Abstract
The ICAM-1/LFA-1 interaction has been clearly demonstrated to play an active role in syncytium formation induced by human immunodeficiency virus type 1 (HIV-1). Since it is known that a high-affinity state of LFA-1 for ICAM-1 can be induced through conformational change, such a high-affinity state may also contribute to the process of syncytium formation. In this study, we have investigated the involvement of the conformational status of LFA-1 in HIV-1-dependent syncytium formation by using the anti-LFA-1 antibody NKI-L16, which is known to activate the high-affinity state. Initial visual observations by light microscopy indeed suggested that the addition of the NKI-L16 antibody led to bigger and more numerous syncytia when different cell lines were tested. To further analyze this NKI-L16-dependent increment of syncytium formation in a quantitative assay, a new luciferase-based assay was developed by using a T-cell line containing an HIV-1 long terminal repeat (LTR)-driven luciferase construct (1G5) in coincubation with an HIV-1-positive cell line (J1.1). Upon fusion, the viral Tat protein could diffuse to the 1G5 cells, leading to a transcriptional increase of the HIV-1 LTR-driven luciferase gene. Initial evaluation of this assay showed a good correlation between the level of syncytium formation determined by microscopic observation and the level of measured luciferase activity. In addition, this assay showed a greater induction of enzymatic activity correlating with syncytium formation in comparison to a similar incubation with the HeLa–CD4–LTR–β-gal indicator cell line. By using this test, NKI-L16 treatment of 1G5/J1.1 cells led to a three- to sevenfold increase in HIV-1 LTR-driven luciferase activity. The syncytium-dependent luciferase activity in NKI-L16-treated cells could be blocked by classical syncytium inhibitors such as soluble CD4, anti-CD4, and anti-gp120 antibodies. Inhibition could also be observed with specific blocking agents for the chemokine receptor CXCR4, as well as with soluble ICAM-1, anti-LFA-1, anti-ICAM-1, and anti-ICAM-2 blocking antibodies, indicating the requirement for the LFA-1/ICAM interaction. Treatment of peripheral blood mononuclear cells with NKI-L16 resulted in a higher level of syncytium formation in the presence of the cell line J1.1. Conversely, when PBMCs were infected with two different syncytium-inducing HIV-1 primary isolates, coincubation with NKI-L16-pretreated 1G5 cells led to higher levels of luciferase activity for both virus isolates. Our results therefore show for the first time a direct role for the LFA-1 high-affinity state in virus-mediated syncytium formation. Based on the demonstration that an increase in ICAM-1 binding is induced by T-cell activation, these data suggest an in vivo involvement of the high-affinity state of LFA-1 in HIV-1-induced syncytium formation. Moreover, syncytia might preferentially occur in lymph nodes, since this microenvironment harbors a high proportion of activated T cells.
Human immunodeficiency virus type 1 (HIV-1), the etiological agent of AIDS, is known to cause a progressive loss of CD4+ T-cell counts. Several virus-mediated pathogenic effects have been postulated to account for the observed CD4+ T-cell depletion. One of the best-described types of HIV-1-induced cell death is syncytium formation, which is characterized by a high rate of cell-to-cell fusion events between uninfected and infected cells, eventually leading to cell death. Although observed in cell culture, few examples of similar observations have been made in vivo and are limited mainly to lymph nodes (including the tonsils) and to the brain region (20, 29, 50).
Syncytium formation is dependent on the interaction between the HIV-1 envelope protein gp120 on the surface of an infected cell and the CD4 receptor molecule of an uninfected cell (15, 39, 40, 45, 55). The interaction of the gp120 molecule with the newly identified α and β chemokine receptors (CXCR4 and CCR5) has also been shown to be of primary importance in syncytium formation (2, 18, 21, 24). Although these interactions seem to suffice under certain conditions, interactions between other cellular constituents such as LFA-1/ICAM-1 have been shown to be very important for the formation of syncytia in cell types in which gp120 expression is limited without necessarily alleviating the requirement for a certain threshold level of gp120/CD4 interaction (31). ICAM-1 is part of a subfamily of cell surface molecules which include ICAM-2 and ICAM-3. ICAM-1 is present mainly on the surface of lymphocytes, granulocytes, monocytes, macrophages, and certain epithelial cells (56). ICAM-2 expression is restricted mainly to the surface of vascular endothelium and of some lymphoid cells (17, 58), while ICAM-3 is limited to leukocytes (16). Their interaction partner, LFA-1, is part of the family of integrins often implicated in intercellular adhesion events. LFA-1 is made of two subunits, CD11a and CD18, and shows restriction in its pattern of expression to hematopoiesis-derived cells. Two distinct conformational states exist for LFA-1, one being of high affinity toward the ligand ICAM-1. The high-affinity conformation of LFA-1 can be induced by stimulating agents such as phytohemagglutinin (PHA), phorbol-12-myristate-13-acetate, divalent ions and several other antibodies specific for surface receptors such as the TCR/CD3 complex, CD2, and major histocompatibility complex type II (MHC-II) (19, 22, 23, 41). Moreover, certain antibodies directed against LFA-1 have been reported to induce this high-affinity state by direct induction of the conformational change (34, 37). This conformational change seems to be dependent on the presence of specific cation such as magnesium (25, 41). This dynamic regulation of integrins allows the cells bearing these molecules to rapidly convert from a nonadherent to an adherent phenotype and vice versa.
The importance of LFA-1/ICAM-1 interaction in HIV-1-mediated syncytium formation has been extensively studied. Antibodies directed against LFA-1 has hence been known to greatly reduce HIV-1-induced cell-to-cell fusion (33, 59). In addition, LFA-1 requirement for syncytium formation has been clearly demonstrated with the use of CD4+ T cells deficient in cell surface expression of LFA-1 (47). Butini and colleagues have further demonstrated the contribution of ICAM-2 and ICAM-3 in the formation of syncytia through LFA-1 interaction (12). It is believed that such a strong interaction might compensate for the low abundance of gp120 by providing a greater cell-to-cell affinity (31). However, no information has yet been gathered on the potential implication of the high-affinity state of LFA-1 in the formation of syncytia.
In this study, we have thus analyzed the outcome of LFA-1 activation on HIV-1-mediated syncytium formation. Using a new luciferase-dependent quantitative assay, we have shown that antibody-mediated LFA-1 activation augmented syncytium formation significantly and that these syncytia were inhibited by blocking agents specific to the gp120/CD4/CXCR4 and the LFA-1/ICAM interactions.
MATERIALS AND METHODS
Cell lines and plasmids.
All cell lines (except for the HeLa–CD4–LTR–β-gal cell line) were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (HyClone Laboratories, Logan, Utah), glutamine (2 mM), penicillin G (100 U/ml), and streptomycin (100 mg/ml). Uninfected CD4+ T cells included Jurkat E6.1 (60), the Jurkat E6.1-derived 1G5 cell line which harbors two copies of a stably transfected plasmid made of the luciferase reporter gene downstream of the HIV-1 long terminal repeat region (1), Sup-T1 (54), PM1 (42) and WE17/10 (61). Jurkat-tat cells, a Jurkat E6.1-derived cell line which expresses the HIV-1 Tat transactivator, were also used (14). We have also used the Jurkat E6.1-derived cell lines J1.1, which is latently infected with HIV-1 (49). OM10.1 is another cell line latently infected with HIV-1, which was initially derived from the promyelocytic cell line HL60 (11). Three chronically infected cell lines derived from the H9 T cell line (43) were obtained by infection with HIV-1IIIB, HIV-1IIIMN, and HIV-1IIIRF. The chronically infected cell line Jurkat-tat/PBS-WT was derived by transfection of pPBS-WT (38) by the DEAE-dextran method. This molecular clone of HIV-1 contains the full-length HIV-1 genome from pSCV21 (30) with the wild-type primer binding site of HXB2D, an infectious molecular clone of HIV-1IIIB (57). The U937-HIV-1IIIB cell line was derived by acutely infecting the monocytic cell line U937 with HIV-1IIIB. An A2.01-derived cell line expressing the HIV-1 gp120 molecule on its surface (A2.01env2.0) was kindly provided by L. Poulin (Centre Hospitalier Universitaire de Québec, Pavillon CHUL) (46). The HeLa–CD4–LTR–β-gal cell line was grown in Dulbecco modified Eagle’s medium supplemented with 10% fetal calf serum, glutamine (2 mM), penicillin G (100 U/ml), and streptomycin (100 mg/ml) in the presence of 200 μg of G418 per ml and 100 μg of hygromycin per ml. This cell line contains stably integrated constructs for CD4 expression and for the β-galactosidase reporter gene under the control of the HIV-1 long terminal repeat (35). pLTRX-LUC was used in transient-transfection experiments and contains a 722-bp XhoI (−644)-HindIII (+78) fragment from HIV-1LAI placed in front of the luciferase reporter gene (kindly provided by O. Schwartz, Unité d’Oncologie Virale, Institut Pasteur, Paris, France) (53).
Antibodies and soluble proteins.
NKI-L16 (anti-CD11a) was obtained from Carl C. Figdor (University Hospital, Nijmegen, The Netherlands) (34), while anti-LFA-1 antibodies MEM30 and MEM83 (anti-CD11a) were kind gifts from Vaclav Horejsi (Institute of Molecular Genetics, Prague, Czech Republic) (5). The anti-ICAM-1 antibody (anti-CD54) RR1/1.1.1 was kindly provided by Robert Rothlein (Boehringer Ingelheim, Ridgefield, Conn.) (52). Anti-ICAM-2 (clone 92C12F) and anti-ICAM-3 (clones ICR-1.1, ICR-3.1, and ICR-5.1) monoclonal antibodies, the latter being specific for its domain 1, were supplied by ICOS Corp. (Bothell, Wash.). Anti-CXCR4 (clone 12G5; no. 3439) and anti-gp120 (anti-V3 loop-neutralizing antibodies from IIIB-V3-13; no. 1727) monoclonal antibodies were supplied by the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases. Plasma-pooled purified human immunoglobulin G (IgG) from HIV-1-infected subjects were also used in our studies. Hybridomas that produce anti-CD4 SIM.2 and SIM.4 antibodies were provided by the National Institutes of Health AIDS Repository Program. SIM.2 recognizes a different epitope from Leu3A, while SIM.4 is specific for the V1 domain of CD4 (44). Antibodies from these producer cell lines and from HIV-1-infected persons were purified on a mAbTrap protein G affinity column as specified by the manufacturer (Pharmacia LKB Biotechnology AB, Uppsala, Sweden). The ICAM-1 fusion protein (sICAM-1) consists of the extracellular part of ICAM-1 fused to the hinge region and constant H-chain domains 2 and 3 of a mouse IgG2b and was purified on an immunoaffinity column. This fusion protein was supplied by E. Lundgren (University of Umeå, Umeå, Sweden) and has been described previously (32). Stromal cell-derived factor 1 (SDF-1, 1-67) was a kind gift from I. Clark-Lewis (Biomedical Research Center, Vancouver, Canada). Recombinant soluble CD4 (sCD4) from R. Sweet (SmithKline Beecham) was also obtained through the NIH AIDS Repository Program.
DEAE-dextran transfection.
Lymphoid cells were transfected as previously described (4). Briefly, 107 cells were washed in TS buffer (137 mM NaCl, 25 mM Tris-HCl [pH 7.4], 5 mM KCl, 0.6 mM Na2HPO4, 0.5 mM MgCl2, 0.7 mM CaCl2) and then incubated for 25 min at room temperature along with 15 μg of pLTRX-LUC in 1 ml of TS buffer containing 500 μg of DEAE-dextran per ml. Afterward, 10 ml of supplemented RPMI 1640 medium was added to the cells along with 100 μM chloroquine and the cells were incubated at 37°C for 45 min. After being pelleted, the cells were resuspended in 10 ml of supplemented RPMI 1640 medium and incubated at 37°C for 24 h.
Syncytium assay.
Uninfected cells were first concentrated to 106 cells/ml. Thereafter, 100 μl of either untransfected or transfected cell suspension was added to a 96-well plate in triplicate. The cells were either left untreated or treated with NKI-L16 (1 μg/ml) or MEM83 (3 μg/ml) prior to incubation at 37°C for 30 min. Following incubation, 100 μl of medium or HIV-1-infected T cells (106 cells/ml) was added. For the experiment where different cell ratios were tested, 1G5 cells were left untreated or incubated with NKI-L16 (1 μg/ml) and then added at different volumes in triplicate to wells to which an adjusted volume of virally infected cells was then added to make up a final volume of 200 μl. For the zidovudine (AZT) experiment, 1G5 cells were pretreated with 1 μM AZT for 90 min before the addition of J1.1 cells. With these cell mixtures, further treatments were also used and included the addition of the following antibodies: anti-gp120 neutralizing antibodies (anti-V3 loop neutralizing antibodies from IIIB-V3-13), purified human pooled anti-HIV-1 antibodies, anti-CD4 (clones SIM.2 and SIM.4), anti-CXCR4 (clone 12G5), anti-LFA-1 (clone MEM30), anti-ICAM-1 (clone RR1/1.1.1), anti-ICAM-2 (clone 92C12F), and anti-ICAM-3 (clones ICR-1.1, ICR-3.1, and ICR-5.1) antibodies. Soluble purified proteins, such as CD4, ICAM-1 and SDF-1, were also added at different concentrations. Mixed cells were then allowed to form syncytia for 12 h (or the indicated time) and were either photographed at a magnification of ×100 with an inverted microscope or lysed in lysis buffer for luciferase counts as previously described (10, 13, 26). Luciferase activity was monitored with a microplate luminometer (MLX; Dynex Technologies, Chantilly, Va.). The HeLa–CD4–LTR–β-gal samples were lysed and evaluated for β-galactosidase activity by using the Dynex luminometer and the Galacto-Light Plus kit as specified by the manufacturer (Tropix, Bedford, Mass.).
Fluorescence-activated cell sorter (FACS) analysis.
Cells were incubated for 30 min on ice with saturating concentrations of monoclonal RR1/1.1.1, 92C12F, or ICR-3.1 antibodies. The cells were washed twice in washing medium and incubated for 30 min on ice with a saturating concentration of secondary antibodies consisting of fluorescein-conjugated goat anti-mouse IgG from Caltag Laboratories (San Francisco, Calif.). Finally, the cells were washed twice in phosphate-buffered saline and were resuspended in 500 μl of phosphate-buffered saline containing 1% (wt/vol) paraformaldehyde prior flow cytometry analysis (EPICS XL; Coulter Corp. Miami, Fla.). Controls consisted of commercial isotype-matched irrelevant murine monoclonal antibodies (Sigma, St. Louis, Mo.).
PBMC isolation, infection, and transfection.
Primary peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by Ficoll-Hypaque density gradient centrifugation. Briefly, 10 ml of venous blood was layered on a Ficoll cushion and centrifuged at 1,500 rpm for 30 min (Sorvall RT6000B; Du Pont Co., Wilmington, Del.). Mononuclear cells at the Ficoll interface were collected and washed twice in Hanks balanced salt solution. The cells were then concentrated at 106 cells/ml, in complete medium supplemented with PHA-P (3 μg/ml) (Sigma) and recombinant human interleukin-2 (rhIL-2) (30 U/ml), for 48 h at 37°C. After incubation, the cells were pelleted, readjusted to 106 cells/ml in complete medium supplemented with rhIL-2 (30 U/ml), and aliquoted in 100-μl samples in triplicate and treated with NKI-L16 (1 μg/ml) or not treated. A syncytium assay was then performed as described above with latently infected J1.1 cells. Syncytium counts were conducted between 24 and 48 h postincubation. In other experiments, PBMCs were infected with two different syncytium-inducing HIV-1 primary isolates (92UG029 and 92UG021). At 7 days postinfection, in the presence of 30 U of rhIL-2 per ml, PBMCs (105 cells) were cocultured with an equal number of untreated or NKI-L16-treated 1G5 cells and evaluated for luciferase activity 12 and 24 h after the start of coculture. The following reagents were obtained through the AIDS Research and Reference Reagent Program: rhIL-2 from Maurice Gately, Hoffman-La Roche Inc. (36), and HIV-1 syncytium-inducing primary isolates 92UG029 and 92UG021. In some experiments, PBMCs were transiently transfected by electroporation as previously described (7).
RESULTS
The interaction between ICAM-1 and LFA-1 is a very important element in HIV-1-mediated syncytium formation in certain cellular contexts. Since LFA-1 can be induced to a high-affinity state for its physiological counterreceptor ICAM-1, we reasoned that such an increase in the affinity of LFA-1 toward ICAM-1 should lead to a larger number of virus-induced syncytia.
High-affinity LFA-1 modulates HIV-1-dependent syncytium formation.
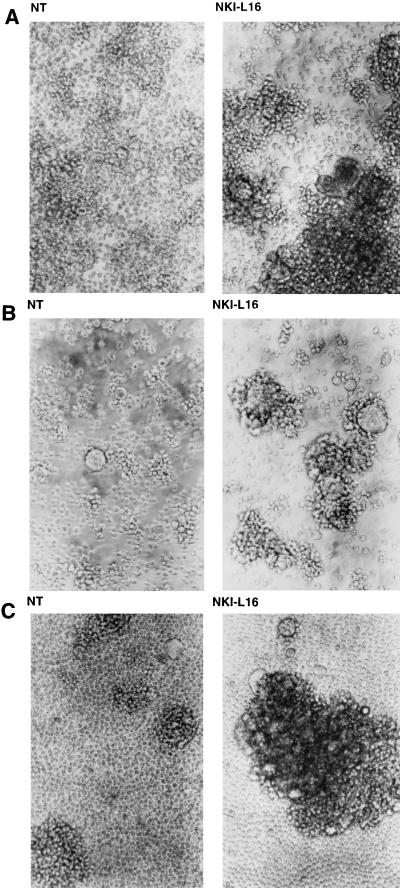
CD4-positive T-lymphoid cell lines (1G5 and Jurkat E6.1) were incubated with NKI-L16, an anti-LFA-1 antibody known to induce the switch of LFA-1 from a low- to a high-affinity state for ICAM-1 (34), and then intermixed for 16 h with HIV-1 latently infected J1.1 or OM10.1 cells. Previous experiments from our group showed that the two uninfected cell lines expressed LFA-1 on their surface (data not shown). As presented in Fig. 1, higher levels of cell aggregation were discernible when NKI-L16 was added to Jurkat E6.1/J1.1 (Fig. 1A), 1G5/J1.1 (Fig. 1B), and 1G5/OM10.1 (Fig. 1C) cell mixtures. Concomitant with an increase in homotypic cellular aggregation was a detectable increase in the overall number of syncytia (Fig. 1). Interestingly, we noticed marked changes in the size of formed syncytia when NKI-L16 was included in the coculture system. The addition of another anti-LFA-1-activating antibody, MEM83, was also found to induce higher levels of homotypic cell aggregation and syncytium formation in these cell settings but to a lower extent than with NKI-L16-incubated cells, which is consistent with previous cell-to-cell conjugate studies (data not shown) (34, 37). Syncytium formation could also be increased in the presence of NKI-L16 when J1.1 cells were incubated with the CD4- and LFA-1-positive cell lines Sup-T1, PM1, and WE17/10 (data not shown). Furthermore, a gp120-expressing cell line, A2.01env2.0, was also found to be more potentially prone to syncytium formation when coincubated with 1G5 in the presence of NKI-L16 (data not shown). These experiments thus suggested a higher rate of HIV-1-dependent syncytium formation upon LFA-1 activation, which was indicated by an increase in both syncytium number and size.
FIG. 1.
NKI-L16 induces higher levels of HIV-1-mediated syncytium formation. J1.1 cells were incubated in the absence or presence of NKI-L16 (1 μg/ml) along with an equal number of uninfected cells, either Jurkat E6.1 (A) or 1G5 (B). Similar experiments were performed with a mixture of OM10.1 and 1G5 cells (C). After 16 h of incubation, cells were observed by light microscopy. Magnification, ×100.
Novel luciferase-based quantitative assay for HIV-1-induced syncytium formation.
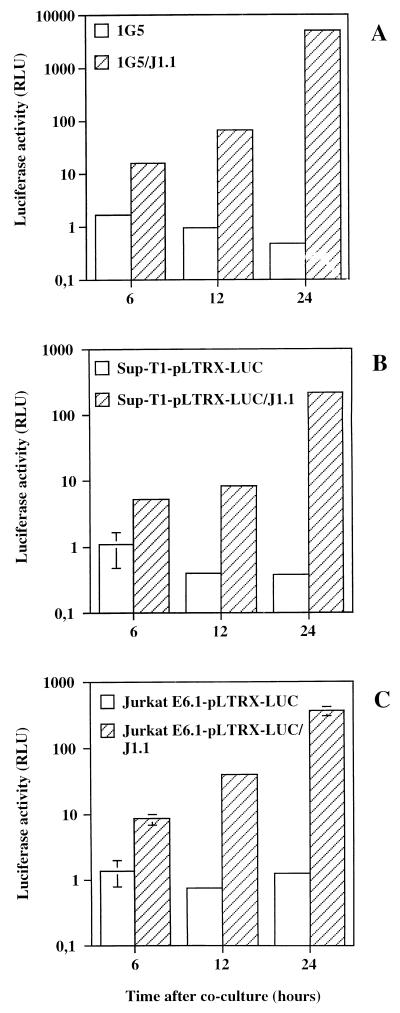
Since syncytium formation seemed to be more pronounced both qualitatively and quantitatively in cells expressing the activated form of LFA-1, we were interested in devising a novel assay for HIV-1-induced syncytium formation which would account for the syncytium number as well as the syncytium size. Although several methods already exist for the estimation of syncytia, one of which was recently developed for the identification of the chemokine receptors (24), we reasoned that a luciferase-based assay would be accurate and sensitive and could easily be quantitative. Other advantages intrinsic to the use of the luciferase reporter gene as an evaluating tool included its capacity to be linearly representative of a given signal over several logs. We further assumed that a luciferase-based assay might be manageable for syncytium quantification by the use of HIV-1 LTR-driven luciferase gene expression. The formation of syncytia would hence result in the transfer of the powerful transactivating viral Tat protein from HIV-1-infected cells to uninfected CD4+ T cells harboring an HIV-1 LTR-driven luciferase reporter gene. We first tested the cell line 1G5, since it stably contained HIV-1 LTR-dependent luciferase constructs and also showed a good increment of the number of syncytia upon treatment with NKI-L16 (Fig. 1). When added along with J1.1 cells, 6 h after coculturing, 1G5 cells showed a significant increase in luciferase activity (10-fold) in comparison to that of 1G5 cells alone. This increase was more pronounced after 12 and 24 h (42- and 3,000-fold, respectively) (Fig. 2A). To see whether different uninfected cells would also generate a similar increase, the pLTRX-LUC plasmid was transiently transfected in Sup-T1 and Jurkat E6.1 cells to which J1.1 cells were added for different periods. These cell lines similarly led to an increase in luciferase activity after incubation with J1.1, which again showed a time-dependent increase. The increases for Sup-T1 cells were 5-, 20-, and 555-fold and those for Jurkat E6.1 cells were 6-, 52-, and 288-fold at 6, 12, and 24 h, respectively (Fig. 2B and C). It should be noted that it is possible that the increase in luciferase activity at later times is mediated partly by HIV-1 particles produced by the J1.1 cell lines infecting the HIV-1 LTR-LUC-containing cell lines (see below).
FIG. 2.
Induction of luciferase activity in a time-dependent fashion by the addition of J1.1 cells to HIV-1 LTR-driven luciferase-containing cell lines. Stably (1G5) (A) and transiently (Sup-T1 or Jurkat E6.1) (B and C) transfected cells were first incubated in the presence of J1.1 and lysed after 6, 12, or 24 h. Luciferase activity was read with a Dynex luminometer apparatus and is shown on a log10 scale. The results are the mean ± standard deviation (SD) for samples studied in triplicate. RLU, relative light units.
To test whether the increase in HIV-1 LTR-driven gene activity was observed with other virally infected cells besides J1.1, several other infected cell lines were mixed with 1G5 cells (Fig. 3). These included the latently infected cell line J1.1, as well as H9/HIV-1IIIB, H9/HIV-1IIIRF, H9/HIV-1IIIMN, and Jurkat-tat/PBS-WT. Upon incubation with 1G5 cells, all tested HIV-1-infected cell lines showed a significant increase in luciferase activity in comparison to 1G5 cells alone, an increase which was also time dependent.
FIG. 3.
Several infected cell lines cocultured with 1G5 cells show increased luciferase activity over time. The 1G5 cell line was incubated along with the infected cell lines J1.1, H9/HIV-1IIIB, H9/HIV-1IIIRF, H9/HIV-1IIIMN, and Jurkat-tat/PBS-WT and lysed after 6 h (A), 12 h (B), or 24 h (C). Luciferase activity was read with a Dynex luminometer apparatus. The results are the mean ± SD for samples studied in triplicate.
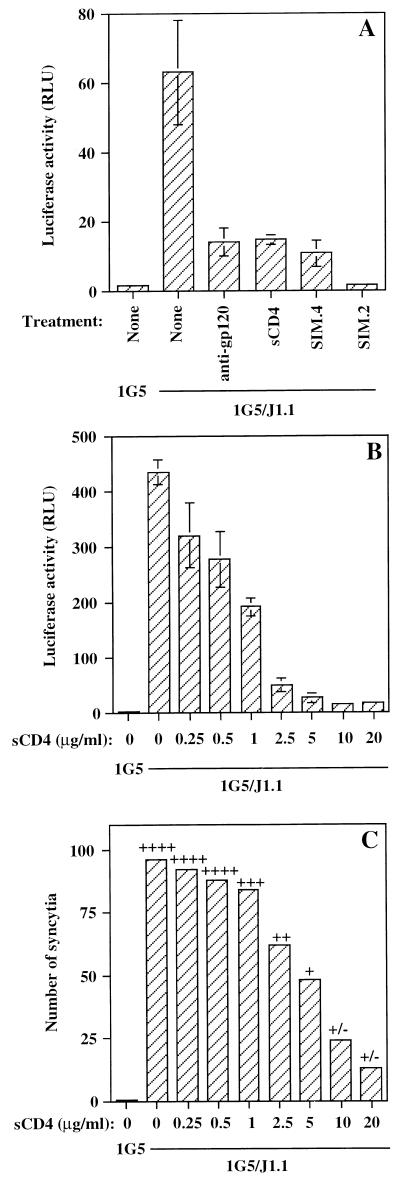
Next, the necessity of the gp120/CD4 interaction for this increase in HIV-1 LTR-dependent luciferase activity was analyzed by using a combination of 1G5 and J1.1 cells. Anti-gp120 neutralizing antibodies, as well as sCD4, were equally active as blocking agents of HIV-1 LTR-driven luciferase activity (Fig. 4A). Moreover, the anti-CD4 antibody SIM.4 was also very potent in blocking the luciferase signal, while SIM.2 resulted in an almost complete inhibition of luciferase activity. It should be noted that a dose-dependent inhibition of HIV-1 LTR-driven luciferase activity was seen with increasing concentrations of sCD4 (Fig. 4B). A final assessment of the reliability of this assay used for syncytium estimation by luciferase activity was performed by a direct comparison between the number of syncytia and luciferase activity when different concentrations of sCD4 were added along with the 1G5/J1.1 cell mix (Fig. 4B and C). A general trend could be observed between the gradual decrease in the number of syncytia at higher sCD4 concentrations and a concomitant diminution in HIV-1 LTR-dependent luciferase activity. In addition, qualitative assessment in terms of the size of syncytia showed a good correlation with luciferase activity. These latter results thus demonstrated that our luciferase-based assay represented an adequate tool for the measurement of HIV-1-mediated syncytium formation.
FIG. 4.
Increases in HIV-1 LTR-driven luciferase activity are blocked by typical inhibitors of syncytium formation and are correlated with syncytium count. (A) Equal numbers of 1G5 and J1.1 cells were incubated along with the following inhibitors: anti-gp120 (20 μg/ml), sCD4 (20 μg/ml), or anti-CD4 antibodies (clones SIM.4 or SIM.2 at 20 μg/ml). The samples were lysed after a 12-h incubation. (B and C) Different concentrations of sCD4 (0.25 to 20 μg/ml) were also added, and after 12 h of incubation, the samples were immediately lysed for monitoring luciferase activity (B) or visual assessment of syncytium number and syncytium size (arbitrarily evaluated from +/− to ++++) was carried out (C). The results are shown as the mean ± SD for samples studied in triplicate.
We also compared the induction of enzymatic activity by J1.1-dependent syncytium formation between the luciferase-based assay (the 1G5 cell line) and a β-galactosidase-based assay by using the cell line HeLa–CD4–LTR–β-gal. Although both assays led to a time-dependent increase in enzymatic activity following coincubation with the cell line J1.1, the 1G5 cell line demonstrated greater fold induction (131.3- and 874.2-fold at 12 and 20 h, respectively) than did the HeLa–CD4–LTR–β-gal cell line (3.8- and 15.7-fold at 12 and 20 h, respectively), suggesting a greater sensitivity of the 1G5 cell line to J1.1-induced syncytium formation.
The luciferase-based assay confirms that the conformational state of LFA-1 promotes HIV-1-mediated syncytium formation.
By using our luciferase-based quantitative syncytium assay, we next attempted to evaluate the increase in the formation of syncytia seen upon addition of NKI-L16 antibody to 1G5 cells. There are several reasons for using this specific cell line along with J1.1 for evaluating the effect of NKI-L16 on luciferase activity. First, these cell lines do not require any transfection before being used for testing. Second, our results indicated that this system was resulting in a high level of HIV-1 LTR-driven luciferase activity. Third, the J1.1 cell line, containing a latent HIV-1 proviral DNA molecule, should constitutively produce very small amounts of virions, and thus new rounds of viral infection should minimally contribute to the overall increase in luciferase activity seen after a 12-h coculture period.
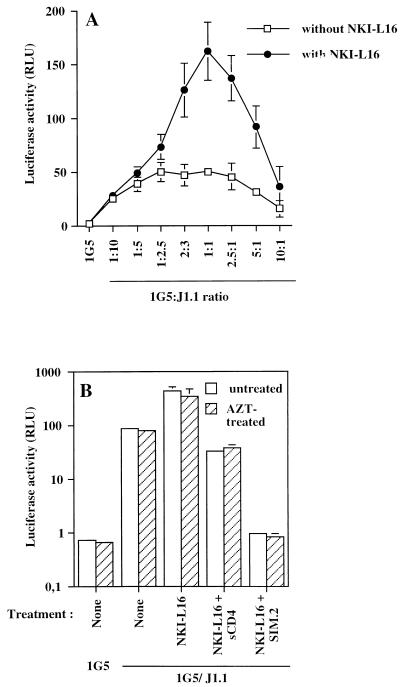
We initially used different ratios of 1G5 to J1.1 cells with or without NKI-L16 to seek the optimal conditions. As shown in Fig. 5A, under all the conditions used, luciferase counts showed greater elevations when NKI-L16 was added to the 1G5/J1.1 cell mixture, which is in accord with our previous data. However, the difference in luciferase activity between untreated and NKI-L16-treated cells achieved by the addition of J1.1 cells were strongest at around a 1:1 cell ratio, which represents the conditions used for the initial testing of NKI-L16-mediated increase in virus-induced syncytium formation (Fig. 1). The optimal time was also investigated, and the peak difference between NKI-L16-treated and untreated cells was found to occur at 12 h (data not shown). At these optimized parameters, the 1G5/J1.1 cell system was generally observed to give an NKI-L16-induced enhancement of luciferase activity ranging between three- and sevenfold. Although the luciferase-based syncytium quantitative assay leads to a certain degree of variation in terms of net luciferase activity, which most probably is a direct consequence of the stochastic nature of syncytium formation, overall induction by the NKI-L16 antibody was shown to be highly reproducible. The use of other HIV-1-infected T cells or uninfected transfected T cells did not give a similarly important difference in luciferase activity, although reporter gene activity was always found to be higher in NKI-L16-treated samples (data not shown). These observations might be reminiscent of the importance of the LFA-1/ICAM-1 interaction for HIV-1-mediated syncytium formation with low-gp120-expressing cell lines like J1.1 in comparison to cells expressing high levels of surface gp120, which do not require these secondary interactions as much.
FIG. 5.
NKI-L16-mediated increase in syncytium formation measured by luciferase activity is optimal at a cell ratio of 1:1 and shows no sensitivity to AZT. (A) 1G5 cells were preincubated or not preincubated with NKI-L16 (1 μg/ml) for 30 min at 37°C. Different cell ratios of 1G5 to J1.1 cells were then incubated in a final volume of 200 μl and lysed after 12 h of incubation. (B) 1G5 cells were pretreated or not pretreated for 90 min at 37°C with AZT (1 μM). Afterward, half of each sample was incubated with NKI-L16 (1 μg/ml) for 30 min. Equal numbers of J1.1 cells were then added with or without sCD4 (20 μg/ml) or anti-CD4 SIM.2 antibodies (20 μg/ml). All the samples were lysed 12 h postincubation. In panel B, luciferase activity is shown on a log10 scale. Luciferase activity was read with a Dynex luminometer apparatus. The results are shown as the mean ± SD for samples studied in triplicate.
We showed in earlier studies that virions, bearing host-derived ICAM-1 on their surface, can infect NKI-L16-treated cells in a more marked fashion (27). It was therefore important to demonstrate that the increase in luciferase activity was not due to low levels of virus production which could more easily infect NKI-L16-incubated 1G5 cells and, after integration, lead to an additive production of Tat proteins within the 12-h window frame. An experiment to investigate this possibility, in which 1G5 cells were first pretreated with AZT and then incubated for 12 h with J1.1 cells in the presence or absence of the NKI-L16 antibody, was thus performed. Clearly, no significant changes in luciferase counts were detected between untreated and AZT-treated samples with or without NKI-L16 addition (Fig. 5B). Blockers such as soluble CD4 or SIM.2 were found to reduce the luciferase activity of untreated and NKI-L16-treated 1G5 cells to a similar extent. These results clearly indicated that HIV-1 LTR-driven luciferase activity detect- ed 12 h after coculture of 1G5 and J1.1 cells was due to fusion events and not to infection by cell-free virions produced by J1.1 cells. Moreover, they suggested that an NKI-L16-mediated increase in HIV-1 LTR-dependent luciferase activity was attributable mostly, if not entirely, to virus-induced syncytium formation.
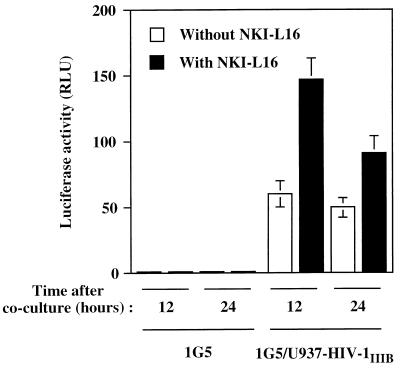
Finally, to determine whether this increase in luciferase activity could also be corroborated with HIV-1-infected monocytoid cells, U937 cells acutely infected with HIV-1IIIB were incubated with 1G5 cells in the presence or the absence of NKI-L16. Similarly to the 1G5/J1.1 cell mixture, 1G5/U937-HIV-1IIIB gave an induction of luciferase activity upon coculture, which was enhanced (2.4-fold) by the addition of NKI-L16 (Fig. 6). In addition, this NKI-L16-mediated increase in syncytium formation was found to be more pronounced after a 12-h incubation than after a 24-h incubation.
FIG. 6.
Enhancement of HIV-1-induced syncytium formation by the activated state of LFA-1 is also seen with monocytoid cells acutely infected with HIV-1. Untreated or NKI-L16-pretreated 1G5 cells were incubated for 12 or 24 h with an equal number of monocytoid U937 cells acutely infected with HIV-1. After lysis, the samples were measured for luciferase activity with a Dynex luminometer. The results are shown as the mean ± SD for samples studied in triplicate.
The chemokine receptor CXCR4 and NKI-L16-mediated increase in syncytium formation.
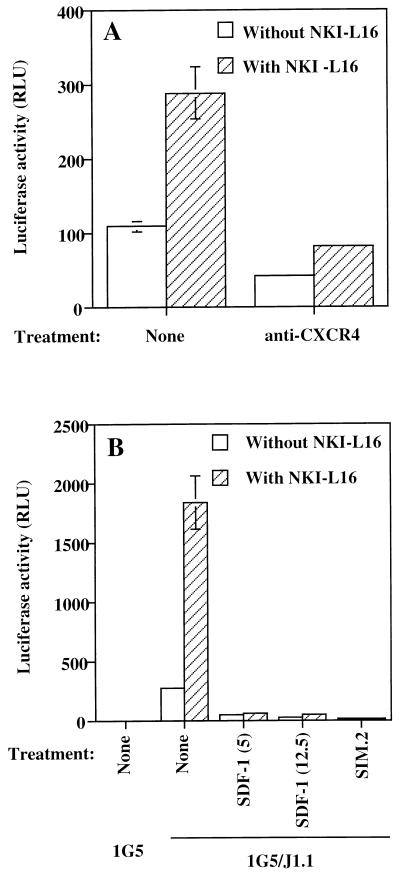
To further show the importance of virus-dependent syncytium formation in the HIV-1 LTR-driven luciferase increase induced by the addition of NKI-L16, antibodies against the chemokine receptor CXCR4 were added to the 1G5/J1.1 cell mixture. As shown in Fig. 7A, whether NKI-L16 was present or not, the addition of anti-CXCR4 antibody resulted in a net loss of luciferase activity. A more complete inhibition of HIV-1-mediated syncytium formation occurred when an NKI-L16-treated or untreated 1G5/J1.1 cell mixture was incubated along with SDF-1, the natural ligand of this chemokine receptor; the same was true when the antibody SIM.2 was used (Fig. 7B).
FIG. 7.
The NKI-L16-induced increase of virus-mediated syncytium formation measured as luciferase activity is sensitive to blocking agents of the chemokine receptor CXCR4. Untreated or NKI-L16-treated 1G5 cells were mixed with the J1.1 cell line, and either anti-CXCR4 antibodies (A) or different concentrations of SDF-1 (5 and 12.5 μg/ml) (B) were added. SIM.2 (20 μg/ml) was also added for a set of samples (B). The samples were lysed 12 h after the start of the coincubation, and luciferase activity was evaluated with a Dynex luminometer. The results are shown as the mean ± SD for samples studied in triplicate.
ICAM-1/LFA-1 and ICAM-2/LFA-1 interactions are essential for the NKI-L16-mediated increase in syncytium formation.
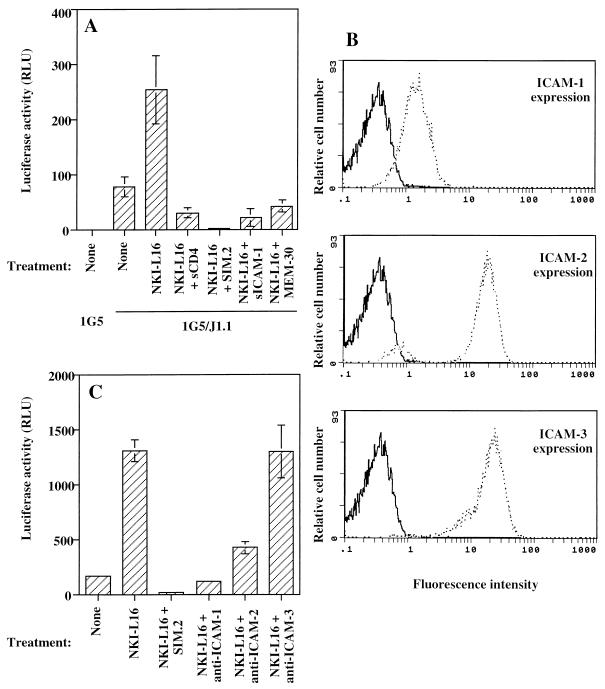
To demonstrate that the ICAM-1/LFA-1 interaction is an important element in NKI-L16-mediated enhancement of HIV-1-dependent syncytium formation, different agents blocking the physical interaction between ICAM-1 and LFA-1 were tested during a 12-h incubation period of 1G5 and J1.1 cells. As shown above, sCD4 and SIM.2 were still potent blockers of HIV-1 LTR-driven luciferase activity and thus of virus-dependent syncytium formation (Fig. 8A). More importantly, soluble ICAM-1 and anti-LFA-1 MEM30 antibody were shown to decrease luciferase activity to a similar extent in the NKI-L16-incubated cell mixture, which was corroborated by visual observation of syncytium formation (Fig. 8A and data not shown). We observed diminished luciferase activity at a level below that of cell mixtures in the absence of NKI-L16, suggesting that ICAM-1/LFA-1 interaction was also important in syncytium formation of 1G5 with J1.1 cells in the absence of NKI-L16.
FIG. 8.
ICAM-1 and ICAM-2 are the main ligands mediating the NKI-L16-induced increase in HIV-1-dependent syncytium formation. (A) Untreated or NKI-L16-pretreated 1G5 cells were incubated with an equal cell number of J1.1 cells in addition to sCD4 (20 μg/ml), anti-CD4 SIM.2 (20 μg/ml), sICAM-1 (2.5 μg/ml), or anti-LFA-1 MEM-30 (20 μg/ml) antibodies. (B) FACS analysis was performed on J1.1 cells by using anti-ICAM-1 (clone RR1/1.1.1), anti-ICAM-2 (clone 92C12F), or anti-ICAM-3 (clone ICR-3.1) antibody and a fluorescein-conjugated goat anti-mouse IgG. Results are shown as an overlay with respect to the isotype-matched antibody control. (C) Untreated or NKI-L16-pretreated 1G5 cells were incubated with an equal number of J1.1 cells in the presence of anti-ICAM-1 (clone RR1/1.1.1), anti-ICAM-2 (clone 92C12F), anti-ICAM-3 (clone ICR-3.1), or anti-CD4 antibodies (clone SIM.2) (all used at 20 μg/ml). Luciferase samples were lysed 12 h after the start of the coincubation. Luciferase activity was evaluated with a Dynex luminometer. The results are shown as the mean ± SD of three independent measurements.
Since the activation of LFA-1 by the NKI-L16 antibody has been shown to be selective in its increase in affinity toward different ICAM molecules (9), we next assessed the implication of the various ICAM molecules in the observed phenomenon. We first evaluated the expression of the different ICAM molecules on the surface of J1.1 cells. As depicted in Fig. 8B, FACS analysis revealed that ICAM-1, ICAM-2, and ICAM-3 are all expressed at relatively high levels on the surface of J1.1 cells and matched the previously described pattern for Jurkat cells (16). These antibodies were next incubated with the 1G5/J1.1 cell mixture for 12 h, and the luciferase activity was determined. Luciferase counts revealed that anti-ICAM-1 and anti-ICAM-2 antibodies were blocking NKI-L16-induced HIV-1 LTR-driven luciferase activity whereas anti-ICAM-3 (ICR3.1) antibodies had no discernible effect (Fig. 8C). Other domain 1-specific anti-ICAM-3 antibodies (ICR-1.1 and ICR-5.1) were also tested and were equally ineffective in blocking NKI-L16-induced syncytium formation (data not shown). These results demonstrated that the NKI-L16-mediated increase in HIV-1-dependent syncytium formation mostly required the ICAM-1 and ICAM-2 counterligands.
The NKI-L16-mediated increase in syncytium formation does not give more resistance against blocking agents.
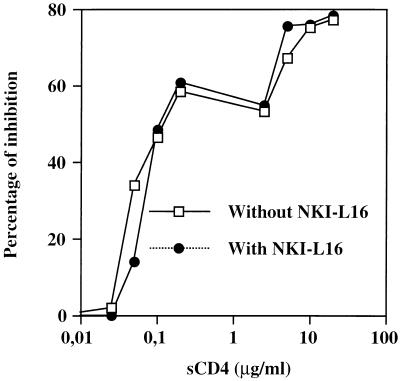
We next wondered if virus-mediated syncytium formation in the presence of NKI-L16 antibodies was more resistant to inhibitory agents. To investigate this, a 1G5-J1.1 cell mixture was incubated with different concentrations of sCD4 in the presence or absence of NKI-L16 (Fig. 9). The addition of sCD4 resulted in a net diminution of luciferase counts, which gradually returned to basal level. However, no net differences were discernible in terms of inhibition in the presence or the absence of NKI-L16. Similar results were obtained when increasing concentrations of plasma-pooled purified IgG isolated from HIV-1-infected subjects were used (data not shown). These results therefore demonstrate that the presence of LFA-1 with a high-affinity state for ICAM-1 did not render 1G5/J1.1 syncytium formation less sensitive to neutralization by sCD4 and human anti-HIV-1 antibodies.
FIG. 9.
The sensitivity of syncytium formation to sCD4 is the same in the presence or absence of NKI-L16. 1G5 cells were preincubated or not preincubated with NKI-L16 (1 μg/ml) for 30 min and then mixed with J1.1 cells in the presence of different concentrations of sCD4 (0.025 to 20 μg/ml) for 12 h. After lysis, the samples were measured for luciferase activity. The results are given as the percent inhibition by a given concentration of sCD4 with respect to the untreated samples and are representative of the mean of three different luciferase counts.
PBMCs are more prone to HIV-1-mediated syncytium formation upon addition of NKI-L16.
We were finally interested in seeing whether antibody-mediated LFA-1 activation could also increase the capacity for syncytium formation when PBMCs were used as partners in our fusion assay. In this set of experiments, visual counts were performed since the luciferase activity was too low upon electroporation of PBMCs with the pLTRX-LUC vector (data not shown). Qualitative evaluation after 24- and 48-h incubations between PBMCs and J1.1 indicated that very few syncytia were present in untreated samples as opposed to NKI-L16-treated samples, which demonstrated higher levels of syncytia paralleled with higher levels of aggregation (Fig. 10). Syncytium counts are presented in Table 1 and reflect the above observations. NKI-L16 treatment of PBMCs resulted in a near-fourfold increase in HIV-1-dependent syncytium number, which was greatly reduced in the presence of sCD4. It should be noted that SIM.2 treatment of the NKI-L16-treated cell mixture resulted in a complete loss of syncytium formation although aggregation was still observed. Our results thus indicated that PBMCs were more efficient in forming HIV-1-mediated syncytia when they expressed LFA-1 with a high-affinity state for ICAM-1 on their surface.
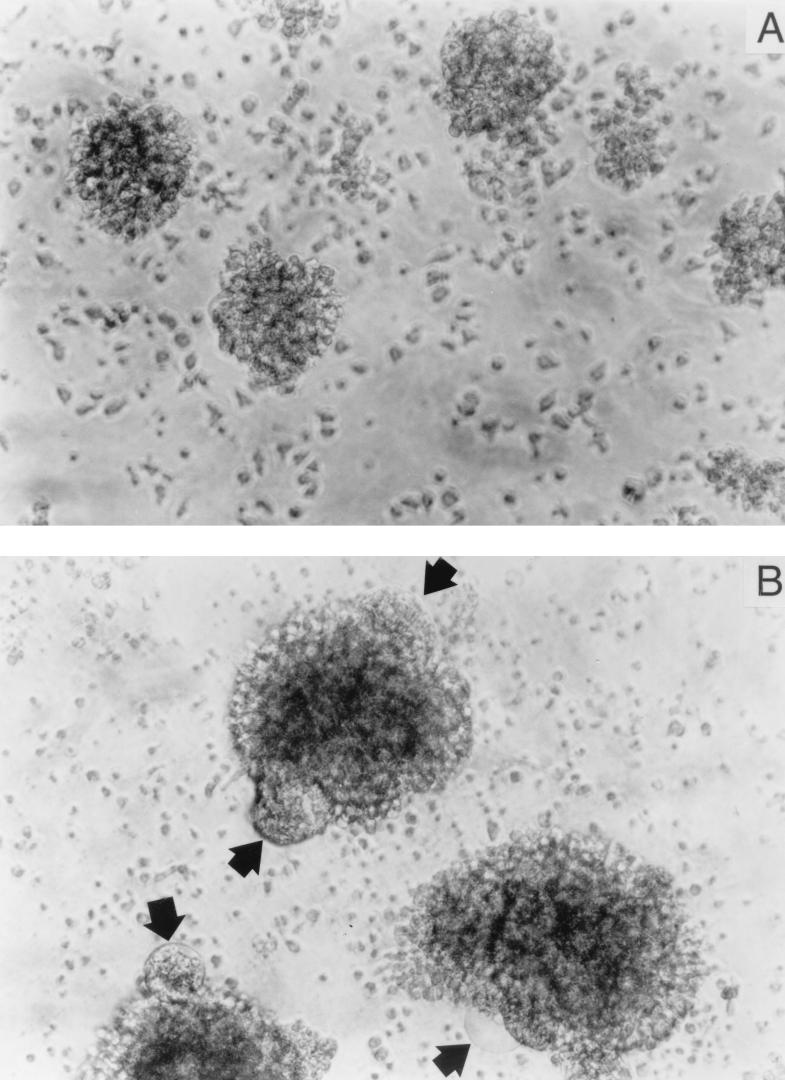
FIG. 10.
NKI-L16 also induces an enhancement of HIV-1-dependent syncytium formation in PBMCs. Ficoll-Hypaque-isolated PBMCs were incubated in the absence (A) or presence (B) of NKI-L16 for 30 min before the addition of an equal number of J1.1 cells. After 36 h of incubation, light microscopy observation was performed and photographs were taken at ×100 magnification. Arrows point to syncytia.
TABLE 1.
HIV-1-induced syncytium formation in PBMCs cocultured with J1.1 cells in the absence or presence of NKI-L16 antibodies
| Cells and conditionsa | No. of syncytia at time after cocultureb:
|
|
|---|---|---|
| 24 h | 48 h | |
| PBMCs/J1.1 | 12 ± 3 | 16 ± 5 |
| PBMCs/J1.1 + NKI-L16 | 33 ± 4 | 60 ± 5 |
| PBMCs/J1.1 + NKI-L16 + sCD4 | 9 ± 5 | 31 ± 7 |
| PBMCs/J1.1 + NKI-L16 + SIM.2 | 0 | 0 |
Equal numbers of PBMCs and J1.1 cells were cocultured in the presence of IL-2.
Results shown are the mean number of syncytia ± SD for triplicate samples.
To further substantiate the LFA-1-dependent increase in syncytium formation in human primary cells, PBMCs acutely infected for 7 days with two different syncytium-inducing HIV-1 primary isolates (92UG029 and 92UG021) were incubated with NKI-L16-pretreated or untreated 1G5 cells. As indicated in Fig. 11, for both clinical isolates, treatment with NKI-L16 led to higher luciferase counts. The higher luciferase values for the 92UG021-infected PBMCs were indeed indicative of a greater level of syncytium formation in these PBMCs, probably because of the higher levels of replication of this isolate. These results demonstrated the higher susceptibility of virally infected PBMCs to syncytium formation with a cell partner bearing the activated LFA-1 form.
FIG. 11.
PBMCs infected with syncytium-inducing HIV-1 primary isolates are more prone to syncytium formation with NKI-L16-treated 1G5 cells. PBMCs infected for 7 days with two different syncytium-inducing HIV-1 primary isolates (92UG029 and 92UG021) were incubated with an equal number of 1G5 cells pretreated or not pretreated with NKI-L16. After 12 or 24 h of coculture, the cells were lysed and measured for luciferase activity with a Dynex luminometer. The results are shown as the mean ± SD of three independent measurements.
DISCUSSION
Interaction between LFA-1 and ICAM-1 has been previously described as important for the formation of HIV-1-induced cell-to-cell fusion. In the present work, we demonstrate for the first time that the expression of LFA-1 with a high-affinity state for ICAM-1 results in a marked enhancement of HIV-1-mediated merging of cellular membranes, also called syncytium formation.
We first set out to optimize a novel luciferase-based syncytium quantitative assay relying on transfer of the potent transactivating viral Tat protein from one latently or chronically HIV-1-infected cell partner to another CD4+ T-cell line transiently or stably transfected with an HIV-1 LTR-driven reporter gene construct. We found that this system was rapid, reproducible, and very sensitive. Furthermore, the system was not found to be cell type specific, because enhancement of HIV-1 LTR-dependent luciferase activity was seen despite the use of different uninfected CD4+ T cells and of different HIV-1-infected monocytoid or T lymphoid cells. We believe that the observed increase in luciferase activity is solely attributable to HIV-1-mediated syncytium formation, since the signal was abolished by several blocking agents such as anti-gp120 antibodies, anti-CXCR4 antibodies, sCD4, anti-CD4 antibodies, and SDF-1. Moreover, the observed increase in luciferase activity was not attributable to new viral infection events per se, since an inhibitory concentration of AZT did not reduce the luciferase activity of NKI-L16-treated samples, at least for the first 12 h of incubation. Finally, luciferase counts were found to increase along with the degree of syncytium formation in a time-dependent fashion.
This new luciferase assay for quantification of syncytia is thus very useful and simple. No transfection or infection is necessary, since mixing 1G5 cells stably carrying HIV-1 LTR-LUC constructs with latently HIV-1-infected J1.1 cells gave reproducible and accurate luciferase measurements. Our assay is less subjective and more precise than simple counting of syncytium numbers because, although there is a relationship between syncytium count and luciferase activity, a linear relation is also apparent when a qualitative assessment of the size of the syncytia is taken into account. In comparison to other previously described similar syncytium-quantifying assays, it also offers the advantage of its lymphoid T-cell setting. Therefore, it is a more representative system for syncytium formation than the T7-dependent syncytium assay or the recently published assay relying on a similar Tat-dependent transactivation of the HIV-1 LTR with the use of the secreted alkaline phosphatase, both of which are based on nonlymphoid T cells (2, 8, 24). Furthermore, we have found that our system is also more sensitive than the classical β-galactosidase MAGI assay (35) for the measurement of syncytium-dependent increase in enzymatic activity. Preliminary results with our system generated satisfying luciferase counts when 1G5 cells were incubated with high-syncytium-forming cell lines after no more than 4 h of coculture (data not shown). Hence, virally induced luciferase counts should minimally contribute to the total count and make the luciferase-based assay more specific to syncytium formation.
Using this system, we have looked at the role of the LFA-1 activation state in HIV-1-induced syncytium formation by measuring luciferase activity. It was logical to believe that conformational change of LFA-1 by the NKI-L16-activating antibody, which is known to increase the LFA-1/ICAM-1 interaction and thus homotypic aggregation, should accentuate virus-mediated syncytium formation. We found that, as expected, the use of NKI-L16 led to a marked enhancement of HIV-1 LTR-driven luciferase activity, which correlated with qualitative determinations of the extent of syncytia by light microscopy. Optimization allowed us to determine that a 1:1 cell ratio (1G5 to J1.1 cells) was most optimal for NKI-L16-mediated stimulation of luciferase activity. With these optimal parameters, it was also found that a similar induction of luciferase and syncytium formation could be observed when using premyelocytic and monocytic cell lines. These optimal parameters are similar to what has been found for most HIV-1-dependent syncytium assays.
The importance of the LFA-1/ICAM-1 interaction was demonstrated when using agents known to abrogate such an interaction (e.g., soluble ICAM-1 and anti-LFA-1 antibodies). These results thus indicate that expression of an activated LFA-1 on the cell surface of the uninfected cellular partner can lead to a significant increase in HIV-1-mediated syncytium formation. It has previously been demonstrated that NKI-L16-mediated change of LFA-1 affinity was directed primarily toward ICAM-1 and ICAM-2 (9). The results of our analyses are in accord with these observations, since we determined that the NKI-L16-induced increase in virally mediated syncytium formation was highly dependent on both ICAM-1 and ICAM-2 but was ICAM-3 independent.
Further analyzes revealed that there was no difference in neutralization of syncytium formation when using either polyclonal human anti-HIV-1 antibodies or sCD4 for cells treated or not treated with NKI-L16. This is in contrast to a previous report showing that inhibitory agents were less efficient in blocking LFA-1/ICAM-1-enhanced syncytium formation than in blocking their LFA-1/ICAM-1 free counterpart (6), an observation which is reminiscent of the demonstrated importance of the ICAM-1 incorporated into HIV-1 virions in terms of their neutralization sensitivity (28, 51). However, in our case, ICAM-1/LFA-1 interaction is taking place both in untreated and NKI-L16-treated cells.
More importantly, PBMCs were found to be increased in the number of syncytia, which was also concomitant with higher cellular aggregation. These observations were taken from coculture experiments with either NKI-L16-treated PBMCs and J1.1 cells or NKI-L16-treated 1G5 cells and PBMCs acutely infected with syncytium-inducing primary isolates of HIV-1. The results thus suggest that HIV-1-mediated syncytium formation in vivo might not only depend on a simple LFA-1/ICAM-1 interaction but also would depend on the state of activation of the LFA-1 molecule.
In light of these results, we are postulating that the probability of encountering HIV-1-mediated syncytium formation events is greater in the constantly activated environment prevailing in secondary lymphoid organs. This assumption is based on the fact that T-cell activation, which takes place primarily in these anatomic sites (48), is subject to greater ICAM-1 binding. In the presence of nonproductively infected T cells (and thus of low levels of surface gp120), which account for most latently infected T cells harboring HIV-1 DNA, syncytium formation might be greatly enhanced. The observed aggregation which results from NKI-L16-induced LFA-1 activation might, in that same sense, induce larger syncytia, which should render them more fragile and more quickly destroyed by the immune system. It is of interest that the architectural destruction of lymph nodes represents one of the anatomic features of this retroviral disease in which HIV-1-mediated syncytium formation could be responsible for anatomical damages seen in such organs. Moreover, the observation that multinucleated giant cells in HIV-1-infected persons have been detected only in lymphoid organs (29) could further suggest a potential role for the conformational state of LFA-1 in virally mediated syncytium formation. In fact, Allen et al. (3) have demonstrated a reduction of the HIV-1 burden in HIV-1-infected patients treated by infusions of anti-LFA-1 antibodies.
In summary, using a novel luciferase-based quantitative assay, we have described a new mode by which an increase in HIV-1-dependent syncytium formation can be achieved by changing the conformational state of LFA-1 for its counterreceptor, ICAM-1. Our results imply that such a phenomenon might be observable in lymph nodes in vivo. This approach should shed light on syncytium-dependent cytopathic effects associated with HIV-1.
ACKNOWLEDGMENTS
We thank M. Dufour for technical assistance in flow cytometry studies and Sachiko Sato and Gilles Robichaud for critical review of the manuscript.
This work was supported by grants to M.J.T. from the Medical Research Council of Canada (MRC) (grant MT-14438 and GR-14500) and the Canadian Foundation for AIDS Research. M.J.T. is supported by a Scholarship award from the Fonds de la Recherche en Santé du Québec. B.B. is the recipient of an institutional Postdoctoral Fellowship from the Centre de Recherche du Pavillon CHUL. J.-F.F. is supported by an MRC Doctoral Fellowship.
REFERENCES
- 1.Aguilar-Cordova E, Chinen J, Donehower L, Lewis D E, Belmont J W. A sensitive reporter cell line for HIV-1 tat activity, HIV-1 inhibitors, and T cell activation effects. AIDS Res Hum Retroviruses. 1994;10:295–301. doi: 10.1089/aid.1994.10.295. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedey P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1959. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Allen A D, Hart D N, Hechinger M K, Slattery M J, Chesson C V, Vidikan P. Leukocyte adhesion molecules as a cofactor in AIDS: basic science and pilot study. Med Hypotheses. 1995;45:164–168. doi: 10.1016/0306-9877(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 4.Barbeau B, Bernier R, Dumais N, Briand G, Olivier M, Faure R, Posner B I, Tremblay M. Activation of HIV-1 LTR transcription and virus replication via NF-κB-dependent and -independent pathways by potent phosphotyrosine phosphatase inhibitors, the peroxovanadium compounds. J Biol Chem. 1997;272:12968–12977. doi: 10.1074/jbc.272.20.12968. [DOI] [PubMed] [Google Scholar]
- 5.Bazil V, Stefanova I, Hilgert I, Kristofova H, Vanek S, Horejsi V. Monoclonal antibodies against leukocytes antigens IV. Antibodies against the LFA-1 (CD11a/CD18) leukocyte-adhesion glycoprotein. Folia Biol. 1990;36:41–50. [PubMed] [Google Scholar]
- 6.Berman P W, Nakamura G R. Adhesion mediated by intercellular adhesion molecule 1 attenuates the potency of antibodies that block HIV-1 gp160-dependent syncytium formation. AIDS Res Hum Retroviruses. 1994;10:585–593. doi: 10.1089/aid.1994.10.585. [DOI] [PubMed] [Google Scholar]
- 7.Bernier R, Tremblay M. Homologous interference resulting from the presence of defective particles of human immunodeficiency virus type 1. J Virol. 1995;69:291–300. doi: 10.1128/jvi.69.1.291-300.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bieniasz P D, Fridell R A, Aramori I, Ferguson S S G, Caron M G, Cullen B R. HIV-1-induced cell fusion is mediated by multiple regions within both the viral envelope and the CCR-5 co-receptor. EMBO J. 1997;16:2599–2609. doi: 10.1093/emboj/16.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binnerts M E, van Kooyk Y, Simmons D L, Figdor C G. Distinct binding of T lymphocytes to ICAM-1, -2, or -3 upon activation of LFA-1. Eur J Immunol. 1994;24:2155–2160. doi: 10.1002/eji.1830240933. [DOI] [PubMed] [Google Scholar]
- 10.Briand G, Barbeau B, Corbeil J, Tremblay M. Enhancement of HIV-1-induced syncytium formation in T cells by the tyrosyl kinase p56lck. Virology. 1997;231:10–19. doi: 10.1006/viro.1997.8518. [DOI] [PubMed] [Google Scholar]
- 11.Butera S T, Perez V L, Wu B-Y, Nabel G J, Folks T M. Oscillation of the human immunodeficiency virus surface receptor is regulated by the state of viral activation in a CD4+ cell model of chronic infection. J Virol. 1991;65:4645–4653. doi: 10.1128/jvi.65.9.4645-4653.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butini L, De Fougerolles A R, Vaccarezza M, Cohen D I, Montroni M, Springer T A, Pantaleo G, Fauci A S. Intercellular adhesion molecules (ICAM)-1 ICAM-2 and ICAM-3 function as counter-receptors for lymphocyte function-associated molecule 1 in human immunodeficiency virus-mediated syncytia formation. Eur J Immunol. 1994;24:2191–2195. doi: 10.1002/eji.1830240939. [DOI] [PubMed] [Google Scholar]
- 13.Cantin R, Fortin J-F, Lamontagne G, Tremblay M. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J Virol. 1997;71:1922–1930. doi: 10.1128/jvi.71.3.1922-1930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caputo A, Sodroski J G, Haseltine W A. Constitutive expression of HIV-1 Tat protein in human Jurkat T cells using a BK virus vector. J Acquired Immune Defic Syndr. 1990;3:372–379. [PubMed] [Google Scholar]
- 15.Dalgleish A G, Beverly P C L, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4(T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 16.de Fougerolles A R, Springer T A. Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J Exp Med. 1992;175:185–190. doi: 10.1084/jem.175.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Fougerolles A R, Stacker S A, Schwarting R, Springer T A. Characterization of ICAM-2 and evidence for a third counter-receptor for LFA-1. J Exp Med. 1991;174:253–267. doi: 10.1084/jem.174.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Mark Hill C, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 19.Diamond M S, Springer T A. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994;4:506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 20.Donaldson Y K, Bell J E, Holmes E C, Hugues E S, Brown H K, Simmonds P. In vivo distribution and cytopathology of variants of human immunodeficiency virus type 1 showing restricted sequence variability in the V3 loop. J Virol. 1994;68:5991–6005. doi: 10.1128/jvi.68.9.5991-6005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 22.Dransfield I, Cabanas C, Craig A, Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dustin M L, Springer T A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 24.Feng Y, Broder C C, Kennedey P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 25.Figdor C G, van Kooyk Y, Keizer G D. On the mode of action of LFA-1. Immunol Today. 1990;11:277–280. doi: 10.1016/0167-5699(90)90112-m. [DOI] [PubMed] [Google Scholar]
- 26.Fortin J-F, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhances viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortin J-F, Cantin R, Tremblay M. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J Virol. 1998;72:2105–2112. doi: 10.1128/jvi.72.3.2105-2112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fortin, J.-F., R. Cantin, M. G. Bergeron, and M. J. Tremblay. Unpublished data.
- 29.Frankel S S, Tenner-Racz K, Wenig B M, Hansen C H, Heffner D, Nelson A M, Pope M, Steinman R M. Active replication of HIV-1 at the lymphoepithelial surface of the tonsil. Am J Pathol. 1997;151:89–96. [PMC free article] [PubMed] [Google Scholar]
- 30.Gottlinger H E, Sodroski J G, Haseltine W A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of HIV-1. Proc Natl Acad Sci USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gruber M F, Webb D S A, Gerrard T L, Mostowski H S, Vujcic L, Golding H. Re-evaluation of the involvement of the adhesion molecules ICAM/LFA-1 in syncytia formation of HIV-infected subclones of a CEM T-cell leukemic line. AIDS Res Hum Retroviruses. 1991;7:45–53. doi: 10.1089/aid.1991.7.45. [DOI] [PubMed] [Google Scholar]
- 32.Hedman H, Lundgren E. Regulation of LFA-1 activity in human B cells. J Immunol. 1992;149:2295–2299. [PubMed] [Google Scholar]
- 33.Hildreth J E K, Orentas R J. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989;244:1075–1078. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- 34.Keizer G D, Visser W, Vliem M, Figdor C C. A monoclonal antibody (NKI-L16) directed against a unique epitope on the α-chain of human leukocyte function-associated antigen 1 induces homotypic cell-cell interactions. J Immunol. 1988;140:1393–1400. [PubMed] [Google Scholar]
- 35.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahm H W, Stein S. Characterization of recombinant human Il-2 with micromethods. J Chromatogr. 1985;326:357–361. doi: 10.1016/s0021-9673(01)87461-6. [DOI] [PubMed] [Google Scholar]
- 37.Landis C R, Bennett R I, Hogg N. A novel LFA-1 activation epitope maps to the I domain. J Cell Biol. 1993;120:1519–1527. doi: 10.1083/jcb.120.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Mak J, Arts E J, Gu Z, Kleiman L, Wainberg M A, Parniak M A. Effects of alterations of primer-binding site sequences on human immunodeficiency virus type 1 replication. J Virol. 1994;68:6198–6206. doi: 10.1128/jvi.68.10.6198-6206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lifson J D, Feinberg M B, Reyes G R, Rabin L, Banapour B, Chakrabarti S, Moss B, Wong-Staal F, Steimer K S, Engleman E G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986;323:725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- 40.Lifson J D, Reyes G R, McGrath M S, Stein B S, Engleman E G. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science. 1986;232:1123–1127. doi: 10.1126/science.3010463. [DOI] [PubMed] [Google Scholar]
- 41.Lub M, van Kooyk Y, Figdor C G. Ins and out of LFA-1. Immunol Today. 1995;16:479–483. doi: 10.1016/0167-5699(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 42.Lusso P, Cocchi F, Balotta C, Markham P D, Louie A, Farci P, Pal R, Gallo R C, Reitz M S., Jr Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1. J Virol. 1995;69:3712–3720. doi: 10.1128/jvi.69.6.3712-3720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mann D L, O’Brien S J, Gilbert D A, Reid Y, Popovic M, Read-Connole E, Gallo R C, Gazdar A F. Origin of the HIV-susceptible human CD4+ cell line H9. AIDS Res Hum Retroviruses. 1989;5:253–255. doi: 10.1089/aid.1989.5.253. [DOI] [PubMed] [Google Scholar]
- 44.McCallus D E, Ugen K E, Sato A I, Williams W V, Weiner D B. Construction of a recombinant bacterial human CD4 expression system producing a bioactive CD4 molecule. Viral Immunol. 1992;5:163–172. doi: 10.1089/vim.1992.5.163. [DOI] [PubMed] [Google Scholar]
- 45.McDougal J S, Kenneky M S, Sligh J M, Cort S P, Mawle A, Nicholson J K A. Binding of HTLV-III/LAV to T4+ cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986;231:382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- 46.Moir S, Poulin L. Expression of HIV env gene in a human T cell line for a rapid and quantifiable cell fusion assay. AIDS Res Hum Retroviruses. 1996;12:811–820. doi: 10.1089/aid.1996.12.811. [DOI] [PubMed] [Google Scholar]
- 47.Pantaleo G, Butini L, Graziosi C, Poli G, Schnittman S M, Greenhouse J J, Gallin J I, Fauci A S. Human immunodeficiency virus (HIV) infection in CD4+ T lymphocytes genetically deficient in LFA-1: LFA-1 is required for HIV-mediated cell fusion but not for viral transmission. J Exp Med. 1991;173:511–514. doi: 10.1084/jem.173.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parrot D M, Wilkinson P C. Lymphocyte locomotion and migration. Prog Allergy. 1981;28:193–284. [PubMed] [Google Scholar]
- 49.Perez V L, Rowe T, Justement J S, Butera S T, June C H, Folks T M. An HIV-1-infected T cell clone defective in IL-2 production and Ca2+ mobilization after CD3 stimulation. J Immunol. 1991;147:3145–3148. [PubMed] [Google Scholar]
- 50.Petito C K, Navia B A, Cho E-S, Jordan B D, George D C, Price R W. Vacuolar myelopathy pathologically resembling subacute combined degeneration in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1985;312:874–879. doi: 10.1056/NEJM198504043121402. [DOI] [PubMed] [Google Scholar]
- 51.Rizzuto C D, Sodroski J G. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J Virol. 1997;71:4847–4851. doi: 10.1128/jvi.71.6.4847-4851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rothlein R, Dustin M L, Marlin S D, Springer T A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137:1270–1274. [PubMed] [Google Scholar]
- 53.Schwartz O, Virelizier J-L, Montagnier L, Hazan U. A microtransfection method using the luciferase-encoding reporter gene for the assay of human immunodeficiency virus LTR promoter activity. Gene. 1990;88:197–205. doi: 10.1016/0378-1119(90)90032-m. [DOI] [PubMed] [Google Scholar]
- 54.Smith S, Shatsky D, Cohen M P S, Warnke R, Link M P, Glader B E. Monoclonal antibody and enzymatic profiles of human malignant T lymphoid cells and derived cell lines. Cancer Res. 1984;44:5657–5660. [PubMed] [Google Scholar]
- 55.Sodroski J, Goh W C, Rosen C, Campbell K, Haseltine W A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. Nature. 1986;322:470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- 56.Springer T A. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 57.Starcich B, Ratner L, Josephs S F, Okamoto T, Gallo R C, Wong-Staal F. Characterization of long terminal repeat sequences of HTLV-III. Science. 1985;227:538–540. doi: 10.1126/science.2981438. [DOI] [PubMed] [Google Scholar]
- 58.Staunton D E, Dustin M L, Springer T A. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989;339:61–64. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- 59.Valentin A, Lundin K, Pararroyo M, Asjö B. The leukocyte adhesion glycoprotein CD18 participates in HIV-1-induced syncytia formation in monocytoid and T cells. J Immunol. 1990;144:934–937. [PubMed] [Google Scholar]
- 60.Weiss A, Imboden J, Shoback D, Strobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci USA. 1984;81:4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willard-Gallo K E, Van de Keere F, Kettmann R. A specific defect in CD3 gamma-chain gene transcription results in loss of T-cell receptor/CD3 expression late after human immunodeficiency virus infection of a CD4+ T-cell line. Proc Natl Acad Sci USA. 1990;87:6713–6717. doi: 10.1073/pnas.87.17.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]