Abstract
Molecular and serological data suggest that noroviruses (NoVs) might be transmitted between humans and domestic carnivores. In this study we screened an age-stratified collection of canine sera (n = 516) by using an ELISA assay based on virus-like particles (VLPs) of human NoVs GII.4 and GIV.1 and carnivore NoVs GIV.2 and GVI.2. Antibodies against GII.4 and GIV.1 human NoVs and GIV.2 and GVI.2 NoVs from carnivores were identified in dog sera (13.0%, 67/516) suggesting their exposure to homologous and heterologous NoVs. Analysis of the trends of age-class prevalence showed a gradual increase in the positive rate from 9.0% and 7.0%, in young dogs <1 year of age to 15.0% in dogs older than 12 years, for GII.4 and GVI.2 NoVs, respectively. A significant difference in the IgG distribution by age classes was observed for GIV.1 NoVs, with the highest rate of antibodies (7.0%) in the age group <1 year and the lowest (1.0%) in the age-classes 7–9 (P = 0.049). High correlation between the reactivity to GII.4 and GVI.2 NoVs was observed, likely due to conserved epitopes in the capsid structure.
Keywords: Noroviruses (NoVs); Genotypes GII.4, GIV.1, GIV.2 and GVI.2; Dogs; Antibodies
1. Introduction
Noroviruses (NoVs) are major human pathogens associated with acute gastroenteritis (Patel et al., 2008). NoVs belong to the genus Norovirus in the family Caliciviridae. The genome of NoVs consists of a single-stranded positive-sense RNA molecule of ~7.5 kb that is organized into three open reading frames (ORFs). ORF1 encodes a polyprotein that is co-translationally cleaved into seven proteins required for replication, while ORF2 encodes the major capsid protein (VP1) and ORF3, a minor capsid protein (VP2) (Green, 2013). Based on the full-length VP1 capsid protein, the Norovirus genus comprises seven genogroups (G), which can be subdivided in at least 40 genotypes (Green, 2013; Vinjé, 2015). Viruses belonging to GI, GII and GIV can infect humans, with GII.4 strains that are the most prevalent worldwide (Green, 2013). NoVs (GIV.2, GVI.1, GVI.2 and GVII) have been also identified in carnivores (Martella et al., 2007, 2008, 2009; Mesquita et al., 2010; Pinto et al., 2012; Tse et al., 2012; Di Martino et al., 2016). Some NoV strains from carnivores are genetically related to human GIV NoVs, suggesting common pathways in their evolution (Martella et al., 2007).
Molecular and serological studies suggest that circulation of NoVs may occur between pets and humans raising concerns of potential cross-species transmission. Human sera may contain specific IgG antibodies against carnivore GIV.2 and GVI.2 NoVs (Mesquita et al., 2013; Di Martino et al., 2014) and in turn, dog sera may contain antibodies against human NoVs of genogroup I and II (Caddy et al., 2015). In a study in Mexico, having dogs in or near home was recognised as a risk factor for acquisition of IgA antibodies specific for NoVs in infants (Peasey et al., 2004). Also, partial capsid sequences of human GII.4 and GII.12 NoVs have been detected in household dogs in contact with human patients affected by NoV gastroenteritis (Summa et al., 2012).
In order to draw a more complete picture of NoV epidemiology in dogs, we screened an age-stratified collection of canine sera by using an enzyme-linked immunosorbent assay (ELISA) based on virus-like particles (VLPs) generated from human NoVs of genotype GII.4 and GIV.1 and from carnivore NoVs of genotype GIV.2 and GVI.2.
2. Materials and methods
2.1. Serum sample collection
A total of 516 serum samples were collected between March 2013 and July 2015 by a convenience sampling of household dogs admitted to veterinary clinics from different Italian regions. The samples were divided on the basis of age groups: <1 year, 1–3 years, 3-year age groups from 4 to 12, and >12 years of age.
2.2. Virus-like particles (VLPs)
The recombinant baculoviruses carrying the genes for the viral capsid proteins of the Hu/NoV/GII.4/MD14512/1987/US, Hu/NoV/GIV.1/SaintCloud/624/1998/US, Lion/NoV/GIV.2/Pistoia/387/06/ITA and Dog/NoV/GVI.2/FD53/2007/ITA were obtained as previously described (Bok et al., 2009; Di Martino et al., 2010, 2014). For large-scale production of VLPs, 100 ml of Spodoptera frugiperda (Sf9) cells (1 × 106 cell/ml) suspension culture were infected with the recombinant baculovirus at a multiplicity of infection of three plaque forming units/cell. The recombinant capsid proteins were concentrated by ultracentrifugation through a 17% sucrose cushion in TEN-buffer (100 mM NaCl; 50 mM Tris-HCl, pH 7.5; 1 mM EDTA) and purified on a discontinuous 20–60% (wt/vol) sucrose gradient. The collected fractions were dialyzed against PBS, and the protein concentration of VLP preparations was determined by measuring the optical density at 280 nm (OD280) and visually by running aliquots on SDS-10% PAGE containing bovine serum albumin (BSA) standards. The presence of VLPs was confirmed by western blotting (WB) and electron microscopy, as previously described (Bok et al., 2009; Di Martino et al., 2010, 2014).
2.3. Enzyme-linked immunoassay (ELISA)
For the development of the antibody-detection ELISA, the supernatant of mock infected cells, GII.4, GIV.1, GIV.2 and GVI.2 VLPs were diluted to a final concentration of 4 μg/ml in carbonate-bicarbonate buffer (0.05 M, pH 9.6) and 100 μl of each antigen were added to the well of a 96-well EIA plate (Costar, Italy). The wells were washed five times with 0.1% Tween-PBS (PBS-T) and then blocked with 200 μl of PBS containing 2% BSA at room temperature for two hours. Each serum sample was tested at the initial dilution of 1:100 and the plates were incubated at 37 °C for 1 h. After incubation with horseradish peroxidase-conjugated goat anti-dog immunoglobulin G (IgG) (Sigma-Aldrich, Italy) at dilution of 1:5000 for 30 min at 37 °C, the reaction was developed with the addition of 100 μl per well of 2,2′-azino-di-(3-ethylbenzthiazo-line-6-sulfonate) (ABTS) substrate. The cut-off point of the ELISA test was established as the mean of the OD405 readings of 50 dog sera negative in WB for each NoV antigen plus 2 standard deviations. For each tested sample a positive/negative ratio (OD405 of VLPs/OD405 of mock infected cells) ≥2.0 was used to evaluate the background binding. All the sera with an OD405 values ≥ 0.5 at the initial dilution of 1:100 were considered positive and titrated in twofold dilutions. Mean ELISA antibody titres were calculated and expressed as the reciprocal of the highest serum dilution with a positive absorbance (OD405 ≥ 0.5) for each NoV antigen.
2.4. Statistical analysis
The data were analysed using Prism Graphpad Software. Fisher’s exact test was used to determine the differences in seroprevalence among the age groups. Pearson’s rank correlation test was applied to assess the correlation among NoV genotype serum titers detected in the tested dogs. A P value of <0.05 was considered statistically significant.
2.5. Evaluation of serological cross-reactivity between GII.4 and GVI.2 NoVs
In order to assess the antigenic relationships between GII.4 and GVI.2 VLPs, we performed blocking assay experiments. Briefly, 6 dog sera positive for both the antigens with titres >1:800 were pre-incubated with optimized concentrations of GII.4 and GVI.2 VLPs (2, 4, 8, 16 μg/ml) for 1 h at 37 °C. After incubation, the sera were tested in ELISA for the presence of GII.4 and GVI.2 antibodies, starting at dilution of 1:100. The possible inter-genogroup serological cross reactivity between GII.4 and GVI.2 VLPs was also investigated in WB analysis. The reactivity of five human sera positive in ELISA for GII.4 with antibodies titers ranging from 1:400 to 1:800, was tested in WB against GII.4 and GVI.2 antigens (at concentrations of 8 μg/ml) using a serum dilution of 1:100. The same experiment was performed using three dog sera positive in ELISA for GVI.2 with titers from 1:400 to 1:800.
3. Results and discussion
Out of 516 dog sera, 13.0% (67/516) reacted with at least one NoV antigen. In detail, 52 sera reacted with the GII.4 VLPs with a prevalence rate of 10.1%, at dilutions ranging from 1:100 to 1:3200. A total of 23 (4.5%) samples reacted with GIV antigens, with titres ranging from 1:100 to 1:800. Of these, 17 (3.3%) resulted positive for both GIV.1 and GIV.2, while 3 (0.58%) sera reacted only with GIV.1 at dilutions from 1:200 to 1:800 and an additional 3 (0.58%) samples reacted only with GIV.2, at final dilution of 1:100. Forty-six (8.9%) sera reacted with GVI.2 VLPs, at dilutions ranging from 1:100 to 1:3200 (Table 1).
Table 1.
IgG antibody titres to GII.4, GIV.1, GIV.2 and GVI.2 VLPs in dog sera. Mean ELISA antibody titres were calculated and expressed as the reciprocal of the highest serum dilution with a positive absorbance (OD405≥0.5) for GII.4, GIV.1, GIV.2 and GVI.2 VLPs.
| Serum dilutions | |||||||
|---|---|---|---|---|---|---|---|
| NoVa VLPsb | 100 (%) | 200 (%) | 400 (%) | 800 (%) | 1600 (%) | 3200 (%) | Total (%) |
| GII.4 | 7/516 (1.4%) | 9/516 (1.7%) | 7/516 (1.4%) | 22/516 (4.3%) | 3/516 (0.6%) | 4/516 (0.8%) | 52/516 (10.1%) |
| GIV.1 | 4/516 (0.8%) | 3/516 (0.6%) | 7/516 (1.4%) | 6/516 (1.2%) | 0/516 (0%) | 0/516 (0%) | 20/516 (3.9%) |
| GIV.2 | 5/516 (1.0%) | 5/516 (1.0%) | 7/516 (1.4%) | 3/516 (0.6%) | 0/516 (0%) | 0/516 (0%) | 20/516 (3.9%) |
| GVI.2 | 5/516 (1.0%) | 5/516 (1.0%) | 12/516 (2.3%) | 16/516 (3.1%) | 4/516 (0.8%) | 4/516 (0.8%) | 46/516 (8.9%) |
NoV, norovirus.
VLPs, virus-like particles.
Analysis of the trends of age-class prevalence by Fisher’s exact test showed a gradual increase in the positive rate from 9.0% and 7.0% in young dogs <1 year of age to 15.0% in dogs older than 12 years for GII.4 and GVI.2 NoVs, respectively. A significant difference in the IgG distribution by age-classes was observed for GIV.1, with the highest rate of antibodies (7.0%) in the group <1 year and the lowest (1.0%) in the age-class 7–9 (P = 0.049). A similar age-related pattern was also found for GIV.2 (Fig. 1).
Fig. 1.
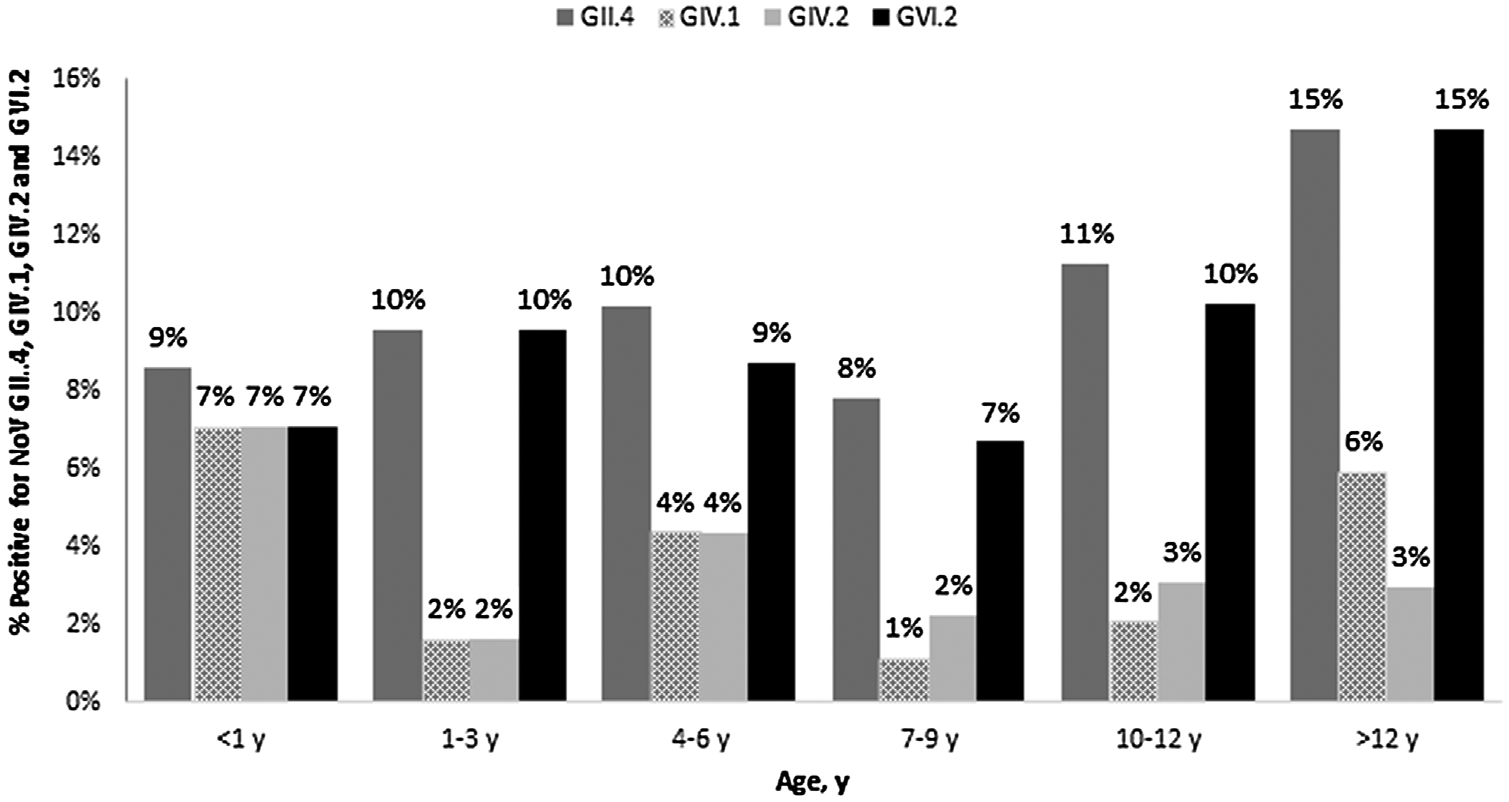
IgG antibodies to GII.4, GIV.1, GIV.2 and GVI.2 VLPs in dog sera of different age groups.
The results obtained are consistent with the hypothesis that dogs may be exposed to both human and carnivore NoVs throughout their life. The magnitude of GII.4 (10.1%) circulation in household dogs was higher in our serological survey than in a previous investigation conducted in UK, where 4.9% and 8.8% of kennel and household dogs, respectively, possessed antibodies for NoVs using a pool of GII.3, GII.4, GII.6 and GII.12 VLPs (Caddy et al., 2015). By converse, the overall prevalence of antibodies against carnivore GIV.2 (3.9%) was lower than that reported (56.6%) in UK (Caddy et al., 2013), but similar to the rate (4.8%) previously reported in an Italian dog population (Di Martino et al., 2010). Furthermore, in our study IgG antibodies reacting only against human GIV.1 VLPs were found in dog sera, suggesting exposure to human GIV NoVs. In general, the seroprevalence for carnivore GVI.2 NoVs revealed in our investigation was lower than the prevalences reported in previous studies (Caddy et al., 2013; Mesquita et al., 2014). In UK (Caddy et al., 2013) the seropositivity for GVI.2 VLPs was 15.9%, while in a multi-centric study conducted in 14 European countries the overall rate was 36.0% (Mesquita et al., 2014).
In our analysis, the majority of the NoV-seropositive dogs showed reactivity for multiple genogroup antigens. Pearson’s rank analysis revealed a high correlation between the levels of antibodies to GIV.1 and GIV.2 antigens (Fig. 2a), while a weak correlation was found when comparing the antibody titers to GIV.1 and to GIV.2 antigens with the antibody titers to GII.4 and to GVI.2 VLPs, respectively (Fig. 2b–e). Of interest, we found a high correlation between the level of antibodies to GII.4 and GVI.2 VLPs (Fig. 2f), in particular in the dog sera that strongly reacted against both antigens (from >1:800 to 1:3200). Previous evidence indicates that GII.4 VLPs are antigenically unrelated to GIV NoVs, whilst antigenic cross-reactivity has been observed between GIV.1 and GIV.2 genotypes (Di Martino et al., 2014).
Fig. 2.
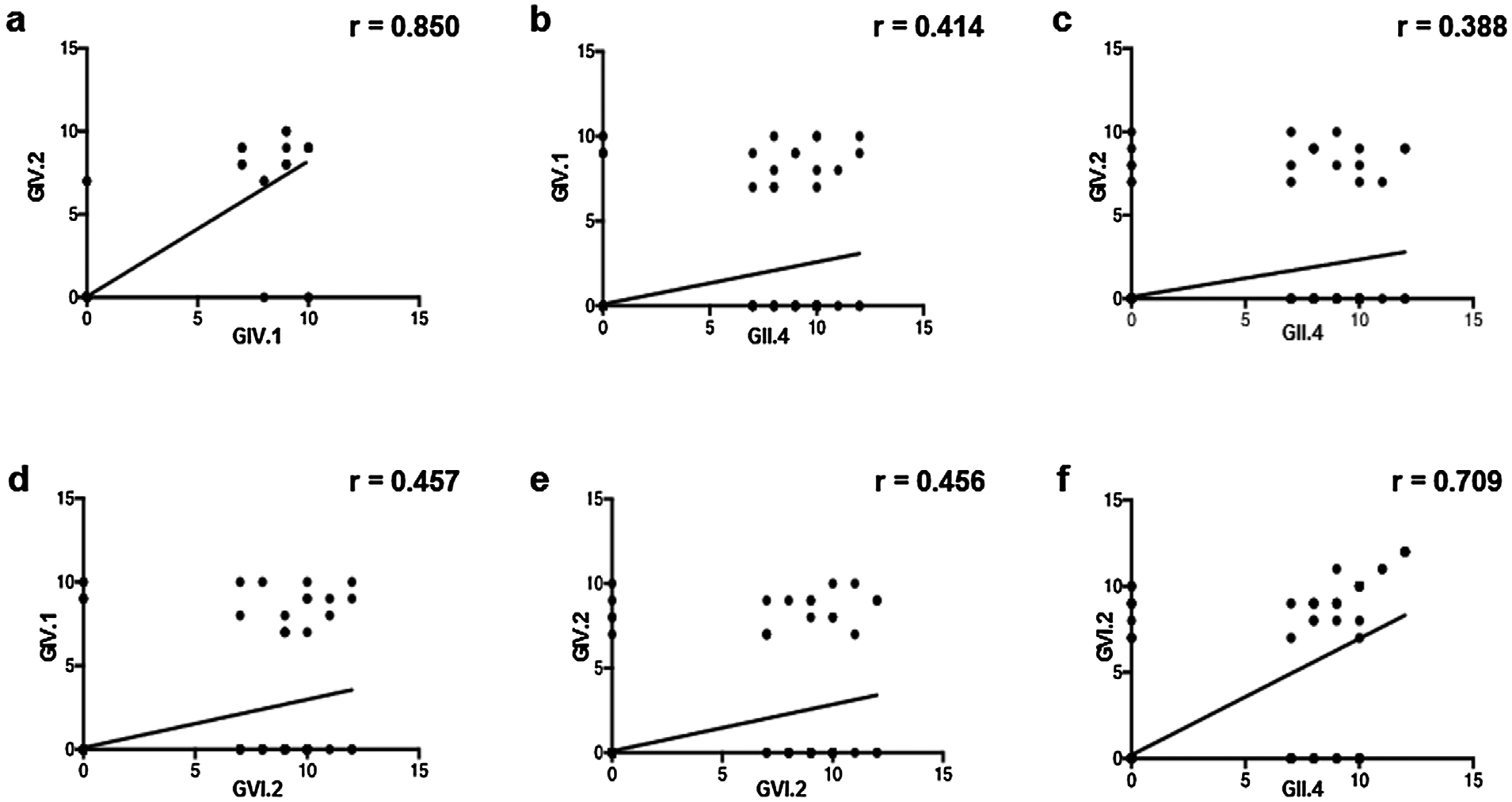
Pearson’s rank analysis of the levels of antibodies among GII.4, GIV.1, GIV.2 and GVI.2 VLPs. Each scatterplot shows the correlation between genotype serum titers detected in the tested dogs (a: GIV.1/GIV.2; b: GII.4/GIV.1; c: GII.4/GIV.2; d: GVI.2/GIV.1; e: GVI.2/GIV.2; f: GII.4/GVI.2). All Pearson ranking values were statistically significant with a p < 0.05.
The antigenic relationships between GII.4 and GVI.2 VLPs was investigated by blocking assay experiments. In our analysis, we found for all the six sera examined that at concentration of 8 μg of each antigen per ml, binding of GVI.2 antibodies was blocked (~50% reduction of OD405 values) by GII.4 VLPs (Fig. 3a), and in turn binding of GII.4 antibodies was blocked by GVI.2 VLPs, although with a ~25% reduction of the OD405 values (Fig. 3b). Furthermore, by testing in WB analysis five human sera positive in ELISA for GII.4 VLPs, all the samples showed reactivity against GVI.2 at the initial dilution of 1:100 (Fig. 3c). A similar reactivity against GII.4 antigen was revealed assessing dog serum samples positive in ELISA for GVI.2 (Fig. 3d). Overall, these findings suggest a strong serologic cross-reactivity between human GII.4 and carnivores GVI.2 NoVs, likely due to the existence of conserved epitopes in the capsid VP1 protein (Parra et al., 2013).
Fig. 3.
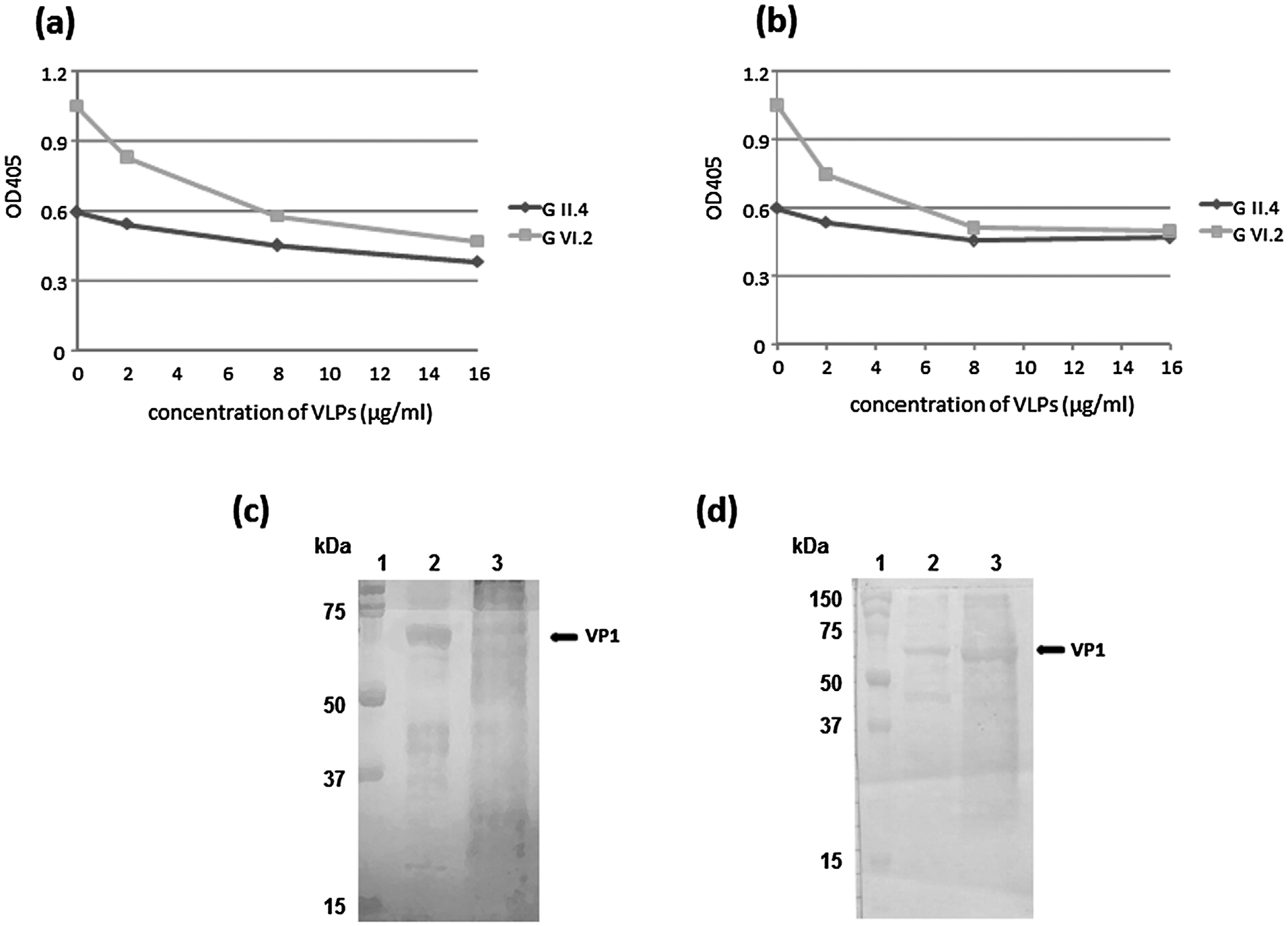
Blocking assay experiment. A canine serum positive for GII.4 and GVI.2 antigens with titres ≥ 1:800 was pre-incubated with GII.4 (a) and GVI.2 (b) VLPs at concentrations of 2, 4, 8 and 16 μg/ml and tested in ELISA for the presence of antibodies against both VLPs. (c) WB analysis of GII.4 and GVI.2 VP1 (at concentration of 8 mg/ml) using a human serum sample positive in ELISA for GII.4 NoV at final dilution of 1:800. (d) WB analysis of GII.4 and GVI.2 VP1 (at concentration of 8 μg/ml) using a dog serum sample positive in ELISA for GVI.2 NoV at final dilution of 1:800. Line 1: Precision Plus protein Standards (Bio-Rad, Italy); line 2: GII.4 VP1; line 3: GVI.2 VP1. Both the sera were tested in WB at dilution of 1:100.
In conclusion, screening of canine sera with multiple NoV antigens demonstrated that dogs are exposed to both human (heterologous) and animal (homologous) NoV strains. Different age-related patterns were observed between the antibody prevalence to GII/GVI and to GIV antigens. Understanding the ecology and dynamics of transmission of NoVs in carnivores will be helpful to assess more precisely if and to which extent pets may pose a risk of infection by homologous and heterologous NoV strains for humans.
Acknowledgements
This study was supported by grants from the University of Teramo, Italy, and by the Italian Ministry of University and Research.
Footnotes
Conflict of interest statement
All Authors declare that there are no financial or other relationships that might lead to a conflict of interest. All authors have seen and approved the manuscript and have contributed significantly to the work.
References
- Bok K, Abente EJ, Realpe-Quintero M, Mitra T, Sosnovtsev SV, Kapikian AZ, Green KY, 2009. Evolutionary dynamics of GII.4 noroviruses over a 34-year period. J. Virol 83, 11890–11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddy S, Emmott E, El-Attar L, Mitchell J, de Rougemont A, Brownlie J, Goodfellow I, 2013. Serological evidence for multiple strains of canine norovirus in the UK dog population. PLoS One 5, e81596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddy SL, de Rougemont A, Emmott E, El-Attar L, Mitchell JA, Hollinshead M, Belliot G, Brownlie J, Le Pendu J, Goodfellow I, 2015. Evidence for human norovirus infection of dogs in the United Kingdom. J. Clin. Microbiol 53, 1873–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino B, Marsilio F, Di Profio F, Lorusso E, Friedrich KG, Buonavoglia C, Martella V, 2010. Detection of antibodies against norovirus genogroup GIV incarnivores. Clin. Vaccine Immunol 17, 180–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino B, Di Profio F, Ceci C, Di Felice E, Green KY, Bok K, De Grazia S, Giammanco GM, Massirio I, Lorusso E, Buonavoglia C, Marsilio F, Martella V, 2014. Seroprevalence of norovirus genogroup IV antibodies among humans, Italy, 2010–2011. Emerg. Infect. Dis 20, 1828–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino B, Di Profio F, Melegari I, Sarchese V, Cafiero MA, Robetto S, Aste G, Lanave G, Marsilio F, Martella V, 2016. A novel feline norovirus in diarrheic cats. Infect. Genet. Evol 38, 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K, et al. , 2013. Caliciviridae: the noroviruses, In: Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA (Eds.), Fields Virology 6th ed. Lippincott Williams & Wilkins, Philadelphia, pp. 583–609. [Google Scholar]
- Martella V, Campolo M, Lorusso E, Cavicchio P, Camero M, Bellacicco AL, Elia G, Greco G, Corrente M, Desario C, Arista C, Banyaj K, Koopmans M, Buonavoglia C, 2007. Norovirus in captive lion cub (Panthera leo). Emerg. Infect. Dis 13, 1071–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V, Lorusso E, Decaro N, Elia G, Radogna A, D’Abramo M, Desario C, Cavalli A, Corrente M, Germinaro CA, Banyai K, Di Martino B, Marsilio F, Carmichael LE, Buonavoglia C, 2008. Detection and molecular characterization of a canine norovirus. Emerg. Infect. Dis 14, 1306–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V, Decaro N, Lorusso E, Radogna A, Moschidou P, Amorisco F, Lucente MS, Desario C, Elia G, Banyai K, Carmichael LE, Buonavoglia C, 2009. Genetic heterogeneity and recombination in canine noroviruses. J. Virol 83, 11391–11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita JR, Barclay L, Nascimento MSJ, Vinjé J, 2010. Novel norovirus in dogs with diarrhea. Emerg. Infect. Dis 16, 980–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita JR, Costantini VP, Cannon JL, Lin SC, Nascimento MS, Vinjé J, 2013. Presence of antibodies against genogroup VI norovirus in humans. Virol. J 10, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita JR, Delgado I, Costantini V, Heenemann K, Vahlenkamp TW, Vinjé J, Nascimento MS, 2014. Seroprevalence of canine norovirus in 14 European countries. Clin. Vaccine Immunol 21, 898–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra GI, Azure J, Fischer R, Bok K, Sandoval-Jaime C, Sosnovtsev SV, Sander P, Green KY, 2013. Identification of a broadly cross-reactive epitope in the inner shell of the norovirus capsid. PLoS One 8, e67592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinjé J, Parashar UD, 2008. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg. Infect. Dis 14, 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peasey AE, Ruiz-Palacios GM, Quigley M, Newsholme W, Martinez J, Rosales G, Jiang X, Blumenthal UJ, 2004. Seroepidemiology and risk factors for sporadic norovirus/Mexico strain. J. Infect. Dis 189, 2027–2036. [DOI] [PubMed] [Google Scholar]
- Pinto P, Wang Q, Chen N, Dubovi EJ, Daniels JB, Millward LM, Buonavoglia C, Martella V, Saif LJ, 2012. Discovery and genomic characterization of noroviruses from a gastroenteritis outbreak in domestic cats in the US. PLoS One 7, e32739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summa M, von Bonsdorff CH, Maunula L, 2012. Pet dogs – a transmission route for human noroviruses? J. Clin. Virol 53, 244–247. [DOI] [PubMed] [Google Scholar]
- Tse H, Lau SK, Chan WM, Choi GK, Woo PC, Yuen KY, 2012. Complete genome sequences of novel canine noroviruses in Hong Kong. J. Virol 86, 9531–9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinjé J, 2015. Advances in laboratory methods for detection and typing of norovirus. J. Clin. Microbiol 53, 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]


