Abstract
The strains of Theiler’s murine encephalomyelitis virus, a picornavirus, are divided into two groups according to their neurovirulence after intracerebral inoculation. The highly virulent GDVII strain causes an acute, fatal encephalomyelitis, whereas the DA strain causes a mild encephalomyelitis followed by a chronic inflammatory demyelinating disease associated with viral persistence. Studies with recombinant viruses showed that the capsid plays the major role in determining these phenotypes. However, the molecular basis for the effect of the capsid on neurovirulence is still unknown. In this paper, we describe a large difference in the patterns of infection of primary neuron cultures by the GDVII and DA strains. Close to 90% of the neurons were infected 12 h after inoculation with the GDVII strain, and the cytopathic effect was complete 24 h postinoculation. In contrast, with the DA strain, viral antigens were not detected in neurons until 24 h postinoculation. Infected neurons accounted for only 2% of the total number of neurons, even 6 days after inoculation. No cytopathic effect was visible, and the cultures could be kept for the same length of time as the noninfected controls. Because the neurovirulence of the GDVII strain has been mapped to the capsid, we examined the role of the capsid in this difference of phenotype. We showed, using recombinant viruses, that the capsid was indeed responsible for the pattern of infection observed in vitro, most likely through its role in viral entry. Thus, the levels of neurovirulence of the GDVII and DA strains correlate with their abilities to infect cultured neurons, and this ability is controlled by the capsid.
Theiler’s murine encephalomyelitis virus (TMEV) is a picornavirus belonging to the cardiovirus subgroup (20–22). Strains of TMEV fall into two groups according to the disease they cause after intracerebral inoculation in mice. One group (the GDVII and FA strains) causes a rapidly fatal encephalomyelitis (30). Strains of the second group (e.g., the DA, BeAn, TO4, and WW strains) are attenuated and cause a biphasic disease. The first phase is a mild encephalomyelitis; it is followed by a chronic inflammatory and demyelinating disease of the spinal cord. This late disease resembles multiple sclerosis in humans (15). It is associated with the persistence of the infection at the sites of the lesions, mainly in macrophages (16) but also in oligodendrocytes (3).
Viral recombinants between the persistent DA or BeAn strain and the virulent GDVII strain have been constructed by several groups in order to map viral genes responsible for persistence. These studies were based on the observation that the GDVII strain was unable to persist in the central nervous systems (CNS) of the rare survivors (14). Recent data from our group, using attenuated GDVII mutants, confirm that the GDVII strain does not have the ability to persist (unpublished data). The data obtained with recombinant viruses by different laboratories are, on the whole, consistent and show that the capsid of the DA or BeAn strain contains the main determinants of persistence (1, 19). Within the capsid, several amino acids involved in viral persistence have been identified (13, 26, 27, 34). They are clustered in a small region at the surface of the capsid (11, 17, 18), and therefore, they delimit a site, made of loops from VP1 and VP2, which is critical for viral persistence.
A virus which establishes a persistent infection must be attenuated. The studies with recombinant viruses referred to above demonstrated that attenuation is controlled mainly by the capsid (1, 6, 10, 19, 32). Indeed, a chimeric GDVII virus whose capsid had been replaced by that of strain DA or BeAn was attenuated, whereas a chimeric DA or BeAn virus with a GDVII capsid was virulent. It should be noted, however, that the 5′ noncoding and L regions of the genome of the GDVII strain also contribute to its neurovirulence (5, 10, 23, 24, 29). The mechanisms behind the neurovirulence of strain GDVII and, in particular, the way in which the capsid determines this phenotype have not been explored yet.
During the first days which follow intracerebral inoculation, the DA and GDVII strains infect predominantly neurons of the brain and the spinal cord. For example, Aubert and Brahic compared the patterns of infection for the two strains in the brains of SJL/J mice 4 or 5 days after inoculation (2). They found that the GDVII strain infects approximately 10 times more cells than the DA strain and that these cells are almost exclusively neurons. They also observed that, besides neurons, the DA strain infects a small number of astrocytes and macrophages/microglial cells. In another study, Simas et al. found that strain GDVII infects mainly neurons, but also some astrocytes, in the CNS of CBA and BALB/c mice (28). Importantly, both studies agreed on the fact that the large majority of cells infected early on by either the GDVII or the DA strain were neurons. Furthermore, these in vivo studies revealed that the number of infected neurons was larger in the case of the GDVII strain than in that of the DA strain. This could be due to differences in the efficacy with which the viruses attach to and enter neurons, differences in the viral yield per infected neuron, or differences in the way the immune response of the host controls the spread of the infection.
In this study, we analyzed the infection of primary cultures of mouse neurons with the DA and GDVII strains and with two viral recombinants between these strains. We report that the GDVII strain infected and killed most of the neurons in the culture within 24 h, whereas the DA strain infected only a minority of cells and did not lyse the culture. This observation correlates with the respective levels of neurovirulence of these strains in vivo. Furthermore, the difference of phenotype was mapped to the viral capsid. Our results also suggest that the control of the infection of neurons by the capsid may occur at an early step of the viral cycle, i.e., binding to the receptor and/or entry into the cell.
MATERIALS AND METHODS
Viruses.
Recombinant viruses R2 and R3 have been described in a previous publication (19). The DA, GDVII, R2, and R3 viruses were grown in baby hamster kidney cells (BHK-21). The viruses used to infect neuron cultures were partially purified as follows. Culture supernatants containing infectious virus were treated with 1% (wt/vol) sodium dodecyl sulfate for 1 h at room temperature. The mixture was then centrifuged at 150,000 × g for 4 h at 21°C, and the pellet was resuspended in 10 mM Tris (pH 7.4). The virus was purified further by centrifugation through a 30% (wt/vol) sucrose cushion for 18 h at 90,000 × g and 21°C. The viral pellet was resuspended in 10 mM Tris (pH 7.4), aliquoted, and stored at −80°C. Infectivity was measured by a standard plaque assay on BHK-21 cell monolayers.
Neuron cultures.
Spinal cords from 13- to 14-day-old embryos of BALB/c mice were dissected. Tissues were kept in L15 medium (Gibco) supplemented with 3.6 mg of glucose/ml during all the steps of dissection and dissociation. Nerve cells were first dissociated with trypsin (0.05% [wt/vol]) for 15 min at 37°C. After inhibition of the trypsin with 10% (vol/vol) fetal calf serum (FCS) and low-speed centrifugation, the cell pellet was resuspended in L15 medium containing 3.6 mg of glucose/ml and 100 μg of DNase/ml. The cells were dissociated further by gentle teasing in this DNase-containing medium. Finally, the cells were collected by low-speed centrifugation through a cushion of 4% (wt/vol) bovine serum albumin and resuspended in the medium described below. They were seeded in 24-well plates onto 12-mm glass coverslips coated with poly-dl-ornithine (6 μg/ml) and laminin (3 μg/ml).
Neurons were cultivated in neurobasal medium (Gibco) supplemented with B27 supplement (Gibco), 2% (vol/vol) FCS, 0.5 mM l-glutamine, 25 μM β-mercaptoethanol, and 25 μM l-glutamate. After 24 h, the cultures were treated with 10 μg of 5′-fluorodeoxyuridine (FUdR), an inhibitor of DNA synthesis in dividing cells, per ml to prevent the overgrowth of nonneuronal cells. Uridine (25 μg/ml) was added to the medium at the same time. The treatment with FUdR and uridine was continued throughout the experiment.
Infection of neuron cultures.
Purified virions were diluted in neurobasal medium and added to the medium of neurons which had been in culture for 7 days. The inoculum was not removed because the cultures did not tolerate a change of medium. Neuron cultures were infected at a multiplicity of infection (MOI) of 5 or 50 PFU per seeded cell, depending on the experiment. Since a large fraction of the neurons that were seeded did not grow, this theoretical MOI was largely underestimated. Therefore, neuron cultures were infected with a large excess of virus.
Cell viability assay.
Cell viability following infection with TMEV was measured by the Alamar blue assay (Interchim). This assay consists of an oxidoreduction indicator that changes color in response to chemical reduction of the growth medium resulting from cell growth. At various times after inoculation, 1/10 volume of Alamar blue reagent was added to the medium of TMEV-inoculated or noninoculated neuron cultures. The cultures were returned to the incubator for 3 h. The absorbance of the medium was then measured, in triplicate, at wavelengths of 570 and 600 nm. The results were expressed as the percent viable cells in inoculated cultures, with the level of viable cells in noninoculated cultures taken as 100%. As a control, the assay was performed on BHK-21 cells infected with TMEV.
Immunofluorescence labeling.
Immunostaining was done directly on cells grown on glass coverslips. The cells were fixed with 4% (wt/vol) paraformaldehyde for 15 min at room temperature. They were permeabilized with 0.1% (vol/vol) Triton X-100, and nonspecific binding sites were blocked by incubation for 30 min with phosphate-buffered saline (PBS) containing 2% (vol/vol) FCS. The first antibody was then added at the indicated dilution and allowed to bind for 30 min. The primary antibodies used as neuron markers were as follows: mouse monoclonal anti-MAP-2 (Sigma; dilution, 1:100), mouse monoclonal anti-neurofilament 200 kDa (NF-200) (Sigma; dilution, 1:200), mouse monoclonal anti-synaptophysin (Boehringer; dilution, 1:10) and rabbit anti-tau antiserum (Sigma; dilution, 1:100). After a wash with PBS, the glass coverslips were treated with the appropriate secondary antibodies. Except for the anti-tau staining, the secondary antibody was an anti-mouse immunoglobulin G (IgG) coupled to fluorescein isothiocyanate (Sanofi Diagnostics Pasteur; dilution, 1:100). For anti-tau staining, the secondary antibody was a biotinylated anti-rabbit IgG (Vector; dilution, 1:400). Incubation with this secondary antibody was followed by several washes in PBS and staining with rhodamine avidin D (Vector; dilution, 1:400). Contaminating astrocytes were detected by immunofluorescence labeling with 5 μg of a mouse monoclonal anti-glial fibrillary acidic protein (GFAP) antibody (Boehringer Mannheim Biochemical)/ml followed by incubation with the anti-mouse IgG coupled to fluorescein isothiocyanate.
TMEV-infected cells were detected with a rabbit hyperimmune serum which binds to capsid antigens with high affinity (dilution, 1:300) (4). This serum, which was raised against purified GDVII virions, recognizes GDVII and DA capsid proteins with equal facility, as shown by Western blotting and immunocytochemistry. The primary antibody was detected with the biotinylated anti-rabbit IgG antibody, and the rhodamine avidin D product described above was used for the detection of the anti-tau antibody.
RESULTS
Description of neuron cultures.
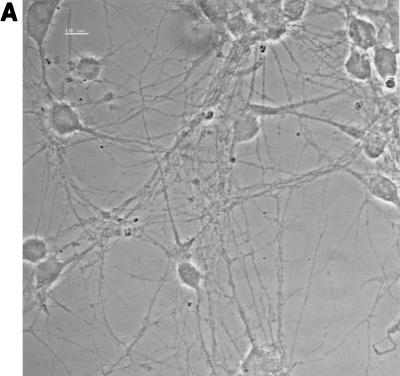
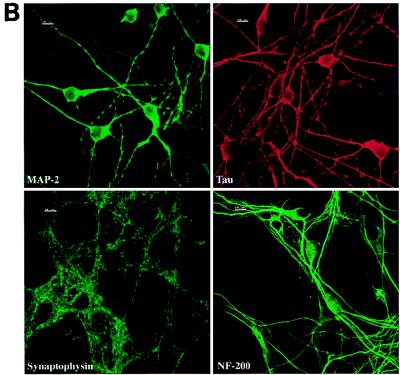
Single-cell suspensions obtained from the spinal cords of mouse embryos were plated on glass coverslips coated with poly-dl-ornithine and laminin. Cytoplasmic processes appeared in the cultures during the first days in vitro (DIV), and a dense network had developed by 7 DIV (Fig. 1A). Neurons were identified in these cultures by indirect immunofluorescence using antibodies directed against the neuronal proteins MAP-2, tau, synaptophysin, and NF-200 (Fig. 1B). These antibodies were tested on control BHK-21 cells, and no staining was observed (data not shown). After 7 DIV, at least 90% of the cells were positive for MAP-2, tau, and synaptophysin. At that time, there was no clear evidence of axonal differentiation, i.e., MAP-2-negative and tau-positive processes. The typical punctuated pattern of synaptophysin immunoreactivity was observed throughout the cell body but was much more abundant along processes, as described previously (7). Neuron cell bodies were frequently observed in large aggregates, although many were isolated. Processes originated from both isolated and clustered neurons. After 7 DIV, the percentage of cells expressing NF-200 was still lower than that of cells expressing MAP-2, tau, or synaptophysin. This percentage increased with time and reached at least 90% after 13 DIV. A few cells in the cultures were astrocytes, as indicated by GFAP-positive staining (data not shown), and a few cells which could not be identified with the markers used were presumably microglial cells or fibroblasts. The neurons could be maintained in culture for 2 weeks. All the experiments described in this paper were performed on neurons cultured for 7 DIV. Similar results were obtained with neurons cultured for 4 or 13 DIV (data not shown).
FIG. 1.
Neuron cultures. (A) Phase-contrast microscopy of neurons after 7 DIV. Bar, 10 μm. (B) Staining of the cultures with antibodies specific for neuronal proteins (immunofluorescence, confocal microscopy). The immunostaining for MAP-2, tau, and synaptophysin was done on cultures after 7 DIV, whereas immunostaining for NF-200 was done after 13 DIV. At least 90% of the cells were positive for these markers. Bars, 10 μm.
It is known that optimal differentiation of neurons can be obtained after long-term culture on top of a feeder monolayer of astrocytes. Our goal was to study specifically the interaction of TMEV with neurons. Therefore, we chose not to use feeder astrocytes. In spite of this, the expression of several neuronal markers (MAP-2, tau, NF-200, and synaptophysin) in our cultures indicated that the neurons had acquired a remarkable level of differentiation.
Cell survival following inoculation with TMEV.
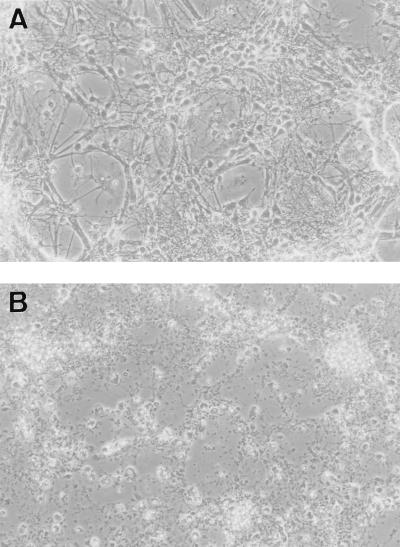
Neurons (7 DIV) were inoculated with the DA or the GDVII strain of TMEV at an MOI greater than 5 PFU/cell (see Materials and Methods for a discussion of the MOI used in these studies). No cytopathic effect was observed in cultures inoculated with the DA strain (Fig. 2A). These cultures could be maintained for as long as 6 days postinoculation without showing cytopathic effect, compared to noninoculated controls. The same result was obtained when the MOI was increased 10-fold. By contrast, the cultures inoculated with the GDVII strain showed a dramatic cytopathic effect during the first 24 or 36 h postinoculation (Fig. 2B).
FIG. 2.
Phase-contrast microscopy of neuron cultures inoculated with DA virus, 4 days postinoculation (A) or GDVII virus, 36 h postinoculation (B). No cytopathic effect was observed in the cultures inoculated with the DA virus, whereas complete cytopathic effect occurred with the GDVII virus.
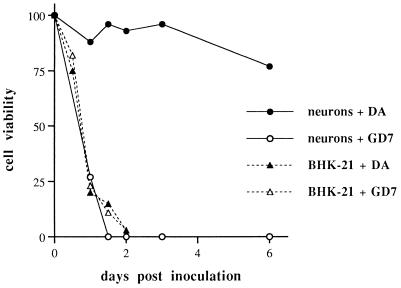
To confirm and quantify these observations, cell viability was measured by the Alamar blue assay as described in Materials and Methods. This assay incorporates a colorimetric growth indicator based on the detection of metabolic activity. Results are presented as levels of viable cells in inoculated wells, expressed as percentages of levels in noninoculated wells (taken as 100%). For example, a viable-neuron level of 96% 3 days after inoculation with the DA strain means that there was no significant difference between inoculated and noninoculated cultures, although there was cell death in both cases, at similar levels, due to the limited time of survival of neurons in culture. The results of a representative experiment are reported in Fig. 3. The percent viable neurons was 27% 24 h after inoculation with the GDVII strain, whereas it remained at about 95% during the 4 days that followed inoculation with the DA strain and dropped to 77% by day 6 after inoculation with this strain. These findings clearly confirmed the drastic difference in cell death caused by the DA and GDVII strains of TMEV in mouse neurons cultured in vitro. As a control, the assay was performed on infected BHK-21 cells. As expected, both viral strains caused rapid cell death within 2 days postinoculation.
FIG. 3.
Quantification of cell viability using the Alamar blue assay. Data are expressed as percent viable cells in inoculated cultures relative to the level of viable cells in noninoculated cultures (taken as 100%).
Permissiveness of cultured neurons for the DA and GDVII strains of TMEV.
The striking difference in neuron death observed after infection with the GDVII and DA strains can be explained in at least two ways. Either there is a major difference in the permissiveness of the neurons for the two viruses or there is a drastic difference in the abilities of the viruses to induce neuron death, regardless of the level of infection. Therefore, we examined the extent of virus replication in the cultures, using immunofluorescence labeling for TMEV capsid antigens.
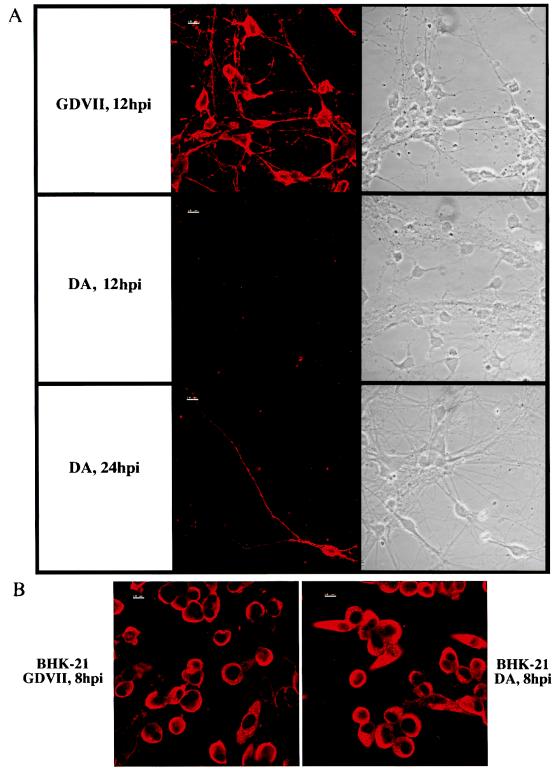
Infected cultures were fixed at different times after inoculation, and infected cells were identified by immunofluorescence staining as described in Materials and Methods. As mentioned in Materials and Methods, the hyperimmune rabbit serum used to detect capsid antigens recognized the GDVII and DA strains with the same efficiency. Figure 4A shows representative fields examined both by phase-contrast microscopy and by immunofluorescence. As early as 8 to 12 h after inoculation with the GDVII strain, the majority of neurons contained viral antigens. At that time, no infected neurons could be detected in the cultures inoculated with the DA strain. Interestingly, the few contaminating nonneuronal cells were positive in both the DA- and GDVII-inoculated cultures (data not shown). From 24 h onward, rare infected neurons could be found in cultures inoculated with the DA strain. An example of these cells is shown in Fig. 4A. As a control, immunofluorescence was performed on BHK-21 cells infected with the DA or GDVII strain. As expected, there was no difference between the two types of infected cultures (Fig. 4B).
FIG. 4.
(A) Immunostaining for TMEV antigens and phase-contrast microscopy of the same fields of neurons. Neurons were inoculated with the GDVII or the DA virus (confocal microscopy). hpi, hours postinfection. (B) Immunostaining for TMEV antigens of infected BHK-21 cells (confocal microscopy).
TMEV antigen-positive neurons were quantitated at different times after inoculation. This was done by counting total neurons and antigen-positive neurons in approximately 20 randomly chosen fields per slide (i.e., between 300 and 500 neurons in total) (Table 1). DA virus-infected neurons represented approximately 2% of the total during 6 days after inoculation. It was very difficult to obtain reliable quantitative data for the GDVII strain because the cells began to die soon after inoculation (12 h) and thus no longer adhered to the coverslips. Nevertheless, more than 50% of the neurons were positive at 8 h postinoculation, and nearly all were positive at 12 or 18 h postinoculation (Fig. 4A and Table 1).
TABLE 1.
Quantification of the number of infected neurons detected by immunofluorescence labeling of viral antigensa
| Time after inoculation | Virus | Total no. of neurons counted | No. (%) of antigen-positive neurons | No. (%) of antigen-positive round cellsb |
|---|---|---|---|---|
| 18 h | GDVII | 154 | 136 (88) | |
| DA | 0 (0) | |||
| 24 h | DA | 386 | 6 (1.5) | 18 (4.7) |
| 48 h | DA | 460 | 10 (2.1) | 32 (6.9) |
| 72 h | DA | 561 | 6 (1) | 40 (7) |
| 6 days | DA | 568 | 13 (2.2) | 83 (14.6) |
An average of 20 fields per slide were chosen at random. Total neurons and antigen-positive neurons were counted. At 18 h postinoculation, many neurons have already been lysed by GDVII, which explains why fewer neurons could be counted.
As described in the text, antigen-positive round cells, with no morphological characteristics of neurons, were observed in cultures infected with DA virus.
In the cultures infected with the DA strain, 5 to 15% of the cells, depending on the time postinoculation, were round cells without processes which were positive for viral antigens (Table 1). These cells could not be identified from their morphology, although they did not look like neurons. They might have been the nonneuronal contaminating cells which were permissive for the DA strain or dead infected neurons, or a mixture of both.
The capsid of TMEV determines its replication pattern in neurons.
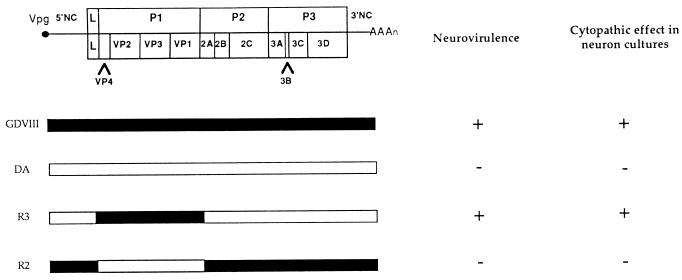
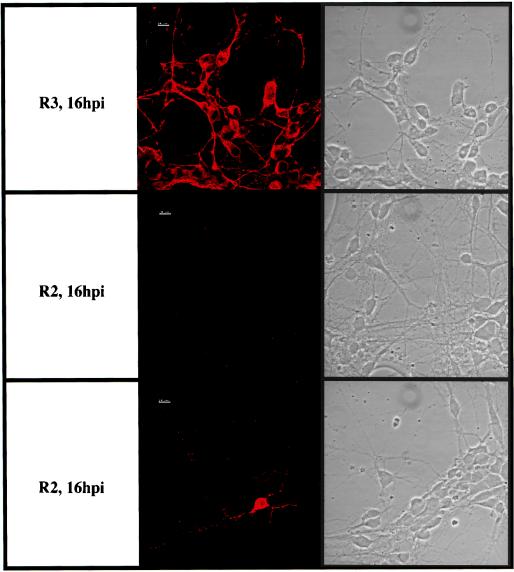
The data reported above showed that the difference in neurovirulence in vivo between the DA and GDVII viruses correlated with the patterns of infection of neurons in culture. By using recombinants between the DA or BeAn strain and the GDVII strain, most of the difference in neurovirulence between these two groups of strains has been mapped to the capsid (1, 6, 10, 19, 32). Thus, it was important to determine whether the capsid also controlled the pattern of infection of cultured neurons. For this purpose, we used the chimeric viruses R2 and R3, which have been described in a previous publication (19). These viruses consist of GDVII and DA viruses in which the genes coding for the capsid and short regions of the flanking L and 2A genes (92 and 85 nucleotides, respectively) have been exchanged. The L protein fragment bears eight differences in amino acids, and the 2A fragment bears four. Virus R2 is a GDVII virus with a DA capsid. It is fully attenuated and persists in the CNS of mice, whereas virus R3, which is a DA virus with a GDVII capsid, is highly virulent and does not persist in survivors (19). Neuron cultures were infected with these recombinant viruses under the same conditions as those described for the DA and GDVII viruses. Virus R3 caused a cytopathic effect indistinguishable from that caused by virus GDVII. No cytopathic effect was visible during several days in the culture inoculated with virus R2, a result similar to that described above for virus DA (Fig. 5). Furthermore, immunofluorescence staining for TMEV antigens gave results almost identical to those described for the GDVII and DA viruses (Fig. 6). Nearly all the neurons were infected with virus R3 (GDVII capsid) 16 h after inoculation. At that time, infected neurons were very rare—fewer than 1% of total neurons—in the cultures inoculated with virus R2 (DA capsid). The only minor difference observed between cultures infected with the DA virus and those infected with virus R2 was that whereas no infected neurons could be detected until 24 h after inoculation with the DA strain, a few infected neurons (1%) were observed 16 h after inoculation with virus R2.
FIG. 5.
Genetic map and phenotype of recombinant viruses R2 and R3. NC, noncoding region. The construction of the chimeric viruses and in vivo neurovirulence studies are from McAllister et al. (19). Mice were inoculated intracerebrally with 104 PFU of attenuated (−) or highly neurovirulent (+) virus. The cytopathic effect in neuron cultures (−, no visible cytopathic effect; +, complete cytopathic effect) was determined by observation with phase-contrast microscopy.
FIG. 6.
Immunostaining for TMEV antigens and phase-contrast microscopy of the same fields of neurons. Neurons were inoculated with virus R2 or R3 (confocal microscopy).
DISCUSSION
The GDVII strain of TMEV is much more neurovirulent than the DA strain. For example, the 50% lethal dose after intracerebral inoculation is 0.7 PFU for the former and 106 PFU for the latter (14). The neurovirulence of strain GDVII is encoded in the 5′ noncoding region (6, 10, 23, 24), the L protein (5), and the capsid. Studies with recombinant viruses between the DA or BeAn strain and the GDVII strain showed that the capsid contains the main determinants of neurovirulence (1, 6, 9, 19, 25). Indeed, exchanging the capsids of the DA (or BeAn) and GDVII viruses led to a large change in neurovirulence. Up to now, the molecular basis for the effect of the capsid on neurovirulence had not been investigated. The capsid could be responsible for differences of viral tropism, differences in the ability of the virus to cause cell death, or differences in the control of the infection by the immune responses of the host. No major difference in the cell tropism has been observed in vivo between the GDVII and DA or BeAn strains during the early phase of infection. In all published studies, neurons represent the large majority of infected cells, although some infection of glial cells has been reported in the brains of DA-infected SJL/J mice (2) and in the CNS of GDVII-infected CBA and BALB/c mice (28). The main difference between the brains of mice inoculated with the GDVII strain of TMEV and those of mice inoculated with the DA strain is the number of neurons which are infected. In a recent study, Aubert and Brahic reported 10 times more infected neurons with the GDVII strain than with the DA strain (2).
In this paper, we report a large difference in the extent of replication of the GDVII and DA strains in primary cultures of mouse neurons. With the GDVII strain, close to 90% of the neurons were infected 12 h postinoculation, whereas neurons infected with the DA strain accounted for only 2% of all neurons even several days postinoculation. Analysis with the recombinant viruses R3 and R2 strongly suggests that the genes coding for the capsid were responsible for the pattern of infection of neurons in vitro, although we cannot formally exclude a role of flanking fragments of the L and 2A genes. Our findings were specific for neurons. Indeed, as shown in Fig. 4B, inoculation of BHK-21 cells at a high MOI (5 or 10 PFU/cell) led to the infection of almost 100% of the cells with both the GDVII and DA viruses. The same result was obtained with the R2 and R3 viruses (data not shown).
We observed the same intensity of fluorescence in neurons which were positive for viral capsid antigens, whether they were infected with the GDVII, DA, R2, or R3 virus (Fig. 4 and 6). Furthermore, Aubert and Brahic found similar levels of viral RNA in GDVII- and DA-infected neurons in vivo (2). These observations suggest that, regardless of the viral strain, viral RNA replication and translation proceeded with similar efficiencies in neurons. This is consistent with the conclusion that the capsid controls the pattern of infection in neurons, since the main role of the capsid is the delivery of the viral genome into the cytoplasm and not the regulation of transcription and replication. Therefore, our data suggest that the control of neuron infection by the capsid occurs at an early stage of the virus cycle, i.e., binding to a receptor and/or entry of the virus in neurons. This conclusion is supported by structural comparisons between the capsids of attenuated (DA and BeAn) and virulent (GDVII) strains of TMEV (33). The main structural variations between these two groups of strains are located at sites that might influence the binding of the virus to its cellular receptor. One can hypothesize that the DA (or BeAn) and GDVII strains do not use the same receptor. Both receptors might be present at the surfaces of BHK-21 cells, explaining the fact that all the strains bind with similar degrees of efficiency on these cells (8). By contrast, the receptor for the DA strain, and not that for the GDVII strain, might be absent, or poorly expressed, at the surfaces of neurons. Our results are also congruent with the hypothesis of Zhou et al. (33). These authors showed a difference in the interaction with sialyllactose between the BeAn and GDVII viruses. This led them to propose that the binding of BeAn virus to sialic acid on non-receptor surface proteins could trap the virus and thus slow down its spread. By contrast, GDVII virus, which does not interact with sialic acid, would bind to the protein component of the functional receptor at a high rate.
Our data did not allow us to conclude whether the DA virus is lytic for neurons in vitro. The proportion of infected neurons remained very low and constant for several days. This could be due to an equilibrium between lysis of infected neurons, production of infectious viruses, and infection of new neurons. Alternatively, the DA virus might not lyse neurons, and the few infected neurons detected 6 days after inoculation might be the same cells that were detected at day 1 postinoculation, i.e., persistently infected neurons. Lysis of neurons in mixed brain cell cultures, or CNS organotypic cultures, following infection with the DA or WW strain of TMEV has been described (12, 31). Nevertheless, in such mixed cell cultures, it is difficult to distinguish between a direct effect of the virus on neurons and an indirect toxic effect mediated by other types of cells which are lysed by the infection. In our neuron cultures infected with the DA virus for 6 days, approximately 15% of the cells were antigen positive and could not be identified. These cells were round and did not look like neurons. They may have represented dead neurons—assuming that several infectious cycles occurred during the 6 days—and/or contaminating nonneuronal infected cells. Even if they were dying neurons, the percentage of neurons infected by the DA strain would amount to a maximum of 17%, as opposed to 90% for the GDVII strain. It is worth mentioning again that no cytopathic effect could be observed by phase-contrast microscopy with the DA strain, whereas the entire culture was lysed by strain GDVII within 24 h. In conclusion, we could not determine if the DA virus causes neuron death in vitro. On the other hand, there is clearly neuronal destruction with neuronophagia in the brains of mice infected with the DA virus. However, it is impossible to distinguish between direct killing by the virus and killing due to the surrounding inflammatory cells.
In summary, we report that the levels of neurovirulence of the GDVII and DA strains of TMEV correlate with their abilities to infect primary neurons in culture. We showed that the viral capsid determines the ability to infect neurons. Our results also suggest that the capsid affects an early stage of the viral cycle, i.e., adsorption and/or entry of the virus in neurons. We propose that our findings account for the enhanced neurovirulence of the GDVII virus compared to that of the DA virus. To our knowledge, we described in this article the first tissue culture system which mimics the drastic difference observed in vivo between the DA and GDVII strains. This system makes it possible to study an important aspect of TMEV pathogenesis at a molecular level.
ACKNOWLEDGMENTS
We thank Emmanuelle Perret for confocal microscopy, Véronique Devignot and Maria-Isabel Thoulouze for advice on neuronal culture, and Mireille Gau for secretarial assistance.
This work was supported by grants from the Centre National de la Recherche Scientifique, the Institut Pasteur Fondation, and the EC Human Capital and Mobility program (contract CHRX-CT94-0670).
REFERENCES
- 1.Adami C, Pritchard A E, Knauf T, Luo M, Lipton H L. A determinant for central nervous system persistence localized in the capsid of Theiler’s murine encephalomyelitis virus by using recombinant viruses. J Virol. 1998;72:1662–1665. doi: 10.1128/jvi.72.2.1662-1665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert C, Brahic M. Early infection of the central nervous system by GDVII and DA strains of Theiler’s virus. J Virol. 1995;69:3197–3200. doi: 10.1128/jvi.69.5.3197-3200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubert C, Chamorro M, Brahic M. Identification of Theiler’s virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb Pathog. 1987;3:319–326. doi: 10.1016/0882-4010(87)90002-7. [DOI] [PubMed] [Google Scholar]
- 4.Brahic M, Haase A T, Cash E. Simultaneous in situ detection of viral RNA and antigens. Proc Natl Acad Sci USA. 1984;81:5445–5448. doi: 10.1073/pnas.81.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calenoff M A, Badshah C S, Dal Canto M C, Lipton H L, Rundell M K. The leader polypeptide of Theiler’s virus is essential for neurovirulence but not for growth in BHK-21 cells. J Virol. 1995;69:5544–5549. doi: 10.1128/jvi.69.9.5544-5549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calenoff M A, Faaberg K S, Lipton H L. Genomic regions of neurovirulence and attenuation in Theiler’s murine encephalomyelitis virus. Proc Natl Acad Sci USA. 1990;87:978–982. doi: 10.1073/pnas.87.3.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron P L, Südhof T C, Jahn R, De Camilli P. Colocalization of synaptophysin with transferrin receptors: implications for synaptic vesicle biogenesis. J Cell Biol. 1991;115:151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fotiadis C, Kilpatrick D R, Lipton H L. Comparison of the binding characteristics to BHK-21 cells of viruses representing the two Theiler’s virus neurovirulence groups. Virology. 1991;182:365–370. doi: 10.1016/0042-6822(91)90683-3. [DOI] [PubMed] [Google Scholar]
- 9.Fu J, Rodriguez M, Roos R P. Strains from both Theiler’s virus subgroups encode a determinant for demyelination. J Virol. 1990;64:6345–6348. doi: 10.1128/jvi.64.12.6345-6348.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu J, Stein S, Rosenstein L, Bodwell T, Routbort M, Semler B L, Roos R P. Neurovirulence determinants of genetically engineered Theiler’s virus. Proc Natl Acad Sci USA. 1990;87:4125–4129. doi: 10.1073/pnas.87.11.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant R A, Filman D J, Fujinami R S, Icenogle J P, Hogle J M. Three-dimensional structure of Theiler’s virus. Proc Natl Acad Sci USA. 1992;89:2061–2065. doi: 10.1073/pnas.89.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graves M C, Bologa L, Siegel L, Londe H. Theiler’s virus in brain cell cultures: lysis of neurons and oligodendrocytes and persistence in astrocytes and macrophages. J Neurosci Res. 1986;15:491–501. doi: 10.1002/jnr.490150406. [DOI] [PubMed] [Google Scholar]
- 13.Jarousse N, Grant R A, Hogle J M, Zhang L, Senkowski A, Roos R P, Michiels T, Brahic M, McAllister A. A single amino acid change determines persistence of a chimeric Theiler’s virus. J Virol. 1994;68:3364–3368. doi: 10.1128/jvi.68.5.3364-3368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipton H L. Persistent Theiler’s murine encephalomyelitis virus infection in mice depends on plaque size. J Gen Virol. 1990;46:169–177. doi: 10.1099/0022-1317-46-1-169. [DOI] [PubMed] [Google Scholar]
- 15.Lipton H L. Theiler’s virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipton H L, Twaddle G, Jelachich M L. The predominant virus antigen burden is present in macrophages in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J Virol. 1995;69:2525–2533. doi: 10.1128/jvi.69.4.2525-2533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo M, He C, Toth K S, Zhang C X, Lipton H L. Three-dimensional structure of Theiler murine encephalomyelitis virus (BeAn strain) Proc Natl Acad Sci USA. 1992;89:2409–2413. doi: 10.1073/pnas.89.6.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo M, Toth K S, Zhou L, Protchard A, Lipton H L. The structure of a highly virulent Theiler’s murine encephalomyelitis virus (GDVII) and implications for determinants of persistence. Virology. 1995;220:246–250. doi: 10.1006/viro.1996.0309. [DOI] [PubMed] [Google Scholar]
- 19.McAllister A, Tangy F, Aubert C, Brahic M. Genetic mapping of the ability of Theiler’s virus to persist and demyelinate. J Virol. 1990;64:4252–4257. doi: 10.1128/jvi.64.9.4252-4257.1990. . (Author’s correction, 67:2427, 1993.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohara Y, Stein S, Fu J, Stillman L, Klaman L, Roos R P. Molecular cloning and sequence determination of DA strain of Theiler’s murine encephalomyelitis viruses. Virology. 1988;164:245–255. doi: 10.1016/0042-6822(88)90642-3. [DOI] [PubMed] [Google Scholar]
- 21.Ozden S, Tangy F, Chamorro M, Brahic M. Theiler’s virus genome is closely related to that of encephalomyocarditis virus, the prototype cardiovirus. J Virol. 1986;60:1163–1165. doi: 10.1128/jvi.60.3.1163-1165.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pevear D C, Calenoff M, Rozhon E, Lipton H L. Analysis of the complete nucleotide sequence of the picornavirus Theiler’s murine encephalomyelitis virus indicates that it is closely related to cardioviruses. J Virol. 1987;61:1507–1516. doi: 10.1128/jvi.61.5.1507-1516.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilipenko E V, Gmyl A P, Masvola S V, Khitrina E V, Agol V I. Attenuation of Theiler’s murine encephalomyelitis virus by modifications of the oligopyrimidine/AUG, tandem, a host-dependent translational cis element. J Virol. 1995;69:864–870. doi: 10.1128/jvi.69.2.864-870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pritchard A E, Calenoff M A, Simpson S, Jensen K, Lipton H L. A single base deletion in the 5′ noncoding region of Theiler’s virus attenuates neurovirulence. J Virol. 1992;66:1951–1958. doi: 10.1128/jvi.66.4.1951-1958.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez M, Roos R P. Pathogenesis of early and late disease in mice infected with Theiler’s virus, using intratypic recombinant GDVII/DA viruses. J Virol. 1992;66:217–225. doi: 10.1128/jvi.66.1.217-225.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato S, Zhang L, Kim J, Jakob J, Grant R A, Wollmann R, Roos R P. A neutralization site of DA strain of Theiler’s murine encephalomyelitis virus important for disease phenotype. Virology. 1996;226:327–337. doi: 10.1006/viro.1996.0660. [DOI] [PubMed] [Google Scholar]
- 27.Senkowski A, Shim B, Roos R P. The effect of Theiler’s murine encephalomyelitis virus (TMEV) VP1 carboxyl region on the virus-induced central nervous system disease. J Neurovirol. 1995;1:101–110. doi: 10.3109/13550289509111014. [DOI] [PubMed] [Google Scholar]
- 28.Simas J P, Dyson H, Fazakerley J K. The neurovirulent GDVII strain of Theiler’s virus can replicate in glial cells. J Virol. 1995;69:5599–5606. doi: 10.1128/jvi.69.9.5599-5606.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein S B, Zhang L, Roos R P. Influence of Theiler’s murine encephalomyelitis virus 5′ untranslated region on translation and neurovirulence. J Virol. 1992;66:4508–4517. doi: 10.1128/jvi.66.7.4508-4517.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theiler M, Gard S. Encephalomyelitis of mice. I. Characteristics and pathogenesis of the virus. J Exp Med. 1940;72:49–67. doi: 10.1084/jem.72.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wroblewska Z, Kim S U, Sheffield W D, Gilden D H. Growth of the WW strain of Theiler virus in mouse central nervous system organotypic culture. Acta Neuropathol. 1979;47:13. doi: 10.1007/BF00698267. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Senkowski A, Shim B, Roos R P. Chimeric cDNA studies of Theiler’s murine encephalomyelitis virus neurovirulence. J Virol. 1993;67:4404–4408. doi: 10.1128/jvi.67.7.4404-4408.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou L, Lin X, Green T J, Lipton H L, Luo M. Role of sialyloligosaccharide binding in Theiler’s virus persistence. J Virol. 1997;71:9701–9712. doi: 10.1128/jvi.71.12.9701-9712.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zurbriggen A, Thomas C, Yamada M, Roos R P, Fujinami R S. Direct evidence of a role for amino acid 101 of VP-1 in central nervous system disease in Theiler’s murine encephalomyelitis virus infection. J Virol. 1991;65:1929–1937. doi: 10.1128/jvi.65.4.1929-1937.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]