Abstract
Background
Schizophrenia is considered to be a disorder of dysconnectivity characterized by abnormal functional integration between distinct brain regions. Different brain connection abnormalities were found to be correlated with various clinical manifestations, but whether a common deficit in functional connectivity (FC) in relation to both clinical symptoms and cognitive impairments could present in first-episode patients who have never received any medication remains elusive.
Objective
To find a core deficit in the brain connectome that is related to both psychopathological and cognitive manifestations.
Methods
A total of 75 patients with first-episode schizophrenia and 51 healthy control participants underwent scanning of the brain and clinical ratings of behaviors. A principal component analysis was performed on the clinical ratings of symptom and cognition. Partial correlation analyses were conducted between the main psychopathological components and resting-state FC that were found abnormal in schizophrenia patients.
Results
Using the principal component analysis, the first principal component (PC1) explained 37% of the total variance of seven clinical features. The ratings of GAF and BACS contributed negatively to PC1, while those of PANSS, HAMD, and HAMA contributed positively. The FCs positively correlated with PC1 mainly included connections related to the insula, precuneus gyrus, and some frontal brain regions. FCs negatively correlated with PC1 mainly included connections between the left middle cingulate cortex and superior and middle occipital regions.
Conclusion
In conclusion, we found a linked pattern of FC associated with both psychopathological and cognitive manifestations in drug-naïve first-episode schizophrenia characterized as the dysconnection related to the frontal and visual cortex, which may represent a core deficit of brain FC in patients with schizophrenia.
Keywords: schizophrenia, untreated, functional connectome, principal correlation analysis, psychopathology
Introduction
Schizophrenia is widely considered to be a disorder of dysconnectivity characterized by abnormal functional integration between distinct brain regions (Pettersson-Yeo et al., 2011). The identification of brain connection abnormalities in patients and their associations with the psychopathological symptoms or cognitive deficits has been of sustained interest (Chen et al., 2021b; Dong et al., 2018; Friston et al., 2016; S. Li et al., 2019; Santo-Angles et al., 2021). However, relevant studies were typically conducted on each domain of symptomology or cognition, respectively (Dietz et al., 2020; Giraldo-Chica et al., 2018; Shukla et al., 2019), while recent transdiagnostic studies on schizophrenia (C. H. Xia et al., 2018) and other mental illness (Caspi et al., 2014; Smith et al., 2020) have identified a general factor of psychopathology that accounted for shared risk among internalizing, externalizing, and thought disorders across diverse samples, which was related to connectivity between the visual association cortex and both the frontoparietal network (FPN) and default mode network (DMN) (Elliott et al., 2018). Regarding schizophrenia, it remains unclear whether a common deficit in functional connectivity (FC) in relation to both clinical symptoms and cognitive impairments could present in first-episode patients who have never received any medication.
Previous studies of brain functional magnetic resonance imaging (fMRI) have revealed that patients with schizophrenia were associated with FC disturbances in multiple systems, including the FPN, cingulo-opercular network, DMN, ventral attention network, and somatosensory network (Dong et al., 2018; van den Heuvel & Fornito, 2014). Aberrant connectivity existed both within and between these networks (Dong et al., 2018), and different connection abnormalities were found to be correlated with various clinical manifestations. For example, abnormalities in the cortico-cerebellar-striatal-thalamic loop, task-positive, and task-negative cortical networks were found related to cognitive impairments in individuals with schizophrenia (Sheffield & Barch, 2016; Yamashita et al., 2020). Dynamic functional network connectivity (FNC) analysis showed that severity of disordered thought and attentional deficits were negatively associated with FNC between anterior DMN and posterior DMN, while the severity of flat affect and bizarre behavior was positively associated with FNC between anterior DMN and the salience network (Hare et al., 2019). In some machine learning studies, between-network connectivity played an essential role in predicting positive and negative symptoms in patients with schizophrenia spectrum illness. Connections contributing to positive symptom prediction mainly involved between-network connectivity in the FPN, dorsal attention network, and motor-sensory region, while connections contributing to negative symptom prediction mainly involved between-network connectivity in the FPN and motor-sensory region (D. Wang et al., 2020). These findings suggest that, although different dimensions of clinical manifestations have specific relevant deficits in the brain network, they may share some common impairment patterns. Moreover, different dimensions of symptoms are inter-correlated rather than independent of each other. For instance, in patients with schizophrenia, cognitive deficits were commonly found to be associated with negative symptoms, and improved cognitive functioning was related to an improvement in positive symptoms (Addington et al., 1991). However, whether there is a linked pattern between general psychopathology and general brain alterations remains unclear. Inconsistent findings on impaired connections and networks might partly result from the effects of antipsychotic treatment (Lesh et al., 2015), which would show adverse effects on brain structure (Luo et al., 2020; Meng et al., 2019; Zeng et al., 2016) and function (F. Li et al., 2016; Yang et al., 2020) in patients with schizophrenia. To minimize the confounding factors potentially derived from medication, studying drug-naïve participants with first-episode schizophrenia might help to elucidate this issue.
Different approaches have been delineated to explore the correlation patterns between brain FC and clinical features, such as principal component analysis (PCA) (Behdinan et al., 2015), partial least squares (Pani et al., 2021), and canonical correlation analysis (Tian et al., 2019; C. H. Xia et al., 2018), while the last two were used for investigating covariate couples. Considering the interpretation, PCA might be preferable since it is commonly used in identifying common parts of a series of variables, which reduces the dimensionality of the data while retaining most of the variation in the data set. By obtaining the principal components (PCs) and their weights of the data through eigen-decomposition of the covariance matrix, PCA can therefore reveal the internal structure of the data and thus better interpret the variables of the data (Ringner, 2008). Previous studies on schizophrenia have adopted PCA to identify clinical features (Carment et al., 2020; Paolini et al., 2016) and then conducted correlation analyses between the PCs and another set of variables (Behdinan et al., 2015).
With these considerations, in this study, we used PCA to identify composite features of different dimensions of clinical behaviors, and then detected their correlation patterns with FC of impaired brain function in drug-naïve participants with first-episode schizophrenia. Our purpose was to identify the potential core correlation pattern linking functional connectome and both psychopathological and cognitive manifestations.
Materials and Methods
Participants
The study was approved by the Research Ethics Committee of West China Hospital of Sichuan University, and all participants, as well as their legal guardians if they were under 18 years old, provided written informed consent before participation. Seventy-five drug-naïve patients with first-episode schizophrenia were recruited from the psychiatric outpatient of The Fourth Hospital of Sichuan Province, and the images were collected at the Radiology Department of West China Hospital of Sichuan University. The diagnosis of schizophrenia was determined with the consensus of two psychiatrists by using the Structured Interview for the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders-5, SCID-5). Illness onset was evaluated by the Nottingham Onset Schedule (Singh et al., 2005) with the information provided by the patients, their family members, and other sources. All patients had never been treated with any antipsychotics or mood stabilizers before participation.
Fifty-one healthy control participants were recruited via poster advertisements from the same areas where patients resided, with similar socioeconomic backgrounds to the patients. All controls were screened to confirm the lifetime absence of psychiatric or neurological illnesses in themselves or their first-degree relatives.
All participants were right-handed. Exclusion criteria for both patients and healthy controls included (i) contraindications for MRI examination, (ii) presence of a neurological disorder examined by a professional neurologist based on medical history and physical examination, (iii) lifetime drug or alcohol abuse or dependency as rated with DSM, and (iv) pregnancy or any significant medical conditions. MRIs of all participants were inspected by an experienced neuroradiologist to exclude those with gross brain abnormalities or visible artifacts.
Psychopathological and Cognitive Assessments
For all patients, the severity of positive, negative, and general psychopathological symptoms was rated by the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987). The global functioning was assessed using Global Assessment of Functioning (GAF) (Jones et al., 1995). Emotional condition was rated by Hamilton Depression Scale (HAMD) (Hamilton, 1960) and Hamilton Anxiety Scale (HAMA) (Hamilton, 1959). General cognitive functioning of both patients and healthy control participants was assessed using the Chinese Version (L. J. Wang et al., 2016) of Brief Assessment of Cognition in Schizophrenia (BACS) scale (Keefe et al., 2004), and the Z-scores for subtests and the composite score were calculated based on the US norms (Keefe et al., 2008).
Image Data Acquisition
All brain images were acquired on a 3.0 T MR scanner (Trio, Siemens Medical Systems, Germany) with a 32-channel head coil, and we collected resting-state functional images and high-resolution 3D T1-weighted and T2-weighted structure images for all participants. The scanning protocols and parameters followed the Human Connectome Project-recommended settings (http://protocols.humanconnectome.org/HCP/3T/imaging-protocols.html).
Resting-state fMRI was acquired using a simultaneous multi-slice multiband echo planner imaging sequence developed by the University of Minnesota with the following parameters: repetition time/echo time (TR/TE) = 700/37.8 ms, flip angle (FA) = 52°, axial field of view (FOV) = 210 × 177 mm, matrix = 100 × 84, slice thickness = 2.1 mm, multiband acceleration factor = 8, and the voxel size = 2.1 × 2.1 × 2.1 mm3. Two runs of phase coding in the right-to-left (RL) and left-to-right (LR) directions were conducted for 5 minutes, respectively, resulting in a total of 10 minute of fMRI scanning. Each run included 415 volumes.
High-resolution T1-weighted and T2-weighted scans were both required for surface reconstruction. The T2-weighted image was required to make accurate pial surfaces, which excluded dura and blood vessels that were isointense to gray matter in the T1-weighted image, and to make myelin maps (Glasser et al., 2013). High-resolution 3D T1-weighted images were acquired using magnetization-prepared rapid gradient-echo sequence with the following parameters: TR/TE/inversion time (TI) = 2400/2.01/1000 ms, FA = 8°, sagittal FOV = 256 × 256 mm, matrix = 320 × 320, 208 axial slices, slice thickness = 0.8 mm, no gap, and the voxel size = 0.8 × 0.8 × 0.8 mm3. High-resolution 3D T2-weighted images were acquired using sampling perfection with application optimized contrasts using different flip angle evolution sequences with the following parameters: TR/TE = 3200/565 ms, sagittal FOV = 256 × 256 mm, matrix = 320 × 320, 224 axial slices, slice thickness = 0.8 mm, no gap, and the voxel size = 0.8 × 0.8 × 0.8 mm3.
All participants were instructed to lie in a supine position with their eyes closed, staying awake and still as much as possible, and trying not to think anything in particular. Earplugs and headphones were provided to block background noise, and sponge pads were used to minimize head movements. After scanning, all the participants were asked whether they fell asleep or wondered anything during scanning and all the participants finally recruited in the study complied with the instructions given before. Participants with a maximal motion or rotation over 2.0 mm or 2.0°, or mean framewise displacement (FD) over 0.2 mm, were excluded. No significant difference was observed in the mean FD between patients and controls (Table 1).
Table 1:
Demographic and clinical ratings of the participants.
| Characteristics | Schizophrenia patients (N = 75) | Healthy controls (N = 51) | t | P value | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age (years) | 28.45 | 9.33 | 24.92 | 2.46 | 3.12 | <0.001 |
| Sex (female/male) | 42/33 | 35/16 | 0.154 | |||
| Education (years) | 11.04 | 3.96 | 17.27 | 1.85 | −12.12 | <0.001 |
| BACS (T-score) | 22.61 | 18.54 | 52.14 | 7.50 | −12.38 | <0.001 |
| GAF | 51.27 | 15.49 | ||||
| HAMA | 10.95 | 7.02 | ||||
| HAMD | 15.53 | 8.63 | ||||
| PANSS | ||||||
| positive | 25.32 | 3.46 | ||||
| negative | 22.56 | 4.69 | ||||
| general | 48.51 | 7.07 | ||||
| PANSS total | 96.39 | 11.99 | ||||
| mFD (RL) | 0.114 | 0.028 | 0.111 | 0.029 | 0.259 | 0.511 |
| mFD (LR) | 0.121 | 0.031 | 0.113 | 0.028 | 1.487 | 0.140 |
Abbreviations: SD, standard deviation. mFD, mean framewise displacement.
Image Processing
The preprocessing procedure followed the minimal preprocessing pipeline for the Human Connectome Project (Glasser et al., 2013), including five major steps: PreFreeSurfer, FreeSurfer, PostFreeSurfer, fMRIVolume, and fMRISurface, performed on FSL (v.6.0.2, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL) and FreeSurfer (v.6.0.0, https://surfer.nmr.mgh.harvard.edu/) software. In brief, distortions related to gradient nonlinearity, head motion, and phase-encoding were corrected, then the images were registered to individuals’ T1-weighted images, and normalized to the MNI space. The output images of fMRIVolume were further denoised using Statistical Parametric Mapping software (SPM12, http://www.fil.ion.ucl.ac.uk/spm/software/spm12/), regressed out white matter and cerebrospinal fluid signals, and corrected for the 24 head motion parameters (i.e. the six rigid-body parameters generated from the realignment step, their first derivatives, and the squares of these 12 parameters). The corrected images were then temporally filtered with band pass 0.01–0.08 Hz to rule out the scanner and physiological noise. The expanded Power brain atlas (Cao et al., 2019) was used to define the nodes, which additionally included six nodes in the bilateral hippocampus, bilateral amygdala, and bilateral ventral striatum based on the Power atlas with 264 nodes (Power et al., 2011). Then, the mean time series for each of the 270 nodes were extracted, and Pearson correlation was performed to generate a 270 × 270 pairwise connectivity matrix for each run with phase coding in the RL or LR direction. Each participant's two connectivity matrices were averaged in the end to generate one final individual FC matrix.
Comparison of FCs between Groups
The statistical analysis for demographic characteristics, clinical scale ratings, and mean FDs of two runs with phase coding RL and LR directions was conducted using SPSS for Windows, v.24.0 (IBM SPSS Statistics for Windows, Armonk, NY, USA). The continuous variables were compared using a two-sample t-test and the categorical variables were analyzed using the Chi-square test. The significance of group differences was set at the threshold of the two-tailed P value <0.05.
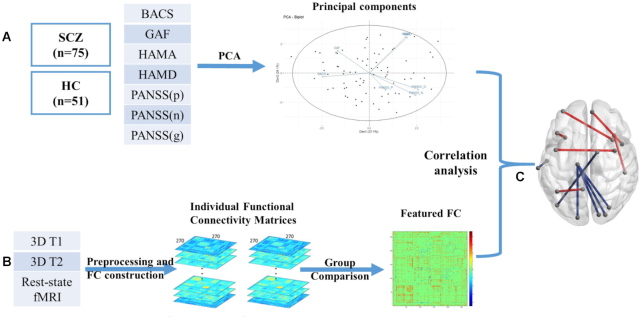
The data processing pipeline for the relationship between FCs and clinical phenotypes was shown in Fig. 1. Between-group comparison of FC was conducted to identify the abnormal functional brain connectivity. We used Gretna toolbox (J. Wang et al., 2015) to conduct the comparison for each FC with age, sex, and years of education as covariates, and those with P values <0.05 were retained for subsequent analysis. Considering our sample size and the number of FCs, we did not correct for multiple comparisons for the heuristic purpose to preliminarily exclude those that were not related to the illness.
Figure 1:
Data processing and analyzing pipeline for detecting the relationship between FCs and clinical phenotypes. (A) PCA of seven clinical scales to obtain clinical PCs. (B) FC construction and inter-group comparison. (C) Correlation analysis between clinical components and the abnormal FC in schizophrenia.
Principal Component Analysis of Clinical Scales
The scores of seven clinical scales (i.e. GAF, HAMD, HAMA, PANSS positive, PANSS negative, PANSS general psychopathological symptoms, and BACS) were converted into z-scores after regressing out covariates including age, sex, and years of education. The scores were then decomposed into a set of PCs using eigen-decomposition of the covariance matrix. PCs were combinations of the seven r-matrices that could represent latent variables of the scales. PC scores were calculated for subsequent analysis in lieu of the original scale scores. PCA was performed and the scree plot and variable map were visualized using FactoMiner package (Lê et al., 2008) in R software. Parallel analysis was conducted to determine the number of PCs to retain and PCs with eigenvalues >1.0 were retained.
Correlation Analysis between Clinical PCs and FCs
To detect FCs that were correlated with behavioral PCs, we performed partial correlation analysis between the abnormal FC in schizophrenia and the first three PCs, with age, sex, years of education, and mean FDs as covariates. We showed FC with P < 0.005 for each PC in the correlation analysis without strict multiple comparisons because our study intended to emphasize patterns of effect sizes rather than the significance of the correlation between any single pair of variables. The connections were visualized using the BrainNet Viewer (M. Xia et al., 2013) (https://www.nitrc.org/projects/bnv/).
Results
Demographic Characteristics
The demographic characteristics are listed in Table 1. The two participant groups did not differ in sex, but age was higher in schizophrenia patients than healthy control participants. As expected, schizophrenia patients have significantly fewer years of education and lower BACS scores (all with P < 0.05). Details are presented in Table 1.
Principal Component Analysis on Clinical Scales
Using the PCA approach, the parallel analysis suggested that the eigenvalues of the first two PCs were >1.0 (Supplementary Fig. S2), so we retained two components for further analysis. As the scree plot (Fig. 2a) showed, PC1 explained 37% of the total variance of all the seven clinical feature and PC2 explained 24%. As the loadings of clinical features in PCs showed (Fig. 2b and Supplementary Table S1), the ratings of GAF and BACS contributed negatively to PC1, while those of PANSS, HAMD, and HAMA contributed positively. The ratings of HAMA and HAMD had relatively high loadings (0.551 and 0.504, respectively) in PC2, so PC2 tended to interpret emotional manifestations than others. Although the eigenvalue of PC3 was <1.0, it explained 15% of the total variance, and we noted that the loading of positive symptoms was high (0.789) in PC3, so we also included this PC in the following analysis to explore the FCs related to the PC, which tended to interpret positive symptoms than others.
Figure 2:
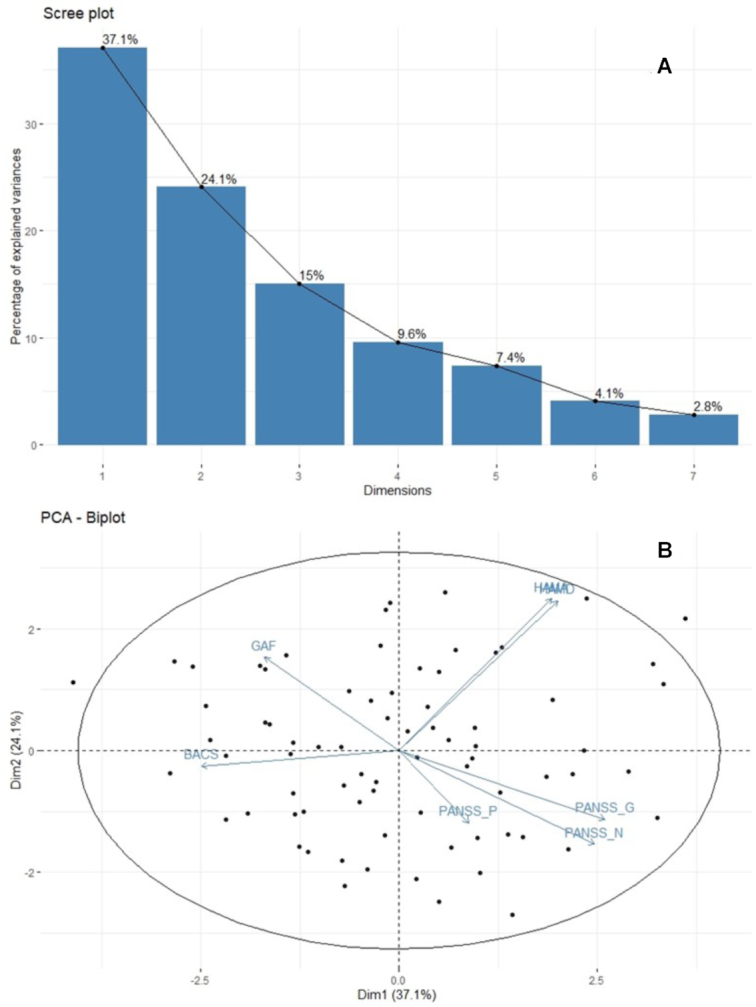
Results of PCA on clinical scales. (A) The scree plot of the percentage of explained variance of each PC. (B) Variables factor map. Arrows represented the loadings of each clinical scales on the first two dimensions and the dots represented individual scores. Abbreviation: Dim, dimension.
The first two components were rotated using Varimax rotation to obtain more interpretable PCs (Supplementary Table S2). However, in the first PC after rotation, the correlation between cognition and depressive symptoms and between depression/anxiety and PANSS general psychopathology, which were common in schizophrenia, were both absent, so we kept our nonrotation PCs for further analysis.
Correlations between FC and Clinical PCs
In total, there were 3732 connections with P < 0.05 in the group comparison (the T-value map is shown in Supplementary Fig. S1). After partial correlation analysis, there were 12 FCs related to PC1, 5 to PC2, and 12 to PC3 with P < 0.005. Details are listed in Supplementary Table S3.
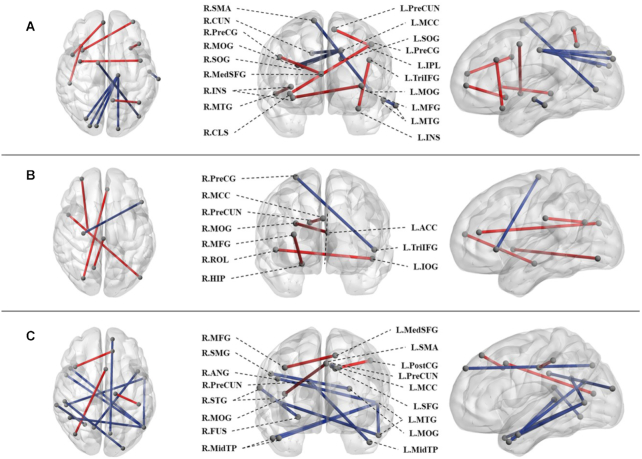
As Fig. 3 showed, the FCs positively correlated with PC1 mainly included connections related to insula, precuneus gyrus (PreCG), and some frontal brain regions. FCs negatively correlated with PC1 mainly included connections between the left middle cingulate cortex (MCC) and superior and middle occipital regions, most of which were between the visual and somatosensory networks according to the modules defined by Power et al. (Power et al., 2011). The FC strength between the left anterior cingulate cortex (ACC) and the right middle occipital gyrus (MOG) was positively correlated with PC2. And the most prominent PC3-related FCs were between left middle temporal gyrus (MTG) and other temporal and occipital regions, between the DMN and dorsal and ventral attention networks as the atlas defined.
Figure 3:
FC related to the first three clinical PCs. The coronal, axial, and sagittal views of the FCs related to (A) PC1, (B) PC2, and (C) PC3. The balls represented nodes of the brain area. Red sticks represented FCs correlated positively with PCs, and the blue ones represented FC correlated negatively with PCs. Abbreviations: R., Right; L., Left, SMA, supplementary motor area; SFG, superior frontal gyrus; CUN, cuneus; SOG, superior occipital gyrus; PreCUN, precuneus; CLS, nucleus claustrum; MedSFG, medial superior frontal gyrus; INS, insula; MFG, middle frontal gyrus. PostCG, postcentral gyrus; SMG, supramarginal gyrus; MidTP, temporal pole of the MTG; FUS, fusiform gyrus; ANG, angular gyrus.
Discussion
In this study, we found a FC pattern that was associated with both psychopathological and cognitive manifestations in drug-naïve first-episode schizophrenia. Within this pattern, in particular, higher connectivity between regions of visual cortices and MCC was associated with better cognition and milder psychopathological ratings, indicating brain connectivity commonly underpinned the psychopathological and cognitive impairments of schizophrenia, and thus might represent core disorganization of brain networks in schizophrenia, whereas, the positive symptoms shared fewer linking patterns with other dimensions of clinical phenotypes, but were related to FC that was relevant to the temporal lobe, especially the left MTG.
While further replication of our findings is warranted in studies with larger samples, our study provided initial evidence for a common network change underlying various domains of clinical phenotypes in patients. The heterogeneity of schizophrenia manifests great severities in patients’ behavioral deficits that were mainly characterized by either positive or negative symptoms, or both, together with different extents of cognitive deficits. To disentangle the neural substrates underlying the clinical manifestations of schizophrenia has been a great challenge but with limited progress. In such efforts, we successfully extracted the general composite underlying different symptoms and cognition, and identified linked brain connectivity patterns that could explain the covariance of these clinical phenotypes, without confounding effects of antipsychotic medication. Although widespread brain dysconnections have been related to clinical manifestations in individuals with schizophrenia (Dong et al., 2018; S. Li et al., 2019; C. H. Xia et al., 2018), in most of these studies, the inter-dependency between different clinical assessments was omitted. The PANSS assessed not only the psychopathological manifestations including positive and negative symptoms but also cognitive behaviors, which was a key factor that was decomposed in a recent machine learning study (Chen et al., 2021a; Chen et al., 2020). Emotion dysregulation including anxiety and depression was also evaluated in PANSS within the general psychopathological symptoms (Kay et al., 1987). Therefore, an association should be noted among different psychopathological and cognitive ratings, and such an association might indicate the primary deficit underlying behavioral problems in schizophrenia. Our findings of dysfunction of visual cortex-related connections might represent the common abnormalities in the functional connections that underpin the primary behavioral abnormalities in early schizophrenia. Detecting this general pattern of brain changes might help achieve early diagnosis of the disorder, and develop targeted therapeutic interference accordingly to prevent further disease progression.
The loadings of clinical features except positive symptoms in PC1 were balanced, so PC1 could represent a composite of the dimensions of clinical phenotypes including cognition, emotion, negative and general psychopathological symptoms, and global functioning. In the identified PC1-related FCs, FCs relevant to PreCG and some frontal brain regions were positively associated with PC1. In addition, BACS and GAF ratings contributed negatively to PC1, while PANSS, HAMA, and HAMD did so positively. We could thus infer that stronger connectivity strength in this FC indicated severe cognitive and functional impairments with more psychopathological and emotional symptoms. Dysfunction of the prefrontal cortex (PFC) has been widely reported in previous studies of schizophrenia (Mubarik & Tohid, 2016). Functional alterations in the PFC are the main substrates underlying impaired cognitive control in schizophrenia (Minzenberg et al., 2010). Apart from cognition, negative symptoms of schizophrenia are also considered to be a psychiatric form of the frontal lobe syndrome (Ziauddeen et al., 2011). Abnormalities in white matter connections between the orbitofrontal cortex and ACC were associated with more severe negative symptoms (Ohtani et al., 2014). Hence, disconnection involving the frontal lobe could be a core abnormality that leads to a variety of clinical manifestations in schizophrenia.
FCs between visual associated occipital areas and MCC were associated negatively PC1, so increased connectivity strength in the PC was in relation to better cognition/function and fewer emotional and psychopathological symptoms. The visual cortex is believed to have a multisensory function beyond visual processing, which directly affects behavior and perception (Murray et al., 2016). Schizophrenia patients have been found with visual perceptual abnormalities (VPAs) (Keane et al., 2018) and visual processing deficits (Turkozer et al., 2019). Furthermore, increased VPAs were strongly correlated with worse hallucinations and earlier age of onset (Keane et al., 2018), while poorer visual processing was associated with greater negative symptoms (Turkozer et al., 2019). FC studies found patients with schizophrenia showed reduced intrinsic visual cortical connectivity that was associated with impaired perceptual closure (van de Ven et al., 2017). Studies using magnetic resonance spectroscopy showed diminished γ-aminobutyric acid (GABA) levels in the visual cortex early in schizophrenia (Yoon et al., 2010), which was not confounded by illness chronicity (Yoon et al., 2020), and such reduced GABA production in cortical interneurons resulted in local circuit dysfunction and information processing deficits (Shaw et al., 2020). Moreover, cortical morphology studies on drug-naïve patients with schizophrenia also showed visual cortical morphological deficits, and these deficits were associated with negative symptoms and global functioning (Adhan et al., 2020). Therefore, our findings of dysconnections involving the visual cortex enhanced the notion of functional abnormalities associated visual cortex underlying the general psychopathology of schizophrenia.
The FC strength between left ACC and right MOG was positively correlated with PC2. ACC was involved in appraisal and expression of negative emotion and generating emotional responses (Etkin et al., 2011). MOG was involved in the processing of cognitive biases by connected with limbic-cortical regions in resting state (Teng et al., 2018) and altered cortical processing of visual contrast was observed during depression (Salmela et al., 2021). Hyper-connectivity between ACC and MOG was also reported in patients with depression, which was consistent with our results. Aberrant FC between these regions might indicate the emotion specific deficit. PC3 has substantial weight of positive symptoms, and most of the FCs were negatively correlated with it. The most prominent PC3-related FC were between left MTG and other temporal and occipital regions, and between the DMN and attention network. Decreased MTG volume (Hu et al., 2013), decreased connectivity in the language network (Zhang et al., 2017), and MTG cortical thinning (Cui et al., 2018) have been found in schizophrenia patients with auditory verbal hallucinations. The ventral stream of the language pathways projected toward MTG is associated with mapping sound onto meaning (Hickok & Poeppel, 2004). Aberrant self-monitoring of the internal speech signal generated by the temporal lobe would lead to the occurrences of auditory verbal hallucinations, indicating that the hypoconnectivity or disrupted connection relevant to temporal speech areas might be critical for the pathological basis of positive symptoms.
We would like to acknowledge some limitations of this study. First, we did not correct for strict multiple comparisons in the correlation analyses considering our sample size and number of FCs. This study tended to emphasize patterns of effect sizes, as opposed to the significance of the correlation between any single pair of variables. Our study was a preliminary attempt to combine different dimensions of symptoms and cognitive domains together to explore the core deficit of brain FC in schizophrenia patients, so the findings should be interpreted cautiously. Second, age was significantly different between patients and healthy control participants. To minimize its effect, we used ages as covariates during the analyses. Third, we used the composite score of BACS instead of its subscales, while the specific cognitive performance and their correlations with brain functional abnormalities of schizophrenia are also of importance, which requires further investigation in future work. Nevertheless, our clinical scales were limited and might not represent comprehensive dimensions of clinical manifestations in patients with schizophrenia. More clinical scales are needed in future studies. Finally, our sample size was relatively small. Future studies with larger samples and follow-up data are needed to confirm our findings and examine the ability of the core network in predicting symptom severity and prognosis.
Conclusion
In conclusion, we found a linked pattern of FC associated with both psychopathological and cognitive manifestations in drug-naïve first-episode schizophrenia characterized as the dysconnection related to the frontal and visual cortex, which may represent a core deficit of brain FC in schizophrenia patients.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (Grant Nos. 8212018014, 82071908, 81761128023, and 82101998), Sichuan Science and Technology Program (Grant Nos. 2021JDTD0002 and 2020YJ0018), Post-Doctor Research Project, West China Hospital, Sichuan University (Grant No. 2020HXBH005), the Fundamental Research Funds for the Central Universities (Grant No. 2020SCU12053), Miaozi Project in Science and Technology Innovation Program of Sichuan Province (Grant No. 2021028), and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Project Nos. ZYYC08001 and ZYJC18020). Dr Lui acknowledges the support from Humboldt Foundation Friedrich Wilhelm Bessel Research Award and Chang Jiang Scholars (Program No. T2019069).
Contributor Information
Hui Sun, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, 610041 Chengdu, China.
Wenjing Zhang, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, 610041 Chengdu, China.
Hengyi Cao, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, 610041 Chengdu, China; Center for Psychiatric Neuroscience, Feinstein Institute for Medical Research, 11030 Manhasset, NY, USA; Division of Psychiatry Research, Zucker Hillside Hospital, 11004 Glen Oaks, NY, USA.
Huaiqiang Sun, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, 610041 Chengdu, China.
Jing Dai, Department of Psychoradiology, Chengdu Mental Health Center, 610031 Chengdu, China.
Siyi Li, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, 610041 Chengdu, China.
Jiaxin Zeng, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, 610041 Chengdu, China.
Xia Wei, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, 610041 Chengdu, China.
Biqiu Tang, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, 610041 Chengdu, China.
Qiyong Gong, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, 610041 Chengdu, China.
Su Lui, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, 610041 Chengdu, China.
Author contributions
Hui S. and W.Z. analyzed and interpreted the data and drafted the work. H.C. devised the methodology and revised the manuscript. Huaiqiang S. preprocessed the imaging data. J.D. made the diagnosis of the participants. S. Li, J.Z., X.W., and B.T. collected the data that included the imaging and clinical scale data. Q.G. and S. Lui contributed to the conception and design of the work and approved the version to be published.
Conflict of Interest
W. Z. and S. Li consult to VeraSci. One of the authors, Dr Qiyong Gong is also the editor-in-chief of Psychoradiology. He was blinded from reviewing or making decisions on the manuscript. The remaining authors have declared no conflict of interest.
Reference
- Addington J, Addington D, Maticka-Tyndale E (1991) Cognitive functioning and positive and negative symptoms in schizophrenia. Schizophr Res. 5:123–34. [DOI] [PubMed] [Google Scholar]
- Adhan I, Lizano P, Bannai Det al. (2020) Visual cortical alterations and their association with negative symptoms in antipsychotic-naive first episode psychosis. Psychiatry Res. 288:112957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behdinan T, Foussias G, Wheeler ALet al. (2015) Neuroimaging predictors of functional outcomes in schizophrenia at baseline and 6-month follow-up. Schizophr Res. 169:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, McEwen SC, Forsyth JKet al. (2019) Toward leveraging human connectomic data in large consortia: generalizability of fMRI-based brain graphs across sites, sessions, and paradigms. Cereb Cortex. 29:1263–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carment L, Khoury E, Dupin Let al. (2020) Common vs. distinct visuomotor control deficits in autism spectrum disorder and schizophrenia. Autism Res. 13:885–96. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DWet al. (2014) The p factor: one general psychopathology factor in the structure of psychiatric disorders?. Clin Psychol Sci. 2:119–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Muller VI, Dukart Jet al. (2021a) Intrinsic connectivity patterns of task-defined brain networks allow individual prediction of cognitive symptom dimension of schizophrenia and are linked to molecular architecture. Biol Psychiatry. 89:308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Patil KR, Weis Set al. (2020) Neurobiological divergence of the positive and negative schizophrenia subtypes identified on a new factor structure of psychopathology using non-negative factorization: an international machine learning study. Biol Psychiatry. 87:282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wensing T, Hoffstaedter Fet al. (2021b) Neurobiological substrates of the positive formal thought disorder in schizophrenia revealed by seed connectome-based predictive modeling. NeuroImage: Clinical. 30:102666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Liu B, Song Met al. (2018) Auditory verbal hallucinations are related to cortical thinning in the left middle temporal gyrus of patients with schizophrenia. Psychol Med. 48:115–22. [DOI] [PubMed] [Google Scholar]
- Dietz MJ, Zhou Y, Veddum Let al. (2020) Aberrant effective connectivity is associated with positive symptoms in first-episode schizophrenia. NeuroImage: Clinical. 28:102444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D, Wang Y, Chang Xet al. (2018) Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 44:168–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott ML, Romer A, Knodt ARet al. (2018) A connectome-wide functional signature of transdiagnostic risk for mental illness. Biol Psychiatry. 84:452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R (2011) Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Brown HR, Siemerkus Jet al. (2016) The dysconnection hypothesis (2016). Schizophr Res. 176:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo-Chica M, Rogers BP, Damon SMet al. (2018) Prefrontal-thalamic anatomical connectivity and executive cognitive function in schizophrenia. Biol Psychiatry. 83:509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JAet al. (2013) The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 80:105–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1959) The assessment of anxiety states by rating. Br J Med Psychol. 32:50–5. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry. 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare SM, Ford JM, Mathalon DHet al. (2019) Salience-default mode functional network connectivity linked to positive and negative symptoms of schizophrenia. Schizophr Bull. 45:892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D (2004) Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 92:67–99. [DOI] [PubMed] [Google Scholar]
- Hu M, Li J, Eyler Let al. (2013) Decreased left middle temporal gyrus volume in antipsychotic drug-naive, first-episode schizophrenia patients and their healthy unaffected siblings. Schizophr Res. 144:37–42. [DOI] [PubMed] [Google Scholar]
- Jones SH, Thornicroft G, Coffey Met al. (1995) A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF). Br J Psychiatry. 166:654–9. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987) The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 13:261–76. [DOI] [PubMed] [Google Scholar]
- Keane BP, Cruz LN, Paterno Det al. (2018) Self-reported visual perceptual abnormalities are strongly associated with core clinical features in psychotic disorders. Front Psychiatry. 9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PDet al. (2004) The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 68:283–97. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD, Goldberg TEet al. (2008) Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res. 102:108–15. [DOI] [PubMed] [Google Scholar]
- Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw. 25:1–18. [Google Scholar]
- Lesh TA, Tanase C, Geib BRet al. (2015) A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiatry. 72:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Lui S, Yao Let al. (2016) Longitudinal changes in resting-state cerebral activity in patients with first-episode schizophrenia: a 1-year follow-up functional MR imaging study. Radiology. 279:867–75. [DOI] [PubMed] [Google Scholar]
- Li S, Hu N, Zhang Wet al. (2019) Dysconnectivity of multiple brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Front Psychiatry. 10:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Lencer R, Hu Net al. (2020) Characteristics of white matter structural networks in chronic schizophrenia treated with clozapine or risperidone and those never treated. Int J Neuropsychopharmacolog. 23:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Li K, Li Wet al. (2019) Widespread white-matter microstructure integrity reduction in first-episode schizophrenia patients after acute antipsychotic treatment. Schizophr Res. 204:238–44. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Firl AJ, Yoon JHet al. (2010) Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology. 35:2590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubarik A, Tohid H (2016) Frontal lobe alterations in schizophrenia: a review. Trends Psychiatry Psychother. 38:198–206. [DOI] [PubMed] [Google Scholar]
- Murray MM, Thelen A, Thut Get al. (2016) The multisensory function of the human primary visual cortex. Neuropsychologia. 83:161–9. [DOI] [PubMed] [Google Scholar]
- Ohtani T, Bouix S, Hosokawa Tet al. (2014) Abnormalities in white matter connections between orbitofrontal cortex and anterior cingulate cortex and their associations with negative symptoms in schizophrenia: a DTI study. Schizophr Res. 157:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani SM, Sabaroedin K, Tiego Jet al. (2021) A multivariate analysis of the association between corticostriatal functional connectivity and psychosis-like experiences in the general community. Psychiatry Res: Neuroimaging. 307:111202. [DOI] [PubMed] [Google Scholar]
- Paolini E, Moretti P, Compton MT (2016) Delusions in first-episode psychosis: principal component analysis of twelve types of delusions and demographic and clinical correlates of resulting domains. Psychiatry Res. 243:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson-Yeo W, Allen P, Benetti Set al. (2011) Dysconnectivity in schizophrenia: where are we now?. Neurosci Biobehav Rev. 35:1110–24. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SMet al. (2011) Functional network organization of the human brain. Neuron. 72:665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringner M (2008) What is principal component analysis?. Nat Biotechnol. 26:303–4. [DOI] [PubMed] [Google Scholar]
- Salmela V, Socada L, Soderholm Jet al. (2021) Reduced visual contrast suppression during major depressive episodes. J Psychiatry Neurosci. 46:E222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo-Angles A, Salvador R, Gomar JJet al. (2021) Interindividual variability of functional connectome in schizophrenia. Schizophr Res. 235:65–73. [DOI] [PubMed] [Google Scholar]
- Shaw AD, Knight L, Freeman TCAet al. (2020) Oscillatory, computational, and behavioral evidence for impaired GABAergic inhibition in schizophrenia. Schizophr Bull. 46:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Barch DM (2016) Cognition and resting-state functional connectivity in schizophrenia. Neurosci Biobehav Rev. 61:108–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Chiappelli JJ, Sampath Het al. (2019) Aberrant frontostriatal connectivity in negative symptoms of schizophrenia. Schizophr Bull. 45:1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Cooper JE, Fisher HLet al. (2005) Determining the chronology and components of psychosis onset: the Nottingham Onset Schedule (NOS). Schizophr Res. 80:117–30. [DOI] [PubMed] [Google Scholar]
- Smith GT, Atkinson EA, Davis HAet al. (2020) The general factor of psychopathology. Ann Rev Clin Psychol. 16:75–98. [DOI] [PubMed] [Google Scholar]
- Teng C, Zhou J, Ma Het al. (2018) Abnormal resting state activity of left middle occipital gyrus and its functional connectivity in female patients with major depressive disorder. BMC Psychiatry. 18:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Zalesky A, Bousman Cet al. (2019) Insula functional connectivity in schizophrenia: subregions, gradients, and symptoms. Biol Psychiatry: Cogn Neurosci Neuroimaging. 4:399–408. [DOI] [PubMed] [Google Scholar]
- Turkozer HB, Hasoglu T, Chen Yet al. (2019) Integrated assessment of visual perception abnormalities in psychotic disorders and relationship with clinical characteristics. Psychol Med. 49:1740–8. [DOI] [PubMed] [Google Scholar]
- van de Ven V, Rotarska Jagiela A, Oertel-Knochel Vet al. (2017) Reduced intrinsic visual cortical connectivity is associated with impaired perceptual closure in schizophrenia. NeuroImage: Clinical. 15:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Fornito A (2014) Brain networks in schizophrenia. Neuropsychol Rev. 24:32–48. [DOI] [PubMed] [Google Scholar]
- Wang D, Li M, Wang Met al. (2020) Individual-specific functional connectivity markers track dimensional and categorical features of psychotic illness. Mol Psychiatry. 25:2119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang X, Xia Met al. (2015) GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Human Neurosci. 9:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LJ, Lin PY, Lee Yet al. (2016) Validation of the Chinese version of brief assessment of cognition in schizophrenia. Neuropsychiatr Dis Treat. 12:2819–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CH, Ma Z, Ciric Ret al. (2018) Linked dimensions of psychopathology and connectivity in functional brain networks. Nat Commun. 9:3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y (2013) BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS ONE. 8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Shimokawa T, Takahashi Set al. (2020) Cognitive functions relating to aberrant interactions between task-positive and task-negative networks: resting fMRI study of patients with schizophrenia. Appl Neuropsychol: Adult. 1–9. [DOI] [PubMed] [Google Scholar]
- Yang C, Zhang W, Yao Let al. (2020) Functional alterations of white matter in chronic never-treated and treated schizophrenia patients. J Magn Reson Imaging. 52:752–63. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Maddock RJ, DongBo Cui Eet al. (2020) Reduced in vivo visual cortex GABA in schizophrenia, a replication in a recent onset sample. Schizophr Res. 215:217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Maddock RJ, Rokem Aet al. (2010) GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 30:3777–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng B, Ardekani BA, Tang Yet al. (2016) Abnormal white matter microstructure in drug-naive first episode schizophrenia patients before and after eight weeks of antipsychotic treatment. Schizophr Res. 172:1–8. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li B, Wang Het al. (2017) Decreased middle temporal gyrus connectivity in the language network in schizophrenia patients with auditory verbal hallucinations. Neurosci Lett. 653:177–82. [DOI] [PubMed] [Google Scholar]
- Ziauddeen H, Dibben C, Kipps Cet al. (2011) Negative schizophrenic symptoms and the frontal lobe syndrome: one and the same?. Eur Arch Psychiatry Clin Neurosci. 261:59–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.