Abstract
Goals:
We aim to explore the relationship between the use of proton pump inhibitors (PPIs) and upper gastrointestinal bleeding (UGIB). We develop a nomogram model to predict mortality in critically ill stroke patients.
Study:
This is a retrospective study based on the MIMIC IV database. We extracted clinical information including demographic data, comorbidities, and laboratory indicators. Univariate and multivariable logistic regressions were used to assess and identify risk factors for the occurrence of UGIB and for the in-hospital mortality of critically ill stroke patients. The resulting model was used to construct a nomogram for predicting in-hospital mortality.
Results:
Five thousand seven hundred sixteen patients from the MIMIC-IV database were included in our analysis. UGIB occurred in 109 patients (1.9%), whereas the PPI use rate was as high as 60.6%. Chronic liver disease, sepsis, shock, anemia, and increased level of urea nitrogen were independent risk factors for the occurrence of UGIB in severe stroke patients. We identified age, heart failure, shock, coagulopathy, mechanical ventilation, continuous renal replacement therapy, antiplatelet drugs, anticoagulation, simplified acute physiology score-II, and Glasgow coma score as independent risk factors for in-hospital mortality in severe stroke patients. The C-index for the final nomograms was 0.852 (95% confidence interval: 0.840, 0.864).
Conclusions:
We found that the overall rate of UGIB in severe stroke patients is low, whereas the rate of PPI usage is high. In our study, PPI was not identified as a risk factor for the occurrence of UGIB and UGIB was not associated with all-cause mortality. More clinical trials are needed to evaluate the benefits of using PPI in critically ill stroke patients.
Key Words: proton pump inhibitor, upper gastrointestinal bleeding, severe stroke, nomogram model
BACKGROUND
Stroke is the second leading cause of disability and death worldwide, and as such it represents a serious threat to public health.1 Ischemic stroke is a common disease caused by cerebrovascular blockage or thrombosis, and it is the most common type of stroke, accounting for approximately 80% of all strokes.2 Acute cerebral stroke is a disease characterized by sudden cerebral ischemia, hemorrhage, or subarachnoid hemorrhage; it is relatively common among patients in intensive care unit (ICU).3 Hemorrhagic stroke is due to aneurysm and vascular malformation, it is mainly manifested as parenchymal hemorrhage and subarachnoid hemorrhage, and it has a high mortality rate. The short-term and long-term prognosis of stroke patients is poor.4,5 In addition to the severity of the disease, the economic costs of treatment and care after stroke are considerable.6
Upper gastrointestinal bleeding (UGIB) is a common complication for patients in ICU. The incidence of stroke with UGIB complications is 1.35% to 2.6%.7,8 According to recent studies, UGIB is correlated with a worse prognosis in acute ischemic stroke patients,7,9 with the occurrence of UGIB increasing the 3-year mortality rate.10 Different studies have reported opposite results about risk factors and the prognosis of UGIB in critically ill stroke patients, and no association between the use of PPI and the incidence of UGIB or increased in-hospital mortality in critically ill stroke patients was observed.11,12
Therefore, we aimed to explore the relationships between PPI use and UGIB and mortality, and between UGIB and mortality in critically ill stroke patients. We used data from a public database (the Medical Information Mart for Intensive Care (MIMIC)-IV database) to explore independent risk factors to better predict of UGIB in patients with acute severe cerebral stroke, and to further individualize treatments.
Study
Data Source
This was a retrospective study based on the MIMIC-IV database (version 1.0). The MIMIC-IV database contains the clinical information of patients who were admitted to the ICUs at the Beth Israel Deaconess Medical Center (BIDMC) between 2008 and 2019. The data held by the MIMIC-IV database have been de-identified and all patient identifiers have been sealed in accordance with the Health Insurance Portability and Accountability Act (HIPAA) Safe Harbor provision. Researchers can apply to use this database without charge. To gain access to the database, we completed relevant training courses at the National Institutes of Health and got the certificate (Record ID: 52116261). This study was approved by the ethics committee of our hospital (Reference: 2022208). Figure 1 shows a flowchart depicting the study protocol.
FIGURE 1.

Flowchart depicting the study population.
Study Population
There were 53150 patients admitted to the ICU in 2008-2019 listed in the MIMIC-IV database. International Classification of Diseases-9 (ICD-9) codes were used to identify patients afflicted by strokes. Then, 10,283 patients diagnosed with ischemic stroke or hemorrhagic stroke or subarachnoid hemorrhage were included in this research. Among those patients, the inclusion criteria were: adult patients (age 18 years and older) with an ischemic stroke or hemorrhage stroke or subarachnoid hemorrhage. The exclusion criteria were: Non-first ICU admission; ICU stay<24 hours or age below 18 years old. Finally, 5716 patients were included in our analysis.
Some Definition
UGIB is diagnosed based on specific symptoms such as hematemesis or melena after admission, with a positive fecal occult blood test. The primary outcome in this study was in-hospital mortality and 30-day mortality.
Data Collection
Data from MIMIC-IV was extracted via Structure query Language. For included patients, we collated data relating to clinical features as follows:
Demographics: sex, age, length of ICU stay.
Treatment: antiplatelet drugs such as aspirin or clopidogrel, parenteral nutrition, use of hormones, use of heparin anticoagulation, PPI (proton-pump inhibitor) therapy, continuous renal replacement therapy (CRRT) and mechanical ventilation.
Comorbidities: hypertension, diabetes, heart failure, atrial fibrillation, peptic ulcer disease, chronic liver disease, sepsis, shock and coagulopathy.
Laboratory test: white blood count (WBC), hemoglobin (HgB), platelets (PLT), creatinine (CR), blood urea nitrogen (BUN), albumin (Alb), total bilirubin (Tbil), prothrombin time (PT), international normalized ratio (INR).
Severity scoring system: simplified acute physiology score (SAPS) II, sequential organ failure assessment (SOFA) score, Glasgow coma score (GCS).
Prognosis: in-hospital mortality.
For all parameters, we used the first values obtained during the first 24 hours in the ICU.
Statistical Analysis
For continuous variables, the Shapiro-Wilk test was used to assess whether the data were normally distributed. Data were expressed as mean±SD when followed a normal distribution. Comparisons between groups were carried out by the Student t test. Data that were not normally distributed were expressed as medians and interquartile ranges. Comparisons between groups were carried out using the Kruskal-Wallis test. Comparisons between groups were carried out with the Chi-squared test for categorical variables. Further, we use univariate and multivariable logistic regression analyses to identify the risk factors for the occurrence of UGIB and mortality in patients with acute cerebral stroke. The final model was used to construct a nomogram for predicting mortality. Statistical significance was defined as P< 0.05, and all analyses were performed using R software, version 4.1.2 and SPSS software, version 25.0.
RESULTS
A Comparison of Clinical Features Across Different Subgroups
We identified 5716 patients with severe stroke from the MIMIC-IV database (Fig. 1). The mean age at the time of enrollment in the study of the included patients was 68.5±15.7 years, and 50.7% of the patients were male. The mean length of ICU stay was 12.3±13.7 days. Amongst this cohort, 1019 patients died in hospital, with an in-hospital mortality rate of 17.8%. UGIB occurred in 109 patients (1.9%), whereas the rate of PPI usage was as high as 60.6%. We divided the patients into two groups: one treated with PPI and the other one not treated with PPI. We observed no significant differences between these two groups for any of the considered features, with the exception of the platelet count (Table 1).
TABLE 1.
Comparison of Clinical Characteristics and Laboratory Tests of Severe Stroke Patients With or Without Use of PPI
| Parameter | Total (n=5716) | PPI (n=3463) | Non-PPI (n=2253) | t/c2/Z | P |
|---|---|---|---|---|---|
| Age (y) | 68.5±15.7 | 68.8±15.6 | 68.3±15.7 | −1.190 | 0.234 |
| Gender (male, %) | 2897 (50.7%) | 1129 (50.1%) | 1768 (51.1%) | 0.486 | 0.499 |
| Length of ICU stay (d) | 12.3±13.7 | 12.4±13.3 | 12.3±13.9 | −0.364 | 0.716 |
| Comorbidities, n (%) | |||||
| Hypertension | 3056 (53.5%) | 1173 (52.1%) | 1883 (54.4%) | 2.930 | 0.087 |
| Diabetes | 1622 (28.4%) | 633 (28.1%) | 989 (28.6%) | 0.144 | 0.719 |
| Heart failure | 1213 (21.2%) | 497 (22.1%) | 716 (20.7%) | 1.563 | 0.221 |
| Atrial fibrillation | 1789 (31.3%) | 720 (32.0%) | 1069 (30.9%) | 0.752 | 0.397 |
| Peptic ulcer disease | 66 (1.2%) | 22 (1.0%) | 44 (1.3%) | 1.034 | 0.375 |
| Chronic liver disease | 347 (6.1%) | 129 (5.7%) | 218 (6.3%) | 0.776 | 0.396 |
| Sepsis | 2354 (41.2%) | 924 (41.0%) | 1430 (41.3%) | 0.045 | 0.847 |
| Shock | 612 (10.7%) | 239 (10.6%) | 373 (10.8%) | 0.038 | 0.861 |
| Coagulopathy | 489 (8.6%) | 184 (8.2%) | 305 (8.8%) | 0.716 | 0.411 |
| Laboratory test | |||||
| WBC (109/L) | 11.2±8.8 | 11.15±9.93 | 11.16±7.90 | 0.058 | 0.954 |
| Hemoglobin (g/L) | 118.6±2.2 | 118.7±2.2 | 118.5±2.2 | −0.381 | 0.703 |
| Platelet (109/L) | 219.0±93.9 | 215.1±86.3 | 221.5±98.5 | 2.519 | 0.012 |
| Serum albumin (g/L) | 34.4±0.6 | 34.4±0.7 | 34.3±0.6 | −0.104 | 0.917 |
| SCr (mg/dL) | 1.22±1.31 | 1.22±1.28 | 1.21±1.33 | −0.164 | 0.869 |
| BUN (mmol/L) | 21.7±16.7 | 21.5±15.6 | 21.8±17.3 | 0.573 | 0.567 |
| Total bilirubin (mmol/L) | 0.82±1.43 | 0.82±1.35 | 0.83±1.48 | 0.087 | 0.930 |
| PT (s) | 14.3±7.3 | 14.3±7.8 | 14.3±7.0 | 0.356 | 0.722 |
| INR | 1.3±0.7 | 1.3±0.9 | 1.3±0.7 | 0.829 | 0.407 |
| Treatment, n (%) | |||||
| Antiplatelet drugs | 3058 (53.5%) | 1210 (53.7%) | 1848 (53.4%) | 0.064 | 0.807 |
| Hormones | 1700 (29.7%) | 676 (30.0%) | 1024 (29.6%) | 0.123 | 0.725 |
| Heparin anticoagulation | 5169 (90.4%) | 2045 (90.8%) | 3124 (90.2%) | 0.489 | 0.491 |
| CRRT | 115 (2.0%) | 37 (1.6%) | 78 (2.3%) | 2.577 | 0.123 |
| Mechanical ventilation | 2381 (41.7%) | 933 (41.4%) | 1448 (41.8%) | 0.091 | 0.784 |
| Severity scoring system | |||||
| SAPS-II | 34.4±12.8 | 34.3±12.6 | 34.5±13.0 | 0.472 | 0.637 |
| SOFA score | 4.4±3.4 | 4.34±3.30 | 4.45±3.44 | 1.203 | 0.229 |
| GCS | 11.1±3.8 | 11.1±3.8 | 11.1±3.8 | −0.389 | 0.697 |
| Prognosis, n (%) | |||||
| Gastrointestinal bleeding | 109 (1.9%) | 38 (1.7%) | 71 (2.1%) | 0.965 | 0.373 |
| In-hospital mortality | 1019 (17.8%) | 384 (17.0%) | 635 (18.3%) | 1.557 | 0.216 |
| 30-day mortality | 980 (17.1%) | 370 (16.4%) | 610 (17.6%) | 1.366 | 0.251 |
BUN indicates blood urea nitrogen; CRRT, continuous renal replacement therapy; GCS, Glasgow coma score; ICU, intensive care unit; INR, international normalized ratio; PPI, proton-pump inhibitor; PT, prothrombin time; SAPS, simplified acute physiology score; SCr, Serum creatinine; SOFA, sequential organ failure assessment; UGIB, upper gastrointestinal bleeding; WBC, White blood cell.
Univariate Logistic Regression Analysis
Table 2 shows the univariate logistics regression analysis for the occurrence of gastrointestinal bleeding and in-hospital mortality in severe stroke patients. As shown in Table 2, risk factors for the occurrence of gastrointestinal bleeding in severe stroke patients are: hypertension, diabetes, heart failure, peptic ulcer disease, chronic liver disease, sepsis, shock, coagulopathy, hemoglobin, serum albumin, SCr, BUN, PT, INR, use of PPI, CRRT, mechanical ventilation, SAPS-II score, SOFA score, and GCS.
TABLE 2.
Univariate Logistics Regression for the Occurrence of Gastrointestinal Bleeding and In-hospital Mortality in Severe Stroke Patients
| Univariate Logistics Regression for the Occurrence of UBGI | Univariate Logistics Regression for In-hospital Mortality | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | P | OR (95% CI) | P |
| Age (y) | 1.007 (0.994, 1.019) | 0.285 | 1.021 (1.016, 1.025) | <0.001 |
| Gender (male) | 1.340 (0.913, 1.967) | 0.135 | 1.003 (0.876, 1.148) | 0.970 |
| Hypertension | 0.606 (0.413, 0.891) | 0.011 | 0.914 (0.798, 1.047) | 0.193 |
| Diabetes | 1.662 (1.127, 2.451) | 0.010 | 1.180 (1.019, 1.367) | 0.027 |
| Heart failure | 2.778 (1.889, 4.085) | <0.001 | 1.688 (1.448, 1.967) | <0.001 |
| Atrial fibrillation | 1.279 (0.863, 1.895) | 0.221 | 1.477 (1.283, 1.700) | <0.001 |
| Peptic ulcer disease | 4.371 (1.721, 11.102) | 0.002 | 1.361 (0.762, 2.430) | 0.297 |
| Chronic liver disease | 5.158 (3.274, 8.128) | <0.001 | 2.311 (1.824, 2.928) | <0.001 |
| Sepsis | 4.689 (3.009, 7.308) | <0.001 | 2.751 (2.393, 3.162) | <0.001 |
| Shock | 5.540 (3.731, 8.226) | <0.001 | 3.552 (2.969, 4.250) | <0.001 |
| Coagulopathy | 1.867 (1.090, 3.200) | 0.023 | 2.635 (2.155, 3.222) | <0.001 |
| WBC | 1.009 (0.998, 1.019) | 0.105 | 1.038 (1.027, 1.050) | <0.001 |
| Hemoglobin | 0.754 (0.694, 0.819) | <0.001 | 0.893 (0.866, 0.921) | <0.001 |
| Platelet | 0.999 (0.997, 1.001) | 0.363 | 0.998 (0.998, 0.999) | <0.001 |
| Serum albumin | 0.474 (0.348, 0.645) | <0.001 | 0.480 (0.420, 0.547) | <0.001 |
| SCr | 1.179 (1.097, 1.267) | <0.001 | 1.137 (1.087, 1.190) | <0.001 |
| BUN | 1.024 (1.018, 1.030) | <0.001 | 1.018 (1.015, 1.022) | <0.001 |
| Total bilirubin | 1.057 (0.963, 1.159) | 0.242 | 1.224 (1.152, 1.300) | <0.001 |
| PT | 1.016 (1.002, 1.031) | 0.030 | 1.017 (1.009, 1.025) | <0.001 |
| INR | 1.189 (1.023, 1.381) | 0.024 | 1.199 (1.100, 1.306) | <0.001 |
| Use of PPI | 0.820 (0.551, 1.220) | 0.327 | 0.915 (0.796, 1.052) | 0.212 |
| Antiplatelet drugs | 1.290 (0.877, 1.898) | 0.196 | 0.511 (0.445, 0.586) | <0.001 |
| Hormones | 1.324 (0.891, 1.966) | 0.165 | 0.983 (0.847, 1.140) | 0.817 |
| Anticoagulation | 2.226 (0.903, 5.484) | 0.082 | 0.318 (0.263, 0.384) | <0.001 |
| CRRT | 4.071 (1.933, 8.575) | <0.001 | 7.344 (5.024, 10.734) | <0.001 |
| Mechanical ventilation | 2.667 (1.792, 3.968) | <0.001 | 6.735 (5.754, 7.896) | <0.001 |
| SAPS-II | 1.044 (1.031, 1.057) | <0.001 | 1.078 (1.071, 1.084) | <0.001 |
| SOFA score | 1.206 (1.159, 1.256) | <0.001 | 1.264 (1.239, 1.289) | <0.001 |
| GCS | 0.934 (0.892, 0.978) | 0.004 | 0.808 (0.794, 0.822) | <0.001 |
| UGIB | / | / | 2.524 (1.692, 3.765) | <0.001 |
/ denotes significance of P values.
BUN indicates blood urea nitrogen; CRRT, continuous renal replacement therapy; GCS, Glasgow coma score.; ICU, intensive care unit; INR, international normalized ratio; PPI, proton-pump inhibitor; PT, prothrombin time; SAPS, simplified acute physiology score; SCr, Serum creatinine; SOFA, sequential organ failure assessment; UGIB, upper gastrointestinal bleeding; WBC, White blood cell.
On the other hand, risk factors for in-hospital mortality in severe stroke patients were: age, diabetes, heart failure, atrial fibrillation, chronic liver disease, sepsis, shock, coagulopathy, WBC, hemoglobin, platelet, serum albumin, SCr, BUN, total bilirubin, PT, INR, antiplatelet drugs, anticoagulation, CRRT, mechanical ventilation, SAPS-II, SOFA score, GCS, and UGIB.
Multivariate Logistic Analysis
Figure 2 shows the results of the multivariable logistic analysis of the occurrence of gastrointestinal bleeding in severe stroke patients. We found that chronic liver disease (OR=1.039, P<0.001), sepsis (OR=2.455, P=0.002), shock (OR=2.041, P=0.004), hemoglobin (OR=0.850, P=0.001), and BUN (OR=1.012, P=0.004) were independent risk factors for the occurrence of gastrointestinal bleeding in severe stroke patients.
FIGURE 2.

Multivariable logistic analysis for the occurrence of gastrointestinal bleeding in severe stroke patients.
Figure 3 presents the multivariable logistic analysis for in-hospital mortality in severe stroke patients. Age (OR=1.018, P<0.001), heart failure (OR=1.418, P=0.004), shock (OR=1.406, P=0.013), coagulopathy (OR=1.609, P=0.001), mechanical ventilation (OR=3.313, P<0.001), CRRT (OR=2.208, P=0.001), antiplatelet drugs (OR=0.464, P<0.001), anticoagulation (OR=0.267, P<0.001), SAPS-II score (OR=1.033, P<0.001), and GCS (OR=0.916, P<0.001) were all independent risk factors for in-hospital mortality in severe stroke patients.
FIGURE 3.
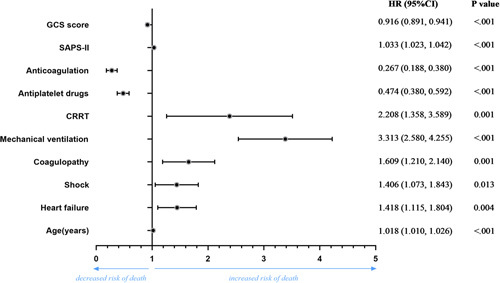
Multivariable logistic analysis for in-hospital mortality in severe stroke patients
Construction and Calibration of Nomogram
Based on the results of the multivariate analyses, we constructed a novel nomogram model to predict survival rates (Fig. 4), containing the 10 independent variables (Fig. 3) we identified as risk factors for in-hospital mortality. The C-index for the nomograms was 0.852 (95% CI: 0.840, 0.864). The highest points in the nomogram were assigned to SAPS-II and age, whereas heart failure and shock level played relatively minor roles. The calibration curve revealed high consistency between predicted and actual risk of death in severe stroke patients (Fig. 5).
FIGURE 4.

Nomogram of risk of death.
FIGURE 5.
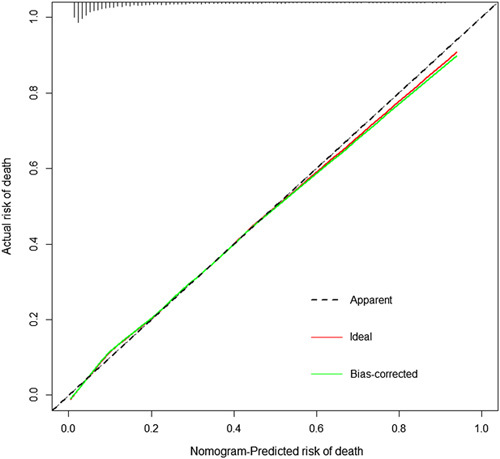
Calibration of nomogram for predicted risk of death.
DISCUSSION
In our study, we noticed that the overall rate of UGIB in severe stroke patients was low (1.9%), which is similar to what was shown in previous studies,7,8 whereas the rate of PPI usage was considerably high (60.6%). To our knowledge, this is the first study to investigate the relationship between UGIB and PPI use in severe stroke patients. We observed no significant differences in the occurrence of UGIB in patients treated with PPI and patients not treated with PPI. Our logistic regression analysis did not identify PPI as a risk factor for the occurrence of UGIB. UGIB itself was not associated with all-cause mortality.
In our study, as shown in Table 2, univariate logistics regression analysis revealed that in severe stroke patients, comorbidities such as hypertension, diabetes, heart failure, peptic ulcer disease, and chronic liver disease were the risk factors for the occurrence of UGIB. Thus, these comorbidities above were important factors. Moreover, according to multivariable logistic analysis, we found that shock (HR=2.041, P=0.004), sepsis (HR=2.445, P=0.002), and chronic liver disease (HR=2.016, P=0.010) were independent risk factors for the occurrence of g UGIB. In conclusion, shock, sepsis, and chronic liver disease were important factors for the occurrence of UGIB in severe stroke patients. Previous studies had reported similar results.8,13 Chronic liver disease is often associated with coagulation disorders secondary to hepatic dysfunction or esophagogastric varices secondary to portal hypertension, which could result in gastrointestinal bleeding. Sepsis and shock often involve gastric mucosal ischemia or edema, which causes gastric mucosal cell damage and leads to gastrointestinal bleeding. Hemoglobin and BUN are associated with the response of gastrointestinal bleeding. These known mechanisms all support the results that patients with one of these comorbidities have an increased risk of UGIB.
Several previous studies have suggested that PPIs were associated with UGIB,14–16 as well with hospital mortality in critically ill patients.17,18 Other studies found no association between PPI use and the incidence of UGIB and increased in-hospital mortality in critically ill stroke patients.11,12 Our study is in agreement with these last observations as we found no evidence of an association between PPI and UGIB and mortality nor between UGIB and mortality in severe stroke patients. This suggests that gastrointestinal bleeding in severe stroke patients cannot be completely avoided using PPIs. In other words, we found that the use of PPI could not significantly reduce the incidence of gastrointestinal bleeding19 nor mortality20 in critically stroke patients. Yet, overutilization of PPIs in ICU is common.21 The use of PPIs increases every year in both Western and Eastern countries.22 Based on our results, we believe that PPIs may not be appropriate for patients at low risk of UGIB. We recommend further clinical trials on large cohorts of patients to evaluate the benefits of using PPI in critically ill patients at low risk of UGIB.23 Better identification of appropriate selection criteria for PPI and comparisons of pharmacologic prevention strategies are warranted.
The pathophysiological mechanism that underlies the association between acute stroke and UGIB is not clear. Previous studies have shown that stress ulcers may develop from what is believed to be vagal hyperactivity, which would result in increased gastric acid and pepsin secretion and damage of gastrointestinal mucosa.24 Hyperactivity of the sympathetic system after acute ischemic stroke tends to induce an excessive catecholamine discharge, and the following vasoconstriction may cause splanchnic hypoperfusion and mucosal ischemia.25 During a stroke, cerebral blood circulation is impaired and a large amount of hormones secreted and stored by the pituitary gland are released, causing the increase of peripheral pituitary hormones. Autonomic nervous dysfunction in stroke patients can not only affect the vasomotion function of the gastric mucosa, leading to local mucosal microcirculation disorder, but also promote the production of a large amount of free oxygen radicals in the digestive tract, impairing the integrity of the mucosa.
Previous studies have indicated that the occurrence of UGIB was correlated with a higher 5-year mortality rate,26 which is in contrast to our findings. The reason for this discrepancy may be that the UGIB rate in the cohort of patients we considered was too low, we also focused on in-hospital mortality rather than 5-year mortality.
As shown in Figure 3, after multivariable logistic analysis, we found that shock (HR=1.406, P=0.013) and heart failure (HR=1.418, P=0.004) were independent risk factors for in-hospital mortality. That is to say, heart failure and shock were important factors for in-hospital mortality in severe stroke patients, which can be explained by the severity of the comorbidities and resulting organ failures. In agreement with previous studies, we confirmed that age,27 heart failure,28 shock, coagulopathy, mechanical ventilation,29 CRRT, antiplatelet drugs, anticoagulation, SAPS-II score, and GCS30 are independent risk factors for in-hospital mortality in severe stroke patients, which can be explained by the severity of the comorbidities and resulting organ failures. We also identified mechanical ventilation as an independent predictor in the survival of individuals with severe stroke.29 This suggests that a severe stroke patient who incurred complications has a higher risk of death. Our identification of risk factors associated with mortality in severe stroke patients could allow physicians to effectively monitor patients most at risk.
We believe our findings are particularly relevant as the study was designed to include a large and diverse population of real-world data. The main outcome of our research is the establishment of a prognostic nomogram, composed of objective indexes, that can predict the mortality risk of ICU stroke patients.
We identified some limitations in our study. First, the study might present selection bias, as it is a single-institution study. Because of the potential selection bias, our model may have limited applicability in other regions. Second, no long-term follow-up events were provided from the MIMIC-IV database, so the functional outcomes and post stroke disposition of patients are unknown. Some stroke patients might not be referred from other hospitals because of the narrow time window for reperfusion therapy. Furthermore, we did not include endoscopic therapy due to serious underlying diseases and unstable vital signs. Endoscopic therapy should be analyzed as an influencing factor in a large sample of ICU population in future. In addition, because we only have information on new-onset UGIB during hospitalization without exact date, it remains unclear whether patients with a longer length of stay are more likely to develop UGIB or if diagnosis of UGIB leads to a longer hospitalization. We were also unable to conduct external validation. Further research with external evidence is therefore needed to validate and consolidate the presented model.
In conclusion, the overall rate of UGIB in severe stroke patients was low, whereas the use rate of PPI was high. PPI was not the risk factor for the occurrence of UGIB. UGIB was also not associated with all-cause mortality. Definitive recommendations need further clinical trials to evaluate benefits of using PPI in critically ill stroke patients with low incidence of UGIB.
ACKNOWLEDGMENTS
The authors acknowledge the role of all patients, investigators, and support staff in conducting this research.
Footnotes
The authors declare that they have nothing to disclose.
Contributor Information
Zengdian Chen, Email: ffczd@163.com.
Weiguo Lin, Email: Linweiguo2022@163.com.
Faqin Zhang, Email: 958756834@qq.com.
Wen Cao, Email: caow1030@163.com.
REFERENCES
- 1.Krishnamurthi RV, Ikeda T, Feigin VL. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the global burden of disease study 2017. Neuroepidemiology. 2020;54:171–179. [DOI] [PubMed] [Google Scholar]
- 2.Randolph SA. Ischemic stroke. Workplace Health Saf. 2016;64:444. [DOI] [PubMed] [Google Scholar]
- 3.McDermott M, Jacobs T, Morgenstern L. Critical care in acute ischemic stroke. Handb Clin Neurol. 2017;140:153–176. [DOI] [PubMed] [Google Scholar]
- 4.Rumalla K, Mittal MK. Gastrointestinal bleeding in acute ischemic stroke: a population-based analysis of hospitalizations in the United States. JStroke Cerebrovasc Dis. 2016;25:1728–1735. [DOI] [PubMed] [Google Scholar]
- 5.Adoukonou T, Agbétou M, Bangbotché R, et al. Long-term mortality of stroke survivors in Parakou: 5-year follow-up. J Stroke Cerebrovasc Dis. 2020;29:104785. [DOI] [PubMed] [Google Scholar]
- 6.Rajsic S, Gothe H, Borba HH, et al. Economic burden of stroke: a systematic review on post-stroke care. Eur J Health Econ. 2019;20:107–134. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y, Xu W, Wang W, et al. Gastrointestinal hemorrhage is associated with mortality after acute ischemic stroke. Curr Neurovasc Res. 2019;16:135–141. [DOI] [PubMed] [Google Scholar]
- 8.Krag M, Perner A, Wetterslev J, et al. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med. 2015;41:833–845. [DOI] [PubMed] [Google Scholar]
- 9.Chen CM, Hsu HC, Chuang YW, et al. Study on factors affecting the occurrence of upper gastrointestinal bleeding in elderly acute stroke patients undergoing rehabilitation. J Nutr Health Aging. 2011;15:632–636. [DOI] [PubMed] [Google Scholar]
- 10.Chou YF, Weng WC, Huang WY. Association between gastrointestinal bleeding and 3-year mortality in patients with acute, first-ever ischemic stroke. J Clin Neurosci. 2017;44:289–293. [DOI] [PubMed] [Google Scholar]
- 11.Shi L, Zhang D. Proton pump inhibitor use before ICU admission is not associated with mortality of critically ill patients. J Clin Pharmacol. 2020;60:860–866. [DOI] [PubMed] [Google Scholar]
- 12.Sun J, Wang B, Cao P, et al. Benefits and risks of stress ulcer prevention with proton pump inhibitors for critical patients: an observational cohort study with 1 972 patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31:539–544. [DOI] [PubMed] [Google Scholar]
- 13.Wang SP, Huang YH. Gastrointestinal hemorrhage after spontaneous subarachnoid hemorrhage: a single-center cohort study. Sci Rep. 2017;7:13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alhazzani W, Alshamsi F, Belley-Cote E, et al. Efficacy and safety of stress ulcer prophylaxis in critically ill patients: a network meta-analysis of randomized trials. Intensive Care Med. 2018;44:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sridharan K, Sivaramakrishnan G, Gnanaraj J. Pharmacological interventions for stress ulcer prophylaxis in critically ill patients: a mixed treatment comparison network meta-analysis and a recursive cumulative meta-analysis. Expert Opin Pharmacother. 2018;19:151–158. [DOI] [PubMed] [Google Scholar]
- 16.Alshamsi F, Belley-Cote E, Cook D, et al. Efficacy and safety of proton pump inhibitors for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis of randomized trials. Crit Care. 2016;20:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyd C, Hassig T, MacLaren R. A pragmatic assessment of proton pump inhibitors vs. histamine type 2 receptor antagonists on clinically important gastrointestinal bleeding and mortality when used for stress ulcer prophylaxis in the ICU. Pharmacotherapy. 2021;41:820–827. [DOI] [PubMed] [Google Scholar]
- 18.Schirmer CM, Kornbluth J, Heilman CB, et al. Gastrointestinal prophylaxis in neurocritical care. Neurocrit Care. 2012;16:184–193. [DOI] [PubMed] [Google Scholar]
- 19.Krag M, Marker S, Perner A, et al. Pantoprazole in patients at risk for gastrointestinal bleeding in the ICU. N Engl J Med. 2018;379:2199–2208. [DOI] [PubMed] [Google Scholar]
- 20.Shrope-Mok SR, Propst KA, Iyengar R. APACHE IV versus PPI for predicting community hospital ICU mortality. Am J Hosp Palliat Care. 2010;27:243–247. [DOI] [PubMed] [Google Scholar]
- 21.Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48:462–469. [DOI] [PubMed] [Google Scholar]
- 22.Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Expert Rev Clin Pharmacol. 2018;11:1123–1134. [DOI] [PubMed] [Google Scholar]
- 23.Jalil BA, El-Kersh K. Enteral nutrition better than proton pump inhibitors? Curr Opin Crit Care. 2019;25:334–339. [DOI] [PubMed] [Google Scholar]
- 24.Chan KH, Mann KS, Lai EC, et al. Factors influencing the development of gastrointestinal complications after neurosurgery: results of multivariate analysis. Neurosurgery. 1989;25:378–382. [DOI] [PubMed] [Google Scholar]
- 25.Schaller BJ, Graf R, Jacobs AH. Pathophysiological changes of the gastrointestinal tract in ischemic stroke. Am J Gastroenterol. 2006;101:1655–1665. [DOI] [PubMed] [Google Scholar]
- 26.Wang T, Zhu D, Kong L, et al. Effect of upper gastrointestinal bleeding on prognosis of middle-aged patients with acute ischemic stroke: a retrospective study. Ann Palliat Med. 2021;10:5494–5501. [DOI] [PubMed] [Google Scholar]
- 27.Hamidon BB, Raymond AA. The risk factors of gastrointestinal bleeding in acute ischaemic stroke. Med J Malaysia. 2006;61:288–291. [PubMed] [Google Scholar]
- 28.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9:105–118. [DOI] [PubMed] [Google Scholar]
- 29.Li XD, Li MM. A novel nomogram to predict mortality in patients with stroke: a survival analysis based on the MIMIC-III clinical database. BMC Med Inform Decis Mak. 2022;22:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Misra UK, Kalita J, Pandey S, et al. Predictors of gastrointestinal bleeding in acute intracerebral haemorrhage. J Neurol Sci. 2003;208:25–29. [DOI] [PubMed] [Google Scholar]


