Abstract
Soybean (Glycine max (L.) Merr.) is an important agricultural crop around the world, and previous studies suggest that honey bees (Apis mellifera Linnaeus) can be a component for optimizing soybean production through pollination. Determining when bees are present in soybean fields is critical for assessing pollination activity and identifying periods when bees are absent so that bee-toxic pesticides may be applied. There are currently several methods for detecting pollinator activity, but these existing methods have substantial limitations, including the bias of pan trappings against large bees and the limited duration of observation possible using manual techniques. This study aimed to develop a new method for detecting honey bees in soybean fields using bioacoustics monitoring. Microphones were placed in soybean fields to record the audible wingbeats of foraging bees. Foraging activity was identified using the wingbeat frequency of honey bees (234 ± 14 Hz) through a combination of algorithmic and manual approaches. A total of 243 honey bees were detected over 10 days of recording in 4 soybean fields. Bee activity was significantly greater in blooming fields than in non-blooming fields. Temperature had no significant effect on bee activity, but bee activity differed significantly between soybean varieties, suggesting that soybean attractiveness to honey bees is heavily dependent on varietal characteristics. Refinement of bioacoustics methods, particularly through the incorporation of machine learning, could provide a practical tool for measuring the activity of honey bees and other flying insects in soybeans as well as other crops and ecosystems.
Keywords: honey bees, soybeans, bioacoustics, monitoring, pollination
Introduction
Soybean (Glycine max (L.) Merr.) is a growing agricultural crop around the world and has become the number one agricultural export of the United States. In 2020, 17.6% of agricultural export value in the United States was attributed to soybeans (USDA FAS 2021). The United States exported $25.7 billion worth of soybeans grown for oil, animal feed, industrial products, and human consumption (USDA FAS 2021), an increase of $7.0 billion from the previous year (USDA FAS 2020). In 2021, soybeans covered 87.6 million acres of agricultural land in the United States, an increase of 5% from the previous year (NASS, USDA 2021). The growing soybean industry is faced with the continued need to optimize resource input and maximize yield output, and many studies suggest that honey bees (Apis mellifera Linnaeus) can be a component for optimizing soybean production through pollination.
Soybeans are self-fertile angiosperms that often exhibit cleistogamy, meaning that self-fertilization occurs within closed flowers (Benitez et al. 2010). Because of this, early publications purported that soybeans are not attractive pollinator plants and are rarely visited by honey bees (Lent 1934, Milum 1940). However, more recent studies have shown that honey bees frequently forage in soybean fields, and cross-pollination by pollinators contributes additional benefits to soybean fruiting. Soybean yield is positively correlated with honey bee visitation, with yield increases ranging from 5.7% to 81% across studies, likely due to differences in experimental methods, soybean varieties, and variables such as time and location (Jaycox 1970, Erickson 1975a, Juliano 1976, Erickson et al. 1978, Kettle and Taylor 1979, Issa et al. 1984, Vila et al. 1992, Santos et al. 2013, Chiari et al. 2005a, Toledo et al. 2011, Milfont et al. 2013, Monasterolo et al. 2015, Blettler et al. 2018, Esquivel et al. 2021, Levenson et al. 2022). Chiari et al. (2005a) demonstrated that pollination by honey bees and pollination by wild pollinators both increased soybean yield. Milfont et al. (2013) demonstrated a yield increase of 11% when open-pollinated soybeans were supplemented with nearby honey bee colonies. Soybean yield also may benefit from the presence of nearby pollinator habitats (Levenson et al. 2022). Together, these studies suggest that optimal soybean production can be achieved through the combined pollination activity of wild pollinators and managed honey bees. Milfont et al. (2013) estimated that honey bee pollination of soybeans could contribute $59.70–$110.50 of profits per hectare, representing a contribution of $6.1–$17.4 billion to the global economy.
Honey bee pollination can have multiple effects on soybean fruiting that may lead to increased yields. Pollination has been shown to increase hybridization (Cutler 1934, Jaycox 1970, Abrams et al. 1978, Chiang and Kiang 1987, Sim and Choi 1993), number of pods (Juliano 1976, Erickson et al. 1978, Pinzauti and Frediani 1980, Moreti et al. 1998, Chiari et al. 2005a, Cunningham-Minnick et al. 2019), number of seeds (Pinzauti and Frediani 1980, Moreti et al. 1998, Blettler et al. 2018, Cunningham-Minnick et al. 2019), pod weight (Juliano 1976, Toledo et al. 2011), fruiting rate (Sim and Choi 1993, Tchuenguem and Dounia 2014, BeaudelaineKengni et al. 2015), healthy seed (Tchuenguem and Dounia 2014, BeaudelaineKengni et al. 2015), and seeds per pod (Pinzauti and Frediani 1980, Issa et al. 1984, Moreti et al. 1998, Milfont et al. 2013, Tchuenguem and Dounia 2014, BeaudelaineKengni et al. 2015). Pollination has also been shown to decrease seed abortion (Erickson et al. 1978, Monasterolo et al. 2015) and flower abortion (Chiari et al. 2005b, Monasterolo et al. 2015).
Some of the earliest studies on the relationship between honey bee pollination and soybean yield reported that yield benefits differ by variety, likely due to variation in bloom characteristics and their resulting attractiveness to honey bees (Erickson 1975a, Issa et al. 1984). Differences between soybean varieties include flower color (ranging from white to violet), growth habit (determinate or indeterminate), maturity group (time from planting to maturity), frequency of cleistogamy, flower size, flower fragrance, number of flowers, nectar volume, and nectar sugar concentration (Erickson 1975b, Benitez et al. 2010, Stowe and Vann 2022). In addition to phenotypic variation across soybean varieties, soybeans produce nectar of varying quality and quantity depending on environmental factors, including temperature (Robacker et al. 1983), time of day (Severson and Erickson 1984, Blettler et al. 2016), soil macronutrients (Robacker et al. 1983), and soil microbiota (BeaudelaineKengni et al. 2015).
Variations in soybean bloom characteristics are correlated with attractiveness to honey bees. Sugar concentrations above 25% and low rates of cleistogamy are generally most attractive to honey bees (Erickson 1975b), whereas flower color has little impact on honey bee foraging (Jaycox 1970, Mason 1979, Severson and Erickson 1984, Chiang and Kiang 1987). Attractiveness also changes over time, with most studies observing peak honey bee activity in soybeans around midday (Jaycox 1970, Issa et al. 1984, Santos et al. 2013, Chiari et al. 2005a, Toledo et al. 2011, BeaudelaineKengni et al. 2015, Blettler et al. 2016). This is likely due to changes in floral nectar quality and quantity throughout the day, with sugar concentration increasing and nectar volume decreasing as the day progresses (Severson and Erickson 1984). Honey bee foraging also peaks in the middle of the month-long blooming period when flower production is at its maximum (Blettler et al. 2016).
Because soybeans are capable of self-pollination—and possibly as a consequence of early studies purporting that honey bees are poor pollinators of soybeans—honey bees are often disregarded as a factor of production, which opens the door for potentially bee-toxic insecticides to be applied during soybean bloom (Jaycox 1970, Milfont et al. 2013). Many soybean insecticides applied during bloom are highly toxic to bees and carry cautionary language to protect bees on the pesticide label. While there have been reports that pyrethroid insecticides repel bees from areas where they have been applied (Rieth and Levin 1988), the pyrethroids cypermethrin and lambda-cyhalothrin were not found to deter honey bee foraging when applied to blooming soybeans (Fagúndez et al. 2016). Pesticides are picked up by foraging honey bees and cause lethal and sublethal effects in the colony, creating a major risk for foraging colonies and reducing the long-term viability of pollination and its associated benefits (Reichelderfer and Caron 1979, Santos et al. 2013, Tosi et al. 2022, Zhao et al. 2022). This both raises legal concerns and prevents growers from fully implementing honey bee pollination into integrated pest and pollinator management (IPPM) frameworks. The conditions for pesticide application in soybeans must be reassessed within the context of honey bee activity to support the long-term sustainability of soybean production.
In order to assess how environmental variables and soybean varieties can predict honey bee foraging in soybeans, we must first be able to detect foraging honey bees across time. There are several existing methods for detecting pollinator activity, mainly through the use of pan traps, visual observation, and manual collection (Portman et al. 2020). Pan traps are brightly colored bowls filled with soapy water that are used as a passive sampling method. Pollinators are attracted to the bright colors, and then become trapped in the liquid. Manual collection methods include targeted netting and sweep netting, and visual observation provides a nonlethal method of insect detection. Pan traps, visual observation, and netting have all previously been used to assess honey bee activity in soybean fields, with visual observation being the most common method (Table 1).
Table 1.
Summary of sampling methods used in previous studies of bee activity in soybeans
| Publication | Methods used |
|---|---|
| Abrams et al. 1978 | Visual observation |
| Sheppard et al. 1979 | Visual observation |
| Issa et al. 1984 | Visual observation |
| Chiang and Kiang 1987 | Visual observation |
| Toledo et al. 2011 | Visual observation |
| Milfont et al. 2013 | Sweep netting |
| Santos et al. 2013 | Visual observation |
| Tchuenguem and Dounia 2014 | Visual observation and targeted netting |
| BeaudelaineKengni et al. 2015 | Visual observation and targeted netting |
| Blettler et al. 2018 | Sweep netting |
| Cunningham-Minnick et al. 2019 | Pan traps |
| St. Clair et al. 2020 | Pan traps |
| Levenson et al. 2022 | Visual observation and targeted netting |
Existing methods for bee detection have drawbacks that make them ineffective for accurate large-scale detection of honey bees in crops. Pan traps tend to favor smaller bees, such as those belonging to the family Halictidae, over larger bees such as honey bees (Portman et al. 2020). Visual observation and netting techniques are time and labor intensive, and these methods are more susceptible to collector bias (Westphal et al. 2008, Portman et al. 2020). In addition, detection methods such as trapping are fatal to the target organisms. A novel method is necessary for accurate bee detection, particularly in large cropping systems like soybeans.
Bioacoustics is a branch of science that focuses on sound production by living organisms, and bioacoustics monitoring is a relatively new method for the detection and identification of species through audio recording and analysis. Deep learning approaches to bioacoustics monitoring are currently being developed for the detection and identification of mosquitoes that vector malaria (Vasconcelos et al. 2019, 2020, Kiskin et al. 2020a, 2020b, Hassall et al. 2021, Khalighifar et al. 2021, Kim et al. 2021) and invasive fruit fly pests (Kalfas et al. 2022). Bioacoustics monitoring has also been combined with machine learning to automate the analysis of birdcall recordings and increase bird monitoring efficiency through the use of the deep neural network BirdNET (Kahl et al. 2021, Toenies and Rich 2021, Wood et al. 2021). A study by Zhang et al. (2017) proposed the use of wingbeat spectrum imaging for insect identification using convolutional neural networks. Similar methods could be used for in-field detection and identification of any insect with a distinct and detectable wingbeat, and honey bees fulfill these criteria by producing an audible wingbeat frequency of 234 ± 14 Hz (Clark et al. 2017). A study by Kawakita and Ichikawa (2019) used machine learning to distinguish bee and wasp species with similar wingbeat frequencies, including A. mellifera, with high precision.
The goal of this study was to develop an effective and efficient bioacoustics method for detecting honey bee activity in soybean fields. To accomplish this, we made audio recordings in soybean fields around the blooming period and used a combination of automated and manual techniques to identify honey bee activity.
Materials and Methods
Study Area
This study was completed in 4 soybean fields near Apple Creek, Ohio (Fig. 1). Fields A and B were planted with the soybean variety Synergy 9720, field C was planted with Synergy 9727, and field D was planted with Synergy 9723 (Table 2). Data were collected over 10 days on 21–24 July, 26–28 July, and 5–7 August 2021, except for field B which yielded no data on 7 August due to a microphone malfunction. For this study, a field was considered to be in bloom during growth stages R2 and R3, and audio was recorded during stage R3. Fields A, B, and D were in bloom 21–24 July and 26–28 July, while only field C was in bloom from 5 to 7 August. All 4 fields were located in predominantly agricultural areas and were surrounded by corn fields, wheat fields, alfalfa fields, additional soybean fields, deciduous forestland, and major and minor roads. An apiary with approximately 10 colonies was located 25 m east of field B, but the locations of other managed apiaries or feral colonies established in wooded areas were not known.
Fig. 1.
Map of study fields designated A–D. Modified from Google Earth Pro 7.3 (2021). Imagery © 2021 Maxar Technologies, State of Ohio/OSIP, USDA/FPAC/GEO.
Table 2.
Characteristics of soybean fields within study area
| Field | Acreage | Variety | Maturity group | Planting date | Surroundings |
|---|---|---|---|---|---|
| A | 29 acres | Synergy 9720 | 2.0 | May 20 | Corn, soybeans, forestland |
| B | 46 acres | Synergy 9720 | 2.0 | May 21 | Alfalfa, corn, soybeans, forestland, minor roads, apiary |
| C | 80 acres | Synergy 9727 | 2.7 | June 15 | Corn, wheat, soybeans, forestland, minor roads |
| D | 25 acres | Synergy 9723 | 2.3 | May 25 | Corn, wheat, soybeans, forestland, minor roads, U.S. Route 250 |
Data Collection
Audio recorders (Sony model ICD-PX370) were attached to 0.635 × 91.44 cm (0.25 × 36 inch) square wooden stakes and protected from wind and rain with high-density foam and 3D-printed white plastic rain covers (https://www.thingiverse.com/thing:5380356, Fig. 2). The recorders were programmed to record audio at the highest microphone sensitivity setting and a bitrate of 48 kbps (mono). One recorder was placed in each field 35 m from an easily accessible field edge. The recorders were left to continuously record environmental audio for 3-day periods, then retrieved for battery replacement. The location of each recorder was marked with a colored flag on top of a stake to aid in retrieval. In blooming fields, the recorders were placed at the same height as the highest blooming nodes beneath the leaf canopy. If the field was not in bloom, the recorders were placed at the same height as the highest non-blooming nodes beneath the leaf canopy. Each recorder’s radius of detection for bee wingbeats was approximately 1 m.
Fig. 2.
Audio recorder in soybeans.
Audio Processing
Audio files were downloaded from audio recorders in MP3 format, downsampled to 16 kHz and converted to WAV files using FFMPEG (Tomar 2006), then split into 1-h segments using the AudioSegment module in the pydub library (v.0.25.1, Robert 2018) using Python (v.3.9.7). Hours of audio recorded before sunrise or after sunset were removed from analysis. Potential bee detections were identified based on a range of audio frequencies corresponding with the second harmonic of the honey bee wingbeat frequency (370–570 Hz) with a duration of 1 s and a threshold of 0.0001 using the “find_rois_cwt” function in the scikit-maad soundscape analysis package in Python (Ulloa et al. 2021). Possible bee detections were output as a CSV file for manual audio assessment.
Manual Audio Assessment
Automated honey bee detections were loaded as labels overlayed on a spectrogram of 1-hour audio sequences and the source of each detected sound was manually identified using Audacity (v.3.1.3, Audacity Team 2022). Audio assessment was completed by listening to the areas of interest and identifying the source of each detection event in a manually curated label file (Table 3). Visual assessment of spectrograms in the areas of interest augmented audio assessment (Fig. 3). The spectrograms were adjusted to show recorded frequencies in the 100–600 Hz range, which included the target frequency for honey bee wingbeats (234 ± 14 Hz) and its second harmonic (468 ± 28 Hz). The gain was set to 35 dB and spectrograms were displayed using Mel scaling to more clearly distinguish target frequencies from background noise. A total of 403 h of audio recordings were manually assessed.
Table 3.
Labels used to categorize audio detections
| Label | Description |
|---|---|
| Bee | Wingbeats of honey bees. Other insect wingbeats were excluded. |
| Insect | Wingbeats of all non-honey bee insects. |
| Combine | Combines and other agricultural equipment. |
| Goose | Canadian geese (Branta canadensis Linn.) calls. |
| Human | Manual setup and takedown of microphones at the start and end of each recording increment. |
| Traffic | Vehicles, excluding agricultural equipment. Ground traffic was not distinguished from air traffic. |
| Other | All sounds not falling into one of the above categories. Includes wind, rain, and minor construction. |
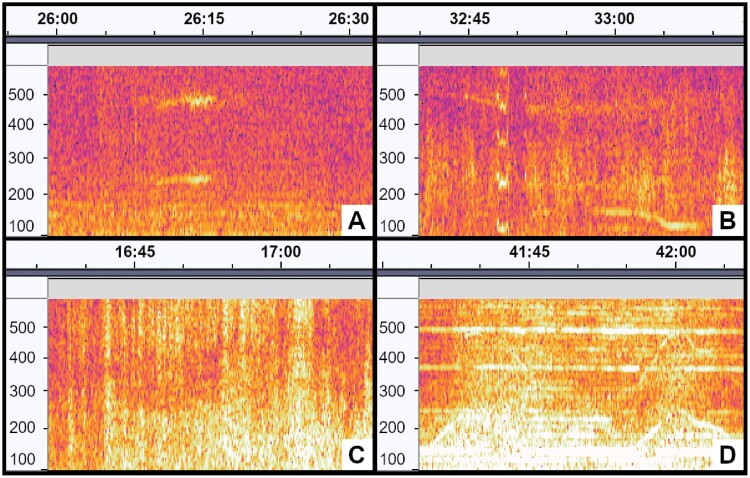
Fig. 3.
Spectrograms of potential bee activity. The x-axis shows time (mm:ss) and the y-axis shows frequency (Hz). Screenshots modified from Audacity (Audacity Team 2022). A) Honey bee wingbeats (230–250 Hz) with second harmonic (460–500 Hz). B) Non-honey bee insect wingbeats (105–125 Hz) with second, third, and fourth harmonics. C) Air traffic. D) Agricultural equipment operating in a neighboring field.
Statistical Analysis
Weather data for the 10 recording dates were obtained from the Ohio State University CFAES Weather System Wooster Station (OSU CFAES 2022), and the correlation between average daily temperature and the number of bee detections was determined using a linear regression analysis and Pearson’s correlation coefficient. Due to non-normal distribution, a Kruskal–Wallis one-way analysis of variance and Dunn’s test were used to determine significant differences in the number of bee detections between soybean varieties, between fields, and between blooming and non-blooming soybeans. All statistical analyses were completed using R statistical software (v.4.0.3, R Core Team 2022) and visualized with the ggplot2 package (Wickham 2016).
Results
The automated audio assessment identified a total of 11,638 potential bee detections over the 10 days of recording (Table 4). Of those detections, 10,307 were produced by vehicles, 130 were produced by insects other than honey bees, and 243 were produced by honey bees. Most bee activity occurred between 10 AM and 5 PM Eastern Daylight Time, with the greatest activity occurring between 1 PM and 4 PM (Fig. 4). Field A had the least amount of recorded bee activity with only 34 bee detections. Fields B and C yielded 52 and 45 bee detections, respectively. Field D yielded 112 bee detections, more than twice the bee activity of any other field. July 21 was the most active day for bees in all 3 fields blooming at that time (Fig. 5). Bee activity was significantly greater in blooming fields than in non-blooming fields (P = 0.001, N = 39, df = 1). Bee activity also differed significantly between soybean varieties (P = 0.004, N = 24, df = 2), with less activity in variety 9720 than in varieties 9723 and 9727, and between fields (P = 0.010, N = 24, df = 3), with less bee activity in fields A and B than in fields C and D. There was no significant correlation between bee detections and average daily temperature (P = 0.107, N = 24, df = 22).
Table 4.
Summary of detection events in each field, identified using the scikit-maad (Ulloa et al. 2021) package in Python followed by manual assessment
| Sound source | |||||||
|---|---|---|---|---|---|---|---|
| Field | Bee | Insect | Farm machinery | Goose | Human | Traffic | Other |
| A | 34 | 4 | 23 | 0 | 30 | 2,354 | 116 |
| B | 45 | 13 | 28 | 0 | 19 | 2,101 | 323 |
| C | 52 | 68 | 844 | 7 | 7 | 2,177 | 202 |
| D | 112 | 45 | 109 | 18 | 13 | 2,671 | 223 |
| Total | 243 | 130 | 1,004 | 25 | 69 | 9,303 | 864 |
Fig. 4.
Bee detections per hour across the 4 study fields pooled over the 10 days of recording (21–24 July, 26–28 July, and 5–7 August 2021).
Fig. 5.
Daily bee detections in each field across all recording dates. No recording was made in field B on 7 August.
Discussion
These results demonstrate that bioacoustics monitoring is a viable method for detecting honey bees and other insects in soybean fields. A comparison of detections during bloom and non-bloom showed that there was more bee activity in each field when it was blooming than when it was not blooming. There were significant differences in bee detections between soybean varieties, with less bee activity in both fields that contained variety 9720. The 130 detected wingbeat frequencies produced by non-honey bee insects were predominantly lower than the target frequency; this aligns with the known wingbeat frequencies of several other insects common to Ohio soybean fields such as Bombus impatiens Cresson (155–205 Hz, Buchwald and Dudley 2010) and Popillio japonica Newman (119 ± 9 Hz, Oertli 1989).
There was no significant correlation between bee detections and average daily temperature. Although a study by Robacker et al. (1983) found that nectar production in soybeans increased with temperature, a different study by Severson and Erickson (1984) found that temperature had no significant effect on nectar production. It is possible that temperature did not impact nectar production in the soybean varieties used in this study and thus had no significant effect on honey bee activity.
Bee detections were not significantly greater in field B than in other fields despite being located next to an apiary. Honey bee foraging in soybeans can decrease when more attractive forage plants are simultaneously blooming in the same area (Silliman et al. 2022). Field B was the only study field adjacent to alfalfa, which also attracts honey bee foragers (Hagler et al. 2011, Haedo et al. 2022) and may have reduced foraging in soybeans. In addition, field B contained variety 9720, which was determined to be the least attractive variety and may have reduced forager interest. Proximity to the apiary could explain why field B still yielded more detections than field A, which also contained variety 9720.
In total, 24 of the 243 bee detections occurred in fields that were not in bloom. Small patches of blooming weeds were present in each field throughout the study, which may have attracted honey bees outside of the soybean blooming period. The difference in bee detections between blooming and non-blooming fields suggests that this weed presence was not large enough or attractive enough to significantly affect honey bee foraging on soybean flowers. It was noted that bee detections in field A exceeded the expected bee activity for non-blooming fields on 6 August, with a total of 10 bee detections. Upon reassessment of the data, it was discovered that 7 of those 10 bee detections occurred in the span of 25 s and were likely produced by the same bee. If this is the case, only 4 bees were detected in field A on this date, which more closely aligns with the number of bee detections on other dates and in other fields during non-bloom. This was the only instance of an individual bee exceeding 3 detections, and most bees were detected only once.
The recorders picked up a wide array of non-bee activities such as traffic, birds, cicadas, and agricultural equipment. By targeting the second harmonic of honey bee wingbeats, the scikit-maad soundscape analysis package was able to exclude cicadas, most birds, and other general noises from detection output. Vehicular noise occupied a similar frequency range to honey bee wingbeats, but the spectrograms produced for vehicular audio were visually distinct from the spectrograms produced for honey bee wingbeats. Despite the high occurrence of traffic detections and the proximity of some fields to major and minor roads, bee activity was still detectable, with only 7 of the 243 manually assessed bee detections overlapping with traffic detections. The only noise source that confounded bee detection was the use of agricultural equipment near fields A and C during the periods of July 21, field C during the periods of July 22, fields C and D during the periods of July 27, fields B and D during periods of July 28, and fields C and D during periods of August 5. Bees could not be detected when agricultural equipment was present due to the sustained duration and intensity of the noise overlapping with bee wingbeat frequencies. However, agricultural equipment was only present for 1 or 2 h on a given day, and enough bees were detected on days when agricultural equipment was present to yield usable data.
This method also proved to be much more time and labor-efficient than other methods with minimal additional costs. The microphones and other materials used were affordable and easily obtained in large quantities, and the software used for manual audio analysis was a free open-source program. This method allowed data to be collected in 4 fields simultaneously by a single researcher, was nonlethal, and was not susceptible to observer bias. It took a total of approximately 8 h to set up and retrieve the microphones before and after the recording periods and 34 h to manually assess the audio data. Altogether, a total of 42 h was needed for a single researcher to collect and analyze 403 h of audio recordings, meaning that only 9% of the total time spent collecting and analyzing the data required human labor. Refinement of this method using machine learning for bee detection could further reduce the amount of time and labor required for manual audio analysis.
These results provide a general picture of when honey bees are most active in soybean fields, and conclusions can be made about when to make pesticide applications. Bee activity greatly decreases after 5 PM in blooming fields, and bees are rarely present in non-blooming fields. Based on these data, pesticide applicators can minimize honey bee exposure to harmful insecticides by only spraying soybean fields when bees are not actively foraging (i) before and after the blooming period or (ii) after 5 PM during the blooming period.
A novel methodology such as audio detection could be used to develop a better IPPM framework for soybean growers that takes honey bee activity into account. Tracking honey bee activity in soybeans across the hours of the day has already been proposed as a means for reducing honey bee exposure to harmful pesticides (Blettler et al. 2016), but this has not been utilized at a commercial scale due to the practical challenges presented by current honey bee detection methods. Bioacoustics monitoring could provide an efficient, effective, and accessible method for determining when honey bees are present in blooming soybean fields, allowing pesticide applicators to better follow pesticide label guidelines and mitigate pesticide exposure. Research to refine this audio detection technology for future implementation in precision agriculture, including the use of advanced machine learning to assist with data analysis and interpretation, is ongoing.
Supplementary Material
Acknowledgments
This work was supported by the Agriculture and Food Research Initiative of the US Department of Agriculture’s National Institute of Food and Agriculture under grant 2019-67013-29297; and the Ohio Agricultural Research and Development Center’s North Central Soybean Research Program under grants OHO01412 and OHO01355-MRF.
Contributor Information
Karlan C Forrester, Department of Entomology, The Ohio State University, 1680 Madison Ave, Wooster, OH 44691, USA.
Chia-Hua Lin, Department of Entomology, The Ohio State University, 1680 Madison Ave, Wooster, OH 44691, USA.
Reed M Johnson, Department of Entomology, The Ohio State University, 1680 Madison Ave, Wooster, OH 44691, USA.
Data Availability
The data that support the findings of this study are openly available in Ag Data Commons at https://doi.org/10.15482/USDA.ADC/1528082.
Author Contributions
Karlan Forrester (Conceptualization [lead], Data curation [equal], Formal analysis [lead], Investigation [lead], Methodology [lead], Project administration [equal], Validation [equal], Visualization [lead], Writing—original draft [lead], Writing—review & editing [equal]), Chia-Hua Lin (Conceptualization [supporting], Funding acquisition [equal], Methodology [supporting], Resources [supporting], Validation [equal], Writing—review & editing [supporting]), and Reed Johnson (Conceptualization [supporting], Data curation [equal], Formal analysis [supporting], Funding acquisition [equal], Methodology [supporting], Project administration [equal], Resources [lead], Software [lead], Supervision [lead], Validation [equal], Visualization [supporting], Writing—review & editing [equal])
References
- Abrams RI, Edwards CR, Harris T.. Yields and cross-pollination of soybeans as affected by honey bees and Alfalfa Leafcutting bees. Am Bee J. 1978:118(8):555–560. [Google Scholar]
- Audacity Team. Audacity®: Free Audio Editor and Recorder. Version 3.1.3. Audacity® software is copyright © 1999-2022 Audacity Team. The name Audacity® is a registered trademark; 2022. http://audacityteam.org/.
- BeaudelaineKengni S, Fohouo F-NT, Ngakou A.. Impact of the foraging activity of Apis mellifera adansonii Latreille (Hymenoptera: Apidae) and Bradyrhizobium fertilizer on pollination and yield components of Glycine max L. (Fabaceae) in the field. Int J Biol Res. 2015:3(2):64–71. 10.14419/ijbr.v3i2.5211 [DOI] [Google Scholar]
- Benitez EP, Khan NA, Matsumura H, Abe J, Takahashi R.. Varietal differences and morphology of Cleistogamy in soybean. Crop Sci. 2010:50(1):185–190. 10.2135/cropsci2009.02.0108 [DOI] [Google Scholar]
- Blettler DC, Fagúndez GA, Caviglia OP.. Contribution of honeybees to soybean yield. Apidologie. 2018:49(1):101–111. 10.1007/s13592-017-0532-4 [DOI] [Google Scholar]
- Blettler DC, Fagúndez GA, Chemez DM.. A study of the foraging schedule of honeybees on soy crops as an agronomical tool to mitigate the effects of agrochemicals. Sci Interfluvius. 2016:7(2):14–28. [Google Scholar]
- Buchwald R, Dudley R.. Limits to vertical force and power production in bumblebees (Hymenoptera: Bombus impatiens). J Exp Biol. 2010:213(3):426–432. 10.1242/jeb.033563 [DOI] [PubMed] [Google Scholar]
- Chiang YC, Kiang YT.. Geometric position of genotypes, honeybee foraging patterns and outcrossing in soybean. Bot Bull Acad Sin. 1987:28(1):1–11. [Google Scholar]
- Chiari WC, Toledo VAA, Ruvolo-Takasusuki MCC, Attencia VM, Costa FM, Kotaka CS, Sakaguti ES, Magalhães HR.. Floral biology and behavior of Africanized honeybees Apis mellifera in soybean (Glycine max L. Merril). Braz Arch Biol Technol. 2005b:48(3):367–378. 10.1590/S1516-89132005000300006 [DOI] [Google Scholar]
- Chiari WC, Toledo VAA, Ruvolo-Takasusuki MCC, Oliveira AJB, Sakaguti ES, Attencia VM, Costa FM, Mitsui MH.. Pollination of soybean (Glycine max L. Merril) by honeybees (Apis mellifera L.). Braz Arch Biol Technol. 2005a:48(1):31–36. 10.1590/S1516-89132005000100005 [DOI] [Google Scholar]
- Clark CJ, Mountcastle AM, Mistick E, Elias DO.. Resonance frequencies of honeybee (Apis mellifera) wings. J Exp Biol. 2017:220(Pt 15):2697–2700. 10.1242/jeb.154609 [DOI] [PubMed] [Google Scholar]
- Cunningham-Minnick MJ, Peters VE, Crist TO.. Nesting habitat enhancement for wild bees within soybean fields increases crop production. Apidologie. 2019:50(6):833–844. 10.1007/s13592-019-00691-y [DOI] [Google Scholar]
- Cutler GH. A simple method for making soybean hybrids. Agron J. 1934:26(3):252–254. 10.2134/agronj1934.00021962002600030016x [DOI] [Google Scholar]
- Erickson EH. Effects of honey bees on yield of three soybean cultivars. Crop Sci Soc Am. 1975a:15(1):84–86. 10.2135/cropsci1975.0011183X001500010025x [DOI] [Google Scholar]
- Erickson EH. Variability of floral characteristics influences honey bee visitation to soybean blossoms. Crop Sci. 1975b:15(6):767–771. 10.2135/cropsci1975.0011183x001500060008x [DOI] [Google Scholar]
- Erickson EH, Berger GA, Shannons JG, Robins JM.. Honey bee pollination increases soybean yields in the Mississippi delta region of Arkansas and Missouri. J Econ Entomol. 1978:71(4):601–603. 10.1093/jee/71.4.601 [DOI] [Google Scholar]
- Esquivel IL, Parys KA, Brewer MJ.. Pollination by non-Apis bees and potential benefits in self-pollinating crops. Ann Entomol Soc Am. 2021:114(2):257–266. 10.1093/aesa/saaa059 [DOI] [Google Scholar]
- Fagúndez GA, Blettler DC, Krumrick CG, Bertos MA, Trujillo CG.. Do agrochemicals used during soybean flowering affect the visits of Apis mellifera L? Span J Agric Res. 2016:14(1):e0301. 10.5424/sjar/2016141-7492 [DOI] [Google Scholar]
- Google Earth Pro 7.3. Wayne County, OH, United States. 40.768247, -81.844195. © 2021 Maxar Technologies, State of Ohio/OSIP, USDA/FPAC/GEO; 2021. Accessed 2022 May 7.
- Haedo JP, Martínez LC, Graffigna S, Marrero HJ, Torretta JP.. Managed and wild bees contribute to alfalfa (Medicago sativa) pollination. Agric Ecosyst Environ. 2022:324:107711. 10.1016/j.agee.2021.107711 [DOI] [Google Scholar]
- Hagler JR, Mueller S, Teuber LR, Machtley SA, Deynze AV.. Foraging range of honey bees, Apis mellifera, in alfalfa seed production fields. J Insect Sci. 2011:11(1):144. 10.1673/031.011.14401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassall KL, Dye A, Potamitis I, Bell JR.. Resolving the identification of weak-flying insects during flight: a coupling between rigorous data processing and biology. Agric For Entomol. 2021:23(4):489–505. 10.1111/afe.12453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa MRC, Velocci MEP, Gonçalves LS, Soares AEE.. Ensaio de polinização em soja (Glicine max) por abelhas Apis mellifera L. In: Anais do 5° Congresso Brasileiro de Apicultura e III Congresso Latino-Ibero-Americano de Apicultura, 23 a 27 de julho de 1980, Viçosa, MG; 1984. p. 241–254.
- Jaycox ER. Ecological relationships between honey bees and soybeans. Am Bee J. 1970:110(10):306–307, 343,–345, 383–385. [Google Scholar]
- Juliano CJ. Polinização entomófila na soja. In: Anais do 4° Congresso Brasileiro de Apicultura. Curitiba (Brasil): CBA, Convênio Incra-FAEP; 1976. p. 235–239. [Google Scholar]
- Kahl S, Wood CM, Eibl M, Klinck H.. BirdNET: a deep learning solution for avian diversity monitoring. Ecol Inf. 2021:61:101236. 10.1016/j.ecoinf.2021.101236 [DOI] [Google Scholar]
- Kalfas I, De Ketelaere B, Beliën T, Saeys W.. Optical identification of fruitfly species based on their wingbeats using convolutional neural networks. Front Plant Sci. 2022:13:812506. 10.3389/fpls.2022.812506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita S, Ichikawa K.. Automated classification of bees and hornet using acoustic analysis of their flight sounds. Apidologie. 2019:50(1):71–79. 10.1007/s13592-018-0619-6 [DOI] [Google Scholar]
- Kettle WD, Taylor OR.. Ecological interactions of honey bees and soybeans. J Kansas Entomol Soc. 1979:52(3):549. [Google Scholar]
- Khalighifar A, Jimenez-Garcia D, Campbell LP, Ahadji-Dabla KM, Aboagye-Antwi F, Ibarra-Juarez LA, Peterson AT.. Application of deep learning to community-science-based mosquito monitoring and detection of novel species. J Med Entomol. 2021:59(1):355–362. 10.1093/jme/tjab161 [DOI] [PubMed] [Google Scholar]
- Kim D, DeBriere TJ, Cherukumalli S, White GS, Burkett-Cadena ND.. Infrared sensors permit rapid recording of wingbeat frequency and bioacoustics species identification of mosquitoes. Sci Rep. 2021:11(1):10042. 10.1038/s41598-021-89644-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskin I, Cobb AD, Wang L, Roberts S.. Humbug Zooniverse: a crowd-sourced acoustic mosquito dataset. In: 2020 IEEE International Conference on Acoustics, Speech, and Signal Processing, 4–8 May 2020, Barcelona, Spain; 2020a. p. 916–920.
- Kiskin I, Zilli D, Li Y, Sinka M, Willis K, Roberts S.. Bioacoustic detection with wavelet-conditioned convolutional neural networks. Neural Comput Appl. 2020b:32(4):915–927. 10.1007/s00521-018-3626-7 [DOI] [Google Scholar]
- Lent CJ. Do bees gather honey from soy beans? Am Bee J. 1934:74(8):340. [Google Scholar]
- Levenson HK, Sharp AE, Tarpy DR.. Evaluating the impact of increased pollinator habitat on bee visitation and yield metrics in soybean crops. Agric Ecosyst Environ. 2022:331:107901. 10.1016/j.agee.2022.107901 [DOI] [Google Scholar]
- Mason CE. Honey bee foraging activity on soybeans in Delaware. In: Proceedings of the Fourth International Symposium on Pollination, Maryland, 1978; 1979. p. 117–122.
- Milfont MO, Rocha EEM, Lima AON, Freitas BM.. Higher soybean production using honeybee and wild pollinators, a sustainable alternative to pesticides and autopollination. Environ Chem Lett. 2013:11(4):335–341. 10.1007/s10311-013-0412-8 [DOI] [Google Scholar]
- Milum VG. Bees and soybeans. Am Bee J. 1940:80(1):22. [Google Scholar]
- Monasterolo M, Musicante ML, Valladares GR, Salvo A.. Soybean crops may benefit from forest pollinators. Agric Ecosyst Environ. 2015:202:217–222. 10.1016/j.agee.2015.01.012 [DOI] [Google Scholar]
- Moreti ACCC, da Silva ECA, Alves MLTMF, da Silva RMB.. Observações sobre a polinização entomófila da cultura de soja (Glycine max Merril). Bol Ind Anim. 1998:55(1):91–94. [Google Scholar]
- NASS, USDA. 2021 Acreage Report; 2021. https://www.nass.usda.gov/Publications/Todays_Reports/reports/acrg0621.pdf [Google Scholar]
- Oertli JJ. Relationship of wing beat frequency and temperature during take-off flight in temperate-zone beetles. J Exp Biol. 1989:145(1):321–338. 10.1242/jeb.145.1.321 [DOI] [Google Scholar]
- OSU CFAES. The Ohio State University CFAES Weather System, Wooster Station; 2022. [accessed 2022 May 2]. https://weather.cfaes.osu.edu/stationinfo.asp?id=1.
- Pinzauti M, Frediani D.. The importance of honeybee pollination in soya bean production. L’Apicoltore Moderno. 1980:71(5):155–160. [Google Scholar]
- Portman ZM, Bruninga-Socolar B, Cariveau DP.. The state of bee monitoring in the United States: a call to refocus away from bowl traps and towards more effective methods. Ann Entomol Soc Am. 2020:113(5):337–342. 10.1093/aesa/saaa010 [DOI] [Google Scholar]
- R Core Team. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2022. http://www.R-project.org. [Google Scholar]
- Reichelderfer KH, Caron DM.. Honey bees and soybean production. Am Bee J. 1979:119(2):107–109. [Google Scholar]
- Rieth JP, Levin MD.. The repellent effect of two pyrethroid insecticides on the honey bee. Physiol Entomol. 1988:13(2):213–218. 10.1111/j.1365-3032.1988.tb00925.x [DOI] [Google Scholar]
- Robacker DC, Flottum PK, Sammataro D, Erickson EH.. Effects of climatic and edaphic factors on soybean flowers and on the subsequent attractiveness of the plants to honey bees. Field Crops Res. 1983:6(4):267–278. 10.1016/0378-4290(83)90067-9 [DOI] [Google Scholar]
- Robert J. Pydub. GitHub; 2018. [accessed 2021 Dec 27]. http://pydub.com/.
- Santos E, Mendoza Y, Vera M, Carrasco-Letelier L, Díaz S, Invernizzi C.. Increase in soybean (Glycine max) production using honey bees (Apis mellifera). Agrociencia-Uruguay. 2013:17(1):81–90. [Google Scholar]
- Severson DW, Erickson EH.. Quantitative and qualitative variation in floral nectar of soybean cultivars in Southeastern Missouri. Environ Entomol. 1984:13(4):1091–1096. 10.1093/ee/13.4.1091 [DOI] [Google Scholar]
- Sheppard WS, Jaycox ER, Parise SG.. Selection and management of honey bees for pollination of soybeans. In: Proceedings of the Fourth International Pollination Symposium, Maryland, 1978; 1979. p. 123–130. [Google Scholar]
- Silliman MR, Schürch R, Malone S, Taylor SV, Couvillon MJ.. Row crop fields provide mid-summer forage for honey bees. Evol Ecol. 2022:12(6):e8979. 10.1002/ece3.8979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim Y-G, Choi Y-E.. Influence of honeybees pollination on soybean yield and yield components. Korean J Appl Entomol. 1993:32(3):271–278. [Google Scholar]
- St. Clair AL, Dolezal AG, O’Neal ME, Toth AL.. Pan traps for tracking honey bee activity-density: a case study in soybeans. Insects. 2020:11(6):366. https://doi.org/10.3390/insects11060366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe KD, Vann R.. North Carolina soybean production guide. Raleigh (NC, USA): NC State Extension Publications; 2022. https://content.ces.ncsu.edu/north-carolina-soybean-production-guide [Google Scholar]
- Tchuenguem FF-N, Dounia. Foraging and pollination behavior of Apis mellifera adansonii Latreille (Hymenoptera: Apidae) on Glycine max L. (Fabaceae) flowers at Maroua. J Res Biol. 2014:4(1):1209–1219. [Google Scholar]
- Toenies M, Rich LN.. Advancing bird survey efforts through novel recorder technology and automated species identification. Calif Fish Wildl J. 2021:107(2):56–70. 10.51492/cfwj.107.5 [DOI] [Google Scholar]
- Toledo VAA, Malerbo-Souza DT, Filho JCS, Pinto AY, Ruvolo-Takasusuki MCC, Chambó ED.. Biodiversity of pollinators and their effect on soybeans grains (Mon Soy 3329 var) production. Revista Varia Scientia Agrároas. 2011:2(1):123–130. [Google Scholar]
- Tomar S. Converting video formats with FFmpeg. Linux J. 2006. https://dl.acm.org/doi/fullHtml/10.5555/1134782.1134792.
- Tosi S, Sfeir C, Carnesecchi E, vanEngelsdorp D, Chauzat M.. Lethal, sublethal, and combined effects of pesticides on bees: a meta-analysis and new risk assessment tools. Sci Total Environ. 2022:844:156857. 10.1016/j.scitotenv.2022.156857 [DOI] [PubMed] [Google Scholar]
- Ulloa JS, Haupert S, Latorre JF, Aubin T, Sueur J.. scikit-maad: an open-source and modular toolbox for quantitative soundscape analysis in Python. Methods Ecol Evol. 2021:12(12):2334–2340. 10.1111/2041-210x.13711 [DOI] [Google Scholar]
- USDA FAS. 2019 United States agricultural export yearbook; 2020. https://fas.usda.gov/sites/default/files/2020-07/2019-export-yearbook.pdf [Google Scholar]
- USDA FAS. 2020 United States agricultural export yearbook; 2021. https://fas.usda.gov/sites/default/files/inline-files/2020-ag-export-yearbook.pdf [Google Scholar]
- Vasconcelos D, Nunes NJ, Gomes J.. An annotated dataset of bioacoustics sensing and features of mosquitoes. Sci Data. 2020:7(1):382. 10.1038/s41597-020-00725-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos D, Nunes N, Ribeiro M, Prandi C, Rogers A.. LOCOMOBIS: a low-cost acoustic-based sensing system to monitor and classify mosquitoes. In: 2019 16th IEEE Annual Consumer Communications & Networking Conference, 11–14 January 2019, Nevada; 2019. [Google Scholar]
- Vila VPV, Martinho MR, Sediyama A, Freire JAH.. Effect of Africanized bees Apis mellifera in the hybridization and productivity of soybeans Glycine max Merill. In: XXXIIe Congres International D’apiculture, Rio de Janeiro, Bresil, 22–28 octobre 1989; 1992. p. 414–415.
- Westphal C, Bommarco R, Carre G, Lamborn E, Morison N, Petanidou T, Potts SG, Roberts SPM, Szentgyorgyi H, Tscheulin T, et al. Measuring bee diversity in different European habitats and biogeographical regions. Ecol Monogr. 2008:78(4):653–671. 10.1890/07-1292.1 [DOI] [Google Scholar]
- Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag; 2016. ISBN 978-3-319-24277-4. https://ggplot2.tidyverse.org. [Google Scholar]
- Wood CM, Kahl S, Chaon P, Peery MZ, Klinck H.. Survey coverage, recording duration and community composition affect observed species richness in passive acoustic surveys. Methods Ecol Evol. 2021:12(5):885–896. 10.1111/2041-210x.13571 [DOI] [Google Scholar]
- Zhang CS, Wang PY, Guo H, Fan GJ, Chen K, Kamarainen JK.. Turning wingbeat sounds into spectrum images for acoustic insect classification. Electron Lett. 2017:53(25):1674–1676. 10.1049/el.2017.3334 [DOI] [Google Scholar]
- Zhao H, Li G, Cui X, Wang H, Liu Z, Yang Y, Xu B.. Review on effects of some insecticides on honey bee health. Pestic Biochem Physiol. 2022:188:105219. 10.1016/j.pestbp.2022.105219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in Ag Data Commons at https://doi.org/10.15482/USDA.ADC/1528082.