ABSTRACT.
A two decades–long epidemic of Rocky Mountain spotted fever in northern México reached the U.S. border city of Tijuana in 2021. Cases were near the city periphery in marginalized areas, some lacking infrastructure such as streets or utilities. We worked in the three census areas where human cases were reported and in 12 additional control Áreas Geoestadisticas Básicas. There were dogs, the primary tick host and Rickettsia rickettsii reservoir, in 76% of homes, with 2.2 owned dogs per home on average, approximately equal numbers of roaming dogs were seen, and 46.2% of owned dogs were allowed to roam in the street. Sixty-eight percent of people had heard of Rocky Mountain spotted fever (RMSF), and 35% self-reported tick infestation, including 19% of homes without dogs. Ticks appeared to move among houses of adjacent neighbors. Of 191 examined dogs, 61.8% were tick-infested, with 6-fold increased odds if they were allowed to roam. Although no dogs were Rickettsia polymerase chain reaction–positive, we found one R. rickettsii– and 11 Rickettsia massiliae–infected ticks. The rickettsial IgG seroprevalence by immunofluorescence antibody assay was 76.4%, associated with unhealthy body condition, adults, dogs with >10 ticks, more dogs being seen in the area, and dogs being permitted in the street. Insufficient medical and canine management resources have contributed to a case fatality rate of RMSF that has exceeded 50% in areas. High canine seroprevalence suggests risks to people and dogs; unfortunately, herd immunity is impeded by high turnover in the canine population owing to the birth of puppies and high death rates. Binational One Health workers should monitor disease spread, enact canine population management and tick eradication, and provide prevention, diagnostic, and treatment support.
INTRODUCTION
Tijuana, Baja California, is a large international city (2.2 million people, metropolitan area of 1,390 km2) with very rapid population growth, immediately abutting the U.S. border in the far northwestern corner of México. There are considerable tracts of poverty in the city, with substandard houses that lack municipal services. Despite large-scale fencing to the north that separates the United States and México, the Tijuana–U.S. borderland crossing is the most heavily visited in the world, with 50 million people crossing per year. In addition, large numbers of would-be immigrants wait near the border in México in sometimes squalid conditions for permission to enter the United States. People cross the border to seek asylum or to transport goods, some live on one side of the border and work on the other, and some cross for tourism. Among the latter groups, travel with pets is common. The size of the Tijuana and San Diego metropolitan districts, their adjacency, and the extensive cross-border traffic suggest that health threats in the region would be shared and that zoonotic disease would be best addressed using international One Health approaches.
Although Rocky Mountain spotted fever (RMSF) has been described since 1910 in at least nine Mexican states,1–3 often in rural areas, a more urban epidemic began in Sonora state over the last two decades and now continues to reemerge across the southwest border region.3–5 It became epidemic in Mexicali in 2008 and has spread farther south and west, including to Tijuana in 2021–2022 and to the smaller towns of Tecate, Ensenada, and San Quintin (Departamento de Epidemiología, Instituto de Servicios de Salud Pública del Estado de Baja California).6 The disease is caused by the bacterium Rickettsia rickettsii and is associated with four North American vector species: the American dog tick (Dermacentor variabilis) in the eastern United States, Dermacentor andersoni in the northwest, and two species (designated lineages) of the brown dog tick (Rhipicephalus sanguineus sensu lato) in the southwestern United States and México.7,8 The preferred host for all life stages of R. sanguineus s.l. is the domestic dog (Canis lupus familiaris), which itself can become ill or die with RMSF and can serve as a reservoir of infection for people.
Rocky Mountain spotted fever is responsible for more human fatalities than any other tickborne disease in North America,9 with a case fatality rate as high as 44% in the Sonora epidemic.10 Frequently, RMSF in this ecology targets children.4,5,10–13 Unfortunately, delays in diagnosis are common both because initial presentation is a nonspecific febrile disease and because of poor access to medical care: Such delays of even a few days increase the case fatality rate considerably. Poverty, stray dogs, and a high burden of brown dog ticks on dogs are important risk factors for human disease.3–5,11–15
Brown dog ticks can reach enormous densities in neighborhoods where free-roaming or stray dogs are abundant,7,16 and unlike many tick species, the brown dog tick thrives in environments with high temperature and low humidity, as is characteristic of Mexicali and Sonora.3,17 Of note, Tijuana, Ensenada, and San Quintin are coastal towns that tend to be both cooler and more humid than cities affected earlier in the epidemic. Climate conditions and the huge impact of international travel on Tijuana suggest that the trajectory of the epidemic there may differ from that of Mexicali and Sonora and that optimal intervention may need to be tailored.
There are numerous unanswered questions about the RMSF epidemic in northern México. Obviously, it is not known how great an impact it will have and how deeply it will penetrate in Tijuana, given that cases to date have tended to be on the eastern and southern peripheries of the city. Whether the disease continues to spread to other cities, including in the United States, is not known. It would be useful to know whether risk factors for disease and true prevalence are similar in Tijuana as in previously impacted cities. Importantly, more data on the impacts of interventions, such as education, dog neutering and acaricide campaigns, and premise treatment, could help inform best and most efficient practices. The goal of this study was to examine, for highly focal areas of elevated tick and Rickettsia risk, the following: 1) the prevalence of tick infestation on dogs, presence of rickettsial DNA in ticks and dogs, and rickettsial antibodies in dogs; 2) risk factors for individual canine cases; 3) environmental and residential patterns associated with high risk; and 4) resident awareness of the risks.
MATERIALS AND METHODS
Study setting.
Data were collected from the city of Tijuana (32.5149°N, 117.0382°W), which has a mean annual rainfall of 373 mm/year, typically in winter, and annual temperatures ranging from 9°C in winter to 26°C in summer. The landscape is often steep and is bisected by deep canyons with a wide elevation range, and some housing and roads along canyon walls or on their floors are subject to landslides and flooding. A map of the study sites is provided in Figure 1A. Prior to sampling, individuals conducting the study were trained on the research protocol, tick bite prevention measures, a COVID-19 protocol, and a personal security protocol. Team members were instructed to wear light-colored clothes to facilitate visualization of any ticks on their person, to also wear a green or yellow Secretaria de Salud vest, and to have an identification badge. When they were sampling, appropriate personal protective equipment, such as nitrile gloves, was worn. Questionnaire data were collected with informed consent, and human subject research was done in compliance with the University of California (UC) Davis and Tijuana General Hospital institutional review boards. Animal sampling was subject to oversight by the UC Davis Institutional Animal Care and Use Committee and the Academic Group of Animal Health.
Figure 1.
Map of Tijuana, México, highlighting sites where we sought brown dog ticks, rickettsial seropositive dogs, and rickettsial polymerase chain reaction–positive dogs and ticks. AGEB = Área Geoestadisticas Básica; R. massiliae = Rickettsia massiliae; RMSF = Rocky Mountain spotted fever; R. rickettsia = Rickettsia rickettsii.
Sampling design.
Fifteen Áreas Geoestadisticas Básicas (AGEBs) were selected for sampling. The AGEBs are census-related geographical delimitations established by the Instituto Nacional de Estadística y Geografía (INEGI). These included three AGEBs in which human cases had been reported and confirmed by the Baja California Secretary of Health in the 12 months preceding the study. The case definition for people9 required clinical signs consistent with RMSF and laboratory confirmation by polymerase chain reaction (PCR) or immunofluorescence assay. For each case AGEB, we randomly chose four control AGEBs located within 3 km of the case AGEB. Because all three of the case AGEBs had a high or very high marginalization status (a measure of “impact of deficiency that the population suffers resulting from the lack of education access, poor housing conditions, and income”18), we required the four controls to comprise two medium- and two high- to very high–marginalization AGEBs. Within each AGEB, two “manzanas” (city blocks) were chosen randomly, and in each, sampling was begun at the northwest-most house and continued in a clockwise direction until five houses had been assessed. In the event that five houses could not be recruited, investigators continued into the adjacent manzana. Inhabitants consented to participate, but they were blinded to the case status of the AGEBs.
Survey tool and specimen collection.
A questionnaire was delivered verbally in Spanish to assess participant awareness of RMSF, ticks in homes, treatment against ticks, and management of any dogs in the home. We assessed the amount of debris on patios (none, a little [defined as covering 1–5% of the patio area], and a lot); whether the home was on a hill, on a dirt road, and within 5 minutes of a canal; and groundcover type. We counted how many dogs were seen during a 15-minute period, including owned dogs on the property and any dogs on the street. We spent 15 minutes per house examining for and collecting all ticks from the patio and exterior walls into 70% ethanol. A maximum of five dogs present per home were examined to determine approximate body mass (<10 kg, 10–20 kg, >20 kg); body condition score (BCS) on a five-point scale (5 representing obesity; Hill’s Pet Nutrition, Topeka, KS); sex; and tick burden (estimated as 0 ticks, 1–10 ticks, >10 ticks). Age was reported as juvenile or adult >1 year old based on information from the owner or by physical examination. A blood sample was collected from any available vein into ethylenediamine tetraacetic acid-treated tubes. Dogs that could not be safely restrained were not sampled for blood. Approximately 1 to 3 mL of blood was collected and kept on ice in a cooler and then frozen at −20°C within 6 hours of collection. A maximum of 20 ticks were collected into 70% ethanol from each infested dog using forceps.
Laboratory testing.
Ticks were identified by species, sex, and age under dissecting microscopy.19–21 DNA was extracted from ticks using the QIAamp 96 DNA QIAcube HT Kit (Qiagen, Valencia, CA). Two 4 mm–diameter stainless steel beads (Spex Certiprep, Metuchen, NJ) were added to samples in 400 µL of Buffer RLT and 40 µL of Proteinase K, and the tissues were homogenized in a GenoGrinder2000 (Spex Certiprep) for 2 minutes at 1,000 strokes per minute. Total nucleic acids were extracted using a QIAcube HT automated nucleic acid workstation (Qiagen). DNA was extracted from dog blood using a blood and tissue kit (Qiagen) following manufacturer’s instructions and eluted in 50 µL of water. Real-time PCR was performed to detect Rickettsia genus DNA,22 and samples were considered positive if the threshold cycle was <40 with a characteristic amplification curve. Pan-Rickettsia PCR-positive samples were subjected to R. rickettsii–specific real-time PCR.23 Three negative water controls and a sequence-confirmed R. rickettsii–positive control were included in each run.
Positive PCR samples were also subjected to conventional PCR and DNA sequencing targeting the 17-kDa protein gene (htrA)24,25 and citrate synthase (gltA).26 All reactions used GoTaq Green Master Mix (Promega, Madison, WI) in 25-µL reactions containing 1.0 M of each primer and 3 µL of template DNA. Results were assessed by electrophoresis and ultraviolet (UV)-transillumination of GelStar–stained (Lonza, Rockland, ME) 1% agarose gels. Bands of the expected size were excised and cleaned with a QIAquick gel extraction kit (Qiagen), and products were sequenced in an ABI Prism 3730 Genetic Analyzer (UC DNA Sequencing Facility, Davis, CA). DNA sequences were manually trimmed and corrected if the nucleotide could be unambiguously determined and then compared with sequences in a large database (GenBank, National Center for Biotechnology Information, Bethesda, MD) by BLAST search.
Blood samples were also subjected to serology using indirect immunofluorescence antibody assays (IFAs). Plasma was diluted at 1/64 in phosphate-buffered saline (PBS) plus heat-inactivated goat serum, gamma globulin–free bovine serum albumin, and 0.01% thimerosal at pH 7.38. Diluted samples were spotted in 25-µL volumes on antigen slides for R. rickettsii (VMRD, Pullman, WA), incubated at 37°C in a humidity chamber for 30 minutes, and washed three times with the PBS mixture. A 1:100 dilution in PBS mixture of fluorescein-conjugated anti-dog IgG heavy and light chain antibodies (KPL, Gaithersburg, MD) was applied to the wells, and the slides were incubated again for 30 minutes at 37°C in moisture. Slides were then washed again three times, and eriochrome black was added as a counterstain in the last wash. Ten percent glycerol (pH 7.4) was spotted on the wells, slides were coverslipped, and then wells were evaluated with a UV microscope. Confirmed positive canine patient sera and water negative controls were incorporated in each run. Seropositive samples were serially diluted 2-fold and tested until the endpoint titer where there was weakened fluorescence.
STATISTICAL ANALYSES
For statistical analysis, data were maintained in Excel (Microsoft, Redmond, WA) and analyzed using program R (R Core Team, 2013). For hypothesis testing, a P-value of ≤ 0.05 was considered significant. Áreas Geoestadisticas Básicas polygons provided by INEGI were plotted in QGIS (www.qgis.org), using the “Map data© OpenStreetMap contributors, Map layer by Esri (Redlands CA).” Maps represented marginalization status, AGEB case and control status, canine seroprevalence and tick abundance, presence of hills and major canals, and sites of particularly high levels of tick infestation.
A series of generalized linear models was constructed as part of testing the significance of candidate risk factors. In general, a forward stepwise model selection process approach was used. Variables with P <0.20 from the univariable analysis and their first-order interactions when appropriate based on expert opinion were considered for multivariable models. Only variables with P <0.05 were maintained in the final models. Collinearity between predictors was assessed using the variance inflation factor (from the car Package in R); only variables with less than a variance inflation factor of 5 were included in the final model. Models that did not converge were rerun with the BOBYQA optimizer set to 100,000 iterations.
A stratified ecological approach was used to assess risk factors for the outcome of whether an AGEB had known cases or was a control. To do this, AGEB-wide exposures for each predictor were created as the average from all surveyed households in that AGEB. Mean percentages of each predictor variable were compared using Welch’s corrected unpaired t-test. Because there were no medium or low marginalization case AGEBs, the analysis was repeated with comparison of only high and very high marginalized areas with and without cases. Assessed predictors were percentage of houses on a hill, within 5 minutes of a canal, with debris on the patio, on a dirt road, with dogs allowed inside, with dogs allowed on the street, and with tick infestation (per owner report) and the average cement coverage, number of dogs at houses, number of dogs seen, number of dogs examined that were allowed on the street, number of respondents familiar with RMSF, and number of houses fumigated at least twice a year.
Mixed logistic regression using the lme4 package in R was performed to assess the binary outcomes of self-reported awareness of RMSF, whether there were any ticks observed on the property, and ticks being reported by the resident inside the home, with the latter two done with univariable analysis only because of low observed infestation levels and a low sample size, respectively. These analyses were conducted at the individual household level. To account for household clustering, a random effect model was fit for the city block. Assessed predictors (for these analyses as well as for canine serostatus and whether dogs were infested with ticks) were as follows: being in a case AGEB, AGEB marginalization status, debris on the patio, being on a hill, being on a dirt road, being near a canal, percentage of cement groundcover, number of people in the home, number of dogs owned, number of dogs observed (inclusive of in the home and on the street), frequency of acaricide use on the home and dog (defined as frequent = multiple times per year, infrequent = 1 or 2 times per year, and rare to never), whether a dog goes inside, and whether owned dogs are allowed in the street. Interactions between owning a dog, allowing the dog in the street, and allowing the dog inside the house were assessed using dummy variables constructed to capture each of the different options. We assessed the relationship between reporting someone in the house having been bitten in the past year and presence of ticks in the home using a Fisher’s exact test. The degree of agreement between observed tick infestation in the patio and self-reported tick infestation in the home was assessed using Cohen’s κ.
Whether dogs were infested with ticks was analyzed at the individual dog level with mixed logistic regression. Infestation levels (0 ticks, 1–10 ticks, >10 ticks) were additionally evaluated using multinomial logistic regression (with the mcgv package in R). Random effects and predictors were included as described above, substituting for whether any dogs were allowed on the street with whether the individual dog was allowed on the street. Additional predictors for these analyses were dog size, sex, BCS, and age. Body condition score was grouped into “healthy” (BCS 2–3) and “unhealthy” (above or below this range) classes.
The canine serostatus analyses were conducted at the individual dog level using the binary outcome of seropositive or seronegative. Adult dogs were analyzed using mixed logistic regression with random effects as described above. Owing to small sample sizes, juvenile dogs were assessed using t-tests and Fisher’s exact tests for numerical and categorical variables, respectively. We used a mixed-effects multinomial analysis to assess significance of endpoint serological titers, categorized as negative, low (64), medium (128), or high (≤256). All predictors were the same as described in the tick infestation analysis.
Several sets of data were summarized but not subjected to hypothesis testing. Positive PCR results, people self-reporting someone in the household as having had RMSF, and self-reported tick bites were not analyzed for risk factors because of their small numbers.
RESULTS
Characteristics of the 150 residences assessed in 15 AGEBs in Tijuana between May 16 and 18, 2022 are given in Table 1 (house characteristics) and Table 2 (dog characteristics). Overall in the study, we examined 227 dogs and collected 808 ticks. Dog ownership was common, with an average of 2.2 dogs/home and 76.0% of homes owning dogs. Dogs roaming near homes were also common, with our team observing 3.37 dogs on average in the neighborhoods of sampled houses (inclusive of owned dogs). All ticks that we collected were confirmed as R. sanguineus s.l. Six individuals, all in control AGEBs, reported that someone in the household previously had had RMSF. Nine percent of people (n = 14) reported either themselves or someone in their household as having been bitten by a tick in the past year, 34.9% (n = 52) reported seeing ticks in the home, and acaricides were used in the environment in 68.0% (n = 102) of homes and on 76.6% (n = 85) of owned dogs. The only factor that differed statistically among case and control AGEBs was that case AGEBs tended to have about twice as many houses on a hill (P = 0.05; Supplemental Table 1).
Table 1.
Characteristics of 15 AGEBs assessed in Tijuana, México, for risk of human and canine Rocky Mountain spotted fever
| AGEB | Marginalization | No. (%) Homes Near Canal | No. (%) Homes on Dirt Road | No. (%) Aware of RMSF | No. Reporting RMSF Case | No. (%) Reporting Tick Bite | Mean ± SD Cement Coverage | No. (%) Houses on Hill | No. (%) Houses Fumigating at least Twice/Year | No. (%) Houses Treating Dogs at least Twice/Year |
|---|---|---|---|---|---|---|---|---|---|---|
| 1* | Very high | 0/8 (0) | 10/10 (100) | 5/10 (50.0) | 0/10 | 0/10 (0) | 24.78 ± 41.33 | 10/10 (100) | 3/10 (30.0) | 3/6 (50.0) |
| 2* | High | 10/10 (100) | 10/10 (100) | 8/10 (80.0) | 0/10 | 1/10 (10.0) | 51.00 ± 36.95 | 7/10 (70.0) | 5/10 (50.0) | 5/10 (50.0) |
| 3* | High | 0/11 (0) | 5/11 (45.5) | 9/11 (81.8) | 0/11 | 0/11 (0) | 42.73 ± 46.23 | 7/11 (63.6) | 10/10 (100) | 8/8 (100) |
| 4 | High | 1/11 (9.1) | 8/11 (72.7) | 7/11 (63.6) | 0/11 | 1/11 (9.1) | 11.82 ± 24.73 | 7/11 (63.6) | 5/11 (45.5) | 2/8 (25.0) |
| 5 | High | 0/10 (0) | 8/10 (80.0) | 8/10 (80.0) | 0/10 | 0/10 (0) | 25.50 ± 35.47 | 0/9 (0) | 3/9 (33.3) | 6/7 (85.7) |
| 6 | High | 0/10 (0) | 0/10 (0) | 6/10 (60.0) | 1/10 | 2/10 (20.0) | 47.50 ± 42.11 | 0/10 (0) | 1/10 (10.0) | 3/7 (42.9) |
| 7 | Very high | 1/9 (20.0) | 10/10 (100) | 9/10 (90.0) | 0/10 | 2/10 (20.0) | 55.60 ± 47.53 | 10/10 (100) | 4/10 (40.0) | 7/8 (87.5) |
| 8 | High | 0/10 (0) | 10/10 (100) | 8/10 (80.0) | 0/10 | 1/10 (10.0) | 10.00 ± 31.62 | 5/10 (50.0) | 1/10 (10.0) | 2/7 (28.6) |
| 9 | Very high | 1/11 (9.1) | 10/11 (90.9) | 7/11 (63.6) | 1/11 | 2/11 (18.2) | 19.55 ± 32.97 | 10/11 (90.9) | 4/10 (40.0) | 4/6 (66.7) |
| 10 | Medium | 0/10 (0) | 0/10 (0) | 9/10 (90.0) | 0/10 | 0/10 (0) | 21.50 ± 41.44 | 0/10 (0) | 5/9 (55.6) | 3/6 (50.0) |
| 11 | Medium | 2/10 (20.0) | 7/10 (70.0) | 4/10 (40.0) | 0/10 | 0/10 (0) | 45.50 ± 48.79 | 2/10 (20.0) | 2/10 (20.0) | 3/7 (42.9) |
| 12 | Medium | 1/9 (11.1) | 8/9 (88.9) | 3/9 (33.3) | 2/9 | 2/9 (22.2) | 20.00 ± 44.72 | 9/9 (100) | 3/9 (33.3) | 3/7 (42.9) |
| 13 | Medium | 0/10 (0) | 1/10 (10.0) | 6/10 (60) | 2/10 | 0/10 (0) | 43.00 ± 41.38 | 3/10 (30.0) | 3/9 (33.3) | 2/5 (40.0) |
| 14 | Medium | 0/10 (0) | 2/10 (20) | 6/10 (60) | 0/10 | 3/10 (30.0) | 40.00 ± 51.64 | 1/10 (10.0) | 4/10 (40.0) | 6/8 (75.0) |
| 15 | Medium | 2/8 (25.0) | 0/8 (0) | 7/8 (87.5) | 0/8 | 0/8 (0) | 51.86 ± 41.91 | 0/8 (0) | 2/8 (25.0) | 3/7 (42.9) |
| All | NA | 19/148 (12.8) | 89/150 (59.3) | 102/150 (68.0) | 6/149 | 14/150 (9.3) | 34.13 ± 41.69 | 71/149 (47.7) | 55/145 (37.9) | 59/106 (55.7) |
AGEB = Área Geoestadisticas Básicas; NA = not applicable; RMSF = Rocky Mountain spotted fever. Case AGEBs were those in which a case of human RMSF had been diagnosed by the Baja California Secretary of Health in the prior 12 months.
These three AGEBs had human cases of RMSF.
Table 2.
Numbers of dogs seen, canine rickettsial seroprevalence, and canine and environmental infestation with brown dog ticks in 15 AGEBs assessed in Tijuana, México, for risk of human and canine Rocky Mountain spotted fever
| AGEB | Marginalization | Mean ± SD Dogs/Home | Mean ± SD Dogs Seen | Canine Seroprevalence | No. (%) Dogs with Ticks | No. (%) Dogs with >10 Ticks | No. (%) Homes with Ticks Inside | Mean ± SD Ticks Outside Home* | No. (%) Homes with Ticks Outside Home |
|---|---|---|---|---|---|---|---|---|---|
| 1† | Very high | 2.70 ± 3.59 | 4.50 ± 5.23 | 10/11 (90.9) | 9/13 (69.2) | 5/13 (38.5) | 0/10 (0) | 0 ± 0 | 0/5 (0) |
| 2† | High | 2.30 ± 1.34 | 2.90 ± 1.52 | 12/16 (75.0) | 8/18 (44.4) | 2/18 (11.1) | 5/10 (50.0) | 0.80 ± 1.75 | 2/10 (20.0) |
| 3† | High | 1.73 ± 1.10 | 4.45 ± 2.07 | 14/16 (87.5) | 8/16 (50.0) | 1/16 (6.3) | 3/11 (27.3) | 0.27 ± 0.90 | 1/11 (9.1) |
| 4 | High | 2.45 ± 1.51 | 4.73 ± 2.97 | 5/12 (41.7) | 14/14 (100) | 6/14 (42.9) | 3/11 (27.3) | 0.78 ± 1.72 | 2/9 (22.2) |
| 5 | High | 1.90 ± 1.60 | 2.30 ± 1.89 | 6/10 (60.0) | 4/11 (36.4) | 1/11 (9.1) | 0/10 (0) | 0 ± 0 | 0/10 (0) |
| 6 | High | 1.30 ± 1.25 | 1.70 ± 1.89 | 4/5 (80.0) | 9/13 (69.2) | 1/13 (7.7) | 3/9 (33.3) | 16.25 ± 35.43 | 2/8 (25.0) |
| 7 | Very high | 2.10 ± 2.23 | 3.90 ± 2.02 | 11/13 (84.6) | 11/19 (57.9) | 6/19 (31.6) | 2/10 (20.0) | 3.30 ± 8.74 | 3/10 (30.0) |
| 8 | High | 2.30 ± 2.54 | 3.70 ± 2.11 | 8/12 (66.7) | 7/13 (53.9) | 6/13 (46.2) | 3/10 (30.0) | 0 ± 0 | 0/10 (0) |
| 9 | Very high | 1.64 ± 1.91 | 2.18 ± 1.66 | 11/12 (91.7) | 7/11 (63.6) | 4/11 (36.4) | 9/11 (81.8) | 4.82 ± 8.39 | 4/11 (36.4) |
| 10 | Medium | 1.30 ± 1.57 | 3.30 ± 2.75 | 7/9 (77.8) | 8/9 (88.9) | 6/9 (66.7) | 6/10 (60.0) | 0.70 ± 1.49 | 2/10 (20.0) |
| 11 | Medium | 1.80 ± 1.87 | 3.30 ± 3.47 | 4/5 (80.0) | 3/7 (42.9) | 2/7 (28.6) | 2/10 (20.0) | 7.11 ± 21.33 | 1/9 (11.1) |
| 12 | Medium | 2.00 ± 1.50 | 3.56 ± 2.40 | 7/10 (70.0) | 12/14 (85.7) | 1/14 (7.1) | 3/9 (33.3) | 2.00 ± 3.38 | 4/8 (50.0) |
| 13 | Medium | 1.30 ± 1.83 | 2.60 ± 2.55 | 6/9 (66.7) | 4/9 (44.4) | 2/9 (22.2) | 4/10 (40.0) | 1.70 ± 3.13 | 4/10 (40.0) |
| 14 | Medium | 3.20 ± 2.70 | 3.70 ± 2.58 | 9/10 (90.0) | 6/12 (50.0) | 4/12 (33.3) | 3/10 (30.0) | 0 ± 0 | 0/8 (0) |
| 15 | Medium | 1.88 ± 1.55 | 3.75 ± 1.75 | 9/11 (81.8) | 8/12 (66.7) | 1/12 (8.3) | 6/8 (75.0) | 0 ± 0 | 0/8 (0) |
| All | NA | 1.99 ± 1.95 | 3.37 ± 2.65 | 123/161 (76.4) | 118/191 (61.8) | 48/191 (25.1) | 52/149 (34.9) | 2.47 ± 10.93 | 25/137 (18.3) |
AGEB = Área Geoestadisticas Básicas; NA = not applicable; RMSF = Rocky Mountain spotted fever. Case AGEBs were those in which a case of human RMSF had been diagnosed by the Baja California Secretary of Health in the prior 12 months.
For two houses, tick numbers were reported only as “>some number,” so these are minimum values.
These three AGEBs had human cases of RMSF.
The majority of people (68.0%, n = 102) surveyed had heard of RMSF (Supplemental Table 2), but there were no differences in this awareness across case or control AGEBs, marginalization status, the environmental factors we assessed, or the number of people in the home. The number of dogs seen (owned and roaming outside) was positively correlated with greater awareness of RMSF (odds ratio [OR] = 1.25, P = 0.01), but the number of owned dogs was not. Having heard of RMSF also was significantly associated with infrequent acaricide use in the environment (OR = 2.71, P = 0.03) and both frequent (OR = 3.63, P = 0.03) and infrequent (OR = 3.15, P = 0.05) use of acaricides on dogs (compared with not using any acaricides). In the multivariable model, dog owners who allowed their dogs inside but not to roam were most likely to report being aware of RMSF (OR = 6.43, P = 0.02) compared with houses where no dogs were seen.
During our visits to each house, tick infestation was assessed from owner self-reports, by researcher assessment of the environment, and on any dogs. In total, 35% of households with sufficient data in this study self-reported tick infestations (52/149; Table 3). Individuals who reported that they or someone in their household had been bitten by a tick were not statistically more likely to report tick infestation (Fisher’s test, P = 0.56). Homes reporting tick infestation included 19.4% of homes (7/36) that did not own dogs. Still, owning a dog increased 7-fold the odds of people seeing ticks in their home compared with homes where no dogs were seen or owned (P = 0.02). No other predictors were statistically significant in the univariable analysis. In the multivariable model, owning a dog (OR = 3.87, P = 0.009) and not living on a dirt road (OR = 2.44, P = 0.04) were retained as positive predictors of reporting a tick infestation.
Table 3.
Mixed-effect logistic regression of residents self-reporting tick infestation of the property and potential risk factors during a survey for brown dog tick infestation and Rocky Mountain spotted fever in Tijuana, México
| Predictor | No. (%) with Ticks/Total | OR | 95% CI | P-Value |
|---|---|---|---|---|
| Univariable Models | ||||
| Case/control | ||||
| Case | 8/31 (25.8) | 0.56 | 0.19–1.64 | 0.29 |
| Control | 44/118 (37.3) | Reference | – | – |
| Marginalization | ||||
| Medium | 24/57 (42.1) | Reference | – | – |
| High | 17/61 (27.9) | 0.5 | 0.20–1.27 | 0.14 |
| Very high | 11/31 (35.5) | 0.71 | 0.23–2.16 | 0.54 |
| Debris | ||||
| None | 13/38 (34.2) | Reference | – | – |
| A little | 25/76 (32.9) | 0.77 | 0.29–2.01 | 0.59 |
| A lot | 10/25 (40.0) | 1.5 | 0.44–5.16 | 0.52 |
| On a hill | ||||
| No | 27/77 (35.1) | Reference | – | – |
| Yes | 25/71 (35.2) | 1.18 | 0.50–2.75 | 0.71 |
| On a dirt road | ||||
| No | 26/60 (43.3) | Reference | – | – |
| Yes | 26/89 (29.2) | 0.52 | 0.24–1.15 | 0.11 |
| Near a canal | ||||
| No | 44/128 (34.4) | Reference | – | – |
| Yes | 8/19 (42.1) | 1.26 | 0.41–3.89 | 0.69 |
| Cement | 1 | 0.99–1.01 | 0.41 | |
| No. of people living in home | 0.96 | 0.84–1.10 | 0.59 | |
| No. of dogs at house | 1.06 | 0.88–1.27 | 0.57 | |
| Total No. of dogs seen | 1.07 | 0.93–1.24 | 0.31 | |
| Ownership and dog presence | ||||
| Owned | 45/114 (39.8) | 6.87 | 1.29–36.64 | 0.02 |
| Seen, not owned | 5/19 (26.3) | 3.28 | 0.46–23.26 | 0.23 |
| No dogs observed | 2/17 (11.8) | Reference | – | |
| Dog allowed inside* | ||||
| Inside | 21/51 (41.2) | 1.19 | 0.47–3.02 | 0.71 |
| Not | 24/62 (38.7) | Reference | – | – |
| Frequency of treating house with acaricide | ||||
| Never or rarely | 17/51 (33.3) | Reference | – | – |
| 1 or 2 times/year | 21/57 (36.8) | 1.11 | 0.47–2.61 | 0.82 |
| Multiple times/year | 12/36 (33.3) | 1 | 0.38–2.67 | 1.0 |
| Frequency of treating dogs with acaricide* | ||||
| Never or rarely | 7/26 (26.9) | Reference | – | – |
| 1 or 2 times/year | 20/41 (48.8) | 3.35 | 0.93–12.06 | 0.06 |
| Multiple times/year | 15/38 (39.5) | 2.14 | 0.59–7.71 | 0.25 |
| Dogs allowed to roam* | ||||
| No | 21/53 (39.6) | Reference | – | – |
| Yes | 21/46 (45.7) | 1.06 | 0.40–2.79 | 0.90 |
| Tick burden on dogs | ||||
| None | 18/44 (40.9) | Reference | – | – |
| 10–20 | 13/39 (33.3) | 0.76 | 0.26–2.21 | 0.61 |
| >10 | 13/23 (56.5) | 2.37 | 0.67–8.37 | 0.18 |
| Has heard of RMSF | ||||
| Yes | 40/101 (39.6) | 2.03 | 0.86–4.75 | 0.10 |
| No | 12/48 (25.0) | Reference | – | – |
| Multivariable Model | ||||
|---|---|---|---|---|
| (Intercept) | – | 0.12 | 0.04–0.36 | <0.001 |
| Owns a dog | 3.87 | 1.40–10.68 | 0.009 | |
| House not on a dirt road | 2.44 | 1.03–5.81 | 0.04 | |
OR = odds ratio; RMSF = Rocky Mountain spotted fever.
Analysis restricted to houses with dogs.
In univariable analyses, the odds of ticks being detected outside a home were higher for houses on a hill (OR = 3.24, P = 0.02; Table 4) but were not associated with other environmental risk factors. No statistically significant relationships were identified between the odds of infestation and owning a dog or the number of dogs owned; however, odds increased as the total number of dogs observed at and around the home increased (OR = 1.25, P = 0.05). The odds of ticks being detected in the environment were significantly elevated if at least one dog surveyed at the house had more than 10 ticks (OR = 9.91, P = 0.01)
Table 4.
Mixed-effect logistic regression of ticks found in external environment of houses and potential risk factors during a survey for brown dog tick infestation and Rocky Mountain spotted fever in Tijuana, México
| Predictor | No. (%) with Ticks/Total | OR | 95% CI | P |
|---|---|---|---|---|
| Univariable Models | ||||
| Case/control | ||||
| Case | 3/26 (11.5) | 0.5 | 0.12–2.17 | 0.36 |
| Control | 22/111 (19.8) | Reference | – | – |
| Marginalization | ||||
| Medium | 11/53 (20.8) | Reference | – | – |
| High | 7/58 (12.1) | 0.52 | 0.17–1.58 | 0.25 |
| Very high | 7/26 (26.9) | 1.4 | 0.41–4.70 | 0.59 |
| Debris | ||||
| None | 7/34 (20.6) | Reference | – | – |
| A little | 13/72 (18.1) | 0.84 | 0.29–2.42 | 0.75 |
| A lot | 2/22 (9.1) | 0.4 | 0.07–2.24 | 0.30 |
| On a hill | ||||
| No | 8/74 (10.8) | Reference | – | – |
| Yes | 17/62 (27.4) | 3.24 | 1.20–8.72 | 0.02 |
| On a dirt road | ||||
| No | 9/56 (16.1) | Reference | – | – |
| Yes | 16/81 (19.8) | 1.38 | 0.49–3.89 | 0.54 |
| Near a canal | ||||
| No | 22/118 (18.6) | Reference | – | – |
| Yes | 3/18 (16.7) | 0.85 | 0.19–3.74 | 0.83 |
| Cement | 1 | 0.99–1.02 | 0.45 | |
| No. of people living in home | 1.05 | 0.85–1.3 | 0.65 | |
| No. of dogs living at house | 1.15 | 0.89–1.49 | 0.30 | |
| Whether home owns a dog | ||||
| No | 20/103 (19.4) | Reference | – | – |
| Yes | 5/34 (14.7) | 1.41 | 0.46–4.30 | 0.54 |
| Total number of dogs seen | 1.25 | 1.00–1.55 | 0.05 | |
| Dogs allowed inside* | ||||
| No | 5/34 (14.7) | Reference | – | – |
| Yes | 11/48 (22.9) | 1.60 | 0.45–5.78 | 0.47 |
| Frequency of treating house with acaricide | ||||
| Rare to never | 9/46 (19.6) | Reference | – | – |
| Once or twice/year | 6/34 (17.7) | 1.04 | 0.35–3.06 | 0.95 |
| Multiple times/year | 10/52 (19.2) | 0.96 | 0.28–3.31 | 0.95 |
| Frequency of treating dogs with acaricide* | ||||
| Rare to never | 3/24 (12.5) | Reference | – | – |
| Once or twice/year | 5/37 (13.5) | 3.03 | 0.23–39.63 | 0.40 |
| Multiple times/year | 9/35 (25.7) | 6.49 | 0.65–65.08 | 0.11 |
| Allowed to roam* | ||||
| No | 7/48 (14.6) | Reference | – | – |
| Yes | 10/42 (23.8) | 2.06 | 0.55–7.70 | 0.28 |
| Tick burden on dogs | ||||
| 0 | 4/42 (9.5) | Reference | – | – |
| 1–10 | 8/33 (24.2) | 4.03 | 0.83–19.59 | 0.08 |
| >10 | 8/21 (38.1) | 9.91 | 1.61–60.9 | 0.01 |
| Has heard of RMSF | ||||
| No | 10/44 (22.7) | Reference | – | – |
| Yes | 15/93 (16.1) | 0.66 | 0.25–1.7 | 0.38 |
OR = odd ratio; RMSF = Rocky Mountain spotted fever.
Analysis restricted to houses with dogs.
A total of 61.8% of dogs examined in the study were infested with ticks. There were multiple risk factors for tick infestation on dogs when examined as a binary outcome (Table 5) and with a multinomial logistic regression (comparing no ticks, 1–10 ticks [light burden], or >10 ticks [heavy burden]; Supplemental Table 3). Owners reported that 46.6% of the dogs that we evaluated were allowed to roam in the street; in univariable analysis, roaming was associated with significantly higher odds (OR = 5.93, P <0.001) of tick infestation and over 7 times the odds of a heavy tick burden versus no ticks (P <0.001). In contrast, dogs at houses where at least one dog was allowed inside were less likely to be infested with ticks (OR = 0.49, P = 0.05). Medium-sized (10- to 20-kg) dogs had higher odds of infestation (OR = 2.27, P = 0.03) than smaller dogs, and higher heavy tick burdens were observed on dogs with an unhealthy BCS compared with healthy dogs (OR = 3.49, P = 0.03). Although female dogs had 2.13 times the odds of experiencing a low tick burden compared with males (P = 0.03), there was no difference in heavy tick burden risk between sexes. Tick burdens on juvenile and adult dogs were comparable. Dogs at houses with lower cement coverage had higher odds of being heavily infested (OR = 0.98, P = 0.002). Dogs had higher odds of being infested (OR = 5.24, P = 0.01) at homes where we documented an environmental tick infestation and 7.77 times the odds of having a heavy tick burden (P = 0.04). Heavy tick burden on dogs occurred more often in homes where acaricide was used in the house occasionally (OR = 3.58, P = 0.03) than in houses that did not use acaricide. Marginally significant but more than 3-fold elevated odds of heavy tick burdens were found for dogs surveyed at houses with high debris levels on patios and houses on hills (P = 0.06). After control for cement coverage and sex, multivariable logistic regression revealed that the strongest predictor of having ticks was whether dogs were allowed to roam (OR = 6.94, P <0.001).
Table 5.
Mixed-effect logistic regression of dogs infested with brown dog ticks and potential risk factors during a survey for proposed risk factors for brown dog tick infestation and Rocky Mountain spotted fever in Tijuana, México
| Predictors | No. (%) with Ticks/Total | OR | 95% CI | P-Value |
|---|---|---|---|---|
| Univariable Models | ||||
| House variables | ||||
| Case/control | ||||
| Case | 25/47 (53.2) | 0.65 | 0.25–1.71 | 0.38 |
| Control | 93/144 (64.6) | Reference | – | – |
| Marginalization | ||||
| Medium | 41/63 (65.1) | Reference | – | – |
| High | 50/85 (58.8) | 0.82 | 0.32–2.12 | 0.68 |
| Very high | 27/43 (62.8) | 1.02 | 0.32–3.21 | 0.98 |
| Debris | ||||
| None | 23/44 (52.3) | Reference | – | – |
| A little | 53/90 (58.9) | 1.36 | 0.57–3.25 | 0.49 |
| A lot | 33/45 (73.3) | 2.54 | 0.87–7.40 | 0.09 |
| On a hill | ||||
| No | 51/93 (54.8) | Reference | ||
| Yes | 66/96 (68.8) | 1.11 | 0.59–2.09 | 0.75 |
| On a dirt road | ||||
| No | 42/64 (65.6) | Reference | – | – |
| Yes | 76/127 (59.8) | 0.8 | 0.34–1.87 | 0.60 |
| Near a canal | ||||
| No | 105/164 (64.0) | Reference | – | – |
| Yes | 11/24 (45.8) | 0.46 | 0.14–1.52 | 0.20 |
| Cement | 0.99 | 0.98–1.00 | 0.03 | |
| Whether ticks were present at house | ||||
| None | 77/140 (55.0) | Reference | – | – |
| Present | 27/32 (84.4) | 5.24 | 1.60–17.19 | 0.006 |
| No. of dogs living at the house | 1.01 | 0.86–1.19 | 0.94 | |
| Total No. of dogs seen | 1.03 | 0.91–1.17 | 0.65 | |
| Some dogs at the house allowed to go inside* | ||||
| No | 69/99 (69.7) | Reference | – | – |
| Yes | 49/92 (53.3) | 0.49 | 0.25–0.99 | 0.05 |
| House infested with ticks (self-reported) | ||||
| No | 69/115 (60.0) | Reference | – | – |
| Yes | 49/76 (64.5) | 1.4 | 0.68–2.87 | 0.36 |
| Frequency of treating house with acaricide | ||||
| Rare to never | 39/59 (66.1) | Reference | – | – |
| Once or twice/year | 49/79 (62.0) | 1.03 | 0.45–2.37 | 0.95 |
| Multiple times/year | 30/47 (63.8) | 0.95 | 0.38–2.39 | 0.92 |
| Frequency of treating dogs with acaricide* | ||||
| Rare to never | 27/37 (73.0) | Reference | – | – |
| Once or twice/year | 29/44 (55.7) | 0.47 | 0.19–1.20 | 0.12 |
| Multiple times/year | 54/97 (65.9) | 0.8 | 0.28–2.33 | 0.69 |
| Dog variables | ||||
| Allowed to roam | ||||
| No | 44/94 (46.8) | Reference | – | – |
| Yes | 67/83 (80.7) | 5.93 | 2.59–13.57 | <0.001 |
| Body mass (kg) | ||||
| <10 | 47/88 (53.4) | Reference | – | – |
| 10–20 | 45/62 (72.6) | 2.27 | 1.07–4.83 | 0.03 |
| >20 | 26/41 (63.4) | 1.31 | 0.56–3.06 | 0.53 |
| Sex | ||||
| Female | 64/95 (67.4) | Reference | – | – |
| Male | 53/95 (55.8) | 0.55 | 0.29–1.06 | 0.08 |
| Body condition | ||||
| Normal (2–3) | 91/156 (58.3) | 2.53 | 0.94–6.84 | 0.07 |
| Abnormal (<2 or >3) | 24/31 (77.4) | Reference | – | – |
| Age | ||||
| Adult | 97/154 (63.0) | – | – | – |
| Juvenile | 21/36 (58.3) | 0.69 | 0.3–1.58 | 0.38 |
| Multivariable models | ||||
| (Intercept) | – | 1.79 | 0.82–3.88 | 0.14 |
| Allowed to roam | 6.94 | 2.87–16.8 | <0.001 | |
| Cement | 0.99 | 0.98–1.00 | 0.02 | |
| Male | 0.42 | 0.19–0.91 | 0.03 | |
OR = odds ratio.
Analysis restricted to houses with dogs.
Three dogs were PCR-positive on pan-rickettsial PCR, although they were negative using the R. rickettsii–specific PCR, and their high cycle thresholds precluded further DNA sequencing and confirmation of infecting species. Two of these dogs were in medium-marginalization and one in high-marginalization AGEBs, all in controls. No to low environmental infestation (zero to five ticks) was detected at the homes. All of the dogs were allowed access to the street; they varied by sex, size, and age, and all were tick-infested. There were no consistent environmental variables, such as being on a hill, being near a canal, having cement, and treating the dog against ticks. There were 12 PCR-positive ticks; DNA seq confirmed that one was infected with R. rickettsii and a second with R. massiliae. The prevalence of R. rickettsii in ticks was 0.12% (0.01–0.80%), the prevalence of R. massiliae was 1.36% (0.76–2.43%), and the prevalence of Rickettsia spp. combined was 1.49% (0.85–2.58%).
The R. rickettsii–infected tick was an adult female collected from the environment in a case in a high-marginalization AGEB, even though residents used acaricide in the home every 3 months. The home had a single dog, which was seropositive and allowed to roam in the street but was not allowed in the house; four other roaming dogs were seen nearby. The ticks with R. massiliae also were adults, both sexes, and from both controls and cases and from all marginalization status AGEBs. Debris was found in 10/11 homes, 8/11 were on hills, 8/11 were on dirt roads, none were near a canal, and on average, cement coverage of the properties was 49.5%. All of the ticks had been removed from dogs, all of which were adults and IFA-positive. Nine of the 11 dogs were allowed on the street, tick burdens were often considerable on these dogs, and 6/11 were males. Figure 1A shows the locations from which PCR-positive ticks were collected.
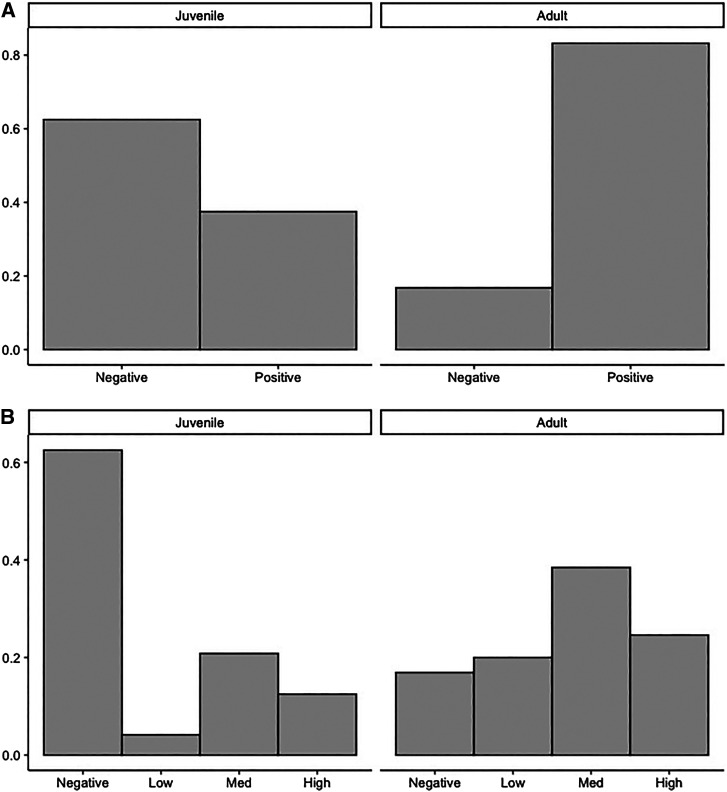
The overall seroprevalence among all tested dogs was 76.4% (Figure 1B). In univariable analyses, when we considered juvenile and adult dogs together, unhealthy BCS was most strongly associated with being seropositive (OR = 13.56, P = 0.02). Only 38% of juvenile dogs tested positive compared with 83% of adult dogs (OR = 0.10, P <0.001; Figure 2A). Given this strong effect of age, we assessed adult and juvenile dogs separately. Among the 24 tested juvenile dogs with seroprevalence data, no predictors were statistically significant (Table 6). There were 131 adult dogs tested by IFA. Adult dogs with an unhealthy BCS had 100% positivity compared with 79% in dogs with a normal BCS. Adult dogs with >10 ticks had 5.48 times higher odds of being seropositive compared with those with no ticks (P = 0.05). The odds of an adult dog testing positive significantly increased with the number of dogs seen in the area (OR = 1.50, P = 0.01). Whether the dog was permitted in the street was associated with a considerable three times—but nonsignificant (P = 0.09)—increase in the odds of positive IFA status. Multivariable analysis was conducted excluding BCS because of ambiguous directionality (infected dogs could have a poor BCS or dogs with a poor BCS could be more susceptible to infection). The minimum adequate model included the total number of dogs seen (OR = 1.50, P = 0.01) and the number of ticks on dogs (>10 versus 0–10; OR = 4.52, P = 0.07). However, we additionally present the model with street access, as it was an important predictor in other studies; its inclusion improved model fit (Akaike information criterion 96 versus 111), and it was a confounder on the effect of tick burden (see Table 6).
Figure 2.
Comparison of rickettsial serology results between juvenile and adult dogs in Tijuana, Mexico. (A) Seroprevalence and (B) Serotiter. Med = medium.
Table 6.
Analyses of relationships between dogs being Rickettsia spp. seropositive and potential risk factors stratified by age during a survey for proposed risk factors for brown dog tick infestation and Rocky Mountain spotted fever in Tijuana, México
| Univariable Predictors | Juvenile Dogs | Adult Dogs | ||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) Testing Seropositive/Total | OR or t-Test | 95% CI | P-Value | No. (%) Testing Seropositive/Total | OR | 95% CI | P-Value | |
| Univariable models | ||||||||
| House variables | ||||||||
| Case/control | ||||||||
| Case | 3/6 (50.0) | 1.94 | 0.20–19.46 | 0.64 | 32/35 (91.4) | 2.81 | 0.68–11.60 | 0.15 |
| Control | 6/18 (33.3) | Reference | – | – | 77/96 (80.2) | Reference | – | – |
| Marginalization | ||||||||
| Medium | 3/7 (42.9) | Reference | – | – | 37/45 (82.2) | Reference | – | – |
| High | 3/13 (23.1) | 0.42 | 0.04–2.55 | 0.61 | 44/56 (78.6) | 0.78 | 0.26–2.34 | 0.65 |
| Very high | 3/4 (75.0) | 3.52 | 0.17–260.91 | 0.55 | 28/30 (93.3) | 3.18 | 0.56–18.03 | 0.19 |
| Debris | ||||||||
| None | 2/5 (40.0) | Reference | – | – | 24/29 (82.8) | Reference | – | – |
| A little | 4/11 (36.4) | 0.87 | 0.06–14.66 | 1 | 53/65 (81.5) | 0.9 | 0.26–3.06 | 0.86 |
| A lot | 2/7 (28.6) | 0.63 | 0.03–13.19 | 1 | 28/31 (90.3) | 1.89 | 0.37–9.62 | 0.44 |
| On a hill | ||||||||
| No | 4/9 (44.4) | Reference | – | – | 51/64 (79.7) | Reference | – | – |
| Yes | 5/15 (33.3) | 0.64 | 0.09–4.78 | 0.68 | 56/65 (86.2) | 1.68 | 0.59–4.78 | 0.33 |
| On a dirt road | ||||||||
| No | 2/7 (28.6) | Reference | – | – | 39/46 (84.8) | Reference | – | – |
| Yes | 7/17 (41.2) | 1.71 | 0.20–22.94 | 0.67 | 70/85 (82.4) | 0.86 | 0.28–2.63 | 0.79 |
| Near a canal | ||||||||
| No | 9/23 (39.1) | Reference | – | – | 92/111 (82.9) | Reference | – | – |
| Yes | 0/1 (0) | 0 | 0–64.94 | 1 | 15/18 (83.3) | 1.05 | 0.22–5.02 | 0.95 |
| Cement | – | t(10) = −1.29 | – | 0.23 | – | 1.01 | 0.99–1.02 | 0.38 |
| Mean ± SD among IFA-pos | 49.00 ± 48.87 | – | – | – | 35.47 ± 42.22 | – | – | – |
| Mean ± SD among IFA-neg | 21.67 ± 40.69 | – | – | – | 26.5 ± 35.02 | – | – | – |
| No. of ticks seen at house | t(6) = −0.92 | – | 0.39 | – | 1.02 | 0.91–1.16 | 0.69 | |
| Mean ± SD among IFA-pos | 4.57 ± 2.33 | – | – | – | 2.37 ± 6.60 | – | – | – |
| Mean ± SD among IFA-neg | 0.67 ± 1.41 | – | – | – | 1.25 ± 3.19 | – | – | – |
| No. of dogs living at house | t(17) = 0.07 | – | 0.95 | – | 1.32 | 0.97–1.78 | 0.07 | |
| Mean ± SD among IFA-pos | 2.56 ± 1.59 | – | – | – | 3.44 ± 2.39 | – | – | – |
| Mean ± SD among IFA-neg | 2.60 ± 1.55 | – | – | – | 2.46 ± 1.47 | – | – | – |
| Total No. of dogs seen | t(12) = 0.41 | – | 0.69 | 1.5 | 1.11–2.02 | 0.008 | ||
| Mean ± SD among IFA-pos | 3.78 ± 2.33 | – | – | – | 4.89 ± 3.11 | – | – | – |
| Mean ± SD among IFA-neg | 4.13 ± 1.41 | – | – | – | 3.09 ± 1.34 | – | – | – |
| Some dogs at the house allowed to go inside | ||||||||
| No | 5/14 (35.7) | Reference | – | – | 51/65 (78.5) | Reference | – | – |
| Yes | 4/10 (40.0) | 1.19 | 0.16–8.5 | 1 | 58/66 (87.9) | 2.06 | 0.75–5.71 | 0.16 |
| House infested with ticks (self-reported) | ||||||||
| No | 6/15 (40.0) | Reference | – | – | 60/72 (83.3) | Reference | – | – |
| Yes | 3/9 (33.3) | 0.76 | 0.09–5.53 | 1 | 49/59 (83.1) | 0.95 | 0.35–2.6 | 0.93 |
| Frequency of treating house with acaricide | ||||||||
| Rare to never | 5/10 (50.0) | Reference | – | – | 24/30 (80.0) | Reference | – | – |
| Once or twice/year | 3/9 (33.3) | 0.36 | 0.01–6.48 | 0.58 | 52/64 (81.0) | 1.07 | 0.33–3.45 | 0.91 |
| Multiple times/year | 1/4 (25.0) | 0.52 | 0.05–4.43 | 0.65 | 29/33 (88.0) | 1.75 | 0.41–7.49 | 0.45 |
| Frequency of treating dogs with acaricide | ||||||||
| Rare to never | 2/6 (33.3) | Reference | – | – | 18/24 (75.0) | Reference | – | – |
| Once or twice/year | 1/5 (20.0) | 0.53 | 0.01–14.52 | 1 | 42/54 (78.0) | 1.19 | 0.35–4.03 | 0.77 |
| Multiple times/year | 5/10 (50.0) | 1.92 | 0.17–30.82 | 0.63 | 40/44 (91.0) | 3.45 | 0.79–15.04 | 0.10 |
| Dog variables | ||||||||
| Allowed to roam | ||||||||
| No | 4/16 (25.0) | Reference | – | 49/61 (80.3) | Reference | – | – | |
| Yes | 4/7 (57.1) | 3.74 | 0.43–38.72 | 0.18 | 54/59 (91.5) | 2.86 | 0.84–9.67 | 0.09 |
| Body mass (kg) | ||||||||
| <10 | 3/12 (25.0) | Reference | – | – | 47/57 (82.5) | Reference | – | – |
| 10–20 | 3/8 (37.5) | 1.75 | 0.17–18.79 | 0.64 | 36/44 (81.8) | 1.02 | 0.34–3.02 | 0.98 |
| >20 | 3/4 (75.0) | 7.64 | 0.43–521.09 | 0.12 | 25/29 (86.2) | 1.37 | 0.36–5.16 | 0.64 |
| Sex | ||||||||
| Female | 4/12 (33.3) | Reference | – | – | 56/66 (84.9) | Reference | – | – |
| Male | 5/12 (41.7) | 1.41 | 0.20–10.36 | 1 | 52/64 (81.3) | 0.75 | 0.28–1.97 | 0.56 |
| Ticks on dog | ||||||||
| No ticks | 4/10 (40.0) | Reference | – | – | 35/45 (77.8) | Reference | – | – |
| 1–10 ticks | – | – | – | – | 37/47 (78.7) | 1.1 | 0.38–3.17 | 0.86 |
| >10 ticks (any ticks present, juvenile) | 5/13 (38.5) | 0.94 | 0.13–7.06 | 1 | 35/37 (94.6) | 5.48 | 1.04–28.93 | 0.05 |
| Body condition | ||||||||
| Normal (2–3) | 7/21 (33.3) | Reference | – | – | 82/104 (78.9) | – | – | – |
| Unhealthy (<2 or >3) | 2/3 (66.7) | 3.76 | 0.17–251.98 | 0.53 | 24/24 (100) | – | – | – |
| Multivariable Models (adults only) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Multivariable predictors | Predictor | OR | 95% CI | P-Value | – | – | – | – |
| Model 1 | (Intercept) | 0.85 | 0.26–2.70 | 0.78 | – | – | – | – |
| Total No. of dogs seen | 1.50 | 1.10–2.05 | 0.01 | – | – | – | – | |
| Ticks on dog (>10) | 4.52 | 0.90–22.82 | 0.07 | – | – | – | – | |
| Model 2 | (Intercept) | 0.97 | 0.26–3.68 | 0.96 | – | – | – | – |
| Total No. of dogs seen | 1.44 | 1.03–2.01 | 0.03 | – | – | – | – | |
| Ticks on dog (>10) | 2.75 | 0.53–14.43 | 0.23 | – | – | – | – | |
| Dog allowed to roam (yes) | 2.10 | 0.59–7.57 | 0.25 | – | – | – | – | |
IFA-neg = immunofluorescence antibody assay–negative; IFA-pos = immunofluorescence antibody assay–positive; OR = odds ratio.
When serology was performed to the endpoint, titers of adult dogs tended to be higher (Figure 2B). When adult and juvenile dogs were grouped, roaming was associated with increased odds of both medium and high antibody titers (OR = 3.78, P = 0.01 and OR = 3.77, P = 0.02, respectively), and an unhealthy BCS was associated with medium (OR = 12.60, P = 0.02) and high titers (OR = 10.9, P = 0.03). After stratification by age, adult dogs had marginally higher odds of having medium serotiters than negative titers in case versus control AGEBs (OR = 3.56, P = 0.06) and a significant 8-fold increase in low relative to no titer (P = 0.03) in highly marginalized AGEBs (Supplemental Table 4). The total number of dogs seen was associated with medium and high titers (OR = 1.56, P = 0.003 and OR = 1.56, P = 0.004, respectively) compared with negative titers. If dogs were allowed to roam, their odds of having a medium titer versus a negative titer was 3.36 (P = 0.05), and high tick burdens were associated with low and medium final serotiters (OR = 5.65, P = 0.04 and OR = 4.85, P = 0.05, respectively).
DISCUSSION
Rocky Mountain spotted fever increasingly impacts urban México, where millions of people and dogs may be at risk of severe or fatal disease, with considerable risk of export to other cities. A common feature of these epidemics appears to be high marginalization and poverty; although Tijuana and other large cities in northern México certainly house people with affluence and stable living situations, there are also thousands of working poor and destitute residents and immigrants. Lack of resources—medical, for tick and dog management, and for environmental improvements—are important barriers to self-protection against RMSF. Responsible agencies have limited resources to intervene, and therefore epidemiological studies identifying key risk factors for brown dog tick infestation and RMSF can assist with campaigns for public health. During our door-to-door surveys, we found high canine rickettsial seroprevalence suggesting shared canine and human risk, areas with extremely tick-infested dogs and environments, and abundant dogs roaming the streets. Although awareness of the disease was high, anti-tick prevention measures were inadequate.
Our data, as well as findings in earlier studies in Arizona, Sonora, and Mexicali,3,7,16,27–31 indicate that roaming dogs and unchecked dog populations increase the risk of RMSF. Likewise, the most important factor influencing risk of RMSF in people and dogs in Tijuana was dogs, specifically those allowed to roam the streets. About three-fourths of all homes had dogs, and there was an average of two dogs per home. About half of the pet dogs were allowed to roam, and typically almost as many roaming dogs were seen at houses as confined pets. Many dogs on the streets were affiliated with a house, with someone providing care to the dogs. This contrasts with truly stray dogs, which do not have a caretaker, although we could not identify all roaming pets versus stray dogs at our site. Individual dogs being allowed to roam was the strongest predictor of tick burden among dogs and was associated with a 7-fold increase in the odds of a heavy tick burden, whereas overall number of dogs, inclusive of roaming dogs, was positively associated with environmental tick infestation. Roaming was also associated with increased seroprevalence and the odds of high serotiters. In contrast, houses where at least one dog was allowed inside were less likely to be infested with ticks.
The majority of dogs were infested with ticks, with heavy infestation in 12% of them, all with brown dog ticks. Dog-specific risk factors for tick infestation included being medium-sized, having an unhealthy BCS, and being female. Risk factors for rickettsial exposure (being seropositive) included having an unhealthy BCS, being an adult, and having a heavy tick burden. In Mexicali, ticks were more common on underconditioned and seropositive dogs, and seropositive dogs tended to have poor body condition.27 It is probable that the poor canine condition is a marker of limited family resources. Importantly, about a fifth of houses that did not own a dog also self-reported a tick infestation, and we found that ticks could move among patios of homes. People who live in brown dog tick–infested environments lacking dogs are even more vulnerable to tickborne disease, as these people represent the only available blood meal for the questing ticks.
We did not confirm any PCR-positive dogs in Tijuana (nor in a prior study in Mexicali27), consistent with the short duration of rickettsemia in these hosts,32 although we found PCR-positive sheltered dogs near the study sites.33 Polymerase chain reactions in three possibly infected dogs were too weak to further characterize. We saw many dogs with high rickettsial antibody titers and overall seroprevalence of 76%, consistent with an active epidemic, compared with 65% in Mexicali27 and 50–70% in Arizona tribal epidemics at their peak.28,31 The high seroprevalence suggests that many dogs may have acquired immunity, contributing to herd immunity. However, turnover in the canine population—due to birth of puppies and high death rates—impedes herd immunity and keeps an epidemic fueled, as we found in Mexicali.34
Unexpectedly, the majority of PCR-positive ticks were infected with R. massiliae, with only one being infected with R. rickettsii. Even where human cases are epidemic, finding PCR-positive ticks is rare with, for example, a low overall R. rickettsii PCR prevalence of ticks in Mexicali of 0.7%, but rising to 6.1% in one particularly highly infected AGEB27 and 3% in an eastern Arizona outbreak.28 The numerous seropositive dogs may have had antibodies to the co-circulating R. massiliae, but the multiple human deaths would have been due to R. rickettsii because R. massiliae is not known to induce fatality. Rickettsia massiliae is considered cosmopolitan35 and is usually detected in R. sanguineus, including in eastern Arizona, California, México, Argentina, and multiple Mediterranean countries,36–47 in sheep from Portugal,35 and in Portuguese and other Mediterranean-area dogs.43,48 Although R. massiliae is not typically associated with severe disease,49 one author suggested that it could be a cause of illness in dogs,36 whereas illness in people was attributed to R. massiliae infection in Palermo, Italy,50 and Tunisia,51 among other locations. The other implications of this pathogen in Tijuana are that it could cross-immunize people and dogs, such that animals exposed to R. massiliae may be less vulnerable to more pathogenic rickettsiae, and that it could reduce female ticks’ fitness or compete with other rickettsiae within the tick.
We found sparse evidence for environmental drivers of rickettsial exposure and tick infestation, probably because the sampling was focused in marginalized neighborhoods in the southern and eastern parts of Tijuana. At the time of the study, cases had only been reported in three AGEBs, and even with careful choice of criteria for controls, we could not cover a very broad spectrum of environmental risk factors. Moreover, six people in control AGEBs told us that there had been a case of RMSF in the home. True cases are likely to be undercounted, and we may have misclassified some of the AGEBs as controls. Houses on hills tended to be more likely to have ticks in the house, and higher tick burdens on dogs occurred where there was less cement, on hills, and with high debris levels, a shared risk factor with Mexicali and Sonora.27,52 However, in Tijuana, these environmental factors are also characteristic of the sprawling, highly impoverished outlying areas where many people live and where RMSF cases are being identified. In Mexicali, higher risk occurred where patios were concrete or related substrate versus grass (which would require financial resources to maintain).27 In Tijuana, many of the neighborhoods we observed were so recent as to not even have concrete or tile and lacking any formal infrastructure such as streets or utilities; here, ticks were found directly in the dirt.
Increasing public awareness can empower people to protect themselves and their pets against RMSF. Two-thirds of people across case and control AGEBs and all marginalization statuses had heard of RMSF (very similar to the 80% in Mexicali), a third of respondents reported having seen ticks in their homes, and one-tenth reported people being bitten by ticks. Although acaricides were widely used in homes and on dogs, we found previously that people used products such as flea spray and herbal remedies with no effect on brown dog ticks.27 Moreover, environmental treatment of brown dog ticks is notoriously challenging,53,54 and infestation often reoccurs. Dogs of owners who reported only occasional use of acaricides in the house tended to have heavier tick burdens. Those who were aware of RMSF still did not tend to use an acaricide in the environment but were more likely to use acaricide on their dogs. However, dog owners’ awareness of RMSF was associated with allowing dogs inside but not to roam, which our data suggest may help protect against RMSF. Often, deaths in local dogs are a harbinger of cases occurring in the people who shared the environment with the dogs.10 Veterinarians could reinforce the importance of acaricide, can help find and identify ticks, and can teach dog owners about rickettsiosis and other tickborne diseases.
This study was subject to several shortcomings. With such high seroprevalence in dogs associated with each level of the various risk factors assessed, we had difficulty finding statistical significance. Another shortcoming is that although we tried to use dog-specific attributes (e.g., age, sex) for outcomes such as tick burden and serostatus, often we only knew whether a dog owner treated at least one of their dogs a particular way but not necessarily which specific dog (e.g., being allowed inside or being treated with acaricide). The concordance of our team finding ticks with owners reporting ticks was only fair and implies that we undercounted ticks. Although it adds to the cost and time, use of dry ice as an attractant would improve tick assessment sensitivity. Lastly, there were too few cases in too few areas for a robust ecological study design.
An epidemic of RMSF appears to be in its early stages in Tijuana. Although human RMSF cases have been detected in only a small set of neighborhoods to date, a high prevalence of seropositive dogs was found almost everywhere we surveyed, and cases and tick bites are likely undercounted. The fact that high-marginalization areas tended to have higher seroprevalence whereas absolute titers trended higher in lower marginalization areas is consistent with an epicenter in the outlying, already identified areas, now beginning to spread. At the root of the epidemic are dogs, serving not only as pets, family members, protection against crime, and sentinels of risk but also as the main host for the ticks and the reservoir of the pathogen. A comprehensive campaign dubbed the “RMSF Rodeo” yielded a substantial but not durable (without repeated intervention) reduction in tick infestation rates on dogs of 1% versus 64% in a control community and no detectable ticks in the environment in a 600-home native American community.16 This campaign featured long-acting acaricide collars on all dogs, monthly treatment of all homes with acaricide, and promotion of dog spaying and neutering and restraint to the homesite. However, such intense intervention would be limited owing to highly limited resources and the enormous scope of the epidemic across multiple cities in México.
In Tijuana, risk identification was optimized by identifying human cases and incorporating surveillance of canine seroprevalence. Future research should evaluate overlap of R. rickettsii and R. massiliae (and potentially other rickettsiae) and cross-reacting serology. Even given this consideration, human case and canine seroprevalence surveillance combined will be more accurate than resident self-reports of tick infestation and bites and much more sensitive than tick and dog PCR. This focus on dog surveillance also would provide necessary data to evaluate canine-focused intervention such as acaricide application, population and movement control, and possible eventual vaccines against rickettsiae or ticks. Nearby Sonora state has experienced almost two decades of epidemic RMSF, almost 3,000 cases and at least 745 fatalities.55 Further spread is possible, exemplified by the finding that seropositive dogs in Imperial County, California, which borders Baja California in México, were significantly closer to a California-Mexico border crossing station than seronegative dogs were.56 Human case numbers in California have doubled over the last four decades, increasing in southern California especially among Hispanic/Latino people, whereas cases in California and Arizona often have either direct travel to México or contact with people from México.57–59 It is imperative that public health workers use evidence from this and other studies to prioritize interventions that can make the greatest impacts and save lives.
Supplemental Materials
ACKNOWLEDGMENTS
We thank Marian Fierro, Rosendo Rojas, and Eduardo Altamirano from the Secretaria de Salud in Baja California and affiliated agencies, Samantha Barnum, and Gloria Edejer for support in the field and laboratory. We also thank Orencio Pérez from the Instituto Nacional de Estadística y Geografía (INEGI) office in Baja California who was instrumental for the sampling design, Ian Erick Núñez-Ramos and Daniela Moreno-Montiel who helped in the acquisition of data and logistics, and the UC Davis Real-Time PCR Research and Diagnostics Core Facility and UC Davis DNA Sequencing Facility for their assistance with sample processing.
Note: Supplemental material appears at www.ajtmh.org.
REFERENCES
- 1. Bustamante M, Varela G, 1943. Una nueva rickettsiosis en México. Existencia de la fiebre manchada americana en los estados de Sinaloa y Sonora. Revista Instituto Salud Enfermidades Tropicales 4: 189–211. [Google Scholar]
- 2. Silva-Goytia R, Elizondo A, 1952. Estudios sobre fiebre manchada en México. IV. Características epidemiológicas de casos de fiebre manchada ocurridos en La Laguna. Medicina Revista Mexicana 32: 569–579. [PubMed] [Google Scholar]
- 3. Álvarez-Hernández G, Roldán JFG, Milan NSH, Lash RR, Behravesh CB, Paddock CD, 2017. Rocky Mountain spotted fever in Mexico: past, present, and future. Lancet Infect Dis 17: e189–e196. [DOI] [PubMed] [Google Scholar]
- 4. Zavala-Castro JE, Dzul-Rosado KR, León JJA, Walker DH, Zavala-Velázquez JE, 2008. An increase in human cases of spotted fever rickettsiosis in Yucatan, Mexico, involving children. Am J Trop Med Hyg 79: 907–910. [PMC free article] [PubMed] [Google Scholar]
- 5. Huerta JDL, Barragán RC, 2008. Fiebre manchada de las Montañas Rocosas en pediatría revisión clínica de una serie de 115 casos. Revista de Enfermedades Infecciosas en Pediatría 21: 4–9. [Google Scholar]
- 6. Zazueta OE, Armstrong PA, Márquez-Elguea A, Milán NSH, Peterson AE, Ovalle-Marroquín DF, Fierro M, Arroyo-Machado R, Rodriguez-Lomeli M, Trejo-Dozal G, 2021. Rocky Mountain spotted fever in a large metropolitan center, Mexico–United States border, 2009–2019. Emerg Infect Dis 27: 1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nicholson WL, Paddock CD, Demma L, Traeger M, Johnson B, Dickson J, McQuiston J, Swerdlow D, 2006. Rocky Mountain spotted fever in Arizona: documentation of heavy environmental infestations of Rhipicephalus sanguineus at an endemic site. Ann NY Acad Sci 1078: 338–341. [DOI] [PubMed] [Google Scholar]
- 8. Eremeeva ME, Zambrano ML, Anaya L, 2011. Rickettsia rickettsii in Rhipicephalus ticks, Mexicali, Mexico. J Med Entomol 48: 418–421. [DOI] [PubMed] [Google Scholar]
- 9. Biggs HM. et al. , 2016. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep 65: 1–44. [DOI] [PubMed] [Google Scholar]
- 10. Álvarez-López DI, Ochoa-Mora E, Heitman KN, Binder AM, Álvarez-Hernández G, Armstrong PA, 2021. Epidemiology and clinical features of Rocky Mountain spotted fever from enhanced surveillance, Sonora, Mexico: 2015–2018. Am J Trop Med Hyg 104: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tinoco-Gracia L, Quiroz-Romero H, Quintero-Martínez M, Rentería-Evangelista T, González-Medina Y, Barreras-Serrano A, Hori-Oshima S, Moro M, Vinasco J, 2009. Prevalence of Rhipicephalus sanguineus ticks on dogs in a region on the Mexico-USA border. Vet Rec 164: 59. [DOI] [PubMed] [Google Scholar]
- 12. Martínez-Medina MÁ, Álvarez-Hernández G, Padilla-Zamudio JG, Rojas-Guerra MG, 2007. Fiebre manchada de las Montañas Rocosas en niños: consideraciones clínicas y epidemiológicas. Gac Med Mex 143: 137–140. [PubMed] [Google Scholar]
- 13. Field-Cortazares J, Seijo JL, 2011. Rickettsiosis in Baja California. Boletín Clínico Hospital Infantil del Estado de Sonora 28: 44–50. [Google Scholar]
- 14. Tinoco-Gracia L, Quiroz-Romero H, Quintero-Martínez M, Rentería-Evangelista T, Barreras-Serrano A, Romano-Osuna M, García-Prieto B, Escárcega-Ávila A, 2009. Prevalencia de infestación de garrapatas (Rhipicephalus sanguineus) en perros y su asociación a factores de riesgo en la Zona Urbana de Mexicali, Baja California, México. VIII Congreso Nacional de Parasitología Veterinaria, October 26–28, 2009, Mérida, Yucatán, México.
- 15. Field-Cortazares J, Moreno JS-y, 2011. Rickettsiosis en Baja California. Bol Clin Hosp Infant Edo Son 28: 44–50. [Google Scholar]
- 16. Drexler N, Miller M, Gerding J, Todd S, Adams L, Dahlgren FS, Bryant N, Weis E, Herrick K, Francies J, 2014. Community-based control of the brown dog tick in a region with high rates of Rocky Mountain spotted fever, 2012–2013. PLoS One 9: e112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoder J, Benoit J, Rellinger E, Tank J, 2006. Developmental profiles in tick water balance with a focus on the new Rocky Mountain spotted fever vector, Rhipicephalus sanguineus. Med Vet Entomol 20: 365–372. [DOI] [PubMed] [Google Scholar]
- 18. Bustos A, 2011. Niveles de marginación: una estrategia multivariada de clasi cación. INEGI. Real Datos Espacio 2: 169–186. [Google Scholar]
- 19. Walker JB, Keirans JE, Horak IG, 2000. The genus Rhipicephalus (Acari, Ixodidae): A Guide to the Brown Ticks of the World. Cambridge, England: Cambridge University Press. [Google Scholar]
- 20. Dantas-Torres F, 2010. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors 3: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nava S, Beati L, Venzal JM, Labruna MB, Szabó MP, Petney T, Saracho-Bottero MN, Tarragona EL, Dantas-Torres F, Silva MMS, 2018. Rhipicephalus sanguineus (Latreille, 1806): neotype designation, morphological re-description of all parasitic stages and molecular characterization. Ticks Tick Borne Dis 9: 1573–1585. [DOI] [PubMed] [Google Scholar]
- 22. Stenos J, Graves SR, Unsworth NB, 2005. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group rickettsiae. Am J Trop Med Hyg 73: 1083–1085. [PubMed] [Google Scholar]
- 23. Kato CY, Chung IH, Robinson LK, Austin AL, Dasch GA, Massung RF, 2013. Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J Clin Microbiol 51: 314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Webb L, Carl M, Malloy DC, Dasch GA, Azad AF, 1990. Detection of murine typhus infection in fleas by using the polymerase chain reaction. J Clin Microbiol 28: 530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tzianabos T, Anderson BE, McDade J, 1989. Detection of Rickettsia rickettsii DNA in clinical specimens by using polymerase chain reaction technology. J Clin Microbiol 27: 2866–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Sousa R, Barata C, Vitorino L, Santos-Silva M, Carrapato C, Torgal J, Walker D, Bacellar F, 2006. Rickettsia sibirica isolation from a patient and detection in ticks, Portugal. Emerg Infect Dis 12: 1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Foley J, Tinoco Gracia L, Rodriguez M, Estrada-Guzman J, Fierro M, Peterson A, Pascoe E, Hori-Oshima S, Paddock CD, Zazueta O, 2019. Unbiased assessment of abundance of Rhipicephalus sanguineus sensu lato ticks, canine exposure to spotted fever group Rickettsia, and risk factors in Mexicali, México. Am J Trop Med Hyg 101: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, Dasch GA, Levin ML, Singleton J, Jr., Zaki SR, 2005. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med 353: 587–594. [DOI] [PubMed] [Google Scholar]
- 29. McQuiston J, Guerra M, Watts M, Lawaczeck E, Levy C, Nicholson W, Adjemian J, Swerdlow D, 2011. Evidence of exposure to spotted fever group rickettsiae among Arizona dogs outside a previously documented outbreak area. Zoon Publ Health 58: 85–92. [DOI] [PubMed] [Google Scholar]
- 30. Wikswo ME, Hu R, Dasch GA, Krueger L, Arugay A, Jones K, Hess B, Bennett S, Kramer V, Eremeeva ME, 2008. Detection and identification of spotted fever group rickettsiae in Dermacentor species from southern California. J Med Entomol 45: 509–516. [DOI] [PubMed] [Google Scholar]
- 31. Demma LJ. et al. , 2006. Serologic evidence for exposure to Rickettsia rickettsii in eastern Arizona and recent emergence of Rocky Mountain spotted fever in this region. Vector Borne Zoonotic Dis 6: 423–429. [DOI] [PubMed] [Google Scholar]
- 32. Greene C, Kidd LB, Breitschwerdt E, Greene C. Infectious Diseases of the Dog and Cat. St. Louis, MO: Elsevier, 259–270. [Google Scholar]
- 33. Backus L, Foley J, Chung C, Virata S, Zazueta OE, López-Pérez A, 2023. Tick-borne pathogens detected in sheltered dogs during an epidemic of Rocky Mountain spotted fever, a One Health challenge. J Am Vet Med Assoc 261: 375–383. [DOI] [PubMed] [Google Scholar]
- 34. López-Pérez A, Orozco L, Zazueta O, Fierro M, Gomez P, Foley J, 2020. An exploratory analysis of demography and movement patterns of dogs: new insights in the ecology of endemic Rocky Mountain-spotted fever in Mexicali, Mexico. PLoS One 15: e0233567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mesquita JR, Santos-Silva S, de Sousa Moreira A, Baptista MB, Cruz R, Esteves F, Vala H, Barradas PF, 2022. Rickettsia massiliae circulation in sheep and attached Rhipicephalus sanguineus in central Portugal. Trop Anim Health Prod 54: 1–7. [DOI] [PubMed] [Google Scholar]
- 36. Beeler E, Abramowicz KF, Zambrano ML, Sturgeon MM, Khalaf N, Hu R, Dasch GA, Eremeeva ME, 2011. A focus of dogs and Rickettsia massiliae–infected Rhipicephalus sanguineus in California. Am J Trop Med Hyg 84: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marquez F, Rodriguez-Liebana J, Soriguer RC, Muniain M, Bernabeu-Wittel M, Caruz A, Contreras-Chova F, 2008. Spotted fever group Rickettsia in brown dog ticks Rhipicephalus sanguineus in southwestern Spain. Parasitol Res 103: 119–122. [DOI] [PubMed] [Google Scholar]
- 38. Eremeeva ME, Bosserman EA, Demma LJ, Zambrano ML, Blau DM, Dasch GA, 2006. Isolation and identification of Rickettsia massiliae from Rhipicephalus sanguineus ticks collected in Arizona. J Appl Environ Microbiol 72: 5569–5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Belkahia H, Selmi R, Zamiti S, Daaloul-Jedidi M, Messadi L, Ben Said M, 2021. Zoonotic Rickettsia species in small ruminant ticks from Tunisia. Front Vet Sci 8: 676896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fernández-Soto P, Pérez-Sánchez R, Díaz Martín V, Encinas-Grandes A, Álamo Sanz R, 2006. Rickettsia massiliae in ticks removed from humans in Castilla y León, Spain. EJCMID 25: 811–813. [DOI] [PubMed] [Google Scholar]
- 41. Scarpulla M, Barlozzari G, Marcario A, Salvato L, Blanda V, De Liberato C, D’Agostini C, Torina A, Macrì G, 2016. Molecular detection and characterization of spotted fever group rickettsiae in ticks from central Italy. Ticks Tick Borne Dis 7: 1052–1056. [DOI] [PubMed] [Google Scholar]
- 42. Barradas PF, Mesquita JR, Mateus TL, Ferreira P, Amorim I, Gärtner F, de Sousa R, 2021. Molecular detection of Rickettsia spp. in ticks and fleas collected from rescued hedgehogs (Erinaceus europaeus) in Portugal. Exp Appl Acarol 83: 449–460. [DOI] [PubMed] [Google Scholar]
- 43. Barradas PF, Mesquita JR, Ferreira P, Amorim I, Gärtner F, 2020. Detection of tick-borne pathogens in Rhipicephalus sanguineus sensu lato and dogs from different districts of Portugal. Ticks Tick Borne Dis 11: 101536. [DOI] [PubMed] [Google Scholar]
- 44. López-Pérez A, Sánchez-Montes S, Foley J, Guzmán-Cornejo C, Colunga-Salas P, Pascoe E, Becker I, Delgado-de la Morae J, Licona-Enriquez J, Suzan G, 2019. Molecular evidence of Borrelia burgdorferi sensu stricto and Rickettsia massiliae in ticks collected from a domestic-wild carnivore interface in Chihuahua, Mexico. Ticks Tick Borne Dis 10: 1118–1123. [DOI] [PubMed] [Google Scholar]
- 45. de Mera IGF, Ruiz-Fons F, de la Fuente G, Mangold AJ, Gortázar C, de la Fuente J, 2013. Spotted fever group rickettsiae in questing ticks, central Spain. Emerg Infect Dis 19: 1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cicuttin GL, Tarragona EL, De Salvo MN, Mangold AJ, Nava S, 2015. Infection with Ehrlichia canis and Anaplasma platys (Rickettsiales: Anaplasmataceae) in two lineages of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) from Argentina. Ticks Tick Borne Dis 6: 724–729. [DOI] [PubMed] [Google Scholar]
- 47. Babalis T, Tselentis Y, Roux V, Psaroulaki A, Raoult D, 1994. Isolation and identification of a rickettsial strain related to Rickettsia massiliae in Greek ticks. Am J Trop Med Hyg 50: 365–372. [DOI] [PubMed] [Google Scholar]
- 48. Movilla R, Altet L, Serrano L, Tabar M-D, Roura X, 2017. Molecular detection of vector-borne pathogens in blood and splenic samples from dogs with splenic disease. Parasit Vectors 10: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bechelli J, Smalley C, Milhano N, Walker DH, Fang R, 2015. Rickettsia massiliae and Rickettsia conorii Israeli spotted fever strain differentially regulate endothelial cell responses. PLoS One 10: e0138830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vitale G, Mansueto S, Rolain J-M, Raoult D, 2006. Rickettsia massiliae human isolation. Emerg Infect Dis 12: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khrouf F, Sellami H, Elleuch E, Hattab Z, Ammari L, Khalfaoui M, Souissi J, Harrabi H, M’ghirbi Y, Tiouiri H, 2016. Molecular diagnosis of Rickettsia infection in patients from Tunisia. Ticks Tick Borne Dis 7: 653–656. [DOI] [PubMed] [Google Scholar]
- 52. Alvarez-Hernandez G, Murillo-Benitez C, del Carmen Candia-Plata M, Moro M, 2015. Clinical profile and predictors of fatal Rocky Mountain spotted fever in children from Sonora, Mexico. Ped Inf Dis J 34: 125–130. [DOI] [PubMed] [Google Scholar]
- 53. Neitz W, 1943. The eradication of the brown dog tick (Rhipicephalus sanguineus Ltr.) from a dog kennel. J S Afr Vet Assoc 14: 90–93. [Google Scholar]
- 54. Dantas-Torres F, 2008. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806)(Acari: Ixodidae): from taxonomy to control. Vet Parasitol 152: 173–185. [DOI] [PubMed] [Google Scholar]
- 55.Secretaría de Salud México, 2023. Boletines epidemiológicos históricos. Sistema de Vigilancia Epidemiológica, Dirección General de Epidemiologia. Available at: https://www.gob.mx/salud/acciones-y-programas/historico-boletin-epidemiologico. Accessed January 12, 2024.
- 56. Estrada I, Balagot C, Fierro M, Kriner P, Iniguez-Stevens E, Kjemtrup A, Foley J, 2020. Spotted fever group rickettsiae canine serosurveillance near the U.S.-Mexico border in California. Zoon Publ Health 67: 148–155. [DOI] [PubMed] [Google Scholar]
- 57. Kjemtrup AM, Padgett K, Paddock CD, Messenger S, Hacker JK, Feiszli T, Melgar M, Metzger ME, Hu R, Kramer VL, 2022. A forty-year review of Rocky Mountain spotted fever cases in California shows clinical and epidemiologic changes. PLoS Negl Trop Dis 16: e0010738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Drexler NA, Yaglom H, Casal M, Fierro M, Kriner P, Murphy B, Kjemtrup A, Paddock CD, 2017. Fatal Rocky Mountain spotted fever along the United States–Mexico border, 2013–2016. Emerg Infect Dis 23: 1621–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. California Department of Public Health , 2022. Vector-Borne Disease Section. Available at: www.cdph.ca.gov/programs/vbds. Accessed January 8, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.