Abstract
Objective:
FLASH irradiation reportedly produces less normal tissue toxicity, while maintaining tumour response. To investigate oxygen’s role in the ‘FLASH effect’, we assessed DNA damage levels following irradiation at different oxygen tensions, doses and dose rates.
Methods:
Samples of whole blood were irradiated (20 Gy) at various oxygen tensions (0.25–21%) with 6 MeV electrons at dose rates of either 2 kGy/s (FLASH) or 0.1 Gy/s (CONV), and subsequently with various doses (0–40 Gy) and intermediate dose rates (0.3–1000 Gy/s). DNA damage of peripheral blood lymphocytes (PBL) were assessed by the alkaline comet assay.
Results:
Following 20 Gy irradiation, lower levels of DNA damage were induced for FLASH, the difference being significant at 0.25% (p < 0.05) and 0.5% O2 (p < 0.01). The differential in DNA damage at 0.5% O2 was found to increase with total dose and dose rate, becoming significant for doses ≥20 Gy and dose rates ≥30 Gy/s.
Conclusion:
This study shows, using the alkaline comet assay, that lower levels of DNA damage are induced following FLASH irradiation, an effect that is modulated by the oxygen tension, and increases with the total dose and dose rate of irradiation, indicating that an oxygen related mechanism, e.g. transient radiation-induced oxygen depletion, may contribute to the tissue sparing effect of FLASH irradiation.
Advances in knowledge:
This paper is first to directly show that FLASH-induced DNA damage is modulated by oxygen tension, total dose and dose rate, with FLASH inducing significantly lower levels of DNA damage for doses ≥20 Gy and dose rates ≥30 Gy/s, at 0.5% O2.
Introduction
Despite important technological advances in dose delivery precision, using image-guided and intensity-modulated radiotherapy, radiation therapy still induces irreversible side-effects. 1 A key restraint on curative cancer care arises from normal tissue toxicity, limiting the total dose a tumour can receive. 2 Current radiation research is focused on developing new treatment modalities, with the aim to reduce the risk of complications arising from radiation treatment, including inducing secondary tumours. 3,4
FLASH radiotherapy (FLASH RT) is a new treatment modality that delivers radiation in a fraction of a second at ultra-high dose rates (≥40 Gy/s), which could revolutionise the field by improving the cancer therapeutic ratio, 2 with heightened recent interest long after pioneering studies of over 40 years ago. 5,6 FLASH RT has been observed to create a differential ‘FLASH effect’, sparing normal tissue while maintaining antitumour efficacy equal to that of conventional dose rate radiotherapy (CONV RT), in mice, 7–10 minipigs and cats. 11 More recently, a study found electron FLASH RT (430–500 Gy/s) as a suitable single fraction treatment alternative for the treatment of canine tumours at low tissue depths (2–3 cm), using a 10 MeV electron beam. 12 In addition to clinical veterinary studies, the first patient (CD30+ T cell cutaneous lymphoma) was treated successfully with FLASH in 2018. 11 However, the mechanisms underpinning the normal tissue sparing properties seen following irradiation at ultra-high dose rates have yet to be elucidated. 13
It has been proposed that FLASH, due to its extremely short ultra-high dose rate delivery of radiation, transiently consumes local oxygen. This in turn better enables the thiol-(RSH)-mediated chemical ‘repair’ of radiation-induced secondary and tertiary organic radicals, so competing with oxygen’s ‘fixation’ of damage. 14–20 Consequently, one likely outcome of FLASH exposure is lower levels of radiation-induced damage formation, including DNA strand breakage, with this contributing to the observed normal tissue sparing effect. Conversely, and in the context of improved therapeutic ratio, tumour tissues that are already hypoxic may not be protected as much from any oxygen consumption/depletion that FLASH induces. 2,8 However, this remains to be experimentally established.
A reduction in cell death in vitro as well as a reduction in neurocognitive damage of irradiated mice in vivo, both dependent on oxygen concentration, have been reported for FLASH compared to CONV RT at the same total dose. 8,19 Furthermore, a recent in vivo study by Cao 21 and co-workers utilised phosphorescence quenching of a water-soluble molecular probe to measure oxygen consumption per unit dose using a 10 MeV electron beam. They identified oxygen depletion as higher in normal tissues (≈2.5 mmHg) than in tumours (≈1.02 mmHg) when irradiating mice with 20 Gy total dose, at ultra-high dose rates (90, 180 and 270 Gy/s); while irradiation of mice with CONV resulted in no change in partial pressures of oxygen. They also carried out oxygen consumption measurements in aqueous solution and found that CONV depletes higher amounts of oxygen (0.19–0.21 mmHg/Gy) than irradiation at ultra-high dose rates (0.16–0.17 mmHg/Gy). However, radiolytic oxygen depletion of a solution in a closed system does not adequately reflect the mechanisms of cellular oxygen depletion and reoxygenation during FLASH and CONV irradiation. 22,23
Further in-vitro studies of FLASH radiolytic oxygen depletion by Khan et al. 24 have shown that the hypoxic core of A549 spheroids may expand under FLASH RT (90 Gy/s) engulfing a large number of well-oxygenated cells, while oxygen is steadily replenished during CONV. They found clonogenic survival to be threefold higher in FLASH irradiated spheroids compared to CONV irradiation; with no difference found in two-dimensional well-oxygenated cultured cells, corroborating earlier studies outlining oxygen as a key determinant of the FLASH effect. 8,19,25,26
In the present study, our aim is to investigate any potential differential in DNA damage in whole blood peripheral blood lymphocytes (PBL), using the alkaline comet assay, following ex-vivo irradiation at FLASH or CONV dose rates, and how this differential effect may be modified by oxygen concentration, total irradiation dose and dose rate.
Methods and materials
The alkaline version of the comet assay [also known as single-cell gel electrophoresis (SCGE)] was performed to assess DNA damage formation in human PBL in fresh whole blood, taken from a single healthy donor, following ex-vivo exposure to FLASH or CONV irradiation. PBLs were chosen primarily for convenience (negating need for cell culture plus the problems/concerns of transporting cultures, and suitable volumes of whole blood samples can be readily obtained by finger pin-prick immediately onsite prior to Comet sample preparation), but do represent a body-wide, systemic normal tissue susceptible to radiation exposure. 27–29 The alkaline version of the comet assay is the most sensitive version of the assay detecting both DNA strand breaks and alkaline-labile sites, with the whole blood PBL being irradiated embedded in thin low melting point agarose gels on glass microscope slides. The agarose gels were prepared by mixing 5 µl whole blood containing EDTA (0.16 mg/100 µl) with 190 µl of 37°C low melting point agarose, with 2 × 80 µl of this mix being used to prepare one gel on two separate slides. For further details of the alkaline comet assay procedure, see Supplementary Material 1.
Varying oxygen concentration
Following slide preparation, the slides were placed in a HypoxyLAB (Oxford Optronix Ltd., Oxford, UK) hypoxia chamber for 2 h set to ≥85% humidity, 20°C. This was performed for five different partial pressures of oxygen (pO2) settings of the chamber; 0.25, 0.5, 0.75, 1 and 3% (2, 4, 6, 8 and 24 mmHg). These setting were varied prior to samples being placed in the chamber through flooding of N2 into the chamber until the desired pO2 was reached, which was controlled by the built-in O2 sensor (calibration and functionality regularly tested through measurements with an Oxylite sensor, Oxford Optronix Ltd.). At the end of the 2 h incubation, slides were placed inside 50 ml centrifuge tubes that had been stored at low oxygen tension overnight prior to use, and tightly sealed with the lid wrapped in nescofilm inside the chamber.
The tubes containing the slides were positioned so that the slide-mounted blood/agarose gels were perpendicular to and facing the beam, then irradiated at room temperature at FLASH (2 kGy/s) or CONV (0.1 Gy/s) dose rates to 20 Gy total dose. 5 min following start of irradiation, the tubes were opened and slides placed into ice cold lysis buffer. Slides irradiated under normoxia (21% O2) were similarly prepared and irradiated in 50 ml centrifuge tubes left at atmospheric O2 then placed into lysis. Unirradiated 21% O2 controls were placed into lysis solution immediately following their preparation plus the time of mock-irradiation to lysis (5 min). Prepared unirradiated 0.5% O2 equilibrated control slides were placed into lysis after all other slides had been irradiated.
Varying irradiation dose
A similar procedure was used to evaluate whether a differential in DNA damage between FLASH (1.5 MGy/s-1.7 kGy/s for a 5–40 Gy delivery) vs CONV (0.1 Gy/s) was modulated by total dose. Slides equilibrated at 0.5% O2 (prepared as described above) were irradiated to a total dose of 5–40 Gy. Slides were placed into ice cold lysis buffer 5 min following start of irradiation (5–20 Gy) or 9 min following start of irradiation (30 and 40 Gy), in order to accommodate the protracted CONV irradiation times. Preliminary studies revealed that the whole blood PBLs used were devoid of any significant repair at room temperature over 40 min.
Varying dose rates
PBL DNA damage levels was also investigated for a variety of intermediate dose rates, ranging from 0.3 Gy/s to 1 kGy/s. Again, slides equilibrated at 0.5% O2 (prepared as described above) were irradiated to a total dose of 20 Gy and placed into ice cold lysis buffer 5 min following the start of irradiation.
Beam characteristics
All irradiations were performed with a FLASH‐optimized in‐house developed linear accelerator (linac), which has been described in more detail elsewhere, 30 delivering electrons of 6 MeV nominal energy with a circular horizontal beam of 5 cm in diameter, with each of the tubes containing the slides placed in contact with the collimation system so that the blood/agarose gels were centred in and perpendicular to the beam (Figure 1).
Figure 1.
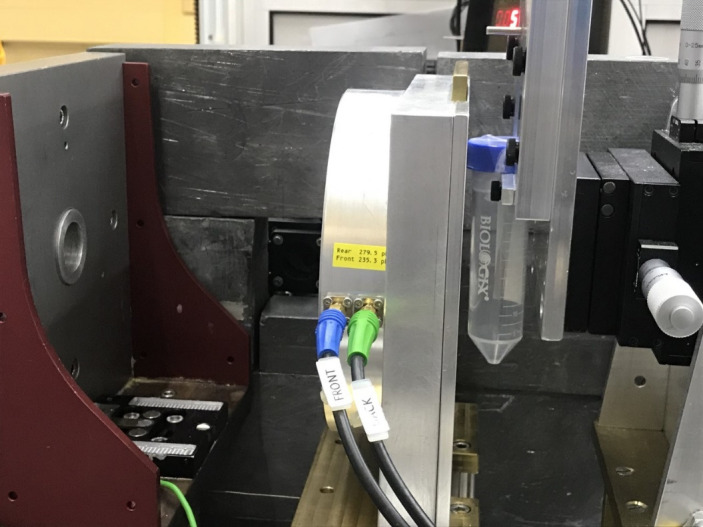
Image of the irradiation setup: each slide was sealed inside a centrifuge tube prior to irradiation (see Materials and Method), with the glass slide-mounted agarose gel containing whole blood sample centred against the collimation system perpendicular to the electron beam.
FLASH irradiation
All FLASH irradiations were performed with pulses of 5 Gy, each with a duration of 3.4 µs (pulse width), a pulse dose rate (or instantaneous dose rate) of 1.5 MGy/s and a pulse repetition frequency of 300 Hz, e.g. 4 pulses were used for a 20 Gy delivery, for a total irradiation time of 0.01 s and an average dose rate of 2 kGy/s.
Conventional irradiation
For conventional irradiation, pulses of 4 mGy were used, each with a duration of 3.4 µs (pulse width), a pulse dose rate (or instantaneous dose rate) of 1.2 kGy/s, a pulse repetition frequency of 25 Hz, and an average dose rate of 0.1 Gy/s, e.g. a 20 Gy delivery had a total irradiation time of 200 s.
Intermediate dose rates
For the eight intermediate dose rates levels, the amplitude of the 3.4 µs electron pulses were controlled by varying the electron gun current on the linac. Also, the pulse repetition frequency was set to 300 Hz for the 5 higher dose rates and at 25 Hz for the three lower dose rates, in order to achieve the desired average dose rates (Table 1).
Table 1.
Irradiation parameters for a 20 Gy delivery at varying average dose rates
| Average dose rate (Gy/s) | Total irradiation time (s) | Pulse repetition frequency (Hz) | Number of pulses (n) | Dose-per-pulse (Gy) | Pulse dose rate (kGy/s) |
|---|---|---|---|---|---|
| 0.1 | 200 | 25 | 5001 | 0.004 | 1.2 |
| 0.3 | 67 | 25 | 1668 | 0.012 | 3.5 |
| 1 | 20 | 25 | 501 | 0.04 | 12 |
| 3 | 6.7 | 25 | 168 | 0.12 | 35 |
| 10 | 2 | 300 | 601 | 0.033 | 98 |
| 30 | 0.67 | 300 | 201 | 0.1 | 29 |
| 100 | 0.2 | 300 | 61 | 0.33 | 96 |
| 300 | 0.067 | 300 | 21 | 0.95 | 280 |
| 1000 | 0.02 | 300 | 7 | 2.9 | 840 |
| 2000 | 0.01 | 300 | 4 | 5 | 1500 |
Dosimetry procedure
Preceding and following irradiation of each experiment and change in beam settings, radiochromic film (GafChromic EBT-XD, Ashland Inc., Covington, KY) was irradiated to verify the dose delivered. Pieces of film (3.3 × 2.3 cm2) were positioned directly on a microscope slide which was placed inside a 50 ml centrifuge tube and positioned as the blood/agarose samples in (and perpendicular to) the beam and irradiated with the defined beam settings. The films were scanned 24 h post-irradiation (Epson Perfection v850 Pro, Seiko Epson Corporation, Nagano, Japan) and the red channel analysed (ImageJ v. 1.52a, Wayne Rasband, NIH). The averaged dose over a 1.2 × 2.0 cm2 central part of the film was recorded. The films had previously been calibrated in a 6 MeV clinical electron beam from a Varian Truebeam linac (Varian Medical Systems Inc., Palo Alto, CA). For online verification of the dose delivery, a toroidal beam charge monitor as well as a beam energy monitor was used. 30 The energy monitor was also used to verify that the electron beam energy was consistently 6 MeV. Our overall uncertainty in dosimetry was estimated to be 4%, including a measured output variation of our FLASH and CONV IR deliveries of within 2%. For intermediate dose rates, due to an increased output variation, the overall uncertainty in dosimetry was estimated to be 8%.
Electrophoresis & comet visualisation/Scoring
The following steps were carried out in the dark, under red light. On removal from lysis, slides were washed twice for 10 min with double distilled water (ddH2O) and incubated in ice-cold electrophoresis buffer for 20 min. Electrophoresis was then performed for 20 min at 30 V (0.8 V/cm) 300 mA. Slides were then incubated in neutralisation buffer for 20 min, washed two times with ddH2O and allowed to dry at 37°C.
Once dry, slides were rehydrated with ddH2O for 20 min then stained with approximately 1 ml propidium iodide (PI) diluted in ddH2O (2.5 µg ml−1) for 25 min, washed two times with ddH2O and dried at 37°C. Comets were visualized at X20 magnification using a fluorescence microscope (Zeiss Axioskop 450; Carl Zeiss, Jena, Germany) fitted with an excitation filter of 515–535 nm, a barrier filter of 590 nm and a 100 W mercury lamp.
Images were captured from the microscope using an attached Stingray F-046B digital camera (Allied Vision Technologies, Stadtroda, Germany) connected to a computer running COMET IV software (Perceptive Instruments, Instem, Cambridge, UK). Comets were captured and scored by randomly selecting 50 comets from the centre of each gel.
Statistical analysis
COMET IV Software calculated % Tail DNA automatically, producing a spreadsheet of data for each slide; % Tail DNA was selected for use as the Comet parameter that best reflects DNA damage. 31 Each individual experiment had up to three internal replicates (constituting three slides = three gels) and each experiment was repeated three times to yield the mean and standard deviation of each test condition. Graphs were created using GraphPad Prism 9 (GraphPad Software, La Jolla, CA).
Statistical analysis was performed using the ‘Analyze’ tool on GraphPad Prism. A two-tailed unpaired t-test was used to assess statistically significant differences (p < 0.05) between % Tail DNA values (FLASH vs CONV) at different oxygen concentrations, at different total doses and various dose rates.
Results
Varying oxygen concentration
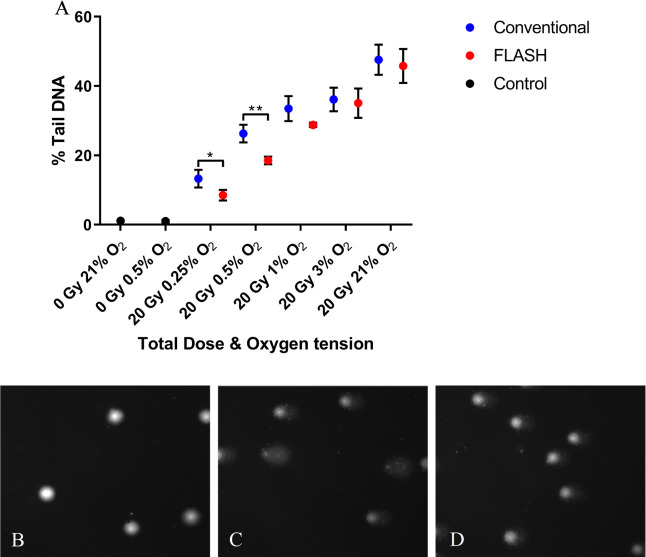
A small (non-significant) difference in DNA damage of PBL was noted following irradiation (20 Gy total dose) when delivered at FLASH (2 kGy/s) vs CONV (0.1 Gy/s) dose rates at 21% O2 (Figure 2), with FLASH inducing a 2% Tail DNA (mean) value lower than CONV. Similarly, a small (non-significant) difference of 1% Tail DNA was observed between FLASH and CONV at 3% O2. At 1% O2, a larger difference (though non-significant) of 5% Tail DNA was observed between FLASH vs CONV irradiation (Figure 2).
Figure 2.
A) Alkaline comet assay measures of peripheral blood lymphocytes (PBL) DNA damage formation (% Tail DNA) following 20 Gy FLASH (2 kGy/s) or conventional dose rate (CONV, 0.1 Gy/s) irradiation over 0.25–21% oxygen tension. Data expressed as the mean % Tail DNA of three slides (n = 3); error bars indicate standard deviation of the means for each experimental condition. Statistical analysis (two-tailed unpaired t-test) FLASH vs CONV revealed significant differences (*, p < 0.05) at 0.25% O2 and (**, p < 0.01) at 0.5% O2. Comet images captured of B) PBL unirradiated at 0.5% O2; C) PBL 20 Gy CONV irradiated at 0.5% O2; D) PBL 20 Gy FLASH irradiated at 0.5% O2. PBL, peripheral blood lymphocyte.
However, significant differences of 8 and 5% Tail DNA were observed between FLASH and CONV at 0.5% O2 (**, p < 0.01) and at 0.25% (*, p < 0.05) (Figure 2). These respective % Tail DNA values correspond to relative DNA damage differences between FLASH and CONV (mean values of %Tail DNA, ) of 0.96, 0.97, 0.85, 0.69, and 0.61 for 21%, 3%, 1%, 0.5%, and 0.25% O2, respectively.
Varying dose
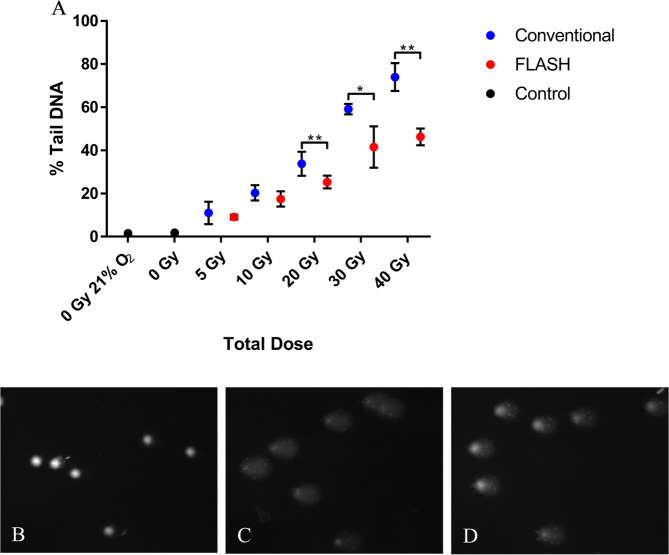
Again, the same difference in PBL DNA damage (8% less Tail DNA) between FLASH and CONV irradiation was observed following 20 Gy irradiation at 0.5% O2 (Figure 3). The difference in DNA damage between FLASH and CONV was seen to increase with total dose delivered. For 5 Gy FLASH, the difference in % Tail DNA (mean value) between FLASH and CONV was 2%. The difference increased with dose to 3% for 10 Gy, and became significant at 20, 30 and 40 Gy with differences of 8%, 18% and 28%, respectively (Figure 3). This corresponds to a relative DNA damage difference between FLASH and CONV (mean values of %Tail DNA, ) of 0.79, 0.85, 0.73, 0.69 and 0.62 for 5, 10, 20, 30 and 40 Gy, respectively.
Figure 3.
A) Alkaline comet assay measures of PBL DNA damage formation (% Tail DNA) following FLASH (2 kGy/s) or conventional dose rate (CONV, 0.1 Gy/s) irradiation to various total doses. Data expressed as the mean % Tail DNA of three slides (n = 3); error bars indicate standard deviation of the means for each experimental condition. Statistical analysis (two-tailed unpaired t-test) FLASH vs CONV revealed no significant differences for doses ≤ 10 Gy. However, statistically significant differences were found for 20, 30 and 40 Gy (**, p < 0.01; *, p < 0.05). Comet images of B) PBL unirradiated 0.5% O2; C) PBL 40 Gy CONV irradiated at 0.5% O2; D) PBL 40 Gy FLASH irradiated at 0.5% O2. PBL, peripheral blood lymphocyte.
Varying dose rates
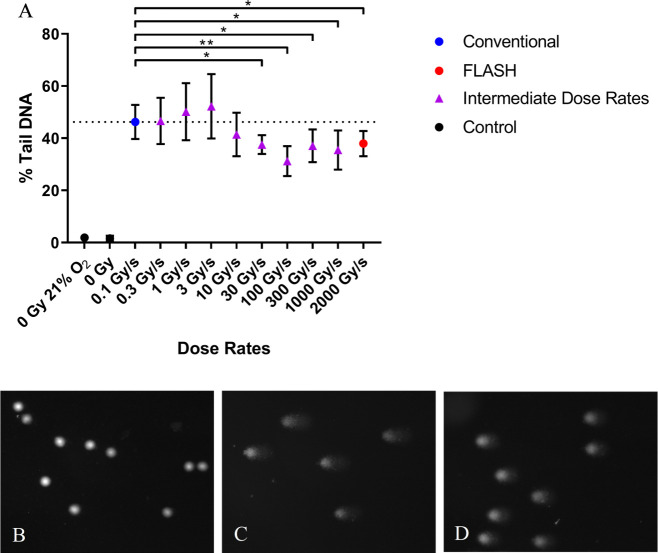
A difference in DNA damage formation (% Tail DNA, mean values) was found for samples exposed to 20 Gy irradiation at CONV dose rate (0.1 Gy/s) and samples exposed to dose rates ≥10 Gy/s, which became significant for dose rates ≥30 Gy/s (Figure 4). Differences in % Tail DNA of 5%, 9%, 15%, 9%, 11% and 8% were found between 0.1 Gy/s and 10, 30, 100, 300, 1000, and 2000 Gy/s, respectively (Figure 4). This corresponds to relative DNA damage differences (mean value of %Tail DNA, ) of 0.83, 0.81, 0.66, 0.79, 0.76 and 0.81 for 10, 30, 100, 300, 1000 and 2000 Gy/s, respectively.
Figure 4.
A) Alkaline comet assay measures of PBL DNA damage formation (% Tail DNA) irradiated to 20 Gy total dose at various dose rates 0.1–2kGy/s. Data expressed as the mean % Tail DNA of three slides (n = 3); error bars indicate standard deviation of the means for each experimental condition. Horizontal dashed line represents % Tail DNA value for 0.1 Gy/s. Statistical analysis (two-tailed unpaired t-test) FLASH vs CONV revealed no significant differences for dose rates ≤ 10 Gy/s. However, statistically significant differences were found between 0.1 Gy/s and dose rates ≥ 30 Gy/s (**, p < 0.01; *, p < 0.05). Comet images of B) PBL unirradiated at 0.5% O2; C) PBL 0.1 Gy/s CONV irradiated at 0.5% O2; D) PBL 100 Gy/s FLASH irradiated at 0.5% O2. PBL, peripheral blood lymphocytes.
Discussion
To date, studies of the FLASH effect and notably the proposed radiolytic consumption of oxygen as a driver of the FLASH sparing of normal tissues, have largely investigated models utilizing water. 32,33 In these studies, it is proposed that the products of water radiolysis (namely hydrated electrons (e aq ⁻) and hydrogen radicals (H•)) react with the dissolved molecular oxygen to produce superoxide anions and perhydroxyl radicals, respectively. However, such reactions will not occur to any significant extent within cells because of the high concentrations of competing scavengers, and such studies have attracted criticism as being inappropriate for the study of FLASH vs CONV exposures. 34,35 A more credible route for the radiolytic consumption of oxygen is via oxygen reacting with radiation-induced secondary and tertiary organic radicals that will occur on the millisecond-or-greater timescale. 35 Therefore, further in vitro work, enabling superior parameter variation and control vs in-vivo studies, is warranted to uncover the likely mechanisms underpinning the benefits of FLASH. This formed the rationale of our ex-vivo irradiations (FLASH vs CONV), varying oxygen concentration, dose, and dose rate to corroborate previous cell survival and in-vivo studies. 8,10,19,36
The alkaline comet assay was chosen as a suitable method to assess DNA damage formation in this study, as it allows for the direct analysis of PBL DNA damage induced following FLASH and CONV irradiation. 37 The assay uses gel electrophoresis of lysed cell nucleoids combined with fluorescence microscopy to allow for the assessment of broken DNA from individual cells. 38 The alkaline version of the assay detects single and double strand breaks and breaks resulting from alkaline labile sites, 39 which were relatively quantified as Tail DNA (%). 31
The comet assay shows a small non-significant sparing of DNA damage for FLASH vs CONV irradiation at 21% O2. A similar non-significant sparing has previously been shown for FLASH in studies of clonogenic survival at 21%, which grows to become significant at lower oxygen concentrations (1–4%), similar to the comet assay data. 19,23,40 Also at 21% O2, Fouillade et al. found that FLASH induces less initial, 53BP1-relevant DNA damage than CONV and that this difference is specific to normal cells, 41 while Adrian et al. found no significant difference in 53BP1-relevant DNA damage but a difference in clonogenic survival that was cell line dependent. 42 In contrast, Buonanno et al. found no statistical difference in clonogenic survival for proton FLASH vs CONV but found a saturation of γH2AX foci formation beyond 10 Gy for the highest dose rate used (1000 Gy/s), an effect that was not seen for lower dose rates (0.05 and 100 Gy/s). 43 Similarly, the comet assay shows sparing of DNA damage for FLASH above 10 Gy, albeit at a lower oxygen concentration of 0.5%.
Transient hypoxia due to radiolytic oxygen depletion following FLASH may account for the significant differential in PBL DNA damage (% Tail DNA) seen at 0.5 and 0.25% O2 vs CONV irradiation (20 Gy total dose delivered), with this sparing effect being modulated by varying the oxygen concentration (Figure 2). When DNA radicals are produced from ionizing radiation, they may react with oxygen to form peroxyl radicals yielding higher levels of permanent DNA damage; but with the caveat that both peroxyl formation and thiol repair may be reversible/not permanent. 15,16,44 Previous studies have measured radiolytic oxygen depletion in aqueous solutions to be around 0.20 mmHg/Gy (i.e. 0.5% or 4 mmHg/20 Gy), which suggests that the 0.5 and 0.25% O2 local oxygen is likely fully consumed in the blood/agarose samples during a 20 Gy delivery, 21,23 through reaction with radiation-induced secondary and tertiary organic radicals. Subsequently, the DNA radicals produced under hypoxia may better undergo thiol mediated chemical repair leading to lower levels of strand break damage formation. 14–20 However, due to the more protracted delivery time for CONV, oxygen is better replenished inside the cell and the oxic conditions maintained. The results from this study meet the expectation that FLASH may induce a local transient hypoxia that may not be visible above or below a certain threshold of oxygen. 18,19,24,45,46 In relation to the results seen in Figure 2, it has been proposed that normal tissue protection following FLASH irradiation in vivo may arise via sparing of hypoxic stem cell niches. 47 These have the potential to elicit a hypoxic response to FLASH irradiation, via oxygen depletion, which may account for conferred radioresistance causing greater stem cell sparing under FLASH vs CONV irradiation.
Increasing irradiation dose rates to ultra-high levels may provide a sparing effect in terms of reducing DNA damage in normal tissues. 10,41 In our study, this was observed in healthy human PBL for dose rates of ≥10 Gy/s, which became significant for dose rates ≥ 30 Gy/s (Figure 4). This is similar to the neurocognitive sparing seen in the mice study by Montay-Gruel et al. 36 Likewise, increasing the total dose delivered to PBL at 0.5% O2 (Figure 3) was also found to create a greater differential between FLASH and CONV DNA damage values (≥20 Gy). Subjecting PBL to ≥20 Gy total dose at 0.5% O2 will sufficiently deplete oxygen, enabling less DNA damage formation under FLASH irradiation in comparison to CONV dose rates (see above).
According to our results, radiolytic oxygen depletion, leading to transient hypoxia may be a contributor for the FLASH survival/sparing effect seen in vitro. 8,19,40 However, radical–radical reactions may also contribute. 34,48 Furthermore, a small sparing effect has also been observed for some cell lines that appears to be unrelated to the oxygen content and thereby not explained by oxygen depletion. 41,42 A differential in immune response 49 (possibly as a consequence of hypoxia) may also contribute to the FLASH sparing effect seen in vivo. Furthermore, considering our data, the FLASH sparing effect seen in vivo in normal tissues at higher oxygen tensions, may only in part be driven by oxygen depletion. It is imperative to truly understand the role of radiolytic oxygen depletion and the ‘FLASH effect’, as to whether it may provide a sparing effect not just to normal tissues but also to solid tumour types that contain sufficient oxygen to produce a lowered radiosensitivity under FLASH irradiation. 19
Conclusions
We have identified an ex-vivo FLASH sparing effect. A differential in DNA damage formation of human PBL between CONV and FLASH irradiation is present at low oxygen tension, significantly at 0.25 and 0.5% O2. We have also shown that this differential in DNA damage is modulated by total dose and dose rate, finding it to increase with total dose delivered and resulting in significantly less DNA damage formation in PBL irradiated at dose rates ≥30 Gy/s in comparison to 0.1 Gy/s. Our findings of FLASH irradiation inducing lower levels of DNA damage meets an expectation of FLASH-induced transient oxygen depletion, and these two factors together (induced hypoxia and/or lower damage levels) may contribute to normal tissue sparing effects of FLASH radiotherapy in vivo.
Supplementary Material
Footnotes
Acknowledgements: We acknowledge Aleksandra Bzura for helpful advice concerning the Comet protocol.
Funding: CRC’s PhD was funded by the Leicester Cancer Research Centre, University of Leicester. This study was also funded by Cancer Research UK (RadNet Grant [C6078/A28736]) and the Medical Research Council (MRC [MC_UU_00001/9]).
Contributor Information
Christian R Cooper, Email: crc23@leicester.ac.uk, Leicester Cancer Research Centre, University of Leicester, Robert Kilpatrick Clinical Sciences Building, Leicester Royal Infirmary, Leicester, UK .
Donald Jones, Email: donald.jones@leicester.ac.uk, Leicester Cancer Research Centre, University of Leicester, Robert Kilpatrick Clinical Sciences Building, Leicester Royal Infirmary, Leicester, UK .
George DD Jones, Email: gdj2@leicester.ac.uk, Leicester Cancer Research Centre, University of Leicester, Robert Kilpatrick Clinical Sciences Building, Leicester Royal Infirmary, Leicester, UK .
Kristoffer Petersson, Email: kristoffer.petersson@oncology.ox.ac.uk, MRC Oxford Institute for Radiation Oncology, University of Oxford, Old Road Campus Research Building, Oxford, UK ; Department of Haematology, Oncology and Radiation Physics, Skåne University Hospital Lund University, Lund, Sweden .
REFERENCES
- 1. Barazzuol L, Coppes RP, van Luijk P . Prevention and treatment of radiotherapy-induced side effects . Mol Oncol 2020. ; 14: 1538 – 54 . doi: 10.1002/1878-0261.12750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bourhis J, Montay-Gruel P, Gonçalves Jorge P, Bailat C, Petit B, et al. . Clinical translation of flash radiotherapy: why and how? Radiother Oncol 2019. ; 139: 11 – 17 . doi: 10.1016/j.radonc.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 3. Zhang Q, Liu J, Ao N, Yu H, Peng Y, et al. . Secondary cancer risk after radiation therapy for breast cancer with different radiotherapy techniques . Sci Rep 2020. ; 10( 1 ): 1220 . doi: 10.1038/s41598-020-58134-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin B, Gao F, Yang Y, Wu D, Zhang Y, et al. . FLASH radiotherapy: history and future . Front Oncol 2021. ; 11: 644400 . doi: 10.3389/fonc.2021.644400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Epp ER, Weiss H, Ling CC . Irradiation of cells by single and double pulses of high intensity radiation: oxygen sensitization and diffusion kinetics . Curr Top Radiat Res Q 1976. ; 11: 201 – 50 . [PubMed] [Google Scholar]
- 6. Hendry JH, Moore JV, Hodgson BW, Keene JP . The constant low oxygen concentration in all the target cells for mouse tail radionecrosis . Radiat Res 1982. ; 92: 172 – 81 . doi: 10.2307/3575852 [DOI] [PubMed] [Google Scholar]
- 7. Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, et al. . Ultrahigh dose-rate flash irradiation increases the differential response between normal and tumor tissue in mice . Sci Transl Med 2014. ; 6: 245 . doi: 10.1126/scitranslmed.3008973 [DOI] [PubMed] [Google Scholar]
- 8. Montay-Gruel P, Acharya MM, Petersson K, Alikhani L, Yakkala C, et al. . Long-term neurocognitive benefits of flash radiotherapy driven by reduced reactive oxygen species . Proc Natl Acad Sci U S A 2019. ; 116: 10943 – 51 . doi: 10.1073/pnas.1901777116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levy K, Natarajan S, Wang J, Chow S, Eggold JT, et al. . Abdominal flash irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice . Sci Rep 2020. ; 10( 1 ): 21600 . doi: 10.1038/s41598-020-78017-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruan J-L, Lee C, Wouters S, Tullis IDC, Verslegers M, et al. . Irradiation at ultra-high (flash) dose rates reduces acute normal tissue toxicity in the mouse gastrointestinal system . Int J Radiat Oncol Biol Phys 2021. ; 111: 1250 – 61 . doi: 10.1016/j.ijrobp.2021.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vozenin M-C, De Fornel P, Petersson K, Favaudon V, Jaccard M, et al. . The advantage of flash radiotherapy confirmed in mini-pig and cat-cancer patients . Clin Cancer Res 2019. ; 25: 35 – 42 . doi: 10.1158/1078-0432.CCR-17-3375 [DOI] [PubMed] [Google Scholar]
- 12. Konradsson E, Arendt ML, Bastholm Jensen K, Børresen B, Hansen AE, et al. . Establishment and initial experience of clinical flash radiotherapy in canine cancer patients . Front Oncol 2021. ; 11: 658004 . doi: 10.3389/fonc.2021.658004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilson JD, Hammond EM, Higgins GS, Petersson K . Corrigendum: ultra-high dose rate (flash) radiotherapy: silver bullet or fool’s gold? Front Oncol 2020. ; 10: 210 . doi: 10.3389/fonc.2020.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adams G . Radiation chemical mechanisms in radiation biology . Adv Radiat Chem 1972. ; 3: 125 – 208 . [Google Scholar]
- 15. von Sonntag C . The Chemical Basis of Radiation Biology . London: : Taylor & Francis; ; 1987. . [Google Scholar]
- 16. von Sonntag C . Free-Radical-Induced DNA Damage and Its Repair . Berlin, Heidelberg: : Springer; ; 2006. . doi: 10.1007/3-540-30592-0 [DOI] [Google Scholar]
- 17. Grimes DR, Partridge M . A mechanistic investigation of the oxygen fixation hypothesis and oxygen enhancement ratio . Biomed Phys Eng Express 2015. ; 1: 045209 . doi: 10.1088/2057-1976/1/4/045209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spitz DR, Buettner GR, Petronek MS, St-Aubin JJ, Flynn RT, et al. . An integrated physico-chemical approach for explaining the differential impact of flash versus conventional dose rate irradiation on cancer and normal tissue responses . Radiother Oncol 2019. ; 139: 23 – 27 . doi: 10.1016/j.radonc.2019.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adrian G, Konradsson E, Lempart M, Bäck S, Ceberg C, et al. . The flash effect depends on oxygen concentration . Br J Radiol 2020. ; 93: 1106 . doi: 10.1259/bjr.20190702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petersson K, Adrian G, Butterworth K, McMahon SJ . A quantitative analysis of the role of oxygen tension in flash radiation therapy . Int J Radiat Oncol Biol Phys 2020. ; 107: 539 – 47 . doi: 10.1016/j.ijrobp.2020.02.634 [DOI] [PubMed] [Google Scholar]
- 21. Cao X, Zhang R, Esipova TV, Allu SR, Ashraf R, et al. . Quantification of oxygen depletion during flash irradiation in vitro and in vivo . Int J Radiat Oncol Biol Phys 2021. ; 111: 240 – 48 . doi: 10.1016/j.ijrobp.2021.03.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wardman P . Comment on: may oxygen depletion explain the flash effect? a chemical track structure analysis . Radiother Oncol 2021. ; 163: 91 – 92 . doi: 10.1016/j.radonc.2021.08.004 [DOI] [PubMed] [Google Scholar]
- 23. Moon EJ, Petersson K, Olcina MM . The importance of hypoxia in radiotherapy for the immune response, metastatic potential and flash-rt . Int J Radiat Biol 2021. ; 1 – 13 . doi: 10.1080/09553002.2021.1988178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khan S, Bassenne M, Wang J, Manjappa R, Melemenidis S, et al. . Multicellular spheroids as in vitro models of oxygen depletion during flash irradiation . Int J Radiat Oncol Biol Phys 2021. ; 110: 833 – 44 . doi: 10.1016/j.ijrobp.2021.01.050 [DOI] [PubMed] [Google Scholar]
- 25. Hornsey S, Bewley DK . Hypoxia in mouse intestine induced by electron irradiation at high dose-rates . Int J Radiat Biol Relat Stud Phys Chem Med 1971. ; 19: 479 – 83 . doi: 10.1080/09553007114550611 [DOI] [PubMed] [Google Scholar]
- 26. Field SB, Bewley DK . Effects of dose-rate on the radiation response of rat skin . Int J Radiat Biol Relat Stud Phys Chem Med 1974. ; 26: 259 – 67 . doi: 10.1080/09553007414551221 [DOI] [PubMed] [Google Scholar]
- 27. Louagie H, Van Eijkeren M, Philippe J, Thierens H, de Ridder L . Changes in peripheral blood lymphocyte subsets in patients undergoing radiotherapy . Int J Radiat Biol 1999. ; 75: 767 – 71 . doi: 10.1080/095530099140113 [DOI] [PubMed] [Google Scholar]
- 28. Petersson K, Gebre-Medhin M, Ceberg C, Nilsson P, Engström P, et al. . Haematological toxicity in adult patients receiving craniospinal irradiation--indication of a dose-bath effect . Radiother Oncol 2014. ; 111: 47 – 51 . doi: 10.1016/j.radonc.2014.01.020 [DOI] [PubMed] [Google Scholar]
- 29. Heylmann D, Ponath V, Kindler T, Kaina B . Comparison of dna repair and radiosensitivity of different blood cell populations . Sci Rep 2021. ; 11( 1 ): 2478 . doi: 10.1038/s41598-021-81058-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berne A, Petersson K, Tullis IDC, Newman RG, Vojnovic B . Monitoring electron energies during flash irradiations . Phys Med Biol 2021. ; 66( 4 ): 045015 . doi: 10.1088/1361-6560/abd672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Møller P, Azqueta A, Boutet-Robinet E, Koppen G, Bonassi S, et al. . Minimum information for reporting on the comet assay (mirca): recommendations for describing comet assay procedures and results . Nat Protoc 2020. ; 15: 3817 – 26 . doi: 10.1038/s41596-020-0398-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jansen J, Knoll J, Beyreuther E, Pawelke J, Skuza R, et al. . Does flash deplete oxygen? experimental evaluation for photons, protons, and carbon ions . Med Phys 2021. ; 48: 3982 – 90 . doi: 10.1002/mp.14917 [DOI] [PubMed] [Google Scholar]
- 33. Boscolo D, Scifoni E, Durante M, Krämer M, Fuss MC . May oxygen depletion explain the flash effect? a chemical track structure analysis . Radiother Oncol 2021. ; 162: 68 – 75 . doi: 10.1016/j.radonc.2021.06.031 [DOI] [PubMed] [Google Scholar]
- 34. Wardman P . Radiotherapy using high-intensity pulsed radiation beams (flash): a radiation-chemical perspective . Radiat Res 2020. ; 194: 607 – 17 . doi: 10.1667/RADE-19-00016 [DOI] [PubMed] [Google Scholar]
- 35. Koch CJ . Re: “a computer modeling study of water radiolysis at high dose rates. relevance to flash radiotherapy.” ahmed alanazi, jintana meesungnoem and jean-paul gerin. radiat res 2021; 195:149-62 . Radiat Res 2021. ; 196: 447 – 48 . doi: 10.1667/RADE-21-00124.1 [DOI] [PubMed] [Google Scholar]
- 36. Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond J-F, et al. . Irradiation in a flash: unique sparing of memory in mice after whole brain irradiation with dose rates above 100gy/s . Radiother Oncol 2017. ; 124: 365 – 69 . doi: 10.1016/j.radonc.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 37. Collins AR . The comet assay for dna damage and repair: principles, applications, and limitations . Mol Biotechnol 2004. ; 26: 249 – 61 . doi: 10.1385/MB:26:3:249 [DOI] [PubMed] [Google Scholar]
- 38. Olive PL, Banáth JP . The comet assay: a method to measure dna damage in individual cells . Nat Protoc 2006. ; 1: 23 – 29 . doi: 10.1038/nprot.2006.5 [DOI] [PubMed] [Google Scholar]
- 39. Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, et al. . Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing . Environ Mol Mutagen 2000. ; 35: 206 – 21 . doi: [DOI] [PubMed] [Google Scholar]
- 40. Tessonnier T, Mein S, Walsh DWM, Schuhmacher N, Liew H, et al. . FLASH dose rate helium ion beams: first in vitro investigations . Int J Radiat Oncol Biol Phys 2021. ; 111: 1011 – 22 . doi: 10.1016/j.ijrobp.2021.07.1703 [DOI] [PubMed] [Google Scholar]
- 41. Fouillade C, Curras-Alonso S, Giuranno L, Quelennec E, Heinrich S, et al. . FLASH irradiation spares lung progenitor cells and limits the incidence of radio-induced senescence . Clin Cancer Res 2020. ; 26: 1497 – 1506 . doi: 10.1158/1078-0432.CCR-19-1440 [DOI] [PubMed] [Google Scholar]
- 42. Adrian G, Konradsson E, Beyer S, Wittrup A, Butterworth KT, et al. . Cancer cells can exhibit a sparing flash effect at low doses under normoxic in vitro-conditions . Front Oncol 2021. ; 11: 686142 . doi: 10.3389/fonc.2021.686142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Buonanno M, Grilj V, Brenner DJ . Biological effects in normal cells exposed to flash dose rate protons . Radiother Oncol 2019. ; 139: 51 – 55 . doi: 10.1016/j.radonc.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O’Neill P, Wardman P . Radiation chemistry comes before radiation biology . Int J Radiat Biol 2009. ; 85: 9 – 25 . doi: 10.1080/09553000802640401 [DOI] [PubMed] [Google Scholar]
- 45. Dewey DL, Boag JW . Modification of the oxygen effect when bacteria are given large pulses of radiation . Nature 1959. ; 183: 1450 – 51 . doi: 10.1038/1831450a0 [DOI] [PubMed] [Google Scholar]
- 46. Berry RJ, Stedeford JB . Reproductive survival of mammalian cells after irradiation at ultra-high dose-rates: further observations and their importance for radiotherapy . Br J Radiol 1972. ; 45: 171 – 77 . doi: 10.1259/0007-1285-45-531-171 [DOI] [PubMed] [Google Scholar]
- 47. Pratx G, Kapp DS . Ultra-high-dose-rate flash irradiation may spare hypoxic stem cell niches in normal tissues . Int J Radiat Oncol Biol Phys 2019. ; 105: 190 – 92 . doi: 10.1016/j.ijrobp.2019.05.030 [DOI] [PubMed] [Google Scholar]
- 48. Labarbe R, Hotoiu L, Barbier J, Favaudon V . A physicochemical model of reaction kinetics supports peroxyl radical recombination as the main determinant of the flash effect . Radiother Oncol 2020. ; 153: 303 – 10 . doi: 10.1016/j.radonc.2020.06.001 [DOI] [PubMed] [Google Scholar]
- 49. Zhang Y, Ding Z, Perentesis JP, Khuntia D, Pfister SX, et al. . Can rational combination of ultra-high dose rate flash radiotherapy with immunotherapy provide a novel approach to cancer treatment? Clin Oncol (R Coll Radiol) 2021. ; 33: 713 – 22 . doi: 10.1016/j.clon.2021.09.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.