Abstract
We previously showed that knockdown or deletion of Fos downstream transcript (FosDT; a stroke-induced brain-specific long noncoding RNA) is neuroprotective. We presently tested the therapeutic potential of FosDT siRNA in rodents subjected to transient middle cerebral artery occlusion (MCAO) using the Stroke Treatment Academic Industry Roundtable criteria, including sex, age, species, and comorbidity. FosDT siRNA (IV) given at 30 min of reperfusion significantly improved motor function recovery (rotarod test, beam walk test, and adhesive removal test) and reduced infarct size in adult and aged spontaneously hypertensive rats of both sexes. FosDT siRNA administered in a delayed fashion (3.5 h of reperfusion following 1 h transient MCAO) also significantly improved motor function recovery and decreased infarct volume. Furthermore, FosDT siRNA enhanced post-stroke functional recovery in normal and diabetic mice. Mechanistically, FosDT triggered post-ischemic neuronal damage via the transcription factor REST as REST siRNA mitigated the enhanced functional outcome in FosDT−/− rats. Additionally, NF-κB regulated FosDT expression as NF-κB inhibitor BAY 11-7082 significantly decreased post-ischemic FosDT induction. Thus, FosDT is a promising target with a favorable therapeutic window to mitigate secondary brain damage and facilitate recovery after stroke regardless of sex, age, species, and comorbidity.
Keywords: Cerebral ischemia, comorbidity, neuroprotection, noncoding RNA, transcriptional regulation, therapy
Introduction
Stroke is a leading cause of mortality and disability worldwide, leaving most survivors with severe long-term disabilities. 1 To preserve normal neural function, tissue plasminogen activator and mechanical thrombectomy are the only approved interventions. 2 However, these therapies are available only to a subset of patients, and the application is limited to 5 h in the case of thrombolytic treatment and up to a day in the case of mechanical thrombectomy. 2
We recently showed that stroke induces extensive spatiotemporal changes in the heterogeneous network of protein-coding RNAs and noncoding RNAs (ncRNAs) that significantly alter the long-term neurologic function and present a unique opportunity for therapeutic targeting.3 –12 Several ncRNAs, including microRNAs (miRNAs) and long noncoding RNAs (lncRNAs), have been recently shown to modulate post-stroke secondary brain damage.6 –9,11,13 This is corroborated by the several RNA-based therapies tested in clinical settings over the past decade, some of which have received FDA approval to treat a range of disorders, including those related to the CNS. 14 LncRNAs, in particular, constitute a substantial percentage of the human transcriptome with cell, tissue, and/or organ-specific expression patterns. 15 Their aberrant expression contributes to a variety of disease etiologies, thereby making them attractive therapeutic targets. 14
We recently showed that a brain-specific, developmentally regulated, and conserved lncRNA called Fos downstream transcript (FosDT) is highly upregulated in rodent brains after focal ischemia. 8 Intriguingly, FosDT-deficient rats grow normally with no detectable phenotypic abnormalities or alterations in the cytoarchitecture of the brain or other peripheral organs. 8 However, FosDT deletion enhanced post-stroke sensorimotor recovery and reduced brain damage through curtailed induction of inflammation, apoptosis, mitochondrial dysfunction, and oxidative stress.8,9 The beneficial effects of FosDT deletion were also observed independent of sex. 8
While the FosDT knockout studies confirm the pathologic role of its induction in post-stroke brain damage, it is essential to therapeutically target it for translation to clinics. Although FosDT siRNA decreased the infarction and promoted better motor function recovery after transient middle cerebral artery occlusion (MCAO), those studies were conducted using only adult male rats and with intracerebral (IC) siRNA delivery. 9 As outcomes after stroke are sex and age-specific, the Stroke Treatment Academic Industry Roundtable (STAIR) and Ischemia Models: Procedural Refinements Of in Vivo Experiments (IMPROVE) provided several guidelines for rigorous testing of stroke therapies in preclinical settings.16 –21 To satisfy those, we currently evaluated the efficacy of FosDT siRNA injected peripherally (intravenous; IV) in adult and aged rats of both sexes by estimating multiple outcome parameters. As STAIR also suggested testing the therapeutic window of opportunity, we tested the effect of delayed administration of FosDT siRNA after focal ischemia in rats. STAIR further stipulates testing a drug in multiple species and in comorbid conditions. Hence, we further tested FosDT siRNA in wild-type and type-2 diabetic mice.
It is important to understand the mechanism of action of any new therapeutic target. We previously showed that FosDT deficiency decreases inflammation, oxidative stress, and mitochondrial damage in the post-stroke brain. We further showed that mechanistically FosDT collaborates with REST-associated chromatin-modifying proteins. 9 This interaction is critical for the induction of REST-downstream genes GRIA2, NF-κB2 and GRIN1, which modulate FosDT-mediated ischemic brain damage. 8 We further evaluated the interaction of FosDT and REST by treating FosDT knockout rats with REST siRNA. To understand the transcriptional control of FosDT, we studied the role of NF-κB as an upstream transcription factor that mediates FosDT induction.
Materials and methods
Focal cerebral ischemia
Animal experimental protocols were approved by the University of Wisconsin Research Animal Resources and Care Committee, and experiments were conducted in compliance with the “Animal Research: Reporting of In Vivo Experiments (ARRIVE)” guidelines. Animals were cared in accordance with the Guide for the Care and Use of Laboratory Animals, U.S. Department of Health and Human Services Publication # 86-23 (revised). 22 Animals were randomly assigned to groups and the outcome measures were evaluated blindly. Adult (3 months) and aged (∼12 months) male and female spontaneously hypertensive rats (SHR) were purchased from Charles River USA. FosDT−/− and FosDT+/+ rats generated on the Sprague Dawley (SD) background were in-house bred. 8 Adult male C57BL/6 mice (3 months) and type-2 diabetic (db/db) mice (3 months) were from Jackson Labs USA. Focal ischemia was induced by transient MCAO using a silicone-coated nylon monofilament (4-0 for rats and 6-0 for mice, Doccol, USA) under isoflurane anesthesia, as described earlier.6,8,9,12,23 –25 MCA was occluded for 60 min in SHR rats and C57BL/6 mice, 90 min for FosDT+/+/FosDT−/− rats, and 35 min for db/db mice.6,8,24 Sham controls underwent the same surgical procedure, except for MCAO. Regional cerebral blood flow (rCBF) and physiological parameters (pH, PaO2, PaCO2, hemoglobin, and blood glucose) were monitored and body temperature was maintained at 37.0 ± 0.5 °C during surgery using a heating blanket.6,8,24 The impact of the estrous cycle on functional outcomes in females was minimized by randomly assigning them to groups. Animals were euthanized between 12 h to 21 days of reperfusion as needed in different experiments. Animals that showed no signs of neurological deficits during the acute phase after MCAO and/or showed a hemorrhage after euthanasia were excluded from the study.6,8,12,24,25
siRNA injections
Rats and mice were injected with a cocktail of 3 FosDT siRNAs or nontargeting negative control siRNA (Cat # 4390844; Life Technologies, USA) at either 30 min, 2 h, or 3.5 h of reperfusion following transient MCAO.6,9,25 The sequences of the FosDT siRNAs used for rats were (5’ to 3’): GGT TCA TTA TTG GAA TTA Att (sense) and TTA ATT CCA ATA ATG AAC Cca (antisense); CCA TGT TCA TTG TCA TGT Att (sense) and TAC ATG ACA ATG AAC ATG Gac (antisense); GAC CAAT ATT AAA CTA AGA tt (sense) and TCT TAG TTT AAT ATT GGT Cat (antisense). The sequences of the FosDT siRNAs used for mice were (5′ to 3′): CAA UAG CUA UAU CCA UGU Att (sense) and UAC AUG GAU AUA GCU AUU Gat (antisense); GGU UCA UUA UUG GAA UUA Att (sense) and UUA AUU CCA AUA AUG AAC Cca (antisense); CAA UUA GAA ACG ACC AAU Att (sense) and UAU UGG UCG UUU CUA AUU Gga (antisense). The siRNAs (25 nmol; 100 µl) were mixed with 50 µl of polyethylene glycol-liposome in vivo transfection reagent (catalog # 5041; Altogen Biosystems, USA) and injected intravenously (IV). Three in vivo grade siRNAs targeting non-overlapping areas of REST were combined to achieve REST knockdown (Catalog #s136124-s136126 Life Technologies, USA).
Inhibition of NF-κB
Adult male FosDT+/+ and FosDT−/− rats were administered intraperitoneal (IP) with NF-κB inhibitor Bay-11-7082 (5 mg/kg dissolved 30 µl of DMSO and 0.9% sterile NaCl). 26 Control animals received an equal volume of vehicle (DMSO and 0.9% sterile NaCl).
Real-time PCR
Total RNA was extracted using the mirVana™ miRNA Isolation Kit (Thermo Fisher Scientific, USA) as per the manufacturer's instructions. FosDT, Fos mRNA, and REST mRNA levels were estimated using the SYBR Green and TaqMan methods, as described earlier.8,9 Relative gene expression was normalized to 18 s rRNA by the comparative Ct method (2−ΔΔCt).
Functional outcomes
Neurological deficits were scored at 3 days and 7 days of reperfusion using the modified neurological scoring system (mNSS) of 0 (no deficit), 1 (forelimb weakness and torso turning to the ipsilateral side when held by the tail), 2 (circling to affected side), 3 (unable to bear weight on the affected side) and 4 (no spontaneous locomotor activity or barrel rolling). 27 Post-ischemic sensory and motor functions were evaluated between days 1 and 21 of reperfusion by rotarod test (cylinder rotating at 8 rpm for 4 min), beam walk test (foot faults while crossing a 120 cm long tapered beam in rats and straight beam in mice) and adhesive sticker removal test as described earlier.6,9,23 All cohorts were screened for baseline sensory and motor activity, followed by 3 days of pretraining.
Lesion volume analysis
Ischemic brain damage was measured from coronal brain sections (40 μm) stained with Cresyl violet and scanned with NIH ImageJ software. Infarct (lesion) volumes were computed by numeric integration of infarct area from serial sections factoring in sectional intervals and corrected for edema.6,7,9,12,28 The volume difference between the ipsilateral and contralateral hemispheres was computed and represented as brain atrophy/lesion volume. 29
Statistical analyses
The Shapiro-Wilks test was used to determine the normality of the data before the application of parametric or nonparametric tests. The Mann–Whitney U-test or ANOVA (one-way/repeated-measures) followed by Sidak’s or Bonferroni's multiple comparisons test were used to determine significance as indicated in the respective figure legends. Statistical analyses were performed using GraphPad Prism software.
Results
Post-ischemic inhibition of FosDT is therapeutically beneficial in both sexes
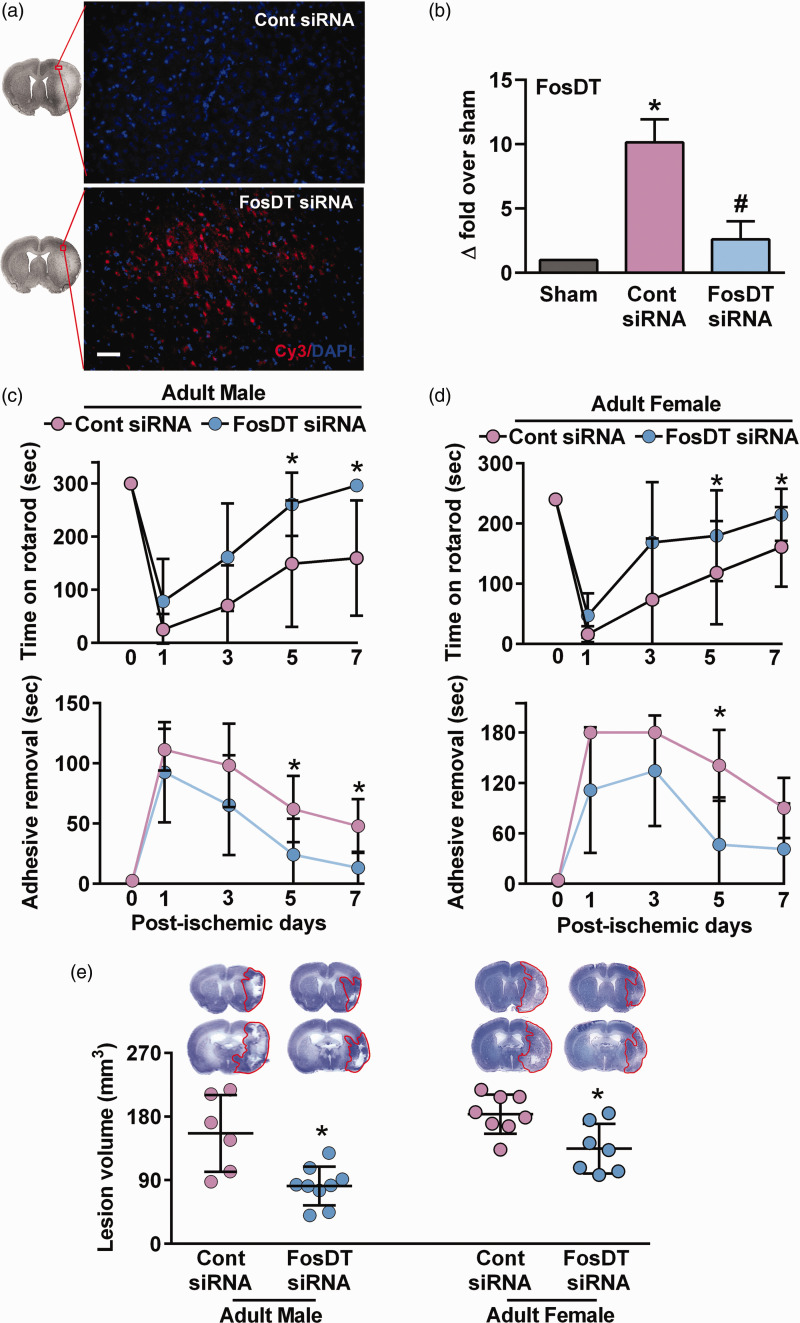
When a Cy3-tagged FosDT siRNA was injected (IV) at 30 min of reperfusion after a 1 h transient MCAO) in adult male rats, significant fluorescence was observed in the peri-infarct cortex at 1 day of reperfusion (Figure 1(a)). FosDT siRNA administration (IV) at 30 min of reperfusion after 1 h transient MCAO resulted in a ∼74% reduction in the cerebral FosDT levels at 1 day of reperfusion compared with control siRNA treated cohort (Figure 1(b)). The FosDT siRNA treatment resulted in a significant reduction in sensorimotor deficits measured by the rotarod test and adhesive removal test between days 1 and 7 of reperfusion in both adult male (Figure 1(c)) and female (Figure 1(d)) rats compared with sex-matched control siRNA treated rats. FosDT siRNA significantly decreased the ischemic lesion volume measured at 7 days of reperfusion in adult males (by 48%; p < 0.05; n = 6–9/group; Figure 1(e)) and females (by 26%; p < 0.05; n = 7–8/group; Figure 1(e)) compared with the sex-matched control siRNA treated cohorts.
Figure 1.
FosDT siRNA protected the brain after focal ischemia in adult rats of both sexes. Cy3-labeled siRNA (red) injected (IV) in adult male rats at 30 min of reperfusion following 1 h transient MCAO was observed in the ipsilateral cortex at 1 day of reperfusion (a). Blue is 4′,6-diamidino-2-phenylindole (DAPI). FosDT siRNA prevented post-ischemic FosDT expression compared to control siRNA assessed at 1 day of reperfusion in the peri-infarct cortex of adult male rats (n = 3–4) (b). *p < 0.05 compared with sham and #p < 0.05 compared with control siRNA by Mann-Whitney U test. FosDT knockdown enhanced motor function recovery assessed by rotarod test (upper) and adhesive removal test (lower) between days 1 and 7 of reperfusion in both male (c) and female (d) rats compared to sex-matched control siRNA cohorts. Representative Cresyl violet-stained sections and lesion volume at day 7 of reperfusion (e). Values are mean ± SD of males (n = 6–9/group) and females (n = 7–8/group). *p < 0.05 compared with control siRNA cohort by repeated-measures ANOVA followed by Bonferroni's multiple comparisons test (c and d) or Mann-Whitney U test. Scale bar = 50 µm.
Post-ischemic FosDT knockdown protected the aged brain
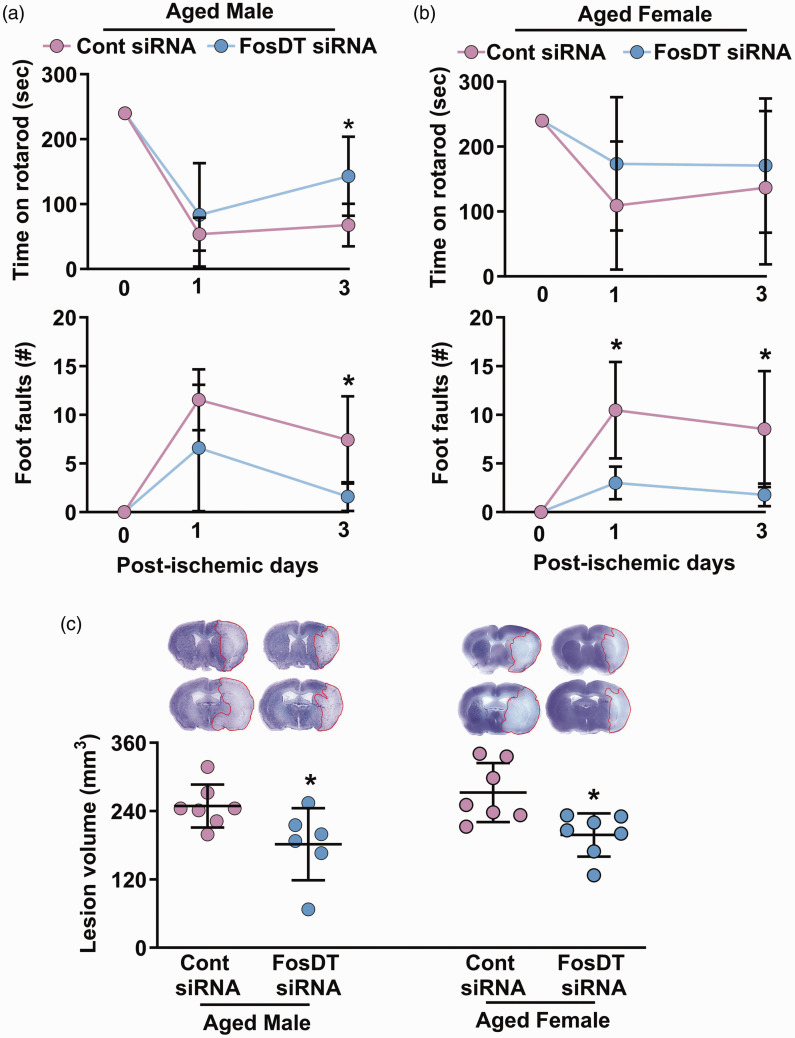
Male and female SHR rats (∼12 months) were used in this experiment as hypertension enhances age-related complexities.30,31 Aged rats of both sexes treated with FosDT siRNA (IV; at 30 min of reperfusion following 1 h transient MCAO) showed significantly improved motor function recovery (rotarod test and beam walk test) at day 1 and day 3 of reperfusion compared with the control siRNA treated sex-matched cohorts (Figure 2(a) and (b)). Aged rats treated with FosDT siRNA also showed significantly smaller infarcts than sex-matched control siRNA treated cohorts (by ∼27%; p < 0.05; n = 6–7/group) (Figure 2(c)).
Figure 2.
Post-ischemic treatment with FosDT siRNA is protective in aged rats. FosDT siRNA administered (IV) at 30 min of reperfusion following MCAO improved motor function (rotarod top and beam walk bottom) between days 1 and 3 of reperfusion in aged (∼12 months) male (a) and female (b) rats. Infarct volume estimated at 3 days of reperfusion was significantly lower in the male and female FosDT siRNA cohorts compared with the sex-matched control siRNA cohorts (c). Values are mean ± SD of n = 6–7/group. *p < 0.05 compared to respective control siRNA group (repeated-measures ANOVA followed by Bonferroni's multiple comparisons test in A and B and Mann-Whitney U test in C).
FosDT siRNA showed a good post-ischemic therapeutic window
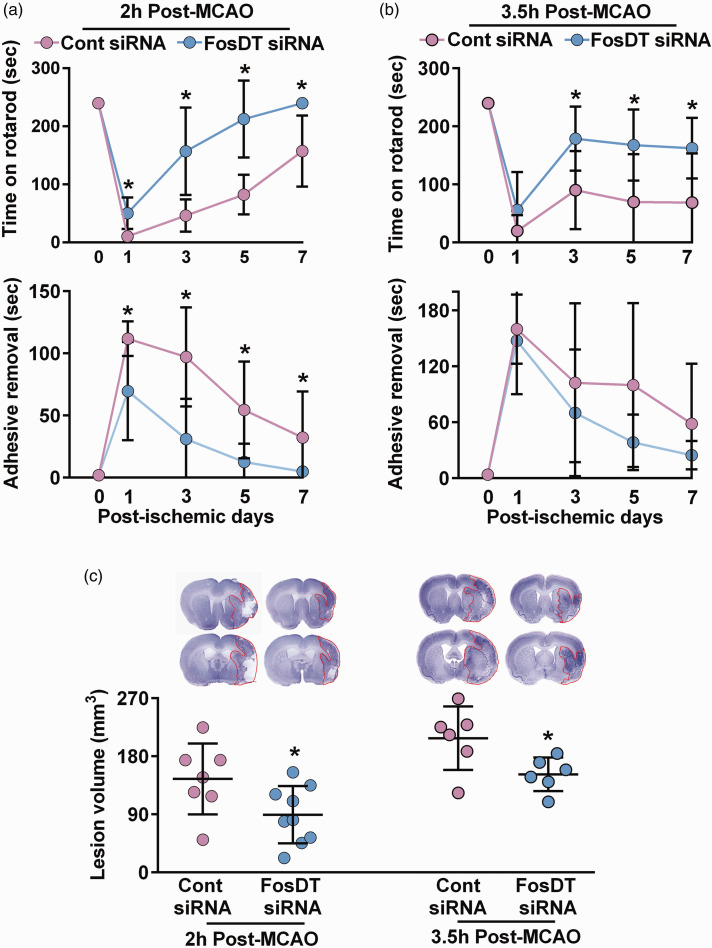
When adult male rats were treated with FosDT siRNA (IV) in a delayed fashion (3 h or 4.5 h after the start of MCAO), they still showed significantly improved motor function recovery between days 1 and 7 of reperfusion (rotarod and adhesive removal tests) compared with the control siRNA treated rats (Figure 3(a) and (b)). Furthermore, delayed administration of FosDT siRNA also resulted in smaller infarcts than control siRNA cohorts (by 38% at 3 h and 27% at 4.5 h) compared with the time-matched control siRNA treated cohorts (p < 0.05; n = 6–9/group) (Figure 3(c)).
Figure 3.
FosDT siRNA has a good therapeutic window after MCAO. When FosDT siRNA was injected at either 2 h or 3.5 h post-MCAO, adult male rats remained on the rotarod for a significantly longer time between days 1 and 7 of reperfusion compared with the control siRNA cohort (a). Mice injected with FosDT siRNA at 2 h, but not 3.5 h, also improved significantly in the adhesive removal test compared with the control siRNA cohort (b). Both cohorts (FosDT siRNA at 2 h and 3.5 h after MCAO) showed significantly smaller infarcts measured at 7 days of reperfusion (c). Values are mean ± SD of n = 6–8/group. *p < 0.05 compared with the respective control siRNA group by repeated-measures ANOVA followed by Bonferroni's multiple comparisons test (a and b) or by Mann-Whitney U test (c).
Post-ischemic protection provided by FosDT silencing is not species-specific
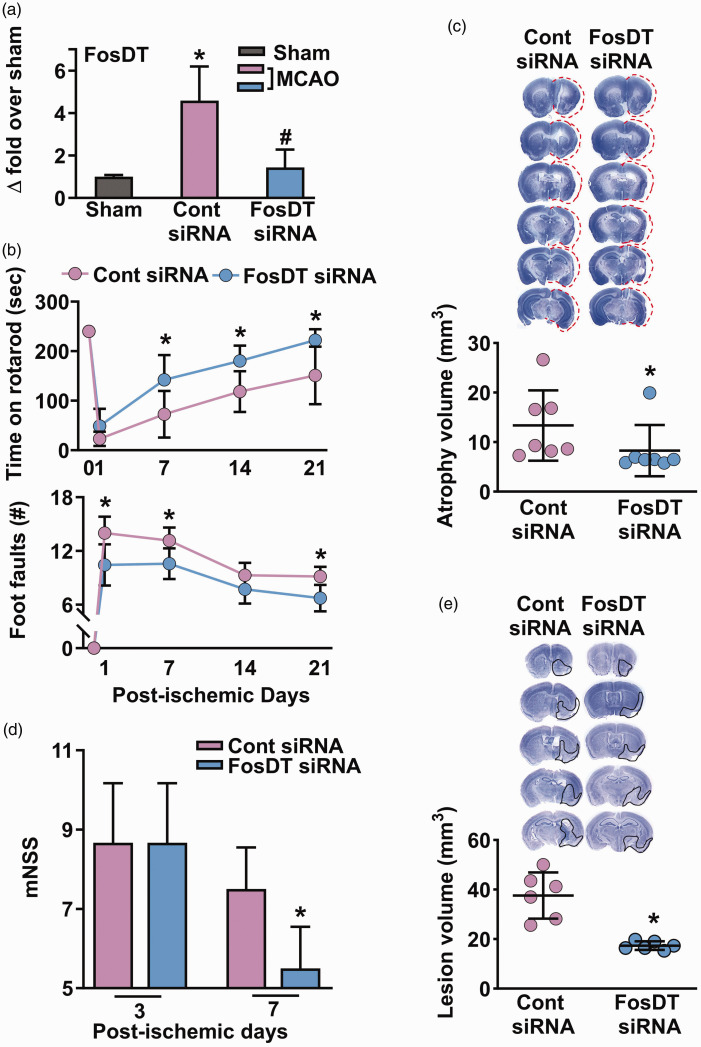
One of the critical criteria for testing preclinical stroke therapy is the inclusion of different species. Adult male C57BL/6 mice subjected to a 1 h transient MCAO showed significantly induced cerebral FosDT expression at 24 h of reperfusion compared to sham control (Figure 4(a)). FosDT siRNA injected (IV) at 30 min of reperfusion after transient MCAO resulted in a ∼68% reduction in FosDT levels at 1 day of reperfusion compared with control siRNA treated cohort (Figure 4(a)). FosDT siRNA treated mice recovered (rotarod and beam walk tests) between days 1 and 21 days of reperfusion following transient MCAO (Figure 4(b)). FosDT siRNA treated mice also showed less brain atrophy/lesion volume at 28 days of reperfusion compared with the control siRNA treated cohort (by ∼38%; p < 0.05; n = 7/group; Figure 4(c)). Post-ischemic IV administration of FosDT siRNA did not cause any overt evidence of toxicity in major peripheral organs (heart, spleen, lung and kidney) in adult male mice (Supplemental Fig. I).
Figure 4.
FosDT siRNA also protected normal and type-2 diabetic mice subjected to transient MCAO. In the peri-infarct cortex of mice subjected to transient MCAO, FosDT expression increased significantly at 24 h of reperfusion compared with sham (a). FosDT siRNA significantly decreased post-ischemic FosDT levels compared with the control siRNA cohort (a). *p < 0.05 compared with sham and #p < 0.05 compared with control siRNA by Mann-Whitney U test (n = 4/group). In adult mice, FosDT siRNA treatment significantly improved motor function recovery between days 1 to 7 (b) and decreased infarct volume (c) at day 7 of reperfusion compared with the control siRNA cohort (b). FosDT siRNA administration also reduced the motor dysfunction (estimated by mNSS) on days 3 and 7 (d) and reduced the infarct volume on day 7 (e) in type-2 diabetic comorbid mice compared with the control siRNA cohort. Values are n = 7/group (a, b, and c) and n = 6/group (d and e). *p < 0.05 compared to the respective control siRNA group by repeated-measures ANOVA followed by Bonferroni's multiple comparisons test (b) or by Mann-Whitney U test (a, c, d, and e).
FosDT siRNA therapy reduced ischemic brain damage in type-2 diabetic mice
Hypertension and diabetes are major comorbidities that exacerbate ischemic brain damage. Above, we showed that FosDT siRNA treatment is therapeutically beneficial in hypertensive rats. In this experiment, we extended that to type-2 diabetic (db/db) mice. The MCAO period was shortened to 35 min since these mice are susceptible to increased mortality and brain damage after stroke. 32 As these mice do not perform in rotarod and beam walk tests, we used mNSS to estimate the post-ischemic functional outcome. In adult male db/db mice, FosDT siRNA (IV at 30 min of reperfusion) significantly reduced neurologic severity scores (Figure 4(d)) and lesion volume (by ∼54%; p < 0.05; n = 6/group; Figure 4(e)) at day 7 of reperfusion following transient MCAO compared with control siRNA treated cohort.
FosDT collaborates with transcription factor REST to promote post-ischemic brain damage
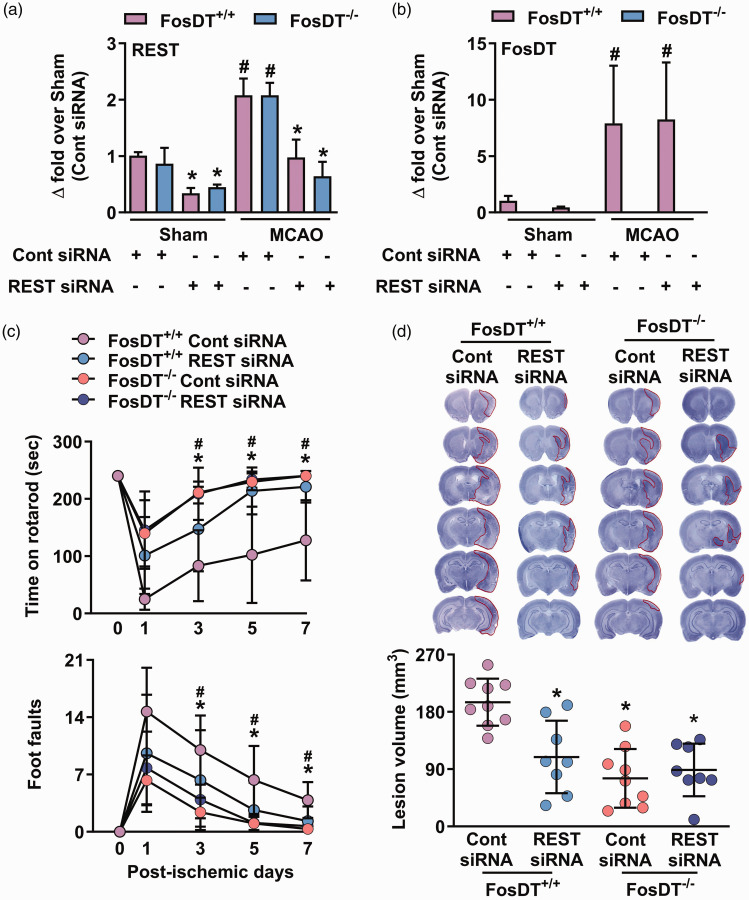
We have previously shown that induction of REST, which silences neural genes by epigenetic remodeling, promotes neurodegeneration following transient MCAO, and its knockdown enhances post-ischemic functional recovery. 33 We further showed that interaction with REST-associated chromatin-modifying proteins is the mechanism of FosDT-induced ischemic brain damage. 9 We currently tested if FosDT influences post-ischemic pathophysiology only via REST or has other partners/targets. Treatment of adult male FosDT+/+ and FosDT−/− rats with REST siRNA at 30 min of reperfusion significantly reduced REST, but not FosDT, expression in both sham and MCAO groups, compared with the respective control siRNA treated cohorts (Figure 5(a) and (b)). Furthermore, both FosDT+/+ and FosDT−/− rats treated with REST siRNA showed a significant reduction in sensorimotor deficits (rotarod and beam walk tests) between days 3 and 7 of reperfusion compared with sex-matched control siRNA treated rats (Figure 5(c)). At 7 days of reperfusion, the FosDT+/+ group treated with REST siRNA showed significantly smaller ischemic lesion size compared to the control siRNA group (by ∼44%; p < 0.05; n = 8-9/group; Figure 5(d)). Additionally, we noticed that deleting FosDT rescued the brain from ischemic damage, but the reduction in brain damage was not further lessened by REST knockdown in FosDT−/− rats compared to FosDT+/+rats indicating that the post-ischemic effects of REST are primarily mediated by FosDT (Figure 5(d)).
Figure 5.
FosDT is essential for REST function. When treated with REST siRNA (IV), expression of REST (a), but not FosDT (b), decreased significantly at 12 h in the peri-infarct cortex of FosDT+/+ and FosDT−/− rats subjected to sham surgery or transient MCAO, compared with respective control siRNA cohort. Values are mean ± SD (n = 3/group). *p < 0.05 compared with the respective control siRNA group and #p < 0.05 compared with the respective sham group by one-way ANOVA followed by Sidak’s multiple comparisons post-test. FosDT−/− rats showed improved motor function recovery and smaller infarcts compared with FosDT+/+ rats (c and d). REST siRNA treatment promoted motor function recovery (c) and smaller infarction (d) in FosDT+/+ rats but not in FosDT−/− rats, compared to respective control siRNA treated cohorts. *p < 0.05 vs FosDT+/+ control siRNA. Values (n = 8–9/group) are mean ± SD.
NF-κB regulates FosDT induction in the ischemic brain
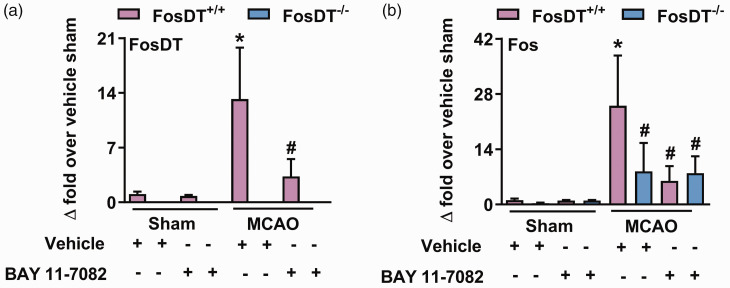
The FosDT gene is cogenic to the Fos gene.8,9 The genomic region transcribing Fos and FosDT carries 48 CpG islands which indicates the proximity to transcription start sites near a promoter. NF-κB is known to bind to this region to control the expression of the Fos gene.34,35 We currently examined if NF-κB is responsible for the post-ischemic induction of FosDT. When FosDT+/+ and FosDT−/− rats were injected with NF-κB inhibitor BAY 11-7082 at 30 min of reperfusion following transient MCAO, both Fos and FosDT levels decreased significantly, indicating that NF-κB controls FosDT expression (Figure 6(a) and (b)). As anticipated, FosDT, but not Fos, was undetectable in FosDT−/− rats (Figure 6(a) and (b)).
Figure 6.
FosDT levels are regulated by NF-κB. When treated with NF-κB inhibitor BAY 11-7082 (IP) at 30 min of reperfusion following transient MCAO, FosDT+/+ rats showed significantly reduced levels of FosDT (a) and Fos mRNA (b) at 12 h of reperfusion compared with vehicle control. FosDT−/− rats showed no FosDT expression (a) and no change in Fos levels (b) by BAY 11-7082 treatment. Values are mean ± SD (n = 4/group). *p < 0.05 compared with FosDT+/+ sham group and #p < 0.05 compared with FosDT+/+ MCAO group treated with the vehicle by one-way ANOVA followed by Sidak’s multiple comparisons post-test.
Discussion
We previously showed that the lncRNA FosDT induced after stroke is a promoter of ischemic brain damage. We presently established the therapeutic potential of preventing FosDT to protect the post-stroke brain by following several STAIR criteria. In brief, our studies show that FosDT siRNA has a good window of opportunity and is efficacious in both sexes and, importantly, in aged and comorbid (type-2 diabetes) animals, and it works irrespective of species after focal ischemia. We further observed that NF-κB is required for FosDT induction and the transcription factor REST is necessary for the FosDT-induced post-ischemic brain damage.
Stroke is known to rapidly induce extensive change in the expression profiles of lncRNAs, and many of them play crucial roles in various aspects of ischemic pathophysiology.5,8,9,11 Hence, targeting lncRNAs is a promising new strategy to minimize post-stroke brain damage and promote functional recovery.8,9,11 STAIR proposed several guidelines for rigorous preclinical testing of stroke therapeutics, including estimating multiple differential outcomes, long-term testing, determining the effectiveness of peripheral and delayed administration of test compounds. 19 We currently showed that FosDT siRNA therapy satisfies these criteria by showing that FosDT siRNA given IV (peripheral administration) promotes motor function recovery as well as better tissue preservation (multiple outcomes) up to 21 days of reperfusion (long-term outcomes). Using a Cy3-tagged siRNA injected IV, we confirmed that peripherally administered FosDT siRNA enters the post-ischemic brain. Additionally, FosDT siRNA administered at a delayed reperfusion time (3 h or 4.5 h after transient MCAO) also significantly improved the motor function recovery and reduced the lesion volume after transient MCAO. However, the efficacy was much better when FosDT siRNA was administered at 3 h than at 4.5 h after transient MCAO.
Multiple preclinical and clinical studies showed that sex affects post-stroke outcomes and treatments.36 –39 This is important since tPA, the only FDA-approved stroke drug, showed sexual dimorphism by significantly enhancing stroke outcomes in females than in males. 39 We observed that FosDT siRNA therapy enhanced functional recovery and protected the brain after focal ischemia in both male and female rats. Older stroke patients have higher severity, morbidity, and reduced recovery than younger stroke patients. 40 Consistent with this, we found that older rats have a more ischemic brain injury and slower post-stroke recovery than young/adult rats. Ideally, therapies that can protect the post-stroke brain in both sexes and, more importantly, in older age groups are preferable. It is exciting to note that FosDT siRNA promoted better neurological recovery and curtailed brain damage in aged mice of both sexes.
STAIR also stipulates testing therapies in multiple species and the present studies showed that FosDT siRNA is efficacious in protecting the post-stroke brain in both rats and mice. Similar to advanced age, comorbidities like hypertension and type-2 diabetes are independent indicators of unfavorable post-stroke functional outcomes and survival.41,42 Both hypertensive rats and type-2 diabetic mice were observed to be protected after stroke by FosDT siRNA therapy. FosDT siRNA also promoted better post-stroke survival in type-2 diabetic mice compared to the control siRNA treated cohort. 32
Genetic manipulation or therapeutic intervention that targets lncRNA can have broader effects on pathophysiological function. However, we found that FosDT deletion had no effect on development, cytoarchitecture and cerebral vasculature. 8 Moreover, targeting FosDT with siRNAs, even at three times the therapeutic dose, has no discernible impact on major body organs.8,9 However, FosDT deletion or FosDT knockdown ameliorated post-stroke functional outcomes independent of sex and age, suggesting the potential of lncRNA therapies.8,9
Epigenetic remodeling controls gene expression in almost all organs during development and disease conditions, including stroke. LncRNAs have emerged as essential epigenetic regulators of gene expression by interacting with chromatin-modifying enzymes, transcription factors, and other transcriptional regulators, thereby shaping the fate of neural cells. 43 We previously showed that FosDT, a brain-specific lncRNA, scaffolds transcription factor REST-associated chromatin-modifying proteins (coREST and Sin3a; corepressors of the REST), which are essential to modulate the transcription of REST downstream genes.9,10 REST-dependent epigenetic remodeling of several genes in neurons plays a critical role in ischemia-induced neuronal death.44,45 The interaction of FosDT with coREST and Sin3a can be reduced through the FosDT knockdown, suggesting that FosDT scaffolding is an essential component for the activity of the REST complex.9,10
Stroke and neurodegenerative diseases lead to REST induction in CNS, which is known to aggravate neuronal injury; REST knockdown decreases brain damage and improves neurologic recovery after stroke.33,44 Our studies show that following focal ischemia, both FosDT and REST are induced, but their induction is not dependent on each other as neither REST knockdown affected FosDT induction nor FosDT knockout curtailed REST upregulation. The extent to which FosDT is indispensable for controlling REST-mediated pathological changes remains uncertain. Thus, we also evaluated the interplay between REST and FosDT in regulating brain damage after stroke. To address this, we administered REST siRNA in FosDT knockout rats. Since REST siRNA did not provide further improvement in FosDT knockout rats, that suggests that FosDT is an essential component of REST complex and function.
Transcription factor NF-κB binds to the locus from which both Fos and FosDT are transcribed.34,35 NF-κB is induced after stroke, and its inhibition reduces ischemic brain damage.46 –48 Additionally, our previous studies showed that NF-ĸB2 induction, which blocks nuclear translocation of NF-κB (RelA/p65) owing to IκB property, is facilitated by suppressing FosDT.46,48 We inhibited NF-κB with BAY 11-7082 (5 mg/kg), and this dose was shown to reduce brain edema more effectively following traumatic brain injury in rats than doses of 1 and 10 mg/kg. 26 By inhibiting NF-κB with BAY 11-7082, we observed a decrease in post-ischemic FosDT induction in FosDT+/+ rats. BAY 11-7082 is an irreversible inhibitor of the NF-κB pathway through inhibiting the phosphorylation of IκB-α. In an inactive form, NF-κB is sequestered in the cytoplasm by inhibitory IκB subunit. However, phosphorylation and degradation of IκB-α are essential for the release of NF-κB from the cytosolic IκB-α/NF-κB complex, leading to nuclear translocation and subsequent activation of the genes containing NF-κB binding sites.49,50 Whether Bay 11-7082 specifically inhibits NF-κB, a previous study has shown that Bay 11-7082 at concentrations of 1.25 μM to 5 μM did not significantly affect the binding of another transcription factor, AP-1 to DNA but it reduced that of NF-κB. 50 We did not evaluate the effect of BAY 11-7082 on CBF. However, we recently reported that FosDT deletion had no noticeable effect on the structure of major cerebral blood vessels, including MCA, ACA, and PCA. Therefore, we do not anticipate that regulating FosDT with BAY 11-7082 will affect CBF. 8 Furthermore, NF-κB inhibition decreased the extent of myocardial ischemia-reperfusion injury and preserved myocardial function in rats. 51 This suggests the strong potential of BAY 11-7082 inhibiting NF-κB. 50 This further indicates that NF-κB is an upstream transcription factor that controls FosDT expression.
Overall, we show that FosDT promotes ischemic brain damage and its knockdown is neuroprotective irrespective of sex, age, species and comorbidity, efficacious when given peripherally, and has a good therapeutic window. Furthermore, NF-κB controls FosDT induction, and once activated, FosDT mediates post-stroke brain damage by collaborating with the REST transcriptional complex. In essence, our studies suggest that FosDT is a novel and promising therapeutic target to reduce secondary brain damage and promote recovery after the stroke.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231212378 for Post-stroke brain can be protected by modulating the lncRNA FosDT by Suresh L Mehta, Bharath Chelluboina, Kahlilia C Morris-Blanco, Saivenkateshkomal Bathula, Soomin Jeong, Vijay Arruri, Charles K Davis and Raghu Vemuganti in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Studies were partly supported by the NIH RO1 NS099531 and the Department of Neurological Surgery, University of Wisconsin-Madison. Dr. Vemuganti is the recipient of a Research Career Scientist award (# IK6BX005690) from the US Department of Veterans Affairs.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: SM, BC, KB, and RV contributed to the conception and design of the study; SM, BC, KB, SB, SJ, VA, and CD contributed to the acquisition and analysis of data; SM and RV drafted the manuscript and edited the text.
Supplementary material: Supplemental material for this article is available online.
ORCID iDs: Bharath Chelluboina https://orcid.org/0000-0001-8834-6484
Charles K Davis https://orcid.org/0000-0002-6342-6565
References
- 1.GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021; 20: 795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du H, Lei H, Ambler G, et al. Intravenous thrombolysis before mechanical thrombectomy for acute ischemic stroke: a meta-analysis. J Am Heart Assoc 2021; 10: e022303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dharap A, Bowen K, Place R, et al. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab 2009; 29: 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dharap A, Nakka VP, Vemuganti R. Altered expression of PIWI RNA in the rat brain after transient focal ischemia. Stroke 2011; 42: 1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dharap A, Nakka VP, Vemuganti R. Effect of focal ischemia on long noncoding RNAs. Stroke 2012; 43: 2800–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim T, Mehta SL, Morris-Blanco KC, et al. The microRNA miR-7a-5p ameliorates ischemic brain damage by repressing alpha-synuclein. Sci Signal 2018; 11: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta SL, Chokkalla AK, Bathula S, et al. MicroRNA miR-7 is essential for post-stroke functional recovery. Transl Stroke Res 2023; 14: 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta SL, Chokkalla AK, Kim T, et al. Long noncoding RNA fos downstream transcript is developmentally dispensable but vital for shaping the poststroke functional outcome. Stroke 2021; 52: 2381–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta SL, Kim T, Vemuganti R. Long noncoding RNA FosDT promotes ischemic brain injury by interacting with REST-associated chromatin-modifying proteins. J Neurosci 2015; 35: 16443–16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dharap A, Pokrzywa C, Vemuganti R. Increased binding of stroke-induced long non-coding RNAs to the transcriptional corepressors Sin3A and coREST. ASN Neuro 2013; 5: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Tang X, Liu K, et al. Long noncoding RNA Malat1 regulates cerebrovascular pathologies in ischemic stroke. J Neurosci 2017; 37: 1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta SL, Chokkalla AK, Bathula S, et al. CDR1as regulates α-synuclein-mediated ischemic brain damage by controlling miR-7 availability. Mol Ther Nucleic Acids 2023; 31: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Cao B, Han D, et al. Long non-coding RNA H19 induces cerebral ischemia reperfusion injury via activation of autophagy. Aging Dis 2017; 8: 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkle M, El-Daly SM, Fabbri M, et al. Noncoding RNA therapeutics – challenges and potential solutions. Nat Rev Drug Discov 2021; 20: 629–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer MK, Niknafs YS, Malik R, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet 2015; 47: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonarow GC, Reeves MJ, Zhao X, et al. Age-related differences in characteristics, performance measures, treatment trends, and outcomes in patients with ischemic stroke. Circulation 2010; 121: 879–891. [DOI] [PubMed] [Google Scholar]
- 17.Gall SL, Donnan G, Dewey HM, et al. Sex differences in presentation, severity, and management of stroke in a population-based study. Neurology 2010; 74: 975–981. [DOI] [PubMed] [Google Scholar]
- 18.Reeves MJ, Bushnell CD, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 2008; 7: 915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savitz SI, Baron JC, Fisher M. Stroke treatment academic industry roundtable X: brain cytoprotection therapies in the reperfusion era. Stroke 2019; 50: 1026–1031. [DOI] [PubMed] [Google Scholar]
- 20.Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl Stroke Res 2013; 4: 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Percie Du Sert N, Alfieri A, Allan SM, et al. The IMPROVE guidelines (ischaemia models: procedural refinements of in vivo experiments). J Cereb Blood Flow Metab 2017; 37: 3488–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Percie Du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol 2020; 18: e3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim T, Mehta SL, Kaimal B, et al. Poststroke induction of alpha-Synuclein mediates ischemic brain damage. J Neurosci 2016; 36: 7055–7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris-Blanco KC, Chokkalla AK, Kim T, et al. High-Dose vitamin C prevents secondary brain damage after stroke via epigenetic reprogramming of neuroprotective genes. Transl Stroke Res 2022; 13: 1017–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chelluboina B, Kim T, Mehta SL, et al. Impact of age and sex on α-Syn (α-Synuclein) knockdown-mediated poststroke recovery. Stroke 2020; 51: 3138–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jayakumar AR, Tong XY, Ruiz-Cordero R, et al. Activation of NF-κB mediates astrocyte swelling and brain edema in traumatic brain injury. J Neurotrauma 2014; 31: 1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bederson JB, Pitts LH, Tsuji M, et al. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 1986; 17: 472–476. [DOI] [PubMed] [Google Scholar]
- 28.Chelluboina B, Chokkalla AK, Mehta SL, et al. Tenascin-C induction exacerbates post-stroke brain damage. J Cereb Blood Flow Metab 2022; 42: 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chokkalla AK, Jeong S, Mehta SL, et al. Cerebroprotective role of N(6)-methyladenosine demethylase FTO (fat mass and obesity-associated protein) after experimental stroke. Stroke 2023; 54: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez CM, Høifødt H, Terry AV., Jr. Spontaneously hypertensive rats: further evaluation of age-related memory performance and cholinergic marker expression. J Psychiatry Neurosci 2003; 28: 197–209. [PMC free article] [PubMed] [Google Scholar]
- 31.Linz W, Jessen T, Becker RH, et al. Long-term ACE inhibition doubles lifespan of hypertensive rats. Circulation 1997; 96: 3164–3172. [DOI] [PubMed] [Google Scholar]
- 32.Tureyen K, Bowen K, Liang J, et al. Exacerbated brain damage, edema and inflammation in type-2 diabetic mice subjected to focal ischemia. J Neurochem 2011; 116: 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris-Blanco KC, Kim T, Bertogliat MJ, et al. Inhibition of the epigenetic regulator REST ameliorates ischemic brain injury. Mol Neurobiol 2019; 56: 2542–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu YC, Huang DY, Shiah SG, et al. Regulation of c-Fos gene expression by NF-κB: a p65 homodimer binding site in mouse embryonic fibroblasts but not human HEK293 cells. PLoS One 2013; 8: e84062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujioka S, Niu J, Schmidt C, et al. NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol Cell Biol 2004; 24: 7806–7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim TH, Vemuganti R. Effect of sex and age interactions on functional outcome after stroke. CNS Neurosci Ther 2015; 21: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber R, Krogias C, Eyding J, et al. Age and sex differences in ischemic stroke treatment in a nationwide analysis of 1.11 million hospitalized cases. Stroke 2019; 50: 3494–3502. [DOI] [PubMed] [Google Scholar]
- 38.Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab 2009; 29: 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sohrabji F, Park MJ, Mahnke AH. Sex differences in stroke therapies. J Neurosci Res 2017; 95: 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roy-O'Reilly M, McCullough LD. Age and sex are critical factors in ischemic stroke pathology. Endocrinology 2018; 159: 3120–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turhan N, Atalay A, Muderrisoglu H. Predictors of functional outcome in first-ever ischemic stroke: a special interest to ischemic subtypes, comorbidity and age. NeuroRehabilitation 2009; 24: 321–326. [DOI] [PubMed] [Google Scholar]
- 42.Simić-Panić D, Bošković K, Milićević M, et al. The impact of comorbidity on rehabilitation outcome after ischemic stroke. Acta Clin Croat 2018; 57: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JT. Epigenetic regulation by long noncoding RNAs. Science 2012; 338: 1435–1439. [DOI] [PubMed] [Google Scholar]
- 44.Noh KM, Hwang JY, Follenzi A, et al. Repressor element-1 silencing transcription factor (REST)-dependent epigenetic remodeling is critical to ischemia-induced neuronal death. Proc Natl Acad Sci U S A 2012; 109: E962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris-Blanco KC, Chokkalla AK, Arruri V, et al. Epigenetic mechanisms and potential therapeutic targets in stroke. J Cereb Blood Flow Metab 2022; 42: 2000–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang W, Potrovita I, Tarabin V, et al. Neuronal activation of NF-kappaB contributes to cell death in cerebral ischemia. J Cereb Blood Flow Metab 2005; 25: 30–40. [DOI] [PubMed] [Google Scholar]
- 47.Nijboer CH, Heijnen CJ, Groenendaal F, et al. Strong neuroprotection by inhibition of NF-kappaB after neonatal hypoxia-ischemia involves apoptotic mechanisms but is independent of cytokines. Stroke 2008; 39: 2129–2137. [DOI] [PubMed] [Google Scholar]
- 48.Schneider A, Martin-Villalba A, Weih F, et al. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med 1999; 5: 554–559. [DOI] [PubMed] [Google Scholar]
- 49.Pierce JW, Schoenleber R, Jesmok G, et al. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem 1997; 272: 21096–21103. [DOI] [PubMed] [Google Scholar]
- 50.Mori N, Yamada Y, Ikeda S, et al. Bay 11-7082 inhibits transcription factor NF-kappaB and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood 2002; 100: 1828–1834. [DOI] [PubMed] [Google Scholar]
- 51.Kim YS, Kim JS, Kwon JS, et al. Bay 11-7082, a nuclear factor-κB inhibitor, reduces inflammation and apoptosis in a rat cardiac ischemia-reperfusion injury model. Int Heart J 2010; 51: 348–353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231212378 for Post-stroke brain can be protected by modulating the lncRNA FosDT by Suresh L Mehta, Bharath Chelluboina, Kahlilia C Morris-Blanco, Saivenkateshkomal Bathula, Soomin Jeong, Vijay Arruri, Charles K Davis and Raghu Vemuganti in Journal of Cerebral Blood Flow & Metabolism