Abstract
BACKGROUND:
Providing analgesia and sedation is an essential component of caring for many mechanically ventilated patients. The selection of analgesic and sedative medications during the COVID-19 pandemic, and the impact of these sedation practices on patient outcomes, remain incompletely characterized.
RESEARCH QUESTION:
What were the hospital patterns of analgesic and sedative use for patients with COVID-19 who received mechanical ventilation (MV), and what differences in clinical patient outcomes were observed across prevailing sedation practices?
STUDY DESIGN AND METHODS:
We conducted an observational cohort study of hospitalized adults who received MV for COVID-19 from February 2020 through April 2021 within the Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS) COVID-19 Registry. To describe common sedation practices, we used hierarchical clustering to group hospitals based on the percentage of patients who received various analgesic and sedative medications. We then used multivariable regression models to evaluate the association between hospital analgesia and sedation cluster and duration of MV (with a placement of death [POD] approach to account for competing risks).
RESULTS:
We identified 1,313 adults across 35 hospitals admitted with COVID-19 who received MV. Two clusters of analgesia and sedation practices were identified. Cluster 1 hospitals generally administered opioids and propofol with occasional use of additional sedatives (eg, benzodiazepines, alpha-agonists, and ketamine); cluster 2 hospitals predominantly used opioids and benzodiazepines without other sedatives. As compared with patients in cluster 2, patients admitted to cluster 1 hospitals underwent a shorter adjusted median duration of MV with POD (β-estimate, −5.9; 95% CI, −11.2 to −0.6; P = .03).
INTERPRETATION:
Patients who received MV for COVID-19 in hospitals that prioritized opioids and propofol for analgesia and sedation experienced shorter adjusted median duration of MV with POD as compared with patients who received MV in hospitals that primarily used opioids and benzodiazepines.
Keywords: acute respiratory failure, analgesia, COVID-19, mechanical ventilation, sedation
Analgesia and sedation are important elements of care for many patients receiving invasive mechanical ventilation (MV). Current practice guidelines emphasize the use of analgesics for mechanically ventilated patients, with administration of nonbenzodiazepine sedatives as needed to achieve a light level of sedation.1 This strategy seeks to minimize pain and discomfort while avoiding complications of oversedation, such as prolonged duration of MV and ICU stay, higher rates of tracheostomy placement, and increased occurrence of delirium.2–7
Prior reports suggest that mechanically ventilated patients with COVID-19 have received deeper than recommended levels of sedation,8,9 with high doses or more frequent administration of opioids and benzodiazepines.10–13 However, analgesia and sedation practices for mechanically ventilated patients with COVID-19 remain incompletely characterized. Selecting specific analgesics and sedatives throughout the COVID-19 pandemic likely has been driven by a multitude of factors, including severity of respiratory failure, use of interventions such as prone positioning and neuromuscular blockade (NMB), elevated triglyceride levels among severely ill patients,14 and medication shortages. In this context, unsupervised clustering of hospitals by analgesic and sedative use is an appealing technique to classify sedation practice patterns without prior knowledge of how hospitals generally select from differing medication classes. Therefore, we sought to use hierarchical clustering to categorize hospital analgesic and sedative practices during the COVID-19 pandemic and to compare patient outcomes across common hospital sedation practices.
Study Design and Methods
This study received approval from the Boston University Medical Center Institutional Review Board on April 16, 2021, as not human subjects research (Identifier: H-41486). This article adheres to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines and checklist.
Data Source and Study Population
The Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS) COVID-19 Registry (ClinicalTrials.gov Identifier: NCT04323787) is an observational, international database of patients hospitalized with COVID-19 at 300 participating sites across 27 countries.15,16 The de-identified patient data in this registry were collected from hospital admission until discharge or death using the Research Electronic Data Capture platform.17,18
The study cohort included adult patients (aged ≥ 18 years) with COVID-19 as documented by positive results on a reverse-transcriptase polymerase chain reaction assay who received invasive MV from February 15, 2020, through April 12, 2021. To focus on institutions with higher-quality data reporting, we evaluated only hospitals that completed outcomes data forms for ≥ 80% of patients.19 We then applied patient-level exclusions by removing individual patients with nonresponses in data fields identifying analgesic or sedative use or in the necessary outcome response fields. To improve the reliability of analgesic and sedative measurement, we applied a hospital-level exclusion to remove hospitals that reported use of opioids in less than 5% of patients (because it would be atypical for such a small percentage of patients mechanically ventilated with COVID-19 to be managed without any opioid analgesia8). After the above criteria were applied, we removed any hospitals with fewer than 10 total patients to increase the likelihood that our models yielded stable hospital-level practice estimates.
Exposures
From the daily summary of the analgesic and sedative medications administered to each patient in the VIRUS database, we calculated the percent of patients at each hospital who received each medication of interest. We used hierarchical clustering to group hospitals based on patterns of analgesic and sedative administration (details in Statistical Analysis); the cluster assignment was the primary exposure of interest. Use of clustering allowed us to distill the diverse use of multiple sedatives and analgesics into interpretable practice patterns. We then could describe common sedation strategies and compare the association of prevailing hospital sedation practices with clinical outcomes. Additionally, we used a hospital-level exposure variable to minimize the risk of confounding by indication.20 This type of bias occurs when a patient’s indication for treatment is associated with both the treatment and the outcome. For example, when a mechanically ventilated patient with COVID-19 has concurrent refractory shock, this may be an indication for specific sedative practices (eg, avoidance of propofol), but the shock also is associated with longer duration of MV. Use of an ecologic exposure can mitigate this type of bias because aggregate hospital practices (eg, the proportion of patients in a hospital receiving specific analgesic or sedative medications) are less likely to be driven by patient-specific indications.20
Given the wide variety of clustering approaches, we compared internal validity and stability across clustering methods and determined that hierarchical clustering demonstrated best performance in our data.21 The hierarchical clustering approach begins with each hospital as a unique observation and pairs it with another hospital that has the most similar analgesia and sedation strategy. This process continues iteratively until all observations have been clustered.22
Outcomes
The primary outcome of interest was the duration of MV. Secondary outcomes included ICU length of stay (LOS), hospital LOS, and in-hospital mortality. All outcomes were defined and established a priori. We considered using ventilator-free days by day 28 as the primary outcome. High mortality in our cohort resulted in a distribution of this potential outcome variable with excessive zeros, because death results in assignment of 0 ventilator-free days. Modeling strategies exist to account for excess zeros in count data (eg, zero-inflated Poisson, zero-inflated negative binomial). However, these models were less appropriate for this cohort because they typically fit data that has excess zeros and is right skewed, whereas ventilator-free days in this cohort demonstrated excess zeros, but otherwise was highly left skewed (e-Fig 1). The main benefit of a ventilator-free day outcome in critical care research is to account for the competing risk of death that can bias assessment of duration outcomes,23 so we applied an alternative analytic strategy (detailed herein) that also accounts for competing risk of death.
Covariates
Because hospital cluster (the primary exposure) was assigned based on a hospital’s selection of specific analgesic and sedative agents, we adjusted for variables that may confound the association between analgesic or sedative choice and duration of MV. These included patient factors such as demographics (age, sex, race or ethnicity, BMI), preexisting conditions (Charlson comorbidities,24 smoking status, and alcohol and substance misuse disorders), severity of acute respiratory failure (use of NMB, use of prone positioning, use of inhaled pulmonary vasodilators, initial ventilator mode, and highest Sequential Organ Failure Assessment score25), and timing in the pandemic (early [2/2020–6/2020] vs later [7/2020–4/2021] based on prior reports that hospital practice patterns in COVID-19 medication use became more standardized after 7/202019). We also included hospital-level covariates of geographic location, a database response field indicating that the hospital was experiencing resource limitations during the patient’s hospitalization (yes vs no; degree or type of resource limitation otherwise unspecified), ICU type (medical, surgical, mixed medical-surgical), ICU nurse to patient ratio (1:1 vs 2:1), total ICU beds in the hospital, and presence of residents or fellows in the ICU (as a surrogate for academic status).
Patient data were missing for variables of Sequential Organ Failure Assessment score and pandemic timing in the final cohort (percent missing shown in Table 1), whereas hospital data were missing for variables of ICU type, ICU nurse to patient ratio, total ICU beds, and presence of residents or fellows (percent missing shown in Table 2). Our primary model included all patient covariates. Notable differential missingness was present in hospital factors (with most data missing from cluster 2 hospitals), so we included only geographic location and lack of hospital resources in our primary model. We then performed a sensitivity analysis including all patient and hospital covariates.
TABLE 1.
Baseline Patient Characteristics Stratified by Sedation Cluster
| Characteristic | Total (N = 1,313) | Sedation Cluster 1 (Opioid and Propofol Predominant; n = 1,060)a | Sedation Cluster 2 (Opioid and Benzodiazepine Predominant; n = 253)a |
|---|---|---|---|
|
| |||
| Age, y | 63 (54–72) | 62 (53–72) | 66 (57–74) |
| Sex, female | 451 (34) | 362 (34) | 89 (35) |
| Race | |||
| White | 536 (41) | 413 (39) | 123 (49) |
| Black | 313 (24) | 233 (22) | 80 (32) |
| Asian | 137 (10) | 124 (12) | 13 (5) |
| Multiracial or other race | 327 (25) | 290 (27) | 37 (15) |
| Hispanic ethnicity | 218 (17) | 181 (17) | 37 (15) |
| BMI, kg/m2 | |||
| Obese (≥ 30) | 616 (47) | 511 (48) | 105 (42) |
| Not obese (< 30) | 642 (49) | 506 (48) | 136 (54) |
| Unknown | 55 (4) | 43 (4) | 12 (5) |
| Charlson comorbidity score | 3 (1–4) | 3 (1–4) | 3 (2–4) |
| Active tobacco use | 69 (5) | 63 (6) | 6 (2) |
| Alcohol misuse disorder | 52 (4) | 49 (5) | 3 (1) |
| Substance misuse disorder | 26 (2) | 24 (2) | 2 (1) |
| Received neuromuscular blockade | 868 (66) | 725 (68) | 143 (57) |
| Underwent prone positioning | 686 (52) | 590 (56) | 96 (38) |
| Received inhaled pulmonary vasodilator | 587 (45) | 497 (47) | 90 (36) |
| Initial ventilator mode | |||
| Volume control | 802 (61) | 635 (60) | 167 (66) |
| Pressure control | 122 (9) | 98 (9) | 24 (10) |
| Other | 389 (30) | 327 (31) | 62 (25) |
| Maximum SOFA scoreb | 9 (6–11) | 8 (6–11) | 9 (6–12) |
| Pandemic timingc | |||
| Early (February 2020-June 2020) | 672 (54) | 511 (50) | 161 (74) |
| Late (July 2020-April 2021) | 570 (46) | 513 (50) | 57 (26) |
Data are presented as No. (%) or median (interquartile range). Percentages do not all sum to 100% because of rounding. SOFA = Sequential Organ Failure Assessment.
See Table 3 for full description of analgesia and sedation practices within clusters.
Missing for 28% of patients.
Missing for 5% of patients.
TABLE 2.
Baseline Hospital Characteristics Stratified by Sedation Cluster
| Characteristic | Total (N = 35 Hospitals) | Sedation Cluster 1 (Opioid and Propofol Predominant; n = 27 Hospitals)a | Sedation Cluster 2 (Opioid and Benzodiazepine Predominant; n = 8 Hospitals)a |
|---|---|---|---|
|
| |||
| Geographic site | |||
| United States | 25 (71) | 21 (78) | 4 (50) |
| International | 10 (29) | 6 (22) | 4 (50) |
| Lack of hospital resources during hospitalization | 9 (26) | 4 (50) | 5 (19) |
| ICU typeb | |||
| Medical ICU | 9 (38) | 9 (41) | 0 (0) |
| Mixed medical-surgical ICU | 7 (29) | 6 (27) | 1 (50) |
| Dedicated COVID-19 ICU | 8 (33) | 7 (32) | 1 (50) |
| ICU nurse to patient ratiob | |||
| 1:1 | 2 (8) | 2 (9) | 0 (0) |
| 1:2 | 20 (83) | 18 (82) | 2 (100) |
| 1: > 2 | 1 (4) | 1 (5) | 0 (0) |
| Other | 1 (4) | 1 (5) | 0 (0) |
| Total ICU bedsb | 25 (16–36) | 24 (16–36) | 40 (30–50) |
| Trainees (residents or fellows) present in the ICUb | 17 (71) | 15 (68) | 2 (100) |
Data are presented as No. (%) or median (interquartile range). Percentages do not all sum to 100% because of rounding.
See Table 3 for full description of analgesia and sedation practices within clusters.
Missing for 31% of hospitals (28% of patients).
We used multiple imputation with chained equations to impute missing data.26 We generated 20 imputed data sets, built regression models in each data set, and pooled effect estimates with the MIANALYZE procedure in SAS (SAS Institute Inc.) to yield primary findings. We conducted a sensitivity analysis using only patients with complete data to evaluate the robustness of findings to assumptions about missingness.
Statistical Analysis
Patient and hospital characteristics were summarized using median (interquartile range [IQR]) for continuous variables and No. (%) for categorical variables. For each hospital, we identified the percentage of patients who ever received propofol, ketamine, opioids (ie, fentanyl, hydromorphone, morphine, or remifentanil), benzodiazepines (ie, lorazepam or midazolam), alpha-agonists (ie, dexmedetomidine or clonidine), and any other sedative or analgesic agent (ie, a combination of pentobarbital plus a response field designated as “other sedative/analgesic”) during the period of MV. We used agglomerative hierarchical clustering with a complete-linkage criterion to cluster hospitals based on the percentage of patients receiving each analgesic and sedative medication or class.22 Optimal number of clusters was determined by a statistical package that evaluates 30 indexes to identify the best scheme across varying combinations of number of clusters, distance measures, and clustering methods.27 After clustering, we described practice patterns of analgesic and sedative use across groups.
We performed multivariable regression analyses to determine the association between sedation-cluster assignment and patient-level outcomes while adjusting for patient-level and facility-level covariates. Because shorter durations of MV and LOS are considered more desirable outcomes, but death shortens observed durations of MV and LOS through an undesirable outcome, we used a placement of death (POD) approach28,29 that assigns patients who die a duration of MV, hospital LOS, and ICU LOS equal to the 99th percentile of each outcome in the cohort. Median regression analysis was used to evaluate the association between sedation cluster and POD duration outcomes, and logistic regression analysis was used to evaluate the association between cluster and mortality. The β-coefficients from median regression represent the expected change in the median of each outcome when comparing a cluster to the reference group, with deaths considered a specific undesirable duration or LOS. We calculated E-values for each outcome, which quantify the strength of association between a theoretical unmeasured confounder, sedation cluster assignment, and outcome that would be needed to move effect estimates to the null.30
Although median regression represented a preferred analytic approach based on the distribution of the duration outcomes, procedures that allow for inclusion of random intercepts within median regression are not developed fully. To evaluate the potential impact of not including hospital of admission as a random intercept, we performed a sensitivity analysis in which we modeled the primary outcome in two distinct linear regression models that did and did not include hospital of admission as a random intercept. We then compared effect estimates and calculated the intraclass correlation coefficient to demonstrate the amount of total variation in the model attributable to the random intercept.
Separately, to explore the relative contribution of mortality and duration of MV when using a POD approach, we conducted additional analyses including (1) logistic regression with hospital of admission as a random intercept and mortality as the outcome and (2) median regression analysis among survivors only with duration of MV as the outcome. Cluster analysis was performed in R version 4.1.2 software (R Foundation for Statistical Computing). All other analyses were completed in SAS version 9.4 software (SAS Institute Inc.).
Results
Study Population
We identified 5,156 adults with SARS-CoV-2 infection who received invasive MV at database hospitals without systemic reporting inefficiencies. After applying exclusion criteria, the final cohort included 1,313 patients across 35 hospitals (Fig 1). Patients had a median age of 63 years (IQR, 54–72 years), 451 patients (34%) were female, 52 patients (4%) had preexisting alcohol misuse disorder, and 26 patients (2%) had preexisting substance misuse disorder. The number of patients each hospital contributed to the analysis ranged from 11 to 179, with most hospitals contributing 15 to 40 patients (e-Table 1).
Figure 1.
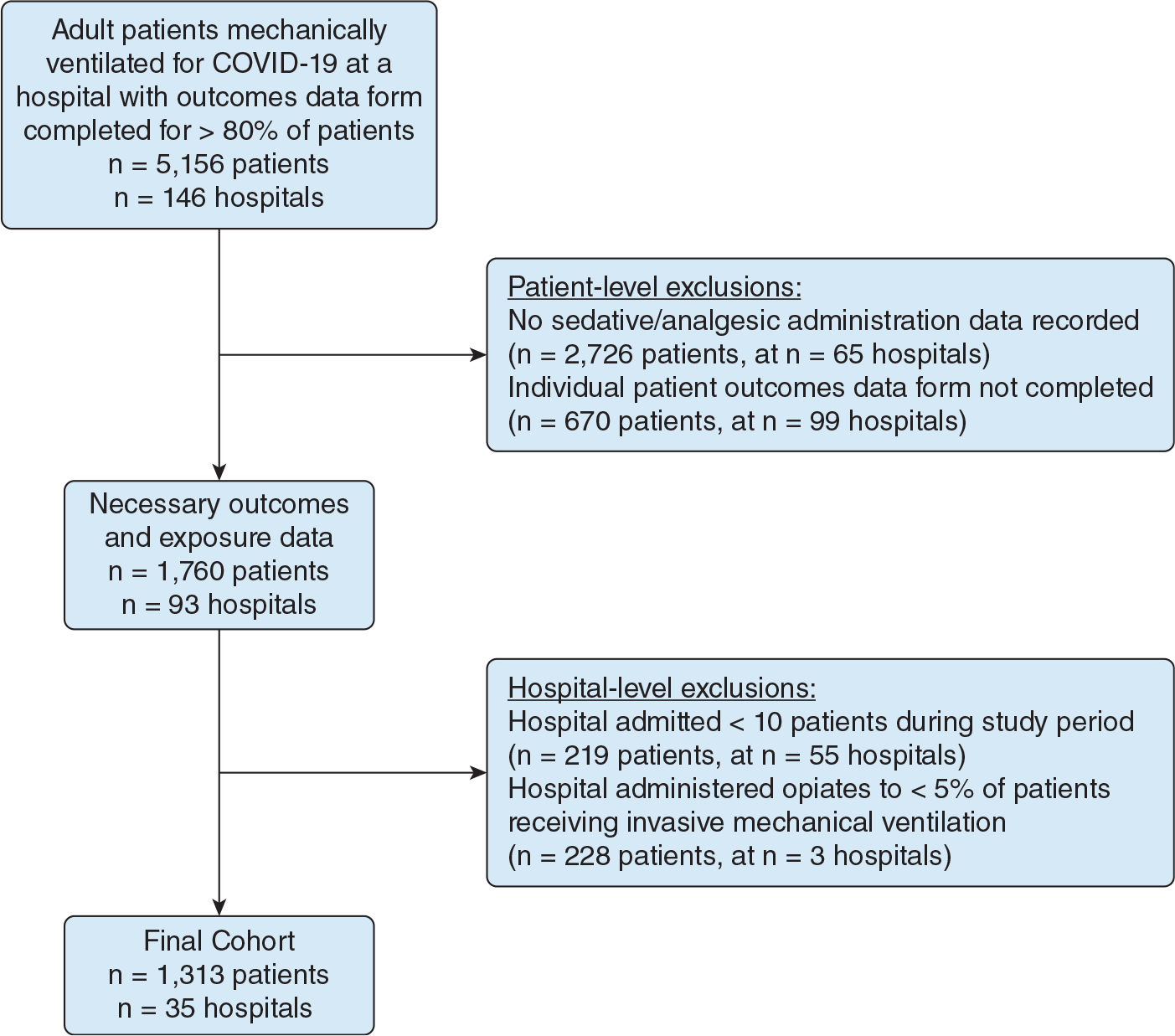
Study flow diagram showing cohort assembly of adult patients with a diagnosis of COVID-19 who received invasive mechanical ventilation.
Sedation Practices by Cluster
The optimal number of hospital clusters based on assessment of hospital-level sedative and analgesic use rates was two (e-Tables 2, 3). After applying hierarchical clustering, 27 hospitals were in cluster 1 and eight hospitals were in cluster 2. Visual representations of the clustering are shown in e-Figure 2.
Tables 1 and 2 show patient and facility characteristics stratified by sedation strategy. As compared with patients in cluster 2 hospitals, patients in cluster 1 more often were identified as Asian or multiracial; were more likely to receive prone positioning, NMB, or inhaled pulmonary vasodilators; and were more likely to be cared for early in the pandemic. Cluster 1 hospitals more often were located in the United States, were more likely to have medical-specific ICUs and experience resource limitations, had fewer ICU beds overall, and were less likely to have trainees present as compared with cluster 2 hospitals. Characteristics were otherwise similar across clusters.
Most patients in both hospital clusters (n = 986 [93%] in cluster 1 vs n = 213 [84%] in cluster 2) received at least one analgesic or sedative agent during the period of MV. Comparable rates of opioid administration were found, with 915 patients (86%) in cluster 1 and 180 patients (71%) in cluster 2 receiving opioids. Rates of specific sedatives and sedative classes are shown in Table 3. For patients admitted to cluster 1 hospitals, propofol was the most commonly used sedative (82% of patients), followed by benzodiazepines (48%), alpha-agonists (34%), and ketamine (23%). Conversely, in cluster 2, benzodiazepines (68%) were the predominant sedative class, followed by propofol (40%). Alpha-agonists (4%) and ketamine (1%) were administered rarely to cluster 2 patients. Hospitals within each cluster showed similar medication use rates (e-Table 4).
TABLE 3.
Percent of Patients Receiving Sedative or Analgesic Medications Stratified by Cluster
| Medication | Total (N = 1,313) | Sedation Cluster 1 (n = 1,060) | Sedation Cluster 2 (n = 253) |
|---|---|---|---|
|
| |||
| Opioidsa | 1,095 (83) | 915 (86) | 180 (71) |
| Benzodiazepinesb | 686 (52) | 514 (48) | 172 (68) |
| Propofol | 969 (74) | 869 (82) | 100 (40) |
| Alpha-agonistsc | 374 (28) | 365 (34) | 9 (4) |
| Ketamine | 242 (18) | 239 (23) | 3 (1) |
| Other sedationd | 180 (14) | 150 (14) | 30 (12) |
| At least 1 sedative or analgesic medication | 1,199 (91) | 986 (93) | 213 (84) |
Data are presented as No. (%).
Includes fentanyl, hydromorphone, morphine, or remifentanil.
Includes lorazepam or midazolam.
Includes dexmedetomidine or clonidine.
Includes pentobarbital or a selection of “other” in sedative data response field.
Patient Outcomes
The median duration of MV for all patients analyzed was 10 days (IQR, 5–18 days). Patients were hospitalized for a median of 18 days (IQR, 11–30 days) and were cared for in the ICU for a median of 13 days (IQR, 7–22 days). In the primary adjusted model (Table 4), admission to a hospital in sedation cluster 1 was associated with a decrease in median duration of MV with POD (β-estimate, −5.9 days; 95% CI, −11.2 to −0.6 days; P = .03; E-value, 2.1). Also, significantly shorter hospital LOS with POD (b-estimate, −8.5 days; 95% CI, −16 to −1 days; P = .03; E-value, 2.2) and ICU LOS with POD (β-estimate, −7.1 days; 95% CI, −12.4 to −1.7 days; P = .01; E-value, 2.4) were found. Mortality was lower in cluster 1 (50%) as compared with cluster 2 (56%; OR, 0.4; 95% CI, 0.2–0.9; P = .03; E-value, 2.5).
TABLE 4.
Association Between Admission to Hospitals Using Predominantly Opioids and Propofol for Analgesia and Sedation (vs Hospitals Using Opioids and Benzodiazepines) and Outcomes in Primary Analysis
| Outcome | Adjusted β-Coefficient (95% CI)a | P Value |
|---|---|---|
|
| ||
| Duration of MV with POD, d | −5.9 (−11.2 to −0.6) | .03 |
| Hospital LOS with POD, d | −8.5 (−16 to −1) | .03 |
| ICU LOS with POD, d | −7.1 (−12.4 to −1.7) | .01 |
|
| ||
| Adjusted OR (95% CI)a | ||
|
| ||
| Mortality | 0.4 (0.2–0.9) | .03 |
LOS = length of stay; MV = mechanical ventilation; POD = placement of death.
Adjusted for demographics (age, sex, race or ethnicity), BMI, preexisting comorbidities (Charlson comorbidity score, alcohol misuse disorder, substance misuse disorder), maximum Sequential Organ Failure Assessment score, initial ventilator mode, additional therapies (use of neuromuscular blockade, prone positioning, inhaled pulmonary vasodilators), pandemic timing during hospitalization, and facility factors (hospital site location, lack of hospital resources during hospitalization).
Findings from the sensitivity analyses (1) using complete case data and (2) including all hospital-level covariates using multiple imputation to address missingness were comparable with those from the primary analysis (Table 5). In sensitivity analyses using a linear regression model to evaluate the potential impact of including hospital of admission as a random effect, we found an intraclass correlation coefficient of 0.02, demonstrating that the random effect of the hospital accounts for only 2% of the total variation seen in the model. Additionally, when building the same model without the random intercept, minimal change in magnitude and direction of effect estimates was found (e-Table 5), although SEs were expectedly larger in models with hospital random intercepts. In sensitivity analysis among only survivors without a POD approach, no statistically significant association was found between sedation cluster assignment and duration of MV (β-estimate, −0.6 days; 95% CI, −3.6 to 2.4 days; P = .7).
TABLE 5.
Association Between Admission to Hospitals Using Predominantly Opioids and Propofol for Analgesia and Sedation (vs Hospitals Using Opioids and Benzodiazepines) and Outcomes in Sensitivity Analyses
| Outcome | Adjusted β-Coefficient or OR (95% CI) | P Value |
|---|---|---|
|
| ||
| Adjusted models using complete cases only | ||
| Duration of MV with POD, d | −6.4 (−13.1 to 0.3)a,b | .06 |
| Hospital LOS with POD, d | −8.7 (−17.8 to 0.4)a,b | .06 |
| ICU length of stay with POD, d | −6.1 (−13 to 0.9)a,b | .09 |
| Mortality | 0.4 (0.2–1.2)a,c | .1 |
| Adjusted models including all hospital-level covariates and using multiple imputation to address missing values | ||
| Duration of MV with POD, d | −3.9 (−9.8 to 2.0)b,d | .19 |
| Hospital LOS with POD, d | −7.0 (−15.0 to 0.9)b,d | .08 |
| ICU LOS with POD, d | −4.4 (−10.4 to 1.7)b,d | .16 |
| Mortality | 0.4 (0.2–0.98)a,c | .04 |
LOS = length of stay; MV = mechanical ventilation; POD = placement of death.
Adjusted for demographics (age, sex, race or ethnicity), BMI, preexisting comorbidities (Charlson comorbidity score, alcohol misuse disorder, substance misuse disorder), maximum Sequential Organ Failure Assessment score, initial ventilator mode, additional therapies (use of neuromuscular blockade, prone positioning, inhaled pulmonary vasodilators), pandemic timing during hospitalization, and facility factors (hospital site location, lack of hospital resources during hospitalization).
β-coefficient.
OR.
Adjusted for all covariates detailed in the table, as well as additional facility factors of ICU type, ICU nursing to patient ratio, total ICU beds, and presence of trainees (residents or fellows in the ICU).
Discussion
In this study of analgesia and sedation practice patterns for adult patients with COVID-19 receiving invasive MV, we found that hospital medication preferences fell broadly into categories of (1) opioids plus propofol, with smaller percentages of patients receiving benzodiazepines, alpha-agonists, and ketamine; or (2) opioids plus benzodiazepines, with smaller percentages of patients receiving propofol and very rare use of any additional agents. This variation in practice was associated with clinical outcomes, with hospitals that predominantly administered opioids plus propofol achieving shorter median duration of MV with POD, shorter median hospital and ICU LOS with POD, and lower mortality.
Findings should be considered in the context of evidence-based guidelines for analgesia and sedation. The Society of Critical Care Medicine Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility and Sleep Disruption in Adult Patients in the ICU state that “pain should be treated before a sedative agent is considered.”1 Hospitals seem to incorporate analgesia regularly into the care of mechanically ventilated patients with COVID-19; our exclusion of hospitals in which < 5% of patients received opioids (out of concern for unreliable data entry) removed only three of 38 hospitals, and 83% of all patients in the final cohort received opioids. Additionally, the Pain, Agitation/Sedation, Delirium, Immobility and Sleep Disruption guidelines recommend the use of either propofol or dexmedetomidine over benzodiazepines for sedation of critically ill mechanically ventilated adults. Our findings align with prior data demonstrating that use of nonbenzodiazepine sedation strategies optimizes patient outcomes.31,32
While redemonstrating the benefits of nonbenzodiazepine sedation, our study simultaneously found that nearly one-quarter of hospitals used primarily benzodiazepines for sedation. Uptake for many evidence-based practices in the ICU remains low,33,34 and findings of this study could be indicative of widespread evidence-to-practice gaps in sedation of mechanically ventilated patients. Multiple elements of the COVID-19 pandemic may have impacted decisions to use particular analgesics and sedatives, including high rates of patient-ventilator dyssynchrony, frequent use of prone positioning and NMB elevated triglyceride levels,14 staffing shortages, and medication shortages. Reviewing facility-level characteristics across clusters in this study suggests that hospitals with medical ICUs (as opposed to mixed or COVID-specific ICUs), fewer total ICU beds, and absence of trainees in the ICU tend to apply the preferred nonbenzodiazepine strategy. Geographic practice trends also may be present, because a higher percentage of cluster 2 (benzodiazepine-predominant) hospitals were located internationally. Future studies examining how these factors—among other hospital-level characteristics—may promote differing analgesia and sedation strategies ultimately could inform implementation efforts seeking to standardize sedation care around evidence-based practices.
Because of the high overall mortality in this cohort (51%), use of a POD approach in our analyses resulted in most patients being assigned the 99th percentile duration of MV, hospital LOS, and ICU LOS. This methodologic approach mitigates the competing risk of death that often hampers the reliability of so-called duration of outcomes35 and is akin to a composite outcome that incorporates mortality and duration of MV. In the context of this composite outcome, we sought to characterize further the relative contribution of mortality to our primary findings. We conducted a multivariable logistic regression analysis that showed decreased risk of mortality among patients treated in cluster 1 hospitals, as well as a sensitivity analysis among only survivors that demonstrated no association between sedation cluster and duration of MV. Acknowledging the limitation of the latter analysis (a notably smaller sample size because of high mortality rates resulting in lower statistical power, as well as possible selection bias), the findings from these two additional analyses suggest that our primary findings likely are driven primarily by differences in mortality between groups.
Notable strengths of this study included a large cohort of mechanically ventilated patients with laboratory-confirmed COVID-19 across a range of hospitals based in the United States and internationally. Sedative practices and patient outcomes during the COVID-19 pandemic likely were impacted by the severity of respiratory failure and supply chain issues, and we addressed these factors by controlling for patient covariates related to acuity of illness and concurrent interventions to treat refractory hypoxemia, as well as facility covariates related to lack of resources at the admitting hospital, pandemic timing at time of admission, and geographic location. The use of a POD approach for duration outcomes mitigates the risk of the competing risk of death, a frequent challenge in observational studies evaluating critical care outcomes. Multiple sensitivity analyses evaluating a priori decisions regarding the primary modeling approach and handling of missing data demonstrated comparable findings to our primary outcome.
The study has potential limitations. Data did not allow us to evaluate the total dose of analgesics and sedatives administered to patients, nor the dosing frequency (eg, continuous infusion vs intermittent bolus), and therefore we cannot completely characterize approaches to analgesic and sedative use. A substantial number of patients were removed from the cohort because of lack of responses in data fields for the primary exposure or outcome. Removal of these patients could have impacted results and may limit generalizability of findings, but patients excluded from the final analytic cohort showed similar demographics, comorbidities, and severity of illness as those included (e-Table 6). Separately, exclusion of hospitals for limited site-level data reporting and fewer than 10 patients meeting inclusion criteria may have affected hospital clustering, primary findings, or both, although these approaches have been applied in prior work within the VIRUS database.19,36 We removed hospitals with very low percentage (< 5%) of mechanically ventilated patients receiving any opioid, which could affect our characterization of sedation practices. However, prior studies among patients with COVID-19 receiving MV demonstrated that opioid analgesia is administered widely (80% of patients received opioid infusions),8 suggesting that such a low percentage of opioid use could represent a limitation in data collection, rather than a description of true practice. Also heterogenous sedation practices may be in place among hospitals outside of the United States, labeled together as international, that could impact findings. The multiple imputation process used to handle missingness is built on the assumption that data are missing at random, which may not be the case in our data. However, multiple imputation is a commonly used technique,26,37–39 and primary findings were comparable across alternative methods of addressing missingness. Different methods of clustering hospitals potentially could affect findings, but we selected the approach that optimized internal validity and stability when clustering our data. Although we accounted for factors at the patient and hospital level that may confound the association between hospital sedation practices and patient outcomes, we were unable to assess other potential confounders (eg, triglyceride measurements, depth of sedation measured by Richmond Agitation Sedation Scale,40,41 or other validated sedation scoring system). However, E-values demonstrated that any theoretical unmeasured confounders would need β-coefficients of 2.1 to 2.4 for duration outcomes or an OR of 2.5 for mortality to move primary effect estimates to the null.
Interpretation
Among mechanically ventilated patients with COVID-19, treatment in hospitals that predominantly used opioids and propofol for analgesia and sedation was associated with a shorter median duration of MV with POD as compared with treatment in hospitals that used primarily opioids and benzodiazepines.
Supplementary Material
Take-home Points.
Study Question:
What analgesic and sedative medications were administered to mechanically ventilated patients with COVID-19, and how did these sedation practices impact patient outcomes?
Results:
Among mechanically ventilated adults with COVID-19, treatment in hospitals that administer predominantly opioids and propofol for analgesia and sedation resulted in shorter duration of mechanical ventilation as opposed to treatment in hospitals using predominantly opioids and benzodiazepines.
Interpretations:
Use of benzodiazepine-predominant sedation strategies persists at some hospitals despite consistent evidence for nonbenzodiazepine approaches; further studies should continue to characterize factors influencing sedation practice ultimately to design interventions that promote evidence-based sedation care.
Funding/Support
This study was supported by the National Center for Research Resources and National Center for Advancing Translational Sciences Clinical and Translational Science Awards, National Institutes of Health [Grant UL1 TR002377 and NIH R01HL151607 (A.J.W)]. The registry is funded in part by the Gordon and Betty Moore Foundation and Janssen Research & Development, LLC. O. G. receives funding from the Agency of Healthcare Research and Quality [Grant R18HS 26609-2]; the National Heart, Lung, and Blood Institute, National Institutes of Health [Grants R01HL 130881 and UG3/UH3HL 141722]; the US Department of Defense [Grant W81XWH]; and the American Heart Association [Rapid Response Grant—Coronavirus Disease 2019]. R. K. receives funding from the National Heart, Lung, and Blood Institute, National Institutes of Health [Grants R01HL 130881 and UG3/UH3HL 141722]; the Gordon and Betty Moore Foundation; and Janssen Research & Development, LLC. A. J. W. receives funding from the National Heart, Lung and Blood Institute, National Institutes of Health [Grants R01HL151607, R01HL139751, and R01HL136660]; the Agency of Healthcare Research and Quality [Grant R01HS026485]; and Boston Biomedical Innovation Center, the National Heart, Lung and Blood Institute, National Institutes of Health [Grant 5U54HL119145-07].
Role of sponsors:
The sponsor had no influence on analysis, interpretation, or reporting of pooled data.
ABBREVIATIONS:
- IQR
interquartile range
- LOS
length of stay
- MV
mechanical ventilation
- NMB
neuromuscular blockade
- POD
placement of death
Footnotes
Collaborators from the Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study COVID-19 Registry Investigator Group appear in e-Appendix 1.
Disclaimer: The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Additional information: The e-Appendix, e-Figures, and e-Tables are available online under “Supplementary Data.”
Financial/Nonfinancial Disclosures
The authors have reported to CHEST Critical Care the following: O. G. receives royalties from Ambient Clinical Analytics. R. K. receives royalties from Ambient Clinical Analytics. A. J. W. receives royalties from UptoDate. None declared (J. M. R., A. C. L., S. B., E. K. Q., M. A. G., K. B., S. Y., V. M. G., V. K.).
Contributor Information
Justin M. Rucci, Pulmonary Center, Department of Medicine, Boston University Chobanian & Avedisian School of Medicine; Center for Healhcare Organization and Implementation Research, VA Boston Healthcare System.
Anica C. Law, Pulmonary Center, Department of Medicine, Boston University Chobanian & Avedisian School of Medicine.
Scott Bolesta, Department of Pharmacy Practice, Nesbitt School of Pharmacy, Wilkes University, Wilkes-Barre, PA.
Emily K. Quinn, Biostatistics and Epidemiology Data Analytics Center, Boston University School of Public Health, University of Massachusetts Chan School of Medicine, Worcester MA.
Michael A. Garcia, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of Washington Medicine Valley Medical Center, Renton, WA.
Ognjen Gajic, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Mayo Clinic, Rochester, MN.
Karen Boman, Society of Critical Care Medicine, Mount Prospect, IL.
Santiago Yus, Department of Intensive Care Medicine, La Paz University Hospital, Madrid, Spain.
Valerie M. Goodspeed, Department of Anesthesia, Critical Care and Pain Medicine, Beth Israel Deaconess Medical Center, Boston, University of Massachusetts Chan School of Medicine, Worcester MA.
Vishakha Kumar, Society of Critical Care Medicine, Mount Prospect, IL.
Rahul Kashyap, Department of Anesthesia and Perioperative Medicine, Mayo Clinic, Rochester, MN.
Allan J. Walkey, Division of Health Systems Science, Department of Medicine, University of Massachusetts Chan School of Medicine, Worcester MA.
References
- 1.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. [DOI] [PubMed] [Google Scholar]
- 2.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000. May;342(20):1471–1477. [DOI] [PubMed] [Google Scholar]
- 3.Treggiari MM, Romand JA, Yanez ND, et al. Randomized trial of light versus deep sedation on mental health after critical illness*. Crit Care Med. 2009;37(9):2527–2534. [DOI] [PubMed] [Google Scholar]
- 4.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008. Jan 12;371(9607):126–134. [DOI] [PubMed] [Google Scholar]
- 5.Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous IV sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114(2):541–548. [DOI] [PubMed] [Google Scholar]
- 6.Anderson A Sedation intensity in the first 48 hours of mechanical ventilation and 180-day mortality: a multinational prospective longitudinal cohort study. J Emerg Med. 2018;55(3):454. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka LMS, Azevedo LCP, Park M, et al. Early sedation and clinical outcomes of mechanically ventilated patients: a prospective multicenter cohort study. Crit Care. 2014;18(4):R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pun BT, Badenes R, Heras La Calle G, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021;9(3):239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanidziar D, Bittner EA. Sedation of mechanically ventilated COVID-19 patients: challenges and special considerations. Anesth Analg. 2020;131(1):e40–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapp CM, Zaeh S, Niedermeyer S, Punjabi NM, Siddharthan T, Damarla M. The use of analgesia and sedation in mechanically ventilated patients with COVID-19 acute respiratory distress syndrome. Anesth Analg. 2020;131(4):e198–e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapaskar N, Colon Hidalgo D, Koo G, et al. Sedation usage in COVID-19 acute respiratory distress syndrome: a multicenter study. Ann Pharmacother. 2022;56(2):117–123. [DOI] [PubMed] [Google Scholar]
- 12.Wongtangman K, Santer P, Wachtendorf LJ, et al. Association of sedation, coma, and in-hospital mortality in mechanically ventilated patients with coronavirus disease 2019-related acute respiratory distress syndrome: a retrospective cohort study. Crit Care Med. 2021;49(9):1524–1534. [DOI] [PubMed] [Google Scholar]
- 13.Luz M, Brandão Barreto B, de Castro REV, et al. Practices in sedation, analgesia, mobilization, delirium, and sleep deprivation in adult intensive care units (SAMDS-ICU): an international survey before and during the COVID-19 pandemic. Ann Intensive Care. 2022;12(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pancholi P, Wu J, Lessen S, et al. Triglyceride levels and their relationship to sedation choice and outcomes in mechanically ventilated patients receiving propofol. Ann Am Thorac Soc. 2023;20(1):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walkey AJ, Kumar VK, Harhay MO, et al. The Viral Infection and Respiratory Illness Universal Study (VIRUS): an international registry of coronavirus 2019-related critical illness. Crit Care Explor. 2020;2(4):e0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walkey AJ, Sheldrick RC, Kashyap R, et al. Guiding principles for the conduct of observational critical care research for coronavirus disease 2019 pandemics and beyond: the Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study Registry. Crit Care Med. 2020;48(11):e1038–e1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia MA, Johnson SW, Bosch NA, et al. Variation in use of repurposed medications among patients with coronavirus disease 2019. From the Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study: Coronavirus Disease 2019 Registry Investigator Group. Crit Care Explor. 2021;3(11):e0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston SC. Combining ecological and individual variables to reduce confounding by indication: case study–subarachnoid hemorrhage treatment. J Clin Epidemiol. 2000. Dec;53(12):1236–1241. [DOI] [PubMed] [Google Scholar]
- 21.Brock G, Pihur V, Datta S, Datta S. clValid: an R package for cluster validation. J Stat Softw. 2008;25(4):1–30. [Google Scholar]
- 22.Everitt B Cluster Analysis. 5th ed. Wiley; 2011. [Google Scholar]
- 23.Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200(7):828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 25.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure: on behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, De A. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. Int J Stat Med Res. 2015;4(3):287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charrad M, Ghazzali N, Boiteau V, Niknafs A. NbClust: an R package for determining the relevant number of clusters in a data set. J Stat Softw. 2014;61(6):1–9. [Google Scholar]
- 28.Lin W, Halpern SD, Prasad Kerlin M, Small DS. A “placement of death” approach for studies of treatment effects on ICU length of stay. Stat Methods Med Res. 2017;26(1):292–311. [DOI] [PubMed] [Google Scholar]
- 29.Anesi GL, Liu VX, Chowdhury M, et al. Association of intensive care unit admission and outcomes in sepsis and acute respiratory failure. Am J Respir Crit Care Med. 2022;205(5):520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. [DOI] [PubMed] [Google Scholar]
- 31.Jakob SM. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307(11):1151. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Jin X, Kang Y, Liang G, Liu T, Deng N. Midazolam and propofol used alone or sequentially for long-term sedation in critically ill, mechanically ventilated patients: a prospective, randomized study. Crit Care Lond Engl. 2014;18(3):R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss CH. Why do we fail to deliver evidence-based practice in critical care medicine? Curr Opin Crit Care. 2017;23(5):400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss CH, Krishnan JA, Au DH, et al. An official American Thoracic Society Research Statement: implementation science in pulmonary, critical care, and sleep medicine. Am J Respir Crit Care Med. 2016;194(8):1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harhay MO, Ratcliffe SJ, Small DS, Suttner LH, Crowther MJ, Halpern SD. Measuring and analyzing length of stay in critical care trials. Med Care. 2019;57(9):e53–e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia MA, Johnson SW, Sisson EK, et al. Variation in use of high-flow nasal cannula and noninvasive ventilation among patients with COVID-19. Respir Care. 2022;67(8):929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. 2016;4(2):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia MA, Rucci JM, Thai KK, et al. Association between troponin I levels during sepsis and postsepsis cardiovascular complications. Am J Respir Crit Care Med. 2021;204(5):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rucci JM, Bosch NA, Quinn EK, Chon KH, McManus DD, Walkey AJ. External validation of a risk score for daily prediction of atrial fibrillation among critically ill patients with sepsis. Ann Am Thorac Soc. 2022;19(4):697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. [DOI] [PubMed] [Google Scholar]
- 41.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003;289(22):2983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


