Abstract
Rationale
Lymphatic transport of peripheral interstitial fluid and dendritic cells (DCs) is important for both adaptive immunity and maintenance of tolerance to self-antigens. Lymphatic drainage can change rapidly and dramatically upon tissue injury or inflammation, and therefore increased fluid flow may serve as an important early cue for inflammation; however, the effects of transmural flow on lymphatic function are unknown.
Objective
Here we test the hypothesis that lymph drainage regulates the fluid and cell transport functions of lymphatic endothelium.
Methods and Results
Using in vitro and in vivo models, we demonstrate that lymphatic endothelium is sensitive to low levels of transmural flow. Basal-to-luminal flow (0.1 and 1 μm/s) increased lymphatic permeability, dextran transport, and aquaporin-2 expression as well as DC transmigration into lymphatics. The latter was associated with increased lymphatic expression of the DC homing chemokine CCL21 and the adhesion molecules ICAM-1 and E-selectin. In addition, transmural flow induced delocalization and downregulation of VE-cadherin and PECAM-1. Flow-enhanced DC transmigration could be reversed by blocking CCR7, ICAM-1, or E-selectin. In an experimental model of lymphedema, where lymphatic drainage is greatly reduced or absent, lymphatic endothelial expression of CCL21 was nearly absent.
Conclusions
These findings introduce transmural flow as an important regulator of lymphatic endothelial function and suggest that flow might serve as an early inflammatory signal for lymphatics, causing them to regulate transport functions to facilitate the delivery of soluble antigens and DCs to lymph nodes.
Keywords: CCL21, ICAM-1, inflammation, lymphedema, in vitro, overhydration
Introduction
Immune functions of lymphatic endothelium include the transport of interstitial fluid to the lymph node, which provides constant sampling of peripheral antigens to antigen-presenting cells (APCs) such as dendritic cells (DCs), macrophages, and B cells residing there,1,2 and cell transport from the periphery to the lymph node.3 These processes are important both for inducing an adaptive immune response as well as maintaining tolerance to self-antigens.4 Despite its importance, the active regulation of fluid and cell transport by lymphatic endothelium is largely unexplored. We asked how the lymphatic endothelium might sense and respond to its local physical environment to regulate these functions, particularly with respect to inflammation and tissue injury.
Arguably, the first events in tissue injury and inflammation include the rapid release of mediators that increase the permeability of the local blood vessels, which leads to influx of plasma fluid and proteins into the interstitium, elevated interstitial fluid pressure, and increased lymph flow.5-8 This is followed by the release of inflammatory cytokines such as tumor necrosis factor (TNF)-α. Recently, several inflammatory cues including TNF-α, TNF-β, and interleukin (IL)-1 as well as pathogenic signals like bacterial lipopolysaccharide (LPS) were shown to influence immune cell traffic into lymphatics by modulating lymphatic endothelial expression of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and endothelial selectin (E-selectin),9-11 which are used by DCs for transmigration into lymphatics.
While the expression of such inflammatory cytokines following tissue injury can take several hours,12 heightened lymph flow increases almost immediately.5-8 We hypothesized that heightened transmural flow could act as an immediate cue for signaling injury or inflammatory conditions to lymphatic endothelium, driving changes in lymphatic endothelial transport functions to modulate fluid and DC trafficking to the draining lymph node. This could potentially act alone or in concert with inflammatory cytokines. Other recent evidence points to fluid shear stress as a modulator of nitric oxide release and lymphatic pump function in contractile lymphatics13, 14, and intraluminal shear stress is well-known to regulate leukocyte adhesion and transmigration events in blood endothelium.15, 16 However, the role of transmural flow on lymphatic endothelium and its transport functions has not been examined to date, and while leukocytes exiting blood vessels experience a high-shear environment before and during their transmigration, leukocytes entering lymphatics do not experience such conditions in the basal interstitium. Because lymphatic capillaries play a different role in inflammation than blood capillaries, their functional responses to environmental cues need to be separately investigated.
Here, using both in vivo and in vitro systems, we demonstrate that transmural flow can stimulate lymphatic endothelium to increase fluid transport and DC transmigration in part by increasing the DC chemoattractant CCL21 and the DC adhesion molecules ICAM-1 and E-selectin, and by decreasing the lymphatic junctional adhesion molecules PECAM-1 and VE-cadherin. Based on these findings we suggest that lymphatic flow is a key mediator of lymphatic function, particularly with respect to DC recruitment and trafficking to the lymph node.
Methods
Cell Lines
Human microvascular LECs were isolated from neonatal foreskin and cultured as described;17 details are given in Supplementary Methods. DCs were derived from peripheral blood mononuclear cells (PBMNCs) of healthy human donors as described18 and matured with 0.2 μg/ml LPS (Invivogen) for 48 h.
Mice
8-10 week old female C57BL/6 and BALB/c mice (Charles River Laboratories) and transgenic C57BL/6 CD45.1 mice and eGFP-BALB/c mice (Jackson Laboratories) were used. All procedures were approved by the Office Vétérinaire Cantonale Vaud, Switzerland.
DC transmigration assay
Details are given in the Supplementary Methods. Briefly, LECs were cultured to confluence on the undersides of collagen-coated 8μm-pore culture inserts. Cell tracker green-labeled human DCs, suspended in 1.8 mg/ml type I collagen with 10% Matrigel, were added to the inserts and transmural flow velocities of 0.1 or 1 μm/s were imposed via a medium pressure head between upper and lower chambers. After 12h, transmigrated DCs were counted. To assess the roles of ICAM-1, E-selectin, or VCAM-1, LECs were pre-incubated for 1h with the appropriate neutralizing antibody that was maintained in the gel and medium for the duration of the experiment. In some experiments, 100 ng/ml TNF-α (R&D Systems) was added. We estimated transmural flow velocities in vitro by measuring flow rates through the inserts at different pressure heads and dividing by the insert cross-sectional area.
In vivo overhydration and adoptive transfer
C57BL/6 CD45.1 and BALB/c mice were subjected to overhydration for 24h by s.c. injection with saline (10-15% of body mass). Mouse weight was measured every 2-3h, and additional saline was injected when necessary to maintain the 10-15% increase in body mass. The overhydration was performed for 24h before conductance measurements or DC transfer. Splenic DCs from C57BL/6 CD45.2 donors were isolated using magnetic sorting with CD11c microbeads (MACS Miltenyi BioTec). Bone marrow DCs from eGFP-BALB/c donors were isolated as described.19 5×105 cells in 10μl were injected i.d. into each foot. Overhydration was maintained for another 12h then draining lymph nodes were isolated and the total number of GFP DCs (in BALB/c mice) or CD45.1+CD11c+ cells (in C57BL/6 mice) was measured by flow cytometry.
Lymphatic conductance
The functional uptake of an injected dextran solution into the initial lymphatics in the tail, as defined by the volume of fluid drained by lymphatics per tissue volume, applied pressure, and time (yielding units of mmHg-1min-1), was determined as described previously20, 21 and detailed in the online Supplementary Methods. This method allows one to differentiate the tissue hydraulic conductivity from the lymphatic conductance and treat them independently from each other.
Mouse tail lymphedema model
Lymphedema was induced as described previously.20, 21 Briefly, a circumferential incision was made through the dermis close to the tail base to sever dermal lymphatic vessels without disturbing major blood vessels. The edges of this incision were then pushed apart with forceps, creating a ∼2 mm gap to delay wound closure. Tissue was excised after 7 days for CCL21 determination of CCL21 expression associated with lymphatic capillaries (LYVE-1+ structures).
Permeability assay
LECs were seeded on the bottom of 0.4μm pore size inserts (Costar) as described above and cultured for 24h. After 12h pretreatment with 0.1 or 1 μm/s transmural flow, flow was stopped by normalizing pressure and 10 μg/ml Cascade blue dextran 3kDa (Invitrogen) in EBM with 2% FBS was added to the upper chamber of the insert. After 4h (static conditions), medium from the bottom side was measured in a Safire 2 plate reader (Tecan) and calibrated to a standard curve to estimate the effective permeability (Peff, μm/sec) as described.22
siRNA transfection of DCs and LECs
siRNA transfection of DCs and LECs was performed using a Nucleofector kit (Amaxa, Lonza) according to the manufacturer's protocol. Control siRNA and siRNA targeting CCR7 on DCs and ICAM-1 on LECs were used (Santa Cruz Biotechnology). Knockdown efficiency was tested for CCR7 in DCs after 24h by real-time PCR (see below) and ICAM-1 in LECs by flow cytometry after 48h and following 12h stimulation with 10 ng/ml TNF-α.
Protein and gene expression
CCL21 secretion from LECs was evaluated by ELISA (R&D Systems) after the collagen/matrigel matrix was removed and digested in Collagenase D (Roche). Gene expression was evaluated after Trizol (Invitrogen) extraction from cells in the matrix when required, cell lysis with RNA lysis-binding solution (Ambion) and RNA extraction (RNAquesous-Micro;Ambion); cDNA synthesis was performed with iScript cDNA Synthesis Kit (BioRad Laboratories). Gene expression was normalized to GAPDH and then to the static control for each experiment. Primer sequences are given in the Supplementary Methods.
Flow cytometry and immunostaining
DCs were isolated from their matrix with collagenase D and analyzed by flow cytometry (CyAN, Dako) using FlowJo software (Tree Star, Inc). For immunostaining, cultured cells or tail skin cryosections were fixed in 2% PFA, and incubated with primary and secondary antibodies (detailed in Supplementary Methods). Actin was visualized using AlexaFluor-647 phalloidin (Invitrogen). Sections were mounted in DAPI-containing Vectashield (Vector Laboratories) and viewed under a LSM 510 META microscope (Zeiss). Image quantification was done in Matlab (Mathworks) and Metamorph 6.3 (Molecular Devices). Huygens (Scientific Volume Imaging, B.V) and Imaris (version 6, Bitplane) were used for isosurface rendering as described.23
Statistical analysis
Kruskal-Wallis with Tukey post-tests or Mann-Whitney U-tests (when comparing only two groups) were used; P≤0.05 was considered significant. Mean ± s.d. are shown in all bar graphs; median ± 95% confidence intervals are shown in box plots.
Results
Transmural flow regulates the fluid transport function of lymphatic endothelium
To determine whether transmural flow regulates fluid transport and influences DC migration into lymphatic vessels (Fig. 1a), we used an in vitro 3D model that recapitulates both the migratory environment of the interstitium and the basal-to-apical fluid flow and DC transmigration directions (Fig. 1b). While transmural flow across lymphatic endothelium has not been measured, interstitial flow has been measured in normal (non-injured) tissues and found to be on the order of 0.1μm/s,24 which is likely to be within the same order of magnitude as bulk-averaged transmural lymph flow velocities (but not actual velocities in cell-cell junctions). In acute inflammation, lymph flow can increase by an order of magnitude or more.6-8 Therefore, we chose to compare bulk transendothelial flow velocities of 0, 0.1, and 1 μm/s to represent static, low flow, and high flow conditions, respectively, which very roughly correlates with measured interstitial flow velocities in normal vs. inflamed tissue24,25 (noting that there is likely to be a large range depending on the animal, tissue, state of activity, type of inflammatory agent, etc).
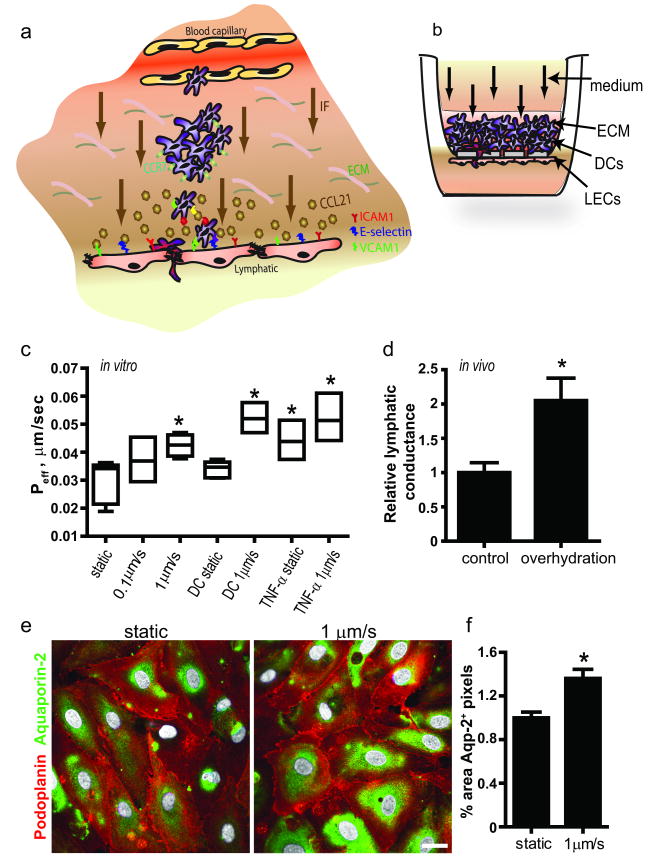
Figure 1. Fluid transport across lymphatic endothelium is increased after transmural flow preconditioning.
a, Schematic showing transmural flow and migration of dendritic cells (DCs) from the extracellular matrix (ECM) across lymphatic endothelium. CCR7+ DCs chemoattract towards CCL21-secreting lymphatic vessels and transmigrate using the adhesion molecules ICAM-1, E-selectin and VCAM-1. b, The in vitro model of the DC-lymphatic microenvironment includes lymphatic endothelial cells (LECs) seeded on the bottom of a porous culture insert and ECM containing DCs through which medium flows at 0.1 or 1μm/s, from the basal to the apical side. c, Effective permeability of LECs to 3 kDa dextran after 12 h preconditioning with transmural flow, DCs, or 100 ng/ml TNF-α. d, Lymphatic conductance (volume drained fluid per tissue volume per time and applied pressure gradient) is roughly doubled in overhydratated vs. control mice. e, Aquaporin-2 expression in vitro under static vs. flow conditions. Bar, 20μm. f, Aquaporin-2 protein expression is increased after 12h transmural flow. *P<0.05 compared to static controls.
We found that after 12 h of 0.1 or 1 μm/s transmural flow preconditioning, the permeability of lymphatic endothelium to 3kDa dextran was increased (Fig. 1c); we note that the permeability was measured under static conditions for 3 h in all cases. The presence of mature DCs did not affect this permeability. Since TNF-α is known to increase blood endothelial permeability,26, 27 we compared the flow effects on LECs with those of TNF-α and found that the flow-enhanced permeability was of a similar magnitude as that due to 100 ng/ml TNF-α (Fig. 1c). In vivo, lymphatic conductance was nearly doubled after 24h overhydration (Fig. 1d). To further assess the impact of transmural flow on lymphatic permeability, we looked at flow-induced changes to Aquaporin-2 channels, which regulate water flux in renal epithelial cells and which are abundant on LECs. Following 1 μm/s transmural flow for 12 h, we observed significantly more aquaporin-2 protein expression in LECs (Fig. 1e-f). These data suggested that lymphatic transport of water and solutes is sensitive to transmural flow.
Flow downregulates and delocalizes VE-cadherin and PECAM-1
Lymphatic cell-cell junctions are composed of vascular endothelial (VE)-cadherin and platelet/endothelial cell adhesion molecule-1 (PECAM-1, CD31) along with other tight junctional molecules including occludin and zona occludens-1 (ZO-1).23 In lymphatic capillaries, VE-cadherin appears as discontinuous ‘buttons’ that pin down overlapping lymphatic endothelial ‘flaps’ expressing PECAM-1.23 In blood endothelium, VE-cadherin and PECAM-1 both help regulate leukocyte extravasation.28-30 Importantly, PECAM-1 and VE-cadherin have been shown, together with VEGFR-2, to comprise a flow mechanosensory complex in blood endothelium, and vessels of PECAM-1-/- mice do not activate NF-κB in response to disturbed flow.31 We therefore wondered whether slow transmural flow could affect these junctional molecules in lymphatic endothelium.
In vitro, we found that the expression patterns of VE-cadherin and PECAM-1 in LEC junctions changed dramatically in response to transmural flow (Fig. 2a). In static conditions, VE-cadherin expression appeared continuous along cell-cell borders, and PECAM-1 was present on overlapping cell-cell surfaces (Fig 2a, yellow arrow). In contrast, under flow conditions, these junctions appeared disorganized (Fig 2a), and the expression of VE-cadherin and PECAM-1 decreased both at the protein (Fig. 2b) and gene (Fig. 2c) levels. Flow also caused the two molecules to become spatially delocalized in both the xy-plane (Fig. 2d) as well as in the z-direction (Fig. 2e-f), where VE-cadherin localized on the basal side relative to PECAM-1 (Fig. 2a and 2f). In vivo, overhydration triggered decreases in expression of lymphatic (LYVE-1+)-associated VE-cadherin and PECAM-1 as compared to those in control conditions (Fig. 2g and Suppl. Fig. IA). In contrast, we found no changes in expression of ZO-1 or occludin (Suppl. Figs IB-D). Furthermore, expression of β-catenin, which binds the cytoplasmic domains of PECAM-1 and VE-cadherin32 and could also be triggered by mechanical stress,31 was unchanged (Suppl. Fig. ID). Therefore, of the junctional molecules examined, only PECAM-1 and VE-cadherin were strongly affected by transmural flow. This was consistent with the increases in permeability observed (Fig. 1), since the organization of VE-cadherins is strongly correlated with vascular permeability in blood endothelium.32
Figure 2. Transmural flow downregulates and delocalizes VE-cadherin and PECAM-1 on lymphatic endothelium.
a, Immunostaining for VE-cadherin (red) and PECAM-1 (green) with 3D reconstruction of lymphatic endothelial cell (LEC) junctions in vitro after 12 h. In static conditions, PECAM-1 appears on overlapping portions of LECs surrounded by VE-cadherin (yellow arrows); in flow conditions, PECAM-1 and VE-cadherin appear delocalized and downregulated, with VE-cadherin localizing to the basal side (lower right). Bar, 20 μm. b, Image quantification shows downregulation of VE-cadherin and PECAM-1 with 1 μm/s flow after 12 h. c, Quantitative PCR for VE-cadherin and PECAM-1 in LECs after 12h treatment with transmural flow or with dendritic cells (DCs). d and e, Colocalization of VE-cadherin and PECAM-1 in the (d) x-y plane and (e) z-plane. f, Relative distributions of VE-cadherin and PECAM-1 show that VE-cadherin becomes localized to the basal surface upon flow activation. g, Quantification of in vivo immunostaining for VE-cadherin and PECAM-1 (associated with LYVE-1+ lymphatic structures) after 24h overhydration (OH).
Transmural flow regulates dendritic cell migration across lymphatic endothelium
We next asked whether transmigration of DCs across lymphatic endothelium was influenced by transmural flow, using our in vitro model (Fig 1b) along with the in vivo model of adoptive transfer of DCs into overhydrated mice. In both cases, 12h was determined to be optimal for observing sufficient numbers of transported DCs (Suppl. Fig II and data not shown). In vitro, transmigration of mature DCs (CD11c+CD86+CCR7+) was higher in 1 μm/s flow vs. static conditions (Fig. 3a). This enhancement (∼3-fold) was similar to that driven by TNF-α, but together, flow and TNF-α synergized to increase DC transmigration 5-fold (Fig. 3a). In vivo, 4 times more adoptively transferred DCs migrated to lymph nodes in overhydrated mice as compared to in control mice (Fig. 3b); however we note that in addition to increased lymphatic flux, overhydration may cause other changes that could directly affect DC migration, and so these results should be considered as complementing the in vitro data. Interestingly, although DCs were frequently observed at the junctional convergence between 2 or 3 adjoining cells (Fig. 3c), as previously reported,33 we infrequently observed in vitro DCs transmigrating through the LEC cytoplasm (Fig. 3d) only under flow conditions. Taken together, these data indicate that the elevated lymph drainage facilitates DC transmigration into lymphatic vessels and that both transcellular and paracellular routes may be used, at least in cultured LECs.
Figure 3. Dendritic cell transmigration across lymphatic endothelium is increased by transmural flow and occurs through both transcellular and paracellular routes.
a, In vitro dendritic cell (DC) transmigration across cultured lymphatic endothelial cells (LECs) after 12 h transmural flow and 100ng/ml TNF-α treatment. b, In vivo DC migration to the draining lymph node (LN) 12 h after adoptive transfer in control vs. overhydrated mice. White and black circles represent data from BALB/c and C57BL/6 mice, respectively. c, Paracellular and d, transcellular transmigration routes of DCs were seen in vitro. Top row: Confocal images of DCs (green, CD11c) in the process of transmigration across LECs (violet, phalloidin; red, VE-cadherin). Right and bottom insets to the first row of images show cross-sections in the y- and x-directions, respectively, with dotted lines indicating the insert membranes. Bar, 10μm. Bottom row: scanning electron micrographs show DC migration through both paracellular (left) and transcellular (right) pathways. Bars 10 μm (left), 4 μm (right).
CCL21 expression and secretion from lymphatic endothelium is flow-dependent
One possible mechanism of flow-enhanced DC trafficking is the modulation of relevant chemokines in response to flow stimulation. Chemokine (C-C motif) ligand 21 (CCL21, SLC or 6CKine) is secreted by lymphatic endothelium to attract mature DCs that express its receptor, C-C chemokine receptor type 7 (CCR7),34. In static culture, LEC expression of CCL21 protein was very low, but transmural flow visibly and significantly enhanced CCL21 protein as determined by immunostaining and ELISA (Fig. 4a-c). In vivo, lymphatic vessels in 7-day lymphedematous skin, which experience greatly reduced flow,20, 21 showed virtually no CCL21 expression compared to skin of control and overhydrated mice (Fig. 4d-e). At the mRNA level, we found time-dependent expression profiles of CCL21, with increased at 6h followed by a decrease at 12h (Fig. 4f). Furthermore, both LECs and CCR7 signaling were required for the flow-enhanced DC transmigration shown earlier (Fig. 1b), since blocking CCR7 or removing LECs reduced DC transmigration to levels seen in static conditions (Fig. 4g).
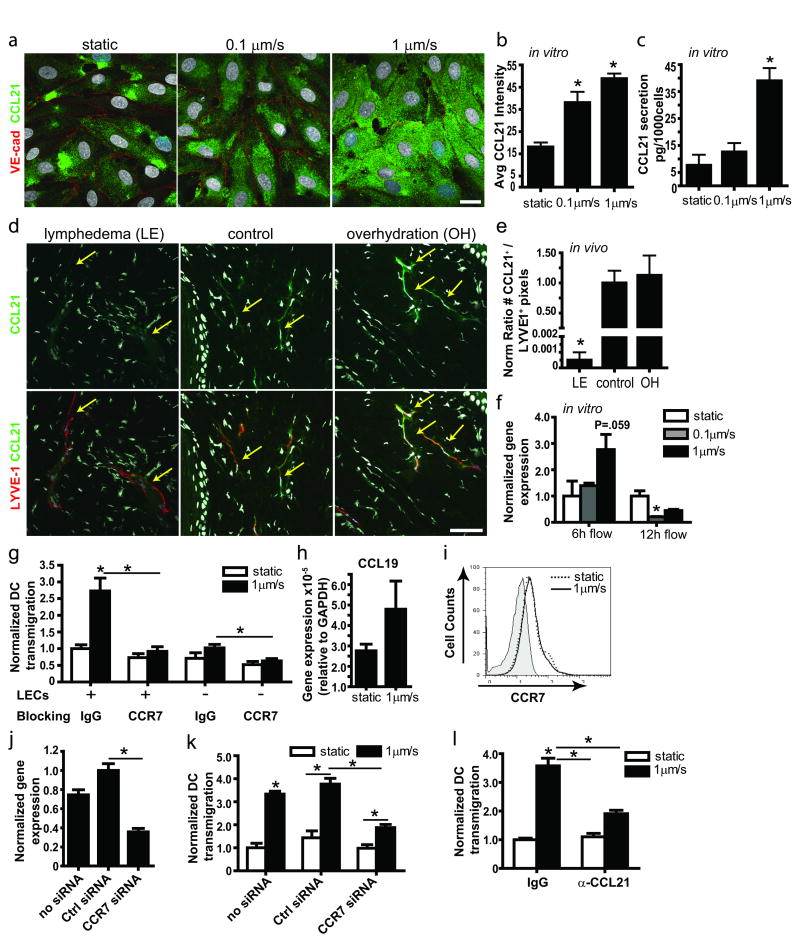
Figure 4. Transmural flow increases CCL21 secretion by lymphatic endothelium.
a, Representative confocal images show CCL21 (green) expressed by lymphatic endothelial cells (LECs) after 12 h exposure to 0 (static), 0.1, and 1μm/sec flow; red, VE-cadherin; bar, 20μm. b, Image quantification of CCL21 protein in cells exposed to 12h flow vs. static conditions. c, CCL21 protein measured by ELISA after 24h culture. d, Representative images of lymphatic vessels (red, LYVE-1) and CCL21 (green) in lymphedematous (LE), control, and overhydrated (OH) skin. Arrows indicate lymphatic vessels; bar, 50μm. e, Quantification of CCL21 staining in cultured LECs after 12h transmural flow. f, Real-time PCR expression after 6h and 12h flow treatment. g, DC transmigration across a LEC monolayer with control IgG or anti-CCR7 blocking antibodies. h, CCL19 gene expression by DCs in 3D cultures after 12h static or flow conditions. i, Representative histogram from flow cytometry showing no differences in CCR7 expression by DCs in static (dotted line) and 12h flow (1μm/sec, solid line) conditions; shaded area shows the negative control. j, Real-time PCR for CCR7 expression in DCs after 24h of siRNA transfection. k, DC transmigration across a LEC monolayer following DC transfection with control or CCR7 siRNA. l, CCL21 blocking inhibits the flow-enhanced DC transmigration across LECs. *P<0.05 compared to static controls.
We note that small increases in LEC-independent DC transmigration were observed under flow conditions (Fig. 4g), reminiscent of flow-induced “autologous chemotaxis” previously described,35 but since autologous CCL19 chemokine secretion by DCs was extremely low (Fig. 4h) and CCR7 expression was unchanged by flow (Fig. 4i), we concluded that the flow-enhanced DC transmigration across LECs was due mainly to LEC activation by flow rather than direct effects on the DCs themselves. To further support the hypothesis that flow-enhanced DC transmigration was LEC CCL21-dependent, we silenced CCR7 in DCs (with ∼60% knockdown, Fig. 4j) and found corresponding knockdown of flow-enhanced effects (Fig. 4k), but no effects on static transmigration levels. Similarly, selective inhibition of CCL21 from LECs following pretreatment with neutralizing antibodies significantly reduced DC transmigration only in flow conditions (Fig. 4l), implying that flow-enhanced DC transmigration was at least partly mediated by LEC-derived CCL21, which increases with flow.
ICAM and E-selectin are regulated by flow to facilitate DC transmigration
While CCL21 secretion provides directional cues for the DC to migrate towards the lymphatic vessel, adhesion molecules may also be required for transmigration into the vessel. ICAM-1, VCAM-1, and E-selectin were previously found to be upregulated on TNF-α–stimulated LECs to facilitate DC transmigration across inflamed lymphatic endothelium,9 suggesting that lymphatic capillaries may sense and respond to inflammatory cytokines via upregulation of leukocyte adhesion molecules. In vitro, we found that 1μm/s transmural flow upregulated protein expression of ICAM-1, VCAM-1, and E-selectin after 12 h (Fig. 5a and 5b). In vivo, only ICAM-1 and E-selectin protein levels were significantly increased on lymphatic endothelium after 24 h overhydration when compared to control conditions (Figs. 5c and Suppl. Fig III); again we note that overhydration may cause other tissue changes as well as increased fluid flow. In addition, quantitative PCR revealed time-dependent gene expression profiles (Fig. 5d); ICAM-1 and VCAM-1 were strongly increased after 6 h flow but decreased to baseline levels at 12h, while E-selectin was increased at both 6 and 12 h but only at 1 μm/s flow.
Figure 5. Lymphatic expression of adhesion molecules is regulated by transmural flow.
a, Immunostaining for ICAM-1, E-selectin and VCAM-1 (green) on cultured lymphatic endothelial cells (LECs) after 12h flow. Bar, 20μm. b, Image quantification from cultured LECs. c, Quantification of ICAM-1+, E-selectin+, and VCAM-1+ pixels associate with lymphatic structures (LYVE-1+) in mouse tail skin sections. d, Normalized gene expression after 6h and 12h flow; black bars show 12h flow in the presence of mature DCs. e, Confocol z-slices after 12h of 1μm/s flow, podoplanin (Podo, red) delineates the cell membrane; bar, 5 μm. *P<0.05 compared to static.
Moreover, when comparing protein and gene expression in cultured LECs under flow and in the presence or absence of mature DCs, we observed that together, flow and DCs caused a synergistic increase in ICAM-1 and E-selectin compared to either flow or DCs alone (Fig 5d, black bars). VCAM-1 expression was not changed upon addition of DCs in either static or flow conditions. ICAM-1 and VCAM-1 were recently shown to be involved in migration of T cells in the basal to apical direction of lymph node-associated lymphatics.36 However, in vitro, all were expressed on both the apical and basal sides of the LEC monolayer (Fig. 5e).
To determine whether the flow-enhanced DC migration was dependent on this upregulation of adhesion molecules, we selectively blocked ICAM-1, E-selectin and VCAM-1 in vitro. Blocking either ICAM-1 or E-selectin alone completely inhibited the flow-enhanced increase in DC migration, while blocking VCAM-1 had no significant effect (Fig 6a). Interestingly, DC transmigration under static conditions was not reduced following blocking antibody treatment (Fig. 6a), consistent with recent findings that DC transmigration into lymphatics could be integrin-independent under steady-state conditions.33
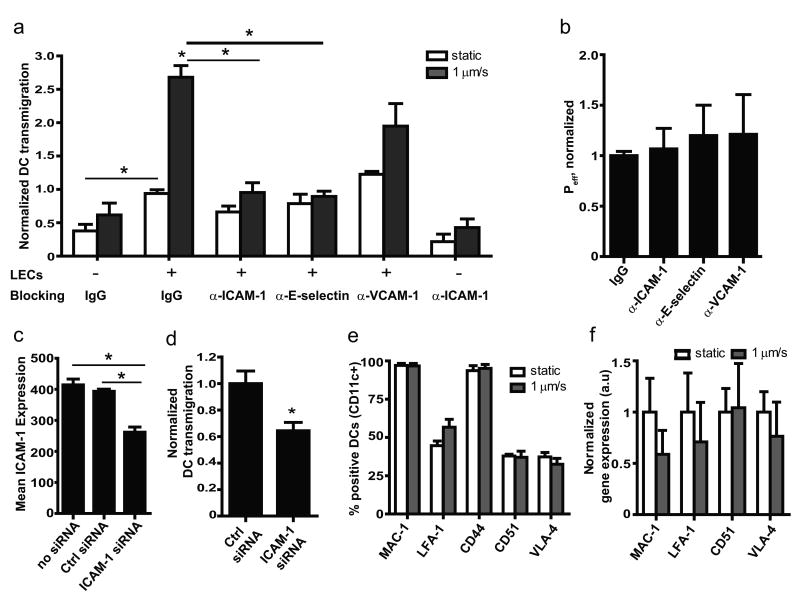
Figure 6. ICAM and E-selectin regulate flow-enhanced, but not static, DC transmigration.
a, Transmigration of dendritic cells (DCs) across lymphatic endothelial cells (LECs) in presence of blocking antibodies after 12h flow (black bars) or static (white bars) conditions. b, No change in effective permeability to dextran of LECs blocked with ICAM-1, E-selectin or VCAM-1 after 12h flow. c, ICAM-1 protein on LECs following ICAM-1 siRNA knockdown; cells were stimulated for 12h with 10ng/ml TNF-α before measurement. d, DC transmigration across ICAM-1-silenced LECs under 1μm/s flow conditions. e and f, DC surface expression by flow cytometry (e) or gene expression by real-time PCR (f) of receptors to ICAM-1, VCAM-1, and E-selectin are not affected by flow.
To determine whether the neutralizing antibodies affected LEC permeability, we measured permeability after 12h flow with concurrent blocking of ICAM-1, E-selectin or VCAM-1 and found no differences in Peff between any groups (Fig 6b). Furthermore, since DCs also express ICAM, we blocked ICAM on DCs in the absence of LECs and found this to have little effect on transmigration (Fig. 6a). In contrast, when ICAM-1 was knocked down ∼40% in LECs (Fig. 6c), flow-enhanced DC transmigration was also decreased about 40% (Fig 6d).
Finally, we assessed whether interstitial flow could influence DC expression of lymphocyte function-associated antigen-1 (LFA-1 or CD11a), macrophage-1 antigen (MAC-1 or CD11b), CD44, and very late antigen-4 (VLA-4 or CD49d), which are the DC-expressed ligands for ICAM-1, E-selectin, and VCAM-1, respectively, as well as CD51 which is involved in PECAM-1 interactions; we found no flow effects on surface expression by flow cytometry (Fig 6e) or gene expression (Fig 6f). Together, these findings suggest that flow-mediated, ICAM-1 and E-selectin-dependent DC transmigration primarily results from changes in the lymphatic endothelium in response to flow.
Discussion
Fluid drainage into lymphatic capillaries can change rapidly and dramatically upon tissue injury or inflammation, and our results demonstrate that the lymphatic endothelium is highly sensitive to this biophysical cue in regards to its fluid and cell transport functions. This likely has the effect of optimizing the delivery of fluid (and presumably soluble antigens) as well as DCs to the draining lymph node after injury or inflammation. Specifically, transmural flow across a lymphatic endothelium led to (1) increased CCL21 secretion, which guides DCs to the lymphatics and lymph nodes;37,38 (2) reorganization and downregulation of PECAM-1 and VE-cadherin, which was consistent with increased lymphatic permeability; and (3) upregulation of ICAM-1 and E-selectin (and to a lesser extent VCAM-1), which facilitate DC transmigration into lymphatic vessels under flow conditions. Moreover, the flow enhancement of DC migration was dependent on CCR7, E-selectin, and ICAM-1.
Following injury or inflammation, the way that local lymphatic capillaries respond may be relevant to the type of immune response subsequently triggered. For example, after subcutaneous antigen delivery, it was shown that MHCII presentation of peptide on DCs in the draining lymph node came in two discrete stages: first from the lymph node-resident DCs that acquired antigen within the lymph node, and second the DCs that had migrated there from the injection site.1 Furthermore, a humoral response can be initiated by lymph node-resident B cells that uptake antigen directly in the node follicles rather than by migrating B cells or exposure to DCs.2 These highlight the importance of lymph flow, which delivers antigen to the lymph node, to adaptive immunity. Whereas increased levels of cytokine production is observed from 4h, peaking at 16h after challenge in a skin lesion model,39 increased interstitial flow is an immediate response to certain types of peripheral inflammation.6 Our data show that both fluid and cell transport are enhanced upon increased flow, and since flow can increase immediately it is likely to be an important mediator of early responses to inflammation.
Recent work has begun to hint at the role of flow on lymphatic endothelial function. Lymphangiogenesis in the adult requires interstitial flow40 and VEGF has a reduced ability to rescue lymphatic function in tissue with poor interstitial flow.41 Fluid stagnation in secondary lymphedema causes lymphatic hyperplasia and decreased Langerhans DC homing to the lymphatics.21 In the lymph node, flow upregulates CCL21,42 presumably to help orchestrate the interactions between naïve T cells and mature DCs, which both express its receptor CCR7. Without flow, CCL21 silences the activity of integrin lymphocyte function associated antigen-1 (LFA-1 or CD18:CD11a), which is expressed by APCs and binds ICAM-1, and this silencing is released after the induction of flow.27 Finally, tumor cells may utilize interstitial flow to home to draining lymphatics.35 Our finding that CCL21 is regulated with flow is consistent with other reports of increased CCL21 expression by lymphoid tissues following viral and bacteria infection, peripheral inflammation, or tumor invasion,43-45 all of which may be accompanied by increased lymph flow.6, 8, 46-48
Lymph flow itself is largely regulated by vascular permeability, and increased lymph flow can be caused by the rapid increase in vascular permeability that occurs in acute inflammation or by the leaky angiogenic vessels in rapidly growing tumors. Molecules associated with regulation of vascular permeability are adherens and tight junctional proteins, particularly VE-cadherin,32 and blocking VE-cadherin causes junctional redistribution and results in elevated permeability.49, 50 We observed that elevated lymphatic permeability induced by transmural flow coincided with VE-cadherin and PECAM-1 downregulation and delocalization on lymphatic endothelium. Furthermore, whereas TNF-α has also been shown to increase vascular permeability,51 flow greatly increased this effect in an apparently additive manner (Fig. 1c). Finally, it is possible that the VE-cadherin and PECAM-1 redistribution that we saw with flow was directly resulting from the mechanical stimulus itself, since these contribute to a mechanosensory complex that regulates the well-described shear stress effects on vascular endothelium.31
The importance of flow-dependent adhesion and transendothelial migration has been widely studied on blood endothelium, but in blood, leukocytes require arrest and firm adhesion against high shear stresses before transmigrating from the lumen to the extracellular matrix, while in lymphatics the transmigration occurs from the matrix into the vessel lumen. In the blood, E-selectin tethers leukocytes while ICAM-1 and VCAM-1 promote stable adhesion of leukocytes to the apical endothelial membrane surface.52, 53 This firm binding to ICAM-1 and VCAM-1 then allows the adherent leukocytes to crawl toward intercellular junctions,54 where additional interactions with the homophilic adhesion molecules PECAM-1 and CD99 and junctional molecules including junctional adhesion molecule 1 (JAM-1) and VE-cadherin promote diapedesis.55 In lymphatics, ICAM-1 and VCAM-1 expression on lymphatic vessels has been reported after TNF-α, LPS, IL-1 and TNF-β stimulation.9,10 Our findings that transmural flow, in the absence of inflammatory cytokines, can upregulate these same molecules points to flow as a biophysical cue of acute inflammation. Furthermore, our demonstration (using blocking antibodies, Fig. 6a) that ICAM-1 and E-selectin were only required for flow-enhanced DC transmigration, but not DC transmigration under static conditions, indicates that the flow-induced upregulation of these molecules on LECs is mechanistically responsible for the enhanced DC transmigration seen under flow conditions. This is consistent with observations of integrin-independent DC transmigration into lymphatics in steady-state conditions.33
These results have important implications for lymphedema as well as for injury and inflammation. As the converse of heightened flow rates in inflammation, lymphedema is characterized by dysfunctional lymphatic transport that leads to an accumulation of interstitial fluid in the affected limb. Edematous tissues have exhibited impaired immune cell scavenging and susceptibility to infection.56 Our findings suggest that the impaired DC trafficking noted in lymphedema21 are likely also dependent on the reduced interstitial flow velocities resulting from downstream lymphatic insufficiencies.
In conclusion, by implicating lymphatic fluid flow as a regulator of lymphatic function, our findings reveal that lymphatic flow is a key mediator of interactions between lymphatic endothelium and DCs. Since increased lymphatic drainage due to blood vessel hyperpermeability occurs immediately upon tissue injury and in acute inflammatory events, our data hint at the possibility that the interstitial fluid flow increase acts as a preconditioning mechanism that affect lymphatic endothelial cell function with respect to immune cell trafficking.
Supplementary Material
Acknowledgments
The authors thank Miriella Pasquier, Didier Foretay, Floyd Sarria, Veronique Borel, and Valentina Triacca for technical assistance.
Sources of Funding. This work was supported by grants from the European Research Commission (DC-LYMPH #206653-2) and the Swiss National Science Foundation (#310010 and #107602).
Non-standard Abbreviations and Acronyms
- DC
dendritic cell
- LEC
lymphatic endothelial cell
- LPS
lipopolysaccharide
Footnotes
Disclosures. None.
References
- 1.Itano AA, Jenkins MK. Antigen presentation to naive CD4 T cells in the lymph node. Nature Immunol. 2003;4:733–739. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- 2.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 4.Förster R, Davalos-Misslitz A, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 5.Bates DO, Hillman NJ, Williams B, Neal CR, Pocock TM. Regulation of microvascular permeability by vascular endothelial growth factors. J Anat. 2002;200:581–597. doi: 10.1046/j.1469-7580.2002.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He C, Young AJ, West CA, Su M, Konerding MA, Mentzer SJ. Stimulation of regional lymphatic and blood flow by epicutaneous oxazolone. J Appl Physiol. 2002;93:966–973. doi: 10.1152/japplphysiol.00212.2002. [DOI] [PubMed] [Google Scholar]
- 7.Brand CU, Hunziker T, Braathen LR. Isolation of human skin-derived lymph: flow and output of cells following sodium lauryl sulphate-induced contact dermatitis. Arch Dermatol Res. 1992;284:123–126. doi: 10.1007/BF00372702. [DOI] [PubMed] [Google Scholar]
- 8.Mullins RJ, Hudgens RW. Increased skin lymph protein clearance after a 6-h arterial bradykinin infusion. Am J Physiol. 1987;253:H1462–1469. doi: 10.1152/ajpheart.1987.253.6.H1462. [DOI] [PubMed] [Google Scholar]
- 9.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawa Y, Ueki T, Hata M, Iwasawa K, Tsuruga E, Kojima H, Ishikawa H, Yoshida S. LPS-induced IL-6, IL-8, VCAM-1, and ICAM-1 expression in human lymphatic endothelium. J Histochem Cytochem. 2008;56:97–109. doi: 10.1369/jhc.7A7299.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ, Skobe M. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol. 2009;183:1767–1779. doi: 10.4049/jimmunol.0802167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci U S A. 1992;89:1398–1402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohlen HG, Wang W, Gashev A, Gasheva O, Zawieja D. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol-Heart Circ Physiol. 2009;297:H1319–H1328. doi: 10.1152/ajpheart.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsunemoto H, Ikomi F, Ohhashi T. Flow-mediated release of nitric oxide from lymphatic endothelial cells of pressurized canine thoracic duct. Jpn J Physiol. 2003;53:157–163. doi: 10.2170/jjphysiol.53.157. [DOI] [PubMed] [Google Scholar]
- 15.Simon SI, Sarantos MR, Green CE, Schaff UY. Leucocyte recruitment under fluid shear: Mechanical and molecular regulation within the inflammatory synapse. Clin Exp Pharmacol Physiol. 2009;36:217–224. doi: 10.1111/j.1440-1681.2008.05083.x. [DOI] [PubMed] [Google Scholar]
- 16.Reinhart-King CA, Fujiwara K, Berk BC. Angiogenesis: In Vitro Systems. Vol. 443. San Diego: Elsevier Academic Press Inc; 2008. Physiologic stress-mediated signaling in the endothelium; pp. 25–44. [DOI] [PubMed] [Google Scholar]
- 17.Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M. Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci U S A. 2002;99:16069–16074. doi: 10.1073/pnas.242401399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anton D, Dabadghao S, Palucka K, Holm G, Yi Q. Generation of dendritic cells from peripheral blood adherent cells in medium with human serum. Scand J Immunol. 1998;47:116–121. doi: 10.1046/j.1365-3083.1998.00284.x. [DOI] [PubMed] [Google Scholar]
- 19.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 20.Swartz MA, Kaipainen A, Netti PA, Brekken C, Boucher Y, Grodzinsky AJ, Jain RK. Mechanics of interstitial-lymphatic fluid transport: theoretical foundation and experimental validation. J Biomech. 1999;32:1297–1307. doi: 10.1016/s0021-9290(99)00125-6. [DOI] [PubMed] [Google Scholar]
- 21.Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res. 2006;72:161–171. doi: 10.1016/j.mvr.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon JB, Raghunathan S, Swartz MA. A tissue-engineered model of the intestinal lacteal for evaluating lipid transport by lymphatics. Biotechnol Bioeng. 2009;103:1224–35. doi: 10.1002/bit.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chary SR, Jain RK. Direct measurment of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc Natl Acad Sci U S A. 1989;86:5385–5389. doi: 10.1073/pnas.86.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–263. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 26.Cinamon G, Grabovsky V, Winter E, Franitza S, Feigelson S, Shamri R, Dwir O, Alon R. Novel chemokine functions in lymphocyte migration through vascular endothelium under shear flow. J Leukoc Biol. 2001;69:860–866. [PubMed] [Google Scholar]
- 27.Woolf E, Grigorova I, Sagiv A, Grabovsky V, Feigelson SW, Shulman Z, Hartmann T, Sixt M, Cyster JG, Alon R. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol. 2007;8:1076–1085. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- 28.Gotsch U, Borges E, Bosse R, Boggemeyer E, Simon M, Mossmann H, Vestweber D. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J Cell Sci. 1997;110:583–588. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- 29.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3:143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 30.Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E, Greenwood J. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci. 2008;121:29–37. doi: 10.1242/jcs.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 32.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 33.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med. 2009;206:2925–35. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–538. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 36.Ledgerwood LG, Lal G, Zhang N, Garin A, Esses SJ, Ginhoux F, Merad M, Peche H, Lira SA, Ding Y, Yang Y, He X, Schuchman EH, Allende ML, Ochando JC, Bromberg JS. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9:42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 37.Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- 38.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 39.Webb EF, Tzimas MN, Newsholme SJ, Griswold DE. Intralesional cytokines in chronic oxazolone-induced contact sensitivity suggest roles for tumor necrosis factor alpha and interleukin-4. J Invest Dermatol. 1998;111:86–92. doi: 10.1046/j.1523-1747.1998.00239.x. [DOI] [PubMed] [Google Scholar]
- 40.Rutkowski JM, Boardman KC, Swartz MA. Characterization of lymphangiogenesis in a model of adult skin regeneration. Am J Physiol Heart Circ Physiol. 2006;291:H1402–1410. doi: 10.1152/ajpheart.00038.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldman J, Conley KA, Raehl A, Bondy DM, Pytowski B, Swartz MA, Rutkowski JM, Jaroch DB, Ongstad EL. Regulation of lymphatic capillary regeneration by interstitial flow in skin. Am J Physiol Heart Circ Physiol. 2007;292:H2176–2183. doi: 10.1152/ajpheart.01011.2006. [DOI] [PubMed] [Google Scholar]
- 42.Tomei A, Siegert S, Britschgi MR, Luther SA, Swartz MA. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node model. J Immunol. 2009;183:4273–4283. doi: 10.4049/jimmunol.0900835. [DOI] [PubMed] [Google Scholar]
- 43.Serra HM, Baena-Cagnani CE, Eberhard Y. Is secondary lymphoid-organ chemokine (SLC/CCL21) much more than a constitutive chemokine? Allergy. 2004;59:1219–1223. doi: 10.1111/j.1398-9995.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 44.Issa A, Le TX, Shoushtari AN, Shields JD, Swartz MA. Vascular endothelial growth factor-C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res. 2009;69:349–357. doi: 10.1158/0008-5472.CAN-08-1875. [DOI] [PubMed] [Google Scholar]
- 45.Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, Ahmed R, Matloubian M. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317:670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- 46.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol. 1989;257:H2059–2069. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- 47.Ruddell A, Harrell MI, Minoshima S, Maravilla KR, Iritani BM, White SW, Partridge SC. Dynamic contrast-enhanced magnetic resonance imaging of tumor-induced lymph flow. Neoplasia. 2008;10:706–713. doi: 10.1593/neo.08342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dafni H, Israely T, Bhujwalla ZM, Benjamin LE, Neeman M. Overexpression of vascular endothelial growth factor 165 drives peritumor interstitial convection and induces lymphatic drain: Magnetic resonance imaging, confocal microscopy, and histological tracking of triple-labeled albumin. Cancer Res. 2002;62:6731–6739. [PubMed] [Google Scholar]
- 49.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Buul JD, Voermans C, van den Berg V, Anthony EC, Mul FP, van Wetering S, van der Schoot CE, Hordijk PL. Migration of human hematopoietic progenitor cells across bone marrow endothelium is regulated by vascular endothelial cadherin. J Immunol. 2002;168:588–596. doi: 10.4049/jimmunol.168.2.588. [DOI] [PubMed] [Google Scholar]
- 51.Angelini DJ, Hyun SW, Grigoryev DN, Garg P, Gong P, Singh IS, Passaniti A, Hasday JD, Goldblum SE. TNF-alpha increases tyrosine phosphorylation of vascular endothelial cadherin and opens the paracellular pathway through fyn activation in human lung endothelia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1232–1245. doi: 10.1152/ajplung.00109.2006. [DOI] [PubMed] [Google Scholar]
- 52.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 53.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 54.Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- 55.Luscinskas FW, Ma S, Nusrat A, Parkos CA, Shaw SK. Leukocyte transendothelial migration: a junctional affair. Semin Immunol. 2002;14:105–113. doi: 10.1006/smim.2001.0347. [DOI] [PubMed] [Google Scholar]
- 56.Olszewski WL, Engeset A, Romaniuk A, Grzelak I, Ziolkowska A. Immune cells in peripheral lymph and skin of patients with obstructive lymphedema. Lymphology. 1990;23:23–33. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.