Abstract
Cortical myelin loss and repair in multiple sclerosis (MS) have been explored in neuropathological studies, but the impact of these processes on neurodegeneration and the irreversible clinical progression of the disease remains unknown. Here, we evaluated in vivo cortical demyelination and remyelination in a large cohort of people with all clinical phenotypes of MS followed up for 5 years using magnetization transfer imaging (MTI), a technique that has been shown to be sensitive to myelin content changes in the cortex.
We investigated 140 people with MS (37 clinically isolated syndrome, 71 relapsing-MS, 32 progressive-MS), who were clinically assessed at baseline and after 5 years and, along with 84 healthy controls, underwent a 3 T-MRI protocol including MTI at baseline and after 1 year. Changes in cortical volume over the radiological follow-up were computed with a Jacobian integration method. Magnetization transfer ratio was employed to calculate for each patient an index of cortical demyelination at baseline and of dynamic cortical demyelination and remyelination over the follow-up period.
The three indices of cortical myelin content change were heterogeneous across patients but did not significantly differ across clinical phenotypes or treatment groups. Cortical remyelination, which tended to fail in the regions closer to CSF (−11%, P < 0.001), was extensive in half of the cohort and occurred independently of age, disease duration and clinical phenotype. Higher indices of cortical dynamic demyelination (β = 0.23, P = 0.024) and lower indices of cortical remyelination (β = −0.18, P = 0.03) were significantly associated with greater cortical atrophy after 1 year, independently of age and MS phenotype. While the extent of cortical demyelination predicted a higher probability of clinical progression after 5 years in the entire cohort [odds ratio (OR) = 1.2; P = 0.043], the impact of cortical remyelination in reducing the risk of accumulating clinical disability after 5 years was significant only in the subgroup of patients with shorter disease duration and limited extent of demyelination in cortical regions (OR = 0.86, P = 0.015, area under the curve = 0.93). In this subgroup, a 30% increase in cortical remyelination nearly halved the risk of clinical progression at 5 years, independently of clinical relapses.
Overall, our results highlight the critical role of cortical myelin dynamics in the cascade of events leading to neurodegeneration and to the subsequent accumulation of irreversible disability in MS. Our findings suggest that early-stage myelin repair compensating for cortical myelin loss has the potential to prevent neuro-axonal loss and its long-term irreversible clinical consequences in people with MS.
Keywords: multiple sclerosis, remyelination, myelin, imaging, neuroprotection, neurodegeneration
Lazzarotto et al. used advanced MRI to study cortical myelin loss and repair in 140 people with multiple sclerosis followed up for 5 years. They conclude that early-stage cortical myelin repair is effective at preventing the long-term accumulation of irreversible clinical disability in people with the disease.
See Mainero et al. (https://doi.org/10.1093/brain/awae083) for a scientific commentary on this article.
See Mainero et al. (https://doi.org/10.1093/brain/awae083) for a scientific commentary on this article.
Introduction
Multiple sclerosis (MS) is an inflammatory disease of the CNS with inflammatory and degenerative components affecting both the white (WM) and grey (GM) matter. The main neuropathological hallmarks of MS include demyelination, inflammation, astrocytic gliosis and neurodegeneration, with neurodegeneration being considered the primary pathological substrate of clinical progression.1 Among these, demyelination is considered the cornerstone of the pathophysiology of MS, and changes in myelin content over time are believed to have a relevant impact on neurodegeneration throughout the disease course.2-5
Endogenous myelin repair in the CNS is a physiological response to a demyelinating event mediated by a population of adult brain resident cells (oligodendrocytes and oligodendrocyte progenitor cells, OPCs), which migrate into areas of demyelination and actively contribute to remyelination.6 While experimental models often exhibit extensive remyelination, neuropathological studies have shown that myelin repair in white matter lesions is limited or ineffective in most people with MS.7 Furthermore, the extent of remyelination in white matter lesions, which varies greatly among patients but can be substantial in the early stages of the disease in some individuals, tends to decrease as the disease progresses, eventually failing and resulting in chronically demyelinated lesions.6-8
Few neuropathological studies have examined the process of remyelination occurring in the cortex of people with MS. These studies, in addition to confirming that also in cortical regions there is a considerable variation across people with MS in the extent of remyelination, have revealed that myelin repair may be more extensive in the cortex compared with WM and develops independently of age and disease duration.9-12 Although the available neuropathological evidence points to the cortex as a particularly relevant region for exploring remyelination, a comprehensive in vivo description of cortical remyelination and its determinants in people with MS, as well as a full understanding of its impact on neurodegeneration and clinical progression, are currently lacking.
Magnetization transfer ratio (MTR), which is an imaging metric sensitive to myelin content,13 has been shown to be lower in demyelinated cortical lesions than normally myelinated cortex in people with MS14-16 and has been employed successfully to generate patient-specific maps of myelin loss and repair in cortical grey matter in in vivo studies.17,18
In this longitudinal retrospective multicentre study, we generated patient-specific MTR-derived maps of cortical myelin content changes to provide the first comprehensive description of myelin loss and repair at the cortical level in a large cohort of people with MS representing all clinical phenotypes. Our objectives were to identify the key factors influencing the extent of these processes in all MS phenotypes and explore the relevance of cortical demyelination and remyelination in predicting cortical atrophy after 1 year and clinical progression after 5 years.
Materials and methods
Subjects and study design
Overall, 180 patients who met the 2010 diagnostic criteria of MS19 were followed up at four European centres of the MAGNIMS consortium (Graz, Milan, Paris, Siena) and enrolled in this retrospective longitudinal clinico-radiological protocol. People with MS were selected according to the following criteria: (i) no history/evidence of neurologic or psychiatric disorders other than MS; (ii) brain MRI including MT imaging (MTI) at baseline and 1-year follow-up; and (iii) application of the same MRI protocol at baseline and at follow-up. Ninety-two sex- and age-matched healthy controls (HCs), selected according to the following criteria, were also included in the study: (i) no history of neurological or psychiatric disorders; (ii) one good quality MRI including MTI; and (iii) (optional) a second MRI including MTI acquired on the same day as the baseline scan, to be employed as a retest MRI.
All people with MS were clinically assessed using the Expanded Disability Status Scale (EDSS)20 at baseline and after 5 years (mean 61.3 ± 7.1 months). Patients were classified into three groups based on their clinical course as defined by the 2010 diagnostic criteria of MS19: clinically isolated syndrome (CIS), relapsing-remitting (RRMS) and progressive (PMS). Clinical progression at 5 years was defined as an increase of the EDSS score compared to baseline of (i) at least 1.5 points if the baseline EDSS score was equal to 0.0; (ii) at least 1.0 point if the baseline EDSS score ranged between 1.0 and 5.5; or (iii) at least 0.5 point if the baseline EDSS score was greater than 6.0.21
HCs were clinically assessed at baseline and underwent the same imaging protocol as patients at study entry, while a subgroup repeated an identical imaging protocol a second time on the same day (10 HCs from Paris and seven from Siena).
Owing to the presence of motion artefacts or the suboptimal quality of some of the collected MRI scans, a total of 40 people with MS and 8 HCs had their images removed from the study. As a result, the analysis was conducted on good-quality MRI scans from a total of 140 people with MS and 84 HCs (Graz: 54 patients and 38 healthy controls; Paris: 60 patients and 39 healthy controls; Siena: 26 patients and 7 healthy controls, Milan: 0 patients, 0 healthy controls) (Table 1).
Table 1.
Demographic, clinical and magnetic resonance characteristics
| People with MS | CIS | RRMS | PMS | HCs | |
|---|---|---|---|---|---|
| n | 140 | 37 | 71 | 32 | 84 |
| n from each centre | Graz: 54 (38.5%) | Graz: 31 (83.8%) | Graz: 23 (32.4%) | Graz: 0 | Graz: 38 |
| Paris: 60 (42.9%) | Paris: 0 | Paris: 28 (39.4%) | Paris: 32 (100%) | Paris: 39 | |
| Siena: 26 (18.6%) | Siena: 6 (16.2%) | Siena: 20 (28.2%) | Siena: 0 | Siena: 7 | |
| Females/Males | 96/44 | 26/11 | 54/17 | 16/16 | 55/29 |
| Age, years, mean ± SD | 38.2 ± 12.2 | 32.4 ± 8.5 | 35.12 ± 10.44 | 51.44 ± 9.75 | 34.3 ± 10.97 |
| Disease duration at baseline, months, mean ± SD | 64.81 ± 67.21 | 10.15 ± 15.77 | 76.31 ± 65.85 | 102.82 ± 71.01 | – |
| Follow-up, months, mean ± SD | 12.52 ± 5.5 | 11.8 ± 2.46 | 11.7 ± 6.66 | 12.72 ± 3.4 | – |
| EDSS at baseline, median (range) | 2.0 (0.0–7.5) | 1.5 (0.0–3.5) | 2.0 (0.0–6.0) | 6.0 (3.0–7.5) | – |
| EDSS at 5 years, median (range) | 2.0 (0.0–8.0) | 1.0 (0.0–5.5) | 2.0 (0.0–7.5) | 6.0 (3.0–8.0) | – |
| Clinical progression, n progressors/non-progressors (%) | 40/96 (29.4%) | 3/30 (9.1%) | 19/52 (26.7%) | 18/14 (56.2%) | – |
| Number of relapses, median (range) | 0 (0–5) | 0 (0–3) | 0 (0–5) | 0 (0–1) | – |
| Type of treatment, n patients | NT = 85; FL = 41; SL = 14 | NT = 29; FL = 8; SL = 0 | NT = 30; FL = 31; SL = 9 | NT = 25; FL = 2; SL = 5 | – |
| T2 lesion volume, mm3, mean ± SD | 14964 ± 18326.39 | 5480.919 ± 6567.432 | 19875.47 ± 22573.17 | 15183 ± 12206.81 | – |
| Cortical atrophy, mean Jacobian ± SD | 0.002819 ± 0.0097 | 0.0038 ± 0.0082 | 0.00463 ± 0.00986 | −0.0023 ± 0.0093 | – |
CIS = clinically isolated syndrome; EDSS = Expanded Disability Status Scale; FL = treated with first-line treatment; HC = healthy control; NT = not treated; PMS = progressive multiple sclerosis; RRMS = relapsing-remitting multiple sclerosis; SD = standard deviation; SL = treated with second-line treatment.
All subjects gave informed written consent before participation in the study, which was approved by the local ethics committees of each participating centre.
Image acquisition
MRI post-processing
MRI anatomical image processing
In patients, T2-weighted lesions were manually contoured on T2-weighted/fluid attenuated inversion recovery (FLAIR) images at both time points by the same expert neurologist (A.L.) using Jim (v6.0, http://www.xinapse.com/) and transformed into binary masks. T2-weighted/FLAIR images were aligned to the corresponding 3D T1-weighted scans using a rigid registration obtained with FLIRT, part of FSL (http://fsl.fmrib.ox.ac.uk/), and the derived transforms were then employed to register lesion masks onto 3D T1-weighted scans. After ‘lesion-filling’ in patients,22 cortical GM and its parcellation according to the Desikan–Killiany atlas, WM, ventricles and peri-pial CSF (pCSF) were segmented in all subjects at both time points on 3D T1-weighted scans using Freesurfer version 6.0 (https://surfer.nmr.mgh.harvard.edu), then manually corrected (A.L.). For each subject, a relative distance map from the pCSF and from the WM was generated using FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/BIANCA/), applying the following formula [distance from pCSF / (distance from pCSF + distance from WM)].
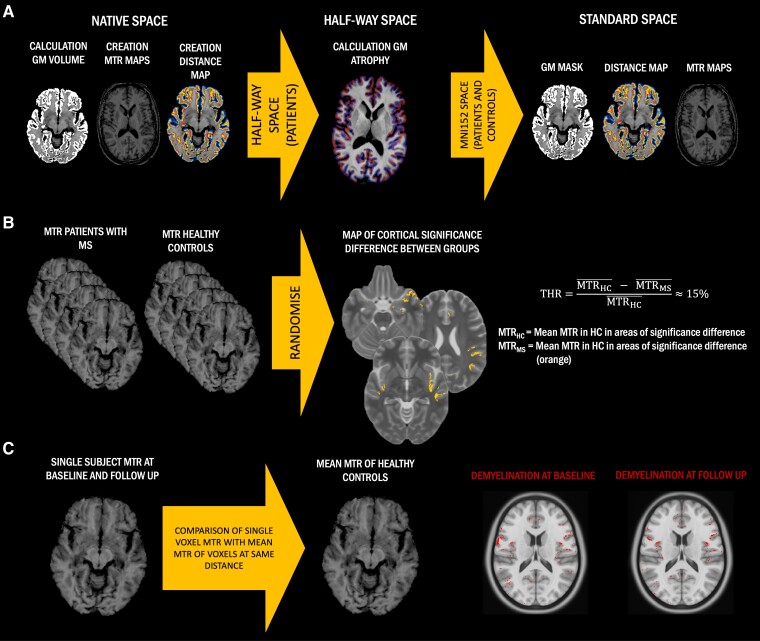
For each patient, the T1-weighted images acquired at the two time points were aligned to each other to create a ‘half-way space’ following the longitudinal image processing of Freesurfer.23 The derived images in ‘half-way space’ in patients, and the baseline T1-weighted images in native space in HCs, were normalized to Montreal Neurological Institute standard space (MNI152) using a non-linear registration with ANTs (http://stnava.github.io/ANTs). Derived transformations were then applied to move all images (including T2-weighted/FLAIR lesion masks, cortical GM masks and distance maps) from native space to MNI152, passing through the half-way space in patients (Fig. 1A).
Figure 1.
Simplified method flow chart. (A) Cross-sectional grey matter (GM) volume, magnetization transfer ratio (MTR) maps and distance maps were created in native space in all subjects and then normalized to standard space, passing through the half-way space in patients. In half-way space, T1-weighted images were used to calculate cortical GM atrophy using the Jacobian integration method. (B) Definition of a threshold (THR) to identify cortical demyelinated voxels in people with multiple sclerosis (pwMS) compared to healthy controls (HCs). Regions of significant group differences between MTR maps of people with MS and HCs were identified through voxel-wise non-parametric permutation-based t-test. For each centre, from these regions of significant difference, mean MTR in people with MS and HCs were extracted and used to calculate a relative percentage difference, which was defined as the threshold for cortical demyelination. (C) Classification of cortical demyelinated voxels in people with MS. We calculated the relative difference between the MTR value of any given cortical voxel in people with MS and the average MTR value of all voxels in HCs localized at the same distance as the given voxel from the external CSF. If this difference was greater than the previously calculated threshold, that cortical voxel was classified as ‘demyelinated’, otherwise as ‘normally myelinated’. As a result, we generated a map of cortical demyelination for each patient at each time point.
Calculation of cortical grey matter volume change over 1 year in patients
Cortical GM masks in the ‘half-way space’ of each time point were used as region of interest (ROI) inputs for the Jacobian integration method to calculate the cortical grey matter volumetric change between the two imaging time points.24 For this purpose, we applied a non-linear registration with ANTs between linearly aligned time points using the 3D T1-weighted images to generate a deformation field describing the local displacement at each voxel that best aligned the two images. We computed the Jacobian of the deformation field at each single voxel, and we then extracted the determinant of the Jacobian, reflecting the magnitude of local volume change at each voxel as a percentage.25 Averaging all the cortical voxels of each patient, we obtained a single value representing the mean magnitude of volume change between the two time points for the whole cortex (Fig. 1A).
Magnetization transfer image processing
Generation of MTR ratio maps
In all subjects at all available time points, MTR maps were generated in native space using the equation MTR = (MToff − MTon) / MToff and rigidly aligned to the corresponding T1-weighted scans using FLIRT (http://fsl.fmrib.ox.ac.uk/). Using the previously derived transformations, MTR maps on T1 space were moved to MNI152, passing through the half-way space in patients (Fig. 1A).
Calculation of centre-specific thresholds for cortical grey matter demyelination at each time point
Regions of significant difference in cortical MTR between patients and HCs of each centre were identified using a voxel-wise permutation-based two-sample t-test, part of the FSL package Randomise (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise) adjusted for age and sex, thresholded at P < 0.05, after threshold-free cluster enhancement correction.18 In these significant regions, we computed the MTR mean relative difference between patients and HCs [(mean MTR in HCs − mean MTR in patients) / mean MTR in HCs], which was employed as the centre-specific threshold (thus accounting for differences among scanners) to classify cortical demyelinated voxels in patients (Fig. 1B). At the end of this step, we obtained three separate thresholds, one for each centre, expressed in the form of percentage of difference in MTR between patients and HCs in cortical regions of significant difference between the two groups.18
Generation of individual maps of demyelination and calculation of indices of myelin content change
For each centre, in each patient at each time point, we then calculated the relative difference between the MTR value of any given cortical voxel and the average MTR value of all voxels in HCs of the same centre localized at the same distance than the given voxel from the pCSF (thus suffering from the same degree of partial volume effect).18 If this difference was greater than the previously calculated centre-specific threshold, that cortical voxel was classified as ‘demyelinated’, otherwise as ‘normally myelinated’. As a result, we generated a binary map of cortical demyelination for each patient at each time point (Fig. 1C).
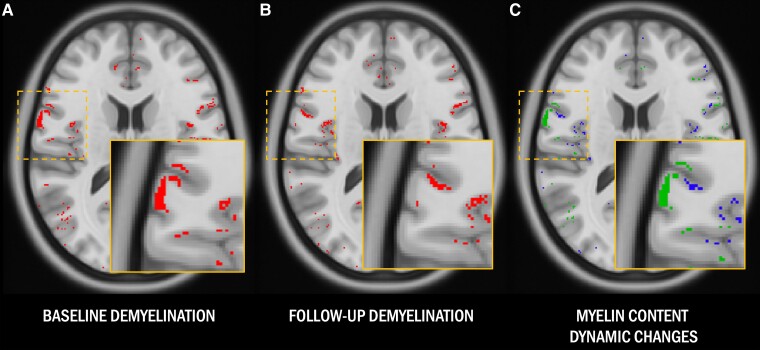
Comparing the patient-specific maps of cortical demyelination at the two time points, we then generated for each patient a map of cortical remyelination and a map of dynamic demyelination over the follow-up period. In particular, each cortical voxel classified as demyelinated at baseline which was then classified as normally myelinated at follow-up was defined as ‘remyelinated’, whereas each cortical voxel classified as normally myelinated at baseline which was then identified as demyelinated at follow-up was considered ‘dynamically demyelinated’ (Fig. 2).
Figure 2.
Magnetization transfer ratio-based myelin content maps. (A and B) Cortical voxels in patients classified as demyelinated compared with healthy controls at baseline and follow-up are highlighted in red. (C) Cortical voxels classified as dynamically demyelinated (i.e. voxels classified as normally myelinated at baseline and demyelinated at follow-up) are highlighted in blue, while cortical voxels classified as remyelinated (i.e. voxels classified as demyelinated at baseline which recovered a normal magnetization transfer ratio signal at follow-up) are displayed in green.
To minimize partial volume effects, only the cortical section of these maps comprised within 25% and 75% of the relative distance between pCSF and WM was retained for further analysis. From the final maps, we extracted three patient-specific indices of cortical myelin content change: (i) the ‘index of cortical demyelination at baseline’, defined as the percentage of cortical voxels classified as demyelinated at baseline over total cortical volume; (ii) the ‘index of cortical dynamic demyelination’, defined as the percentage of cortical voxels classified as normally myelinated at baseline, which were then identified as demyelinated at the second time point over the volume of cortical voxels classified as normally myelinated at baseline; and (iii) the ‘index of cortical remyelination’, defined as the percentage of cortical voxels classified as demyelinated at baseline, which was then identified as normally myelinated at the second time point over the volume of cortical voxels classified as demyelinated at baseline.
Then, in each patient and in each cortical region defined based on the Desikan–Killiany atlas, we calculated a regional index of dynamic cortical demyelination, defined as the number of cortical voxels dynamically demyelinating during the follow-up in each cortical region over the total volume of that specific cortical area, and a regional index of cortical remyelination, defined as the number of cortical voxels remyelinating during the follow-up in each cortical region over the total volume of the voxels of that specific cortical area defined as demyelinated at baseline.
Generation of cortical indices of myelin content change from artificially downsampled MTR images
To investigate the impact of centre-specific MTI resolutions on the cortical indices of myelin content change, we artificially downsampled all the MTI maps of patients and healthy controls from Paris and Graz, which had original resolutions of 1 × 1 × 2 mm3 and 0.86 × 0.86 × 3 mm, respectively, to match the lowest resolution used across the three centres, as seen in the Siena cohort (with a resolution of 1 × 1 × 3 mm3).
Once we downsampled all the MTI maps of patients and healthy controls to a uniform spatial resolution of 1 × 1 × 3 mm3, we replicated the same post-processing steps that were originally applied to the images at their native resolution. Specifically, we generated MTR maps at baseline and at follow-up, calculated centre-specific thresholds for cortical grey matter demyelination at each time point, generated individual cortical demyelination maps for each time point, and finally computed patient-specific ‘downsampled’ indices of cortical demyelination at baseline, as well as cortical dynamic demyelination and cortical remyelination.
Calculation of the indices of myelin content change in the inner and outer bands of the cortex
In each patient, the three individual maps of cortical myelin content change were first overlaid onto the distance map in standard space. Then, using 50% of the relative distance from the pial surface and WM as a parting line on the cortical distance maps, we defined an inner band (closer to the white matter) and an outer band (closer to pCSF) of the cortex. Then, in each patient, we calculated the mean index of cortical demyelination at baseline, dynamic demyelination and remyelination in each of these two bands.
White matter T2-weighted lesion maps of demyelination and white matter indices of myelin content change
Following a previously published method,26 we calculated a centre-specific threshold for WM T2-weighted lesion demyelination based on the relative per cent difference between the mean MTR of white matter lesions in people with MS and the mean MTR of the white matter of healthy controls. Then we compared the MTR of each given voxel in T2-weighted lesions in patients with the mean MTR of all the voxels of healthy controls localized at the same distance from ventricular CSF (therefore potentially affected to the same extent by partial volume). If the difference between these two values was greater than the previously calculated centre-specific threshold, that given voxel was classified as demyelinated.
In each patient with MS, three individual maps of T2-weighted lesion myelin content change were generated: (i) an individual map of T2-weighted lesion demyelinated voxels at baseline; (ii) an individual map of T2-weighted lesion demyelinating voxels at follow-up, defined as lesional voxels classified as normally myelinated at baseline and as demyelinated at follow-up; and (iii) an individual map of T2-weighted lesion remyelinating voxels at follow-up, defined as lesional voxels classified as demyelinated at baseline and as normally myelinated at follow-up.
From these three maps, we extracted the indices of T2-weighted lesion demyelination at baseline, dynamic demyelination and remyelination, defined as follows: (i) ‘index of T2-weighted lesion demyelination at baseline’, defined as the percentage of demyelinated T2-weighted lesion voxels at baseline over total T2-weighted lesion volume; (ii) ‘index of T2-weighted lesion dynamic demyelination’, defined as the percentage of demyelinated T2-weighted lesion voxels dynamically demyelinating over the follow-up over total T2-weighted lesion volume; and (iii) ‘index of T2-weighted lesion remyelination’, defined as the percentage of demyelinated T2-weighted lesion voxels remyelinating over the follow-up over total T2-weighted lesion volume.
Evaluation of misclassification errors
To cross-validate the classification of each voxel, we performed a leave-one-out test on HCs for whom a baseline and a follow-up MT acquisition were available as a test-retest.
Each healthy control at each time point (test and retest) was compared to the other healthy subjects of the same centre to create two maps of ‘cortical demyelination’, the first generated from the test scan and the second from the re-test scan, following the same procedure employed in patients. From the comparison of these maps, we generated a map of ‘pseudo-cortical dynamic demyelination’ and of ‘pseudo-cortical remyelination’ from which we extracted three fictitious indices of cortical myelin content change which reflected the misclassification error in the three corresponding indices in people with MS.
Statistical analysis
Statistical analysis was performed using R version 4.0.2 (https://www.r-project.org/). A two-sided P-value <0.05 was considered significant. Corrections for multiple comparisons were performed using false discovery rate.
To investigate the impact of centre-specific MTR resolutions on the cortical indices of myelin content change, we assessed the correlation between the three indices of cortical myelin content change calculated from the native resolution images and the corresponding indices calculated from the downsampled images (referred to as the ‘downsampled’ indices of cortical demyelination at baseline, cortical dynamic demyelination, and cortical remyelination) using the Pearson’s correlation coefficient.
Using one-way ANOVA and Tukey’s honest significance test, we investigated which cortical regions showed significantly higher regional indices of dynamic cortical demyelination and remyelination compared with the others.
Linear regression models, adjusted for centre and time between scans, were employed to estimate the differences in the three indices of cortical myelin content change and cortical atrophy across clinical phenotypes and among treatment-based patient subgroups. These models were also employed to investigate the associations between the three indices of cortical myelin content change.
The association between age, disease duration, T2-weighted lesion volume at baseline, T2-weighted lesion volume change over the follow-up, the indices of T2-weighted lesion myelin content change and the three indices of cortical myelin content change was assessed using linear regression models adjusted for centre and time between scans.
Differences in the percentage of cortical voxels demyelinated at baseline, dynamically demyelinated and remyelinated over the follow-up between the outer and the inner bands of the cortex were investigated with a Wilcoxon signed-rank test.
To estimate the association between the three indices of cortical myelin content change and cortical atrophy at 1 year, a linear regression model adjusted for centre, time between scans, sex, age, disease duration, MS clinical phenotypes, number of relapses during the 5-year follow-up and T2-weighted lesions volume was employed.
To assess the association between the indices of cortical demyelination at baseline, dynamic demyelination and remyelination and clinical progression over 5 years, we performed a logistic regression model including the three indices of myelin content change as independent variables, the patient classification in ‘clinically stable’ or ‘clinically progressing’ at 5 years as dependent variable, and centre, time between scans, sex, age, disease duration, MS clinical phenotype, number of relapses during the 5-year follow-up, cortical atrophy at 1 year and T2-weighted lesions volume as confounding factors. To test the hypothesis of a combined modulating effect of the extent of cortical demyelination at baseline and disease duration on the impact of cortical remyelination on clinical progression, we ran an additional logistic regression model, including the interaction term between disease duration, the index of cortical demyelination at baseline and the index of cortical remyelination as independent variable.
Beta coefficients and odds ratios (OR) are reported along with their P-values for each predictor, as appropriate. Adjusted-R2 and areas under the curve (AUC) are reported as an estimation of the quality of the prediction for linear and logistic models, respectively. Likelihood ratio tests (LRT) were applied to test the difference between logistic regression models with and without the interaction term.
Results
Of the 140 people with MS included in this study, 37 presented with a CIS, 71 with RRMS and 32 with PMS (5 secondary progressive MS, 27 primary progressive MS). During the follow-up, 12 CIS patients evolved to RRMS. At study entry, 41 were treated with a first-line disease-modifying treatment (DMT) (8 CIS, 31 RRMS, 2 PMS) and 14 with a second-line DMT (9 RRMS, 5 PMS). During the 5-year follow-up, 26 people with MS initiated a first-line DMT (10 CIS and 16 RRMS) and 3 switched from a first-line to a second-line DMT (3 CIS, who evolved to RRMS).
Forty patients experienced clinical progression over 5 years (3 CIS, 19 RRMS and 18 PMS), 96 were clinically stable (30 CIS, 52 RRMS and 14 PMS) and for 4 patients, clinical data at 5-year follow-up were not available.
Thirty-nine people with MS (8 CIS, 29 RRMS and 2 PMS) experienced at least one clinical relapse during the 5-year follow-up. Among the 40 people with MS presenting a clinical progression over the 5-year follow-up, 16 experienced at least one clinical relapse during the same interval (see Table 1 for further demographic characteristics).
The mean MTR values in cortical areas showing significant group differences between patients and healthy controls were 34.7% units (pu), 27.2 pu and 24.7 pu in patients from Paris, Graz and Siena, respectively, and 40.4 pu, 32.0 pu and 29.0 pu in healthy controls from Paris, Graz and Siena, respectively. This resulted in mean per cent differences of 14.5%, 14.1% and 13.9%. To account for variations between centres and maintain a conservative approach, we rounded the threshold to 15%.
A misclassification error < 1% was obtained from the leave-one-out test performed on HCs in both test-retest cohorts of Paris and Siena. Out of all cortical voxels, only 0.47% and 0.52% were misclassified as demyelinated at baseline, 0.21% and 0.23% as dynamically demyelinated and 0.30% and 0.38% as remyelinated in the Paris and Siena cohorts of HCs, respectively.
We observed significant correlations between the indices of cortical demyelination at baseline, cortical dynamic demyelination and cortical remyelination calculated on the downsampled images of the Paris and the Graz cohorts and the corresponding indices calculated from the images at their native resolution: index of cortical demyelination at baseline: Pearson’s rho = 0.96, P ≤ 2×10−16; index of cortical dynamic demyelination: Pearson’s rho = 0.98, P ≤ 2×10−16; index of cortical remyelination: Pearson’s rho = 0.94, P ≤ 2×10−16.
Heterogenous cortical myelin content changes but extensive cortical remyelination
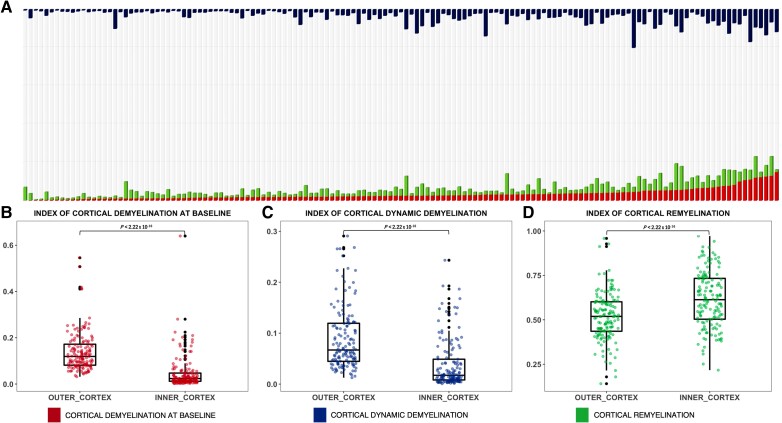
In patients, the indices of cortical myelin content change were characterized by a wide inter-subject variability, with the percentage of cortical demyelinated voxels at baseline ranging from 0.4% to 20% of total cortical voxels, the percentage of dynamically demyelinated voxels ranging from 0.3% to 20% of normally myelinated voxels at baseline and remyelinated voxels ranging from 14% to 95% of demyelinated cortical voxels at baseline (Fig. 3A). Considering separately each of the MS clinical phenotypes, we confirmed the same heterogeneity: in CIS from 1% to 14% (demyelination at baseline), from 0.4% to 9% (dynamic demyelination) and from 20% to 75% (remyelination); in RRMS from 0.7% to 20% (demyelination at baseline), from 0.3% to 20% (dynamic demyelination) and from 14% to 95% (remyelination); in PMS from 0.4% to 14% (demyelination at baseline), from 0.4% to 16% (dynamic demyelination) and from 26% to 89% (remyelination).
Figure 3.
The three indices of cortical myelin content change are largely heterogeneous across patients and affected by the distance from CSF. (A) Cortical myelin content change is represented by a bar plot in which each bar represents the cortex of a single patient: cortical voxels classified as demyelinated at baseline and follow-up are highlighted in red; cortical voxels classified as remyelinated are in green; and cortical voxels classified as dynamically demyelinated are in blue. Bars are sorted based on demyelination at baseline. B–D show the differences between the inner and outer cortex in the extent of cortical demyelination (B), dynamic demyelination (C) and remyelination (D).
Despite this heterogeneity, we found that in half of our cohort, cortical demyelination at baseline and dynamic demyelination affected more than 5% and 2.9% of the cortex, respectively. Moreover, we found that in half of our cohort cortical remyelination was extensive, with myelin repair between the two time points occurring in more than half of the demyelinated cortical areas at baseline (median value for the index of cortical remyelination = 50%).
A significantly greater extent of cortical dynamic demyelination over the follow-up in comparison with all the other cortical areas was found in frontal and temporal regions (P < 0.05), specifically in pars orbitalis, frontal pole, lateral orbito-frontal cortex, precentral cortex, rostral-middle frontal cortex, superior frontal cortex, entorhinal cortex, rostral-anterior cingulate, middle temporal areas and temporal pole. We also found a significantly greater extent of cortical remyelination in frontal and temporal regions (P < 0.05), specifically in lateral and medial orbito-frontal cortex, pars opercularis and pars orbitalis, fusiform cortex, middle and inferior temporal cortex and temporal pole.
There were no significant differences found in the index of cortical demyelination at baseline, the index of dynamic cortical demyelination, the index of cortical remyelination or in terms of cortical atrophy across MS clinical phenotypes, nor across treatment at baseline and follow-up or between individuals with MS who did or did not undergo a change in DMT during the 1-year follow-up (P > 0.05).
Cortical remyelination occurs independently of age and disease duration in multiple sclerosis
A greater extent of cortical demyelination at baseline was associated with a greater extent of dynamic demyelination over the follow-up (β = 0.53, P = 6.09 × 10−10), while no significant association was found between the indices of cortical demyelination (at baseline and dynamic) and the index of cortical remyelination (P = 0.32 and P = 0.93, respectively).
While none of the three indices of cortical myelin content change was associated with age (P > 0.34), we found that longer disease duration was significantly associated with a greater amount of demyelination at baseline (β = 0.52, P = 2.08 × 10−7) and with a greater amount of dynamic demyelination over the follow-up (β = 0.34, P = 0.002). The index of cortical remyelination was not associated with disease duration (β = −0.08, P = 0.33).
A greater T2-weighted lesions volume was associated with a greater extent of cortical demyelination at baseline (β = 0.18, P = 0.03), a greater extent of dynamic demyelination (β = 0.17, P = 0.02) and with a lower extent of cortical remyelination (β = −0.03, P = 0.004). No association was found between the three indices of cortical myelin content change and T2-weighted lesions volume change over 1 year (P > 0.09).
Cortical and T2-weighted lesions myelin content changes are associated and complementary processes
A greater extent of demyelination at baseline and dynamic demyelination over the follow-up in cortical regions was associated with a greater extent of demyelination at baseline and dynamic demyelination over the follow-up occurring in T2-weighted lesions (demyelination at baseline: β = 0.28, P < 0.001, R2 = 0.46, P < 0.001; dynamic demyelination over the follow-up: β = 0.40, P < 0.001, R2 = 0.59, P < 0.001). A greater extent of remyelination in cortical regions was associated with a greater extent of remyelination in T2-weighted lesions (β = 0.43, P < 0.001, R2 = 0.21, P < 0.001).
In regions closer to peri-pial CSF, demyelination is severe and remyelination fails
In the outer cortical band compared to the inner cortical band, we found a significantly higher index of cortical demyelination at baseline (+10%, P < 0.001) and of cortical dynamic demyelination (+6%, P < 0.001) and a significantly lower index of cortical remyelination (−11%, P < 0.001) (Fig. 3B–D).
The extent of changes in cortical myelin content predicts cortical atrophy development over 1 year
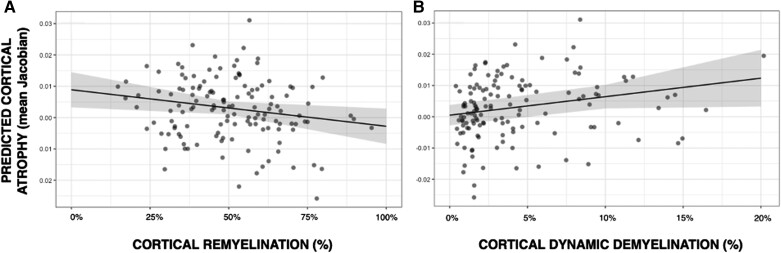
A higher index of dynamic demyelination (β=0.23, P = 0.024) and a lower index of cortical remyelination (β=−0.18, P = 0.03) were significantly associated with greater cortical atrophy development over 1 year, independently of baseline demyelination (P = 0.14), sex (P = 0.25), age (P = 0.10), disease duration (P = 0.67), MS clinical phenotype (P = 0.13), number of relapses during the 5-year follow-up (P = 0.59) and T2-weighted lesions volume (P = 0.52). The whole model explained 14% of the GM atrophy variance (adjusted-R2 = 0.142, P = 0.0015) (Fig. 4).
Figure 4.
Association between the indices of cortical myelin content change and cortical atrophy development over 1 year. (A) Predicted cortical grey matter (GM) atrophy according to the extent of cortical remyelination: a greater extent of cortical remyelination predicts lower cortical atrophy over 1 year. (B) Predicted cortical GM atrophy according to the extent of cortical dynamic demyelination: a greater extent of cortical dynamic demyelination predicts greater cortical atrophy over 1 year.
Cortical demyelination at baseline predicts clinical progression at 5 years in multiple sclerosis
A greater index of cortical demyelination at baseline (OR = 1.2; P = 0.043), the MS phenotype (PMS: OR = 4.25, P = 0.002) and the number of relapses (OR = 1.9; P = 0.026) were significantly associated with a greater probability of clinical progression at 5 years independently of the index of cortical dynamic demyelination (OR = 1.05, P = 0.27), the index of remyelination (OR = 0.9, P = 0.64), sex, disease duration, T2-weighted lesions volume and cortical atrophy developing over 1 year (P > 0.31) (AUC of the model = 0.77).
Early cortical remyelination is associated with a lower probability of clinical progression
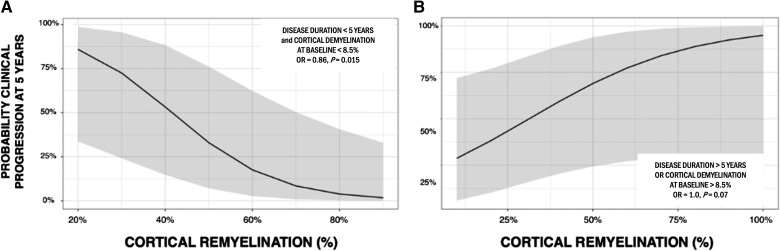
While no association was found between the index of cortical remyelination and clinical progression at 5 years in the whole patient cohort (P = 0.64), the interaction term between cortical remyelination, the index of cortical demyelination at baseline and disease duration significantly predicted long-term clinical progression (P = 0.03, AUC of the model = 0.82; difference between the models with and without interaction: LRT P = 0.033). In other words, a greater extent of cortical remyelination was associated with a lower probability of clinical progression at 5 years only in the subgroup of people with MS with a limited amount of cortical demyelination at baseline and a shorter disease duration. To test the predictive value of the index of cortical remyelination on clinical progression, using the mean values as thresholds, we defined a subgroup of people with MS presenting an extent of cortical demyelination at baseline lower than 8.5% and a disease duration shorter than 5 years. In this subgroup (n = 57, 23 CIS, 24 RRMS, 10 PMS), the failure of cortical remyelination significantly contributed to clinical progression at 5 years (OR = 0.86, P = 0.015), independently of the extent of cortical demyelination (at baseline and over the follow-up), sex, age, MS clinical phenotype, clinical relapses over the follow-up, T2-weighted lesions volume and GM atrophy developing over 1 year (P > 0.14). In these patients, according to the model, an increase of 30% in the index of cortical remyelination reduced by 47% the risk of clinical progression at 5 years (model AUC 0.93; sensitivity 80%; specificity 92%) (Fig. 5A). Conversely, in people with MS with more than 8.5% of cortical voxels classified as demyelinated at baseline or a disease duration longer than 5 years, no significant predictive value of cortical remyelination on clinical progression was found (P > 0.05) (Fig. 5B).
Figure 5.
Association between the index of cortical remyelination and the probability of clinical progression after 5 years. (A) Predicted probabilities of clinical progression in the multiple sclerosis (MS) cohort characterized by a limited extent of cortical demyelination at baseline and a disease duration shorter than 5 years. (B) Predicted probabilities of clinical progression in the MS cohort characterized by an extent of cortical demyelination at baseline greater than 8.5% or a disease duration longer than 5 years.
Discussion
In this multicentre study, we generated patient-specific maps of cortical myelin content change based on MTI in a large population of patients with all clinical phenotypes of MS to explore the key determinants of myelin loss and repair at the cortical level and to investigate the role of these processes in neurodegeneration and clinical progression. Our findings indicate that cortical myelin loss increases significantly as the disease duration progresses, but we also discovered that extensive spontaneous cortical myelin repair occurs in half of people with MS and in the majority of demyelinated cortical areas, regardless of age or disease duration. Furthermore, we established that cortical demyelination is a predictive factor for short-term cortical atrophy and long-term clinical progression in all people with MS. Importantly, our research demonstrated that cortical remyelination, which often fails in the regions closer to pCSF, effectively prevents cortical volume loss and long-term clinical disability in patients at early stages of the disease and with a limited extent of myelin loss affecting cortical regions.
We demonstrated that cortical myelin loss and repair are heterogeneous across all clinical MS phenotypes, confirming the variable extent of cortical demyelination and compensatory mechanisms of myelin repair previously observed in post-mortem MS brains and smaller-scale MTR studies.10,17,18 Our findings also align with previous evidence from quantitative ultra-high field MRI studies, which have revealed a heterogeneous extent of myelin loss and repair across cortical lesions in MS, since the earliest stages of the disease.27 Despite the wide across-patient heterogeneity in the extent of myelin content changes, our findings provide evidence that spontaneous cortical myelin repair is a generally extensive process in people with MS, regardless of their clinical phenotype. Specifically, we showed that at least 50% of demyelinated cortical areas were effectively repaired in half of the patients of our cohort. This is the first in vivo confirmation of the neuropathological and experimental evidence that indicates an extensive process of myelin repair occurring in cortical regions, consistently observed in post-mortem MS brains and animal models of this disease.10-12 Importantly, our data demonstrate that the extent of cortical remyelination in people with MS is not influenced by chronological age, disease duration, or clinical phenotype. This finding further validates in vivo the neuropathological evidence that people with MS retain a substantial potential for cortical remyelination throughout their life and disease course.10-12 This specific propensity of demyelinated cortical regions to undergo remyelination may be associated with the relatively preserved density of OPCs in cortical lesions, combined with minimal astrogliosis and low expression of extracellular molecular matrix molecules that inhibit OPCs differentiation and myelin repair in cortical areas.11,12 Interestingly, we observed that the indices of myelin loss and repair in the cortex and the T2-weighted lesions of patients with MS are significantly associated with each other, yet the strength of this association appears limited. Consequently, while the processes of demyelination and remyelination in these two brain areas appear to be associated, their interdependence seems limited, suggesting that these processes likely have complementary roles in the physiopathology of the disease.18 As far as the regional distribution of these processes is concerned, we found that the regional indices of dynamic cortical demyelination and remyelination were significantly higher in frontal and in temporal regions. While our findings regarding the spatial distribution of dynamic cortical demyelination align with previous evidence indicating a higher number of cortical lesions in these specific cortical regions,28,29 the significantly greater extent of remyelination observed in these same areas may reveal for the first time a more efficient remyelination process occurring in these regions. However, it is equally plausible that this increased level of remyelination is only detectable in cortical regions undergoing a more extensive demyelination process.
We demonstrated that the processes of demyelination and remyelination at the cortical level are consistently modulated by the distance from the CSF. Specifically, the outer band of the cortical ribbon displayed a more pronounced extent of demyelination and a lower extent of remyelination compared with the rest of the cortex. One possible explanation for the increase in myelin loss and failure of myelin repair in cortical regions near the CSF is linked to the pathogenic role of chronic meningeal inflammation and CSF inflammatory profiles in causing cortical damage in MS.30-33 Specifically, post-mortem studies of MS cases with high levels of meningeal inflammation and cortical demyelination revealed profound CSF inflammatory changes characterized by B-cell-linked inflammatory mediators such as CXCL13, CXCL10, LTα, interleukin 6 and 10, as well as proinflammatory cytokines such as interferon-γ, tumor necrosis factor and interleukin 2.34,35 These inflammatory mediators, possibly in combination with other cyto- and/or myelinotoxic soluble CSF factors,36 are believed to diffuse towards the outer cortical regions, inducing and sustaining a ‘surface-in’ direct and/or microglia-mediated pattern of cortical damage, consisting of demyelination, chronic inflammation31,35,36 and failing myelin repair.37,38 Supporting this CSF-centred hypothesis, multiple lines of evidence have demonstrated a similar ‘surface-in’ gradient of chronic demyelination,39 failed myelin repair, and microglia activation in periventricular regions, chronically exposed to CSF inflammatory changes.39,40 Notably, this periventricular gradient of tissue damage is closely associated with the size and degree of inflammation of the choroid plexus, which is part of the blood–CSF–brain barrier and plays a crucial role in regulating CNS immunosurveillance.41,42
Our results indicate that all myelin dynamics in cortical regions play a crucial role in the cascade of events leading to neurodegeneration. Specifically, we observed that, in all clinical phenotypes of MS, an increase in the extent of cortical demyelination and a failure in cortical remyelination are both associated with greater cortical atrophy at 1 year. Importantly, this relationship remained consistent regardless of age, disease duration, baseline extent of cortical demyelination, and WM T2-weighted lesion volume. This finding suggests that, throughout the course of the disease, the irreversible loss of neurons at the cortical level may result from the combination of a persistent demyelinating environment and an insufficient compensatory process of myelin repair in cortical regions.2,43 Moreover, these findings align with previous evidence from experimental studies, indicating that remyelination plays a critical protective role for neurons, prolonging the survival and preventing both anterograde and retrograde degeneration of previously demyelinated axons.4,44,45
Our clinical results further support the hypothesis that neurodegeneration is closely associated with the accumulation of cortical demyelination, but also shed light on the potential and limitations of cortical remyelination in preventing the accumulation of clinical disability. Specifically, we observed that the extent of cortical demyelination measured at baseline significantly predicted the probability of clinical progression after 5 years in the entire cohort. Conversely, our data revealed that cortical myelin repair was effective in reducing the probability of clinical progression after 5 years across all MS phenotypes, but this benefit was limited to patients with a shorter disease duration and a limited extent of cortical demyelination. This evidence suggests that, while cortical remyelination can occur at any stage of the disease, including advanced stages, its ability to prevent the progressive accumulation of clinical disability is limited by long disease duration and extensive cortical demyelination. In other terms, during the early years of the disease course, when the extent of cortical demyelination is still limited, the process of cortical myelin repair appears highly effective in compensating for myelin loss, protecting neurons from neurodegeneration and, consequently, preventing the long-term accumulation of clinical disability, irrespective of the clinical phenotype. However, as the disease progresses and the cortex experiences extensive demyelination, the process of myelin repair appears to lose its effectiveness in providing adequate protection to the axons, which then degenerate over the years leading to the irreversible accrual of clinical disability.
Some limitations may challenge the interpretation of our results, with the primary concern being the variability in MTR resolution across centres and the generally suboptimal resolution of MT images. Although we showed that artificially modifying the resolution of MTR scans did not significantly impact the calculation of cortical myelin content change indices, we cannot rule out the possibility that the suboptimal resolution of MTR maps may have partially affected our measurement of cortical myelin content changes. Another critical factor to consider when interpreting our results is the limited specificity of MTR in capturing changes in myelin content. In particular, the evidence from MRI-pathological studies suggesting myelin as the most significant correlate of MTR, does not inherently imply that MTR can be used as a reliable biomarker for assessing myelin content changes, and in particular myelin repair.16 MTR changes may not exclusively measure alterations in myelin content, but also reflect other processes, including inflammation, oedema, neuronal loss, mitochondrial damage and pathological changes in iron content as suggested by previous neuropathological evidence.14,16 Consequently, the MTR changes we interpret as suggestive of cortical demyelination and remyelination could potentially arise from other pathological processes, particularly from the increase and resolution of oedema and innate immune cell inflammation, which can be extensive in the cortical regions of people with MS.46
However, we are confident that the positive changes in cortical MTR values we have measured in our patients for the most part reflect myelin repair, since axonal damage and loss, which may also affect the MTR signal at the cortical level14 do not spontaneously recover in MS, and since the amount of inflammation is typically less pronounced at the cortical level compared to the white matter in this disease.47 Although our methods to measure remyelination have not been validated against pathology, the reproducibility of our results in multicentre data and the limited number of misclassification errors in generating the maps of cortical myelin content changes support the robustness of our findings. Another relevant limitation of this study is the unavailability of sequences to measure the extent and localization of focal cortical lesions, which are the cortical regions showing a particularly strong association between MTR values and myelin content in MS.16 Other limitations include the lack of specific sequences to assess the extent and the impact of partial remyelination, which is a common finding in histopathological studies in MS brains,12,48 and to quantify chronic inflammation.40 Future studies using quantitative ultra-high field MRI techniques, which previously allowed measuring myelin content changes in the cortex of individuals with MS with the best possible resolution and correlating these changes with radiological, clinical, and cognitive scores, have the potential to address several of these limitations and to open new perspectives in the in vivo exploration of cortical changes in MS.27,49-52 Finally, our investigation of the relationship between repair failure and neurodegeneration would have been significantly improved by the measure of other biomarkers of neurodegeneration such as blood neurofilament levels53 and by the detection of individual patterns of CSF-derived inflammatory mediators,32 which were not available in this cohort.
In conclusion, our study highlights the critical role of cortical myelin dynamics in the process of neurodegeneration and clinical progression in MS. We have demonstrated that effective myelin repair during the early stages of the disease may significantly reduce the probability of developing short-term cortical atrophy and clinical progression at 5 years. Therefore, our results suggest that therapeutic interventions aimed at preventing the accumulation of demyelination (such as disease-modifying therapies) together with emerging treatments aimed at promoting remyelination early in the course of the disease may be most effective in preventing long-term clinical progression in MS. This approach may have a transformative impact on the evolution of the disease in the long-term, by effectively preventing neurodegeneration and its irreversible clinical consequences. Furthermore, our results indicate that in future clinical trials investigating novel promyelinating therapies, the use of individual profiles of cortical myelin repair based on MTR should be considered as outcome measures and for patient stratification. This approach could maximize the likelihood of identifying effective treatments to enhance myelin repair and improve outcomes for people with MS in the near future.
Acknowledgements
Authors are members of the MAGNIMS network (Magnetic Resonance Imaging in MS; https://www.magnims.eu/), which is a group of European clinicians and scientists with an interest in undertaking collaborative studies using MRI methods in multiple sclerosis, independent of any other organization and is run by a steering committee whose members are F. Barkhof (Amsterdam), N. de Stefano (Siena), J. Sastre-Garriga (Barcelona, Co-Chair), O. Ciccarelli (London), C. Enzinger (Graz), M. Filippi (Milan), Claudio Gasperini (Rome), L. Kappos (Basel), J. Palace (Oxford), H. Vrenken (Amsterdam), À. Rovira (Barcelona), M.A. Rocca (Milan, Co-Chair) and T. Yousry (London).
Contributor Information
Andrea Lazzarotto, Department of Neuroscience, Sorbonne Université, Paris Brain Institute, CNRS, Inserm, 75013 Paris, France; AP-HP, Hôpital Universitaire Pitié-Salpêtrière, 75013 Paris, France; Padova Neuroscience Center, University of Padua, 35122 Padua, Italy.
Mariem Hamzaoui, Department of Neuroscience, Sorbonne Université, Paris Brain Institute, CNRS, Inserm, 75013 Paris, France.
Matteo Tonietto, Université Paris-Saclay, CEA, CNRS, Inserm, BioMaps, Service Hospitalier Frédéric Joliot, 91400 Orsay, France; Roche Pharma Research & Early Development, F. Hoffmann-La Roche Ltd., CH-4070 Basel, Switzerland.
Anne-Laure Dubessy, AP-HP, Hôpital Universitaire Pitié-Salpêtrière, 75013 Paris, France.
Michael Khalil, Department of Neurology, Medical University of Graz, 8036 Graz, Austria.
Lukas Pirpamer, Department of Neurology, Medical University of Graz, 8036 Graz, Austria; Medical Image Analysis Center (MIAC) and Department of Biomedical Engineering, University of Basel, CH-4051 Basel, Switzerland.
Stefan Ropele, Department of Neurology, Medical University of Graz, 8036 Graz, Austria.
Christian Enzinger, Department of Neurology, Medical University of Graz, 8036 Graz, Austria.
Marco Battaglini, Department of Medicine, Surgery and Neuroscience, University of Siena, 53100 Siena, Italy.
Maria Laura Stromillo, Department of Medicine, Surgery and Neuroscience, University of Siena, 53100 Siena, Italy.
Nicola De Stefano, Department of Medicine, Surgery and Neuroscience, University of Siena, 53100 Siena, Italy.
Massimo Filippi, Neuroimaging Research Unit, Institute of Experimental Neurology, Division of Neuroscience, Neurology Unit, IRCCS San Raffaele Scientific Institute, 20132 Milan, Italy; Vita-Salute San Raffaele University, 20132 Milan, Italy.
Maria Assunta Rocca, Neuroimaging Research Unit, Institute of Experimental Neurology, Division of Neuroscience, Neurology Unit, IRCCS San Raffaele Scientific Institute, 20132 Milan, Italy; Vita-Salute San Raffaele University, 20132 Milan, Italy.
Paolo Gallo, Padova Neuroscience Center, University of Padua, 35122 Padua, Italy; Multiple Sclerosis Centre of Veneto Region, 35128 Padua, Italy.
Claudio Gasperini, San Camillo-Forlanini Hospital, 00152 Rome, Italy.
Bruno Stankoff, Department of Neuroscience, Sorbonne Université, Paris Brain Institute, CNRS, Inserm, 75013 Paris, France; AP-HP, Hôpital Universitaire Pitié-Salpêtrière, 75013 Paris, France.
Benedetta Bodini, Department of Neuroscience, Sorbonne Université, Paris Brain Institute, CNRS, Inserm, 75013 Paris, France; AP-HP, Hôpital Universitaire Pitié-Salpêtrière, 75013 Paris, France.
the MAGNIMS Study Group:
F Barkhof, N de Stefano, J Sastre-Garriga, O Ciccarelli, C Enzinger, M Filippi, Claudio Gasperini, L Kappos, J Palace, H Vrenken, À Rovira, M A Rocca, and T Yousry
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Funding
A.L. was supported by Università Italo-Francese (Vinci Fellowship no. C2-111) for jointly supervised PhD thesis. B.B. was supported by ANR MNP2008-007125. Additional funding was obtained from Fondation pour l'aide à la recherche sur la sclérose en plaques, and Fondation pour la Recherche Médicale. Assistance Publique des Hôpitaux de Paris sponsored the study (Paris Cohort).
Competing interests
A.L. has received funding for travel and speaker honoraria from Merck Serono, Roche, Novartis and Sandoz. None related to the present work. M.H. has nothing to disclose. M.T. is an employee of and owns stocks or stock options in F. Hoffmann-La Roche Ltd. A.-L.D. has nothing to disclose. M.K. has received speaker honoraria from Bayer, Novartis, Merck, Biogen Idec and Teva Pharmaceutical Industries Ltd. and serves on scientific advisory boards for Biogen Idec, Merck Serono, Roche, Novartis, Bristol-Myers Squibb and Gilead. He received research grants from Biogen and Novartis. L.P. has nothing to disclose. S.R. has received honoraria from Axon Neuroscience, QPS, and NeuroScios for consulting services. C.E. has received funding for travel and speaker honoraria from Biogen, Bayer Schering, Merck Serono, Novartis, Shire, Genzyme and Teva Pharmaceutical Industries Ltd., Sanofi-Aventis; research support from Merck Serono, Biogen, and Teva Pharmaceutical Industries Ltd./Sanofi-Aventis; and has served on scientific advisory boards for Bayer Schering, Biogen, Celgene, Merck Serono, Novartis, Roche and Teva Pharmaceutical Industries Ltd./Sanofi-Aventis. M.B. has nothing to disclose. M.L.S. has nothing to disclose. N.D. has received honoraria from Biogen-Idec, Bristol Myers Squibb, Celgene, Genzyme, Immunic, Merck Serono, Novartis, Roche and Teva for consulting services, speaking and travel support. He serves on advisory boards for Merck, Novartis, Biogen-Idec, Roche and Genzyme, Immunic and he has received research grant support from the Italian MS Society. M.F. is Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Neurological Sciences, and Radiology; received compensation for consulting services from Alexion, Almirall, Biogen, Merck, Novartis, Roche, Sanofi; speaking activities from Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, Janssen, Merck-Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and TEVA; participation in Advisory Boards for Alexion, Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Genzyme, Takeda; scientific direction of educational events for Biogen, Merck, Roche, Celgene, Bristol-Myers Squibb, Lilly, Novartis, Sanofi-Genzyme; he receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Italian Ministry of Health, and Fondazione Italiana Sclerosi Multipla. M.A.R. received consulting fees from Biogen, Bristol Myers Squibb, Eli Lilly, Janssen, Roche; and speaker honoraria from AstraZaneca, Biogen, Bristol Myers Squibb, Bromatech, Celgene, Genzyme, Horizon Therapeutics Italy, Merck Serono SpA, Novartis, Roche, Sanofi and Teva. She receives research support from the MS Society of Canada, the Italian Ministry of Health, and Fondazione Italiana Sclerosi Multipla. She is Associate Editor for Multiple Sclerosis and Related Disorders. P.G. has received grants and personal fees from Merck Serono, Biogen Idec, Genzyme Sanofi, grants and personal fees from Novartis, grants from University of Padua, Department of Neurosciences DNS, grants from Veneto Region of Italy, grants from Italian Association for Multiple Sclerosis (AISM), grants from Italian Ministry of Public Health, during the conduct of the study. C.G. has received fees as invited speaker or travel expenses for attending meeting from Biogen, Merck Serono, Teva, Mylan, Sanofi, Novartis, Genzyme, none related to the present work. B.S. has received grants and personal fees for lectures from Roche, Sanofi-Genzyme, Merck-Serono and Janssen, personal fees for lectures from Novartis, Biogen and Teva, all outside of the submitted work. B.B. has received funding for travel and speaker honoraria from Novartis, Genzyme, Roche and Merck Serono, and she received research support from Biogen, none related to the present work.
References
- 1. Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;214:183–193. [DOI] [PubMed] [Google Scholar]
- 2. Kuhlmann T, Moccia M, Coetzee T, et al. . Multiple sclerosis progression: Time for a new mechanism-driven framework. Lancet Neurol. 2023;22:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Correale J, Marrodan M, Ysrraelit MC. Mechanisms of neurodegeneration and axonal dysfunction in progressive multiple sclerosis. Biomedicines. 2019;1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lubetzki C, Zalc B, Williams A, Stadelmann C, Stankoff B. Remyelination in multiple sclerosis: From basic science to clinical translation. Lancet Neurol. 2020;19:678–688. [DOI] [PubMed] [Google Scholar]
- 5. Kiljan S, Preziosa P, Jonkman L, et al. . Cortical axonal loss is associated with both gray matter demyelination and white matter tract pathology in progressive multiple sclerosis: Evidence from a combined MRI-histopathology study. Mult Scler. 2021;27:380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Franklin RJM, Goldman SA. Glia disease and repair-remyelination. Cold Spring Harb Perspect Biol. 2015;7:a020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patrikios P, Stadelmann C, Kutzelnigg A, Rauschka H. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129(Pt. 12):3165–3172. [DOI] [PubMed] [Google Scholar]
- 8. Patani R, Balaratnam M, Vora A, et al. . Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol Appl Neurobiol. 2007;33:277–287. [DOI] [PubMed] [Google Scholar]
- 9. Werkman IL, Lentferink DH, Baron W. Macroglial diversity: White and grey areas and relevance to remyelination. Cell Mol Life Sci. 2021;278:143–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strijbis EMM, Kooi E-J, van der Valk P, Geurts JJG. Cortical remyelination is heterogeneous in multiple sclerosis. J Neuropathol Exp Neurol. 2017;76:390–401. [DOI] [PubMed] [Google Scholar]
- 11. Albert M, Antel J, Brück W, Stadelmann C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 2007;17:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang A, Staugaitis SM, Dutta R, et al. . Cortical remyelination: A new target for repair therapies in multiple sclerosis. Ann Neurol. 2012;272:918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mancini M, Karakuzu A, Cohen-Adad J, Cercignani M, Nichols TE, Stikov N. An interactive meta-analysis of MRI biomarkers of myelin. Elife. 2020;9:e61523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol. 2004;56:407–415. [DOI] [PubMed] [Google Scholar]
- 15. Chen JT-H, Easley K, Schneider C, et al. . Clinically feasible MTR is sensitive to cortical demyelination in MS. Neurology. 2013;80:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moccia M, van de pavert S, Eshaghi A, et al. . Pathologic correlates of the magnetization transfer ratio in multiple sclerosis. Neurology. 2020;95:e2965–e2976. [DOI] [PubMed] [Google Scholar]
- 17. Derakhshan M, Caramanos Z, Narayanan S, Arnold DL, Collins DL. Surface-based analysis reveals regions of reduced cortical magnetization transfer ratio in patients with multiple sclerosis: A proposed method for imaging subpial demyelination. Hum Brain Mapp. 2014;35:3402–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lazzarotto A, Tonietto M, Poirion E, et al. . Clinically relevant profiles of myelin content changes in patients with multiple sclerosis: A multimodal and multicompartment imaging study. Mult Scler. 2022;28:1881–1890. [DOI] [PubMed] [Google Scholar]
- 19. Polman CH, Reingold SC, Banwell B, et al. . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology. 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 21. University of California, MS-EPIC Team, Cree B, Hollenbach J, et al. . Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85:653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chard DT, Jackson JS, Miller DH, Wheeler-Kingshott CAM. Reducing the impact of white matter lesions on automated measures of brain gray and white matter volumes. J Magn Reson Imaging. 2010;32:223–228. [DOI] [PubMed] [Google Scholar]
- 23. Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakamura K, Guizard N, Fonov VS, Narayanan S, Collins DL, Arnold D. Jacobian integration method increases the statistical power to measure gray matter atrophy in multiple sclerosis. Neuroimage Clin. 2013;4:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolinksy JS, Hauser SL, Kappos L, et al. . Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult Scler J. 2019;25:1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tonietto M, Poirion E, Lazzarotto A, et al. . Periventricular remyelination failure in multiple sclerosis: A substrate for neurodegeneration. Brain. 2023;146:182–194. [DOI] [PubMed] [Google Scholar]
- 27. Barletta V, Herranz E, Treaba CA, et al. . Quantitative 7-tesla imaging of cortical myelin changes in early multiple sclerosis. Front Neurol. 2021;12:714820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Calabrese M, Battaglini M, Giorgio A, et al. . Imaging distribution and frequency of cortical lesions in patients with multiple sclerosis. Neurology. 2010;75:1234–1240. [DOI] [PubMed] [Google Scholar]
- 29. Beck ES, Maranzano J, Luciano NJ, et al. . Cortical lesion hotspots and association of subpial lesions with disability in multiple sclerosis. Mult Scler. 2022;28:1351–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mezydlo A, Treiber N, Ullrich Gavilanes EM, et al. . Remyelination by surviving oligodendrocytes is inefficient in the inflamed mammalian cortex. Neuron. 2023;111:1748–1759.e8. [DOI] [PubMed] [Google Scholar]
- 31. Magliozzi R, Howell OW, Reeves C, et al. . A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010;68:477–493. [DOI] [PubMed] [Google Scholar]
- 32. Magliozzi R, Howell O, Vora A, et al. . Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130(Pt 4):1089–1104. [DOI] [PubMed] [Google Scholar]
- 33. Pirko I, Lucchinetti CF, Sriram S, Bakshi R. Gray matter involvement in multiple sclerosis. Neurology. 2007;68:634–642. [DOI] [PubMed] [Google Scholar]
- 34. Magliozzi R, Howell OW, Nicholas R, et al. . Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann Neurol. 2018;83:739–755. [DOI] [PubMed] [Google Scholar]
- 35. Howell OW, Reeves CA, Nicholas R, et al. . Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134(Pt 9):2755–2771. [DOI] [PubMed] [Google Scholar]
- 36. Magliozzi R, Hametner S, Facchiano F, et al. . Iron homeostasis, complement, and coagulation cascade as CSF signature of cortical lesions in early multiple sclerosis. Ann Clin Trans Neurol. 2019;6:2150–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klotz L, Antel J, Kuhlmann T. Inflammation in multiple sclerosis: Consequences for remyelination and disease progression. Nat Rev. 2023;19:305–320. [DOI] [PubMed] [Google Scholar]
- 38. Pardini M, Brown JW, Magliozzi R, Reynolds R, Chard DT. Surface-in pathology in multiple sclerosis: A new view on pathogenesis? Brain. 2021;144:1646–1654. [DOI] [PubMed] [Google Scholar]
- 39. Magliozzi R, Fadda G, Brown RA, et al. . “Ependymal-in” gradient of thalamic damage in progressive multiple sclerosis. Ann Neurol. 2022;92:670–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poirion E, Tonietto M, Lejeune F, et al. . Structural and clinical correlates of a periventricular gradient of neuroinflammation in multiple sclerosis. Neurology. 2021;96:e1865–e1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ricigliano V, Louapre C, Poirion E, et al. . Imaging characteristics of choroid plexuses in presymptomatic multiple sclerosis: A retrospective study. Neurol Neuroimmunol Neuroinflamm. 2022;9:e200026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ricigliano V, Morena E, Colombi A, et al. . Choroid Plexus enlargement in inflammatory multiple sclerosis: 3.0-T MRI and translocator protein PET evaluation. Radiology. 2021;301:166–177. [DOI] [PubMed] [Google Scholar]
- 43. Mey MG, Mahajan KR, DeSilva TM. Neurodegeneration in multiple sclerosis. WIREs Mech Dis. 2023;15:e1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mei F, Lehmann-Horn K, Shen Y, et al. . Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. Elife. 2016;5:e18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Irvine KA BW. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131(6):1464–1477. [DOI] [PubMed] [Google Scholar]
- 46. Herranz E, Giannì C, Louapre C, et al. . Neuroinflammatory component of gray matter pathology in multiple sclerosis. Ann Neurol. 2016;80:776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peterson JW, Bö L, Mörk S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. Volume. 2001;50:389–400. [DOI] [PubMed] [Google Scholar]
- 48. Klaver R, Popescu V, Voorn P, et al. . Neuronal and axonal loss in normal-appearing gray matter and subpial lesions in multiple sclerosis. J Neuropathol Exp Neurol. 2015;74:453–458. [DOI] [PubMed] [Google Scholar]
- 49. Granberg T, Fan Q, Treaba CA, et al. . In vivo characterization of cortical and white matter neuroaxonal pathology in early multiple sclerosis. Brain. 2017;140:2912–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Petracca M, El Mendili MM, Moro M, et al. . Laminar analysis of the cortical T1/T2-weighted ratio at 7T. Neurol Neuroimmunol Neuroinflamm. 2020;7:e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mangeat G, Badji A, Ouellette R, et al. . Changes in structural network are associated with cortical demyelination in early multiple sclerosis. Hum Brain Mapp. 2018;39:2133–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Louapre C, Govindarajan ST, Giannì C, et al. . The association between intra- and juxta-cortical pathology and cognitive impairment in multiple sclerosis by quantitative T2* mapping at 7T MRI. Neuroimage Clin. 2016;12:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Benkert P, Meier S, Schaedelin A, et al. . Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: A retrospective modelling and validation study. Lancet Neurol. 2022;21:246–257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.