Abstract
Donor safety is crucial for living donor liver transplantation (LDLT), and sufficient liver regeneration significantly affects outcomes of living donors. This study aimed to investigate clinical factors associated with liver regeneration in living donors. The study retrospectively reviewed 380 living donors who underwent liver donation at Chang Gung Memorial Hospital in Linkou. The clinical characteristics and medical parameters of donors were analyzed and compared according to liver donation graft type. There were 355 donors (93.4%) with right hemi-liver donations and 25 donors (6.6%) with left hemi-liver donations. Left hemi-liver donors had a higher body mass index (BMI) and a larger ratio of remnant liver volume (RLV) to total liver volume (TLV). However, the 2 groups showed no significant difference in the liver regeneration ratio. The type of remnant liver (P < .001), RLV/body weight (P = .027), RLV/TLV (P < .001), serum albumin on postoperative day 7 and total bilirubin levels on postoperative day 30 were the most significant factors affecting liver regeneration in living donors. In conclusion, adequate liver regeneration is essential for donor outcome after liver donation. The remnant liver could eventually regenerate to an adequate volume similar to the initial TLV before liver donation. However, the remnant left hemi-liver had a faster growth rate than the remnant right hemi-liver in donors.
Keywords: liver regeneration, liver transplantation, living donor, outcome
1. Introduction
Living donor liver transplantation (LDLT) is a promising treatment for end-stage liver disease and is much more common than deceased-donor liver transplantation in Asia.[1,2] In this scenario, donor safety is paramount in this major surgical procedure because living donors are healthy individuals. Generally, the remnant liver undergoes rapid regenerations to regain its volume and functional capacity after partial liver donation.[3] However, sufficient liver regeneration significantly impacts the clinical outcome of both the recipient and donor, relying on rapid liver regeneration to reach the correct liver mass and maintain sufficient physiological function.[4]
Numerous studies have analyzed postoperative biochemical and demographic factors that may influence liver regeneration after hepatectomy.[5–8] However, the association between long-term liver regeneration and postoperative biochemical factors based on the type of partial liver donation has rarely been investigated. This study investigated the clinical features of living donors who underwent partial liver donation to determine factors associated with liver regeneration after liver donation and improve donor safety.
2. Materials and methods
The clinical data of 380 living donors who underwent hepatectomy for partial liver donation in the Organ Transplantation Institute, Chang Gung Memorial Hospital at Linkou, Taiwan, from January 2012 to January 2018, were retrospectively analyzed. With the approval of the Institutional Review Board, all medical records of living donors included in the study were retrospectively reviewed. Written informed consent from donors was waived due to the study retrospective nature. All the data was anonymized or maintained with confidentiality, and the study was performed in accordance with the ethical standards of the Declaration of Helsinki.
Generally, both donor and recipient were thoroughly surveyed by biochemical analysis, imaging scan and immunologic tests before liver transplantation. The selection of graft type for LDLT should be based on a comprehensive consideration of donor vascular anatomy, estimated donor functional liver remnant, and expected graft to recipient body weight ratio (GRWR). Of note, it would be better that an estimated GRWR could be >0.8%. Briefly, the left hemi-liver graft was accepted only if the estimated GRWR could reach 0.8%, otherwise right hemi-liver graft would be utilized in our institute. All LDLT procedures involving donor hepatectomy and recipient operations were performed using standard techniques as previously described.[9,10]
Donor hepatectomies were performed by traditional laparotomy approach for all living donors of the study. Specifically, the middle hepatic vein was not included in the right or left hemi-liver graft during donor hepatectomies for the sake of donor safety. Additionally, the necessity of reconstruction of middle hepatic vein tributaries in the hemi-liver graft was based on congestion in the venous drainage area as previous report from our institute.[11] The clinical characteristics of the donors, including pre- and postoperative radiological imaging studies and biochemical profiles, were collected. The 380 donors were classified into 2 groups according to the type of liver graft for comparison.
Liver volume was calculated using computed tomography (CT) volumetric assessment. All donors were regularly followed up postoperatively for liver function and volume at the institute. Generally, liver CT is performed at 1, 6, and 12 months after donor hepatectomy. The liver regeneration rate was calculated as the percentage of postoperative liver volume on each examination relative to the preoperative volume. The liver growth ratio was calculated as the increase in remnant liver volume (RLV) divided by the initial RLV.
All statistical analyses were performed using Statistical Package for the Social Sciences version 24 (IBM, Armonk, NY). A P < .05 was considered statistically significant.
3. Results
The clinical characteristics of living donors who underwent partial liver graft donation are summarized in Table 1. There were 194 male and 186 female donors, and the median age at liver donation was 31 years (range: 18–58 years). The median body mass index (BMI) of the donors was 22.6 kg/m2 (range: 15.9–30.8 kg/m2), and the number of right hemi-liver donations (n = 355, 93.4%) was higher than that of left hemi-liver donations (n = 25, 6.6%). Most living donors (76.1%) were recipients’ adult sons or daughters. None of the donors had severe fatty liver, and the median liver-to-spleen ratio of the donors was 1.19 (range: 1.13–1.24). The median ratio of the RLV to the total liver volume (TLV) was 50.7% (range: 34.1–84.4%).
Table 1.
Clinical characteristics of living donors.
| Characteristics | Donors (n = 380) |
|---|---|
| Age (yr), median (range) | 31 (18–58) |
| Gender | |
| Male | 194 (51.1%) |
| Female | 186 (48.9%) |
| BMI, median (range) | 22.6 (15.9–30.8) |
| Donation of graft type | |
| Right hemi-liver | 355 (93.4%) |
| Left hemi-liver | 25 (6.6%) |
| Relationship of donor to recipient | |
| Parent | 6 |
| Couple | 35 |
| Daughter/Son | 289 |
| Sibling | 31 |
| Cousin | 16 |
| Other | 3 |
| Preoperative estimate liver volume (cm3) | |
| Total liver volume | 1286 (810–2792) |
| Future remnant liver volume | 652 (344–1839) |
BMI = body mass index.
Table 2 compares clinical features and liver volume variation according to graft type donation. The left hemi-liver donors had higher BMI and a larger RLV to TLV ratio than the right hemi-liver donors. The major complications for the right hemi-liver donors was 1.9% (7/355), including 2 grade I with wound infections, 2 grade II with long-term hyperbilirubinemia, and 3 grade III requiring surgical intervention for internal hemorrhage (n = 2) and removal of retained drainage tube (n = 1) according to Clavien-Dindo classification of surgical complications.[12] There was no surgical complication occurred to left hemi-liver donor in the study. Additionally, clinical features and outcomes of recipients related to graft type were shown in the supplemental Table 1, http://links.lww.com/MD/M29 and Figure 1, http://links.lww.com/MD/M28. The clinical features were mostly no significant difference between the 2 groups, but the outcome of recipients was relative suboptimal in the group with left hemi-liver graft.
Table 2.
Comparison of clinical features and liver regeneration of donors based on graft type donation.
| Character | Right hemi-liver donor (n = 355) | Left hemi-liver donor (n = 25) | P value |
|---|---|---|---|
| Age | 31 (25–37) | 33 (25–44.5) | .222 |
| BMI | 22.3 (20.4–24.5) | 24.8 (23.2–25.9) | <.001 |
| Liver-Spleen ratio | 1.20 (1.14–1.24) | 1.17 (1.13–1.20) | .100 |
| Liver volume (cm3) | |||
| Preoperative TLV | 1273 (1132–1452) | 1524 (1437–1847) | <.001 |
| Estimated RLV | 681 (599–774) | 1147 (1057–1349) | <.001 |
| RLV-BW (%) | 1.21 (1.05–1.35) | 1.87 (1.62–1.97) | <.001 |
| RLV-TLV (%) | 53 (49–57) | 75 (72–80) | <.001 |
| Clavien-Dindo grade complications | 1.000 | ||
| I | 2 (0.6%) | 0 | |
| II | 2 (0.6%) | 0 | |
| III | 3 (0.8%) | 0 | |
| IV–V | 0 | 0 | |
| Liver volume after operation | |||
| 1 mo | 951 (857–1103) | 1168 (1061–1362) | <.001 |
| 6 mo | 1072 (946–1227) | 1381 (1194.5–1479) | <.001 |
| 12 mo | 1113 (1001–1302) | 1486 (1252–1569) | <.001 |
| Liver regeneration ratio (%)* | |||
| 1 mo | 76 (70–83) | 77 (71–84) | .451 |
| 6 mo | 85 (78–91) | 86 (82–94) | .205 |
| 12 mo | 89 (83–96) | 89 (82–95) | .870 |
| Liver growth ratio (%) | |||
| 1 mo | 42 (28–60) | 3 (−6–15) | <.001 |
| 6 mo | 59 (42–80) | 20 (4–29) | <.001 |
| 12 mo | 69 (49–87) | 18 (10–33) | <.001 |
BMI = body mass index, BW = body weight, RLV = remnant liver volume, TLV = total liver volume.
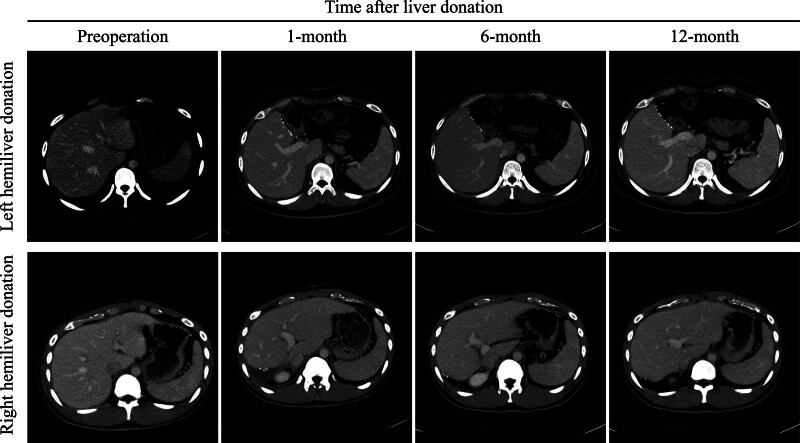
Figure 1.
Dynamic computerized tomography assessment of liver volume regarding remnant right and left liver.
The liver regeneration ratio regarding postoperative liver volume related to preoperative TLV showed no significant difference between the 2 groups (Fig. 1). However, the liver growth rate was faster in right hemi-liver donors than in left hemi-liver donors. The remnant liver eventually grows to an adequate liver volume nearly similar to the initial TLV before liver donation in all donors. Moreover, variables including age, gender, and BMI were matched on the basis of propensity score model with 1 left hemi-liver donor to 2 right hemi-liver donors for comparison. As a result, patients selected from the propensity score matching also showed a faster liver growth rate as compared with left hemi-liver donors (Fig. 2).
Figure 2.
The variation of liver volume after liver donation according to the type of remnant liver. The growth rate of the left remnant hemi-liver was usually faster than that of the right hemi-liver. However, the remnant liver would eventually regenerate to the initial total liver volume before liver donation in the right and left hemi-liver. Patients selected by the propensity score matching also showed a similar liver growth rate.
Factors associated with regeneration in the remnant liver were further analyzed. On univariate and correlation analysis, the type of remnant liver, RLV, RLV/body weight (BW), RLV/TLV, higher serum levels of total bilirubin, lower serum levels of alanine transaminase, albumin on postoperative day 7, and total bilirubin levels on postoperative day 30 were found to be significant factors affecting the rate of liver regeneration (Table 3). Of these factors, the type of remnant liver with right hemi-liver donation (P < .001), lower RLV corrected to BW regarding RLV/BW(P = .027) and RLV/TLV(P < .001), lower serum albumin on postoperative day 7, and higher total bilirubin levels on postoperative day 30 were the most significant factors affecting liver regeneration in logistic regression analysis (Table 4).
Table 3.
Correlation analysis of liver regeneration ratio after liver donation.
| Variables | 1-mo | 6-mo | 12-mo | |||
|---|---|---|---|---|---|---|
| Correlation coefficient | P value | Correlation coefficient | P value | Correlation coefficient | P value | |
| Age | 0.01 | .91 | −0.06 | .14 | 0.01 | .77 |
| Gender | 0.03 | .50 | 0.02 | .63 | −0.04 | .43 |
| BMI | 0.05 | .23 | 0.18 | <.001 | 0.01 | .83 |
| Liver-Spleen ratio | −0.01 | .88 | −0.04 | .65 | 0.05 | .58 |
| Type of remnant liver | −0.03 | .56 | 0.05 | .31 | 0.02 | .70 |
| Liver graft weight | −0.03 | .48 | 0.05 | .23 | −0.01 | .75 |
| RLV | −0.12 | .003 | −0.4 | <.001 | −0.13 | .005 |
| RLV-BW | −0.13 | .001 | −0.41 | <.001 | −0.12 | .007 |
| RLV-TLV | −0.06 | .13 | −0.32 | <.001 | −0.11 | .02 |
| Serum liver enzymes | ||||||
| Postoperative d 2 | ||||||
| AST | −0.02 | .60 | 0.02 | .70 | 0.00 | .89 |
| ALT | −0.01 | .87 | 0.11 | .01 | 0.04 | .45 |
| Alk-P | −0.04 | .40 | 0.04 | .40 | −0.02 | .73 |
| Total bilirubin | −0.02 | .69 | 0.00 | .98 | 0.07 | .88 |
| Albumin | −0.02 | .59 | −0.05 | .25 | −0.03 | .48 |
| Prothrombin time, INR | −0.02 | .67 | −0.03 | .44 | 0.01 | .91 |
| Postoperative d 7 | ||||||
| AST | 0.02 | .64 | −0.03 | .50 | −0.01 | .78 |
| ALT | 0.03 | .49 | 0.03 | .44 | 0.01 | .81 |
| Alk-P | −0.01 | .89 | 0.03 | .44 | 0.00 | .93 |
| Total bilirubin | 0.03 | .45 | 0.12 | .01 | 0.02 | .60 |
| Albumin | 0.00 | .95 | 0.01 | .76 | −0.01 | .88 |
| Prothrombin time, INR | 0.00 | .99 | 0.00 | .96 | 0.00 | .99 |
| Postoperative d 30 | ||||||
| AST | 0.01 | .80 | 0.05 | .24 | 0.04 | .33 |
| ALT | 0.02 | .60 | 0.09 | .04 | 0.04 | .36 |
| Alk-P | −0.003 | .95 | 0.07 | .11 | 0.07 | .15 |
| Total bilirubin | 0.01 | .88 | −0.02 | .64 | −0.03 | .51 |
| Albumin | 0.01 | .76 | −0.04 | .40 | −0.02 | .73 |
| Prothrombin time, INR | −0.05 | .21 | −0.04 | .37 | −0.06 | .23 |
Alk-P = alkaline phosphatase, ALT = alanine amino transferase, AST = aspartate amino transferase, BMI = body mass index, BW = body weight, INR = international normalized ratio, RLV = remnant liver volume, TLV = total liver volume.
Table 4.
Multivariate analysis of factors related to liver growth ratio after liver donation.
| Variables | B | 95% CI of B | P values |
|---|---|---|---|
| Type of remnant liver | 0.277 | 0.302~−0.587 | <.001 |
| RLV-BW | −0.137 | −0.314~−0.019 | .027 |
| RLV-TLV | −0.908 | −4.733~−3.445 | <.001 |
| Total bilirubin (POD 7) | 0.067 | −0.084~−0.003 | .07 |
| Albumin (POD 7) | 0.091 | 0.020~0.159 | .012 |
| Total bilirubin (POD 30) | −0.118 | −0.156~−0.040 | .001 |
B = standardized regression coefficient, BW = body weight, CI = confidence interval, POD = postoperative day, RLV = remnant liver volume, TLV = total liver volume.
4. Discussion
Liver regeneration capability led to the evolution of LDLT. By understanding the predictive factors of liver regeneration, optimizing the LDLT process and preventing lethal complications in living donors may be possible. In the present study, a rapid regeneration rate of the left remnant liver was observed as compared to that of the right remnant liver in living liver donors. This study also identified numerous important predictive factors, including the type of remnant liver, RLV/BW, RLV/TLV, and postoperative total bilirubin and albumin values that might affect liver regeneration in living liver donors.
Generally, the remaining liver tissue is highly regenerated in the early period after donation.[8,13–15] The remnant liver, whether right or left hemi-liver donated, regenerated to approximately 80% of the initial TLV in the first month and 90% in 6 to 12 months postoperatively. In contrast, the growth rate of the regenerated volume in right hemi-liver donors was faster than that in left hemi-liver donors, even when the resected graft volumes were greater in the right hemi-liver donors. This demonstrates the high regenerative potential of liver tissue in healthy individuals. However, the selection of the graft type should also consider the disease severity of the recipient. Although right hemi-liver grafts usually provide sufficient graft size to recipients, the safety of living donors remains paramount.
The remnant liver has been proven to impact liver regeneration after liver resection in healthy donors and patients with pathological conditions. A previous study showed that serious complications are more likely to occur when the donor has a small residual liver volume.[7,8,16,17] A greater extent of liver resection may increase the release of cytokines and growth factors, promoting more remarkable regeneration of the remnant liver.[3] Theoretically, the threshold RLV/TLV ratio is usually not <35% in living donors.[1] In our study, RLV/BW and RLV/TLV were significant factors in liver regeneration. One study demonstrated that RLV/BW accurately assessed the functional limit of hepatectomy, with a suggestive threshold of 0.5% in patients without a cirrhotic liver.[14] However, other studies have rarely mentioned RLV/BW compared to the RLV/TLV ratio. Both were considered surrogate markers of the metabolic demand for liver regeneration in our study.
After decades of investigation, the mechanisms of liver regeneration have been well described in animal models; however, a gap remains between animal models and clinical practice. Bile acids are essential for liver regeneration, as they can promote hepatocyte division by binding to fibroblast growth factor receptors.[18] However, bile acid levels could not be checked regularly, and the serum level of total bilirubin might be another marker of bile juice stimulation. Accordingly, this study found that elevated postoperative bilirubin levels were associated with rapid liver regeneration in both the univariate and multivariate analyses.
Numerous studies have reported similar results regarding the importance of bilirubin levels.[6,18,19] Patients who underwent extended hemihepatectomy with external biliary drainage had lower levels of liver regeneration than those who underwent extended hemihepatectomy without external biliary drainage, and lower bilirubin levels were observed in the drainage group.[19] In contrast, elevated postoperative bilirubin levels are traditionally considered a surgical complication of major hepatectomy. However, the acceptable range of bilirubin levels in living liver donors or recipients may be undetermined because bile acid is a potential promoter of liver regeneration.
Additionally, the regenerative mechanism of the liver is a process of “hepatostat” that through various pathways to ensure the stability and propriety of the liver- to- bodyweight ratio for body homeostasis.[20] Although numerous factors might affect the liver regeneration, a great and healthy underlying liver quality is necessary for the success of this process. Noteworthy, aging liver and hepatic steatosis need to be considered for marginal donors that is not only affecting the outcome of recipients but also risky for living donors after hepatectomy.[21] Despite no absolute cutoff value of age for liver donation, studies have shown that donor ages beyond 60 years are associated with lower regenerative capacity and graft survival.[22–25] Therefore, the institute including the study rarely accepted donors older than 60 years of age.
Moreover, a liver-to-spleen ratio of < 1.1 might be considered as fatty liver, and should be excluded from liver donor during pre-transplantation survey.[26] Hepatic steatosis is also at increased risk of perioperative complications to both donor and recipient. Therefore, a living donor with obesity and severe steatosis should not be accepted for liver donation for LDLT.
However, this study may be limited by its retrospective nature at a single center. Numerous remarkable observations could also provide additional information to guide clinical decision-making in LDLT. Generally, each potential donor should be comprehensively screened for medical conditions and liver status, which may affect the donor and recipient postoperative outcomes. Importantly, the safety of living donors should be prioritized in LDLT.
5. Conclusion
Liver regeneration and adequate liver function volume are associated with the prognoses of both recipient and donors. This study showed that right hemi-liver donation, low RLV/BW ratio, and postoperative bilirubin and albumin serum levels were essential factors related to liver regeneration after hepatectomy in living donors. The growth rate of the remnant liver was usually faster in right hemi-liver donations than in left hemi-liver donations. However, the remnant liver would eventually regenerate to an adequate liver volume nearly similar to the initial TLV before liver donation in the right or left hemi-liver donation.
Author contributions
Data curation: Hao-Chien Hung, Jin-Chiao Lee, Chih-Hsien Cheng, Yu-Chao Wang, Chen-Fang Lee, Ting-Jung Wu, Hong-Shiue Chou.
Formal analysis: Tsung-Han Wu.
Investigation: Wei-Cheng Wang.
Supervision: Wei-Chen Lee.
Writing – review & editing: Kun-Ming Chan.
Supplementary Material
Abbreviations:
- BMI
- body mass index
- BW
- body weight
- CT
- computed tomography
- GRWR
- graft to recipient body weight ratio
- LDLT
- living donor liver transplantation
- RLV
- remnant liver volume
- TLV
- total liver volume
WWC and WTH contributed equally to this work.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
This work was partly supported by grants from the Taiwan Ministry of Health and Welfare (MOHW111-TDU-B-221-014009) to W.-C. Lee and K.-M. Chan.
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
How to cite this article: Wang W-C, Wu T-H, Hung H-C, Lee J-C, Cheng C-H, Wang Y-C, Lee C-F, Wu T-J, Chou H-S, Chan K-M, Lee W-C. Liver regeneration of living donor after liver donation for transplantation: Disparity in the left and right remnant liver. Medicine 2024;103:14(e37632).
Contributor Information
Wei-Cheng Wang, Email: b9002072@cgmh.org.tw.
Tsung-Han Wu, Email: wutj5056@cgmh.org.tw.
Hao-Chien Hung, Email: mp0616@cgmh.org.tw.
Jin-Chiao Lee, Email: weichen@cgmh.org.tw.
Chih-Hsien Cheng, Email: chengcchj@cgmh.org.tw.
Yu-Chao Wang, Email: b9002072@cgmh.org.tw.
Chen-Fang Lee, Email: weichen@cgmh.org.tw.
Ting-Jung Wu, Email: wutj5056@cgmh.org.tw.
Hong-Shiue Chou, Email: chouhs@cgmh.org.tw.
Wei-Chen Lee, Email: weichen@cgmh.org.tw.
References
- [1].Chen CL, Kabiling CS, Concejero AM. Why does living donor liver transplantation flourish in Asia? Nat Rev Gastroenterol Hepatol. 2013;10:746–51. [DOI] [PubMed] [Google Scholar]
- [2].Pillai VG, Chen CL. Living donor liver transplantation in Taiwan-challenges beyond surgery. Hepatobil Surg Nutr. 2016;5:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–6. [DOI] [PubMed] [Google Scholar]
- [4].Lauterio A, Di Sandro S, Concone G, et al. Current status and perspectives in split liver transplantation. World J Gastroenterol. 2015;21:11003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lisman T, Porte RJ. Mechanisms of platelet-mediated liver regeneration. Blood. 2016;128:625–9. [DOI] [PubMed] [Google Scholar]
- [6].Ibis C, Asenov Y, Akin M, et al. Factors affecting liver regeneration in living donors after hepatectomy. Med Sci Monit. 2017;23:5986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mohapatra N, Sinha PK, Sasturkar SV, et al. Preoperative alanine aminotransferase and remnant liver volume predict liver regeneration after live donor hepatectomy. J Gastrointest Surg. 2020;24:1818–26. [DOI] [PubMed] [Google Scholar]
- [8].Reichman TW, Sandroussi C, Azouz SM, et al. Living donor hepatectomy: the importance of the residual liver volume. Liver Transpl. 2011;17:1404–11. [DOI] [PubMed] [Google Scholar]
- [9].Chan KM, Wu TH, Cheng CH, et al. Inferior outcomes associated with the coexistence of hepatocellular carcinoma recurrence and hepatic virus reinfection after living donor liver transplantation. J Gastrointest Surg. 2020;24:353–60. [DOI] [PubMed] [Google Scholar]
- [10].Wu TH, Wang YC, Cheng CH, et al. Outcomes associated with the intention of loco-regional therapy prior to living donor liver transplantation for hepatocellular carcinoma. World J Gastrointest Surg. 2020;12:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chan KM, Cheng CH, Wu TH, et al. Clinical strategy for the reconstruction of middle hepatic vein tributaries in right liver living donor liver transplantation. World J Surg. 2014;38:2927–33. [DOI] [PubMed] [Google Scholar]
- [12].Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Akamatsu N, Sugawara Y, Tamura S, et al. Regeneration and function of hemiliver graft: right versus left. Surgery. 2006;139:765–72. [DOI] [PubMed] [Google Scholar]
- [14].Truant S, Oberlin O, Sergent G, et al. Remnant liver volume to body weight ratio > or =0.5%: a new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg. 2007;204:22–33. [DOI] [PubMed] [Google Scholar]
- [15].Yokoi H, Isaji S, Yamagiwa K, et al. Donor outcome and liver regeneration after right-lobe graft donation. Transpl Int. 2005;18:915–22. [DOI] [PubMed] [Google Scholar]
- [16].Hsu HW, Tsang LL, Ou HY, et al. Donor outcomes after liver donation in adult to adult living donor liver transplantation. Transplant Proc. 2018;50:2588–92. [DOI] [PubMed] [Google Scholar]
- [17].Lo CM. Complications and long-term outcome of living liver donors: a survey of 1,508 cases in five Asian centers. Transplantation. 2003;75(3 Suppl):S12–5. [DOI] [PubMed] [Google Scholar]
- [18].Forbes SJ, Newsome PN. Liver regeneration - mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol. 2016;13:473–85. [DOI] [PubMed] [Google Scholar]
- [19].Otao R, Beppu T, Isiko T, et al. External biliary drainage and liver regeneration after major hepatectomy. Br J Surg. 2012;99:1569–74. [DOI] [PubMed] [Google Scholar]
- [20].Michalopoulos GK. Hepatostat: liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65:1384–92. [DOI] [PubMed] [Google Scholar]
- [21].Puri P, Kumar A, Qaleem M. Donor evaluation protocol for live and deceased donors. J Clin Exp Hepatol. 2024;14:101217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Adam R, Karam V, Delvart V, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675–88. [DOI] [PubMed] [Google Scholar]
- [23].Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–90. [DOI] [PubMed] [Google Scholar]
- [24].Pascher A, Sauer IM, Walter M, et al. Donor evaluation, donor risks, donor outcome, and donor quality of life in adult-to-adult living donor liver transplantation. Liver Transpl. 2002;8:829–37. [DOI] [PubMed] [Google Scholar]
- [25].Wakabayashi H, Nishiyama Y, Ushiyama T, et al. Evaluation of the effect of age on functioning hepatocyte mass and liver blood flow using liver scintigraphy in preoperative estimations for surgical patients: comparison with CT volumetry. J Surg Res. 2002;106:246–53. [DOI] [PubMed] [Google Scholar]
- [26].Iwasaki M, Takada Y, Hayashi M, et al. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78:1501–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.