Abstract
Addition of heparin to the virus culture inhibited syncytial plaque formation due to respiratory syncytial virus (RSV). Moreover, pretreatment of the virus with heparinase or an inhibitor of heparin, protamine, greatly reduced virus infectivity. Two anti-heparan sulfate antibodies stained RSV-infected cells, but not noninfected cells, by immunofluorescence. One of the antibodies was capable of neutralizing RSV infection in vitro. These results prove that heparin-like structures identified on RSV play a major role in early stages of infection. The RSV G protein is the attachment protein. Both anti-heparan sulfate antibodies specifically bound to this protein. Enzymatic digestion of polysaccharides in the G protein reduced the binding, which indicates that heparin-like structures are on the G protein. Such oligosaccharides may therefore participate in the attachment of the virus.
Respiratory syncytial virus (RSV), which belongs to the Pneumovirus genus of the Paramyxoviridae family, is the major cause of acute lower respiratory tract illness in infants and young children (17). Its envelope contains two glycoproteins, G and F, that are responsible, respectively, for virus attachment to the cell and for cell fusion (20). A third protein, named SH, has an unknown function. Although it enhanced the fusion process when coexpressed with the F and G glycoproteins (13), it was recently shown, by using a virus without SH, that the SH protein is dispensable for the fusion function (4). The G protein has unusual features compared to other paramyxovirus glycoproteins. The RSV G protein is synthesized as a precursor (36 kDa) (12, 28, 34) which is modified by the addition of N-linked sugars to form an intermediate of 45 kDa. These sugars convert to the complex type, and then O-linked sugars are added to yield a mature molecule of approximately 90 kDa (8, 35). Because of its high serine, threonine, and proline content, the RSV G protein has been described as mucin like. Such proteins are secreted by epithelial cells (2). The structure of the fusion protein (F) is similar to that of other paramyxoviruses (6, 31). The F protein is N glycosylated, as shown by tunicamycin treatment. It contains 13% N-glycans (19) and is palmitylated (7). It has five or six potential N-glycosylation sites, one of which is on the F1 subunit. The SH protein is present in RSV-infected cells in nonglycosylated and glycosylated forms with a variable degree of glycosylation (1, 25).
The precise structure of RSV oligosaccharides and the functional role of the F and SH carbohydrates in infectivity are still not well defined. By using inhibitors of N- or O-linked glycosylations or endoglycosidases, partially glycosylated intermediates of the G protein have been generated and virus infectivity has been shown to be greatly reduced after removal of N- or O-linked oligosaccharides (19). Moreover, it has been shown that O-linked carbohydrates are necessary for the binding of most anti-G protein antibodies (26). Thus, oligosaccharides contribute either directly or indirectly to antigenic sites on viral glycoproteins.
Sulfated polysaccharides, including heparin, were found to inhibit RSV cytopathogenicity, and it has been suggested that these inhibitors interfere with virus-cell binding and/or virus-cell fusion (14). Heparin is composed of heterogeneous-sized sequences of alternating hexuronic acid and glucosamine. In addition, the degree of sulfatation and acetylation varies in these sequences. We further investigated, by using a variety of inhibition assays, on which protagonist of the interaction, the virus or the cell, heparin acts.
MATERIALS AND METHODS
Virus strains and cell cultures.
Human RSV (Long strain) was propagated in HEp-2 cells grown in Eagle basal medium (modified) with Hanks salts (HBME) supplemented with 2 mM l-glutamine, 2.08-g/ml sodium bicarbonate, 105 U of penicillin per liter, and 100 mg of streptomycin per liter.
Antibodies.
Monoclonal antibodies to RSV were produced as previously described (3). RS-A412 recognizes the G protein, and RS-18B2 recognizes the fusion protein. Three anti-heparan sulfate monoclonal antibodies were used throughout this study. Anti-HSPG (heparan sulfate proteoglycan) antibody BMS4056 was purchased from Boehringer Ingelheim Bioproducts, Ingelheim, Germany. Antibodies F58-10E4 and HepSS-1 were purchased from Seikagaku, Tokyo, Japan.
Purification of the G protein.
The G protein was purified from an RSV-infected culture supernatant by affinity chromatography using antibody RS-A412 (32).
Compounds.
Heparin was obtained from Leo Pharmaceutical (Ballerup, Denmark). Heparin chains are heterogeneous in size with a mean value of around 50 monosaccharide residues and a molecular mass of 5 to 25 kDa. Low-molecular-weight heparin (Fragmine) and protamine were purchased from Pharmacia (Uppsala, Sweden) and Sanofi Choay (Gentilly, France), respectively. Fragmine is a mixture of short chains (around 18 residues) and has a molecular mass of around 6 kDa. Protamine was dissolved in a glucose-cresol solution. An identical solvent was prepared and used as a control. Heparinase II (heparin lyase II [no assigned EC number]), heparinase III (heparin lyase III, heparitinase I [EC 4.2.2.8]), chondroitinase ABC (EC 4.2.2.4), and bovine kidney heparan sulfate were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Inhibition of RSV-induced plaque formation by heparin, Fragmine, or heparan sulfate.
Twofold serial dilutions (300 μl) of heparin, Fragmine, or heparan sulfate in HBME were mixed with an equal volume of virus (700 PFU/ml) and incubated for 1 h at 37°C. Mixtures were then allowed to adsorb to cells grown in six-well tissue culture plates for 2 h at 37°C. The inoculum was then removed, and the cells were overlayered with 3 ml of HBME containing 0.5% agarose. Five days later, the plates were stained with 0.5 mg of neutral red per well. Viral plaques were counted after further incubation at 37°C for 4 h. Fifty percent inhibitory concentrations (IC50s) were determined by calculating the efficiency of virus plaque formation relative to that of an untreated infected control. In all assays carried out in vitro, syncytium detection was done as described here.
Heparin treatment of cell monolayers.
HEp-2 cells were incubated with serially diluted heparin or heparan sulfate for 1 h at 37°C. The cells were then thoroughly washed and inoculated with RSV. Two hours later, cells were overlaid with 0.5% agarose in HBME.
Activity of heparin. (i) Effect of incubation time.
In the first experiment, diluted heparin (1 IC80) in HBME was incubated in vials at 37°C with the virus (700 PFU/ml) for various periods. HBME was added in the same way to the virus in control vials. These heparin-virus and HBME-virus mixtures were then allowed to adsorb for 2 h at 37°C to monolayers of HEp-2 cells grown in six-well plates. After adsorption of the virus, the inoculum was removed and the cells were overlaid with 3 ml of HBME containing 0.5% agarose. In the second experiment, a virus inoculum was allowed to adsorb to cells for 2 h at 37°C, and heparin (1 IC80) was added at various times during this adsorption period, which was always the same, i.e., 2 h. The cells were then thoroughly washed before being overlaid with HBME agarose as described above.
(ii) Effect of temperature.
The virus was allowed to adsorb to HEp-2 cells for 1.5 or 2 h at 37°C and for 1, 2, or 2.5 h at 4°C. The inoculum was then removed, and heparin (1 IC80) was added to the wells and left at 37°C until neutral red staining. No heparin was added to control wells.
Effects of heparinase, chondroitinase, and protamine on RSV infection.
(i) Serial dilutions (300 μl of each) of heparinase (0.1 to 10 U/ml), chondroitinase (0.017 to 170 mIU/ml [170 mIU of chondroitinase is equivalent to 100 U of heparinase]), protamine (0.1 to 10 IU/ml), or protamine solvent were mixed with an equal volume of virus (700 PFU/ml) and incubated for 1 h in vials at 37°C. Inhibitor-virus mixtures were then allowed to adsorb to cells for 2 h at 37°C. (ii) The same dilutions of inhibitors were incubated with cells for 1 h at 37°C before virus infection (700 PFU/ml). Cells were thoroughly washed before virus infection. After adsorption of the virus, the inoculum was removed and the cells were overlaid with HBME-agarose.
Immunofluorescence.
RSV-infected and noninfected HEp-2 cells were fixed on slides in acetone and incubated first with the anti-HSPG antibody (purified immunoglobulin, 100 μg/ml), F58-10E4, or HepSS-1 (all were diluted 1:50). Fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin (diluted 1:100; Pasteur Diagnostics) or fluorescein isothiocyanate-conjugated goat anti-mouse polyvalent immunoglobulin (diluted 1:100; Sigma) was then added.
ELISA. (i) Antibody binding.
The binding of the three anti-heparan sulfate antibodies was assessed by indirect enzyme-linked immunosorbent assay (ELISA). The wells of microtiter plates were coated overnight at room temperature either with intact RSV-infected HEp-2 cells or with intact noninfected HEp-2 cells in phosphate-buffered saline (PBS). Nonspecific binding sites in the wells were saturated by incubation with 2% nonfat milk for 30 min at 37°C. Antibodies were incubated in the wells for 1 h at 37°C. Bound antibodies were then detected with peroxidase-conjugated anti-mouse immunoglobulin (1:1,000).
(ii) Sandwich ELISA.
An anti-HSPG antibody (diluted 1:5 in PBS) or antibody F58-10E4 (diluted 1:10 in PBS) was used to coat microtiter plate wells and incubated overnight at room temperature. Binding sites in the wells were saturated by incubation with 3% nonfat milk for 30 min at 37°C. Several dilutions of the purified G protein in PBS were then added to the wells and incubated for 1 h at 37°C. Bound protein was then detected with peroxidase-conjugated antibody RS-A412 or RS-18B2. To check reaction specificity, the G protein was treated with heparinase or heparitinase (10 IU/well) for 1 h at 37°C before assessment of binding to the F58-10E4 antibody. In this assay, the protein was diluted in 0.1 M phosphate buffer (pH 8.2).
Neutralization assay.
Long RSV (5 × 103 PFU) or parainfluenza 3 virus was mixed for 1 h with anti-HSPG antibody (diluted from 10 to 2.5 μg/ml). Monolayers of HEp-2 cells in six-well plates were then infected. Syncytia were counted after neutral red staining. Neutralization was recorded when the mean reciprocal of the antibody dilution gave 50% plaque reduction compared to the well infected without antibody. In a parallel experiment, monolayers of HEp-2 cells were treated with the anti-HSPG antibody and then washed with PBS before infection with RSV or parainfluenza 3 virus.
RESULTS
IC50 determination.
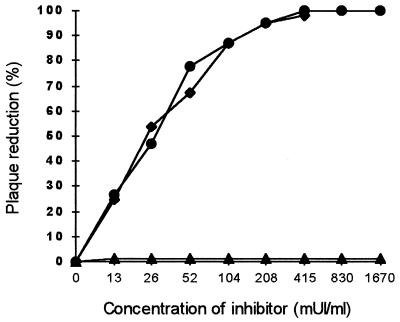
We first examined the inhibitory effects of heparin, Fragmine, and heparan sulfate on RSV infection in vitro. Serially diluted reagents were incubated with RSV prior to inoculation onto the cells (Fig. 1). The IC50s of heparin and Fragmine were similar at about 0.024 IU/ml. Heparin is composed of heterogeneous-sized sequences. Low-molecular-weight heparin, i.e., Fragmine, was also inhibitory. Thus, heparin with a molecular size below 8 kDa is still active. These short sequences, which bear the antithrombotic properties of heparin, are also able to inhibit RSV infection. No inhibition of plaque formation was observed, whatever the dilution of heparan sulfate used in the assay. In contrast to heparin, heparan sulfate had no inhibitory effect when this procedure was used.
FIG. 1.
Effects of various concentrations of heparin, Fragmine, and heparan sulfate on RSV-induced plaque formation. Various concentrations of heparin (•), Fragmine (⧫), and heparan sulfate (▴) were incubated for 1 h at 37°C with 700 PFU of RSV per ml, and the ability to reduce virus infectivity was then assessed. mUI, international milliunits.
HEp-2 cell treatment with heparin or heparan sulfate before infection with RSV.
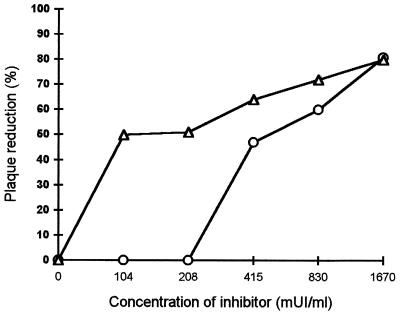
HEp-2 cells were then exposed to heparin or heparan sulfate to determine how these reagents are able to reduce infectivity. As shown in Fig. 2, cell treatment with heparin before inoculation reduced infectivity in a dose-dependent manner. As we obtained some inhibition, we can say that binding of these reagents to the cells is not completely reversible by washing, in particular when a higher concentration of heparin (more than 0.45 IU/ml) is used. However, at low concentrations, the inhibition obtained after cell treatment was lower than when heparin was added simultaneously with the virus. In this case, washing removed a major part of the unbound heparin. The viruses then occupy the free binding sites, and the virus can adsorb to the cell surface without any free competing heparin. On the contrary, when an inoculum with heparin is added to the cell culture, the virus and heparin compete simultaneously for cell surface area, which leads to 100% inhibition.
FIG. 2.
Effect of the treatment of cells with various concentrations of heparin (○) and heparan sulfate (▵) on RSV infectivity. Cells were washed before infection. mUI, international milliunits.
Heparan sulfate activity followed a distinct pattern (Fig. 1 and 2). Pretreatment of the cells with heparan sulfate also led to inhibition of infectivity. However, when an inoculum was added with heparan sulfate, leading to simultaneous competition between the virus and heparan sulfate for cell surface area, complete infection resulted. Thus, heparan sulfate has an inhibitory effect on RSV infection only if this reagent has already bound to the cell surface. It may reflect the fact that binding to the cells by the virus is much quicker than that of heparan sulfate.
Determination of the stage of infection at which heparin is active.
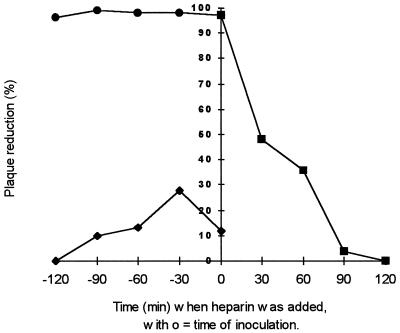
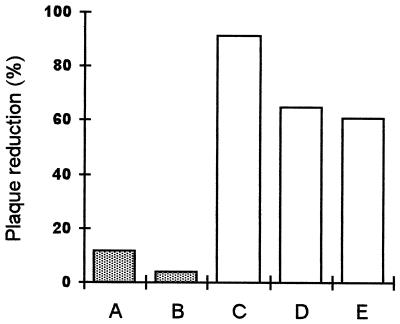
Heparin was first preincubated with the virus before infection. We observed complete inhibition of plaque formation, whatever the length of the contact time (Fig. 3). Even when the virus and heparin were added simultaneously to the cells, virus infection was abolished (Fig. 3, • and first ■). No variation due to the incubation at 37°C before inoculation was noticed in the control (Fig. 3, ⧫), so the diminution of the number of syncytia could not be attributed at all to preincubation of the inoculum. Heparin was then added at different times during adsorption. We observed a variation in the number of syncytia depending on the length of the contact time. The longer the contact time, the more the number of syncytia decreased (Fig. 3, ■). When heparin was added after 90 min of adsorption at 37°C and then left for 30 min and the cells were subsequently washed, the number of syncytia was the same as in the control. This suggests that heparin only interferes with the first steps of infection. To check this, we added heparin after 1.5 h of adsorption at 37°C and left the heparin during culture under agarose. No decrease in the number of syncytia was observed compared to controls. Thus, heparin was no longer capable of inhibiting infectivity. To distinguish between the effect on virus-cell binding and that on the fusion process, heparin was added during adsorption performed at either 37 or 4°C. Cells were washed before being overlaid with agarose. When adsorption was done at 4°C, only partial inhibition was obtained (60%) (Fig. 4). Heparin affects only early events of infection, which are completed in 90 min at 37°C but not at 4°C.
FIG. 3.
Effects of heparin added to RSV during different periods before inoculation or during inoculation. Symbols: ⧫, virus preincubated at 37°C for different times without heparin before inoculation; •, virus preincubated at 37°C for different times with heparin before inoculation; ■, heparin added at different times during inoculation, followed by washing of the cells before addition of agarose.
FIG. 4.
Percent plaque reduction was determined per well after adsorption for 1.5 h at 37°C (A), 2 h at 37°C (B), 1 h at 4°C (C), 2 h at 4°C (D), and 2.5 h at 4°C (E). Heparin was added after adsorption and left during culture under agarose in the assays. No heparin was added after adsorption of the virus in the controls.
Effect of heparinase on RSV before adsorption to HEp-2 cells.
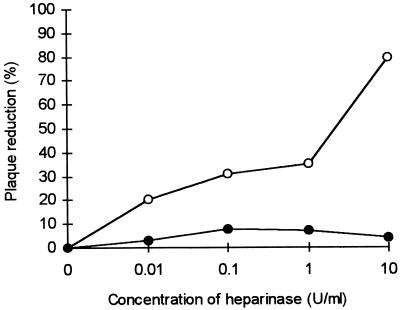
The virus was treated for 2 h with different concentrations of heparinase (Fig. 5). This treatment with progressively higher concentrations of heparinase decreased the number of syncytia (Fig. 5, ○). On the other hand, pretreatment of the cells with heparinase did not affect virus infectivity (Fig. 5, •). Preincubation of the virus with heparinase had an effect on its infectivity only at concentrations of heparinase greater than about 0.5 U/ml. With heparinase at 10 U/ml, we obtained 80% plaque reduction. These results suggest that heparinase is able to destroy a heparin-like structure on the virus, thus leading to a decrease of virus infectivity.
FIG. 5.
Effect of heparinase treatment on RSV before adsorption. Symbols: ○, virus treated with heparinase before infection of cells with RSV; •, cells pretreated with heparinase and then washed before infection with RSV.
Effect of chondroitinase on RSV before adsorption to HEp-2 cells.
To check the specificity of the inhibition observed with heparinase treatment, the same kind of assay was performed with chondroitinase ABC. This enzyme degrades chondroitin sulfate but not heparin or heparan sulfate (30). No decrease of infectivity was noted, whatever the enzyme concentration used (data not shown).
Effect of protamine on adsorption of RSV to HEp-2 cells.
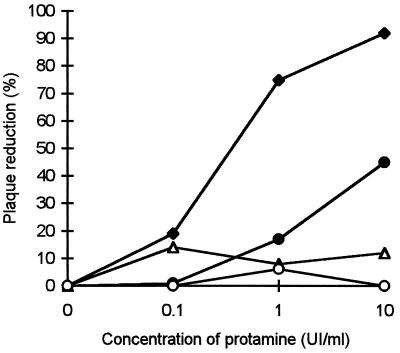
RSV was treated with protamine to inhibit heparin-like moieties on its surface (Fig. 6). A similar assay was done on cells as a control. With this inhibitor of heparin, the number of syncytia was reduced by 80% (from 1- to 10-IU/ml protamine) (Fig. 6, ⧫). Incubation of the virus with protamine solvent did not affect virus infectivity (Fig. 6, ▵). However, pretreatment of the cells with protamine led to a moderate decrease in infectivity (Fig. 6, •), which could be due to a nonspecific interaction between protamine and the cell surface. These results confirm that RSV infection is affected by inhibition of heparin-like structures on the virus surface.
FIG. 6.
Effect of protamine treatment on RSV before adsorption. Symbols: ⧫, virus treated with protamine before infection of cells with RSV. Symbols: •, cells pretreated with protamine and then washed before infection with RSV; ▵, virus treated with protamine solvent (glucose-cresol solution) before infection of cells with RSV; ○, cells pretreated with protamine solvent (glucose-cresol solution) and then washed before infection with RSV. UI, international units.
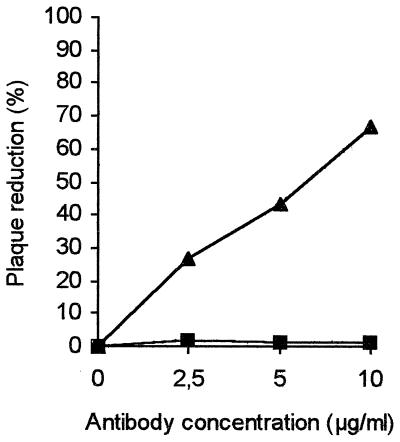
Neutralizing activity of anti-HSPG antibody.
As heparin was shown to be a competitor of RSV infection, an anti-HSPG antibody was investigated for its capacity to neutralize RSV infection in vitro. The anti-HSPG antibody titer determined in a neutralization assay of RSV infection was 7.8 μg/ml (Fig. 7). Pretreatment of HEp-2 cells with the anti-HSPG antibody did not affect RSV infectivity. No cytotoxicity was observed at the dilutions used in the assay. On the other hand, the anti-HSPG antibody was not capable of neutralizing the parainfluenza 3 virus (Fig. 7). Parainfluenza 3 virus was shown not to be susceptible to heparin (29). This assay confirms the specificity of the neutralization of RSV by the anti-HSPG antibody.
FIG. 7.
Neutralizing effect of anti-HSPG antibody on RSV infectivity (▴) or on parainfluenza 3 virus infectivity (■).
Immunoreactivity of RSV with anti-heparan sulfate antibodies. (i) Immunofluorescence.
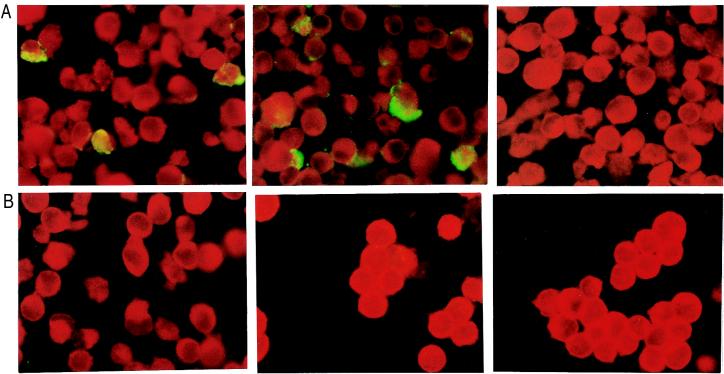
Because HSPGs, which are similar to heparin, are present on the surfaces of most cells, we expected that these antibodies would also stain HEp-2 cells. In fact, two of the antibodies, the anti-HSPG antibody and F58-10E4, stained only RSV-infected cells (Fig. 8). No staining was obtained with the third anti-heparan antibody, HepSS-1. RSV thus has heparin-like structures at its surface; these are different from the glycans that are probably carried by cells. HepSS-1 recognizes low-sulfated species of heparan sulfate. These are structurally different from heparin. Residues recognized by the anti-HSPG antibody and F58-10E4 are exhibited on RSV.
FIG. 8.
RSV-infected (A) or noninfected (B) HEp-2 cells stained with anti-HSPG antibody (left), F58-10E4 (middle), or HepSS-1 (right). Magnification; ×400. A positive reaction is indicated by green fluorescence.
(ii) ELISA.
We obtained the same results by ELISA as by immunofluorescence. A stronger signal was detected with F58-10E4. We checked that the anti-heparan sulfate antibodies reacted with heparin and heparan sulfate by competitive ELISA of infected cells. F58-10E4 antibody binding was strongly inhibited by heparin and heparan sulfate; anti-HSPG antibody binding was more strongly inhibited by heparan sulfate than by heparin (data not shown).
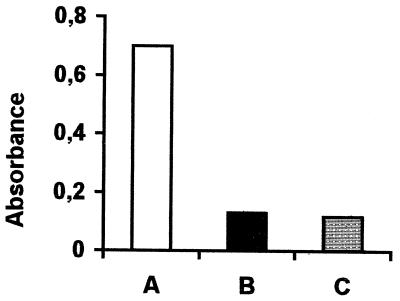
As heparin was shown to inhibit early events of infection, we carried out a sandwich ELISA involving the G protein to see if this protein was directly involved. The anti-HSPG and F58-10E4 antibodies strongly reacted with the purified G protein. The binding of the G protein was antigen dose dependent (data not shown). No signal was detected with the labeled RS-18B2 antibody, which recognizes the F protein. As a weaker signal was obtained after treatment of the G protein with heparinase or heparitinase, we conclude that the binding to the G protein involved heparin-like structures (Fig. 9).
FIG. 9.
Binding of purified G protein (A), heparinase-treated G protein (B), and heparitinase-treated G protein (C) to antibody F58-10E4. The signal/background ratio was above 2.
DISCUSSION
The data presented in this report confirm and extend previous observations showing the antiviral activity of heparin (14). In the study of Hosoya et al., heparin and the virus were added to the cells at the same time. In our study, we carried out assays which allowed us to make a distinction between effects on the cells and on the virus. We showed that heparin is an inhibitor of RSV infection. By using either the labeled virus or the labeled, purified G protein, it has been shown that binding to HEp-2 cells is not saturable and seems to be of low affinity (33). This is corroborated by our data which suggest that heparin may bind to the cell surface more rapidly than does the G protein. Indeed, heparin inhibited viral infection even when added to the cells at the same time as a viral inoculum. In our assays, heparin was an inhibitor of viral infection only for a certain time, 90 min at the most, when infection started at 37°C. Therefore, inhibition by heparin is efficient only during the very early events of infection. It is generally assumed that virus-cell binding can occur at 37 or 4°C and that the fusion process needs a temperature of 37°C to take place (5). Our assay did not allow us to discriminate between attachment and fusion. If no inhibition of infectivity had been observed after a long period of adsorption at 4°C, we could have concluded that heparin acts only on the attachment process. However, the addition of heparin led to partial inhibition. Thus, some viruses had already entered the cells and heparin could no longer affect them. At 37°C, total infection was already obtained after 90 min of adsorption. Therefore, heparin-sensitive steps of infection occur quickly at 37°C but proceed more slowly at 4°C. It is also possible that there is a supplementary stage of infection that occurs only at 37°C and that it is sensitive to heparin. Heparin competes with heparin-like moieties to inhibit an early stage of infection, probably a virion-cell surface interaction.
Heparin is a glycosaminoglycan composed of a sequence of alternating hexuronic acid and glucosamine components. Heparinase attacks heparin and produces fragments of varied lengths by cutting after glucosamine when it has a N-sulfated or O-sulfated group in position 6 only. This enzyme cuts also, but more slowly, if N-acetylated derivatives are the substrates and does not cut at all when N-unsubstituted amino sugars, apart from O-sulfated derivatives, are the substrates (9, 24). It has an unusual effect, as it hydrolyzes both →4)-iduronic and →4)-glucuronic linkages of glucosamine (1→. The iduronic acid may be 2-O sulfated. This enzyme acts on both heparan sulfate and heparin but not on any other glucosaminoglycans (21). In our study, after treatment of the virus with heparinase, we obtained a great loss of infectivity, i.e., 80%. Heparinase cleaves glycosidic linkages present in heparin and in heparan sulfate, while heparitinase selectively cleaves linkages present in nonsulfated parts of heparan sulfate. Heparitinase cleaves glucosamine (1→4)-glucuronic acid linkages, which is more common within heparan sulfate sequences than in heparin. It needs glucosamine N-acetylation or N-sulfatation but tolerates only O-sulfatation of C-6 in the amino sugar. Such structural features correspond especially to heparan sulfate (21). The binding of the G protein to antibody F58-10E4 was altered by digestion with both heparinase and heparitinase. However, differences in the in vitro inhibitory effects of heparin and heparan sulfate indicate that specific structures on these oligosaccharides could be of importance in the functional activity of RSV. The heparan sulfate group differs from the heparin group because it is more acetylated and less sulfated than heparin. Thus, heparin has a higher total polyanionic charge. Although our results give additional information on the kind of sugars present on RSV, it is difficult to assess the precise structure of these oligosaccharides without further biochemical characterization.
The inhibitory effect of heparin on RSV infection has already been reported. Recently, a mechanism different from that which we have described has been proposed, suggesting that heparin inhibits virus infectivity by binding to the G protein (18). Isolation of the G protein on heparin-Sepharose CL6B supported this hypothesis. However, we also harvested the G protein by using Sepharose CL6B as a control. This isolation was probably the result of nonspecific binding of the G protein to Sepharose CL6B, independently of heparin (data not shown). Other members of the family Paramyxoviridae (measles virus and paramyxoviruses) are not at all susceptible to inhibitors such as sulfated polysaccharides, dextran sulfate, and heparin (14, 29, 36). At first, the G glycoprotein was thought to be equivalent to the HN protein. However, the G protein differs radically from the glycoprotein reported in other paramyxoviruses. The G protein lacks hemagglutinating and neuraminidase activities (27). The absence of these activities suggests that, in contrast to paramyxoviruses such as Sendai virus, attachment does not occur via sialic acid residues present on the host cells. Moreover, the RSV G protein is heavily glycosylated. Far more of its molecular weight is due to carbohydrates than to an amino acid backbone. Not only is the amount of oligosaccharides greater, but the fundamental difference between RSV and the other members of the family Paramyxoviridae is that they are mainly O-linked oligosaccharides. Thus, it is not surprising that the binding of RSV to cells via its attachment protein involves different residues than for the other paramyxoviruses and that inhibitors such as dextran sulfate and heparin do not work on paramyxovirus.
Few studies have correlated RSV oligosaccharides and functional properties. After treatment of virions with O-glycanase, which removed O-linked oligosaccharides, only a small difference in electrophoretic mobility was observed, but there was a 97% reduction of infectivity (19). We also obtained a substantial reduction in infectivity after virus treatment with heparinase and showed that the G protein carries heparin-like sugars. Little information is available on how the G protein mediates virus attachment. A model of the three-dimensional structure of the G protein has recently been published (23). In this model, close to the putative receptor binding site (15), there are two regions bearing potential sites for O-glycosylations, regions which are therefore mucin like. Heparin-like structures, which are O-linked polysaccharides, seem to play a role in the attachment of RSV to the cell surface. They are therefore presumably close to the receptor binding site. They could participate in the virus-cell interaction as a stabilizer of the protein conformation needed for virus-cell contact. It could also be that an initial interaction occurs by means of oligosaccharides and that a second interaction, probably more specific and irreversible, involves a protein sequence.
Most monoclonal antibodies to the G protein need O-linked oligosaccharides to bind the RSV G protein (26). Of the 18 used in the study of Palomo et al., 8 were of the immunoglobulin M type; these 8 recognized the O-glycosylated or mature form of the G protein but not the precursor. Moreover, six of the eight were neutralizing (10, 26). Thus, sugars, particularly O-linked sugars, are important for the functional properties of the G protein. The neutralizing activity of the anti-HSPG antibody highlights the importance of oligosaccharides in infectivity. In addition, expression of the G gene has been obtained in a prokaryotic system (22) and has been shown to elicit much lower levels of neutralizing antibodies than the native G protein. It could be, in part, that the antibodies induced by a glycosylated G protein are able to link carbohydrate moieties, neutralizing the virus because they inhibit binding of the G protein to the cell surface, either directly or by steric inhibition.
In the study described here, heparin completely inhibited infectivity, which led to the supposition that blocking of attachment blocks infection. However, an RSV mutant without the G protein grew well in Vero cells (16). This virus bears several mutations which may be compensatory. It may also be, as suggested by Karron et al. (16), that cell surface lectins on Vero cells serve as an alternate receptor. On the other hand, this RSV mutant was only slightly infectious for humans. Thus, a difference in the lectins on the Vero cell surface and the human respiratory epithelium may exist. The antigenicity of the G protein is associated with the infected cell type (11). The RSV G protein from infected human tracheal biopsies exhibited no difference in electrophoretic mobility from that from HEp-2 cells, unlike the G protein obtained from some other cell lines. (Vero cells were not tested in that study.) The authors deduced that the G glycoprotein of RSV grown in HEp-2 cells is similar to the G protein found in the RSV-infected respiratory tract. Thus, heparin-like structures may also be found in infected human tracheal epithelium and not be cell-specific glycosylations. Further characterization is needed to determine if these glycans are also important during RSV infection in vivo.
ACKNOWLEDGMENTS
This work was supported by grants from the INSERM, the CNAMTS (no. 4API09), and the Conseil Régional de Bourgogne.
REFERENCES
- 1.Anderson K, King A M Q, Lerch R A, Wertz G W. Polylactosaminoglycan modification of the respiratory syncytial virus small hydrophobic (SH) protein: a conserved feature among human and bovine respiratory syncytial viruses. Virology. 1992;191:417–430. doi: 10.1016/0042-6822(92)90203-2. [DOI] [PubMed] [Google Scholar]
- 2.Apostolopoulos V, McKenzie I F C. Cellular mucins: targets for immunotherapy. Crit Rev Immunol. 1994;14:293–309. doi: 10.1615/critrevimmunol.v14.i3-4.40. [DOI] [PubMed] [Google Scholar]
- 3.Bourgeois C, Corvaisier C, Bour J B, Kohli E, Pothier P. Use of synthetic peptides to locate neutralizing antigenic domains on the fusion protein of respiratory syncytial virus. J Gen Virol. 1991;72:1051–1058. doi: 10.1099/0022-1317-72-5-1051. [DOI] [PubMed] [Google Scholar]
- 4.Bukreyev A, Whitehead S S, Murphy B R, Collins P L. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J Virol. 1991;71:8973–8982. doi: 10.1128/jvi.71.12.8973-8982.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choppin P W, Scheid A. The role of viral glycoproteins in adsorption, penetration and pathogenicity of viruses. Rev Infect Dis. 1980;2:40–61. doi: 10.1093/clinids/2.1.40. [DOI] [PubMed] [Google Scholar]
- 6.Collins P L, Huang Y T, Wertz G W. Nucleotide sequence of the gene encoding the fusion (F) glycoprotein of human respiratory syncytial virus. Proc Natl Acad Sci USA. 1984;81:7683–7687. doi: 10.1073/pnas.81.24.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins P L, Mottet G. Post-translational processing and oligomerization of the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol. 1991;72:3095–3101. doi: 10.1099/0022-1317-72-12-3095. [DOI] [PubMed] [Google Scholar]
- 8.Collins P L, Mottet G. Oligomerization and post-translational processing of glycoprotein G of human respiratory syncytial virus: altered O-glycosylation in the presence of brefeldin A. J Gen Virol. 1992;73:849–863. doi: 10.1099/0022-1317-73-4-849. [DOI] [PubMed] [Google Scholar]
- 9.Desai U R, Wang H, Linhardt R J. Specificity studies on the heparin lyases from Flavobacterium heparinum. Biochemistry. 1993;32:8140–8145. doi: 10.1021/bi00083a012. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Barreno B, Palomo C, Penas C, Delgado T, Perez-Brena P, Melero J A. Marked differences in the antigenic structure of human respiratory syncytial virus F and G glycoproteins. J Virol. 1989;63:925–932. doi: 10.1128/jvi.63.2.925-932.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Beato R, Martinez I, Franci C, Real F X, Garcia-Barreno B, Melero J A. Effect upon glycosylation and antigenicity of human respiratory syncytial virus G glycoprotein. Virology. 1996;221:301–309. doi: 10.1006/viro.1996.0379. [DOI] [PubMed] [Google Scholar]
- 12.Gruber C, Levine S. Respiratory syncytial virus polypeptides. IV. The oligosaccharides of the glycoproteins. J Gen Virol. 1985;66:417–432. doi: 10.1099/0022-1317-66-3-417. [DOI] [PubMed] [Google Scholar]
- 13.Heminway B R, Yu Y, Tanaka Y, Gustafson E, Bernstein J M, Galinski M S. Analysis of respiratory syncytial virus F, G, and SH proteins in cell fusion. Virology. 1994;200:801–805. doi: 10.1006/viro.1994.1245. [DOI] [PubMed] [Google Scholar]
- 14.Hosoya M, Balzarini J, Shigeta S, De Clercq E. Differential inhibitory effects of sulfated polysaccharides and polymers on the replication of various myxoviruses and retroviruses, depending on the composition of the target amino acid sequences of the viral envelope glycoproteins. Antimicrob Agents Chemother. 1991;35:2515–2520. doi: 10.1128/aac.35.12.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson P R, Spriggs M K, Olmsted R A, Collins P L. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci USA. 1987;84:5625–5629. doi: 10.1073/pnas.84.16.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karron R A, Buonagurios D A, Georgius A F, Whitehead S S, Adamus J E, Clements-Mann M L, Harris D O, Randolph V B, Udem S A, Murphy B R, Sidhu M S. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci USA. 1997;97:13961–13966. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H W, Arrobio J O, Brandt C D, Jeffries B C, Pyles G, Reid J L, Chanock R M, Parrot R H. Epidemiology of RSV infection in Washington, D.C. Importance of virus in different respiratory tract disease syndromes and temporal distribution of infection. Am J Epidemiol. 1973;98:216–225. doi: 10.1093/oxfordjournals.aje.a121550. [DOI] [PubMed] [Google Scholar]
- 18.Krusat T, Streckert H J. Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch Virol. 1997;142:1247–1254. doi: 10.1007/s007050050156. [DOI] [PubMed] [Google Scholar]
- 19.Lambert D M. Role of oligosaccharides in the structure and function of respiratory syncytial virus glycoproteins. Virology. 1988;164:458–466. doi: 10.1016/0042-6822(88)90560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine S, Klaiber-Franco R, Paradiso P R. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J Gen Virol. 1987;68:521–524. doi: 10.1099/0022-1317-68-9-2521. [DOI] [PubMed] [Google Scholar]
- 21.Linhardt R J, Turnbull J E, Wang H M, Loganathan D, Gallagher J T. Examination of the substrate specificity of heparin and heparan sulfate lyases. Biochemistry. 1990;29:2611–2617. doi: 10.1021/bi00462a026. [DOI] [PubMed] [Google Scholar]
- 22.Martin-Gallardo A, Fleischer E, Doyle S A, Arumugham R, Collins P L, Hildreth S W, Paradiso P R. Expression of the G glycoprotein gene of human respiratory syncytial virus in Salmonella typhimurium. J Gen Virol. 1993;74:453–458. doi: 10.1099/0022-1317-74-3-453. [DOI] [PubMed] [Google Scholar]
- 23.Melero J A, Garcia-Barreno B, Martinez I, Pringle C R, Cane P A. Antigenic structure, evolution and immunobiology of human respiratory syncytial virus attachment (G) protein. J Gen Virol. 1997;78:2411–2418. doi: 10.1099/0022-1317-78-10-2411. [DOI] [PubMed] [Google Scholar]
- 24.Moffat C F, McLean M W, Long W F, Williamson F B. Heparinase II from Flavobacterium heparinum. HPLC analysis of the saccharides generated from chemically modified heparins. Eur J Biochem. 1991;202:531–541. doi: 10.1111/j.1432-1033.1991.tb16405.x. [DOI] [PubMed] [Google Scholar]
- 25.Olmsted R A, Collins P L. The 1A protein of respiratory syncytial virus is an integral membrane protein present as multiple, structurally distinct species. J Virol. 1989;63:2019–2029. doi: 10.1128/jvi.63.5.2019-2029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palomo C, Garcia-Barreno B, Penas C, Melero J A. The G protein of human respiratory syncytial virus: significance of carbohydrate side-chains and the C-terminal end to its antigenicity. J Gen Virol. 1991;72:669–675. doi: 10.1099/0022-1317-72-3-669. [DOI] [PubMed] [Google Scholar]
- 27.Richman A V, Pedreira F A, Tarauso N M. Attemps to demonstrate hemagglutination and hemadsorption by respiratory syncytial virus. Appl Microbiol. 1971;21:1099–1100. doi: 10.1128/am.21.6.1099-1100.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satake M, Coligan J E, Elango N, Norrby E, Venkatesan S. Respiratory syncytial virus envelope glycoprotein (G) has a novel structure. Nucleic Acids Res. 1985;13:7795–7812. doi: 10.1093/nar/13.21.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schols D, De Clercq E, Balzarini J, Baba M, Witvrouw M, Hosoya M, Andrei G, Snoeck R, Neyts J, Pauwels R, Nagy M, Györgyi-Edelényi J, Machovich R, Horvath I, Löw M, Görög S. Sulphated polymers are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, respiratory syncytial virus, and toga-, arena- and retroviruses. Antiviral Chem Chemother. 1990;1:233–240. [Google Scholar]
- 30.Schomburg D, Salzmann M, editors. Enzyme handbook. Class 4: lyases. Berlin, Germany: Springer-Verlag KG; 1990. [Google Scholar]
- 31.Spriggs M K, Olmsted R A, Venkatesan S, Coligan J E, Collins P L. Fusion glycoprotein of human parainfluenza type 3: nucleotide sequence of the gene, direct identification of the cleavage-activation site, and comparison with other paramyxoviruses. Virology. 1986;152:241–251. doi: 10.1016/0042-6822(86)90388-0. [DOI] [PubMed] [Google Scholar]
- 32.Walsh E E, Schlesinger J J, Brandriss M W. Purification and characterization of GP90, one of the envelope glycoproteins of respiratory syncytial virus protein. J Gen Virol. 1984;66:761–767. doi: 10.1099/0022-1317-65-4-761. [DOI] [PubMed] [Google Scholar]
- 33.Walter A, Downing L A. Respiratory syncytial virus interactions with host cell membranes: an enigma among the Paramyxoviridae. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 363–383. [Google Scholar]
- 34.Wertz G W, Collins P L, Huang Y, Gruber C, Levine S, Ball L A. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci USA. 1985;82:4075–4079. doi: 10.1073/pnas.82.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wertz G W, Krieger M, Ball L A. Structure and cell surface maturation of the attachment glycoprotein of human respiratory syncytial virus in a cell line deficient in O glycosylation. J Virol. 1989;63:4767–4776. doi: 10.1128/jvi.63.11.4767-4776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White J, Matlin K, Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981;89:674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]