Abstract
Objective
To evaluate the prognostic effect of pre-operative contrast-enhanced ultrasound (CEUS) features on intrahepatic cholangiocarcinoma (ICC) after percutaneous microwave ablation (MWA).
Methods:
A total of ICC 29 patients (average age 56.34 ± 9.78 years old, 33~75 years old) underwent MWA from March 2012 to December 2020, with a total of 58 lesions (0.5–8.1 cm, mean diameter, 2.68 ± 1.59 cm), and their pre-operative CEUS images and clinical data were collected and reviewed. Survival rate, local progression rate, intra- and extrahepatic metastasis rate were evaluated. Uni- and multivariate analysis were used to analyze the prognostic factors affecting the survival of ICC patients with pre-operative CEUS features.
Results:
The median follow-up time after MWA was 18.43 months (4.17–93.13 months). 1-, 2-, and 3-year OS rates were 64.4%, 48.1% and 48.1%; 6-, 12-, 18-, 24-, 36-, 48-, and 60-month local progress and extrahepatic metastasis rates were 0.0%, 4.0%, 17.7%, 17.7%, 17.7%, 17.7%, 17.7% and 3.4%, 21.5%, 32.7%, 45.6%, 55.2%, 55.2% and 77.6%, respectively. Uni- and multivariate analysis showed that post-operative extrahepatic metastasis was an important factor for long-term survival of ICC patients after MWA (p = 0.006, 0.01), and Rim-enhancement feature of pre-operative CEUS was identified as an independent predictor of post-operative extrahepatic metastasis and long-term survival (p = 0.02, 0.02).
Conclusion
Rim-enhancement feature of pre-operative CEUS is a predictor high post-operative extrahepatic metastasis and poor prognosis through distant microvascular metastasis after MWA of ICC patients.
Advances in knowledge:
This study determined the important CEUS features of ICC and analyzed their impact on the prognosis of ICC patients after MWA, providing scientific guidance for better clinical treatment in the future.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is a highly malignant epithelial tumor originating in the bile duct and characterized by differentiation of bile duct cells. 1 ICC is the second most common primary liver cancer, which is highly invasive, with a poor prognosis and a short survival period. Treatment options for ICC patients are limited because of the challenge of early diagnosis and the limited efficacy of chemotherapy or radiotherapy because ICC is a heterogeneous malignancies. 2
Over the past decade, significant advances have been made in the diagnosis, staging, and treatment of ICC. 3 Image-guided ablations, such as radiofrequency ablation and microwave ablation (MWA), have been used in the treatment of ICC patients due to their low invasiveness and acceptable clinical outcomes, and have been well used as an acceptable option for surgical resection. 4 Nevertheless, treatment is challenging, and cancer recurrence is the main problem after treatment, leading to low survival rates. 5 Recent studies have found that despite aggressive treatment, tumor recurrence is frequent and long-term oncology outcomes are poor. A single treatment regimen is unlikely to make significant progress in improving outcomes for ICC patients, and multimodal treatment concept is clearly recommended. 6,7 Reduce recurrence rates by prioritizing early systemic treatment for aggressive cancers. 8,9 Therefore, pre-operative invasive identification of ICC patients is the key to provide scientific treatment basis for future clinical work.
Ultrasound has the advantages of safety, easy to repeat, and relatively low cost. 10 In particular, compared with CT and MRI, contrast-enhanced ultrasound (CEUS) has the advantage of real-time observation of enhanced patterns. It can capture transient changes in vascular structure, enhancement time and dynamic changes during enhancement. 11 The characteristics of CEUS can reflect tumor enhancement mode and level, blood supply type, vascular invasion, etc. Blood supply type and degree of microvascular invasion have relatively accurate predictive value for tumor recurrence. In addition, it is also closely related to the pathological type and differentiation degree of tumor. Generally, different pathological type and differentiation degree of tumor can predict the prognosis of tumor. 12,13
However, there are no relevant literature reports on the prediction of the prognosis of ICC patients after MWA by CEUS features. Therefore, on the basis of previous reports, this study determined the important CEUS features of ICC and analyzed their impact on the prognosis of ICC patients after MWA, providing scientific guidance for better clinical treatment in the future.
Methods and materials
Patients
This study was approved by the institutional review board, and informed consent was obtained from each patient.
Inclusion criteria: (1) patients with ICC pathologically diagnosed from March 2012 to December 2020; (2) the tumor is a newly diagnosed lesion without any other treatment; (3) pre-operative CEUS examination was performed; (4) voluntary MWA; (5) able to tolerate the operation; (6) plasma prothrombin time ≤ 25 s, prothrombin activity ≥ 40%, platelet count ≥ 40×109 l−1.
Exclusion criteria: (1) severe jaundice, abdominal fluid accumulation, coagulation dysfunction, or patients with acute infection; (2) patients with severe liver and kidney functional damage; (3) pregnant and unco-operative patients; (4) patients with incomplete images or lost follow-up.
This was a retrospective study, which was approved by the Ethics Committee of PLA General Hospital, and all patients provided written informed consent before clinical examination.
Instrument
The KY-2000 microwave therapeutic instruments developed by Nanjing Kangyou Microwave Energy Application Research Institute are adopted. The operating frequency is 2450 and 915 MHz, respectively, and the output power is 1–100 W. 2450 MHz was selected when the tumor was ≤2 cm, and 915 MHz was selected when the tumor was >2 cm. Built-in water cooling circulation system to prevent excessive rod temperature. 15 G implantable water-cooled microwave antenna, rod length 18 cm, antenna external surface with antistick processing, can effectively avoid the antenna and tissue adhesion may occur during the ablation process. The microwave instrument is equipped with a 21 G temperature measuring needle, which can be placed in a predetermined position under the guidance of ultrasound to monitor the therapeutic temperature in real time during the MWA process.
The color Doppler LOGIQ E9 instrument (GE, Milwaukee, USA) is adopted, the frequency of the transducer is 2.0–5.0 Hz, and the mechanical index is 0.12–0.18.
CEUS
All patients received CEUS before MWA. Conventional ultrasound examination was performed before CEUS to determine the location, number, size, formation, internal echo, blood supply and relationship with surrounding structures. The contrast agent was sulfur hexafluoride microbubbles (SonoVue, Bracco, Italy). The microbubbles were prepared by dissolving 24.98 mg dry powder in 5 ml NaCl at a concentration of 8 μL ml−1. For each image set, 2.4 ml of contrast suspension was injected via a super-jugular indwelling needle preinserted into the elbow within 3–5 s, followed by a 5 ml infusion of normal saline. When the contrast agent is injected, the timing device is activated and the dynamic image is recorded. The enhancement of the lesion and surrounding liver tissue was observed within 4–6 min. If the results were not satisfactory, repeat the above steps. Inject the contrast agent multiple times, at least 15 min apart.
Interpretation of CEUS images
According to the guidelines of the European Union Association for Medical and Biological Ultrasound, 14 two experienced CEUS physicians evaluated pre-operative contrast-enhanced ultrasound features of ICC, to determine the intensities and patterns of different stages of lesions. When the two physicians do not agree, the superior doctor will be asked for further judgment. The arterial phase (10–30 s), portal phase (31–120 s), and delayed phase (121–360 s) after CEUS were identified.
The features on CEUS were recorded and characterized as follows: (1) the number of lesions; (2) maximum diameter of the target lesion; (3) shape of the target lesion; (4) boundary of the lesion; (5) enhancement level in the arterial/portal/late phase (hyper/iso/hypo); (6) enhancement patterns of the lesion in the arterial phase (rim/homogeneous/in homogeneous/others); (7) time to enhanced commencement; (8) washout time (within 60 s or not); 15 (9) duration of enhancement (washout time time to enhanced commencement); (10) tumor supply artery (defined as an artery extending from the surrounding liver parenchyma into the tumor); 16 (11) peripheral circular artery (defined as an annularstrip artery around the tumor in the arterial phase); 16 (12) tumor capsule (defined as an enhancement line that surrounds the tumor during the portal venous phase); 17 (13) intratumoral vein (defined as straight vessel branches extending through the mass during the portal venous and late phase); 18 (14) boundary of the intratumoral nonenhanced area (if it was present); and (15) marked washout (defined as the lesion appearing as a uniform black defect within the enhanced liver parenchyma). 15
MWA procedure
All patients underwent pre-operative blood routine, urine routine, blood coagulation function, liver function, chest X-ray and electrocardiogram to fully understand the physical condition of the patients. Basic clinical data including age and sex were recorded. Laboratory tests included hepatitis status, alpha-fetoprotein (AFP) levels, and CA199 levels.
The patient was placed in lateral decubitus position, and the puncture point and direction were determined under ultrasonic guidance. After disinfection, napkin laying and local anesthesia, a small incision of 2~3 mm was made at the puncture point. The 18G biopsy needle was entered under ultrasonic guidance, and 2~3 tissues were taken, and the microwave needle was placed into the liver tumor along the original incision. Microwave radiation was started after the breathing of the patients was stabilized after intravenous anesthesia, and the ablation power was generally 40–65 W, and the ablation time was 200–2460 s. Change the power or extend the transmission time if necessary. During the treatment, the range of strong echo was monitored in real time. When it covered and exceeded the tumor and the peritulotumor temperature reached the target temperature, microwave radiation was stopped, and the needle passage was cauterized routinely during the process of needle withdrawal to prevent needle passage bleeding and needle passage implantation metastasis. Ambulate ECG, respiration and blood pressure monitoring were given throughout the operation. Monitor blood pressure and vital signs after surgery, and pay attention to abdominal pain or other discomfort.
Follow-up and outcomes measures
On the third day after the operation, CEUS, enhanced CT and/or enhanced MRI were performed by an experienced sonographer to assess the inactivation of the tumor. If residual tumor was found, microwave therapy was performed again, and follow-up was performed if no residual tumor was found. The follow-up period was calculated from the time the patient completed ablation. Efficacy was assessed by enhanced imaging after ablation. Follow-up was conducted at 1, 3, 6 months, and then every 6 months. All patients were followed up until death or July 1, 2021.
The end points of the study were progression-free survival (PFS) and overall survival (OS). Local tumor recurrence was defined as the presence of enhanced lesions at or near the previous MWA site. New contrast-enhanced lesions in the intra- or extrahepatic sites were considered new metastases and were defined as intra- and extrahepatic progression, respectively. Disease progression includes local recurrence, intra- and extrahepatic progression. The PFS calculates from the date of the first MWA treatment in the ICC to the date of disease progression or the last date of follow-up. OS from the date of first MWA treatment in ICC to the date of death or the date of last follow-up. The mortality associated with thermal ablation was death within 30 days after ablation. Serious complications such as guidance to a sick or disabled, care level increased, people court again, and to extend the length of time. 19
Statistics
All results were analyzed using SPSS 21.0 (IBM, Chicago, IL). The quantitative data were expressed as mean ± standard deviation, and qualitative data were expressed as frequency. PFS and OS were estimated by the Kaplan–Meier method. Univariate analyses were performed using log-rank test. Cox model was used for multivariate analysis. For all analyses, p values was bilateral and <0.05 was considered statistically significant.
Results
Patients’ characteristics
According to the inclusion criteria of this study, a total of 55 patients were included in this study, including 23 patients with incomplete CEUS images, 3 patients lost to follow-up and the remaining 29 patients. A total of 29 patients with ICC were included in this study. There were 22 males and 7 females. The patients were 33~75 years old (mean age, 56.34 ± 9.78 years), five were ≤45 years old, 14 were 46–60 years old, and 10 were >60 years old. A total of 58 lesions were treated in 29 patients, including 15 patients with single lesions and 14 patients with multiple lesions. The diameter of lesions was 0.5–8.1 cm (mean diameter, 2.68 ± 1.59 cm), among which 44 lesions were ≤ 3.0 cm, 13 lesions were 3.1–5.0 cm, and five lesions were > 5.0 cm. The lesions were divided into safe sites and dangerous sites according to whether the distance from the tumor to the important organs (gastrointestinal tract, gallbladder, bile duct, etc.) was less than or equal to 5 mm. Among them, 36 lesions were located in safe sites and 22 lesions were located in dangerous sites. All 29 patients were pathologically confirmed to have ICC, including 0 cases of highly differentiated type, 11 cases of moderately differentiated type, 12 cases of poorly differentiated type, and 6 cases of undifferentiated type. There were 10 patients with hepatitis B, one patient with hepatitis C, 6 patients with cirrhosis, and 23 patients without cirrhosis. According to child-pugh classification, all are grade A; AFP ≤ 20 µg l−1 in 27 cases, >20 µg l−1 in 2 cases, CA199 ≤35 U ml−1 in 18 cases, >35 U ml−1 in 11 cases. Among them, 8 patients had extrahepatic metastasis before operation. Patients’ primary characteristics are shown in Table 1.
Table 1.
Patients’ primary characteristics
| Characteristics | Number |
|---|---|
| Total | 29 |
| Age (years), mean (range) | 56 (33–75) |
| ≤45 years | 5 |
| 46–60 years | 14 |
| >60 years | 10 |
| Gender | |
| Female | 7 |
| Male | 22 |
| Hepatitis | |
| HBV | 10 |
| HCV | 1 |
| Liver cirrhosis | |
| Yes | 6 |
| No | 23 |
| Comorbidities | |
| Yes | 16 |
| No | 13 |
| AFP | |
| ≤20 µg l−1 | 27 |
| >20 µg l−1 | 2 |
| CA19-9 | |
| ≤35 U ml−1 | 18 |
| >35 U ml−1 | 11 |
| Tumor number, total | 58 |
| Single | 15 |
| Multiple | 14 |
| Tumor size | |
| ≤3.0 cm | 44 |
| 3.1–5.0 cm | 13 |
| >5.0 cm | 5 |
| Tumor lesions | |
| Safe | 36 |
| Dangerous | 22 |
| Differentiation | |
| High | 0 |
| Moderate | 11 |
| Low | 12 |
| Without | 6 |
| Follow-up (months), median (range) | 18.43 (4.17–93.13) |
AFF, alpha-fetoprotein.
Survival outcomes
All patients enrolled in this study were completely inactivated once by MWA. Four patients progressed after a median follow-up of 18.43 months (4.17–93.13 months). 14 of them were fatal. Median PFS after MWA was 18.43 months (95% CI 18.33–33.61 months); PFS rates after 6, 12, 18 and 24 months were 82.2%, 64.4%, 48.1% and 48.1%, respectively. The median OS was 18.43 months (95% CI 18.33–33.61 months). OS rates after 1, 2 and 3 years were 64.4%, 48.1% and 48.1%. The local progress rates after 6, 12, 18, 24, 36, 48 and 60 months were 0.0%, 4.0%, 17.7%, 17.7%, 17.7%, 17.7%, 17.7%, respectively. The intrahepatic metastasis rates after 6, 12, 18, 24, 36, 48 and 60 months were 3.4%, 11.5%, 20.4%, 35.7%, 48.4%, 48.4%, 86.3%, respectively. The extrahepatic metastasis rates after 6, 12, 18, 24, 36, 48 and 60 months were 3.4%, 21.5%, 32.7%, 45.6%, 55.2%, 55.2% and 77.6%, respectively.
Prognostic factors
To evaluate the prognostic factors for the survival prognosis of ICC patients treated by MWA. according to univariate analysis of all clinical indicators (gender, age, tumor number, maximum tumor diameter, tumor location, AFP level, CA199 level, extrahepatic metastasis, tumor differentiation type, hepatitis type, cirrhosis, comorbidities, local progression, post-operative intra- and extrahepatic metastases), post-operative extrahepatic metastasis was a risk factor affecting the survival of the ICC patients after MWA (p = 0.006); according to multivariate analysis, AFP level, tumor size, tumor differentiation type and post-operative extrahepatic metastasis were risk factors affecting the survival of the ICC patients after MWA (p < 0.05). In addition, there was no significant difference in the rate of local progression and intrahepatic metastasis after MWA among the groups with preoperative CEUS features (p > 0.05). Therefore, the presence of post-operative extrahepatic metastases is an independent risk factor for the survival of ICC patients after MWA. The results of multivariate analysis are shown in Table 2.
Table 2.
The results of multivariate analysis using cox proportional hazards model
| Factors | Post-operative extrahepatic metastasis | OS | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| AFP | 33.60 (0.00–6452.20) | 3.91 | 3.57 (1.42–29.87) | 0.02* |
| Tumor size | 7.56 (3.76–7.12) | 0.01* | 4.84 (1.33–24.00) | 0.001* |
| Tumor differentiation | 4.65 (9.77–931.87) | 0.01* | 5.24 (4.78–40.11) | 0.01* |
| Postoperative extrahepatic metastasis | _ | _ | 20.41 (1.84–226.74) | 0.01* |
| Rim-enhancement | 4.00 (0.00–0.72) | 0.02* | 0.05 (0.00–0.66) | 0.02* |
| Peripheral circular artery | 0.84 (0.03–193.78) | 2.31 | 8.27 (2.13–3071.75) | 0.001* |
| Marked washout | 3.20 (0.00–4.83) | 0.04* | 5.65 (0.00–18056.61) | 0.04* |
| Intratumoral vein | 18.67 (0.00–3678.24) | 1.30 | 0.32 (0.04–2.37) | 0.27 |
AFP, alpha-fetoprotein ; CI, confidence interval; HR, hazard ratio; OS, overall survival.
We examined 14 CEUS features of ICC: irregular shape, hyper-enhanced in arterial phase, hypo/ iso-enhanced in arterial phase, hypo-enhanced in portal phase, hypo-enhanced in late phase, rim-enhancement, early washout (<60 s), duration of enhancement (<30 s), tumor supply artery, peripheral circular artery or tumor capsule, intratumoral vein, obscure boundary of tumor, obscure boundary of intratumoral non-enhanced area, Marked washout was included in univariate analysis. Univariate analysis results show that rim-enhancement significantly affects OS after MWA (p = 0.02).
Factors with p values less than 0.500 from the univariate analysis were then subjected to multivariate analysis to determine independent prognostic factors for OS and post-operative extrahepatic metastasis. Marked washout, intratumoral vein, peripheral circular artery and rim-enhancement were included in the multivariate analysis of OS and post-operative extrahepatic metastasis after MWA, and the results showed that rim-enhancement, peripheral circular artery and marked washout were identified as independent prognostic factors for OS (p = 0.02, 0.001, 0.04); rim-enhancement and marked washout were identified as independent prognostic factors for post-operative extrahepatic metastasis (p = 0.02, 0.04). So, rim-enhancement is an independent risk factor affecting the survival of ICC patients after MWA, while marked washout is a potential risk factor affecting the survival of ICC patients after MWA.
A Kaplan–Meier survival analysis showed the OS of ICC patients after MWA without rim-enhancement was significantly longer than that of rim-enhancement patients (p = 0.001). The survival curve is shown in Figure 1.
Figure 1.
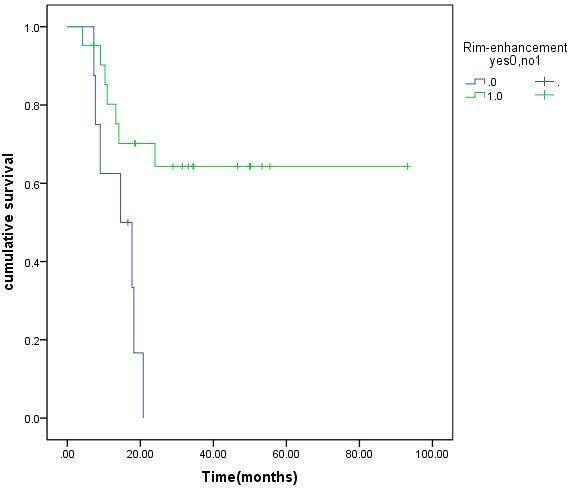
Cumulative OS curves with or without rim-enhancement in 29 patients with ICC treated by MWA. ICC, intraclass coefficient; MWA, microwave ablation; OS, overall survival.
Therefore, ICC patients with rim-enhancement of pre-operative CEUS are more likely to have distant metastasis, high rate of extrahepatic metastasis and poor long-term survival after MWA.
Discussion
ICC accounts for 10–15% of all primary liver cancer, second only to HCC, 20 and its incidence is on the rise globally, 21 with high invasiveness and low survival rate. Unfortunately, only about 20–40% of ICC patients are able to receive surgical treatment, and post-operative recurrence rates are high, as high as 60%. 22 Non-operative treatments, such as chemotherapy and radiotherapy, have limited survival benefits. 23
Due to its minimally invasive nature and reliable effectiveness, recent guidelines also indicate that ablation can provide a survival benefit for ICC patients if surgical treatment is not available. 24 However, although RFA has been the most studied, MWA has been frequently reported in recent years as a safe and effective treatment for ICC patients. 4,25
This retrospective study showed that the survival time of ICC patients was comparable to that after radical resection, 26 suggesting that MWA was less invasive, safe and effective than surgical treatment for ICC. Among the 29 ICC patients in this study, 4 patients developed local tumor progression and died during the follow-up period, so PFS was consistent with OS and lower than Zhang. 4 All patients enrolled in this study were completely inactivated once by MWA and had a low rate of local progression. These results indicate that ultrasound-guided percutaneous MWA has better local tumor inactivation and lower local progression rate in ICC patients. This study mainly analyzed the prognostic factors of survival in ICC patients treated with MWA. We did not only univariate analysis, but also multivariate analysis. First of all, uni- and multivariate analysis showed that there was no significant difference in the rate of local progression and intrahepatic metastasis after MWA among the groups with pre-operative CEUS features (p > 0.05). This indicates that MWA can achieve local complete inactivation of tumor through local expanded ablation, with a low rate of local progression. It is a good treatment method for ICC, and has a good ability of local inactivation of tumor, just like surgery. However, the survival of ICC patients is still poor after treatment, The low rate of local tumor progression is not an important factor affecting the long-term survival of ICC patients, which may be related to the special biological characteristics of ICC itself.
Second, this group of studies showed that the presence of post-operative extrahepatic metastases is an independent risk factor for the survival of ICC patients after MWA (p < 0.05). Post-operative liver cancer is prone to intrahepatic recurrence and extrahepatic metastasis, which is an important reason for poor prognosis of liver cancer. 27 This suggests that the occurrence of post-operative extrahepatic metastasis is an important factor affecting the poor prognosis of ICC patients with MWA. As a result, we can speculate that even if the tumor is completely inactivated by MWA again and local recurrence is low, the patients will soon develop distant metastasis due to their biological characteristics, and the high rate of post-operative extrahepatic metastasis leads to poor long-term survival of the patients. Therefore, it is critical to identify which biological characteristics of ICC patients are associated with the occurrence and timing of post-operative extrahepatic metastases. Identifying the biological behavior of malignant tumors can help to develop targeted interventions to improve the prognosis of patients. CEUS can be used to evaluate the microvascular perfusion in tumors, and its enhanced features can reflect the biological characteristics of tumors to a certain extent.
Third, we conducted uni- and multivariate analyses of the association between the enhanced features of CEUS before treatment and the prognostic survival after MWA in ICC patients. Univariate analysis results show that rim-enhancement significantly affects OS after MWA (p = 0.02), multivariate analysis showed that rim-enhancement, Peripheral circular artery and marked washout were identified as independent prognostic factors for OS (p = 0.02, 0.001, 0.04); Rim-enhancement and marked washout were identified as independent prognostic factors for post-operative extrahepatic metastasis (p = 0.02, 0.04). According to the literature, rim-enhancement means that there are many cancer cells around the lesion, rich blood supply, and it is related to the walk along the bile duct. 28 There is a close correlation between the type of tumor blood supply and the imaging characteristics of CEUS, 29 marked washout means that the tumor’s blood supply is mainly arterial with increased vascular permeability. 30 Therefore, ICC patients with rim-enhancement and marked washout characteristics of pre-operative CEUS may have microvascular metastasis after MWA, resulting in the occurrence of extrahepatic metastasis, which will affect the long-term survival of patients and lead to poor prognosis. As we know, as reported in previous studies, the important CEUS features that distinguish HCC from ICC include rim-enhancement, early washout (<60 s) and marked washout, which are three CEUS features favorable to ICC. 31 Therefore, rim-enhancement not only contributes to the diagnosis of ICC, but also is an important predictor of long-term survival in ICC patients after MWA treatment. In clinical practice, it is worth our attention that ICC patients with rim-enhancement features of pre-operative CEUS should be closely followed up for early detection and early management, whether after MWA or surgery. In order to reduce or delay the occurrence of post-operative extrahepatic metastasis, post-operative systemic comprehensive therapy, such as targeted therapy and immunotherapy, can be combined to improve the long-term survival of ICC patients and benefit them. In addition, for ICC patients with rim-enhancement features of pre-operative CEUS, can measures to reduce tumor blood supply be assisted before MWA? Therefore, how to reduce or delay the occurrence of post-operative extrahepatic metastasis is the key to improve the long-term survival of patients with ICC patients with rim-enhancement features of pre-operative CEUS, which is the direction of our future research.
In addition, multiple clinical indicators were included in the multivariate analysis, and the multivariate results showed that age, the maximum tumor size and tumor differentiation were also important factors affecting the OS of ICC patients treated by MWA (p < 0.05). The results showed that smaller tumor size and higher tumor differentiation were predictors of OS elongation. Smaller tumors and higher tumor differentiation are thought to represent less aggressive tumor biology and are therefore associated with improved survival. 13 In the case of the patient with the longest survival (93.13 months) in this study, the maximum diameter of the lesion was 1.9 cm, which is exactly to verify this conclusion. Therefore, the earlier ICC is detected, the smaller the tumor and the better the prognosis of the patient.
Our study has several limitations. First, this is a retrospective study. In the future work, prospective research is necessary. Second, we included a small number of cases in the study, some cases were incomplete and angiographic data were lost, which reminded us that it is very important to manage and retain the precious images of patients in the future work, so that we can get better scientific research results. Third, the study is a single-center study, which increases the subjectivity of the study and can be interfered with by the physician’s level of treatment. Therefore, further multicenter, large sample prospective studies are needed.
Conclusions
In conclusion, ultrasonic-guided percutaneous MWA is a good method for local tumor inactivation in ICC patients, with a low post-operative local recurrence rate, and is not an important factor affecting the long-term survival of ICC patients. Post-operative extrahepatic distant metastasis is an important factor affecting the long-term survival of ICC patients. ICC patients with rim-enhancement feature of pre-operative CEUS are more likely to have distant metastasis through microvessels after MWA, resulting in high post-operative extrahepatic metastasis rate and poor long-term survival. Therefore, how to reduce or delay the occurrence of post-operative extrahepatic metastasis is the key to improve the long-term survival of patients with ICC patients with rim-enhancement features of pre-operative CEUS, which is the direction of our future research.
Footnotes
Acknowledgments: This work was supported by Grants 12126607, 91859201, 81971625, 82030047 from the National Scientific Foundation Committee of China and from the Medical Science and Technology Project of Henan Province (No.LHGJ20210348), Science and Technology Development Project of Henan Province (212102311107).
Contributor Information
Xiaohui Wang, Email: xhhyykl@126.com, Department of Interventional Ultrasound, The Fifth Medical Center of PLA General Hospital, Beijing, China ; Department of Ultrasound, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China .
Ping Liang, Email: liangping301@126.com, Department of Interventional Ultrasound, The Fifth Medical Center of PLA General Hospital, Beijing, China .
Jie Yu, Email: jiemi301@163.com, Department of Interventional Ultrasound, The Fifth Medical Center of PLA General Hospital, Beijing, China .
Jun-dong Yao, Email: beijing301yjd@126.com, Department of Interventional Ultrasound, The Fifth Medical Center of PLA General Hospital, Beijing, China .
Fang-ying Fan, Email: beijing301ffy@126.com, Department of Interventional Ultrasound, The Fifth Medical Center of PLA General Hospital, Beijing, China .
Xiaoling Yu, Email: beijing301lfy@126.com, Department of Interventional Ultrasound, The Fifth Medical Center of PLA General Hospital, Beijing, China .
Zhi-gang Cheng, Email: beijing301czg@126.com, Department of Interventional Ultrasound, The Fifth Medical Center of PLA General Hospital, Beijing, China .
Zhi-yu Han, Email: beijing301hzy@126.com, Department of Interventional Ultrasound, The Fifth Medical Center of PLA General Hospital, Beijing, China .
Fang-yi Liu, Department of Interventional Ultrasound, The Fifth Medical Center of PLA General Hospital, Beijing, China .
Jian-ping Dou, Email: beijing301djp@126.com, Department of Interventional Ultrasound, The Fifth Medical Center of PLA General Hospital, Beijing, China .
REFERENCES
- 1. Lim JH . Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings . American Journal of Roentgenology 2003. ; 181: 819 – 27 . doi: 10.2214/ajr.181.3.1810819 [DOI] [PubMed] [Google Scholar]
- 2. Sato K, Baiocchi L, Kennedy L, Zhang W, Ekser B, et al. . Current advances in basic and translational research of cholangiocarcinoma . Cancers (Basel) 2021. ; 13( 13 ): 3307 . doi: 10.3390/cancers13133307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ejaz A, Cloyd JM, Pawlik TM . Advances in the diagnosis and treatment of patients with intrahepatic cholangiocarcinoma . Ann Surg Oncol 2020. ; 27: 552 – 60 . doi: 10.1245/s10434-019-07873-z [DOI] [PubMed] [Google Scholar]
- 4. Zhang K, Yu J, Yu X, Han Z, Cheng Z, et al. . Clinical and survival outcomes of percutaneous microwave ablation for intrahepatic cholangiocarcinoma . Int J Hyperthermia 2018. ; 34: 292 – 97 . doi: 10.1080/02656736.2017.1327678 [DOI] [PubMed] [Google Scholar]
- 5. Padthaisong S, Phetcharaburanin J, Klanrit P, Li JV, Namwat N, et al. . Integration of global metabolomics and lipidomics approaches reveals the molecular mechanisms and the potential biomarkers for postoperative recurrence in early-stage cholangiocarcinoma . Cancer Metab 2021. ; 9: 30 . doi: 10.1186/s40170-021-00266-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maier CF, Zhu L, Nanduri LK, Kühn D, Kochall S, et al. . Patient-derived organoids of cholangiocarcinoma . Int J Mol Sci 2021. ; 22( 16 ): 8675 . doi: 10.3390/ijms22168675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beal EW, Cloyd JM, Pawlik TM . Surgical treatment of intrahepatic cholangiocarcinoma: current and emerging principles . J Clin Med 2020. ; 10( 1 ): E104 . doi: 10.3390/jcm10010104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akateh C, Ejaz AM, Pawlik TM, Cloyd JM . Neoadjuvant treatment strategies for intrahepatic cholangiocarcinoma . World J Hepatol 2020. ; 12: 693 – 708 . doi: 10.4254/wjh.v12.i10.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cloyd JM, Ejaz A, Pawlik TM . ASO author reflections: advances in the multidisciplinary management of intrahepatic cholangiocarcinoma . Ann Surg Oncol 2020. ; 27: 2866 – 67 . doi: 10.1245/s10434-020-08635-y [DOI] [PubMed] [Google Scholar]
- 10. Kitano M, Yamashita Y, Kamata K, Ang TL, Imazu H, et al. . Working group for the international consensus guidelines for contrast-enhanced harmonic endoscopic ultrasound. the asian federation of societies for ultrasound in medicine and biology (afsumb) guidelines for contrast-enhanced endoscopic ultrasound . Ultrasound Med Biol 2021. ; 47: 1433 – 47 . doi: 10.1016/j.ultrasmedbio.2021.01.030 [DOI] [PubMed] [Google Scholar]
- 11. Kim TK, Jang HJ . Contrast-enhanced ultrasound in the diagnosis of nodules in liver cirrhosis . World J Gastroenterol 2014. ; 20: 3590 – 96 . doi: 10.3748/wjg.v20.i13.3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo HL, Zheng X, Cheng MQ, Zeng D, Huang H, et al. . Contrast-enhanced ultrasound for differentiation between poorly differentiated hepatocellular carcinoma and intrahepatic cholangiocarcinoma . J Ultrasound Med 2021. . doi: 10.1002/jum.15812 [DOI] [PubMed] [Google Scholar]
- 13. Deng S, Jiang Q, Wang Y, Lu X, Zhang Y . Relationship between quantitative contrast-enhanced ultrasonography parameters and angiogenesis in primary small hepatocellular carcinoma: a retrospective study . Medicine (Baltimore) 2021. ; 100( 27 ): e26489 . doi: 10.1097/MD.0000000000026489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, et al. . Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (ceus) in the liver - update 2012: a wfumb-efsumb initiative in cooperation with representatives of afsumb, aium, asum, flaus and icus . Ultrasound Med Biol 2013. ; 39: 187 – 210 . doi: 10.1016/j.ultrasmedbio.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 15. Piscaglia F, Wilson SR, Lyshchik A, Cosgrove D, Dietrich CF, et al. . American college of radiology contrast enhanced ultrasound liver imaging reporting and data system (ceus li-rads) for the diagnosis of hepatocellular carcinoma: a pictorial essay . Ultraschall Med 2017. ; 38: 320 – 24 . doi: 10.1055/s-0042-124661 [DOI] [PubMed] [Google Scholar]
- 16. Catalano O, Nunziata A, Lobianco R, Siani A . Real-time harmonic contrast material-specific us of focal liver lesions . Radiographics 2005. ; 25: 333 – 49 . doi: 10.1148/rg.252045066 [DOI] [PubMed] [Google Scholar]
- 17. Schellhaas B, Görtz RS, Pfeifer L, Kielisch C, Neurath MF, et al. . Diagnostic accuracy of contrast-enhanced ultrasound for the differential diagnosis of hepatocellular carcinoma: esculap versus ceus-li-rads . Eur J Gastroenterol Hepatol 2017. ; 29: 1036 – 44 . doi: 10.1097/MEG.0000000000000916 [DOI] [PubMed] [Google Scholar]
- 18. Xu HX, Lu MD, Liu GJ, Xie XY, Xu ZF, et al. . Imaging of peripheral cholangiocarcinoma with low-mechanical index contrast-enhanced sonography and sonovue: initial experience . J Ultrasound Med 2006. ; 25: 23 – 33 . doi: 10.7863/jum.2006.25.1.23 [DOI] [PubMed] [Google Scholar]
- 19. Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, et al. . Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update . Radiology 2014. ; 273: 241 – 60 . doi: 10.1148/radiol.14132958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Razumilava N, Gores GJ . Cholangiocarcinoma . Lancet 2014. ; 383: 2168 – 79 . doi: 10.1016/S0140-6736(13)61903-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khan SA, Toledano MB, Taylor-Robinson SD . Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma . HPB (Oxford) 2008. ; 10: 77 – 82 . doi: 10.1080/13651820801992641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM . Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis . JAMA Surg 2014. ; 149: 565 – 74 . doi: 10.1001/jamasurg.2013.5137 [DOI] [PubMed] [Google Scholar]
- 23. Park SY, Kim JH, Yoon HJ, Lee IS, Yoon HK, et al. . Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma . Clin Radiol 2011. ; 66: 322 – 28 . doi: 10.1016/j.crad.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 24. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, et al. . Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma . J Hepatol 2014. ; 60: 1268 – 89 . doi: 10.1016/j.jhep.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 25. Yang H, Cheng Z, Han Z, Liu F, Yu X, et al. . Assessment of the outcomes of intrahepatic cholangiocarcinoma after ultrasound-guided percutaneous microwave ablation based on albumin-bilirubin grade . Cardiovasc Intervent Radiol 2021. ; 44: 261 – 70 . doi: 10.1007/s00270-020-02637-9 [DOI] [PubMed] [Google Scholar]
- 26. Jutric Z, Johnston WC, Hoen HM, Newell PH, Cassera MA, et al. . Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the national cancer database . HPB (Oxford) 2016. ; 18: 79 – 87 . doi: 10.1016/j.hpb.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saab S, Zhou K, Chang EK, Busuttil RW . De novo hepatocellular carcinoma after liver transplantation . J Clin Transl Hepatol 2015. ; 3: 284 – 87 . doi: 10.14218/JCTH.2015.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palmieri VO, Santovito D, Marano G, Minerva F, Ricci L, et al. . Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma . Radiol Med 2015. ; 120: 627 – 33 . doi: 10.1007/s11547-014-0494-9 [DOI] [PubMed] [Google Scholar]
- 29. Huang JY, Li JW, Ling WW, Li T, Luo Y, et al. . Can contrast enhanced ultrasound differentiate intrahepatic cholangiocarcinoma from hepatocellular carcinoma? World J Gastroenterol 2020. ; 26: 3938 – 51 . doi: 10.3748/wjg.v26.i27.3938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sporea I, Săndulescu DL, Şirli R, Popescu A, Danilă M, et al. . Contrast-enhanced ultrasound for the characterization of malignant versus benign focal liver lesions in a prospective multicenter experience - the srumb study . J Gastrointestin Liver Dis 2019. ; 28: 191 – 96 . doi: 10.15403/jgld-180 [DOI] [PubMed] [Google Scholar]
- 31. Chen Y, Zhu Y, Chen K, Wang H, Zhang W, et al. . Differentiation between hepatocellular carcinoma and intrahepatic cholangiocarcinoma using contrast-enhanced ultrasound: a systematic review and meta-analysis . Clin Hemorheol Microcirc 2021. ; 79: 293 – 309 . doi: 10.3233/CH-211145 [DOI] [PubMed] [Google Scholar]


