Abstract
This systematic review, meta-analysis, and novel time course analysis examines microvascular failure in the treatment of acute ischemic stroke (AIS) patients undergoing endovascular therapy (EVT) and/or thrombolytic administration for stroke management. A systematic review and meta-analysis following PRIMSA-2020 guidelines was conducted along with a novel curve-of-best fit analysis to elucidate the time-course of microvascular failure. Scopus and PubMed were searched using relevant keywords to identify studies that examine recanalization and reperfusion assessment of AIS patients following large vessel occlusion. Meta-analysis was conducted using a random-effects model. Curve-of-best-fit analysis of microvascular failure rate was performed with a negative exponential model. Twenty-seven studies with 1151 patients were included. Fourteen studies evaluated patients within a standard stroke onset-to-treatment time window (≤6 hours after last known normal) and thirteen studies had an extended time window (>6 hours). Our analysis yields a 22% event rate of microvascular failure following successful recanalization (95% CI: 16–30%). A negative exponential curve modeled a microvascular failure rate asymptote of 28.5% for standard time window studies, with no convergence of the model for extended time window studies. Progressive microvascular failure is a phenomenon that is increasingly identified in clinical studies of AIS patients undergoing revascularization treatment.
Keywords: Hypoperfusion, microvascular failure, no-reflow, revascularization, ischemic stroke
Introduction
Acute ischemic stroke (AIS) is a leading cause of death and long-term disability in the United States.1,2 While advances in recanalization strategies have led to increased rates of successful recanalization up to >85%,3 –6 they have not translated to an equivalent improvement in functional clinical outcomes. One possibility for this limited recovery is that beyond vessel occlusion, cerebral ischemia and reperfusion unleash a complex physiological response that involves robust thrombo-inflammatory processes that are not targeted by revascularization therapy7 –14 and result in progressive microvascular dysfunction and secondary tissue injury.15 –18 Growing recognition of the importance of reperfusion as distinct from recanalization9,10,14 has led to recent interest in the phenomenon of progressive microvascular failure as a potential treatment target for mitigating the disparity between recanalization success and motor and cognitive recovery in patients.
Progressive microvascular failure is a time-dependent phenomenon that evolves following successful recanalization in LVO stroke as determined by angiography, in which patients demonstrate incomplete reperfusion on post-revascularization perfusion imaging studies. Unlike the no-reflow phenomenon, progressive microvascular failure does not encompass embolization of original thrombus fragments into the distal vasculature that may present as impaired cerebral blood flow on angiography following LVO revascularization.19,20 Stalled neutrophils 21 , microvascular pericyte constriction22,23, and deleterious vascular remodeling secondary to acute neuroinflammation 24 are proposed as the underlying mechanisms of progressive microvascular failure that are compatible with the pathophysiology timeline identified in existing AIS studies.8,25 The loss of capillary integrity and autoregulation results in progressive vasoconstriction and ultimately flow arrest.26,27 The resulting circulatory failure is defined as hypoperfusion in the vascular territory that is distal to the site of recanalization, and does not include ischemic injury associated with embolization of inciting thrombus that may be directly associated with the revascularization intervention. 20 At a molecular level, elevated proinflammatory cytokines (IL-1, IL-6)28 –30, increased neutrophil-to-leukocyte ratio 31 , and increased Egr-1 expression 32 have been associated with the structural and functional microvascular dysfunction resulting in impaired perfusion. 25 Progressive microvascular failure has largely been characterized as a microcirculatory phenomenon that localizes to arterioles and capillaries. However, venule obstruction secondary to leukocyte adhesion and subsequent thrombus formation has been identified in middle cerebral artery stroke, and supports a more generalized microcirculatory dysfunction not confined to vessel subtypes.20,33
Following recanalization, the prognostic value of reperfusion and its relationship to clinical and functional outcomes has been difficult to characterize. 9 Hypoperfusion volume in AIS patients has been associated with early neurologic decline at 72 hours of stroke onset. 34 Recent pooled analysis from three RCTs35 –37 has provided Class II evidence that hypoperfusion, in the setting of complete revascularization, was independently associated with early functional decline at 24 hours and poor functional outcomes at 90-days (modified Rankin Scale score ≥3) that is independent of final infarct volume. 7 However, clinical studies that examine progressive microvascular failure have generally been limited in scope to static, associative relationships between reperfusion and patient outcomes that do not necessarily track the temporal evolution of post-intervention reperfusion.7,38,39
There is heterogeneity with respect to the definition of progressive microvascular failure and effective reperfusion which necessitates a structured approach to evaluating the phenomenon in clinical studies. The overarching goal of this systematic review and meta-analysis is to evaluate the frequency of progressive microvascular failure across studies that and clarify its time course across the included studies, which are generally limited to a single post-recanalization reperfusion assessment that precludes the temporal analysis of progressive microvascular dysfunction. This approach clarifies the temporal dimension of microvascular failure in AIS, a novel contribution to the emergent study of progressive microvascular dysfunction and its timeline in ischemia/reperfusion injury. Based on prior clinical studies of microvascular dysfunction in AIS13,38, we expect that there will be a time-dependent course of progressive microvascular failure following revascularization therapy, that likely evolves between 30 minutes and five days from angiographically-confirmed recanalization.8,10,40 The rate of change of microvascular dysfunction may be affected by revascularization method, reperfusion imaging modality, and time window of intervention.8,13,38,41,42
Methods
Eligibility criteria
Studies that evaluated the degree of recanalization and reperfusion following EVT, intravenous thrombolysis, or a combined approach (EVT + thrombolytic) for the treatment of acute ischemic stroke were included in the analysis. Table 1 summarizes the relevant inclusion and exclusion criteria for studies used during the screening process. The methodology for quantification of the extent of recanalization and reperfusion varies in the existing literature of microvascular failure. 13 As operational definitions of progressive microvascular failure are evolving and reflect technological advancement in the ability to capture impaired reperfusion on imaging studies, no singular definition of successful reperfusion was implemented as a screening criterion. Instead, an article was included if the proportion of patients who had microvascular failure (patients with successful or complete recanalization without reperfusion) could be determined with the respective criteria and thresholds outlined in the study’s methodology. To avoid mischaracterization of progressive microvascular failure with the distal thrombus embolization associated with no-reflow, we excluded studies that evaluated downstream macrovascular occlusion following initial revascularization. With respect to recanalization, measures of patency at the site of occlusion (e.g. AOL) and patency of vasculature distal to the site of occlusion (e.g. TICI) were both considered valid if tissue-level microvascular reperfusion was determined using a validated methodology with a different and discrete threshold.43 –47 Non-English manuscripts, editorials, abstracts, and case reports were not included in the systematic review. Studies that assessed the microvascular failure in the same cohort of patients were not included to avoid double-counting of patient data and ensure the meta-analysis of microvascular failure rate was limited to unique patients.
Table 1.
Inclusion and exclusion criteria for study screening using the PRISMA-2020 framework for systematic reviews.
| Inclusion criteria |
|
|
|
|
|
|
| Exclusion criteria |
|
|
|
|
|
|
|
|
AOL: Arterial Occlusive Lesion (AOL) Recanalization score; ASPECTS: The Alberta stroke program early CT Score; MTT: mean transit time; rCBF: relative cerebral blood flow; rCBV: relative cerebral blood volume; TICI: Thrombolysis in Cerebral Infarction Scale; TIMI: Thrombolysis in Myocardial Infarction Scale; Tmax: Time-to-Maximum.
Information and data sources, search methodology
PubMed and Scopus were searched using free-text and MeSH terms relevant to microvascular failure, including “no-reflow”, “microvascular failure”, “recanalization without reperfusion”, “thrombolytic therapy”, “perfusion imaging”, “cerebral revascularization”, “thrombectomy”, “fibrinolytic agents”, and their relevant synonyms in appropriate combinations. No filters were used during the initial search process.
Article selection and data collection
Two independent co-authors (TT, EFS) sourced articles from the aforementioned databases and compiled them in a Microsoft Excel database. All duplicate articles were removed and studies in the database were subsequently screened for eligibility. Article titles and abstracts were initially reviewed for relevance to the systematic review and meta-analysis. Then, articles were reviewed in full to observe if appropriate the eligibility criteria were met and outcomes of interest were included in the study. Studies that met all screening criteria were then discussed among co-authors. In any instances of disagreement regarding the inclusion of a specific study, results were discussed with an additional co-author (ESC). Data from included studies including acute revascularization intervention type, imaging modality used to assess recanalization and reperfusion status, and time of assessment of reperfusion were recorded. Summary data from each study were gathered and pooled into the Microsoft Excel database, and did not represent an aggregation of individual patient data. If there were any conflicted data values for a given study, an additional researcher (ESC) was involved in confirming the appropriate data entry value.
Assessment of risk of bias
The National Institutes of Health Quality Assessment Tools were used to evaluate bias across included case series, cohort studies, and meta-analyses. ROB-2 and ROBINS-I tools were used for randomized and non-randomized studies respectively to systematically assess for risk of bias in the included studies based on the Cochrane methodology outlined in Version 6.3 of the Cochrane Handbook for Systematic Reviews of Interventions. 48
Determination of overall “low”, “medium”, “high”, or “critical” risk of bias for randomized trials using ROB-2 and ROBINS-I was made using the algorithms provided by Cochrane based on aggregation of rater sub-scores across the five and seven domains of bias respectively (refer to Cochrane Handbook for specific calculation of overall bias algorithms based on domain sub-scores). 48 Thresholds for overall bias calculated using NIH quality assessment tools for case series, cohort studies, and meta-analyses were made using two-rater determination based on the tool-specific “General Guidance” instructions developed to determine a study’s internal validity. 49 If there was disagreement between the ratings by the two reviewers (TT, EFS), final scoring was evaluated by a third reviewer (ESC) as a tie-breaker. 49 Peters’ linear regression test for funnel plot asymmetry was used to assess for small study effects in the meta-analysis.
Statistical analysis
RStudio software version 4.0.1 (RStudio Team (2020). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA) was used for the meta-analysis, including the use of “meta”, “metafor”, “drc”, and “ggplot2” packages.50 –52 MATLAB software version: 9.13.0 (R2022b; The MathWorks Inc. Natick, MA) with the “Statistics and Machine Learning Toolbox” was used for curve-fitting of a two-parameter negative exponential model of microvascular failure rate from over time from successful recanalization for the included studies. Random-effects modeling was selected for estimating effect sizes across the included studies to account for heterogeneity in trial design, patient samples, and recanalization interventions (Supplemental Table 1).
To compare the number of patients who demonstrated progressive microvascular failure, the effect size for a given study was reported as the proportion of patients with successful recanalization without reperfusion. A patient was deemed to have microvascular failure if they had successful recanalization without reperfusion using the criteria outlined by a given study. The meta-analysis calculated a pooled effect size and 95% confidence interval. Between-study heterogeneity was reported using I2 statistic 53 to evaluate the percentage variability in effect sizes not attributable to sampling error and τ2 to quantify the variance of the true effect sizes. Subgroup analyses were conducted by recanalization method, imaging modality used to assess reperfusion status, and time window of revascularization using a mixed-effects model, with an independent or common estimate of τ2 used for analyses with subgroup size k> or ≤5 respectively. 54 Meta-regression of microvascular failure rate against study characteristics (time window, reperfusion assessment timepoint, recanalization method) was conducted using a mixed-effects, maximum likelihood-fitted model.
Results
Systematic review
The systematic review follows PRIMSA-2020 guidelines for systematic review reporting. 55 Using PubMED and Scopus, 299 articles were identified using a comprehensive list of search terms selected to identify records associated with progressive microvascular failure in the setting of AIS (Figure 1). Records were screened based on title and abstract, and 69 records were excluded due to abstract irrelevance, two duplicate records were removed, and one record was removed as it was not published in the English language. Subsequently, 227 reports were sought for retrieval, and one was not retrieved after extensive search. The search yielded 226 reports that were assessed for eligibility in the screening process, with 5 preclinical studies and 186 studies with the outcomes of interest not reported. Eleven review articles and seven case reports were further removed during the screening process. Additionally, 14 additional studies were sourced primarily from citation and reference analysis from the original study sample. Following exclusion of reports in this subset that did not evaluate microvascular failure in unique patients and review articles, ten of these studies were ultimately included in the analysis. Ultimately, 27 studies were included in the systematic review and meta-analysis.
Figure 1.
PRISMA-2020 flowchart of studies describing the identification, screening, and inclusion process.
Figure 2 identifies the risk of publication bias of randomized and non-randomized studies that were included in the systematic review. Of the 27 studies included, two were deemed to have high levels of overall bias as determined by the appropriate risk-of-bias tool. No included studies had critical levels of overall bias that precluded their downstream analysis in the systematic review and meta-analysis.
Figure 2.
Risk of publication bias assessed with NIH Quality Assessment Tools for case series, cohort studies, meta-analyses and Cochrane ROB-2 and ROBINS-I risk-of-bias assessment tools for randomized & non-randomized studies. Proportion of n = 27 studies in systematic review with low, medium, or high risk-of-bias with size of subgroup in each risk category.
Table 2 summarizes the characteristics of each study, including study size, imaging modalities used to assess patients, and relevant timepoints of interventions.
Table 2.
Characteristics of included studies and recanalization and reperfusion outcomes of AIS patients undergoing revascularization treatment.
| Study (Name, Year) | Number of patients with successful recanalization | Stroke onset-to-treatment delay (Standard ≤6 h, Extended >6 h) (h) | Acute revascularization treatment | Ancillary revascularization treatment | Recanalization criteria | Imaging modality used to assess reperfusion |
Reperfusion assessment time (t= hrs since recanalization) |
Microvascular failure criteria | % Microvascular failure (absolute # pts in parentheses) |
|---|---|---|---|---|---|---|---|---|---|
| Ng FC et al. (2021) 7 | 130 | Standard (<4.5) | EVT +/− tPA | EXTEND-IA, EXTEND-IA TNK : stenting of external carotid artery may require antiplatelet agent use, otherwise antiplatelet/anticoagulant not permitted within first 24 hours after endovascular therapy | mTICI 3-2c | CTP/PWI | 24 | Regions of visually demonstrable persistent hypoperfusion on rCBV or flow maps with >15% asymmetry | 25% (33) |
| Baird et al. (1994) 56 | 4 | Extended (4–24) | IA Streptokinase (SK) | 20/22 SK-treated patients and 33/35 control subjects received aspirin treatment | partial or complete | SPECT | 24 | Count rate <12% defined as hypoperfusion based on perfusion deficit index (cm^3) = (counts in voxel unaffected hemisphere - counts in hypoperfused voxel / Counts in unaffected hemisphere) * voxel size | 25% (1) |
| Yasaka et al. (1998) 57 | 8 | Standard (<4) | IV SK or placebo | All patients received 100 mg aspirin within 4 hours of SK or placebo. No subjects received anticoagulants within 48 hours of SK / placebo. | partial or complete | SPECT | 24 | Reperfusion <25% by volume on the second vs. first SPECT | 50% (4) |
| Khatri et al. (2005) 46 | 43 | Standard (<3) | IV+IA tPA (no EVT) | N/A | AOL II/III | DSA (TIMI) | 0 | TIMI 2/3 | 23% (10) |
| Albers et al. (2006) 58 | 19 | Standard (3–6) | IV tPA | N/A | partial or complete | PWI | 3-6 | <30% and <10 ml reduction in PWI lesion volume | 21% (4) |
| De Silva et al. (2009) 8 | 13 | Standard (3–6) | IV tPA | N/A | partial or complete (TIMI2-3) | PWI | 72-120 | <90% reduction in magnetic resonance perfusion-weighted imaging lesion volume | 31% (4) |
| Soares et al. (2010) 14 | 13 | Standard (<6) | IV tPA +/− EVT, or no treatment | N/A | partial or complete | CTP | 25 | MTT reperfusion index <75% | 38% (5) |
| Bivard et al. (2013) 59 | 48 | Standard (<6) | IV tPA or no treatment | N/A | complete | ASL | 24 | Pixel intensity <= −2 standard deviations (SD) of mean normal pixel density from healthy hemisphere | 0% (0) |
| Eilaghi et al. (2013) 9 | 59 | Standard (<4.5) | IV tPA or no treatment | N/A | partial or complete (TIMI2-3) | CTP | < or = 24 | Absolute time to maximum reperfusion index < 58.7% | 24% (14) |
| Horsch et al. (2015) 60 | 83 | Extended (<9) | IV tPA or no treatment | N/A | complete | CTP | 72 | Perfusion deficit (focal asymmetry on CBF, CBV, MTT, or TTP map, matching MCA flow territory) on follow-up CTP vs. baseline | 40% (33) |
| Cho et al. (2015) 10 | 13 | Standard (<6) | IV tPA or no treatment | N/A | partial or complete (AOLII-III) | PWI | <6 | No acute reperfusion = (volume of reperfused voxels at 3 hrs/perfusion lesion volume at t = 0) <50% | 0% (0) |
| Carbone et al. (2019) 61 | 39 | Extended (<8) | IV tPA +/− EVT, or no treatment | N/A | partial or complete (TIMI2-3) | CTP | 24 | Reperfusion index : percentage reductionof baseline MTT lesion at 24 h | 38% (15) |
| Ter Schiphorst et al. (2021) 13 | 33 | Extended (<24) | EVT +/− tPA | N/A | mTICI 3-2c | ASL | 24 | ≥40% reduction in cerebral blood flow (CBF) affecting anatomical regions of the affected hemisphere on 24-h ASL maps | 3% (1) |
| Khalil et al. (2018) 62 | 6 | Extended (1–24) | IV tPA | N/A | TIMI 1-3 | BOLD delay + DSC | 25 | BOLD delay. Peak of Kernel density plot over time (between 10–20 s), represents hypoperfused tissue | 50% (3) |
| Potreck et al. (2021) 63 | 38 | Extended (0–24) | EVT +/− tPA | N/A | TICI2b-3 | DSC | 21 | Visual assessment of perfusion maps with “predominantly hypoperfused infarct core” vs. healthy contralateral hemisphere | 18% (7) |
| Kosior et al. (2019) 11 | 16 | Extended (24) | EVT +/− tPA | 16/50 patients were receiving antiplatelet agents and 9/50 patients were on anticoagulant treatment. Timing / dose of ancillary treatment not specified relative to EVT. | All | DSA | 0 | Prolonged Tmax and MTT | 19% (3) |
| Rubiera et al. (2020) 40 | 94 | Extended (<9) | EVT +/− tPA | N/A | TICI3 | CTP | 0.5 | Tmax > 6 second volume | 43% (40) |
| Zhao et al. (2022) 64 | 170 | Extended (<9) | EVT +/− tPA | 43/170 patients were receiving antiplatelet agents prior to stroke. Timing / dose of antiplatelet agent relative to EVT not specified. | TICI3 | TCD | < or = 24 | Highest quartile MCA-PI | 26% (45) |
| Tomsick et al. (2008) 65 | 81 | Standard (<3) | IV tPA or IV + IA tPA | N/A | TICI2b-3 | DSA | 0 | TICI < 2 | 6% (5) |
| Ng FC et al. (2018) 39 | 53 | Standard (<6) | EVT +/− tPA | N/A | TICI2b-3 | TCD | 24–48 | Gosling Pulsatility Index (asymmetrically increased, >20% interside difference) | 28% (15) |
| Sah et al. (2019) 67 | 22 | Extended (<12) | EVT +/− tPA or antithrombotic treatment | N/A | TICI3 | MRI | 5, 24 | DWI lesion growth | 0% (0) |
| Gilberti et al. (2017) 68 | 68 | Standard (< 6) | EVT +/− tPA | 6/68 patients were receiving warfarin anticoagulation at time of stroke. Time/dosing of warfarin relative to EVT not specified. | TICI 2 b/3 | CT/MRI | < or = 24 | Moderate-severe LA (van Swietwen scale 2-4) | 34% (23) |
| Parsons et al. (2009) 69 | 17 | Standard (< 6) | IV tenecteplase | N/A | partial or complete (TIMI2-3) | CTP/PWI | 24 | <80% reperfusion (% reduction in baseline–24-hour MTT lesion) | 24% (4) |
| Lu et al. (2021) 70 | 39 | Extended (<10) | EVT +/− tPA | 30/36 patients demonstrating hypoperfusioon ASL imaging and 16/18 patients with “nonhypoperfusion” on imaging were receiving aspirin therapy at time of stroke. Aspirin dosage unknown. | TICI 2 b/3 | ASL | 96 | Hypoperfusion on ASL (no specified threshold) | 15% (6) |
| Yu et al. (2018) 71 | 23 | Extended (N/A) | EVT or tPA | N/A | TICI 2 b/3 | ASL | 6.4 | auto-RPS < 6. Refer to paper for proprietary auto-RPS categorization | 48% (11) |
| Okazaki et al. (2016) 72 | 10 | Standard (< 4.5) | IV thrombolytic | N/A | partial or complete (AOL II-III) | ASL | 24-72 | TIMI ≤ 2 | 20% (2) |
| Nael et al. (2013) 73 | 20 | Extended (<24) | EVT or IA tPA or stent placement | N/A | TICI 2a-3 | ASL/DSC | 0 | Visually perceptible increased TTP and decreased CBF on DSC & decreased perfusion on ASL-CBF vs. surrounding brain tissue | 40% (8) |
AOL: primary arterial occlusive lesion scale; CTA: CT angiography; CTP: CT perfusion; DSA: digital subtraction angiography; DSC: dynamic susceptibility contrast; EVT: endovascular therapy; MRP: magnetic resonance perfusion; PWI: perfusion-weighted imaging; rCBV: relative cerebral blood volume; SK: streptokinase; SPECT: single photon emission CT; TCD: transcranial Doppler; TICI: Treatment in Cerebral Ischemia score; TIMI: Thrombolysis in Myocardial Infarction score; tPA: tissue plasminogen activator.
Meta-analysis and regression
The results of the meta-analysis identified 1151 unique patients with available recanalization and perfusion data (Figure 3). The pooled proportion of patients that had progressive microvascular failure (patients with successful recanalization without reperfusion) was 0.22 (95% CI: 0.16-0.30, I2 = 53%, τ2 = 0.71). Bias from small-study effects was examined using Peters’ regression test (t = 0.51, p = 0.62, df = 22), indicating no significant plot asymmetry. After excluding studies with a high degree of bias (n = 2) as determined with the appropriate risk-of-bias tool, the adjusted pooled proportion of patients with progressive microvascular failure was 0.24 (95% CI: 0.18-0.31, I2 = 57%, τ2 = 0.45; Supplemental Figure 1).
Figure 3.
Meta-analysis of proportion of patients demonstrating inadequate reperfusion following recanalization (progressive microvascular failure phenomenon) for the treatment of AIS. Random-effects model used for pooled effect size estimation.
Subgroup analysis revealed that neither recanalization method used (thrombolytic-alone k = 13, EVT + thrombolytic combined treatment, k = 14; Q = 1.00, p = 0.32) nor imaging modality used to assess reperfusion (F = 1.73, p = 0.16) demonstrated significant inter-group differences in the proportion of patients demonstrating progressive microvascular failure. When stratified by standard time window (k = 14) versus extended time window (k = 13) for revascularization, subgroup analysis revealed no significant difference in the proportion of patients with microvascular failure (Q = 0.68, p = 0.41). Meta-regression of microvascular failure rate against study characteristics indicated that time in hours following recanalization (p < 0.05), recanalization method (p < 0.01) and the interactive effect between recanalization method and time following revascularization (p < 0.001) are individually significant predictors of progressive microvascular failure rate (Table 3).
Table 3.
Mixed-effects meta-regression model of 27 included studies that examines microvascular failure rate effect size against recanalization method, time window of intervention, and time of reperfusion assessment.
| Regressor a | Regression coefficient ( ) b | 95% confidence interval | p-value |
|---|---|---|---|
| Time of reperfusion assessment (hours) | 0.02 (0.01) | (−2.31–0.87) | 0.021 |
| Recanalization method c | 0.99 (0.35) | (0.02–0.03) | 0.005 |
| Time window c | 0.08 (0.33) | (0.30–1.68) | 0.816 |
| Reperfusion time * Time window Interaction | −0.01 (0.01) | (−0.02–0.01) | 0.567 |
| Reperfusion time * Recanalization method Interaction | −0.03 (0.01) | (−0.05–0.01) | 0.001 |
| R 2 90.77%Breusch-Pagan test (Ho = error variances are equal, H1: error variances are not equal): p = 0.73 suggests no critical heteroskedasticity in the model | |||
Mixed effects meta-regression model with the form using maximum likelihood approach for pooling effect sizes (microvascular failure rate) from included studies. indicates observed microvascular failure rate for a given study, represents the regression coefficient for selected study characteristics in their respective units of measurement or dummy variable value of ‘1’, represents sampling error for a given study’s deviation from true effect size, captures between-study heterogeneity in effect size. * denotes interaction term between two study variables.
Logit-transformed regression effect size coefficient estimates (standard error of estimate in parentheses).
Dummy variables for recanalization method (1= Endovascular therapy, 0 = Thrombolytic alone) and time window [Standard Time Window (≤6 hours) = 1, Extended Time Window (>6 hours) = 0].
Microvascular failure rate analysis
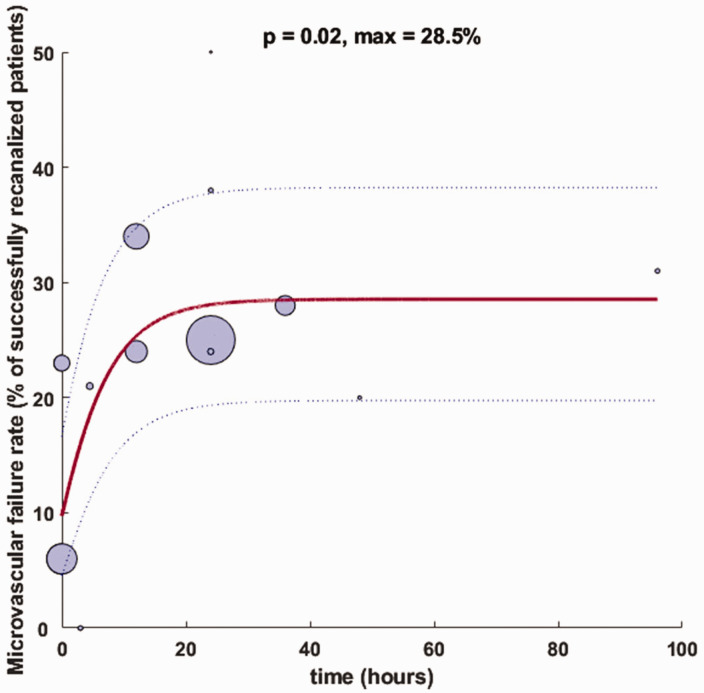
A two-parameter negative exponential curve with of best fit was generated in MATLAB with the form where , and (Figure 4). Individual proportion of microvascular failure rates were arcsine transformed to adjust for the non-normal distribution of percentages for curve fitting. Curve fit as determined by the F-statistic for overall significance of model coefficients indicated a significant fit (p = 0.02) for standard time window studies and no overall significance for extended time window studies (p = 0.90). A linear model with the form of extended time window studies was not significant (p = 0.66). There was no significant difference in model intercept coefficients for standard versus extended time window studies (p = 0.11, t = 1.65).
Figure 4.
Negative exponential curve-of-best-fit for microvascular failure of standard time window studies. Bubble size represents sample size of patients with successful recanalization as defined by the authors for each respective study, with curve estimates (solid blue) weighted by study sample sizes and confidence bands (dashed blue lines) representing the 95% confidence interval. Bubble location on x-axis indicates midpoint time of reperfusion assessment.
Discussion
This systematic review and meta-analysis indicates that microvascular failure in AIS is a pathophysiologic phenomenon that is increasingly captured in clinical stroke studies, with over 70% of studies included in our analysis published within the last ten years. The reported pooled proportion of AIS patients demonstrating microvascular failure (patients with successful recanalization without reperfusion) of 22% (95% CI: 16–30%) was consistent with previous reviews of the phenomenon, with Dalkara et al. 38 noting a composite 26% (95% CI: 19–35%) event rate of recanalization without reperfusion, based on the analysis of seven studies included in our meta-analysis. In a more recent analysis of eleven studies, ter Schiphorst and colleagues 13 note a range of 0–81% for the event rate of hypoperfusion in the setting of recanalization, which is adjusted to 21– 40% after excluding studies that utilized DSA as the method of reperfusion assessment 46 and used a partial recanalized patient as an example of microvascular failure. 56 Varying exclusion criteria based on degree of recanalization (e.g. TICI 2 b-3 versus TICI 2c-3) and imaging modalities used to assess reperfusion are controversial parameters in the determination of microvascular failure.
As there are no consensus operational definitions of successful microvascular reperfusion based on a specific imaging modality 42 , we aim to present a comprehensive view of progressive microvascular failure in our current analysis that represents the breadth of imaging parameters and thresholds for effective reperfusion that is reflective of real-world AIS trial design. The existence of progressive microvascular failure itself is controversial 7 due to difficulties at evaluating recanalization and reperfusion status without invasive angiography in earlier studies. Future standardization of reperfusion cut-offs, imaging protocols, and post-processing analyses will enable robust between-study comparison of the microvascular failure phenomenon.
Two studies were determined to have a high risk of bias due to partially missing outcome data that did not affect the determination of progressive microvascular failure rate and unclear case definitions.56,59 Exclusion of these studies results in an adjusted pooled proportion of patients that was not significantly different than the unadjusted meta-analysis. The I2 range between 50–60% suggests that there is a moderate degree of study heterogeneity that is robust after exclusion of studies with a high degree of bias, and is likely associated with the variable reperfusion imaging modalities and thresholds used across studies (Supplemental Table 1). 48 Even with the exclusion of the Baird et al. 56 study, which may be associated with low precision due to the small subsample of patients with recanalization (n = 4), the degree of variability across studies not attributed to sampling error did not decrease substantially.
A singular time point of reassessment of reperfusion following recanalization, a common feature across prior studies of microvascular dysfunction in AIS, does not necessarily capture the temporal evolution of impaired flow downstream of revascularized LVO. The limitations of imaging modalities used to evaluate cerebral blood flow, including contrast toxicity and radiation concerns, have precluded the serial assessment of reperfusion following recanalization that is necessary to characterize the dynamic process of microvascular failure and elucidate its underlying mechanism.70,74,75 Multiple data collection points with non-invasive imaging techniques may enable clinicians to differentiate pathologic microvascular failure from physiologic responses of cerebrovasculature to ischemia/reperfusion injury over clinically relevant time horizons.10,76
Our negative exponential curve-of-best-fit indicates that there may be a more pronounced increase in microvascular failure rate within the first 24 hours after recanalization with a tapering off beyond that point. This time trend is pronounced and significant for studies with a standard time window of revascularization (p = 0.02; Figure 4) versus no clear or significant relationship between microvascular failure rate and time for studies with an extended intervention window (p = 0.90; Figure 5). The modeled asymptote of 28.5% for standard-time window studies is in-line with a previously reported composite rate of recanalization without reperfusion across seven studies, six of which had a standard time window of treatment initiation. 38 This maximum value estimates a steady-state microvascular failure rate across standard time window studies included in our analysis as time passes from successful recanalization.
Figure 5.
(a) Bubble plot of microvascular failure rate of extended time window studies (>6 hrs from LKN; k = 13) over time from recanalization in hours. Bubble size represents sample size of patients with successful recanalization as defined by the authors for each respective study and (b) Boxplot for extended time window studies. Box represents the median and interquartile range of microvascular failure rate for extended time window studies and whiskers indicate the 95% confidence interval.
In contrast, both linear and negative exponential models of microvascular failure rate over time for extended time window studies were not significant. Figure 5 demonstrates the substantial variation in microvascular failure rate over time in this study subgroup. Progressive microvascular failure following successful recanalization in extended time window studies may not show a significant time trend due to the underlying pathophysiology of microcirculatory dysfunction; dynamic changes in the decoupling of successful recanalization and effective reperfusion may be attributed to structural and functional changes in cerebral microvasculature that are more pronounced in patients with early intervention.8,10,41
Previous studies have suggested that recanalization and reperfusion rates decline as time from revascularization therapy passes.8,41 This proposed time course may be the result of microvascular events such as pericyte disruption, endothelial cell dysfunction, and stalled neutrophils that are most prominent in the first 24 hours after revascularization, with more static changes thereafter.33,38,42,77 Animal studies indicate that both extrinsic compressive forces from perivascular edema and endothelial cell swelling and intravascular obstruction secondary to platelet activation, leukocyte adhesion, and fibrin deposition are rapidly activated as early as one hour following LVO. 33 The rapid appearance of activated platelets and subsequent increase and persistence of leukocyte adhesion factors including P-selectin, E-selectin, and intercellular adhesion moelcule-1 (ICAM-1) on activated endothelium at 24 hours following vessel occlusion78,79 may form the pathophysiologic basis of increasing progressive microvascular failure over this time period.
These interrelated sequelae likely contribute to the cortical spreading depression, vasoconstrictive effect associated with elevated extracellular K+, and cycles of hypoperfusion and hyperemia that result in secondary tissue injury following revascularization.76,80 Likewise, the delayed appearance of pro-angiogenic factors after 24 hours, such as the biphasic elevation of ang-2 transcripts at 24 hours and 14 days following focal ischemic and increase in tie-2 mRNA transcripts beginning at 24 hours in a rat model81,82, may counteract the aberrant microcirculatory response following recanalization, and form the basis of the “tapering-off” effect in microvascular failure observed beyond 24 hours. However, these phenomena have yet to be clarified in human AIS patients.
Meta-regression of included studies using a robust mixed-effects model demonstrates that recanalization method and its interaction with time of reperfusion assessment are significant estimators of microvascular failure rate and may be critical factors that should be considered in quantitative analyses that examine microvascular failure in the extended time window. The lack of significant fit of our curve-fitting analysis for extended interventions compared to standard time window studies supports the preliminary evidence that time of intervention following stroke onset may influence post-revascularization outcomes.10,83 Future studies that specifically examine patients with an extended time window of intervention (treatment initiation 6–24 hours from LKN) will clarify if stroke treatment time window mediates differential rates of microvascular failure over time.
The analysis of progressive microvascular failure over time is inherently limited by the singular time point of reperfusion assessment for the majority of studies. An analysis of microvascular failure rate over time across studies is affected by variability in study methodology, reperfusion imaging modality, and operational definitions of successful reperfusion (Table 2) that may limit generalizability of an implied time trend.
Differentiation of progressive microvascular failure versus progressive microvascular ischemia is also limited by technical consideration; it can be difficult to evaluate the microvasculature at the desired temporal and spatial resolution with current imaging modalities and metabolic analysis techniques. 38 Given the meta-analysis study methodology involves summary data from each study using respective thresholds for reperfusion that vary by imaging modality and associated post-processing software, interpolating common attributes among microvascular failure events may result in ecological fallacy. Patient-level factors including age, sex, and NIHSS at time of presentation were not uniformly reported across included studies. These relevant parameters were omitted as potential covariates in meta-analysis and meta-regression due to the cumulative effect of reporting bias that may impact downstream analysis following data aggregation. However, the findings of our meta-analysis are limited due to the inherent omitted-variable bias and associated confounding that may be attributed to the inconsistent reporting of these parameters and affect the progression of microvascular failure.
Variability in imaging modality itself, in addition to reperfusion assessment time point, may contribute to the observed variability in microvascular failure rate in AIS patients. CT perfusion (CTP) is one of the most common imaging modalities used to assess reperfusion status in AIS patients given cost considerations and limitations of MR-based approaches in emergent settings and resource-limited settings. 84 Our systematic review includes five studies in which CTP is the primary assessment tool of reperfusion and two studies in which it is combined with perfusion-weighted imaging (PWI). Magnetic resonance-based PWI studies offer a radiation free alternative to CT-based approaches, which may be contraindicated in certain clinical scenarios due to contrast and radiation toxicity and a lengthy washout period that precludes serial imaging. 85
Arterial spin labeling (ASL) is a quantitative, non-invasive functional MRI (fMRI) technique using voxel-wise comparison that does not require gadolinium contrast enhancement, unlike PWI, which may be preferential in patients with severe renal impairment or those with repetitive prior perfusion imaging.70,74,75 Compared to PWI, hyperperfusion on ASL has demonstrated better information about penumbral salvage and was a better predictor of early clinical outcome, 59 indicating that it may be an effective marker of tissue reperfusion with positive early clinical correlation for acute ischemic stroke patients. While CTP and PWI have been the most common imaging techniques used in prior investigations of microvascular failure, between-study heterogeneity is likely to increase in future meta-analyses of this phenomenon as novel modalities such as ASL and BOLD-delay are used to circumvent gadolinium contrast toxicity and radiation concerns associated with serial imaging.
Conclusion
In terms of future considerations of microvascular failure in clinical care and therapeutic development, our analysis suggests that the biomarkers, imaging studies, and clinical evaluations needed to evaluate the phenomenon are increasingly included in the study of post-recanalization AIS patients. However, the literature is inconsistent in the reporting of the component measures and imaging outcomes that are necessary to determine microvascular dysfunction. In total, 186 studies were excluded from the analysis as the component variables needed to calculate microvascular failure rate in the study were not present. Most of these studies included the measurement of both recanalization and reperfusion using validated methodologies in their trial design, but did not explicitly report the subset of patients with partial or successful recanalization without perfusion on post-treatment imaging.
While advanced perfusion imaging has been leveraged extensively in the pre-recanalization setting to optimize revascularization strategy for a given patient, it has been used sparingly following treatment to identify reperfusion dynamics in post-revascularized brain tissue. Serial imaging throughout the pre- and post-recanalization phases may provide prognostic information regarding microvascular failure dynamics that may guide the ongoing development of experimental therapeutics, including complement inhibition86,87 and targeted cerebral edema therapies 88 , that may target the neuroinflammatory basis of progressive microcirculatory dysfunction and potentially minimize secondary injury.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231216766 for Progressive microvascular failure in acute ischemic stroke: A systematic review, meta-analysis, and time-course analysis by Thilan Tudor, Eleonora F Spinazzi, Julia E Alexander, Grace K Mandigo, Sean D Lavine, Jack Grinband and E Sander Connolly Jr in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD
Thilan Tudor https://orcid.org/0009-0002-6349-2818
Supplementary material
Supplemental material for this article is available online.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics – 2014 update: a report from the American Heart Association. Circulation 2014; 129: e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics – 2019 update: a report from the American Heart Association. Circulation 2019; 139: e56–e528. [DOI] [PubMed] [Google Scholar]
- 3.Bala F, Ospel J, Mulpur B, et al. Infarct growth despite successful endovascular reperfusion in acute ischemic stroke: a meta-analysis. AJNR Am J Neuroradiol 2021; 42: 1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babadjouni RM, Walcott BP, Liu Q, et al. Neuroprotective delivery platforms as an adjunct to mechanical thrombectomy. Neurosurg Focus 2017; 42: E4. [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Hill MD, Saver JL, et al. Challenges and opportunities of endovascular stroke therapy. Ann Neurol 2016; 79: 11–17. [DOI] [PubMed] [Google Scholar]
- 6.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 7.Ng FC, Churilov L, Yassi N, et al. Prevalence and significance of impaired microvascular tissue reperfusion despite macrovascular angiographic reperfusion (no-reflow). Neurology 2022; 98: e790–e801. [DOI] [PubMed] [Google Scholar]
- 8.De Silva DA, Fink JN, Christensen S, et al. Assessing reperfusion and recanalization as markers of clinical outcomes after intravenous thrombolysis in the echoplanar imaging thrombolytic evaluation trial (EPITHET). Stroke 2009; 40: 2872–2874. [DOI] [PubMed] [Google Scholar]
- 9.Eilaghi A, Brooks J, d'Esterre C, et al. Reperfusion is a stronger predictor of good clinical outcome than recanalization in ischemic stroke. Radiology 2013; 269: 240–248. [DOI] [PubMed] [Google Scholar]
- 10.Cho T-H, Nighoghossian N, Mikkelsen IK, et al. Reperfusion within 6 hours outperforms recanalization in predicting penumbra salvage, lesion growth, final infarct, and clinical outcome. Stroke 2015; 46: 1582–1589. [DOI] [PubMed] [Google Scholar]
- 11.Kosior JC, Buck B, Wannamaker R, et al. Exploring reperfusion following endovascular thrombectomy. Stroke 2019; 50: 2389–2395. [DOI] [PubMed] [Google Scholar]
- 12.Yoo R-E, Yun TJ, Yoo DH, et al. Monitoring cerebral blood flow change through use of arterial spin labelling in acute ischaemic stroke patients after intra-arterial thrombectomy. Eur Radiol 2018; 28: 3276–3284. [DOI] [PubMed] [Google Scholar]
- 13.Ter Schiphorst A, Charron S, Hassen WB, et al. Tissue no-reflow despite full recanalization following thrombectomy for anterior circulation stroke with proximal occlusion: a clinical study. J Cereb Blood Flow Metab 2021; 41: 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soares BP, Tong E, Hom J, et al. Reperfusion is a more accurate predictor of follow-up infarct volume than recanalization: a proof of concept using CT in acute ischemic stroke patients. Stroke 2010; 41: e34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson DA, Hallenbeck JM. Acute focal ischemia-induced alterations in MAP2 immunostaining: description of temporal changes and utilization as a marker for volumetric assessment of acute brain injury. J Cereb Blood Flow Metab 1996; 16: 170–174. [DOI] [PubMed] [Google Scholar]
- 16.Dawson DA, Ruetzler CA, Hallenbeck JM. Temporal impairment of microcirculatory perfusion following focal cerebral ischemia in the spontaneously hypertensive rat. Brain Res 1997; 749: 200–208. [DOI] [PubMed] [Google Scholar]
- 17.Zhang ZG, Chopp M, Goussev A, et al. Cerebral microvascular obstruction by fibrin is associated with upregulation of PAI-1 acutely after onset of focal embolic ischemia in rats. J Neurosci 1999; 19: 10898–10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okada Y, Copeland BR, Fitridge R, et al. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke 1994; 25: 1847–1853. discussion 1853–1854. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe R, Charron T, Puley G, et al. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 2008; 117: 3152–3156. [DOI] [PubMed] [Google Scholar]
- 20.Desilles J-P, Syvannarath V, Di Meglio L, et al. Downstream microvascular thrombosis in cortical venules is an early response to proximal cerebral arterial occlusion. J Am Heart Assoc 2018; 7: e007804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Amki M, Glück C, Binder N, et al. Neutrophils obstructing brain capillaries are a major cause of no-reflow in ischemic stroke. Cell Rep 2020; 33: 108260. [DOI] [PubMed] [Google Scholar]
- 22.Yemisci M, Gursoy-Ozdemir Y, Vural A, et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 2009; 15: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 23.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Wang Y, Akamatsu Y, et al. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog Neurobiol 2014; 115: 138–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperring CP, Savage WM, Argenziano MG, et al. No-reflow post-recanalization in acute ischemic stroke: mechanisms, measurements, and molecular markers. Stroke 2023; 54: 2472–2480. [DOI] [PubMed] [Google Scholar]
- 26.Nanobashvili J, Neumayer C, Fuegl A, et al. Development of “no-reflow” phenomenon in ischemia/reperfusion injury: failure of active vasomotility and not simply passive vasoconstriction. Eur Surg Res 2003; 35: 417–424. [DOI] [PubMed] [Google Scholar]
- 27.Guldbrandsen HO, Staehr C, Iversen NK, et al. Does src kinase mediated vasoconstriction impair penumbral reperfusion? Stroke 2021; 52: e250–e258. [DOI] [PubMed] [Google Scholar]
- 28.Murray KN, Girard S, Holmes WM, et al. Systemic inflammation impairs tissue reperfusion through endothelin-dependent mechanisms in cerebral ischemia. Stroke 2014; 45: 3412–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mechtouff L, Bochaton T, Paccalet A, et al. Association of interleukin-6 levels and futile reperfusion after mechanical thrombectomy. Neurology 2021; 96: e752–e757. [DOI] [PubMed] [Google Scholar]
- 30.Mechtouff L, Bochaton T, Paccalet A, et al. A lower admission level of interleukin-6 is associated with first-pass effect in ischemic stroke patients. J Neurointerv Surg 2022; 14: 248–251. [DOI] [PubMed] [Google Scholar]
- 31.Weyland CS, Vey JA, Mokli Y, et al. Full reperfusion without functional independence after mechanical thrombectomy in the anterior circulation: performance of prediction models before versus after treatment initiation. Clin Neuroradiol 2022; 32: 987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi J-H, Park S-W, Kapadia R, et al. Role of transcription factors in mediating post-ischemic cerebral inflammation and brain damage. Neurochem Int 2007; 50: 1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab 2003; 23: 879–894. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M, Zhu W, Ma Y, et al. Early neurological deterioration and hypoperfusion volume ratio on arterial spin labeling in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 2021; 30: 105885. [DOI] [PubMed] [Google Scholar]
- 35.Campbell BCV, Mitchell PJ, Churilov L, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK part 2 randomized clinical trial. JAMA 2020; 323: 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell BC, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before endovascular thrombectomy (EXTEND-IA TNK): a multicenter, randomized, controlled study. Int J Stroke 2018; 13: 328–334. [DOI] [PubMed] [Google Scholar]
- 37.Goyal M, Demchuk AM, Hill MD. Endovascular therapy for ischemic stroke. N Engl J Med 2015; 372: 2366. [DOI] [PubMed] [Google Scholar]
- 38.Dalkara T, Arsava EM. Can restoring incomplete microcirculatory reperfusion improve stroke outcome after thrombolysis? J Cereb Blood Flow Metab 2012; 32: 2091–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng FC, Coulton B, Chambers B, et al. Persistently elevated microvascular resistance postrecanalization. Stroke 2018; 49: 2512–2515. [DOI] [PubMed] [Google Scholar]
- 40.Rubiera M, Garcia-Tornel A, Olivé-Gadea M, et al. Computed tomography perfusion after thrombectomy: an immediate surrogate marker of outcome after recanalization in acute stroke. Stroke 2020; 51: 1736–1742. [DOI] [PubMed] [Google Scholar]
- 41.Wunderlich MT, Goertler M, Postert T, Competence Network Stroke et al. Recanalization after intravenous thrombolysis: does a recanalization time window exist? Neurology 2007; 68: 1364–1368. [DOI] [PubMed] [Google Scholar]
- 42.Mujanovic A, Ng F, Meinel TR, et al. No-reflow phenomenon in stroke patients: a systematic literature review and meta-analysis of clinical data. Int J Stroke 2023; 17474930231180434. [DOI] [PubMed] [Google Scholar]
- 43.Angermaier A, Langner S. Recanalization and reperfusion in acute stroke – more often different than alike. Clin Neuroradiol 2016; 26: 375–376. [DOI] [PubMed] [Google Scholar]
- 44.Mahon BR, Nesbit GM, Barnwell SL, et al. North American clinical experience with the EKOS MicroLysUS infusion catheter for the treatment of embolic stroke. AJNR Am J Neuroradiol 2003; 24: 534–538. [PMC free article] [PubMed] [Google Scholar]
- 45.Pierre Gobin Y, Starkman S, Duckwiler GR, et al. Merci 1. Stroke 2004; 35: 2848–2854. [DOI] [PubMed] [Google Scholar]
- 46.Khatri P, Neff J, Broderick JP, IMS-I Investigators et al. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke 2005; 36: 2400–2403. [DOI] [PubMed] [Google Scholar]
- 47.Arnold M, Schroth G, Nedeltchev K, et al. Intra-arterial thrombolysis in 100 patients with acute stroke due to middle cerebral artery occlusion. Stroke 2002; 33: 1828–1833. [DOI] [PubMed] [Google Scholar]
- 48.Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3, https://training.cochrane.org/handbook/current (2022, accessed 19 December 2022).
- 49.National Heart, Lung, and Blood Institute, Research Triangle Institute International. Study Quality Assessment Tools. NHLBI, NIH, www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (2021, accessed 15 February 2023).
- 50.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft 2010; 36: 1–48. [Google Scholar]
- 51.Wickham H. ggplot2. New York, NY: Springer, 2009. [Google Scholar]
- 52.Onofri A. The broken bridge between biologists and statisticians: a blog and R package, www.statforbiology.com (2020, accessed 5 January 2023). [Google Scholar]
- 53.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 54.Borenstein M, Higgins JPT. Meta-analysis and subgroups. Prev Sci 2013; 14: 134–143. [DOI] [PubMed] [Google Scholar]
- 55.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baird AE, Donnan GA, Austin MC, et al. Reperfusion after thrombolytic therapy in ischemic stroke measured by single-photon emission computed tomography. Stroke 1994; 25: 79–85. [DOI] [PubMed] [Google Scholar]
- 57.Yasaka M, O'Keefe GJ, Chambers BR, et al. Streptokinase in acute stroke: effect on reperfusion and recanalization. Australian streptokinase trial study group. Neurology 1998; 50: 626–632. [DOI] [PubMed] [Google Scholar]
- 58.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006; 60: 508–517. [DOI] [PubMed] [Google Scholar]
- 59.Bivard A, Stanwell P, Levi C, et al. Arterial spin labeling identifies tissue salvage and good clinical recovery after acute ischemic stroke. J Neuroimaging 2013; 23: 391–396. [DOI] [PubMed] [Google Scholar]
- 60.Horsch AD, Dankbaar JW, Niesten JM, et al. Predictors of reperfusion in patients with acute ischemic stroke. AJNR Am J Neuroradiol 2015; 36: 1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carbone F, Busto G, Padroni M, et al. Radiologic cerebral reperfusion at 24 h predicts good clinical outcome. Transl Stroke Res 2019; 10: 178–188. [DOI] [PubMed] [Google Scholar]
- 62.Khalil AA, Villringer K, Filleböck V, et al. Non-invasive monitoring of longitudinal changes in cerebral hemodynamics in acute ischemic stroke using BOLD signal delay. J Cereb Blood Flow Metab 2020; 40: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Potreck A, Mutke MA, Weyland CS, et al. Combined perfusion and permeability imaging reveals different pathophysiologic tissue responses after successful thrombectomy. Transl Stroke Res 2021; 12: 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao W, Liu R, Yu W, et al. Elevated pulsatility index is associated with poor functional outcome in stroke patients treated with thrombectomy: a retrospective cohort study. CNS Neurosci Ther 2022; 28: 1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomsick T, Broderick J, Carrozella J, et al. Revascularization results in the interventional management of stroke II trial. AJNR Am J Neuroradiol 2008; 29: 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 67.Sah RG, d'Esterre CD, Hill MD, et al. Diffusion-weighted imaging lesion growth occurs despite recanalization in acute ischemic stroke: implications for future treatment trials. Int J Stroke 2019; 14: 257–264. [DOI] [PubMed] [Google Scholar]
- 68.Gilberti N, Gamba M, Premi E, et al. Leukoaraiosis is a predictor of futile recanalization in acute ischemic stroke. J Neurol 2017; 264: 448–452. [DOI] [PubMed] [Google Scholar]
- 69.Parsons MW, Miteff F, Bateman GA, et al. Acute ischemic stroke: imaging-guided tenecteplase treatment in an extended time window. Neurology 2009; 72: 915–921. [DOI] [PubMed] [Google Scholar]
- 70.Lu S-S, Cao Y-Z, Su C-Q, et al. Hyperperfusion on arterial spin labeling MRI predicts the 90-day functional outcome after mechanical thrombectomy in ischemic stroke. J Magn Reson Imaging 2021; 53: 1815–1822. [DOI] [PubMed] [Google Scholar]
- 71.Yu S, Ma SJ, Liebeskind DS, et al. ASPECTS-based reperfusion status on arterial spin labeling is associated with clinical outcome in acute ischemic stroke patients. J Cereb Blood Flow Metab 2018; 38: 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okazaki S, Griebe M, Gregori J, et al. Prediction of early reperfusion from repeated arterial spin labeling perfusion magnetic resonance imaging during intravenous thrombolysis. Stroke 2016; 47: 247–250. [DOI] [PubMed] [Google Scholar]
- 73.Nael K, Meshksar A, Liebeskind DS, et al. Quantitative analysis of hypoperfusion in acute stroke: arterial spin labeling versus dynamic susceptibility contrast. Stroke 2013; 44: 3090–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bardin T, Richette P. Nephrogenic systemic fibrosis. Curr Opin Rheumatol 2010; 22: 54–58. [DOI] [PubMed] [Google Scholar]
- 75.Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363: 768–774. [DOI] [PubMed] [Google Scholar]
- 76.Kloner RA, King KS, Harrington MG. No-reflow phenomenon in the heart and brain. Am J Physiol Heart Circ Physiol 2018; 315: H550–H562. [DOI] [PubMed] [Google Scholar]
- 77.Chen J, Zhang Z, Chen L, et al. Correlation of changes in leukocytes levels 24 hours after intravenous thrombolysis with prognosis in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 2018; 27: 2857–2862. [DOI] [PubMed] [Google Scholar]
- 78.Okada Y, Copeland BR, Mori E, et al. P-selectin and intercellular adhesion molecule-1 expression after focal brain ischemia and reperfusion. Stroke 1994; 25: 202–211. [DOI] [PubMed] [Google Scholar]
- 79.Haring HP, Berg EL, Tsurushita N, et al. E-selectin appears in nonischemic tissue during experimental focal cerebral ischemia. Stroke 1996; 27: 1386–1391; discussion 1391–1392. [DOI] [PubMed] [Google Scholar]
- 80.Ayata C, Lauritzen M. Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol Rev 2015; 95: 953–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin TN, Wang CK, Cheung WM, et al. Induction of angiopoietin and tie receptor mRNA expression after cerebral ischemia-reperfusion. J Cereb Blood Flow Metab 2000; 20: 387–395. [DOI] [PubMed] [Google Scholar]
- 82.Lin TN, Nian GM, Chen SF, et al. Induction of tie-1 and tie-2 receptor protein expression after cerebral ischemia-reperfusion. J Cereb Blood Flow Metab 2001; 21: 690–701. [DOI] [PubMed] [Google Scholar]
- 83.Shahid AH, Abbasi M, Larco JLA, et al. Risk factors of futile recanalization following endovascular treatment in patients with large‐vessel occlusion: systematic review and meta‐analysis. Svin 2022; 2: e000257. [Google Scholar]
- 84.Campbell BCV, Christensen S, Levi CR, et al. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke 2012; 43: 2648–2653. [DOI] [PubMed] [Google Scholar]
- 85.Schaefer PW, Barak ER, Kamalian S, et al. Quantitative assessment of core/penumbra mismatch in acute stroke: CT and MR perfusion imaging are strongly correlated when sufficient brain volume is imaged. Stroke 2008; 39: 2986–2992. [DOI] [PubMed] [Google Scholar]
- 86.Clarke AR, Christophe BR, Khahera A, et al. Therapeutic modulation of the complement cascade in stroke. Front Immunol 2019; 10: 1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma Y, Liu Y, Zhang Z, et al. Significance of complement system in ischemic stroke: a comprehensive review. Aging Dis 2019; 10: 429–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yao Y, Zhang Y, Liao X, et al. Potential therapies for cerebral edema after ischemic stroke: a mini review. Front Aging Neurosci 2020; 12: 618819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231216766 for Progressive microvascular failure in acute ischemic stroke: A systematic review, meta-analysis, and time-course analysis by Thilan Tudor, Eleonora F Spinazzi, Julia E Alexander, Grace K Mandigo, Sean D Lavine, Jack Grinband and E Sander Connolly Jr in Journal of Cerebral Blood Flow & Metabolism