Abstract
Introduction
Intrauterine infection with Ureaplasma species (U.spp.) is mostly a result of vaginal colonization with subsequent ascending infection and is associated with adverse pregnancy outcome. Little is known about rates and risk factors for ascending infection. Aim of the current study was to analyse the frequency of ascending U.spp. infection in vaginally colonized pregnant women delivering preterm and subsequent short- and long-term outcome of infants.
Methods
Women delivering ≤32 weeks of gestation with available data on vaginal U.spp. colonization in early pregnancy as well as amniotic and placental colonization screening during caesarean section were included. Neonatal short- and long-term outcome was analysed depending on vaginal and intrauterine colonization.
Results
Seventy-two women giving birth to 104 preterm infants were included. Intrauterine microbial invasion was found in 23/72 (31.9%) pregnancies. The most commonly detected organisms were U.spp. (52.2%), followed by E. coli (21.7%) and Enterococcus faecalis (17.4%). Intrauterine growth of U.spp. occurred exclusively after previous vaginal colonization in early pregnancy (42/72; 58.3%) and was found in 12/42 (28.6%) cases. Ascending U.spp. infection mainly occurred in pregnancies delivering <28 weeks after preterm rupture of membranes or preterm labour (9/17, 52.3%). Intrauterine detection of U.spp., but not vaginal colonization, was associated with a significantly higher rate of severe intraventricular haemorrhage, retinopathy of prematurity, bronchopulmonary dysplasia, and unfavourable psychomotor outcome.
Conclusion
Ascending U.spp. infection after previous vaginal colonization occurred in almost one-third of pregnancies delivering ≤32 weeks, with particularly high rates in those <28 weeks, and was associated with adverse outcome of preterm infants.
Keywords: Ureaplasma species, Vaginal colonization, Ascending infection, Intrauterine colonization, Neonatal outcome
Introduction
Preterm birth is the major cause of neonatal mortality and morbidity. Around 40% of preterm births are associated with intrauterine infection, mostly resulting from ascending vaginal infections [1, 2]. Bacteria colonizing the lower vaginal tract are able to ascend into the uterine compartment, where they can subsequently activate local inflammatory pathways [2]. One of these vaginal commensals is Ureaplasma species (U.spp.) [3]. The association between intrauterine U.spp. infection and spontaneous preterm birth is well supported by literature. Moreover, the U.spp.-induced inflammatory response might contribute to neonatal injury [4, 5].
In an earlier multi-centre study, we were able to show that not only intrauterine but also vaginal colonization in early pregnancy, which can be routinely screened for, is associated with adverse pregnancy outcome [6, 7]. However, due to the high rate of vaginal colonization in pregnant women, ranging between 30 and 60% [3, 5, 6], vaginal U.spp. colonization cannot be used as a prognostic marker for preterm delivery.
Scarce data exists on the rate of ascending infection and invasion of the amniotic membranes and placenta after vaginal U.spp. colonization [8, 9]. Therefore, aim of the current study was to analyse the frequency of ascending U.spp. infection in vaginally colonized pregnant women delivering preterm and subsequent short- and long-term outcome of infants.
Methods
Study Design
A total of 4,330 pregnant women were enrolled in an earlier prospective observational multi-centre study and screened for vaginal U.spp. colonization in order to investigate a potential association between vaginal U.spp. colonization in early pregnancy and adverse outcome [6, 7]. Ureaplasma spp. colonization was diagnosed via DNA extraction from vaginal swabs and PCR analysis was performed as previously described and published by Mallard et al. [6, 10]. Since PCR analyses were performed for study purposes only, no macrolide eradication therapy was performed based on the results.
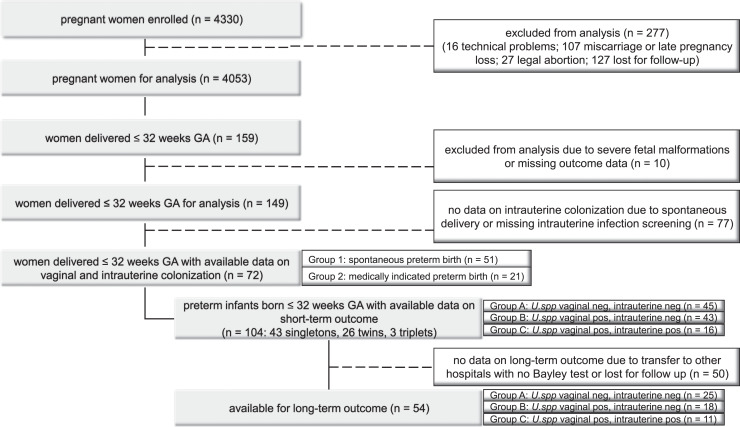
In the current study, we aimed at investigating the frequency of ascending U.spp. infection in the aforementioned study cohort. Women delivering ≤32 weeks of gestational age (wGA) with available data on amniotic and placental colonization as described below were included. Depending on the indication for preterm caesarean delivery, women were divided into two groups: group 1: “spontaneous preterm birth,” women with caesarean section after preterm premature rupture of membranes (pPROM) or preterm labour (PL) with no response to the indicated therapy; group 2: “medically indicated preterm birth,” women with caesarean section due to HELLP syndrome, preeclampsia, intrauterine growth retardation, or pathologic Doppler ultrasound (Fig. 1).
Fig. 1.
Patient flow chart.
Intrauterine Infection Screening Program
Based on earlier published data [11], a comprehensive infection screening program is performed as part of our routine protocol to improve the detection rate of intrauterine infections. During caesarean section ≤32 wGA, it is intended to obtain amniotic and placental biopsies under sterile conditions which are analysed for aerobic and anaerobic bacteria, U.spp., and fungi using culture techniques. Growth of contaminants (Staphylococcus epidermidis, Propionibacterium acnes, or Lactobacillus spp.) was considered as negative results. In women delivering vaginally, no amniotic and placental biopsies are obtained.
Clinical Outcome
Neonatal short- and long-term outcome was analysed depending on vaginal and intrauterine detection of U.spp.: group A: vaginal and intrauterine negative; group B: vaginal positive and intrauterine negative; group C: vaginal and intrauterine positive results. Data on short-term outcome were obtained from patients electronic medical records and defined as previously described [7]. The routine long-term follow-up program at our level III perinatal centre includes a standardized test using Bayley Scales of Infant Development at 24 months adjusted age for all preterm infants born ≤32 wGA [7]. Based on German norms, patients were classified as normal (≥85), mildly impaired (70–84, <1 SD), moderately impaired (69–55, <2 SDs), or severely impaired (<55, <3 SDs) [12]. Normal outcome and mild impairment were categorized as “favourable outcome,” moderate impairment and severe impairment as “unfavourable outcome.” Bayley testing was not performed in infants transferred to peripheral hospitals before discharge.
Statistical Analysis and Ethics
Statistical analyses were performed using SPSS, version 23 or later (IBM Corporation) with p values <0.05 considered statistically significant. Quantitative data are expressed as median (interquartile range) and qualitative data as counts (percentages). Differences between groups were summarized using descriptive statistics with the Mann-Whitney U and Kruskal-Wallis tests for continuous variables and the χ2 test (or variants thereof) for categorical variables. Linear regression was used to evaluate the association between outcome adjusted for the covariate GA at birth, multiple birth status, as well as statistically significant short-term morbidities (severe intraventricular haemorrhage [IVH] and any retinopathy of prematurity [ROP]) and colonization group.
The study was approved by the Ethics Committees of the Medical University of Vienna (EK 655/2008) and the City of Vienna (EK 09-120-VK). All participants gave written informed consent prior to their participation.
Results
Study Population
A total of 159 pregnant women included in the previous multi-centre study delivered between 23+0 and 32+6 wGA, whereof 10 cases were excluded due to severe foetal malformations (n = 7) or missing outcome data (n = 3). Intrauterine microbiological results were available in 72 cases with caesarean section (43 singletons, 26 twins, 3 triplets), resulting in 104 preterm infants with known status of intrauterine colonization (Fig. 1). Of those 72 pregnancies with available intrauterine microbiological results (study group), caesarean section was performed after pPROM (n = 35) or PL (n = 16) with no response to the indicated therapy in 51 cases (group 1), and due to other indications in 21 cases (group 2).
Intrauterine Microbial Growth
Intrauterine growth was found in 23/72 (31.9%) pregnancies delivering via caesarean sections ≤32 wGA, mainly in group 1 pregnancies (22/23). The organisms most commonly detected were U.spp. (12/23, 52.2%), followed by E. coli (5/23, 21.7%) and Enterococcus faecalis (4/23, 17.4%). Details on microorganisms found in the amniotic membranes or placenta in the two groups are shown in Table 1.
Table 1.
Microbiological results of amniotic and placental biopsies at caesarean section, depending on the indication for preterm birth
| Spontaneous preterm birth (group 1) | Medically indicated preterm birth (group 2) | |
|---|---|---|
| Pregnancies | 51 | 21 |
| U.spp. only | 7 (13.7) | 0 (0) |
| Coinfection U.spp. and other pathogen | 5a (9.8) | 0 (0) |
| Other pathogens | 10b (19.6) | 1c (4.8) |
| None or contaminants | 29 (56.9) | 20 (95.2) |
Data are presented as n (%).
U.spp., Ureaplasma species.
a1x E. coli, 1x Enterococcus faecalis, 2x Mycoplasma spp., 1x Streptococcus spp.
b3x E. coli, 2x Enterococcus faecalis, 2x Streptococcus spp., 1x Fusobacterium, 1x Mycoplasma spp. and Proteus mirabilis, 1x Candida spp.
c1x E. coli and Enterococcus faecalis.
Ascending Infection with Ureaplasma spp.
In total, 42/72 (58.3%) women in the study group were screened positive for vaginal U.spp. colonization in the first trimester of pregnancy, 31/51 (60.1%) in group 1 and 11/21 (52.4%) in group 2. Of the 42 women with vaginal U.spp. colonization, 38 were positive for U. parvum, three for U. urealyticum, and one woman was colonized with both biovars.
Ureaplasma spp. was found in the amniotic membranes or placenta in 12/42 cases, thus, the total rate of intrauterine ascending infection in vaginally colonized pregnant women delivering via caesarean section ≤32 wGA was 28.6%. Intrauterine U.spp. infection was found exclusively in group 1 pregnancies (12/31, 38.7%; Table 2). Ten women were vaginally colonized with U. parvum and two with U. urealyticum. In none of the women negative for vaginal U.spp. colonization in early pregnancy, U.spp. was detected in the amniotic membranes or placenta at delivery. Intrauterine detection of U.spp. was highest in women in the spontaneous preterm birth group delivering <28 wGA (9/17, 52.3%; Table 2).
Table 2.
Vaginal and intrauterine Ureaplasma spp. detection rates depending on the indication for preterm caesarean section
| Total | Spontaneous preterm birth (group 1) | Medically indicated preterm birth (group 2) | |||
|---|---|---|---|---|---|
| Pregnancies | 72 | 51 | 21 | ||
| Vaginal colonization | 42 (58.3) | 31 (60.1) | 11 (52.4) | ||
| Intrauterine U.spp. infection (rate-ascending infection) | 12 (28.6) | 12 (38.7) | 0 (0) | ||
| Subgroup | <28 wGA | ≥28 wGA | <28 wGA | ≥28 wGA | |
|---|---|---|---|---|---|
| Pregnancies | 31 | 41 | 25 | 26 | 21 |
| Vaginal colonization | 19 (61.3) | 23 (56.1) | 17 (68) | 14 (53.4) | 0 (0) |
| Intrauterine U.spp. infection (rate-ascending infection) | 9 (47.4) | 3 (13.1) | 9 (52.3) | 3 (21.4) | 0 (0) |
Data are presented as n (%).
U.spp., Ureaplasma species; wGA, weeks of gestational age.
When examining subgroups depending on the reason for caesarean section in group 1, we observed that 20/35 (57.1%) women with pPROM were vaginally colonized with U.spp., and among this subset, U.spp. was found in the amniotic membranes or placenta of 8 individuals (40%). Eleven out of 16 (68.8%) women with caesarean section after PL with no response to the indicated therapy were vaginally colonized with U.spp. and intrauterine U.spp. colonization was found in 4 cases (36.4%). We could not identify any statistically significant risk factor for ascending U.spp. infection, although the rate of vaginal candidiasis was higher in women with ascending U.spp. infection (7/12, 58.3%) compared to women without ascending infection (9/30, 30%, p = 0.158).
There was no significant difference regarding vaginal/intrauterine U.spp. colonization status between singleton and multiple pregnancies (p = 0.383). Moreover, in our study group, no differences in intrauterine colonization status within each set of multiples were detected. Within the 3 sets of triplets, there was no intrauterine U.spp. colonization. Four of the 12 pregnancies with intrauterine detection of U.spp. were twin pregnancies, resulting in 16 infants exposed to intrauterine U.spp.
Neonatal Short-Term Outcome
Clinical characteristics as well as short-term outcome of preterm infants in relation to the U.spp. colonization status of the mother are summarized in Table 3. Significantly more infants in group C (vaginal and intrauterine detection of U.spp.) were diagnosed with severe IVH, any ROP, and bronchopulmonary dysplasia compared to groups A and B. Severe IVH and ROP remained significant after correction for gestational age (IVH: p = 0.005, ROP: p = 0.016). Necrotising enterocolitis was significantly more often diagnosed in group C compared to group A.
Table 3.
Clinical characteristics and short-term outcome of the study group depending on vaginal and intrauterine Ureaplasma spp. colonization status
| U. spp. detection vaginal/intrauterine | Total | Group A U.spp. neg/neg | Group B U.spp. pos/neg | Group C U.spp. pos/pos | p value |
|---|---|---|---|---|---|
| Infants, n (%) | 104 (100.0) | 45 (43.3) | 43 (41.3) | 16 (15.4) | |
| Clinical characteristics | |||||
| Gestational age, wGA | 28.9 (27.1–31.0) | 28.9 (27.6–30.6) | 30.0 (27.4–31.1) | 26.1 (25.9–29.9) | 0.005 |
| Weight, g | 1,110 (840–1,389) | 1,125 (975–1,353) | 1,180 (840–1,410) | 851 (763–1,155) | 0.086 |
| Male sex | 57 (54.8) | 27 (60.0) | 19 (44.2) | 11 (68.8) | 0.157 |
| Antenatal steroids | 102 (98.1) | 44 (97.8) | 42 (97.7) | 16 (100.0) | 0.830 |
| Singleton pregnancy | 42 (40.4) | 16 (35.6) | 18 (41.9) | 8 (50.0) | 0.580 |
| Cord blood-pH | 7.32 (7.29–7.36) | 7.33 (7.29–7.39) | 7.32 (7.29–7.34) | 7.31 (7.27–7.35) | 0.401 |
| Apgar 1 | 8 (7–8) | 8 (7–8) | 8 (6–8) | 8 (7–8) | 0.066 |
| Apgar 5 | 8 (8–9) | 9 (8–9) | 8 (8–9) | 8 (8–9) | 0.121 |
| Apgar 10 | 9 (9–9) | 9 (9–9) | 9 (9–9) | 9 (9–9) | 0.104 |
| Short-term outcome | |||||
| Ventilation 1st week | 25 (24.0) | 9 (20.0) | 9 (20.9) | 7 (43.8) | 0.133 |
| Any IVH | 17 (16.5) | 6 (13.3) | 6 (14.0) | 5 (31.3) | 0.224 |
| Severe IVH | 8 (7.8) | 1 (2.3) | 2 (4.7) | 5 (31.3) | 0.001a* |
| NEC | 7 (7.1) | 1 (2.3) | 3 (7.3) | 3 (20.0) | 0.071b |
| Any ROP | 24 (24.2) | 7 (15.9) | 9 (22.5) | 8 (53.3) | 0.013a# |
| Severe ROP | 5 (5.1) | 1 (2.3) | 2 (5.0) | 2 (13.3) | 0.240 |
| BPD 28 daysc | 39 (38.6) | 17 (38.6) | 13 (31.0) | 9 (60.0) | 0.140 |
| BPD 36 weeksc | 6 (6.1) | 2 (4.7) | 0 (0.0) | 4 (26.7) | 0.001a° |
Data are presented as n (%)/(IQR).
U.spp., Ureaplasma species; wGA, weeks of gestational age; IQR, interquartile range; g, gram; EOS, early-onset sepsis; IVH, intraventricular haemorrhage; ROP, retinopathy of prematurity; BPD, bronchopulmonary dysplasia; NEC, necrotising enterocolitis.
a p value <0.05 group U.spp. vaginal positive/intrauterine positive compared to group U.spp. vaginal negative/intrauterine negative as well as to group U.spp. vaginal positive/intrauterine negative.
b p value <0.05 group U.spp. vaginal positive/intrauterine positive compared to group U.spp. vaginal negative/intrauterine negative.
cOnly patients surviving 28 days of life (n = 101)/36 weeks of adjusted age (n = 98), respectively, are included.
*p value = 0.005 after correction for gestational age.
# p value = 0.016 after correction for gestational age.
° p value = 0.072 (ns) after correction for gestational age.
Neonatal Long-Term Outcome
Long-term outcome data were available for 54 infants (Table 4). Overall, favourable motor outcome at 24 months adjusted age was found in 41/48 (85.4%) survivors and favourable cognitive outcome in 33/48 (68.8%) neonates.
Table 4.
Long-term outcome at 24 months depending on vaginal and intrauterine Ureaplasma spp. colonization status
| U. spp. detection vaginal/intrauterine | total | Group A U.spp. neg/neg | Group B U.spp. pos/neg | Group C U.spp. pos/pos | p value | |
|---|---|---|---|---|---|---|
| Outcome, n (%) | 54 (100) | 25 (46.3) | 18 (33.3) | 11 (20.4) | ||
| Death | 6 (5.8) | 2 (4.4) | 3 (7.0) | 1 (6.3) | 0.875 | |
| Motor outcome in survivors | ||||||
| Favourable outcome | >85 | 31 (64.6) | 13 (56.5) | 13 (86.7) | 5 (50.0) | <0.001a* |
| 70–84 | 10 (20.8) | 9 (39.1) | 1 (6.7) | 0 (0.0) | ||
| Unfavourable outcome | 69–55 | 4 (8.3) | 0 (0.0) | 0 (0.0) | 4 (40.0) | |
| <55 | 3 (6.3) | 1 (4.4) | 1 (6.7) | 1 (10.0) | ||
| Cognitive outcome in survivors | ||||||
| Favourable outcome | >85 | 23 (47.9) | 13 (56.5) | 8 (53.3) | 2 (20.0) | 0.173 |
| 70–84 | 10 (20.8) | 4 (17.4) | 4 (26.7) | 2 (20.0) | ||
| Unfavourable outcome | 69–55 | 6 (12.5) | 4 (17.4) | 0 (0.0) | 2 (20.0) | |
| <55 | 9 (18.8) | 2 (8.7) | 3 (20.0) | 4 (40.0) | ||
Data are presented as n (%).
U.spp., Ureaplasma species; a, age.
a p value <0.05 group U.spp. vaginal positive/intrauterine positive compared to group U.spp. vaginal negative/intrauterine negative as well as to group U.spp. vaginal positive/intrauterine negative.
*p value = 0.009 after correction for gestational age, multiple birth status, severe IVH, and any ROP.
There was a statistically significant difference between groups for motor outcome (p < 0.001). Significantly more infants with intrauterine detection of U.spp. (group C) had an unfavourable motor outcome (5/10, 50%) compared to group A (1/23, 4.4%) and group B (1/15, 6.7%). In order to adjust for significant group differences (clinical characteristics as well as neonatal short-term morbidities; Table 3) as well as multiple birth status (not significantly different between groups), results were controlled for gestational age, severe IVH, any ROP, and multiple birth status and remained statistically significant (p = 0.009). Unfavourable motor outcome was independent of the vaginal colonization status of mothers in early pregnancy.
For cognitive outcome, there was no statistically significant difference between groups. However, there was a trend towards an increased rate of unfavourable outcome in infants of group C (6/10, 60%) compared to infants of groups A (6/23, 26.1%) and B (3/15, 20%).
Discussion
Intrauterine infection is the most common cause for preterm birth; however, it is mostly undetected until pPROM or onset of PL [1]. Although non-invasive screening for U.spp. colonization in the vaginal tract is feasible, the prognostic benefit is low, since most women with vaginal colonization deliver at term and reasons for ascending infection with potential subsequent preterm delivery are not clear. In the present study, we evaluated the rate of ascending U.spp. infection in vaginally colonized pregnant women delivering preterm via caesarean section and associated short- and long-term outcome of preterm infants.
Microbial growth in the intrauterine compartment was detected in 31.9% of pregnant women delivering via caesarean section ≤32 wGA, with U.spp. being the most common organism. Ureaplasma spp. and other intrauterine microbials were mainly present in the spontaneous preterm birth group with only one case of bacterial growth in the placenta in the medically indicated preterm birth group. All cases of ascending U.spp. infection in vaginally colonized pregnant women were found in the spontaneous preterm birth group. The rate of intrauterine detection of U.spp. was highest in women in the spontaneous preterm birth group delivering <28 wGA.
To the best of our knowledge, there are little data on rates of ascending U.spp. infections in pregnancy. Kafetzis cultured nose, throat, and tracheal aspirate from infants of vaginally colonized mothers and found transmission rates to the newborn of 17% in term and 33% in preterm infants, with the highest rate of 60% in infants with a birth weight <1,000 g [9]. Abele-Horn et al. [8] found transmission rates of 37% in term and up to 95% in very low-birth-weight infants. In a recent review, Viscardi [13] reported almost half of preterm infants ≤32 wGA being colonized with U.spp. in one or more compartments (respiratory, blood, cerebrospinal fluid) [13], which is in line with our data.
Analysis of short- and long-term outcome of our cohort revealed a statistically significantly increased risk for severe IVH, any ROP, and unfavourable motor outcome at 24 months adjusted age in preterm infants with intrauterine detection of U.spp. compared to infants with negative results, independent of gestational age and neonatal morbidities. Moreover, a trend for a higher risk of bronchopulmonary dysplasia and necrotising enterocolitis as well as worse cognitive outcome was observed in these patients. Results of the current study suggest that the increased risk for adverse short- and long-term outcome is only true for preterm infants with ascending U.spp. infection during pregnancy.
Although the pathomechanisms are largely unknown, Ureaplasmas are increasingly considered as pathogens for neonatal central nervous system injury [14]. It is known, that Ureaplasmas are able to induce a chronic inflammation in the intrauterine compartment and the subsequent host immune response might contribute to the pathogenesis of neonatal brain damage [5]. Viscardi et al. [15] demonstrated that in colonized preterm infants, U.spp. can invade from the respiratory tract into the bloodstream, cross the immature blood-brain barrier, and be associated with central nervous inflammation, which illustrates that brain injury may also be generated by direct impact of local U.spp. central nervous system infection. These data are supported by several studies by the group of Glaser et al. [14] in vivo as well as in the ovine model showing that Ureaplasmas seem to weaken host immune defence strategies and impair the blood-brain barrier function which facilitates chronic neuroinflammation [14, 16]. Normann et al. [17] demonstrated in a mouse model that antenatal exposure to U.spp. can induce central microgliosis, delayed myelination, and disturbed brain development by decreasing neurons in the neocortex. Therefore, it seems plausible that intrauterine U.spp. exposure can contribute to neonatal brain injury and white matter disease. Clinical data regarding long-term outcome after intrauterine U.spp. exposure are rare and existing studies report contradictory results. A small Japanese study did not find any association between vaginal U.spp. colonization and long-term outcome, while results of the present analysis confirm data of an earlier study of our research group showing an association between intrauterine U.spp. at birth and adverse psychomotor outcome at a 24-month adjusted age [18, 19].
The identification of risk factors for ascending U.spp. infections remains challenging. Previous studies could not consistently demonstrate why these commensals can lead to ascending infections with colonization of the amniotic membranes and the placenta but stay clinically silent in most cases, and approaches to explain a potential pathomechanism are controversial. Much work has been done into investigating the pathogenicity of different Ureaplasma biovars or serovars, the composite of the vaginal microbiome or coinfections with other bacteria. In our previous study, we found that U. parvum serovar 3 was associated with the highest risk of preterm birth and this risk was significantly increased if colonization with U. parvum was accompanied by bacterial vaginosis [20]. Payne et al. [21], who screened pregnant women for vaginal U.spp. and Candida spp., found that the odds ratio for preterm birth increased when Candida albicans was found alongside with U. parvum compared to U. parvum alone [21], while Farr et al. [22] found that vaginal candidosis alone is no significant risk factor for spontaneous preterm birth. Our current data showed higher rates of vaginal candidosis in women with ascending U.spp. infection compared to women without ascending infection, however, numbers are small and data were not statistically significant. These findings are in line with the hypothesis that alterations of the vaginal microbiome, e.g., a Lactobacillus-poor microbiome, accompanied by a high abundance of U.spp. as described by DiGiulio et al. [23], might increase the risk for ascending infection and adverse pregnancy outcomes [24, 25]. This could be explained by the protective effect of a healthy, mainly lactobacilli-driven microbiome in keeping an acid milieu and preventing other organisms to overgrow and ascend into the intrauterine department [26, 27]. In a paper on ascending bacterial infection, the group by Romero proposed that the risk for ascending infection is not dependent on the type of bacteria present in the vagina but on the capacity of the cervix to control them, and that this anatomical and immunological barrier is altered in the presence of viral infections [28]. Similarly, Pavlidis et al. [29] demonstrated the crucial role of the cervical epithelium as a barrier against ascending infection in a mouse model.
A major limitation of our study is the relatively small number of cases, particularly regarding infants with 24-month follow-up data. However, preterm infants included in this study derived from more than 4,000 pregnancies included in the original multi-centre study, which demonstrates the difficulty in obtaining a study group large enough to satisfactorily answer these questions with enough statistical power. Moreover, in this study setting we did not assess intra-amniotic cytokine levels, which does not allow to differentiate between intrauterine U.spp. colonization and intrauterine infection and inflammation. A very recent paper, however, suggests that abundant “colonizers” do not exist in the placenta and the womb in normal, non-pathogenic circumstances [30].
Conclusion
In summary, we found a high rate of intrauterine growth of U.spp. at delivery after previous vaginal colonization in pregnancies delivering via caesarean sections after pPROM or PL ≤32 wGA, with particularly high rates in those delivering <28 wGA. Only infants born after vaginal U.spp. colonization and subsequent ascending infection with intrauterine detection of U.spp. had a statistically significantly increased risk of adverse short- and long-term outcomes.
Statement of Ethics
The study was approved by the Ethics Committees of the Medical University of Vienna (EK 655/2008) and the City of Vienna (EK 09-120-VK). All participants gave their written informed consent prior to participation in the study.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The study did not receive any funding.
Author Contributions
Conceptualization/design and supervision/oversight: J.R.-B., A.B., and K.G. Methodology: J.R.-B., R.F., A.F., B.W., and A.B. Investigation: J.R.-B., R.F., A.F., and B.W. Data curation: J.R.-B. and K.G. Formal analysis: K.G. Resources: A.B.
Funding Statement
The study did not receive any funding.
Data Availability Statement
Data are not publicly available due to ethical reasons. Further enquiries can be directed to the corresponding author.
References
- 1. Bastek JA, Gómez LM, Elovitz MA. The role of inflammation and infection in preterm birth. Clin Perinatol. 2011;38(3):385–406. [DOI] [PubMed] [Google Scholar]
- 2. Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31(3):553–84. [DOI] [PubMed] [Google Scholar]
- 3. DiGiulio DB, Romero R, Kusanovic JP, Gómez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64(1):38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Motomura K, Romero R, Xu Y, Theis KR, Galaz J, Winters AD, et al. Intra-amniotic infection with ureaplasma parvum causes preterm birth and neonatal mortality that are prevented by treatment with clarithromycin. mBio. 2020;11(3):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silwedel C, Speer CP, Glaser K. Ureaplasma-associated prenatal, perinatal, and neonatal morbidities. Expert Rev Clin Immunol. 2017;13(11):1073–87. [DOI] [PubMed] [Google Scholar]
- 6. Rittenschober-Böhm J, Waldhoer T, Schulz SM, Stihsen B, Pimpel B, Goeral K, et al. First trimester vaginal ureaplasma biovar colonization and preterm birth: results of a prospective multicenter study. Neonatology. 2018;113:1–6. [DOI] [PubMed] [Google Scholar]
- 7. Rittenschober-Böhm J, Habermüller T, Waldhoer T, Fuiko R, Schulz SM, Pimpel B, et al. Maternal vaginal ureaplasma spp. colonization in early pregnancy is associated with adverse short- and long-term outcome of very preterm infants. Children. 2021;8(4):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abele-Horn M, Peters J, Genzel-Boroviczény O, Wolff C, Zimmermann A, Gottschling W. Vaginal Ureaplasma urealyticum colonization: influence on pregnancy outcome and neonatal morbidity. Infection. 1997;25(5):286–91. [DOI] [PubMed] [Google Scholar]
- 9. Kafetzis DA, Skevaki CL, Skouteri V, Gavrili S, Peppa K, Kostalos C, et al. Maternal genital colonization with Ureaplasma urealyticum promotes preterm delivery: association of the respiratory colonization of premature infants with chronic lung disease and increased mortality. Clin Infect Dis. 2004;39(8):1113–22. [DOI] [PubMed] [Google Scholar]
- 10. Mallard K, Schopfer K, Bodmer T. Development of real-time PCR for the differential detection and quantification of Ureaplasma urealyticum and Ureaplasma parvum. J Microbiol Methods. 2005;60(1):13–9. [DOI] [PubMed] [Google Scholar]
- 11. Witt A, Berger A, Gruber CJ, Petricevic L, Apfalter P, Worda C, et al. Increased intrauterine frequency of Ureaplasma urealyticum in women with preterm labor and preterm premature rupture of the membranes and subsequent cesarean delivery. Am J Obstet Gynecol. 2005;193(5):1663–9. [DOI] [PubMed] [Google Scholar]
- 12. Fuiko R, Oberleitner-Leeb C, Klebermass-Schrehof K, Berger A, Brandstetter S, Giordano V. The impact of norms on the outcome of children born very-preterm when using the bayley-III: differences between US and German norms. Neonatology. 2019;116(1):29–36. [DOI] [PubMed] [Google Scholar]
- 13. Viscardi RM. Ureaplasma species: role in neonatal morbidities and outcomes. Arch Dis Child Fetal Neonatal. 2014;99(1):F87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Silwedel C, Speer CP, Härtel C, Glaser K. Ureaplasma-driven neuroinflammation in neonates: assembling the puzzle pieces. Neonatology. 2020;117(6):665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Viscardi RM, Hashmi N, Gross GW, Sun CC, Rodriguez A, Fairchild KD. Incidence of invasive ureaplasma in VLBW infants: relationship to severe intraventricular hemorrhage. J Perinatol. 2008;28(11):759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silwedel C, Hütten MC, Speer CP, Härtel C, Haarmann A, Henrich B, et al. Ureaplasma-driven neonatal neuroinflammation: novel insights from an ovine model. Cell Mol Neurobiol. 2023;43(2):785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Normann E, Lacaze-Masmonteil T, Eaton F, Schwendimann L, Gressens P, Thébaud B. A novel mouse model of Ureaplasma-induced perinatal inflammation: effects on lung and brain injury. Pediatr Res. 2009;65(4):430–6. [DOI] [PubMed] [Google Scholar]
- 18. Suzuki Y, Horie K, Yada Y, Kono Y, Hirashima C, Usui R, et al. Vaginal Ureaplasma species increase chorioamnionitis in very preterm infants with preterm premature rupture of the membranes at < 28 weeks of gestation. Eur J Clin Microbiol Infect Dis. 2018;37(12):2371–80. [DOI] [PubMed] [Google Scholar]
- 19. Berger A, Witt A, Haiden N, Kaider A, Klebermasz K, Fuiko R, et al. Intrauterine infection with Ureaplasma species is associated with adverse neuromotor outcome at 1 and 2 years adjusted age in preterm infants. J Perinat Med. 2009;37:72–8. [DOI] [PubMed] [Google Scholar]
- 20. Rittenschober-Bohm J, Waldhoer T, Schulz SM, Pimpel B, Goeral K, Kasper DC, et al. Vaginal Ureaplasma parvum serovars and spontaneous preterm birth. Am J Obstet Gynecol. 2019;220(6):594.e1–9. [DOI] [PubMed] [Google Scholar]
- 21. Payne MS, Ireland DJ, Watts R, Nathan EA, Furfaro LL, Kemp MW, et al. Ureaplasma parvum genotype, combined vaginal colonisation with Candida albicans, and spontaneous preterm birth in an Australian cohort of pregnant women. BMC Pregnancy Childbirth. 2016;16(1):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farr A, Kiss H, Holzer I, Husslein P, Hagmann M, Petricevic L. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet Gynecol Scand. 2015;94(9):989–96. [DOI] [PubMed] [Google Scholar]
- 23. DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A. 2015;112(35):11060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Freitas AC, Chaban B, Bocking A, Rocco M, Yang S, Hill JE, et al. The vaginal microbiome of pregnant women is less rich and diverse, with lower prevalence of Mollicutes, compared to non-pregnant women. Sci Rep. 2017;7(1):9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Donders GG, Van Bulck B, Caudron J, Londers L, Vereecken A, Spitz B. Relationship of bacterial vaginosis and mycoplasmas to the risk of spontaneous abortion. Am J Obstet Gynecol. 2000;183(2):431–7. [DOI] [PubMed] [Google Scholar]
- 26. Vinturache AE, Gyamfi-Bannerman C, Hwang J, Mysorekar IU, Jacobsson B; Preterm Birth International Collaborative PREBIC . Maternal microbiome: a pathway to preterm birth. Semin Fetal Neonatal Med. 2016;21(2):94–9. [DOI] [PubMed] [Google Scholar]
- 27. Harada K, Tanaka H, Komori S, Tsuji Y, Nagata K, Tsutsui H, et al. Vaginal infection with Ureaplasma urealyticum accounts for preterm delivery via induction of inflammatory responses. Microbiol Immunol. 2008;52(6):297–304. [DOI] [PubMed] [Google Scholar]
- 28. Racicot K, Cardenas I, Wünsche V, Aldo P, Guller S, Means RE, et al. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J Immunol. 2013;191(2):934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pavlidis I, Spiller OB, Sammut Demarco G, MacPherson H, Howie SEM, Norman JE, et al. Cervical epithelial damage promotes Ureaplasma parvum ascending infection, intrauterine inflammation and preterm birth induction in mice. Nat Commun. 2020;11(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kennedy KM, de Goffau MC, Perez-Muñoz ME, Arrieta MC, Bäckhed F, Bork P, et al. Questioning the fetal microbiome illustrates pitfalls of low-biomass microbial studies. Nature. 2023;613(7945):639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are not publicly available due to ethical reasons. Further enquiries can be directed to the corresponding author.