The clinicopathological approach to traumatic brain injury
The clinicopathological method
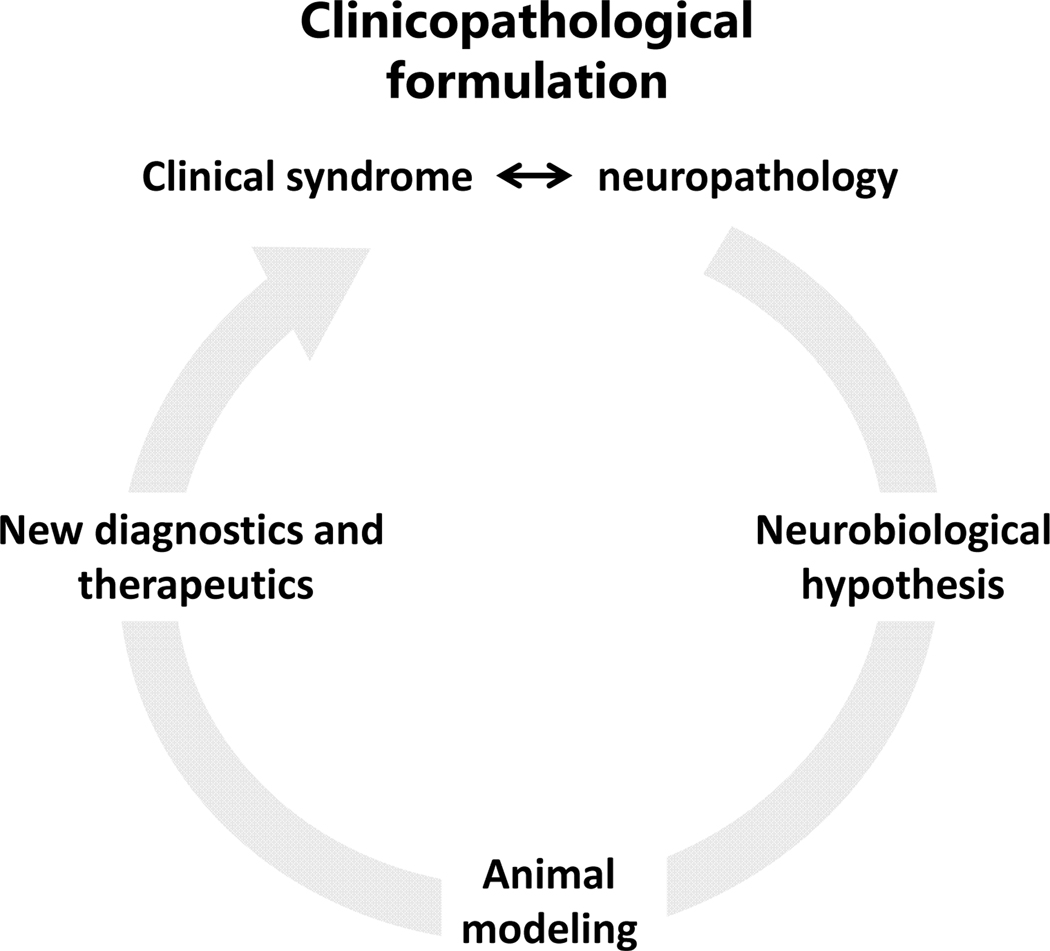
Traumatic brain injury (TBI) is a common problem. In the US alone, it is estimated that there are close to 3 million new cases of TBI requiring medical attention annually. TBI is not a disease per se but a calamity that causes a number of primary lesions and triggers secondary events responsible for transient, static and, rarely, progressive illness. These morbidities are conventionally classified by cause, specific pathology, and clinical severity or outcome. The correspondence among these variables is complex and it is usually more informative to describe TBI based on more than one of them, for example cause and mechanism or mechanism and outcome. Unfortunately, the latter is poorly represented in the literature, especially from long-run samples, and this is a serious limitation especially for moderate-severe TBI that results in chronic disease. A useful approach to conceptualize TBI-related disease is the classical clinicopathological method that links bedside clinical observations and prognoses to findings from the examination of patient tissues and then extends pathological hypotheses to models of disease (1)(Fig. 1). Although the complexity of TBI does not lend itself to straightforward correlations between clinical symptoms and brain pathology, let alone correlations between clinical events and hypotheses or models, the clinicopathological method can be used to outline key issues, track progress, and point out remaining challenges in the behavioral neuroscience and neuropsychiatry of TBI.
Figure 1.
General clinicopathological schema. The problem always starts with the patient. Clinical presentations are first linked to specific pathologies (in the case of neuropsychiatry, neuropathologies), from which hypotheses emerge and then get tested in laboratory models. From the latter emerge more refined hypotheses or diagnostic and therapeutic ideas that take the inquiry from the bench back to bedside. The cycle can be repeated many times to further understanding of the disease and improved diagnostics and therapeutics.
Acute problems associated with moderate-severe TBI involve elevations in intracranial pressure and other neurological and systemic pathophysiologies that cause serious, life-threatening illness managed in critical care settings. This topic is only superficially treated here because our focus is chronic TBI that often becomes referred to the neuropsychiatrist or neuropsychologist. Mild TBI or concussion, a very common problem in the community, is typically a transient illness with good prognosis. Chronic TBI is often associated with significant disability because of permanent damage to brain circuitry and possibly progressive disease and includes: focal contusions often caused by low-impact falls, diffuse axonal injury (DAI) due to rotational acceleration forces as it occurs in motor vehicle crashes, TBI due to repeat concussions e.g. from boxing or other collision/contact sports, and blast injury to brain, an old problem that has resurfaced in the wake of recent US conflicts in Iraq and Afghanistan. Below is a brief account of neuropathology and pathobiology or modeling of these problems with an explanation of their behavioral significance.
Focal TBI: contusions
Low-impact blunt TBI causes focal damage to brain and meninges, including hematomas and parenchymal contusions. Penetrating TBI may also be viewed as an extensive focal TBI determined by the ballistics of the projectile and complex interface phenomena involving the skull. Contusions are common lesions caused by falls, strike-by or -against events and motor vehicle accidents and occur at both the site of impact (coup) and the diametrically opposite site (contrecoup). Striking the immobile head with blunt force, e.g. with a weapon, causes skull fractures and coup lesions at the site of the impact, whereas if the moving head strikes a firm fixed surface there is predominance of contrecoup lesions regardless of impact location. Contrecoup injuries tend to occur at ventral frontal and temporopolar locations for complex mechanical and perhaps also vascular reasons (Fig. 2).
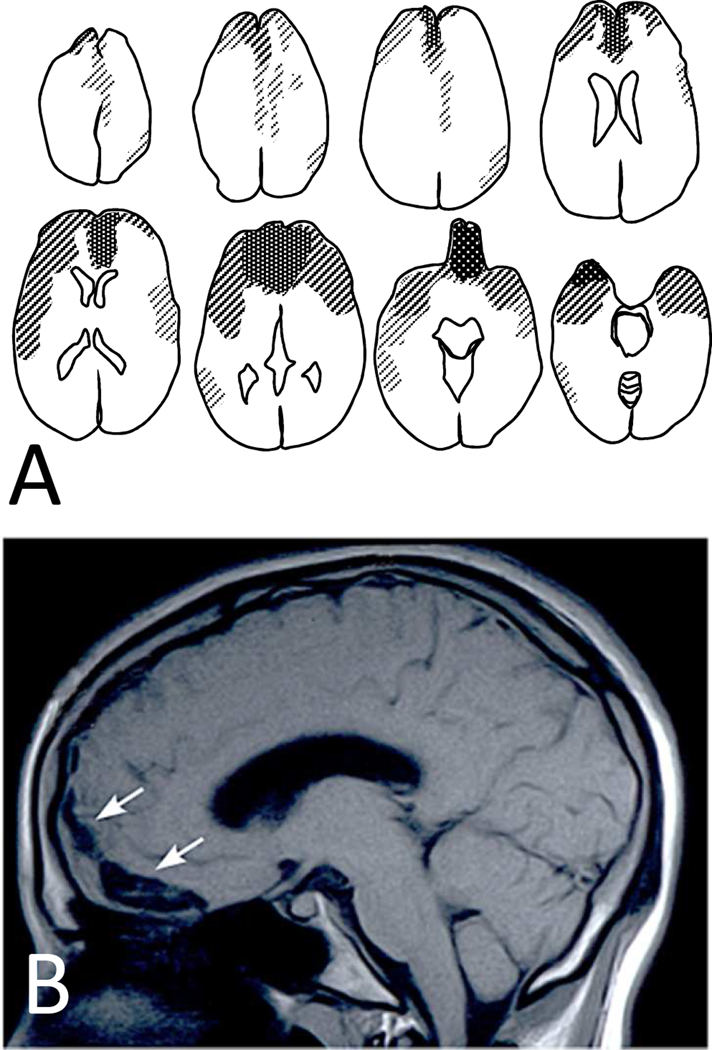
Figure 2.
In brain contusions, concentration of pathology is in orbitofrontal and temporopolar regions. When such cases survive, they often get referred to neuropsychiatric clinics because of chronic and pervasive affective, social, and cognitive changes. Panel A is a compound diagram of brain scans from 28 cases with frontal contusions seen in the Neuropsychiatry Program at Sheppard Pratt. Panel B is a representative case from a 20-year-old patient with orbitofrontal contusion (arrows) resulting from a fall. The patient presented with intact general intelligence but impulsive and inappropriate behaviors causing him to be expelled from college and live a marginal life at home.
The brain contusion is hemorrhagic necrosis of brain tissue that ranges from multiple triangular hemorrhagic lesions near the cortical surface to frank parenchymal hemorrhage and is commonly associated with subarachnoid and intraventricular bleeding. Contusions may evolve in the first few hours or days after injury and some, for example the ones associated with expanding hemorrhage and herniation of the compressed hippocampus and medulla may cause significant secondary morbidity and death. By virtue of their anatomical distribution at the frontotemporal olfactory paralimbic zone, contrecoup lesions typically cause complex “frontal” symptomatology with prominent behavioral and social impairments (see the section on Behavioral and Cognitive Neurology). Focal neurological signs are atypical or may be absent.
The pathobiology of contusion overlaps extensively with that of stroke and involves massive neuronal cell death, as characterized in pioneering work in the 1990s. Proceeding via mechanical and anoxic mechanisms, both neuronal necrosis and apoptosis are encountered, with the former predominating at the center of the contusion whereas apoptosis may prevail at the penumbra. Contusions have been replicated in rodents mainly with the controlled cortical impact model, that has been extremely popular despite two key differences from the clinical scenario, including the exposed brain and the dorsal impact that is ectopic to the fronto-temporal location of clinical contusions (2)(Fig. 3).
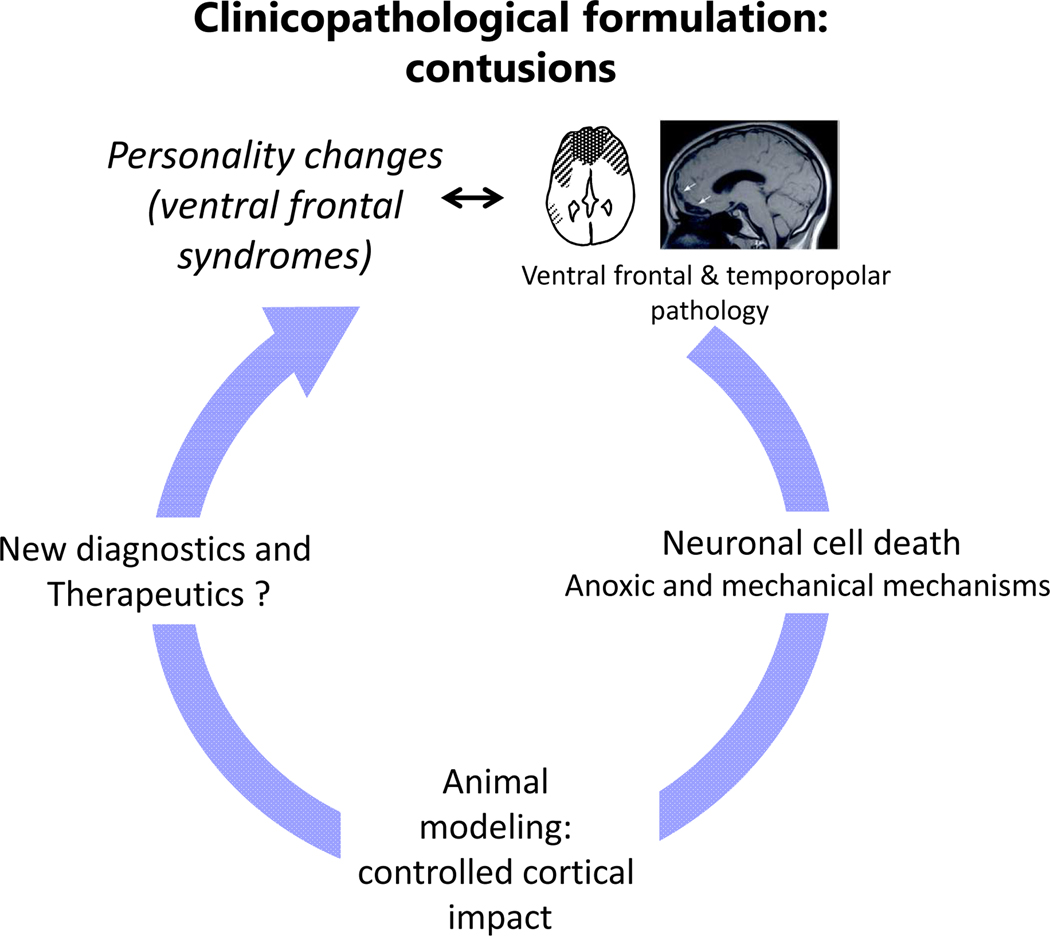
Figure 3.
Clinicopathological schema as it applies to brain contusions. For the general idea, see Fig. 1. Although significant progress has been made in hypotheses and animal models, and perhaps also diagnostics using structural imaging and CSF or serum biomarkers, we still don’t have evidence-based therapies for the main pathologies or the chronic neuropsychiatric impairments.
Diffuse blunt TBI: diffuse axonal injury
In contrast to brain contusions that primarily damage neuronal perikarya, traumatic (diffuse) axonal injury (DAI) is a primary axonal perturbation that occurs very rapidly, usually as a result of rotational acceleration of the head in the course of motor vehicle crashes and high-impact falls (3). DAI leads to multifocal axonal disruption accompanied by focal micro- hemorrhages and, in severe cases, gross shearing lesions in large white matter tracts, especially corpus callosum, and at the gray-white matter junction in the form of gliding contusions (Fig. 4). Widely varying in severity, DAI is a common denominator in all types of TBI, from concussions to blast and chronic traumatic encephalopathy (CTE, see below). The gyrencephalic human brain may be especially prone to DAI, in part because of the sheer bulk of white matter but perhaps also the complex three-dimensional organization of intertwined white matter tracts (4). When it comes to clinicopathological correlations, DAI has been difficult to study compared to contusions, perhaps because of a wider range of severity, variance in anatomical distribution, and the historical use of low-resolution imaging such as CT and conventional MRI. These problems aside, DAI is usually a frontal pathology much like contusions, but involves white matter and is predominantly medial-dorsal, whereas contusions involve gray matter and are ventral (5, 6). With some notable exceptions, most studies have linked DAI with poor outcomes (7, 8) and a whole host of neuropsychiatric symptoms in the cognitive/executive behavioral and affective domains that, at least qualitatively, do not differ much from the symptoms associated with contusions (see the section on Behavioral and Cognitive Neurology). There may be associated motor, cerebellar and other focal symptoms and signs.
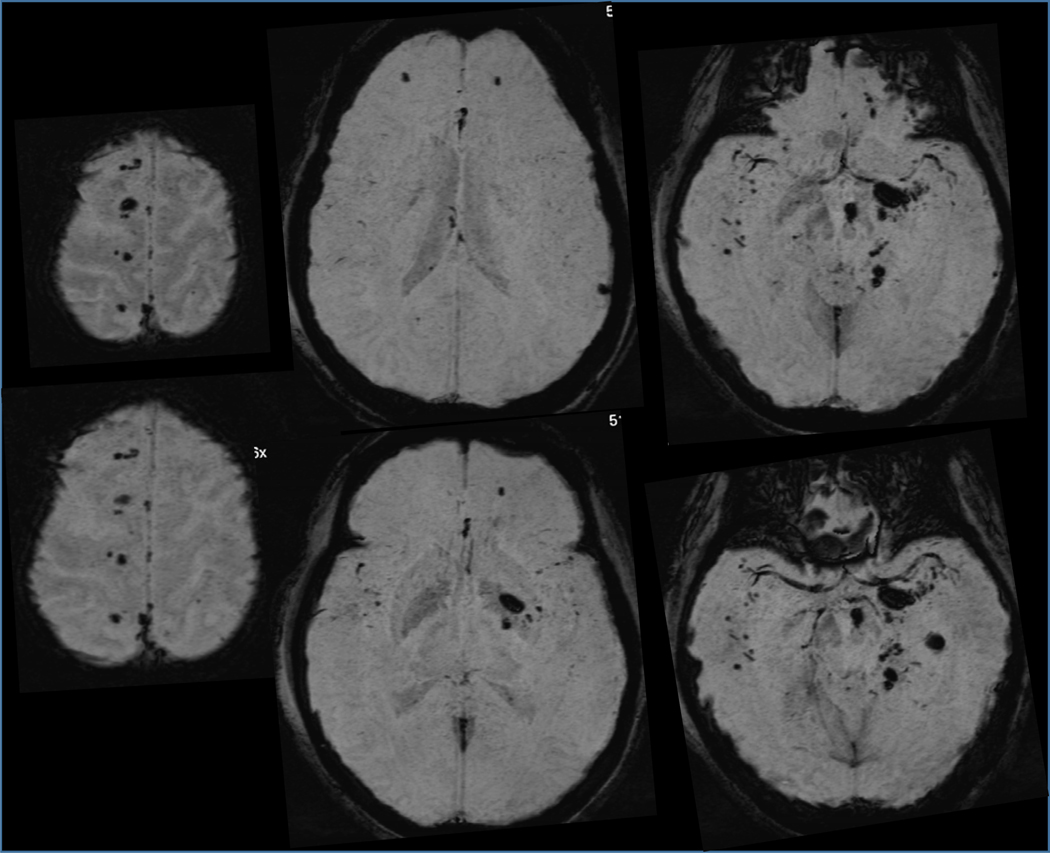
Figure 4.
Despite high mortality in severe cases, may patients with DAI survive. This is a susceptibility weighted brain MRI sequence from this 24-year-old woman, an unbelted driver who was ejected from her vehicle on crash. Brain shows numerous magnetic field-distorting, black-appearing microbleeds from the structural disruption of small vessels, also marking the sites of colocalized axonal injury. The patient had some cognitive deficits and organic personality changes with mood lability, but adjusted reasonably well to a domestic setting. Confluence of lesions in the left internal capsule/peduncle/pons caused right-side weakness.
The mechanisms of DAI have received considerable attention by biologists and bioengineers alike. Engineers tend to associate DAI with the viscoelastic properties of axons, i.e. the concept that axons behave as viscous materials under slow tensile strength and as elastic entities when exposed to rapid tensile elongation (“time-dependent strain”). The former condition may either leave the axonal structure intact or even cause accelerated axonal growth during development due to microtubule polymerization, whereas the latter causes breakage of microtubules leading to undulations, swellings, and axon bulbs. Diffuse axonal injury entails both structural and functional alterations. Some investigators have emphasized the early role of fracture of microtubules, i.e. the stiffest part of axon structure, caused by ultra-rapid shearing or tensile deformation. Microtubule breakdown causes an arrest in anterograde transport of organelles and molecules followed by local accumulation and axonal swelling, then presumably passive separation of the mechanically unsustainable volume and secondary axotomy with the formation of axon bulbs and retraction balls. Additional primary structural effects may include mechanoporation of the axolemma and neurofilament compaction and breakdown. There are closely associated biochemical events such as the influx of Ca2+ followed by activation of calcium-dependent cysteine proteases (calpains, caspases) and phosphatases that further degrade the neuronal cytoskeleton (9).
A disadvantage in studying DAI is that it is not easy to reproduce in rodents that are experimental animals of choice, although we and others have made some progress (10–13). Based on this work, we have recently reformulated the problem of traumatic axonal injury based on evidence for active, as contrasted to passive, mechanisms, such as the highly conserved self-destruction signals associated with Wallerian degeneration (4). Many investigators have emphasized the role of neuroinflammation in both the acute and chronic phase of axonopathy post-TBI, but the existence of both protective and detrimental sides to innate and systemic immune response and the protean nature of microglial phenotypes complicate the interpretation of related findings.
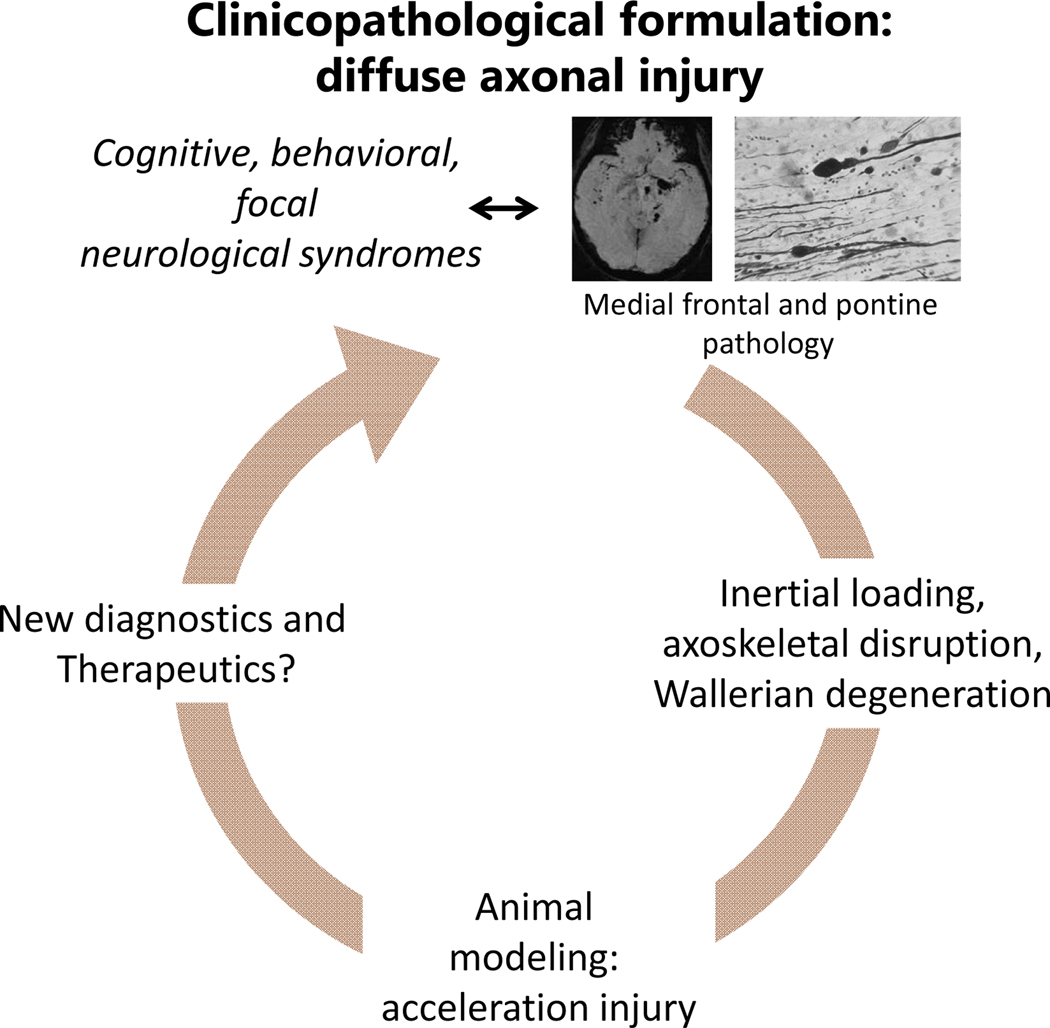
An important aspect of DAI, especially for the clinician, is its secondary effects on populations of neurons with injured axons or damaged inputs. Such effects can lead to retrograde and orthograde (transsynaptic) degeneration proceeding downstream or upstream in the neural circuit. For example, in the first weeks to months after severe acceleration injury, there is progressive reduction in brain size with some selectivity for particular brain regions, although gray and white matter atrophy may not be exactly in anatomical register with each other (14, 15)(Fig. 5). Advances in cellular and molecular neuropathology, primarily based on axotomy models and especially the biology of Wallerian degeneration, may shed light into the nature and time course of these secondary changes especially in the white matter, and help establish windows for potential interventions (see the section on Relevance of the Molecular Neuropathology of White Matter)(Fig. 6).
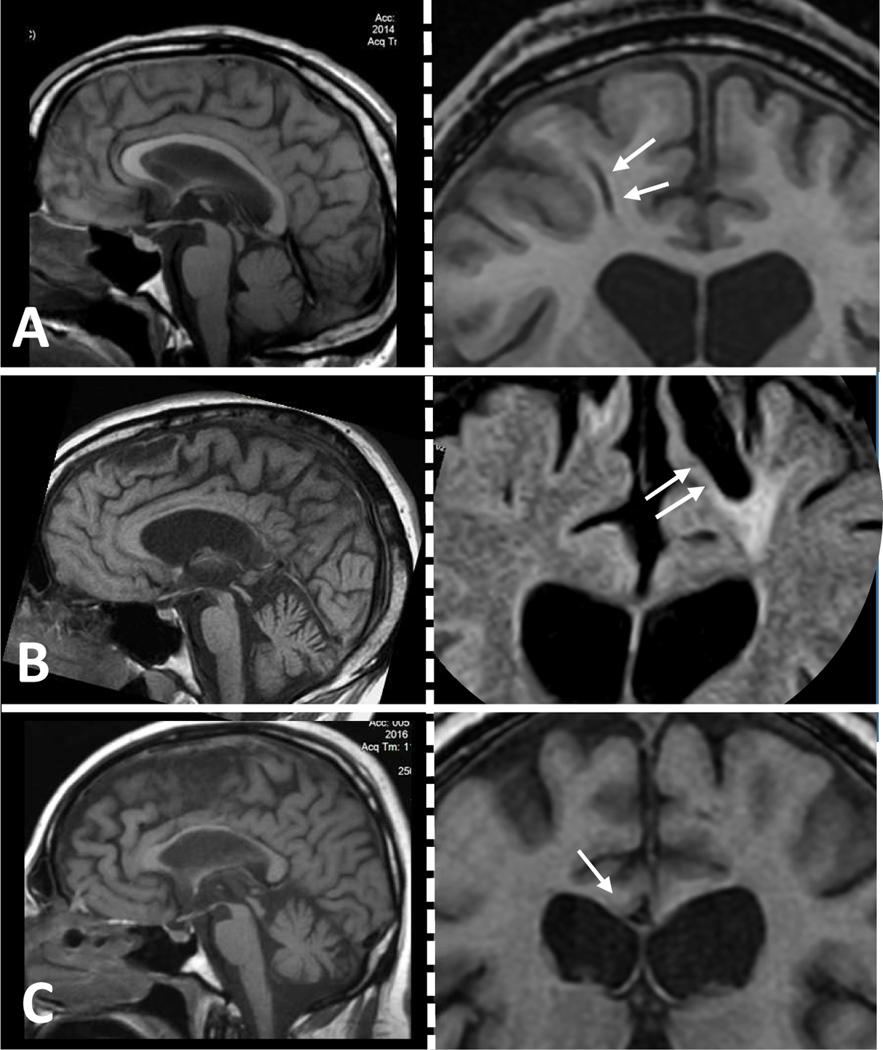
Figure 5.
These three cases of moderate to severe TBI with DAI show progressive atrophy years after injury. (A-C) In each one of the three cases, left-side panels contain midsagittal T1 sequences to illustrate atrophy of the corpus callosum and right-side panels contain corresponding frontal coronal T1 or FLAIR sequences showing evidence of severe DAI as indicated with gliding contusions (arrows, A and B) or gross shearing in the corpus callosum (arrow in C). A: 30 yo, 1.5 years post-TBI; B: 40 yo, 18 years post-TBI; C: 35 yo, 2 years post-TBI.
Figure 6.
Clinicopathological schema for DAI. For the general idea, see Fig. 1. Although pathology is well characterized and the phenomenon is reasonably well understood especially from the bioengineering point of view, animal models are still in development. Imaging and perhaps CSF or serum markers help with diagnosis, but there are no evidence-based treatments for the main pathology or chronic neuropsychiatric deficits.
Repetitive mild TBI and chronic traumatic encephalopathy
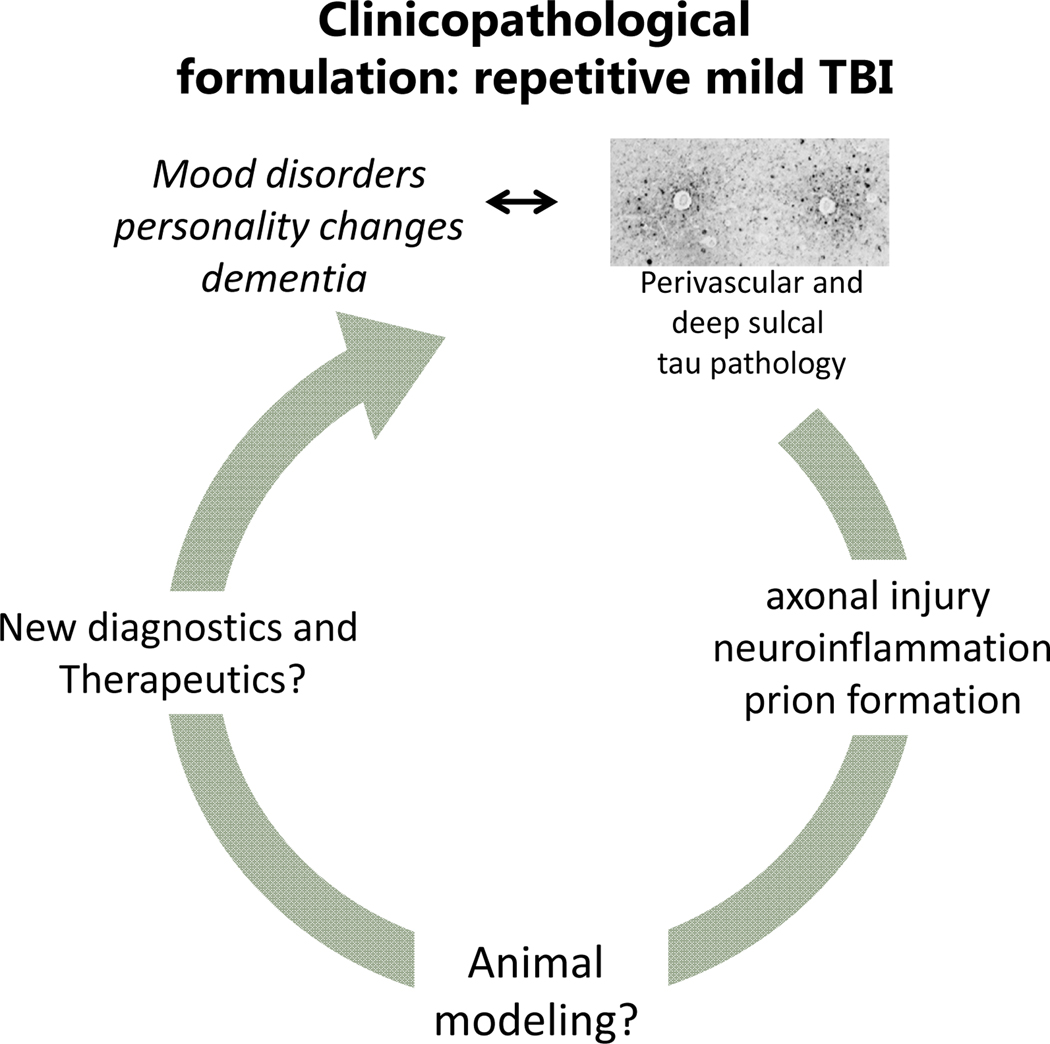
Repeat concussion, associated with specific life styles or medical problems such as chronic involvement with collision and contact sports, partner or child abuse (exposure to repeat violence), epilepsy (falls) and autism (head banging) may have a cumulative effect on the brain. This effect distinguishes repetitive from single concussions that typically have benign outcome. It has been argued that “subconcussive” injuries may also add to the outcome, in other words, the key factor may be the total burden of the life style, rather than individual “index” concussions. Repeat concussions are associated with two main clinical presentations: one is post-concussive syndrome (see the section on The Problem of Mild TBI: Post-Concussive Syndrome). The other is chronic traumatic encephalopathy (CTE), a progressive neurodegenerative disease featured by tau and 43 kDa transactive response (TAR) DNA-binding protein (TDP-43) inclusions and neuronal cell loss in neocortex and the limbic system. TBI-related neurodegeneration has been historically linked to boxing under the name “punch drunk” and “dementia pugilistica” before called CTE in the late 1950s (16–18). In the era of immunohistochemistry, the problem has been redefined as a type of tau proteinopathy associated not only with boxing but also other collision and contact sports, especially American football (19, 20). Tau inclusions in neurons and astrocytes especially around arterioles at the depths of the sulci are the hallmark of the condition. These features distinguish CTE from classical degenerative tauopathies like Alzheimer’s disease (AD), frontotemporal degeneration (FTD) and progressive supranuclear palsy (PSP), although the boundaries are not always clear and there has been no follow-up to recently established consensus diagnostic criteria (21). It is thought that the disease begins in frontal cortex and then progresses caudally.
CTE has been touted as a neuropsychiatric disease with severe mood and behavioral symptoms even in the early stages (see the section on The Problem of Athletic Concussions)(20). However, despite considerable efforts by investigators in Boston University to define clinicopathological correlations and stages of progression based on the gold standard of Braak staging in Alzheimer’s disease, the natural course of CTE remains elusive. This task requires prospective studies and equal chance of including cases with relatively good outcomes (22). Our present understanding of the problem is retrospective, predominantly from brains of boxers and increasingly also brains of professional football players many of whom died prematurely due to suicide or unusual accidents, e.g. accidental gunshot in the course of gun cleaning or falling from the back of a moving track while chasing a fiancé. In the brains of older football veterans, distinction with other more common forms of age-associated degeneration is not always easy.
Proteinopathy, i.e. the acquisition of abnormal conformations by brain proteins and their tendency to aggregate, is increasingly recognized as a common problem across neurodegenerative disease and, for some investigators, the cause of these disorders. In fact, neurodegenerative diseases are often classified on the basis of corresponding aggregated proteins, for example Aβ in AD (“amyloidosis”), synuclein in Parkinson’s disease, Lewy body dementia and multiple systems atrophy (“synucleopathies”), TDP-43, tau and FUS (fused in sarcoma) in FTD and amyotrophic lateral sclerosis (ALS), TDP-43, SOD1 (superoxide dismutase 1) and ubiquitin in ALS, tau in progressive supranuclear palsy, etc. If proteinopathies are the cause of corresponding neurodegenerative diseases (still an unproven claim), the question arises how such protein configurations lead to widespread neuronal death. One of the prevailing theories is the formation of prions, i.e. seeding of misfolded proteins in stable conformational states and then spreading from one part of the brain to another. While misfolded proteins self-template into such seeds, they can also hetero-template by nucleating (“corrupting”) normal proteins. In such a fashion seeds increase in number and size and abnormal proteins take on fibrillar forms, disrupt normal neuronal function, and cause neuronal death. Proteinopathy may coexist with axonal injury and neuroinflammation and be caused by them, for example via excess tau accumulation, aberrant hydrolysis by serum-born enzymes or excessive phosphorylation of tau within neuronal perikarya (23)(Fig. 7).
Figure 7.
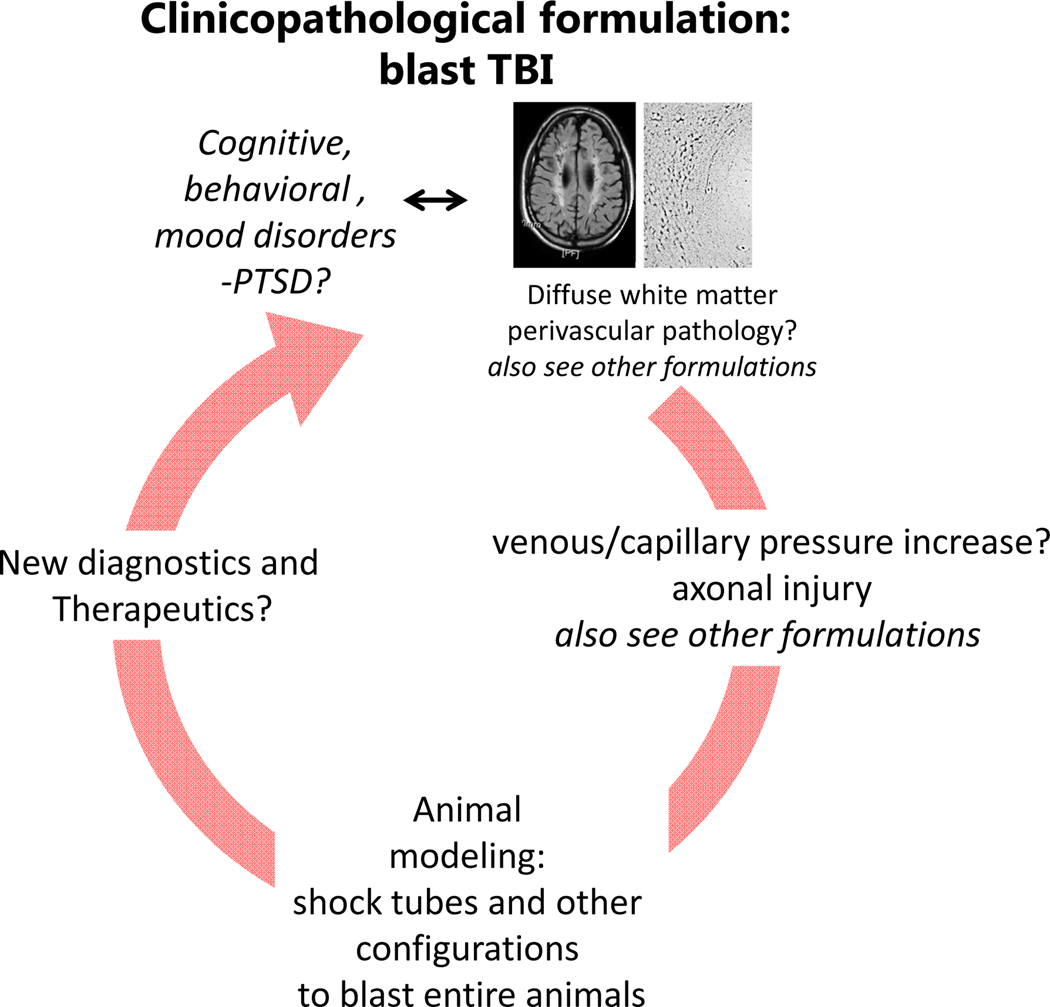
Clinicopathological schema for blast TBI. The problem is little understood, especially when it comes to primary blast from the overpressure wave. Part of the problem is the complex clinical picture that does not lend itself to straightforward correlations with pathology.
Blast TBI
Blast TBI is a complex injury (24). Besides the presumed primary effects of overpressure caused by the shock wave, there may be secondary injuries from the forceful bullet-like mobilization of mobile elements and debris (shrapnel) against the victim and tertiary injuries from the displacement of the body by the blast wind causing contrecoup contusions and DAI from rotational acceleration. There may also be quaternary injuries including flash burns from the intense heat of the explosion that may enhance the injurious impact of other force, as well as asphyxiation and respiratory damage from the inhalation of toxic substances. The intense heat can exacerbate the noxious effects of all other mechanisms. Although the secondary and tertiary components of blast TBI are identical to other types of TBI reviewed here, the neuropathology related to the primary effect of blast has not been well characterized. Part of the problem is lack of sufficient high-quality autopsy material, especially from long-term survivors of blast injuries and the very rarity of isolated primary blast events except during professional training with explosives or shoulder-fired weapons. Recent work from our group on brains of veterans with history of blast exposure has shown the presence of a peculiar type of traumatic axonal injury in the frontal lobe in a characteristic arrangement of lesions at sub-millimeter distance from arterioles forming 3D honeycombs (25). Such lesions colocalize with reactive microglia, a marker of active neuroinflammation. These configurations appear to be distinct from the large fronts of axonal undulations and bulbs seen in classical DAI and from axonal abnormalities seen on other types of TBI or in the case of opiate overdose (25). As in the case of repeat concussions, blast TBI is associated with numerous psychiatric symptoms but also tainted by very significant brain-mind dilemmas right at the border of the clinicopathological model (see below).
Animal models of primary blast have generated evidence consistent with a sharp rise in central venous pressure and axonal injury in discrete white matter tracts with associated microglial activation. Shielding of the thorax and abdomen, but not brain, prevents blood-brain barrier disruption, neuroinflammation, and axonal injury (24). This evidence, taken together with the periarteriolar pattern of axonal injury in the brains of blast-injured veterans is suggestive of an “internally” driven injury during which central venous pressure elevation is transmitted upwards to brain sinuses and, perhaps, to venules, with back-loading of the capillaries and at least transient brain edema. The pulse of overpressure and brain edema may compress the parenchyma onto the stiffest part of the organ that is the arterioles, endowed as they are with muscular walls and containing blood under pressure. Pulsatile deformation of tissue against the arterioles may explain the periarteriolar distribution of diffuse axonal injury (Fig. 8).
Figure 8.
Clinicopathological schema for repetitive mild TBI. As in the case of blast, the relationship between repetitive mild TBI exposure and pathology is not straightforward, although some lesions, like the perivascular tau accumulation indicated here, have certain degree of specificity. There are no satisfactory animal models or diagnostic and therapeutic breakthroughs at this time.
Neuropsychiatric morbidities associated with TBI
Overview
This review is not meant to be a comprehensive examination of neuropsychiatric disorders associated with TBI which we recently reviewed elsewhere (26). Some important conditions like TBI-associated PTSD are separately dealt with in the section on The Dilemma of Mild TBI versus PTSD. The term “neuropsychiatric” here refers to conditions in which neurology and, by extension, neuroscience, is necessary or very helpful in the understanding and management of psychiatric morbidities. In general, neuropsychiatric presentations associated with TBI, although generally related to the clinicopathological models examined in Part 1, they do not have 1:1 correlation with cause of TBI, severity, or location and type of pathology. Contusions and DAI correlate better with specific neuropsychiatric syndromes, but in the case of blast and repeat concussions the clinicopathological model has significant limitations. Overall, TBI survivors have substantially higher rates of psychiatric disorders compared to the general population (27) and these disturbances are leading causes of disability and poor quality of life in chronic TBI. As further explained in the section on Behavioral and Cognitive Neurology, this relationship can be explained by the fact that CNS regions and circuits involved in emotional regulation, behavioral control, and high-order cognitive operations are all affected in TBI (Fig. 9). It should be noted that TBI has a bidirectional correlation with psychiatric illness: TBI increases the rate of psychiatric morbidity but psychiatric morbidity is also a risk factor for TBI. Therefore, assigning causality in psychiatric symptoms emerging after TBI is not always straightforward: in many cases, TBI is merely an index event.
Figure 9.
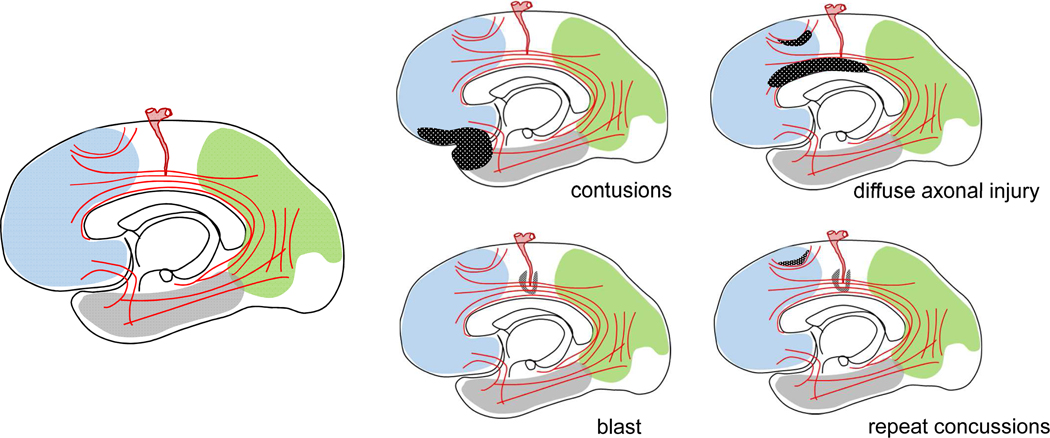
This is a sketch of important anatomical elements involved in TBI-associated neuropathology. These include associative cortical areas in the frontal, parietal and temporal lobes, local and associative white matter bundles, and medium-small size arteries supplying the brain. Diagram on the left shows main anatomy. Diagrams on the right show the involvement of the previous anatomical elements in the four main types of TBI-associated neuropathology namely contusions, DAI, blast, and repeat concussions: In contusion, there is focal damage in the frontal and temporal associative cortex. In DAI, there is multifocal involvement of both local and associative white matter tracts. Blast injury and repeat concussions involve perivascular injuries and repeat concussions also affect gray matter at the depth of sulci.
Besides core neuropsychiatric problems reviewed in this section, patients surviving moderate-severe TBI injury experience the injury as catastrophic illness with dramatic and often permanent changes in their lives, they are exposed to continued chronic stress with hospitalizations, endless physician visits and the need for chronic rehabilitation, sometimes legal battles over compensation, and they seek meaning in the new reality set after the injury, often preoccupied with who they were and what they were doing before TBI of what was going on at the time of the injury.
Behavioral and cognitive neurology
As indicated in the sketches of neuropathologies associated with the 4 main types of TBI (sans concussion) (Fig. 9), the regions more commonly affected are: the frontotemporal paralimbic zone and associated neocortical sites; and the central white matter that hosts a number of longitudinal, commissural, cortico-subcortical and U-fiber systems associated with both local and large-scale networks including basal ganglionic-thalamocortical loops. For the purpose of a neuropsychiatric paper, the involvement of frontal/frontotemporal regions, cortical associative and commissural tracts, and basal ganglia-thalamocortical loops is crucial because the afflicted regions/pathways and associated networks underlie important behavioral, cognitive, and social functions. Functional MRI has been extensively used to demonstrate some of these secondary effects but there is also increasing popularity in high-resolution structural imaging, i.e. diffusion tensor imaging, despite the substantial inter-individual variance and a need to establish better baselines (28). Functional studies of large-scale networks have demonstrated impairments in the default mode network, salience network and basal ganglia-thalamocortical loops engaging the caudate (29–32). In the case of DAI, there seems to be correspondence between functional disconnection of networks and structural disintegration of the underlying tracts (33, 34).
Despite a plethora of structural and functional MRI studies, the transsynaptic pathological effects of focal or diffuse lesions on other brain regions have not been directly explored. Recent evidence in our laboratory suggests that fronto-temporal contusions have a rather restricted secondary (retrograde) impact on ventrolateral thalamus and limbic structures such as the hippocampus and amygdala, whereas diffuse axonal injury has widespread secondary transsynaptic effects in cortex (35).
Much of the psychiatric morbidity associated with TBI, especially disinhibition, aggression and other changes in personality (see below) as well as executive dysfunction falls under the rubric of “frontal lobe syndrome” that remains a diagnostic entry on ICD10 (36). The problem is known for almost two centuries and, in the English-speaking literature, was popularized in the classical case report of Harlow on Phineas Gage. With the revival of connectional neuroanatomy in the 80s, especially the concept of cortico-subcortical circuits engaging basal ganglia and thalamus (37), the generic frontal lobe syndrome was further specified into distinct anatomo-functional entities based on parallel segregated circuits. One schema that we have found clinically useful is the subdivision into the orbitofrontal lesion pattern with behavioral disinhibition, the dorsolateral lesion pattern with executive dysfunction, and the anterior cingulate lesion pattern with apathy (Fig. 10)(38). A recent cluster analysis largely agrees with the functional aspects of this schema and supports the existence of four distinct “trends” in personality disturbances arising from frontal lobe lesions: dysregulation of both emotions and behavior, low emotion and energy (corresponding to apathy), distress/anxiety, and executive impairments that tend to be associated with all other conditions (39). This analysis elevates executive dysfunction as the central, if not the driving, problem in the so-called frontal lobe syndrome. An interesting related development from animal models is evidence for selective vulnerability of inhibitory interneurons, raising the question whether executive dysfunction has some relationship with loss of local inhibitory control.
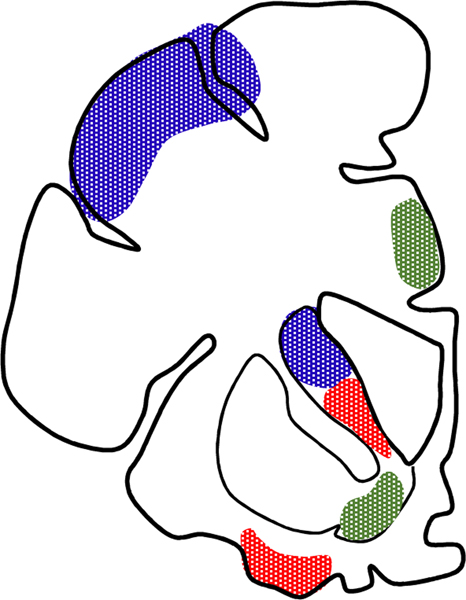
Figure 10.
One of the earlier conceptualizations of frontal circuits underlying specific cognitive or behavioral syndromes are based on the Alexander, DeLong and Strick schema of parallel basal ganglia-thalamocortical loops. This sketch is based on Cummings’s adaptation of this schema for behavioral neurology and showcases cortical and neostriatal regions involved in the dorsolateral prefrontal syndrome characterized by executive dysfunction (in blue), regions involved in the “anterior cingulate syndrome” manifesting with psychomotor retardation and apathy (green) and the orbitofrontal syndrome marked by changes in personality and social and interpersonal deficits (red). In the latter case, although the initial formulation involved the lateral orbitofrontal cortex, it is likely that medial orbitofrontal cortex is more relevant for these types of deficits.
Ever since Lishman’s pioneer study on WWII patients with penetrating injuries (40), the right hemisphere has been suspected to have a special role in psychiatric morbidity after TBI. Although the relationship has not been sufficiently addressed in the existing literature, the majority of patients with moderate-severe TBI followed in the Neuropsychiatry Program at Sheppard Pratt have either right-selective or right-predominant lesions (41). These patients present with a mixture of affective lability, anosognosia, inappropriate behaviors, aprosodia and deficits in pragmatics, and neglect. This syndrome is very much in keeping with the earlier conceptualizations of Heilman (42) and is likely to attract a lot of interest in the near future.
Select neuropsychiatric disorders associated with TBI
Organic personality changes
Personality change due to TBI is a diagnostic term used to describe stable changes (what we like to call “unstably stable”) in affective or behavioral disposition that emerge in the aftermath of TBI and usually fall into several discrete categories featured by irritability/aggression, impulsivity/ disinhibition, mood lability, and apathy (an admixture of blunted affect and lack of motivation or will). Rates range from 10–70% depending on the severity of TBI and time interval since TBI, with moderate-severe TBI predictably having the lion’s share (43). In some individuals, these changes may represent an accentuation of previous personality traits and in others the emergence of new traits. Collateral history from family members and longitudinal follow-up are crucial for the establishment of the diagnosis. The problem of apathy deserves special comment. Although classified in DSMV under personality changes, it may also be viewed as some form of mood disorder. It is a common problem post TBI with prevalence in the range of 10–70 % (26). It is typically associated with frontal pathology or with dysfunction in circuits involving the anterior cingulate, thalamus and mesencephalic-pontine dorsal tegmentum related to the arousal system (44).
Mood disorders
The relationship between TBI and mood disorders is known for a very long time (45). TBI-associated depression is the most common and involves heterogeneous conditions ranging from adjustment disorders with depressed mood to prolonged, persistent sadness sometimes associated with anhedonia, vegetative signs and symptoms and executive dysfunction. These conditions are very common after TBI, with a one-year incidence of 25-% to 50%, with a lifetime prevalence of 26% to 64% (46). A prior history of mood disorders and poor social functioning are well-established risk factors. Neuropathology favors anterior frontal locations (47, 48). TBI-associated depression is often expressed outward as aggression (48). Mania after TBI is less frequent that depression but higher than in the general population and may be preferentially associated with pathology in the right hemisphere (49).
Dementia and cognitive impairments
Impairments in cognition are extremely common after moderate-severe TBI and are the best prognosticators of loss of independence and inability to return to work (26). Severe injuries are associated with worse cognitive outcomes but there are significant individual differences depending on pre-injury functioning, intellect, and other individual factors. Allowing for transient severe changes associated with alterations in level of consciousness in the acute phase and an ensuing delirious phase (50) that may last for weeks to months in moderate-severe injuries, stable cognitive impairments are settled by 1–2 years post-TBI. Such impairments involve nearly all cognitive domains including attention, memory, visual-spatial processing, language, social cognition, and executive functioning. The most common impairments are in the speed of information processing, attention, and working memory. Patients usually complain about poor recall, but the underlying problems have more to do with impairments in speed of information processing, attention, and working memory. Frontal-type deficits in planning, cognitive flexibility, and reasoning also contribute to TBI-associated amnesia. Awareness deficits after TBI are also common, may be related to right hemisphere pathology, and profoundly impair the ability of the patient to engage with treatment and rehabilitative efforts (26). Finally, there is evidence, still debated, that moderate-severe and perhaps repeat mild TBI may be risk factors for late-onset neurodegenerative dementias including AD, PD and LBD (51–54).
Behavior dyscontrol syndrome
This term is often used to describe a mixture of behavioral, mood, and executive problems manifesting as irritability-anger-aggression, impulsivity, affective lability or pathological laughter and crying, and impaired attention and judgment. They are, in a sense, the amalgamation of problems in other neuropsychiatric domains, but they deserve special mentioning because of their episodic acuity and associated management difficulties that require neuropsychiatric expertise and multidisciplinary interventions. Such presentations often culminate in classical catastrophic reactions in the form of elopement, assaultiveness, or suicidal gestures.
The problem of mild TBI: post-concussive syndrome
A minority of patients estimated at 10–20% do not do well even after a single concussion. The symptoms of this so-called post-concussive syndrome (PCS) overlap with these immediately after concussion and include mood changes such as depression and irritability, cognitive symptoms such as decreased attention/concentration and often impaired memory, various degrees of executive dysfunction, and other symptoms such as headache, insomnia, dizziness/vertigo, tinnitus, light and noise sensitivity, fatigue, and problems with coordination. Some of these cases may have DAI (26). Although the symptoms of concussion per se as often termed post-concussive syndrome (PCS) in the literature, here we refer to lingering symptoms a month or longer after the index event. Persistence of post-concussive symptoms for several months may betray major mental illness (e.g. major depression, PTSD), adverse effects of medications, substance abuse, and embellishment of symptoms or malingering associated with ongoing litigation.
Blast TBI and the “brain-mind” dilemma in understanding and managing TBI
The legacy of “shell shock”
Blast TBI, whose neuropathology was briefly reviewed in the section on Blast TBI in this article, is a 100-year old problem that first surfaced in the trench warfare of the western front in WWI. Trinitrotoluene-filled artillery shells were used in abandon in the battles of Somme, Ypres and Verdun and caused a tremendous number of casualties with minor military gains. What made the medical news, however, was the appearance of an illness that was initially dubbed “Not Yet Diagnosed Nervous”, “Neurasthenia”, or “Shell shock” and led to a million of soldiers being discharged home for recuperation after the battle of Somme alone. Many were British. The way the problem was handled in Great Britain is an interesting episode in the history of medicine and exemplifies the tension between Neurology and Psychiatry that continues up to this day: Soldiers sent to Maudsley Hospital in London, that was under the influence of the neuropathologist Frederick Mott, were treated as neurological cases; in contrast, patients sent to Moss Side Military Hospital outside Liverpool were managed as hysteria under the spreading influence of dynamic psychiatry and psychoanalysis (55). The reasons for this dilemma should become apparent to any clinician who reviews archival video clips or photographs (56). A matter-of-fact approach of Gordon Holmes dealt effectively with the problem with “immediate treatment”, an intensive rest and support regimen, in the battle of Passchendaele. The nosological problem, however, was eventually pushed aside when, for reasons unrelated to science or medicine, all cases of shell shock were administratively thrown out by the British government as incidents of malingering. The problem resurfaced later, especially in the Yugoslav war in the 1990s and then the post-9/11 deployments in the Middle East. Although the Vietnam war also generated a great deal of psychiatric morbidity much of which came to be known as PTSD in DSMIII, blast TBI was not common in that conflict. In recent wars in Iraq and Afghanistan, blast TBI has come to be known as the “signature” medical problem, although the bulk of TBI suffered by over 380,000 of US warfighters of these cohorts is due to blunt, not blast, forces, and most TBI incidents have taken place during training and other activities in garrison. The reason blast TBI is covered in some detail in this paper is not only because the neurology versus psychiatry (or “brain-mind”) tension is one of the main issues in clinical neuropsychiatry. It also illustrates the fact that, in contrast to the more abstract clinicopathological entities, real patients present with problems that also reflect their idiosyncrasies and their past psychiatric and non-psychiatric histories, and these problems may be comorbid with other conditions at the time of assessment. The clinicopathological formula works well when it comes to clarifying nosology and planning research, but the situation at the bedside is more complicated. For example, in the veteran cohorts from post-9/11 deployments, besides TBI we also encounter post-traumatic stress, chronic pain, pre-existing and emergent mood disorders, substance abuse, and adjustment disorders. The neuropsychiatrist or general psychiatrist is asked to evaluate and treat patients who present with a combination of problems with which he or she should feel comfortable. The section on Clinical Presentations Associated with Blast TBI from Recent American Wars below introduces three representative complex cases from patients exposed to the battlefields of OIF and OEF (military code names for the Iraq and Afghanistan wars) treated in the Neuropsychiatry Program of Sheppard Pratt in the period 2009–2019.
Clinical presentations associated with blast TBI from recent American wars
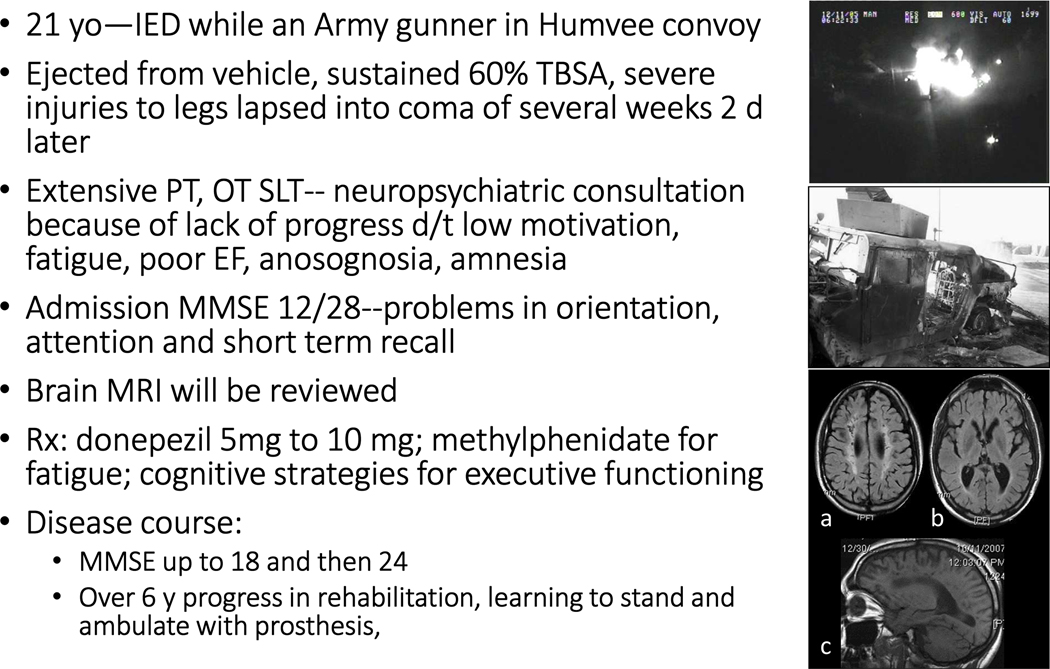
Figure 12 and Boxes 1–2 contain the vignettes of three cases presenting with various combinations of blast TBI and psychiatric and general medical comorbidities. Cases illustrate the prototype of a “purely organic” patient with history of severe blast TBI with burns but no prior psychiatric history (Figure 12), a patient example that combines blast exposure with significant prior psychiatric history (Box 1), and a patient example with a history of blast exposure but presentation consistent with classical PTSD (Box 2). In the first case, there was plenty of documentation of the index event from a surveillance balloon that captures a significant quaternary effect (fire from the explosion resulting in burns, top left; what was left from the military vehicle (Humvee) is shown on bottom left); we also had access to brain MRI images including a transverse FLAIR image showing diffuse white matter signal and punch lesions in centrum semiovale/coronal radiata (top right), diffuse brain atrophy on transverse T1 (middle right) and white matter rarefactions in sagittal T1 (bottom).
Figure 12.
Mixed Blast injury
Box 1. Repeat blast TBI/?PTSD.
40 yo Army Captain referred from military base because of mood lability with suicidal and homicidal ideation, rage attacks with numerous altercations, insomnia, headaches, “memory loss”
Multiple IED blast exposures in OIF, unclear LOC
Concussion from abuse by father; at least one concussion from bar fight
Chronic depression, suicide attempt at age 10, binge drinking
Chronic aggressiveness prior to TBI
Neuro exam normal. MMSE 29/30. Aggressive, homicidal or escape obsessions. Hypervigilant—able to describe in detail every other person in the waiting room as well as escape planning. No nightmares, flashbacks, or reviving.
MRI normal. No heme signal on SWI
Rx: valproate and quetiapine for mood stabilization; CBT for depression
Disease course: After 14 m of care improved, then lost to f-u
Box 2. Blast TBI/PTSD.
44 yo retired police officer, contractor in Afghanistan referred for “anxiety and PTSD symptoms”.
Fragmented sleep with nightmares, mild memory problems, periods of “confusion”, hypervigilance, hyperacusis, irritable depression, impulsivity, generalized and panic anxiety. Hearing loss with tinnitus, balance problems and pain in neck, shoulders and lower back. On venlafaxine for depression, and Lyrica and Flector patch for chronic pain.
In sustained firefight where his partner was killed. Two months later ambushed while driving an armored truck; IED exploded under the vehicle. Struck in head by falling debris while exiting the vehicle and dazed.
No prior psychiatric history. Family history of drug addiction and depression. Childhood exposure to domestic violence between parents.
Neuropsychological testing: deficits in verbal fluency, memory, response inhibition, EF, processing speed. Affect constricted and intense, anxious mood.
Brain MRI unremarkable.
Rx: Continue antidepressant and muscle relaxants. Refer for trauma-specific psychotherapy.
The dilemma of mild TBI versus PTSD
More than 80% of cases of TBI in veterans of recent wars are cases of mild TBI. When patients with such histories are referred for neuropsychiatric assessment, a common differential diagnostic issue is mild TBI versus PTSD. This issue has attracted considerable research interest as well as R&D investment in medical technologies to help separate the two conditions. As shown in Box 3, most symptoms in mild TBI have neuropsychiatric signatures and most of them, including some non-behavioral symptoms, are also encountered in PTSD. Although greater clarity on formulation is always desirable, it is important to remember that these conditions may very well coexist. In fact, TBI in itself is also psychological trauma and, in addition, a risk factor for PTSD (57). It is also possible that the explosive surprise associated with blast but also the killing or maiming of comrades because of the power of explosion increase the likelihood of trauma, as illustrated in the case of Box 2, although data on this topic are conflicting. The neuropsychiatric complexity of these cohorts is further exemplified by a significant increase in suicide rate among veteran and military active-duty personnel between 2001 and today, with a human toll of close to 20 of veteran suicides a day, amounting to a striking 15% of all suicides (58). The high rates of PTSD and suicide as well as other psychiatric morbidities in these veteran cohorts have attracted a lot of interest and various theories have been proposed, including that of preexisting trauma and mental illness in recruits of recent years that increase the risk of PTSD with or without deployment (59). The case of Box 1 illustrates that relationship.
Box 3. Mild TBI versus PTSD.
| Cognitive/Behavioral, mTBI | Non-behavioral, mTBI |
|---|---|
| • Cognitive | • Headache |
| • Memory problems ✔ | • Dizziness |
| • Attention problems ✔ | • Fatigue ✔ |
| • Executive difficulties | • Vision problems |
| • Bradyphrenia | • Photophobia-phonophobia ✔ |
| • Dysnomia | |
| • Emotional | |
| • Low mood ✔ | More PTSD specific |
| • Irritability and angry outbursts | • Flashbacks |
| • Anxiety ✔ | • Nightmares |
| • Sleep disturbances ✔ | • Overalertness |
| • Personality changes | • Sense of foreshortened future |
| • Lability ✔ | |
| • Impulsivity | |
| • Apathy ✔ |
From the neurobiological perspective, PTSD symptoms are thought to reflect dysfunction in at all three large-scale networks involving the frontal lobe, i.e. the salience network engaging dorsal anterior cingulate cortex and insula, the central executive network engaging dorsolateral prefrontal cortex, and the default mode network engaging the medial prefrontal and posterior parietal cortex (60, 61). The first is thought to be responsible for hyper-or hypo-arousal, the second for the executive dysfunction commonly associated with PTSD, and the third with a disturbed sense of self that is common in PTSD. Relevant cortical areas, for example the dorsal medial and ventral medial prefrontal cortex are injured in several types of TBI described here, including blast TBI. It is therefore reasonable to assume that TBI can only make some of these PTSD-specific abnormalities worse, especially in the area of executive dysfunction, and this may contribute to catastrophic reactions and suicidal or non-suicidal self-injury.
Nosological and clinical dilemmas associated with repetitive mild TBI and CTE
The problem of athletic concussions
The main reason behind current interest in CTE is the exposure of young Americans, primarily male but also increasingly female, to athletic injuries in the course of contact and collision sports. Besides the 2,000 or so active NFL members, there are over 5 million children over 6 and adolescents who play recreational football, over 1 million high school football players, and 70,000 college football players. As also noted in the section on Repetitive Mild TBI and Chronic Traumatic Encephalopathy, neuropathological papers with retrospective exploration of medical histories of brain donors, mostly from retired NFL players, have emphasized the role of psychiatric symptoms in these subjects including mood disorders (especially depression), personality changes, and cognitive impairments that, in some cases, progress to dementia (20). In most cases of young brains that came to autopsy cause of death was suicide followed by self-inflicted accidents.
Nosological ambiguities in CTE
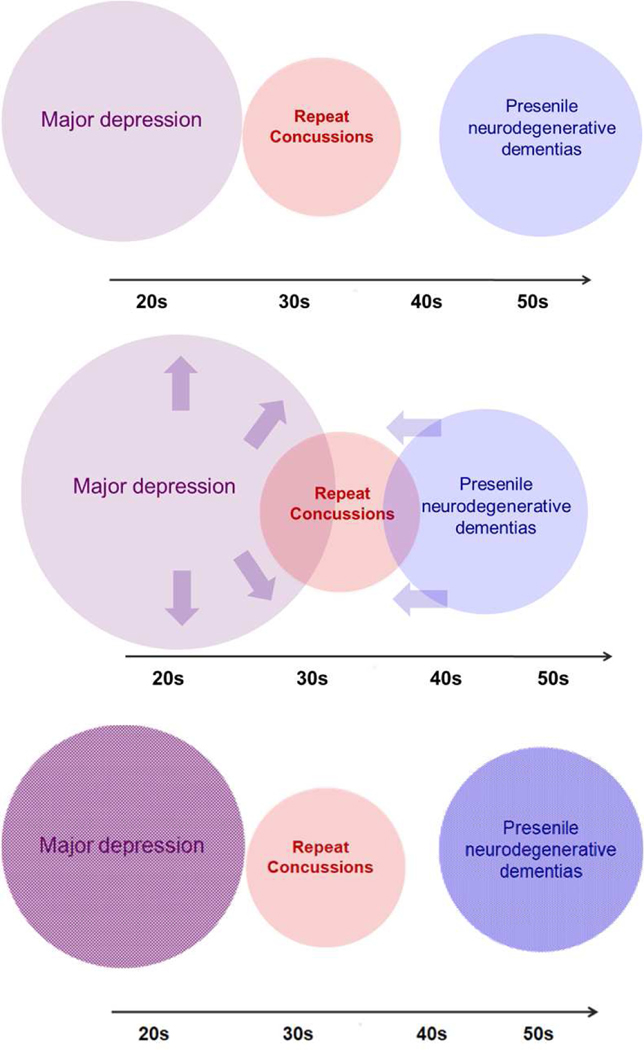
The proposed early occurrence of psychiatric illness, primarily depression and personality changes, in the course of CTE raises some important clinical questions. Psychiatric illness, especially depressive illness, is fairly common in the age range of young athletes, is quite prevalent, is often externalized especially in males, and goes unreported because of the associated stigma. In addition, major depression and its variants are commonly caused or triggered by TBI. If early CTE is featured by mood and behavioral symptoms, is the clinical presentation of depression in young collision and contact sports athletes the manifestation of a separate disease (CTE) or merely that of the common idiopathic depressive illness? On the other end of the age spectrum, how can one distinguish between cognitive and behavioral symptoms associated with early-onset neurodegenerative disease, e.g. the behavioral variant of frontotemporal degeneration, and CTE? In the former case, are we dealing with a mere increase in depression risk that is to be expected with TBI? In the latter, are we faced with an accelerated “incubation” of early-onset neurodegeneration that would have happened anyway, especially in genetically predisposed individuals (Fig. 11)? On the pathology side, is tau accumulation, at least up to a point, a cause of disease or a marker of TBI exposure?
Figure 11.
The prominence of neuropsychiatric symptomatology in CTE and the questionable specificity of neuropathology raise important nosological questions on the differentiation from common mood disorders and presenile dementias, especially of the frontotemporal type. Top diagram shows the sets of repeat concussions (red), common mood disorders like major depression (purple) and presenile neurodegenerative dementias (blue) along the age continuum. Size of sets is grossly representative of prevalence. One possibility is that repetitive mild TBI increases the risk of other neuropsychiatric conditions in exposed patients including major depression (as TBI is known to do), or presenile neurodegenerative disorders (middle). Another possibility is that repetitive mild TBI has a pathoplastic effect: without increasing the incidence of major depression or accelerating a neurodegenerative process, it modifies depressive manifestations in the direction of aggressive or other acting out or it adds a TBI-specific marker, for example perivascular and deep sulcal pathology, to an independent neurodegenerative process (bottom).
These problems have not been solved and cannot be adequately addressed in current retrospective research, especially because most of this work is based on autopsy brains from patients many of whom, for one reason or another, had poor outcomes (depression, executive/impulse control problems, motor neuron disease). More work is needed to separate between CTE and other, more common, conditions, and this work requires prospective cohort studies aided by careful clinical characterization and, ideally, input by biomarkers. Unfortunately, despite substantial progress in molecular tau imaging, specific biomarkers are not available at this point. One idea might be to use the prevalent and early neuropsychiatric symptoms as clinical “biomarkers”, but there is only a dearth of neuropsychiatrists with TBI expertise and often the distinction between idiopathic and secondary (“organic”) psychiatric illness is subtle (Box 4). Still, such distinctions might be useful in prospective cohorts and, as many of us have stressed in professional meetings and panels, there is urgent need for a greater presence of psychiatrists familiar with the topic.
Box 4. Differentiating idiopathic from organic.
| IDIOPATHIC ILLNESS | “ORGANIC” ILLNESS |
|---|---|
| • Depression • Short-lived mania • Psychotic episodes with organized delusions • Mood congruent • With schizophrenic features • Relative paucity of cognitive deficits • Relative paucity of neurological signs |
• Apathy without depression • Impulsivity without mania • Aggressiveness without mania or delusional thinking • Social regression without thought disorganization • Confabulatory thinking • Incongruency between affect and mood • Cognitive deficits • Focal or diffuse neurological signs |
A key question in CTE is why only some athletes develop it and others not. For now, the conventional wisdom is that the risk rises with concussive burden: in the case of boxing, the “bad” boxer who cannot protect himself from the punches of the opponent; in the case of football, either players who suffer the greatest number of hits like linebackers and linemen, or players who endure the most severe blows like running backs and quarterbacks. However, the most significant correlate in severity of tauopathy is age, not symptom severity, indicating that the relationship between TBI burden and disease is complex. Genetic and other predispositions or important anamnestic events have not been addressed in the literature as of yet.
Clinical dilemmas associated with repetitive mild TBI
Concerns raised in the previous section pose clinical dilemmas that have been addressed in a recent letter signed by international experts in the field (22). For example, if a psychiatrist examines a college football athlete player who presents with major depression (with or without alcohol or other substance abuse) and the patient asks if the mood change is the first stage in a disease that may eventually render him demented, how does the clinician respond? Does he or she engage in discussion of prognosis? Moreover, what does the psychiatrist do and how does he or she manage the patient?
Based on the available evidence, our opinion is that a clinician should treat the patient as a case of common mood disorder with generally recommended interventions, e.g. antidepressants and cognitive-behavioral therapy. We would not engage in discussion of CTE and focus, instead, on the prognosis of a single mood episode, even stating that such discussions are premature and the likelihood that the presentation has anything to do with CTE is extremely low. We would also bring up issues related to return to play or continued play and briefly discuss relevant recommendations from professional organizations. If the clinician is not familiar with TBI or these recommendations, he or she may refer the patient for more expert advice.
Relevance of the molecular neuropathology of white matter
Many common neuropsychiatric disorders including neurodegenerative and vascular dementias and the mood, behavioral and cognitive syndromes associated with TBI are associated with white matter pathology (Fig. 9). In the case of TBI, the role of white matter in in large measure related to the high frequency of DAI, a primary white matter lesion even in mild TBI (4).Protecting the white matter, besides managing risk factors such as preventing atherosclerotic vascular disease, falls and motor vehicle accidents, is predicated on a better understanding of the molecular and cellular biology of the axon. Perhaps the most important issue is the vulnerability of the axon to Wallerian degeneration.
Wallerian degeneration is a complex molecular program of axonal self-destruction activated by a wide range of injurious insults. Detailed studies on animal models and postmortem human brains indicate that this type of partial injury may be the principal molecular pathology in DAI. The seminal discovery of the slow WD mice (Wlds), in which transected axons do not degenerate but survive and function independently for weeks, has established the axon as a biological entity separate from the neuronal cell body and has shifted emphasis on the NAD salvage pathway and key synthetic or catabolizing enzymes, such as NAMNT and SARM1 (4). Another key program is the MAPK stress cascade that transmits retrograde injury signals to the cell body (4). Recent discoveries in our laboratory and elsewhere have revealed that the decision that commits axons to degeneration is temporally separated from the time of injury, a critical window that allows potentially effective pharmacological interventions and opens up new therapeutic opportunities for TBI.
Conclusions
Chronic TBI is a common medical problem resulting from moderate-severe and perhaps repetitive mild TBI. The varied combination of causes, mechanisms, comorbidities and antecedent medical, including psychiatric, disorders, make for extremely variable presentations at bedside that call for special expertise. Although historically the domain of neuropsychology, there has been increasing recognition of clinical syndromes that resemble those associated with idiopathic mental illness and may empirically respond to the same pharmacological agents, although there are very few well-controlled trials. Long-term patient outcomes depend primarily on cognitive, behavioral and social level of functioning. There is also the recent epidemic of athletic and military TBI in which psychiatric problems predominate, including suicidality in the latter. For the above reasons, traumatic brain injury a prime domain for neuropsychiatry. In addition, partly because the timing of the insult is known and TBI-associated pathologies affect brain systems underlying cognitive and complex behavioral operations, TBI is also a favorite subject for behavioral neuroscience and a model for other neuropsychiatric disorders. Because certain types of TBI appear to increase the risk of age-associated neurodegeneration, TBI is also interesting as an incubator for new ideas for conditions like ALS and Parkinson’s and Alzheimer’s disease.
Synopsis.
Traumatic brain injury (TBI) is a calamity of various causes, pathologies, and extremely varied and often complex clinical presentations. Because of its predilection for brain systems underlying cognitive and complex behavioral operations, it may cause chronic and often severe psychiatric illness that requires expert management. This is more so for the modern epidemic of athletic and military brain injuries that is dominated by psychiatric symptoms. Past medical, including psychiatric, history, and comorbidities are important and extremely relevant for formulation and management. TBI is a model for other neuropsychiatric disorders and may serve as an incubator of new ideas for neurodegenerative disease.
Key Points.
Chronic TBI, usually as a result of moderate-severe and perhaps repetitive mild TBI, is a prototypical neuropsychiatric illness
Long-term patient outcomes after moderate-severe TBI depend primarily on cognitive, behavioral and social level of functioning and the neuropsychiatrist is best equipped to assess and manage deficits in these domains
The previous fact is more accentuated in athletic and military injuries, in which psychiatric morbidity (or comorbidity) is paramount, including suicidality in the latter
Traumatic brain injury is a “niche” for clinical neuroscience research linking neuropathology to network dysfunction and network dysfunction to symptoms, much like Alzheimer’s disease was in the 80s
Traumatic brain injury is a model for other neuropsychiatric conditions especially neurodegenerative diseases of the brain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Charcot JM, Harris R. Clinical lectures on diseases of the nervous system. London; New York: Tavistock/Routledge; 1991. lxviii, xviii, 438 p. p. [Google Scholar]
- 2.Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39(3):253–62. [DOI] [PubMed] [Google Scholar]
- 3.Moe HK, Limandvik Myhr J, Moen KG, Haberg AK, Skandsen T, Vik A. Association of cause of injury and traumatic axonal injury: a clinical MRI study of moderate and severe traumatic brain injury. J Neurosurg. 2019:1–9. [DOI] [PubMed]
- 4.Koliatsos VE, Alexandris AS. Wallerian degeneration as a therapeutic target in traumatic brain injury. Curr Opin Neurol. 2019;32(6):786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moenninghoff C, Kraff O, Maderwald S, Umutlu L, Theysohn JM, Ringelstein A, et al. Diffuse axonal injury at ultra-high field MRI. PLoS One. 2015;10(3):e0122329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su E, Bell M. Diffuse Axonal Injury. In: Laskowitz D, Grant G, editors. Translational Research in Traumatic Brain Injury. Frontiers in Neuroscience. Boca Raton (FL)2016. [Google Scholar]
- 7.van Eijck MM, Schoonman GG, van der Naalt J, de Vries J, Roks G. Diffuse axonal injury after traumatic brain injury is a prognostic factor for functional outcome: a systematic review and meta-analysis. Brain Inj. 2018;32(4):395–402. [DOI] [PubMed] [Google Scholar]
- 8.Ubukata S, Ueda K, Sugihara G, Yassin W, Aso T, Fukuyama H, et al. Corpus Callosum Pathology as a Potential Surrogate Marker of Cognitive Impairment in Diffuse Axonal Injury. J Neuropsychiatry Clin Neurosci. 2016;28(2):97–103. [DOI] [PubMed] [Google Scholar]
- 9.Smith DH, Hicks R, Povlishock JT. Therapy development for diffuse axonal injury. J Neurotrauma. 2013;30(5):307–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greer JE, McGinn MJ, Povlishock JT. Diffuse traumatic axonal injury in the mouse induces atrophy, c-Jun activation, and axonal outgrowth in the axotomized neuronal population. J Neurosci. 2011;31(13):5089–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Hamm RJ, Povlishock JT. Traumatic axonal injury in the optic nerve: evidence for axonal swelling, disconnection, dieback, and reorganization. J Neurotrauma. 2011;28(7):1185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsbie DS, Ziogas NK, Xu L, Kim BJ, Ge Y, Patel AK, et al. Targeted disruption of dual leucine zipper kinase and leucine zipper kinase promotes neuronal survival in a model of diffuse traumatic brain injury. Mol Neurodegener. 2019;14(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziogas NK, Koliatsos VE. Primary Traumatic Axonopathy in Mice Subjected to Impact Acceleration: A Reappraisal of Pathology and Mechanisms with High-Resolution Anatomical Methods. J Neurosci. 2018;38(16):4031–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewan Feltrin F, Zaninotto AL, Guirado VMP, Macruz F, Sakuno D, Dalaqua M, et al. Longitudinal changes in brain volumetry and cognitive functions after moderate and severe diffuse axonal injury. Brain Inj. 2018;32(10):1208–17. [DOI] [PubMed] [Google Scholar]
- 15.Ubukata S, Oishi N, Sugihara G, Aso T, Fukuyama H, Murai T, et al. Transcallosal Fiber Disruption and its Relationship with Corresponding Gray Matter Alteration in Patients with Diffuse Axonal Injury. J Neurotrauma. 2019;36(7):1106–14. [DOI] [PubMed] [Google Scholar]
- 16.Critchley M. Medical aspects of boxing, particularly from a neurological standpoint. Br Med J. 1957;1(5015):357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973;3(3):270–303. [DOI] [PubMed] [Google Scholar]
- 18.Martland HS. Punch drunk. J Amer Med Assoc. 1928;91:1103–7. [Google Scholar]
- 19.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57(1):128–34; discussion −34. [DOI] [PubMed] [Google Scholar]
- 20.McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(Pt 1):43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131(1):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart W, Allinson K, Al-Sarraj S, Bachmeier C, Barlow K, Belli A, et al. Primum non nocere: a call for balance when reporting on CTE. Lancet Neurol. 2019;18(3):231–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koliatsos VE, Xu L. The Problem of Neurodegeneration in Cumulative Sports Concussions: Emphasis on Neurofibrillary Tangle Formation. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Frontiers in Neuroengineering. Boca Raton (FL) 2015. [PubMed] [Google Scholar]
- 24.Koliatsos VE, Xu L, Ryu J, Ziogas N. A Modern Clinicopathological Approach to Traumatic Brain Injury. Conn’s Translational Neuroscience. 2017:467–87.
- 25.Ryu J, Horkayne-Szakaly I, Xu L, Pletnikova O, Leri F, Eberhart C, et al. The problem of axonal injury in the brains of veterans with histories of blast exposure. Acta Neuropathol Commun. 2014;2:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao V, Koliatsos V, Ahmed F, Lyketsos C, Kortte K. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin Neurol. 2015;35(1):64–82. [DOI] [PubMed] [Google Scholar]
- 27.Deb S, Lyons I, Koutzoukis C, Ali I, McCarthy G. Rate of psychiatric illness 1 year after traumatic brain injury. Am J Psychiatry. 1999;156(3):374–8. [DOI] [PubMed] [Google Scholar]
- 28.Ljungqvist J, Nilsson D, Ljungberg M, Sorbo A, Esbjornsson E, Eriksson-Ritzen C, et al. Longitudinal study of the diffusion tensor imaging properties of the corpus callosum in acute and chronic diffuse axonal injury. Brain Inj. 2011;25(4):370–8. [DOI] [PubMed] [Google Scholar]
- 29.Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, et al. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109(12):4690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, et al. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci. 2011;31(38):13442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Simoni S, Grover PJ, Jenkins PO, Honeyfield L, Quest RA, Ross E, et al. Disconnection between the default mode network and medial temporal lobes in post-traumatic amnesia. Brain. 2016;139(Pt 12):3137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Simoni S, Jenkins PO, Bourke NJ, Fleminger JJ, Hellyer PJ, Jolly AE, et al. Altered caudate connectivity is associated with executive dysfunction after traumatic brain injury. Brain. 2018;141(1):148–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bigler ED. Traumatic brain injury, neuroimaging, and neurodegeneration. Front Hum Neurosci. 2013;7:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes JP, Bigler ED, Verfaellie M. Traumatic Brain Injury as a Disorder of Brain Connectivity. J Int Neuropsychol Soc. 2016;22(2):120–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziogas N, Castillo M, Ryu J, Pletnikova O, Troncoso J, Koliatsos V. Retrograde Degeneration of Corticothalamic Circuits after Traumatic Contusions in Ventral Frontal Lobes. J Neurotraum. 2017;34(13):A135–A6. [Google Scholar]
- 36.Stuss DT. Traumatic brain injury: relation to executive dysfunction and the frontal lobes. Curr Opin Neurol. 2011;24(6):584–9. [DOI] [PubMed] [Google Scholar]
- 37.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. [DOI] [PubMed] [Google Scholar]
- 38.Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50(8):873–80. [DOI] [PubMed] [Google Scholar]
- 39.Barrash J, Stuss DT, Aksan N, Anderson SW, Jones RD, Manzel K, et al. “Frontal lobe syndrome”? Subtypes of acquired personality disturbances in patients with focal brain damage. Cortex. 2018;106:65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lishman WA. Brain damage in relation to psychiatric disability after head injury. Br J Psychiatry. 1968;114(509):373–410. [DOI] [PubMed] [Google Scholar]
- 41.Winkler AE, Koliatsos VE. Right Hemisphere Syndrome in the Real World of Traumatic Brain Injury: Three Longitudinal Cases Seen in the Neuropsychiatry Program at Sheppard Pratt Health System. J Neuropsych Clin N. 2019;31(3):E27–E8. [Google Scholar]
- 42.Heilman KM, Bowers D, Valenstein E, Watson RT. The right hemisphere: neuropsychological functions. J Neurosurg. 1986;64(5):693–704. [DOI] [PubMed] [Google Scholar]
- 43.Stefan A, Mathe JF, group S. What are the disruptive symptoms of behavioral disorders after traumatic brain injury? A systematic review leading to recommendations for good practices. Ann Phys Rehabil Med. 2016;59(1):5–17. [DOI] [PubMed] [Google Scholar]
- 44.Starkstein SE, Pahissa J. Apathy following traumatic brain injury. Psychiatr Clin North Am. 2014;37(1):103–12. [DOI] [PubMed] [Google Scholar]
- 45.Meyer A. The anatomical facts and clinical varieties of traumatic insanity. Am J Insanity. 1904;60(3):373–441. [DOI] [PubMed] [Google Scholar]
- 46.Jorge RE, Arciniegas DB. Mood disorders after TBI. Psychiatr Clin North Am. 2014;37(1):13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jorge RE, Robinson RG, Arndt SV, Starkstein SE, Forrester AW, Geisler F. Depression following traumatic brain injury: a 1 year longitudinal study. J Affect Disord. 1993;27(4):233–43. [DOI] [PubMed] [Google Scholar]
- 48.Jorge RE, Robinson RG, Moser D, Tateno A, Crespo-Facorro B, Arndt S. Major depression following traumatic brain injury. Arch Gen Psychiatry. 2004;61(1):42–50. [DOI] [PubMed] [Google Scholar]
- 49.Jorge RE, Robinson RG, Starkstein SE, Arndt SV, Forrester AW, Geisler FH. Secondary mania following traumatic brain injury. Am J Psychiatry. 1993;150(6):916–21. [DOI] [PubMed] [Google Scholar]
- 50.Sherer M, Nakase-Thompson R, Yablon SA, Gontkovsky ST. Multidimensional assessment of acute confusion after traumatic brain injury. Arch Phys Med Rehab. 2005;86(5):896–904. [DOI] [PubMed] [Google Scholar]
- 51.Crane PK, Gibbons LE, Dams-O’Connor K, Trittschuh E, Leverenz JB, Keene CD, et al. Association of Traumatic Brain Injury With Late-Life Neurodegenerative Conditions and Neuropathologic Findings. JAMA Neurol. 2016;73(9):1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiner MW, Crane PK, Montine TJ, Bennett DA, Veitch DP. Traumatic brain injury may not increase the risk of Alzheimer disease. Neurology. 2017;89(18):1923–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003;74(7):857–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gardner RC, Langa KM, Yaffe K. Subjective and objective cognitive function among older adults with a history of traumatic brain injury: A population-based cohort study. PLoS Med. 2017;14(3):e1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones E. Shell Shock at Maghull and the Maudsley: Models of Psychological Medicine in the UK. J Hist Med All Sci. 2010;65(3):368–95. [DOI] [PubMed] [Google Scholar]
- 56.Wonderful Shell Shock Recovery (1914-1918): British Pathe; [video clip]. Available from: https://www.youtube.com/watch?v=S7Jll9_EiyA.
- 57.Yurgil KA, Barkauskas DA, Vasterling JJ, Nievergelt CM, Larson GE, Schork NJ, et al. Association Between Traumatic Brain Injury and Risk of Posttraumatic Stress Disorder in Active-Duty Marines. Jama Psychiat. 2014;71(2):149–57. [DOI] [PubMed] [Google Scholar]
- 58.Annual Suicide Report: US Department of Defense; 2018. [Available from: https://www.dspo.mil/Portals/113/2018%20DoD%20Annual%20Suicide%20Report_FINAL_25%20SEP%2019_508c.pdf.
- 59.Manners JL, Forsten RD, Kotwal RS, Elbin RJ, Collins MW, Kontos AP. Role of Pre-Morbid Factors and Exposure to Blast Mild Traumatic Brain Injury on Post-Traumatic Stress in United States Military Personnel. J Neurotrauma. 2016;33(19):1796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yehuda R, Hoge CW, McFarlane AC, Vermetten E, Lanius RA, Nievergelt CM, et al. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015;1:15057. [DOI] [PubMed] [Google Scholar]
- 61.Fenster RJ, Lebois LAM, Ressler KJ, Suh J. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat Rev Neurosci. 2018;19(9):535–51. [DOI] [PMC free article] [PubMed] [Google Scholar]