Abstract
Psychomotor slowing is a frequent symptom of schizophrenia. Short-interval intracortical inhibition assessed by transcranial magnetic stimulation demonstrated inhibitory dysfunction in schizophrenia. The inhibitory deficit results from additional noise during information processing in the motor system in psychosis. Here, we tested whether cortical inhibitory dysfunction was linked to psychomotor slowing and motor network alterations.
In this cross-sectional study, we included 60 patients with schizophrenia and psychomotor slowing determined by the Salpêtrière Retardation Rating Scale, 23 patients without slowing and 40 healthy control participants. We acquired single and double-pulse transcranial magnetic stimulation effects from the left primary motor cortex, resting-state functional connectivity and diffusion imaging on the same day. Groups were compared on resting motor threshold, amplitude of the motor evoked potentials, as well as short-interval intracortical inhibition. Regression analyses calculated the association between motor evoked potential amplitudes or cortical inhibition with seed-based resting-state functional connectivity from the left primary motor cortex and fractional anisotropy at whole brain level and within major motor tracts.
In patients with schizophrenia and psychomotor slowing, we observed lower amplitudes of motor evoked potentials, while the short-interval intracortical inhibition/motor evoked potentials amplitude ratio was higher than in healthy controls, suggesting lower cortical inhibition in these patients. Patients without slowing also had lower amplitudes of motor evoked potentials. Across the combined patient sample, cortical inhibition deficits were linked to more motor coordination impairments. In patients with schizophrenia and psychomotor slowing, lower amplitudes of motor evoked potentials were associated with lower fractional anisotropy in motor tracts. Moreover, resting-state functional connectivity between the primary motor cortex, the anterior cingulate cortex and the cerebellum increased with stronger cortical inhibition. In contrast, in healthy controls and patients without slowing, stronger cortical inhibition was linked to lower resting-state functional connectivity between the left primary motor cortex and premotor or parietal cortices.
Psychomotor slowing in psychosis is linked to less cortical inhibition and aberrant functional connectivity of the primary motor cortex. Higher neural noise in the motor system may drive psychomotor slowing and thus may become a treatment target.
Keywords: motor inhibition, SICI, catatonia, resting-state fMRI, diffusion imaging
Lefebvre et al. demonstrate that psychomotor slowing in psychosis is linked to less cortical inhibition and aberrant functional connectivity of the primary motor cortex. They conclude that higher levels of neural noise in the motor system may drive psychomotor slowing and could represent a potential treatment target.
Introduction
Schizophrenia is a devastating disorder, characterized by eight symptom dimensions, i.e. delusions, hallucinations, disorganization, negative symptoms, abnormal psychomotor behaviour, impaired cognition, depression and mania.1 Symptoms are thought to arise from aberrant brain connectivity at multiple levels.2,3
Abnormal psychomotor behaviour may include a general slowing of fine and gross motor behaviour, i.e. psychomotor slowing, but also specific motor phenomena such as dyskinesia, parkinsonism or catatonia.4-6 Motor abnormalities can be observed in chronic patients, but also in treatment-naïve first episode psychosis patients or even in subjects at risk for psychosis.5,7-11 Finally, motor abnormalities severely impair social and community functioning and predict poor clinical outcome.12-14
Psychomotor slowing is frequent in schizophrenia and thought to arise from poor movement preparation, altered motor coordination, impaired movement execution as well as complex interactions with altered emotion processing.15-23,24 The phenomenon can be assessed with clinical rating scales, instrumental measures such as actigraphy, or neurocognitive tests.6,15 Within schizophrenia, we found a subgroup with severe psychomotor slowing that presented with reduced physical activity, slower gait and compromised dexterity.24 Thus, a proportion of psychosis patients presents with severely altered behaviour, pointing to distinct pathobiology in subjects with psychomotor slowing.
Neuroimaging studies suggest multiple alterations in the motor circuitry in schizophrenia in general,25 including reduced grey matter in premotor areas, higher structural connectivity between cortical and subcortical motor areas,26 or higher resting-state functional connectivity (rsFC) between premotor/motor cortices and basal ganglia, thalamus, as well as cerebellum.27-29 In addition, specific alterations in the motor circuit were linked to hypokinetic motor abnormalities in psychosis.4,25,30 For example, patients with psychomotor slowing in the context of catatonia had higher resting-state cerebral blood flow in the supplementary motor area (SMA), higher fractional anisotropy (FA) in the left cortico-spinal tract, higher thalamo-cortical rsFC, and less flexible shifts in resting-state networks.27,31-36 However, the direction of rsFC changes between thalamus and primary motor cortex (M1) is debated. While some linked motor inhibition and negative symptoms to thalamocortical hyperconnectivity,27,37 others reported lower connectivity with psychomotor slowing,38 triggering the assumption that psychomotor slowing was linked to hypoconnectivity in the motor circuit.39
Currently, the field still struggles to relate the observed neural alterations in psychosis to pathophysiology or specific symptoms. Particularly, the physiology of the motor and premotor cortices remains unknown. Higher neural activity or higher rsFC in the premotor and motor cortices may arise from either too much excitatory action or too little inhibitory feedback. One key feature of schizophrenia pathobiology is noisy signalling in the cortex as evidenced by the excitation-inhibition imbalance, particularly in the frontal cortex, which is thought to result from impaired inhibitory action.40 In fact, reduced cortical inhibition seemed to be associated with more noise in the motor system.41-44 Indeed, post-mortem studies have substantiated reduced GABAergic tone in schizophrenia.45 For example, reduced GABA-related mRNA expression was detected in multiple cortex regions, including M1.46 In addition, in vivo spectroscopy studies indicated reduced levels of GABA in schizophrenia,45 while a SPECT study suggested reduced GABA receptor density in M1 in catatonia.47
Single and double pulse transcranial magnetic stimulation (TMS) experiments allow testing M1 physiology.48 The amplitude of motor evoked potential (MEP) informs on the excitability of corticospinal neurons and the influence of GABAergic and glutamatergic tone.49 The short-interval intracortical inhibition (SICI) paradigm applies two stimuli over M1, where the first subthreshold stimulus triggers a reduced response to a subsequent stimulus. SICI resembles another indirect in vivo probe of GABAA-related activity.45,49 Higher cortical inhibition seems to be associated with better motor execution and coordination.50-53 A substantial body of evidence suggests reduced SICI in schizophrenia across different stages of this disorder.49,54,55 But the association of this inhibitory impairment with symptoms remains unclear.
Few studies linked SICI to brain imaging measures in schizophrenia.56-58 Less inhibition was associated with lower FA in the left corona radiata57 and lower rsFC between left M1 and bilateral medial prefrontal cortex, right insula, as well as left cerebellum.56 In fact, the effect of white matter properties on SICI was mediated by rsFC in schizophrenia. Still, the contribution of motor symptoms, such as psychomotor slowing, remains unknown.
Given that schizophrenia is associated with (i) alterations in the motor circuitry including higher neural resting-state activity in the sensorimotor cortex; and (ii) lower GABAergic tone and lower intracortical inhibition in the motor cortex, we may speculate that these alterations are most pronounced in patients with current psychomotor slowing, who may present distinct pathobiology.24 Thus, this study aimed to test the differences in cortical excitability measures between healthy controls and patients with schizophrenia with or without psychomotor slowing. Furthermore, we probed whether cortical excitability measures were linked to specific alterations of structural and functional brain connectivity in schizophrenia. We hypothesized that SICI was particularly reduced in patients with psychomotor slowing and that corticospinal tract metrics would correlate with MEP amplitudes. Finally, we expected this difference to be pronounced when the patients were classified according to the presence of catatonia, which is associated with extreme forms of psychomotor slowing.4
Materials and methods
Participants
This cross-sectional study included baseline data of 123 participants from the double-blind, randomized, placebo-controlled trial OCoPS-P (Overcoming Psychomotor Slowing in Psychosis; ClinicalTrials.gov Identifier: NCT03921450). The study was conducted at the University Hospital of Psychiatry and Psychotherapy in Bern, Switzerland between March 2019 and October 2022. Participants included three groups: 60 patients with schizophrenia and psychomotor slowing (PS) according to the Salpêtrière Retardation Rating Scale59 (SRRS, total score ≥ 15), 23 patients with schizophrenia without psychomotor slowing (non-PS, SRRS score < 15), and 40 age and gender-matched healthy controls (HC) (Table 1). The study protocol adhered to the Declaration of Helsinki and was approved by the local ethics committee (KEK-BE 2018-02164). Inclusion criteria encompassed being right-handed and aged between 18 and 60 years. In addition, patients needed to fulfil the DSM-5 criteria for a schizophrenia spectrum disorders (more details given in the ‘Clinical characteristics’ section). General exclusion criteria were active substance dependence except for nicotine, neurological disorders impacting motor behaviour, severe brain injury with consecutive loss of consciousness, and contra-indications for magnetic resonance scans and TMS acquisition, i.e. metallic objects in the body or pregnancy. Occasional cannabis consumers were included but were asked to halt consumption in the 24 h prior to MRI and TMS acquisition. Additional exclusion criteria for HC were a history of any psychiatric disorder or any first-degree relative with psychosis. A proportion of this sample was included in reports on grey matter structure in catatonia or structural brain correlates of formal thought disorder.60,61
Table 1.
Demographic and clinical sample characteristics
| HC | PS | Non-PS | Tests | P-value | |
|---|---|---|---|---|---|
| n for cortical excitability | 40 | 60 | 23 | – | |
| Subset for DTI, n | 38 | 48 | 18 | – | |
| Subset for VBM, n | 40 | 51 | 23 | – | |
| Subset for BOLD resting-state, n | 37 | 51 | 23 | – | |
| Age in years, mean ± SD | 37 ± 13 | 37 ± 13 | 33 ± 12 | F(2,120) = 0.29 | 0.746 |
| Sex | 19 M | 31 M | 11 M | χ2 (2, n = 123) = 3.5 | 0.740 |
| Education in years, mean ± SD | 16 ± 3 | 13 ± 2 | 13 ± 2 | F(2,120) = 22.94 | <0.001* |
| Duration of illness in years, mean ± SD | – | 10.7 ± 10.3 | 8.7 ± 10.7 | W = 281 | 0.650 |
| PANSS Total, mean ± SD | – | 81.2 ± 16.9 | 65.5 ± 14.6 | W = 348 | <0.001* |
| PANSS Positive, mean ± SD | – | 15.7 ± 5.1 | 16.3 ± 4.6 | W = 763 | 0.456 |
| PANSS Negative, mean ± SD | – | 24.6 ± 6.2 | 15.9 ± 4.9 | W = 176 | <0.001* |
| Antipsychotic OLZ eq. in mg, mean ± SD | – | 16.2 ± 11.1 | 15.1 ± 10.5 | W = 653 | 0.714 |
| Benzodiazepines, n | – | 13 | 2 | – | |
| Valproic acid, n | – | 2 | 4 | – | |
| Antidepressant, n | – | 2 | 1 | – | |
| Lithium, n | – | 1 | 0 | – | |
| SRRS | – | 23.4 ± 5.6 | 8.4 ± 2.8 | W = 0 | <0.001* |
| BFCRS | – | 6.1 ± 4.6 | 1.3 ± 1.7 | W = 157 | <0.001* |
| UPDRS III | – | 21.9 ± 12.4 | 9.0 ± 6.3 | W = 237 | <0.001* |
| NES | – | 17.1 ± 10.4 | 10.0 ± 5.6 | W = 352 | <0.001* |
| NES motor coordination | – | 2.1 ± 2.2 | 0.6 ± 2.2 | W = 329.5 | <0.001* |
BFCRS = Bush-Francis Catatonia Rating Scale; BOLD = blood oxygen level-dependent; DTI = diffusion tensor imaging; HC = healthy controls; M = male; NES = neurological evaluation scale; non-PS = patients without psychomotor slowing; OLZ eq. = olanzapine equivalent; PANSS = Positive And Negative Syndrome Scale; PS = patients with psychomotor slowing; SD = standard deviation; SRRS = Salpêtrière Retardation Rating Scale; UPDRS III = Unified Parkinson’s Disease Rating Scale Part III; VBM = voxel-based morphometry.
*Significant P-value.
Measures
Clinical characteristics
The diagnosis of schizophrenia spectrum disorders (schizophrenia, schizoaffective or schizophreniform disorders) was given according to DSM-5 using the Structured Clinical Interview for DSM-5 (SCID-5) and clinical case files. Symptom severity was measured with the Positive and Negative Syndrome Scale (PANSS).62 We assessed motor abnormalities such as psychomotor slowing with SRRS,59 catatonia using the Bush-Francis Catatonia Rating Scale (BFCRS)63 and the neurological soft signs with the Neurological Evaluation Scale (NES).64 All patients were ON antipsychotic medication at the time of testing and mean olanzapine equivalents (OLZ eq.) were calculated according to Leucht et al.65 Few patients also received benzodiazepines (n = 15), antidepressants (n = 3), lithium or other mood stabilizers (n = 7).
Cortical excitability assessment using transcranial magnetic stimulation
The subjects were seated in a comfortable reclining chair during the whole procedure. We used single- and paired-pulse TMS (Magstim Inc.) with a figure-of-eight coil to deliver the stimulations and surface electrodes to record muscle activity (Dantec® Keypoint®). To target the left M1, the standard TMS procedure66 is based on the localization of the ‘hand motor hotspot’, i.e. the scalp position where TMS induces the largest MEPs in the first dorsal interosseus muscle. After the hotspot identification, we first determined the resting motor threshold (RMT). The RMT was defined as the minimal stimulus intensity (percentage of the maximal stimulator output) that produced MEPs > 50 µV in peak-to-peak amplitude in at least 5 of 10 trials with the recorded muscle fully relaxed.66 Then, we identified the test stimulus threshold (TS), i.e. the minimum stimulus intensity that generated MEPs of 0.5–2.5 mV peak-to-peak amplitude in 5 of 10 trials, i.e. the stimulation intensity was adjusted to obtain stable baseline MEPs of 0.5–2.5 mV. On average, this represented an intensity of 120% RMT. We acquired 15 trials using the TS and averaged the 15 recorded peak-to-peak amplitudes to obtain the MEP amplitude. This measure reflects the general excitability of M1. Finally, we measured the SICI using a paired-pulse TMS paradigm with 1 and 3 ms interstimulus intervals. For each SICI trial, a subthreshold conditioning stimulus (80% RMT) was followed by a suprathreshold stimulus (TS). Owing to the large interindividual variability in cortical inhibition exploration,67 we used a standard method56,58,68 by acquiring 12 trials for each interstimulus interval and averaged the 24 recorded peak-to-peak amplitudes to obtain the SICI amplitude. Interstimulus intervals of 1 and 3 ms share similar cortical inhibition patterns.67
The ratio between SICI and MEP amplitude is used to quantify cortical inhibition. Ratios <1 indicate cortical inhibition; the smaller the ratio, the stronger the cortical inhibition.
MRI acquisition procedures
On the same day as the cortical excitability measurements, we performed multimodal neuroimaging at the translational imaging centre of the Swiss Institute for Translational and Entrepreneurial Medicine, Bern, Switzerland. Three neuroimaging markers were acquired: functional connectivity using blood oxygenation level-dependent (BOLD) resting-state functional MRI (rsfMRI), grey matter density using voxel-based morphometry, and structural connectivity using diffusion-weighted images (DWI). The MRI scans were acquired on a 3 T Prisma MRI whole-body scanner using a 20-channel radio-frequency head coil (Siemens). Participants lay horizontally in the magnetic resonance scanner and their arms rested beside their trunk. We placed foam pads around the participants’ head and instructed them to avoid head motion and to not fall asleep.
The MRI protocol encompassed four sequences: a T1-weighted MP2RAGE, a field map scan, a diffusion-weighted scan and a BOLD rsfMRI scan (Supplementary material, Section A).
MRI processing
Diffusion-weighted imaging: structural connectivity
We performed a voxel-wise statistical analysis of FA using tract-based spatial statistics (TBSS) with FSL 6.04 (https://fsl.fmrib.ox.ac.uk/).69,70 To evaluate the association of cortical excitability parameters with structural integrity of the motor pathways, we used tractography and computed mean FA for the six main motor-related fibre bundles connecting bilateral SMA, M1 and pathways of motor output [precentral connections via the corpus callosum (CC-precentral); CC-SMA; left and right corticospinal tract (CST), left and right thalamo-precentral connections], using the Quantitative Imaging Toolkit (QIT,71https://cabeen.io/qitwiki).
BOLD resting-state functional connectivity
The preprocessing of the BOLD resting-state data was similar to the one described in Walther et al.72 (Supplementary material, Section B). An absolute head motion involving translation >2 mm or rotation >2°, as well as a mean frame-wise displacement (FD) (calculated as defined by Power et al.73) >0.5 mm were used as exclusion criteria (n = 1).
We used the preprocessed rsfMRI data to explore the seed-to-voxel connectivity with a seed (10 mm sphere) created over the left M1 (x = −37, y = −24, z = 56, covering the motor hand knob to reflect the scalp motor hotspot used as the TMS target for the cortical excitability acquisition).
Voxel-based morphometry: grey matter density
We used the DARTEL VBM algorithm with SPM 12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12), following the standard procedure established by Ashburner74 to process the grey matter volume and obtained an MRI normalized modulated grey matter smoothing with a 6 mm full-width at half-maximum (FWHM) Gaussian kernel.
Statistics
The demographic data were compared between groups using ANOVA, Wilcoxon and chi-squared tests. The analyses were performed in three ways: (i) categorical group comparisons [healthy controls (HC), all patients with schizophrenia, psychomotor slowing (PS) and non-psychomotor slowing (non-PS)]; (ii) dimensional associations between MRI measurements and general M1 excitability (MEP amplitude) or cortical inhibition (SICI/MEP amplitude) across the whole sample to explore mechanisms that could be similar irrespective of the clinical status, and within each group to explore mechanisms that are linked to the disorder; and (iii) dimensional associations with measures of psychomotor slowing (a) correlations within all patients with schizophrenia considering the motor abnormalities as a continuum; and (b) correlations within each patient subgroup, i.e. PS and non-PS).
In all analyses, age was used as a covariate. In addition, for analyses focusing on patients only, current antipsychotic dosage (OLZ eq.) was also used as a covariate. Finally, MRI analyses required specific additional covariates depending on the modality. For structural connectivity and grey matter density (GMD) analyses, total intracranial volume was used as an additional covariate whereas for the rsFC analyses, the mean framewise displacement was used. We refrained from comparing the associations of cortical excitability parameters with the neuroimaging markers between groups due to the complex interpretation. Instead, we exclusively performed the associations analyses within each group.
Cortical excitability
We used R (https://www.r-project.org/about.html) to perform the cortical excitability analyses. We ran ANCOVAs to compare the cortical excitability measures [RMT, TS, MEP amplitude (general M1 excitability) and ratio SICI/MEP amplitude (cortical inhibition)] between the three groups (PS, non-PS and HC). We used Tukey post hoc tests.
To explore the association between cortical inhibition and impaired motor coordination in schizophrenia, we performed a Kendall-tau partial correlation analysis between the ratio SICI/MEP amplitude and an expert rating scale on motor coordination deficits, the NES motor coordination subscore. A P-value < 0.05 was considered statistically significant.
MRI measurements
Structural connectivity
For tractography, we extracted the mean FA values of each of the six fibre bundles and compared them between the groups. We also performed Kendall-tau partial correlations between the FA and cortical excitability measures within each group. A qFDR < 0.05 was considered statistically significant.
In TBSS, we compared the whole brain FA between the groups. We also performed a regression analysis of whole-brain FA with the MEP amplitude as well as the ratio SICI/MEP amplitude within the whole sample as well as within each group (HC, all schizophrenia, PS, non-PS). The level of significance was set at PFWEcorrected < 0.05, using a threshold-free cluster enhancement (TFCE) with 5000 randomized permutations.
Resting-state functional connectivity
We used the CONN toolbox for seed-to-voxel analysis. Within each group, we explored (i) the rsFC with left M1 as the seed; and (ii) the association between the rsFC seeded on left M1 and the cortical excitability measures: MEP amplitude as well as the ratio SICI/MEP amplitude. We set a cluster-forming threshold of P = 0.005 and qFDR < 0.05 for the cluster-wise threshold.
Grey matter density
For voxel-based morphometry (VBM) analysis, we used an absolute threshold of 0.1 to ensure the inclusion of grey matter voxels with a probability ≥0.1 of being grey matter. We first compared the whole brain GMD between the groups. Then, to evaluate the association between local GMD and cortical excitability, we used a second-level multiple regression model within each group. We set a cluster-forming threshold of P = 0.005 and qFDR < 0.05 for the cluster-wise threshold.
Exploratory analyses in catatonia
Finally, we classified all psychosis patients into those with and without catatonia according to the BFCRS screening instrument (scoring at least 1 on two of the first 14 items of the BFCRS).63 We repeated analyses with this classification to check whether findings on slowing and catatonia were consistent.
Results
Cortical excitability
The three groups presented similar RMT (intensity of ∼40%) and TS (intensity of ∼50%) (Table 2). Despite similar motor thresholds, both PS and non-PS patients had reduced MEP amplitudes (i.e. M1 excitability) compared to healthy controls. Likewise, we noted a group difference in cortical inhibition with less inhibition in psychomotor slowing compared to HC. However, there was no difference between the two patient groups in any cortical excitability measure. Results were similar when adding medication as covariate to the model (Fig. 1A). We found no difference in SICI (cortical inhibition) between patients treated with clozapine and those without (Supplementary material, Section C).
Table 2.
Between-groups comparison of cortical excitability measurements
| RMT (%) | TS (%) | MEP amplitude (µV) | Ratio SICI/MEP amplitude | |
|---|---|---|---|---|
| PS (mean ± SD) | 41 ± 7 | 50 ± 9 | 967.6 ± 555.4 | 0.45 ± 0.20 |
| Non-PS (mean ± SD) | 42 ± 8 | 51 ± 9 | 840.6 ± 358.2 | 0.45 ± 0.30 |
| HC (mean ± SD) | 39 ± 1 | 47 ± 9 | 1357 ± 581.9 | 0.31 ± 0.20 |
| Main ANCOVA controlling for age | ||||
| Tests | F(2,120) = 1.71 | F(2,120) = 1.62 | F(2,120) = 9.01 | F(2,120) = 3.24 |
| P-value | 0.186 | 0.203 | <0.001* | 0.043* |
| Post hoc P-values | ||||
| PS versus HC | 0.304 | 0.328 | 0.003* | 0.048* |
| Non-PS versus HC | 0.201 | 0.224 | <0.001* | 0.100 |
| ANCOVA only in patients only controlling for age and medication | ||||
| Tests | F(1,81) = 0.35 | F(1,81) = 0.28 | F(1,81) = 1.03 | F(1,81) = 0.001 |
| P-value | 0.560 | 0.600 | 0.310 | 0.990 |
HC = healthy controls; MEP = motor evoked potential; non-PS = patients without psychomotor slowing; PS = patients with psychomotor slowing; RMT = resting motor threshold; SICI = short-interval intracortical inhibition; TS = test stimulus.
*Significant P-value.
Figure 1.
Cortical excitability measurements related to motor abnormalities. (A) Cortical excitability measurements related to psychomotor slowing. Box and whisker plots of transcranial magnetic stimulation (TMS) threshold (RMT and TS), MEP and ratio SICI/MEP amplitude between groups. (B) Cortical excitability measurements related to catatonia. Box and whisker plots of TMS threshold (RMT and TS), MEP and ratio SICI/MEP amplitude between groups. The centre line represents the median value, the lower bound of the box represents the 25th percentile, the upper bound of the box the 75th percentile, and the whiskers represent 3× the interquartile range. *P < 0.05, **P < 0.01, ***P < 0.001. (C) Association between cortical inhibition and motor coordination. Correlation plot between the cortical inhibition and the motor coordination subscore of the NES. The solid line is the ‘line of best fit’, a line that minimizes the vertical distances between the data-points and the line itself. The ‘best fit line’ is a useful way of representing the linear trend. The grey shading around the blue line represents the 95% confidence interval around the line of best fit. HC = healthy controls; MEP = motor evoked potential; NES = neurological evaluation scale; non-PS = patients without psychomotor slowing; PS = patients with psychomotor slowing; RMT = resting motor threshold; SICI = short-interval cortical inhibition; TS = test stimulus.
Across all patients with schizophrenia, we observed a significant positive association between SICI/MEP ratio and the NES motor coordination subscore (tau = 0.15, P = 0.047) (Fig. 1C), suggesting more coordination deficits in subjects with lower cortical inhibition. This association was not significant in either of the patient groups separately (PS or non-PS).
Diffusion tensor imaging
Comparison of fractional anisotropy between groups
Tractography within bundles
The mean FA within the CC-SMA bundle was lower in PS than in both HC and non-PS at trend level (F = 2.4, P = 0.09; HC-PS: t = −2.16, P = 0.07; HC-non-PS: t = 0.2, P = 0.9; PS-non-PS: t = −1.9, P = 0.07). However, the five other bundles showed similar mean FA between the groups (F < 0.3, P > 0.5) (Fig. 2C).
Figure 2.
Association of fractional anisotropy with cortical excitability. (A) whole brain tract-based spatial statistics (TBSS) comparison between groups [healthy controls (HC), patients with psychomotor slowing (PS) and patients without psychomotor slowing (non-PS)]. Clusters in red indicate significant differences between the groups at TFCE PFWE< 0.05. The fractional anisotropy (FA) skeleton is displayed in green. (B) Association between FA (whole brain TBSS) and motor evoked potential (MEP) amplitude. Clusters in red indicate significant positive associations between FA and MEP at PFWE < 0.05. The FA skeleton is displayed in green. (C) Comparison of the mean FA values of the corpus callosum supplementary motor area (CC-SMA) bundles between the groups. (D) Association between FA in motor bundles and MEP amplitude within the groups. This figure displays the correlation plots between the MEP amplitude and the FA within the six major motor bundles. In each plot the solid line is the ‘line of best fit’, a line that minimizes the vertical distances between the data-points and the line itself. The best fit line’ is a useful way of representing the linear trend. The grey shading around the blue line represents the 95% confidence interval around the line of best fit. CST = corticospinal tract; FA = fractional anisotropy; HC = healthy controls; L = left; MEP amp = motor evoked potential amplitude; non-PS = patients without psychomotor slowing; PS = patients with psychomotor slowing; R = right; SCZ = schizophrenia patients; SMA = supplementary motor area; thal = thalamus.
Whole-brain tract-based spatial statistics
At the whole brain level, PS showed lower FA than HC in the hippocampus area and inferior longitudinal fasciculus. PS also had lower FA than non-PS in the hippocampus area, inferior and superior longitudinal fasciculi, inferior fronto-occipital fasciculus, forceps major and CST. No differences were observed between non-PS and HC (TFCE PFWE < 0.05) (Fig. 2A).
Association of FA and cortical excitability measurements
No associations between white matter microstructure and cortical inhibition were observed, neither in the TBSS nor the tractography analysis in any group.
Tractography within bundles
The tractography across the six fibre bundles showed a significant positive association between FA of the CC-precentral, left CST and bilateral thalamo-precentral tracts and the MEP amplitude across all participants. However, this effect was strongly driven by the PS group (all tau > 0.219, qFDR < 0.031) probably due to the large variation in the FA values within the different bundles compared to the other groups (Fig. 2D).
Whole-brain tract-based spatial statistics
At the whole-brain level, the TBSS analyses demonstrated a significant positive association between the MEP amplitude and FA within the sensorimotor pathway (including the CST, frontal aslant tract, the pre/motor, sensory and parietal corpus callosum segments75) for all participants, all schizophrenia patients and separately for both PS and HC (TFCE PFWE < 0.05). Non-PS did not show significant association between whole brain mean FA and the MEP amplitude (Fig. 2B).
Seed-based (left M1) resting-state functional connectivity
Comparison of the seed-based resting-state functional connectivity between groups
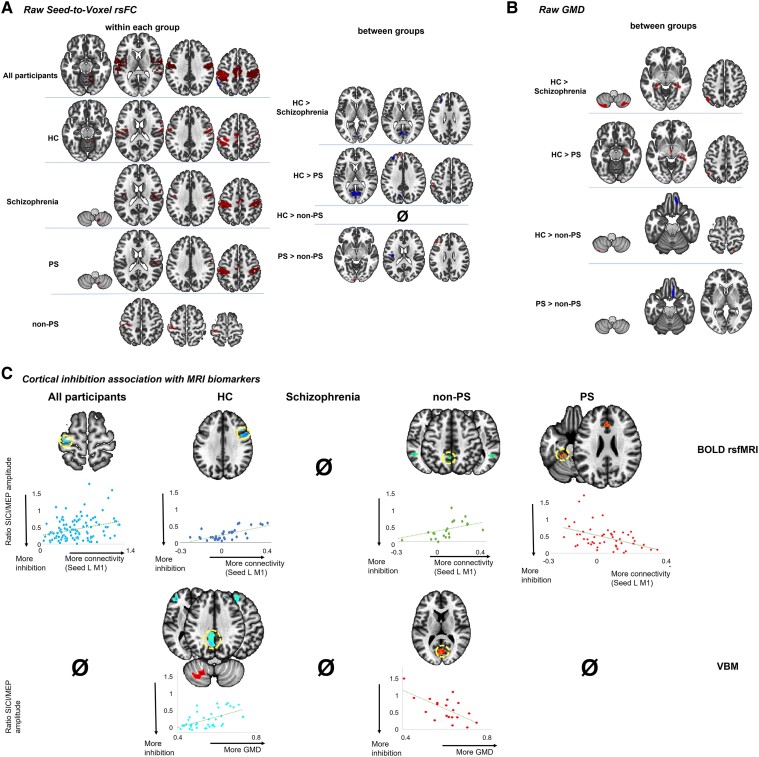
Within all participants, left M1 was highly connected with the other key areas of the motor network (bilateral motor/premotor areas including the SMA and the right cerebellum). This pattern seemed highly preserved across the HC and patients with schizophrenia. However, comparisons between groups revealed connectivity differences between HC and patients with schizophrenia (between left M1 and parietal as well as prefrontal cortices), which is highly driven by PS (Fig. 3A and Table 3).
Figure 3.
Association between cortical inhibition and functional connectivity and grey matter density within and between the groups (all participants, controls, all patients with schizophrenia, psychomotor slowing and no psychomotor slowing) cluster-forming threshold of P = 0.005 and qFDR < 0.05 for the cluster-wise threshold. (A) The seed-to-voxel resting-state functional connectivity (rsFC) seeded on left (L) M1 within and between each group. (B) The grey matter density (GMD) within and between each group. (C) The association between the SICI/MEP ratio and the strength of the functional connectivity (top) or the GMD (bottom) within each group. The scatterplots reflect the association between cortical inhibition and the neuroimaging markers within the highlighted (yellow circle) brain area. BOLD = blood oxygen level-dependent; HC = healthy controls; MEP = motor evoked potential; non-PS = patients without psychomotor slowing; PS = patients with psychomotor slowing; SICI = short-interval cortical inhibition; VBM = voxel-based morphometry.
Table 3.
Cluster information related to rsfMRI and VBM analyses
| Group | Cluster | Size | Cluster PFWE | Cluster PFDR | Area |
|---|---|---|---|---|---|
| Functional connectivity between groups | |||||
| Schizophrenia > HC | −08 −70 12 | 229 | <0.001 | <0.001 | L occipital cortex (BA17)/L parietal cortex (BA 7) |
| Schizophrenia > HC | −28 40 28 | 115 | <0.010 | <0.010 | L anterior prefrontal cortex (BA 10) |
| HC > PS | −14 50 40 | 107 | 0.013 | <0.010 | L DLPFC (BA 9) |
| 04 36 −20 | 75 | 0.100 | 0.023 | R orbitofrontal cortex (BA 11) | |
| −52 −34 20 | 64 | 0.212 | 0.042 | L supramarginal cortex (BA 40) | |
| PS > HC | −06 −70 12 | 742 | <0.001 | <0.001 | Occipital cortex (BA17)/L parietal cortex (BA 7) |
| −28 40 28 | 265 | <0.001 | <0.001 | L anterior prefrontal cortex (BA 10) | |
| −30 −40 −32 | 102 | 0.018 | <0.010 | L cerebellum (I, VI) | |
| −30 −44 −48 | 59 | 0.292 | 0.049 | L cerebellum (VIII) | |
| −02 −46 −18 | 58 | 0.312 | 0.049 | L cerebellum (vermis IV–V) | |
| PS > non-PS | −32 34 20 | 164 | <0.001 | <0.001 | L DLPFC (BA 46) |
| −06 −96 −06 | 77 | 0.114 | 0.038 | L secondary visual area (BA 18) | |
| Non-PS > PS | −36 −14 16 | 92 | 0.045 | 0.021 | L insula (BA 13) |
| rsFC association with cortical inhibition | |||||
| More inhibition == lower connectivity | |||||
| All participants | −26 −24 62 | 106 | 0.014 | 0.010 | L PM (BA 6), L M1 (BA 4) |
| HC | 54 12 40 | 189 | <0.001 | <0.001 | R PM (BA 6) |
| Non-PS | −04 −50 48 | 159 | <0.001 | <0.001 | Precuneous |
| Non-PS | −52 −54 12 | 107 | <0.010 | <0.005 | L angular gyrus |
| Non-PS | 48 −60 22 | 69 | 0.088 | 0.037 | R angular gyrus |
| More inhibition == higher connectivity | |||||
| PS | −02 28 26 | 117 | 0.012 | <0.001 | L ACC (BA32) |
| PS | −28 −24 −34 | 96 | 0.039 | 0.016 | L cerebellum IV–V |
| GMD between groups | |||||
| HC > Schizophrenia | 40 −41 −5 | 4063 | 0.036 | 0.016 | R hippocampus/R parahippocampus |
| −24 −36 −4 | 3136 | 0.121 | 0.034 | L hippocampus/L parahippocampus | |
| 26 −69 −57 | 3254 | 0.104 | 0.034 | R cerebellum (VIII) | |
| −44 −70 −57 | 4229 | 0.029 | 0.016 | L cerebellum (VIII) | |
| HC > PS | −6 −8 19 | 3610 | 0.065 | 0.028 | L caudate/L thalamus/L putamen |
| 40 −38 −8 | 4292 | 0.027 | 0.028 | R hippocampus/R parahippocampus | |
| Non-PS > HC | 8 21 −31 | 3493 | 0.129 | 0.041 | R orbitofrontal cortex (BA 11) |
| Non-PS > PS | 9 20 −36 | 6635 | <0.010 | <0.010 | orbitofrontal cortex (BA 11) |
| GMD association with cortical inhibition | |||||
| More inhibition == lower GMD | |||||
| HC | −10 −57 34 | 3503 | 0.020 | 0.029 | L dorsal parietal cortex (BA 31) |
| HC | 1 34 43 | 3377 | 0.024 | 0.029 | SMA (BA 6) |
| More inhibition == higher GMD | |||||
| HC | −23 −62 −46 | 3604 | 0.020 | 0.029 | L cerebellum VIIIa |
| Non-PS | −5 −78 3 | 4133 | 0.002 | 0.002 | L occipital cortex |
ACC = anterior cingulate cortex; BA = Broadmann area; DLPFC = dorsolateral prefrontal cortex; GMD = grey matter density; HC = healthy controls; L = left; M1 = primary motor cortex; non-PS = patients without psychomotor slowing; PM = premotor cortex; PS = patients with psychomotor slowing; R = right; rs = resting-state; rsFC = resting-state functional connectivity; rsfMRI = resting state functional MRI; SMA = supplementary motor area; VBM = voxel-based morphometry.
Association of resting-state functional connectivity with cortical excitability measures
We found no associations between the seed-based rsFC and the MEP amplitude in any group. In contrast, across all participants, stronger cortical inhibition was linked to lower rsFC between the left M1 and the left M1/premotor cortex. In HC, more cortical inhibition was associated with lower left M1-right premotor cortex [PM, Broadmann area (BA) 6] rsFC. Interestingly, while in the entire group of patients with schizophrenia a significant association between left M1 and cortical inhibition was lacking, PS and non-PS groups showed an opposing pattern. In PS, stronger cortical inhibition (lower SICI/MEP amplitude) was associated with higher rsFC between left M1-left anterior cingulate cortex (ACC, BA32) and left M1-left cerebellum IV–V. But in non-PS, more inhibition was linked to lower connectivity between left M1 and parietal cortex (bilateral precuneus and angular cortex) (Fig. 3C and Table 3).
Grey matter density
Comparison of the grey matter density between groups
The between-group differences revealed that HC had higher GMD than schizophrenia patients in bilateral cerebellum (II and VII), bilateral hippocampus, parahippocampus, amygdala, and left parietal cortex. Specifically, the differences in limbic and parietal GMD appeared to be due to decreased GMD in PS. Non-PS showed higher GMD than HC and PS in the orbitofrontal cortex (Fig. 3B and Table 3).
Association of grey matter density with cortical excitability measures
MEP amplitudes were unrelated to GMD in all groups. There were no significant associations between cortical inhibition and GMD within all participants or within schizophrenia patients. Conversely, stronger cortical inhibition was associated with lower GMD in the SMA (BA 6) and the left dorsal parietal cortex (BA 31) in HC and with higher GMD in the left cerebellum VIIIa and the left occipital cortex in non-PS. However, no association appeared between GMD and cortical inhibition in PS (Fig. 3C and Table 3).
Exploratory analysis on catatonia
As an exploratory analysis, we classified the patients into those with and without current catatonia based on the BFCRS (details of these analyses are provided in Supplementary material, Section D). Similar to the results observed with the psychomotor slowing classification, patients with schizophrenia with or without catatonia had smaller MEP amplitudes (reduced excitability) and lower cortical inhibition than HC. Moreover, the patients without catatonia had an intermediate level of cortical inhibition between the controls and the patients with catatonia (Fig. 1B and Table 2). At the rsFC level, the patients without catatonia had similar association patterns as HC (more inhibition associated with decreased left M1-right PM rsFC). However, in the patients with catatonia, more inhibition was associated with increased left M1-SMA and left M1-right cerebellum (vermis VI) rsFC (Supplementary material, Section D).
Discussion
Motor abnormalities and psychomotor slowing specifically, are frequent symptoms of psychosis but their nature remains widely unknown.5 To understand the contribution of motor cortex physiology to psychomotor slowing in psychosis, this study tested cortical excitability and brain connectivity measures in patients with schizophrenia with (PS) and without (non-PS) psychomotor slowing, as well as in HC. We hypothesized that inhibitory deficits would be most pronounced in psychomotor slowing and linked to aberrant connectivity with M1. While TS and RMT were similar across groups, patients had lower MEP amplitudes than HC suggesting reduced general M1 excitability. Furthermore, psychomotor slowing had higher SICI/MEP ratios compared to HC, pointing towards impaired inhibition in psychomotor slowing. This group had distinct neural associations with excitability measures, e.g. lower MEP amplitudes with lower FA in the main motor fibre tracts, while less intracortical inhibition was linked to lower resting-state connectivity between from cerebellum or ACC and left M1. Finally, when patients were classified according to catatonia, results were even more pronounced, suggesting a difference in intracortical inhibition between catatonia and non-catatonia. Furthermore, in catatonia we found lower inhibition to be linked to reduced rsFC between contralateral SMA or cerebellum and left M1. In summary, psychomotor slowing in psychosis is associated with aberrant cortical excitability and inhibition and brain connectivity, suggesting specific pathobiology most likely associated with GABAergic deficits.
Our findings on cortical excitability corroborate and extend prior reports. In line with most studies, we noted no change in RMT in schizophrenia.49 Similar to Tang et al.,76 we found reduced MEP amplitudes in both schizophrenia groups. Furthermore, our MEP results are numerically similar to studies that found no difference from HC.56 However, other prior results on MEP have been mixed.49 Most previous studies reported reduced SICI or increased SICI/MEP ratios in schizophrenia across all stages of the disorder, indicating impaired intracortical inhibition pointing towards GABAergic deficits.41,49,54,55 For our patient groups, SICI/MEP ratios were similar to prior studies,56,57,77,78 whereas others found lower ratios in HC. For the first time, impaired inhibition was specifically found in patients with psychomotor slowing compared to HC. PS and non-PS did not differ from each other in SICI/MEP ratios. However, when we stratified patients according to catatonia, patients with catatonia had more pronounced SICI deficits than patients without catatonia at trend level and the difference between catatonia and HC became even clearer. Thus, the TMS data suggest a specific inhibitory deficit in patients with schizophrenia and hypokinetic motor abnormalities, i.e. psychomotor slowing and catatonia.39,79,80 This would fit well with the notion of aberrant GABAergic activity in motor cortices in catatonia,47 but also to abnormal resting-state hyperactivity in schizophrenia with psychomotor slowing or catatonia.27,31,33 Cerebellum, SMA and basal ganglia are known to be part of a complex inhibitory motor network that could cause aberrant inhibition of motor coordination, execution and planning, particularly when this network is driven to hyperactivity.4,25,81-85 We may speculate that the dysfunction of GABAergic interneurons may drive tonic cortical hyperactivity in SMA and M1. However, the between-patient comparisons were slightly underpowered and larger samples are required to substantiate this finding. Interestingly, in patients, we detected a correlation between diminished intracortical inhibition and increased coordination deficits (Fig. 1C). Thus, inhibitory dysfunction in M1 was directly linked to impaired motor coordination.
In the group comparisons, PS had lower FA in motor pathways and more rsFC from M1 to prefrontal cortical areas than non-PS. PS also differed from HC in FA and rsFC. Thus, the group not only has distinct behavioural patterns,24 but also aberrant neural connectivity in the motor system.27,86
One study tested the neuroimaging correlates of intracortical inhibition deficits in schizophrenia, reporting lower inhibition to be associated with reduced FA in the left corona radiata and lower rsFC to the bilateral medial prefrontal cortex, left cerebellum and right insula.56,57 In PS, we found similar associations with SICI in resting-state connectivity from left cerebellum lobule VI–V but also the dorsal ACC to left M1: less inhibition indicated lower rsFC. However, this pattern differed clearly from that in HC and non-PS, in whom we detected associations in the opposite direction: less inhibition with increased rsFC to the contralateral PM (HC) or bilateral inferior parietal lobes (non-PS). Thus, normally intracortical inhibition would be facilitated by reduced rsFC from the contralateral premotor cortex, arguing that less information flow from the contralateral premotor cortex would enable M1 to have more intracortical inhibition, which is relevant for a good excitation/inhibition balance. By contrast, patients with psychomotor slowing appear to lack these mechanisms and instead have connectivity patterns from ACC and cerebellum to M1 that seem to restore M1 inhibitory tone by increasing rsFC to M1. Both ACC and cerebellar lobules IV–V have, among other functions, been implicated in movement control87-89 and show clear rsFC to M1.90,91
In contrast to a previous study,56,57 we failed to link SICI to white matter indices. However, this study is the first to find cortical excitability (MEP amplitude) strongly linked to white matter microstructure. This association is somehow expected in the major motor output fibres that transport the signal from left M1 to the muscles. Particularly, linear associations between increased FA and increased MEP amplitude were seen mostly in PS, e.g. in left CST, bilaterally in the tract connecting M1 and thalamus, and in the interhemispheric callosal connection between bilateral M1. The latter was the only pathway in which also HC had a significant association, whereas non-PS had no association at all. In an earlier probabilistic fibre tracking study, we reported that patients had stronger probabilities of structural connectivity between bilateral thalamus and M1 in schizophrenia compared to HC.26 Furthermore, in white matter underneath the SMA and M1, we reported higher FA values to correlate with lower physical activity in patients with schizophrenia.92 Thus, there is indirect evidence supporting a role for MEP amplitude and motor pathway white matter alterations in aberrant motor behaviour in psychosis.25 We found specifically reduced excitability and reduced inhibition in M1 in psychomotor slowing, suggesting a dual dysfunction including glutamatergic and GABAergic neurotransmission in the motor circuits. Previous studies with magnetic resonance spectroscopy found reduced GABA concentrations in occipital cortex and ACC, but not in the basal ganglia.93,94 While neuroimaging studies were less clear, post-mortem studies reported reduced GABA in schizophrenia. Furthermore, TMS studies found SICI was reduced in schizophrenia across all stages of the disorder.45,55 SICI is thought to reflect to GABAA activity, which in turn is important for behaviour and functional activation in motor cortices.95 In fact, SICI reductions in schizophrenia were reported during motor tasks, such as grip maintenance or stop signal inhibition, indicating more noise in the motor system in psychosis.41,42 Our findings also align with previous studies demonstrating a link between neurological soft signs in psychosis and increased cerebello-frontal rsFC,96 as well as glutamatergic dysfunction.97
In summary, our findings add to the growing literature on excitation/inhibition imbalance in schizophrenia,40 suggesting a role in aberrant motor behaviour.
The present study included a moderately sized group of severely ill patients for close clinical phenotyping, TMS experiments and MRI. Experiments were conducted on the same day as the MRI scans with consistent timing across the entire sample. However, some limitations of the current study require discussion. First, the non-PS group was comparably smaller than the PS and control groups, which may have led to lower statistical power for the group comparisons. Second, our patients were mostly ill and thus receiving medication. We controlled for medication dosage but cannot rule out any impact of medication on either cortical excitability measures or MRI parameters. However, we could not control for cumulated lifetime antipsychotic exposure or for the duration of the current medication. Still, SICI measures may be influenced more by symptoms than by medication.49 The lack of significant difference between the PS and non-PS groups for the cortical excitability measurement could be explained by the aforementioned reasons or variability of scores in the expert ratings. Future studies need to be performed using larger samples for both patient groups and classification methods based on instrumental measures to provide a better overview. During the MRI acquisition, the participants were only asked to avoid motion and sleep. A careful monitoring as well as a precise instruction might have reduced intra-individual variability in the state of mind during the resting-state scans. Finally, our HC were closely matched for age and sex to our patient groups but presented slightly higher SICI/MEP ratios and MEP amplitudes than in some of the previous literature.
In summary, this study demonstrates reduced excitability and diminished intracortical inhibition in the motor cortex of patients with schizophrenia and psychomotor slowing. The inhibition deficit is linked to poor motor coordination and altered rsFC in the motor circuit. This excitation/inhibition imbalance within the motor circuitry might give rise to psychomotor abnormalities in psychoses.
Supplementary Material
Acknowledgements
We thank Danai Alexaki and Daniel Baumann Gama for conducting some of the clinical assessments.
Contributor Information
Stephanie Lefebvre, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, 3000 Bern, Switzerland; Translational Imaging Center (TIC), Swiss Institute for Translational and Entrepreneurial Medicine, 3000 Bern, Switzerland.
Gwendolyn Gehrig, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, 3000 Bern, Switzerland.
Niluja Nadesalingam, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, 3000 Bern, Switzerland; Translational Imaging Center (TIC), Swiss Institute for Translational and Entrepreneurial Medicine, 3000 Bern, Switzerland.
Melanie G Nuoffer, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, 3000 Bern, Switzerland; Translational Imaging Center (TIC), Swiss Institute for Translational and Entrepreneurial Medicine, 3000 Bern, Switzerland; Graduate School for Health Sciences, University of Bern, 3000 Bern, Switzerland.
Alexandra Kyrou, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, 3000 Bern, Switzerland.
Florian Wüthrich, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, 3000 Bern, Switzerland; Translational Imaging Center (TIC), Swiss Institute for Translational and Entrepreneurial Medicine, 3000 Bern, Switzerland.
Sebastian Walther, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, 3000 Bern, Switzerland; Translational Imaging Center (TIC), Swiss Institute for Translational and Entrepreneurial Medicine, 3000 Bern, Switzerland.
Data availability
The dataset presented in this article is not readily available because some participants did not provide consent to data sharing. Requests to access the datasets should be directed to S.L., stephanie.lefebvre@unibe.ch.
Funding
This study was funded by the Swiss National Science Foundation (grant 182469 to S.W.).
Competing interests
S.W. has received honoraria from Janssen, Lundbeck, Mepha and Neurolite.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Heckers S, Barch DM, Bustillo J, et al. Structure of the psychotic disorders classification in DSM-5. Schizophr Res. 2013;150:11–14. [DOI] [PubMed] [Google Scholar]
- 2. Seitz-Holland J, Wojcik JD, Cetin-Karayumak S, et al. Cognitive deficits, clinical variables, and white matter microstructure in schizophrenia: A multisite harmonization study. Mol Psychiatry. 2022;27:3719–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sapienza J, Bosia M, Spangaro M, et al. Schizophrenia and psychedelic state: Dysconnection versus hyper-connection. A perspective on two different models of psychosis stemming from dysfunctional integration processes. Mol Psychiatry. 2023;28:59–67. [DOI] [PubMed] [Google Scholar]
- 4. Walther S, Stegmayer K, Wilson JE, Heckers S. Structure and neural mechanisms of catatonia. Lancet Psychiatry. 2019;6:610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walther S, Strik W. Motor symptoms and schizophrenia. Neuropsychobiology. 2012;66:77–92. [DOI] [PubMed] [Google Scholar]
- 6. van Harten PN, Walther S, Kent JS, Sponheim SR, Mittal VA. The clinical and prognostic value of motor abnormalities in psychosis, and the importance of instrumental assessment. Neurosci Biobehav Rev. 2017;80:476–487. [DOI] [PubMed] [Google Scholar]
- 7. Pappa S, Dazzan P. Spontaneous movement disorders in antipsychotic-naive patients with first-episode psychoses: A systematic review. Psychol Med. 2009;39:1065–1076. [DOI] [PubMed] [Google Scholar]
- 8. Peralta V, Campos MS, De Jalon EG, Cuesta MJ. Motor behavior abnormalities in drug-naive patients with schizophrenia spectrum disorders. Mov Disord. 2010;25:1068–1076. [DOI] [PubMed] [Google Scholar]
- 9. Peralta V, Cuesta MJ. Motor abnormalities: From neurodevelopmental to neurodegenerative through “functional” (neuro)Psychiatric disorders. Schizophr Bull. 2017;43:956–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whitty PF, Owoeye O, Waddington JL. Neurological signs and involuntary movements in schizophrenia: Intrinsic to and informative on systems pathobiology. Schizophr Bull. 2009;35:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walther S, van Harten PN, Waddington JL, et al. Movement disorder and sensorimotor abnormalities in schizophrenia and other psychoses—European consensus on assessment and perspectives. Eur Neuropsychopharmacol. 2020;38:25–39. [DOI] [PubMed] [Google Scholar]
- 12. Nadesalingam N, Chapellier V, Lefebvre S, et al. Motor abnormalities are associated with poor social and functional outcomes in schizophrenia. Compr Psychiatry. 2022;115:152307. [DOI] [PubMed] [Google Scholar]
- 13. Sambataro F, Fritze S, Rashidi M, et al. Moving forward: Distinct sensorimotor abnormalities predict clinical outcome after 6 months in patients with schizophrenia. Eur Neuropsychopharmacol. 2020;36:72–82. [DOI] [PubMed] [Google Scholar]
- 14. Pieters LE, Nadesalingam N, Walther S, van Harten PN. A systematic review of the prognostic value of motor abnormalities on clinical outcome in psychosis. Neurosci Biobehav Rev. 2022;132:691–705. [DOI] [PubMed] [Google Scholar]
- 15. Osborne KJ, Walther S, Shankman SA, Mittal VA. Psychomotor slowing in schizophrenia: Implications for endophenotype and biomarker development. Biomark Neuropsychiatry. 2020;2:100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morrens M, Hulstijn W, Sabbe B. Psychomotor slowing in schizophrenia. Schizophr Bull. 2007;33:1038–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Docx L, Morrens M, Bervoets C, et al. Parsing the components of the psychomotor syndrome in schizophrenia. Acta Psychiatr Scand. 2012;126:256–265. [DOI] [PubMed] [Google Scholar]
- 18. Liddle PF, Morris DL. Schizophrenic syndromes and frontal lobe performance. Br J Psychiatry. 1991;158:340–345. [DOI] [PubMed] [Google Scholar]
- 19. Peralta V, Cuesta MJ. Negative parkinsonian, depressive and catatonic symptoms in schizophrenia: A conflict of paradigms revisited. Schizophr Res. 1999;40:245–253. [DOI] [PubMed] [Google Scholar]
- 20. Peralta V, Cuesta MJ. Motor features in psychotic disorders. I. Factor structure and clinical correlates. Schizophr Res. 2001;47(2–3):107–116. [DOI] [PubMed] [Google Scholar]
- 21. Peralta V, Cuesta MJ. Neuromotor abnormalities in neuroleptic-naive psychotic patients: Antecedents, clinical correlates, and prediction of treatment response. Compr Psychiatry. 2011;52:139–145. [DOI] [PubMed] [Google Scholar]
- 22. Telfer S, Shivashankar S, Krishnadas R, McCreadie RG, Kirkpatrick B. Tardive dyskinesia and deficit schizophrenia. Acta Psychiatr Scand. 2011;124:357–362. [DOI] [PubMed] [Google Scholar]
- 23. Ungvari GS, Goggins W, Leung SK, Lee E, Gerevich J. Schizophrenia with prominent catatonic features (‘catatonic schizophrenia’) III. Latent class analysis of the catatonic syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:81–85. [DOI] [PubMed] [Google Scholar]
- 24. Nadesalingam N, Lefebvre S, Alexaki D, et al. The behavioral mapping of psychomotor slowing in psychosis demonstrates heterogeneity among patients suggesting distinct pathobiology. Schizophr Bull. 2023;49:507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walther S. Psychomotor symptoms of schizophrenia map on the cerebral motor circuit. Psychiatry Res. 2015;233:293–298. [DOI] [PubMed] [Google Scholar]
- 26. Bracht T, Schnell S, Federspiel A, et al. Altered cortico-basal ganglia motor pathways reflect reduced volitional motor activity in schizophrenia. Schizophr Res. 2013;143(2–3):269–276. [DOI] [PubMed] [Google Scholar]
- 27. Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV. Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia Spectrum disorders. Schizophr Bull. 2017;43:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Viher PV, Docx L, Van Hecke W, et al. Aberrant fronto-striatal connectivity and fine motor function in schizophrenia. Psychiatry Res Neuroimaging. 2019;288:44–50. [DOI] [PubMed] [Google Scholar]
- 29. Woodward ND, Heckers S. Mapping thalamocortical functional connectivity in chronic and early stages of psychotic disorders. Biol Psychiatry. 2016;79:1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mittal VA, Bernard JA, Northoff G. What can different motor circuits tell US about psychosis? An RDoC perspective. Schizophr Bull. 2017;43:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walther S, Schappi L, Federspiel A, et al. Resting-State hyperperfusion of the supplementary motor area in catatonia. Schizophr Bull. 2017;43:972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Viher PV, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Walther S. Altered diffusion in motor white matter tracts in psychosis patients with catatonia. Schizophr Res. 2020;220:210–217. [DOI] [PubMed] [Google Scholar]
- 33. Foucher JR, Zhang YF, Roser M, et al. A double dissociation between two psychotic phenotypes: Periodic catatonia and cataphasia. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:363–369. [DOI] [PubMed] [Google Scholar]
- 34. Wasserthal J, Maier-Hein KH, Neher PF, et al. Multiparametric mapping of white matter microstructure in catatonia. Neuropsychopharmacology. 2020;45:1750–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sambataro F, Hirjak D, Fritze S, et al. Intrinsic neural network dynamics in catatonia. Hum Brain Mapp. 2021;42:6087–6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hirjak D, Rashidi M, Kubera KM, et al. Multimodal magnetic resonance imaging data fusion reveals distinct patterns of abnormal brain structure and function in catatonia. Schizophr Bull. 2020;46:202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martino M, Magioncalda P, Yu H, et al. Abnormal resting-state connectivity in a substantia Nigra-related striato-thalamo-cortical network in a large sample of first-episode drug-naive patients with schizophrenia. Schizophr Bull. 2017;44:419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Magioncalda P, Martino M, Conio B, et al. Intrinsic brain activity of subcortical-cortical sensorimotor system and psychomotor alterations in schizophrenia and bipolar disorder: A preliminary study. Schizophr Res. 2020;218:157–165. [DOI] [PubMed] [Google Scholar]
- 39. Northoff G, Hirjak D, Wolf RC, Magioncalda P, Martino M. All roads lead to the motor cortex: Psychomotor mechanisms and their biochemical modulation in psychiatric disorders. Mol Psychiatry. 2021;26:92–102. [DOI] [PubMed] [Google Scholar]
- 40. Howes OD, Shatalina E. Integrating the neurodevelopmental and dopamine hypotheses of schizophrenia and the role of cortical excitation-inhibition balance. Biol Psychiatry. 2022;92:501–513. [DOI] [PubMed] [Google Scholar]
- 41. Lindberg PG, Teremetz M, Charron S, et al. Altered cortical processing of motor inhibition in schizophrenia. Cortex. 2016;85:1–12. [DOI] [PubMed] [Google Scholar]
- 42. Carment L, Dupin L, Guedj L, et al. Neural noise and cortical inhibition in schizophrenia. Brain Stimul. 2020;13:1298–1304. [DOI] [PubMed] [Google Scholar]
- 43. Teremetz M, Amado I, Bendjemaa N, Krebs MO, Lindberg PG, Maier MA. Deficient grip force control in schizophrenia: Behavioral and modeling evidence for altered motor inhibition and motor noise. PLoS One. 2014;9:e111853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carment L, Dupin L, Guedj L, et al. Impaired attentional modulation of sensorimotor control and cortical excitability in schizophrenia. Brain. 2019;142:2149–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taylor SF, Tso IF. GABA abnormalities in schizophrenia: A methodological review of in vivo studies. Schizophr Res. 2015;167(1–3):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: Investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jannati A, Oberman LM, Rotenberg A, Pascual-Leone A. Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacology. 2023;48:191–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. di Hou M, Santoro V, Biondi A, Shergill SS, Premoli I. A systematic review of TMS and neurophysiological biometrics in patients with schizophrenia. J Psychiatry Neurosci. 2021;46:E675–E701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gilbert DL, Isaacs KM, Augusta M, Macneil LK, Mostofsky SH. Motor cortex inhibition: A marker of ADHD behavior and motor development in children. Neurology. 2011;76:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. He JL, Fuelscher I, Enticott PG, Teo W-P, Barhoun P, Hyde C. Interhemispheric cortical inhibition is reduced in young adults with developmental coordination disorder. Front Neurol. 2018;9:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cardellicchio P, Hilt PM, Olivier E, Fadiga L, D'Ausilio A. Early modulation of intra-cortical inhibition during the observation of action mistakes. Sci Rep. 2018;8:1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Loomes M, Tran DMD, Chowdhury NS, Birney DP, Harris JA, Livesey EJ. Is cortical inhibition in primary motor cortex related to executive control? Cortex. 2023;160:100–114. [DOI] [PubMed] [Google Scholar]
- 54. Radhu N, de Jesus DR, Ravindran LN, Zanjani A, Fitzgerald PB, Daskalakis ZJ. A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clin Neurophysiol. 2013;124:1309–1320. [DOI] [PubMed] [Google Scholar]
- 55. Rogasch NC, Daskalakis ZJ, Fitzgerald PB. Cortical inhibition, excitation, and connectivity in schizophrenia: A review of insights from transcranial magnetic stimulation. Schizophr Bull. 2014;40:685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Du X, Choa FS, Chiappelli J, et al. Aberrant middle prefrontal-motor Cortex connectivity mediates motor inhibitory biomarker in schizophrenia. Biol Psychiatry. 2019;85:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Du X, Kochunov P, Summerfelt A, Chiappelli J, Choa FS, Hong LE. The role of white matter microstructure in inhibitory deficits in patients with schizophrenia. Brain Stimul. 2017;10:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hare SM, Du X, Adhikari BM, et al. Mapping local and long-distance resting connectivity markers of TMS-related inhibition reduction in schizophrenia. Neuroimage Clin. 2021;31:102688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Widlocher D, Ghozlan A. The measurement of retardation in depression. In: Hindmarch I, Sotnier PD, eds. Human psychopharmacology: Measures and methods. Wiley; 1989:1–22. [Google Scholar]
- 60. Walther S, Nadesalingam N, Nuoffer M, Kyrou A, Wuthrich F, Lefebvre S. Structural alterations of the motor cortex and higher order cortical areas suggest early neurodevelopmental origin of catatonia in schizophrenia. Schizophr Res. 2022;263:131–138. [DOI] [PubMed] [Google Scholar]
- 61. Maderthaner L, Pavlidou A, Lefebvre S, et al. Neural correlates of formal thought disorder dimensions in psychosis. Schizophr Bull. 2023;49(Suppl_2):S104–S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 63. Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93:129–136. [DOI] [PubMed] [Google Scholar]
- 64. Buchanan RW, Heinrichs DW. The neurological evaluation scale (NES): A structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res. 1989;27:335–350. [DOI] [PubMed] [Google Scholar]
- 65. Leucht S, Samara M, Heres S, et al. Dose equivalents for second-generation antipsychotic drugs: The classical mean dose method. Schizophr Bull. 2015;41:1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rossini PM, Burke D, Chen R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Du X, Summerfelt A, Chiappelli J, Holcomb HH, Hong LE. Individualized brain inhibition and excitation profile in response to paired-pulse TMS. J Mot Behav. 2014;46:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gordon PC, Valiengo L, de Paula VJR, et al. Changes in motor cortical excitability in schizophrenia following transcranial direct current stimulation. Prog Neuropsychopharmacol Biol Psychiatry. 2019;90:43–48. [DOI] [PubMed] [Google Scholar]
- 69. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–790. [DOI] [PubMed] [Google Scholar]
- 70. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 71. Cabeen RP, Laidlaw DH, Toga AW. Quantitative imaging toolkit: Software for interactive 3D. Visualization, processing, and analysis of neuroimaging datasets. In: Proceedings of International Society for Magnetic Resonance in Medicine (ISMRM). ISMRM; 2018:abstract #2854.
- 72. Walther S, Lefebvre S, Conring F, et al. Limbic links to paranoia: Increased resting-state functional connectivity between amygdala, hippocampus and orbitofrontal cortex in schizophrenia patients with paranoia. Eur Arch Psychiatry Clin Neurosci. 2021;272:1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ashburner J. Computational anatomy with the SPM software. Magn Reson Imaging. 2009;27:1163–1174. [DOI] [PubMed] [Google Scholar]
- 75. Radwan AM, Sunaert S, Schilling K, et al. An atlas of white matter anatomy, its variability, and reproducibility based on constrained spherical deconvolution of diffusion MRI. Neuroimage. 2022;254:119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tang Y, Zhang T, Edelman B, et al. Prolonged cortical silent period among drug-naive subjects at ultra-high risk of psychosis. Schizophr Res. 2014;160(1–3):124–130. [DOI] [PubMed] [Google Scholar]
- 77. Wobrock T, Schneider M, Kadovic D, et al. Reduced cortical inhibition in first-episode schizophrenia. Schizophr Res. 2008;105(1–3):252–261. [DOI] [PubMed] [Google Scholar]
- 78. Hasan A, Wobrock T, Grefkes C, et al. Deficient inhibitory cortical networks in antipsychotic-naive subjects at risk of developing first-episode psychosis and first-episode schizophrenia patients: A cross-sectional study. Biol Psychiatry. 2012;72:744–751. [DOI] [PubMed] [Google Scholar]
- 79. Walther S, Vladimirova I, Alexaki D, et al. Low physical activity is associated with two hypokinetic motor abnormalities in psychosis. J Psychiatr Res. 2022;146:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hirjak D, Kubera KM, Wolf RC, Northoff G. Going back to kahlbaum's psychomotor (and GABAergic) origins: Is catatonia more than just a motor and dopaminergic syndrome? Schizophr Bull. 2020;46:272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Aron AR. From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69:e55–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kasess CH, Windischberger C, Cunnington R, Lanzenberger R, Pezawas L, Moser E. The suppressive influence of SMA on M1 in motor imagery revealed by fMRI and dynamic causal modeling. Neuroimage. 2008;40:828–837. [DOI] [PubMed] [Google Scholar]
- 83. Mink JW. The basal ganglia: Focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. [DOI] [PubMed] [Google Scholar]
- 84. Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107:8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Houk JC, Wise SP. Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: Their role in planning and controlling action. Cereb Cortex. 1995;5:95–110. [DOI] [PubMed] [Google Scholar]
- 86. Walther S, Heckers S. Mapping psychomotor behavior in the brain. JAMA Psychiatry. 2024;81:7–8. [DOI] [PubMed] [Google Scholar]
- 87. Grillner S, El Manira A. Current principles of motor control, with special reference to vertebrate locomotion. Physiol Rev. 2020;100:271–320. [DOI] [PubMed] [Google Scholar]
- 88. Moberget T, Ivry RB. Prediction, psychosis, and the cerebellum. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:820–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Merel J, Botvinick M, Wayne G. Hierarchical motor control in mammals and machines. Nat Commun. 2019;10:5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bernard JA, Seidler RD, Hassevoort KM, et al. Resting state cortico-cerebellar functional connectivity networks: A comparison of anatomical and self-organizing map approaches. Front Neuroanat. 2012;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Marek S, Siegel JS, Gordon EM, et al. Spatial and temporal organization of the individual human cerebellum. Neuron. 2018;100:977–993.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Walther S, Federspiel A, Horn H, et al. Alterations of white matter integrity related to motor activity in schizophrenia. Neurobiol Dis. 2011;42:276–283. [DOI] [PubMed] [Google Scholar]
- 93. Thakkar KN, Rosler L, Wijnen JP, et al. 7 T proton magnetic resonance spectroscopy of gamma-aminobutyric acid, glutamate, and glutamine reveals altered concentrations in patients with schizophrenia and healthy siblings. Biol Psychiatry. 2017;81:525–535. [DOI] [PubMed] [Google Scholar]
- 94. Rowland LM, Kontson K, West J, et al. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull. 2013;39:1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Stagg CJ. Magnetic resonance spectroscopy as a tool to study the role of GABA in motor-cortical plasticity. Neuroimage. 2014;86:19–27. [DOI] [PubMed] [Google Scholar]
- 96. Cai XL, Wang YM, Wang Y, et al. Neurological soft signs are associated with altered cerebellar-cerebral functional connectivity in schizophrenia. Schizophr Bull. 2021;47:1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cai XL, Pu CC, Zhou SZ, et al. Anterior cingulate glutamate levels associate with functional activation and connectivity during sensory integration in schizophrenia: A multimodal (1)H-MRS and fMRI study. Psychol Med. 2022;53:4904–4914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset presented in this article is not readily available because some participants did not provide consent to data sharing. Requests to access the datasets should be directed to S.L., stephanie.lefebvre@unibe.ch.