Abstract
BACKGROUND:
Arterial hyperoxemia may cause end-organ damage secondary to the increased formation of free oxygen radicals. The clinical evidence on postoperative lung toxicity from arterial hyperoxemia during cardiopulmonary bypass (CPB) is scarce, and the effect of arterial partial pressure of oxygen (Pao2) during cardiac surgery on lung injury has been underinvestigated. Thus, we aimed to examine the relationship between Pao2 during CPB and postoperative lung injury. Secondarily, we examined the relationship between Pao2 and global (lactate), and regional tissue malperfusion (acute kidney injury). We further explored the association with regional tissue malperfusion by examining markers of cardiac (troponin) and liver injury (bilirubin).
METHODS:
This was a retrospective cohort study including patients who underwent elective cardiac surgeries (coronary artery bypass, valve, aortic, or combined) requiring CPB between April 2015 and December 2021 at a large quaternary medical center. The primary outcome was postoperative lung function defined as the ratio of Pao2 to fractional inspired oxygen concentration (Fio2); P/F ratio 6 hours following surgery or before extubation. The association between CPB in-line sample monitor Pao2 and primary, secondary, and exploratory outcomes was evaluated using linear or logistic regression models adjusting for available baseline confounders.
RESULTS:
A total of 9141 patients met inclusion and exclusion criteria, and 8429 (92.2%) patients had complete baseline variables available and were included in the analysis. The mean age of the sample was 64 (SD = 13), and 68% were men (n = 6208). The time-weighted average (TWA) of in-line sample monitor Pao2 during CPB was weakly positively associated with the postoperative P/F ratio. With a 100-unit increase in Pao2, the estimated increase in postoperative P/F ratio was 4.61 (95% CI, 0.71–8.50; P = .02). Our secondary analysis showed no significant association between Pao2 with peak lactate 6 hours post CPB (geometric mean ratio [GMR], 1.01; 98.3% CI, 0.98–1.03; P = .55), average lactate 6 hours post CPB (GMR, 1.00; 98.3% CI, 0.97–1.03; P = .93), or acute kidney injury by Kidney Disease Improving Global Outcomes (KDIGO) criteria (odds ratio, 0.91; 98.3% CI, 0.75–1.10; P = .23).
CONCLUSIONS:
Our investigation found no clinically significant association between Pao2 during CPB and postoperative lung function. Similarly, there was no association between Pao2 during CPB and lactate levels, postoperative renal function, or other exploratory outcomes.
KEY POINTS.
Question: Is higher arterial oxygen partial pressure (Pao2) during cardiopulmonary bypass (CPB) associated with worse postoperative lung function?
Findings: We found a weak positive association between in-line sample Pao2 during CPB and postoperative P/F ratio, with only a 5-point increase in P/F ratio for a 100-unit increase in Pao2 (mm Hg).
Meaning: There is no clinically meaningful adverse effect of hyperoxemia during CPB on postoperative lung function in adult patients undergoing coronary artery bypass, valve, or combined procedures.
Excess oxygen in tissues (hyperoxia) and blood (hyperoxemia) leads to the generation of free oxygen radicals, which cause cytotoxicity through lipid peroxidation.1 Prolonged exposure to very high fractional inspired oxygen concentration (Fio2) (≥0.9) in animals causes severe hyperoxic acute lung injury that is usually fatal.2–5 In neonates, exposure to hyperoxia at birth is associated with bronchopulmonary dysplasia, retinopathy of prematurity, and neonatal hemolytic anemia.6 Aside from the harmful effects of high-inspired Fio2 on the lungs, arterial hyperoxemia following resuscitation from cardiac arrest has been associated with an increased risk of in-hospital mortality.7 However, hyperoxemia is commonly used during cardiopulmonary bypass (CPB) to prevent intraoperative hypoxic injury and minimize gaseous embolization, although there is insufficient evidence to support such practice. Essentially, arterial oxygen partial pressure (Pao2) management during CPB is highly variable between and within institutions, because there is currently no evidence to support or guide appropriate Pao2 targets while on CPB. Studies in cardiac surgical patients showed conflicting findings on the effects of hyperoxemia on outcomes with some reporting reduction in cardiac index, as well as worsened microcirculatory perfusion heterogeneity,8–10 and others showing no increased risk of adverse cardiovascular or neurological outcomes.11,12 Lungs lose approximately 99% of blood supply during CPB relaying solely on bronchial artery contribution and are, therefore, vulnerable to ischemia-reperfusion injury, which is potentially aggravated by hyperoxemia. However, the effect of CPB-related hyperoxemia on postoperative lung function remains under investigation.10,13,14
Thus, the primary objective of this study was to explore the relationship between Pao2 during CPB and postoperative lung injury among patients undergoing elective cardiac surgery. Since hyperoxemia has also been associated with peripheral vasoconstriction,15 accordingly, our secondary objectives were to explore the relationship between Pao2 during CPB and global tissue malperfusion by measuring lactate levels, as well as individual end-organ perfusion by examining acute kidney injury (AKI) using Kidney Disease Improving Global Outcomes (KDIGO) stage. We further explored the proposed effect of hyperoxemia on perfusion of other organs by examining postoperative troponin and bilirubin levels.
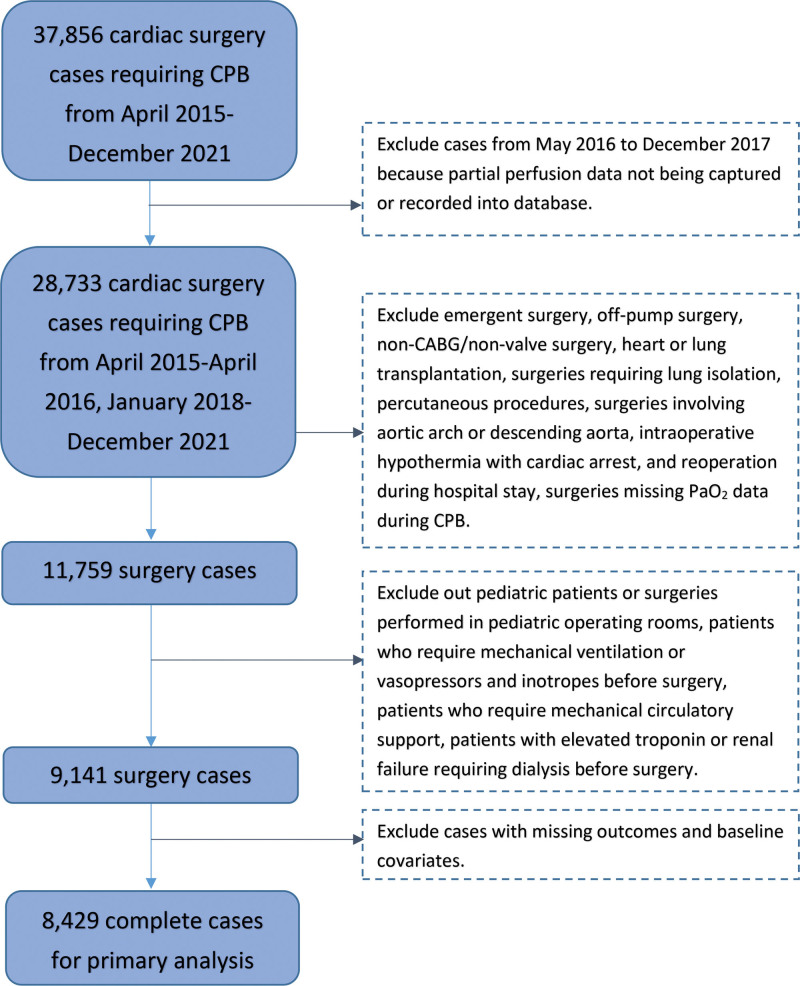
Figure.
Patient flow chart. CABG indicates coronary artery bypass grafting; CPB, cardiopulmonary bypass; Pao2, arterial oxygen partial pressure.
We hypothesized that a higher level of arterial Pao2 during CPB is associated with worse postoperative lung function measured by the ratio of Pao2 in mm Hg to Fio2 (in %) (Pao2/Fio2; P/F). Similarly, for our secondary objective, we hypothesized that higher arterial Pao2 levels during CPB are associated with worse global tissue perfusion while on CPB, and worse postoperative kidney function.
METHODS
The study was approved by Cleveland Clinic institutional review board with written informed consent waived. Baseline demographics, preoperative and intraoperative confounding variables as well as primary, secondary, and exploratory outcome variables were obtained from electronic medical records, Perioperative Health Documentation System, the Cleveland Clinic Quality and Patient Safety Institute Registry, and Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database (versions 2.81, 2.9, and 4.20.2). A data analysis and statistical plan was written and filed with the institutional review board before data were accessed.
Patients
All adult (>18 years old) patients who underwent elective cardiac surgery (coronary artery bypass, valve, aortic, or combined) requiring CPB between April 2015 and December 2021 were included in the study. We excluded pediatric patients or surgeries performed in pediatric OR suites (due to missing in-line Pao2 data), patients undergoing emergency (to ensure availability of data), off-pump cardiac and percutaneous procedures (no exposure to CPB), lung and heart transplants (removing patients receiving immunosuppression), surgeries requiring lung isolation, involving aortic arch or descending aorta, intraoperative hypothermia with cardiac arrest, and reoperations during the hospital stay. We also excluded patients who required mechanical ventilation or vasopressors and inotropes before surgery, patients who required mechanical circulatory support, and those with elevated troponin or renal failure requiring dialysis before surgery. These exclusions were applied to generate a more homogenous population, and because of their association with outcomes. Surgeries between April 2016 and December 2017 were also excluded because of missing perfusion data (Figure).
Perioperative Ventilation and CPB Management
Lung-protective ventilation was used in the perioperative period. Specifically, usual ventilation strategy included the use of pressure control ventilation mode with pressures adjusted to achieve tidal volume of 4 to 8 mL/kg ideal body weight (plateau pressure ≤30 mm Hg), respiratory rate of 8 to 25 titrated to arterial pH goal of 7.35 to 7.45, positive end expiratory pressure 5 to 8 mm Hg, and Fio2 to maintain Spo2 92% to 96%, or Pao2 ≥65 mm Hg. Patients were extubated in the intensive care unit (ICU) if they met the following criteria: were not on high-dose vasopressors or inotropes, had temperature ≥36°C, chest tube output ≤50 mL/h for 2 hours, were awake and following commands, passed spontaneous breathing trial, and were able to lift head for >5 seconds.
No specific CPB protocol was followed during the study period. In general, institutional approach during the study period was to provide normothermic CPB, with target ≥CI 2.2 L/min/m2, and mean arterial pressures between 60 and 80 mm Hg depending on patient age.
Outcome Variables
The primary outcome was postoperative lung function defined for this study as the P/F ratio measured 6 hours after ICU admission or immediately before extubation if extubated before the 6-hour mark. The 6 hours were selected because most patients in this selected population were expected to meet extubation criteria by 6 hours following ICU admission and would have at least 1 arterial blood gas drawn. An arbitrary P/F ratio increase of at least 50 for a 100-unit increase in Pao2 would be considered clinically significant by the authors.
Secondary outcomes were: (1) end-organ perfusion and function, as measured by lactate levels (peak and mean lactate) within 6 hours post CPB; and (2) AKI (defined by KDIGO criteria; increase in serum creatinine by ≥0.3 mg/dL within 48 hours, an increase in serum creatinine to ≥1.5× baseline within the last 7 days).
Exploratory outcomes were: (1) respiratory complications, defined as a collapsed composite of STS outcomes, including postoperative reintubation during the hospital stay, prolonged ventilation, pneumonia, pleural effusion requiring drainage, and pneumothorax requiring intervention; (2) cardiac injury using troponin levels on postoperative day (POD) 1; and (3) liver injury using PODs 1, 3, and 7 total bilirubin.
Exposure Variable
Arterial Pao2 during CPB obtained from perfusion in-line sample monitor and used as a time-weighted average (TWA) was selected as the exposure variable. The in-line sample monitor provides minute-by-minute Pao2 data from the CPB circuit, and TWA accounts for the level of hyperoxia over the specified period (referred to as Pao2 in the following text). Sensitivity analysis was performed using Pao2 from arterial blood gas drawn from an indwelling arterial line (radial, brachial, or femoral) during CPB instead of TWA of Pao2 from the in-line sample monitor to observe whether there would be a difference in results with 2 different methods of Pao2 monitoring during CPB. Furthermore, sensitivity analysis was performed to examine whether changing the timeframe of the exposure variable limiting the exposure timeframe to reperfusion period of CPB (from “after cross-clamp release” to “off bypass” event or 15 minutes after cross-clamp release whichever was earlier) instead of during the whole CPB period affected results. This was done because sudden hyperoxia exposure of previously ischemic organs such as lungs during reperfusion may lead to more severe ischemia-reperfusion injury.14
Statistical Analysis
Primary Analysis
We first drew a scatter plot and observed a linear relationship between TWA in-line Pao2 and postoperative P/F ratio. Pearson correlation between Pao2 and postoperative P/F ratio was reported. Then, the association between TWA in-line Pao2 and postoperative P/F ratio was evaluated using a linear regression model, with all the available baseline confounders in Table 1 (patient demographics, comorbidities, preoperative medications, preoperative laboratory measurements, pre-CPB arterial Pao2, and relevant intraoperative factors) adjusted directly in the model. Diagnostic plots (residuals versus fitted plot, Q-Q plot, and scale-location plot) were drawn to assess the linear regression assumptions (linearity, normality, and homoscedasticity of residuals). Variance inflation factor (VIF) was calculated to assess collinearity. An added variable plot with locally estimated scatterplot smoothing (LOESS) line was drawn to show if the association between TWA in-line Pao2 and P/F ratio was indeed linear and if the slope stays relatively constant over the entire Pao2 range after adjustment for potential confounders.
Table 1.
Demographic and Baseline Characteristics of the Population
| Patient variable | Missing | Summary statistics, N = 9141 |
|---|---|---|
| Age (y) | 64 ± 13 | |
| Male | 6206 (68) | |
| BMI (kg/m2) | 1 | 29 ± 6 |
| Race | ||
| White | 8264 (90) | |
| Black | 312 (4) | |
| Other | 565 (6) | |
| ASA physical classification | ||
| III | 751 (8) | |
| IV | 8390 (92) | |
| Comorbidities | ||
| Endocarditis | 368 (4) | |
| Hypertension | 3 | 6698 (73) |
| Atrial fibrillation or flutter | 106 | 2320 (26) |
| Left ventricular dysfunction | 216 | |
| Normal | 7888 (88) | |
| Mild | 670 (8) | |
| Moderate | 289 (3) | |
| Moderate-severe | 44 (<1) | |
| Severe | 34 (<1) | |
| Myocardial infarction | 9 | 1049 (12) |
| Pulmonary hypertension | 610 (7) | |
| Chronic lung disease | 3 | 1788 (20) |
| Moderate or severe mitral insufficiency | 2894 (32) | |
| Carotid artery disease | 1 | 473 (5) |
| Peripheral vascular disease | 547 (6) | |
| Diabetes mellitus | 1 | 1902 (21) |
| Stroke | 19 | 557 (6) |
| Preoperative medication | ||
| Aspirin | 2 | 3027 (33) |
| Beta Blocker | 5912 (65) | |
| ACE or ARB | 4093 (45) | |
| Statin | 2915 (32) | |
| Diuretic | 8619 (94) | |
| Inhaled steroids | 538 (6) | |
| Inhaled bronchodilator | 1054 (12) | |
| Preoperative laboratory measurements | ||
| Hematocrit (%) | 357 | 42 [39, 45] |
| Creatinine (mg/dL) | 37 | 0.98 [0.83, 1.14] |
| Surgery characteristics | ||
| Surgery type | ||
| Aortic valve procedure | 3186 (35) | |
| Mitral valve procedure | 2353 (26) | |
| CABG | 1559 (17) | |
| Aortic resection, replacement, or anastomosis | 1237 (14) | |
| Other valve surgery | 806 (9) | |
| Pre-CPB arterial Pao2 | 9 | 151 [112, 189] |
| CPB length (min) | 93 [68, 127] | |
| Total fluid (mL) | 2900 [2250, 3550] | |
| Total blood transfusion (unit) | 0 [0, 1] | |
| Ultrafiltration | 998 (11) | |
Data are summarized as median [Q1, Q3], mean ± SD, or N (%).
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; ASA, American Society of Anesthesiologists; BMI, body mass index; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; Pao2, arterial oxygen partial pressure.
Sensitivity Analyses
To test the robustness of our findings, we assessed the association between (1) average of Pao2 from arterial blood gas and postoperative P/F ratio and (2) TWA of Pao2 from in-line sample monitor during reperfusion period and postoperative P/F ratio using linear regression models, with all the available baseline confounders listed in Table 1.
Subanalysis
We assessed the association between TWA in-line Pao2 and postoperative P/F ratio in high-risk patients (defined as any patient >80 years old, any patient with BMI >35, any patient with moderate or severe left ventricle dysfunction, any patient with pulmonary hypertension, chronic obstructive pulmonary disease, diabetes mellitus, estimated glomerular filtration rate <30, any surgery for endocarditis, any surgery with CPB duration >2 hours) through a linear regression model, with all the available baseline confounders listed in Table 1.
Secondary Analyses
The association of Pao2 with AKI was assessed in a logistic regression model, with direct adjustment for potential confounders, and the association with mean and peak lactate level (after log-transformation to achieve normal distribution) was assessed through linear regression models with direct adjustment for all confounders listed in Table 1.
Exploratory Analyses
The association of Pao2 with respiratory complications was assessed through a logistic regression model with direct adjustment for confounders. The effect of Pao2 on POD 1 troponin levels (after log-transformation to achieve normal distribution) and PODs 1, 3, and 7 total bilirubin (after log-transformation to achieve normal distribution) was assessed through linear regression models with direct adjustment for all confounders listed in Table 1.
Sample Size Justification
During the study time frame, we expected to have 10,000 patients who underwent elective cardiac surgeries on CPB. Assuming complete data was available on 80% of the patients, we would have 8000 surgeries available for analysis. Based on a data query, Pao2 during CPB was relatively normally distributed with a mean ± standard deviation (SD) of 340 ± 50, while the P/F ratio on POD 1 had a mean ± SD of 365 ± 110. With the available sample size, at a significance level of 0.05, we would have had >99% power to test a partial correlation between Pao2 during CPB and postoperative P/F of −0.1 or stronger.
All analyses were completed using 2-sided tests with significance levels of 0.05 (0.05 for the primary outcome, 0.017 for each secondary outcome [ie, 0.05/3, Bonferroni correction], and 0.05 for each exploratory outcome). Analyses were completed using R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria), and sample size justification was completed using PROC POWER multiple regression and correlation procedure in SAS version 9.4 or newer (SAS Institute). This article adheres to the applicable STROBE guidelines.
RESULTS
A total of 9141 patients met inclusion and exclusion criteria, and 8429 (92.2%) had complete baseline variables available and were included in this analysis (Figure). The mean age of the sample was 64 (SD = 13), 68% were men (n = 6208), and 35% had aortic valve procedure (n = 3186). Summary statistics of patient demographics, comorbidities, preoperative medication, preoperative laboratory measurements, and intraoperative factors are presented in Table 1.
TWA in-line Pao2 during CPB was relatively normally distributed with a mean ± SD of 345 ± 48 mm Hg. Pearson correlation between Pao2 and postoperative P/F ratio was 0.09 (CI, 0.07–0.11; P < .001). Pao2 was weakly positively associated with postoperative P/F ratio, though without clinical significance. With a 100-unit increase in TWA of in-line Pao2, the estimated increase in postoperative P/F ratio was 4.61 (95% CI, 0.72–8.50; P = .020) (Table 2). Coefficients of the full model were reported in Supplemental Digital Content 1, Table 1, http://links.lww.com/AA/E448, and added variable plot was reported in Supplemental Digital Content 2, Figure 1, http://links.lww.com/AA/E449.
Table 2.
Association Between Pao2 During CPB and Postoperative P/F Ratio
| Exposure | Slope (95% CI)a | P value |
|---|---|---|
| Primary analysis | ||
| Time-weighted average of in-line Pao2 during CPB | 4.61 (0.71–8.50) | .020 |
| Sensitivity analyses | ||
| Time-weighted average of in-line Pao2 during reperfusion | 1.05 (22122.96 to 5.06) | .61 |
| Average of arterial Pao2 during CPB | 6.87 (1.63–12.1) | .010 |
Primary analysis assessed the association between TWA of in-line Pao2 during CPB and postoperative P/F ratio using a linear regression model, with all the baseline potential confounders in Table 1 adjusted for directly in the model. Sensitivity analyses assessed (1) the association between TWA of in-line Pao2 during reperfusion and postoperative P/F ratio and (2) the association between the average of arterial Pao2 during CPB and postoperative P/F ratio using linear regression models, with all the baseline confounders in Table 1 adjusted for directly in the models.
Abbreviations: CI, confidence interval; CPB, cardiopulmonary bypass; Pao2, arterial oxygen partial pressure; TWA, time-weighted average.
The slope coefficient estimate is the estimated change in postoperative P/F ratio for each 100-unit change in TWA of in-line Pao2. The CI is the estimated 95% confidence interval for change in postoperative P/F ratio for each 100-unit change in TWA of in-line Pao2. P values <.05 were considered significant for primary and sensitivity analyses.
The 2 sensitivity analyses performed to test the robustness of our findings revealed that: (1) the average of Pao2 (from indwelling arterial catheter blood gas) was also weakly positively associated with the postoperative P/F ratio. With a 100-unit increase in the average of arterial Pao2, the estimated increase in postoperative P/F ratio was 6.87 (95% CI, 1.63–12.1; P = .010); and (2) Pao2 during limited reperfusion period was not associated with postoperative P/F ratio (95% CI, −2.96 to 5.06; P = .61) (Table 2).
The subanalysis revealed that Pao2 was weakly positively associated with postoperative P/F ratio in high-risk patients, though without clinical significance. With a 100-unit increase in TWA of in-line Pao2, the estimated increase in postoperative P/F ratio was 6.15 (95% CI, 2.26–10.0; P = .016) (Supplemental Digital Content 3, Table 2, http://links.lww.com/AA/E450).
Our secondary analysis revealed that higher Pao2 was not associated with peak lactate 6 hours post CPB (geometric mean ratio, 1.01; 98.3% CI, 0.98–1.03; P = .55), average lactate 6 hours post CPB (GMR, 1.00; 98.3% CI, 0.97–1.03; P = .93), or AKI (OR, 0.91; 98.3% CI, 0.75–1.10; P = .23) (Table 3).
Table 3.
Association Between Pao2 During CPB and Post CPB Lactate Level (Average and Peak) and AKI
| Outcome | GMR/OR (98.3% CI)a | P value |
|---|---|---|
| Lactate levels | ||
| Peak lactate (mmol/L, after log-transformation) 6 h post CPB | 1.01 (0.98–1.03)GMR | .55 |
| Average lactate (mmol/L, after log-transformation) 6 h post CPB | 1.00 (0.97–1.03)GMR | .93 |
| AKI (KDIGO) | 0.91 (0.75–1.10)OR | .23 |
Secondary analyses assessed the association between TWA of in-line Pao2 during CPB and (1) post CPB lactate levels (peak and average lactate 6 h post CPB) and (2) AKI. The effect of in-line Pao2 during CPB on post CPB lactate levels (after log-transformation) was assessed through linear regression models with direct adjustment of all potential baseline confounders in Table 1. The effect of in-line Pao2 during CPB on AKI was assessed through a logistic regression model with direct adjustment of all potential baseline confounders in Table 1.
Abbreviations: AKI, acute kidney injury; CI, confidence interval; CPB, cardiopulmonary bypass; GMR, geometric mean ratio; KDIGO, Kidney Disease Improving Global Outcomes; OR, odds ratio; Pao2, arterial oxygen partial pressure; TWA, time-weighted average.
The GMRs estimate the change in ratio of geometric means for post CPB lactate levels for each 100-unit change in TWA of in-line Pao2. The OR estimates the change in odds of AKI for each 100-unit change in TWA of in-line Pao2. 98.3% CI was reported, and P values <.017 were considered significant for secondary analyses.
The exploratory analysis revealed that higher Pao2 was not associated with postoperative respiratory complications (OR, 0.87; 95% CI, 0.73–1.03; P = .11), POD 1 troponin levels (GMR, 0.98; 95% CI, 0.95–1.02; P = .34), POD 1 total bilirubin (GMR, 1.02; 95% CI, 1.00–1.05; P = .07), POD 3 total bilirubin (GMR, 0.99; 95% CI, 0.97–1.01; P = .38), and POD 7 total bilirubin (GMR, 1.01; 95% CI, 0.98–1.05; P = .44) (Table 4).
Table 4.
Association Between Pao2 During CPB and Respiratory Complications, Cardiac Injury, and Liver Injury
| Outcome | GMR/OR (95% CI)a | P value |
|---|---|---|
| Respiratory complications | 0.87 (0.73–1.03)OR | .11 |
| Cardiac injury | ||
| POD 1 troponin levels (ng/mL, after log-transformation) | 0.98 (0.95–1.02)GMR | .34 |
| Liver injury | ||
| POD 1 total bilirubin (mg/dL, after log-transformation) | 1.02 (1.00–1.05)GMR | .07 |
| POD 3 total bilirubin (mg/dL, after log-transformation) | 0.99 (0.97–1.01)GMR | .38 |
| POD 7 total bilirubin (mg/dL, after log-transformation) | 1.01 (0.98–1.05)GMR | .44 |
Exploratory analyses assessed the association between TWA of in-line Pao2 during CPB and (1) respiratory complications, (2) cardiac injury using POD 1 troponin levels, and (3) liver injury using POD 1, 3, and 7 total bilirubin. The effect of in-line Pao2 during CPB on respiratory complications was assessed through a logistic regression model with direct adjustment of all potential baseline confounders in Table 1. The effect of in-line Pao2 during CPB on POD 1 troponin levels (after log-transformation) and POD 1, 3, and 7 total bilirubin (after log-transformation) was assessed through linear regression models with direct adjustment of all potential baseline confounders in Table 1.
Abbreviations: CI, confidence interval; CPB, cardiopulmonary bypass; GMR, geometric mean ratio; OR, odds ratio; Pao2, arterial oxygen partial pressure; TWA, time-weighted average.
The OR estimates the change in odds of respiratory complications for each 100-unit change in TWA of in-line Pao2. The GMRs estimate the change in ratio of geometric means for POD 1 troponin levels and POD 1, 3, and 7 total bilirubin for each 100-unit change in TWA of in-line Pao2. 95% CI was reported, and P values <.05 were considered significant for exploratory analyses.
Respiratory complications outcome is defined as a composite outcome of postoperative reintubation during the hospital stay, prolonged ventilation, pneumonia, pleural effusion requiring drainage, and pneumothorax requiring intervention.
DISCUSSION
We found no clinically meaningful association between hyperoxemia during CPB and postoperative pulmonary or other end-organ function. Considering normal P/F ratios ranging between 400 and 500 mm Hg, and ratios ≤300 mm Hg being used in the current definition of acute respiratory distress syndrome, the association between Pao2 during CPB and postoperative P/F ratio observed in this study is not clinically significant (only 5-point increase in P/F ratio for a 100-unit increase in Pao2). Based on currently available animal studies, as well as the known effect of hyperoxia on free oxygen radical formation, we anticipated opposite results but failed to demonstrate harm from higher Pao2 levels during CPB. A potential reason for this may be that despite our adjustment for known significant confounders in the multivariable analysis, postoperative lung function was still affected by many other unrecorded intraoperative and immediate postoperative events and management decisions confounding our results. While lung function was selected as the primary outcome because of potential aggravation of ischemia-reperfusion injury in the setting of hyperoxemia during CPB, it is also possible that, unlike other organs, lung exposure to high arterial oxygen level during CPB is minimal because collapsed lungs are generally not perfused and receive minimal blood flow via bronchial arteries. The lack of association between Pao2 during CPB and end-organ function also does not support the commonly perceived protective effect of higher concentration of dissolved oxygen during CPB. The lack of harmful effect of higher arterial Pao2 demonstrated in this study is in concordance with post hoc analysis of the CARDIOX trial, which primarily examined the effect of hyperoxemia during CPB on the occurrence of cardiovascular complications after cardiac surgery.11,12 Our findings may also be explained by the length of exposure to hyperoxemia. The average CPB time may be insufficient to cause significant harmful effect from hyperoxemia itself compared to longer exposures such as during extracorporeal circulatory membrane oxygenation (ECMO) support as demonstrated in a recent retrospective analysis examining Pao2 levels for at least 48 hours and showing an association between higher Pao2 on ECMO with increased 28-day mortality.16 Furthermore, there is no universal definition of hyperoxemia, and the lack of harmful association in our study could also be explained by the fact that most patients were exposed to higher than physiological arterial Pao2 levels during CPB (mean ± SD, 345 ± 48).
Our secondary outcomes focused on demonstrating the potentially hypothesized effect of hyperoxia during CPB on peripheral vasoconstriction and subsequent end-organ malperfusion during CPB as reflected in lactate levels (overall perfusion surrogate), and postoperative AKI (most sensitive to malperfusion during CPB).15,17 Again, we showed no harm with increasing Pao2 levels, which is in concordance with limited existing supporting evidence.13 A larger randomized controlled trial examining this specific outcome would be beneficial since older, albeit underpowered studies suggested worse renal outcomes and overall morbidity with hyperoxic CPB management attributed to worsened rheological properties of blood in the hyperoxic environment.18
Similar trends have been observed with our exploratory outcomes with no apparent harmful association of higher arterial Pao2 during CPB with other respiratory complications, and cardiac or liver injury.
Considering the sufficient body of evidence on hyperoxia and ischemia-reperfusion injury in other settings, and no strong evidence of harm in the setting of cardiac surgery, future studies should potentially focus on higher-risk cardiac surgical populations and longer exposures to hyperoxemia (ECMO).
Limitations
This is a single-center retrospective analysis, and our results may not be generalizable or applicable to all cardiac surgical populations (not applicable to types of procedures that were excluded); however, minimizing differences in CPB management enabled a better focus on the exposure variable. Our study did not adjust for perioperative Fio2 delivered via ventilator because the accuracy of that data was unreliable. However, because arterial Pao2 during bypass is solely determined by perfusionists’ practice and preferences, while Fio2 is determined by anesthesiologists and intensivists involved in patient care, patients were likely well balanced on this unadjusted variable.
Conclusions
This study explored the relationship between hyperoxemia during CPB and postoperative lung injury and end-organ damage. Hyperoxemia was not associated with lung injury or end-organ damage. Future studies should examine additional periods of hyperoxemia and populations at risk from hyperoxemia to inform CPB management strategies that would minimize postoperative complications.
DISCLOSURES
Name: Marta Kelava, MD, MS.
Contribution: This author provided substantial contributions to the conception and design of the work; analysis and interpretation of data for the work; drafting the work; and final approval of the version to be published.
Name: Adam J. Milam, MD, PhD.
Contribution: This author provided substantial contributions to the conception and design of the work, drafting the work, and final approval of the version to be published.
Name: Junhui Mi, MS.
Contribution: This author provided substantial contributions to the acquisition, analysis, and interpretation of the data for the work, drafting the work, and final approval of the version to be published.
Name: Andrej Alfirevic, MD, FASE.
Contribution: This author provided substantial contributions to the conception and design of the work, drafting the work, and final approval of the version to be published.
Name: Patrick Grady, CCP.
Contribution: This author provided substantial contributions to the conception and design of the work, drafting the work, and final approval of the version to be published.
Name: Shinya Unai, MD.
Contribution: This author provided substantial contributions to the conception and design of the work, drafting the work, and final approval of the version to be published.
Name: Haytham Elgharably, MD.
Contribution: This author provided substantial contributions to the conception and design of the work, drafting the work, and final approval of the version to be published.
Name: Kenneth McCurry, MD.
Contribution: This author provided substantial contributions to the conception and design of the work, drafting the work, and final approval of the version to be published.
Name: Marijan Koprivanac, MD, MS.
Contribution: This author provided substantial contributions to the conception and design of the work, analysis and interpretation of data for the work, drafting the work, and final approval of the version to be published.
Name: Andra Duncan, MD, FASE.
Contribution: This author provided substantial contributions to the conception and design of the work, analysis and interpretation of data for the work, drafting the work, and final approval of the version to be published.
This manuscript was handled by: Stefan G. De Hert, MD.
Supplementary Material
GLOSSARY
- ACE
- angiotensin converting enzyme
- AKI
- acute kidney injury
- ARB
- angiotensin receptor blocker
- ASA
- American Society of Anesthesiologists
- BMI
- body mass index
- CABG
- coronary artery bypass grafting;
- CI
- confidence interval
- CPB
- cardiopulmonary bypass
- ECMO
- extracorporeal circulatory membrane oxygenation
- EMR
- electronic medical record;
- FIO2 =
- fractional inspired oxygen concentration
- GMR
- geometric mean ratio
- ICU
- intensive care unit
- KDIGO
- Kidney Disease Improving Global Outcomes
- LOESS
- locally estimated scatterplot smoothing
- OR
- odds ratio
- Pao2
- arterial oxygen partial pressure
- POD
- postoperative day
- SD
- standard deviation
- STROBE
- Strengthening the Reporting of Observational Studies in Epidemiology
- STS
- Society of Thoracic Surgeons
- TWA
- = time-weighted average
- VIF
- variance inflation factor
Funding: None.
Reprints will not be available from the authors.
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
REFERENCES
- 1.Buechter DD. Free radicals and oxygen toxicity. Pharm Res. 1988;5:253–260. [DOI] [PubMed] [Google Scholar]
- 2.Ellman PI, Alvis JS, Tache-Leon C, et al. Hyperoxic ventilation exacerbates lung reperfusion injury. J Thorac Cardiovasc Surg. 2005;130:1440. [DOI] [PubMed] [Google Scholar]
- 3.Rodgers JL, Iyer D, Rodgers LE, Vanthenapalli S, Panguluri SK. Impact of hyperoxia on cardiac pathophysiology. J Cell Physiol. 2019;234:12595–12603. [DOI] [PubMed] [Google Scholar]
- 4.Makena PS, Luellen CL, Balazs L, et al. Preexposure to hyperoxia causes increased lung injury and epithelial apoptosis in mice ventilated with high tidal volumes. Am J Physiol Lung Cell Mol Physiol. 2010;299:L711–L719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care. 2013;58:123–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrone S, Bracciali C, Di Virgilio N, Buonocore G. Oxygen use in neonatal care: a two-edged sword. Front Pediatr. 2017;4:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellomo R, Bailey M, Eastwood GM, et al. ; Study of Oxygen in Critical Care (SOCC) Group. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue T, Ku K, Kaneda T, Zang Z, Otaki M, Oku H. Cardioprotective effects of lowering oxygen tension after aortic unclamping on cardiopulmonary bypass during coronary artery bypass grafting. Circ J. 2002;66:718–722. [DOI] [PubMed] [Google Scholar]
- 9.Ihnken K, Winkler A, Schlensak C, et al. Normoxic cardiopulmonary bypass reduces oxidative myocardial damage and nitric oxide during cardiac operations in the adult. J Thorac Cardiovasc Surg. 1998;116:327–334. [DOI] [PubMed] [Google Scholar]
- 10.Joachimsson PO, Sjoberg F, Forsman M, Johansson M, Ahn HC, Rutberg H. Adverse effects of hyperoxemia during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1996;112:812–819. [DOI] [PubMed] [Google Scholar]
- 11.Abou-Arab O, Huette P, Martineau L, et al. Hyperoxia during cardiopulmonary bypass does not decrease cardiovascular complications following cardiac surgery: the cardiox randomized clinical trial. Intensive Care Med. 2019;45:1413–1421. [DOI] [PubMed] [Google Scholar]
- 12.Abou-Arab O, Huette P, Guilbart M, Dupont H, Guinot PG. Hyperoxia during cardiopulmonary bypass does not increase respiratory or neurological complications: a post hoc analysis of the cardiox study. Br J Anaesth. 2020;125:e400–e401. [DOI] [PubMed] [Google Scholar]
- 13.McGuinness SP, Parke RL, Drummond K, et al. A multicenter, randomized, controlled phase IIb trial of avoidance of hyperoxemia during cardiopulmonary bypass. Anesthesiology. 2016;125:465–473. [DOI] [PubMed] [Google Scholar]
- 14.Anaya-Prado R, Toledo-Pereyra LH, Lentsch AB, Ward PA. Ischemia/reperfusion injury. J Surg Res. 2002;105:248–258. [DOI] [PubMed] [Google Scholar]
- 15.Smit B, Smulders YM, van der Wouden JC, Oudemans-van Straaten HM, Spoelstra-de Man AME. Hemodynamic effects of acute hyperoxia: systematic review and meta-analysis. Crit Care. 2018;22:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moussa MD, Beyls C, Lamer A, et al. Early hyperoxia and 28-day mortality in patients on venoarterial ECMO support for refractory cardiogenic shock: a bicenter retrospective propensity score-weighted analysis. Crit Care. 2022;26:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spoelstra-de Man AM, Smit B, Oudemans-van Straaten HM, Smulders YM. Cardiovascular effects of hyperoxia during and after cardiac surgery. Anaesthesia. 2015;70:1307–1319. [DOI] [PubMed] [Google Scholar]
- 18.Belboul AN, Ericson L, Thornbolm KS, Roberts DG, William-Oisson G. The effect of hyperoxia during cardiopulmonary bypass on blood cell rheology and postoperative morbidity associated with cardiac surgery. JECT 1991;23:43–48. [Google Scholar]