Abstract

With the increasing oil demand, more attention has been paid to enhancing oil recovery in old oil fields. CO2 flooding is popular due to its high oil displacement efficiency and ability to reduce greenhouse gas emissions. Laboratory experiments and on-site application cases have shown that the minimum miscibility pressure has a greater impact on CO2 flooding than other factors. If the reservoir pressure is below the minimum miscible pressure, then there is CO2 immiscible flooding. Both theoretical analysis and experimental results show that the recovery rate of CO2 miscible flooding is 2–5 times higher than that of immiscible flooding. If the reservoir pressure is increased by water flooding before CO2 injection, it is easily limited by the physical property parameters. Therefore, accurately determining and effectively reducing the minimum mixing pressure has become the focus of research. Currently, there are two types of methods for determining the minimum miscible pressure: experimental and theoretical methods. The experimental method is generally considered more accurate, including the slim tube test, rising bubble apparatus, and vanishing interfacial tension, etc. However, it is worth noting that the minimum miscibility pressure is dynamically changing, and there will be high economic costs if measured repeatedly through experimental methods during reservoir development. Therefore, it is recognized that the minimum mixing pressure can be determined at any time using theoretical calculation of initial data, which will reduce economic and time costs to a high degree. In this paper, the theoretical calculation method is divided into empirical correlation, state equation, and artificial intelligence algorithm. The techniques for reducing the minimum miscibility pressure can be classified into two categories: miscible solvents and surfactant methods. The miscible solvent method can be further divided into monocomponent and polycomponent methods. This paper compares the advantages and disadvantages of the existing techniques for measuring and reducing MMP and selects the best method.
1. Introduction
The process of petroleum production is complex. Oil field development has traditionally been divided into three stages. Primary recovery refers to relying only on the reservoir’s own pressure for displacement. Secondary recovery usually involves the injection of water to maintain the reservoir pressure. Tertiary recovery is that developing remaining oil using various enhanced oil recovery (EOR) technologies such as heavy oil thermal recovery and chemical and gas injection based on secondary recovery to further increase oil production. The rational application of EOR technology could enable the efficient utilization of old oil fields. With the development of industry, a large amount of CO2 emission has led to global warming, which has created a serious problem with how to deal with greenhouse gases. To address this issue, the CO2 EOR technology, which has two distinguished advantages, has been noticed by scholars. On the one hand, the application of CO2 flooding can store CO2 in the reservoir and reduce CO2 emissions, playing a significant role in environmental protection. On the other hand, except for low cost, it has great potential to improve oil recovery for CO2 flooding, which can bring significant economic benefits.1−4
Although the prospect of the application of CO2 flooding is promising, it should be considered that CO2 flooding is usually divided into two categories: CO2 miscible flooding and CO2 immiscible flooding. The oil displacement efficiency was directly influenced by the degree of miscibility.
Both theoretical analysis and experimental results have indicated that the recovery rate of CO2 miscible flooding is 2–5 times higher than that of immiscible flooding. Given this, the CO2-EOR is mainly driven by miscible displacement in the United States and other countries.5 The project of CO2 flooding application mainly depends on accurately determining and reducing the minimum miscible pressure (MMP).6 It is necessary to have a comprehensive understanding of MMP because it is a crucial parameter for selecting reservoirs as well as for selecting models for simulating or predicting reservoir performance in CO2 flooding. The definition of MMP varies among scholars. Some define it as the optimal pressure required to inject gas into hydrocarbon fluids to achieve first contact miscibility,7 while others define it as the minimum pressure required to achieve miscibility of oil and injected gas.8 In general, MMP is determined by crude oil composition and reservoir conditions.9 Through a large number of investigations, we found that the type of crude oil can affect the level of MMP. However, due to the different classification criteria, there are many evaluation criteria for crude oil types. In this paper, the effect of the crude oil type on MMP is analyzed from the perspective of crude oil composition. Crude oil composition can be divided into: volatile components, C1; intermediate components, C2–C6; and heavy components, C7+. The volatile components and heavy components were positively correlated with MMP, and intermediate components were negatively correlated with MMP. As for the importance of MMP, it is mainly reflected in two aspects. One is that the determination of the injection method depends on MMP.10 The other is that the part oil displacement efficiency of CO2 gas injection highly depends on MMP.11 CO2 miscible flooding can only be achieved when the pressure is higher than MMP, thereby enhancing oil recovery.12,13 If the injection pressure is lower than MMP, miscible flooding will not occur, and only immiscible flooding can be achieved.14 In conclusion, as one of the key parameters affecting gas injection for improving oil recovery, MMP has great significance for reservoir exploitation.15
First, this article reviews the measurement methods for MMP, which consist of experimental and theoretical calculation methods. Second, the technology of reducing the minimum miscible pressure is illustrated, which mainly includes the miscible solvent and surfactant methods. Finally, the advantages and disadvantages of each method are discussed, and the corresponding suggestions are given for accurately determining and efficiently reducing MMP.
2. MMP Determining Methods
Injecting gas at a pressure lower than the MMP can lead to immiscible displacement, resulting in a decrease in oil recovery. Conversely, injection under an excessively high pressure can increase process costs and pose healthy risks. The process of miscible injection could be made more effective, economical, and feasible by accurately estimating the MMP.16 Currently, there are mainly two methods for measuring MMP, including experimental and theoretical calculation methods.
2.1. MMP Laboratory Methods
The experimental method is generally considered the most accurate and reliable method.17 Under laboratory conditions, methods for determining MMP include slim tube test (STT), rising bubble apparatus (RBA), vanishing interfacial tension (VIT), X-ray computerized tomography (CT), sonic response method (SRM), magnetic resonance imaging (MRI), fast fluorescence-based microfluidic (FFBM), oil droplet volume measurement (ODVM), pressure–composition diagrams (PCDs), vapor density method, and expansion extraction method (VDM), etc.18–20
2.1.1. Slim Tube Test (STT)
The STT was first proposed by Stalkup in 1983,21 which currently has become the most commonly used method in the laboratory for MMP. It aims to study the effect of phase on displacement efficiency by eliminating reservoir heterogeneity, gravity overlap, and viscous fingering.22
The experimental device is shown in Figure 1. The main operational process is as follows: First, the crude oil is injected into a spiral slim tube until it becomes saturated, and the temperature is adjusted to match that of the reservoir. Next, the back pressure regulator is adjusted at the outlet of the slim tube to maintain a fixed pressure. Finally, gas is gradually injected to displace the crude oil. Please refer to reference (23) for a detailed illustration of the process. It is important to clean the slim tube before each test. If asphaltene precipitates during the experiment, then cleaning may be particularly challenging. Furthermore, STT involves many operating steps, but the MMP predicted by STT is the most reliable.
Figure 1.
Schematic diagram of the STT.
Ahmad et al.24 tested the uniqueness and repeatability of MMP determination in three different samples of Oman crude oil (crude oil compositions of L-721, MZE, and N are shown in Table 1) using three different coil lengths of equal diameter and three different injection speeds, repeating the process 55 times.
Table 1. Crude Oil Samples Composition.
| Component | Sample L-721 | Sample MZE | Sample N |
|---|---|---|---|
| N2 | 0.22 | 0.89 | 0.54 |
| CO2 | 0.29 | 0.21 | 0.30 |
| H2S | 0.00 | 0.00 | 0.01 |
| C1 | 28.51 | 34.91 | 18.66 |
| C2 | 5.77 | 3.69 | 4.64 |
| C3 | 5.42 | 2.45 | 4.79 |
| i-C4 | 2.09 | 1.04 | 1.56 |
| n-C4 | 4.03 | 1.85 | 3.39 |
| i-C5 | 2.80 | 1.25 | 1.66 |
| n-C5 | 2.87 | 1.41 | 2.06 |
| C6 | 5.40 | 3.08 | 5.10 |
| C7+ | 42.60 | 49.22 | 57.29 |
| Total | 100.00 | 100.00 | 100.00 |
Figure 2 shows the schematic diagram of the STT device. Ahmad et al.24 made several improvements to the STT device. To ensure constant measurement of permeability and porosity, they tightly wrapped 1/4’ OD and 100 mesh sand particles by three different lengths of 12, 18, and 24 m spiral tubes. The liquid storage tank and gas storage tank are placed in an oven outside the slim tube to maintain the required temperature of the reservoir. Additionally, a back pressure regulator (BPR) is added at the outlet of the tube to control pressure reduction. A check valve is installed at the outlet to prevent crude oil and injected gas from returning to the liquid storage tank (CV-01) and the gas storage tank (CV-02).
Figure 2.

Modified schematic diagram of STT. Reprinted with permission from ref (24). Copyright 2016 Elsevier.
Ahmad et al.24 found that the error of MMP predicted by the improved STT was approximately 2% through collecting 55 sets of experimental data measured by STT. This indicates that the MMP measured by STT is more accurate. Zhang et al.25 analyzed the MMP measured by STT and discovered a linear relationship between MMP and reservoir temperature. With every 10 °F increase in temperature, MMP increased by approximately 130 psi. Moreover, Ahmad et al.24 found that oil displacement performance and recovery increased with longer coil length when using the same crude oil samples. Further studies have shown that a coil length of 40 feet can achieve the best result.20
Scholars have studied the effect of injection rate on MMP and found that viscous fingering can be promoted by increasing the gas injection rate.20 Furthermore, the displacement speed is greater with a smaller diameter of the slim tube and smaller porosity, resulting in a smaller MMP. Although the porosity and permeability of the samples in STT may not precisely reflect the actual reservoir conditions, the MMP obtained by STT can accurately reflect the actual situation of the measured oil and gas system. Therefore, it is important to consider the cost and time consumption of using STT. However, STT has some drawbacks, such as being expensive and time-consuming. Some STT experiments may take 2–6 weeks to estimate MMP.26
2.1.2. Rising Bubble Apparatus (RBA)
RBA was proposed in the 1980s as an alternative to STT. It requires less crude oil and CO2, which reduces costs and time.27,28 The schematic diagram of the RBA experimental apparatus is shown in Figure 3. The device features a flat glass tube that is vertically installed in a high-pressure sight in a temperature-controlled bath. The glass tube is flat to allow better observation of bubbles rising in opaque oil. To record the evolution of the rising bubble shape permanently, a videotape was installed on the rack parallel to the bubble path in the visual acuity chart. The device magnifies the view of the bubbles and records their ascent on the videotape for subsequent inspection. The following are the main steps of the operation procedure:
Figure 3.
Schematic of the RBA.
Initially, the sight gauge with the glass tube and the hollow needle was all filled with distilled and deionized water. Then, oil is injected downward into the flat glass tube, displacing the water. At the end of this step, the lower circular portion of the glass tube is filled with water, while the remainder of the tube contains oil. The pressure inside of the sight gauge can be adjusted to the desired level by the addition or removal of water. After the pressure is set, a bubble of gas is formed at the tip of the hollow needle in the water phase. When the buoyant force lifting the bubble exceeds the adhesive forces holding the bubble to the needle, the bubble rises through the water, through the water–oil interface, and up through the column of oil. The behavior of the rising bubble is recorded on video tape. After one or more bubbles have risen through the oil, the contaminated oil can be replaced with fresh oil. After completing the experiment, it was found that RBA required 1–2 h for each determination of MMP (excluding preparation time) for the gasification or condensation system, and the pressure at which the miscible flooding occurred could be visually observed. However, it takes 1–2 weeks for each measurement of MMP using the slim tube method. In addition, Elsharkawy et al.23 analyzed the relationship between MMP results measured by the device and the temperature, finding that MMP measured by RBA had a linear relationship with temperature. On average, for every 10 °F increase in temperature, MMP increased by about 150 psi.
The determination of MMP by RBA is rapid, but some scholars believe that there is a certain degree of subjectivity involved due to its reliance on naked-eye observation. Although RBA can provide quantitative information on composition change and displacement efficiency, its reliability needs further verification.29,30 In this regard, some scholars have proposed the Rapid Pressure Increase (RPI) method as an improvement to RBA. The main principle of the RPI method is to identify the MMP as the point corresponding to the change in the ratio of pressure increase to volume reduction after the initial pressure drop in the CO2-crude oil system. It is important to note that volume reduction refers to the decrease in the volume of CO2 dissolved in the crude oil when complete miscibility occurred. In comparison to RBA, RPI can achieve reasonable results quickly in the experimental process without disrupting the CO2-crude oil system18
To enhance the reliability of RBA determination of MMP, some scholars have proposed new qualitative and quantitative evidence. The qualitative basis is bubble breakup (BBU), while the quantitative basis includes the bubble rise height (BRH) and bubble rise velocity (BRV). This evidence has been verified by experiments, and it has been found that BBU has strong applicability. This is because BBU is relatively independent from experimental factors such as glass tube height and bubble size but sensitive to experimental conditions such as test pressure and gas composition. The BBU qualitative criterion is easier to apply than the existing four qualitative criteria, namely, bubble shape, size, color, and rising height.31
2.1.3. Vanishing Interfacial Tension (VIT)
MMP is determined experimentally by measuring the decrease of the CO2-oil interfacial tension (IFT) with increasing pressure and extrapolating the trend to zero.32 It can also be understood that when CO2 and crude oil reach complete miscible flooding the IFT between them will completely disappear, and the pressure at which the IFT becomes 0 is regarded as MMP. VIT can be used to determine the miscible conditions of various CO2-crude oil systems18 and to obtain MMP values from the measured IFT equilibrium data.33
The method is considered good due to its quantitative measurability, time-saving nature, simplicity, and low cost. The measurement of MMP in CO2 miscible flooding involves determining the IFT of a high-pressure system, which can be achieved by using the hanging drop method. This involves measuring the shape parameters of droplets suspended on the top of a capillary probe and applying the surfactant selection method proposed by Andreas et al.34 It is important to note that the instruments for testing IFT cannot measure the IFT when complete miscibility is reached, and the miscibility should not be observed only when the IFT is zero. Therefore, the extrapolation method is used to calculate the MMP when the interfacial tension is zero. To determine MMP, the VIT method can be used and is easy, rapid, and economical. However, it is important to note that this method can be greatly affected by subjective factors. Several studies have shown that the accuracy of MMP measured by VIT is low, with an overestimation of MMP.26 The composition of the mixture used by VIT also affects the accuracy of the measured MMP.35 Therefore, further demonstration of the accuracy is required. However, recent studies have indicated that the MMP measured by VIT are consistent with those measured by STT and RBA, with a difference of only 5% to 8%.32
VIT is capable of measuring IFT in high-temperature and -pressure conditions up to 10,000 psi and 300 °C. When measuring MMP in the 10,000 psi range, it is recommended to use a high-resolution charge-coupled device (CCD) camera to capture an image of the droplet. This image could be transmitted to the computer supported with drop shape analysis software based on the axisymmetric droplet shape analysis (ADSA) technique. The software fits a set of theoretical differential equations to the experimental droplet profile to evaluate its shape. A profile optimization algorithm is applied to calculate the IFT along with the drop volume. This method provides a more accurate calculation of IFT.36 If experimental conditions do not allow the use of VIT, alternative instruments can be used to determine IFT. For instance, VIT is suitable for determining MMP in the South Sumatra Basin oil field in Indonesia37 and the Oman oil field. The experimental device used to determine MMP in the Oman oil field is shown in Figure 4, and the specific experimental process is detailed in ref (23).
Figure 4.
Schematic diagram of the VIT.
The calculation of MMP by VIT reveals that MMP is less dependent on the crude oil composition. Additionally, the higher the molecular weight of the heavier components in crude oil, the higher the MMP value that can be obtained. Temperature significantly affects MMP, and there is a linear relationship between MMP and temperature.38,39
2.1.4. Other Determining Methods
2.1.4.1. X-ray Computerized Tomography (CT)
Under the influence of MMP, the oil–gas interface disappears as the pressure increases until it reaches MMP, at which point the interface completely vanishes and the two phases mix. The entire process can be captured by the micro-CT scanning system by increasing the pressure at any time. Therefore, CT technology (the experimental device is shown in Figure 5) can directly determine MMP. The device can adjust the temperature and measure MMP at the required temperature.40
Figure 5.

Schematic diagram of the CT. Reprinted with permission from ref (40). Copyright 2015 Springer Nature.
2.1.4.2. Sonic Response Method (SRM)
However, CT technology has higher capital and operating expenses as well as potential health risks. In comparison, SRM (as shown in Figure 6) avoids radiation exposure, reducing health risks, and provides accurate volume distribution of the two fluids and their interfaces during the experiment without invading the system. However, SRM is deficient since it can only determine miscibility based on the disappearance of the boundary between two fluids under increasing pressure at constant temperature. This method does not provide an accurate determination of the MMP.18
Figure 6.

Schematic diagram of the SRM.
2.1.4.3. Magnetic Resonance Imaging (MRI)
At a fixed temperature, MRI (Figure 7) can be used to obtain proton density images of n-decane at various pressures. The average image intensity of the decane phase is then plotted as a curve, which shows an exponential relationship with pressure. When CO2 and n-decane are mixed into a single phase, the average intensity of the mixed phase reaches zero, excluding background noise. Currently, the MMP value can be determined by identifying the intersection of the exponential curve and the zero-intensity line. However, due to the requirement of a high current supply for the experimental equipment, the cost of MRI is significant. In addition, the density of hydrocarbon fluids can also be measured by MRI intensity. For example, if the mechanism of condensed gas prevails as a result of the dissolution of gas in oil, the oil density decreases, thereby decreasing the intensity of MRI in the oil phase.41
Figure 7.
Schematic diagram of the MRI. Reprinted with permission from ref (41). Copyright 2016 Elsevier.
2.1.4.4. Fast Fluorescence Based Microfluidic (FFBM), Oil Droplet Volume Measurement (ODVM), and Pressure–Composition Diagrams (PCDs)
If FFBM (the experimental device shown in Figure 8) is used to qualitatively detect MMP, the measurement time is only about 30 min. This method has the advantage of measuring MMP at the nanoscale.19 The ODVM (the schematic diagram shown in Figure 9)42 is similar to the VIT. However, instead of measuring the IFT of oil and gas, the ODVM method measures the change in droplet volume with pressure and time. It is proposed based on the thermodynamics principle that the gas/oil miscibility process occurring at the minimum pressure condition is not an instantaneous process but takes a period of contact and mass transfer process between each other to achieve complete miscibility. At the MMP point, the oil droplet will gradually shrink and finally mix with the surrounding CO2 fluid. Taking advantage of the quick but accurate quantitative volume measurement technique in a state-of-the-art drop shape analyzer, the MMP of the studied two-phase system could therefore be determined as the pressure under which the pendent oil volume in the CO2 environment continuously decreases with a certain criteria speed.43 Therefore, ODVM can be considered a time-consuming method for determining MMP. The ODVM is employed to determine the MMPs of the CO2/oil system with water present under different temperature levels by Cui et al. The results show the water presence could obviously reduce the MMPs of the CO2/n-C16H34 system by up to 1.4 MPa.42
Figure 8.

Schematic diagram of the FFBM. Photograph courtesy of ref (19). Copyright 2024.
Figure 9.

Schematic diagram of the ODVM. 1: Oil sample; 2: hand wheel; 3: piston pump; 4: computer; 5: CCD camera; 6: pressure gauge; 7: test chamber; 8: capillary burette; 9: illumination source; 10: temperature control unit; 11: CO2 tank; 12: intermediate container; 13: hand wheel; 14: piston pump; 15: water supply for CO2 pressurization; 16: gas inlet; 17: gas outlet; 18: liquid outlet. Reprinted with permission from ref (42). Copyright 2023 Elsevier.
PCD (the schematic diagram shown in Figure 10) plots the phase state of the fluid in the visual high-pressure grid at the reservoir temperature by measuring the composition represented by the mole fraction of the injected gas. Miscibility occurs outside the phase line, while immiscibility occurs within the phase line. The pressure at which oil and gas fluids become completely miscible at reservoir temperature and a specific composition can be calculated using the pressure–composition diagram (PCD). However, PCD is seldom used due to its high cost, requirement for a large amount of fluid, and potential for experimental errors.19
Figure 10.
Typical PCD.
2.1.4.5. Vapor Density Method (VDM)45
The vapor density method is a dynamic experimental technique that can establish the relationship between the density of the injected gas phase and the pressure. Additionally, it can determine the MMP of gas and crude oil by utilizing their solubility characteristics. CO2 can be injected into a container containing experimental oil samples multiple times, and the vapor density can be measured after each injection cycle for a period of time. Equilibrium is reached when the pressure and vapor density of the system remain stable. The vapor density is then measured to create a vapor density curve based on the pressure and corresponding vapor density. The point of sudden change in the gas phase density on the curve is the MMP that requires measurement. This method is frequently used to test at low temperatures and then determine the MMP at high temperatures. The test time is short, taking only a few hours. While this approach saves time and reduces costs, its repeatability is not strong.
In this section, the characteristics of experimental methods for the determination of the MMP have been illustrated. When we need to use the experimental method to determine MMP, we should consider the actual situation of the reservoir and other factors such as the difficulty of operation, economic cost, and time cost. For example, as an international standard method, STT has a high accuracy in the determination of MMP, but it takes a long time and has high requirements for instruments. RBA spends less time, but the accuracy is easily affected by human factors. VIT can adapt to high temperatures and high pressures, but it is also susceptible to human factors. MRI can measure the density of hydrocarbon fluid, but the experimental cost is high. To provide a more concise introduction of the experimental methods, this paper gives a comparison table of the advantages and disadvantages of the commonly used experimental methods (see Table 2)
Table 2. Comparison of Characteristics of Several Commonly Used Methods for Determining MMP.
| Laboratory Methods | Advantages | Disadvantages |
|---|---|---|
| STT | International common determination method. More accurate. Repeatability. | Time consuming. Require higher precision of the instrument. |
| RBA | Rapid determination of MMP. Require lower precision of the instrument. | Seriously affected by human factors. When the temperature is lower than 40 °C, the error is large. Reliability needs to be verified. |
| VIT | Experimental time is short. Suitable for high-temperature and -pressure state. | Seriously affected by human factors. Application limitations. |
| CT | Adjust the temperature. Observe MMP intuitively. | High cost. Potential health risks. |
| SRM | Avoid radiation exposure. Reduce health risks. | MMP can be measured only when the constant temperature pressure is increased. |
| MRI | Measure density of hydrocarbon fluid. | Experimental equipment consumes too much electricity. High cost. |
| FFBM | Determination time is short. MMP can be determined at the nanometer scale. | There can be no human intervention. |
| VDM | Direct determination of the relationship between the density and the pressure of the injected gas. | Limitation is large. Only the MMP at low temperature can be measured. |
This section outlines the characteristics of experimental methods determining the MMP. When selecting a method, factors such as reservoir conditions, operational difficulty, economic cost, and time constraints should be taken into account. As an internationally recognized method, STT has high accuracy in determining MMP. Moreover, RBA experiments are short, but their accuracy is easily influenced by human factors. However, it is time-consuming and requires advanced instruments. VIT can adapt to high temperatures and pressures but is also susceptible to human factors. MRI can measure the density of hydrocarbon fluids, but the experimental cost is high. Finally, Table 2 compares the advantages and disadvantages of common experimental methods.
2.2. MMP Theoretical Calculation Methods
Experimental methods are generally considered accurate and reliable for determining the MMP, but it is important to note that the MMP is a dynamic process. Dividing the miscible region solely based on the MMP measured in the initial experiment is inaccurate.46 This is because the miscibility of the injected gas is largely dependent on the injection pressure and equipment limitations.47 Additionally, the experimental method is costly, time-consuming, and susceptible to human error. Therefore, determining the MMP through experimentation multiple times is currently challenging. To enable oil fields determination of MMP at any time, scholars have attempted to use theoretical calculation methods due to their cost-effectiveness, speed, and stability.48 The prediction of MMP through theoretical derivation can save time and money. The difference between the MMP predicted by the theoretical calculation method and the MMP measured by the experimental method is still within an acceptable range. The MMP of crude oil can be rough estimated by using the theoretical calculation method. Currently, the methods for estimating MMP through theoretical calculation include the empirical correlation, equation of state, and artificial intelligence algorithm.
2.2.1. Empirical Correlation (EC)
The simplest method to measure MMP among theoretical methods is to use EC calculation.49 This method can be easily used by other researchers.50 The accuracy of the ECs generally increases with the mathematical complexity of the equation.51 Three main factors affect MMP in CO2 flooding: temperature, crude oil composition, and injected gas composition. All empirical correlations are based on these three factors.52 However, each EC has a different focus, resulting in different applicable conditions.53 This work provides specific forms of some ECs.
2.2.1.1. Johnson and Pollin (J-P) Correlation54
| 1 |
For Pure CO2: I = 1.2762, α = 18.9, and β = 0.285. Pmm is the MMP, in MPa; T is reservoir temperature, in °C; Pcin is the critical pressure of injected gas, in MPa; Tcin is the critical temperature of injected gas, in °C; M is the average molecular weight of crude oil, in g/mol; Min is the molecular weight of the injected gas, in g/mol; and K, I is a constant (depends on reservoir physical properties).
2.2.1.2. Glaso Correlation55
The Glaso Correlation is proposed based on the work of Benham et al. It takes into account the effect of medium components on the MMP. Glaso discovered that when the molar percentage of the medium component exceeded 18% it had no effect on the MMP. Therefore, Glaso used the 18% molar percentage as the boundary for the medium component and proposed two relationships.
1. When the molar percentage of medium components in crude oil is less than 18%:
| 2 |
2. When the molar percentage of medium components in crude oil is greater than 18%:
| 3 |
MC7+ is the molecular weight of C7+ in degassed oil; FR is the molar content of C2–C6 in reservoir fluid; T is temperature, in °F; and Pmm is MMP, in psia.
2.2.1.3. Alston et al.56 Correlation
Alston et al. discovered that the reservoir temperature and C5+ components have a significant effect on the MMP for most reservoirs. The influence of volatile and intermediate components on MMP is not clear. Heavy components are better suited for establishing a correlation to calculate MMP. Therefore, they proposed an EC to predict pure CO2 and live oil MMP.
| 4 |
MC5+ is the molecular weight of fractions above pentane, in g/mol; nvol is the molar fraction of volatile components (C1 and N2) in crude oil; nmint is the molar fraction of the intermediate component (C2–C4, CO2, and H2S); and TR is reservoir temperature, in °F.
2.2.1.4. The Petroleum Recovery Institute (PRI) Correlation57
The Petroleum Recovery Institute gives two correlations for the prediction of the CO2 MMP.
The first EC PRII:
| 5 |
where R = 1.8T + 492.
The second EC PRIII:
| 6 |
where T is reservoir temperature.
2.2.1.5. Yelling and Metcalfe Correlation58
Yelling and Metcalfe proposed an EC for predicting the MMP of CO2-crude oil systems based on the reservoir temperature in 1998:
| 7 |
where T is reservoir temperature.
Due to the continuous improvement of relevant theories, the number of ECs has increased. However, different ECs possess distinct characteristics. Blindly using them can result in a significant discrepancy between predicted and actual MMP. Therefore, when applying an EC, it is crucial to carefully select the appropriate one based on the actual reservoir conditions. Using Daqing Oilfield as a case study, Zhao et al.57 compared the MMP of crude oil and CO2 in the test area predicted by the EC. Table 3 shows that the MMP values calculated by each EC were significantly different. Among them, the PRII model had the smallest prediction error with a relative error of 1.09%. Therefore, the PRII model can be applied to predict the MMP in other blocks of the test area.
Table 3. MMP Prediction Results Comparison Table.
| Determining Methods | MMP (MPa) | Relative error value (%) |
|---|---|---|
| STT | 29.10 | 0.00 |
| Johnson and Pollin | 26.58 | 8.66 |
| Glaso | 42.60 | 46.40 |
| Alston | 21.95 | 24.58 |
| PRII | 28.78 | 1.09 |
| PRIII | 21.52 | 26.04 |
2.2.2. Equation of State (EOS)
In comparison to the EC, the EOS can categorize the complex multicomponent system into three groups: light component C1, intermediate component C2–C6, and heavy component C7+ systems.59 While the EC has certain advantages, the oil industry prefers EOS as it can provide results that are almost consistent with the experimental method.60 EOS is a calculation method based on state equation and system phase equilibrium theory, which can accurately calculate MMP. Based on the cubic EOS proposed by van der Waals in 1873, scholars have developed new EOS, including RK-EOS, SRK-EOS, and PR-EOS. The equations are given in the following sections.
2.2.2.1. van der Waals EOS61
In 1873, van der Waals established the van der Waals equation of state (VdW-EOS) based on the ideal gas equation of state, taking into account intermolecular forces. The introduction of VdW-EOS significantly improves the predictive ability of the ideal gas state equation. The equation’s specific form is
| 8 |
V is the thermal motion volume of the system; a is the molecular attraction constant; b is the molecular repulsion constant; and R is the gas constant.
Pure CO2 injection:
| 9 |
Impure CO2 injection:
| 10 |
a are the gravitational parameters; b is the Co-volume; R is the gas constant; T is reservoir temperature; V is the total volume; and ci is the molar fraction of component i.
The proposed equation presents a two-parameter contrast state principle that accurately describes the phase behavior of equilibrium gas–liquid two-phase systems. However, it only provides a simple correction to the ideal gas model, disregarding the actual molecular geometry and molecular force field asymmetry as well as the influence of temperature on intermolecular attraction and repulsion.
2.2.2.2. RK-EOS62
In 1949, Redlich and Kwong proposed a modification to the VdW-EOS by introducing a functional relationship between the gravitational parameter ‘a’ and temperature ‘T’. This modification resulted in the development of the Redlich–Kwong equation of state (RK-EOS). Subsequently, numerous examples were used to verify the accuracy of the RK-EOS. The study found that correcting the VdW-EOS by changing parameter a was more effective than changing parameter b. Additionally, RK-EOS had a higher prediction accuracy than VdW-EOS. The specific form of RK-EOS was not mentioned.
| 11 |
where a =
0.42748 ; b = 0.08664
; b = 0.08664 ; R is gas constant; TC is critical temperature; and PC is critical pressure.
; R is gas constant; TC is critical temperature; and PC is critical pressure.
RK-EOS is also known as a cubic equation of state because it can be transformed into a cubic polynomial of the molar volume and compressibility factor. The cubic form of RK-EOS is as follows.
| 12 |
| 13 |
Pure CO2 injection:
| 14 |
Impure CO2 injection:
| 15 |
| 16 |
The equation considers the impact of molecular density and temperature on intermolecular gravity and incorporates the temperature to modify the gravitational term. However, the equation still relies on TC and PC to determine the two parameters a and b, which means that RK-EOS is still restricted to extremely simple hard spherical nonpolar symmetric molecules.
2.2.2.3. SRK-EOS63
In 1961, Pitzer introduced the concept of eccentric factor ω from the perspective of molecular physics. (ω = −lg(Prs)Tr = 0.7 – 1), and Soave introduced the eccentricity factor proposed by Pitzer as the third parameter into EOS and improved it to obtain SRK-EOS.
In 1961, Pitzer introduced the concept of the eccentricity factor ω from a molecular physics perspective. The eccentricity factor reflects the degree to which the interaction force between two molecules deviates from the force between their molecular centers. In 1972, Soave incorporated Pitzer’s proposed eccentricity factor as the third parameter into EOS and improved it to obtain SRK-EOS, which was highly successful. The introduction of the eccentricity factor has enabled the establishment of the three-parameter corresponding state principle, thereby improving the two-parameter corresponding state principle theory proposed by van der Waals. The specific form of SRK-EOS is
| 17 |
where
| 18 |
;
; ω =  ; ω is eccentricity factor; TC is critical temperature;
; ω is eccentricity factor; TC is critical temperature; 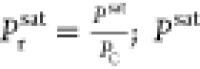 is the saturation
pressure at Tr= 0.7;
and PC is critical pressure.
is the saturation
pressure at Tr= 0.7;
and PC is critical pressure.
The cubic form of SRK-EOS is as follows
| 19 |
Pure CO2 injection:
| 20 |
Impure CO2 injection:
| 21 |
| 22 |
kij is the binary interaction coefficient that characterizes the degree of interaction between components i and j.
Compared with RK-EOS, SRK-EOS incorporates a temperature function α(T,ω) to better account for the impact of complex molecular systems, such as hydrocarbons, on PVT phase behavior.
2.2.2.4. PR-EOS64,65
In 1976, Peng and Robinson noted that SRK-EOS had poor accuracy in predicting physical properties and liquid volume characteristics of systems containing strong polar components. To address this issue, they used different fitting functions to fit experimental data and obtained different values for α. As a result, they established the PR equation of state (PR-EOS). Compared to SRK-EOS, PR-EOS provides improved predictions of fluid density. PR-EOS is widely used due to its simplicity and ability to accurately predict the phase equilibrium of mixtures. The specific form of PR-EOS is
| 23 |
where
; and
The cubic form of PR-EOS is as follows:
| 24 |
It should be noted that for the same system the results of pure CO2 injection and impure CO2 injection using PR-EOS are similar to those obtained using SRK-EOS, except for the value of kij. In 1978, Peng and Robinson discovered that α is influenced not only by the fitting function but also by the eccentricity factor ω. Therefore, they further adjusted α based on the magnitude of ω. The modified α fitting correlation takes a specific form as follows
 |
25 |
The cubic EOS mentioned above, in combination with various mixing rules, can be used to calculate the phase equilibrium of a CO2–crude oil system. For instance, Choubineh et al.66 selected crude oil samples from the bottom of an Iranian reservoir, with a crude oil sample gravity of approximately 30° API. They utilized the SRK-EOS to estimate the PVT properties of the crude oil samples and obtained accurate results, demonstrating the high precision of the SRK-EOS. However, EOS still has some limitations. For instance, the criteria for judging the miscible function of EOS are unclear, and it is less sensitive to crude oil composition and reservoir temperature.50
2.2.3. Artificial Intelligence Algorithm (AIA)
When determining MMP, experimental methods are more reliable but can be costly and time-consuming.67 Theoretical methods, such as EC and EOS, can be advantageous for reducing costs. However, companies involved in CO2-EOR seek a faster and more accurate method for determining MMP.68 Compared to the aforementioned methods, AIA exhibits superior robustness, speed, and accuracy, and are capable of modeling complex variable relationships.4 To calculate MMP using AIA, three main factors are taken into account: injected gas composition, crude oil composition, and temperature. The collected data will be divided into a training set and a test set. The training set is used to select the most suitable method for calculating MMP, and the accuracy of the method is verified using the data in the test set. For cases with fewer data sets, cross-validation can be used. AIAs are divided into machine learning (ML) and deep learning (DL). ML builds models by training data and discovering patterns and rules. DL is a special machine learning technology that uses deep neural networks to autonomously learn data features. Furthermore, it has a broad range of applications and high practical value.69 These advantages have made it a popular choice among scholars who have conducted research on predicting MMP using AIA.
Emera et al.70 identified reservoir temperature and injected gas composition as key parameters and used a genetic algorithm (GA) to predict the corresponding MMP. The results demonstrate that GA can be used to predict MMP with an acceptable level of accuracy, even in the absence of a large number of accurately measured MMP data.
Shokrollahi et al.71 proposed using a least-squares support vector machine (LSSVM) to predict the MMP of pure and impure CO2. The model was constructed and evaluated using approximately 147 data sets from the literature that employed experimental methods to determine MMP values and the corresponding gas/oil composition information. The results indicate that the proposed model significantly outperforms all existing methods and provides predictions that align with experimental data. Furthermore, the model can simulate the actual physical change trend of MMP with the five most important input parameters, including reservoir temperature, molecular weight of C7+, hydrogen sulfide, and nitrogen concentration.
Sayyad et al.48 utilized particle swarm optimization (PSO) to optimize the initial and bias values of the artificial neural network (ANN) and predict MMP. The optimized model resulted in a lower average absolute deviation (AAD) and a higher coefficient of determination (R2).
Hemmati-Sarapardeh et al.72 proposed a reliable model to predict MMP using a feedforward artificial neural network. The model takes into account the reservoir temperature, crude oil composition, and injected gas composition as input parameters. The results demonstrate that this model is more accurate and reliable than the existing models. Furthermore, correlation factors indicate that the reservoir temperature has the most significant impact on MMP.
Valluri et al.73 established a prediction model for MMP based on the power law and compared its accuracy with previously established EC. The results indicate that the power law model is more accurate. If the reservoir temperature and geothermal gradient as well as the composition of the produced fluid in the block are known, the power law model can be used to quickly and accurately determine the feasibility of miscible CO2 injection into the reservoir, provided that the reservoir pressure is known.
Hamdi et al.74 used heavy hydrocarbon molecular weight, reservoir temperature, volatile matter, and intermediate components as input variables, and MMP as the output variable. They employed an ANFIS model to predict the MMP, and the accuracy was estimated using the root-mean-square error (RMSE). The simulation results indicate that the ANFIS model has higher accuracy (average RMSE = 1.846) and a wider application range than the traditional correlation method (RMSE = 4.25). It is worth noting that ANFIS is a faster and more cost-effective method than the experimental approach. Among all ANFIS models, the hybrid algorithm that uses the Gaussian membership function to optimize ANFIS has the highest accuracy with an RMSE of 1.44.
Dargahi-Zarandi et al.75 compared the predicted MMP values using three different algorithms: data processing combination algorithm (GMDH), adaptive enhanced support vector regression (AdaBoost SVR), and multilayer perceptron (MLP). The results showed that the AdaBoost SVR model had the highest accuracy with an average absolute percentage relative error (AAPRE) value of 3.09% and a root-mean-square error (RMSE) value of 0.9 MPa.
Sinha et al.76 proposed a hybrid method based on random forest (RF) regression, which is defined as a super learner model. The model can solve the problem of inaccurate MMP prediction caused by excessive injection pressure (>4000 psia). It can also be used as a tool to quickly check the quality of existing experimental data in the absence of experimental data for predicting MMP.
Al-Khafaji et al.77 compared the predicted MMP values of the model using five machine learning algorithms: Multiple linear regression (MLR), Support vector regression (SVR), Decision trees (DT), Random Forest (RF), and K-nearest neighbors (KNN). They evaluated the effects of different parameters on MMP through sensitivity analysis using two types of data: literature and specific PVT reports from Iraqi oil fields. The study concluded that SVR is a suitable method for predicting the MMP with smaller data sets. Among the five algorithms tested, DT had the smallest error index and the highest determination coefficient, making it the best method for predicting the MMP. MLR had low accuracy in predicting MMP in the high-pressure range. KNN, on the other hand, has a low complexity and is suitable for various training models. The composition of the injection gas has the greatest influence on the MMP, accounting for approximately 46%. This is followed by reservoir temperature, C6+ molecular weight, C5+ molecular weight, C7+ molecular weight, and volatile and intermediate components.
Lv et al.69 compared several machine learning models, including extreme gradient boosting (XGBoost), categorical boosting (CatBoost), light gradient boosting machine (LGBM), random forest (RF), deep multilayer neural network (deep MLN), deep belief network (DBN), and convolutional neural network (CNN), to predict the AARD of MMP. The study found that the empirical formula has an AARD of approximately 19% for better prediction effect. In contrast, the CatBoost model has an AARD of only 1.34%, demonstrating its reliability and a wide range of applications in the database.
Liu et al.78 integrated seven baseline machine learning models, including SVM, KNN, CART, LRR, Ridge, Lasso, Elastic, and EC models, into a Stacking Model. The performance of the Stacking Model was evaluated using MAE, RMSE, and R2. The results indicate that the Stacking Model has strong robustness and high accuracy with an R2 value of 0.98 and an MAE as low as 0.62. The model can make more accurate predictions of the MMP of the CO2-crude oil system, although its calculation time increases.
In this study, various AIAs were evaluated for their ability to estimate MMP. These algorithms included the data processing combination algorithm (GMDH), adaptive enhanced support vector regression (AdaBoost SVR), multilayer perceptron (MLP),75 genetic algorithm (GA), particle swarm optimization (PSO), imperialist competitive algorithm (ICA), ant colony algorithm (ACO), and differential evolution algorithm (DE).79 AIAs including over algorithms and their hybrid models have been utilized to predict MMP values, resulting in accurate predictions.
This section introduces several commonly used theoretical methods for calculating the MMP. To determine the MMP of the reservoir more accurately, a combination of experimental and theoretical calculation methods can be used. However, the MMP predicted by theoretical calculation methods is comparable to that measured by experimental methods. As reservoir exploitation progresses, the MMP is subject to change. Repeatedly determining MMP through experimental methods can be costly in terms of both time and money. Therefore, it is advisable to use theoretical calculation methods to predict MMP during reservoir exploitation. When the MMP is determined using theoretical calculation methods, it is important to consider the specific situation of the reservoir and choose the appropriate calculation method accordingly. Table 4 compares the advantages of the various theoretical methods.
Table 4. Comparison Table of the Theoretical Methods.
| Theoretical Calculation Methods | Advantages | Disadvantages |
|---|---|---|
| ECM | Easily obtained. Low application difficulty. | Different application range. Low accuracy. |
| EOS | Accurately simulate the miscible process. Low cost. | Large calculation. Need to be solved iteratively. |
| AIA | Save calculation time. Higher prediction accuracy. Better generalization performance. | Need a large amount of data. If the amount of data is small, it is prone to overfitting or underfitting. |
3. MMP Reducing Techniques
When the formation pressure of the reservoir is below the MMP, miscible flooding of CO2 cannot be fully realized. In such cases, only CO2 immiscible flooding with a low displacement efficiency can be used to further expand the swept volume and improve the local displacement efficiency, which will lead to higher oil recovery,80 CO2 miscible flooding should be implemented as much as possible. To achieve this goal, two methods have been proposed: (1) increasing the gas injection pressure to reach the MMP and (2) reducing MMP by altering the composition of the injected gas.26
Due to the fact that the MMP of some reservoirs may exceed the formation fracture pressure, achieving CO2 miscible flooding using the first method can be challenging and costly.81 Additionally, research has shown that the addition of alcohol reagents and surfactants can effectively reduce MMP by 1–9.4 MPa.82 Therefore, this review focuses on the second method. The reduction technologies for common MMP are mainly divided into two categories: miscible solvents and surfactants. The miscible solvents are based on extracting light hydrocarbons from crude oil, increasing the solubility, promoting crude oil expansion, reducing oil–water interfacial tension, and other oil displacement mechanisms. The addition of surfactants is based on reducing the interfacial tension between oil and water.83
3.1. Miscible Solvents
The method principle is as follows: a slug of a certain size of miscible solvent is injected into the reservoir. The miscible solvent gradually diffuses and its leading edge completely mixes with the crude oil after full contact, forming a mixed zone of the two fluids. The front end of the mixing zone is in contact with the crude oil, and the end of the mixing zone is in contact with the injected CO2. Under lower -pressure conditions, the mixed fluid of crude oil, miscible solvent, and CO2 achieves miscibility, forming a miscible zone. The miscible solvent reduces the miscible pressure of the displacement front. The miscible zone may disappear gradually during the displacement process. The actual situation should be determined based on the size of the miscible solvent injection slug.84 If the size of the miscible solvent injection slug is large, then only the front part of the miscible solvent slug will mix with the crude oil to form a mixing zone. The miscible solvent at the back end of the slug will still exist in its own separate phase state. Currently, the CO2 injection is in direct contact with the rear of the miscible solvent slug, creating a miscible zone of CO2 and miscible solvent. This zone also helps to reduce the miscible pressure of the displacement front. This work summarizes various methods of reducing MMP using the miscible solvent method. The methods are categorized into monocomponent miscible solvents and polycomponent miscible solvents based on the injected solvent’s components.
3.1.1. Monocomponent Miscible Solvents
Monocomponent miscible solvents mainly refer to the injection of low molecular hydrocarbons, such as benzene, alkanes, liquefied petroleum gas (LPG), etc. They can increase the content of light hydrocarbons in crude oil and enhance the extraction effect of CO2 on intermediate hydrocarbons (C5–C12), which can achieve a more efficient miscibility enhancement effect.8 Salari et al.60 investigated the effect of adding small molecular hydrocarbons, such as benzene, toluene, and xylene, to the injected gas. The experimental results indicate that the inclusion of aromatic hydrocarbons can considerably decrease the MMP. Toluene, in particular, has a more pronounced effect.
The addition of benzene and other small molecular hydrocarbons can impact MMP. Similarly, the addition of alkanes can also affect MMP, but it is not necessarily used to reduce it. Nitrogen or methane in CO2 can significantly increase the formation pressure to achieve the required pressure for the miscible phase. However, ethane and higher molecular weight hydrocarbons in CO2 may reduce MMP.85
To confirm the reliability of this conclusion, Choubineh et al.66 utilized the eclipse reservoir simulator to investigate the impact of nitrogen, methane, ethane, and propane on MMP. The findings are presented in Figure 11.
Figure 11.

MMP versus carbon dioxide (percent) for four binary mixtures of nitrogen, methane, ethane, and propane with CO2. (a) C1 mixed with CO2 in the gas injection stream. (b) C2 mixed with CO2 in the gas injection stream. (c) N2 mixed with CO2 in the gas injection stream. (d) C3 mixed with CO2 in the gas injection stream. Reprinted with permission from ref (66). Copyright 2019 Petroleum Science.
The study concluded that increasing the ethane content did not have a significant impact on MMP. However, adding a small amount of propane was found to be more effective in reducing MMP.66 Chinese scholars Tang et al.86 also discovered that MMP is proportional to the molar content of methane and nitrogen in crude oil during CO2 flooding. MMP decreases as the concentrations of methane and nitrogen in crude oil decrease. Within a certain range, the concentration of methane and nitrogen has a linear relationship with MMP. In their study of the Cooper Basin reservoir in Australia, Bon et al.87 discovered that the addition of a small amount of C5+ to CO2 can significantly reduce the MMP from 23.7 to 19.8 MPa.
Some scholars do not fully support the conclusion above. They believe that ethane can also reduce MMP. For instance, Javid et al.88 predicted the MMP of the CO2-crude oil system after injecting nitrogen, methane, and ethane into two crude oil samples from the Abu Dhabi carbonate reservoir using the component simulation method. They found that ethane was more effective in reducing MMP, and the MMP of the first crude oil sample decreased by 100 psi/10 mol % after injecting ethane.
Currently, many oil fields opt for direct discharge of crude oil-associated gas due to economic benefits rather than collecting and utilizing it. However, better utilization of crude oil-associated gas can not only effectively utilize resources but also play a role in protecting the environment. In light of this, scholars propose adding processed oilfield-associated gas to CO2 and injecting it into the ground for miscible flooding.
Peng et al.89 determined the MMP between CO2 and crude oil using a high-temperature and -pressure interfacial tension meter. The hanging drop and extrapolation methods were employed in VIT. The results indicate that MMP can be reduced by adding a certain proportion of liquefied petroleum gas (LPG) to the CO2. MMP decreased gradually with the decrease in the CO2 content (after adding LPG), and the addition of about 38% LPG reduced MMP to 68.88% of the original. Jeong et al.90 conducted numerical simulation experiments on crude oil in the Webern reservoir using CMG software to explore the effect of LPG injection on CO2 flooding enhanced oil recovery. The MMP of the CO2-crude oil system was predicted by PR-EOS, and the simulation results are presented in Figure 12.
Figure 12.

Variation of the MMP with the temperature.
Figure 12 shows that MMP decreases as the LPG concentration increases. This reduction in MMP increases the likelihood of implementing a CO2 miscible flooding in the reservoir, which significantly improves oil recovery. Scholars at home and abroad have studied LPG and found that it has a certain effect on reducing the MMP of oil and gas in two phases.
3.1.2. Polycomponent Miscible Solvents
Polycomponent miscible solvents are primarily hydrocarbon-based due to their wider range of applications. These solvents are formed through the interaction of oxygen atoms with CO2 and include alcohols, ethers, ketones, and esters etc.
Permadi et al.91 found that low-carbon alcohols, such as methanol and ethanol, can enhance the extraction performance of CO2, thereby reducing MMP.
To confirm the reliability of this conclusion, both domestic and foreign scholars have conducted numerous experiments and obtained the following results:
Methanol, ethanol, n-hexane, n-octane, petroleum ether, gasoline, and four different light oils were selected as miscible solvents. Zhang et al.84 tested the antihypertensive effect of miscible solvents using a self-developed high-temperature and high-pressure interfacial tension tester. The results showed that n-octane and petroleum ether were the most effective in reducing MMP.
Yang et al.92 measured the IFT of the CO2-crude oil system and the CO2-crude oil and 5% alcohol mixture system at different pressures but the same temperature. They used a visual high-temperature and high-pressure autoclave. Please refer to Figure 13 for the results.
Figure 13.

Comparison of interfacial tensions between the CO2 + crude oil system and CO2 + crude oil with 5% alcohols mixture system under different pressures at 343.15 K. Reprinted with permission from ref (92). Copyright 2019 Elsevier.
At the same temperature, as the pressure increases, the IFT of both systems gradually decreases. When the pressure is lower than 13.5 MPa, the IFT of both systems decreases sharply with increasing pressure. However, when the pressure is higher than 13.5 MPa, the rate of decrease in IFT gradually decreases with increasing pressure, and the IFT of the second system is significantly lower than that of the first system. After adding a 5% alcohol mixture to the crude oil, the MMP between CO2 and crude oil decreased by 9.21%.
Saira et al.93 utilized the VIT method to measure the IFT of a pure CO2-crude oil system and an alcohol-treated CO2-crude oil system at 70 °C and varying pressures. The results showed that the MMP was reduced by 0.2 and 1.1 MPa after methanol and ethanol treatment, respectively. Ethanol was found to have a better antihypertensive effect than methanol. Furthermore, the addition of methanol or ethanol greatly improved CO2’s ability to promote crude oil expansion.
In summary, alcohol reagents can have a positive impact on reducing the MMP of the CO2-crude oil system and have great potential for application in the CO2-EOR. However, it should be noted that alcohol reagents may not always be the optimal choice for reducing MMP in different physical reservoirs. In some cases, ethers, ketones, and esters may have better MMP reduction effects.
Liu et al.94 investigated the CO2-crude oil system’s MMP using the high-temperature and high-pressure interfacial tensiometer with the VIT on a crude oil sample from the H block in X oilfield. This block belongs to a low permeability, high temperature, and high salinity reservoir. The study also compared the effectiveness of alkanes, alcohols, petroleum ethers, and oil-soluble surfactants in reducing MMP. It was discovered that four types of reagents have the ability to reduce MMP levels. Among these, petroleum ether with a boiling range of 30–60 °C was found to be the most effective, reducing MMP levels by 12.17%.
Novriansyah et al.37 established a VIT device to determine the original MMP of two crude oil samples from the Air Benakat reservoir in the south of Sumatra Basin, Indonesia. The VIT device was established under high-temperature and high-pressure conditions, with measurements taken in the pressure range of 3000 psi and the temperature range of 300 °C. Subsequently, methanol, ethanol, and acetone were added to the CO2-crude oil system, and the MMP was measured in the corresponding samples after altering the experimental environment. The results indicate that acetone is more effective in reducing MMP, regardless of the temperature or crude oil composition. Moreover, the greater the amount of acetone injected, the more pronounced the reduction in MMP.
In summary, miscible solvents can decrease the MMP of the CO2-crude oil system to some extent. However, this method requires a large volume of miscible solvent injection, which is costly and has limited application due to hydrocarbon gases. Therefore, it is essential to develop more economical and effective methods to reduce MMP.95
3.2. Surfactants
Surfactants are commonly used to promote the mixing of oil and water and reduce IFT between the two phases due to their unique amphiphilic structure.96 The mechanism by which surfactants reduce IFT is as follows: when surfactants accumulate spontaneously at the interface between the two phases, the interaction between the CO2-philic end and the CO2 becomes similar to the interaction between the lipophilicity end and crude oil. This reduces the polarity difference between the two phases and the asymmetric force applied by the two phases, resulting in a decrease in the IFT of the oil-gas in two phases. The addition of surfactants during CO2 flooding can increase the solubility of CO2 in crude oil and reduce the IFT of the CO2-crude oil system. This, in turn, reduces the MMP and improves oil recovery.97
In recent years, oil companies have sought more cost-effective ways to enhance oil recovery due to frequent fluctuations in oil prices.98 The method of adding surfactants has gained attention due to its small dosage, low economic cost, and wide field application. Furthermore, the inclusion of surfactants can significantly decrease the MMP of the CO2-crude oil system, with a reduction range of approximately 2.3–27.4%.99 Therefore, numerous domestic and international scholars have conducted extensive experimental studies to verify the effectiveness of adding surfactants to the CO2-crude oil system. The following are some of the research findings:
Wu et al.100 conducted a study to address the issue of high MMP preventing CO2 miscible flooding in certain blocks of Zhongyuan Oilfield. They compared the effectiveness of five antimixing agents, ethylene glycol butyl ether, isopropyl citrate, alkyl phenol polyoxyethylene ether, Span 80, and mixed benzene, in reducing MMP in a CO2-crude oil system. The crude oil sample used in the study was obtained from Zhongyuan Oilfield Qiao 18–10, and the experiments were conducted at 100 °C. The experimental results indicate that ethylene glycol monobutyl ether is more effective in reducing MMP compared to isopropyl citrate and Span 80. Additionally, the addition of ethylene glycol monobutyl ether with a mass fraction of 0.5% can reduce IFT by 17.66%. To enhance the reduction of MMP, a new compound system chemical agent was designed (a mixture of benzene with a mass fraction of 0.3% and ethylene glycol butyl ether with a mass fraction of 0.2%). The MMP was determined using the VIT method before and after the addition of the chemical agent. The results showed that the new compound system chemical agent reduced the MMP between CO2 and crude oil by 17.86%.
Yang et al.101 developed two new surfactants for use in microemulsion of CO2-water systems. The surfactants have a fluorinated alkane chain and nonfluorinated OAc (acetoxy) chain as the CO2-philic end (fluorinated alkane for comparison) and alkane structure as the lipophilic end. The surfactants are peracetyl glucose dodecyl ester molecule and citric acid triisopropyl ester molecule. The study revealed that both surfactants exhibit a positive mixing effect, with peracetyl glucose dodecyl ester demonstrating the most significant impact. The addition of peracetyl glucose dodecyl ester resulted in a 27.47% reduction in MMP between CO2 and crude oil.
Achieving CO2 miscible flooding can be challenging in Malaysia due to the high reservoir temperature, particularly when maintaining MMP within the range of 2300–4380 psi. Qayyimah et al.102 added fatty acid methyl ester (FAME) extracted from rubber seed oil (FAME composition analysis is shown in Table 5) to crude oil samples from the refinery of Malaysia National Petroleum Corporation in Malacca, Malaysia (crude oil composition is shown in Table 6) and determined MMP after the experiment by STT. The experimental results indicate that the addition of 10% FAME can decrease the MMP of the CO2-crude oil system by 15%.
Table 5. FAME Composition Analysis for Rubber Seed Oil Methyl Ester.
| Formula | IUPAC name | Composition (%) |
|---|---|---|
| C17H32O2 | Methyl palmitoleate | 0.50 |
| C17H34O2 | Methyl palmitate | 12.56 |
| C19H34O2 | Methyl linoleate | 32.73 |
| C19H36O2 | Methyl oleate | 8.68 |
| C19H38O2 | Methyl octadecanoate | 9.56 |
Table 6. Composition of Crude Oil.
| Component | Mole fraction (%) |
|---|---|
| Pentane | 2.6 |
| Hexane | 4.38 |
| Heptanes | 1.15 |
| Octanes | 2.27 |
| Nonanes | 5.05 |
| Decanes | 6.68 |
| Undecanes plus | 77.87 |
| Total | 100 |
| Molecular weight (kg mol–1) | 224.46 |
Qayyimah et al.103 added FAME to two crude oil samples from Petronas Refinery, Melaka, Malaysia, and used the VIT method to determine MMP in the CO2-crude oil system. The experimental results, shown in Figure 14, indicate that the addition of FAME to crude oil can effectively reduce MMP. The use of alkoxy FAME is more effective than that of methyl laurate.
Figure 14.

IFT between CO2 and crude oils with FAME at different pressures. Reprinted with permission from ref (103). Copyright 2017 Springer Singapore.
Sun et al.104 added isobutyl citrate with a slug size of 0.3% HCPV to crude oil samples from the YSL oilfield. The MMP between CO2 and crude oil was measured by using the STT method before and after adding the surfactant. The results showed that the MMP decreased from 32.4 to 30.1 MPa, a decrease of 2.3 MPa. At this stage, the oil recovery rate was the highest during the miscible flooding process with an oil recovery rate of 17.22%, which was 7.97% higher than that of the simple CO2 flooding scheme.
Guo et al.105 designed an oil-soluble surfactant, CAE, to address the issue of MMP being much higher than the formation pressure in low permeability reservoirs, which prevents CO2 immiscible displacement. They conducted two groups of displacement experiments at a temperature of 85 °C and a pressure of 22.64 MPa, using different concentrations of CAE and pure CO2 flooding. The experimental results indicate that the optimal injection concentration of CAE is 0.2%. At a CAE concentration of 0.2%, the measured MMP is 6.1 MPa lower than that of pure CO2 flooding. This demonstrates CAE’s effective ability to reduce MMP. The application of CAE preslug displacement can significantly improve oil recovery.
Luo et al.106 used the pendant drop method combined with axisymmetric drop shape analysis (ADSA) technology to measure the IFT of the CO2-crude oil system. They determined the MMP by using the VIT method. The effect of the addition of propoxylated surfactant CiPOj (where Ci = tail chain hydrocarbons, POj = oxygen-containing allyl, i = 12, j = 4∼9) and the addition of pentane or ethanol on the reduction of IFT in the CO2-crude oil system was subsequently compared. The results indicate that the addition of 0.5 wt % CiPOj resulted in a significantly greater reduction in IFT compared to the addition of 20 wt % pentane or 5 wt % ethanol. The comparison is illustrated in Figure 15.
Figure 15.
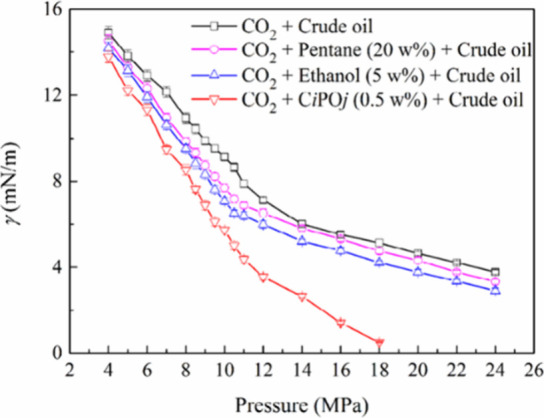
Measured interfacial tension between CO2 and crude oil with different additives at different pressures and temperatures of 333 K.
To address the issue of high MMP of CO2 flooding in the beach-bar sand reservoir of Shengli Oilfield, Zhang107 employed a compound chemical system consisting of self-made DYJ-13 chemical agent and solubilizer S6. The system was added to the crude oil sample of the Fan 142–9–5 well in the Fan 142 block at a mass fraction of 3%. The MMP of the CO2-crude oil system was then determined using the STT method after the addition of chemical surfactants. The study revealed a decrease in MMP from 31.65 to 24.60 MPa, representing a 22% decrease of 7.05 MPa.
Due to the low reservoir maturity, strong reservoir heterogeneity, extremely low reservoir permeability, and MMP of 29.6 MPa, which is far higher than the reservoir pressure in some blocks, Zhao et al.108 selected two surfactants, isobutyl citrate and isoamyl citrate, to reduce the MMP between CO2 and crude oil in the study area. The effect of reducing the MMP by the STT method was determined. The experimental results indicate that increasing the size of the chemical reagent injection slug leads to a significant reduction in MMP, although the reduction becomes less pronounced as the slug size increases. The optimal injection slug size for the chemical reagent is 0.003 PV (pore volume). At this size, the MMP of isobutyl citrate decreased by 6.1 MPa, and the MMP of isoamyl citrate decreased by 5.5 MPa, representing decreases of 20.61% and 18.58%, respectively. The results indicate that the addition of isobutyl citrate can significantly reduce MMP, resulting in a crude oil recovery increase of 6.5%–11.6% compared to the scheme without isobutyl citrate injection. Therefore, isobutyl citrate is recommended as the most effective chemical reagent for reducing the MMP.
Almobarak et al.109 investigated the effect of surfactants and multicomponent miscible solvents on reducing MMP in CO2-crude oil systems containing methane at different temperatures. The study found that surfactants can reduce MMP by 9% at 373 K, while polycomponent miscible solvents have no significant effect on MMP.
Sun et al.110 investigated the ability of different volume fractions of the gas-soluble surfactant JS to reduce MMP. The experimental results showed that the original MMP between CO2 and crude oil was 22.05 MPa. After injection of JS with a volume fraction of 3% and 20%, the content of heavy components in crude oil decreased by 25.07% and 48.21%, respectively. The average viscosity of crude oil decreased by 61.51% and 96.88%, respectively. Under the same pressure, the IFT between crude oil and CO2 was reduced by 30.36%, while the MMP decreased to 19.95 and 17.14 MPa for CO2 and crude oil, respectively, representing a decrease of 9.52% and 22.27%.
All in all, there are two main categories of methods for reducing the MMP: miscible solvents and surfactants. Miscible solvents can be further divided into monocomponent and polycomponent miscible solvents, depending on the injected solvent’s component. Table 7 displays the types of mechanisms for MMP reduction and their respective characteristics.
Table 7. Comparison of Advantages and Disadvantages of the MMP Reduction Methods.
| Reducing Techniques | Advantages | Disadvantages | |
|---|---|---|---|
| Miscible Solvents | Monocomponent Miscible Solvents | Efficiently promote CO2 flooding to be miscible. | High cost. MMP may increase sharply during the displacement process. |
| Polycomponent Miscible Solvents | Effect of reducing IFT is better. | Large amount of injection. Economic benefit is low. | |
| Surfactants | Less dosage. Effect of reducing MMP is remarkable. Low cost. | It is easy to cause chromatographic separation. The selection of reagents needs to evaluate the adaptability to reservoirs. | |
4. Results and Discussion
There are several experimental determination methods in this review, including STT, RBA, VIT, CT, SRM, MRI, FFBM, ODVM, and VDM. First, STT is an international common determination method with high accuracy and repeatability, but it is time-consuming and requires high-precision instruments. Second, RBA has a short determination time and low instrument requirements, but it is seriously affected by human factors. Third, VIT is a cost-effective and time-efficient method suitable for high-temperature and high-pressure conditions. However, it is subject to human factors and has limitations. Next, CT allows for temperature adjustment and visual observation of the MMP, but it is expensive and poses potential health risks. Then, SRM has the advantage of avoiding radiation to reduce health risks. However, it can only measure the MMP when the constant temperature pressure increases. In addition, MRI can measure the density of hydrocarbon fluids. Nevertheless, the experimental equipment required for this method consumes too much electricity, resulting in higher costs. Furthermore, FFBM has the capability to measure MMP at the nanoscale in a short amount of time. However, it is prone to human error and, therefore, cannot be used with human intervention. Finally, VDM can directly measure the relationship between injected meteorological density and pressure but is limited to measuring MMP at low temperatures.
There are three main theoretical calculation methods: ECM, EOS, and AIA in this review. ECM is advantageous due to its ease of obtaining and applicability to oilfield use, but its range of application is limited. To calculate the MMP, it is necessary to select and compare the results of different ECMs based on the specific characteristics of the reservoir. In addition, EOS can simulate the miscible process, but it requires iterative solving, which is time-consuming. AIA is a popular method due to its shorter calculation time, higher prediction accuracy, and better generalization performance. However, there is an important defect that needs a large amount of data to ensure accuracy. Insufficient data may result in inaccurate calculations, leading to overfitting or underfitting phenomena.
There are two types of solvents for the miscible solvent method: monocomponent and polycomponent. Monocomponent solvents, such as benzene, alkanes, and LPG, promote CO2 flooding to be miscible, but their cost is high and may lead to the increase in MMP during the displacement process. Polycomponent solvents include alcohols, ethers, and ketones. Alcohols can reduce the MMP between the CO2-crude oil system by 9.21%, which is effective in reducing MMP. However, it is recommended to add ethers or ketones to the CO2-crude oil system to reduce MMP under high-temperature and high-pressure conditions. Therefore, it is not advisable to add alcohols to the CO2-crude oil system under such conditions.
This review illustrates the characteristics of surfactants by enumerating numerous cases. Compared with the miscible solvent method, it offers several advantages, including lower dosage, a significant reduction in MMP, and higher economic benefits. It involves synthesizing a new material from existing materials using technical means to reduce the IFT between CO2-crude oil. However, it can also cause chromatographic separation. When selecting reagents for adaptability evaluation, it is recommended to sample them from the reservoir in advance.
5. Conclusion
Considering CO2 flooding, the efficiency of crude oil recovery is related to the achievement of miscible flooding. This paper discusses the methods for measuring and reducing the MMP, which is crucial for CO2 miscible flooding. The conclusion drawn from this investigation is listed as follows:
-
(1)
Although the STT method has the drawbacks of high cost and time consumption, it provides the highest accuracy among existing laboratory methods for measuring MMP. Therefore, the STT method is recommended for obtaining a more precise MMP value.
-
(2)
In terms of the research content, it can be concluded that artificial intelligence algorithms have become the prevailing method for calculating MMP in recent years. The AIA is valued for short calculation time and various methods to fit a correlation suitable for calculating the MMP of the reservoir based on its physical parameters such as temperature, crude oil composition, and injected gas composition. Generally speaking, its R2 can reach 85% or more, indicating a better fitting effect and high prediction accuracy.
-
(3)
If miscible solvents are used to reduce MMP, it is more economical to add monocomponent miscible solvents. Among the monocomponent miscible solvents that can be added, alcohols have a better effect on reducing MMP.
-
(4)
To reduce MMP, surfactants can be added to the CO2-crude oil system, which has been proven effective. In terms of the economic benefit, it is more appropriate to add surfactant to the reservoir.
Acknowledgments
This work is supported by the Natural Science Foundation of Heilongjiang Province No. LH2020E013.
The authors declare no competing financial interest.
References
- Pereponov D.; Tarkhov M.; Dorhjie D. B.; Rykov A.; Filippov I.; Zenova E.; Krutko V.; Cheremisin A.; Shilov E. Microfluidic Studies on Minimum Miscibility Pressure for N-Decane and CO2. Energies 2023, 16 (13), 4994. 10.3390/en16134994. [DOI] [Google Scholar]
- Kwon S.; Lee W. Parameter estimation in multiple contact CO2 miscibility simulation with uncertain experimental core flooding data. Korean Journal of Chemical Engineering 2012, 29, 750–755. 10.1007/s11814-011-0264-5. [DOI] [Google Scholar]
- Lashkarbolooki M.; Eftekhari M. J.; Najimi S.; Ayatollahi S. Minimum miscibility pressure of CO2 and crude oil during CO2 injection in the reservoir. Journal of Supercritical Fluids 2017, 127, 121–128. 10.1016/j.supflu.2017.04.005. [DOI] [Google Scholar]
- Hou Z.; Su H.; Wang G. Study on Minimum Miscibility Pressure of CO2–Oil System Based on Gaussian Process Regression and Particle Swarm Optimization Model. J. Energy Resour. Technol. 2022, 144 (10), 103002. 10.1115/1.4053949. [DOI] [Google Scholar]
- Qin J.; Han H.; Liu X. Application and enlightenment of carbon dioxide flooding in the United States of America. Petroleum Exploration and Development 2015, 42 (2), 232–240. 10.1016/S1876-3804(15)30010-0. [DOI] [Google Scholar]
- Rezaei M.; Eftekhari M.; Schaffie M.; Ranjbar M. A CO2-oil minimum miscibility pressure model based on multi-gene genetic programming. Energy exploration & exploitation 2013, 31 (4), 607–622. 10.1260/0144-5987.31.4.607. [DOI] [Google Scholar]
- Clark P.; Toulekima S.; Sarma H.. A miscibility scoping study for gas injection into a high-temperature volatile oil reservoir in the Cooper Basin, Australia. In SPE Asia Pacific Oil and Gas Conference and Exhibition Perth, Australia, 20–22 October 2008; SPE. 10.2118/116782-MS. [DOI]
- Guo P.; Zhang W.; Jia B. N. Research progress of assistants for reducing CO2 crude oil minimum miscible pressure. Petroleum Reservoir Evaluation and Development 2022, 12 (5), 726–733. [Google Scholar]
- Bui L. H. Near miscible CO2 application to improve oil recovery. Doctoral dissertation, University of Kansas: Lawrence, KS, USA, 2010. https://kuscholarworks.ku.edu/handle/1808/6962. [Google Scholar]
- Ayoub M. A.; Tackie-Otoo B. N.; Zulkefli S. H. B. The combined effects of the minimum miscibility pressure and injection rate variations on recovery of co2 flooding in sandstone reservoir. Journal of Petroleum Exploration and Production Technology 2022, 12 (11), 2899–2913. 10.1007/s13202-022-01480-7. [DOI] [Google Scholar]; https://link.springer.com/article/10.1007/s13202-022-01480-7.
- Yuan H.; Johns R. T.; Egwuenu A. M.; Dindoruk B.. Improved MMP correlations for CO2 floods using analytical gas flooding theory. In SPE Improved Oil Recovery Conference; 2004; pp SPE-89359. 10.2118/89359-MS. [DOI]
- Belhaj H.; Abukhalifeh H.; Javid K. Miscible oil recovery utilizing N2 and/or HC gases in CO2 injection. J. Pet. Sci. Eng. 2013, 111, 144–152. 10.1016/j.petrol.2013.08.030. [DOI] [Google Scholar]
- Perera M.; Gamage R.; Rathnaweera T.; Ranathunga A.; Koay A.; Choi X. A Review of CO2-Enhanced Oil Recovery with a Simulated Sensitivity Analysis. Energies 2016, 9 (7), 481. 10.3390/en9070481. [DOI] [Google Scholar]
- Seyyedi M.; Sohrabi M. Assessing the Feasibility of Improving the Performance of CO 2 and CO 2 – WAG Injection Scenarios by CWI. Ind. Eng. Chem. Res. 2018, 57 (34), 11617–11624. 10.1021/acs.iecr.8b02000. [DOI] [Google Scholar]
- Ahmadi M. A.; Zendehboudi S.; James L. A. A reliable strategy to calculate minimum miscibility pressure of CO2-oil system in miscible gas flooding processes. Fuel 2017, 208, 117–126. 10.1016/j.fuel.2017.06.135. [DOI] [Google Scholar]
- Shokir E. M. E.-M. CO2–oil minimum miscibility pressure model for impure and pure CO2 streams. J. Pet. Sci. Eng. 2007, 58 (1–2), 173–185. 10.1016/j.petrol.2006.12.001. [DOI] [Google Scholar]
- Zhang K.; Gu Y. Two different technical criteria for determining the minimum miscibility pressures (MMPs) from the slim-tube and coreflood tests. Fuel 2015, 161, 146–156. 10.1016/j.fuel.2015.08.039. [DOI] [Google Scholar]
- Czarnota R.; Janiga D.; Stopa J.; Wojnarowski P.; Kosowski P. Minimum miscibility pressure measurement for CO2 and oil using rapid pressure increase method. Journal of CO2 Utilization 2017, 21, 156–161. 10.1016/j.jcou.2017.07.007. [DOI] [Google Scholar]
- Nguyen P.; Mohaddes D.; Riordon J.; Fadaei H.; Lele P.; Sinton D. Fast fluorescence-based microfluidic method for measuring minimum miscibility pressure of CO2 in crude oils. Analytical chemistry 2015, 87 (6), 3160–3164. 10.1021/ac5047856. [DOI] [PubMed] [Google Scholar]
- Safaei A.; Kazemzadeh Y.; Riazi M. Mini Review of Miscible Condition Evaluation and Experimental Methods of Gas Miscible Injection in Conventional and Fractured Reservoirs. Energy Fuels 2021, 35 (9), 7340–7363. 10.1021/acs.energyfuels.0c04384. [DOI] [Google Scholar]
- Stalkup F. I. Jr Status of miscible displacement. Journal of Petroleum Technology 1983, 35 (04), 815–826. 10.2118/9992-PA. [DOI] [Google Scholar]
- Ahmed T. H.Prediction of CO2 minimum miscibility pressures. In SPE Latin America and Caribbean Petroleum Engineering Conference Buenos Aires, Argentina, 27–29 April 1994; p SPE. 10.2118/27032-MS. [DOI]
- Elsharkawy A. M.; Poettmann F. H.; Christiansen R. L. Measuring CO 2 minimum Miscibility Pressures: Slim-Tube or Rising-Bubble Method?. Energy Fuels 1996, 10 (2), 443–449. 10.1021/ef940212f. [DOI] [Google Scholar]
- Ahmad W.; Vakili-Nezhaad G.; Al-Bemani A. S.; Al-Wahaibi Y. Uniqueness, repeatability analysis and comparative evaluation of experimentally determined MMPs. J. Pet. Sci. Eng. 2016, 147, 218–227. 10.1016/j.petrol.2016.06.023. [DOI] [Google Scholar]
- Zhang N.; Wei M.; Bai B. Statistical and analytical review of worldwide CO2 immiscible field applications. Fuel 2018, 220, 89–100. 10.1016/j.fuel.2018.01.140. [DOI] [Google Scholar]
- Almobarak M.; Wu Z.; Zhou D.; Fan K.; Liu Y.; Xie Q. A review of chemical-assisted minimum miscibility pressure reduction in CO2 injection for enhanced oil recovery. Petroleum 2021, 7 (3), 245–253. 10.1016/j.petlm.2021.01.001. [DOI] [Google Scholar]
- Christiansen R. L.; Haines H. K. Rapid Measurement of Minimum Miscibility Pressure With the Rising-Bubble Apparatus. SPE Reservoir Engineering 1987, 2 (04), 523–527. 10.2118/13114-PA. [DOI] [Google Scholar]
- Nobakht M.; Moghadam S.; Gu Y. Determination of CO 2 minimum Miscibility Pressure from Measured and Predicted Equilibrium Interfacial Tensions. Ind. Eng. Chem. Res. 2008, 47 (22), 8918–8925. 10.1021/ie800358g. [DOI] [Google Scholar]
- Thomas F. B.; Zhou X. L.; Bennion D. B.; Bennion D. W. A Comparative Study of RBA, P-x, Multicontact And Slim Tube Results. J. Can. Pet. Technol. 1994, 10.2118/94-02-02. [DOI] [Google Scholar]
- Zhou D.; Orr F. M. An Analysis of Rising Bubble Experiments to Determine Minimum Miscibility Pressures. SPE J. 1998, 3, 19–25. 10.2118/30786-PA. [DOI] [Google Scholar]
- Zhang K.; Gu Y. New qualitative and quantitative technical criteria for determining the minimum miscibility pressures (MMPs) with the rising-bubble apparatus (RBA). Fuel 2016, 175, 172–181. 10.1016/j.fuel.2016.02.021. [DOI] [Google Scholar]
- Teklu T. W.; Alharthy N.; Kazemi H.; Yin X.; Graves R. M.. Vanishing Interfacial Tension Algorithm for MMP Determination in Unconventional Reservoirs. SPE Western North American and Rocky Mountain Joint Meeting. SPE Western North American and Rocky Mountain Joint Meeting, Denver, Colorado; 2014. 10.2118/169517-MS. [DOI]
- Rezk M. G.; Foroozesh J. Phase behavior and fluid interactions of a CO2-Light oil system at high pressures and temperatures. Heliyon 2019, 5 (7), e02057 10.1016/j.heliyon.2019.e02057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreas J. H.; Hauser E. A.; Tucker W. B. Boundary tension by pendant drops. J. Phys. Chem. 1938, 42, 1001–1019. 10.1021/j100903a002. [DOI] [Google Scholar]
- Jessen K.; Orr F. M. On Interfacial-Tension Measurements to Estimate Minimum Miscibility Pressures. SPE Reservoir Evaluation & Engineering 2008, 11 (05), 933–939. 10.2118/110725-PA. [DOI] [Google Scholar]
- Samara H.; Al-Eryani M.; Jaeger P. The role of supercritical carbon dioxide in modifying the phase and interfacial properties of multiphase systems relevant to combined EOR-CCS. Fuel 2022, 323, 124271 10.1016/j.fuel.2022.124271. [DOI] [Google Scholar]
- Novriansyah A.; Bae W.; Park C.; Permadi A. K.; Sri Riswati S. Ketone Solvent to Reduce the Minimum Miscibility Pressure for CO2 Flooding at the South Sumatra Basin, Indonesia. Processes 2020, 8 (3), 360. 10.3390/pr8030360. [DOI] [Google Scholar]
- Ibrahim A. F.; Abdelgawad K. Z.; Al-Anazi A.; Al Hamad J. S. Effect of Crude Oil Properties on the Interfacial Tension of Crude Oil/CO2 Under HPHT Conditions. Arabian Journal for Science and Engineering 2023, 48 (7), 9269–9286. 10.1007/s13369-022-07291-6. [DOI] [Google Scholar]
- Hemmati-Sarapardeh A.; Ayatollahi S.; Ghazanfari M.-H.; Masihi M. Experimental Determination of Interfacial Tension and Miscibility of the CO 2 – Crude Oil System; Temperature, Pressure, and Composition Effects. Journal of Chemical & Engineering Data 2014, 59 (1), 61–69. 10.1021/je400811h. [DOI] [Google Scholar]
- Liu Y.; Jiang L.; Tang L.; Song Y.; Zhao J.; Zhang Y.; Wang D.; Yang M. Minimum miscibility pressure estimation for a CO 2/n-decane system in porous media by X-ray CT. Experiments in Fluids 2015, 56, 1–9. 10.1007/s00348-015-2025-4. [DOI] [Google Scholar]
- Liu Y.; Jiang L.; Song Y.; Zhao Y.; Zhang Y.; Wang D. Estimation of minimum miscibility pressure (MMP) of CO2 and liquid n-alkane systems using an improved MRI technique. Magn. Reson. Imaging 2016, 34 (2), 97–104. 10.1016/j.mri.2015.10.035. [DOI] [PubMed] [Google Scholar]
- Cui P.; Liu Z.; Cui X.; Li Y.; Du D. Impact of Water on Miscibility Characteristics of the CO2/n-Hexadecane System using the Pendant Drop Shape Analysis Method. Arabian Journal of Chemistry 2023, 16, 105038 10.1016/j.arabjc.2023.105038. [DOI] [Google Scholar]
- Cui X.; Zheng L.; Liu Z.; Cui P.; Du D. Determination of the minimum miscibility pressure of the CO2/oil system based on quantification of the oil droplet volume reduction behavior. Colloids Surf., A 2022, 653, 130058 10.1016/j.colsurfa.2022.130058. [DOI] [Google Scholar]
- Harmon R. A.; Grigg R. B. Vapor-density measurement for estimating minimum miscibility pressure. SPE reservoir engineering 1988, 3 (04), 1215–1220. 10.2118/15403-PA. [DOI] [Google Scholar]
- Li N.; Tian J.; Tan X. H.; et al. Microscopic sweeping characteristics of CO2 flooding in low permeability reservoirs. Fault-Block Oil & Gas Field 2015, 22 (2), 237–239. [Google Scholar]
- Ahmadi M.-A.; Ebadi M. Fuzzy Modeling and Experimental Investigation of Minimum Miscible Pressure in Gas Injection Process. Fluid Phase Equilib. 2014, 378, 1–12. 10.1016/j.fluid.2014.06.022. [DOI] [Google Scholar]
- Sayyad H.; Manshad A. K.; Rostami H. Application of hybrid neural particle swarm optimization algorithm for prediction of MMP. Fuel 2014, 116, 625–633. 10.1016/j.fuel.2013.08.076. [DOI] [Google Scholar]
- Hao Y. M.; Chen Y. M.; Yu H. L.. Determination and prediction of minimum miscibility pressure in CO2 flooding. Editorial Department of Petroleum Geology and Recovery Efficiency, 2005, 12 ( (6), ). http://yqdzycsl.cnjournals.com/pgreen/article/abstract/050621.
- Chen G.; Gao H.; Fu K.; Zhang H.; Liang Z.; Tontiwachwuthikul P. An improved correlation to determine minimum miscibility pressure of CO2–oil system. Green Energy & Environment 2020, 5 (1), 97–104. 10.1016/j.gee.2018.12.003. [DOI] [Google Scholar]
- Hassan A.; Elkatatny S.; Abdulraheem A. Intelligent Prediction of Minimum Miscibility Pressure (MMP) During CO2 Flooding Using Artificial Intelligence Techniques. Sustainability 2019, 11 (24), 7020. 10.3390/su11247020. [DOI] [Google Scholar]
- Ye A. P. Experience formula of determining minimum miscible pressure in CO2 flooding. Fault-Block Oil & Gas Field 2009, 05, 75–76. [Google Scholar]
- Shuai W.; Taichao W.; Yunyan G.; Hao L.; Yang X. Summary of prediction methods for minimum miscible pressure of CO2 flooding. Petrochemical Industry Technology 2019, 26 (11), 52. [Google Scholar]
- Johnson J. P.; Pollin J. S.. Measurement and correlation of CO2 miscibility pressures. In SPE Improved Oil Recovery Conference; Tulsa, OK, USA, 17–20 April 1981; p SPE. 10.2118/9790-MS. [DOI] [Google Scholar]
- Glaso Ø. Generalized minimum miscibility pressure correlation. Society of Petroleum Engineers Journal 1985, 25 (06), 927–934. 10.2118/12893-PA. [DOI] [Google Scholar]
- Alston R. B.; Kokolis G. P.; James C. F. CO2 minimum Miscibility Pressure: A Correlation for Impure CO2 Streams and Live Oil Systems. Society of Petroleum Engineers Journal 1985, 25 (02), 268–274. 10.2118/11959-PA. [DOI] [Google Scholar]
- Zhao Y. J.; Song K. P.; Fan G. J. The Adaptability Research of Experience Formula Method for Predicting The Minimum Miscible Pressure in Test Area. Mathematics In Practice And Theory 2017, 18, 285–289. [Google Scholar]
- Yellig W. F.; Metcalfe R. S. Determination and Prediction of CO2 minimum Miscibility Pressures (includes associated paper 8876). Journal of Petroleum Technology 1980, 32 (01), 160–168. 10.2118/7477-PA. [DOI] [Google Scholar]
- Yurkiw F.J.; Flock D.L. A Comparative Investigation of Minimum Miscibility Pressure Correlations For Enhanced Oil Recovery. J. Can. Pet. Technol. 1994, 10.2118/94-08-04. [DOI] [Google Scholar]
- Salari Sardari F.; Khorsand Movaghar M. R. A simulation approach to achieve the best miscible enrichment in gas flooding and chemical injection process for enhanced oil recovery: Slim Tube Simulation for Minimum Miscible Enrichment. Asia-Pacific Journal of Chemical Engineering 2017, 12 (2), 230–246. 10.1002/apj.2067. [DOI] [Google Scholar]
- Van der Waals J. D.Over de Continuiteit van den Gas-en Vloeistoftoestand. Sijthoff 1873, 1. [Google Scholar]
- Redlich O.; Kwong J. N. On the thermodynamics of solutions. V. An equation of state. Fugacities of gaseous solutions.. Chem. Rev. 1949, 44 (1), 233–244. 10.1021/cr60137a013. [DOI] [PubMed] [Google Scholar]
- Soave G. Equilibrium constants from a modified Redlich-Kwong equation of state. Chemical engineering science 1972, 27 (6), 1197–1203. 10.1016/0009-2509(72)80096-4. [DOI] [Google Scholar]
- Peng D. Y.; Robinson D. B. A new two-constant equation of state. Industrial & Engineering Chemistry Fundamentals 1976, 15 (1), 59–64. 10.1021/i160057a011. [DOI] [Google Scholar]
- Robinson D. B.; Peng D. Y.. The characterization of the heptanes and heavier fractions for the GPA Peng-Robinson programs. Gas processors association 1978. [Google Scholar]
- Choubineh A.; Helalizadeh A.; Wood D. A. The impacts of gas impurities on the minimum miscibility pressure of injected CO2-rich gas–crude oil systems and enhanced oil recovery potential. Petroleum Science 2019, 16 (1), 117–126. 10.1007/s12182-018-0256-8. [DOI] [Google Scholar]
- Teklu T. W.; Ghedan S. G.; Graves R. M.; Yin X.. Minimum Miscibility Pressure Determination: Modified Multiple Mixing Cell Method. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012. 10.2118/155454-MS. [DOI]
- Chen H.; Zhang C.; Jia N.; Duncan I.; Yang S.; Yang Y. A machine learning model for predicting the minimum miscibility pressure of CO2 and crude oil system based on a support vector machine algorithm approach. Fuel 2021, 290, 120048 10.1016/j.fuel.2020.120048. [DOI] [Google Scholar]
- Lv Q.; Zheng R.; Guo X.; Larestani A.; Hadavimoghaddam F.; Riazi M.; Hemmati-Sarapardeh A.; Wang K.; Li J. Modelling minimum miscibility pressure of CO2-crude oil systems using deep learning, tree-based, and thermodynamic models: Application to CO2 sequestration and enhanced oil recovery. Sep. Purif. Technol. 2023, 310, 123086 10.1016/j.seppur.2022.123086. [DOI] [Google Scholar]
- Emera M. K.; Sarma H. K. Use of genetic algorithm to estimate CO2–oil minimum miscibility pressure—A key parameter in design of CO2 miscible flood. J. Pet. Sci. Eng. 2005, 46 (1–2), 37–52. 10.1016/j.petrol.2004.10.001. [DOI] [Google Scholar]
- Shokrollahi A.; Arabloo M.; Gharagheizi F.; Mohammadi A. H. Intelligent model for prediction of CO2 – Reservoir oil minimum miscibility pressure. Fuel 2013, 112, 375–384. 10.1016/j.fuel.2013.04.036. [DOI] [Google Scholar]
- Hemmati-Sarapardeh A.; Ghazanfari M.-H.; Ayatollahi S.; Masihi M. Accurate determination of the CO2 -crude oil minimum miscibility pressure of pure and impure CO 2 streams: A robust modelling approach. Canadian Journal of Chemical Engineering 2016, 94 (2), 253–261. 10.1002/cjce.22387. [DOI] [Google Scholar]
- Valluri M. K.; Mishra S.; Schuetter J. An improved correlation to estimate the minimum miscibility pressure of CO 2 in crude oils for carbon capture, utilization, and storage projects. J. Pet. Sci. Eng. 2017, 158, 408–415. 10.1016/j.petrol.2017.08.059. [DOI] [Google Scholar]
- Hamdi Z.; Chenxi D.. Accurate Prediction of CO2 minimum Miscibility Pressure Using Adaptive Neuro-Fuzzy Inference Systems. In Proceedings of the SPE Gas & Oil Technology Showcase and Conference, Dubai, United Arab Emirates, 21–23 October 2019; p D022S026R002. 10.2118/198553-MS. [DOI]
- Dargahi-Zarandi A.; Hemmati-Sarapardeh A.; Shateri M.; Menad N. A.; Ahmadi M. Modeling minimum miscibility pressure of pure/impure CO2-crude oil systems using adaptive boosting support vector regression: Application to gas injection processes. J. Pet. Sci. Eng. 2020, 184, 106499 10.1016/j.petrol.2019.106499. [DOI] [Google Scholar]
- Sinha U.; Dindoruk B.; Soliman M. Prediction of CO2 minimum Miscibility Pressure Using an Augmented Machine-Learning-Based Model. SPE Journal 2021, 26 (04), 1666–1678. 10.2118/200326-PA. [DOI] [Google Scholar]
- Al-Khafaji H. F.; Meng Q.; Hussain W.; Mohammed R. K.; Harash F.; AlFakey S. A. Predicting minimum miscible pressure in pure CO2 flooding using machine learning: Method comparison and sensitivity analysis. Fuel 2023, 354, 129263 10.1016/j.fuel.2023.129263. [DOI] [Google Scholar]
- Liu B.; Wang Z.; Jin Y.; Ge Z.; Xu C.; Liu H.; Chen H. Novel Way to Predict the MMP of a CO2–Oil System Using Stacking Models. Energy Fuels 2023, 37 (2), 935–944. 10.1021/acs.energyfuels.2c03033. [DOI] [Google Scholar]
- Karkevandi-Talkhooncheh A.; Rostami A.; Hemmati-Sarapardeh A.; Ahmadi M.; Husein M. M.; Dabir B. Modeling minimum miscibility pressure during pure and impure CO2 flooding using hybrid of radial basis function neural network and evolutionary techniques. Fuel 2018, 220, 270–282. 10.1016/j.fuel.2018.01.101. [DOI] [Google Scholar]
- Dindoruk B.; Johns R.; Orr F. M. Measurement and Modeling of Minimum Miscibility Pressure: A State-of-the-Art Review. SPE Reservoir Evaluation & Engineering 2021, 24 (02), 367–389. 10.2118/200462-PA. [DOI] [Google Scholar]
- Almobarak M.; Holmes S.; Wood C. D.; Myers M. B.; Saeedi A.; Xie Q. Effect of Functional Groups on Chemical-Assisted MMP Reduction of a Methane-Oil System. Energy Fuels 2021, 35 (18), 14519–14526. 10.1021/acs.energyfuels.1c01479. [DOI] [Google Scholar]
- Janna F.; Le-Hussain F. Effectiveness of modified CO2 injection at improving oil recovery and CO2 storage—Review and simulations. Energy Reports 2020, 6, 1922–1941. 10.1016/j.egyr.2020.07.008. [DOI] [Google Scholar]
- Hu W.; Wei Y.; Bao J. Development of the theory and technology for low permeability reservoirs in China. Petroleum Exploration and Development 2018, 45 (4), 685–697. 10.1016/S1876-3804(18)30072-7. [DOI] [Google Scholar]
- Zhang G. D.; Liu J. Y.; Liu Y. L.; et al. Study on reducing miscibility pressure in CO2 flooding by miscible solvent. Special Oil & Gas Reservoirs 2013, 20 (2), 115. [Google Scholar]
- Stalkup F. I. Carbon Dioxide Miscible Flooding: Past, Present, And Outlook for the Future. Journal of Petroleum Technology 1978, 30 (08), 1102–1112. 10.2118/7042-PA. [DOI] [Google Scholar]
- Tang A. N. G.; Xuemei Z. H. A. O.; Yang W. A. N. G. Analysis of influence factor of minimum miscible pressure of CO2. Reservoir Evaluation and Development 2018, 8 (4), 42–45. [Google Scholar]
- Bon J.; Sarma H. K.; Theophilos A. M.. An investigation of minimum miscibility pressure for CO2-rich injection gases with pentanes-plus fraction. In SPE international improved oil recovery conference in Asia Pacific. In Proceedings of the SPE International Improved Oil Recovery Conference in Asia Pacific, Kuala Lumpur, Malaysia; 5–6 December 2005; pp SPE–97536. 10.2118/97536-MS. [DOI]
- Javid K.; Khalifeh H. A.; Belhaj H.; Haroun M.. Improving MMP by Co-Injection of Miscible CO2 With Abu Dhabi Crude Oil. Volume 6: Polar and Arctic Sciences and Technology; Offshore Geotechnics; Petroleum Technology Symposium, V006T11A005, 2013. 10.1115/OMAE2013-10258. [DOI]
- Peng C.; Liu J. Y.; Zhang G. D.; Peng Y. J.; Guo K. A new method of reducing the miscible-phase pressure of CO2 flooding. Journal of Chongqing University of Science and Technology (Natural Science edition) 2012, 14 (1), 48–51. [Google Scholar]
- Jeong M. S.; Lee K. S. Simulation Study on Miscibility Effect of CO 2 /Solvent Injection for Enhanced Oil Recovery at Nonisothermal Conditions. Mathematical Problems in Engineering 2016, 2016, 1–9. 10.1155/2016/2562971. [DOI] [Google Scholar]
- Permadi A. K.; Pratama E. A.; Hakim A. L. L.; Widi A. K.; Abdassah D. The Effect of Carbonyl and Hydroxyl Compounds Addition on CO2 Injection through Hydrocarbon Extraction Processes. Applied Sciences 2021, 11 (1), 159. 10.3390/app11010159. [DOI] [Google Scholar]
- Yang Z.; Wu W.; Dong Z.; Lin M.; Zhang S.; Zhang J. Reducing the minimum miscibility pressure of CO2 and crude oil using alcohols. Colloids Surf., A 2019, 568, 105–112. 10.1016/j.colsurfa.2019.02.004. [DOI] [Google Scholar]
- Saira S.; Yin H.; Le-Hussain F.. Using Alcohol-Treated CO2 to Reduce Miscibility Pressure during CO2 Injection. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Virtual, 17–19 November 2020; p D013S101R012. 10.2118/202341-MS. [DOI]
- Liu J.; Sun L.; Li Z.; Wu X. Experimental Study on Reducing CO2–Oil Minimum Miscibility Pressure with Hydrocarbon Agents. Energies 2019, 12 (10), 1975. 10.3390/en12101975. [DOI] [Google Scholar]
- Li Z.; Xi L.; Zhang C.; Wang M. Progress of Reducing Miscibility Pressure between CO 2 and Crude Oil by CO 2-soluble Surfactant. Oilfield Chemistry 2020, 37 (4), 745–751. 10.19346/j.cnki.1000-4092.2020.04.030. [DOI] [Google Scholar]
- Ma C.; Dou X.; Liu Z.; Liao P.; Zhu Z.; Liu K.; Huang J. Application and Mechanism of a Novel CO2-Oil Miscible Flooding Agent, CAA8-X. Acta Physico-Chimica Sinica 2021, 38 (8), 201201. 10.3866/PKU.WHXB202012019. [DOI] [Google Scholar]
- Li L.; Zhou X.; Wang R.; Zhang X.; Ma S.; Su Y.; Wang C.; Luo W.; Sun H. Microscopic experiment study on mechanisms of oil-gas interaction and CO2 -surfactant flooding with different temperatures and pressures. Journal of CO2 Utilization 2023, 69, 102389 10.1016/j.jcou.2022.102389. [DOI] [Google Scholar]
- Bera A.; Belhaj H. Ionic liquids as alternatives of surfactants in enhanced oil recovery—A state-of-the-art review. J. Mol. Liq. 2016, 224, 177–188. 10.1016/j.molliq.2016.09.105. [DOI] [Google Scholar]
- Lv W.; Gong H.; Li Y.; Li Z.; Dong M. The potential and mechanism of nonionic polyether surfactants dissolved in CO2 to improve the miscibility of CO2–hydrocarbon systems. Fuel 2022, 326, 125012 10.1016/j.fuel.2022.125012. [DOI] [Google Scholar]
- Wu S.; He J.; Li Z. Z.; Lin W. M.; Wang Q. T.; Wu F. Y.; Zhang J. J. Studies on the chemical agent system for reducing in the minimal miscibility pressure of CO2 flooding. China Science paper 2015, 10 (18), 2161–2164. [Google Scholar]
- Shen Y.; Lin H.; Zhao L.; Zeng Z.; Gong Y.; Guo R.; Min F. Hydrocarbon MigrationAccumulation Characteristics and PoolForming Patterns in OverlapErosion Zones in Northwestern Margin of Junggar Basin. Xinjiang Petroleum Geology 2015, 36 (5), 1. 10.7657/XJPG20150501. [DOI] [Google Scholar]
- Qayyimah M. A.; Awang M.; Yusup S. Fatty acid methyl ester from rubber seed oil as additives in reducing the minimum miscibility pressure of CO2 and crude oil. Journal of Applied Sciences 2016, 16 (11), 542–548. 10.3923/jas.2016.542.548. [DOI] [Google Scholar]
- Qayyimah M. A.; Awang M.. Palm Fatty Acid Methyl Ester in Reducing Interfacial Tension in CO2–Crude Oil Systems. In ICIPEG 2016: Proceedings of the International Conference on Integrated Petroleum Engineering and Geosciences; Springer Singapore, 2017; pp 217–227. 10.1007/978-981-10-3650-7_18. [DOI]
- Sun N.; Baihe F. Study on reducing the minimum miscibility pressure (MMP) of CO2 flooding by miscible solvent method. Liaoning Chem. Ind. 2017, 2, 135–136. [Google Scholar]
- Guo P.; Hu Y.; Qin J.; Li S.; Jiao S.; Chen F.; He J. Use of oil-soluble surfactant to reduce minimum miscibility pressure. Petroleum Science and Technology 2017, 35 (4), 345–350. 10.1080/10916466.2016.1259630. [DOI] [Google Scholar]
- Luo H.; Zhang Y.; Fan W.; Nan G.; Li Z. Effects of the Non-ionic Surfactant (C i PO j) on the Interfacial Tension Behavior between CO 2 and Crude Oil. Energy Fuels 2018, 32 (6), 6708–6712. 10.1021/acs.energyfuels.8b01082. [DOI] [Google Scholar]
- Zhang L. Development of chemical system for reducing minimum miscible pressure during CO2 flooding. Editorial Department of Petroleum Geology and Recovery Efficiency 2020, 27 (1), 45–49. [Google Scholar]
- Zhao Y.; Fan G.; Song K.; Li Y.; Chen H.; Sun H. The experimental research for reducing the minimum miscibility pressure of carbon dioxide miscible flooding. Renewable and Sustainable Energy Reviews 2021, 145, 111091 10.1016/j.rser.2021.111091. [DOI] [Google Scholar]
- Almobarak M.; Wu Z.; Myers M. B.; Wood C. D.; Al Maskari N. S.; Liu Y.; Rommerskirchen R.; Saeedi A.; Xie Q. Chemical-assisted minimum miscibility pressure reduction between oil and methane. J. Pet. Sci. Eng. 2021, 196, 108094 10.1016/j.petrol.2020.108094. [DOI] [Google Scholar]
- Sun D.; Zhang G.; Peng X. Experiment on the mechanism of enhancing oil recovery by CO2 flooding with gas- soluble demixing agent. Special Oil & Gas Reservoirs 2022, 29 (1), 134–140. [Google Scholar]







