Abstract
Objective
A growing body of literature has focused on the neural mechanisms of depression. Our goal was to conduct a systematic review on the white matter microstructural differences in adolescents with depressive disorders vs adolescents without depressive disorders.
Method
We searched PubMed and PsycINFO for publications on August 3, 2022 (original search conducted in July 2021). The review was registered on PROSPERO (registration number: CRD42021268200), and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed. Eligible studies were original research papers comparing diffusion tensor/spectrum imaging findings in adolescents with vs without depression (originally ages 12-19 years, later expanded to 11-21 years). Studies were excluded if they focused on depression exclusively in the context of another condition, used only dimensional depressive symptom assessment(s), or used the same dataset as another included publication.
Results
The search yielded 575 unique records, of which 14 full-text papers were included (824 adolescents with depression and 686 without depression). The following white matter regions showed significant differences in fractional anisotropy in at least 3 studies: uncinate fasciculus, cingulum, anterior corona radiata, inferior fronto-occipital fasciculus, and corpus callosum (genu and body). Most studies reported decreased, rather than increased, fractional anisotropy in adolescents with depression. Limitations include the possibility for selective reporting bias and risk of imprecision, given the small sample sizes in some studies.
Conclusion
Our systematic review suggests aberrant white matter microstructure in limbic-cortical-striatal-thalamic circuits, and the corpus callosum, in adolescents with depression. Future research should focus on developmental trajectories in depression, identifying sources of heterogeneity and integrating findings across imaging modalities.
Key words: depression/depressive disorders, major depressive disorder, diffusion tensor imaging, adolescence
It has been estimated that 2.9 million adolescents between the ages of 12 and 17 years have experienced 1 or more major depressive episode(s) during the prior year, according to the 2020 National Survey on Drug Use and Health (NSDUH) survey, summarized on the National Institute of Mental Health website.1 Depression starting in childhood or adolescence can have a significant impact on development, functioning, and adult life, including increased risk of morbidity and mortality in adulthood (increased risk of depressive and/or anxiety disorders,2 psychosocial impairement,3 and suicidality4). Adolescent depression develops during an important period for brain maturation. Brain changes during adolescence in typical development include synaptic pruning, increased myelination, and neurotransmitter changes,5,6 as well as increases in white matter volumes and inverted U-shaped gray matter volume trajectories across different brain regions.7 It has been noted that many psychiatric disorders, including depression, emerge in adolescence.8 Furthermore, the clinical features of depression in children and adolescents can differ from those in adults. For example, per the DSM-5,9 depressed mood or anhedonia (or both) are required for diagnosis of major depressive disorder (MDD); yet, in children and adolescents with MDD, the mood can be irritable (rather than depressed).
Prior studies have investigated the neural mechanisms of depression in adolescents and have suggested structural and functional dysconnectivity,10, 11, 12, 13 along with heterogeneity in neuroimaging findings.14 Functional connectivity disruptions in adolescent depression have been demonstrated between frontal lobe regions (dorsolateral prefrontal cortex, ventromedial prefrontal cortex, orbitofrontal cortex) and temporal and/or limbic regions (anterior cingulate, amygdala, insula).11, 12, 13 These regions are part of 3 larger resting-state networks implicated in adolescent depression: the cognitive control network (CCN), the salience network (SN), and the default mode network (DMN) (reviewed by Chahal et al.14). Functional connectivity disruptions in adolescent depression have also been highlighted in several reviews,13,15,16 including 2 systematic reviews.15,16
In contrast to the emerging evidence on functional connectivity, less is understood about white matter microstructural correlates of adolescent depression. One early systematic review (from 2011)16 summarized studies using multiple imaging modalities including diffusion tensor imaging (DTI), although additional publications have emerged over the last decade. Studies using DTI have shown alterations in white matter integrity/structural connectivity of fronto-limbic connections (including the uncinate fasciculus, cingulum), fronto-frontal (including the genu and body of the corpus callosum), and fronto-thalamic (anterior thalamic radiation) white matter regions, as compared to those in typically developing children (TDCs).17, 18, 19, 20, 21 A meta-analysis (using the ENIGMA DTI pipeline) showed no white matter microstructural differences between adolescents with vs without depression.22 However, notably, the authors had not used a systematic review approach to identify and summarize prior published literature on the topic, and instead pooled data across multiple research groups. A very recent systematic review and meta-analysis described decreased fractional anisotropy (FA) in adolescents and young adults with MDD (as compared to healthy controls [HC]) in 3 clusters, spanning the corpus callosum, left anterior thalamic radiation (ATR), left corticospinal tract (CST), and the right frontal orbito-polar tract (including portions of the right uncinate fasciculus [UNC] and right inferior fronto-occipital fasciculus [IFO]). However, the authors did not focus exclusively on the adolescence period but combined studies of adolescents and young adults.23 Although the mean age for the MDD group across all studies was 23 years,23 the age range of some of the included papers was fairly broad and outside ranges typically considered to be adolescence (ie, age ranges of 18-50 years24 or 20-41 years25).
The goal of our systematic review was to perform a comprehensive literature search and to summarize the research on the white matter microstructural correlates of adolescent depression. We reviewed the literature focusing on depression in the adolescent period and using diffusion tensor imaging.
Method
Inclusion Criteria
Our initial set of inclusion criteria focused on the age range for adolescence of 12 to 19 years. After an initial review of the literature, it was determined that several papers used an expanded age range, and a decision was made to expand the age range using the definition of the adolescent period (ages 11-21 years) of the American Academy of Pediatrics26 to maximize the impact of the review. The resulting inclusion criteria for the articles for this systematic review were the following: (1) human participant studies of adolescents aged 11-21 years with any depressive disorder (as defined in DSM-5 or any prior DSM or International Classification of Diseases [ICD] classification) vs adolescents without depression (ie, without any depressive disorder); (2) neuroimaging method: diffusion tensor imaging or diffusion spectrum imaging; (3) articles written in the English language; and (4) primary, original data papers.
Exclusion Criteria
The exclusion criteria were as follows: (1) studies presenting combined data of adolescents and young adults or combined data of adolescents and younger children, unless the results for adolescents and adults or adolescents and younger children are presented separately; (2) studies focusing on depression exclusively in the context of another specified psychiatric or neurological condition such as bipolar disorder, traumatic brain injury, eating disorder(s), posttraumatic stress disorder (on the other hand, co-occurring conditions would be allowed as long as the primary inclusion criteria of the reviewed study was depression); and (3) studies primarily using a dimensional assessment of depressive symptoms rather than focusing on groups of adolescents with depression (based on formal clinical DSM or ICD diagnosis) vs adolescents without depression.
Search Strategy
A combination of the following search terms were used: imaging of the white matter (“diffusion” or “tensor” or “tractography” or “TBSS” or “tract-based spatial statistics”) and depress∗, and adolescen∗. Per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,27 2 separate online databases were searched, including PubMed and PsycINFO (Appendix 1). The search was originally conducted on July 21, 2021, and was updated August 3, 2022, to incorporate the most recently published literature. Records (abstract and title) were reviewed independently by 2 reviewers (P.R. and V.M. for the initial search, and P.R. and C.L. for the updated search) who determined which records met criteria for review of the full paper. The lists of full papers were compared and discussed by the 2 reviewers, and any discrepancies were resolved.
Per the PRISMA guidelines,27 the 2 reviewers independently reviewed the full papers and determined whether these meet criteria for inclusion in the systematic review. The reviewers compared their lists of articles to be included in the systematic review, and any discrepancies were resolved (if needed, by a third reviewer, D.P.D.). In addition, the 2 reviewers independently examined the reference lists of the selected full-text articles and identified any additional references that might be relevant for the systematic review. The reviewers discussed these lists and resolved any discrepancies; after independent assessment of any additional selected full-text articles by the 2 reviewers, the 2 reviewers discussed the finalized list of articles to be included in the systematic review and resolved any discrepancies (if needed, by a third reviewer, D.P.D.).
Critical Appraisal
Initially, studies were classified into whole-brain studies (tract-based spatial statistics [TBSS], whole-brain voxel-based studies) vs region of interest (ROI) studies/tractography, although several additional types of analyses were identified (including TBSS-ROI and connectome-based analyses). Our plan was that if a sufficient number of studies using similar methodology were identified, a meta-analysis would be conducted (see Data Collection and Synthesis section below), and publication bias would be assessed via funnel plots for each studied brain region.
Data Collection and Synthesis
Data and results from the selected full-text papers were abstracted and summarized in tables, focusing on differences in indices of white matter microstructural organization between adolescents with MDD and HC. Only results reaching statistical significance (p < .05) were summarized. Of note, we initially focused on fractional anisotropy (FA), as it is the most widely reported measure, and later, in a post hoc fashion, summarized results for axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD) as well. If 4 or more references using the same analysis type (eg, tractography for specific white matter tracts, or whole-brain studies reporting the coordinates of clusters of voxels displaying statistically significant differences between adolescents with depression vs without depression) were identified, a meta-analysis of the tract or whole-brain data would be conducted. We selected a goal of minimum of 4 papers because results from at least 4 studies would be needed to conduct a sensitivity analysis (to determine whether a single study may be driving a significant result in the meta-analysis).28
Results
Search Results Summary
The search of PubMed and PsycINFO yielded 415 and 295 references, respectively (Figure 1 flowchart). After removal of identical references, 575 records remained. After independent review of the title and abstract (by 2 reviewers) and discussion of the selection of records, 73 records were deemed to meet criteria for review of the full paper. After independent review of the papers by the 2 reviewers and discussion of selections, 17 full-text papers were initially determined to meet criteria for inclusion in the systematic review, although 3 records were excluded subsequently: one for reporting the measures of myelin imaging but not DTI measures, and 2 for reporting results on the same dataset that was already included in another selected report (by P.R. and D.P.D. consensus). Therefore, the results of 14 full-text papers were summarized in this systematic review. After independent review of the reference lists (by P.R. and V.M. for the initial search, or by P.R. and C.L. for the updated search) of the selected full-text articles and discussion of the selected lists, no additional references were identified. Table 117, 18, 19, 20, 21, 22,29, 30, 31, 32, 33, 34, 35, 36 summarizes the final list of records included in the Systematic Review.
Figure 1.
Flow Chart of the Systematic Review
Note:Reports excluded for the following other reasons: paper on subthreshold depression (n = 2); prospective prediction of depression based on baseline imaging data (n = 1); no results of depressive diagnosis vs controls presented (n = 2); no DTI/DSI tract metrics presented (n = 1); and use of the same dataset as another already included publication (n = 2). The template for the figure was adapted from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.27“Identification of studies via other methods” was created to comply with the PRISMA guidelines (see Method section), and included search of the references of studies included in the review and of Zhou et al.23
Table 1.
Summary of Demographics and Clinical Characteristics
| Authors, year, reference | Participant characteristics |
Psychiatric assessments and characteristics |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group(s) | Age range, y | Depression (n) | HC (n) | Average age, y (age SD), depression | Sex (F/M), depression | Average age, y (age SD), HC | Sex (F/M), HC | Race / ethnicity | Sample overlap with another included study | SES | Diagnostic details (for depression) | Depression severity (scale type) | Co-occurring psychiatric conditions | Psychiatric medication use (Y/N) | |
| Lee et al. (2021)29 | MDD, HC | 13-18 | 31 | 27 | 15.03 (1.45) | 19 F, 12 M | 15.96 (1.02) | 22 F, 5 M | N/A | N/A | N/A | K-SADS-PL | CDI, HDRS | Y | N (initially) |
| Wu et al. (2020)18 | MDD, HC | 13-17 | 36 | 37 | 15.6 (1.27) | 24 F, 12 M | 15.6 (1.30) | 19 F, 18 M | N/A | N/A | N/A | K-SADS-PL | HAMD-17 | N | N |
| van Velzen et al. (2020)22 | MDD, HC | 12-21 | 372 | 290 | 15.53 (1.55) | 255 F, 117 M | 14.6 (1.64) | 192 F, 98 M | N/A | N/A | N/A | Differs across 20 testing sites | BDI-II, HDRS-17 | Y | Y |
| Cullen et al. (2020)30 | MDD, HC | 12-19 | 44 | 37 | 15.9 (2.02) | 11 F, 33 M | 16.3 (2.12) | 12 F, 25 M | N/A | N/A | Y | K-SADS-PL | BDI-II, CDRS-R, IDAS | Y | N (except stimulants) |
| Chu et al. (2018)31 | MDD, HC | 15-19 | 52 | 27 | 15.62 (1.56) | 41 F, 11 M | 16.32 (2.1) | 19 F, 8 M | N/A | N (but 2 excluded publications) | N/A | N/A | N/A | N/A | N/A |
| Chang et al. (2018)32 | SZ, BD, MDD, HC | 13-18 | 45 | 43 | N/A (not provided for ages 13-18 y) | N/A (not provided for ages 13-18 y) | N/A (not provided for ages 13-18 y) | N/A (not provided for ages 13-18 y) | N/A | N/A | N/A | K-SADS-PL | HAMD-17, HDRS | Y | Y |
| Tymofiyeva et al. (2017)33 | MDD, HC | 13-17 | 57 | 41 | 16.2 (1.3) | 33 F, 24 M | 16 (1.4) | 25 F, 16 M | N/A | Y (LeWinn et al.17 51 MDD and 39 HC subjects overlapped) | Y | K-SADS-PL | CDRS-R, RADS-2 | N/A | Y |
| Geng et al. (2016)34 | MDD, HC | 13-17 | 26 | 31 | 15.6 (1.27) | 19 F, 7 M | 15.6 (1.38) | 17 F, 14 M | N/A | N/A | N/A | K-SADS-PL | HAMD-17 | N/A | N |
| LeWinn et al. (2014)17 | MDD, HC | 13-17 | 52 | 42 | 16.2 (0.2) | 31 F, 21 M | 16 (0.2) | 26 F, 16 M | Y | Y (Tymofiyeva et al.33) | Y | K-SADS-PL | CDRS-R, RADS-2 | Y | N |
| Aghajani et al. (2014)35 | MDD, HC | 13-19 | 25 | 21 | 15.6 (1.4) | 21 F, 4 M | 14.7 (1.6) | 18 F, 3 M | N/A | N/A | N/A | Clinical assessment (by child and adolescent psychiatrists) | CDI, RCADS, YSR, CBCL | Y | N |
| Henderson et al. (2013)21 | MDD, HC | 13-20 | 17 | 16 | 16.8 (2.2) | 8 F, 9 M | 16.4 (1.4) | 10 F, 6 M | Y | N/A | N/A | K-SADS-PL, | CDRS-R, BDI-II | Y | N |
| Bessette et al. (2014)19 | MDD, HC | 13-19 | 31 | 31 | 17.1 (1.88) | 24 F, 7 M | 17.0 (2.4) | 19 F, 12 M | Y | N/A | N/A | K-SADS-PL, SCID-IV | BDI-II | Y | Y |
| Cullen et al. (2010)20 | MDD, HC | 15-19 | 14 | 14 | 16.79 (1.29) | 10 F, 4 M | 16.81 (1.5) | 8 F, 6 M | Y | N/A | N/A | K-SADS-PL | BDI-II | Y | Y |
| Wu et al. (2022)36 | MDD, ADHD, HC | N/A | 22 | 29 | 15.91 (1.80) | 18 F, 4 M | 15.24 (1.60) | 16 F, 13 M | N/A | N/A | N/A | Participants meeting criteria per DSM-5 | MADRS, CBCL-AAA | N/A | Y |
Note: ADHD = attention-deficit/hyperactivity disorder; BD = bipolar disorder; BDI-II = Beck Depression Inventory–Second Edition; CBCL = Child Behavior Checklist; CDI = Children’s Depression Inventory; CDRS-R = Children’s Depression Rating Scale, Revised; DSM-5 = Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; F = female; HAMD-17 = Hamilton Rating Scale for Depression; HC = healthy controls; HDRS = Hamilton Depression Rating Scale; IDAS = Inventory of Depression and Anxiety Symptoms; K-SADS-PL = Schedule for Affective Disorders and Schizophrenia for School-Age Children: Present and Lifetime version; M = male; MADRS = Montgomery–Åsberg Depression Rating Scale; MDD = major depressive disorder; N/A = not reported or not assessed; RADS-2 = Reynolds Adolescent Depression Scale, Second Edition; RCADS = Revised Child Anxiety and Depression Scale; SCID-IV = Structured Clinical Interview for DSM-IV; SES = socioeconomic status; SZ = schizophrenia; Y = yes; YSR = youth self-report.
Demographic Characteristics and Diagnostic Classification
A total of 824 adolescents with depression and 686 without depression were included across the 14 studies (Table 1). Several of the studies (6 of 13) included predominantly female participants (two-thirds or more of the participants with MDD were female). Of note, few reports (4 of 14 studies) explicitly described the race and ethnicity of their participants (Table 1). Socioeconomic status (SES) was evaluated and reported in 3 of the 14 studies (Table 1).
All included studies focused on diagnosis of MDD. The diagnosis was established through the Schedule for Affective Disorders and Schizophrenia for School Age Children–Present and Lifetime version (K-SADS-PL)37 in the majority of the studies (10 of 14); other studies reported using clinical assessment (by child and adolescent psychiatrists) (n = 1); differing methods across sites (including the K-SADS-PL for some of the sites in van Velzen et al.,22 n = 1); referenced participants meeting DSM-5 criteria for major depressive disorder (MDD) (n = 1), or no description provided of diagnostic method (n = 1). Nine of the 14 papers reported co-occurring psychiatric conditions (in addition to MDD); 1 study reported lack of co-occurring psychiatric conditions, and 4 studies did not discuss whether participants had co-occurring disorders.
Types of Neuroimaging Analyses
A diverse set of neuroimaging data analysis methods was used across studies (Table 2). Per our protocol, a meta-analysis was planned if at least 4 studies using similar methodology were identified for each white matter region (as including 4 or more studies could allow for sensitivity analysis to be conducted as described in Aoki et al.28). However, for ROI or tractography methods, there were no more than 4 studies using the same analysis method (combination of Table 2 and Table 3). The TBSS method was used in more than 4 studies but the way the data were presented in the papers did not allow for a meta-analysis of reported coordinates (as the number of studies reporting coordinates of significant clusters was deemed insufficient for analysis using GingerALE).38
Table 2.
Summary of Diffusion Tensor Imaging (DTI) Data Acquisition Parameters and Data Analysis Approaches
| Authors, year, reference | Field, T | No. of directions | Voxel size, mm3 | Coordinate system/atlas | Analysis software | Analysis type (TBSS, whole brain tractography, ROI, VBM) |
FA | AD | RD | MD | ADC | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VBM | TBSS | ROIa | Tractography | |||||||||||
| Lee et al. (2021)29 | 3T | 64 | 2 × 2 × 2 | MNI spaceb | FSL | - | V | - | - | V | V | V | V | - |
| Wu et al. (2020)18 | 3T | 25 | 3 × 3 × 3q | MNI spaceb | FSL, MRIcron | - | - | V | - | V | - | - | - | - |
| van Velzen et al. (2020)22 | Variesc | Variesc | Variesa | Variesc | FSL | - | V | V | V | V | V | V | - | |
| Cullen et al. (2019)30 | 3T | 30 | 2 × 2 × 2 | Standard template (Tracula) | FSL, FreeSurfer | - | V | - | V | V | - | - | - | - |
| Chu et al. (2018)31 | 3T | 30 | 2 × 2 × 2 | Desikan–Killiany atlas | FSL, HCP pipeline | - | - | V | - | V | V | V | V | V |
| Chang et al. (2018)32 | 3T | 25 | 2 × 2 × 2 | MNI spaceb | VBM8 toolbox, SPM8 | V | - | - | - | V | - | - | - | - |
| Tymofiyeva et al. (2017)33 | 3T | 30 | 1.875 × 1.875 × 2.5 | MNI space | FSL | - | - | V | - | V | - | - | - | - |
| Geng et al. (2016)34 | 3T | 25 | 2 × 2 × 2 | MNI spaceb | FSL, MRIcron | - | - | V | - | V | - | - | - | - |
| LeWinn et al. (2014)17 | 3T | 30 | 1.875 × 1.875 × 2.5 | MNI spaceb | FSL | - | V | - | - | V | V | V | - | - |
| Aghajani et al. (2014) 35 | 3T | 32 | 2.3 × 2.3 × 2.3 | FMRIBIB57 FA space | FSL | - | V | V | - | V | V | V | V | - |
| Henderson et al. (2013)21 | 3T | 12 | 2.5mm (slice thickness) | MNI space | FSL | - | V | - | - | V | V | V | V | - |
| Bessette et al. (2014)19 | 3T | 12 | 1.6 × 1.6 × 3.0 | MNI space | FSL | - | V | - | - | V | - | - | - | - |
| Cullen et al. (2010)20 | 3T | 30 | 2 × 2 × 2 | MNI space | FSL (Probtrackx) | - | V | - | V | V | - | - | - | - |
| Wu et al. (2022)36 | 3T | 13 | N/A | MINI space | FSL | - | V | - | - | V | - | - | - | - |
Note: Hyphens indicates the absence of the specified type of analysis. AD = axial diffusivity; ADC = apparent diffusion coefficient; FA = fractional anisotropy; FSL = FMRIB Software Library; HCP = Human Connectome Project; MD = mean diffusivity; MNI = Montreal Neurological Institute; N/A = not reported; RD = radial diffusivity; ROI = region of interest; SPM = Statistical Parametric Mapping; T = Tesla; TBSS = tract-based spatial statistics; VBM = voxel-based morphometry.
Some ROI analyses use the TBSS-ROI method.
JHU ICBM-DTI-81 / ICBM-DTI-81 WM labels.
Differs across 20 testing sites.
Table 3.
Summary of the Comparison of Fractional Anisotropy (FA) Values in Major Depressive Disorder as Compared to Healthy Controls (HC) Participants
| UNC- L | UNC-R | UNC-B | CGC-L | CGC-R | CGC-B | CGH-L | CGH-R | CGH-B | ATR-L | ATR-R | ATR-B | ACR-L | ACR-R | ACR-B | IC-L | IC-R | ALIC-B | IFO-L | IFO-R | IFO-B | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lee et al. (2021)29 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |||||||||||||||
| Wu et al. (2020)18 | ↓ | - | |||||||||||||||||||
| van Velzen et al. (2020)22 | - | - | - | - | - | - | |||||||||||||||
| Cullen et al. (2020)30 | ↑(u) | - | ↑(u) | ↑(u) | - | - | - | - | |||||||||||||
| Geng et al. (2016)34 | |||||||||||||||||||||
| LeWinn et al. (2014)17 | ↓ | ↓ | - | - | ↓ | ↓ | ↓ | ↓ | ↓ | ||||||||||||
| Aghajani et al. (2014)35 | ↑ | ||||||||||||||||||||
| Henderson et al. (2013)21 | ↓ (u) | ↑(u) | |||||||||||||||||||
| Bessette et al. (2014)19 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ||||||||||||
| Cullen et al. (2010)20 | ↓(u) | ↓(u) | ↓(u) | ↓(u) | ↓(u) | ||||||||||||||||
| Wu et al. (2022)36 | |||||||||||||||||||||
| Total Number | 6 | 3 | 4 | 3 | 2 | 2 | 1 | 3 | 2 | 2 | 2 | 4 |
| SLF-L | SLF-R | SLF-B | ILF-L | ILF-R | ILF-B | CST-L | CST-R | CST-B | SCR-L | SCR-R | SCR-B | Fornix-B | GCC | BCC | SCC | EC-L | EC-R | PTR-L | PTR-R | PTR-B | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lee et al. (2021)29 | ↑ | ↑ | ↑ | ||||||||||||||||||
| Wu et al. (2020)18 | |||||||||||||||||||||
| van Velzen et al. (2020)22 | - | - | - | - | ↓(u) | - | |||||||||||||||
| Cullen et al. (2020)30 | - | - | - | - | - | - | |||||||||||||||
| Geng et al. (2016)34 | ↓ | ||||||||||||||||||||
| LeWinn et al. (2014)17 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||||||
| Aghajani et al. (2014)35 | ↓ | ||||||||||||||||||||
| Henderson et al. (2013)21 | |||||||||||||||||||||
| Bessette et al. (2014)19 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ||||||||||||||
| Cullen et al. (2010)20 | ↓(u) | ||||||||||||||||||||
| Wu et al. (2022)36 | |||||||||||||||||||||
| Total Number | 1 | 1 | 2 | 2 | 1 | 3 | 5 | 2 | 2 | 1 |
Note: ACR = anterior corona radiata; ALIC = anterior limb of the internal capsule; ATR = anterior thalamic radiation; BCC = body of the corpus callosum; CGC = cingulate (dorsal portion); CGH = cingulate (ventral portion); CST = corticospinal tract; EC = external capsule; GCC = genu of the corpus callosum; IC = internal capsule; IFO = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; SCR = superior corona radiata; SLF = superior longitudinal fasciculus; PTR = posterior thalamic radiation; SCC = splenium of the corpus callosum; UNC = uncinate fasciculus. ↓ Indicates that decreased FA was found in MDD as compared to HC; ↑ Indicates that increased FA was found in MDD as compared to HC. “-” Indicates that no significant difference was found between the MDD and HC groups and specific results for each tract/region were listed in the corresponding paper. “u” Indicates significant p value (uncorrected); p value was not significant after correction for multiple comparisons
Summary of the Results for FA of the Selected Full-Text Papers
The majority of the studies reported decreased FA in adolescents with depression across many regions (Table 3, Table S1, available online). The last row of Table 3 summarizes the number of studies that showed a difference in FA in each white matter region/tract (via any analysis method) in adolescents with MDD vs controls. At least 3 of the 14 studies reported the following regions: uncinate fasciculus (left and right), cingulum (CGC [left and right], anterior corona radiata [right], inferior fronto-occipital fasciculus [right], and genu and body of the corpus callosum) (Figure 2,39,40 Table 3). The results using a less stringent criterion of 2 (or more) of 14 studies (reporting altered FA in the specific regions) are described in the supplemental results (Supplement 1, available online). Notably, because of the disparate analysis methods, the results of a subset of the studies could not be summarized in the format listed in Table 3 and are included in Table S1, available online.
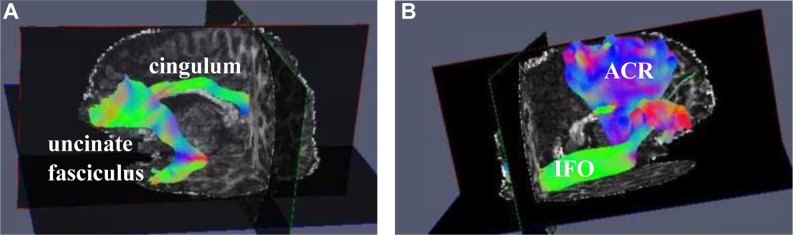
Figure 2.
Visual Representation of Some of the White Matter Regions (Implicated in Adolescent Depression in the Current Systematic Review) in a 12-Year-Old Girl With Major Depressive Disorder (MDD)
Note:(A) Cingulum and uncinate fasciculus. (B) Inferior fronto-occipital fasciculus (IFO) and anterior corona radiata (ACR). (Not visualized in this figure: genu and body of corpus callosum). Neuroimaging data for this participant with MDD was acquired as part of a project on irritability (R01MH111542, PI: Dr. Daniel Dickstein). MDD was diagnosed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children: Present and Lifetime version (K-SADS-PL). Data were processed using FSL39and QIT.40
Summary of the Results for AD, MD, and RD of the Selected Full-Text Papers
The majority of the papers (8 of 14) included in this review did not report results for AD, RD, and MD (Tables S1-S4, available online). The last row of Tables S1-S4 (available online), summarizes the number of studies that showed a significant difference in AD, RD, or MD in each white matter region/tract in adolescents with MDD vs controls. At least 2 of the 6 studies (focusing on additional DTI indices beyond FA) reported the following regions: left and right uncinate fasciculus (for RD), and genu and body of the corpus callosum (for RD and MD) (Tables S3 and S4, available online). Again, because of the disparate analysis methods, the results of 1 study could not be summarized in the format listed in Tables S2-S4, available online, and are included in Table S1, available online.
Associations of WM Indices With Biological Sex
The majority of the included papers did not evaluate whether the white matter correlates of adolescent depression differ across male and female participants (n = 12 of 14 papers). The paper by van Velzen et al.22 assessed diagnosis by sex interaction. The authors reported a statistically significant difference (after multiple comparison correction) in the uncinate fasciculus RD in adolescents with MDD vs controls that was found only in male participants. Bessette et al.19 described different patterns of MDD differences in male vs female participants. Namely, female participants had lower FA in several regions, including the right thalamus, right ATR, cerebellar tracts, left cingulum, corpus callosum, bilateral orbitofrontal, left inferior frontal gyrus, and left UNC, whereas male participants had decreased FA in the left thalamus, left inferior frontal pole, left inferior longitudinal fasciculus (ILF), and right IFO. However, of note, the study of Bessette et al.19 included only a small number of male participants with MDD (n = 7, along with 24 female participants with MDD).
Critical Appraisal
All selected papers (n = 14) reported results for FA, but less than half of the publications (6 of 14) reported results for at least 1 additional DTI measure (Table 2). The papers used diverse analysis methods: whole-brain white matter analyses (eg, TBSS), region of interest (ROI)–based analyses, TBSS-ROI, tractography, or connectome-based approaches, with some of studies using more than 1 analysis method (Table 2). The results of the papers that referenced white matter regions consistent with the nomenclature used in the MRI Atlas of White Matter41 are summarized in Table 3. Please note that each region listed in columns for white matter region may in fact represent different portions of the white matter region: for example, the TBSS-ROI method would result in a few core voxels included for a WM region, whereas tractography would summarize FA/DTI measures across the entire white matter tract.
Quality Control Procedures of Included Studies
The majority of the included papers described incorporating motion and/or eddy current correction in their DTI pre-processing methods (13 of 14, except for Lee et al.29). Five studies explicitly discussed including an inhomogeneity correction.17,20,30,31,33 Three studies used outlier detection and removal.17,20,33 Four studies explicitly described checking the quality of the data or results (by visual inspection).19,20,29,33
Study Risk of Bias
Selective Reporting
Selective reporting could be a limitation of the reports summarized in the current systematic review. In general, statistically significant results may be more likely to be reported or published. The majority of the summarized studies reported a difference in FA of at least 1 white matter region between adolescents with depression as compared to typically developed controls (10 of 14).
Risk of Imprecision
In addition, there is a risk of imprecision in the context of relatively low numbers of participants included in some of the summarized studies (eg, less than 20 participants per group were noted in 2 of the 14 studies). Considering that our systematic review did not focus on the study of interventions or clinical trials, other selective bias domains such as allocation concealment, use of random sequence generation, blinding of participants, outcomes assessment, or study attrition are not relevant.
Effect Size Multiplicity
Of note, 2 of the studies had partial overlap of participants (in both the MDD and control groups)17,33 (Tymofiyeva et al.33 reported having 51 MDD and 39 HC overlapping participants with LeWinn et al.17). This introduces the issue that the 2 reports are not independent of each other, and if meta-analysis were to be carried out on these data, multiplicity and statistical dependency would need to be recognized and appropriately handled.42 It should be noted that because of the divergent analysis methods that were used in the 2 papers,17,33 the data from the 2 reports could not be summarized in the same format or using the same DTI variables in Table 3: the majority of the papers, including LeWinn et al.17 (but not Tymofiyeva et al.33) used similar enough approaches to render summary in Table 3 (TBSS, ROI, tractography), whereas Tymofiyeva et al.33 used a connectome analysis approach. If future meta-analyses focus on the topic of interest of the current systematic review, researches could consider using integrative approaches to dealing with these reports with 2 overlapping samples, given the distinctness of reported white matter regions or reports (see Lopez-Lopez et al.42 for suggestions on approaches for handling effect size multiplicity).
Discussion
Our systematic literature search included 14 full-text papers for summary and discussion (824 adolescents with depression and 686 without depression). All of the described papers focused on MDD, and the majority of the studies demonstrated reduced fractional anisotropy in adolescents with MDD as compared to adolescents without MDD. The reported specific white matter tracts or regions varied across studies. At least 3 separate studies demonstrated FA alterations in patients with MDD (as compared to HC) for the following limbic and cortical circuits: limbic system (uncinate fasciculus, cingulum); and long-range connections to or from the frontal cortex (anterior corona radiata, inferior fronto-occipital fasciculus, genu, and body of the corpus callosum).
The finding that the majority of the summarized studies reported reduced FA in adolescents with MDD is not surprising, and supports the notion that dysconnectivity across brain regions may be a feature of adolescent depression. Notably, a meta-analysis in adults with MDD reported lower FA (as compared to that in controls) in a relatively widespread set of white matter regions: cingulum, corpus callosum, corona radiata, inferior fronto-occipital fasciculus, internal capsule, fornix, superior fronto-occipital fasciculus, and sagittal stratum.22 These regions largely overlap with the white matter regions highlighted by the current systematic review on white matter correlates of adolescent depression, suggesting that adolescent depression may have structural connectivity alterations similar to depression in adults. Yet, it should be noted that the same meta-analysis22 did not find differences in white matter tracts in adolescents with MDD (discussed further below). Prior literature has also pointed to aberrant limbic-cortical-striatal-thalamic circuit based on functional connectivity in adolescent depression, including between regions in the frontal lobe (dorsolateral prefrontal cortex, ventromedial prefrontal cortex, orbitofrontal cortex) and temporal lobe and/or limbic regions (anterior cingulate, amygdala, insula).11, 12, 13
The majority of the summarized studies focused only on FA, although several studies assessed additional DTI measures, including AD, MD, and RD. Additional measures can be helpful in characterizing the nature of the microstructural white matter alterations. AD reflects the diffusion along the direction of the fiber tract, whereas RD correlates with diffusion perpendicular to the main orientation of the fiber tract. Of note, increased RD was reported in 2 of the 3 studies that showed an alteration in the body of the corpus callosum (BCC) (Table S4, available online), which could be suggestive of decreased myelination in adolescents with MDD as compared to HC. Given the role of the corpus callosum as the major white matter tract connecting the 2 hemispheres, it is possible that information is less efficiently relayed across the hemispheres in adolescents with MDD.
One prominent feature across the included studies is the heterogeneity of sample characteristics (age, co-occurring disorders), analysis methods, and reported results. Although the age range for the systematic review was 11 to 21 years, the specific range varied across studies: from fairly narrow (13-17 years) to a wider age range (12-21 years) (Table 1). Notably, the wider age range (12-21 years) spans a dynamic period of development, and it is possible that using this wider age range may be 1 contributing factor to the finding of a lack of statistically significant differences between adolescents with MDD vs controls in van Velzen et al.22 (especially if the trajectories of MDD and TDC differ). On the other hand, another meta-analysis focusing on an even wider age range (across adolescents and young adults with ASD, with mean age across studies of 23 years, and a maximum age in 1 of the included studies of 50 years) described decreased FA in MDD (compared to HC in the corpus callosum, left ATR, and left CST, right frontal orbito-polar tract (including portions of the right UNC and right IFO).23 These WM regions were also highlighted (among additional regions) by our current systematic review. Of note, our review includes a more than 4-fold larger number of participants with MDD across studies (even though we have a narrower age range): 824 adolescents with MDD and 686 HC (in the current review), as compared to 205 with MDD (adolescents and young adults) and 194 HC in Zhou et al.23 (which is likely secondary to Zhou et al.23 not using robust methods for systematic review such as following the PRISMA guidelines implemented in our current review). Van Velzen et al.,22 on the other hand, did not conduct a systematic review of published literature, but instead pooled available data in adolescent and adult MDD across multiple research groups.
The majority of the included studies reported that their participants with MDD had co-occurring psychiatric disorders. The most frequently co-occurring disorders in adolescent MDD are anxiety disorders; for example, generalized anxiety disorder (GAD) was found in 41% of the adolescents with MDD in Henderson et al.21 and some adolescents with MDD also had attention-deficit/hyperactivity disorder (ADHD) (eg, 6% in Henderson et al.21; 9.6% in LeWinn et al.17; 13.6% in Cullen et al.30). The neural mechanisms of ADHD and anxiety disorders also include altered connectivity, in partially overlapping albeit somewhat distinct networks.43,44 Thus, it is possible that when different studies of adolescent MDD include varying proportions of adolescents with co-occurring disorders (eg, ranging from 0% in Wu et al.18 to 13.6% with ADHD in Cullen et al.30 and 41% with GAD in Henderson et al.21), divergent and/or not fully overlapping white matter regions would be implicated across studies. Future research could focus on differentiating whether and how much of the heterogeneity found across studies (including lack of finding of a difference between participants with MDD and controls) may be at least partially associated with the neural mechanisms of co-occurring psychiatric disorders.
In light of the heterogeneity of neuroimaging findings across studies (along with notable heterogeneity of clinical symptoms of depression across individuals with MDD), 1 future direction could be to classify youth with adolescent MDD into subgroups based on their structural connectivity/patterns of alterations of microstructural organization in different tracts and to compare the clinical symptoms of the subgroups of youth with MDD. Notably, researchers have used resting-state functional magnetic resonance imaging (fMRI) to identify clusters of subtypes of altered functional connectivity in adults with MDD, and have explored how these subgroups differ in clinical characteristics.45, 46, 47 For example, Drysdale et al.45 found that 4 distinct patterns of functional connectivity (in limbic and fronto-striatal networks) corresponded to different clinical symptom subtypes of depression. Wang et al.47 identified 2 subtypes of adults with MDD (insomnia-dominated vs anhedonia-dominated), which in turn was associated with distinct neural patterns of connectivity alterations (hyperconnectivity in the ventral attention network or hypoconnectivity in subcortical and dorsal attention networks, respectively). Chahal et al.14 identified the approach of mapping the correspondence between specific clinical features of depression and brain connectivity patterns as an important step that could facilitate targeted prevention, assessment, and treatment of adolescent depression in the future (ie, precision mental health).14
Yet, another recent body of literature should also be kept in mind when thinking about heterogeneity of brain–psychiatric phenotype correlations. Challenges in reproducibility of neuroimaging findings have been highlighted by a recent analysis conducted by Marek et al.48 of neuroimaging data (specifically, resting state functional connectivity and cortical thickness measures) vs cognitive/behavioral/clinical phenotypes. The authors concluded that thousands of participants may be needed to reliably detect brain-wide–psychiatric phenotype correlations of small effect sizes. It is particularly important to highlight this work, as some of the early studies (identified in our systematic review) have rather small sample sizes, including 1 study with 14 participants in the MDD group and another with 17) (Table 1). Small sample sizes, as discussed by Marek et al.,48 create the risk of inflated effect sizes. Publication bias is another likely confounding factor, as publication bias can be associated with overestimation of small effect sizes.49,50 Moreover, Marek et al.48 discussed that future research should focus on within-participant study designs (including intervention studies) rather than cross-sectional or observational study designs.
In addition, researchers have tried to classify participants into diagnostic groups (MDD vs controls) based on brain cortical and subcortical measures (cortical thickness and surface area, subcortical volumes), white matter microstructure, and/or functional connectivity, and have found relatively low (or at-chance) accuracy of classification.50, 51, 52 Some studies have also attempted to tease apart contributions from demographic and clinical variables, such as sex, age of onset of MDD, acute vs chronic MDD, medication use, and number of episodes, and have similarly found very small effect sizes and/or low (or at-chance) classification accuracy.50, 51, 52 Although this research is an important initial step, it appears to be insufficient in accounting for sources of variability in clinical and neural phenotypes. These studies highlight further the challenges posed by heterogeneity as well as the importance of deep phenotyping and multi-modal imaging.51,52
One notion (building upon clinical work and research) would be to try to create models of pathways to health and disease. This idea would align well with what clinicians already do when evaluating and creating treatment recommendations for individual patients with MDD. For example, clinicians may create a “biopsychosocial formulation” for an individual patient, considering risk and protective factors in the biological, psychological, and social domains.53 Biological factors may include family history of mood and anxiety disorders, medical conditions such as hypothyroidism, vitamin D deficiency, insomnia, sleep apnea, and substance use; psychological factors may include specific perceptions, thoughts, and beliefs of the individual; and the social domain may include sociocultural factors, family, and community supports for the patient. Protective factors may consist of support networks available to the individual, engagement in meaningful activities, hopes and goals for the future, exercise, and healthy diet. A thorough understanding of these factors for an individual patient are therefore, in turn, important for creating specific recommendations for care, including recommendations for psychotherapy, lifestyle modifications, additional social supports, and medications.
Interestingly, meta-analyses of some of these risk and protective factors have shown associations with white matter integrity.54, 55, 56, 57 For example, a recent meta-analysis described correlation between physical activity, cardiorespiratory fitness, and exercise and microstructural integrity of the corpus callosum, and the anterior limb of internal capsule, with effect sizes of 0.345 and 0.198, respectively.55 Another meta-analysis found that increased obesity measurements were related to reduced FA in the genu of the corpus callosum.57 A meta-analysis of white matter correlates of relatives of patients with severe mental disorders (MDD, bipolar disorder, and/or schizophrenia) showed decreased FA in the genu and splenium of the corpus callosum in relatives of patients as compared to controls, although a specific effect size was not reported.56 Lim et al.54 concluded, in a meta-analysis, that history of childhood maltreatment was associated with reduced FA in the anterior corpus callosum, along with the fornix, anterior thalamic radiation, optic radiations, the inferior longitudinal fasciculus, and inferior frontal-occipital fasciculus. Notably, these regions are similarly implicated in MDD in our current systematic review.
A possible future direction for research could be a focus on deep phenotyping, in both the clinical and neuroimaging domains of individuals.51,52 Deep neuroimaging phenotyping could include repeated measures of the same individual (eg, multiple measures during an MDD episode, and during remissions), using multi-modal imaging modalities, along with detailed assessments of current and past risk and protective factors associated with depression in both individuals with MDD and healthy controls. This approach aligns well with the within-participant study designs suggested by Marek et al.,48 as well as the important goal of bringing “results down to the level of the individual.”58 As pointed out by White,58 different pathways may exist (leading to similar behavioral phenotypes), and individuals have unique brains, shaped and influenced over time by genetic, epigenetic, environmental, and random factors.
There are several limitations of the current systematic review. A relatively small number of publications have focused on adolescent depression and met our inclusion criteria. Moreover, many of the studies had relatively small sample sizes (as low as 14 or 17 in the MDD group in some studies), which may have given rise to inflated effect sizes (in light of the paper by Marek et al.48) and are vulnerable to publication bias. Furthermore, the studies included in the current systematic review used a variety of analysis methods, had different sample characteristics (including different rates of co-occurring psychiatric disorders), and focused on MDD. Future studies could consider longitudinal study designs, focusing on developmental trajectories of depression (ie, separately focusing across childhood, adolescence, and adulthood), taking into account biological sex and exploring sex-by-diagnosis interactions. Such work, paired with careful tracking of co-occurring psychiatric disorders, medical conditions, clinical characteristics (including distinct depression subtypes and psychological factors), medication use, and genetic and environmental factors such as diet, exercise, history of trauma, and childhood adversity) could do the following: (1) lay the foundation for understanding microstructural organization across development in detail in MDD (along with other co-occurring psychiatric disorders that may emerge prior to or after the onset of MDD) as compared to typical development; (2) explore contributions to variation within and across participants from biological, psychological, and social risk and protective factors (such as diet, exercise, socio-economic status, medication use, psychotherapy, co-occurring psychiatric disorders, medical conditions, and biological sex); which, in turn (3) could help to establish personalized medicine approaches (for example, as proposed/envisioned in Chahal et al.14), including guiding which treatment approach may be optimal for a particular patient and predicting treatment response.
Footnotes
This article was reviewed under and accepted by Ad Hoc Editor Tonya White, MD, PhD.
The authors have reported funding from the American Academy of Child and Adolescent Psychiatry (AACAP) Pilot Research Award for Junior Faculty and Child and Adolescent Psychiatry Fellows (PI: Dr. Petya Radoeva; Mentor: Dr. Daniel Dickstein); Grant support from R01MH111542 (PI: Dr. Daniel Dickstein) and NIH S10OD025181 (PI: Dr. Jerome Sanes) supported the data acquisition and analysis (as part of a study of irritability) for the participant with MDD, for whom specific white matter regions were presented in Figure 2; K23MH128466 (PI: Dr. Petya Radoeva; Primary Mentor: Dr. Noah Philip; Co-Mentors: Dr. Daniel Dickstein, Dr. Sean Deoni, Dr. Jeffrey Hunt, Dr. Carla Mazefsky, and Dr. Stephen Sheinkopf); and Dr. Noah Philip is supported in part by P20 GM130452 and I50 RX002864. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the National Institutes of Health.
The results of this systematic review were presented as a poster at the American Academy of Child and Adolescent Psychiatry and Canadian Academy of Child and Adolescent Psychiatry 69th Annual Meeting; October 17-22, 2022; Toronto, Ontario, Canada.
This work has been prospectively registered: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=268200.
Author Contributions
Conceptualization: Radoeva, Milev, Hunt, Deoni, Sheinkopf, Mazefsky, Philip, Dickstein
Data curation: Radoeva, Milev, Legere
Formal analysis: Radoeva, Milev, Legere
Fundingacquisition: Radoeva, Dickstein
Investigation: Radoeva, Milev, Legere, Mazefsky, Dickstein
Methodology: Radoeva, Milev, Hunt, Philip, Dickstein
Project administration: Radoeva, Milev, Hunt, Legere, Philip, Dickstein
Resources: Radoeva
Supervision: Radoeva, Hunt, Deoni, Sheinkopf, Philip, Dickstein
Validation: Radoeva, Milev, Legere, Dickstein
Visualization: Radoeva, Milev, Legere
Writing – original draft: Radoeva
Writing – review and editing: Radoeva, Milev, Hunt, Legere, Deoni, Sheinkopf, Mazefsky, Philip, Dickstein
Disclosure: Dr. Mazefsky has reported royalties from Oxford University Press. Dr. Philip has received clinical trial support (through federal contracts) from Neurolief and Wave Neuro in the past three years. He has served on the scientific advisory board of Pulvinar Neuro. Drs. Radoeva, Hunt, Deoni, Sheinkopf, and Dickstein and Messrs. Milev and Legere have reported no biomedical financial interests or potential conflicts of interest.
Supplemental Material
References
- 1.National Institute of Mental Health Major depression. 2022. https://www.nimh.nih.gov/health/statistics/major-depression
- 2.Pine D.S., Cohen P., Gurley D., Brook J., Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 3.Melvin G.A., Dudley A.L., Gordon M.S., Ford S., Taffe J., Tonge B.J. What happens to depressed adolescents? A follow-up study into early adulthood. J Affect Disord. 2013;151(1):298–305. doi: 10.1016/j.jad.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Levy J.C., Deykin E.Y. Suicidality, depression, and substance abuse in adolescence. Am J Psychiatry. 1989;146(11):1462–1467. doi: 10.1176/ajp.146.11.1462. [DOI] [PubMed] [Google Scholar]
- 5.Wahlstrom D., Collins P., White T., Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn. 2010;72(1):146–159. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giedd J.N., Blumenthal J., Jeffries N.O., et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 7.Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Psychiatric Association . Fifth ed. American Psychiatric Association; 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 10.Lichenstein S.D., Verstynen T., Forbes E.E. Adolescent brain development and depression: a case for the importance of connectivity of the anterior cingulate cortex. Neurosci Biobehav Rev. 2016;70:271–287. doi: 10.1016/j.neubiorev.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cullen K.R., Westlund M.K., Klimes-Dougan B., et al. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry. 2014;71(10):1138–1147. doi: 10.1001/jamapsychiatry.2014.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connolly C.G., Ho T.C., Blom E.H., et al. Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. J Affect Disord. 2017;207:86–94. doi: 10.1016/j.jad.2016.09.026. S0165-0327(16)31054-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connolly C.G., Wu J., Ho T.C., et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013;74(12):898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chahal R., Gotlib I.H., Guyer A.E. Research review: brain network connectivity and the heterogeneity of depression in adolescence—a precision mental health perspective. J Child Psychol Psychiatry Allied Discip. 2020 doi: 10.1111/jcpp.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerestes R., Davey C.G., Stephanou K., Whittle S., Harrison B.J. Functional brain imaging studies of youth depression: a systematic review. Neuroimage Clin. 2014;4:209–231. doi: 10.1016/j.nicl.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hulvershorn L.A., Cullen K., Anand A. Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging Behav. 2011;5(4):307–328. doi: 10.1007/s11682-011-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeWinn K.Z., Connolly C.G., Wu J., et al. White matter correlates of adolescent depression: structural evidence for frontolimbic disconnectivity. J Am Acad Child Adolesc Psychiatry. 2014;53(8):899–909. doi: 10.1016/j.jaac.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu F., Tu Z., Sun J., et al. Abnormal functional and structural connectivity of amygdala-prefrontal circuit in first-episode adolescent depression: a combined fMRI and DTI study. Front Psychiatry. 2020;10:983. doi: 10.3389/fpsyt.2019.00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bessette K.L., Nave A.M., Caprihan A., Stevens M.C. White matter abnormalities in adolescents with major depressive disorder. Brain Imaging Behav. 2014;8(4):531–541. doi: 10.1007/s11682-013-9274-8. [DOI] [PubMed] [Google Scholar]
- 20.Cullen K.R., Klimes-Dougan B., Muetzel R., et al. Altered white matter microstructure in adolescents with major depression: a preliminary study. J Am Acad Child Adolesc Psychiatry. 2010;49(2):173–183. doi: 10.1097/00004583-201002000-00011. 00004583-201002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson S.E., Johnson A.R., Vallejo A.I., Katz L., Wong E., Gabbay V. A preliminary study of white matter in adolescent depression: relationships with illness severity, anhedonia, and irritability. Front Psychiatry. 2013;4:152. doi: 10.3389/fpsyt.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Velzen L.S., Kelly S., Isaev D., et al. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol Psychiatry. 2020;25(7):1511–1525. doi: 10.1038/s41380-019-0477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou L., Wang L., Wang M., et al. Alterations in white matter microarchitecture in adolescents and young adults with major depressive disorder: a voxel-based meta-analysis of diffusion tensor imaging. Psychiatry Res Neuroimaging. 2022;323 doi: 10.1016/j.pscychresns.2022.111482. [DOI] [PubMed] [Google Scholar]
- 24.Guo W.B., Liu F., Xue Z.M., et al. Altered white matter integrity in young adults with first-episode, treatment-naive, and treatment-responsive depression. Neurosci Lett. 2012;522(2):139–144. doi: 10.1016/j.neulet.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Ma N., Li L., Shu N., et al. White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. Am J Psychiatry. 2007;164(5):823–826. doi: 10.1176/ajp.2007.164.5.823. [DOI] [PubMed] [Google Scholar]
- 26.Hardin A.P., Hackell J.M. Committee On Practice and Ambulatory Medicine, Age limit of pediatrics. Pediatrics. 2017;140(3):1–2. doi: 10.1542/peds.2017-2151. e20172151. [DOI] [PubMed] [Google Scholar]
- 27.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed) 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aoki Y., Abe O., Nippashi Y., Yamasue H. Comparison of white matter integrity between autism spectrum disorder subjects and typically developing individuals: a meta-analysis of diffusion tensor imaging tractography studies. Mol Autism. 2013;4(1) doi: 10.1186/2040-2392-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J.H., Chi S., Ko M., et al. Prospective study on microstructure in medication-naive adolescents with first-episode major depressive disorder. J Affect Disord. 2021;293:268–275. doi: 10.1016/j.jad.2021.06.048. S0165-0327(21)00634-0 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Cullen K.R., Brown R., Schreiner M.W., et al. White matter microstructure relates to lassitude but not diagnosis in adolescents with depression. Brain Imaging Behav. 2020;14(5):1507–1520. doi: 10.1007/s11682-019-00078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu S.H., Lenglet C., Schreiner M.W., Klimes-Dougan B., Cullen K., Parhi K.K. Anatomical biomarkers for adolescent major depressive disorder from diffusion weighted imaging using SVM Classifier. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2018;2018:2740–2743. doi: 10.1109/EMBC.2018.8512852. [DOI] [PubMed] [Google Scholar]
- 32.Chang M., Womer F.Y., Edmiston E.K., et al. Neurobiological commonalities and distinctions among three major psychiatric diagnostic categories: a structural MRI study. Schizophr Bull. 2018;44(1):65–74. doi: 10.1093/schbul/sbx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tymofiyeva O., Connolly C.G., Ho T.C., et al. DTI-based connectome analysis of adolescents with major depressive disorder reveals hypoconnectivity of the right caudate. J Affect Disord. 2017;207:18–25. doi: 10.1016/j.jad.2016.09.013. S0165-0327(16)30586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geng H., Wu F., Kong L., et al. Disrupted structural and functional connectivity in prefrontal-hippocampus circuitry in first-episode medication-naive adolescent depression. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0148345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aghajani M., Veer I.M., van Lang N.D., et al. Altered white-matter architecture in treatment-naive adolescents with clinical depression. Psychol Med. 2014;44(11):2287–2298. doi: 10.1017/S0033291713003000. [DOI] [PubMed] [Google Scholar]
- 36.Wu S.J., Hsu J.W., Huang K.L., Bai Y.M., Tu P.C., Chen M.H. Functional dysconnectivity of cerebellum and attention networks in emotional dysregulation shared between attention deficit hyperactivity disorder and major depressive disorder: a multimodal imaging study. CNS Spectr. 2022:1–8. doi: 10.1017/S1092852922000876. [DOI] [PubMed] [Google Scholar]
- 37.Kaufman J., Birmaher B., Brent D., et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 38.Eickhoff S.B., Laird A.R., Grefkes C., Wang L.E., Zilles K., Fox P.T. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith S.M., Jenkinson M., Woolrich M.W., et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. S1053-8119(04)00393-3. [DOI] [PubMed] [Google Scholar]
- 40.Cabeen R.P., Laidlaw D.H., Ruggieri A., Dickstein D.P. Preliminary mapping of the structural effects of age in pediatric bipolar disorder with multimodal MR imaging. Psychiatry Res Neuroimaging. 2018;273:54–62. doi: 10.1016/j.pscychresns.2017.12.006. S0925-4927(17)30159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori S., Oishi K., Faria A.V. White matter atlases based on diffusion tensor imaging. Curr Opin Neurol. 2009;22(4):362–369. doi: 10.1097/WCO.0b013e32832d954b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Lopez J.A., Page M.J., Lipsey M.W., Higgins J.P.T. Dealing with effect size multiplicity in systematic reviews and meta-analyses. Res Synthesis Methods. 2018 doi: 10.1002/jrsm.1310. [DOI] [PubMed] [Google Scholar]
- 43.Saletin J.M., Jackvony S., Rodriguez K.A., Dickstein D.P. A coordinate-based meta-analysis comparing brain activation between attention deficit hyperactivity disorder and total sleep deprivation. Sleep. 2019;42(3) doi: 10.1093/sleep/zsy251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao M., Yang F., Zhang Y., He Z., Su L., Li L. White matter abnormalities in adolescents with generalized anxiety disorder: a diffusion tensor imaging study. BMC Psychiatry. 2014;14:41. doi: 10.1186/1471-244X-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drysdale A.T., Grosenick L., Downar J., et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dinga R., Schmaal L., Penninx B., et al. Evaluating the evidence for biotypes of depression: methodological replication and extension of. Neuroimage Clin. 2019;22 doi: 10.1016/j.nicl.2019.101796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y., Tang S., Zhang L., et al. Data-driven clustering differentiates subtypes of major depressive disorder with distinct brain connectivity and symptom features. Br J Psychiatry. 2021;219(5):606–613. doi: 10.1192/bjp.2021.103. [DOI] [PubMed] [Google Scholar]
- 48.Marek S., Tervo-Clemmens B., Calabro F.J., et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603(7902):654–660. doi: 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Algermissen J., Mehler D.M.A. May the power be with you: are there highly powered studies in neuroscience, and how can we get more of them? J Neurophysiol. 2018;119(6):2114–2117. doi: 10.1152/jn.00765.2017. [DOI] [PubMed] [Google Scholar]
- 50.Belov V., Erwin-Grabner T., Gonul A.S., Amod A.R., et al. Multi-site benchmark classification of major depressive disorder using machine learning on cortical and subcortical measures. arxiv. 2022 doi: 10.48550/arXiv.2206.08122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stolicyn A., Harris M.A., Shen X., et al. Automated classification of depression from structural brain measures across two independent community-based cohorts. Hum Brain Mapp. 2020;41(14):3922–3937. doi: 10.1002/hbm.25095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winter N.R., Leenings R., Ernsting J., et al. Quantifying deviations of brain structure and function in major depressive disorder across neuroimaging modalities. JAMA Psychiatry. 2022;79(9):879–888. doi: 10.1001/jamapsychiatry.2022.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schotte C.K., Van Den Bossche B., De Doncker D., Claes S., Cosyns P. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depress Anxiety. 2006;23(5):312–324. doi: 10.1002/da.20177. [DOI] [PubMed] [Google Scholar]
- 54.Lim L., Howells H., Radua J., Rubia K. Aberrant structural connectivity in childhood maltreatment: a meta-analysis. Neurosci Biobehav Rev. 2020;116:406–414. doi: 10.1016/j.neubiorev.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Maleki S., Hendrikse J., Chye Y., et al. Associations of cardiorespiratory fitness and exercise with brain white matter in healthy adults: a systematic review and meta-analysis. Brain Imaging Behav. 2022;16(5):2402–2425. doi: 10.1007/s11682-022-00693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu M., Zhang W., Hochwalt P., et al. Structural connectivity associated with familial risk for mental illness: a meta-analysis of diffusion tensor imaging studies in relatives of patients with severe mental disorders. Hum Brain Mapp. 2022;43(9):2936–2950. doi: 10.1002/hbm.25827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daoust J., Schaffer J., Zeighami Y., Dagher A., Garcia-Garcia I., Michaud A. White matter integrity differences in obesity: a meta-analysis of diffusion tensor imaging studies. Neurosci Biobehav Rev. 2021;129:133–141. doi: 10.1016/j.neubiorev.2021.07.020. [DOI] [PubMed] [Google Scholar]
- 58.White T. Behavioral phenotypes, stochastic processes, entropy, evolution, and individual variability: toward a unified field theory for neurodevelopment and psychopathology. Aperture Neuro. 2022;2:1–3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.