Abstract
Introduction
Sucroferric oxyhydroxide (SO), a non-calcium, chewable, iron-based phosphate binder (PB), effectively lowers serum phosphorus (sP) concentrations while reducing pill burden relative to other PBs. To date, SO studies have largely examined treatment-experienced, prevalent hemodialysis populations. We aimed to explore the role of first-line SO initiated during the first year of dialysis.
Methods
We retrospectively analyzed deidentified data from adults receiving in-center hemodialysis who were prescribed SO monotherapy within the first year of hemodialysis as part of routine clinical care. All patients continuing SO monotherapy for 12 months were included. Changes from baseline in sP, achievement of sP ≤5.5 and ≤4.5 mg/dL, and other laboratory parameters were analyzed quarterly for 1 year.
Results
The overall cohort included 596 patients, 286 of whom had a dialysis vintage ≤3 months. In the 3 months preceding SO initiation, sP rapidly increased (mean increases of 1.02 and 1.65 mg/dL in the overall cohort and incident cohort, respectively). SO treatment was associated with significant decreases in quarterly sP (mean decreases of 0.26–0.36; p < 0.0001 for each quarter and overall). While receiving SO, 55–60% of patients achieved sP ≤5.5 mg/dL and 21–24% achieved sP ≤4.5 mg/dL (p < 0.0001 for each quarter and overall vs. baseline). Daily PB pill burden was approximately 4 pills. Serum calcium concentrations increased and intact parathyroid hormone concentrations decreased during SO treatment (p < 0.0001 vs. baseline).
Conclusions
Among patients on hemodialysis, initiating SO as a first-line PB resulted in significant reductions in sP while maintaining a relatively low PB pill burden.
Keywords: Incident hemodialysis, Hyperphosphatemia, Phosphate binder, Sucroferric oxyhydroxide
Introduction
End-stage kidney disease (ESKD) markedly increases the risk of elevated serum phosphorus (sP) concentrations, which are associated with adverse outcomes, particularly cardiovascular disease [1–3]. A relationship between elevated sP and increased mortality has been observed in both prevalent and incident dialysis populations, extending to sP concentrations at dialysis initiation [1–5]. Hyperphosphatemia has been identified as a leading modifiable contributor to mortality in ESKD [3].
Current Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend lowering elevated sP concentrations in patients receiving dialysis toward the “normal range,” whereas the National Kidney Foundation guidelines, the Kidney Disease Outcomes Quality Initiative (K/DOQI), recommend targeting concentrations 3.5–5.5 mg/dL [2, 6]. Three broad strategies are available to help reduce sP: dietary changes, phosphate binders (PBs), and intensified dialysis [2]. Clinicians have multiple approved PBs in their armamentarium which can be broadly categorized as calcium-based or non-calcium-based. Although all have demonstrated efficacy at reducing sP, other aspects of their clinical profiles, including formulation, dose, tolerability, and safety, can vary. Despite availability of these agents, the proportion of hemodialysis patients with sP >4.5 and >5.5 mg/dL has remained largely unchanged over the last decade at ∼65 and ∼35%, respectively [7, 8].
Owing to the absence of compelling head-to-head trials, it has been recommended that selection of a PB is based on the safety and efficacy of the agent, potential drug interactions, cost, and patient preference [9, 10]. Given the risk of increased serum calcium concentrations with calcium-based PBs, these agents should generally be avoided or used with dose restriction in patients with hypercalcemia or evidence of vascular or soft-tissue calcification [2, 6]. As many PBs are associated with a high pill burden, accounting for an average of half of the total daily pill burden for hemodialysis patients, experts also recommend consideration of pill burden and patient adherence when prescribing a PB [9–11]. As a result of residual kidney function, incident hemodialysis populations may have improved physiologic phosphate control (and less need for pharmacotherapy) at the start of dialysis. As residual kidney function declines over time, the need for pharmacotherapy increases [12–14].
Sucroferric oxyhydroxide (SO, 500 mg per tablet; Velphoro®, Fresenius Medical Care Renal Therapies Group, Waltham, MA, USA) is a non-calcium, chewable, iron-based PB that effectively lowers sP while reducing pill burden relative to other PBs. To date, studies have largely examined the effects of SO in prevalent hemodialysis populations previously treated with other PBs [14–21]. This study aimed to explore the role of SO as a first-line PB among patients in their first year of dialysis.
Methods
Data Source and Patient Population
Using a retrospective study design, we extracted deidentified demographic, clinical, and prescription data from the Fresenius Kidney Care (FKC) data warehouse and pharmacy database. Adult (≥18 years) in-center hemodialysis patients were included if they were prescribed SO monotherapy as a part of routine clinical care within the first year of hemodialysis, between May 2018 and December 2019. Eligible patients had no record of prior PB therapy and had a laboratory sP concentration from 91 days prior to SO initiation. The primary analysis included patients who continued SO monotherapy for 12 months (overall cohort), with a subgroup of patients with a dialysis vintage ≤3 months at SO start (incident cohort). Observation periods were divided into consecutive 91-day intervals. The 3 months (−3 months, −2 months, and −1 month) before SO prescription was considered baseline (BL) and 12 months (M1–M12) of SO prescription was defined as follow-up (Q1–Q4). In a sensitivity analysis among patients who discontinued SO within 12 months, sP was compared between baseline and follow-up, excluding time periods when patients were not on SO. This study was reviewed by an independent review board (New England Independent Review Board (NEIRB)/WCG IRB, Needham, MA, USA), and was granted an exempt status determination under the common rule and applicable guidance because of its purely observational nature and use of only deidentified data.
Data Assessment and Clinical Parameters
Patient-level demographics (age, gender, race, and ethnicity), body mass index, dialysis vintage, Charlson Comorbidity Index, primary cause of kidney failure, and diagnosis of diabetes and congestive heart failure were evaluated at baseline. Clinical and laboratory variables included markers of mineral and bone disorder (sP, calcium, and intact parathyroid hormone [iPTH]) and nutritional and clearance parameters (albumin, body weight, equilibrated normalized protein catabolic rate [enPCR], and equilibrated Kt/V). The mean daily prescribed number of SO pills was recorded for each patient. We also extracted hemoglobin concentrations, iron indices (ferritin and transferrin saturation [TSAT]), and the use and dosage of relevant medications (cinacalcet, etelcalcetide, oral/intravenous vitamin D, iron sucrose, and erythropoiesis-stimulating agents).
Laboratory tests were measured monthly except for hemoglobin, which was measured weekly, and serum ferritin and iPTH, which were measured quarterly per standard FKC practice. Concomitant medication use was evaluated quarterly. Given the targeted phosphorus levels recommended by the K/DOQI and KDIGO guidelines, two definitions of in-range sP concentrations, ≤5.5 mg/dL and ≤4.5 mg/dL, were used.
Statistical Analysis
Baseline characteristics are presented as the mean ± standard deviation (SD) for continuous variables and number of patients (percentage) for categorical variables. Quarterly and monthly means of continuous data were calculated using mixed-effects linear regression. Summary statistics are presented as least-squares (LS) mean (standard error [SE]) with comparisons across treatment periods (overall p values) and between baseline and follow-up periods. Cochran’s Q test and the McNemar χ2 test were used for significance testing of categorical variables. A subgroup analysis was performed in patients with a dialysis vintage ≤3 months at SO initiation. Two-tailed p values <0.05 were considered statistically significant. Analyses were conducted with SAS (SAS® Enterprise Guide® 8.3, SAS Institute Inc., Cary, NC, USA).
Results
Baseline Patient Characteristics
Patient disposition for the primary analysis (overall cohort; N = 596), subgroup analysis (incident cohort; N = 286), and sensitivity analysis (sensitivity cohort; N = 559) are described in online supplementary figure 1 (for all online suppl. material, see https://doi.org/10.1159/000535754). Baseline characteristics for the overall and incident cohorts are summarized in Table 1. The overall cohort was 44% female, with a mean age of approximately 60 years and a mean dialysis vintage of 4 months at SO initiation. In the incident cohort, the mean dialysis vintage was 1.5 months.
Table 1.
Patient characteristics at baseline
| Characteristic | Overall cohort (N = 596) | Patients with HD vintage ≤3 months (N = 286) |
|---|---|---|
| Age, years | 59.8±13.4 | 59.2±13.6 |
| Dialysis vintage, months | 4.0±3.1 | 1.5±0.8 |
| Post-dialysis BMI, kg/m2 | 30.4±8.1 | 30.6±8.1 |
| Charlson Comorbidity Index | 4.0±1.5 | 3.9±1.3 |
| Female, n (%) | 263 (44.1) | 115 (40.2) |
| Race, n (%) | ||
| White | 337 (56.5) | 168 (58.7) |
| Black/African American | 227 (38.1) | 104 (36.4) |
| Other | 7 (1.2) | 4 (1.4) |
| Unknown | 25 (4.2) | 10 (3.5) |
| Hispanic/Latino | 57 (9.6) | 37 (12.9) |
| Primary cause of kidney failure, n (%) | ||
| Diabetes mellitus | 318 (53.4) | 149 (52.1) |
| Hypertension | 187 (31.4) | 91 (31.8) |
| Glomerulonephritis | 23 (3.9) | 13 (4.5) |
| Polycystic kidney disease | 12 (2.0) | 4 (1.4) |
| Other | 55 (9.2) | 28 (9.8) |
| Unknown | 1 (0.2) | 1 (0.3) |
| Medical history, n (%) | ||
| Diabetes mellitus | 358 (60.1) | 172 (60.1) |
| Congestive heart failure | 123 (20.6) | 58 (20.3) |
Summary estimates are presented as the mean ± standard deviation or number (%) of patients.
BMI, body mass index; HD, hemodialysis.
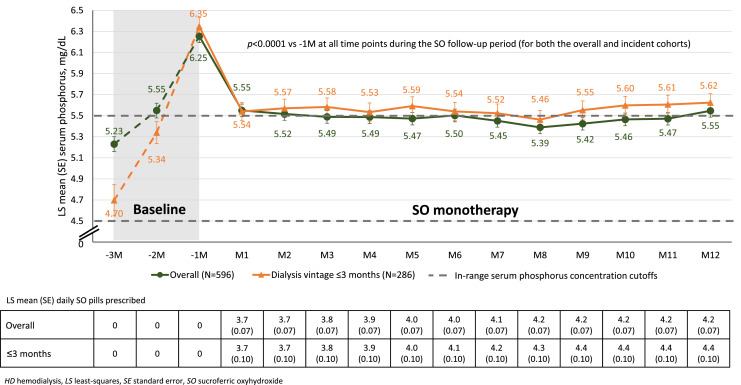
As shown from the dotted lines in Figure 1, in the 3 months preceding SO initiation (baseline), sP rapidly increased. There was an LS mean increase in sP of 1.02 mg/dL and 1.65 mg/dL from month −3 to month −1 in the overall and incident cohorts, respectively. Most patients had sP ≤5.5 mg/dL in month −3 (70% and 84% for overall and incident cohorts, respectively), whereas no more than 25% in each group had sP ≤5.5 mg/dL in month −1 (online suppl. Table 1). In the 3 months preceding SO initiation, significant increases in serum calcium and serum albumin concentrations and enPCR were also seen (online suppl. Table 1).
Fig. 1.
Monthly changes in LS mean sP in overall patients and patients with HD vintage ≤3 months.
Overall Cohort
Concentrations of sP at baseline and over the 1-year follow-up are shown in Table 2. At baseline, the mean (SE) sP concentration was 5.78 (0.05) mg/dL and 43% of patients had sP ≤5.5 mg/dL. Three months after initiating SO, patients exhibited a mean decrease in sP of 0.26 mg/dL (p < 0.0001 vs. baseline). Significant reductions were maintained throughout the follow-up period. More than 55% of patients exhibited sP ≤5.5 mg/dL within 3 months of initiating SO (p < 0.0001 vs. baseline), and a majority of the cohort met this criteria throughout the follow-up period. Similarly, whereas fewer than 6% of patients had sP ≤4.5 mg/dL prior to SO initiation, more than 20% of patients attained such concentrations by Q1 and at each follow-up quarter thereafter (p < 0.0001 for all vs. baseline). The mean number of prescribed pills per day of SO increased from 3.7 in Q1 to 4.2 in Q4.
Table 2.
Temporal changes in clinical parameters and CKD-MBD medication use in overall patients (N = 596)
| Parameter | Baseline | Follow-up | p value | |||
|---|---|---|---|---|---|---|
| –1Q; ref | Q1 | Q2 | Q3 | Q4 | ||
| CKD-MBD biochemical markers | ||||||
| sP, mg/dL | 5.78 (0.05) | 5.52 (0.05)*** | 5.49 (0.05)*** | 5.42 (0.05)*** | 5.50 (0.05)*** | <0.0001 |
| sP ≤5.5, mg/dL | 43.3 | 55.2*** | 57.5*** | 60.2*** | 58.0*** | <0.0001 |
| sP ≤4.5, mg/dL | 5.9 | 20.6*** | 21.0*** | 24.2*** | 22.7*** | <0.0001 |
| Serum calcium, mg/dL | 8.90 (0.02) | 8.97 (0.02)*** | 8.96 (0.02)*** | 8.96 (0.02)*** | 8.92 (0.02) | <0.0001 |
| iPTH, pg/mL | 535 (15) | 482 (15)*** | 475 (15)*** | 461 (15)*** | 469 (15)*** | <0.0001 |
| CKD-MBD medications | ||||||
| PB pills/day | 0 | 3.7 (0.06) | 3.9 (0.06) | 4.1 (0.06) | 4.2 (0.06) | NA |
| Cinacalcet use | 8.2 | 14.8*** | 17.1*** | 18.6*** | 21.3*** | <0.0001 |
| Home cinacalcet dose, mg/day | 27.5 (3.4) | 38.4 (2.9)** | 42.4 (2.7)*** | 43.3 (2.7)*** | 46.7 (2.6)*** | <0.0001 |
| In-center cinacalcet dose, mg/administration | 21.8 (4.0) | 34.3 (3.4)*** | 43.1 (3.3)*** | 57.1 (3.3)*** | 65.6 (3.2)*** | <0.0001 |
| Etelcalcetide use | 0.5 | 1.2* | 3.0** | 4.0*** | 5.4*** | <0.0001 |
| Etelcalcetide dose, μg/administration | 4.03 (0.92) | 4.19 (0.69) | 4.94 (0.55) | 5.76 (0.51)* | 6.18 (0.48)* | 0.003 |
| Any vitamin D use | 61.2 | 70.1*** | 74.3*** | 77.7*** | 82.4*** | <0.0001 |
| Oral active vitamin D usea | 49.5 | 56.4*** | 58.6*** | 60.1*** | 63.8*** | <0.0001 |
| IV active vitamin D useb | 13.8 | 15.8* | 17.6** | 19.1*** | 20.5*** | <0.0001 |
| Nutritional and clearance parameters | ||||||
| Serum albumin, g/dL | 3.68 (0.01) | 3.79 (0.01)*** | 3.84 (0.01)*** | 3.86 (0.01)*** | 3.85 (0.01)*** | <0.0001 |
| Predialysis weight, kg | 89.3 (1.0) | 89.8 (1.0)*** | 90.5 (1.0)*** | 90.9 (1.0)*** | 91.2 (1.0)*** | <0.0001 |
| enPCR, g/kg/day | 0.86 (0.01) | 0.91 (0.01)*** | 0.92 (0.01)*** | 0.91 (0.01)*** | 0.91 (0.01)*** | <0.0001 |
| eKt/V | 1.43 (0.01) | 1.44 (0.01) | 1.46 (0.01)* | 1.46 (0.01)* | 1.46 (0.01)* | 0.05 |
Values are presented as least-squares mean (standard error) or %. p values compare the summary estimates across time with -Q1 as the reference. Overall p values were calculated using linear mixed-effects regression (continuous variables) or Cochran’s Q test (categorical variables). CKD-MBD, chronic kidney disease-mineral and bone disorder; eKt/V, equilibrated Kt/V; enPCR, equilibrated normalized protein catabolic rate; iPTH, intact parathyroid hormone; IV, intravenous; NA, not applicable; PB, phosphate binder; sP, serum phosphorus.
*p < 0.05; **p < 0.001; ***p < 0.0001 (vs. baseline).
aIncludes calcitriol and doxercalciferol.
bIncludes doxercalciferol, calcitriol, and paricalcitol.
Given the marked changes between −3 months and −1 month, we also examined monthly changes in sP during SO therapy (Fig. 1). Within 1 month of initiating SO, sP decreased from an LS mean of 6.25 mg/dL to an LS mean of 5.55 mg/dL (p < 0.0001). Sustained reductions in sP were observed at each month of follow-up (p < 0.0001 vs. −1 month).
In the overall cohort, serum calcium concentrations increased and iPTH concentrations decreased during SO treatment (p < 0.0001 vs. baseline). These changes occurred in tandem with significant increases in cinacalcet use and dose, etelcalcetide use and dose, and both oral and intravenous vitamin D use (Table 2). Across all follow-up periods, patients demonstrated improved nutritional profiles as assessed by serum albumin concentrations and enPCR (p < 0.0001 vs. baseline for each follow-up period and overall). Changes in anemia and iron indices as well as anemia therapies are summarized in online supplementary Table 2. Serum ferritin concentrations and TSAT increased progressively through Q4 (p < 0.0001 vs. baseline for each follow-up period and overall).
Incident Cohort
As observed in the overall cohort, initiation of SO among patients with a dialysis vintage ≤3 months resulted in significant reductions in sP (Table 3). Three months after initiating SO, sP decreased by an average of 0.28 mg/dL (p < 0.0001 vs. baseline), and significant reductions in sP continued through subsequent follow-up time points. While receiving SO, a majority of patients had sP ≤5.5 mg/dL (p < 0.0001 vs. baseline) and ∼20% had sP ≤4.5 mg/dL (p < 0.0001 vs. baseline). The average daily dose of SO increased from 3.7 pills/day (Q1) to 4.4 pills/day (Q4).
Table 3.
Temporal changes in clinical parameters and CKD-MBD medication use in patients with HD vintage ≤3 months (N = 286)
| Parameter | Baseline | Follow-up | p value | |||
|---|---|---|---|---|---|---|
| –1Q; ref | Q1 | Q2 | Q3 | Q4 | ||
| CKD-MBD biochemical markers | ||||||
| sP, mg/dL | 5.84 (0.07) | 5.56 (0.07)*** | 5.56 (0.07)*** | 5.51 (0.07)*** | 5.61 (0.07)** | <0.0001 |
| sP ≤5.5, mg/dL | 36.4 | 53.1*** | 56.8*** | 57.2*** | 55.1*** | <0.0001 |
| sP ≤4.5, mg/dL | 6.6 | 22.0*** | 21.1*** | 21.8*** | 19.3*** | <0.0001 |
| Serum calcium, mg/dL | 8.75 (0.03) | 8.96 (0.03)*** | 8.95 (0.03)*** | 8.96 (0.03)*** | 8.92 (0.03)*** | <0.0001 |
| iPTH, pg/mL | 554 (20) | 476 (19)*** | 459 (19)*** | 448 (19)*** | 452 (19)*** | <0.0001 |
| CKD-MBD medications | ||||||
| PB pills/day | 0 | 3.7 (0.09) | 4.0 (0.09) | 4.3 (0.09) | 4.4 (0.09) | NA |
| Cinacalcet use | 5.2 | 11.2** | 15.4*** | 17.1*** | 17.8*** | <0.0001 |
| Home cinacalcet dose, mg/day | 22.6 (4.8) | 34.2 (3.5)* | 36.2 (3.3)* | 41.0 (3.3)*** | 44.7 (3.2)*** | <0.0001 |
| In-center cinacalcet dose, mg/administration | 11.5 (7.3) | 19.9 (4.5) | 30.6 (4.1)* | 50.9 (4.0)*** | 64.5 (3.9)*** | <0.0001 |
| Etelcalcetide use | 0 | 0.3 | 2.1 | 2.8 | 3.5 | <0.0001 |
| Total vitamin D use | 57.7 | 71.3*** | 74.1*** | 76.9*** | 82.9*** | <0.0001 |
| Oral active vitamin D usea | 49.0 | 58.7** | 59.8*** | 59.4** | 65.0*** | <0.0001 |
| IV active vitamin D useb | 9.8 | 14.7** | 16.8*** | 18.5*** | 19.2*** | <0.0001 |
| Nutritional and clearance parameters | ||||||
| Serum albumin, g/dL | 3.58 (0.02) | 3.77 (0.02)*** | 3.84 (0.02)*** | 3.87 (0.02)*** | 3.87 (0.02)*** | <0.0001 |
| Predialysis weight, kg | 90.4 (1.5) | 90.3 (1.5) | 90.9 (1.5)*** | 91.4 (1.5)*** | 91.9 (1.5)*** | <0.0001 |
| enPCR, g/kg/day | 0.83 (0.01) | 0.91 (0.01)*** | 0.93 (0.01)*** | 0.92 (0.01)*** | 0.93 (0.01)*** | <0.0001 |
| eKt/V | 1.41 (0.02) | 1.44 (0.02) | 1.45 (0.02) | 1.44 (0.02) | 1.46 (0.02)* | 0.22 |
Values are presented as least-squares mean (standard error) or %. p values compare summary estimates across time with -Q1 as the reference. Overall p values were calculated using linear mixed-effects regression (continuous variables) or Cochran’s Q test (categorical variables).
CKD-MBD, chronic kidney disease-mineral and bone disorder; eKt/V, equilibrated Kt/V; enPCR, equilibrated normalized protein catabolic rate; HD, hemodialysis; iPTH, intact parathyroid hormone; IV, intravenous; NA, not applicable; PB, phosphate binder; sP, serum phosphorus.
*p < 0.05; **p < 0.001; ***p < 0.0001 (vs. baseline).
aIncludes calcitriol and doxercalciferol.
bIncludes doxercalciferol, calcitriol, and paricalcitol.
Patients in the incident cohort demonstrated LS mean 0.81 mg/dL reduction in sP during the first month of SO monotherapy. Significant reductions in sP were observed for each of the 12 on-treatment months (p < 0.0001 vs. −1 month; Fig. 1). At months 2–12, LS mean sP was slightly higher (0.04–0.14 mg/dL) in the incident cohort than in the overall cohort. Similarly, mean daily PB pill burdens were also slightly higher in the incident dialysis subgroup during the last 6 months of follow-up.
Patients in the incident cohort had lower baseline serum calcium concentrations than those in the overall cohort (8.75 mg/dL vs. 8.90 mg/dL). Patients new to dialysis exhibited a mean 0.21 mg/dL increase in serum calcium between baseline and Q1 (p < 0.0001; Table 3). Thereafter, serum calcium concentrations remained relatively stable (between 8.95 and 8.92 mg/dL from Q2 through Q4). SO initiation was also accompanied by significant decreases in iPTH and significant increases in cinacalcet use, vitamin D use, serum albumin, and enPCR. Baseline serum ferritin concentrations, TSAT, hemoglobin concentrations, and ESA use were all lower in the incident cohort than in the overall cohort (online suppl. Table 2). During follow-up, increases in these parameters generally paralleled those seen in the overall cohort. At Q4, iron and anemia endpoints were similar between the 2 cohorts.
Sensitivity Analysis among Patients Who Discontinued SO
Of the 1,155 patients initiating first-line SO monotherapy during the study period, 559 (48%) were excluded from the primary analyses (online suppl. Fig. 1). Reasons for SO discontinuation included death (n = 152; 27.2%), transfer to other dialysis facility (n = 67; 12.0%), recovery of kidney function (n = 16; 2.9%), kidney transplant (n = 13; 2.3%), relocation out of the USA (n = 3; 0.5%), and other (including lost to follow-up [n = 7; 1.3%]). For the remaining 301 (53.8%) patients, the reason for SO monotherapy discontinuation was unknown/unrecorded. Among patients who discontinued SO monotherapy prior to the end of the 12-month follow-up period, the mean (SE) baseline sP concentration was 5.81 (0.05) mg/dL. Prior to SO discontinuation, the last (on-treatment) sP concentration was 5.66 (0.05) mg, representing 0.15 mg/dL reduction (p = 0.0006).
Discussion
In this retrospective analysis of nearly 600 hemodialysis patients initiating SO monotherapy as first-line PB therapy within the first year of hemodialysis, treatment was associated with significantly improved sP concentrations and a marked increase in the proportion of patients meeting guideline-recommended sP cutoffs. Specifically, at Q1, the mean sP decreased from a -Q1 baseline of 5.78 mg/dL to 5.52 mg/dL (p < 0.0001), and there was a 250% increase in the proportion of patients with sP ≤4.5 mg/dL (p < 0.0001). Such control was maintained through 1 year of treatment (Q4: 5.50 mg/dL; p < 0.0001) and achieved with a mean daily PB pill burden of approximately 4 pills. Based on a prior analysis, 3.75 tablets of SO provides approximately the phosphorus binding equivalency of 10 sevelamer tablets and 9 calcium acetate tablets [22].
In the 3 months prior to SO therapy, sP concentration increased rapidly. In the overall cohort, the mean sP concentration increased to 6.25 mg/dL in the month prior to initiation; this represented a more than 1 mg/dL increase from 2 months earlier. In the subgroup of patients with a dialysis vintage ≤3 months, the rise in sP was more rapid and sP reached even higher concentration before SO initiation (from 4.70 mg/dL at −3 months to 6.35 g/dL at −1 month). The underlying reasons for these rapid increases are likely multifactorial. Because increases in sP occurred in tandem with increases in serum albumin, serum calcium, and enPCR, we propose that dietary improvements, specifically, increases in protein intake, played a contributory role. Differences between the overall and incident cohorts decreased over time, with both groups exhibiting similar profiles 1 year after initiation of SO (i.e., 12–24 months after initiation of dialysis).
Because even very low glomerular filtration rates can contribute to endogenous clearance of phosphorus [13, 14], changes in residual kidney function likely impacted phosphorus control during our study. Although the trajectory of residual kidney function decline in hemodialysis has not been well studied [23] and measures of residual function were not collected in this study, we would expect kidney function to decline over time. As such, endogenous phosphorus clearance would be expected to be reduced (1) longitudinally across the study period (from BL to Q4) and (2) among patients with a longer dialysis vintage (i.e., 3–12 months) at baseline. Finally, the initiation of dialysis is associated with multiple changes in medication and titration of dialysis prescriptions, which can impact the phosphorus balance. Regardless of the underlying cause(s), the observed rapid changes in sP prior to SO initiation highlight the need for routine monitoring such that appropriate therapy, dietary and/or pharmacologic, can be initiated. For those patients not yet receiving kidney replacement therapy, the development of hyperphosphatemia has been proposed as an indicator to initiate dialysis [24].
This represents the first study dedicated to examining the role of SO as a first-line PB therapy in an incident hemodialysis population. The Velphoro Evaluation of Real-life Safety, Effectiveness, and Adherence (VERIFIE) study included approximately 500 patients naïve to treatment with PBs prior to starting SO, but all patients had a dialysis vintage ≥6 months (mean vintage, 4.3 years) [25]. No subgroup analyses examining the clinical profile of first-line SO in that trial have been published. Ramos et al. [26] examined the real-world effectiveness of SO in nearly 1,100 European hemodialysis patients, including 188 who received SO as first-line PB monotherapy. In that subgroup, 3 months of SO therapy was associated with a mean sP reduction of approximately 0.38 mg/dL (from 5.67 mg/dL to 5.29 mg/dL) and a 33% increase in the proportion of patients with sP ≤5.5 mg/dL (from 49.5% to 65.8%). The large sample size of the current study extends the findings of these prior, smaller studies and highlights the unique phosphate management needs of the incident dialysis population.
This study was not designed to examine the clinical decision to prescribe SO as a first-line therapy; such determinants likely include both clinical and nonclinical factors. During the same time period as the present study, 48,453 patients in the FKC database were initiated on a first-line non-SO PB (calcium acetate, sevelamer, ferric citrate, and lanthanum carbonate) within the first year of hemodialysis. The mean baseline (i.e., pre-PB) sP concentration of these patients was 5.42 mg/dL (vs. 5.78 mg/dL in the present analysis). Although these data suggest that SO is being selected for patients with more severe hyperphosphatemia, temporal trends suggest physicians are increasingly prescribing SO to a broader group of patients. In prior real-world cohorts switched from other PBs to SO, mean baseline sP concentrations have reduced over time. In data from April 2014 to March 2015, Coyne et al. [18] studied a cohort with a mean baseline sP concentration of 6.93 mg/dL. Using data from May 2018 to May 2019, Rhee et al. [14] and Kendrick et al. [27] studied cohorts with mean baseline sP concentrations of 6.39 mg/dL and 6.38 mg/dL, respectively. These sP concentrations were similar to −1 month concentrations observed in the present analysis (6.25 mg/dL). Reasons for the progressively lower threshold for initiating SO are unclear but may include patient preference, increasing clinical experience with SO, and/or changes in access to SO.
When considering therapies to lower sP in ESKD, the need for long-term control is paramount. Gong and colleagues found a 3.2% reduced risk of mortality for each month of phosphorus control [28]. Such data highlight the need for early and prolonged reduction of sP. It is recognized that patient satisfaction with a PB can impact adherence, sP concentrations, and ultimately, the risk of death [29, 30]. Data on the impact of PB use and its effect on mortality among incident dialysis patients are scant. In a prospective observational study of approximately 8,600 incident hemodialysis patients, PB treatment was independently associated with an 18–30% relative reduction in 1-year mortality risk [31].
As described above, we examined the profile of SO in a previously understudied population, incident hemodialysis patients receiving SO as a first-line PB therapy. Nonetheless, several methodological limitations should be considered when interpreting the results. The retrospective and uncontrolled nature of the study precludes any conclusions regarding causality between SO therapy and changes in laboratory and clinical markers. Although we analyzed nutritional markers, the data extracted provide no information on the dietary guidance provided to patients. We also are unable to determine the reasons for the initiation of PB therapy, let alone the rationale for selecting SO. Although the results of the sensitivity analysis were directionally consistent with the primary analysis, the mean magnitude SO-associated sP reductions was smaller among patients who discontinued SO (0.14 mg/dL vs. 0.28 mg/dL at Q4). Information on the reasons for discontinuation is not available and may have included adverse effects/reduced tolerability, nonadherence, insurance coverage, out-of-pocket costs, and lack of effectiveness. As such, caution is warranted when generalizing the results to patients who did not complete the 1-year SO follow-up. Finally, we cannot draw any inferences regarding the safety and tolerability of SO from the available data.
In conclusion, initiating SO as a first-line PB within the first year of hemodialysis was associated with significant reductions in sP. Between 55% and 60% of patients achieved sP ≤5.5 mg/dL and between 21% and 24% achieved sP ≤4.5 mg/dL. Such results were achieved with a low PB pill burden (mean of 3.7–4.2 pills/day) and support the use of SO as a first-line PB in patients on hemodialysis.
Acknowledgment
Medical writing and editing support was provided by Adam Perahia, MD, of NorthStar Strategic Consulting, LLC, via funding by Fresenius Medical Care.
Statement of Ethics
This study was reviewed by the New England Independent Review Board (NEIRB)/WCG IRB and determined to be exempted under the common rule and applicable guidance. Due to the anonymous and purely observational nature of the study, the need for informed consent was waived by an independent institutional review board (New England Independent Review Board (NEIRB)/WCG IRB, Needham, MA, USA; Work Order 17-1396220-1).
Conflict of Interest Statement
M.Z., L.H.F., and M.S.A. are employees of Fresenius Medical Care, Global Medical Office. L.H.F. and M.S.A. have share options/ownership in Fresenius Medical Care. SMS receives consultancy fees from OPKO Health, Vifor Pharma, Amgen, Ardelyx, and Fresenius Medical Care Renal Therapies Group and research funding from Abbott, Amgen, Ardelyx, OPKO Health, Reata Pharmaceuticals, and Vifor Pharma.
Funding Sources
The study was supported by Fresenius Medical Care.
Author Contributions
Research conception and study design, data interpretation, and manuscript preparation and critical review: J.A.M., M.Z., L.H.F., M.S.A., and S.M.S.; data analysis: M.Z. and L.H.F.; and provision of clinical insights: J.A.M., M.S.A., and S.M.S. All authors gave approval of the version to be published, have agreed on the journal to which the article has been submitted, and agreed to be accountable for all aspects of the work.
Funding Statement
The study was supported by Fresenius Medical Care.
Data Availability Statement
The data underlying the findings described in this manuscript are proprietary and not publicly available. Further inquiries can be directed to Dr. Michael S. Anger at Michael.Anger@freseniusmedicalcare.com.
Supplementary Material
References
- 1. Fernández-Martín JL, Martínez-Camblor P, Dionisi MP, Floege J, Ketteler M, London G, et al. Improvement of mineral and bone metabolism markers is associated with better survival in haemodialysis patients: the COSMOS study. Nephrol Dial Transplant. 2015;30(9):1542–51. [DOI] [PubMed] [Google Scholar]
- 2. Kidney Disease Improving Global Outcomes KDIGO CKD-MBD Update Work Group . KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease – mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7(1):1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCullough PA. Phosphate control: the next frontier in dialysis cardiovascular mortality. Cardiorenal Med. 2021;11(3):123–32. [DOI] [PubMed] [Google Scholar]
- 4. Owaki A, Inaguma D, Aoyama I, Inaba S, Koide S, Ito E, et al. Serum phosphate level at initiation of dialysis is associated with all-cause mortality: a multicenter prospective cohort study. Ren Fail. 2018;40(1):475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noordzij M, Korevaar JC, Boeschoten EW, Dekker FW, Bos WJ, Krediet RT. The Kidney Disease Outcomes Quality Initiative (K/DOQI) guideline for bone metabolism and disease in CKD: association with mortality in dialysis patients. Am J Kidney Dis. 2005;46(5):925–32. [DOI] [PubMed] [Google Scholar]
- 6. National Kidney Foundation . K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):S1–S201. [PubMed] [Google Scholar]
- 7. DOPPS Practice Monitor . Serum phosphorus (most recent), categories. https://www.dopps.org/DPM-HD/DPMSlideBrowser.aspx?type=Topic&%3bid=11 [Accessed 1 March 2023]. [Google Scholar]
- 8. Dwyer JP, Kelepouris E. New directions in phosphorus management in dialysis. J Ren Nutr. 2023;33(1):12–6. [DOI] [PubMed] [Google Scholar]
- 9. Vervloet MG. Hyperphosphataemia: which phosphate binder? Nephrol Dial Transplant. 2018;33(7):1091–3. [DOI] [PubMed] [Google Scholar]
- 10. Sekar A, Kaur T, Nally JV, Rincon-Choles H, Jolly S, Nakhoul GN. Phosphorus binders: the new and the old, and how to choose. Cleve Clin J Med. 2018;85(8):629–38. [DOI] [PubMed] [Google Scholar]
- 11. Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rhee H, Yang JY, Jung WJ, Shin MJ, Yang BY, Song SH, et al. Significance of residual renal function for phosphate control in chronic hemodialysis patients. Kidney Res Clin Pract. 2014;33(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Penne EL, van der Weerd NC, Grooteman MP, Mazairac AHA, van den Dorpel MA, Nubé MJ, et al. Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(2):281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rhee CM, Zhou M, Woznick R, Mullon C, Anger MS, Ficociello LH. A real-world analysis of the influence of age on maintenance hemodialysis patients: managing serum phosphorus with sucroferric oxyhydroxide as part of routine clinical care. Int Urol Nephrol. 2023;55(2):377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Floege J, Covic AC, Ketteler M, Rastogi A, Chong EMF, Gaillard S, et al. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int. 2014;86(3):638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Floege J, Covic AC, Ketteler M, Mann JF, Rastogi A, Spinowitz B, et al. Long-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant. 2015;30(6):1037–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coyne DW, Sprague SM, Vervloet M, Ramos R, Kalantar-Zadeh K. Sucroferric oxyhydroxide for hyperphosphatemia: a review of real-world evidence. J Nephrol. 2022;35(3):875–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coyne DW, Ficociello LH, Parameswaran V, Anderson L, Vemula S, Ofsthun NJ, et al. Real-world effectiveness of sucroferric oxyhydroxide in patients on chronic hemodialysis: a retrospective analysis of pharmacy data. Clin Nephrol. 2017;88(8):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kendrick J, Parameswaran V, Ficociello LH, Ofsthun NJ, Davis S, Mullon C, et al. One-year historical cohort study of the phosphate binder sucroferric oxyhydroxide in patients on maintenance hemodialysis. J Ren Nutr. 2019;29(5):428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gray K, Ficociello LH, Hunt AE, Mullon C, Brunelli SM. Phosphate binder pill burden, adherence, and serum phosphorus control among hemodialysis patients converting to sucroferric oxyhydroxide. Int J Nephrol Renovasc Dis. 2019;12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalantar-Zadeh K, Ficociello LH, Parameswaran V, Athienites NV, Mullon C, Kossmann RJ, et al. Changes in serum albumin and other nutritional markers when using sucroferric oxyhydroxide as phosphate binder among hemodialysis patients: a historical cohort study. BMC Nephrol. 2019;20(1):396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gutekunst L. An update on phosphate binders: a dietitian’s perspective. J Ren Nutr. 2016;26(4):209–18. [DOI] [PubMed] [Google Scholar]
- 23. Steinwandel U, Kheirkhah H, Davies H. Residual renal function: how fast does the residual urine output function decline in the first year of haemodialysis? – a scoping review. Front Nephrol. 2021;1:808909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu YA, Lee SY, Lin HY, Liu YC, Kao HK, Chen YC, et al. Serum phosphate as an additional marker for initiating hemodialysis in patients with advanced chronic kidney disease. Biomed J. 2015;38(6):531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vervloet MG, Boletis IN, de Francisco ALM, Kalra PA, Ketteler M, Messa P, et al. Real-world safety and effectiveness of sucroferric oxyhydroxide for treatment of hyperphosphataemia in dialysis patients: a prospective observational study. Clin Kidney J. 2021;14(7):1770–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramos R, Chazot C, Ferreira A, Di Benedetto A, Gurevich K, Feuersenger A, et al. The real-world effectiveness of sucroferric oxyhydroxide in European hemodialysis patients: a 1-year retrospective database analysis. BMC Nephrol. 2020;21(1):530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kendrick JB, Zhou M, Ficociello LH, Parameswaran V, Mullon C, Anger MS, et al. Serum phosphorus and pill burden among hemodialysis patients prescribed sucroferric oxyhydroxide: one-year follow-up on a contemporary cohort. Int J Nephrol Renovasc Dis. 2022;15:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gong N, Xiao Z, Zhang F, Zhong X, He Y, Yi Z, et al. Duration of serum phosphorus control associated with overall mortality in patients undergoing peritoneal dialysis. Kidney Dis. 2020;6(6):434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cozzolino M, Galassi A, Ciceri P. Phosphate binders in dialysis: better satisfied than sorry. Clin Kidney J. 2021;14(8):1859–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCullough K, Port FK, de Sequera P, Rayner H, Pecoits-Filho R, Walpen S, et al. European hemodialysis patient satisfaction with phosphate binders is associated with serum phosphorus levels: the Dialysis Outcomes and Practice Patterns Study. Clin Kidney J. 2021;14(8):1886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Isakova T, Gutiérrez OM, Chang Y, Shah A, Tamez H, Smith K, et al. Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol. 2009;20(2):388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying the findings described in this manuscript are proprietary and not publicly available. Further inquiries can be directed to Dr. Michael S. Anger at Michael.Anger@freseniusmedicalcare.com.