Abstract
The lungs are a major organ site of cytomegalovirus (CMV) infection, pathogenesis, and latency. Interstitial CMV pneumonia represents a critical manifestation of CMV disease, in particular in recipients of bone marrow transplantation (BMT). We have employed a murine model for studying the immune response to CMV in the lungs in the specific scenario of immune reconstitution after syngeneic BMT. Control of pulmonary infection was associated with a vigorous infiltration of the lungs, which was characterized by a preferential recruitment and massive expansion of the CD8 subset of α/β T cells. The infiltrate provided a microenvironment in which the CD8 T cells differentiated into mature effector cells, that is, into functionally active cytolytic T lymphocytes (CTL). This gave us the opportunity for an ex vivo testing of the antigen specificities of CTL present at a relevant organ site of viral pathogenesis. The contribution of the previously identified immediate-early 1 (IE1) nonapeptide of murine CMV was evaluated by comparison with the CD3ɛ-redirected cytolytic activity used as a measure of the overall CTL response in the lungs. The IE1 peptide was detected by pulmonary CTL, but it accounted for a minor part of the response. Interestingly, no additional viral or virus-induced antigenic peptides were detectable among naturally processed peptides derived from infected lungs, even though infected fibroblasts were recognized in a major histocompatibility complex-restricted manner. We conclude that the antiviral pulmonary immune response is a collaborative function that involves many antigenic peptides, among which the IE1 peptide is immunodominant in a relative sense.
Effective control by the immune system is a hallmark of cytomegalovirus (CMV) infection. Accordingly, human CMV disease is a medical problem restricted to the immunologically immature or immunocompromised host (for a review, see reference 21). Murine models have implicated natural killer (NK) cells and CD8 T cells in the control of CMV infection. While NK cells mediate early protection in genetically resistant mouse inbred strains (4, 5, 31, 51), CD8 T cells establish enduring protective memory and function as principal antiviral effectors in susceptible strains (31). Specifically, in the BALB/c strain, major histocompatibility complex (MHC) class I-restricted antiviral CD8 T cells resolve acute murine CMV infection and prevent lethal CMV disease (45; for a review, see reference 27). In a model of experimental bone marrow transplantation (BMT), reconstitution of CD8 T cells proved to be essential for the prevention of lethal murine CMV pathogenesis in multiple organs (36). Furthermore, preemptive experimental cytoimmunotherapy by adoptive transfer of antiviral CD8 T cells limited the burden of latent viral genome and thereby reduced the risk of virus recurrence (54). Since efficient reconstitution of CD8 T cells after clinical BMT is of positive prognostic value for a control of human CMV (46, 48), experimental BMT in the susceptible BALB/c mouse strain is likely to be a relevant model for studying the immune response to CMV in the specific context of immunological reconstitution after BMT.
The established role of CD8 T cells in immunity to murine as well as human CMV contrasts with the recent finding that these viruses have both evolved manifold immune evasion mechanisms that interfere at various steps in the MHC class I pathway of antigenic peptide presentation in the infected cell (reviewed in reference 19). Downregulation of MHC class I cell surface expression should result in enhanced susceptibility to NK cells (22). Notably, by expressing the respective viral class I homologs, human as well as murine CMVs have acquired the potential to evade control by NK cells as well (14, 47).
Effective in vivo control of CMV by CD8 T cells implies a leakiness of molecular immune evasion. It is a known but so far insufficiently understood phenomenon that the immune response to virus infections is often focused on a limited number of immunodominant peptides. To become immunodominant, a viral peptide must be superior to other potentially antigenic peptides in passing through all the critical steps in the pathway of antigen processing and presentation, namely, efficient generation by protein cleavage at the proteasome, transport into the endoplasmic reticulum, high-affinity binding to the presenting MHC class I molecule, and transport of the assembled MHC-peptide complex to the cell surface. Viral immune evasion mechanisms place further obstacles in the way of candidate peptides. Accordingly, an immunodominant peptide must be one that also overcomes or circumvents the evasion strategies of the virus more efficiently than others do. Therefore, immune evasion and peptide immunodominance are likely to be linked phenomena.
Specifically, although the genome of murine CMV comprises ca. 170 open reading frames (37) with the capacity to encode numerous antigenic peptides for any MHC haplotype, an antigen expressed during the immediate-early (IE) phase of the viral replication cycle proved to be immunodominant in BALB/c mice (42, 43). The immunodominant antigen was identified as a nonapeptide with the sequence YPHFMPTNL, derived from the regulatory IE1 protein pp89 and presented by the MHC class I molecule Ld (13, 44). Its significance in protection against lethal murine CMV disease has been documented by the protective efficacy of a vaccinia virus recombinant expressing the IE1 nonapeptide selectively (12). Apparently, if a limited number or, in the extreme, only a single “privileged” antigenic peptide overcomes the immune evasion strategies of the virus, this will suffice for effective antiviral control by CD8 T cells.
The central question of how many different viral peptides are involved in the in vivo immune response to acute CMV infection has remained unanswered to date because lymphocytes derived from lymphoid tissues did not exert an ex vivo cytolytic activity (40). Current knowledge thus rests on cytolytic T-lymphocyte lines (CTLL) propagated in culture under conditions that entail the risk of arbitrary selection.
We demonstrate here that pulmonary infiltrates that develop after BMT and concurrent murine CMV infection provide a microenvironment for the differentiation of CD8 T cells into functional cytolytic T lymphocytes (CTL). This gave us for the first time the opportunity to study the specificity of CTL that are operative at a relevant organ site of CMV pathogenesis, namely, the lungs. CTL isolated from the pulmonary infiltrates were tested directly with naturally processed peptides derived from the infected lungs. The result was surprising. Although the pulmonary CTL displayed a high cytolytic activity and lysed infected target cells at all stages of the viral replicative cycle, this cytolytic activity could not be quantitatively attributed to immunodominant peptides.
MATERIALS AND METHODS
BMT and concurrent CMV infection.
Syngeneic BMT was performed by using female BALB/c (H-2d) mice at the age of 8 weeks as donors and recipients of bone marrow (BM) cells (BMC). Hematoablative conditioning of the recipients was performed by total-body γ-irradiation with a single dose of 6 Gy from a 137Cs source (OB58; Buchler, Braunschweig, Germany). This irradiation is equivalent to a 50% lethal dose determined on day 30. Donor femoral and tibial BMC were obtained as described previously (35), and the indicated doses were injected intravenously into the tail vein of the recipients at ca. 6 h after the irradiation. Murine BM contains T-cell receptor (TCR) α/β-expressing T cells in an amount that is at the detection limit of cytofluorometry. To exclude a contamination of donor BMC by mature donor CD8 T cells, depletion was performed by three treatment cycles with a rat anti-murine CD8 monoclonal antibody (MAb), clone YTS 169.4 (9), and magnetic beads coated with sheep anti-rat immunoglobulin (Ig) antibody (Ab) (Dynabeads M-450; Dynal, Oslo, Norway) at a bead-to-cell ratio of 2:1. The efficacy of depletion was controlled in parallel with BMC to which 5% CD8 T cells had been added. Mice were infected subcutaneously in the left hind footpad with 105 PFU of purified murine CMV, strain Smith ATCC VR-194, at ca. 2 h after BMT.
Two-color IHC for the simultaneous analysis of tissue infection and pulmonary infiltrates.
Lung tissue was fixed by in situ perfusion of the pulmonary vascular tree with phosphate-buffered saline (PBS; pH 7.4) containing formalin (4%, vol/vol). Alveolar spaces were distended by instillation of the fixative into the trachea. The lungs were then excised and processed by standard procedures for the preparation of paraffin-embedded tissue. Two-micrometer-thick sections were dewaxed in xylene and either stained with hematoxylin and eosin (HE) by standard procedures or used for the detection of T cells and infected lung cells by two-color immunohistochemistry (IHC). For IHC, sections were pretreated with trypsin solution (1.25 mg/ml) at 37°C for 15 min. Incubation in a microwave oven at 300 W for 2 min was used to enhance the signals by the unmasking of antigens. Endogenous peroxidase activity was blocked by an incubation for 30 min at 20°C in 0.5% (vol/vol) hydrogen peroxide in methanol-PBS (1:1). To saturate unspecific binding sites, slides were overlaid for 20 min at 20°C with a 1:10 dilution of normal goat serum in PBS. T cells were labeled by an incubation of the sections for 1 h with a rat IgG1 MAb, clone CD3-12 (no. BT 01 260 003 0; Biotrend, Cologne, Germany), directed against the 14-amino-acid peptide ERPPPVPNPDYEPC that represents a conserved cytoplasmic epitope shared by human and murine CD3ɛ (24). The staining was performed by the ABC method, by using a biotinylated goat anti-rat Ig Ab (no. 12112D; Pharmingen, San Diego, Calif.) at a 1:100 dilution in PBS for 30 min, followed by detection with an avidin-biotin-peroxidase complex (Vectastain ABC kit standard PK-4000; Vector Laboratories, Burlingame, Calif.) and diaminobenzidine tetrahydrochloride (no. D-5637; Sigma, Munich, Germany) as the substrate. The staining was enhanced by ammonium nickel sulfate hexahydrate (no. 09885; Fluka, Neu-Ulm, Germany), resulting in a black precipitate. The slides were then incubated for 20 min with normal rabbit serum, diluted 1:10 with PBS. The intranuclear viral IE1 protein pp89 was labeled by incubation for 1 h with MAb CROMA 101 (murine IgG1; kindly provided by S. Jonjic, University of Rijeka, Rijeka, Croatia), followed by staining with rabbit anti-mouse Ig Ab (no. Z-0259; Dako, Hamburg, Germany) and the alkaline phosphatase–anti-alkaline phosphatase (APAAP) complex (no. A-7827; Sigma) with new fuchsin as the substrate, yielding a brilliant red precipitate. Counterstaining was performed with Mayer’s hemalum.
Isolation of interstitial mononuclear leukocytes from pulmonary infiltrates.
Leukocytes were isolated from the lung parenchyma by using a modification of the method described by Holt et al. (23). Mice were lethally anesthetized by inhalation of carbon dioxide. For removal of intravascular leukocytes, the vascular bed of the lungs was perfused with 5 to 10 ml of PBS devoid of Ca2+ and Mg2+, containing heparin (10 U/ml). Alveolar leukocytes were removed by bronchoalveolar lavage. The lungs were then excised under careful stereomicroscopic control to exclude the peritracheal lymph nodes and were washed in Dulbecco’s modified Eagle’s medium (DMEM; high glucose [no. 41965-039; Gibco BRL, Eggenstein, Germany]) supplemented with 10% (vol/vol) fetal calf serum, penicillin, and streptomycin. The trachea, bronchi, bronchioles, and hilar lymph nodes were discarded. The remaining lung parenchyma was minced, and a single cell suspension was prepared by collagenase-DNase digestion. Usually, the tissue from three to five lungs was incubated for 1 h at 37°C with 15 ml of supplemented DMEM, which contained type I collagenase (200 U/ml; Biochrom, Berlin, Germany) and type I DNase (DN-25, 50 μg/ml; Sigma). It is important to note that the specific activity and individual batch of the collagenase proved to be critical. The incubation was performed in an Erlenmeyer glass bottle with constant stirring. Clumps were resolved by passage through a steel mesh. After washing, the cells were resuspended in RPMI medium (no. 31870-025; Gibco BRL) supplemented with 5% (vol/vol) fetal calf serum, penicillin, streptomycin, 10 mM HEPES, 50 μM 2-mercaptoethanol, and 2 mM l-glutamine. Mononuclear leukocytes were enriched by density gradient centrifugation for 30 min at 760 × g on Ficoll (1.077 g/ml; Sigma).
Three-color cytofluorometric analysis of interstitial pulmonary T lymphocytes.
Three-color cytofluorometric analysis was performed with a FACSort (Becton Dickinson, San Jose, Calif.) by using CellQuest software (Becton Dickinson) for data processing. The cells retrieved from the Ficoll interphase were labeled with the directly conjugated MAbs TCR α/β-phycoerythrin (TCR α/β-PE) (clone H57-597; Pharmingen), CD4-RED613 (clone H129.19; Gibco BRL), and CD8-fluorescein isothiocyanate (CD8-FITC) (clone 53-6.7; Becton Dickinson). A threshold was set in the forward scatter to exclude events of the size of erythrocytes, and each analysis was then based on 105 events representing viable cells. A lymphocyte gate was set in the forward versus side scatter plot. In addition, the analysis was restricted to T cells by setting a gate on signals with positive PE fluorescence. The overlap in the emission spectra of the three dyes was compensated. The distribution of CD4 and CD8 expression among the T cells is documented by RED613 (ordinate) and FITC (abscissa) fluorescence intensity dot plots. Yields of CD4 and CD8 T cells were calculated from the absolute yield of Ficoll interphase cells and the percentages of cells in the various gates.
Quantitation of infectious virus in the lungs.
The titer of infectious virus was determined by a plaque assay performed with centrifugal enhancement of infectivity as described previously (45). In brief, the lung parenchyma was frozen and thawed to disrupt the cells, and appropriate dilutions of the homogenate were used to infect permissive murine embryofetal fibroblasts (MEF) in close-to-confluence cultures at a centrifugal force of 1,000 × g for 30 min at 20°C. Plaques in the MEF monolayer were counted 4 to 5 days later. The virus titers represent titers per organ and are expressed as PFU* to indicate the centrifugal enhancement.
Assays of cytolytic activity. (i) Effector cells.
CTL activity was determined ex vivo for the interstitial pulmonary leukocytes. Depletion of CD4 and CD8 T cells was performed by the standard procedure of complement-mediated lysis by using MAbs anti-CD4 (clone YTS 191.1) and anti-CD8 (clone YTS 169.4), respectively (9). A long-term CTLL specific for the IE1 peptide YPHFMPTNL presented by the MHC class I molecule Ld (38, 44) served to monitor the processing and presentation of this peptide. This reference CTLL was obtained by restimulation of virus-specific memory T cells and was maintained in the presence of recombinant interleukin-2 essentially as described previously (38), with some modifications. In brief, spleen cells derived from latently infected, immune BALB/c mice at >3 mo after primary infection were restimulated weekly with 10−9 M of the synthetic IE1 peptide. The CTLL reached monospecificity after the third restimulation.
(ii) Target cells.
P815 cells (DBA/2-derived mastocytoma cells; H-2d) were used as 51Cr-labeled target cells for monitoring antigenic peptides and for the determination of the cytolytic activity of activated T cells by the TCR- or CD3ɛ-redirected lysis assay (28, 53). P815 cells transfected with the human B7-1/CD80 cDNA (2) (P815-B7 cells) were used to engage resting T lymphocytes (2, 3). The P815-B7 cells were propagated in culture medium containing 1 mg of G418 per ml, and cell surface expression of B7 was monitored by cytofluorometry with FITC-conjugated mouse MAb anti-human B7-1 (IgM; clone BB1 [no. 33514; Dianova, Hamburg, Germany]). For assaying peptide-specific cytolytic activity, P815 or P815-B7 cells were incubated for 15 min at 20°C with synthetic IE1 peptide or with high-performance liquid chromatography (HPLC) fractions containing naturally processed peptides. Excess peptide was removed by washing the cells before the cytolytic assay. For redirected lysis assays, 51Cr-labeled P815 or P815-B7 cells were preincubated for 15 min at 20°C with optimal doses of hamster MAb (IgG) specific for either murine CD3ɛ (clone 145-2C11; Boehringer, Mannheim, Germany), murine TCR α/β (clone H57-597; Dianova), or murine TCR γ/δ (clone GL3; Dianova). The presentation of antigenic peptides during the infection of permissive cells was tested with second-passage MEF infected with a multiplicity of infection of 4 PFU* per cell. Differential gene expression defining the IE, early (E), and late (L) phases of the CMV replicative cycle was achieved by metabolic inhibitors as specified previously (39, 41). A standard 4-h 51Cr-release assay was performed with 103 target cells per 0.2-ml microwell. Data represent the mean percentage of specific lysis from three replicate cultures.
Isolation of endogenously processed peptides from infected lungs.
Peptides were acid extracted from tissue by the method developed by Rötzschke et al. (49, 50) with modifications (16). In brief, lung parenchyma was homogenized in DMEM and the homogenate was acidified to pH 2 by using 5% (vol/vol) trifluoroacetic acid (TFA). After sonification, particulate components were removed by ultracentrifugation for 45 min at 100,000 × g. Low-molecular-weight fractions obtained by size exclusion chromatography (Sephadex G-25 column; Pharmacia, Freiburg, Germany) were concentrated to a volume of 1 ml by solid-phase extraction (SepPak C18 reversed-phase unit; Waters, Eschborn, Germany) and vacuum centrifugation. Separation of peptides was performed by HPLC with the PepS (Pharmacia) reversed-phase column. Specifically, 0.5 ml of the concentrated SepPak eluate was loaded on the HPLC column, and peptides were eluted at a flow rate of 0.8 ml per min on a linear acetonitrile gradient: solution A, 0.1% (vol/vol) TFA; solution B, 70% (vol/vol) acetonitrile and 0.1% (vol/vol) TFA. The gradient was generated as follows: min 0 to 4, 25% solution B; min 4 to 18, linear increase to 90% solution B; min 18 to 22, 90% solution B; min 22 to 27, linear decrease to 25% solution B; and min 27 to 30, 25% solution B. Fractions of 0.8 ml were aliquoted and lyophilized for storage. The efficacy of peptide extraction proved to be 10% when tested with lung homogenate supplemented with the synthetic IE1 peptide. This extraction efficacy was taken into account in the calculation of peptide yields.
RESULTS
High doses of syngeneic BMC prevent lethal murine CMV disease.
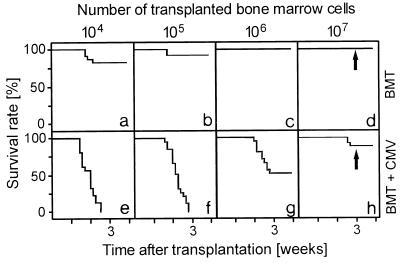
After hematoablative treatment of 8-week-old BALB/c mice with 6 Gy of γ-irradiation, BMT performed with low doses of syngeneic BMC was sufficient to accomplish hematopoietic reconstitution and to prevent mortality (Fig. 1a to d). Throughout the BMC titration, a concurrent infection with murine CMV significantly reduced the survival rates (Fig. 1e to h). Lethal CMV infection is associated with histopathology in multiple organs (36) combined with a BM aplasia, referred to as CMV aplastic anemia (32, 35). As we have shown recently, murine CMV infection directly interferes with the engraftment of a BM transplant in the BM stroma of the recipients (55). Notably, however, the lethal course of CMV infection was prevented in a high proportion of recipients if reconstitution was performed with a large dose of syngeneic BMC (Fig. 1h). Survivors in this experimental group should thus reveal the protective antiviral principle operative after syngeneic BMT.
FIG. 1.
Syngeneic BMT with high doses of BMC prevents lethal CMV disease. Kaplan-Meier survival plots documenting the influence of the number of transplanted BMC on the survival rates (ordinate) as a function of time (abscissa) after BMT (a to d) or BMT and concurrent murine CMV infection (e to h) for groups of 20 recipients are shown. The arrows mark the time point of the histological analysis depicted in Fig. 2.
T-cell infiltrates confine foci of murine CMV infection in the lungs.
Previous data have indicated two possibly connected mechanisms of protection by MHC-matched BMT. First, high doses of BMC were found to protect against virus-induced functional deficiency of BM stroma, with the result of successful BM repopulation. Notably, this part of protection proved to be unrelated to control of BM infection (55). Second, depletion of newly formed CD8 T cells, but not of CD4 T cells, during the process of hematolymphopoietic reconstitution resulted in lethal murine CMV disease (36, 54) characterized by dramatically enhanced virus replication and consequent viral histopathology in almost all vital organs (36). It is therefore logical to conclude that successful BM repopulation leads to successful reconstitution of antiviral CD8 T cells, which then protect against organ disease by controlling the infection in tissues. If this scenario were true, we should see T cells in action at a relevant organ site of CMV disease. For testing this prediction, we have selected the lungs, since interstitial pneumonia is a relevant manifestation of CMV disease, clinically (58) as well as in murine models (45, 52).
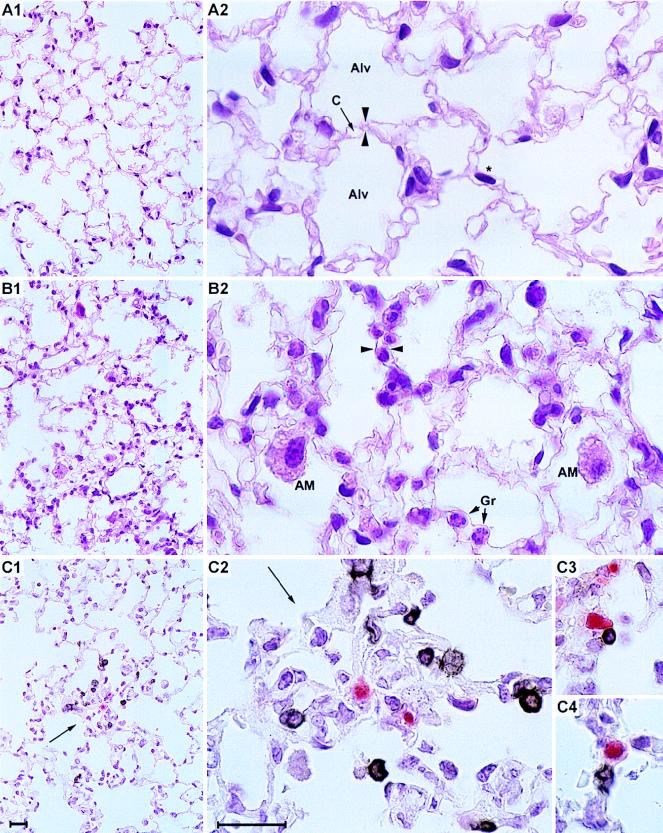
Three weeks after protective BMTs were performed with a large dose of BMC, the lung tissue histologies for survivors among uninfected and infected BMT recipients were compared (Fig. 2A and B, respectively, with experimental conditions corresponding to Fig. 1, arrows). In the uninfected BMT recipients, the lung tissue displayed a healthy architecture with no signs of inflammation (Fig. 2A1 [overview] and A2 [detail]). By contrast, survivors in the infected group experienced an interstitial pneumonia characterized by a widening of the alveolar septa and an interstitial as well as alveolar leukocyte infiltrate in which interstitial granulocytes and alveolar macrophages in addition to the mononuclear interstitial infiltrate cells were prominent (Fig. 2B1 [overview] and B2 [detail]). Two-color IHC was used to visualize the localization of infiltrating CD3ɛ-positive T cells in topographic relation to infected lung tissue cells. Notably, the infiltrating CD3ɛ-expressing T cells were found not to be randomly distributed; apparently, they had been specifically attracted to the foci of infection (Fig. 2C1 [overview] and C2 to C4 [details]). This strict colocalization strongly suggests a role for infiltrating T cells in the confinement and eventual resolution of pulmonary infection.
FIG. 2.
In situ colocalization of CD3ɛ-positive T lymphocytes and foci of infection. (A) Histology of the lung tissue 3 weeks after BMT with no infection (corresponding to data shown in Fig. 1d). (A1) Overview; (A2) detail. HE staining. Alv, alveoli; C, capillary. An asterisk indicates an elongated nucleus of a parenchymal lung cell; opposed arrowheads indicate an alveolar septum. Note the absence of infiltrates and the normal architecture of the lung tissue. (B) Pathohistology of the lung tissue 3 weeks after BMT and murine CMV infection (corresponding to data shown in Fig. 1h). (B1) Overview; (B2) detail. HE staining. AM, alveolar macrophages; Gr, granulocytes. Opposed arrowheads indicate a widened alveolar septum with interstitial mononuclear leukocytes. Note the infiltration and the widening of the alveolar septa. (C) Detection of infiltrating CD3ɛ-positive lymphocytes and of infected cells 3 weeks after BMT and murine CMV infection, corresponding to the HE-stained tissue shown in panel B. Infected cells are visualized by red (APAAP-new fuchsin) IHC staining of the intranuclear viral IE1 protein. Infiltrating lymphocytes are visualized by black (ABC-diaminobenzidine-nickel) IHC staining of CD3ɛ antigen. (C1) Overview; (C2) Detail of panel C1. The arrow indicates a focus of infection, surrounded by CD3ɛ-positive infiltrating cells. (C3 and C4) CD3ɛ-positive cells located in intimate proximity to infected cells. Bars, 20 μm.
Pulmonary T-cell infiltrates are dominated by CD8 T cells.
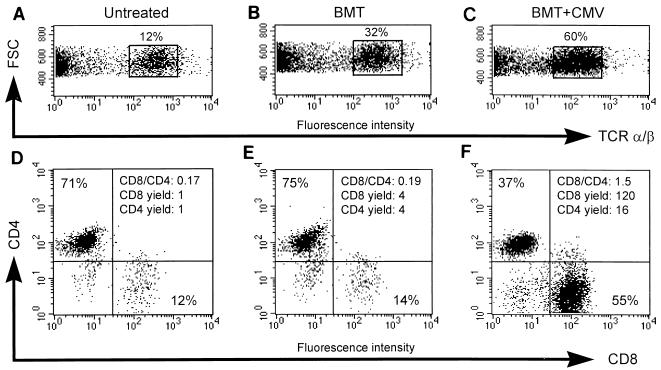
The subset composition of T cells in the pulmonary infiltrates was determined by three-color cytofluorometric analysis (for comparison of TCR α/β, CD4, and CD8 expression) of mononuclear leukocytes isolated from lung tissue 4 weeks after BMT, with normal lung tissue included in the analysis as a reference. The data for one transplantation are shown as an example (Fig. 3), and all other transplantations were analyzed accordingly. It is worth noting that a two-color cytofluorometric analysis (for comparison of TCR α/β and CD3ɛ expression) identified the CD3ɛ-positive cells as α/β T lymphocytes (data not shown). Infection was associated with a vigorous and preferential recruitment of CD8 T cells, as reflected by a marked increase in both the CD8/CD4 ratio and the yield of CD8 T cells. In comparison to normal lung tissue, the yield of pulmonary T cells was found to be moderately increased in lung tissue from mice after BMT with no CMV infection, but in this case with no subset preference. The expansion of the CD8 T-cell pool in the infected lung tissue was massive indeed, being 120-fold larger than that in normal lung tissue and 30-fold larger than that in uninfected lung tissue after BMT. In conclusion, CMV infection engages preferentially the CD8 subset of α/β T lymphocytes.
FIG. 3.
Preferential enrichment of CD8 T cells in lung infiltrates. Three-color cytofluorometric analysis of interstitial pulmonary T lymphocytes was done. After perfusion and bronchoalveolar lavage were done for groups of three to five mice, mononuclear leukocytes were recovered from the lung parenchyma by collagenase-DNase digestion and were enriched by Ficoll gradient centrifugation. A lymphocyte gate was set in the forward versus side scatter plot (data not shown). (A and D) Pulmonary lymphocytes recovered as a control from lung tissue of untreated, adult BALB/c mice; (B and E) pulmonary lymphocytes recovered from lung tissue 4 weeks after performance of BMT with 107 syngeneic BMC; (C and F) pulmonary lymphocytes recovered from lung tissue 4 weeks after BMT (same conditions as those described for panels B and E) and concurrent murine CMV infection; (A to C) dot plot of forward scatter (FSC, ordinate) versus PE (TCR α/β, abscissa) fluorescence intensity, with TCR α/β cells enclosed in a rectangle and their percentage among the cells in the lymphocyte gate indicated; (D to F) dot plots of RED613 (CD4, ordinate) versus FITC (CD8, abscissa) fluorescence intensities for TCR α/β-expressing cells. The percentages of CD4 and CD8 T cells are given in the respective quadrants, and the CD8/CD4 ratio is indicated in the upper right quadrant. The yield of CD8 and CD4 T cells is expressed as a multiple of the respective yield from normal, uninfected lungs.
CD8 T-cell infiltration of the lungs coincides with the resolution of pulmonary infection.
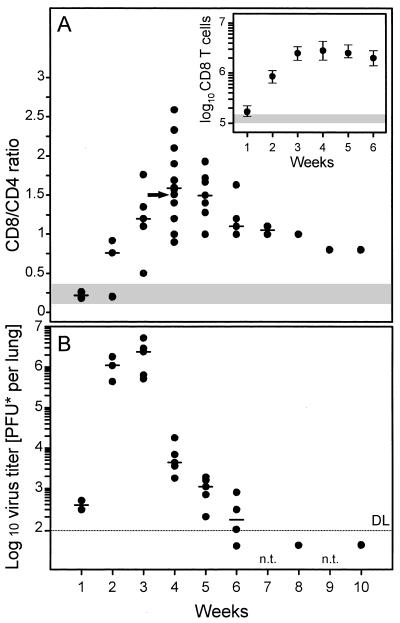
The kinetics of CD8/CD4 ratios compiled from independent but analogous transplantations revealed a peak of CD8-subset predominance 4 weeks after BMT and infection (Fig. 4A). In absolute terms, the number of pulmonary CD8 T cells had increased to 1.8 × 106 to 4.4 × 106 per lung, as compared to 0.10 × 106 to 0.16 × 106 per lung after BMT with no infection (Fig. 4A, inset). The infiltration resolved only slowly, and even after 10 weeks, the CD8/CD4 ratio was found to be significantly above the range observed for uninfected lungs.
FIG. 4.
The peak of virus production in the lungs precedes the peak of infiltration. (A) Kinetics of the infiltration expressed as CD8/CD4 cell ratios documenting the preferential engagement of CD8 T cells. A cumulative plot of results compiled over a period of 2 years from 12 independent but analogous transplantations is shown. Each dot represents one time point of one transplantation. The median values are indicated by dashes. An arrow marks the particular transplantation and time point for which the determination of the ratio is documented as an example in Fig. 3. The shaded region indicates the range of CD8/CD4 cell ratios observed 4 weeks after BMT with no infection. The inset shows the median values of the absolute numbers of CD8 T cells per lung. Ranges are indicated by vertical bars. The shaded region indicates the range of the CD8 T-cell yields obtained 4 weeks after BMT with no infection. (B) Kinetics of virus production in the lungs. Each dot represents the median value of the virus titers of five mice of one transplantation and time point. The median values for independent transplantations are indicated by dashes. DL and dotted line, detection limit of the assay; n.t.: not tested.
Virus production in the lungs preceded the T-cell infiltration and reached its peak after 3 weeks (Fig. 4B). Virus titers declined sharply thereafter in coincidence with the rise in infiltration. In contrast to the slow resolution of the infiltrates, the acute infection of the lungs was cleared between 6 and 8 weeks after infection. In conclusion, we infer from these data that infection of the lungs recruits the CD8 T cells to the lungs and that the infiltrating CD8 T cells then control the pulmonary infection.
Ex vivo cytolytic activity of CD8 T cells in pulmonary infiltrates.
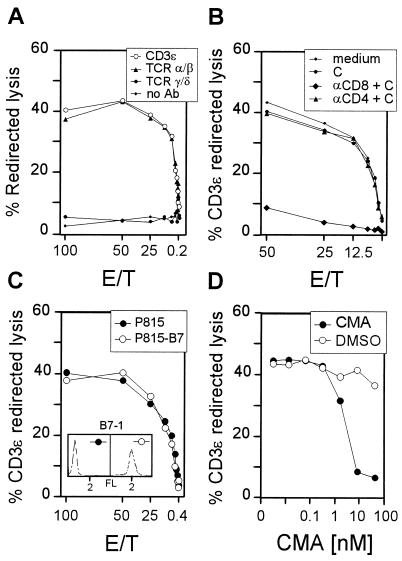
Redirected-lysis assays (28, 53) can serve to monitor the collective cytolytic activity of a polyclonal CTL population for which the antigen specificities are not all known. In essence, in this type of assay, the cytolytic effector phase is triggered by bridging effector cells and target cells via a MAb bound by an Fc receptor on the target cells and directed against a component of the TCR-CD3 complex on the T cells. We used this approach here to determine the total cytolytic activity in the pulmonary infiltrates, which may include CTL specific for virus-encoded or virus-induced peptides as well as CTL activated by the infection but unrelated in specificity. For a representative transplantation, redirected lysis was measured at the peak of CD8 T-cell infiltration, and the phenotype of the effector cells was determined (Fig. 5).
FIG. 5.
Characterization of the pulmonary effector cells of CD3ɛ-redirected lysis. Throughout, pulmonary lymphocytes were isolated from lung infiltrates at 4 weeks after BMT and murine CMV infection. E/T, effector/target cell ratio. (A) Redirected lysis was assayed with P815 target cells carrying Fc receptor-bound antibodies directed against murine CD3ɛ, TCR α/β, or TCR γ/δ. (B) The pulmonary infiltrate cell population was depleted of either CD8- or CD4-positive lymphocytes by treatment with the respective MAbs and complement before the assay of CD3ɛ-redirected lysis. (C) The state of activity of pulmonary lymphocytes was tested by providing B7-1 on the target cells for the B7-CD28 costimulatory interaction. The inset shows the cytofluorometric analysis of B7-1 expression by the P815-B7 transfectant and the absence of B7-1 on parental P815. FL, log10 FITC fluorescence intensity. (D) Sensitivity of CD3ɛ-redirected lysis by pulmonary lymphocytes to concanamycin A (CMA), with P815 as the target (E/T, 100). The solvent dimethyl sulfoxide (DMSO) was titrated as a control.
There is consensus that NK cells do not express membrane CD3, with the exception of CD3ζ (for reviews, see references 29 and 30). We therefore performed the assay with an antibody specifically directed against CD3ɛ. Accordingly, and also because the Fc receptor-expressing P815 mastocytoma cells are resistant to NK cell-mediated lysis, NK cells were not detected. The CD3ɛ-positive T cells located in the inflammatory foci sequestering infected lung cells (Fig. 2) were thus found to include cytolytically active effector cells (Fig. 5A).
Membrane CD3ɛ is expressed by T lymphocytes carrying TCR α/β or TCR γ/δ. Since γ/δ T lymphocytes take part in the late pulmonary immune response to influenza virus (7), the possibility that γ/δ T lymphocytes contribute to the pulmonary immune response to murine CMV had to be considered. Substitution of anti-CD3ɛ MAb with MAbs directed against the respective TCRs in the redirected-lysis assay identified the pulmonary effector cells as T lymphocytes expressing TCR α/β (Fig. 5A). It is important to note that P815 cells did not detect any cytolytic activity in the absence of redirecting antibodies. To discriminate between the CD8 and the CD4 subpopulations of α/β T lymphocytes, which can both be cytolytic, pulmonary T lymphocytes were depleted of either subset before the assay of CD3ɛ-redirected lysis was performed. This approach identified the vast majority of effector cells as CD8-positive T lymphocytes (Fig. 5B). Azuma and colleagues (2, 3) have shown by the approach of redirected lysis that engagement of the CD3-TCR complex on small, resting T cells is insufficient to trigger cytolytic activity, unless target cells are equipped with B7 to permit the B7-CD28 costimulatory signal. By contrast, activated T cells do not require a B7-CD28 interaction to exert a cytolytic effector function. Lysis of B7-negative P815 mastocytoma cells by pulmonary lymphocytes in the assay of CD3ɛ-redirected lysis thus identified the effector cells as activated cells (Fig. 3C). Notably, provision of B7 by using the transfectant P815-B7 as the target did not recruit a higher number of effector cells. This suggests that all pulmonary T lymphocytes represented sufficiently activated cells. Finally, the cytolytic activity proved to be sensitive to concanamycin A (Fig. 5D), which indicates selective usage of the perforin pathway of cytolysis (25). Accordingly, the CD3ɛ-redirected lysis was not enhanced by Fas expressed on transfectant P815-Fas (data not shown). In summary, the lung infiltrates contained mature CTL with the phenotype CD3+ TCR α/β+ CD8+.
CTL specific for a single antigenic viral peptide are detectable in pulmonary infiltrates.
To evaluate the individual contribution of an antigenic viral peptide to the in situ antiviral CTL response, the total CTL activity must be known for use as a reference. Peptide-pulsed or virus-infected target cells do not provide a correct reference value because we do not know all antigenic peptides and because infected cells do not simultaneously present all relevant peptides at any stage in the viral replication cycle. This is particularly true for cells infected with murine or human CMV, since antigenic peptide presentation is modulated by the immune evasion mechanisms of these viruses (19). In addition, the cell type selected as the target cell for the in vitro cytolytic assay is certainly not representative of all the various infectable cell types present in host tissues (36). Therefore, the assay of CD3-TCR-redirected lysis described above currently represents the closest approach to the cytolytic activity of a polyclonal ex vivo CTL population.
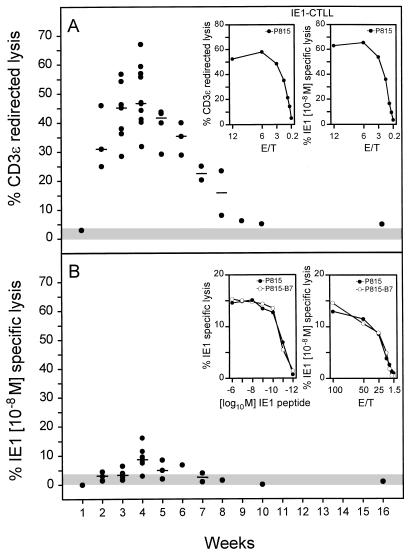
The kinetics of CD3ɛ-redirected lysis in pulmonary infiltrates after BMT and infection (Fig. 6A) essentially paralleled the kinetics of CD8 T-cell infiltration, with the notable difference that the cytolytic activity returned to control levels after clearance of the productive infection (compare Fig. 6A and 4). Thus, cytolytic activity more closely reflects antiviral activity than infiltration does. Notably, throughout the kinetics, pulmonary lymphocytes isolated after BMT from the lungs of uninfected recipients were not cytolytic. These data demonstrate that redirected lysis precisely reflects the antiviral immune response and is a powerful tool for monitoring a cytolytic response in vivo.
FIG. 6.
Cytolytic activity of pulmonary T lymphocytes. (A) Kinetics of cytolytic activity in lung infiltrates as determined by CD3ɛ-redirected lysis for an effector/target cell ratio (E/T) of 100, with P815 mastocytoma cells as targets. Dots represent results compiled from independent but analogous transplantations. The median values are marked by dashes. The shaded area represents the activity measured with pulmonary lymphocytes recovered from lung tissue 4 weeks after BMT with no infection. This activity never exceeded the upper 95% confidence limit of the spontaneous lysis measured in the absence of effector cells. To serve as a positive reference, CD3ɛ-redirected lysis and murine CMV IE1 peptide-specific lysis are compared for an IE1 peptide-specific long-term CTLL in the insets. (B) Kinetics of the IE1 peptide-specific cytolytic activity in lung infiltrates for an E/T of 100. Target cells were P815 pulsed with a saturating concentration [10−8 M] of the synthetic IE1 peptide, a nonapeptide of the sequence YPHFMPTNL. Symbols are as described for panel A. IE1 peptide dose dependence of IE1-specific cytolytic activity of pulmonary effector cells recovered 4 weeks after BMT and infection is shown in the left inset. P815-B7 transfectants and parental P815 served as target cells at an E/T of 100. Titration of the pulmonary effector cells is shown in the right inset. Target cells were pulsed with a saturating concentration [10−8 M] of synthetic IE1 peptide.
In previous work, the IE1 nonapeptide YPHFMPTNL presented by the MHC class I molecule Ld has been classified as an immunodominant antigenic peptide of murine CMV in the host MHC haplotype H-2d (reviewed in reference 26). The pulmonary infiltrates gave us for the first time the opportunity to test the contribution of this particular specificity to the CD8 T-cell response at a relevant site of CMV pathogenesis (Fig. 6B). The IE1 nonapeptide was indeed recognized in most experiments, at least at the peak of the infiltration. This is remarkable because it is the first demonstration of CMV peptide-specific CTL detectable ex vivo without in vitro interleukin-2-mediated expansion or restimulation by antigen. However, the data also make it obvious that recognition of this single peptide accounts only for a minor fraction of the CTL activity in the infiltrates (Fig. 6, compare panels A and B).
That CD3ɛ-redirected lysis is not generally more efficient than peptide-specific lysis is documented for an IE1 peptide-specific CTLL (IE1-CTLL) used as effector cells. Monospecificity of a CTL population is reflected by a congruence between peptide-specific lysis and redirected lysis (Fig. 6A, insets). Pulmonary CTL could be heterogeneous with respect to TCR affinity for the presented IE1 peptide, and hence a target pulsed with a 10−8 M concentration of the peptide, which is a saturating concentration for IE1-CTLL, may not be adequate for all IE1-specific CTL in the polyclonal pulmonary CTL population. However, the IE1-specific lysis by pulmonary infiltrate cells could not be enhanced by pulsing the target cells with higher concentrations of synthetic IE1 peptide (Fig. 6B, left inset). Further, the target recognition by pulmonary CTL might depend on affinity enhancement by accessory interactions or on costimulatory signalling. This was not the case, since expression of B7-1 on the target cells did not cause any enhancement of lysis (Fig. 6B, insets). Finally, a significant contribution of the Fas-Fas ligand pathway inducing apoptosis was excluded by the finding that expression of Fas on P815-Fas transfectants as target cells did not enhance the IE1 peptide-specific activity (data not shown). In conclusion, the pulmonary CTL are not IE1 monospecific but must comprise additional specificities.
Kinetics of IE1 peptide processing in infected lung cells.
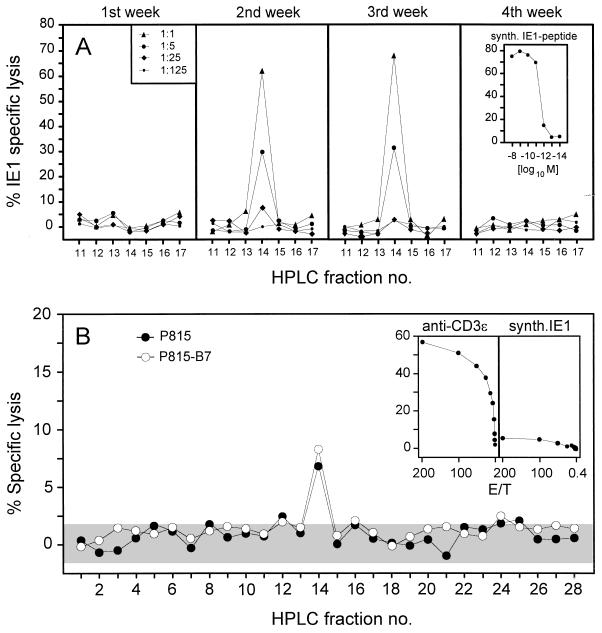
One explanation for the relatively low quantitative representation of IE1 peptide-specific CTL in pulmonary infiltrates could be an insufficient processing of this peptide within infected lung cells. Previous work has shown that virus productivity is not the only requirement for in vivo peptide processing but that gamma interferon (IFN-γ) plays a critical role (16). We addressed this question directly by isolating the peptide from infected lung tissue at weekly intervals after BMT and infection (Fig. 7A). The IE1-CTLL was used as a very sensitive probe capable of detecting the IE1 peptide with a detection limit of 10−12 M (Fig. 7A, right inset). The IE1 peptide was detected in the lung tissue at 2 and 3 weeks, which precisely corresponds to the peak of virus productivity in the lungs (Fig. 4B). This indicates that IFN-γ was not a limiting factor in the lung infiltrates. Accordingly, the lack of detectable peptide in week 4 is explained by the 100-fold decrease in virus productivity after week 3. At the peak of infection, the number of processed IE1 peptides in the lungs, as calculated by comparative titration with the synthetic IE1 nonapeptide as a standard, was ca. 4 × 1010 molecules per whole organ (data not shown). This is a high yield compared with data on the amount of the IE1 peptide in organs described for other conditions of murine CMV infection (16). In conclusion, IE1 antigen processing was not a limiting factor for the CTL response in the lung infiltrates. Most likely, this conclusion also applies to the processing of other potential antigens in the lungs.
FIG. 7.
Antigen processing and presentation in infected lungs. (A) Kinetics of IE1 peptide processing in infected lungs after BMT. Naturally processed peptides were acid extracted from lung tissue and separated by HPLC. The HPLC fractions were used at the dilutions indicated in the left panel for the exogenous peptide pulsing of P815 target cells. The presentation of the IE1 peptide by the targets, and thus the presence of the IE1 peptide in the respective HPLC fraction, was probed with the IE1-specific CTLL at an effector/target cell ratio (E/T) of 10. The sensitivity of the CTLL tested by titration of the synthetic IE1 nonapeptide YPHFMPTNL is shown in the inset. (B) Spectrum of all antigenic peptides, viral and nonviral, presented in the infected lungs and detected by pulmonary effector cells. HPLC fractions (undiluted 0.1 ml thereof) from the separation performed in week 3 after BMT and infection (see panel A) were used to pulse P815 or P815-B7 target cells for testing the specificity of pulmonary CTL that were isolated at the peak of infiltration. The assay was performed at an E/T of 200. The shaded area indicates the 95% confidence limits (two sided) of the spontaneous lysis. The cytolytic activities of the pulmonary CTL in the CD3ɛ-redirected assay and on P815 target cells pulsed with 10−8 M of the synthetic IE1 peptide are shown in the insets.
The IE1 peptide is the only lung-derived antigenic peptide that is detected by pulmonary CTL.
Since the IE1 peptide is efficiently processed in the lungs, its low recognition could reflect an insufficient presentation by lung cells, possibly due to the immune evasion mechanisms operating in CMV-infected cells (19). However, a general deficiency in peptide presentation within pulmonary infiltrates appeared to us unlikely in view of the high CD3ɛ-redirected CTL activity. Accordingly, other antigenic peptides should account for the difference between the high CD3ɛ-redirected CTL activity and the low IE1 peptide-specific CTL activity. These postulated additional antigenic peptides need not necessarily be virus-encoded peptides but could be virus-induced cellular peptides or even self peptides presented by uninfected cells in response to signals from the particular microenvironment of the inflammatory infiltrate. Specifically, cytokines in the infiltrate could lead to a bystander recruitment and activation of CD8 T cells of multiple specificities unrelated to murine CMV. HPLC fractions from lung cell extracts include all antigenic peptides processed in the lungs. Testing these fractions with pulmonary CTL should therefore reveal the presence of further antigenic peptides, regardless of whether these peptides are virus encoded. Surprisingly, pulmonary CTL failed to detect peptides, except for a minute activity in fraction 14, the position at which the IE1 peptide elutes (Fig. 7B). Controls for the activity of the pulmonary CTL used in this experiment included CD3ɛ-redirected activity as well as the activity against target cells pulsed with a saturating concentration of synthetic IE1 peptide (Fig. 7B, insets). Importantly, screening of the HPLC fractions with target P815-B7 did not reveal additional antigenic peptides (Fig. 7B), which is in accordance with the previous conclusion that the pulmonary CD8 T cells were already sufficiently activated and did not require B7-CD28 costimulation.
In conclusion, the immunodominant virus-encoded IE1 peptide did not dominate the pulmonary CTL response in an absolute sense, but it was the only peptide that became individually visible.
Pulmonary CTL recognize infected cells in all phases of the viral replicative cycle.
From the apparent failure in the detection of antigenic peptides except IE1, one could surmise that the strong signalling provided by CD3ɛ cross-linking in the redirected-lysis assay engages functionally irrelevant bystander T cells that are competent for cytolysis but not specific for MHC-presented peptides. Alternatively, the antiviral CTL response could be composed of so many specificities that only the immunodominant IE1 peptide reaches the detection limit of the assay.
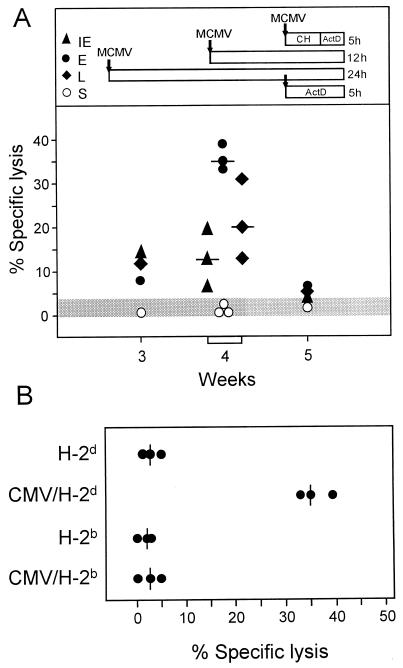
In permissive fibroblasts infected in vitro, the IE1 peptide of murine CMV is not presented during the E phase of the viral replicative cycle (11, 39). As a consequence, if IE1-specific CTL were the only virus-specific effector cells in the pulmonary infiltrates, infected fibroblasts would not be recognized during the E phase. We therefore tested the pulmonary CTL with infected MEF as target cells used in the three main kinetic stages of CMV infection, known as IE phase, E phase, and L phase (41) (Fig. 8A). Target cells infected with the same dose of inoculum virus in the presence of actinomycin D were included in the analysis as a control for antigen presentation caused by exogenous loading of the MHC class I pathway with virion structural (S) proteins in the absence of viral gene expression (41). In accordance with the kinetics of lung infiltration and of CD3ɛ-redirected lysis (Fig. 4A and 6A), lytic activity of pulmonary CTL against virus-infected, syngeneic MEF reached a maximum at 4 weeks after BMT and infection. Notably, viral gene expression was required for recognition, and the infected cells were lysed in all three phases of the viral replicative cycle. Surprisingly, the E-phase target cell proved to be the most susceptible. The E-phase-specific pulmonary effector cells were characterized as CD8-positive T cells (data not shown), which is in accordance with previous phenotyping of E-phase- and L-phase-specific CTL (40).
FIG. 8.
Recognition of infected target cells by pulmonary CTL. (A) Kinetics of cytolytic activity specific for the phases of the viral replicative cycle. Lung infiltrate cells were isolated at the indicated time points after BMT and infection and tested for cytolytic activity against infected MEF at an effector/target cell ratio (E/T) of 200. Data for week 4 are compiled from three analogous transplantations. Median values are indicated by dashes. MEF were infected with 4 PFU* per cell and used for the cytolytic assay in the three phases of viral gene expression: IE phase, cycloheximide (CH; 50 μg/ml) added for 3 h, replaced by actinomycin D (ActD; 5 μg/ml) for 2 h; E phase, no inhibitor for 12 h; L phase, no inhibitor for 24 h. S indicates exogenous loading of virion proteins from inoculum doses of 4 to 40 PFU* per cell, with ActD (5 μg/ml) added for 5 h. The shaded area represents the upper 95% confidence limit of the highest spontaneous lysis, which was that of the late-phase target. (B) MHC restriction of the E-phase-specific lysis. Pulmonary effector cells were recovered 4 weeks after BMT and infection and tested at an E/T of 200 on syngeneic BALB/c (H-2d) and allogeneic C57BL/6 (H-2b) MEF, which were either left uninfected or infected with murine CMV under E-phase conditions.
One could still argue that the pulmonary CTL may lyse the infected cells by an MHC-unrestricted mechanism. We therefore tested the MHC restriction of the E-phase-specific recognition by the pulmonary CTL. It is important to note that BALB/c (H-2d) MEF and C57BL/6 (H-2b) MEF are equally permissive for murine CMV infection (18). Uninfected MEF of either haplotype were not lysed, and lysis of infected MEF was restricted to the syngeneic H-2d haplotype (Fig. 8B).
In conclusion, the pulmonary CTL did recognize infected target cells in an MHC-restricted manner, even though this recognition could not be attributed to detectable antigenic peptides.
DISCUSSION
Interstitial pneumonia is the most critical manifestation of CMV disease in the immunocompromised host after BMT. Understanding the immune response in the lungs is therefore a key to the understanding of CMV pneumonia. We have addressed this medically important issue in a murine model of experimental BMT. The data presented herein have shown a massive infiltration and expansion of CD8 T cells in the infected lungs of survivors. The pulmonary infiltrates were found to provide a microenvironment in which antiviral CD8 T cells mature into cytolytically active effector CTL that can be analyzed for their specificity without any in vitro propagation. We emphasize this because of its novelty in CMV immunology. So far, analysis of CTL specificities to CMV has been limited to the analysis of CTL generated in vitro from sensitized precursors or from memory cells (reviewed in reference 26). Any method of in vitro cultivation unavoidably entails a risk of arbitrary selection and of unspecific activation by lymphokines. This caveat must be taken into account whenever the immunodominance of particular viral proteins is discussed. The use of pulmonary ex vivo CTL gave us a chance to reevaluate own previous conclusions regarding the immunodominance of the murine CMV IE1 protein pp89 and the antigenic peptide derived thereof, the Ld-presented IE1 nonapeptide YPHFMPTNL (13, 42, 44). The specificity analysis of the pulmonary CTL clearly modified our view as far as the contribution of this peptide to the antiviral CTL response in the lungs is concerned. Its contribution is in fact detectable but is low in an absolute sense. However, this finding does not prove previous data wrong. Among the HPLC-separated naturally processed peptides derived from infected lungs, which include viral as well as nonviral peptides, the viral IE1 peptide was found to be the only antigenic peptide that became individually detectable at the peak of the antiviral immune response. This finding emphasizes the relative immunodominance of the IE1 peptide.
To evaluate the quantitative contribution of the IE1 peptide, it was necessary to find a measure of the overall CTL response in the pulmonary infiltrates. We have chosen CD3-TCR complex-redirected lysis, a method that bypasses the requirement of MHC-peptide recognition by triggering cytolytic activity of effector cells via the cross-linking of surface CD3 or TCR (28, 53). The data indicate that CD3ɛ-redirected lysis is a very sensitive and specific means to monitor an in vivo immune response. Notably, there was absolutely no noise activity within the pulmonary lymphocyte population isolated from the lungs of uninfected BMT recipients. Apparently, the CD3ɛ-redirected lysis that was detected in lung infiltrates of infected recipients indeed reflected a response to the infection. Most importantly, CD3ɛ-redirected lysis vanished with the resolution of the productive infection, even though the infiltrates and elevated CD8/CD4 ratios persisted for several weeks after clearance of the virus. Thus, the kinetics of CD3ɛ-redirected lysis describes the time period of antiviral activity more accurately than the infiltration does. The recently described approach of cytofluorometric quantitation of peptide-specific T cells by using soluble tetrameric MHC class I-peptide complexes (1) will undoubtedly be helpful for enumerating the CD8 T cells capable of binding the IE1 peptide. However, as shown by Gallimore et al. (15) and discussed by McMichael and O’Callaghan (33), quantitation of antigen-binding T cells does not necessarily reveal the functional potential of a T-cell population, since precursors as well as exhausted T cells can bind antigen. The contribution of the IE1 peptide to the overall CTL response in the infected lungs is therefore adequately evaluated by the ex vivo functional assays employed here.
The cytolytic effector cells detected by CD3ɛ-redirected lysis were clearly generated in response to the infection, but this does not necessarily imply that they were all specific for viral peptides. Based on the discrepancy between a strong in vivo response to viruses and a low frequency of virus antigen-specific T cells estimated by in vitro limiting dilution assays, it was previously assumed that most of the response observed in vivo resulted from cytokine-mediated bystander activation of T cells with unrelated TCR specificities (57). However, this view was recently revised by a number of reports indicating that the low cloning efficacy in the limiting dilution assays had led to a significant underestimation of the virus-specific response (for an overview and commentary, see reference 33). Specifically, by using T-cell staining with tetrameric MHC class I-peptide complexes, 50 to 70% of the T cells activated by lymphocytic choriomeningitis virus proved to be virus specific at the peak of the in vivo immune response (34), and T cells specific for an immunodominant lytic cycle peptide of Epstein-Barr virus were found to comprise 40% or more of all CD8 T cells in the peripheral blood of individuals with acute infectious mononucleosis (6).
The question of whether CD3ɛ-redirected lysis and MHC-peptide-specific lysis detect effector cells of comparable maturities is of importance for the interpretation of our results. One might argue that a strong signalling provided by the CD3 cross-linking could trigger cytolytic activity in otherwise immature cells and thus lead to an overestimation of the total cytolytic activity in the infiltrates. As a consequence, the contribution of the viral IE1 nonapeptide would be underestimated. However, as shown previously by Azuma et al. (2, 3), the CD3 cross-linking does not trigger a cytolytic effector function in CTL precursors unless the target cells express B7-1 to permit the B7-CD28 costimulatory signal. In our study, the pulmonary CTL did exert their effector function in the absence of the B7-CD28 interaction. Moreover, provision of B7-1 on the target cells did not engage more cells in the CD3ɛ-redirected lysis. This finding indicates that the effector cells in the pulmonary infiltrate represented mature CTL that were independent of costimulatory signals.
Another possible explanation for the difference between CD3ɛ-redirected lysis and MHC-peptide-specific lysis was provided by the work of Zheng and Liu (59) showing that a low-affinity TCR ligand, namely, a peptide derived from the influenza virus nucleoprotein, failed to trigger the cytolytic effector phase unless the target cells expressed B7-1 for affinity enhancement. Accordingly, our CTL assay on B7-negative P815 cells could have missed low-affinity peptide-TCR interactions, whereas CD3ɛ-redirected lysis bypasses this interaction and is therefore independent of TCR ligand affinity. However, this attractive explanation does not apply here, since expression of B7-1 by the target cells did not enhance the IE1 peptide-specific lysis and since no further antigenic peptides were identified with target P815-B7 in the HPLC fractions of naturally processed peptides derived from infected lungs (Fig. 7B).
From the screening of the HPLC fractions, one might conclude that only one antigenic peptide was presented during murine CMV infection, an interpretation that would fit well the immune evasion mechanisms described for this virus (19). However, this is clearly not the case. As a result of the expression of immune evasion genes in the early (E) phase of the viral replication cycle, the IE1 protein is processed but the IE1 peptide is not presented in infected fibroblasts during the E phase (11, 39). However, the same E-phase targets have been recognized by an Ld-restricted CTL clone specific for an antigen encoded in the EcoRI-F fragment of the murine CMV genome (11). In agreement with this previous work, pulmonary CTL were found in this study to recognize E-phase targets in an MHC-restricted manner (Fig. 8). Furthermore, there exist a number of MHC-restricted CTL clones that are specific for peptides generated from murine CMV virion structural proteins by exogenous loading of the class I pathway of antigen processing and presentation (38).
Why is the IE1 peptide immunodominant? For murine CMV, currently known immune evasion genes are expressed in the E phase of the viral replication cycle (10, 56, 60). This offers a plausible explanation for the relative immunodominance of the IE1 nonapeptide that is processed and presented before the expression of these evasion genes. The situation may be different for human CMV. The processing of the 72-kDa IE protein of human CMV appears to be selectively blocked by pp65, an abundant virion matrix protein that enters the cell during virus penetration (17).
The MHC-restricted recognition of infected E-phase target cells and the fact that the IE1 nonapeptide accounts only for a minor fraction of the strong and protective pulmonary CTL response to murine CMV imply a leakiness of immune evasion. The existence of multiple immune evasion mechanisms operating at different steps in the MHC class I pathway of antigen processing and presentation is indeed indicative of a leakiness at least of the mechanisms that operate early in the pathway. Since lysis by CTL can be triggered by a very small amount of presented peptide molecules (8), a minor leakiness of the immune evasion mechanisms might be sufficient for lysis to occur. In addition, enhancement of class I expression by IFN-γ has been shown to counteract immune evasion of murine CMV by overriding the retention of the assembled MHC class I-peptide complex in a cis-Golgi compartment (20). This mechanism is likely to be operative in the infected lungs, since pulmonary infiltrates contain activated CD8 T cells known to be very effective IFN-γ producers.
From all the above evidence and arguments, we propose that the polyclonal antiviral CTL in the lung infiltrates recognize a multitude of subdominant antigenic peptides, which together constitute the strong activity detected in the assay of CD3ɛ-redirected lysis. In the E phase of the viral replication cycle, many viral genes are expressed. Recognition of E-phase target cells in the absence of detectable peptides may thus also reflect a collaborative function involving a group of many subdominant peptides. The presentation of the IE1 peptide precedes the expression of the immune evasion genes of murine CMV. We propose that this “head-start” advantage confers a relative immunodominance to the IE1 nonapeptide.
ACKNOWLEDGMENTS
We thank Milorad Susa and Liane Dreher, Ulm, Germany, for contributions earlier in this project and Thomas Ruppert, Munich, Germany, for advice regarding peptide isolation. The transfectant P815-B7 was generously provided by L. L. Lanier, DNAX, Palo Alto, Calif. S. Jonjic, Rijeka, Croatia, helped by supplying MAb CROMA 101.
This work was supported by grants to M. J. Reddehase by the Deutsche Forschungsgemeinschaft, projects RE 712/3-2 and RE 712/4-1.
REFERENCES
- 1.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotype analysis of antigen-specific T lymphocytes. Science (Washington, DC) 1996;274:94–96. [Google Scholar]
- 2.Azuma M, Cayabyab M, Buck D, Philipps J H, Lanier L L. CD28 interaction with B7 costimulates primary allogeneic proliferative responses and cytotoxicity mediated by small, resting T lymphocytes. J Exp Med. 1992;175:353–360. doi: 10.1084/jem.175.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azuma M, Cayabyab M, Phillips J H, Lanier L L. Requirements for CD28-dependent T cell-mediated cytotoxicity. J Immunol. 1993;150:2091–2101. [PubMed] [Google Scholar]
- 4.Bancroft G J, Shellam G R, Chalmer J E. Genetic influence on the augmentation of natural killer (NK) cells during murine cytomegalovirus infection: correlation with patterns of resistance. J Immunol. 1981;126:988–994. [PubMed] [Google Scholar]
- 5.Bukowski J F, Woda B A, Welsh R M. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J Virol. 1984;52:119–128. doi: 10.1128/jvi.52.1.119-128.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callan M F C, Tan L, Annels N, Ogg G S, Wilson J D K, O’Callaghan C A, Steven N, McMichael A J, Rickinson A B. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carding S R, Allan W, Kyes S, Hayday A, Bottomly K, Doherty P C. Late dominance of the inflammatory process in murine influenza by γδ T cells. J Exp Med. 1990;172:1225–1231. doi: 10.1084/jem.172.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christinck E R, Luscher M A, Barber B H, Williams D B. Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis. Nature (London) 1991;352:67–70. doi: 10.1038/352067a0. [DOI] [PubMed] [Google Scholar]
- 9.Cobbold S P, Jayasuriya A, Nash A, Prospero T D, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature (London) 1984;312:252–254. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 10.Del Val M, Hengel H, Häcker H, Hartlaub U, Ruppert T, Lucin P, Koszinowski U H. Cytomegalovirus prevents antigen presentation by blocking the transport of peptide-loaded major histocompatibility complex class I molecules into the medial-Golgi compartment. J Exp Med. 1992;176:729–738. doi: 10.1084/jem.176.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Val M, Münch K, Reddehase M J, Koszinowski U H. Presentation of CMV immediate-early antigen to cytolytic T lymphocytes is selectively prevented by viral genes expressed in the early phase. Cell. 1989;58:305–315. doi: 10.1016/0092-8674(89)90845-3. [DOI] [PubMed] [Google Scholar]
- 12.Del Val M, Schlicht H-J, Volkmer H, Messerle M, Reddehase M J, Koszinowski U H. Protection against lethal cytomegalovirus infection by a recombinant vaccine containing a single nonameric T-cell epitope. J Virol. 1991;65:3641–3646. doi: 10.1128/jvi.65.7.3641-3646.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Val M, Volkmer H, Rothbard J B, Jonjic S, Messerle M, Schickedanz J, Reddehase M J, Koszinowski U H. Molecular basis for cytolytic T-lymphocyte recognition of the murine cytomegalovirus immediate-early protein pp89. J Virol. 1988;62:3965–3972. doi: 10.1128/jvi.62.11.3965-3972.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrell H E, Vally H, Lynch D M, Fleming P, Shellam G R, Scalzo A A, Davis-Poynter N J. Inhibition of natural killer cells by a cytomegalovirus MHC class I homologue in vivo. Nature (London) 1997;386:510–514. doi: 10.1038/386510a0. [DOI] [PubMed] [Google Scholar]
- 15.Gallimore A, Glithero A, Godkin A, Tissot A C, Plückthun A, Elliot T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geginat G, Ruppert T, Hengel H, Holtappels R, Koszinowski U H. IFN-γ is a prerequisite for optimal antigen processing of viral peptides in vivo. J Immunol. 1997;158:3303–3310. [PubMed] [Google Scholar]
- 17.Gilbert M J, Riddell S R, Plachter B, Greenberg P D. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature (London) 1996;383:720–722. doi: 10.1038/383720a0. [DOI] [PubMed] [Google Scholar]
- 18.Harnett G B, Shellam G R. Variation in murine cytomegalovirus replication in fibroblasts from different mouse strains in vitro: correlation with in vivo resistance. J Gen Virol. 1982;62:39–47. doi: 10.1099/0022-1317-62-1-39. [DOI] [PubMed] [Google Scholar]
- 19.Hengel H, Koszinowski U H. Interference with antigen processing by viruses. Curr Opin Immunol. 1997;9:470–476. doi: 10.1016/s0952-7915(97)80097-0. [DOI] [PubMed] [Google Scholar]
- 20.Hengel H, Lucin P, Jonjic S, Ruppert T, Koszinowski U H. Restoration of cytomegalovirus antigen presentation by gamma interferon combats viral escape. J Virol. 1994;68:289–297. doi: 10.1128/jvi.68.1.289-297.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho M. Cytomegaloviruses. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 1351–1364. [Google Scholar]
- 22.Höglund P, Sundbäck J, Olsson-Alheim M Y, Johansson M, Salcedo M, Öhlen C, Ljunggren H-G, Sentman C L, Kärre K. Host MHC class I gene control of NK-cell specificity in the mouse. Immunol Rev. 1997;155:11–28. doi: 10.1111/j.1600-065x.1997.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 23.Holt P G, Degebrodt A, Venaille T, O’Leary C, Krska K, Flexman J, Farrell H, Shellam G, Young P, Penhale J, Robertson T, Papadimitriou J M. Preparation of interstitial lung cells by enzymatic digestion of tissue slices: preliminary characterization by morphology and performance in functional assays. Immunology. 1985;54:139–147. [PMC free article] [PubMed] [Google Scholar]
- 24.Jones M, Cordell J L, Beyers A D, Tse A G D, Mason D Y. Detection of T and B cells in many animal species using cross-reactive anti-peptide antibodies. J Immunol. 1993;150:5429–5435. [PubMed] [Google Scholar]
- 25.Kataoka T, Shinohara N, Takayama H, Takaku K, Kondo S, Yonehara S, Nagai K. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- 26.Koszinowski U H, del Val M, Reddehase M J. Cellular and molecular basis of the protective immune response to cytomegalovirus infection. Curr Top Microbiol Immunol. 1990;154:189–220. doi: 10.1007/978-3-642-74980-3_8. [DOI] [PubMed] [Google Scholar]
- 27.Koszinowski U H, Reddehase M J, Jonjic S. The role of T-lymphocyte subsets in the control of cytomegalovirus infection. In: Thomas D B, editor. Viruses and the cellular immune response. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 429–445. [Google Scholar]
- 28.Kranz D M, Tonegawa S, Eisen H N. Attachment of an anti-receptor antibody to non-target cells renders them susceptible to lysis by a clone of cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1984;81:7922–7926. doi: 10.1073/pnas.81.24.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanier L L, Corliss B, Phillips J H. Arousal and inhibition of human NK cells. Immunol Rev. 1997;155:145–154. doi: 10.1111/j.1600-065x.1997.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 30.Lanier L L, Spits H, Philipps J H. The developmental relationship between NK cells and T cells. Immunol Today. 1992;13:392–395. doi: 10.1016/0167-5699(92)90087-N. [DOI] [PubMed] [Google Scholar]
- 31.Lathbury L J, Allan J E, Shellam G R, Scalzo A A. Effect of host genotype in determining the relative roles of natural killer cells and T cells in mediating protection against murine cytomegalovirus infection. J Gen Virol. 1996;77:2605–2613. doi: 10.1099/0022-1317-77-10-2605. [DOI] [PubMed] [Google Scholar]
- 32.Mayer A, Podlech J, Kurz S, Steffens H-P, Maiberger S, Thalmeier K, Angele P, Dreher L, Reddehase M J. Bone marrow failure by cytomegalovirus is associated with an in vivo deficiency in the expression of essential stromal hemopoietin genes. J Virol. 1997;71:4589–4598. doi: 10.1128/jvi.71.6.4589-4598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMichael A J, O’Callaghan C A. A new look at T cells. J Exp Med. 1998;187:1367–1371. doi: 10.1084/jem.187.9.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 35.Mutter W, Reddehase M J, Busch F W, Bühring H-J, Koszinowski U H. Failure in generating hemopoietic stem cells is the primary cause of death from cytomegalovirus disease in the immunocompromised host. J Exp Med. 1988;167:1645–1658. doi: 10.1084/jem.167.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Podlech J, Holtappels R, Wirtz N, Steffens H-J, Reddehase M J. Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J Gen Virol. 1998;79:2099–2104. doi: 10.1099/0022-1317-79-9-2099. [DOI] [PubMed] [Google Scholar]
- 37.Rawlinson W D, Farrell H E, Barrell B G. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol. 1996;70:8833–8849. doi: 10.1128/jvi.70.12.8833-8849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddehase M J, Bühring H-J, Koszinowski U H. Cloned long-term cytolytic T-lymphocyte line with specificity for an immediate-early membrane antigen of murine cytomegalovirus. J Virol. 1986;57:408–412. doi: 10.1128/jvi.57.1.408-412.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddehase M J, Fibi M R, Keil G M, Koszinowski U H. Late-phase expression of a murine cytomegalovirus immediate-early antigen recognized by cytolytic T lymphocytes. J Virol. 1986;60:1125–1129. doi: 10.1128/jvi.60.3.1125-1129.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reddehase M J, Keil G M, Koszinowski U H. The cytolytic T lymphocyte response to the murine cytomegalovirus. I. Distinct maturation stages of cytolytic T lymphocytes constitute the cellular immune response during acute infection of mice with the murine cytomegalovirus. J Immunol. 1984;132:482–489. [PubMed] [Google Scholar]
- 41.Reddehase M J, Keil G M, Koszinowski U H. The cytolytic T lymphocyte response to the murine cytomegalovirus. II. Detection of virus replication stage-specific antigens by separate populations of in vivo active cytolytic T lymphocyte precursors. Eur J Immunol. 1984;14:56–61. doi: 10.1002/eji.1830140111. [DOI] [PubMed] [Google Scholar]
- 42.Reddehase M J, Koszinowski U H. Significance of herpesvirus immediate early gene expression in cellular immunity to cytomegalovirus infection. Nature (London) 1984;312:369–371. doi: 10.1038/312369a0. [DOI] [PubMed] [Google Scholar]
- 43.Reddehase M J, Mutter W, Münch K, Bühring H-J, Koszinowski U H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987;61:3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddehase M J, Rothbard J B, Koszinowski U H. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature (London) 1989;337:651–653. doi: 10.1038/337651a0. [DOI] [PubMed] [Google Scholar]
- 45.Reddehase M J, Weiland F, Münch K, Jonjic S, Lüske A, Koszinowski U H. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985;55:264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reusser P, Riddell S R, Meyers J D, Greenberg P D. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–1380. [PubMed] [Google Scholar]
- 47.Reyburn H T, Mandelboim O, Vales-Gomez M, Davis D M, Pazmany L, Strominger J L. The class I homologue of human cytomegalovirus inhibits attack by natural killer cells. Nature (London) 1997;386:514–517. doi: 10.1038/386514a0. [DOI] [PubMed] [Google Scholar]
- 48.Riddell S R, Watanabe K S, Goodrich J M, Li C R, Agha M E, Greenberg P D. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science (Washington, DC) 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 49.Rötzschke O, Falk K, Deres K, Schild H, Norda M, Metzger J, Jung G, Rammensee H-G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature (London) 1990;348:252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- 50.Rötzschke O, Falk K, Wallny H-J, Faath S, Rammensee H-G. Characterization of naturally occurring minor histocompatibility peptides including H-4 and H-Y. Science (Washington, DC) 1990;249:283–287. doi: 10.1126/science.1695760. [DOI] [PubMed] [Google Scholar]
- 51.Scalzo A A, Fitzgerald N A, Simmons A, LaVista A B, Shellam G R. Cmv-1, a genetic locus that controls murine cytomegalovirus replication in the spleen. J Exp Med. 1990;171:1469–1483. doi: 10.1084/jem.171.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shanley J D, Pesanti E L. The relation of viral replication to interstitial pneumonitis in murine cytomegalovirus lung infection. J Infect Dis. 1985;151:454–458. doi: 10.1093/infdis/151.3.454. [DOI] [PubMed] [Google Scholar]
- 53.Spits H, Yssel H, Leeuwenberg J, de Vries J E. Antigen-specific cytotoxic T cell and antigen-specific proliferating T cell clones can be induced to cytolytic activity by monoclonal antibodies against T3. Eur J Immunol. 1985;15:88–91. doi: 10.1002/eji.1830150117. [DOI] [PubMed] [Google Scholar]
- 54.Steffens H-P, Kurz S, Holtappels R, Reddehase M J. Preemptive CD8 T-cell immunotherapy of acute cytomegalovirus infection prevents lethal disease, limits the burden of latent viral genomes, and reduces the risk of virus recurrence. J Virol. 1998;72:1797–1804. doi: 10.1128/jvi.72.3.1797-1804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steffens H-P, Podlech J, Kurz S, Angele P, Dreis D, Reddehase M J. Cytomegalovirus inhibits the engraftment of donor bone marrow cells by downregulation of hemopoietin gene expression in recipient stroma. J Virol. 1998;72:5006–5015. doi: 10.1128/jvi.72.6.5006-5015.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thäle R, Szepan U, Hengel H, Geginat G, Lucin P, Koszinowski U H. Identification of the mouse cytomegalovirus genomic region affecting major histocompatibility complex class I molecule transport. J Virol. 1995;69:6098–6105. doi: 10.1128/jvi.69.10.6098-6105.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tough D F, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science (Washington, DC) 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 58.Winston D J, Ho W G, Champlin R E. Cytomegalovirus infections after bone marrow transplantation. Rev Infect Dis. 1990;12:S776–S792. doi: 10.1093/clinids/12.supplement_7.s776. [DOI] [PubMed] [Google Scholar]
- 59.Zheng P, Liu Y. Costimulation by B7 modulates specificity of cytotoxic T lymphocytes: a missing link that explains some bystander T cell activation. J Exp Med. 1997;186:1787–1791. doi: 10.1084/jem.186.10.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ziegler H, Thäle R, Lucin P, Muranyi W, Flohr T, Hengel H, Farell H, Rawlinson W, Koszinowski U H. A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity. 1997;6:57–66. doi: 10.1016/s1074-7613(00)80242-3. [DOI] [PubMed] [Google Scholar]