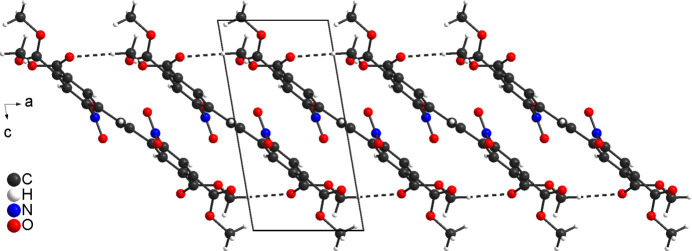
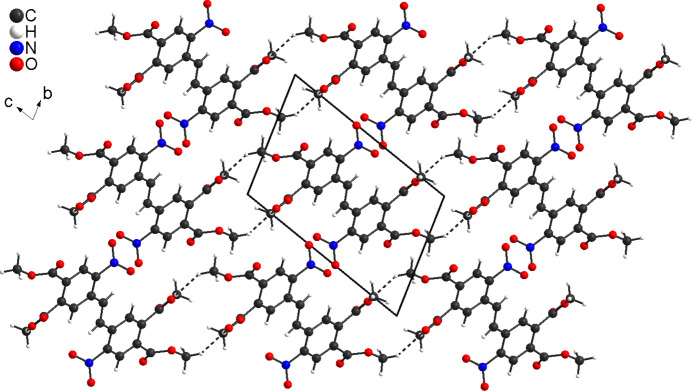
In the crystal structure of the title compound the two phenyl rings are coplanar, whereas the nitro and the two methyl ester groups are rotated out of the ring plane. The molecules are linked by intermolecular C—H⋯O hydrogen bonding into a tri-periodic network.
Keywords: crystal structure, synthesis, stilbene derivative, diazocine
Abstract
The title compound, C22H18N2O12, was obtained as a by-product during the planned synthesis of 1,2-bis(2-nitro-4,5-dimethyl phthalate)ethane by oxidative dimerization starting from dimethyl-4-methyl-5-nitro phthalate. To identify this compound unambiguously, a single-crystal structure analysis was performed. The asymmetric unit consists of half a molecule that is located at a centre of inversion. As a result of symmetry restrictions, the molecule shows an E configuration around the double bond. Both phenyl rings are coplanar, whereas the nitro and the two methyl ester groups are rotated out of the ring plane by 32.6 (1), 56.5 (2) and 49.5 (2)°, respectively. In the crystal, molecules are connected into chains extending parallel to the a axis by pairs of C—H⋯O hydrogen bonds that are connected into a tri-periodic network by additional C—H⋯O hydrogen-bonding interactions.
1. Chemical context
In recent years, molecular photoswitches have gained much attraction because of their wide range of potential applications, e.g. as photoresponsive materials (Pang et al., 2019 ▸) or as drugs (Kobauri et al., 2023 ▸). Bridged azobenzenes, so-called diazocines, are photoswitches, in which the thermodynamically stable Z isomer can be reversibly converted to the metastable E isomer through irradiation with visible light of different wavelengths (Fig. 1 ▸). Compared to azobenzenes, these compounds exhibit superior photophysical properties such as well-separated absorption bands, high quantum yields and high switching efficiencies (Siewertsen et al., 2009 ▸). Additionally, the light-driven E/Z isomerization leads to a reversible molecular movement between the bent, sterically demanding Z, and the stretched E isomer (Moormann et al., 2019 ▸), which can be used for reversible expansion and contraction between polymer strands (Burk et al., 2023 ▸) or reversible receptor–substrate binding (Cabré et al., 2019 ▸; Ewert et al., 2022 ▸).
Figure 1.
Light-induced reversible isomerization between the thermodynamically stable Z and the metastable E isomer of the parent diazocine with different wavelengths in the visible range. In addition, thermal relaxation leads to re-isomerization.
The general synthesis of diazocines usually includes two key reactions: the formation of the ethylene unit and the azo group. Common synthesis strategies for C—C linkage include an oxidative dimerization (Moormann et al., 2017 ▸), a Sonogashira cross-coupling (Maier et al., 2019 ▸), Wittig reaction (Samanta et al., 2012 ▸) or organolithium-mediated reductive couplings (Li et al., 2020 ▸). In contrast, N—N formation is usually achieved by reductive/oxidative coupling starting from dinitro/diamino compounds (Moormann et al., 2017 ▸; Maier et al., 2019 ▸; Klockmann et al., 2021 ▸) or by a Cu-catalysed cascade reaction using diiodide compounds (Li et al., 2020 ▸). Unfortunately, late-stage functionalization after formation of the diazocine ring is difficult. Therefore, substituents have to be introduced at an earlier stage in synthesis, ideally before the oxidative C—C bond-formation stage.
Along these lines, we aimed at the synthesis of a tetramethylester substituted diazocine with two ester groups each in the meta and para positions to the azo group. After ester hydrolysis, the carboxylic acids were converted to the cyclic anhydrides, which were reacted with different amines to yield the corresponding imides. The tetraester, therefore, is an ideal precursor for further functionalization of the diazocine chromophore.

Starting from commercially available 4-methylphthalic anhydride, we carried out nitration and esterification reactions according to literature procedures (Hao et al., 2019 ▸) yielding dimethyl-4-methyl-5-nitro phthalate (1, Fig. 2 ▸). Dimerization of 1 by oxidative C—C bond formation was achieved through consecutive addition of potassium tert-butoxide and bromine in tetrahydrofuran yielding a crude product. According to 1H NMR spectroscopy, the raw material contained a structurally similar by-product in addition to the expected main product 1,2-bis(2-nitro-4,5-dimethyl phthalate)ethane (2, Fig. 2 ▸). From vapour diffusion experiments of the crude product, we obtained crystals of the pure product, which were characterized by single crystal structure analysis, proving that (E)-1,2-bis(2-nitro-4,5-dimethyl phthalate)ethene, C22H18N2O12, (3) has formed as by-product (Fig. 2 ▸).
Figure 2.
Reaction scheme to obtain the title compound (3) as a by-product.
2. Structural commentary
The asymmetric unit of 3 consists of half of a molecule that is located at a centre of inversion (Fig. 3 ▸). As a result of symmetry restrictions, the molecule shows the E configuration around the double bond, which can be traced back to steric hindrance. Both phenyl rings are oriented in a coplanar fashion (Fig. 4 ▸). The nitro group is rotated out of the phenyl ring plane by 32.6 (1)°, whereas the dihedral angles between the six-membered ring and the two methyl ester groups amount to 56.5 (2) and 49.5 (2)°, respectively.
Figure 3.
Crystal structure of the title compound with labelling and displacement ellipsoids drawn at the 50% probability level. [Symmetry code: (i) −x, −y + 1, −z + 1.]
Figure 4.
Side view of the title compound showing the torsion of the nitro and the ester groups.
3. Supramolecular features
In the crystal of 3, the molecules are connected into chains by centrosymmetric pairs of C—H⋯O hydrogen bonds between the methyl hydrogen atom H11C and the carbonyl oxygen atom O5 (Fig. 5 ▸). The C—H⋯O angle is close to linearity, indicating that this is a significant interaction (Table 1 ▸). These chains propagate parallel to the a axis, with each chain surrounded by six neighbouring chains (Fig. 6 ▸). The chains are additionally linked into a tri-periodic network by centrosymmetric pairs of C—H⋯O hydrogen bonds between the methyl hydrogen atom H9B and the carbonyl O atoms O5, forming 16-membered rings that are located around centres of inversion (Fig. 6 ▸). The corresponding O⋯H distance and the C–H⋯O angle point to a weaker interaction (Table 1 ▸). There is one additional C—H⋯O hydrogen bond but with a significant longer O⋯H distances (Table 1 ▸), which consolidates the packing. Finally, the molecules are arranged in a way that phenyl rings of neighbouring molecules are parallel but the ring planes are shifted relative to each other and the distance between the centroids of the six-membered rings amount to 4.144 (1) Å, which does not point to significant π–π interactions (Fig. 7 ▸).
Figure 5.
Crystal structure of the title compound along the b axis in a view of the hydrogen-bonded chains. Intermolecular C—H⋯O hydrogen bonding is shown as dashed lines.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C9—H9B⋯O5i | 0.98 | 2.42 | 3.308 (2) | 150 |
| C11—H11A⋯O3ii | 0.98 | 2.52 | 3.3650 (19) | 144 |
| C11—H11C⋯O5iii | 0.98 | 2.39 | 3.364 (2) | 173 |
Symmetry codes: (i)
 ; (ii)
; (ii)
 ; (iii)
; (iii)
 .
.
Figure 6.
Crystal structure of the title compound in a view along the a axis. Intermolecular C—H⋯O hydrogen bonding is shown as dashed lines.
Figure 7.
View of two neighbouring molecules. The distance between the centroids of the six-membered rings is given.
4. Database survey
A search of the CSD (version 5.43, last update March 2023, Groom et al., 2016 ▸) using CONQUEST (Bruno et al., 2002 ▸) revealed that thousands of stilbene derivatives are reported. With only nitro groups in an ortho-position, only three hits are found, including trans-1,1′-(ethene-1,2-diyl)-bis(2-nitrobenzene [1,2-bis(2-nitrophenyl)ethene] or trans-2,2′-dinitrostilben (refcodes WIXJIZ and WIXJIZ01, Bulatov & Haukka, 2019 ▸; Blelloch et al., 2021 ▸). In addition, a hydrate of trans-1,1′-(ethene-1,2-diyl)-bis(4-carboxylato-2-nitrobenzene) (refcode JAWYIS, Song et al., 2017 ▸) matches the search criterion. Finally, there is one zinc carboxylate compound with carboxylate groups in the 4-position (refcode BOZYOG, Li et al. 2014 ▸). With each two carboxylate or ester groups in ortho positions to each other, no hits are found. In fact, there is no compound reported in the CCDC that is more closely related to the title compound.
5. Synthesis and crystallization
General
Dimethyl-4-methyl-5-nitro phthalate (1) was prepared according to the literature (Hao et al., 2019 ▸) starting from 4-methylphthalic anhydride (> 98%), which was purchased from TCI. Potassium tert-butoxide (> 97%) was purchased from TCI and bromine (99%) from Thermo Scientific. Tetrahydrofuran (99.9%) was purchased from Fisher Scientific and dried using the solvent purification system PureSolv MD 5 from Inert Corporation.
Synthesis
Under a nitrogen atmosphere, dimethyl-4-methyl-5-nitro phthalate (1, 10.0 g, 39.5 mmol) was dissolved in dry tetrahydrofuran (330 ml) and cooled to 263 K. Potassium tert-butoxide (5.76 g, 51.3 mmol) was added in one portion. The reaction mixture was stirred for 30 s, whereupon bromine (2.02 ml, 39.5 mmol) was immediately added. After complete addition, the reaction mixture was stirred at 263 K for 10 min and then quenched with ice. The precipitate was filtered off, washed with the smallest possible amount of ice-cold ethyl acetate and dried in vacuo. The crude product was obtained as a pale-yellow solid.
Crystallization
Single crystals of 3 were obtained by vapour diffusion using chloroform/methanol as solvent/antisolvent.
6. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. C-bound hydrogen atoms were positioned with idealized geometry (methyl H atoms allowed to rotate but not to tip) and were refined isotropically with U ĩso(H) = 1.2 U eq(C) (1.5 for methyl hydrogen atoms) using a riding model.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C22H18N2O12 |
| M r | 502.38 |
| Crystal system, space group | Triclinic, P

|
| Temperature (K) | 100 |
| a, b, c (Å) | 5.9454 (2), 7.9543 (3), 12.0673 (4) |
| α, β, γ (°) | 72.124 (3), 79.661 (3), 86.052 (3) |
| V (Å3) | 534.25 (3) |
| Z | 1 |
| Radiation type | Cu Kα |
| μ (mm−1) | 1.12 |
| Crystal size (mm) | 0.19 × 0.08 × 0.02 |
| Data collection | |
| Diffractometer | XtaLAB Synergy, Dualflex, HyPix |
| Absorption correction | Multi-scan (CrysAlis PRO; Rigaku OD, 2022 ▸) |
| T min, T max | 0.791, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 5274, 2211, 2042 |
| R int | 0.020 |
| (sin θ/λ)max (Å−1) | 0.639 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.043, 0.125, 1.09 |
| No. of reflections | 2211 |
| No. of parameters | 165 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.37, −0.30 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989024002676/wm5712sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989024002676/wm5712Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989024002676/wm5712Isup3.cml
CCDC reference: 2342598
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
This work was supported by the State of Schleswig-Holstein.
supplementary crystallographic information
Crystal data
| C22H18N2O12 | Z = 1 |
| Mr = 502.38 | F(000) = 260 |
| Triclinic, P1 | Dx = 1.561 Mg m−3 |
| a = 5.9454 (2) Å | Cu Kα radiation, λ = 1.54184 Å |
| b = 7.9543 (3) Å | Cell parameters from 3255 reflections |
| c = 12.0673 (4) Å | θ = 3.9–78.8° |
| α = 72.124 (3)° | µ = 1.12 mm−1 |
| β = 79.661 (3)° | T = 100 K |
| γ = 86.052 (3)° | Plate, colourless |
| V = 534.25 (3) Å3 | 0.19 × 0.08 × 0.02 mm |
Data collection
| XtaLAB Synergy, Dualflex, HyPix diffractometer | 2211 independent reflections |
| Radiation source: micro-focus sealed X-ray tube, PhotonJet (Cu) X-ray Source | 2042 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.020 |
| Detector resolution: 10.0000 pixels mm-1 | θmax = 80.1°, θmin = 3.9° |
| ω scans | h = −7→7 |
| Absorption correction: multi-scan (CrysAlisPro; Rigaku OD, 2022) | k = −9→6 |
| Tmin = 0.791, Tmax = 1.000 | l = −15→15 |
| 5274 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.043 | H-atom parameters constrained |
| wR(F2) = 0.125 | w = 1/[σ2(Fo2) + (0.071P)2 + 0.2093P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.09 | (Δ/σ)max < 0.001 |
| 2211 reflections | Δρmax = 0.37 e Å−3 |
| 165 parameters | Δρmin = −0.30 e Å−3 |
| 0 restraints |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.0359 (3) | 0.5721 (2) | 0.51241 (15) | 0.0281 (3) | |
| H1 | −0.031345 | 0.684945 | 0.481716 | 0.034* | |
| C2 | 0.2167 (3) | 0.55372 (19) | 0.58634 (13) | 0.0233 (3) | |
| C3 | 0.3159 (3) | 0.69811 (19) | 0.60246 (13) | 0.0227 (3) | |
| C4 | 0.4711 (3) | 0.6807 (2) | 0.67872 (13) | 0.0231 (3) | |
| H4 | 0.530524 | 0.782718 | 0.687774 | 0.028* | |
| C5 | 0.5389 (3) | 0.5136 (2) | 0.74157 (13) | 0.0228 (3) | |
| C6 | 0.4506 (3) | 0.36589 (19) | 0.72551 (13) | 0.0232 (3) | |
| C7 | 0.2901 (3) | 0.3868 (2) | 0.65094 (14) | 0.0240 (3) | |
| H7 | 0.228245 | 0.284508 | 0.643620 | 0.029* | |
| N1 | 0.2610 (2) | 0.88106 (17) | 0.53758 (12) | 0.0251 (3) | |
| O1 | 0.2657 (2) | 0.99488 (15) | 0.58698 (11) | 0.0322 (3) | |
| O2 | 0.2220 (2) | 0.91252 (15) | 0.43721 (11) | 0.0326 (3) | |
| C8 | 0.6966 (3) | 0.50015 (19) | 0.82811 (13) | 0.0232 (3) | |
| O3 | 0.8602 (2) | 0.59305 (14) | 0.80816 (10) | 0.0283 (3) | |
| O4 | 0.62797 (19) | 0.37663 (14) | 0.93005 (9) | 0.0258 (3) | |
| C9 | 0.7826 (3) | 0.3411 (2) | 1.01503 (15) | 0.0302 (4) | |
| H9A | 0.933156 | 0.306373 | 0.979732 | 0.045* | |
| H9B | 0.722258 | 0.245232 | 1.085262 | 0.045* | |
| H9C | 0.796944 | 0.447755 | 1.037561 | 0.045* | |
| C10 | 0.5267 (3) | 0.1811 (2) | 0.78464 (14) | 0.0259 (3) | |
| O5 | 0.3990 (2) | 0.05833 (16) | 0.82506 (13) | 0.0407 (3) | |
| O6 | 0.75063 (19) | 0.17046 (14) | 0.78393 (10) | 0.0261 (3) | |
| C11 | 0.8374 (3) | −0.0025 (2) | 0.84384 (17) | 0.0332 (4) | |
| H11A | 0.806798 | −0.087882 | 0.804802 | 0.050* | |
| H11B | 0.761355 | −0.039755 | 0.926402 | 0.050* | |
| H11C | 1.002594 | 0.003374 | 0.840751 | 0.050* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0292 (8) | 0.0238 (8) | 0.0394 (9) | 0.0083 (6) | −0.0167 (7) | −0.0170 (6) |
| C2 | 0.0255 (7) | 0.0214 (7) | 0.0276 (7) | 0.0048 (5) | −0.0089 (6) | −0.0128 (6) |
| C3 | 0.0273 (7) | 0.0172 (7) | 0.0268 (7) | 0.0060 (5) | −0.0088 (6) | −0.0099 (5) |
| C4 | 0.0268 (7) | 0.0186 (7) | 0.0279 (7) | 0.0021 (5) | −0.0088 (6) | −0.0110 (6) |
| C5 | 0.0243 (7) | 0.0207 (7) | 0.0264 (7) | 0.0035 (5) | −0.0081 (6) | −0.0100 (6) |
| C6 | 0.0255 (7) | 0.0188 (7) | 0.0276 (7) | 0.0033 (5) | −0.0082 (6) | −0.0093 (6) |
| C7 | 0.0268 (7) | 0.0187 (7) | 0.0316 (8) | 0.0030 (5) | −0.0105 (6) | −0.0124 (6) |
| N1 | 0.0276 (6) | 0.0196 (6) | 0.0319 (7) | 0.0046 (5) | −0.0117 (5) | −0.0107 (5) |
| O1 | 0.0396 (7) | 0.0196 (6) | 0.0455 (7) | 0.0080 (4) | −0.0185 (5) | −0.0169 (5) |
| O2 | 0.0418 (7) | 0.0262 (6) | 0.0326 (6) | 0.0038 (5) | −0.0183 (5) | −0.0071 (5) |
| C8 | 0.0282 (7) | 0.0166 (7) | 0.0289 (7) | 0.0053 (5) | −0.0105 (6) | −0.0110 (6) |
| O3 | 0.0309 (6) | 0.0226 (6) | 0.0356 (6) | 0.0005 (4) | −0.0138 (5) | −0.0102 (4) |
| O4 | 0.0327 (6) | 0.0202 (5) | 0.0272 (5) | 0.0016 (4) | −0.0126 (4) | −0.0069 (4) |
| C9 | 0.0366 (8) | 0.0265 (8) | 0.0311 (8) | 0.0045 (6) | −0.0171 (7) | −0.0085 (6) |
| C10 | 0.0306 (8) | 0.0193 (7) | 0.0319 (8) | 0.0021 (6) | −0.0137 (6) | −0.0094 (6) |
| O5 | 0.0386 (7) | 0.0223 (6) | 0.0605 (8) | −0.0023 (5) | −0.0246 (6) | −0.0018 (5) |
| O6 | 0.0297 (6) | 0.0185 (5) | 0.0333 (6) | 0.0066 (4) | −0.0129 (4) | −0.0097 (4) |
| C11 | 0.0373 (9) | 0.0200 (8) | 0.0443 (9) | 0.0099 (6) | −0.0173 (7) | −0.0084 (7) |
Geometric parameters (Å, º)
| C1—C1i | 1.383 (3) | N1—O1 | 1.2316 (17) |
| C1—H1 | 0.9500 | N1—O2 | 1.2208 (18) |
| C1—C2 | 1.488 (2) | C8—O3 | 1.2065 (19) |
| C2—C3 | 1.406 (2) | C8—O4 | 1.3335 (19) |
| C2—C7 | 1.403 (2) | O4—C9 | 1.4497 (18) |
| C3—C4 | 1.388 (2) | C9—H9A | 0.9800 |
| C3—N1 | 1.4710 (19) | C9—H9B | 0.9800 |
| C4—H4 | 0.9500 | C9—H9C | 0.9800 |
| C4—C5 | 1.386 (2) | C10—O5 | 1.202 (2) |
| C5—C6 | 1.398 (2) | C10—O6 | 1.3268 (19) |
| C5—C8 | 1.5000 (19) | O6—C11 | 1.4515 (18) |
| C6—C7 | 1.394 (2) | C11—H11A | 0.9800 |
| C6—C10 | 1.502 (2) | C11—H11B | 0.9800 |
| C7—H7 | 0.9500 | C11—H11C | 0.9800 |
| C1i—C1—H1 | 119.4 | O2—N1—C3 | 118.67 (12) |
| C1i—C1—C2 | 121.20 (18) | O2—N1—O1 | 123.86 (13) |
| C2—C1—H1 | 119.4 | O3—C8—C5 | 123.87 (14) |
| C3—C2—C1 | 123.55 (13) | O3—C8—O4 | 124.94 (14) |
| C7—C2—C1 | 121.09 (13) | O4—C8—C5 | 111.17 (13) |
| C7—C2—C3 | 115.26 (13) | C8—O4—C9 | 115.16 (12) |
| C2—C3—N1 | 121.38 (13) | O4—C9—H9A | 109.5 |
| C4—C3—C2 | 123.52 (13) | O4—C9—H9B | 109.5 |
| C4—C3—N1 | 115.10 (12) | O4—C9—H9C | 109.5 |
| C3—C4—H4 | 120.2 | H9A—C9—H9B | 109.5 |
| C5—C4—C3 | 119.57 (13) | H9A—C9—H9C | 109.5 |
| C5—C4—H4 | 120.2 | H9B—C9—H9C | 109.5 |
| C4—C5—C6 | 118.99 (13) | O5—C10—C6 | 123.44 (14) |
| C4—C5—C8 | 117.98 (13) | O5—C10—O6 | 124.79 (14) |
| C6—C5—C8 | 122.98 (13) | O6—C10—C6 | 111.75 (13) |
| C5—C6—C10 | 122.09 (13) | C10—O6—C11 | 115.35 (12) |
| C7—C6—C5 | 120.29 (13) | O6—C11—H11A | 109.5 |
| C7—C6—C10 | 117.60 (13) | O6—C11—H11B | 109.5 |
| C2—C7—H7 | 118.9 | O6—C11—H11C | 109.5 |
| C6—C7—C2 | 122.30 (13) | H11A—C11—H11B | 109.5 |
| C6—C7—H7 | 118.9 | H11A—C11—H11C | 109.5 |
| O1—N1—C3 | 117.41 (12) | H11B—C11—H11C | 109.5 |
| C1i—C1—C2—C7 | −11.9 (3) | C1—C2—C7—C6 | −176.69 (14) |
| C1i—C1—C2—C3 | 171.9 (2) | C4—C3—N1—O2 | 146.08 (15) |
| C7—C2—C3—C4 | −1.7 (2) | C2—C3—N1—O2 | −33.6 (2) |
| C1—C2—C3—C4 | 174.69 (15) | C4—C3—N1—O1 | −31.3 (2) |
| C7—C2—C3—N1 | 177.99 (13) | C2—C3—N1—O1 | 148.99 (15) |
| C1—C2—C3—N1 | −5.6 (2) | C4—C5—C8—O3 | −43.2 (2) |
| C2—C3—C4—C5 | 1.5 (2) | C6—C5—C8—O3 | 139.53 (16) |
| N1—C3—C4—C5 | −178.24 (13) | C4—C5—C8—O4 | 134.90 (14) |
| C3—C4—C5—C6 | 0.7 (2) | C6—C5—C8—O4 | −42.34 (19) |
| C3—C4—C5—C8 | −176.63 (13) | O3—C8—O4—C9 | −8.0 (2) |
| C4—C5—C6—C7 | −2.5 (2) | C5—C8—O4—C9 | 173.88 (12) |
| C8—C5—C6—C7 | 174.67 (14) | C7—C6—C10—O5 | −40.6 (2) |
| C4—C5—C6—C10 | 176.16 (14) | C5—C6—C10—O5 | 140.64 (18) |
| C8—C5—C6—C10 | −6.6 (2) | C7—C6—C10—O6 | 137.89 (15) |
| C5—C6—C7—C2 | 2.3 (2) | C5—C6—C10—O6 | −40.8 (2) |
| C10—C6—C7—C2 | −176.45 (14) | O5—C10—O6—C11 | −4.0 (2) |
| C3—C2—C7—C6 | −0.2 (2) | C6—C10—O6—C11 | 177.52 (13) |
Symmetry code: (i) −x, −y+1, −z+1.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C9—H9B···O5ii | 0.98 | 2.42 | 3.308 (2) | 150 |
| C11—H11A···O3iii | 0.98 | 2.52 | 3.3650 (19) | 144 |
| C11—H11C···O5iv | 0.98 | 2.39 | 3.364 (2) | 173 |
Symmetry codes: (ii) −x+1, −y, −z+2; (iii) x, y−1, z; (iv) x+1, y, z.
References
- Blelloch, N. D., Mitchell, H. T., Greenburg, L. C., Van Citters, D. W. & Mirica, K. A. (2021). Cryst. Growth Des. 21, 6143–6154.
- Brandenburg, K. & Putz, H. (1999). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Bruno, I. J., Cole, J. C., Edgington, P. R., Kessler, M., Macrae, C. F., McCabe, P., Pearson, J. & Taylor, R. (2002). Acta Cryst. B58, 389–397. [DOI] [PubMed]
- Bulatov, E. & Haukka, M. (2019). Dalton Trans. 48, 3369–3379. [DOI] [PubMed]
- Burk, M. H., Hagenbusch, D., Arndt, C., Pott, L., Hauck, M., Drewes, J., Rehders, S., Strunskus, T., Hartig, T., Selhuber-Unkel, C., Adelung, R., Schütt, F., Herges, R., Faupel, F. & Schröder, S. (2023). http://doi.org/10.26434/chemrxiv-2023-15rbd.
- Cabré, G., Garrido-Charles, A., González-Lafont, À., Moormann, W., Langbehn, D., Egea, D., Lluch, J. M., Herges, R., Alibés, R., Busqué, F., Gorostiza, P. & Hernando, J. (2019). Org. Lett. 21, 3780–3784. [DOI] [PubMed]
- Ewert, J., Heintze, L., Jordà-Redondo, M., von Glasenapp, J.-S., Nonell, S., Bucher, G., Peifer, C. & Herges, R. (2022). J. Am. Chem. Soc. 144, 15059–15071. [DOI] [PubMed]
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Hao, M., Liu, T., Xiao, Y., Ma, L.-K., Zhang, G., Zhong, C., Chen, Z., Luo, Z., Lu, X., Yan, H., Wang, L. & Yang, C. (2019). Chem. Mater. 31, 1752–1760.
- Klockmann, F., Fangmann, C., Zender, E., Schanz, T., Catapano, C. & Terfort, A. (2021). ACS Omega, 6, 18434–18441. [DOI] [PMC free article] [PubMed]
- Kobauri, P., Dekker, F. J., Szymanski, W. & Feringa, B. L. (2023). Angew. Chem. Int. Ed. 62, e202300681. [DOI] [PubMed]
- Li, S., Deng, L.-X., He, J.-J., Liu, H. & Zeng, H.-P. (2014). Wuji Huaxue Xuebao, 30, 2401.
- Li, S., Eleya, N. & Staubitz, A. (2020). Org. Lett. 22, 1624–1627. [DOI] [PubMed]
- Maier, M. S., Hüll, K., Reynders, M., Matsuura, B. S., Leippe, P., Ko, T., Schäffer, L. & Trauner, D. (2019). J. Am. Chem. Soc. 141, 17295–17304. [DOI] [PubMed]
- Moormann, W., Langbehn, D. & Herges, R. (2017). Synthesis, 49, 3471–3475.
- Moormann, W., Langbehn, D. & Herges, R. (2019). Beilstein J. Org. Chem. 15, 727–732. [DOI] [PMC free article] [PubMed]
- Pang, X., Lv, J., Zhu, C., Qin, L. & Yu, Y. (2019). Adv. Mater. 31, 1904224. [DOI] [PubMed]
- Rigaku OD (2022). CrysAlis PRO. Rigaku Oxford Diffraction, Yarnton, England.
- Samanta, S., Qin, C., Lough, A. J. & Woolley, G. A. (2012). Angew. Chem. Int. Ed. 51, 6452–6455. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Siewertsen, R., Neumann, H., Buchheim-Stehn, B., Herges, R., Näther, C., Renth, F. & Temps, F. (2009). J. Am. Chem. Soc. 131, 15594–15595. [DOI] [PubMed]
- Song, L., Hui, L., Linxin, D. & Xiangdong, Q. (2017). Z. Krist. New Cryst. Struct. 232, 411–412.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989024002676/wm5712sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989024002676/wm5712Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989024002676/wm5712Isup3.cml
CCDC reference: 2342598
Additional supporting information: crystallographic information; 3D view; checkCIF report