Abstract
Background
Data on the care of Asian patients with lung cancer in the US are limited; however, lung cancer is the leading cause of cancer death in this population.
Methods
Demographics, low-dose computed tomography (LDCT) screening, disease characteristics, and treatment history were compared between Asian and White patients newly diagnosed with lung cancer from 2014 to 2019 identified from Tufts Medical Center cancer registry. The influence of race on presenting stage was assessed via ordinal logistic regression. Time to treatment initiation (TTI) and overall survival (OS) were analyzed via log-rank tests. The impact of race on OS was evaluated via multivariable Cox regression.
Results
Asian patients (N = 144) were more likely to prefer non-English languages, use interpreters, be never-smokers, and harbor EGFR alterations, compared to White patients (N = 472), and to be diagnosed with later-stage lung cancer (odds ratio: 2.14, P < .001), had longer median TTI (early stage: 2.30 vs. 1.43 months, P = .035; curative stage: 1.88 vs. 1.20 months, P = .041) and more often did not receive cancer-directed therapy (12.6% vs. 5.7%, P = .01). Screening LDCT was done only in 11.9% of Asian and 21.4% of White patients (P = .20) who would have met screening criteria prior to diagnosis (N = 215). Median OS was similar between Asian and White patients (not reached vs. 74.8 months, P = .17). Multivariable Cox model suggested better OS for Asian patients (hazard ratio: 0.57, P = .01).
Conclusion
In our study, Asian patients presented with later-stage lung cancer, had treatment delays, and more often did not receive treatment, compared to White patients, yet did not have inferior survival.
Keywords: lung cancer, Asian, screening, time to treatment, survival, cancer care disparities
This article evaluates quality of care for Asian and White patients with lung cancer, assessing for differences in disease detection, presentation, initial treatment, and outcomes in the era of novel therapy.
Implications for Practice.
Quality of care of Asian patients with lung cancer in the US is not well studied. Compared to White patients, Asian patients presented with later-stage lung cancer, more often did not receive treatment, and had delayed treatment in early and curative stage settings, yet had similar survival. In both Asian and White patients with lung cancer, relatively few had low-dose computed tomography screening. Relatively favorable survival outcomes can mask cancer care disparities Asian patients experienced at the start of their illness course. Further work to identify and overcome racial care gaps throughout the trajectory of lung cancer is needed.
Introduction
Lung cancer is the leading cause of cancer death worldwide.1 In the US, lung cancer accounted for approximately 23% of all cancer deaths in 2020.2 An estimated 238 340 new cases and 127 070 deaths are predicted to occur from lung cancer in the US in 2023.3 The US Preventive Services Task Force (USPSTF) has recommended annual low-dose computed tomography (LDCT) screening for lung cancer for asymptomatic high-risk individuals since 2013.4,5 Alongside earlier detection of lung cancer, lung cancer treatments have advanced dramatically over the last decade. With targeted therapies and immune checkpoint inhibitors (ICIs), overall survival (OS) for patients with lung cancer has improved significantly, including in advanced stage disease.6 Questions remain as to whether such improvements in lung cancer screening, care, and outcomes are distributed equitably across various demographic groups.
Asian Americans are a heterogeneous group, and “Asian” refers to those who can trace their origin to any of more than 20 countries of East Asia, Southeast Asia, and the Indian subcontinent. Comprising approximately 6% of the US population,7,8 this group grew from 10.5 million in 2000 to 18.9 million in 2019 and are projected to exceed 35 million by 2060, representing the fastest-growing racial/ethnic group in the US.8 Cancer, as opposed to heart disease, is the leading cause of death in this group, accounting for approximately 25% of deaths in 2018.9 Lung cancer specifically is the leading cause of cancer-related mortality in Chinese, Japanese, and Vietnamese women, and men across all Asian American ethnicities.10 Yet, research on the care of Asian patients with lung cancer in the US is scarce.
Tufts Medical Center (TMC) is a tertiary care hospital that serves a large proportion of Asian patients in the greater Boston area. We sought to evaluate the quality of care in Asian and White patients with lung cancer, assessing for differences in disease detection, presentation, initial treatment, and outcomes in the era of novel therapy.
Materials and Methods
Patients
This study was approved by the Institutional Review Board of TMC. All patients with a new diagnosis of lung cancer between January 01, 2014 and December 31, 2019 were identified from the TMC cancer registry. Patients who self-identified as Asian or Non-Hispanic White (referred to as “White” in this text) were included. All other races/ethnicities and those who presented with recurrent, progressive, or refractory disease were excluded. Other baseline demographics, lung cancer screening data, tumor characteristics, frontline treatment course, and clinical outcomes were extracted from the electronic medical record. Data were collected through May 31, 2022.
Variables
Patient variables including age at diagnosis, sex, race, zip code, preferred language, interpreter use at first oncologic clinic encounter, and smoking status were collected. Age was treated as a continuous variable. Zip codes were correlated with geographic annual median household income according to American Community Survey 2010-2014.11 Income levels were categorized into 4 groups: $0-$50k, $50k-$100k, $100k-$150k, and $150k-$200k annually. Smoking status was obtained from documentation entered at the first clinic encounter and was categorized as never, current, or former smokers. Pack-year history and quit year were collected for current and former smokers. Any use of LDCT prior to diagnosis of lung cancer was counted as undergoing lung cancer screening. Date of diagnosis, stage at diagnosis (according to the contemporary American Joint Committee on Cancer staging system), brain metastasis status at diagnosis, histologic type, any detected tumor mutations, and programmed cell death ligand 1 expression tumor proportion score (PD-L1 TPS) were collected. Stage was grouped into stages I, II, III, or IV. Histologic type was noted and further grouped into non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), or other. PD-L1 TPS was grouped into <1%, 1%-49%, ≥50%, as assessed per PD-L1 IHC 22C3 pharmDx assay. Frontline treatment modality, start date for any cancer-directed treatment, and date of last follow-up or death were additionally collected.
Statistical Analysis
Baseline clinical information was summarized via descriptive statistical analyses. Median/range were used for continuous variables, and frequency/percentage for categorical variables. Mann-Whitney U test and Chi-squared/Fisher’s exact tests were used to compare continuous and categorical variables, respectively, between Asian and White patients. Ordinal logistic regression was used to evaluate the influence of race on presenting stage. Time to treatment initiation (TTI) was defined as time from the date of procedure from which diagnosis was made to the first cancer-directed treatment initiation. Excluding patients who underwent diagnostic surgery, TTI was evaluated with the Kaplan-Meier method and compared using log-rank tests. OS was defined as time from diagnosis to death. Those who remained alive at the time of last follow-up or who were lost to follow up were considered censored. OS was compared using log-rank tests and univariable and multivariable Cox regression analyses were performed to assess the impact of race on OS. Statistical significance was met if the 2-sided P-value was ≤.05. Statistical analyses were performed with R software (version 4.1.1).
Results
Baseline characteristics of 144 Asian and 472 White patients are as summarized (Table 1). Asian patients were older (median age 72 vs. 69 years, P < .001), included more males (74.3% vs. 43.6%, P < .001) and never-smokers (31.3% vs. 9.6%, P < .001) than White patients. Of 45 Asian never-smokers, women comprised 75.6%; of 45 White never-smokers, women comprised 64.4%. No significant difference in geographic annual median household income was found between the groups. Asian patients had greater linguistic diversity (non-English languages: 7 vs. 4) with less preference for English (11.8% vs. 98.7%). The most common non-English primary languages for Asian patients were Cantonese, Mandarin, Taishanese, and Vietnamese. Interpreter use was more common for Asian patients (43.1% vs. 0.6%, P < .001), however, was not documented for 68 of 127 Asian patients whose primary language was not English.
Table 1.
Baseline demographics and clinical characteristics (N = 616).
| Characteristic | Asian (N = 144) | White (N = 472) | P-value |
|---|---|---|---|
| Patient characteristics | |||
| Age | <.001 | ||
| Median [Min, Max] | 71.5 [48.0, 97.0] | 68.5 [21.0, 96.0] | |
| Sex | <.001 | ||
| Female | 37 (25.7%) | 266 (56.4%) | |
| Male | 107 (74.3%) | 206 (43.6%) | |
| Annual geographic median household income | .06 | ||
| 0-$50k | 14 (9.7%) | 57 (12.1%) | |
| $50k-100k | 124 (86.1%) | 369 (78.2%) | |
| $100k-150k | 5 (3.5%) | 44 (9.3%) | |
| $150k-200k | 1 (0.7%) | 2 (0.4%) | |
| Language | <.001 | ||
| English | 17 (11.8%) | 466 (98.7%) | |
| Albanian | 0 (0%) | 1 (0.2%) | |
| Bosnian | 0 (0%) | 3 (0.6%) | |
| Burmese | 1 (0.7%) | 0 (0%) | |
| Cambodian | 2 (1.4%) | 0 (0%) | |
| Cantonese | 91 (63.2%) | 0 (0%) | |
| Italian | 0 (0%) | 1 (0.2%) | |
| Japanese | 1 (0.7%) | 0 (0%) | |
| Mandarin | 17 (11.8%) | 0 (0%) | |
| Polish | 0 (0%) | 1 (0.2%) | |
| Taishanese | 10 (6.9%) | 0 (0%) | |
| Vietnamese | 5 (3.5%) | 0 (0%) | |
| Interpreter use | <.001 | ||
| No | 82 (56.9%) | 469 (99.4%) | |
| Yes | 62 (43.1%) | 3 (0.6%) | |
| Smoking status | <.001 | ||
| Never | 45 (31.3%) | 45 (9.6%) | |
| Current | 35 (24.3%) | 171 (36.4%) | |
| Former | 64 (44.4%) | 254 (54.0%) | |
| Pack years | .12 | ||
| Median [Min, Max] | 40.0 [0.500, 165] | 40.0 [0.200, 150] | |
| Tumor characteristics | |||
| Histology | .002 | ||
| Adenocarcinoma | 102 (70.8%) | 245 (51.9%) | |
| Squamous cell carcinoma | 19 (13.2%) | 106 (22.5%) | |
| Adenosquamous carcinoma | 0 (0%) | 7 (1.5%) | |
| Large cell carcinoma | 0 (0%) | 3 (0.6%) | |
| NSCLC, not otherwise specified | 6 (4.2%) | 16 (3.4%) | |
| SCLC | 10 (6.9%) | 64 (13.6%) | |
| Other | 7 (4.9%) | 31 (6.6%) | |
| Major histologic type | .06 | ||
| NSCLC | 127 (88.2%) | 377 (79.9%) | |
| SCLC | 10 (6.9%) | 64 (13.6%) | |
| Others | 7 (4.9%) | 31 (6.6%) | |
| Stage at diagnosis | <.001 | ||
| Stage I | 25 (17.4%) | 137 (29.0%) | |
| Stage II | 12 (8.3%) | 47 (10.0%) | |
| Stage III | 18 (12.5%) | 89 (18.9%) | |
| Stage IV | 89 (61.8%) | 199 (42.2%) | |
| Brain metastasis at diagnosis | .32 | ||
| No | 109 (77.9%) | 371 (78.6%) | |
| Yes | 31 (21.1%) | 81 (17.2%) | |
| Driver mutation | <.001 | ||
| Absent | 39 (27.1%) | 120 (25.4%) | |
| Present | 67 (46.5%) | 140 (29.7%) | |
| Not assessed | 38 (26.4%) | 212 (44.9%) | |
| PD-L1 expression Tumor Proportion Score | .12 | ||
| TPS <1% | 39 (27.1%) | 117 (24.8%) | |
| TPS 1%-49% | 16 (11.1%) | 64 (13.6%) | |
| TPS ≥50% | 27 (18.8%) | 52 (11.0%) | |
| Not assessed/not available | 62 (43.1%) | 239 (50.6%) |
Abbreviations: NSCLC: non-small cell lung cancer; SCLC: small cell lung cancer; PD-L1: programmed cell death ligand 1.
Adenocarcinoma was more common in Asian patients (70.8% vs. 51.9%), while squamous cell carcinoma (13.2% vs. 22.5%) and SCLC (6.9% vs. 13.6%) were more common in White patients (Table 1). Driver mutations were more often found in Asian patients (46.5% vs. 29.7%, P < .001). Of 207 patients with detected oncogenic mutations, the top 3 mutations in Asian patients were EGFR (61.2%), TP53 (14.9%), and BRAF or KRAS (7.5%), versus KRAS (39.2%), TP53 (29.3%), and EGFR (15.0%) in White patients (Supplementary Table S1). PD-L1 TPS results were available for half of the patients (51.1%). No difference in PD-L1 TPS distribution was found between Asian and White patients (P = .12).
More Asian patients presented with stage IV disease (61.8% vs. 42.2%). No significant difference was noted in presence of brain metastasis at diagnosis (21.1% vs. 17.2%, P = .32). Asian patients were 2.14 times (P < .001) more likely to be diagnosed with lung cancer at later stage than White patients by multivariable ordinal logistic regression (Table 2). A lower percentage of Asian patients (29.2%, 42/144) compared to White patients (36.7%, 173/472) would have met eligibility criteria for lung cancer screening by the 2013 USPSTF recommendation prior to diagnosis; however, this was not statistically significant (P = .12). Among these 215 patients who would have met lung cancer screening criteria, screening LDCT was done in a lower percentage of Asian than White patients (11.9% vs. 21.4%, P = .20), although again this was not statistically significant (Table 3).
Table 2.
Univariable and multivariable ordinal logistic regressions for associations between race and lung cancer presenting stage.
| Variable | Regression | Lung cancer stage at diagnosis (I, II, III, IV) | ||||
|---|---|---|---|---|---|---|
| Coefficient effect | Odds ratio | 95% confidence interval | P-value | |||
| Race | Asian | Unadjusted regression | 0.74 | 2.10 | [1.46, 3.05] | <.001 |
| White | Reference | |||||
| Asian | Adjusted regression* | 0.76 | 2.14 | [1.43.3.24] | <.001 | |
| White | Reference | |||||
*Adjusted for age, sex, income (recategorized into 3 levels: <$50k, $50k-100k, >$100k), major histologic type (NSCLC, SCLC, others), and smoking status (never, current, former).
Table 3.
Low-dose computed tomography scan (LDCT) for lung cancer screening in patients who would have met screening criteria prior to diagnosis.
| LDCT | Asian (N = 42 of 144) | White (N = 173 of 472) | P-value |
|---|---|---|---|
| .20 | |||
| No | 37 (88.1%) | 136 (78.6%) | |
| Yes | 5 (11.9%) | 37 (21.4%) |
For the 469 patients included for TTI analysis (Supplementary Fig. S1), Asian patients tended toward longer median TTI versus White patients (1.23 vs. 0.97 months, P = .06). When stratified by stage, Asian patients were found with longer median TTI compared to White patients in early stage (stages I + II: 2.30 vs. 1.43 months, P = .035, Fig. 1A) and curative stage disease (stages I + II + III: 1.88 vs. 1.20 months, P = .041, Fig. 1B), and no difference in median TTI was found in advanced (stages III + IV, Fig. 1C) or metastatic stage disease (Fig. 1D).
Figure 1.
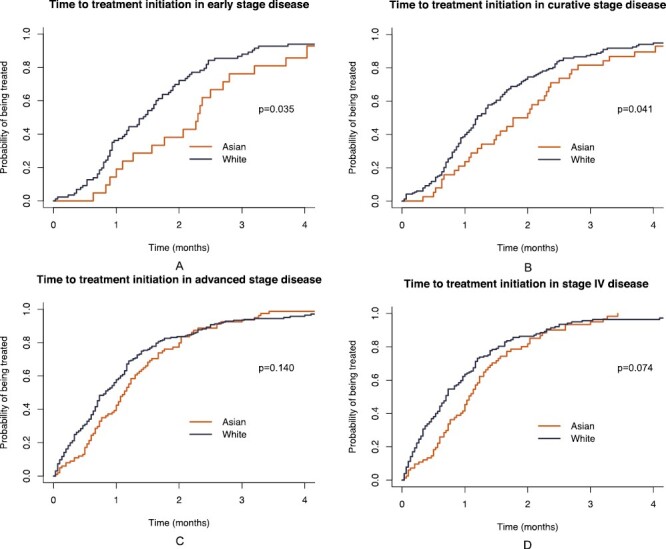
Cumulative incidence estimates of time from diagnosis to treatment initiation (TTI) by race in different stages of lung cancer. (A) Early stage (stages I + II, N = 109): median TTI was 2.30 months in Asian vs. 1.43 months in White patients, P = .035; (B) Curative stage (stages I + II + III, N = 207): median TTI was 1.88 months in Asian vs. 1.20 months in White patients, P = .041; (C) Advanced stage (stages III + IV, N = 360): median TTI was 1.13 months in Asian vs. 0.83 months in White patients, P = .140; (D) stage IV (N = 262): median TTI was 1.07 months in Asian vs. 0.70 months in White patients, P = .074
Of 137 Asian and 441 White patients with evaluable frontline management data after diagnosis, Asian patients were found more often not to receive cancer-directed therapy (12.6% vs. 5.7%, P = .01); these patients include those who declined cancer-directed treatment, received best supportive care or palliative care only or hospice care, per available medical records. However, whether these patients received other unconventional/alternative therapies directed against cancer (eg, Traditional Chinese Medicine, energy healing therapy) is unknown.
Frontline treatments according to presenting stage of lung cancer (curative vs. metastatic stage) were examined in the 534 patients who received cancer-directed therapy (Table 4). Asian patients with metastatic lung cancer were found more often to receive upfront targeted therapy (25.4% vs. 5.6%, P < 0.001) compared to White patients, but frontline immunotherapy treatment (either given alone or as part of chemoimmunotherapy) was not different between the 2 groups (18.3% vs. 10.5%, P = .154).
Table 4.
Frontline cancer-directed treatment in curative (stages I, II, III) and metastatic (stage IV) lung cancer (N = 534).
| Asian (N = 118) | White (N = 416) | |||
|---|---|---|---|---|
| Frontline treatment | Curative (N = 47) | Metastatic (N = 71) | Curative (N = 254) |
Metastatic (N = 162) |
| Chemoimmunotherapy | 0 (0%) | 7 (9.9%) | 2 (0.8%) | 12 (7.4%) |
| Chemoradiation | 10 (21.3%) | 6 (8.5%) | 40 (15.7%) | 14 (8.6%) |
| Chemotherapy | 4 (8.5%) | 13 (18.3%) | 22 (8.7%) | 40 (24.7%) |
| Immunotherapy | 1 (2.1%) | 6 (8.5%) | 3 (1.2%) | 5 (3.1%) |
| Radiation | 3 (6.4%) | 16 (22.5%) | 23 (9.1%) | 58 (35.8%) |
| Surgery | 28 (59.6%) | 5 (7.0%) | 164 (64.6%) | 24 (14.8%) |
| Targeted therapy | 1 (2.1%) | 18 (25.4%) | 0 (0%) | 9 (5.6%) |
Of overall 608 patients with OS data, no difference was seen in median OS between Asian and White patients (not reached [NR] vs. 74.83 months, P = .17, Fig. 2A). Median OS remained similar in early stage disease (NR vs. 90.40 months, P = .22, Fig. 2B); however, Asian patients’ OS advantage became statistically significant in advanced stage (55.4 vs. 35.2 months, P = .046, Fig. 2C) and metastatic stage (44.8 vs. 13.8 months, P = .002, Fig. 2D) settings. Multivariable Cox regression suggested that Asian patients had superior OS, adjusting for age, sex, income, smoking status, histology and stage (hazard ratio [HR]: 0.57, P = .01, Table 5).
Figure 2.
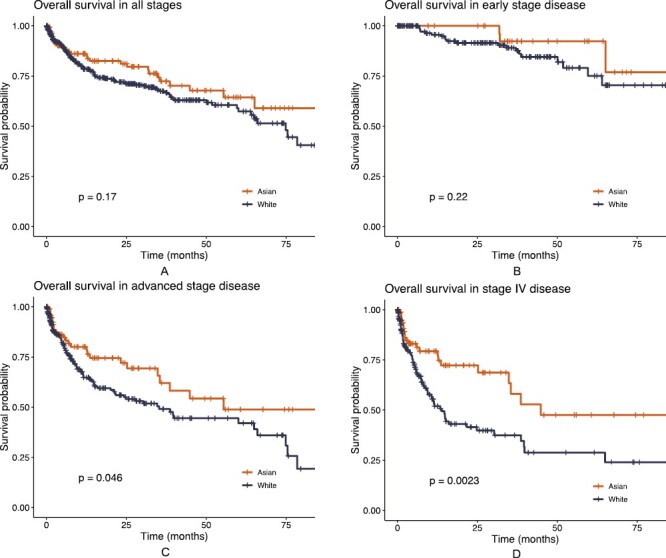
Kaplan-Meier curves for overall survival (OS) by race. (A) All stages of lung cancer (N = 608): median OS was not reached (NR) in Asian vs. 74.83 months in White patients, P = .17; (B) Early stage (stages I + II, N = 216): median OS was NR in Asian vs. 90.40 months in White patients, P = .22; (C) Advanced stage (stages III + IV, N = 392): median OS was 55.4 months in Asian vs. 35.2 months in White patients, P = .046; (D) Stage IV (N = 285): median OS was 44.8 months in Asian vs. 13.8 months in White patients, P = .002.
Table 5.
Univariable and multivariable Cox regressions for overall survival by race.
| Regression | Variable | Coefficient | Hazard ratio | 95% confidence interval | P-value |
|---|---|---|---|---|---|
| Crude Cox regression | Asian | −0.29 | 0.75 | [0.34, 1.17] | .17 |
| White | Reference | ||||
| Adjusted Cox regression* | Asian | −0.56 | 0.57 | [0.36, 0.89] | .01 |
| White | Reference |
Statistically significant P-values ≤.05.
*Adjusted for stage (I, II, III, IV), age, sex, income (recategorized into 3 levels: <$50k, $50k-100k, >$100k), major histologic type (NSCLC, SCLC, others), and smoking status (never, current, former).
Discussion
In our study, Asian patients were more likely to present with later-stage lung cancer, had longer median TTI in early/curative stage disease, and more often did not receive cancer-directed therapy, compared to White patients. Multivariable Cox regression suggested superior OS in Asian patients. To the best of our knowledge, this is the first study to characterize lung cancer screening status and early management with survival outcomes of Asian patients with lung cancer in the era of novel therapies.
While tobacco use contributes to the majority of lung cancers,12 non-smokers comprise 10%-20% of US patients (15.7% women vs. 9.5% men) with lung cancer,13,14 and more than 30% of patients in Asia with lung cancer,15 half of which are women.16 Asian Americans in aggregate have the lowest cigarette smoking rate (9.0%), with women having an even lower prevalence (4.6%), compared to White (16.6%) individuals.17 In Asian never-smokers with lung cancer, adenocarcinoma is the predominant disease histology, with increased rates of EGFR sensitizing mutations (60%-78%) and ALK rearrangements (5%-10%).16,18-20 Factors contributing to lung cancer pathogenesis in Asian never-smokers may include exposure to second-hand smoke, cooking fumes, hazardous air pollutants, and inherited genetic susceptibility.7,15 Our findings consistently showed that more Asian than White patients with lung cancer were never-smokers with adenocarcinoma, a majority of whom were female.
Only about a third of patients in our study would have met lung cancer screening criteria prior to diagnosis, and screening LDCT was done in even fewer patients (11.9% of Asian vs. 21.4% of White patients). Cancer screening rates in Asian Americans are lower than those of other racial/ethnic groups for breast, cervical, and colorectal cancers.21-23 Systemic barriers, such as limited access to medical interpreters, may contribute to this disparity.24 Data regarding lung cancer screening status in Asian patients in the US are limited. One retrospective study done in California reported that 11.1% of eligible Asian individuals received orders for screening LDCT during 2010-2016.25 While difficult to contextualize our results in such a data-scarce setting, our observation that LDCT screening had been done in a numerically smaller proportion of Asian versus White patients with lung cancer suggests failure of contemporary LDCT eligibility criteria to capture Asian patients at risk for lung cancer, including nonsmokers.
In our study, Asian patients were more than twice as likely as White patients to be diagnosed with lung cancer at a later stage. Percentages of later-stage lung cancer diagnoses among Asian, Hispanic, Black, AI/AN, and White patients were reported to be 58.8%, 55.9%, 54.5%, 51.4%, and 50.0%, respectively.26 Asian immigrants with lung cancer have been found with prolonged symptomatology and have presented more frequently with stage III/IV disease.27 Increased frequency of oncogene addicted tumors may contribute to later-stage presentation in Asian patients.28-30 Linguistic difficulties and financial considerations may impede timely access to care. Patients’ health literacy levels, attitudes regarding western medicine, stigma associated with cancer, and religious beliefs could also play a role.31-33 As fewer Asian patients may meet eligibility criteria for screening LDCT, this group’s specific lung cancer risk may not be captured adequately by screening guidelines. This is a significant missed opportunity to detect lung cancer at earlier, curative stage in Asian patients, which if done, presumably may lead to better overall survival outcomes potentially exceeding that of White patients.
With early and curative stage lung cancer, Asian patients suffered disparity with longer TTI compared to White patients. Furthermore, the percentage of Asian patients who did not receive any known cancer-directed therapy was double that of White patients. Previously, Zhang et al showed that White patients were more likely to receive definitive therapy in lung cancer (OR = 1.178).34 As literature is limited, hypotheses regarding contributing factors for a prolonged TTI in Asian patients can be generated from real world experience. Language barriers may result in delayed communication and prolonged time for a clinical encounter or procedure to be arranged and completed. Culturally mediated factors may also contribute. Family may hesitate to share a diagnosis of cancer with the patient or vice versa.35 In contrast to patient-centered care in Western countries, a family-centered decision-making process is often preferred in Asian patients, which may require extended appointments to accommodate caregivers.7
Asian patients in our study did not have inferior survival compared to White patients. In both advanced and metastatic stage, a significant OS advantage was seen in Asian patients. This differs from Finlay et al’s older finding that the 2-year survival rate was significantly lower in Asian patients with lung cancer.27 National data from 2012 to 2018 showed that the 5-year relative survival for Asian Americans and Pacific Islanders with lung cancer has improved to 26.0%, compared to 22.9% for White individuals.36 In a study of patients in California with NSCLC from 1991 to 2005, Ou et al found that being Asian, compared with non-Asian, is a favorable prognostic factor for OS independent from smoking status (HR = 0.86, P < .0001). In alignment with our findings, Ou et al also found that Asian compared to non-Asian patients with stage III and IV NSCLC had significantly better 1-year and 5-year survival rates.37
The survival advantage with Asian race/ethnicity in patients with advanced lung cancer could perhaps be explained by the higher percentage of nonsmokers, associated with fewer smoking-related comorbidities and greater likelihood of harboring an EGFR mutation amenable to targeted therapy. While up to 20% of White patients with NSCLC carry an EGFR mutation, reported EGFR mutation frequency in Asian patients with lung cancer ranges from 30% to over 50%.38-40 Further study of a subset of patients from mainland China found the EGFR mutation frequency to be 50.2%.41 In our study, the EGFR mutation frequency in Asian patients, a large proportion of whom spoke Chinese languages, was 61.2%. Our observed EGFR mutation frequency of 15% in White patients matched that reported in literature. In our study, Asian patients with metastatic lung cancer were more likely than White patients to receive upfront targeted therapy, likely contributing to the observed OS results. EGFR tyrosine kinase inhibitors (TKIs) have more than doubled progression-free survival (PFS) in comparison with chemotherapy,42,43 with osimertinib shown to be associated with longer OS in the first-line treatment of EGFR-mutated advanced NSCLC, versus comparator TKIs.44
Frontline immunotherapy treatment was not significantly different between Asian and White patients with metastatic lung cancer in our study. Since 2016 when pembrolizumab monotherapy demonstrated dramatic OS benefit compared with platinum-based chemotherapy in patients with untreated advanced NSCLC with PD-L1 TPS ≥ 50% without EGFR/ALK alterations,45 various other ICIs and combinations have been approved in frontline treatment settings with continued significant survival improvements in metastatic disease.46,47 In 2018, consolidation durvalumab became the standard of care for patients with locally advanced, unresectable NSCLC after definitive chemo-radiation, conferring significant improvement in PFS and OS.48,49 Whether racial differences exist in the efficacy of ICIs for treatment of lung cancer is uncertain. A meta-analysis showed that Asian patients with cancer receiving PD-1/PD-L1 inhibitor-based therapy have significantly improved survival benefit than non-Asian patients, with pooled PFS HR 0.78 and OS HR 0.84.50 Our finding of survival advantage in Asian patients with advanced lung cancer may derive in part from treatment benefits of frontline immunotherapy.
Our study has several limitations. It is a single-institution retrospective study. Asian patients were taken in aggregate, and more nuanced analysis was not done with respect to the heterogeneous Asian ethnicities. In addition, this analysis was unable to distinguish Asian immigrants versus US-born Asian persons. The impacts of immigration and acculturation could not be accounted for. Yet, as the Asian patients in our study were generally older, non-English speaking and lived around Boston’s Chinatown, participants may be more representative of Asian immigrants, and the results more applicable to foreign-born Asian persons. Furthermore, some potential confounders were not assessed such as patients’ educational level and performance status. Finally, TTI did not include the time from the first abnormal imaging prompting further diagnostic workup to the date of procedure confirming lung cancer diagnosis in this study.
Differences in patterns of lung cancer screening, diagnosis, and initial management between Asian and White patients in our study intimates potential racial disparities and complex interactions with cultural factors that contribute to quality of care and that deserve additional study. To build a more nuanced understanding of whether lung cancer screening, testing and treatments are being delivered and received equitably with regard to Asian patients, additional research into physician- and medical establishment-specific factors, and culturally mediated beliefs and preferences in managing cancer is warranted. Assessment of risk factors other than smoking status that may be incorporated into lung cancer screening, and improved access to biomarker testing at diagnosis of lung cancer for Asian patients would be valuable. In addition, our study prompts a search for more meaningful outcome measures in assessments of cancer care equity besides OS, which is limited in its reflection of racial disparities experienced throughout the cancer care trajectory starting from prior to diagnosis.
Conclusion
In this study, although Asian patients were more likely to be diagnosed with advanced lung cancer and less likely to receive cancer-directed treatment, compared to White patients, overall survival was similar. Asian patients with advanced/metastatic disease even demonstrated improved survival, likely due to having unique lung cancer biology responsive to upfront targeted therapy or immunotherapy. Such relatively favorable survival outcomes mask the cancer care disparities Asian patients experienced at the start of their illness course, given low rates of lung cancer screening and longer delay prior to treatment initiation. Educational efforts should be implemented to raise awareness of existing racial cancer care gaps for those already affected, those vulnerable, the healthcare professionals caring for patients from affected and vulnerable communities, and the greater public. Increased vigilance in discerning disparities and more work with disaggregated data on Asian patients with lung cancer are needed to inform culturally sensitive, practical and sustainable interventions to overcome barriers of cancer care equity.
Supplementary Material
Supplementary material is available at The Oncologist online.
Acknowledgements
The authors respectfully express special thanks to the patients at Tufts Medical Center whose experiences contributed to this work. The authors also thank Dr. Lori Pai, MD (thoracic medical oncologist at Tufts Medical Center) for her valuable comments.
Contributor Information
Xiao Hu, Division of Hematology-Oncology, Department of Medicine, Tufts Medical Center, Boston, MA, USA.
John W Melson, Division of Hematology-Oncology, Department of Medicine, Tufts Medical Center, Boston, MA, USA.
Stacey S Pan, Division of Hematology-Oncology, Department of Medicine, Tufts Medical Center, Boston, MA, USA.
Yana V Salei, Deparment of Medicine, Tufts Medical Center, Boston, MA, USA.
Yu Cao, Division of Hematology-Oncology, Department of Medicine, Tufts Medical Center, Boston, MA, USA.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors indicate no financial or other Conflict of Interest relationships.
Author Contributions
Conception/Design: X.H., J.W.M., Y.C. Provision of study materials or patients: Y.C.
Collection and/or assembly data: All authors. Data analysis and interpretation: All authors. Manuscript writing: X.H., J.W.M., Y.C. Final approval of manuscript: All authors.
Data Availability
The data that support the findings of this study are available on reasonable request to the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Prior Presentations
9/30/2022 (2022 ASCO Quality Care Symposium, poster presentation): Hu X, Melson J, Pan S, Salei Y, Cao Y. Quality of care of Asian and White patients with lung cancer: Single-institution study. Published online September 30, 2022. J Clin Oncol 40, 2022 (suppl 28; abstr 115). DOI: 10.1200/JCO.2022.40.28_suppl.115
References
- 1. International Agency for Research on Cancer. GLOBOCAN lung cancer facts sheet 2020. Accessed 23 March 2023. https://gco.iarc.fr/today/home
- 2. Centers for Disease Control and Prevention. An update on cancer deaths in the United States. Atlanta GUDoHaHS, Centers for Disease Control and Prevention, Division of Cancer Prevention and Control; 2022. [Google Scholar]
- 3. American Cancer Society. Key statistics for lung cancer. Accessed 23 March 2023. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
- 4. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. 10.7326/M13-2771 [DOI] [PubMed] [Google Scholar]
- 5. Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arbour KC, Riely GJ.. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322(8):764-774. 10.1001/jama.2019.11058 [DOI] [PubMed] [Google Scholar]
- 7. Lee RJ, Madan RA, Kim J, Posadas EM, Yu EY.. Disparities in cancer care and the Asian American population. Oncologist. 2021;26(6):453-460. 10.1002/onco.13748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Budiman A, Ruiz NG.. Key facts about Asian Americans, a diverse and growing population. Accessed 04 August 2022. https://www.pewresearch.org/fact-tank/2021/04/29/key-facts-about-asian-americans/
- 9. Centers for Disease Control and Prevention. Leading causes of death, males, non-hispanic Asian, United States, 2018. Accessed 4 August 2022. https://www.cdc.gov/healthequity/lcod/men/2018/nonhispanic-asian/index.htm
- 10. Thompson CA, Gomez SL, Hastings KG, et al. The burden of cancer in Asian Americans: a report of national mortality trends by Asian ethnicity. Cancer Epidemiol Biomarkers Prev. 2016;25(10):1371-1382. 10.1158/1055-9965.EPI-16-0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bureau USC. Accessed 23 March 2023. https://data.census.gov/
- 12. Walser T, Cui X, Yanagawa J, et al. Smoking and lung cancer: the role of inflammation. Proc Am Thorac Soc. 2008;5(8):811-815. 10.1513/pats.200809-100TH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. Lung cancer among people who never smoked. Accessed 6 August 2022. https://www.cdc.gov/cancer/lung/nonsmokers/index.htm
- 14. Siegel DA, Fedewa SA, Henley SJ, Pollack LA, Jemal A.. Proportion of never smokers among men and women with lung cancer in 7 US States. JAMA Oncol. 2021;7(2):302-304. 10.1001/jamaoncol.2020.6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou F, Zhou C.. Lung cancer in never smokers-the East Asian experience. Transl Lung Cancer Res. 2018;7(4):450-463. 10.21037/tlcr.2018.05.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ha SY, Choi SJ, Cho JH, et al. Lung cancer in never-smoker Asian females is driven by oncogenic mutations, most often involving EGFR. Oncotarget. 2015;6(7):5465-5474. 10.18632/oncotarget.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jamal A, Phillips E, Gentzke AS, et al. Current cigarette smoking among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(2):53-59. 10.15585/mmwr.mm6702a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247-4253. 10.1200/JCO.2009.22.6993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958-967. 10.1056/NEJMoa0904554 [DOI] [PubMed] [Google Scholar]
- 20. Taron M, Ichinose Y, Rosell R, et al. Activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor are associated with improved survival in gefitinib-treated chemorefractory lung adenocarcinomas. Clin Cancer Res. 2005;11(16):5878-5885. 10.1158/1078-0432.CCR-04-2618 [DOI] [PubMed] [Google Scholar]
- 21. Jun J. Cancer/health communication and breast/cervical cancer screening among Asian Americans and five Asian ethnic groups. Ethn Health. 2020;25(7):960-981. 10.1080/13557858.2018.1478952 [DOI] [PubMed] [Google Scholar]
- 22. Ma GX, Shive SE, Wang MQ, Tan Y.. Cancer screening behaviors and barriers in Asian Americans. Am J Health Behav. 2009;33(6):650-660. 10.5993/ajhb.33.6.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ibaraki AY, Hall GCN, Sabin JA.. Asian American cancer disparities: the potential effects of model minority health stereotypes. Asian Am J Psychol. 2014;5(1):75-81. 10.1037/a0036114 [DOI] [Google Scholar]
- 24. Dang J, Lee J, Tran JH, et al. The role of medical interpretation on breast and cervical cancer screening among Asian American and Pacific Islander women. J Cancer Educ. 2010;25(2):253-262. 10.1007/s13187-010-0074-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li J, Chung S, Wei EK, Luft HS.. New recommendation and coverage of low-dose computed tomography for lung cancer screening: uptake has increased but is still low. BMC Health Serv Res. 2018;18(1):525. 10.1186/s12913-018-3338-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Underwood JM, Townsend JS, Tai E, et al. Racial and regional disparities in lung cancer incidence. Cancer. 2012;118(7):1910-1918. 10.1002/cncr.26479 [DOI] [PubMed] [Google Scholar]
- 27. Finlay GA, Joseph B, Rodrigues CR, Griffith J, White AC.. Advanced presentation of lung cancer in Asian immigrants: a case-control study. Chest. 2002;122(6):1938-1943. 10.1378/chest.122.6.1938 [DOI] [PubMed] [Google Scholar]
- 28. Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer. 2012;118(18):4502-4511. 10.1002/cncr.27409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shin DY, Na II, Kim CH, Park S, Baek H, Yang SH.. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol. 2014;9(2):195-199. [DOI] [PubMed] [Google Scholar]
- 30. Hendriks LE, Smit EF, Vosse BA, et al. EGFR mutated non-small cell lung cancer patients: more prone to development of bone and brain metastases?. Lung Cancer. 2014;84(1):86-91. 10.1016/j.lungcan.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 31. Liu XH, Zhong JD, Zhang JE, Cheng Y, Bu XQ.. Stigma and its correlates in people living with lung cancer: a cross-sectional study from China. Psychooncology. 2020;29(2):287-293. 10.1002/pon.5245 [DOI] [PubMed] [Google Scholar]
- 32. OncLive. More can be done for Asian American lung cancer patients. Accessed 26 March 2023. https://www.onclive.com/view/more-can-be-done-for-asian-american-lung-cancer-patients
- 33. Tung W-C, Li Z.. Pain beliefs and behaviors among Chinese. Home Health Care Manag Pract. 2015;27(2):95-97. [Google Scholar]
- 34. Zhang C, Zhang C, Wang Q, et al. Differences in stage of cancer at diagnosis, treatment, and survival by race and ethnicity among leading cancer types. JAMA Netw Open. 2020;3(4):e202950. 10.1001/jamanetworkopen.2020.2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen MS, Lee RJ, Madan RA, et al. Charting a path towards Asian American cancer health equity: a way forward. J Natl Cancer Inst. 2022;114(6):792-799. 10.1093/jnci/djac055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. National Cancer Institute S E, and end results lung and bronchus: SEER 5-year relative survival rates, 2012-2018. Accessed 24 March 2023. https://seer.cancer.gov/statistics-network/explorer/application.html?site=47&data_type=4&graph_type=5&compareBy=race&chk_race_6=6&chk_race_5=5&chk_race_4=4&chk_race_9=9&chk_race_8=8&series=9&sex=1&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&advopt_show_count=on&hdn_view=1&advopt_show_apc=on&advopt_display=2#resultsRegion1
- 37. Ou SH, Ziogas A, Zell JA.. Asian ethnicity is a favorable prognostic factor for overall survival in non-small cell lung cancer (NSCLC) and is independent of smoking status. J Thorac Oncol. 2009;4(9):1083-1093. 10.1097/JTO.0b013e3181b27b15 [DOI] [PubMed] [Google Scholar]
- 38. Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154-162. 10.1097/JTO.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yatabe Y, Kerr KM, Utomo A, et al. EGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic survey. J Thorac Oncol. 2015;10(3):438-445. 10.1097/JTO.0000000000000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou W, Christiani DC.. East meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Cancer. 2011;30(5):287-292. 10.5732/cjc.011.10106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shi Y, Li J, Zhang S, et al. Molecular epidemiology of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology - mainland China subset analysis of the PIONEER study. PLoS One. 2015;10(11):e0143515. 10.1371/journal.pone.0143515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 43. Karachaliou N, Fernandez-Bruno M, Bracht JWP, Rosell R.. EGFR first- and second-generation TKIs-there is still place for them in EGFR-mutant NSCLC patients. Transl Cancer Res. 2019;8(Suppl 1):S23-S47. 10.21037/tcr.2018.10.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41-50. 10.1056/NEJMoa1913662 [DOI] [PubMed] [Google Scholar]
- 45. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 46. Cancer Research Institute. Immunotherapy for lung cancer. Accessed 07 August 2022. https://www.cancerresearch.org/cancer-types/lung-cancer
- 47. Onoi K, Chihara Y, Uchino J, et al. Immune checkpoint inhibitors for lung cancer treatment: a review. J Clin Med. 2020;9(5):1362. 10.3390/jcm9051362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350. 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 49. Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):1301-1311. 10.1200/JCO.21.01308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peng L, Qin BD, Xiao K, et al. A meta-analysis comparing responses of Asian versus non-Asian cancer patients to PD-1 and PD-L1 inhibitor-based therapy. Oncoimmunology. 2020;9(1):1781333. 10.1080/2162402X.2020.1781333 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on reasonable request to the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


