Abstract
Objectives:
Modern radiotherapy (RT) techniques require careful delineation of the target. There is no particular RT contouring guideline for patients receiving neoadjuvant chemotherapy (NACT). In this study, we examined the distribution of pre-chemotherapy clinically positive nodal metastases.
Methods:
We explored the coverage rate of the RTOG breast contouring guideline by deformable fusion of 18-fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) scan. We retrospectively evaluated neoadjuvant chemotherapy patients. All PET-CT images were imported into the planning software. We combined the planning CT and the CT images of PET-CT with rigid and then a deformable registration. We manually contoured positive lymph nodes on the CT component of the PET-CT data set and transferred them to planning CT after fusion. We evaluated whether previously contoured lymphatic CTVs, according to the RTOG breast atlas, include GTV-LNs.
Results:
All breast cancer patients between October 2018 and February 2021 were evaluated from the electronic database. There were 142 radiologically defined positive lymph nodes in 31 patients who were irradiated after NACT. Most LNs (70%) were in the level I axilla. Only 71.1% (n:101) of the whole lymph nodes in 10 patients were totally covered, 22.5% (n:32) partially covered and 6.4% %(n:9) totally undercovered.
Conclusions:
The extent of regional nodal areas in the RTOG atlas may be insufficient to cover positive lymph nodes adequately. For patients with nodal involvement undergoing neoadjuvant chemotherapy, PET-CT image fusions can be helpful to be sure that positive lymph nodes are in the treatment volume.
Advances in knowledge:
RTOG contouring atlas may be insufficient to cover all involved lymph nodes after NACT. For patients with nodal involvement undergoing neoadjuvant chemotherapy, PET-CT image fusions may help to be sure that positive lymph nodes are in the treatment volume.
Introduction
Traditional two-dimensional (2D) breast and regional nodal irradiation (RNI) plans treat large fields. Conformal techniques such as three-dimensional (3D), intensity-modulated radiotherapy (IMRT) require careful definition and delineation of the target. Suboptimal nodal coverage may lead to marginal failures. The considerable interobserver variation regarding the regional nodal region exists in breast radiotherapy. 1 Consensus groups have issued several contouring atlases to reduce interobserver variability and increase consistency. 2,3
The contouring atlases were designed for primarily surgically operated patients and intended to reproduce anatomic boundaries of areas historically included in 2D field-based RNI. 2,3 The current contouring volumes for regional lymph nodes are similar for both early-stage and locally advanced tumors. A specific radiotherapy contouring guideline for patients receiving NACT does not exist. In recent years, an essential body of data showed that ASTRO and ESTRO contouring atlases do not cover the entire lymphatic drainage system because a significant part of the LN recurrences occurs outside the recommended borders. 4,5 A profound understanding of the lymphatic metastases pattern and localization is essential. In one review, it was suggested that for patients with nodal involvement undergoing neoadjuvant chemotherapy, image fusion with the baseline PET-CT could be helpful to ensure adequate coverage of involved lymph nodes. 5
Image registration, whether rigid registration or deformable registration, is a frequent procedure used in the different parts of RT: contouring, planning, image segmentation, image guidance, treatment response assessment, re-planning, and plan adaptation. 6 Rigid registration uses only rigid translations and rotations between frames of reference, while deformable registration can provide a non-linear registration of each point in the images.
PET-CT is a standard component of initial staging in locally advanced breast cancer before NACT in our institution. In this study, we examined the distribution of pre-chemotherapy radiologically positive nodal metastases in PET-CT. We explored the coverage rate of the RTOG breast contouring guideline by deformable fusion of PET-CT scan.
Methods and materials
Our institutional ethics committee approved this study date on 01/18/2021 and numbered ASM-EK-21/137. We retrospectively reviewed our breast cancer database and identified regional nodal irradiated patients. The study cohort included only neoadjuvant chemotherapy patients whose pretreatment PET-CT is available. All patients had undergone breast and axillary ultrasound, PET-CT for the initial staging procedure. Breast magnetic resonance imaging is not routine and is done with the decision of breast tumor board for some patients. An experienced specialist in nuclear medicine analyzed all the PET-CT images. All nodes are discussed with nuclear medicine specialist with the help of breast imaging modalities. According to the radiological standards in axillary ultrasound or PET-CT, an ultrasound-guided lymph node biopsy was done if it is not clearly defined as positive or negative. All neoadjuvant treatment decisions were made on a multidisciplinary breast cancer board.
The target volume after NACT was whole breast or chest wall depending on the surgery, axilla level I-III and supraclavicular (SCV) lymph node region irradiation in all patients. Some of the patients had internal mammary node (IMN) irradiation but were excluded from analyses because of not the subject of this study. Depending on the treating physician, some of the patients underwent IMN irradiation but were not analyzed as it is not the subject of this study.
Planning CT images of patients immobilized in the supine position with their arms above their heads in a vacuum bed were obtained with a slice thickness of 2.5 mm on the Discovery RT (General Electric (GE) Healthcare, Milwaukee WI, USA). Treatment plans were created using the Radixact System (version X9, Accuray Inc., Sunnyvale CA, USA) with the Helical IMRT method in Precision Treatment Planning System (TPS) (version 2.0.0.1, Accuray Inc., Sunnyvale CA, USA). A field width of 2.5 cm, a pitch of 0.215, and a modulation factor between 2.3 and 2.8 were used in the planning. Some delineated dummy structures were marked as 'never' or 'exit only' to reduce bilateral lung and heart doses during optimization. The prescribed dose was 50 Gy in 25 fractions for all patients. Boost doses were 10–16 Gy in 5–8 fractions, depending on the patient’s age and tumor grade for breast-conserving surgery. All plans were delivered with the daily megavoltage CT scans (MVCT).
All PET-CT images were imported into the planning software (Eclipse 15.5, Varian Medical Systems, Palo Alto, CA). The planning CT and the CT images of PET-CT were first combined with rigid fusion using adjacent ribs and vertebrae as target structures. After that, a deformable registration was performed using regions of interest, defined by involved lymph node regions, including pectoralis minor muscle and ribs. SmartAdapt® module of the Eclipse was used for deformable registration. All fusions are done by a radiation oncologist (RO) (*.*.) and visually checked by another RO (*.*.) and a medical physicist (E.K.).
Previously contoured lymphatic CTV volumes are independently checked by an experienced RO (*.*.) for compliance with the RTOG breast atlas. PET-CT images and reports are revived and FDG avid lymph nodes were manually contoured as GTV-LN on the CT of the corresponding PET-CT data set and transferred to planning CT after fusion. It was evaluated whether lymphatic CTVs which were previously contoured according to the RTOG breast atlas, include GTV-LNs (Figure 1). If the whole GTV volume is within the lymphatic CTV this is determined as totally covered. In the presence of any volume coming out of lymphatic CTV, it is defined as partially covered and if it is completely outside it is defined as totally undercovered. The contouring of GTV-LNs was done by the same RO (*.*.) and the decision of coverage was made by different two ROs (*.*, *.*.) simultaneously and recorded. All steps are summarized in Figure 2.
Figure 1.
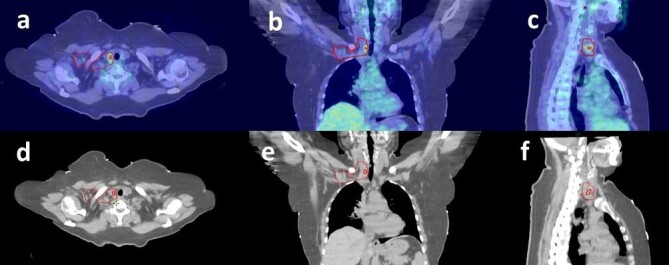
An example of previously contoured lymphatic CTV volumes and one supraclavicular lymph node after deformable registration in axial (a), coronal (b), and sagittal (c) images. Manually contoured GTV-LN on the CT of the corresponding PET-CT data set and transferred to the planning CT in axial (d), coronal (e), and sagittal (f) images.
Figure 2.
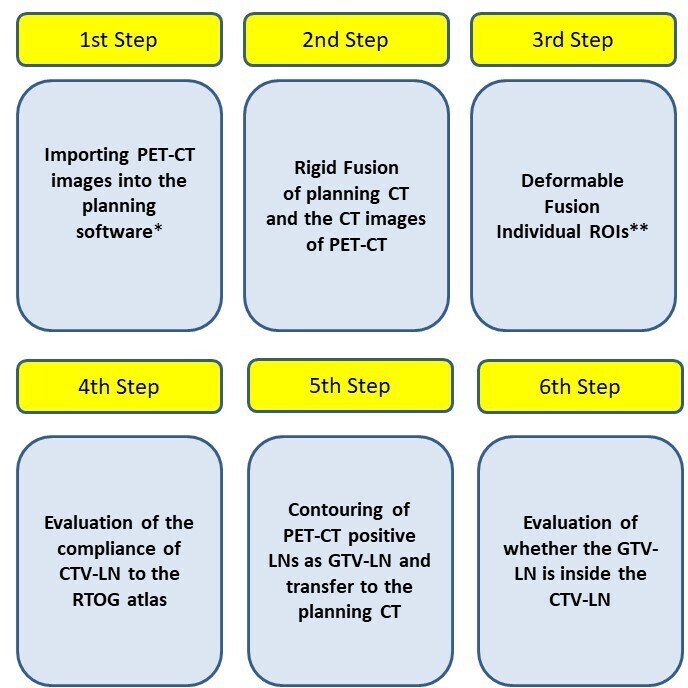
Schematic summary of study steps.
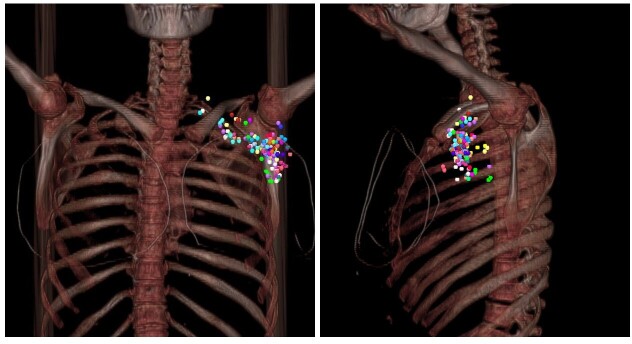
Additionally, for better visualization, all lymph nodes were mapped manually using CT imaging of a patient who had undergone left-sided breast conservation surgery and sentinel node biopsy (Figure 3). All lymph nodes were plotted on the left side of the representative patient to allow uniformity. A 5-mm-diameter circle was placed at the epicenter of each lymph node at the equivalent location on the reference images. The vasculature, ribs, pectoralis minor, and sternocleidomastoid muscle were used for orientation. The partially covered LNs spotted on the RTOG nodal CTV border regardless of the volume outside.
Figure 3.
Clinically positive lymph node distribution on digitally reconstructed radiographs (DRRs). Anteroposterior (AP) and lateral view. Each color represents a different patient.
Results
Between October 2018 and February 2021, 45 consecutive patients were treated with chest wall or breast and peripherical lymphatic irradiation after NACT. PET-CT data could not be obtained in 6 of 45 patients. Eight patients were excluded due to limited axillary irradiation and, 31 patients were eligible for further evaluation.
Patient and treatment details are summarized in Table 1. Median 4 2–5,7,8 lymph nodes were removed after sentinel lymph node biopsy (SLNB); 8 (5–15) after selective axillary dissection and 17 (7-41) after axillary dissection (AD). 7 out of 13 patients had an extracapsular extension (ECE) in residual lymph nodes. The arm positions were up in 29 PET-CT scans and down in two patients. Seventeen patients had seroma in the axilla due to surgery.
Table 1.
Patient and treatment characteristics
| Number | |
|---|---|
| Primary tumor irradiation | |
| Breast | 27 |
| Chest wall | 4 |
| Laterality | |
| Left | 13 |
| Right | 18 |
| Molecular subtype | |
| Luminal A | 1 |
| Luminal B | 19 |
| Her2 Type | 7 |
| Triple Negative | 4 |
| Axillary sampling before neoadjuvant treatment | |
| Yes | 15 |
| No | 16 |
| Surgical Axillary Intervention | |
| ALND: 14 | 14 |
| Selective ALND: 7 | 7 |
| SLNB: 10 | 10 |
| Lymph Node Status After Surgery | |
| ypN0 | 14 |
| ypN1 | 10 |
| ypN2 | 3 |
| pN1mi | 1 |
| pN0 (i+) | 3 |
ALND, Axillary lymph node dissection; SLNB, Sentinel lymph node biopsy.
There were 142 FDG avid positive lymph nodes in 31 patients. The geographic distribution of the nodes is listed in detail in Table 2. Most LNs (70%) were in the level I axilla. 71.1% (n:101) of the whole lymph nodes were totally covered, 22.5% (n:32) partially covered and 6.4% (n:9) totally undercovered. Detailed coverage percentage according to the lymph node region is listed in Table 3. All lymph nodes were inside the boundaries of the CTVs only in 10 patients. These patients had relatively low positive LNs median 2 (between 1 and 8) compared to missing coverage patients with a median of 5 (between 1 and 14) LNs.
Table 2.
Positive Lymph Node Distribution According to PET-CT Before Neoadjuvant Treatment
| LN location | Patients with Positive LNs (n) | Number of Positive LN (%) |
|---|---|---|
| Axillary-Level I | 29 | 98 (70) |
| Axillary-Level II | 12 | 30 (21.4) |
| Axillary-Level III | 5 | 9 (6.4) |
| Supraclavicular | 3 | 3 (2.2) |
LN, lymph node.
Table 3.
Lymph Node Coverage According to RTOG Breast Atlas
| Totally covered LNs (n) (%) | Partially covered LNs (n) (%) and geographical sites of undercoverage | Totally undercovered LNs (n) (%) and geographical sites of undercoverage | |||
|---|---|---|---|---|---|
| Axillary-Level 1 | 69 (70,4) | 21 (21,4) | five
anterior four anterolateral six posterolateral four posterior one lateral one medial |
8 (8,2) | four
posterior three posterolateral one caudal |
| Axillary-Level 2 | 23 (76,7) | 7 (23,3) | posteriomedial | 0 (0) | - |
| Axillary-Level 3 | 8 (88,9) | 1 (11,1) | cranial | 0 (0) | - |
| Supraclavicular | 1 (33,3) | 1 (33,3) | posterior | 1 (33,3) | posterior |
LN: lymph node.
Axillary level III had the highest coverage percentage with 88.9% of totally covered LNs and no totally undercovered LN. The most common geographical missing was seen in the lateral region in axillary levels I and II. Figure 4 shows LN locations relative to the surrounding structures and RTOG breast cancer atlas nodal target volume on condensed axial CT slices of a representative patient.
Figure 4.
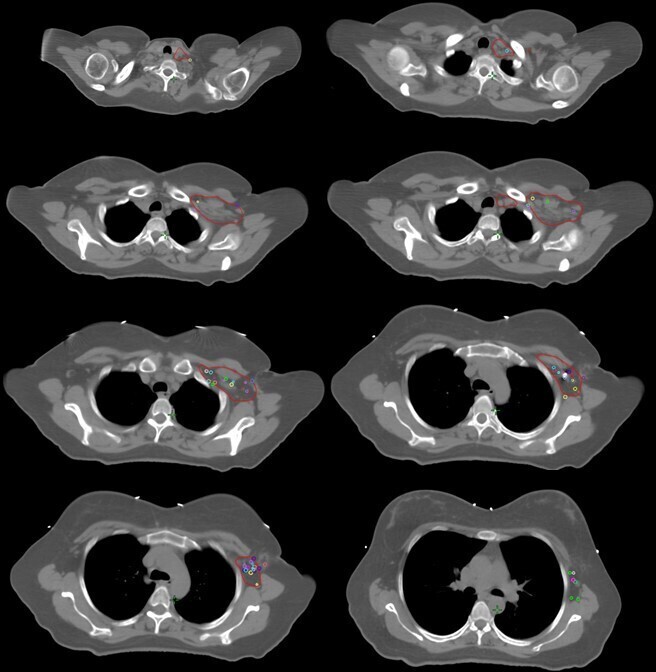
Condensed axial views of clinically positive lymph nodes (each color represents a different patient) and in relation with the Radiation Therapy Oncology Group (RTOG)- axillary level I-III and supraclavicular clinical target volume (CTV; red).
Twenty-four patients were alive without disease. One patient has died from COVID. Three of them lost in follow-up. Three patients developed metastasis. These patients who developed metastases have at least one partially covered or totally undercovered LNs.
Discussion
In the modern radiotherapy era, optimizing the target volume to balance adequate coverage of target structures and sparing normal tissue for potential toxicities is crucial. In light of current literature, it is not known that RT field best suits neoadjuvant systemic treatment in breast cancer patients. It is recommended to determine the adjuvant radiotherapy target volume after NACT according to the maximal disease stage at the time of diagnosis and pathology results after NACT. 9 However, specific radiotherapy contouring guideline for patients receiving NACT does not exist. Our study showed that the extent of regional nodal areas in the standard RTOG breast contouring atlas might be insufficient in covering initially positive lymph nodes.
In the patients with the complete nodal response after NACT, this undercoverage may be clinically negligible. Nevertheless, 13 out of 31 patients had residual disease in lymph nodes in our series, and more than half of the patients had ECE. It may be more critical for these patients to carefully identify the initial positive areas and make sure they are fully covered.
Borm and colleagues have found that lymph node metastasis patterns may differ with the extent of the disease. 10 Positive supraclavicular and internal mammary lymph nodes are more frequent in recurrent breast cancer than the primary disease. Furthermore, more importantly, RTOG and ESTRO atlases inadequately cover all these involved LNs. RTOG Atlas has more coverage than ESTRO atlas, especially in the supraclavicular region with more extension in the cranial border. 2,3 The authors in ESTRO guidelines mentioned that these atlases are not intended for cases with locally advanced disease, and delineation in these cases should be individualized. 3 In cases with pathological nodes in level 3, they suggest extending the cranial border of Level 4, which is contoured as SCV in RTOG with an additional 10–20 mm margin to the pathological node to define CTV.
NACT is used for locally advanced, inflammatory breast cancer, larger tumors, and unfavorable tumor profiles. These patients potentially have more extensive lymph node number, volume, and staging; therefore, anatomical distribution of these lymph nodes may differ from primary surgically treated breast cancer patients.
Clinical results are not the subject of this study, but we observed three metastases in the study cohort. All these patients have undercovered LNs. However, we cannot attribute these metastases directly to lymph node undercoverage because one has triple-negative histology, and complete response was seen in only one of them after NACT. These biological features play essential roles in disease nature. However, we should pay attention to covering all lymph nodes.
A more comprehensive Radiotherapy Comparative Effectiveness (RADCOMP) atlas was proposed in the RTOG 3509/3510 protocol RADCOMP trial, which investigated photon and proton irradiation in nonmetastatic breast cancer receiving RNI. 11 The main differences between these atlases are the posterior neck volume, which extends the posterolateral border of SCV. Posterior neck volume is created according to the recurrence patterns from the retrospective series. This atlas can better suit the patient with only one supraclavicular lymph node outside the nodal CTV volume.
In one study, Kowalski compared RADCOMP, RTOG, and ESTRO atlases according to the PET-CT positive lymph nodes dosimetric coverage rate. 12 All plans adequately covered low axillary lymph nodes. The RADCOMP-based VMAT and proton therapy plans provide improved coverage in high axillary, supraclavicular, and IMN regions. We did not look at the dosimetric impact of these under coverages in our trial. These undercovered LN areas may not be clinically meaningful after adding PTV margins to the nodal CTVs.
Image registration is a mathematical tool with limited or no biological information involved in the process. There are limitations in compensating for significant changes in pose and patients' anatomy after surgery. 13
The main limitation of this study is the retrospective nature with a limited patient and lymph node number, especially in the supraclavicular area. We excluded IMN and coverage of this area which is critical for this NACT receiving study population. We do not investigate the dose coverage of these undercovered LNs. After adding the PTV margin to the nodal CTVs, this undercoverage may become clinically meaningless. Also, we do not consider the impact of the nodal response to NACT and the extent of axillary surgery. It is not clear that nodal CTV should cover the whole GTV-LN in cases of ypN0 after ALND.
Regional nodal irradiation in the setting of involved lymph nodes can require more comprehensive treatment volumes. Trials (Alliance A011202 trial (ClinicalTrials.gov identifier: NCT01901094) 14 ; the NSABP B-51/RTOG 1304 trial (ClinicalTrials.gov identifier: NCT01872975) 15 are studying the optimal axillary surgery and radiotherapy after NACT. According to treatment response, molecular subtype, genetic information, and surgery type, personalized treatment fields are necessary.
Conclusion
It is not known that current atlases are enough to cover clinically node-positive lymph nodes after NACT. For patients with nodal involvement undergoing neoadjuvant chemotherapy, PET-CT image fusions may help to be sure that positive lymph nodes are in the treatment volume.
Contributor Information
Menekse Turna, Email: menekse.turna@gmail.com, Department of Radiation Oncology, Anadolu Medical Center, Gebze, Kocaeli, Turkey .
Rashad Rzazade, Email: rashadr84@gmail.com, Department of Radiation Oncology, Anadolu Medical Center, Gebze, Kocaeli, Turkey .
Mehmet Doğu Canoğlu, Email: dogu.canoglu@anadolusaglik.org, Department of Radiation Oncology, Anadolu Medical Center, Gebze, Kocaeli, Turkey .
Esra Küçükmorkoç, Email: ekmorkoc@gmail.com, Department of Radiation Oncology, Anadolu Medical Center, Gebze, Kocaeli, Turkey .
Nadir Küçük, Email: nadir.kucuk@anadolusaglik.org, Department of Radiation Oncology, Anadolu Medical Center, Gebze, Kocaeli, Turkey .
Hale Başak Çağlar, Email: hale.caglar@anadolusaglik.org, Department of Radiation Oncology, Anadolu Medical Center, Gebze, Kocaeli, Turkey .
REFERENCES
- 1. Li XA, Tai A, Arthur DW, Buchholz TA, Macdonald S, et al. . Variability of target and normal structure delineation for breast cancer radiotherapy: an rtog multi-institutional and multiobserver study . Int J Radiat Oncol Biol Phys 2009. ; 73: 944 – 51 . doi: 10.1016/j.ijrobp.2008.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. NRG Oncology Contouring Atlas, Templates and Tools. Breast Cancer Atlas . Internet]. c2021 . 2021. . Available from : https://www.nrgoncology.org/Portals/0/Scientific%20Program/CIRO/Atlases/BreastCancerAtlas_corr.pdf?ver=2018-04-18-144201-270
- 3. Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC, et al. . ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer . Radiother Oncol 2015. ; 114: 3 – 10 : S0167-8140(14)00524-6 . doi: 10.1016/j.radonc.2014.11.030 [DOI] [PubMed] [Google Scholar]
- 4. Gentile MS, Usman AA, Neuschler EI, Sathiaseelan V, Hayes JP, et al. . Contouring guidelines for the axillary lymph nodes for the delivery of radiation therapy in breast cancer: evaluation of the rtog breast cancer atlas . Int J Radiat Oncol Biol Phys 2015. ; 93: 257 – 65 : S0360-3016(15)00732-4 . doi: 10.1016/j.ijrobp.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 5. Loganadane G, Truong PT, Taghian AG, Tešanović D, Jiang M, et al. . Comparison of nodal target volume definition in breast cancer radiation therapy according to rtog versus estro atlases: a practical review from the transatlantic radiation oncology network (trone) . Int J Radiat Oncol Biol Phys 2020. ; 107: 437 – 48 : S0360-3016(20)31030-0 . doi: 10.1016/j.ijrobp.2020.04.012 [DOI] [PubMed] [Google Scholar]
- 6. Brock KK, Mutic S, McNutt TR, Li H, Kessler ML, et al. . Use of image registration and fusion algorithms and techniques in radiotherapy: report of the aapm radiation therapy committee task group no. 132 . Med Phys 2017. ; 44: e43 – 76 . doi: 10.1002/mp.12256 [DOI] [PubMed] [Google Scholar]
- 7. Chang JS, Byun HK, Kim JW, Kim KH, Lee J, et al. . Three-dimensional analysis of patterns of locoregional recurrence after treatment in breast cancer patients: validation of the estro consensus guideline on target volume . Radiother Oncol 2017. ; 122: 24 – 29 : S0167-8140(16)34358-4 . doi: 10.1016/j.radonc.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 8. Jing H, Wang S-L, Li J, Xue M, Xiong Z-K, et al. . Mapping patterns of ipsilateral supraclavicular nodal metastases in breast cancer: rethinking the clinical target volume for high-risk patients . Int J Radiat Oncol Biol Phys 2015. ; 93: 268 – 76 : S0360-3016(15)03169-7 . doi: 10.1016/j.ijrobp.2015.08.022 [DOI] [PubMed] [Google Scholar]
- 9. NCCN Clinical Practice Guidelines Breast Cancer. Version 3.2021-March 29,2021 . Internet]. c2021 . 2021. . Available from : https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- 10. Borm KJ, Voppichler J, Düsberg M, Oechsner M, Vag T, et al. . FDG/pet-ct-based lymph node atlas in breast cancer patients . Int J Radiat Oncol Biol Phys 2019. ; 103: 574 – 82 : S0360-3016(18)33541-7 . doi: 10.1016/j.ijrobp.2018.07.2025 [DOI] [PubMed] [Google Scholar]
- 11. . NRG Oncology RADCOMP Breast Atlas . [ Internet]. c2021 [cited 2021. July 27 ]. Available from: https://www.nrgoncology.org/Portals/0/Scientific%20Program/CIRO/Atlases/RADCOMP/RADCOMP%20Breast%20Atlas%20v.3%20-%20bigreduced.pdf?ver=2020-08-01-140849-360
- 12. Kowalski ES, Feigenberg SJ, Cohen J, Fellows Z, Vadnais P, et al. . Optimal target delineation and treatment techniques in the era of conformal photon and proton breast and regional nodal irradiation . Pract Radiat Oncol 2020. ; 10: 174 – 82 : S1879-8500(19)30359-5 . doi: 10.1016/j.prro.2019.11.010 [DOI] [PubMed] [Google Scholar]
- 13. Barber J, Yuen J, Jameson M, Schmidt L, Sykes J, et al. . Deforming to best practice: key considerations for deformable image registration in radiotherapy . J Med Radiat Sci 2020. ; 67: 318 – 32 . doi: 10.1002/jmrs.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trials C . A Randomized Phase III Clinical Trial Evaluating Post-Mastectomy Chestwall and Regional Nodal XRT and Post-Lumpectomy Regional Nodal XRT in Patients with Positive Axillary Nodes Before Neoadjuvant Chemotherapy Who Convert to Pathologically Negative Axillary Nodes After Neoadjuvant Chemotherapy . Internet]. c2021 . 2021. . Available from : https://clinicaltrials.gov/ct2/show/NCT01872975
- 15. Trials C . A Randomized Phase III Trial Comparing Axillary Lymph Node Dissection to Axillary Radiation in Breast Cancer Patients (cT1-3 N1) Who Have Positive Sentinel Lymph Node Disease After Neoadjuvant Chemotherapy . Internet]. c2021 . 2021. . Available from : https://clinicaltrials.gov/ct2/show/NCT01901094