Abstract
Granular hydrogels are formed through the packing of hydrogel microparticles and are emerging for various biomedical applications, including as inks for 3D printing, substrates to study cell-matrix interactions, and injectable scaffolds for tissue repair. Granular hydrogels are suited for these applications due to their unique properties including inherent porosity, shear-thinning and self-healing behavior, and tunable design. The characterization of their material properties and biological response involves technical considerations that are unique to modular systems like granular hydrogels. Here, we describe detailed methods that can be used to quantitatively characterize the rheological behavior and porosity of granular hydrogels using reagents, tools, and equipment that are typically available in biomedical engineering laboratories. In addition, we detail methods for 3D cell invasion assays using multicellular spheroids embedded within granular hydrogels and describe steps to quantify features of cell outgrowth (e.g., endothelial cell sprouting) using standard image processing software. To illustrate these methods, we provide examples where features of granular hydrogels such as the size of hydrogel microparticles and their extent of packing during granular hydrogel formation are modulated. Our intent with this resource is to increase accessibility to granular hydrogel technology and to facilitate the investigation of granular hydrogels for biomedical applications.
Keywords: Microgels, Injectable, Biomaterials, Angiogenesis, Sprouting, Microscopy
Graphical Abstract:

1. Introduction:
Granular hydrogel materials consisting of densely packed hydrogel particles have been broadly applied to many fields for decades. For example, in food science, fluid gels (gelled microparticles suspended in a continuous non-gelled polymer phase) have been used as thickeners and stabilizers.1,2 In another example, super absorbent polymer (SAP) particles have been used in many industries, including agriculture, health products, and concrete buildings, where dry polymeric particles are introduced to a wet surface in order to absorb and retain water, forming a granular gel consisting of water-swollen packed particles.3 Recently, jammed hydrogel microparticles have even been explored to tune light scattering through windows, with the potential to reduce building energy consumption.4
In recent years, there has been growing interest in the use of packed hydrogel microparticles (i.e., “granular hydrogels”) as biomaterials for biomedical applications (Fig. 1). Granular hydrogels consist of hydrogel microparticles (i.e., “microgels”) that are tens to hundreds of microns in diameter and packed into a jammed state.5 Due to their granular nature and the ability of particles to flow under force, these biomaterials can be shear-thinning and self-healing, permitting injection into tissues and extrusion for 3D printing.6 The shear-thinning and self-healing behavior of granular hydrogels has also been utilized to create granular gel suspension baths for embedded printing techniques.7 In addition, the microscale-porosity within the interstitial space between particles allows for cell invasion as well as diffusion of nutrients and therapeutics in drug delivery applications.8,9 To further leverage the modularity of granular hydrogels, different microgel populations can be combined into a single granular hydrogel scaffold, allowing the tuning of properties including degradation, mechanical moduli, and drug delivery.8,10,11 Due to these unique properties, granular hydrogels are being explored for many biomedical applications, including in vivo tissue repair, in vitro cell culture, and extrusion bioprinting.
Fig. 1:

Overview of granular hydrogels for biomedical applications. (a) Methods to fabricate microgels. (b) Methods to assemble granular hydrogels through microgel packing. (c) Characterization of granular hydrogel properties including rheological behavior, porosity, and cell response. (d) Applications of granular hydrogels for extrusion printing, in vitro cell culture, and in vivo tissue repair.
There are numerous methods to fabricate microgels for the construction of granular hydrogels (Fig. 1a).12 For example, droplet-generating microfluidic devices have been widely utilized to fabricate microgels with homogenous size and shape.13–15 The process involves using a flow-focusing microfluidic device to pinch off homogeneous droplets of hydrogel precursor solution, which are subsequently crosslinked downstream (e.g., photo-crosslinking, chemical stimuli, temperature change) to form stable microgels. Batch emulsions have also been utilized to form microgels, where hydrogel precursor solution is introduced into an immiscible fluid (e.g., oil) while stirring, resulting in dispersed phase droplet formation.16–18 These droplets are then crosslinked to form stable microgels with a heterogenous size distribution. Electro-spraying has also been explored for microgel fabrication, where hydrogel precursor solution is electro-sprayed into an oil bath for rapid formation of heterogenous droplets that are then crosslinked to form stable microgels.19–21 These microgel fabrication techniques all make use of water-in-oil emulsions to form spherical microgel building blocks for granular hydrogels.
Additional approaches have been explored to fabricate microgels without the need for an emulsion, to avoid requisite steps for the removal of the oil and surfactant. For example, mechanical fragmentation has been used to form jagged, heterogenous microgel particles from a hydrogel crosslinked in bulk. Fragmentation to form microgels can be performed by the pushing of bulk hydrogels through a mesh22–24 or via the passing of bulk hydrogels through a series of smaller and smaller gauge needles.12,25 In another example, alginate microgels can be fabricated by flowrate-controlled dropping of alginate precursor solution into a bath of calcium chloride, resulting in ionically crosslinked alginate microgels for granular hydrogel assembly.26 Recently, gelatin microgels have also been fabricated using complex coacervation between positively-charged gelatin and negatively-charged acacia gum.10
To assemble microgels after their fabrication into a granular hydrogel, microgels must be jammed together (Fig. 1b). Centrifuging is often used to pack microgels into a granular scaffold, where varying centrifuge speed can influence the degree of microgel packing.20,27,28 To further increase packing between microgel particles, granular hydrogels can be fabricated using vacuum-driven filtration, where an aqueous suspension of microgels is added to a membrane filter with sub-micron pore sizes, and vacuum force is used to remove interstitial solvent.29–31 To avoid the need for external forces (i.e., centrifugal, vacuum pressure), microgel packing by gravitational settling can also be used.32 Lastly, microgels can be dehydrated after fabrication, and the aqueous resuspension of dried microgel particles can be used for granular hydrogel assembly.23,33–35
For many biomedical applications, the flowability and self-healing behavior of granular hydrogels is important (Fig. 1c). For example, injectable granular hydrogels have been utilized in vivo for multiple tissue repair applications, including bone healing,34 repair of damaged brain tissue after stroke,36,37 axonal nerve outgrowth,13 enhanced wound healing,15,27 and to repair cardiac tissue after myocardial infarction.11,38 Further, granular hydrogels have been utilized as extrusion printing inks, leading to the formation of microporous structures with shape and structural stability.10,17,20,25,29,39 For many of these studies, inter-particle crosslinking is used to stabilize the granular hydrogel structures, including with photocrosslinking, click reactions, enzymatic annealing, and physical interactions. For all of these applications, characterization of granular hydrogel rheological properties (i.e., storage and loss moduli, strain-yielding, shear-thinning, self-healing recovery) is crucial for materials design and application.
The microscale porosity present within the microgel interstitial space is another major advantage of granular hydrogel biomaterials. Granular hydrogels have been explored for in vitro cell and spheroid culture.25,28,33,40–43 Often, cells or spheroids are placed in the microgel interstitial space, using the microgel surfaces as a substrate for invasion, proliferation, and outgrowth (Fig. 1c). Here too, granular hydrogels are often stabilized by inter-particle crosslinking to create scaffolds with structural integrity for in vitro studies. For these applications, it is important to fully characterize the material porosity (i.e., void space, pore number, pore size, pore interconnectivity), as well as cell behavior in the pore space (i.e., invasion distance, spheroid outgrowth). In-depth characterization of granular hydrogels using the techniques described above allows for their rational design towards target applications (Fig. 1d).
Herein, we investigate a model granular hydrogel system and describe methods for the preparation of granular hydrogels, as well as the characterization of granular hydrogel rheological behavior, porosity, and cell invasion. The granular hydrogel is comprised of microgels fabricated from hyaluronic acid (HA) modified with norbornene groups (Nor-HA) and crosslinked with dithiol crosslinkers. Photocrosslinked Nor-HA microgels are fabricated using a flow-focusing microfluidic device, and flowrates are varied to form small (~65 μm), medium (~120 μm), and large (~200 μm) microgels. Microgels are packed by either centrifugation at varied speeds or vacuum-driven filtration to assemble granular hydrogels. We investigate the rheological behavior, including storage and loss moduli, strain-yielding, and self-healing, using oscillatory shear rheometry. We investigate porosity using both 2D (confocal imaging) and 3D (IMARIS) methods to characterize void space, pore density, and pore size distribution. Further, we investigate cell invasion into granular hydrogels using embedded spheroids containing endothelial cells in vitro, analyzing sprout length and tortuosity within the granular hydrogels as outcomes. The system described herein is only a representative example of a granular hydrogel biomaterial, and the characterization methods described can be utilized with a broad range of granular hydrogels with varied microgel compositions and features. We organize this work into four main methods sections, including 1) granular hydrogel preparation, 2) rheology, 3) porosity, and 4) cell invasion assays.
2. Method 1: Granular hydrogel preparation
Granular hydrogels are assembled through the packing of hydrogel microparticles (i.e., microgels). Microgels can be fabricated using several methods including but not limited to microfluidic droplet generation, water-in-oil emulsion, bulk gel fragmentation, lithography, and electro-spraying.5 These methods vary in processing times, throughput, polymer compatibility, and fabricated microgel features. Packing of microgels to form granular hydrogels can also be performed in several ways such as gravitational settling, centrifugation, or vacuum jamming. In this section, we describe how microgels of varying sizes can be fabricated using a flow-focusing microfluidic device. We also describe steps to assemble granular hydrogels with varying degrees of packing using either centrifugation or vacuum-driven removal of the liquid phase.
2.1. Materials
2.1.1. Reagents
Norbornene modified hyaluronic acid (Nor-HA; MW ~60 kDa, ~14% of repeat units modified, synthesis described elsewhere with reagents available from Sigma-Aldrich)44
Light mineral oil (e.g., Catalogue # O121-1, Fisher Scientific)
Surfactant (e.g., Span-80, Catalogue # S6760, Sigma-Aldrich)
Tween-20 (Catalogue # P9416, Sigma-Aldrich)
High molecular weight Fluorescein isothiocyanate-dextran (FITC-dextran average mol wt. 2,000,000 e.g., Catalogue # FD2000S-1G)
2.1.2. Equipment
Omnicure S1500 UV lamp
Microfluidic device (replica molded from custom-designed mold printed through Protolabs)
Connective tubing (e.g., Saint-Gobain Catalogue # 02-587-1A)
Buechner funnel
Membrane filter (e.g., Durapore® Membrane Filter, 0.22 μm Catalogue # GVWP04700, Merck Millipore)
1.5 mL microcentrifuge tubes (e.g., Fisherbrand™ Premium Microcentrifuge Tubes Catalogue # 05-408-129, Fisher Scientific)
2.2. Procedure
2.2.1. Step 1: Microgel Fabrication
In our studies, we adopt a microfluidics approach due to its advantages including homogeneity of microgel sizes produced and the ease with which microgel size can be varied by tuning experimental parameters. Our laboratory has expertise in using photo-crosslinkable HA-based polymeric biomaterials, and Nor-HA that crosslinks via a thiol-ene reaction under UV light, which is used in this study (Fig. 2a). Nor-HA is synthesized as previously described.44 In addition to HA, the microfluidics approach to fabricating microgels has been shown to be compatible with a number of other photo-crosslinkable polymers including poly(ethylene glycol)-based (e.g., PEG-diacrylate) and gelatin-based (e.g., gelatin methacrylamide (Gel-MA)) materials.42
Fig. 2:

Fabrication and processing of microgels to make granular hydrogels. (a) Chemical structure of the norbornene-modified hyaluronic acid (Nor-HA) polymer used to make microgels in this study. (b) Schematic showing single channel microfluidic droplet generation of Nor-HA microgels that are crosslinked via thiol-ene reaction off-chip using a UV source. (c) Representative images of fluorescently labeled microgels of varying sizes in the range of ~65 μm (SMALL), ~120 μm (MEDIUM), and ~200 μm (LARGE) diameters, achieved through changing oil and polymer flow rate ratios during droplet generation on a microfluidic device. (d) Distribution of microgel diameters within each size group. (e) Schematic showing the steps involved in washing microgels from oil through to a microgel suspension. (f) Schematic showing methods used to achieve granular hydrogels with different degrees of packing (low, high, and very high). Note: The following methods on rheology, porosity, and cell invasion are applicable to other microgel types (e.g., chemistry, size) and fabrication methods (e.g., batch emulsion, fragmentation); this figure illustrates the approach used for the representative experimental data shown.
Whereas sophisticated chips with high-throughput capabilities have been employed, in our studies we use a simple single channel flow-focusing microfluidic device as previously described29 (Fig. 2b). Briefly, this device with polymer and oil channel diameters of 100 μm was formed through replica molding of a 3D-printed master using PDMS. Inlet and outlet channels were created using 1 mm biopsy punches. The device was plasma bonded to a glass slide and blunt stainless steel needles were inserted to the inlet and outlet channels.
To begin, dissolve Nor-HA in PBS (final concentration: 3 % w/v) and mix in dithiothreitol (DTT) crosslinker and Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) photoinitiator. Separately, add Span-80 surfactant (2% v/v) into light mineral oil and mix vigorously, then remove bubbles via centrifugation. Transfer both the polymer and oil mixtures into separate syringes and load them onto syringe pumps. Flow rates for both phases should be optimized to obtain stable droplet generation. The ratio of the oil to polymer flow rates can be varied to change droplet size, where a higher ratio will generate smaller droplets. Connect one end of a piece of tubing to the microfluidic outlet channel and place the other end inside a collection vessel such as a conical tube. Position a UV source set at 20 mW/cm2 directly on the tubing between the device outlet and collection tube. It is important to ensure that microgel crosslinking occurs off-chip, i.e., no part of the device itself should be exposed to UV as this can crosslink the polymer in the channels and cause the device to clog. In our studies, we optimize flow rates to obtain three sizes of microgels that we term SMALL (~65 μm; oil: 150 μL/min, polymer: 4 μL/min), MEDIUM (~120 μm; oil: 100 μL/min, polymer 4 μL/min), and LARGE (~200 μm; oil: 75 μL/min, polymer: 5 μL/min) (Fig. 2c). It is important to note that exact flow rates depend on several variables including device design, syringe size used, and polymer viscosity, and should therefore be optimized by the user for a specific composition. Images of microgels can be obtained using a light or fluorescence microscope and microgel diameters can be determined using standard software such as ImageJ. Size distribution graphs of the microgel batches show relatively monodisperse populations (Fig. 2d). A wider distribution is observed for larger microgels likely due to viscous instability caused by increasing the flow rate of the polymer phase.
Critical Step microgel crosslinking:
Concentration of crosslinker (DTT) must be optimized for each polymer batch with varying degrees of functional group modification. Too low a concentration leads to weakly crosslinked networks that can cause microgels to fracture during subsequent washing and processing steps.
Critical Step polymer behavior in microfluidic channels:
Many inherent properties of polymers can influence their suitability for microfluidic-based droplet generation including viscosity, degree of modification, and temperature sensitivity. It is therefore recommended to benchmark the microfluidic behavior of any newly used polymer with one that has been previously reported in literature to work well with this technique, or alternatively to improvise on the microfluidic device design or setup to accommodate for the unique properties of a polymer.
2.2.2. Step 2: Microgel washing and packing
For downstream applications, microgels should first be washed to remove any oil and surfactant (Fig. 2e). To do this, transfer the microgels to microcentrifuge tubes with about 200 μL of microgels per tube. Pellet the microgels at high centrifugation speeds (5000-10,000 rcf) and aspirate the oily supernatant phase. Resuspend the pellet in ~ 1 mL PBS containing 0.1% v/v Tween-20 and incubate on a vortex shaker for 10 minutes; this helps to capture tiny oil droplets that are invisible to the naked eye. Centrifuge and aspirate the supernatant. Resuspend microgels in PBS and transfer to a clean tube. Repeat the wash steps with surfactant and with plain PBS multiple times if the sample looks unclean.
Granular hydrogels are formed by either centrifuging the microgel suspension to form a cohesive pellet or by vacuum jamming to remove the liquid phase (Fig. 2f). Transfer the microgel suspension into a microcentrifuge tube and centrifuge at either low (1000 rcf) or high (18,000 rcf) speeds for 5 minutes. Then carefully remove the supernatant using a pipette. To achieve very high packing, place a membrane filter on a Buechner funnel positioned on a Buechner flask with a connected vacuum line. Pipette the microgel suspension onto the membrane filter and allow the vacuum to remove the liquid phase; in most cases this happens within 0–60 seconds of adding the sample. With a spatula, collect the granular sample and use for downstream applications.
3. Method 2: Rheological characterization of granular hydrogels
Rheology can be used to characterize the flow behavior of granular hydrogels.12 Investigating this behavior is crucial in the design of granular hydrogels for injectable and extrudable applications, where shear-thinning and self-healing behavior is desired. We have previously described methods to assess the rheological behavior of injectable hydrogels.45 In this section, we describe methods to assess the rheological properties of granular hydrogels, drawing attention to critical adjustments and differences from the rheometer setup to those methods for traditional bulk hydrogels. We present these methods by investigating the rheological differences in granular hydrogels formed from microgels with various sizes and different degrees of packing.
3.1. Materials
3.1.1. Reagents
Granular hydrogel samples
Phosphate buffered saline (e.g., DPBS, no calcium, no magnesium Catalogue # 14190235)
Deionized water
3.1.2. Equipment
Rheometer (e.g., AR 2000 EX, TA Instruments)
Parallel plate geometry
Peltier plate stage
Spatula
1.5 mL microcentrifuge tubes (e.g., Fisherbrand™ Premium Microcentrifuge Tubes Catalogue # 05-408-129, Fisher Scientific)
3.2. Procedure
3.2.1. Step 1: Rheometer setup and calibration
The following steps are specific to the TA Instrument AR 2000 EX rheometer. Setup and calibration instructions should be followed as provided by the rheometer manufacturer. First, turn on the air compressor and close the vent valve. When the pressure reaches 30 PSI, turn on the AR 2000 EX rheometer. Open the instrument control software on the computer and calibrate the system inertia. Attach the Peltier plate stage to the rheometer, leaving the temperature setting at room temperature (25 °C). If desired, the temperature stage can be set to 37 °C to mimic physiological temperature. Attach the parallel plate geometry to the rheometer. Calibrate the geometry inertia, rotational mapping, and zero gap height.
Critical step: Using parallel plate geometry.
The geometry attached to the rheometer can greatly impact the rheological measurements obtained. For granular systems, parallel plate geometry is most appropriate, as larger gap heights can be used to accommodate larger particle sizes compared to cone-and-plate configurations. In these studies, we used a 20 mm steel plate geometry. In general, the gap height of the parallel plate geometry should be sufficiently larger than the diameter of an individual microgel particle to ensure that the rheometer is measuring the bulk macroscale properties of the granular hydrogels instead of the microscale properties of individual particles. In our studies, the gap height was set to 10x the size of the average microgel particle.
3.2.2. Step 2: Sample loading
Prior to loading the granular hydrogel sample, determine the approximate volume of sample you will need for each rheometer run. This is determined by considering the size of your microgel particles. For example, if the microgel diameter is ~60 μm, a gap height of at least ~600 μm (ten times the diameter) is best for rheometry. Considering the 20 mm diameter of the parallel plate geometry, the volume of granular hydrogel to be loaded onto the rheometer is about ~190 μL. Using a spatula, place the approximate volume of granular hydrogel required onto the center of the Peltier plate (Fig. 3a). It is best to avoid air bubbles in the granular hydrogel whenever possible. Lower the geometry to the desired gap height. If conducting extended rheometry runs (i.e., more than ~30 min), excess PBS droplets can be added around the granular hydrogel (at least 2 cm away from the sample to avoid contact with the hydrogel) on the Peltier plate to keep the sample hydrated. Alternatively, mineral oil can also be used as a sealant to prevent sample dehydration.
Fig. 3:
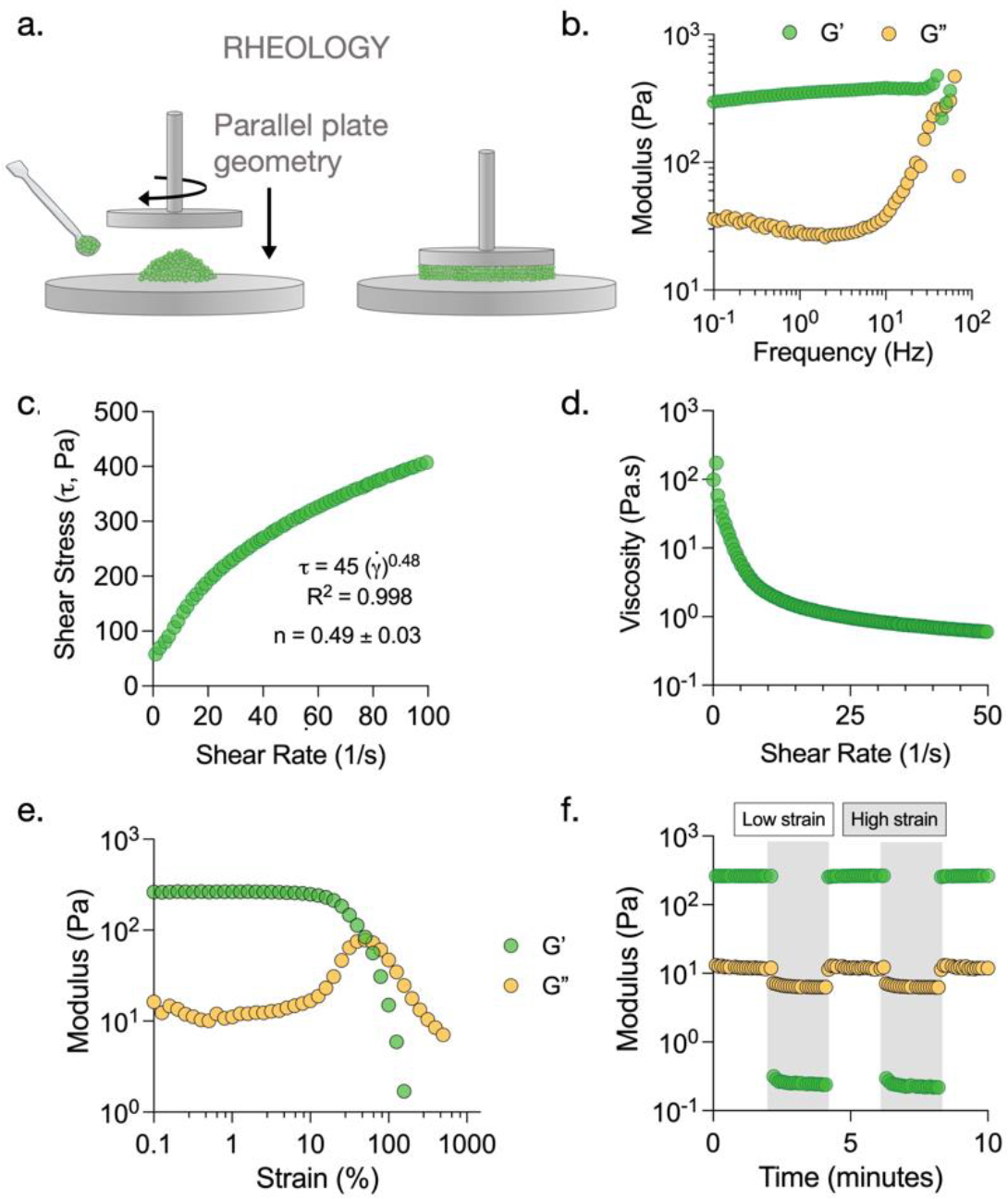
Rheological characterization of granular hydrogels. (a) Oscillatory shear rheometry with parallel plate geometry is used to characterize rheological behavior of granular hydrogels. (b) Representative frequency sweep performed from 0.1 Hz to 100 Hz at a constant strain of 1% shows a linear trend in storage modulus (G’) at low frequencies. (c) Representative plot of shear stress vs. shear rate fitted to a power law with an exponent of 0.49 indicating shear-thinning behavior of granular hydrogels. (d) Representative plot showing reduction in sample viscosity with increasing shear rate. (e) Representative strain sweep (0.1 % to 500 %) showing sample yielding (G” > G’) at high strains. (f) Representative time sweep showing strain yielding and self-healing behavior of granular hydrogels when assessed through regimes of 0.5% (unshaded) and 500% (shaded) strain.
Critical step: Proper filling of sample under the geometry.
Ensure that the granular hydrogel is loaded evenly under the geometry and not over-filled or under-filled. Ideally, the granular hydrogel should form a perfect disc under the parallel plate geometry (Fig. 3a). If under-filled, raise the geometry and add more granular hydrogel, then lower the geometry. If over-filled, use a pipette tip or spatula to remove excess granular hydrogel.
3.2.3. Step 3: Data acquisition
In the instrument control software, add the procedure to be used for granular hydrogel analysis. These tests may vary depending on the granular hydrogel sample and information of interest. For the studies performed herein, we use the following procedure, which takes about ~25 min from start to finish:
Time Sweep, 0.5% strain, 1 Hz, 2 min
Frequency sweep, 0.1–100 Hz, 1% strain, 20 points per decade, 8 min
Time Sweep, 0.5% strain, 1 Hz, 2 min
High strain (cyclic strain) Time Sweep, 500% strain, 1 Hz, 2 min
Low strain (cyclic strain) Time Sweep, 0.5% strain, 1 Hz, 2 min
Repeat steps 2 and 3 an additional time
- Strain Sweep, 0.05 – 500% strain
- This step is conducted at a constant frequency of 1 Hz, and a sample interval of 10 points per decade.
Shear ramp, 0.01 – 100 (1/s)
3.2.4. Step 4: Data analysis
Export the raw data from the instrument software and analyze/plot using a software of choice (e.g., Excel, GraphPad Prism). The storage (G’, Pa) and loss (G”, Pa) moduli can be determined at low strain (0.5%) in the initial Time Sweep. The range in which the material exhibits linear trend in properties can be determined from the frequency sweep (Fig. 3b). Non-Newtonian flow behavior can be investigated by fitting shear stress vs. shear rate data to a power law model, γ̇ = K(γ̇)n, where γ̇ is the shear stress, K is the consistency index, γ̇ is the shear rate, and n is the power law index.46 Shear-thinning behavior is indicated by a power law index less than 1 (i.e., n < 1) (Fig. 3c). Alternate models could be used for fitting data, such as the Herschel-Bulkley model to account for critical shear stress needed to begin flowing. Shear-thinning behavior is also described by a reduction in viscosity with an increase in shear rate (Fig. 3d). The yield strain (%) can be determined from the Strain Sweep, as the strain at which G” becomes greater than the G’ value (Fig. 3e). Self-healing can be quantified by measuring the percent recovery of G’ before and after shearing at high strain (Fig. 3f).
3.2.5. Anticipated Results
For these investigations, we prepare granular hydrogels and characterize with rheology for various parameters such as shear recovery and shear yielding (Fig. 4a). Specific formulations included granular hydrogels with various degrees of packing (Fig. 4b) and various microgel diameters (Fig. 4c). All granular hydrogels exhibit self-healing behavior, as storage moduli are completely recovered after cycles of high strain. We find that increasing the degree of packing using a constant microgel size of ~120 μm results in an increase in storage modulus from ~80 Pa at low packing (centrifugation), ~270 Pa at high packing (centrifugation), and ~520 Pa at very high packing (vacuum filtration). Further, the yield strain also increases as the degree of packing increases. When changing microgel size at a constant degree of packing achieved via centrifugation at 18,000 rcf, we find that increasing the microgel diameter results in a decrease in storage modulus from ~480 Pa for small microgels to ~250 Pa for large microgels. Similar trends are observed in yield strain, as medium and large microgels result in a yield strain of ~50%, whereas small microgels lead to a yield strain of ~80%.
Fig. 4:
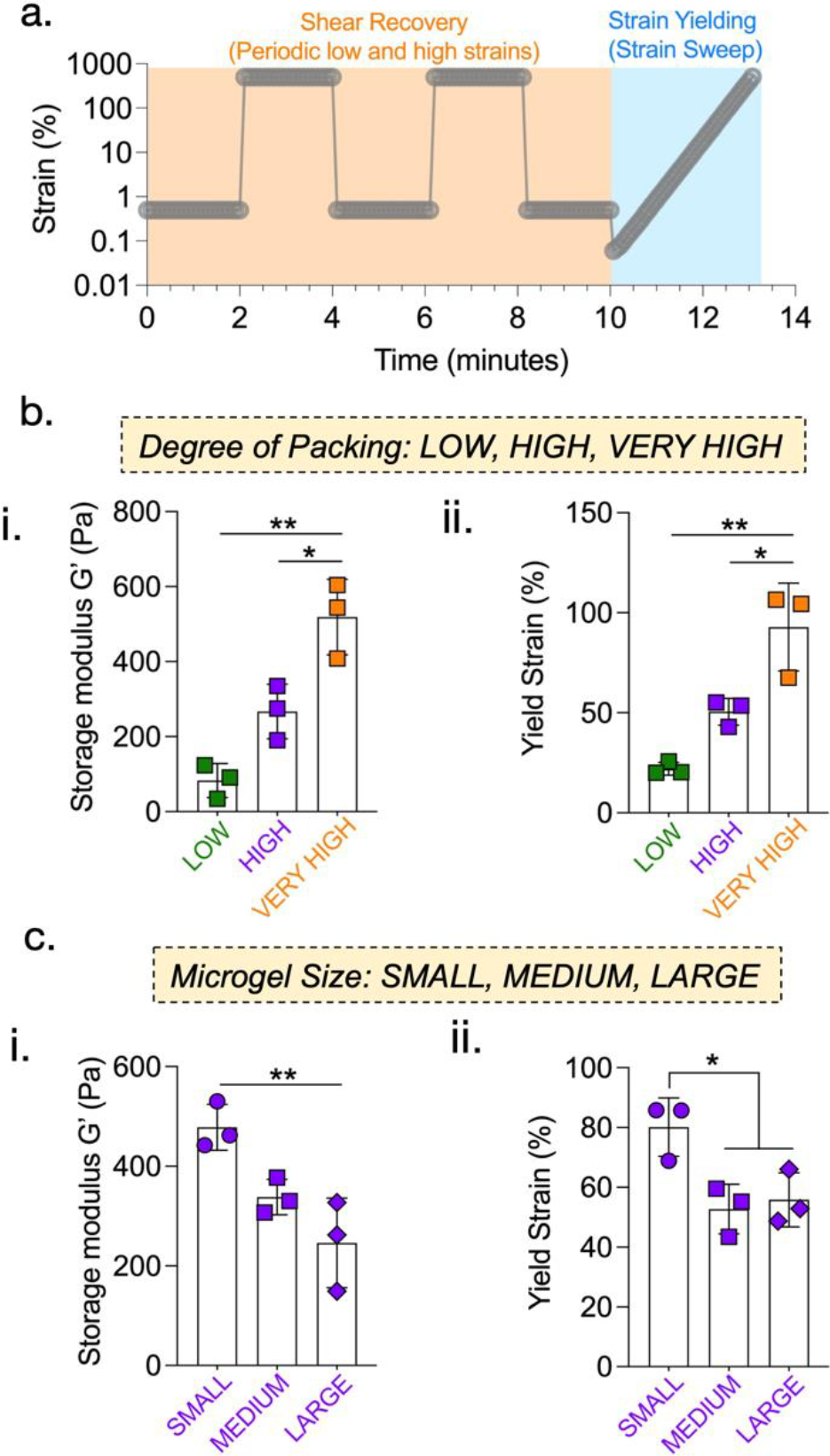
Effect of degree of packing and microgel size on rheological behavior of granular hydrogels. (a) Programmed regimes of varying strain rates to determine moduli, strain recovery, and strain yielding properties. Microgel packing and size influence the rheological behavior of granular hydrogels. Storage moduli (G’ derived at 0.5% strain) (b.i) increase significantly with constant microgel size and increasing degree of packing (LOW: ~80 Pa; HIGH: ~270 Pa; VERY HIGH: ~520 Pa), but (c.i) decrease with constant degree of packing and increasing microgel size (SMALL: ~480 Pa; MEDIUM: ~340 Pa; LARGE: ~250 Pa). Yield strains (b.ii) increase significantly with packing (LOW: ~22 %; HIGH: ~50 %; VERY HIGH: ~93 %), but (c.ii) decrease from the smallest to the largest microgel size (SMALL: ~82 %; MEDIUM: ~53 %; LARGE: ~55 %). [*p<0.05; **p<0.01]
4. Method 3: Porosity characterization of granular hydrogels
Granular hydrogels have inherent porosity consisting of interconnected pores due to the interstitial spaces that exist between microgels. Although evaluating 3D interconnected pores with standard techniques can be challenging, 2D porosity can be evaluated qualitatively and quantitatively using commonly used reagents, microscopes, and image processing software. In this section, we describe methods to characterize the porosity, pore size, and number of pores in granular hydrogels. Granular hydrogels with varying porosities can be obtained by changing microgel size and/or degree of packing, and the methods described can be used to quantitatively characterize features and compare among groups. Finally, we validate the values for porosity obtained using 2D analysis by comparing with values obtained with 3D analysis using specialized software (IMARIS).
4.1. Materials
4.1.1. Reagents
High molecular weight FITC-Dextran (e.g., Fluorescein isothiocyanate-dextran, average mol wt 2,000,000 Catalogue # FD2000S-1G)
Phosphate buffered saline (e.g., DPBS, no calcium, no magnesium, Catalogue # 14190235)
Polydimethylsiloxane PDMS elastomer (e.g., SYLGARD™ 184 Silicone Elastomer Kit, Catalogue # 2065622, Ellsworth Adhesives)
4.1.2. Equipment
Vortex (e.g., Catalogue# 02-215-414, Thermo Fisher Scientific) with microcentrifuge tube holder (e.g., Catalogue# 11-676-363, Thermo Fisher Scientific)
Spatula
Microcentrifuge (e.g., Eppendorf Refrigerated Microcentrifuge 5417R, Eppendorf)
Buchner funnel
Membrane filter (e.g., Durapore® Membrane Filter, 0.22 μm Catalogue # GVWP04700, Merck Millipore)
1.5 mL microcentrifuge tubes (e.g., Fisherbrand™ Premium Microcentrifuge Tubes Catalogue # 05-408-129, Fisher Scientific)
Glass coverslip (e.g., Micro Cover Glasses Catalogue # 72200-31, Electron Microscopy Sciences)
Objective slides (e.g., Fisherbrand™ Economy Plain Glass Micro Slides Catalogue # 12-549-3, Fisher Scientific)
6 mm biopsy punch (e.g., Integra™ Miltex™ Standard Biopsy Punches Catalogue # 12-460-412, Fisher Scientific)
Confocal Microscope (e.g., SP5 Leica)
Computer with ImageJ and IMARIS software
4.2. Procedure
4.2.1. Step 1: Sample preparation
Start by preparing PDMS sample holders. These can be prepared in bulk ahead of time and trimmed as necessary at the time of sample preparation. To prepare PDMS sample holders, follow SYLGARD 184 manufacturer instructions. Typically, this involves combining the base elastomer with the curing agent in a 10:1 ratio in a disposable paper cup and rigorously mixing using a 1000 μL pipette tip or a plastic spatula. The mixture is then degassed, such as by placing inside a desiccator connected to a vacuum line to remove air bubbles. After visually confirming removal of air bubbles, the mixture is cast onto empty petri dishes (e.g., diameter 15 mm) or well plates. When casting, it can be helpful to note the weight of material poured so that the thickness of the final cured PDMS sample can be adjusted in later iterations. For imaging granular hydrogel porosity, the target thickness should be 1.5–2 mm. Curing can be performed at 37 °C overnight or at higher temperatures (60–80 °C) for 2 hours. Cured PDMS can be peeled from the dish/plate and trimmed to size using a blade. A rectangular trim is most appropriate since it can be easily placed onto a standard objective slide. Use a biopsy punch to create a through-hole in the PDMS slab. When placed on an objective slide, the hole becomes the sample holder. Multiple holes can be punched into the same slab; however, these should be placed far apart from each other to prevent sample cross-contamination or spillover. Gently press the PDMS slab against the objective slide to remove any bubbles and to form an air-tight seal.
Prepare a solution of high molecular weight FITC-dextran in PBS (5 mg/mL). This solution must be kept in the dark and is stable at room temperature for several months (Fig. 5a(i)). In a microcentrifuge tube, suspend a sample of washed, non-fluorescent microgels in the FITC-dextran solution (Fig. 5a(ii)). Place the sample in a tube holder on a vortex at low speed for 15 minutes. Assemble the granular hydrogel with the desired degree of packing using either centrifugation or vacuum filtration (Fig. 5a(iii)). In our studies, we use three degrees of packing: (1) low packing achieved via centrifugation at 1000 rcf; (2) high packing achieved via centrifugation at 18000 rcf; (3) very high packing achieved via vacuum filtration. Ready the sample holder as described above, then use a spatula to collect the sample from the microcentrifuge tube or membrane filter and transfer to the PDMS sample holder (Fig. 5a(iv)). A small pipette tip can be used to push the sample off the spatula and into the sample holder, but care must be taken to prevent air bubbles from forming. Seal the sample by gently placing a glass coverslip on top of the PDMS slab and pushing lightly if necessary to flatten. The glass coverslip serves two purposes: (1) to prevent the sample from drying during imaging, and (2) to present a surface where a water droplet can be placed without affecting the sample when using a water-immersion objective lens.
Fig. 5:
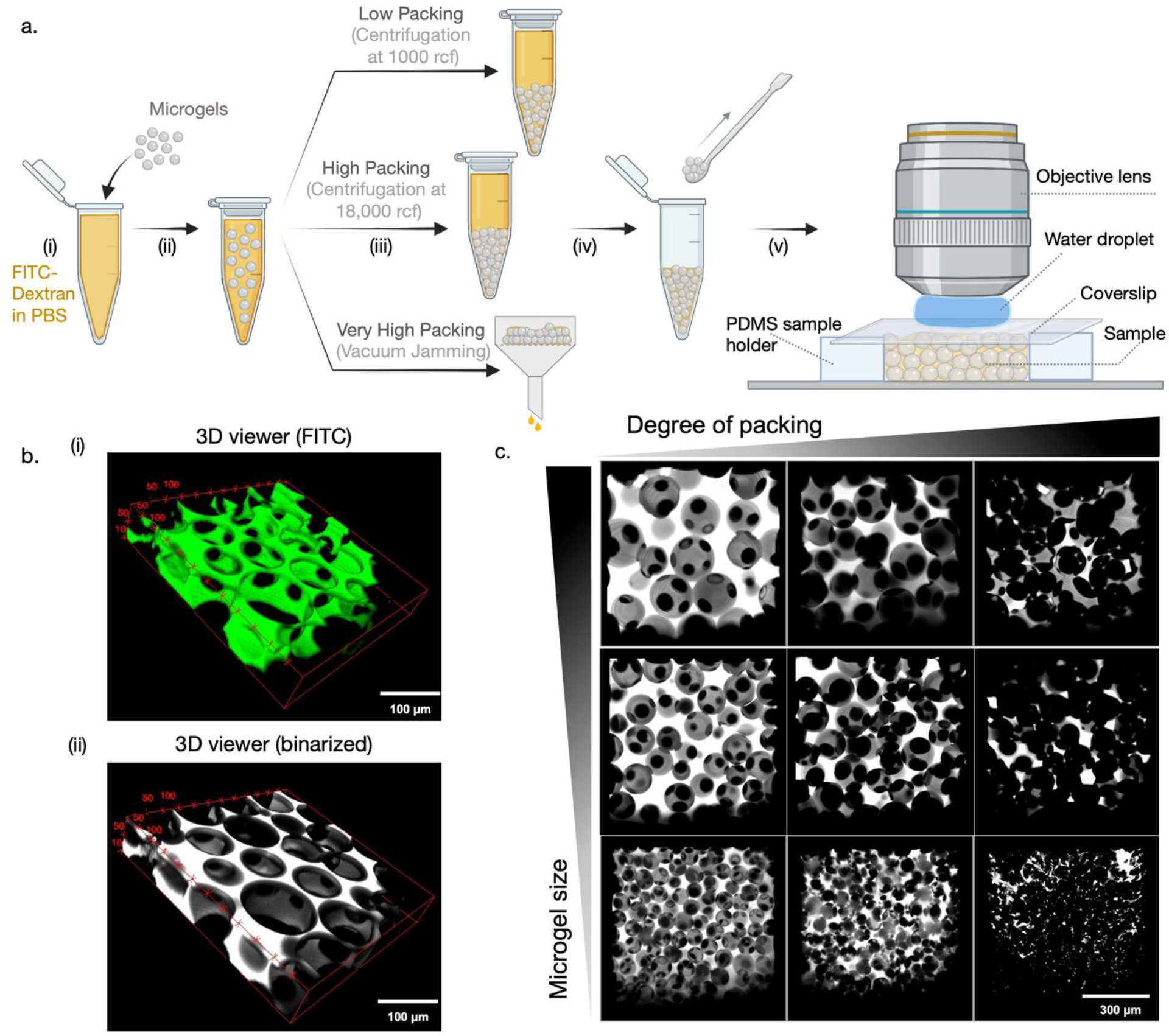
Sample preparation and imaging interstitial pores in granular hydrogels. (a) Schematic showing steps involved in the preparation of granular hydrogel samples infiltrated with FITC-dextran solution to visualize pores. (b) Volumetric stacks ((i) FITC and (ii) binarized) imaged using confocal microscopy to generate 3D reconstructions of the porous network. (c) Representative maximum projections of granular hydrogels showing the effect of changing microgel size and degree of packing on pores.
Critical Step: granular hydrogel assembly.
After centrifugation at low speeds, the granular hydrogel pellet may appear to be loosely held together. Make sure that most of the supernatant is aspirated using a pipette. Start by using a 1000 μL pipette tip and switch to smaller 200 μL (or lower) pipette tips with narrower ends to remove small volumes. Due to the conical shape of the microcentrifuge tube, samples can have subtle differences in degree of packing across their volume. To normalize this, use a pipette tip to evenly mix the pellet, then use a spatula to collect the sample. With vacuum filtration, do not leave the sample on the membrane filter for too long as this runs the risk of the sample drying out.
4.2.2. Step 2: Confocal microscope setup and Imaging
Samples in our studies were imaged using a Leica SP5 upright confocal microscope with a 25x water immersion objective lens, but the technique is generalizable to other microscopes with z-stack capabilities. To begin imaging, switch on the microscope and lasers (e.g., the Argon laser for imaging FITC) and allow some time for the laser to warm up. Place the objective slide with the sample on the microscope stage and switch on brightfield mode. Use the eyepiece and the x-y-z stage controls to bring the sample into focus and choose a region of interest (ROI). With a high frequency and low-resolution setting, switch to live view with the correct laser mode selected and adjust pinhole, laser power, and gain settings until an optimal fluorescent signal is achieved. An optimal signal is one where the non-fluorescent microgels appear dark and there is a clearly defined boundary between the dark microgels and the bright fluorescent signals originating from the interstitial pores. It is also important to note that the penetration depth of the laser is limited due to absorption of the excitation energy along the beam path and scattering by the sample itself. Therefore, it is strongly advised to use the z-axis stage controls to note the depth at which signals start to become blurry. Imaging at and beyond this depth should be avoided due to a sharp decrease in signal intensity. When settings have been optimized, use the z-stack acquisition panel to mark the beginning and end of a volumetric z-stack at the chosen ROI. Choose a z-step size between 5 and 10 μm to ensure that microscale features are not excluded and a high-resolution image can be reconstructed during image processing. In our studies, imaging is carried out at a frequency of 200 Hz and a resolution of 1024 × 1024 pixels, and each ROI is composed of at least 20 slices with a z-step size between 5 and 7 μm. The z-step size can be adjusted accordingly for samples with small feature sizes (e.g., small step sizes when small microgels are used).
Critical Step: laser power.
High laser power, gain, or pinhole settings can lead to bleaching of the fluorescence signal. It is important to start with low laser power, gain, and narrow pinhole settings when switching to live view and then adjust signals accordingly.
4.2.3. Step 3: Image processing and analysis with ImageJ
Confocal files can be processed using standard software such as ImageJ as well as specialized software such as IMARIS. In our studies, we use the built-in functions and plugins in ImageJ to reconstruct 3D views of the sample, generate maximum projection images, and compute pore features. ImageJ is an open-source software that can be downloaded free of charge from https://imagej.nih.gov/ij/. To begin image processing, load the confocal file directly into ImageJ and choose the image stack of interest. The scroll bar at the bottom can be used to browse through the individual images within the stack. The threshold function (Image > Adjust > Threshold) can be used to binarize the stack using one of several algorithms available within the thresholding option window; in our studies the Huang and Otsu algorithms provide an accurate overlap with the confocal image. The 3D viewer plugin (https://imagej.nih.gov/ij/plugins/3d-viewer/) can be used to visualize volumetric renderings of the image stack in either default or binarized modes (Fig. 5b). Using the 3D viewer tool, we compare how interstitial porosity changes across a set of 9 samples: i.e., microgels of three different sizes that are packed at three different degrees of packing. We observe that increasing microgel size leads to the formation of larger interconnected pores, whereas increasing the degree of packing reduces the size and number of pores (Fig. 5c).
4.2.4. Step 4: Quantification of porosity, pore size, and number of pores
By following simple steps that use built-in functions, ImageJ can be used to quantify the porosity, pore area, and number of pores in granular hydrogel samples (Fig. 6a). To quantify porosity, convert confocal stacks to 8-bit format (Image > Type > 8-bit), then adjust thresholds using the method describe above, and apply the smooth function (Process > Smooth) (Fig. 6b). In general, a few large interstitial pores will be observed at lower packing and with a larger microgel size, and many small interstitial pores will be observed at higher packing and a smaller microgel size (Fig. 6c). Use the analyze particles function (Analyze > Analyze Particles…) to obtain quantitative values describing the pores (white regions) within each 2D slice of the confocal stack. It is important to note that small artifacts are always present due to variations in pixel intensities, particularly along the boundaries of microgels and interstitial pores. In our experience most of these artifacts are smaller than 5 μm2 in size. To avoid counting these artifacts as pores, assign a size range of 5 μm2-Infinity in the pop-up options window. The pop-up summary table will list the number of pores (Count) and porosity (%Area) of each slice in the stack. The pop-up results table will list the size (Area) of every pore in the stack. For each region of interest, a porosity value can be obtained by taking the mean of the porosity of each slice in the stack. This should be repeated for multiple regions of interest across multiple samples to get an average porosity value for each group. In our studies, the porosity varies from 2–40% across the 9 groups characterized (Fig. 6d). To confirm the validity of the porosity values obtained using the 2D analysis method, we perform 3D reconstruction of confocal stacks using the Surfaces feature in a specialized software called IMARIS (Fig. 6g). Powerful 3D rendering algorithms available in IMARIS allow accurate reconstruction of confocal stacks to compute numerous quantitative parameters including volumes of 3D objects enclosed by the created surfaces (Fig. 6h). 3D porosity values can be obtained by dividing the sum of all objects’ volumes by the total volume of the confocal stack. This quantification reveals no significant differences between quantified 3D and 2D porosity values for samples prepared with ~ 100 μm sized microgels (Fig. 6i).
Fig. 6:
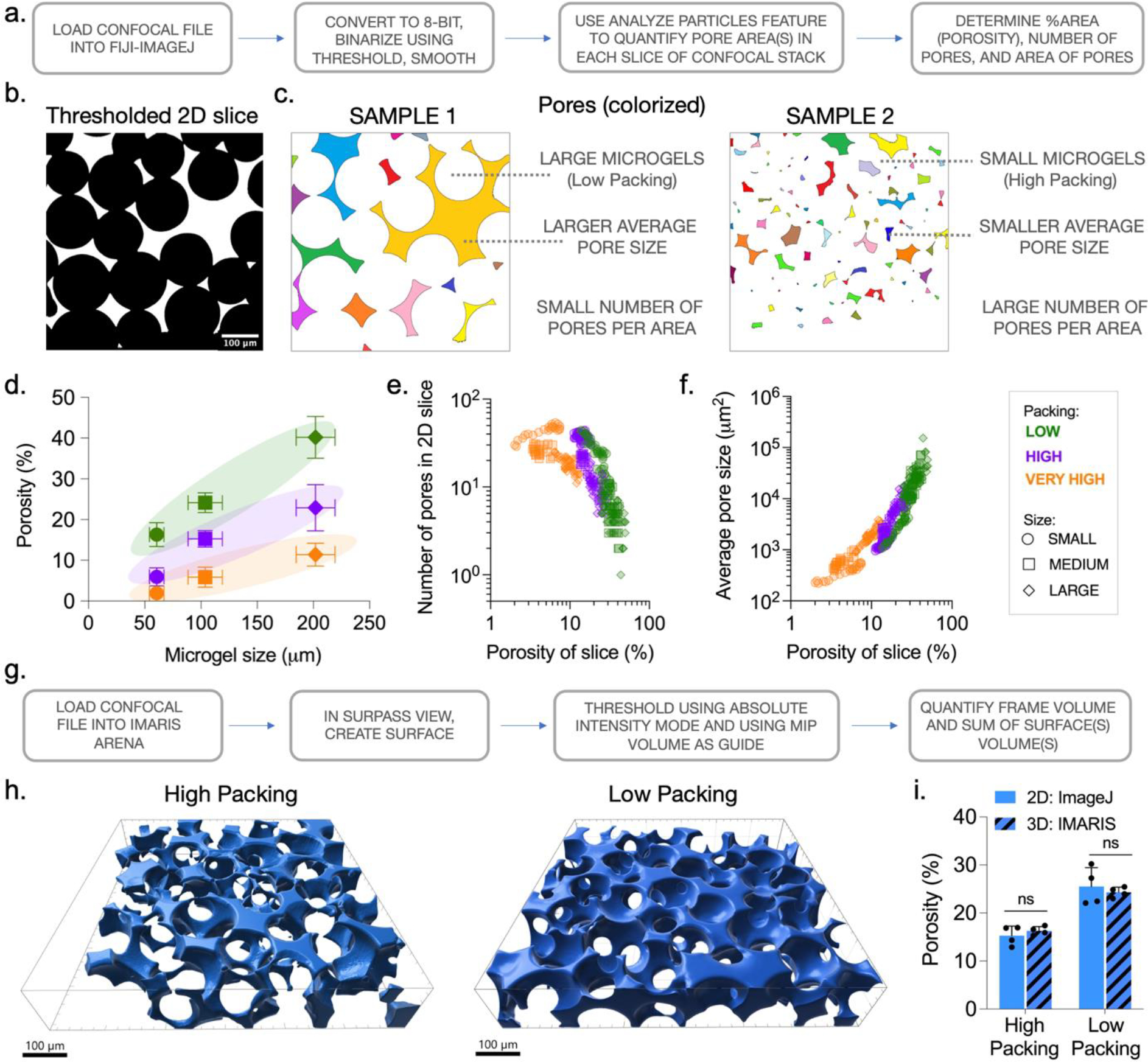
Quantifying porosity of granular hydrogels. (a) Workflow to quantify 2D slice porosity from confocal stacks using FIJI/ImageJ. (b) Individual 2D slices from confocal stacks can be thresholded based on FITC intensity using standard image processing software (e.g., ImageJ). (c) Segmented pores (colorized to aid visualization) can vary in size and number across granular hydrogel samples prepared with varying microgel size and packing. (d) Combinatorial modulation of microgel size (SMALL, MEDIUM, LARGE) and packing (LOW, HIGH, VERY HIGH) result in granular hydrogels with overall porosities ranging from ~2 % to ~40 %. Analysis of confocal stacks across multiple formulations highlights the influence of changing microgel size and packing on (e) number of pores in a 2D slice and (f) pore size. (g) Workflow to quantify 3D volumetric porosity from confocal stacks using IMARIS. (h) 3D reconstruction of confocal stacks using IMARIS to visualize pores (blue) in HIGH and LOW packed granular hydrogels. (i) Quantified values of overall pore volume with IMARIS closely match the quantified values obtained from 2D slices processed with ImageJ.
4.3. Anticipated Results
In our studies we observe interesting trends between the microgel size and degree of packing used to assemble granular hydrogels, and the porosity, average pore size, and the number of pores obtained through structural characterization. Low packing of larger microgels results in high porosity granular hydrogels, whereas very high packing of smaller microgels results in very low porosities (Fig. 6d). Further, trends are observed when values for the number of pores and average pore size obtained from 2D slices are plotted against slice porosity across 9 groups (Fig. 6e,f). Specifically, slice porosity negatively correlates with the number of pores per slice and positively with average pore size. It should be noted that since pores are defined by the interstitial spaces between enclosing microgels, pores with unique architectures and features may be formed when using microgels of different shapes or size distributions (e.g., polygonal fragmented microgels, anisotropic rod-shaped microgels, or a polydisperse mixture of microgels). Additionally, porosity can be modulated by incorporating degradable microgels prior to packing. In vitro degradation can be tracked through microscopy or fluorescent detection of encapsulated dye that is released into surrounding medium.
5. Method 4: Cell Invasion Assay
Cell invasion into implanted biomaterials is crucial for integration with host tissue and to promote tissue repair. Whereas traditional bulk hydrogels have nanoscale pores that block cells from invading, granular hydrogels have interconnected microscale pores that cells and vessels can invade into, potentially accelerating tissue repair. Before performing in vivo tests in animal models, it can be helpful to perform in vitro assays to determine how properties of granular hydrogels influence cell invasion. In this section, we describe an in vitro assay that combines microgels, multicellular spheroids, and an interstitial matrix to study how microgel size and packing affect the invasion of lumen-containing sprouts into interstitial spaces (Fig. 7a).
Fig. 7:

3D assay to evaluate the invasion of endothelial cells and sprouts into granular hydrogels. (a) Schematic showing key components of the sprout invasion assay. (b) Spheroids of HUVECs and MSCs are formed using microwell arrays. (c) Interstitial matrix is optimized by varying the type and concentration of crosslinker (non-degradable DTT or MMP-degradable) to support sprouting from spheroids. Schematics showing steps involved in the preparation of spheroid-embedded granular hydrogel samples (d) without and (e) with infiltrated interstitial matrix. (f) Without interstitial matrix, cells either adhere and spread on the surface of neighboring microgels (+RGD) or remain part of the spheroid (−RGD); however, with interstitial matrix, robust sprout formation and invasion are observed.
5.1. Materials
5.1.1. Reagents
Cell Culture:
Trypsin (e.g., Trypsin 0.05% EDTA, Catalogue # 25300054, Invitrogen)
Penicillin-Streptomycin (e.g., Catalogue # 15140122, Invitrogen)
Phosphate buffered saline (e.g., DPBS, no calcium, no magnesium Catalogue # 14190235)
Endothelial Cell Growth Medium-2 (EGM™-2) BulletKit™, (CC-3156 & CC-4176), Lonza CC-3162
Cell growth media (e.g., Alpha-MEM, Catalogue # 12561056, Invitrogen)
Live/Dead staining kit (e.g., Catalogue # L3224, ThermoFisher Scientific)
Recombinant human vascular endothelial growth factor (VEGF-165 e.g., Catalogue # 293-VE, R&D Systems)
Phorbol 12-myristate 13-acetate (PMA) (e.g., Catalogue # P8139, Merck)
Sphingosine-1-phosphate (S1P) (e.g., Catalogue # 62570, Cayman Chemical)
Staining:
Phalloidin Alexa Fluor 647 (e.g., A22287, ThermoFisher Scientific)
Fixative (e.g., 4% paraformaldehyde Catalogue# 30450002 bioPLUS™, bioWworld)
Permeabilizing agent (e.g., Triton X-100, Catalogue # 93443, Sigma Aldrich)
Blocking solution (e.g., Fetal Horse Serum Catalogue # SRX0960, SEROX)
Spheroid Formation
PDMS elastomer (e.g., SYLGARD™ 184 Silicone Elastomer Kit, Catalogue # 2065622, Ellsworth Adhesives)
Agarose (e.g., UltraPure™ Agarose, Catalogue # 16-500-100, ThermoFisher Scientific)
Human mesenchymal stromal cells (MSCs) (can be primary cells isolated from donated sample tissue, or purchased from commercial supplier)
Human umbilical vein endothelial cells (HUVECs) (e.g., Catalogue # C2519A, Lonza)
Hexane
Silane (e.g., Trichloro(1H,1H,2H,2H-perfluorooctyl)silane, Catalogue # 448931 Sigma-Aldrich)
Interstitial Matrix (IM)
Custom-ordered RGD peptide (GCGYGRGDSPG, Genscript)
MMP-degradable peptide (GCNSVPMSMRGGSNCG, Genscript)
DTT (Dithiothreitol; Catalogue # D0632, Sigma-Aldrich)
Nor-HA
5.1.2. Equipment
6-well plates
X-acto precision knife (e.g., Office Depot Catalogue # 930248)
Omnicure S1500 UV lamp
Spatula
1.5 mL microcentrifuge tubes (e.g., Fisherbrand™ Premium Microcentrifuge Tubes Catalogue # 05-408-129, Fisher Scientific)
Microwell array master (e.g., AggreWell™ 400, Catalogue # 34421, STEMCELL Technologies)
Glass coverslips (e.g., Micro Cover Glasses Catalogue # 72200-31, Electron Microscopy Sciences)
Objective slides (e.g., Fisherbrand™ Economy Plain Glass Micro Slides, Catalogue # 12-549-3, Fisher Scientific)
Microcentrifuge (e.g., Eppendorf Refrigerated Microcentrifuge 5417R, Eppendorf)
1 mL plastic syringes (e.g., Tuberculin Syringe BD Catlogue # 309659)
Cell microsieves 20 μm mesh size (Catalogue# NSB; BioDesign Inc.)
5.2. Procedure
5.2.1. Step 1: Microwell array formation
Microwell arrays are a reliable and high-throughput method to form spheroids with reproducible size and high viability. Single use microwell arrays can be purchased directly from commercial suppliers or made in-house. In our studies, we fabricate microwell arrays in-house using negative molds prepared by casting on a commercially purchased product (master). Start by treating the surface of the master array with silane vapor (e.g., by pouring a few drops of silane in a weigh boat placed next to the master inside a desiccator connected to a vacuum line) for 30 minutes. Separately, prepare PDMS by mixing base elastomer, curing agent, and hexane. In our studies we mix 2.5g base elastomer with 0.25 g curing agent and 1 mL hexane; this generates enough PDMS to pour over one well of a 6-well format master array. Combine these components in a 50 mL conical tube and mix vigorously using a vortex. Keep lid closed to prevent evaporation until the silane-treated master is ready. Pour the PDMS over the master array and degas to remove air bubbles. After complete removal of bubbles, bake the master array at 55–70 °C for 2 hours. Peel off the negative mold from the master array and allow it to cool down to room temperature. Separately, dissolve agarose powder in PBS at a concentration of 4 % w/v by heating in a microwave oven and mixing intermittently. Pour the dissolved agarose into a well plate and use the PDMS negative as a stamp, pushing down gently on the agarose. This step should ideally be performed in the cell-culture hood using sterile well plates. Transfer the well plate to a fridge and allow the agarose to solidify for 2 hours. Once cooled, peel away the PDMS negative mold and sterilize the agarose under a germicidal UV lamp. Pour PBS and transfer to incubator. The PDMS molds are stable for many months, but wear and tear can cause loss of microscale features that are then transferred to the agarose array and ultimately influence spheroid formation. Therefore, it is strongly advised to regularly check the quality of the agarose microwell arrays under a light microscope and make new PDMS negative molds as necessary.
Critical Step: safety protocols.
Silane and hexane are neurotoxins and irritants. Work with these chemicals should be performed inside a fume hood. Follow the appropriate health and safety guidelines provided by your institution and noted on the chemical safety data sheets.
5.2.2. Step 2: Spheroid formation
Spheroids are formed by pipetting a 2:1 mixture of HUVECs and MSCs, respectively, directly onto the agarose microwell array (Fig. 7b(i)). Briefly, HUVECs and MSCs should be cultured in parallel and expanded to large enough numbers to be sufficient for seeding onto the microwell array. Final cell numbers will depend on the desired size of the spheroids. This should be optimized by each lab as it depends on the size and number of microwells on the master array, which can vary between commercial suppliers. If fewer spheroids are required, a larger agarose microwell array can be cut down to the size of smaller well-plates using a blade or X-acto knife. Calculate how many cells are required for the experiment. Use trypsin to detach HUVECs and MSCs from their culture flasks and count the cells using an automatic cell counter or a hemocytometer after staining with Trypan blue. Combine the two cell populations according to the ratio described above and centrifuge to form a pellet. Using a pipette, resuspend the pellet in a mixture of endothelial growth media and alpha-MEM (2:1 corresponding to the ratio of cells) and carefully add to the well containing the agarose microwell array. In the array, cells self-assemble into spheroids within 24 hours and can be harvested by simple pipetting-based dispersion (Fig. 7b(ii)). Spheroid size, determined by analyzing light microscope images on ImageJ, is typically very uniform except for a few outliers that might be caused by two adjacent spheroids fusing together (Fig. 7b(iii)). To assess viability, incubate spheroids with components of the Live/Dead kit (Calcein-AM: live; Ethidium homodimer-1: dead). To enable effective diffusion of the dyes through the dense spheroid, it is recommended to incubate at 4 °C for up to 3 hours on a slow orbital shaker platform. The low temperature slows down cellular metabolism without causing loss of viability and allows sufficient time for the diffusion of dyes to the inner portion of the spheroids (Fig. 7b(iv)). A fluorescence microscope can be used to image live and dead cells and determine spheroid viability.
5.2.3. Step 3: Interstitial matrix (IM) preparation
The interstitial matrix (IM) that occupies the interstitial pores of the granular hydrogel provides the appropriate physicochemical cues to support endothelial sprouting from embedded spheroids. In our studies, we use Nor-HA as the IM base material, but with properties that are significantly different to the material used to make microgels. Parameters screened and optimized in our studies include Nor-HA concentration, RGD peptide concentration, and type and concentration of crosslinker (DTT or MMP-degradable). Screening experiments are performed by mixing spheroids in the IM solution containing a photo-initiator (LAP) and crosslinking under UV (20 mW/cm2) for 2 minutes inside a cut-off syringe barrel to form bulk hydrogels, before transferring to a well plate, washing with PBS and culturing in stimulatory media (EGM-2 bullet kit + VEGF (100 ng/mL) + S1P (500 nM) + PMA (600 ng/mL)).47 When spheroids are embedded in bulk Nor-HA hydrogel that is crosslinked with a non-degradable crosslinker (DTT) and cultured for three days in stimulatory media, no significant sprouting is observed (Fig. 7c(i–ii)). In comparison, when spheroids are embedded in Nor-HA crosslinked with an MMP-degradable crosslinker, robust sprouting is observed (Fig. 7c(iii–iv)). Notably, both IM materials have comparable mechanics (Fig. 7c(v)). The optimized IM that supported robust spheroid sprouting is composed of Nor-HA (1.8 % w/v), RGD peptide (0.5 mM), and MMP-degradable peptide crosslinker (3.5 mM), along with LAP (0.05 % w/v) as the photoinitiator.
5.2.4. Step 4: Construct assembly and culture
To study cell invasion, microgels, spheroids, and the IM solution are combined and assembled into a construct that is then cultured in stimulatory media for three days. If it is desired to simultaneously analyze both the microgels and the cells invading through their interstitial spaces, it is recommended to incorporate a fluorescent dye (e.g., high molecular weight FITC-dextran) during microgel fabrication. A complementary dye should then be used to stain cells (e.g., Alexa Fluor 647 phalloidin).
The ratio of spheroids to microgels should be optimized through trial runs and empirical observations. A higher ratio can lead to interactions between and potential fusion of adjacent or neighboring spheroids, which complicates downstream quantitative analysis. On the other hand, a lower ratio can present fewer regions of interest for imaging per sample. To investigate sprouting behavior in the absence of an interstitial matrix, pack the microgels via centrifugation and carefully aspirate the supernatant. In this experiment, microgels with and without RGD are investigated. Microgels with RGD are fabricated by incubation with a cysteine containing RGD peptide and photoinitiator for 30 minutes with mechanical agitation, followed by exposure to UV for 5 minutes and washing multiple times to remove excess unbound RGD peptide. Add the spheroids directly to the microgel pellet and distribute homogenously by mixing with a pipette tip (Fig. 7d). Because there is no interparticle crosslinking between microgels to hold this mixture together, transfer it to a PDMS mold (similar to the sample holder used for confocal microscopy described above) and flatten using a spatula. To prevent microgels from flowing away when incubated with culture media, a flexible microsieve (20 μm mesh size) can be glued onto the edges of the PDMS mold. A reservoir for culture media can be formed by gluing on another piece of PDMS with a cut-off hole on top of the mesh. This setup will prevent microgels from dispersing and therefore maintain porosity while still allowing diffusion of media and nutrients. In this experimental setup (i.e., without IM), we observe that spheroid behavior depends on the presence of cell-adhesive peptides on the surfaces of microgels. When RGD is present, individual cells migrate out of spheroids and adhere to the surface of neighboring microgels, but no sprout formation is observed (Fig. 7f). In the absence of RGD peptides, no migration is observed, but the spheroid visibly grows over the culture period.
To investigate sprouting behavior in the presence of interstitial matrix, resuspend a pellet of microgels in the IM solution and place on the vortex shaker for 15 minutes. Centrifuge the mixture to pack the microgel and aspirate excess IM solution in the supernatant. Microgels will pellet due to differences in the concentration (microgels: 3% w/v; IM: 1.8% w/v) and different centrifugation speeds will lead to different degrees of packing and thus different interstitial space volumes (equivalent to porosity when IM is not present). Add the spheroids to the pelleted microgels+IM mixture and homogenously distribute by mixing with a pipette tip. Use a spatula to transfer the mixture into a pre-fabricated PDMS mold or a cut-off syringe. Use the spatula to flatten the transferred mixture while taking care to avoid bubble formation. Place a glass slide on the mold/syringe and exposure the mixture to UV (20 mW/cm2) for 2 minutes. Peel off the PDMS mold or in the alternate setup use the syringe plunger to push out the construct. Using a spatula, transfer the construct to a well plate and wash with PBS to remove any unreacted LAP. Finally, add stimulatory media and incubate at 37 °C for three days with media changes every day (Fig. 7e). Robust sprouting and cell invasion is observed from spheroids regardless of whether the microgels contain RGD in constructs containing IM (Fig. 7f). In granular hydrogels, sprouts are tortuous and show robust branching. This is in contrast to bulk hydrogels where sprouts extend perpendicularly from the spheroid body and show less branching.
Critical Step 1: packing limitations.
It is not possible to pack the microgels+IM mixture using vacuum-driven filtration (i.e., very high packing) without blocking the membrane filter due to the relatively high viscosity of the interstitial matrix solution compared to PBS. Different centrifugation speeds can be used to vary the degree of microgel packing and thus porosity.
Critical Step 2: sterility.
For the cell invasion assay, care should be taken to work under sterile conditions. Dry materials like lyophilized Nor-HA can be sterilized under the germicidal UV lamp for 1 hour. Peptides are typically packed inside vials and supplied in sterile form; these should only be opened inside the cell culture hood.
Critical Step 3: photo-sensitive IM.
When working with the interstitial matrix solution, care should be taken to reduce exposure to ambient light to avoid premature crosslinking. Microcentrifuge tubes should be wrapped in aluminum foil if necessary and to transport to and from the microcentrifuge.
5.2.5. Step 5: Sample fixation and staining
After three days of culture, wash constructs with PBS and fix the sample for 30 minutes on an orbital shaker using 4% paraformaldehyde (PFA) while working inside a well-ventilated fume hood. Wash away excess PFA with PBS and permeabilize the sample using 0.1% v/v Triton-X in PBS. Wash samples with PBS before blocking with 3% fetal horse serum in PBS for one hour at room temperature on an orbital shaker. Constructs can then be stained (e.g., with phalloidin to stain F-Actin following manufacturer’s instructions). Additional staining can be performed with anti-CD31 antibody to label and observe the positions of endothelial cells along the sprouts, as reported recently.48
5.2.6. Step 6: Imaging and image processing
Imaging can be performed using a confocal microscope by transferring the construct into a prefabricated PDMS mold, similar to the sample holder used for confocal microscopy described above, and covering with a glass coverslip. The brightfield mode and the eyepiece can be used to locate spheroids of interest. For our studies, all imaging is carried out using a Leica SP5 upright microscope with a 25x water immersion objective lens (Fig. 8a). Microgels are fluorescent due to incorporated FITC-dextran, and cells are stained with Alexa Fluor 647 phalloidin. Using the z-stack acquisition mode, mark the starting and ending positions that correspond to the sprouts nearest to the objective lens and the mid-point of the spheroid, respectively. Frequently, sprouts can extend several hundred microns from the surface of the spheroid to the surface of the construct. Imaging these embedded spheroids presents a challenge due to the focal depth limitations of the microscope. It is recommended to identify spheroids that lie closer to the surface of the construct so that sharp images can be obtained. Care should be taken to image single spheroids and the sprouts emerging from these spheroids and to avoid imaging ROIs with fused spheroids. To capture detailed features, smaller z-step sizes (e.g., 2–5 μm) should be assigned for confocal stacks.
Fig. 8:
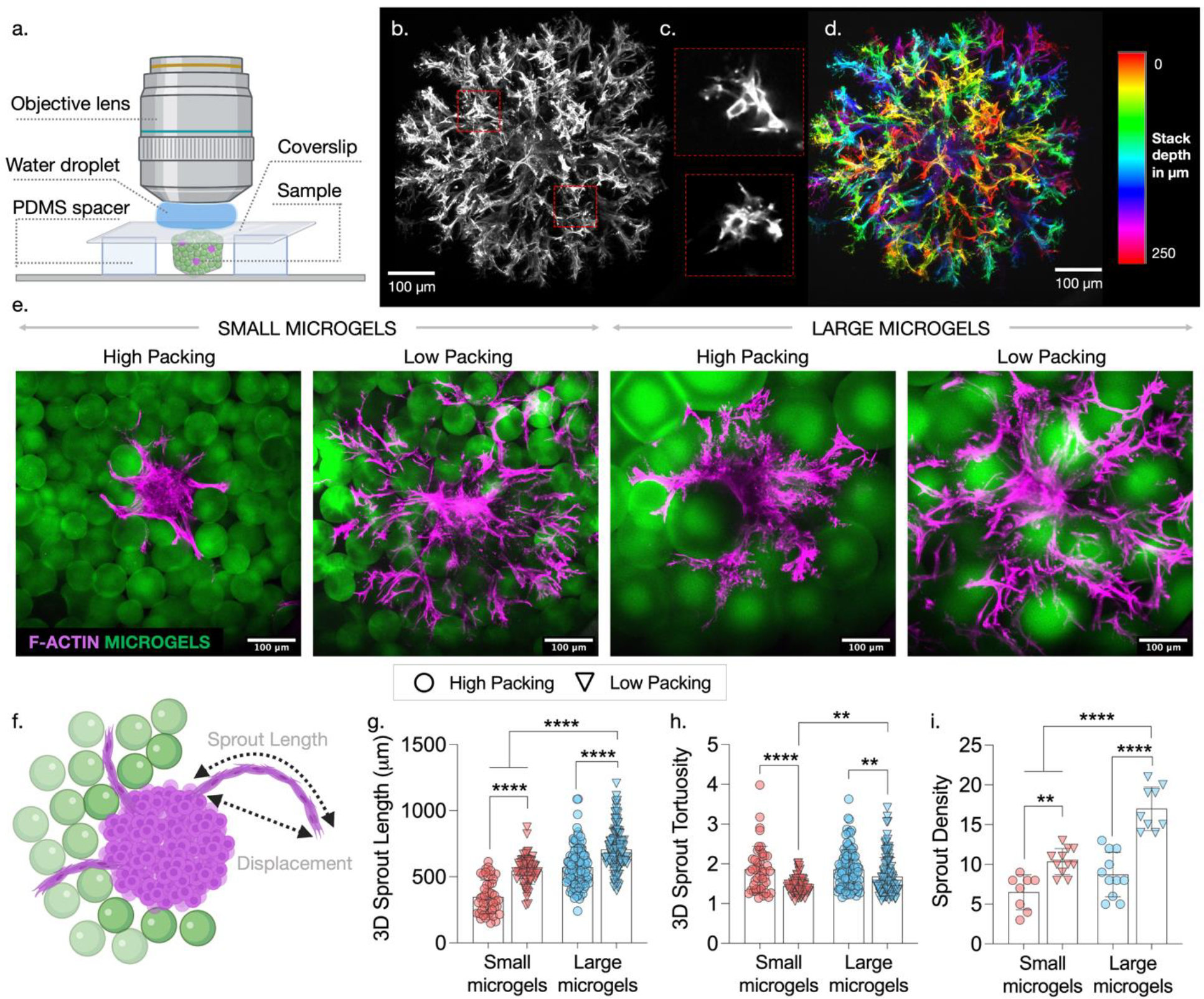
Qualitative and quantitative characterization of sprout invasion. (a) Schematic showing setup to image fixed and stained samples using confocal microscope. (b) Maximum projection of confocal stacks showing 3D sprouts extending from a single spheroid. (c) Lumen containing sprouts are observed. (d) Color-coded maximum projection of confocal stack showing sprouts extending in 3D. (e) Representative confocal images showing the effect of varying microgel size and packing on the sprout invasion. (f) Schematic showing 3D sprout length and displacement. Analysis of confocal stacks using ImageJ to quantify (g) 3D sprout length, (h) 3D sprout tortuosity (ratio of sprout length to displacement), and (i) sprout density per spheroid. [**p<0.01; ****p<0.0001]
Confocal stacks can be processed with standard image processing software like ImageJ. After loading the confocal file and selecting the stack of interest, use the scroll bar to browse through the stack to determine which images will best capture the sprouts. Create a maximum projection image, which is essentially a composite of all the images in the stack, by going to Image > Stack > Z Project (Fig. 8b). This function can also be used to create maximum projection images of smaller portions of the stack by assigning the start and stop slices in the options window. To generate images of specific features of interest such as lumen-containing sprouts, duplicate a selected region of the stack by marking a ROI using one of the area selection tools on the main ImageJ window and duplicating it. Then use the Z Project function with the appropriate start and stop slice positions (Fig. 8c). To highlight the volumetric nature of the maximum projection images, images in the stack can be depth-encoded by going to Image > Hyperstack > Temporal color code and choosing the desired color-coding scheme (Fig. 8d).
Several quantitative parameters can be determined from confocal stacks using ImageJ. For example, sprouts can be traced along the z-stack using the Manual Tracking Tool (Plugins > Tracking > Manual Tracking). This results in a table of xyz coordinates marking each position identified with the tracking tool. Quantitative description of the sprouting behavior (e.g., sprout length and sprout tortuosity) can be computed from the xyz coordinates. To do this, start by loading the confocal file into ImageJ and choosing the stack of interest. Only using the channel that shows cell staining, browse through the stack to determine if continuous sprouts can be tracked. Then navigate to the Manual Tracking Tool and follow instructions on the pop-up window. Although this is a laborious process, it is one that allows 3D length and tortuosity to be determined from a series of 2D slices. To quantify sprout density (i.e., the number of sprouts that emerge per spheroid), create maximum projections of confocal stacks including slices that contain the position of the spheroid and the radially extending sprouts. Then, manually count the number of sprouts around the spheroid perimeter. One caveat with this method is that it is challenging to count sprouts that emerge along the z-direction, especially immediately around the spheroid. However, considering that spheroids are embedded within a 3D granular hydrogel and that gradients of stimulatory growth factors exist in all directions, radial sprout density is a reliable representation of overall sprout density.
5.2.7. Anticipated Results
Using the methods described above, we investigate how sprouting behavior and cell invasion varies due to changes in granular hydrogel properties and interstitial spacing through synergistically changing microgel size and degree of packing (Fig. 8e). Because sprouting behavior is only observed when IM is incorporated (as shown in figure 7), all the experiments described henceforth are conducted in constructs containing IM. Although a high level of sprout branching and invasion is observed, interstitial spacing has a significant impact as evidenced by quantitative characterization of sprout invasion. The xyz coordinates obtained from the manual tracking tool described above are used to compute total sprout length and the sprout displacement using the formula for calculating distance between points (sqrt((x2-x1)2 + (y2-y1) 2 + (z2-z1) 2)) (Fig. 8f). 3D sprout length is lowest in lowest interstitial spacing samples (small microgels, high packing) and highest in highest interstitial spacing samples (large microgels, low packing) (Fig. 8g). Tortuosity is determined by calculating the arc-chord ratio (i.e., dividing total sprout length by sprout displacement). More tortuous sprouts are observed in high packing groups likely due to restricted interstitial spaces that cause cells to maneuver around to find paths of least resistance (Fig. 8h). Sprouts are significantly less tortuous in low packing groups where microgels are spaced further apart with larger interstitial spaces for cells to sprout through. Interestingly, among the low packing groups, sprouts are least tortuous with small microgels, likely because the smaller size creates more available interstitial openings and paths for cells to invade through. Sprout density is higher in low packing groups and with larger microgels, likely due to interstial spaces that are large enough to facilitate multiple sprouts and fewer closed pores (Fig. 8i). It should be noted that while IM is a necessary element for the in vitro assay to study endothelial cell sprouting, its inclusion is not envisioned for in vivo applications where acellular granular hydrogels are used to promote endogenous cell and vessel invasion.48 Endogenous proteins from blood or body fluids are likely to be absorbed onto the surface of injected microgels and play a role similar to IM.
6. Summary
Granular hydrogels have increased in popularity for biomedical applications because they combine many desirable properties into a highly tunable and modular design. Through rigorous characterization of their properties, granular hydrogel design can be optimized for specific applications including for 3D printing, in vitro evaluation of cell-matrix interactions, and in vivo delivery for tissue repair. We describe the use of oscillatory shear rheometry to characterize the moduli, shear thinning, recovery, and yielding properties of granular hydrogel samples, showing how these values are affected by changes in microgel size and degree of packing. Compared to traditional bulk hydrogels, granular hydrogels have inherent porosity due to interstitial spaces that exist between microgels. This porosity permits invasion of cells and blood vessels and enhances integration of the injected hydrogel with host tissue. We describe methods to quantify the porosity of granular hydrogels using standard imaging and processing that provides insights into pore size and the number of pores in addition to overall porosity, showing how these features are affected by changes in microgel size and degree of packing. Importantly, we show that despite the complex 3D interconnected porosity in granular hydrogels, standard and widely accessible methods such as the ones described here provide an accurate assessment of porosity when validated against more sophisticated 3D analyses. These pore features can be tuned to guide cell behavior, which can be evaluated through in vitro studies to screen the relationship between cell behavior and granular hydrogel properties. Although the adhesion and proliferation of single cells has been described before, evaluation of collective endothelial sprouting has been challenging to study. We describe methods to visualize and quantify sprouting behavior in constructs containing spheroids surrounded by an interstitial matrix and embedded in packed granular hydrogels. This includes methods that use standard image processing to quantify sprout length, tortuosity, and density, showing how these metrics of cell behavior change in response to changes in microgel size and degree of packing.
7. Acknowledgements:
This work was supported by the German Research Foundation (QA 58/1-1 to T.H.Q.), the National Science Foundation through the Center for Engineering MechanoBiology STC (CMMI: 15-48571), the UPenn MRSEC program (DMR-1720530), and a graduate research fellowship (to V.G.M) and the National Institutes of Health (R01AR077362 to J.A.B.). The authors thank the Cell and Developmental Biology (CDB) Microscopy Core at the University of Pennsylvania for use of IMARIS software. Schematics used in figures were created with Biorender.com.
8. References:
- (1).Garrec DA; Norton IT Understanding Fluid Gel Formation and Properties. J. Food Eng. 2012, 112, 175–182. [Google Scholar]
- (2).Garrec DA; Norton IT Kappa Carrageenan Fluid Gel Material Properties. Part 2: Tribology. Food Hydrocoll. 2013, 33, 160–167. [Google Scholar]
- (3).Zhang W; Wang P; Liu S; Chen J; Chen R; He X; Ma G; Lei Z Factors Affecting the Properties of Superabsorbent Polymer Hydrogels and Methods to Improve Their Performance: A Review. J. Mater. Sci. 2021, 56, 16223–16242. [Google Scholar]
- (4).Li XH; Liu C; Feng SP; Fang NX Broadband Light Management with Thermochromic Hydrogel Microparticles for Smart Windows. Joule 2019, 3, 290–302. [Google Scholar]
- (5).Daly AC; Riley L; Segura T; Burdick JA Hydrogel Microparticles for Biomedical Applications. Nat. Rev. Mater. 2020, 5, 20–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Riley L; Schirmer L; Segura T Granular Hydrogels: Emergent Properties of Jammed Hydrogel Microparticles and Their Applications in Tissue Repair and Regeneration. Curr. Opin. Biotechnol. 2019, 60, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bhattacharjee T; Zehnder SM; Rowe KG; Jain S; Nixon RM; Sawyer WG; Angelini TE Writing in the Granular Gel Medium. Sci. Adv. 2015, 1, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).de Rutte JM; Koh J; Di Carlo D Scalable High-Throughput Production of Modular Microgels for In Situ Assembly of Microporous Tissue Scaffolds. Adv. Funct. Mater. 2019, 29, 1–10. [Google Scholar]
- (9).Bhattacharjee T; Angelini TE 3D T Cell Motility in Jammed Microgels. J. Phys. D. Appl. Phys. 2019, 52. [Google Scholar]
- (10).Seymour AJ; Shin S; Heilshorn SC 3D Printing of Microgel Scaffolds with Tunable Void Fraction to Promote Cell Infiltration. Adv. Healthc. Mater. 2021, 2100644, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Mealy JE; Chung JJ; Jeong HH; Issadore D; Lee D; Atluri P; Burdick JA Injectable Granular Hydrogels with Multifunctional Properties for Biomedical Applications. Adv. Mater. 2018, 30. [DOI] [PubMed] [Google Scholar]
- (12).Muir VG; Qazi TH; Shan J; Groll J; Burdick JA Influence of Microgel Fabrication Technique on Granular Hydrogel Properties. ACS Biomater. Sci. Eng. 2021, 7, 4269–4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hsu RS; Chen PY; Fang JH; Chen YY; Chang CW; Lu YJ; Hu SH Adaptable Microporous Hydrogels of Propagating NGF-Gradient by Injectable Building Blocks for Accelerated Axonal Outgrowth. Adv. Sci. 2019, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Zoratto N; Di Lisa D; de Rutte J; Sakib MN; Alves e Silva AR; Tamayol A; Di Carlo D; Khademhosseini A; Sheikhi A In Situ Forming Microporous Gelatin Methacryloyl Hydrogel Scaffolds from Thermostable Microgels for Tissue Engineering. Bioeng. Transl. Med. 2020, 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Pruett L; Ellis R; McDermott M; Roosa C; Griffin D Spatially Heterogeneous Epidermal Growth Factor Release from Microporous Annealed Particle (MAP) Hydrogel for Improved Wound Closure. J. Mater. Chem. B 2021, 9, 7132–7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Jivan F; Yegappan R; Pearce H; Carrow JK; McShane M; Gaharwar AK; Alge DL Sequential Thiol-Ene and Tetrazine Click Reactions for the Polymerization and Functionalization of Hydrogel Microparticles. Biomacromolecules 2016, 17, 3516–3523. [DOI] [PubMed] [Google Scholar]
- (17).Hirsch M; Charlet A; Amstad E 3D Printing of Strong and Tough Double Network Granular Hydrogels. Adv. Funct. Mater. 2021, 31. [Google Scholar]
- (18).Caldwell AS; Rao VV; Golden AC; Bell DJ; Grim JC; Anseth KS Mesenchymal Stem Cell-Inspired Microgel Scaffolds to Control Macrophage Polarization. Bioeng. Transl. Med. 2021, No. March, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Xin S; Wyman OM; Alge DL Assembly of PEG Microgels into Porous Cell-Instructive 3D Scaffolds via Thiol-Ene Click Chemistry. Adv. Healthc. Mater. 2018, 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Xin S; Chimene D; Garza JE; Gaharwar AK; Alge DL Clickable PEG Hydrogel Microspheres as Building Blocks for 3D Bioprinting. Biomater. Sci. 2019, 7, 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Jivan F; Alge DL Bio‐Orthogonal, Site‐Selective Conjugation of Recombinant Proteins to Microporous Annealed Particle Hydrogels for Tissue Engineering. Adv. Ther. 2020, 3, 1900148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Gehlen DB; Jürgens N; Omidinia-Anarkoli A; Haraszti T; George J; Walther A; Ye H; De Laporte L Granular Cellulose Nanofibril Hydrogel Scaffolds for 3D Cell Cultivation. Macromol. Rapid Commun. 2020, 41, 1–10. [DOI] [PubMed] [Google Scholar]
- (23).Sinclair A; O’Kelly MB; Bai T; Hung HC; Jain P; Jiang S Self-Healing Zwitterionic Microgels as a Versatile Platform for Malleable Cell Constructs and Injectable Therapies. Adv. Mater. 2018, 30, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Hsu CC; George JH; Waller S; Besnard C; Nagel DA; Hill EJ; Coleman MD; Korsunsky AM; Cui Z; Ye H Increased Connectivity of HiPSC-Derived Neural Networks in Multiphase Granular Hydrogel Scaffolds. Bioact. Mater. 2021, No. February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Zhang H; Cong Y; Osi AR; Zhou Y; Huang F; Zaccaria RP; Chen J; Wang R; Fu J Direct 3D Printed Biomimetic Scaffolds Based on Hydrogel Microparticles for Cell Spheroid Growth. Adv. Funct. Mater. 2020, 30, 1–10. [Google Scholar]
- (26).Jeon O; Lee YB; Hinton TJ; Feinberg AW; Alsberg E Cryopreserved Cell-Laden Alginate Microgel Bioink for 3D Bioprinting of Living Tissues. Mater. Today Chem. 2019, 12, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Griffin DR; Weaver WM; Scumpia PO; Di Carlo D; Segura T Accelerated Wound Healing by Injectable Microporous Gel Scaffolds Assembled from Annealed Building Blocks. Nat. Mater. 2015, 14, 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Truong NF; Kurt E; Tahmizyan N; Lesher-Pérez SC; Chen M; Darling NJ; Xi W; Segura T Microporous Annealed Particle Hydrogel Stiffness, Void Space Size, and Adhesion Properties Impact Cell Proliferation, Cell Spreading, and Gene Transfer. Acta Biomater. 2019, 94, 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Highley CB; Song KH; Daly AC; Burdick JA Jammed Microgel Inks for 3D Printing Applications. Adv. Sci. 2019, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Shin M; Song KH; Burrell JC; Cullen DK; Burdick JA Injectable and Conductive Granular Hydrogels for 3D Printing and Electroactive Tissue Support. Adv. Sci. 2019, 6, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Mendes BB; Daly AC; Reis RL; Domingues RMA; Gomes ME; Burdick JA Injectable Hyaluronic Acid and Platelet Lysate-Derived Granular Hydrogels for Biomedical Applications. Acta Biomater. 2021, 119, 101–113. [DOI] [PubMed] [Google Scholar]
- (32).Li F; Truong VX; Fisch P; Levinson C; Glattauer V; Zenobi-Wong M; Thissen H; Forsythe JS; Frith JE Cartilage Tissue Formation through Assembly of Microgels Containing Mesenchymal Stem Cells. Acta Biomater. 2018, 77, 48–62. [DOI] [PubMed] [Google Scholar]
- (33).Zhang J; Xin W; Qin Y; Hong Y; Xiahou Z; Zhang K; Fu P; Yin J “All-in-One” Zwitterionic Granular Hydrogel Bioink for Stem Cell Spheroids Production and 3D Bioprinting. Chem. Eng. J. 2021, 430, 132713. [Google Scholar]
- (34).Cai B; Zou Q; Zuo Y; Mei Q; Ma J; Lin L; Chen L; Li Y Injectable Gel Constructs with Regenerative and Anti-Infective Dual Effects Based on Assembled Chitosan Microspheres. ACS Appl. Mater. Interfaces 2018, 10, 25099–25112. [DOI] [PubMed] [Google Scholar]
- (35).Morley CD; Tordoff J; O’Bryan CS; Weiss R; Angelini TE 3D Aggregation of Cells in Packed Microgel Media. Soft Matter 2020, 16, 6572–6581. [DOI] [PubMed] [Google Scholar]
- (36).Nih LR; Sideris E; Carmichael ST; Segura T Injection of Microporous Annealing Particle (MAP) Hydrogels in the Stroke Cavity Reduces Gliosis and Inflammation and Promotes NPC Migration to the Lesion. Adv. Mater. 2017, 29, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Darling NJ; Xi W; Sideris E; Anderson AR; Pong C; Carmichael ST; Segura T Click by Click Microporous Annealed Particle (MAP) Scaffolds. Adv. Healthc. Mater. 2020, 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Fang J; Koh J; Fang Q; Qiu H; Archang MM; Hasani-Sadrabadi MM; Miwa H; Zhong X; Sievers R; Gao DW; et al. Injectable Drug-Releasing Microporous Annealed Particle Scaffolds for Treating Myocardial Infarction. Adv. Funct. Mater. 2020, 30, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Xin S; Deo KA; Dai J; Pandian NKR; Chimene D; Moebius RM; Jain A; Han A; Gaharwar AK; Alge DL Generalizing Hydrogel Microparticles into a New Class of Bioinks for Extrusion Bioprinting. Sci. Adv. 2021, 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Xin S; Gregory CA; Alge DL Interplay between Degradability and Integrin Signaling on Mesenchymal Stem Cell Function within Poly(Ethylene Glycol) Based Microporous Annealed Particle Hydrogels. Acta Biomater. 2020, 101, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Xin S; Dai J; Gregory CA; Han A; Alge DL Creating Physicochemical Gradients in Modular Microporous Annealed Particle Hydrogels via a Microfluidic Method. Adv. Funct. Mater. 2020, 30, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Sheikhi A; de Rutte J; Haghniaz R; Akouissi O; Sohrabi A; Di Carlo D; Khademhosseini A Microfluidic-Enabled Bottom-up Hydrogels from Annealable Naturally-Derived Protein Microbeads. Biomaterials 2019, 192, 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Caldwell AS; Campbell GT; Shekiro KMT; Anseth KS Clickable Microgel Scaffolds as Platforms for 3D Cell Encapsulation. Adv. Healthc. Mater. 2017, 6, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Gramlich WM; Kim IL; Burdick JA Synthesis and Orthogonal Photopatterning of Hyaluronic Acid Hydrogels with Thiol-Norbornene Chemistry. Biomaterials 2013, 34, 9803–9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Chen MH; Wang LL; Chung JJ; Kim YH; Atluri P; Burdick JA Methods to Assess Shear-Thinning Hydrogels for Application As Injectable Biomaterials. ACS Biomater. Sci. Eng. 2017, 3, 3146–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Colby R Official Symbols and Nomenclature of The Society of Rheology. J. Rheol. (N. Y. N. Y). 2013, 57, 1047–1055. [Google Scholar]
- (47).Song KH; Highley CB; Rouff A; Burdick JA Complex 3D-Printed Microchannels within Cell-Degradable Hydrogels. Adv. Funct. Mater. 2018, 28, 1–10. [Google Scholar]
- (48).Qazi TH; Wu J; Muir VG; Weintraub S; Gullbrand SE; Lee D; Issadore D; Burdick JA Anisotropic Rod‐Shaped Particles Influence Injectable Granular Hydrogel Properties and Cell Invasion. Adv. Mater. 2021, 2109194. [DOI] [PMC free article] [PubMed] [Google Scholar]


