Abstract
Objective:
Primary open-angle glaucoma (POAG) is a degenerative optic neuropathy disease which has somewhat similar pathophysiology to Alzheimer’s disease (AD). This study aims to determine the presence of medial temporal atrophy and parietal lobe atrophy in patients with POAG compared to normal controls using medial temporal atrophy (MTA) scoring and posterior cortical atrophy (PCA) scoring system on T1 magnetization-prepared rapid gradient-echo.
Methods:
50 POAG patients and 50 normal subjects were recruited and an MRI brain with T1-magnetization-prepared rapid gradient-echo was performed. Medial temporal lobe and parietal lobe atrophy were by MTA and PCA/Koedam scoring. The score of the PCA and MTA were compared between the POAG group and the controls.
Results:
There was a significant statistical difference between PCA score in POAG and the healthy control group (p-value = 0.026). There is no statistical difference between MTA score in POAG compared to the healthy control group (p-value = 0.58).
Conclusion:
This study suggests a correlation between POAG and PCA score. Potential application of this scoring method in clinical diagnosis and monitoring of POAG patients.
Advances in knowledge:
The scoring method used in AD may also be applied in the diagnosis and monitoring of POAG
MRI brain, specifically rapid volumetric T1 spoiled gradient echo sequence, may be applied in POAG assessment.
Introduction
Primary open-angle glaucoma (POAG) is a degenerative optic neuropathy disease and chronic in nature, characterized by concurrent loss of retinal ganglion cells and their axons, resulting in optic nerve cupping and visual field loss. Visual impairment caused by glaucoma is irreversible, and early detection is paramount. POAG detection can be challenging as it tends to be asymptomatic before advanced visual field loss. 1 Glaucoma is thought to be primarily related to outflow impairment in the anterior segment of the eye globe, which causes an increased intraocular pressure (IOP) that results in secondary optic nerve damage in the posterior segment. However, it seems that the development of glaucoma is not just restricted to elevated IOP and may occur in patients with moderate and low IOP. 2 In addition, it has been observed that the optic nerve damage continues to progress despite treatment of IOP reduction in patients with POAG. 3,4 Hence the cause of POAG is more complex than a mere increase in IOP, and more evidence suggest that POAG is indeed a primary neurodegenerative process. 4,5
The underlying pathophysiological process of the development of POAG is somewhat similar to Alzheimer’s disease (AD) in many ways. 6–10 There is active research on establishing links between POAG and AD at this juncture. Various studies have shown the increased comorbidity between glaucoma and AD, 11,12 which led some to suggest that glaucoma is another type of ‘ocular AD’ 13 and others to describe AD as 'cerebral glaucoma’. 14 A significant amount of epidemiological, animal study, and immunohistochemically data support the link between the two entities. However, limited exploration has been done using structural brain imaging as a research tool on POAG to link it to AD.
It is an established fact that brain atrophy detected on neuroimaging is the hallmark of AD. Parietal lobe and temporal lobe atrophies, in particular, are observed in the early stage of AD. Posterior cortical atrophy (PCA) or Koedam score and medial temporal atrophy (MTA) score are established scoring systems used to assess parietal lobe and temporal lobe atrophies.
To the best of our knowledge, no studies have been done specifically to investigate the parietal lobe atrophy and medial temporal lobe atrophy among patients with POAG, which by proving this may strengthen the evidence that POAG and AD are of the same entities. If similar changes in neuroimaging are observed in POAG, it may potentially be used as an imaging biomarker for clinical diagnosis, potentially influencing the current approach to treat patients with POAG. The specific objective of the study was to determine the presence of medial temporal atrophy, and parietal lobe atrophy on patients with POAG compared to normal controls using MTA scoring and PCA scoring system on T1-magnetization-prepared rapid gradient-echo (MPRAGE).
Methods
Sample recruitment
This study was conducted at UiTM Medical Specialist Centre, Selangor, involving 50 patients diagnosed with POAG and 50 healthy subjects. The patients were recruited via convenient sampling through the clinics. All subjects gave written consent. To exclude patients with mild cognitive impairment or symptomatic AD, all patients should not have had any significant complaint of any problems with memory or cognition. The inclusion criteria include age ≥30 years old, Mini Mental State Examination (MMSE) score ≥28, and free from any confirmed past and current medical history of ischaemic heart disease, heart failure, stroke, and central neurodegenerative diseases. The institutional research ethics committee approved the study.
Glaucoma evaluation
An ophthalmologist screened all patients prior to recruitment, including slit lamp exam, gonioscopy, intraocular pressure measurement using Goldmann applanation tonometry, and optic nerve Optical Coherence Tomography images. All patients then underwent Humphrey Matrix Visual Field test (Carl Zeiss Meditec, Jena, Germany). POAG diagnosis and subsequent treatment were made based on the Malaysia Clinical Practice Guidelines Second edition (June 2017). All recruited POAG patients received the standard of care throughout this study.
MRI data acquisition
Magnetic resonance images were acquired in a 1.5 Tesla (Siemens Healthcare AG, Erlangen, Germany) MAGNETOM AERA scanner using a 20-channel phased-array head coil as a magnetic resonance signal receiver. Participants were positioned in lying position. T1-weighted 3D MPRAGE was performed in the axial plane with 1 mm thickness covering the whole brain. Sequence parameters used for MP-RAGE were TR/TE = 1900/2.67 ms, a field of view of 240 × 240 x 170, data matrix of 256 × 256×176, and isotropic voxel size of 1 × 1 x 1 mm. 3D MP-RAGE protocol of 1 mm slice thickness is chosen in this study because of its capability for multiplanar reconstructions (MPR) into other planes without diminishing the resolution. The MRI examination is performed within 1 month from the glaucoma evaluation date, with an average treatment duration of 2 weeks when imaging is performed.
Image processing and evaluation
Image assessment on each sample was made based on a consensus of the two radiologists (both 8 years in experience) who were blinded to clinical information and diagnosis. The assessment was made in a research setting.
Image interpretation was made on Siemens Syngo software (Siemens Healthcare AG, Erlangen, Germany). The evaluations of atrophy were done on PCA and MTA scorings and the optic nerve diameter (Figure 1). The visual rating for the PCA/Koedam scoring system is based on the following anatomical landmark in three different orientation images reconstructed from T1 MPRAGE on sagittal, axial, and coronal views. The posterior lobe atrophy is graded using the 4-point rating scale; Grade 0 = no atrophy; Grade 1 = mild widening of the sulci without evident volume loss of the gyri; Grade 2 = substantial widening of the sulci and volume loss of the gyri; Grade 3 = severe end-stage atrophy (Figure 2). On the other hand, the MTA score was performed using a coronal image reconstructed by MPR. It is an assessment of the hippocampus at the level of the anterior pons. Scoring was made based on three features: 1) width of the choroid fissure, 2) width of the temporal horn of the lateral ventricle, and 3) height of the hippocampus. The results in a score of 0 to 4. Grade 0 = no cerebrospinla fluid (CSF) is visible around the hippocampus, Grade 1: choroid fissure is slightly widened, Grade 2: moderate widening of the choroid fissure, mild enlargement of the temporal horn and mild loss of hippocampal height, Grade 3: marked widening of the choroid fissure, moderate enlargement of the temporal horn, and moderate loss of hippocampal height, Grade 4: marked widening of the choroid fissure, marked enlargement of the temporal horn, and the hippocampus is markedly atrophied, and internal structure is lost. Lastly, the optic nerve was assessed, and measurements were made on coronal images of the ON at 10 mm behind the eye globes using Siemens Syngo software. The measurements include the optic nerve’s surface area (SA) with and without the sheath.
Figure 1.
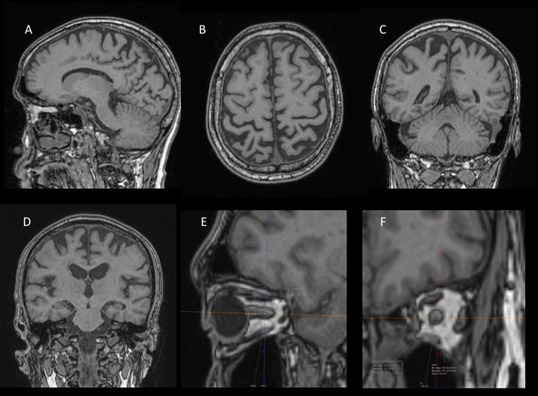
Selected T1-MPRAGE images demonstrating sagittal (A), axial (B), and coronal (C) images used for PCA scoring, selected coronal image (D) for MTA scoring, and focused images (E & F) for optic nerve surface area measurement. The above images show PCA scoring of 2 and MTA score of 0. MPRAGE, magnetization-prepared rapid gradient-echo; MTA, medial temporal atrophy; PCA, posterior cortical atrophy.
Figure 2.
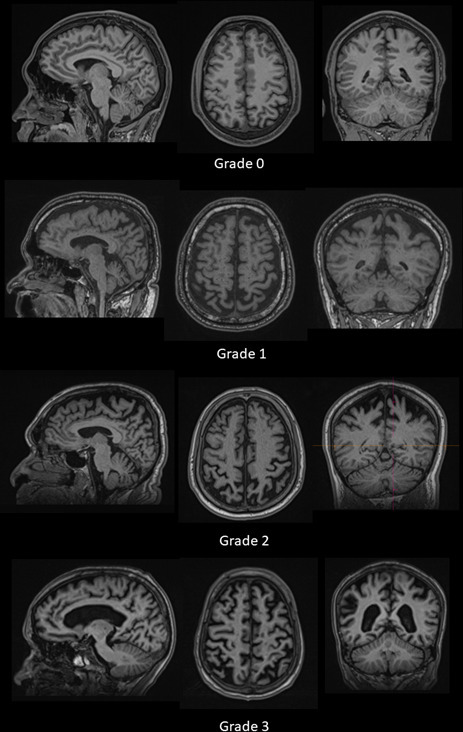
Example cases of the MRI brain in sagittal, axial, and coronal views with grading as per PCA/ Koedam scoring system. PCA, posterior cortical atrophy.
Statistical analysis
The score of the PCA and MTA were tabulated and compared between the POAG group and the controls. As to compare the median differences of PCA and MTA between POAG patients and the control group, the data were analyzed using the Mann–Whitney Wilcoxon Test. Spearman’s Rank Correlation method was further performed to determine the effect of demographic factors and basic clinical information of patients with POAG. The mean difference of the optic nerve surface area are compared between the two groups using t-test analysis. p-value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS v. 26.0.
Results
A total 50 POAG patients and normal subjects (control patients) with the age range of (62.44 9.83) and (55.63 9.94) respectively, were evaluated. Out of the total 100 patients, most are in the age range of 51–80 years old (controls (67%), cases (92%)).
The demographic details comparing the two groups of the sample were summarized in Table 1. It showed no significant difference between the two groups in terms of the demographic aspects and basic clinical information of smoking history, hypertension, dyslipidemia, and diabetes
Table 1.
Sociodemographic information including history of smoking, hypertension, dyslipidaemia and diabetes mellitus of both patients with POAG and healthy controls
| VARIABLE | POAG (%) | HEALTHY CONTROL (%) | p-VALUE |
|---|---|---|---|
| Gender | |||
| Male | 26 (52%) | 28 (56%) | |
| Female | 24 (48%) | 22 (44%) | 0.69 |
| Mean age ± SD | 62.44 ± 9.83 | 55.62 ± 9.94 | |
| Race | |||
| Malay | 24 (48%) | 33 (66%) | |
| Chinese | 14 (28%) | 10 (20%) | |
| Indian | 12 (24%) | 7 (14%) | 0.068 |
| Smoking | |||
| Yes | 3 (6%) | 8 (16%) | |
| No | 47 (94%) | 42 (84%) | 0.112 |
| Hypertension | |||
| Yes | 19 (38%) | 23 (46%) | |
| No | 31 (62%) | 27 (54%) | 0.42 |
| Dyslipidemia | |||
| Yes | 19 (38%) | 26 (56%) | |
| No | 31 (62%) | 24 (48%) | 0.162 |
| Diabetic mellitus | |||
| Yes | 16 (32%) | 21 (42%) | |
| No | 34 (68%) | 29 (58%) | 0.303 |
POAG, primary open-angle glaucoma; SD, standard deviation.
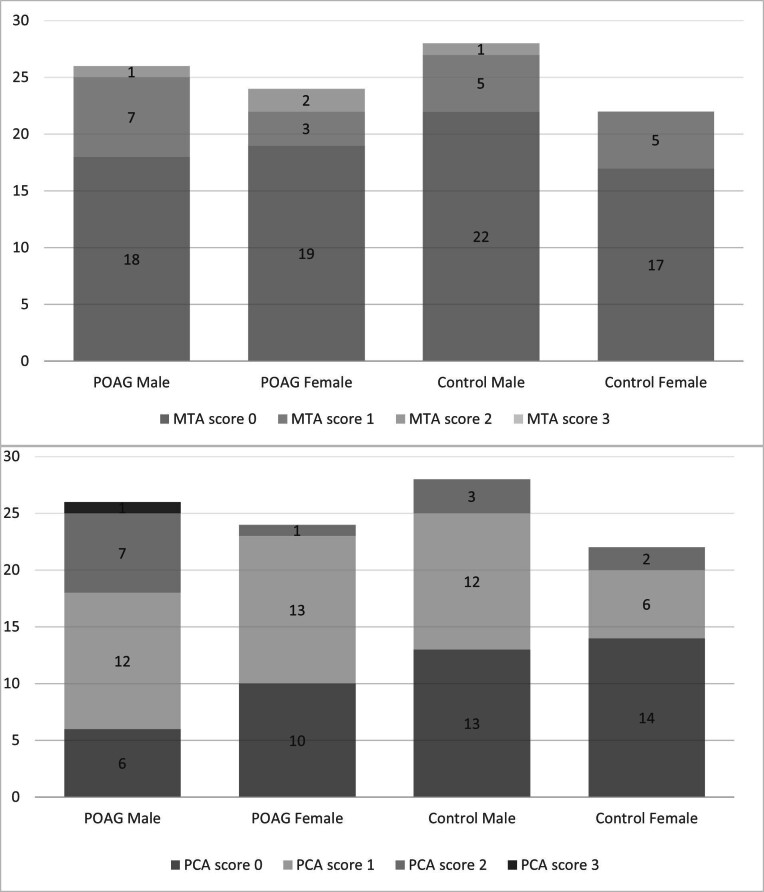
Most patients with POAG had PCA scores of 1 followed by 0, 2 and 3. Whereas for normal subjects, most had PCA scores of 0, followed by 1, and 2 (Figure 3). None of the two groups score 4. PCA scores of 3 are only observed in POAG patients with age group >50 years old. There is no significant correlation between the PCA score and basic clinical information (smoking history, hypertension, diabetes, and dyslipidemia). The median value of PCA score was 1.00 (±0.75) for POAG and 0.00 (±0.67) for healthy control. Therefore, on average, the POAG patients score 1 for PCA and 0 for control subjects. In addition, there was a statistical difference in PCA score between POAG and the healthy control group (p-value = 0.026).
Figure 3.
Bar chart showing PCA and MTA scores of patients with POAG vs normal controls according to gender. MTA, medial temporal atrophy; PCA, posterior cortical atrophy; POAG, primary open-angle glaucoma.
On the other hand, for MTA score, most patients with POAG and normal controls score 0 followed by 1 and 2, as shown in Figure 3. None of the two groups score 3. Score of 2 is only seen in age group >50 years old. The median value of MTA score was 0 for both POAG and healthy control with standard deviation of 0.59 and 0.48, respectively. There was no statistical difference in the MTA score between POAG and the healthy control group (p-value = 0.58) (Table 2). In addition, there is no correlation between the MTA score and demographic variables and the basic clinical information.
Table 2.
Median of the PCA and MTA scores (±SD, standard deviation) as well as the mean (±SD) of SA of the ONs with and without sheath
| POAG | CONTROL | p-value | |
|---|---|---|---|
| Median PCA score | 1 ± 0.75 | 0 ± 0.67 | 0.026 |
| Median MTA score | 0 ± 0.59 | 0 ± 0.48 | 0.58 |
| SA of ON without sheath (average) | 5.12 ± 1.60 | 5.73 ± 1.40 | 0.046 |
| SA of ON with sheath | 20.86 ± 4.52 | 19.43 ± 3.98 | 0.096 |
MTA, medial temporal atrophy; ON, optic nerve; PCA, posterior cortical atrophy; POAG, primary open-angle glaucoma; SA, surface area; SD, standard deviation.
The mean values of the IOP of the right and left eyes of patients with POAG were 14.04 ± 3.22 and 13.76 ± 3.45, respectively. While the mean values of IOP of the right and left eyes of the normal subjects were 13.27 ± 4.58 and 13.32 ± 4.72, respectively. It is important to note that the IOP for patients with POAG in this study were not the baseline status before treatment. On average, the SA of the optic nerve contributed to differences in glaucoma prevalence significantly (p = 0.046). This was indeed an important finding confirming the POAG groups had some degree of optic nerve atrophy when compared to the control group. Expectedly, when the sheath is included (i.e. measurement was inclusive of the surrounding CSF), no significant differences were seen between the two groups.
Discussion
Most of the established methods of glaucoma assessment with regards to neuroimaging are confined to the visual pathway. The optic nerve consists of white matter tissues surrounded by fat when located at the intraorbital segment. Fat produces hypersignal intensity, whereas optic nerve produces low signal intensities on T 2 weighted images, which makes the optic nerves easily distinguishable in MRI. 15 Quantification of the optic nerve was facilitated by the excellent contrast of the nerve against its surroundings on MRI. 16
Previous effort to measure optic nerve diameter has been made using imaging modalities, namely the ultrasound, CT scan, and MRI, confirming optic nerve thinning. 15,17–19 Likewise, our subanalysis of the optic nerve in the form of SA revealed significantly lower size indicating atrophy in patients with POAG when compared to normal controls. The right and left optic nerve mean SA were 5.17 ± 1.61 mm and 5.07 ± 1.72 mm, respectively. On T1-MPRAGE, the optic nerve appears iso-intense to the brain parenchyma and is surrounded by hypointense CSF fluid within the optic nerve sheath. The contrast of signal intensities between the optic nerve and CSF allowed for adequate assessment and measurement.
Many researchers had proposed that the medial temporal lobe was an early site of pathological involvement in AD. 20 Structural neuroimaging offers great potential in discriminating AD from other type of dementia. 21 By evaluating the medial temporal lobe atrophy using MRI, it has an excellent capability to differentiate AD from dementia with Lewy body and vascular cognitive impairment. 21 Medial temporal lobe atrophy improved the ability to detect patients at high risk for Alzheimer’s type dementia among those with minor cognitive impairment when combined with age and memory function. 22 Various techniques of structural neuroimaging for medial temporal lobe atrophy using MRI are available - namely, volumetric assessment, linear assessment, and visual qualitative rating. 23 Visual assessment is much less time-consuming than volumetry and easily applicable in clinical practice, with an acceptable within and between rater reliability. 24
In addition to MTA being a feature of AD, prominent atrophy in the brain’s posterior (parietal lobe) regions is also frequently found, particularly the precuneus and posterior cingulate gyrus 25,26 . Typical and atypical AD presentations were characterized with prominent posterior atrophy (PA) 27 . The unique metabolic, connectivity, and vascular features of the posterior cingulate cortex and precuneus predispose a risk of neurodegenerative processes 28 . Furthermore, in a subset of patients with pathologically proven AD, PA may be present in the absence of marked atrophy in the medial temporal lobes. A review by Jacobs et al 29 also suggested using MRI to demonstrate the precuneus and posterior cingulate gyrus as areas most relevant in the parietal lobe for the early detection of AD. This is likely why PCA score was found to be significantly different between POAG and normal subjects in our study. This was in line with Wang et al, who reported brain morphological alteration reflecting glaucoma severity. 30
Visual rating method for detection of atrophy shown to be a quick and reproducible in a clinical setting, compared to more labor intensive techniques like automated volumetric analysis 27 . Among several imaging methods used to determine specific areas in the brain, observation of the posterior cingulate cortex and parietal sulci using MRI images on the sagittal plane has been suggested to be the most practical approach to demonstrate structural brain changes in the parietal lobe 31,32 . Furthermore, measurements taken of the MRI images with a widening of the marginal part of the cingulate sulcus or posterior cingulate sulcus and the parieto-occipital sulci relative to surrounding sulci have been proposed be an indication of volume loss of the underlying parietal lobe 31 . The measurement of parietal lobe atrophy using this visual rating scale adds value to the discrimination of AD from controls and other dementias on MRI. 27
To the best of our knowledge, studies looking at brain atrophy in specific locations such as temporal and parietal lobes concerning POAG have never been performed before. This semi-quantitative visual scoring is preferred in the current study due to its simplicity and its potential straightforward application on day-to-day reporting. T1-MPRAGE or its equivalent is usually readily available sequence or technique to any purchased MRI machine. Whereas advanced technique measuring volume, such as voxel-based monometry (VBM), is not readily available and are mainly obtained and used in established research centers. The focus of atrophy on the medial temporal lobe and parietal lobe using visual scoring methods for the patient with POAG in our study is based on the postulation of a possible linkage between POAG and AD. Early AD shows increased MTA and PCA scores before the atrophic changes become generalized in advanced cases.
This study showed no significant difference in MTA scores between the two groups (p-value = 0.58). The early process of diseases may not show significant volume loss and may not be well detected visually. There is still a possibility that early neurodegenerative processes are also present in medial temporal in patients with POAG but are too minimal to cause any discriminating choroidal fissure and temporal horn widening, the fundamental basis used in the visual MTA scoring system.
A whole-brain approach looking at brain atrophy using VBM was used in a previous study on patients with POAG. Zikou et al used a conventional VBM and diffusion tension imaging and examined the visual pathway in patients with POAG who showed a thinning of the left temporal lobe. 33 They concluded that neurodegenerative changes beyond the optic pathway could be found in patients with POAG. However, a more focused study on temporal and parietal lobes should be performed given that they are the primary locations of early degenerative processes in patients with AD. A whole-brain approach such as VBM may have limitations on this aspect.
It is pertinent to note; however, there are published articles that suggested no correlation between AD and POAG, 34,35 or if a correlation exists, it may be due to chance. 36 Hence, it is presumptuous to assume direct association from our study alone. More research is needed, especially at the molecular or genetics level, to investigate if any direct linkages present between AD and POAG.
Our study has several limitations. Firstly, other confounding factors that were not strictly excluded could potentially cause brain atrophy and hence influence the reading. Diseases such as epilepsy, certain drug toxicity, alcohol consumption, previous infection or insult that may cause cerebral atrophy to some degree. Secondly, MMSE was used to exclude patients with cognitive impairment particularly those who potentially has an underlying early AD. MMSE is a test of global mental status. 37 It has a pooled sensitivity of 79.8%, a specificity of 81.3%, a positive-predictive value of 86.3%, and a negative-predictive value of 73.0%. 38 Other tests such as the Isaacs Set Test for verbal fluency and semantic memory, the Free and Cued Selective Reminding Test for verbal episodic memory could have been added to improve the accuracy. 39 Thirdly, although MTA and PCA scoring is a validated semi-quantitative technique to assess for brain atrophy in specific areas, they are still subjected to the individual performance of interpretation and errors. It is likely to be less reproducible than the full volumetric quantitative method, comparatively. Another limitation is that the IOP measured in study participants may not be the baseline as in some cases, as treatment has already started prior to the MRI examination. Lastly, we did not recruit the AD population for direct comparison with the POAG population. Hence, disease matched correlation and comparison cannot be established.
Other advanced imaging modalities that can be used to specifically explore the medial temporal lobe and parietal lobe for patients with POAG are diffusion tensor imaging with functional tractography, which primarily assesses the neuronal integrity and microstructure detailing the diffusivity and anisotropy of water molecules. The prime anatomical region of investigation for DTI in POAG patients was the optic nerve, with result showing an increase in radial diffusivity but a reduction of fractional anisotropy. 40,41 Studies on patients with POAG specifically looking at the parietal lobe and medial temporal lobes are limited; hence would be an interesting area of research.
Conclusion
Our data showed a significant difference in PCA scoring between POAG and healthy patients using a rapid 3D T1 spoiled gradient echo sequence compared with normal subjects. However, no significant association between MTA and POAG. Hence, a potential relationship may exist between AD and POAG to a certain extent, which is unlikely causal. However, more research is needed to investigate further the association between the two diseases and MRI brain usage in POAG.
Footnotes
Acknowledgements: The authors are thankful to Sushil Kumar, Norhafni Razali, Iskandar Supardi, Basri Saidi and Nur Ismaliza Ismail for thier assistance in data collection and provision of expertise that greatly assisted the reasearch.
Funding: Research Development Grant Scheme; 600-RMI/RAGS 5/3 (58/2014)
Patient consent: Participants' consent was obtained prior to enrolment into the study.
Ethics approval: Approved by the Institutional Review Board (UiTM Research Ethics Committee)
Contributors: All authors have contributed equally to the study and manuscript preparation. Hazlenah Hanafiah performed the statistical analysis. Bushra Johari and Nazimah Ab Mumin prepared the figures.
Contributor Information
Mohammad Hanafiah, Email: mhanafiah8804@gmail.com, Department of Radiology, Sunway Medical Centre, Selangor, Malaysia .
Bushra Johari, Email: bushra0271@yahoo.com, Department of Radiology, Universiti Teknologi MARA, Sungai Buloh, Selangor, Malaysia .
Nazimah Ab Mumin, Email: nazimah_mumin@uitm.edu.my, Department of Radiology, Universiti Teknologi MARA, Sungai Buloh, Selangor, Malaysia .
Azlan Azha Musa, Email: azlanazha@uitm.edu.my, Department of Ophtalmology, Universiti Teknologi MARA, Sungai Buloh, Selangor, Malaysia .
Hazlenah Hanafiah, Email: hazlenahh@uitm.edu.my, Statistics Unit, Universiti Teknologi MARA Sabah Branch, Kota Kinabalu Campus, Kota Kinabalu, Malaysia .
REFERENCES
- 1. . Allingham RR, Damji KF, Freedman SF, Moroi SE, Rhee DJ, Shields MB. Shields textbook of glaucoma : Lippincott Williams & Wilkins; ; 2012. . [Google Scholar]
- 2. Wostyn P, Audenaert K, De Deyn PP . Alzheimer’s disease-related changes in diseases characterized by elevation of intracranial or intraocular pressure . Clin Neurol Neurosurg 2008. ; 110: 101 – 9 . doi: 10.1016/j.clineuro.2007.10.011 [DOI] [PubMed] [Google Scholar]
- 3. Fechtner RD, Weinreb RN . Mechanisms of optic nerve damage in primary open angle glaucoma . Surv Ophthalmol 1994. ; 39: 23 – 42 . doi: 10.1016/s0039-6257(05)80042-6 [DOI] [PubMed] [Google Scholar]
- 4. Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, et al. . Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial . Arch Ophthalmol 2002. ; 120: 1268 – 79 . doi: 10.1001/archopht.120.10.1268 [DOI] [PubMed] [Google Scholar]
- 5. Klein BE, Klein R, Sponsel WE, Franke T, Cantor LB, et al. . Prevalence of glaucoma. the beaver dam eye study . Ophthalmology 1992. ; 99: 1499 – 1504 . doi: 10.1016/s0161-6420(92)31774-9 [DOI] [PubMed] [Google Scholar]
- 6. Guo L, Duggan J, Cordeiro MF . Alzheimer’s disease and retinal neurodegeneration . Curr Alzheimer Res 2010. ; 7: 3 – 14 . doi: 10.2174/156720510790274491 [DOI] [PubMed] [Google Scholar]
- 7. Guo L, Salt TE, Luong V, Wood N, Cheung W, et al. . Targeting amyloid-beta in glaucoma treatment . Proc Natl Acad Sci U S A 2007. ; 104: 13444 – 49 . doi: 10.1073/pnas.0703707104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kountouras J, Zavos C, Gavalas E, Boziki M, Chatzopoulos D, et al. . Normal-tension glaucoma and alzheimer’s disease: helicobacter pylori as a possible common underlying risk factor . Med Hypotheses 2007. ; 68: 228 – 29 . doi: 10.1016/j.mehy.2006.07.008 [DOI] [PubMed] [Google Scholar]
- 9. Parisi V . Correlation between morphological and functional retinal impairment in patients affected by ocular hypertension, glaucoma, demyelinating optic neuritis and alzheimer’s disease . Seminars in Ophthalmology 2003. ; 18: 50 – 57 . doi: 10.1080/08820530390897855 [DOI] [PubMed] [Google Scholar]
- 10. Wostyn P, Audenaert K, De Deyn PP . Alzheimer’s disease and glaucoma: is there a causal relationship? Br J Ophthalmol 2009. ; 93: 1557 – 59 . doi: 10.1136/bjo.2008.148064 [DOI] [PubMed] [Google Scholar]
- 11. Bayer AU, Ferrari F, Erb C . High occurrence rate of glaucoma among patients with alzheimer’s disease . Eur Neurol 2002. ; 47: 165 – 68 . doi: 10.1159/000047976 [DOI] [PubMed] [Google Scholar]
- 12. Tamura H, Kawakami H, Kanamoto T, Kato T, Yokoyama T, et al. . High frequency of open-angle glaucoma in japanese patients with alzheimer’s disease . J Neurol Sci 2006. ; 246: 79 – 83 . doi: 10.1016/j.jns.2006.02.009 [DOI] [PubMed] [Google Scholar]
- 13. McKinnon SJ . Glaucoma: ocular alzheimer’s disease? Front Biosci 2003. ; 8: s1140 - 56 . doi: 10.2741/1172 [DOI] [PubMed] [Google Scholar]
- 14. Wostyn P, Audenaert K, De Deyn PP . Alzheimer’s disease: cerebral glaucoma? Med Hypotheses 2010. ; 74: 973 – 77 . doi: 10.1016/j.mehy.2009.12.019 [DOI] [PubMed] [Google Scholar]
- 15. Kashiwagi K, Okubo T, Tsukahara S . Association of magnetic resonance imaging of anterior optic pathway with glaucomatous visual field damage and optic disc cupping . J Glaucoma 2004. ; 13: 189 – 95 . doi: 10.1097/00061198-200406000-00003 [DOI] [PubMed] [Google Scholar]
- 16. Chen J, Bushman FD, Lewis JD, Wu GD, Li H . Structure-constrained sparse canonical correlation analysis with an application to microbiome data analysis . Biostatistics 2013. ; 14: 244 – 58 . doi: 10.1093/biostatistics/kxs038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boles Carenini B, Tettoni E, Brogliatti B . CT and a echography of optic nerve in glaucoma . Acta Ophthalmol Scand 2002. ; 80: 40 – 41 . doi: 10.1034/j.1600-0420.80.s236.23.x [DOI] [PubMed] [Google Scholar]
- 18. Dichtl A, Jonas JB . Echographic measurement of optic nerve thickness correlated with neuroretinal rim area and visual field defect in glaucoma . Am J Ophthalmol 1996. ; 122: 514 – 19 . doi: 10.1016/s0002-9394(14)72111-7 [DOI] [PubMed] [Google Scholar]
- 19. Beatty S, Good PA, McLaughlin J, O’Neill EC . Echographic measurements of the retrobulbar optic nerve in normal and glaucomatous eyes . Br J Ophthalmol 1998. ; 82: 43 – 47 . doi: 10.1136/bjo.82.1.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, et al. . Atrophy of medial temporal lobes on mri in “probable” alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates . J Neurol Neurosurg Psychiatry 1992. ; 55: 967 – 72 . doi: 10.1136/jnnp.55.10.967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burton EJ, Barber R, Mukaetova-Ladinska EB, Robson J, Perry RH, et al. . Medial temporal lobe atrophy on mri differentiates alzheimer’s disease from dementia with lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis . Brain 2009. ; 132: 195 – 203 . doi: 10.1093/brain/awn298 [DOI] [PubMed] [Google Scholar]
- 22. Visser PJ, Verhey FRJ, Hofman PAM, Scheltens P, Jolles J . Medial temporal lobe atrophy predicts alzheimer’s disease in patients with minor cognitive impairment . J Neurol Neurosurg Psychiatry 2002. ; 72: 491 – 97 . doi: 10.1136/jnnp.72.4.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wahlund LO . Magnetic resonance imaging and computed tomography in alzheimer’s disease . Acta Neurol Scand 1996. ; 94: 50 – 53 . doi: 10.1111/j.1600-0404.1996.tb00373.x [DOI] [PubMed] [Google Scholar]
- 24. Wahlund L-O, Julin P, Lindqvist J, Scheltens P . Visual assessment of medical temporal lobe atrophy in demented and healthy control subjects: correlation with volumetry . Psychiatry Res 1999. ; 90: 193 – 99 . doi: 10.1016/s0925-4927(99)00016-5 [DOI] [PubMed] [Google Scholar]
- 25. Frisoni GB, Testa C, Sabattoli F, Beltramello A, Soininen H, et al. . Structural correlates of early and late onset alzheimer’s disease: voxel based morphometric study . J Neurol Neurosurg Psychiatry 2005. ; 76: 112 – 14 . doi: 10.1136/jnnp.2003.029876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galton CJ, Patterson K, Xuereb JH, Hodges JR . Atypical and typical presentations of alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases . Brain 2000. ; 123 Pt 3: 484 – 98 . doi: 10.1093/brain/123.3.484 [DOI] [PubMed] [Google Scholar]
- 27. Koedam ELGE, Lehmann M, van der Flier WM, Scheltens P, Pijnenburg YAL, et al. . Visual assessment of posterior atrophy development of a mri rating scale . Eur Radiol 2011. ; 21: 2618 – 25 . doi: 10.1007/s00330-011-2205-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKee AC, Au R, Cabral HJ, Kowall NW, Seshadri S, et al. . Visual association pathology in preclinical alzheimer disease . J Neuropathol Exp Neurol 2006. ; 65: 621 – 30 . doi: 10.1097/00005072-200606000-00010 [DOI] [PubMed] [Google Scholar]
- 29. Jacobs HIL, Van Boxtel MPJ, Jolles J, Verhey FRJ, Uylings HBM . Parietal cortex matters in alzheimer’s disease: an overview of structural, functional and metabolic findings . Neurosci Biobehav Rev 2012. ; 36: 297 – 309 . doi: 10.1016/j.neubiorev.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 30. Wang J, Li T, Sabel BA, Chen Z, Wen H, et al. . Structural brain alterations in primary open angle glaucoma: a 3t mri study . Sci Rep 2016. ; 6( 1 ): 18969 . doi: 10.1038/srep18969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barkhof F, Polvikoski TM, van Straaten ECW, Kalaria RN, Sulkava R, et al. . The significance of medial temporal lobe atrophy: a postmortem mri study in the very old . Neurology 2007. ; 69: 1521 – 27 . doi: 10.1212/01.wnl.0000277459.83543.99 [DOI] [PubMed] [Google Scholar]
- 32. Kirby E, Bandelow S, Hogervorst E . Visual impairment in alzheimer’s disease: a critical review . J Alzheimers Dis 2010. ; 21: 15 – 34 . doi: 10.3233/JAD-2010-080785 [DOI] [PubMed] [Google Scholar]
- 33. Zikou AK, Kitsos G, Tzarouchi LC, Astrakas L, Alexiou GA, et al. . Voxel-based morphometry and diffusion tensor imaging of the optic pathway in primary open-angle glaucoma: a preliminary study . AJNR Am J Neuroradiol 2012. ; 33: 128 – 34 . doi: 10.3174/ajnr.A2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ou Y, Grossman DS, Lee PP, Sloan FA . Glaucoma, alzheimer disease and other dementia: a longitudinal analysis . Ophthalmic Epidemiol 2012. ; 19: 285 – 92 . doi: 10.3109/09286586.2011.649228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kessing LV, Lopez AG, Andersen PK, Kessing SV . No increased risk of developing alzheimer disease in patients with glaucoma . J Glaucoma 2007. ; 16: 47 – 51 . doi: 10.1097/IJG.0b013e31802b3527 [DOI] [PubMed] [Google Scholar]
- 36. Keenan TDL, Goldacre R, Goldacre MJ . Associations between primary open angle glaucoma, alzheimer’s disease and vascular dementia: record linkage study . Br J Ophthalmol 2015. ; 99: 524 – 27 . doi: 10.1136/bjophthalmol-2014-305863 [DOI] [PubMed] [Google Scholar]
- 37. Folstein MF, Folstein SE, McHugh PR . “Mini-mental state”. a practical method for grading the cognitive state of patients for the clinician . J Psychiatr Res 1975; 12: 189–98. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 38. Mitchell AJ . A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment . J Psychiatr Res 2009. ; 43: 411 – 31 . doi: 10.1016/j.jpsychires.2008.04.014 [DOI] [PubMed] [Google Scholar]
- 39. Isaacs B, Kennie AT . The set test as an aid to the detection of dementia in old people . Br J Psychiatry 1973. ; 123: 467 – 70 . doi: 10.1192/bjp.123.4.467 [DOI] [PubMed] [Google Scholar]
- 40. Garaci FG, Bolacchi F, Cerulli A, Melis M, Spanò A, et al. . Optic nerve and optic radiation neurodegeneration in patients with glaucoma: in vivo analysis with 3-t diffusion-tensor mr imaging . Radiology 2009. ; 252: 496 – 501 . doi: 10.1148/radiol.2522081240 [DOI] [PubMed] [Google Scholar]
- 41. Hui ES, Q-l F, editors . ( n.d .). Diffusion tensor MR study of optic nerve degeneration in glaucoma . In : 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; IEEE . [DOI] [PubMed] [Google Scholar]