ABSTRACT
The tumor microenvironment comprises both tumor and non-tumor stromal cells, including tumor-associated macrophages (TAMs), endothelial cells, and carcinoma-associated fibroblasts. TAMs, major components of non-tumor stromal cells, play a crucial role in creating an immunosuppressive environment by releasing cytokines, chemokines, growth factors, and immune checkpoint proteins that inhibit T cell activity. During tumors develop, cancer cells release various mediators, including chemokines and metabolites, that recruit monocytes to infiltrate tumor tissues and subsequently induce an M2-like phenotype and tumor-promoting properties. Metabolites are often overlooked as metabolic waste or detoxification products but may contribute to TAM polarization. Furthermore, macrophages display a high degree of plasticity among immune cells in the tumor microenvironment, enabling them to either inhibit or facilitate cancer progression. Therefore, TAM-targeting has emerged as a promising strategy in tumor immunotherapy. This review provides an overview of multiple representative metabolites involved in TAM phenotypes, focusing on their role in pro-tumoral polarization of M2.
KEYWORDS: Cancer, tumor microenvironment, metabolites, TAM, polarization
Introduction
Cancer, a leading cause of death worldwide, is not simply a genetic disease. The tumor microenvironment (TME), encompassing both cellular and acellular components, plays an important role in tumor initiation, growth, invasion, metastasis, and resistance to treatment. According to the classical theory, oncogenic mutations in malignant cells initiate cancer. This is accompanied by the release of various intercellular communicators, including cytokines, chemokines, and vesicles, which recruit and adapt to surrounding non-transformed cells, resulting in TME formation and close interaction with cancer cells (Balkwill et al. 2012). Chronic inflammation or wound-healing processes in an abnormal microenvironment can also trigger oncogenic signals and drive tumor development (Mantovani et al. 2008; Capp 2017; Deng et al. 2019; Todoric and Karin 2019).
Recently, immunotherapy encompassing checkpoint inhibitors (Pardoll 2012; Abril-Rodriguez and Ribas 2017) and adoptive cell therapy (D'Aloia et al. 2018; June and Sadelain 2018) has shown promise in cancer treatment, demonstrating durable clinical responses (Sharma P et al. 2011). Nevertheless, in most cancer types, some patients are either unresponsive or resistant to therapy (Bagley and O'Rourke 2020; Meric-Bernstam et al. 2021). Investigations into tumor-induced immunosuppressive mechanisms to overcome immunotherapeutic resistance have identified multiple suppressor cell populations within the TME. Notably, tumor-associated macrophages (TAMs) account for a major component of immune cell types in the TME. Plasticity is a hallmark of macrophages, allowing them to respond to environmental cues by exhibiting different forms of polarization, including the pro-inflammatory M1 and the anti-inflammatory M2 phenotypes (Zhou et al. 2014; Locati et al. 2020). M1 macrophages have tumor-killing properties, whereas M2 macrophages have anti-inflammatory properties that may indirectly promote tumor growth (Aras and Zaidi 2017). Therefore, TAMs have been proposed as novel emerging targets for immunomodulatory therapies in cancers (Cassetta and Kitamura 2018; Zhang M et al. 2018).
Despite the generation of diverse types of metabolites in the TME through metabolic pathways and their profound influence the tumor developmental process, the metabolites have been considered as mere metabolic waste or detoxification products and thus overlooked. However, recent evidence has revealed new and important oncogenic roles of metabolites within malignant tumors (Huang S et al. 2021; Martinez-Reyes and Chandel 2021).
TME composition
The TME is a crucial factor in augmenting the efficiency of cancer therapy. The vigorous interaction of cancer cells with the cellular and non-cellular components of TME promotes progression and metastasis, increasing resistance to anticancer therapy. Cancer cells are surrounded by fibroblasts, endothelial cells, pericytes, and immune cells, such as T and B cells, macrophages, neutrophils, and dendritic cells, critically involved in the promotion or suppression of tumor growth. Paracrine signaling mediates intercellular communication by secreting chemokines, cytokines, and growth factors within the TME.
The highly complex and heterogeneous TME, formed by the recruitment of non-cancerous host cells and remodeling of the vasculature and extracellular matrix (ECM), including collagen, hyaluronan, laminin, and fibronectin, is regulated by cancer cells (Jahanban-Esfahlan et al. 2017; Jahanban-Esfahlan et al. 2018; Lee TW and Lee 2022). Critical networks for intercellular communication in the TME encompass various cell-to-cell interactions, including paracrine signaling, through the production of cytokines, growth factors, and chemokines (Shi et al. 2019; Song et al. 2020; Jin Y et al. 2023). Adhesion molecules, such as integrins, selectins, and cadherins, regulate tumor growth and suppression through signal transduction by promoting cell-to-cell interactions and cell–ECM adhesion (Kechagia et al. 2019; Yeini et al. 2021; Hwang et al. 2023). The ECM plays a pivotal role in the storage of secreted molecules, promoting adhesion and migration and activating intercellular communication. The interaction between cancer cells and the ECM establishes a dynamic reciprocity between neoplastic cells and the tumor stroma within the TME (Gonzalez-Avila et al. 2020; Li et al. 2020).
Exosomes originating from different cancer cells communicate with non-cancerous host cells, including immune cells, endothelial cells, and cancer-associated fibroblasts, thereby promoting tumor invasion (Huang XY et al. 2020), migration (Chen C et al. 2023), and immune escape (Liu J et al. 2020). Exosomal secretion of long non-coding RNAs from cancer cells influences various biological processes, such as metastasis (Yao et al. 2022) and tumor growth (Ni et al. 2023). Extracellular vesicles contain different molecules, including RNA, DNA, metabolites, and proteins, which act as bioactive cargoes that affect target cells and mediate intercellular communication (Dong et al. 2023; Serrati et al. 2023; Uemura et al. 2023; Zhang Y et al. 2023).
Metabolic reprogramming of cancer cells is a hallmark of malignancy. It allows cancer cells to adapt to hypoxic and nutrient-poor environments and alters their metabolism to maintain growth and proliferation (Ward and Thompson 2012; Bhagat et al. 2019; Watson et al. 2021; Jin HR et al. 2023). Tumor cells influence the metabolic environment within the TME by consuming nutrients and producing metabolites (Elia and Haigis 2021). These metabolites regulate transcriptional responses and modulate the expression of target genes via transactivation or transrepression domains (Angelin et al. 2017; Feng et al. 2017; Kuang et al. 2017; Walton et al. 2018). Furthermore, the metabolic reprogramming of tumor-infiltrating cells leads to differentiation and acquisition of effector functions (Stone et al. 2019; Wang H et al. 2020; Han et al. 2023; Wang Z et al. 2023).
TAM
Macrophages, the main components of immune infiltrates, can efficiently infiltrate tumors and are abundant within the TME (Lee H et al. 2022; Cassetta and Pollard 2023). Concurrent with the growing understanding of TAM function, its clinical significance in solid tumors is widely being studied. Elevated TAM infiltration correlates with advanced tumor stages, as observed in esophageal (Li et al. 2019), ovarian (Yuan et al. 2017), breast (Qiu et al. 2018), and pancreatic cancers (Di Caro et al. 2016). In addition, higher TAM densities are related to poor patient outcomes (Qian and Pollard 2010; Medrek et al. 2012; Wang XL et al. 2016; Wu P et al. 2016; Kitano et al. 2018; Li et al. 2019; Wang C et al. 2022) owing to their facilitation of tumor growth, invasion, metastasis, angiogenesis, and drug resistance (Mantovani 1978; De Palma and Lewis 2013; Cassetta and Pollard 2018). Initially, the tissue-resident macrophages were thought to be derived from blood monocytes (Volkman and Gowans 1965); however, it is now known that they arise either from circulating monocytes or from primitive macrophages originating in the embryonic yolk sac and fetal liver (Ginhoux and Guilliams 2016). Therefore, TAMs can be derived from both the infiltration of tumors by circulating monocytes and tissue-resident macrophages from the surrounding tissues (Loyher et al. 2018; Laviron et al. 2022).
As mentioned earlier, activated macrophages are frequently classified into two phenotypes depending on their polarization: pro-inflammatory M1 and anti-inflammatory M2 (Mills et al. 2000; Mantovani et al. 2002). M2 macrophages can be further subdivided into four subtypes based on changes in their microenvironment and stimuli: M2a, M2b, M2c, and M2d (Roszer 2015). Among these subtypes, M2d macrophages (sometimes called TAMs) are involved in angiogenesis and tumor progression, induced by adenosine A2 receptors and leukemia inhibitory factor (an IL-6 cytokine). They also contribute to the secretion of vascular endothelial growth factor (VEGF), IL-10, and TGF-β (Roszer 2015). In general, M1 macrophages promote inflammatory responses against invading pathogens and inhibit tumor progression, while M2 macrophages exert an immunosuppressive phenotype that favors tissue repair and tumor progression (Bingle et al. 2002; Lewis and Pollard 2006; Zaynagetdinov et al. 2011; Seth et al. 2017). Thus, it is reasonable to assume that most pro-tumoral TAMs are M2-like macrophages, and their reprogramming into M1-like macrophages is a promising therapeutic approach for cancer treatment (Zhang J, Zhou, et al. 2022). However, cancer-associated inflammation induced by M1-like macrophages triggers heightened inflammation, continuous cell proliferation, angiogenesis, and the survival of damaged or transformed cells, resulting in neovascularization and rapid tumor expansion (Coussens and Werb 2002). Moreover, M1-like TAMs have been found to increase tumor cell mobility and facilitate cancer metastasis (Wang H et al. 2014; Xiao et al. 2018).
TAM polarization
Although M1-like and M2-like classifications in TAM studies are predominant (Mantovani et al. 2002; Mantovani et al. 2013), it is important to consider that macrophage polarization only refers to the state of macrophage activation at a particular time-point. Moreover, because of plasticity, the polarization state of macrophages is not fixed and can change based on the integration of multiple signals from the TME (Bardi et al. 2018; Boutilier and Elsawa 2021; Laviron et al. 2022).
TAM polarization is regulated by cytokines, chemokines, growth factors, and other signals derived from the TME (Qian and Pollard 2010). M1-like TAMs are activated by IFN-γ, TNF-α, and GM-CSF. They express CD68, CD80, and CD86 and secrete cytokines including IL-1β, IL-6, IL-12, IL-23, CXCL9, and CXCL10. In contrast, M2-like TAMs are stimulated by CSF-1, C–C motif ligand 2 (CCL2), IL-10, or TGF-β. They express CD163, CD204, CD206, and stabilin-1, and secrete IL-10, IL-12, TGF-β, CCL17, CCL18, CCL22, and CCL24 (Lewis and Pollard 2006; Algars et al. 2012; Biswas et al. 2013; Ruffell and Coussens 2015; Koelzer et al. 2016; Jeannin et al. 2018).
Recently, TAM markers that are not defined by conventional M1 and M2 polarization but are associated with prognostic factors have been reported. For instance, the CXCL9:SPP1 ratio in TAMs of patients with head and neck squamous cell carcinoma is strongly associated with prognosis (Bill et al. 2023). Additionally, CCL8 and SIGLEC1 expressing TAMs in breast cancer indicate shorter disease-specific survival (Cassetta et al. 2019).
The two most well-documented factors among these are CSF-1 and CCL2, which act as macrophage recruiters and M2 stimulators (Mantovani and Sica 2010; Xu R et al. 2019), (Poh and Ernst 2018). The main factor influencing M2 macrophage polarization is the interaction of chemokine/cytokine receptors with their ligands present within the TME, leading to the activation of several signaling pathways, including the PI3 K/AKT, JAK/STAT6, STAT3, and TGF-β/SMAD-dependent pathways (Gao et al. 2022; Kerneur et al. 2022).
Epigenetic alterations are universal features of all human cancers and are now known to interact with genetic alterations to influence cancer phenotypes (Sharma S et al. 2010; Baylin and Jones 2016). Epigenetic regulators reshape chromatin structures, pack the genome, and alter gene expression patterns without altering the DNA code. These alterations involve DNA methylation, post-translational modifications of histone proteins, chromatin remodeling, non-coding RNAs, and other chromatin components (Baylin and Jones 2016; Cheng et al. 2019). Because epigenetic modifications play a significant role in macrophage polarization (Hoeksema and de Winther 2016; Kapellos and Iqbal 2016), pharmacological modulators or inhibitors can protect TAMs from M2 polarization and tumor progression (de Groot and Pienta 2018; Niu et al. 2022).
Macrophages are highly sensitive to changes in metabolite concentrations (O'Neill 2011; Tannahill et al. 2013; Kim S et al. 2014), substrates (Pesce et al. 2009; Rodriguez-Prados et al. 2010), certain lipids (El Kasmi et al. 2013), oxygen tension (Pfau et al. 2004; Fumagalli et al. 2015), environmental pH (Wu H et al. 2019), tissue osmolality (Ip and Medzhitov 2015), and other molecular components of the microenvironment (El Kasmi et al. 2014). Therefore, additional non-cytokine pathways are required for macrophage polarization (Colegio et al. 2014; El Kasmi et al. 2014) along with the primary cytokine-mediated pathway.
TME metabolites regulate TAM
Lactate
Cancer cells produce lactate through glycolysis. The released lactate engages in metabolic symbiosis with other cells in the TEM, where it enters the TCA cycle and generates ATP through oxidative phosphorylation. In addition, lactate can influence macrophages towards pro-angiogenic and anti-inflammatory phenotypes through several mechanisms. Pyruvate and lactate compete with α-ketoglutarate for the prolyl hydroxylases of HIF-1α (Lu et al. 2002; Kim MJ et al. 2023). Hydroxylated HIF-1α is ubiquitinated and subsequently undergoes proteasomal degradation (Lu et al. 2005). Therefore, heightened pyruvate and lactate levels inhibit the proline hydroxylation of HIF-1α, leading to its stabilization in a hypoxia-independent manner, thereby increasing the expression of anti-inflammatory Arg1 and VEGF in TAMs (Bohn et al. 2018).
In addition to HIF-1α, lactate can stabilize HIF-2α and endow it with the pro-angiogenic phenotype of TAMs (Liu N et al. 2019). Furthermore, lactate downregulates the expression of ATP6V0d2, a vacuolar ATPase involved in the lysosomal-mediated degradation of HIF-2α, in TAMs through the mTORC1-mediated inhibition of the transcription factor EB. Decreased lysosomal degradation of HIF-2α, coupled with increased tumor incidence, was observed in Atp6v0d2−/− mice. A correlation between ATP6V0d2 expression and the survival rate has also been reported in patients with lung adenocarcinoma (Liu N et al. 2019).
The lactate within TAMs can participate in the post-translational modification of proteins, and lactate-derived lactylation of histone lysine residues has emerged as a novel epigenetic modification that induces the expression of genes related to the pro-tumorigenic polarization of macrophages, such as those of arginase 1, HIF-1α and VEFG-A (Zhang D et al. 2019). However, the process mechanism as well as enzymes involved in the addition or removal of lactate remain unknown.
Instead of entering cells via the monocarboxylate transporter (MCT), lactate can act on G-protein-coupled receptor 132 (Gpr132) on the plasma membrane and initiate signaling for the M2 polarization of TAMs (Chen P et al. 2017). Gpr132 is a lactate-signaling sensor that forms an interplay between cancer cells and macrophages within the acidic environment of the TME. Induction and stimulation of Gpr132 in macrophages by tumor-derived lactate promotes adhesion, migration, and invasion of breast cancer cells in vitro and in vivo (Chen P et al. 2017). Patients with breast cancer exhibit a positive correlation between Gpr132 expression, M2 macrophages, metastasis, and poor prognosis, making lactate-Gpr132 signaling a potential therapeutic target (Chen P et al. 2017). The macrophage odorant receptor Olfr78 is a heterodimeric partner of Gpr132 that senses lactate in the TME and generates pro-tumoral M2 TAMs (Vadevoo et al. 2021). In Gpr132-mediated signaling, the cAMP-mediated expression of inducible cAMP early repressor may be involved in the pro-angiogenic, pro-tumorigenic phenotype of TAMs. This is because of the increased anti-inflammatory expression of Arg1, VEGF-A, and HIF-1α in macrophages (Bohn et al. 2018).
Lactate activates the ERK/STAT3 axis, mediates M2 polarization, and is responsible for angiogenesis, along with cancer cell migration and invasion. The inhibition of ERK/STAT3 in MCF7 breast cancer cells reduced macrophage M2 polarization and decreased breast cancer proliferation and angiogenesis in vitro. Growth of the implanted tumor cells and progression and angiogenesis were inhibited in vivo as well (Mu et al. 2018).
TCA cycle intermediates
Succinate
Succinate, present in large quantities in tumors, is a byproduct of either hypoxia-induced glycolysis in tumor cells or reduced succinate dehydrogenase activity because of genetic mutations and/or epigenetic regulation (King et al. 2006; Killian et al. 2014; Richter et al. 2016; Wu J-Y et al. 2020). Within cells, succinate stabilizes and activates HIF-1α by competing with ketoglutarate to inhibit HIF–prolyl hydroxylase activity (Selak et al. 2005). Various types of cancer cells, including those of lung, breast, and colon cancers, secrete succinate, which directs TAM polarization towards the M2 phenotype and contributes to cancer progression as a metabolic signal (Wu J-Y et al. 2020; Trauelsen et al. 2021). Succinate in the TEM can bind to succinate receptor 1 (SUCNR1), also known as Gpr91, in macrophages. SUCNR1 is highly expressed on the surface of immature dendritic cells and macrophages and is involved in the succinate-driven recruitment of macrophages. Inhibition by anti-SUCNR1 antibodies reduces the migration of succinate-treated macrophages. Succinate-induced SUCNR1 activation leads to intracellular signaling events in TAMs. The PI3K–HIF-1α axis is a potential downstream mediator of SUCNR1, which induces a pro-angiogenic phenotype in TAMs. Succinate-induced TAMs enhance cancer cell migration in vitro and promote cancer metastasis in vivo (Wu J-Y et al. 2020). Succinate increases the expression of M2 type macrophages, such as Arg1, Fizz1, and Mgl1/2, within TAMs in a dose-dependent manner. Conversely, SUCNR1 inhibition using small interfering RNA or inhibition of PI3 K by LY294002 suppresses their expression (Wu J-Y et al. 2020).
α-Ketoglutarate
α-Ketoglutarate is an essential co-substrate of HIF–prolyl hydroxylases (Epstein et al. 2001; Fong and Takeda 2008) that can affect HIF-1α degradation. This induces the M1 polarization of macrophages. α-Ketoglutarate is also involved in the M2 polarization of macrophages by facilitating fatty acid oxidation and jumonji domain-containing protein 3-dependent epigenetic changes in genes involved in M2 polarization (Liu P-S et al. 2017). The ratio of α-ketoglutarate to succinate may be a factor driving M1 and M2 polarization, as low and high ratios of α-ketoglutarate to succinate are related to the M1 and M2 polarization of macrophages, respectively (Liu P-S et al. 2017).
Itaconate
Itaconate, a cis-aconitate metabolite, has recently emerged as an important regulator of immunity and inflammation (Feng et al. 2023). It inhibits succinate dehydrogenase, thereby blocking the production of reactive oxygen species (ROS) from complex 1 and leading to the inhibition of HIF-1α activity and interferon-1β production. Inhibition of M2 polarization by itaconate was demonstrated through the inhibition of the JAK1/STAT6 pathway and succinate dehydrogenase, which increased succinate levels (Runtsch et al. 2022).
Adenosine
Adenosine is produced in many different types of tumors, and high levels in the TME suppress local antitumor immune responses (Antonioli et al. 2013). Tumor cells express the ecto-5′-nucleotidase, CD73, which hydrolyzes extracellular ATP, ADP, and AMP to adenosine that acts on adenosine receptors (Montalbán Del Barrio et al. 2016). Hypoxia and HIF-1α induce transcription of CD73 (Kobayashi et al. 2000; Synnestvedt et al. 2002), and upregulation of CD73 in hypoxic TME causes accumulation of extracellular adenosine (Hatfield et al. 2014). Four distinct adenosine receptors, A1, A2A, A2B, and A3, are G-protein-coupled transmembrane receptors that are differentially expressed in various cell types, including cancer, endothelial, immune, and inflammatory cells (Eltzschig et al. 2012; Haskó and Pacher 2012). Tumor-protective effects are associated with A2A and A2B receptors that stimulate adenylyl cyclase, elevate intracellular cAMP levels, and lead to the activation of cAMP response element-binding (CREB) protein (Ohta et al. 2006). The adenosine pathway within cells creates a TME with tumor-protective effects. Deletion of CD73 or the A2A receptor has been shown to reduce tumor growth, inhibit metastasis, and evoke a potent antitumor immune response in mice (Stagg et al. 2011; Beavis et al. 2013; Cekic et al. 2014). Expression of A2A and A2B receptors is heightened in activated macrophages, and they are predominantly involved in M1 and M2 macrophage activation, respectively (Csoka et al. 2012). Adenosine inhibits the pro-inflammatory consequences of M1 activation, such as the secretion of TNF-α and IL-12 (Hasko et al. 2000), and polarizes macrophages into M2-like macrophages that produce anti-inflammatory IL-10 to suppress immune cells and produce VEGF to support angiogenesis (Csoka et al. 2012). Thus, metabolic alterations within the TME that facilitate adenosine accumulation in interstitial spaces may cause immunosuppression by facilitating M2 polarization.
The A2A receptor and adenosine have been implicated in the proliferation of macrophages, high levels of which are correlated with poor prognosis in patients with hepatocellular carcinoma (Wang J, Wang, et al. 2021). These proliferating macrophages, which constitute a major macrophage pool in the TME of hepatocellular carcinoma, are poorly differentiated and immunosuppressive. Tumor-stimulated TAMs secrete GM-CSF, which acts on TAMs to induce the A2A receptor in an autocrine manner, thereby allowing tumor-derived adenosine to promote the proliferation and activation of TAMs through A2A and the PI3 K/Akt and MEK/ERK pathways (Wang J, Wang, et al. 2021). Because adenosine exhibits pro-tumorigenic effects and protection from antitumor immune responses, modulation of macrophage function via adenosine or adenosine receptor-targeted therapies may be beneficial in cancer treatment.
Lipid metabolites
Prostaglandin E2
Prostaglandin E2 (PGE2) is derived from arachidonic acid through cyclooxygenase (COX) and PGE2 synthase (PGES) and is synthesized in most types of cells. COX-1, expressed constitutively, is linked to cytosolic PGES, whereas COX-2 is functionally associated with microsomal PGES-2, which is rapidly induced by pro-inflammatory stimuli (Weigert et al. 2018). PGE2 is a pro-inflammatory and pro-tumorigenic factor and is a major prostaglandin responsible for both inflammatory responses and tumor progression. Various cancer cells, including both established cell lines and human samples, produce high amounts of PGE2, which is involved in tumor formation, progression, metastasis, and immune evasion (Stolina et al. 2000; Eruslanov et al. 2009; Wang D et al. 2015; Nagaraja et al. 2016; Hsu et al. 2017; Zang et al. 2017). The significance of PGE2 in tumors treated with aspirin and COX-2 inhibitors has been studied in human cancers, including mesothelioma and colorectal cancer, as well as in mouse models with implanted cancer cells (DeLong et al. 2003; Haas et al. 2006; Nan et al. 2015). PGE2 acts on cancer cells and other cell types in the TME in a paracrine manner. It binds to the G protein-coupled receptors EP1, EP2, EP3, and EP4 to transfer signals that induce angiogenesis, lymphangiogenesis, epithelial–mesenchymal transition, and immune evasion (Dehne et al. 2017). Recent studies have reported that PGE2 influences the transformation of M1 macrophages into immunosuppressive M2 macrophages (Bi et al. 2020; Wang J, Liu, et al. 2021; Xu M et al. 2021; Wang W et al. 2023). COX-2 in TAMs is upregulated by molecules from cancer cells and produces PGE2, which induces the pro-tumorigenic M2 phenotype in TAMs (Inaba et al. 2003; Weigert et al. 2018). Multiple factors from apoptotic cancer cells, including sphingosine-1-phosphate and lysosphatidylcholine, recruit macrophages to the tumor and stimulate them to upregulate COX-2 and mPGES-2, besides producing PGE2 in a feed forward manner (Dehne et al. 2017).
The crosstalk between macrophages and tumor cells contributes to hepatocellular carcinoma progression. TAM-derived PGE2 upregulates tumor ubiquitin-like proteins with PHD and ring finger domain 1 (UHRF1), an oncogene that is highly expressed in hepatocellular carcinoma. UHRF1 recruits histone 3 lysine 9 methyltransferase, leading to epigenetic changes in the cancer genome. Methylation of the Krüppel-like factor promoter decreases KLF6 expression, which correlates with hepatocellular carcinoma progression. UHRF1 also increases CSF-1 expression, which in turn recruits and activates TAMs, thus forming a reciprocal loop within the TME to promote hepatocellular carcinoma growth and tumor metastasis (Zhang J, Zhang, et al. 2022).
Sphingosine-1-phosphate
Sphingosine-1-phosphate (S1P) is a bioactive metabolite that regulates various cellular processes. S1P produced by cancer cells is a pro-survival and pro-tumorigenic factor that induces tumor cell transformation, viability, migration, and angiogenesis (Pyne Nigel et al. 2012). S1P released by apoptotic breast cancer cells polarizes macrophages toward an M2-like phenotype (Weigert et al. 2007). The effects of S1P on tumor cells, macrophages, and subsequent tumor growth have been reported in various studies. Although the effects of these components remain unexplored, enzymes involved in the production of S1P and S1P receptors are important regulators of macrophage polarization (Weigert et al. 2019).
Polyunsaturated fatty acids
Polyunsaturated fatty acids, such as linoleic acid, can act as fatty acid ligands in serous ovarian carcinoma ascites, activating PPARβ/δ and thus promoting the polarization of TAMs into an M2 phenotype (Schumann et al. 2015). Moreover, tumor cells promote lipid accumulation in TAMs, which also polarizes them towards the M2 phenotype (Qiao et al. 2023).
Amino acids
Depletion of glucose and non-essential amino acid (NEAA) affects immunity and is often observed in the TME. Due to continual glucose uptake and lactate secretion, glucose is rapidly depleted in the TME (Ho et al. 2015; Kamphorst et al. 2015), thus suppressing inflammatory macrophage differentiation and simultaneous promotion of M2 macrophage polarization (Schworer et al. 2019). Amino acids are the primary source of carbon for proliferating cells (Hosios et al. 2016). NEAAs, including glutamine, serine, and cysteine, may be depleted within tumors (Kamphorst et al. 2015). Depletion of cysteine and serine in the TME promotes ROS accumulation to toxic levels in inflammatory macrophages and suppresses M1 activity during chronic antitumor responses (Schworer et al. 2019).
Conclusion
The focus of cancer research and treatment has shifted from a primary emphasis on the cancer itself to a more comprehensive approach centered on the TME, recognizing its growing importance in understanding cancer biology. Despite the recognition, the effectiveness of therapeutic approaches specifically targeting TME cells or pathways remains unsatisfactory. Therefore, identification and targeting of immunosuppressive factors within the TME may provide valuable insights into the mechanisms underlying tumor formation and progression.
TAMs represent a distinct population of immune cells abundant in the TME (Cassetta and Pollard 2023). Inhibiting TAM recruitment (Mantovani and Allavena 2015) and reprogramming of the phenotype from M2 to M1 (Ugel et al. 2015) have emerged as potential therapeutic strategies for effectively arresting tumor growth and preventing metastasis.
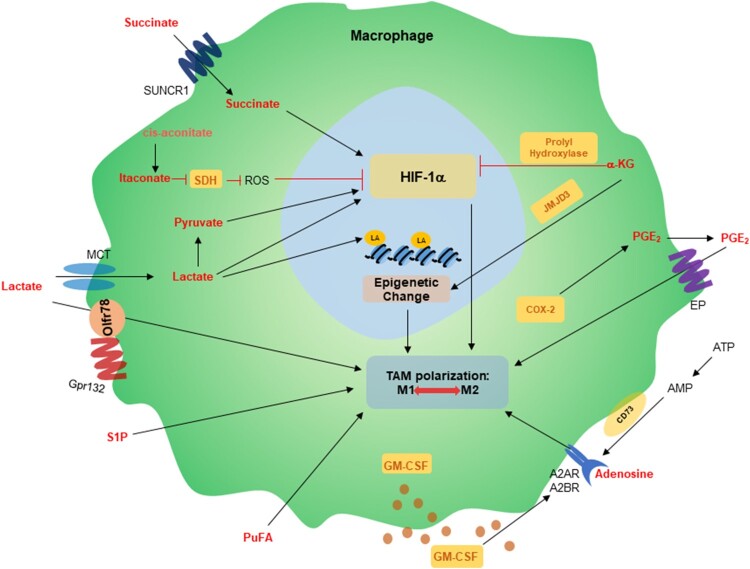
While extracellular nutrients are crucial in providing necessary components for cell growth, the metabolites generated during this process can directly affect neighboring stromal cells, including TAMs. This is achieved by activating signal transduction cascades, thereby exerting paracrine effects (Oh et al. 2010; Lorendeau et al. 2015; Schworer et al. 2019) (Figure 1).
Figure 1.
Metabolites generated in the tumor microenvironment affect TAM polarization. Schematic shows the roles of metabolites in macrophages. Metabolites can either stabilize or degrade HIF-1α, which induces TAM polariztion. Lactate-derived lactylation of histone lysine residues promotes tumorigenic polarization of macrophages. A2AR, A2A adenosine receptor; A2BR, A2B adenosine receptor; COX, cyclooxygenase; EP, E prostanoid receptor; Gpr132, G Protein-Coupled Receptor 132; JMJD3, jumonji domain-containing protein-3; PGE2, prostaglandin E2; PuFA, polyunsaturated fatty acids; S1P, sphingosine-1-phosphate; SDH, succinate dehydrogenase; SUNCR1, succinate receptor; TAM, tumor-associated macrophage.
Although no known metabolite modulators act specifically on TAMs, various cancer metabolites mentioned earlier can affect the polarization of macrophages. Therefore, inhibitors of enzymes that produce metabolites or antagonists of transporters or receptors can be used as immunomodulating agents to target TAMs. For instance, lactate dehydrogenase inhibitors such as oxamate (Zhao et al. 2015; Yu et al. 2020), gossypol (Van Poznak et al. 2001; Rani and Kumar 2016), and galloflavin (Manerba et al. 2012), or MCT inhibitors such as 7ACC2 (Corbet et al. 2018), CHC (Sonveaux et al. 2008), and DIDS (Amorim et al. 2015), can prevent lactate-induced polarization of TAMs. NF-56-EJ40 is a SUCNR1 inhibitor that can prevent immune cell migration and inhibit succinate-induced M2 polarization of TAMs (Trauelsen et al. 2021). In addition, oleclumab (Bendell et al. 2023) and durvalumab (Lim et al. 2022), inhibitors of CD73 that produces adenosine from AMP, and AZD4635 (Atif et al. 2022; Lim et al. 2022), a selective A2AR antagonist, can inhibit adenosine-induced M2 polarization. Celecoxib, a selective COX-2 inhibitor, can also modulate the polarization of TAMs by inhibiting the production of PGE2 (Song et al. 2016; Xun et al. 2021). Thus, the development of metabolite modulators and antagonists of transporters or receptors that can specifically act on TAMs is expected to promote antitumor immune responses by modulating the TME.
Funding Statement
This study was supported by the National Research Foundation of Korea (NRF) [grant numbers: NRF-2022R1F1A1069937 (S.W.K.), NRF-2021R1A2C1012480 (Y-A. M.), and NRF-2022R1F1A1073025 (H.S.K.)]; and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea [grant number: HI22C1989].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abril-Rodriguez G, Ribas A.. 2017. SnapShot: immune checkpoint inhibitors. Cancer Cell. 31(6):848–848.e1. doi: 10.1016/j.ccell.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Algars A, Irjala H, Vaittinen S, Huhtinen H, Sundstrom J, Salmi M, Ristamaki R, Jalkanen S.. 2012. Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer. 131(4):864–873. doi: 10.1002/ijc.26457. [DOI] [PubMed] [Google Scholar]
- Amorim R, Pinheiro C, Miranda-Goncalves V, Pereira H, Moyer MP, Preto A, Baltazar F.. 2015. Monocarboxylate transport inhibition potentiates the cytotoxic effect of 5-fluorouracil in colorectal cancer cells. Cancer Lett. 365(1):68–78. doi: 10.1016/j.canlet.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Angelin A, Gil-de-Gomez L, Dahiya S, Jiao J, Guo L, Levine MH, Wang Z, Quinn WJ, 3rd, Kopinski PK, Wang L, et al. 2017. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25(6):1282–1293.e7. doi: 10.1016/j.cmet.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonioli L, Blandizzi C, Pacher P, Haskó G.. 2013. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer. 13(12):842–857. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- Aras S, Zaidi MR.. 2017. TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer. 117(11):1583–1591. doi: 10.1038/bjc.2017.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atif M, Alsrhani A, Naz F, Ullah MI, Alameen AAM, Imran M, Ejaz H.. 2022. Adenosine A(2A) receptor as a potential target for improving cancer immunotherapy. Mol Biol Rep. 49(11):10677–10687. doi: 10.1007/s11033-022-07685-7. [DOI] [PubMed] [Google Scholar]
- Bagley SJ, O'Rourke DM.. 2020. Clinical investigation of CAR T cells for solid tumors: lessons learned and future directions. Pharmacol Ther. 205:107419. doi: 10.1016/j.pharmthera.2019.107419. [DOI] [PubMed] [Google Scholar]
- Balkwill FR, Capasso M, Hagemann T.. 2012. The tumor microenvironment at a glance. J Cell Sci. 125(Pt 23):5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- Bardi GT, Smith MA, Hood JL.. 2018. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine. 105:63–72. doi: 10.1016/j.cyto.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylin SB, Jones PA.. 2016. Epigenetic determinants of cancer. Cold Spring Harb Perspect Biol. 8(9). doi: 10.1101/cshperspect.a019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavis PA, Divisekera U, Paget C, Chow MT, John LB, Devaud C, Dwyer K, Stagg J, Smyth MJ, Darcy PK.. 2013. Blockade of A2A receptors potently suppresses the metastasis of CD73 + tumors. Proc Natl Acad Sci U S A. 110(36):14711–14716. doi: 10.1073/pnas.1308209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendell J, LoRusso P, Overman M, Noonan AM, Kim DW, Strickler JH, Kim SW, Clarke S, George TJ, Grimison PS, et al. 2023. First-in-human study of oleclumab, a potent, selective anti-CD73 monoclonal antibody, alone or in combination with durvalumab in patients with advanced solid tumors. Cancer Immunol Immunother. 72(7):2443–2458. doi: 10.1007/s00262-023-03430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat TD, Von Ahrens D, Dawlaty M, Zou Y, Baddour J, Achreja A, Zhao H, Yang L, Patel B, Kwak C, et al. 2019. Lactate-mediated epigenetic reprogramming regulates formation of human pancreatic cancer-associated fibroblasts. Elife. 8. doi: 10.7554/eLife.50663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi C, Fu Y, Zhang Z, Li B.. 2020. Prostaglandin E2 confers protection against diabetic coronary atherosclerosis by stimulating M2 macrophage polarization via the activation of the CREB/BDNF/TrkB signaling pathway. FASEB J. 34(6):7360–7371. doi: 10.1096/fj.201902055R. [DOI] [PubMed] [Google Scholar]
- Bill R, Wirapati P, Messemaker M, Roh W, Zitti B, Duval F, Kiss M, Park JC, Saal TM, Hoelzl J, et al. 2023. CXCL9:SPP1 macrophage polarity identifies a network of cellular programs that control human cancers. Science. 381(6657):515–524. doi: 10.1126/science.ade2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingle L, Brown NJ, Lewis CE.. 2002. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 196(3):254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Allavena P, Mantovani A.. 2013. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 35(5):585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- Bohn T, Rapp S, Luther N, Klein M, Bruehl TJ, Kojima N, Aranda Lopez P, Hahlbrock J, Muth S, Endo S, et al. 2018. Tumor immunoevasion via acidosis-dependent induction of regulatory tumor-associated macrophages. Nat Immunol. 19(12):1319–1329. doi: 10.1038/s41590-018-0226-8. [DOI] [PubMed] [Google Scholar]
- Boutilier AJ, Elsawa SF.. 2021. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. 22(13). doi: 10.3390/ijms22136995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capp JP. 2017. Tissue disruption increases stochastic gene expression thus producing tumors: cancer initiation without driver mutation. Int J Cancer. 140(11):2408–2413. doi: 10.1002/ijc.30596. [DOI] [PubMed] [Google Scholar]
- Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, Soong DYH, Cotechini T, Anur P, Lin EY, et al. 2019. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell. 35(4):588–602.e10. doi: 10.1016/j.ccell.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassetta L, Kitamura T.. 2018. Macrophage targeting: opening new possibilities for cancer immunotherapy. Immunology. 155(3):285–293. doi: 10.1111/imm.12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassetta L, Pollard JW.. 2018. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 17(12):887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- Cassetta L, Pollard JW.. 2023. A timeline of tumour-associated macrophage biology. Nature Reviews Cancer. 23(4):238–257. doi: 10.1038/s41568-022-00547-1. [DOI] [PubMed] [Google Scholar]
- Cekic C, Day Y-J, Sag D, Linden J.. 2014. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 74(24):7250–7259. doi: 10.1158/0008-5472.CAN-13-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu L, Si C, Xu Y, Wu X, Wang C, Sun Z, Kang Q.. 2023. Exosomal circTUBGCP4 promotes vascular endothelial cell tipping and colorectal cancer metastasis by activating Akt signaling pathway. J Exp Clin Cancer Res. 42(1):46. doi: 10.1186/s13046-023-02619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Zuo H, Xiong H, Kolar MJ, Chu Q, Saghatelian A, Siegwart DJ, Wan Y.. 2017. Gpr132 sensing of lactate mediates tumor–macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci USA. 114(3):580–585. doi: 10.1073/pnas.1614035114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, He C, Wang M, Ma X, Mo F, Yang S, Han J, Wei X.. 2019. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct Target Ther. 4:62. doi: 10.1038/s41392-019-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Brokowski CN, Eisenbarth CE, Phillips SC, M G, et al. 2014. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 513(7519):559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbet C, Bastien E, Draoui N, Doix B, Mignion L, Jordan BF, Marchand A, Vanherck JC, Chaltin P, Schakman O, et al. 2018. Interruption of lactate uptake by inhibiting mitochondrial pyruvate transport unravels direct antitumor and radiosensitizing effects. Nat Commun. 9(1):1208. doi: 10.1038/s41467-018-03525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z.. 2002. Inflammation and cancer. Nature. 420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Jr., Gause WC, Leibovich SJ, et al. 2012. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 26(1):376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aloia MM, Zizzari IG, Sacchetti B, Pierelli L, Alimandi M.. 2018. CAR-T cells: the long and winding road to solid tumors. Cell Death Dis. 9(3):282. doi: 10.1038/s41419-018-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot AE, Pienta KJ.. 2018. Epigenetic control of macrophage polarization: implications for targeting tumor-associated macrophages. Oncotarget. 9(29):20908–20927. doi: 10.18632/oncotarget.24556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehne N, Mora J, Namgaladze D, Weigert A, Brüne B.. 2017. Cancer cell and macrophage cross-talk in the tumor microenvironment. Curr Opin Pharmacol. 35:12–19. doi: 10.1016/j.coph.2017.04.007. [DOI] [PubMed] [Google Scholar]
- DeLong P, Tanaka T, Kruklitis R, Henry AC, Kapoor V, Kaiser LR, Sterman DH, Albelda SM.. 2003. Use of cyclooxygenase-2 inhibition to enhance the efficacy of immunotherapy. Cancer Res. 63(22):7845–7852. [PubMed] [Google Scholar]
- Deng S, Clowers MJ, Velasco WV, Ramos-Castaneda M, Moghaddam SJ.. 2019. Understanding the complexity of the tumor microenvironment in K-ras mutant lung cancer: finding an alternative path to prevention and treatment. Front Oncol. 9:1556. doi: 10.3389/fonc.2019.01556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Caro G, Cortese N, Castino GF, Grizzi F, Gavazzi F, Ridolfi C, Capretti G, Mineri R, Todoric J, Zerbi A, et al. 2016. Dual prognostic significance of tumour-associated macrophages in human pancreatic adenocarcinoma treated or untreated with chemotherapy. Gut. 65(10):1710–1720. doi: 10.1136/gutjnl-2015-309193. [DOI] [PubMed] [Google Scholar]
- Dong S, Liu X, Bi Y, Wang Y, Antony A, Lee D, Huntoon K, Jeong S, Ma Y, Li X, et al. 2023. Adaptive design of mRNA-loaded extracellular vesicles for targeted immunotherapy of cancer. Nat Commun. 14(1):6610. doi: 10.1038/s41467-023-42365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia I, Haigis MC.. 2021. Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism. Nat Metab. 3(1):21–32. doi: 10.1038/s42255-020-00317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kasmi KC, Anderson AL, Devereaux MW, Vue PM, Zhang W, Setchell KD, Karpen SJ, Sokol RJ.. 2013. Phytosterols promote liver injury and Kupffer cell activation in parenteral nutrition-associated liver disease. Sci Transl Med. 5(206):206ra137. doi: 10.1126/scitranslmed.3006898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kasmi KC, Pugliese SC, Riddle SR, Poth JM, Anderson AL, Frid MG, Li M, Pullamsetti SS, Savai R, Nagel MA, et al. 2014. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol. 193(2):597–609. doi: 10.4049/jimmunol.1303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Sitkovsky MV, Robson SC.. 2012. Purinergic signaling during inflammation. N Engl J Med. 367(24):2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, et al. 2001. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 107(1):43–54. doi: 10.1016/S0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Eruslanov E, Kaliberov S, Daurkin I, Kaliberova L, Buchsbaum D, Vieweg J, Kusmartsev S.. 2009. Altered expression of 15-hydroxyprostaglandin dehydrogenase in tumor-infiltrated CD11b myeloid cells: a mechanism for immune evasion in cancer. J Immunol. 182(12):7548–7557. doi: 10.4049/jimmunol.0802358. [DOI] [PubMed] [Google Scholar]
- Feng J, Read OJ, Dinkova-Kostova AT.. 2023. Nrf2 in TIME: the emerging role of nuclear factor erythroid 2-related factor 2 in the tumor immune microenvironment. Mol Cells. 46(3):142–152. doi: 10.14348/molcells.2023.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Yang H, Zhang Y, Wei H, Zhu Z, Zhu B, Yang M, Cao W, Wang L, Wu Z.. 2017. Tumor cell-derived lactate induces TAZ-dependent upregulation of PD-L1 through GPR81 in human lung cancer cells. Oncogene. 36(42):5829–5839. doi: 10.1038/onc.2017.188. [DOI] [PubMed] [Google Scholar]
- Fong GH, Takeda K.. 2008. Role and regulation of prolyl hydroxylase domain proteins. Cell Death Differ. 15(4):635–641. doi: 10.1038/cdd.2008.10. [DOI] [PubMed] [Google Scholar]
- Fumagalli S, Perego C, Pischiutta F, Zanier ER, Simoni D, G M.. 2015. The ischemic environment drives microglia and macrophage function. Front Neurol. 6:81. doi: 10.3389/fneur.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Liang Y, Wang L.. 2022. Shaping polarization of tumor-associated macrophages in cancer immunotherapy. Front Immunol. 13:888713. doi: 10.3389/fimmu.2022.888713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Guilliams M.. 2016. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 44(3):439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Avila G, Sommer B, Garcia-Hernandez AA, Ramos C.. 2020. Matrix metalloproteinases’ role in tumor microenvironment. Adv Exp Med Biol. 1245:97–131. doi: 10.1007/978-3-030-40146-7_5. [DOI] [PubMed] [Google Scholar]
- Haas AR, Sun J, Vachani A, Wallace AF, Silverberg M, Kapoor V, Albelda SM.. 2006. Cycloxygenase-2 inhibition augments the efficacy of a cancer vaccine. Clin Cancer Res. 12(1):214–222. doi: 10.1158/1078-0432.CCR-05-1178. [DOI] [PubMed] [Google Scholar]
- Han S, Bao X, Zou Y, Wang L, Li Y, Yang L, Liao A, Zhang X, Jiang X, Liang D, et al. 2023. d-lactate modulates M2 tumor-associated macrophages and remodels immunosuppressive tumor microenvironment for hepatocellular carcinoma. Sci Adv. 9(29):eadg2697. doi: 10.1126/sciadv.adg2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C.. 2000. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 14(13):2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- Haskó G, Pacher P.. 2012. Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol. 32(4):865–869. doi: 10.1161/ATVBAHA.111.226852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield SM, Kjaergaard J, Lukashev D, Belikoff B, Schreiber TH, Sethumadhavan S, Abbott R, Philbrook P, Thayer M, Shujia D, et al. 2014. Systemic oxygenation weakens the hypoxia and hypoxia inducible factor 1alpha-dependent and extracellular adenosine-mediated tumor protection. J Mol Med (Berl). 92(12):1283–1292. doi: 10.1007/s00109-014-1189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, Tsui YC, Cui G, Micevic G, Perales JC, et al. 2015. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell. 162(6):1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeksema MA, de Winther MP.. 2016. Epigenetic regulation of monocyte and macrophage function. Antioxid Redox Signal. 25(14):758–774. doi: 10.1089/ars.2016.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosios AM, Hecht VC, Danai LV, Johnson MO, Rathmell JC, Steinhauser ML, Manalis SR, Vander Heiden MG.. 2016. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev Cell. 36(5):540–549. doi: 10.1016/j.devcel.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HR J, Wang J, Wang ZJ, Xi MJ, Xia BH, Deng K, Yang JL.. 2023. Lipid metabolic reprogramming in tumor microenvironment: from mechanisms to therapeutics. J Hematol Oncol. 16(1):103. doi: 10.1186/s13045-023-01498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HH, Lin YM, Shen CY, Shibu MA, Li SY, Chang SH, Lin CC, Chen RJ, Viswanadha VP, Shih HN, et al. 2017. Prostaglandin E2-induced COX-2 expressions via EP2 and EP4 signaling pathways in Human LoVo colon cancer cells. Int J Mol Sci. 18(6):1132. doi: 10.3390/ijms18061132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Wang Z, Zhao L.. 2021. The crucial roles of intermediate metabolites in cancer. Cancer Manag Res. 13:6291–6307. doi: 10.2147/CMAR.S321433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XY, Huang ZL, Huang J, Xu B, Huang XY, Xu YH, Zhou J, Tang ZY.. 2020. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res. 39(1):20. doi: 10.1186/s13046-020-1529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang PY, Mathur J, Cao Y, Almeida J, Ye J, Morikis V, Cornish D, Clarke M, Stewart SA, Pathak A, et al. 2023. A Cdh3-beta-catenin-laminin signaling axis in a subset of breast tumor leader cells control leader cell polarization and directional collective migration. Dev Cell. 58(1):34–50 e39. doi: 10.1016/j.devcel.2022.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba T, Sano H, Kawahito Y, Hla T, Akita K, Toda M, Yamashina I, Inoue M, Nakada H.. 2003. Induction of cyclooxygenase-2 in monocyte/macrophage by mucins secreted from colon cancer cells. Proc Natl Acad Sci U S A. 100(5):2736–2741. doi: 10.1073/pnas.0435410100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip WK, Medzhitov R.. 2015. Macrophages monitor tissue osmolarity and induce inflammatory response through NLRP3 and NLRC4 inflammasome activation. Nat Commun. 6:6931. doi: 10.1038/ncomms7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanban-Esfahlan R, Seidi K, Banimohamad-Shotorbani B, Jahanban-Esfahlan A, Yousefi B.. 2018. Combination of nanotechnology with vascular targeting agents for effective cancer therapy. J Cell Physiol. 233(4):2982–2992. doi: 10.1002/jcp.26051. [DOI] [PubMed] [Google Scholar]
- Jahanban-Esfahlan R, Seidi K, Zarghami N.. 2017. Tumor vascular infarction: prospects and challenges. Int J Hematol. 105(3):244–256. doi: 10.1007/s12185-016-2171-3. [DOI] [PubMed] [Google Scholar]
- Jeannin P, Paolini L, Adam C, Delneste Y.. 2018. The roles of CSFs on the functional polarization of tumor-associated macrophages. FEBS J. 285(4):680–699. doi: 10.1111/febs.14343. [DOI] [PubMed] [Google Scholar]
- Jin Y, Cai Q, Wang L, Ji J, Sun Y, Jiang J, Wang C, Wu J, Zhang B, Zhao L, et al. 2023. Paracrine activin B-NF-kappaB signaling shapes an inflammatory tumor microenvironment in gastric cancer via fibroblast reprogramming. J Exp Clin Cancer Res. 42(1):269. doi: 10.1186/s13046-023-02861-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, Sadelain M.. 2018. Chimeric Antigen Receptor Therapy. N Engl J Med. 379(1):64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, Vander Heiden MG, Miller G, Drebin JA, Bar-Sagi D, et al. 2015. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 75(3):544–553. doi: 10.1158/0008-5472.CAN-14-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapellos TS, Iqbal AJ.. 2016. Epigenetic control of macrophage polarisation and soluble mediator gene expression during inflammation. Mediators Inflamm. 2016:6591703. doi: 10.1155/2016/6591703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kechagia JZ, Ivaska J, Roca-Cusachs P.. 2019. Integrins as biomechanical sensors of the microenvironment. Nat Rev Mol Cell Biol. 20(8):457–473. doi: 10.1038/s41580-019-0134-2. [DOI] [PubMed] [Google Scholar]
- Kerneur C, Cano CE, Olive D.. 2022. Major pathways involved in macrophage polarization in cancer. Front Immunol. 13:1026954. doi: 10.3389/fimmu.2022.1026954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian JK, Miettinen M, Walker RL, Wang Y, Zhu YJ, Waterfall JJ, Noyes N, Retnakumar P, Yang Z, Smith WI, Jr., et al. 2014. Recurrent epimutation of SDHC in gastrointestinal stromal tumors. Sci Transl Med. 6(268):268ra177. doi: 10.1126/scitranslmed.3009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Lee H, Thoudam CD, Kang T, Harris HJ, Lee RA, K I.. 2023. The role of pyruvate metabolism in mitochondrial quality control and inflammation. Mol Cells. 46(5):259–267. doi: 10.14348/molcells.2023.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Hwang J, Jung XJ, Cha YH, Kim HS, H K.. 2014. Global metabolite profiling of synovial fluid for the specific diagnosis of rheumatoid arthritis from other inflammatory arthritis. PLoS One. 9(6):e97501. doi: 10.1371/journal.pone.0097501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Selak MA, Gottlieb E.. 2006. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 25(34):4675–4682. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- Kitano Y, Okabe H, Yamashita YI, Nakagawa S, Saito Y, Umezaki N, Tsukamoto M, Yamao T, Yamamura K, Arima K, et al. 2018. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer. 118(2):171–180. doi: 10.1038/bjc.2017.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Zimmermann H, Millhorn DE.. 2000. Chronic hypoxia enhances adenosine release in rat PC12 cells by altering adenosine metabolism and membrane transport. J Neurochem. 74(2):621–632. doi: 10.1046/j.1471-4159.2000.740621.x. [DOI] [PubMed] [Google Scholar]
- Koelzer VH, Canonica K, Dawson H, Sokol L, Karamitopoulou-Diamantis E, Lugli A, Zlobec I.. 2016. Phenotyping of tumor-associated macrophages in colorectal cancer: impact on single cell invasion (tumor budding) and clinicopathological outcome. Oncoimmunology. 5(4):e1106677. doi: 10.1080/2162402X.2015.1106677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang R, Jahangiri A, Mascharak S, Nguyen A, Chandra A, Flanigan PM, Yagnik G, Wagner JR, De Lay M, Carrera D, et al. 2017. GLUT3 upregulation promotes metabolic reprogramming associated with antiangiogenic therapy resistance. JCI Insight. 2(2):e88815. doi: 10.1172/jci.insight.88815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviron M, Petit M, Weber-Delacroix E, Combes AJ, Arkal AR, Barthelemy S, Courau T, Hume DA, Combadiere C, Krummel MF, et al. 2022. Tumor-associated macrophage heterogeneity is driven by tissue territories in breast cancer. Cell Rep. 39(8):110865. doi: 10.1016/j.celrep.2022.110865. [DOI] [PubMed] [Google Scholar]
- Lee H, Park SJ, Hong S, Lim SW, Kim S.. 2022. Deletion of IP6K1 in mice accelerates tumor growth by dysregulating the tumor-immune microenvironment. Anim Cells Syst (Seoul). 26(1):19–27. doi: 10.1080/19768354.2022.2029560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TW, Lee KM.. 2022. ECM1 is associated with endocrine resistance in ER(+) breast cancers. Anim Cells Syst (Seoul). 26(3):99–107. doi: 10.1080/19768354.2022.2083235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CE, Pollard JW.. 2006. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 66(2):605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Li J, Xie Y, Wang X, Li F, Li S, Li M, Peng H, Yang L, Liu C, Pang L, et al. 2019. Prognostic impact of tumor-associated macrophage infiltration in esophageal cancer: a meta-analysis. Future Oncol. 15(19):2303–2317. doi: 10.2217/fon-2018-0669. [DOI] [PubMed] [Google Scholar]
- Li J, Xu X, Jiang Y, Hansbro NG, Hansbro PM, Xu J, Liu G.. 2020. Elastin is a key factor of tumor development in colorectal cancer. BMC Cancer. 20(1):217. doi: 10.1186/s12885-020-6686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EA, Bendell JC, Falchook GS, Bauer TM, Drake CG, Choe JH, George DJ, Karlix JL, Ulahannan S, Sachsenmeier KF, et al. 2022. Phase Ia/b, open-label, multicenter study of AZD4635 (an adenosine A2A receptor antagonist). as monotherapy or combined with durvalumab, in patients with solid tumors. Clin Cancer Res. 28(22):4871–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wu S, Zheng X, Zheng P, Fu Y, Wu C, Lu B, Ju J, Jiang J.. 2020. Immune suppressed tumor microenvironment by exosomes derived from gastric cancer cells via modulating immune functions. Sci Rep. 10(1):14749. doi: 10.1038/s41598-020-71573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Luo J, Kuang D, Xu S, Duan Y, Xia Y, Wei Z, Xie X, Yin B, Chen F, et al. 2019. Lactate inhibits ATP6V0d2 expression in tumor-associated macrophages to promote HIF-2alpha-mediated tumor progression. J Clin Invest. 129(2):631–646. doi: 10.1172/JCI123027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P-S, Wang H, Li X, Chao T, Teav T, Christen S, Di Conza G, Cheng W-C, Chou C-H, Vavakova M, et al. 2017. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. 18(9):985–994. doi: 10.1038/ni.3796. [DOI] [PubMed] [Google Scholar]
- Locati M, Curtale G, Mantovani A.. 2020. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 15:123–147. doi: 10.1146/annurev-pathmechdis-012418-012718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorendeau D, Christen S, Rinaldi G, Fendt SM.. 2015. Metabolic control of signalling pathways and metabolic auto-regulation. Biol Cell. 107(8):251–272. doi: 10.1111/boc.201500015. [DOI] [PubMed] [Google Scholar]
- Loyher PL, Hamon P, Laviron M, Meghraoui-Kheddar A, Goncalves E, Deng Z, Torstensson S, Bercovici N, Baudesson de Chanville C, Combadiere B, et al. 2018. Macrophages of distinct origins contribute to tumor development in the lung. J Exp Med. 215(10):2536–2553. doi: 10.1084/jem.20180534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A.. 2005. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 280(51):41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- Lu H, Forbes RA, Verma A.. 2002. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 277(26):23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- Manerba M, Vettraino M, Fiume L, Di Stefano G, Sartini A, Giacomini E, Buonfiglio R, Roberti M, Recanatini M.. 2012. Galloflavin (CAS 568-80-9): a novel inhibitor of lactate dehydrogenase. ChemMedChem. 7(2):311–317. doi: 10.1002/cmdc.201100471. [DOI] [PubMed] [Google Scholar]
- Mantovani A. 1978. Effects on in vitro tumor growth of murine macrophages isolated from sarcoma lines differing in immunogenicity and metastasizing capacity. Int J Cancer. 22(6):741–746. doi: 10.1002/ijc.2910220617. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P.. 2015. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 212(4):435–445. doi: 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F.. 2008. Cancer-related inflammation. Nature. 454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M.. 2013. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 229(2):176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A.. 2010. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 22(2):231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A.. 2002. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23(11):549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Martinez-Reyes I, Chandel NS.. 2021. Cancer metabolism: looking forward. Nat Rev Cancer. 21(10):669–680. doi: 10.1038/s41568-021-00378-6. [DOI] [PubMed] [Google Scholar]
- Medrek C, Ponten F, Jirstrom K, Leandersson K.. 2012. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meric-Bernstam F, Larkin J, Tabernero J, Bonini C.. 2021. Enhancing anti-tumour efficacy with immunotherapy combinations. Lancet. 397(10278):1010–1022. doi: 10.1016/S0140-6736(20)32598-8. [DOI] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM.. 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 164(12):6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- Montalbán Del Barrio I, Penski C, Schlahsa L, Stein RG, Diessner J, Wöckel A, Dietl J, Lutz MB, Mittelbronn M, Wischhusen J.. 2016. Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages - a self-amplifying, CD39- and CD73-dependent mechanism for tumor immune escape. 4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Shi W, Xu Y, Xu C, Zhao T, Geng B, Yang J, Pan J, Hu S, Zhang C, et al. 2018. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle. 17(4):428–438. doi: 10.1080/15384101.2018.1444305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraja AS, Dorniak PL, Sadaoui NC, Kang Y, Lin T, Armaiz-Pena G, Wu SY, Rupaimoole R, Allen JK, Gharpure KM, et al. 2016. Sustained adrenergic signaling leads to increased metastasis in ovarian cancer via increased PGE2 synthesis. Oncogene. 35(18):2390–2397. doi: 10.1038/onc.2015.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan H, Hutter CM, Lin Y, Jacobs EJ, Ulrich CM, White E, Baron JA, Berndt SI, Brenner H, Butterbach K, et al. 2015. Association of aspirin and NSAID use with risk of colorectal cancer according to genetic variants. JAMA. 313(11):1133–1142. doi: 10.1001/jama.2015.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Q, Zhang H, Shi X, Li X.. 2023. Exosomal lncRNA HCG18 contributes to cholangiocarcinoma growth and metastasis through mediating miR-424-5p/SOX9 axis through PI3 K/AKT pathway. Cancer Gene Ther. 30(4):582–595. doi: 10.1038/s41417-022-00500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Chen J, Qiao Y.. 2022. Epigenetic modifications in tumor-associated macrophages: a new perspective for an old foe. Front Immunol. 13:836223. doi: 10.3389/fimmu.2022.836223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM.. 2010. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 142(5):687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, et al. 2006. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 103(35):13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA. 2011. A critical role for citrate metabolism in LPS signalling. Biochem J. 438(3):e5–e6. doi: 10.1042/BJ20111386. [DOI] [PubMed] [Google Scholar]
- Palma D, Lewis MEC.. 2013. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 23(3):277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Pardoll DM. 2012. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, Kasmi E, Smith KC, Thompson AM, Cheever RW, Murray AW, Wynn PJ, A T.. 2009. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 5(4):e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau JC, Schneider JC, Archer AJ, Sentissi J, Leyva FJ, Cramton J.. 2004. Environmental oxygen tension affects phenotype in cultured bone marrow-derived macrophages. Am J Physiol Lung Cell Mol Physiol. 286(2):L354–L362. doi: 10.1152/ajplung.00380.2002. [DOI] [PubMed] [Google Scholar]
- Poh AR, Ernst M.. 2018. Targeting macrophages in cancer: from bench to bedside. Front Oncol. 8:49. doi: 10.3389/fonc.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne Nigel J, Tonelli F, Lim Keng G, Long Jaclyn S, Edwards J, Pyne S.. 2012. Sphingosine 1-phosphate signalling in cancer. Biochem Soc Trans. 40(1):94–100. doi: 10.1042/BST20110602. [DOI] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW.. 2010. Macrophage diversity enhances tumor progression and metastasis. Cell. 141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X, Hu Z, Xiong F, Yang Y, Peng C, Wang D, Li X.. 2023. Lipid metabolism reprogramming in tumor-associated macrophages and implications for therapy. Lipids Health Dis. 22(1):45. doi: 10.1186/s12944-023-01807-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu SQ, Waaijer SJH, Zwager MC, de Vries EGE, van der Vegt B, Schroder CP.. 2018. Tumor-associated macrophages in breast cancer: innocent bystander or important player? Cancer Treat Rev. 70:178–189. doi: 10.1016/j.ctrv.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Rani R, Kumar V.. 2016. Recent update on human lactate dehydrogenase enzyme 5 (hLDH5) inhibitors: a promising approach for cancer chemotherapy. J Med Chem. 59(2):487–496. doi: 10.1021/acs.jmedchem.5b00168. [DOI] [PubMed] [Google Scholar]
- Richter S, Klink B, Nacke B, de Cubas AA, Mangelis A, Rapizzi E, Meinhardt M, Skondra C, Mannelli M, Robledo M, et al. 2016. Epigenetic mutation of the succinate dehydrogenase C promoter in a patient with two paragangliomas. J Clin Endocrinol Metab. 101(2):359–363. doi: 10.1210/jc.2015-3856. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L.. 2010. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 185(1):605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- Roszer T. 2015. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Coussens LM.. 2015. Macrophages and therapeutic resistance in cancer. Cancer Cell. 27(4):462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runtsch MC, Angiari S, Hooftman A, Wadhwa R, Zhang Y, Zheng Y, Spina JS, Ruzek MC, Argiriadi MA, McGettrick AF, et al. 2022. Itaconate and itaconate derivatives target JAK1 to suppress alternative activation of macrophages. Cell Metab. 34(3):487–501.e8. doi: 10.1016/j.cmet.2022.02.002. [DOI] [PubMed] [Google Scholar]
- Schumann T, Adhikary T, Wortmann A, Finkernagel F, Lieber S, Schnitzer E, Legrand N, Schober Y, Nockher WA, Toth PM, et al. 2015. Deregulation of PPARbeta/delta target genes in tumor-associated macrophages by fatty acid ligands in the ovarian cancer microenvironment. Oncotarget. 6(15):13416–13433. doi: 10.18632/oncotarget.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schworer S, Vardhana SA, Thompson CB.. 2019. Cancer metabolism drives a stromal regenerative response. Cell Metab. 29(3):576–591. doi: 10.1016/j.cmet.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E.. 2005. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 7(1):77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Serrati S, Di Fonte R, Porcelli L, De Summa S, De Risi I, Fucci L, Ruggieri E, Marvulli TM, Strippoli S, Fasano R, et al. 2023. Circulating extracellular vesicles are monitoring biomarkers of anti-PD1 response and enhancer of tumor progression and immunosuppression in metastatic melanoma. J Exp Clin Cancer Res. 42(1):251. doi: 10.1186/s13046-023-02808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Csizmadia E, Hedblom A, Vuerich M, Xie H, Li M, Longhi MS, Wegiel B.. 2017. Deletion of lactate dehydrogenase-A in myeloid cells triggers antitumor immunity. Cancer Res. 77(13):3632–3643. doi: 10.1158/0008-5472.CAN-16-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Wagner K, Wolchok JD, Allison JP.. 2011. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 11(11):805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Kelly TK, Jones PA.. 2010. Epigenetics in cancer. Carcinogenesis. 31(1):27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Gao W, Lytle NK, Huang P, Yuan X, Dann AM, Ridinger-Saison M, DelGiorno KE, Antal CE, Liang G, et al. 2019. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature. 569(7754):131–135. doi: 10.1038/s41586-019-1130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Liu T, Shi C, Zhang X, Chen X.. 2016. Bioconjugated manganese dioxide nanoparticles enhance chemotherapy response by priming tumor-associated macrophages toward M1-like phenotype and attenuating tumor hypoxia. ACS Nano. 10(1):633–647. doi: 10.1021/acsnano.5b06779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Yeku OO, Rafiq S, Purdon T, Dong X, Zhu L, Zhang T, Wang H, Yu Z, Mai J, et al. 2020. Tumor derived UBR5 promotes ovarian cancer growth and metastasis through inducing immunosuppressive macrophages. Nat Commun. 11(1):6298. doi: 10.1038/s41467-020-20140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, Saedeleer D, Kennedy CJ, Diepart KM, Jordan C, F B, et al. 2008. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 118(12):3930–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, Smyth MJ.. 2011. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 71(8):2892–2900. doi: 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, Zhu L, Kronenberg M, Miller PW, Portanova J, et al. 2000. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 164(1):361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- Stone SC, Rossetti RAM, Alvarez KLF, Carvalho JP, Margarido PFR, Baracat EC, Tacla M, Boccardo E, Yokochi K, Lorenzi NP, et al. 2019. Lactate secreted by cervical cancer cells modulates macrophage phenotype. J Leukoc Biol. 105(5):1041–1054. doi: 10.1002/JLB.3A0718-274RR. [DOI] [PubMed] [Google Scholar]
- Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP.. 2002. Ecto-5'-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 110(7):993–1002. doi: 10.1172/JCI0215337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. 2013. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 496(7444):238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todoric J, Karin M.. 2019. The fire within: cell-autonomous mechanisms in inflammation-driven cancer. Cancer Cell. 35(5):714–720. doi: 10.1016/j.ccell.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Trauelsen M, Hiron TK, Lin D, Petersen JE, Breton B, Husted AS, Hjorth SA, Inoue A, Frimurer TM, Bouvier M, et al. 2021. Extracellular succinate hyperpolarizes M2 macrophages through SUCNR1/GPR91-mediated Gq signaling. Cell Rep. 35(11):109246. doi: 10.1016/j.celrep.2021.109246. [DOI] [PubMed] [Google Scholar]
- Uemura T, Kawashima A, Jingushi K, Motooka D, Saito T, Nesrine S, Oka T, Okuda Y, Yamamoto A, Yamamichi G, et al. 2023. Bacteria-derived DNA in serum extracellular vesicles are biomarkers for renal cell carcinoma. Heliyon. 9(9):e19800. doi: 10.1016/j.heliyon.2023.e19800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugel S, De Sanctis F, Mandruzzato S, Bronte V.. 2015. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest. 125(9):3365–3376. doi: 10.1172/JCI80006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadevoo SMP, Gunassekaran GR, Lee C, Lee N, Lee J, Chae S, Park JY, Koo J, Lee B.. 2021. The macrophage odorant receptor Olfr78 mediates the lactate-induced M2 phenotype of tumor-associated macrophages. Proc Natl Acad Sci U S A. 118(37):e2102434118. doi: 10.1073/pnas.2102434118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Poznak C, Seidman AD, Reidenberg MM, Moasser MM, Sklarin N, Van Zee K, Borgen P, Gollub M, Bacotti D, Yao TJ, et al. 2001. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial. Breast Cancer Res Treat. 66(3):239–248. doi: 10.1023/A:1010686204736. [DOI] [PubMed] [Google Scholar]
- Volkman A, Gowans JL.. 1965. The origin of macrophages from bone marrow in the rat. Br J Exp Pathol. 46(1):62–70. [PMC free article] [PubMed] [Google Scholar]
- Walton ZE, Patel CH, Brooks RC, Yu Y, Ibrahim-Hashim A, Riddle M, Porcu A, Jiang T, Ecker BL, Tameire F, et al. 2018. Acid suspends the circadian clock in hypoxia through inhibition of mTOR. Cell. 174(1):72–87.e32. doi: 10.1016/j.cell.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Lin Y, Zhu H, Zhou Y, Mao F, Huang X, Sun Q, Li C.. 2022. The prognostic and clinical value of tumor-associated macrophages in patients with breast cancer: a systematic review and meta-ANALYSIS. Front Oncol. 12:905846. doi: 10.3389/fonc.2022.905846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Fu L, Sun H, Guo L, DuBois RN.. 2015. Prostaglandin E2 promotes colorectal cancer stem cell expansion and metastasis in mice. Gastroenterology. 149(7):1884–1895.e4. doi: 10.1053/j.gastro.2015.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Franco F, Tsui YC, Xie X, Trefny MP, Zappasodi R, Mohmood SR, Fernandez-Garcia J, Tsai CH, Schulze I, et al. 2020. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat Immunol. 21(3):298–308. doi: 10.1038/s41590-019-0589-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang X, Li X, Fan Y, Li G, Guo C, Zhu F, Zhang L, Shi Y.. 2014. CD68(+)HLA-DR(+) M1-like macrophages promote motility of HCC cells via NF-kappaB/FAK pathway. Cancer Lett. 345(1):91–99. doi: 10.1016/j.canlet.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu Y, Ding H, Shi X, Ren H.. 2021. Mesenchymal stem cell-secreted prostaglandin E(2) ameliorates acute liver failure via attenuation of cell death and regulation of macrophage polarization. Stem Cell Res Ther. 12(1):15. doi: 10.1186/s13287-020-02070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang Y, Chu Y, Li Z, Yu X, Huang Z, Xu J, Zheng L.. 2021. Tumor-derived adenosine promotes macrophage proliferation in human hepatocellular carcinoma. J Hepatol. 74(3):627–637. doi: 10.1016/j.jhep.2020.10.021. [DOI] [PubMed] [Google Scholar]
- Wang W, Liang M, Wang L, Bei W, Rong X, Xu J, Guo J.. 2023. Role of prostaglandin E2 in macrophage polarization: insights into atherosclerosis. Biochem Pharmacol. 207:115357. doi: 10.1016/j.bcp.2022.115357. [DOI] [PubMed] [Google Scholar]
- Wang XL, Jiang JT, Wu CP.. 2016. Prognostic significance of tumor-associated macrophage infiltration in gastric cancer: a meta-analysis. Genet Mol Res. 15(4). doi: 10.4238/gmr15049040. [DOI] [PubMed] [Google Scholar]
- Wang Z, Dai Z, Zhang H, Liang X, Zhang X, Wen Z, Luo P, Zhang J, Liu Z, Zhang M, et al. 2023. Tumor-secreted lactate contributes to an immunosuppressive microenvironment and affects CD8 T-cell infiltration in glioblastoma. Front Immunol. 14:894853. doi: 10.3389/fimmu.2023.894853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Thompson CB.. 2012. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 21(3):297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, Menk AV, Rittenhouse NL, DePeaux K, Whetstone RD, et al. 2021. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. 591(7851):645–651. doi: 10.1038/s41586-020-03045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert A, Olesch C, Brüne B.. 2019. Sphingosine-1-Phosphate and Macrophage Biology—How the Sphinx Tames the Big Eater [Review]. Front Immunol. 10. English. doi: 10.3389/fimmu.2019.01706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert A, Strack E, Snodgrass RG, Brüne B.. 2018. mPGES-1 and ALOX5/-15 in tumor-associated macrophages. Cancer Metastasis Rev. 37(2):317–334. doi: 10.1007/s10555-018-9731-3. [DOI] [PubMed] [Google Scholar]
- Weigert A, Tzieply N, Av K, Johann AM, Schmidt H, Geisslinger G, Brüne B.. 2007. Tumor cell apoptosis polarizes macrophages—role of sphingosine-1-phosphate. Mol Biol Cell. 18(10):3810–3819. doi: 10.1091/mbc.e06-12-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Yin Y, Hu X, Peng C, Liu Y, Li Q, Huang W, Huang Q.. 2019. Effects of environmental pH on macrophage polarization and osteoimmunomodulation. ACS Biomater Sci Eng. 5(10):5548–5557. doi: 10.1021/acsbiomaterials.9b01181. [DOI] [PubMed] [Google Scholar]
- Wu J-Y, Huang T-W, Hsieh Y-T, Wang Y-F, Yen C-C, Lee G-L, Yeh C-C, Peng Y-J, Kuo Y-Y, Wen H-T, et al. 2020. Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol Cell. 77(2):213–227.e215. [DOI] [PubMed] [Google Scholar]
- Wu P, Wu D, Zhao L, Huang L, Chen G, Shen G, Huang J, Chai Y.. 2016. Inverse role of distinct subsets and distribution of macrophage in lung cancer prognosis: a meta-analysis. Oncotarget. 7(26):40451–40460. doi: 10.18632/oncotarget.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M, Zhang J, Chen W, Chen W.. 2018. M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. J Exp Clin Cancer Res. 37(1):143. doi: 10.1186/s13046-018-0815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Wang X, Li Y, Geng X, Jia X, Zhang L, Yang H.. 2021. Arachidonic acid metabolism controls macrophage alternative activation through regulating oxidative phosphorylation in PPARgamma dependent manner. Front Immunol. 12:618501. doi: 10.3389/fimmu.2021.618501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Li Y, Yan H, Zhang E, Huang X, Chen Q, Chen J, Qu J, Liu Y, He J, et al. 2019. CCL2 promotes macrophages-associated chemoresistance via MCPIP1 dual catalytic activities in multiple myeloma. Cell Death Dis. 10(10):781. doi: 10.1038/s41419-019-2012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun X, Zhang C, Wang S, Hu S, Xiang X, Cheng Q, Li Z, Wang Y, Zhu J.. 2021. Cyclooxygenase-2 expressed hepatocellular carcinoma induces cytotoxic T lymphocytes exhaustion through M2 macrophage polarization. Am J Transl Res. 13(5):4360–4375. [PMC free article] [PubMed] [Google Scholar]
- Yao J, Hua X, Shi J, Hu X, Lui K, He K, Mai J, Lan T, Lu M.. 2022. LncRNA THEMIS2-211, a tumor-originated circulating exosomal biomarker, promotes the growth and metastasis of hepatocellular carcinoma by functioning as a competing endogenous RNA. FASEB J. 36(4):e22238. [DOI] [PubMed] [Google Scholar]
- Yeini E, Ofek P, Pozzi S, Albeck N, Ben-Shushan D, Tiram G, Golan S, Kleiner R, Sheinin R, Israeli Dangoor S, et al. 2021. P-selectin axis plays a key role in microglia immunophenotype and glioblastoma progression. Nat Commun. 12(1):1912. doi: 10.1038/s41467-021-22186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Yin Y, Yi Y, Cheng Z, Kuang W, Li R, Zhong H, Cui Y, Yuan L, Gong F, et al. 2020. Targeting lactate dehydrogenase A (LDHA) exerts antileukemic effects on T-cell acute lymphoblastic leukemia. Cancer Commun (Lond). 40(10):501–517. doi: 10.1002/cac2.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Zhang J, Li D, Mao Y, Mo F, Du W, Ma X.. 2017. Prognostic significance of tumor-associated macrophages in ovarian cancer: a meta-analysis. Gynecol Oncol. 147(1):181–187. doi: 10.1016/j.ygyno.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Zang S, Ma X, Wu Y, Liu W, Cheng H, Li J, Liu J, Huang A.. 2017. PGE(2) synthesis and signaling in malignant transformation and progression of human hepatocellular carcinoma. Hum Pathol. 63:120–127. doi: 10.1016/j.humpath.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Zaynagetdinov R, Sherrill TP, Polosukhin VV, Han W, Ausborn JA, McLoed AG, McMahon FB, Gleaves LA, Degryse AL, Stathopoulos GT, et al. 2011. A critical role for macrophages in promotion of urethane-induced lung carcinogenesis. J Immunol. 187(11):5703–5711. doi: 10.4049/jimmunol.1100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al. 2019. Metabolic regulation of gene expression by histone lactylation. Nature. 574(7779):575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang H, Ding X, Hu J, Li Y, Zhang J, Wang H, Qi S, Xie A, Shi J, et al. 2022. Crosstalk between macrophage-derived PGE(2) and tumor UHRF1 drives hepatocellular carcinoma progression. Theranostics. 12(8):3776–3793. doi: 10.7150/thno.69494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhou X, Hao H.. 2022. Macrophage phenotype-switching in cancer. Eur J Pharmacol. 931:175229. doi: 10.1016/j.ejphar.2022.175229. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kim JA, Huang AY.. 2018. Optimizing tumor microenvironment for cancer immunotherapy: beta-glucan-based nanoparticles. Front Immunol. 9:341. doi: 10.3389/fimmu.2018.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liang F, Zhang D, Qi S, Liu Y.. 2023. Metabolites as extracellular vesicle cargo in health, cancer, pleural effusion, and cardiovascular diseases: an emerging field of study to diagnostic and therapeutic purposes. Biomed Pharmacother. 157:114046. doi: 10.1016/j.biopha.2022.114046. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Han F, Yang S, Wu J, Zhan W.. 2015. Oxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: involvement of the Akt-mTOR signaling pathway. Cancer Lett. 358(1):17–26. doi: 10.1016/j.canlet.2014.11.046. [DOI] [PubMed] [Google Scholar]
- Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, Li J.. 2014. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 26(2):192–197. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]