, 133.2, 132.9, 128.4, 128.3, 128.1, 127.8, 126.9, 126.4, 126.3, 121.2, 121.1, 119.5, 119.4, 105.5, 105.3, 105.2, 105.0, 82.2, 78.5, 56.8, 37.2, 30.7, 28.1 ppm; IR (neat) 1714 cm–1; HRMS (CI) m/z: [M + H]+ calcd for [C26H27F3NO5]+ ([M + H]+) 490.1836; found 490.1837. [α]D20 = −1.52 (c 1.0, CHCl3).
tert-Butyl-(R)-(naphthalen-2-ylmethoxy)(4-oxo-4-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-1-(2,4,5-trifluorophenyl)butan-2-yl)carbamate (14)
3-(Trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride (156 mg, 0.53 mmol, 1.5 equiv), EDC·HCl (114 mg, 0.74 mmol, 2.1 equiv), and DMAP (180 mg, 1.48 mmol, 4.2 equiv) were dissolved in dichloromethane (5 mL) under an argon atmosphere. A solution of (R)-3-((tert-butoxycarbonyl)((4-nitrobenzyl)oxy)amino)-4-(2,4,5-trifluorophenyl)butanoic acid (4) (172 mg, 0.35 mmol) in dichloromethane (5 mL) was added dropwise to the reaction mixture at room temperature. The reaction was stirred for 16 h until the TLC analysis showed that the reaction was complete. After completion of the reaction, the residue was quenched with 1 N HCl and acidified to pH = 2. The residue was extracted with dichloromethane (20 mL × 2), dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, hexane/ethyl acetate = 1:1) to afford compound 14 as a white solid (mp 84 °C, 198 mg, 85% yield); 1H NMR (400 MHz, CD3OD) δ 7.77–7.87 (m, 4H), 7.43–7.56 (m, 3H), 7.15–7.28 (m, 1H), 7.03 (m, 1H), 4.69–5.03 (m, 5H), 3.85–4.27 (m, 3H), 3.58 (br s, 1H), 2.46–2.99 (m, 4H), 1.32 (d, J = 8.2 Hz, 9H) ppm; 13C NMR (101 MHz, CD3OD) δ 170.1, 169.9, 157.7, 156.7, 151.1, 150.7, 150.1, 128.0, 127.8, 127.4, 126.3, 126.2, 122.0, 119.4, 119.1, 105.2, 104.9, 104.7, 81.8, 77.5, 56.9, 43.3, 43.1, 41.9, 41.1, 38.6, 38.3, 37.9, 37.7, 35.5, 30.4, 27.0 ppm; IR (neat) 1748, 1706 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C32H32F6N5O4]+ ([M + H]+) 664.2358; found 664.2363. [α]D20 = −7.08 (c 1.0, CHCl3).
(R)-tert-Butyl-(4-oxo-4-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-1-(2,4,5-trifluorophenyl)butan-2-yl)carbamate (15)
To a solution of tert-butyl-(R)-(naphthalen-2-ylmethoxy)(4-oxo-4-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-1-(2,4,5-trifluorophenyl)butan-2-yl)carbamate (14) (100 mg, 0.15 mmol, 1 equiv) in EtOAc (1.5 mL) was added a catalytic amount of acetone-washed (5 × 1 mL) Raney-Ni (4200; slurry in water; active catalyst; Sigma-Aldrich). The reaction mixture was stirred under a hydrogen atmosphere at room temperature. After stirring for 2 h, the reaction mixture was filtered through celite pad and washed with EtOAc. The filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography (Hx:EtOAc = 1:1) to afford molecule 15 as a white solid (mp 192 °C, 57 mg, 75% yield); 1H NMR (400 MHz, CDCl3) δ 7.02–7.09 (m, 1H), 6.82–6.91 (m, 1H), 5.25–5.25 (m, 1H), 4.98–5.10 (m, 1H), 4.91 (s, 1H), 3.95–4.25 (m, 5H), 2.61–2.95 (m, 4H), 1.34 (s, 9H) ppm; 13C NMR (201 MHz, CDCl3) δ 169.9, 169.5, 156.1 (dd, J = 243.2, 6.4 Hz), 155.2, 150.2, 148.9 (dt, J = 250.6, 13.1 Hz) 146.7 (dd, J = 244.8, 11.1 Hz), 143.7 (q, J = 41.3 Hz), 143.5 (q, J = 42.9 Hz), 119.1 (t, J = 16.7 Hz), 118.2 (qd, J = 270.2, 9.5 Hz), 105.42 (dd, J = 27.8, 21.5 Hz), 79.9, 79.7, 60.4, 48.4, 48.2, 43.6, 43.2, 42.6, 41.8, 39.3, 38.1, 36.8, 33.0, 29.7, 28.2 ppm; IR (neat) 1679, 1670 cm–1; HRMS (FAB) m/z: [M + H]+ calcd for [C21H24F6N5O3]+ ([M + H]+) 508.1783; found 508.1797. [α]D20 = +22.0 (c 1.0, CHCl3).
The analytical data obtained for compound 15 were found to be in agreement with those for the sample reported in the literature.8d
(R)-Sitagliptin·HCl (1)
(R)-tert-Butyl-(4-oxo-4-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-1-(2,4,5-trifluorophenyl)butan-2-yl)carbamate (15) (60 mg, 0.12 mmol) was dissolved in 0.5 M HCl in methanol (3 mL) and stirred at room temperature. The reaction was stirred for 3 h until the TLC analysis showed that the reaction was complete. After completion of the reaction, the residue was solidified with diethyl ether and hexane to afford sitagliptin (1) hydrochloride as a white solid (mp 171 °C, 26 mg, 92% yield); 1H NMR (500 MHz, DMSO-d6) δ 8.36 (s, 3H, NH3), 7.57–7.63 (m, 1H), 7.46–7.53 (m, 1H), 4.81–4.99 (m, 2H), 4.25–4.27 (m, 1H), 3.85–4.16 (m, 3H), 3.70–3.76 (m, 1H), 2.76–3.12 (m, 4H) ppm; 13C NMR (126 MHz, DMSO-d6) δ 168.7, 156.2 (dd, J = 243.5, 11.4 Hz) 150.8, 150.7, 148.4 (dt, J = 247.3, 13.6 Hz), 145.9 (dd, J = 242.0, 9.9 Hz), 142.5 (q, J = 38.8 Hz), 142.4 (q, J = 39.2 Hz), 120.1 (ddd, J = 18.3, 5.7, 4.2 Hz), 119.8 (dd, J = 19.5, 6.3 Hz), 118.4 (q, J = 270.1 Hz), 105.9 (dd, J = 29.1, 21.3 Hz), 47.8, 47.7, 43.5, 43.0, 41.7, 41.0, 38.5, 37.5, 34.5, 34.2, 30.9, 30.8 ppm; IR (neat) 1745, 1707 cm–1; HRMS (EI) m/z: [M]+ calcd for [C16H15F6N5O]+ ([M]+) 407.1179; found 407.1175. [α]D20 = −21.6 (c 0.7, CH3OH).
Computational Method
To suggest a plausible transition state, calculations were carried out using the Jaguar v10.7 of the Schrodinger Suite with density-functional theory. All of the initial geometries of each species were optimized to respective minimum energy geometries using our previous method. DFT calculations of the species using the B3LYP-D3 functional and 6-31++G(d,p) basis set optimized individual molecules. After combining the catalyst with enone 7 and carbamate 8, the relaxed coordinate scan was conducted in order to examine the best dihedral angle of the catalysts N63-C61-C66-C71 (−06.121 to −136.121 with 5 increments of 9 steps) to make pi–pi interactions. The most stable complex chosen from the scan was more optimized under the functional ωB97X-D method with the basis set 6-31++G(d,p) at the gas phase.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2022R1A6A1A03046247) and the BK21 Plus Program in 2023.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c10080.
Spectral data of all new compounds including 1H, 13C{1H} NMR spectra, HPLC analysis graphs under optimized conditions, and computational data for Michael addition to 7f (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Diabetes Programme, 2023. http://www.who.int/diabetes/en/.
- Drucker D. J.; Nauck M. A. The incretin system: glucagon-like peptide-1receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–705. 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- Thornberry N. A.; Gallwitz B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4(DPP-4). Best Pract. Res., Clin. Endocrinol. Metab. 2009, 23, 479–486. 10.1016/j.beem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Kim D.; Wang L.; Beconi M.; Eiermann G. J.; Fisher M. H.; He H.; Hickey G. J.; Kowalchick J. E.; Leiting B.; Lyons K.; Marsilio F.; McCann M. E.; Patel R. A.; Petrov A.; Scapin G.; Patel S. B.; Sinha; Roy R.; Wu J. K.; Wyvratt M. J.; Zhang B. B.; Zhu L.; Thornberry N. A.; Wever A. (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 2005, 48, 141–151. 10.1021/jm0493156. [DOI] [PubMed] [Google Scholar]
- Pan X.; Li X.; Lu Q.; Yu W.; Li W.; Zhang Q.; Deng F.; Liu F. Efficient synthesis of sitagliptin phosphate, a novel DPP-IV inhibitor, via a chiral aziridine intermediate. Tetrahedron Lett. 2013, 54, 6807–6809. 10.1016/j.tetlet.2013.09.136. [DOI] [Google Scholar]
- a Subbaiah C. S.; Haq W. Efficient stereocontrolled synthesis of sitagliptin phosphate. Tetrahedron: Asymmetry 2014, 25, 1026. 10.1016/j.tetasy.2014.06.001. [DOI] [Google Scholar]; b Dey S.; Sudalai A. A concise enantioselective synthesis of (R)-selegiline, (S)-benzphetamine and formal synthesis of (R)-sitagliptin via electrophilic azidation of chiral imide enolates. Tetrahedron: Asymmetry 2015, 26, 67. 10.1016/j.tetasy.2014.11.014. [DOI] [Google Scholar]; c Fistikci M.; Gundogdu O.; Aktas D.; Secen H.; Sahin M. F.; Altundas R.; Kara Y. Novel and enantioselective syntheses of (R)- and (S)-3-hydroxy-4-(2,4,5-trifluorophenyl)butanoic acid: a synthon for sitagliptin and its derivatives. Tetrahedron 2012, 68, 2607. 10.1016/j.tet.2012.01.095. [DOI] [Google Scholar]; d Lin K.; Cai Z.; Zhou W. Practical and economical approach to synthesize sitagliptin. Synth. Commun. 2013, 43, 3281–3286. 10.1080/00397911.2013.773353. [DOI] [Google Scholar]; e Kang S. K.; Cho G. H.; Leem H. J.; Soh B. K.; Sim J.; Suh Y.-G. A highly stereoselective and efficient synthesis of enantiomerically pure sitagliptin. Tetrahedron: Asymmetry 2017, 28, 34–40. 10.1016/j.tetasy.2016.10.010. [DOI] [Google Scholar]; f Gutierrez O.; Metil D.; Dwivedi N.; Gudimalla N.; Chandrashekar E. R. R.; Dahanukar V. H.; Bhattacharya A.; Bandichhor R.; Kozlowski M. C. Practical, Asymmetric Route to Sitagliptin and Derivatives: Development and Origin of Diastereoselectivity. Org. Lett. 2015, 17, 1742–1745. 10.1021/acs.orglett.5b00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savile C. K.; Janey J. M.; Mundorff E. C.; Moore J. C.; Tam S.; Jarvis W. R.; Colbeck J. C.; Krebber A.; Fleitz F. J.; Brands J.; et al. Biocatalytic Asymmetric Synthesis of Chiral Amines from Ketones Applied to Sitagliptin Manufacture. Science 2010, 329, 305. 10.1126/science.1188934. [DOI] [PubMed] [Google Scholar]
- a Dey S.; Gadakh S. K.; Ahuja B. B.; Kamble S. P.; Sudalai A. Pd-catalyzed reductive cleavage of Nsingle bondN bond in dibenzyl-1-alkylhydrazine-1,2-dicarboxylates with PMHS: application to a formal enantioselective synthesis of (R)-sitagliptin. Tetrahedron Lett. 2016, 57, 684–687. 10.1016/j.tetlet.2015.12.116. [DOI] [Google Scholar]; b Bae H. Y.; Kim M. J.; Sim J. H.; Song C. E. Direct Catalytic Asymmetric Mannich Reaction with Dithiomalonates as Excellent Mannich Donors: Organocatalytic Synthesis of (R)-Sitagliptin. Angew. Chem., Int. Ed. 2016, 55, 10825–10829. 10.1002/anie.201605167. [DOI] [PubMed] [Google Scholar]; c Gao H.; Yu J.; Ge C.; Jiang Q. Practical Asymmetric Synthesis of Sitagliptin Phosphate Monohydrate. Molecules 2018, 23, 1440. 10.3390/molecules23061440. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Hayama N.; Kuramoto R.; Földes T.; Nishibayashi K.; Kobayashi Y.; Pápai I.; Takemoto Y. Mechanistic Insight into Asymmetric Hetero-Michael Addition of α,β-Unsaturated Carboxylic Acids Catalyzed by Multifunctional Thioureas. J. Am. Chem. Soc. 2018, 140, 12216–12225. 10.1021/jacs.8b07511. [DOI] [PubMed] [Google Scholar]
- a Hansen K. B.; Balsells J.; Dreher S.; Hsiao Y.; Kubryk M.; Palucki M.; Rivera N.; Steinhuebel D.; Armstrong J. D. III; Askin D.; Grabowski E. J. First Generation Process for the Preparation of the DPP-IV Inhibitor Sitagliptin. Org. Process Res. Dev. 2005, 9, 634–639. 10.1021/op0500786. [DOI] [Google Scholar]; b Hansen K. B.; Hsiao Y.; Zu F.; Rivera N.; Clausen A.; Kubryk M.; Krska S.; Rosner T.; Simmons B.; Balsells J.; Ikemoto N.; Sun Y.; Spindler F.; Malan C.; Grabowski E. J. J.; Armstrong J. D. III Highly Efficient Asymmetric Synthesis of Sitagliptin. J. Am. Chem. Soc. 2009, 131, 8798–8804. 10.1021/ja902462q. [DOI] [PubMed] [Google Scholar]; c Bao H.; Bayeh L.; Tambar U. K. Catalytic Enantioselective Allylic Amination of Olefins for the Synthesis of ent-Sitagliptin. Synlett 2013, 24, 2459. 10.1055/s-0033-1340079. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zhou S.; Wang J.; Chen X.; Aceña J. L.; Soloshonok V. A.; Liu H. Chemical Kinetic Resolution of Unprotected β-Substituted β-Amino Acids Using Recyclable Chiral Ligands. Angew. Chem., Int. Ed. 2014, 53, 7883. 10.1002/anie.201403556. [DOI] [PubMed] [Google Scholar]; e Sreenivasulu K.; Chaudhari P. S.; Achanta S.; Sud A.; Dahanukar V.; Cobley C. J.; Llewellyn-Beard F.; Bandichhor R. Synthesis of (R)-3-(tert-Butoxycarbonylamino)-4-(2,4,5-trifluorophenyl)butanoic Acid, a Key Intermediate, and the Formal Synthesis of Sitagliptin Phosphate. Chem. - Asian J. 2020, 15, 1605–1608. 10.1002/asia.202000092. [DOI] [PubMed] [Google Scholar]; f Sudhakaran S.; Shinde P. G.; Aratikatla E. K.; Kaulage S. H.; Rana P.; Parit R. S.; Kavale D. S.; Senthilkumar B.; Punji B. Nickel-Catalyzed Asymmetric Hydrogenation for the Synthesis of a Key Intermediate of Sitagliptin. Chem. - Asian J. 2022, 17, e202101208 10.1002/asia.202101208. [DOI] [PubMed] [Google Scholar]; g MacNeil C. S.; Zhong H.; Pabst T. P.; Shevlin M.; Chirik P. J. Cationic Bis(phosphine) Cobalt(I) Arene Complexes as Precatalysts for the Asymmetric Synthesis of Sitagliptin. ACS Catal. 2022, 12, 4680–4687. 10.1021/acscatal.2c01059. [DOI] [Google Scholar]
- Lee J.; Ban J. W.; Kim J.; Yang S.; Lee G.; Dhorma L. P.; Kim M.-h.; Ha M. W.; Hong S.; Park H.-g. Asymmetric Phase-Transfer Catalytic aza-Michael Addition to Cyclic Enone: Highly Enantioselective and Diastereoselective Synthesis of Cyclic 1,3-Aminoalcohols. Org. Lett. 2022, 24, 1647–1651. 10.1021/acs.orglett.2c00192. [DOI] [PubMed] [Google Scholar]
- Raveendra B.; Maity S.; Das B. G.; Ghorai P. Organocatalytic, Enantioselective Synthesis of 1- and 3-Substituted Isochromans via Intramolecular Oxa-Michael Reaction of Alkoxyboronate: Synthesis of (+)-Sonepiprazole. J. Org. Chem. 2015, 80, 7008–7018. 10.1021/acs.joc.5b00719. [DOI] [PubMed] [Google Scholar]
- The corresponding quinidine based catalyst (10) gave (S)-11f that was converted to (S)-Sitagliptin.
- See an example of the enhanced stereoselectivity by removal of C(3)-allyl group in cinchona alkaloid derived catalyst;Fanourakis A.; Williams B. D.; Paterson K. J.; Phipps R. J. Enantioselective Intermolecular C–H Amination Directed by a Chiral Cation. J. Am. Chem. Soc. 2021, 143, 10070–10076. 10.1021/jacs.1c05206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Chan L. K. M.; Poole D. L.; Shen D.; Healy M. P.; Donohoe T. J. Rhodium-Catalyzed Ketone Methylation Using Methanol Under Mild Conditions: Formation of α-Branched Products. Angew. Chem., Int. Ed. 2014, 53 (3), 761–765. 10.1002/anie.201307950. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Feng F.-F.; Li S.; Cheung C. W.; Ma J.-A. Chiral β-Keto Propargylamine Synthesis via Enantioselective Mannich Reaction of Enamides with C-Alkynyl N-Boc N,O-Acetals. Org. Lett. 2019, 21 (20), 8419–8423. 10.1021/acs.orglett.9b03181. [DOI] [PubMed] [Google Scholar]; c Rana N. K.; Huang H.; Zhao J. C. -G. Highly Diastereodivergent Synthesis of Tetrasubstituted Cyclohexanes Catalyzed by Modularly Designed Organocatalysts. Angew. Chem., Int. Ed. 2014, 53 (29), 7619–7623. 10.1002/anie.201404072. [DOI] [PubMed] [Google Scholar]
- Lee J.; Kim M.; Jew S.; Park H.; Jeong B.-S. Phase-Transfer Catalytic Aza-Michael Addition of Tert-Butyl Benzyloxycarbamate to Electron-Deficient Olefins. Chem. Commun. 2008, 1932–1934. 10.1039/b800130h. [DOI] [PubMed] [Google Scholar]
- For detailed, see Supporting Information.; a Kamachi T.; Yoshizawa K. Enantioselective Alkylation by Binaphthyl Chiral Phase-Transfer Catalysts: A DFT-Based Conformational Analysis. Org. Lett. 2014, 16, 472–475. 10.1021/ol4033545. [DOI] [PubMed] [Google Scholar]; b Prakash G. K. S.; Wang F.; Rahm M.; Zhang Z.; Ni C.; Shen J.; Olah G. A. The Trifluoromethyl Group as a Conformational Stabilizer and Probe: Conformational Analysis of Cinchona Alkaloid Scaffolds. J. Am. Chem. Soc. 2014, 136, 10418–10431. 10.1021/ja504376u. [DOI] [PubMed] [Google Scholar]; c Ullah Z.; Kim K.; Venkanna A.; Kim H.; Kim M. I.; Kim M.-h. Plausible Pnicogen Bonding of epi-Cinchonidine as a Chiral Scaffold in Catalysis. Front. Chem. 2021, 9, 669515 10.3389/fchem.2021.669515. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Chai J. D.; Head-Gordon M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. 10.1039/b810189b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.