Abstract
Mouse hepatitis virus receptor (MHVR) is a murine biliary glycoprotein (Bgp1a). Purified, soluble MHVR expressed from a recombinant vaccinia virus neutralized the infectivity of the A59 strain of mouse hepatitis virus (MHV-A59) in a concentration-dependent manner. Several anchored murine Bgps in addition to MHVR can also function as MHV-A59 receptors when expressed at high levels in nonmurine cells. To investigate the interactions of these alternative MHVR glycoproteins with MHV, we expressed and purified to apparent homogeneity the extracellular domains of several murine Bgps as soluble, six-histidine-tagged glycoproteins, using a baculovirus expression system. These include MHVR isoforms containing four or two extracellular domains and the corresponding Bgp1b glycoproteins from MHV-resistant SJL/J mice, as well as Bgp2 and truncation mutants of MHVR and Bgp1b comprised of the first two immunoglobulin-like domains. The soluble four-domain MHVR glycoprotein (sMHVR[1-4]) had fourfold more MHV-A59 neutralizing activity than the corresponding soluble Bgp1b (sBgp1b) glycoprotein and at least 1,000-fold more neutralizing activity than sBgp2. Although virus binds to the N-terminal domain (domain 1), soluble truncation mutants of MHVR and Bgp1b containing only domains 1 and 2 bound virus poorly and had 10- and 300-fold less MHV-A59 neutralizing activity than the corresponding four-domain glycoproteins. In contrast, the soluble MHVR glycoprotein containing domains 1 and 4 (sMHVR[1,4]) had as much neutralizing activity as the four-domain glycoprotein, sMHVR[1-4]. Thus, the virus neutralizing activity of MHVR domain 1 appears to be enhanced by domain 4. The sBgp1b[1-4] glycoprotein had 500-fold less neutralizing activity for MHV-JHM than for MHV-A59. Thus, MHV strains with differences in S-glycoprotein sequence, tissue tropism, and virulence can differ in the ability to utilize the various murine Bgps as receptors.
Mouse hepatitis viruses (MHV) are a group of coronaviruses that can cause diarrhea, hepatitis, immunological dysfunction, acute and chronic neurological disorders, or subclinical infections in mice (3, 14, 62). In vitro, MHV strains readily infect many murine cell lines, usually causing cell fusion and lysis. MHV infection is initiated by binding of the viral spike, a trimer of 180-kDa S glycoproteins (11, 16), to a receptor glycoprotein on the cell membrane, followed by S-mediated fusion of the viral envelope with the cell membrane (60). The first receptor identified for MHV, MHVR (also called Bgp1a [24, 44, 65, 66]), is a biliary glycoprotein (Bgp) in the carcinoembryonic antigen (CEA) family of the immunoglobulin (Ig) superfamily (7, 53, 65). MHVR consists of four Ig-like extracellular domains, a transmembrane domain, and either a long or a short cytoplasmic tail (24, 42). MHVR is expressed at the portals of virus entry on the apical membranes of intestinal and respiratory epithelial cells, on the luminal surfaces of endothelial cells, and in lymphoid cells, macrophages, liver, and spleen (15, 20, 31). Bgps can function as cell adhesion molecules and may have signal transducing activity (41, 61). Recognition of MHVR by the viral spike glycoprotein is the initial determinant of species specificity and tissue tropism of MHV infection, although subsequent steps in the virus life cycle, such as membrane fusion, virus uncoating, and replication, can also affect susceptibility to MHV infection (1, 13, 18, 24, 57, 69). The viral spike protein and an anti-MHVR monoclonal antibody (MAb CC1) that blocks virus infection of mouse cells both bind to the N-terminal Ig-like domain of MHVR (referred to in this report as domain 1) (25, 30).
In addition to MHVR, several other murine glycoproteins in the CEA family can also serve as receptors for MHV strain A59 (MHV-A59) when they are expressed at high levels in MHV-resistant nonmurine cells (22, 70, 71). These receptor glycoproteins include an isoform of MHVR consisting of domains 1 and 4 (MHVR[1,4]) (42, 70), homologous glycoproteins that have four or two Ig-like domains derived from MHV-resistant SJL/J mice (referred to as Bgp1b[1-4] and Bgp1b[1,4], respectively) (22, 42, 67, 71), the two-domain Bgp2 glycoprotein (44), and brain CEA, a member of the pregnancy-specific glycoprotein family (9). SJL/J mice are homozygous for the Bgp1b allele. They are much more resistant to infection by MHV-A59 than BALB/c mice, and Bgp1b binds virus poorly relative to MHVR (3, 4, 6, 19, 47, 71). However, when recombinant Bgp1b is expressed at high levels in MHV-resistant hamster (BHK) cells or in an embryonic fibroblast cell line derived from MHV-resistant SJL/J mice, these cells become susceptible to infection by MHV-A59, suggesting that receptor density may also affect cellular susceptibility to infection (22, 71). The principal differences between MHVR and Bgp1b lie in domain 1, which differs in 28 of its 108 amino acids (65, 67, 71). The Bgp2 and bCEA glycoproteins are much less efficient receptors than MHVR (9, 22, 44). The outcome of MHV infection depends on the strain of MHV, mouse strain and age, and the dose and route of administration of the virus (4, 18, 57, 63). The different spike glycoproteins of various MHV strains may interact differently with the Bgp receptor glycoproteins, possibly affecting the tissue tropisms and virulence of the virus strains in the same host (13, 28, 48, 51, 70).
Ig-related glycoproteins are specific receptors for poliovirus, rhinovirus, and human immunodeficiency virus (HIV) (reviewed in references 33, 45, 50, and 55), and soluble versions of these membrane glycoproteins have been used to study virus binding and uncoating. A truncated, soluble, anchorless form of the poliovirus receptor neutralizes virus by binding to and eliciting structural changes in the viral capsid (37, 68). Soluble forms of ICAM-1, the major receptor for human rhinoviruses, also neutralize infectious virus by inducing conformational changes in the virion that resemble the normal process of uncoating (32, 34, 46). The binding of soluble CD4 to soluble HIV gp120 spike glycoprotein elicits conformational changes in both gp120 and CD4 that are required for viral fusion at the cell membrane (17). Several soluble forms of MHVR and Bgp1b have been used to investigate the interactions between MHV and its receptors. A soluble, truncated glycoprotein consisting of MHVR domain 1 alone blocks MHV-A59 infection in vitro (23), and a fusion protein consisting of MHVR domain 1 with the Fc domain of IgG showed differences in binding and induction of conformational change in the spike glycoproteins of two MHV-JHM variants (30). Furthermore, unpurified soluble two-domain MHVR[1,4] neutralizes MHV-JHM infectivity 500-fold better than the corresponding soluble two-domain Bgp1b[1,4] (48).
This report describes the expression of a soluble, anchorless MHVR glycoprotein by using a recombinant vaccinia virus and the expression of five murine Bgp glycoproteins, plus two two-domain (domains 1 and 2) truncation mutants of MHVR and Bgp1b, as anchorless, soluble glycoproteins by using a baculovirus expression system. These glycoproteins were purified to apparent homogeneity and used to compare their MHV-A59 binding activities and neutralizing activities for MHV-A59 and MHV-JHM.
MATERIALS AND METHODS
Cells and viruses.
Spodoptera frugiperda (Sf9) cells (Invitrogen, Carlsbad, Calif.) were maintained at 27°C in TC-100 medium (JRH BioSciences, Lenexa, Kans.) with 10% fetal bovine serum (FBS; Gemini Bioproducts, Calabasas, Calif.) and 2% antibiotics (penicillin, streptomycin, and amphotericin B; Gibco/BRL, Gaithersburg, Md.). The 17 clone 1 line of spontaneously transformed BALB/c 3T3 fibroblasts, L2 cells (22), and African green monkey kidney cells (Vero-76; American Type Culture Collection) were maintained in Dulbecco’s modified Eagle’s minimal essential medium (DMEM, Gibco/BRL) with 10% FBS and 2% antibiotics at 37°C and 5% CO2.
MHV-A59 was propagated and plaque assayed in 17 clone 1 cells (27). The MHV-JHM strain used in this study has the spike glycoprotein sequence of isolate MHV-4/DL/Cl-2 described by Rowe et al. (52). Vaccinia virus strain WR (kindly provided by B. Moss, National Institutes of Health, Bethesda, Md.) was propagated in CV-1 cells and used as the parent virus for vac-MHVR, which expresses soluble, anchorless four-domain MHVR (49).
Antibodies.
Anti-MHVR MAb CC1 binds to the N-terminal domain of MHVR but not Bgp1b (25), blocks virus binding to MHVR, and prevents virus infection of murine cells (24, 56, 66). MAb CC1 and a control IgG1 MAb directed against an irrelevant antigen (the B subunit of cholera toxin) were used as supernatant media from hybridoma cultures. Polyclonal rabbit anti-MHVR antiserum 655 was prepared by inoculation with MHVR glycoprotein purified from Swiss Webster mouse liver by affinity chromatography with MAb CC1 (66). In immunoblots, anti-MHVR antiserum 655 detects both MHVR and Bgp1b glycoproteins but not Bgp2 (24). The polyclonal goat antibody AO4 directed against purified MHV-A59 spike glycoprotein was previously described (59).
Soluble MHVR from recombinant vaccinia virus.
The recombinant vaccinia virus, vac-sMHVR, that encodes a soluble four-domain MHVR glycoprotein [truncated at amino acid 420, just prior to the transmembrane domain; designated sMHVR(vac)] is described in reference 49. Vero cells were inoculated with vac-sMHVR at a multiplicity of infection (MOI) of 10, and culture medium containing the secreted 106-kDa sMHVR(vac) glycoprotein was collected 48 h postinoculation. The sMHVR(vac) glycoprotein was concentrated as a 50 to 90% ammonium sulfate precipitate and then separated from serum proteins by preparative isoelectric focusing in a pH 3 to 10 gradient in a Rotofor apparatus (Bio-Rad, Hercules, Calif.). Fractions (pH 3 to 3.5) containing sMHVR(vac) glycoprotein were identified by dot blot analysis with MAb CC1 and then pooled and concentrated by ultrafiltration with a Centricon-10 (Amicon, Beverly, Mass.) before further purification by gel filtration on a Superose-6 column (Pharmacia, Piscataway, N.J.).
Recombinant baculoviruses expressing six-histidine-tagged soluble receptor glycoproteins.
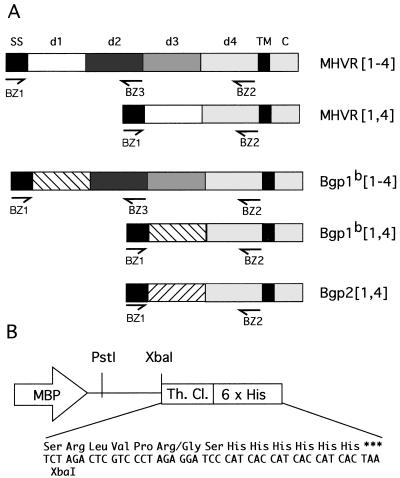
The baculovirus expression vector pAcMP2 (PharMingen, San Diego, Calif.) was digested with XbaI and BamHI, and preannealed oligonucleotides BZ4 and BZ5 (CTAGACTCGTCCCTAGAGGATCCCATCACCATCACCATCACTAA and TCTTCGTGATGGTGATGGTGATGGGATCCTCTAGGGACGAGT; all oligonucleotides from Gibco/BRL) were ligated in to create pAcMP2(TH). The inserted oligonucleotides encoded a consensus thrombin cleavage site and six histidine residues that would be coupled to the carboxyl termini of proteins encoded by cDNAs ligated in frame into the XbaI site. The cDNA sequences encoding the leader and four extracellular Ig-like domains of MHVR (24) or Bgp1b (42) were amplified by using Pfu polymerase (Stratagene, La Jolla, Calif.) and oligonucleotides BZ1 and BZ2 (GCACTGCAGACCATGGAGCTGGCCTCAGCA and CGCGTGTCTAGAGAGGCCTCCTTGTGTTGG), which added PstI and XbaI restriction sites to the 5′ and 3′ ends, respectively, to allow cloning into pAcMP2(TH). The resulting constructs, called pAcMP2(TH)-MHVR[1-4] and pAcMP2(TH)-Bgp1b[1-4], produced soluble, six-histidine-tagged glycoproteins called sMHVR[1-4] and sBgp1b[1-4], respectively. Similarly, we made three additional constructs that combined pAcMP2(TH) with Pfu-amplified products encoding the leader sequence and first two extracellular domains of MHVR and Bgp1b (oligonucleotides BZ1 and BZ3 [CGCGCGTCTAGAAGGGGGATATAATCGGGGT]) and the leader sequence and two extracellular Ig-like domains of Bgp2 (44) (oligonucleotides BZ1 and BZ13 [CGCGCTCTAGAGGGGACATCTGAAATGTCATTG]). The anchorless, six-histidine-tagged proteins encoded by these constructs are called sMHVR[1,2], sBgp1b[1,2], and sBgp2[1,4], respectively. Two additional constructs, pAcMP2(TH)-sMHVR[1,4] and pAcMP2(TH)-sBgp1b[1,4], were generated by combining pAcMP2(TH) with the products made by amplifying cDNAs encoding the naturally occurring 1,4 splice variants of MHVR and Bgp1b (42), using oligonucleotides BZ1 and BZ2. The soluble, six-histidine-tagged glycoproteins encoded by these constructs are called sMHVR[1,4] and sBgp1b[1,4], respectively. The baculovirus expression constructs were cotransfected with BaculoGold DNA (PharMingen) into Sf9 cells, and progeny viruses were plaque purified according to the manufacturer’s instructions. Diagrams of the constructs are shown in Fig. 1.
FIG. 1.
Construction of the baculovirus expression vectors that express soluble, six-histidine-tagged MHVR and Bgp glycoproteins. (A) cDNAs encoding MHVR and related Bgp glycoproteins illustrate the cloning strategy used to express the signal sequence (SS) and Ig-like extracellular domains (d1, etc.) as soluble proteins. The transmembrane domain (TM) and cytoplasmic domain (C) were not amplified. Pfu polymerase and sequence-specific primers (arrows) introduced restriction sites at the 5′ (PstI) and 3′ (XbaI) ends of PCR-amplified fragments to allow cloning into the modified baculovirus expression vector. (B) The baculovirus expression vector pAcMP2 was modified by adding a consensus thrombin cleavage (Th. Cl.) site, a six-histidine tail, and a stop codon. Amplified DNA fragments encoding the extracellular domains of MHVR and Bgp were ligated in frame, using PstI and XbaI. All constructs were confirmed by sequencing. Expression in Sf9 cells is driven by the major basic protein promoter (MBP).
Purification of soluble, six-histidine-tagged glycoproteins.
Large-scale (0.5- to 1-liter) infections were done in the UCHSC Cancer Center Tissue Culture Core Facility, using spinner-adapted Sf9 cells inoculated at an MOI of 1 and grown in SF-900 II serum-free medium (Gibco/BRL) for 72 h. The culture medium was clarified by centrifugation (10,000 × g, 30 min) and filtration (0.22-mm-pore-size filter; Millipore, Bedford, Mass.) prior to two 20-fold overnight dialyses at 4°C against Tris-buffered saline (TBS; 25 mM Tris [pH 7.6], 150 mM NaCl). The dialysate (approximately 1 mg of total protein per ml) was then loaded onto a 5-ml HiTrap metal affinity column (Pharmacia) previously charged with 10 ml of 100 mM NiSO4. After washing with TBS containing 5% glycerol (TBSG), bound proteins were eluted with a 0 to 500 mM gradient of imidazole (Sigma, St. Louis, Mo.) in TBSG. Fractions containing soluble recombinant receptor glycoproteins were identified by immunoblot analysis, pooled, diluted with 3 volumes of 25 mM Tris (pH 7.6)–5% glycerol, loaded onto an HR 5/5 MonoQ column (Pharmacia), and eluted at 100 to 150 mM NaCl in a 50 to 300 mM NaCl gradient. After analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and staining with Coomassie brilliant blue G (Sigma), fractions containing purified receptor glycoproteins were pooled and stored at −80°C.
Because sBgp2 did not bind to MonoQ resin, the Bgp2 fractions from the nickel affinity column were pooled, dialyzed against phosphate-buffered saline (PBS) containing glycerol (PBSG; 25 mM sodium phosphate [pH 7.6], 50 mM NaCl, 5% glycerol), and loaded onto an HR 5/5 MonoS column (Pharmacia). sBgp2 was eluted with a 50 to 300 mM NaCl gradient in 25 mM sodium phosphate (pH 7.6)–5% glycerol and dialyzed against TBSG prior to storage.
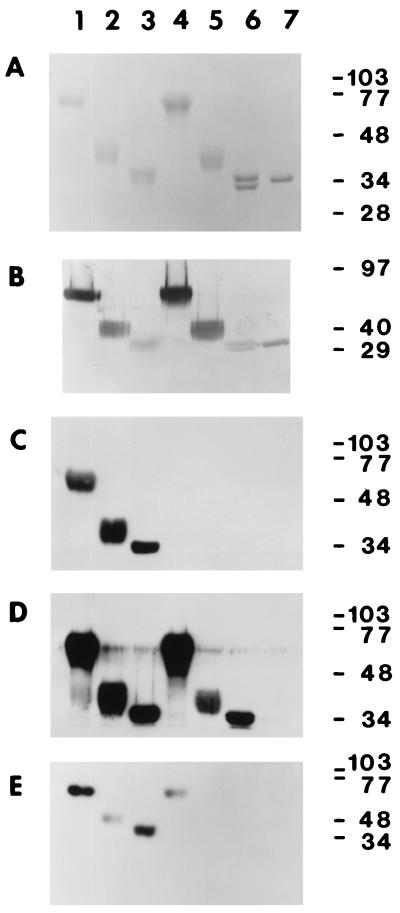
The molecular weights of the purified soluble glycoproteins were estimated by comparison to the migration of known protein standards (Gibco/BRL), and protein concentrations were determined by using the Micro BCA assay (Pierce, Rockford, Ill.) with a bovine serum albumin (BSA) standard. Glycosylation of the purified glycoproteins was detected with a GlycoTrack kit (Oxford Glycosystems, Rosedale, N.Y.). Typical yields from a 1-liter culture were 1 to 2 mg of purified receptor glycoprotein. The glycoproteins were purified to apparent homogeneity based on the absence of other bands in SDS-polyacrylamide gels stained with either Coomassie blue (Fig. 3A) or silver (data not shown).
FIG. 3.
Characterization of the purified, soluble receptor glycoproteins. In each panel, 0.5-μg aliquots of purified sMHVR[1-4] (lane 1), sMHVR[1,2] (lane 2), sMHVR[1,4] (lane 3), sBgp1b[1-4] (lane 4), sBgp1b[1,2] (lane 5), sBgp1b[1,4] (lane 6), and sBgp2[1,4] (lane 7) were separated by SDS-PAGE (12% gel) and stained with Coomassie blue (A) or transferred to Immobilon-P for further analysis (B to E). In panel B, the protein blot was probed for total carbohydrate. The immunoreactivities of the purified glycoproteins with anti-MHVR MAb CC1 and polyclonal anti-MHVR antiserum 655 are shown in panels C and D, respectively. Panel E shows the relative MHV-A59 binding activities of the purified glycoproteins determined by VOPBA. Sizes are indicated in kilodaltons.
For some experiments, the six-histidine tag was removed from 200 μg of purified sMHVR[1-4] by cleavage with 0.1 U of biotinylated thrombin (Novagen, Madison, Wis.) at 16°C for 16 h. The cleaved receptor was then purified by passage through streptavidin-agarose (Novagen) to remove the biotinylated thrombin and then a nickel-charged HiTrap column to remove cleaved six-histidine tags and any remaining uncleaved His-tagged sMHVR[1-4]. The flowthrough from these columns was concentrated by MonoQ chromatography, and fractions containing sMHVR[1-4] were dialyzed against TBSG and frozen.
Immunoblots and virus binding assays.
The soluble receptor glycoproteins (500 ng of each) and prestained molecular weight standards (Bio-Rad) were resolved by SDS-PAGE (12% gels), transferred to Immobilon-P membranes (Millipore), and blocked overnight at 4°C in TBST (25 mM Tris [pH 7.6], 150 mM NaCl, 0.1% Tween 20) supplemented with 5% powdered milk; all subsequent steps used TBST with 0.5% milk. Primary antisera, either anti-MHVR MAb CC1 or rabbit polyclonal serum 655 were used at a 1:4,000 dilution and incubated for 1 h. Duplicate blots were probed with control MAb IgG1 or normal rabbit serum. After washing, the blots were incubated for 30 min with a 1:4,000 dilution of the appropriate horseradish peroxidase-conjugated secondary antibody, either goat anti-mouse IgG (Cappel, Durham, N.C.) or donkey anti-rabbit IgG (Amersham, Arlington Heights, Ill.). Bound horseradish peroxidase-antibody complexes were detected with Renaissance Chemiluminescence Reagent and Reflection autoradiography film (DuPont/NEN, Boston, Mass.).
For virus overlay protein blot analysis (VOPBA), blots of purified receptor glycoproteins were blocked overnight at 4°C with TBST containing 2% BSA and then incubated for 1 h with 20 ml of MHV-A59 (107 PFU/ml). Bound virus was detected with anti-MHV-A59 spike antiserum AO4 and 125I-protein A (10 μCi/μg, 105 dpm/ml; DuPont/NEN) and visualized by autoradiography (6). Brush border membranes (BBM) purified from the small intestines of adult BALB/c mice were positive controls for virus binding, and BSA and BBM from adult SJL/J mice were negative controls (data not shown) (6).
Virus neutralization assays.
Dilutions of supernatant medium from vac-sMHVR-inoculated cells or purified sMHVR(vac) were preincubated with 2 × 104 PFU of MHV-A59 for 15 min at 37°C prior to inoculating onto L2 cells in 96-well culture plates. After 1 h at 37°C, the monolayers were washed and refed. At 7 h postinoculation, the cell monolayers were washed with PBS, fixed in methanol at −20°C, and air dried. The amount of viral nucleocapsid (N) protein in each well was measured by enzyme-linked immunosorbent assay as previously described (23). Neutralization of MHV by sMHVR(vac) was calculated by comparing the amounts of N antigen in wells inoculated with MHV-A59 preincubated with sMHVR(vac) with that in control wells infected with MHV-A59 preincubated with buffer alone.
Virus neutralization by baculovirus-expressed receptor glycoproteins was done with purified proteins serially diluted in TBSG containing 0.1 mg of BSA per ml (Sigma). A 180-μl volume of diluted receptor was mixed with 30 μl (5,000 PFU) of MHV-A59 and incubated at 37°C for 1 h. The mixture was then diluted 10- or 100-fold with DMEM–10% FBS and immediately inoculated onto 17 clone 1 cells in six-well plates (0.3 ml per well, three wells per assay point). After adsorption for 1 h at 37°C, the inocula were replaced with 3 ml of DMEM containing 4% serum and 0.95% Noble agar (Difco). After 2 days at 37°C, the plates were overlaid with 1 ml of medium containing 0.95% Noble agar and 0.01% neutral red. Control plates were inoculated with MHV-A59 and incubated for 1 h at 37°C with TBSG-BSA (without soluble receptor protein). The percent virus neutralization by receptor glycoproteins was calculated as 100 − [(number of experimental plaques/number of control plaques) × 100]. The results were graphed and fitted to a sigmoid curve, using the Origin 4.1 (Microcal, Northampton, Mass.) program. The amount of each soluble receptor that neutralized 50% of the virus is called the 50% neutralizing dose (ND50). Table 1 reports the average values, standard deviations, and number of experiments used for each determination. For the soluble receptors that caused less than 50% neutralization at the highest concentrations tested, the ND50 was approximated by extrapolation from the available data, and a minimum value is given in Table 1. Representative experiments are illustrated in Fig. 4 and 5. The significance (P) of the differences in virus neutralizing activities of two soluble glycoproteins was calculated by one-way analysis of variance.
TABLE 1.
Characterization of the purified soluble receptor glycoproteins
| Protein | Predicted molecular mass (kDa)a | No. of potential N-linked glycosylation sitesa | Molecular mass(es) (kDa) determined by SDS-PAGE | ND50 (nM)b vs
|
|
|---|---|---|---|---|---|
| MHV-A59 | MHV-JHM | ||||
| sMHVR[1-4] | 48 | 16 | 60–70 | 0.7 ± 0.6 (13) | 2.2 ± 1.3 (4) |
| sMHVR[1,2] | 29 | 9 | 37–42 | 9.9 ± 3 (2) | ND |
| sMHVR[1,4] | 28 | 5 | 32–36 | 0.83 ± 0.3 (4) | 2.1 ± 0.7 (3) |
| sBgp1b[1-4] | 48 | 15 | 60–70 | 3.1 ± 1.5 (9) | >1,000 |
| sBgp1b[1,2] | 29 | 8 | 36–39 | >1,000 | ND |
| sBgp1b[1,4] | 28 | 4 | 32, 34 | >1,000 | >1,000 |
| sBgp2[1,4] | 27 | 4 | 33 | >1,000 | ND |
Based on predicted amino acid sequences.
Average ND50 ± standard deviation of the number of experiments indicated in parentheses. ND, not determined.
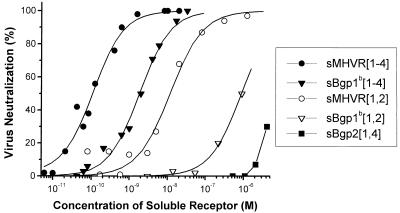
FIG. 4.
The soluble MHVR-related glycoproteins expressed by baculovirus differ in the ability to neutralize MHV-A59. MHV-A59 (5,000 PFU) virions were preincubated with serial dilutions of the five purified soluble receptor glycoproteins. The surviving virions were quantitated by plaque assays, and the percent neutralization is plotted against the molar concentration of the purified glycoproteins. These data are from a representative experiment. The ND50s are summarized in Table 1.
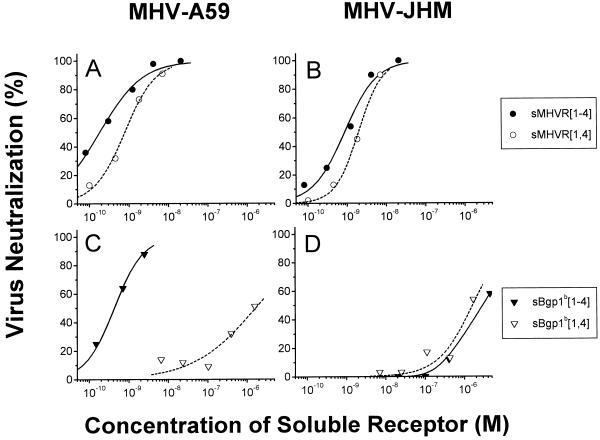
FIG. 5.
Soluble receptor glycoproteins show different neutralizing activities for MHV-A59 and MHV-JHM. The plaque assay described in the legend to Fig. 4 was used to determine the virus neutralizing activities of sMHVR[1-4] and sMHVR[1,4] against MHV-A59 (A) and MHV-JHM (B) and of sBgp1b[1-4] and sBgp1b[1,4] against MHV-A59 (C) and MHV-JHM (D). The ND50s are reported in Table 1.
RESULTS
MHV-A59 neutralization activity of sMHVR(vac) expressed in Vero cells from a recombinant vaccinia virus.
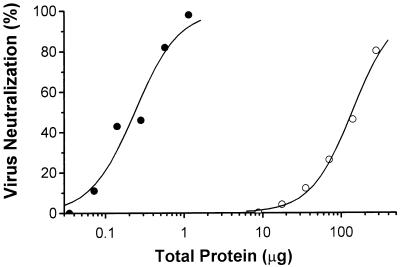
The vaccinia virus expression system was used to produce a secreted, anchorless four-domain MHVR glycoprotein, sMHVR(vac), which is recognized by MAb CC1 and binds MHV-A59 in a VOPBA (49). Figure 2 shows that both unpurified sMHVR(vac) and highly purified sMHVR(vac) neutralized MHV-A59 in a concentration-dependent manner. The purified sMHVR(vac) (calculated ND50 = 20 nM) showed a 1,000-fold increase in specific activity over the crude sMHVR(vac) supernatant.
FIG. 2.
The soluble MHVR[1-4] expressed by a recombinant vaccinia virus neutralizes MHV-A59. MHV-A59 (10,000 PFU) was preincubated with the unpurified culture medium of vac-MHVR-infected Vero cells (open circles) or with highly purified sMHVR(vac) (closed circles) prior to inoculation of L2 cells. MHV-A59 replication was monitored by an MHV N-protein-specific MAb, and the percent neutralization was calculated as described in Materials and Methods. The ND50 of sMHVR(vac) is estimated to be 20 nM.
Characterization of six-histidine-tagged, soluble murine receptor glycoproteins expressed by baculovirus.
We constructed seven baculovirus expression vectors which encoded soluble, six-histidine-tagged MHVR-related receptor glycoproteins: sMHVR[1-4], sMHVR[1,4], sMHVR[1,2], sBgp1b[1-4], sBgp1b[1,4], sBgp1b[1,2], and sBgp2[1,4] (Fig. 1). The secreted receptor glycoproteins were purified to apparent homogeneity from the Sf9 cell supernatant by nickel affinity and ion-exchange chromatography. The purified proteins migrated as broad bands, except for sBgp1b[1,4], which migrated as two distinct bands (Fig. 3A). The molecular weights of the glycoproteins were higher than expected based on their predicted amino acid compositions, probably due to glycosylation; all stained positively for carbohydrate (Fig. 3B). Electrophoresis in nondenaturing gels and size exclusion chromatography showed that each of the purified glycoproteins migrated as a single peak corresponding to a monomer of the expected size for an Ig-like molecule (data not shown).
The sMHVR[1-4], sMHVR[1,2], and sMHVR[1,4] glycoproteins were recognized in immunoblots by anti-MHVR MAb-CC1 (Fig. 3C), which recognizes domain 1 of MHVR but not Bgp1b or Bgp2 (25). Rabbit polyclonal anti-MHVR antiserum 655, which recognizes both MHVR and Bgp1b proteins (65), reacted with the six soluble proteins derived from MHVR and Bgp1b cDNAs (Fig. 3D). As expected, sBgp2[1,4] was not recognized by either MAb CC1 or 655, although this protein did react with a Bgp2 specific antiserum kindly provided by N. Beauchemin (McGill Cancer Center, Montreal, Quebec, Canada) (data not shown). Blots probed with control antisera showed no bands (data not shown). Thus, the antigenicities of the purified soluble, recombinant glycoproteins expressed in insect cells correspond to those of the anchored receptor glycoproteins naturally expressed in murine tissues and to recombinant murine Bgps expressed in nonmurine cell lines (22, 23, 65, 66).
VOPBA (6) was used to compare MHV-A59 virus binding to the purified soluble receptor glycoproteins (Fig. 3E). sMHVR[1-4] and sMHVR[1,4] bound virus better than the two-domain sMHVR[1,2], suggesting that domains 2 and 4 have different effects on the virus binding activity of domain 1. The soluble sBgp1b[1-4] glycoprotein bound less MHV-A59 than sMHVR[1-4], and neither sBgp1b[1,2], sBgp1b[1,4], nor sBgp2[1,4] showed any MHV-A59 binding activity (Fig. 3E).
Virus neutralization activities of purified soluble receptor glycoproteins produced by recombinant baculovirus.
The soluble recombinant receptor glycoprotein sMHVR[1-4] expressed in insect cells has the same four domains as sMHVR(vac), the glycoprotein expressed in mammalian cells by using recombinant vaccinia virus. To assay the biological activity of baculovirus-expressed, purified protein, 5,000 PFU of MHV-A59 virus was incubated at 37°C for 1 h with dilutions of sMHVR[1-4]. The remaining infectious virus was assayed by plaquing on murine 17 clone 1 cells (Fig. 4). Different lots of purified, baculovirus-expressed glycoprotein had 20- to 200-fold more virus neutralizing activity than vaccinia virus-expressed sMHVR(vac). The six-histidine tag had no effect on the strong virus neutralization activity of sMHVR[1-4], as removal of the tag did not affect virus neutralization activity (data not shown). We estimate that the number of sMHVR[1-4] molecules at the ND50 (0.7 nM, 8.4 × 109 molecules/assay) is approximately 10-fold greater than the estimated number of spike molecules (approximately 9 × 108 spike proteins/assay, estimated as 5,000 PFU/assay × 300 virions/PFU × 200 peplomers/virion × 3 spike proteins/peplomer). The higher yield, ease of purification, and enhanced biological activity of sMHVR[1-4] in comparison to sMHVR(vac) justified the use of the baculovirus system for the production of the other six soluble receptor glycoproteins.
Although each of the purified baculovirus-expressed murine glycoproteins tested neutralized MHV-A59 in a concentration-dependent manner, there were highly reproducible differences between the ND50s of the various receptor glycoproteins (Table 1; representative experiments are shown in Fig. 4 and 5). sMHVR[1-4], the glycoprotein that corresponds to the anchored four-domain MHVR of MHV-A59-susceptible BALB/c mice, had the greatest virus neutralizing activity (ND50 = 0.7 ± 0.6 nM). In contrast, the soluble four-domain receptor glycoprotein sBgp1b[1-4], derived from MHV-A59-resistant SJL/J mice, had fourfold less MHV-A59 neutralizing activity than sMHVR[1-4] (P < 0.001). Figure 5A shows that sMHVR[1,4], which corresponds to the natural two Ig-like domain isoform of MHVR generated by alternative mRNA splicing, has approximately the same MHV-A59 neutralizing activity as sMHVR[1-4]. In marked contrast, the MHV-A59 virus neutralization activity of the natural two-domain sBgp1b[1,4] was at least 300-fold less than that of the corresponding four-domain sBgp1b[1-4] (Fig. 5C; Table 1).
Truncated MHVR and Bgp1b glycoproteins containing only domains 1 and 2, sMHVR[1,2] and sBgp1b[1,2], had approximately 10- and 300-fold, respectively, less MHV-A59 neutralization activity than the corresponding soluble four-domain glycoproteins (Fig. 4; Table 1). These results suggest that domains 3 and/or 4 of the four-domain receptor glycoproteins sMHVR[1-4] and sBgp1b[1-4] are required for maximal virus neutralization activity. The soluble Bgp2 glycoprotein, sBgp2[1,4], had the least MHV-A59 neutralizing activity (ND50 >> 1 μM) of all of the purified recombinant murine glycoproteins tested (Fig. 4).
Although both MHV-A59 and MHV-JHM can use anchored MHVR as a receptor, the anchored Bgp1b is a good receptor for MHV-A59 but not for MHV-JHM (13, 48, 69, 71). We therefore compared the MHV-JHM neutralization activities of the purified anchorless sMHVR[1-4], sMHVR[1,4], sBgp1b[1-4], and sBgp1b[1,4] glycoproteins (Fig. 5B and D; Table 1). The sMHVR[1-4] and sMHVR[1,4] glycoproteins had approximately threefold (P < 0.05) less neutralizing activity for MHV-JHM than for MHV-A59 (Fig. 5A and B; Table 1). In contrast, the four-domain sBgp1b[1-4] glycoprotein had at least 300-fold less virus neutralizing activity for MHV-JHM than for MHV-A59, while the sBgp1b[1,4] glycoprotein had very little neutralizing activity for either MHV-JHM or MHV-A59 (Fig. 5C and D; Table 1).
DISCUSSION
Initial studies of the MHV-A59 neutralization activities of murine Bgp-related glycoproteins used affinity-purified, anchorless MHVR glycoprotein, sMHVR(vac), expressed in mammalian cells by vaccinia virus (Fig. 2). This sMHVR(vac) glycoprotein effectively neutralized MHV-A59 infectivity (ND50 = 20 nM) and served as a benchmark for our studies using purified soluble receptor proteins expressed in insect cells. The sMHVR[1-4] glycoprotein expressed in insect cells and purified to apparent homogeneity had 20-fold more MHV-A59 neutralizing activity (ND50 = 0.7 nM) than sMHVR(vac). Thus, soluble, biologically active sMHVR[1-4] glycoprotein is produced in insect cells by infection with recombinant baculovirus even though the pattern of glycosylation in insect cells differs from that in animal cells (35, 36, 58). This finding is consistent with the evidence that glycosylation of domain 1 of MHVR is not essential for MHV-A59 receptor activity (21). Therefore, because of the high yield and biological activity of the receptor glycoprotein expressed in insect cells, the recombinant baculovirus system was used to produce the other soluble murine Bgps for MHV-A59 virus binding and neutralizing studies.
MHV-A59 virus bound strongly to purified sMHVR[1-4] and sMHVR[1,4], less well to sMHVR[1,2] and sBgp1b[1-4], and not at all to sBgp1b[1,2], sBgp1b[1,4], or sBgp2 (Fig. 3E). In VOPBAs, virus binding depends upon the affinity of the virus-receptor interaction and the renaturation of the receptor glycoprotein after SDS denaturation (6). Despite this dependence on protein refolding, the rank order of virus binding activity among the soluble receptors in VOPBAs correlates with the virus neutralization activities of the soluble receptors and with the receptor activities of the corresponding anchored murine Bgp glycoproteins expressed at high levels in BHK cells (44, 64). Although the four-domain receptor glycoprotein sMHVR[1-4] bound more virus than sBgp1b[1-4], the virus binding activity of sBgp1b[1-4], derived from MHV-resistant SJL/J mice, was unexpected. Our previous studies using intestinal BBM from MHV-A59-susceptible adult BALB/c and MHV-A59-resistant SJL/J mice showed that anchored MHVR[1-4] binds MHV-A59 in VOPBA, but anchored Bgp1b[1-4] does not (6). However, an embryonic SJL/J mouse fibroblast line that expresses Bgp1b and is resistant to infection with MHV-A59 becomes susceptible to infection when additional recombinant anchored Bgp1b[1-4] is expressed (22). This finding suggests that MHV-A59 can bind to high concentrations of Bgp1b. The MHV-A59 binding activity of purified sBgp1b[1-4] observed in Fig. 3E probably results from a higher density of Bgp1b on blots of highly purified receptor in comparison to blots of SJL/J BBM.
Quantitative analysis of the MHV-A59 neutralizing activities of the MHVR-related soluble glycoproteins expressed in insect cells revealed at least a 1,000-fold difference between the most and least active glycoproteins (Fig. 4 and 5; Table 1). Overall, for each of the soluble receptor glycoproteins, these data correlated well with the virus receptor activity of the corresponding anchored glycoproteins (22, 44, 48, 64, 71). This finding demonstrates that the soluble receptors produced in insect cells are suitable for in vitro studies of virus-receptor interactions.
Our experiments show that virus neutralization activity is primarily determined by the primary amino acid sequence of domain 1, in that each soluble MHVR glycoprotein had more activity than the corresponding soluble Bgp1b glycoprotein (Fig. 4 and 5; Table 1). Domain 1 of Bgp1b differs from that of MHVR in 28 of its 108 amino acids, while domain 1 of Bgp2 is even more divergent (51 of 108 amino acids). Many of these amino acid differences lie in the putative C-C′ loop, C′ beta sheet, and C′-C" loop, which includes the MHV binding site (51, 64). In contrast, the amino acid sequences of domains 2, 3, and 4 of MHVR and Bgp1b are remarkably similar (five differences in 282 amino acids) (42). Thus, the primary sequences in domain 1 of sMHVR[1-4], sBgp1b[1-4], and sBgp2 probably account for the observed differences in their MHV-A59 binding and neutralizing activities.
These experiments also show that domains 2, 3, and 4 modulate the virus binding and neutralizing activities of domain 1. The sMHVR[1,2] glycoprotein had markedly less MHV-A59 binding activity and had 10-fold less MHV-A59 neutralizing activity than sMHVR[1-4] and sMHVR[1,4]. Similarly, anchored MHVR[1,2] is a poor receptor in comparison to MHVR[1-4] and MHVR[1,4], even when expressed at high levels in BHK cells (64). One major difference between the structures of Ig-related proteins that can affect their functions is the nature of the interdomain junctions (2, 40). The junction between domains 1 and 2 of MHVR may have a different degree of flexibility than the junction between domains 1 and 4, which might affect the virus binding and neutralizing activities of domain 1. Perhaps domain 4, which is common to both sMHVR[1-4] and sMHVR[1,4], maintains domain 1 in an optimal conformation for virus binding and neutralization. While sBgp1b[1-4] had some MHV-A59 binding and neutralizing activity, both the sBgp1b[1,4] and sBgp1b[1,2] glycoproteins had very little neutralizing activity. The amino acid differences in domain 1 of Bgp1b may affect its interactions with other domains in addition to its direct effect on virus binding and neutralizing activities. Future structural studies of the purified proteins will allow comparison of the Ig-like domains of MHVR and Bgp1b and their interdomain junctions.
Several previous studies compared the relative MHV receptor activities of various anchored receptor glycoproteins, using transiently transfected cell lines to express the recombinant receptor glycoproteins (22, 48, 51, 64, 70, 71). However, infection in these assays depends on the strain and MOI of the virus, the percentage of cells transfected, and the surface density of the expressed receptor glycoprotein in addition to its intrinsic receptor activity (10, 29, 30). This report provides a quantitative comparison of the virus neutralizing activities of soluble forms of the receptor glycoproteins. If, as seems likely, the neutralizing activities of the soluble receptors provide a more sensitive assay for the differences between receptor glycoproteins than cells transfected with anchored receptors, our data predict that anchored Bgp1b[1,4] would be a very poor receptor for MHV-A59 compared with anchored Bgp1b[1-4], while anchored MHVR[1,4] and MHVR[1-4] both would have the highest receptor activity. This may help explain why adult SJL/J mice are resistant to MHV-A59 infection (4, 39, 57), even though Bgp1b[1-4] has only fourfold less virus neutralizing activity than MHVR[1-4]. In BALB/c mice, both MHVR[1-4] and MHVR[1,4] would serve as effective receptors, while the SJL/J mouse would have only one functional receptor, Bgp1b[1-4], since Bgp1b[1,4] probably has little receptor function. Susceptibility to infection may require that both the two- and four-domain receptors be functional receptors. Since Bgps may exist in the cell membrane as homodimers or heterodimers (5, 26), in BALB/c mice both the two-domain and four-domain homodimers and heterodimers may be functional, while in the SJL mouse only the four-domain Bgp1b[1-4] homodimer may be functional. Unfortunately, the expression patterns of various isoforms of MHVR and Bgp1b in particular cell types have not yet been determined (38, 44).
Soluble receptors may neutralize virus by competing with cellular receptor for binding to the viral spike protein and/or by inducing conformational changes in virus attachment proteins that mimic the initial events of virus entry and uncoating. Soluble receptor glycoproteins that neutralize the infectivity of HIV, rhinovirus, and poliovirus induce conformational changes in HIV gp120 and the picornavirus capsids (8, 12, 32, 34, 37, 43). Binding of an MHVR domain1-IgG Fc chimera to MHV-JHM results in the dissociation of the S1 and S2 subunits of the spike glycoprotein, a process that may resemble the natural entry process for MHV (30, 54, 60) and serves as a model for neutralization by soluble receptors. Fusion of MHV with the host cell membrane probably involves some sort of receptor-induced conformational change in S to bring the lipid bilayers close enough to fuse.
The different tissue tropisms and virulence of MHV strains may reflect differences in the ability to utilize the various receptor isoforms expressed on different murine tissues. We compared the neutralizing activities of the soluble receptors for MHV-JHM and MHV-A59. The sMHVR[1-4] and sMHVR[1,4] glycoproteins neutralized both virus strains quite well (Fig. 5A and B; Table 1), and the corresponding anchored MHVR glycoproteins are functional receptors for both MHV-A59 and MHV-JHM (47, 51, 70). In contrast, sBgp1b[1-4] had at least 300-fold less neutralizing activity for MHV-JHM than for MHV-A59. This quantitative difference in the susceptibility of MHV-A59 and MHV-JHM to neutralization by purified sBgp1b[1-4] in our in vitro assay is consistent with the observation that MHV-A59 could infect an SJL/J cell line (PSJLSV) that expresses Bgp1b, while MHV-JHM could not infect these cells (71). The two-domain sBgp1b[1,4] glycoprotein did not neutralize either MHV-A59 or MHV-JHM. This finding confirms and extends the observation of Ohtsuka et al. that soluble Bgp1b[1,4] blocked MHV-JHM infection of DBT cells 500-fold less efficiently than soluble MHVR[1,4] (48). Thus, the quantitative studies on the neutralization of murine coronaviruses by purified soluble receptor glycoproteins provide insight into the entry mechanisms and receptor specificities of various MHV strains and mutants. Such studies may elucidate how receptor selectivity influences the tissue tropism and virulence of different MHV strains.
ACKNOWLEDGMENTS
We thank Nicole Beauchemin, Dianna Blau, Aurelio Bonavia, Carlos Catallano, Dina Tresnan, Jeanne Schickli, and David Wentworth for helpful discussions and review of the manuscript. We thank Thomas Chamberlain and John Schneider for excellent technical assistance and Kurt Christiansen and Suzanne Meintzer of the UCHSC Cancer Center Core (supported by NCI grant P30-CA46934) for assistance with the baculovirus expression system.
This work was supported by NIH grants AI25231 and AI26075.
REFERENCES
- 1.Asanaka M, Lai M M. Cell fusion studies identified multiple cellular factors involved in mouse hepatitis virus entry. Virology. 1993;197:732–741. doi: 10.1006/viro.1993.1649. [DOI] [PubMed] [Google Scholar]
- 2.Banfield M J, King D J, Mountain A, Brady R L. VL:VH domain rotations in engineered antibodies: crystal structures of the Fab fragments from two murine antitumor antibodies and their engineered human constructs. Proteins. 1997;29:161–171. doi: 10.1002/(sici)1097-0134(199710)29:2<161::aid-prot4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Barthold S W. Mouse hepatitis virus: biology and epizootiology. In: Bhatt P N, Jacoby R O, Morse III H C, New A E, editors. Viral and mycoplasma infections of laboratory rodents. Effects on biomedical research. Orlando, Fla: Academic Press; 1986. pp. 571–601. [Google Scholar]
- 4.Barthold S W, Beck D S, Smith A L. Mouse hepatitis virus nasoencephalopathy is dependent upon virus strain and host genotype. Arch Virol. 1986;91:247–256. doi: 10.1007/BF01314284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates P A, Luo J, Sternberg M J. A predicted three-dimensional structure for the carcinoembryonic antigen (CEA) FEBS Lett. 1992;301:207–214. doi: 10.1016/0014-5793(92)81249-l. [DOI] [PubMed] [Google Scholar]
- 6.Boyle J F, Weismiller D G, Holmes K V. Genetic resistance to mouse hepatitis virus correlates with absence of virus-binding activity on target tissues. J Virol. 1987;61:185–189. doi: 10.1128/jvi.61.1.185-189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brümmendorf T, Rathjen F G. Introduction. In: Sheterline P, editor. Cell adhesion molecules 1: immunoglobulin superfamily. London, England: Academic Press; 1994. pp. 951–962. [PubMed] [Google Scholar]
- 8.Bryn R A, Sekigawa I, Chamow S M, Johnson J S, Gregory T J, Capon D J, Groopman J E. Characterization of in vitro inhibition of human immunodeficiency virus by purified recombinant CD4. J Virol. 1989;63:4370–4375. doi: 10.1128/jvi.63.10.4370-4375.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen D S, Asanaka M, Yokomori K, Wang F, Hwang S B, Li H P, Lai M M. A pregnancy-specific glycoprotein is expressed in the brain and serves as a receptor for mouse hepatitis virus. Proc Natl Acad Sci USA. 1995;92:12095–12099. doi: 10.1073/pnas.92.26.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Madden V J, Bagnell C R, Jr, Baric R S. Host-derived intracellular immunization against mouse hepatitis virus infection. Virology. 1997;228:318–332. doi: 10.1006/viro.1996.8402. [DOI] [PubMed] [Google Scholar]
- 11.Collins A R, Knobler R L, Powell H, Buchmeier M J. Monoclonal antibodies to murine hepatitis virus-4 (strain JHM) define the viral glycoprotein responsible for attachment and cell-cell fusion. Virology. 1982;119:358–371. doi: 10.1016/0042-6822(82)90095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colston E, Racaniello V R. Soluble receptor-resistant poliovirus mutants identify surface and internal capsid residues that control interaction with the cell receptor. EMBO J. 1994;13:5855–5862. doi: 10.1002/j.1460-2075.1994.tb06930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compton S R. Enterotropic strains of mouse coronavirus differ in their use of murine carcinoembryonic antigen-related glycoprotein receptors. Virology. 1994;203:197–201. doi: 10.1006/viro.1994.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Compton S R, Barthold S W, Smith A L. The cellular and molecular pathogenesis of coronaviruses. Lab Anim Sci. 1993;43:15–28. . (Erratum, 43:203.) [PubMed] [Google Scholar]
- 15.Coutelier J-P, Godfraind C, Dveksler G S, Wysocka M, Cardellichio C B, Nöel H, Holmes K V. B lymphocyte and macrophage expression of carcinoembryonic antigen-related adhesion molecules that serve as receptors for murine coronavirus. Eur J Immunol. 1994;24:1383–1390. doi: 10.1002/eji.1830240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delmas B, Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J Virol. 1990;64:5367–5375. doi: 10.1128/jvi.64.11.5367-5375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denisova G, Raviv D, Mondor I, Sattentau Q J, Gershoni J M. Conformational transitions in CD4 due to complexation with HIV envelope glycoprotein gp120. J Immunol. 1997;158:1157–1164. [PubMed] [Google Scholar]
- 18.Dindzans V J, Skamene E, Levy G A. Susceptibility/resistance to mouse hepatitis virus strain 3 and macrophage procoagulant activity are genetically linked and controlled by two non-H2-linked genes. J Immunol. 1986;137:2355–2360. [PubMed] [Google Scholar]
- 19.Dveksler, G. 1998. Unpublished data.
- 20.Dveksler G S, Basile A A, Cardellichio C B, Beauchemin N, Dieffenbach C W, Holmes K V. Expression of MHV-A59 receptor glycoproteins in susceptible and resistant strains of mice. Adv Exp Med Biol. 1993;342:267–272. doi: 10.1007/978-1-4615-2996-5_41. [DOI] [PubMed] [Google Scholar]
- 21.Dveksler G S, Basile A A, Cardellichio C B, Holmes K V. Mouse hepatitis virus receptor activities of an MHVR/mph chimera and MHVR mutants lacking N-linked glycosylation of the N-terminal domain. J Virol. 1995;69:543–546. doi: 10.1128/jvi.69.1.543-546.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dveksler G S, Dieffenbach C W, Cardellichio C B, McCuaig K, Pensiero M N, Jiang G S, Beauchemin N, Holmes K V. Several members of the mouse carcinoembryonic antigen-related glycoprotein family are functional receptors for the coronavirus mouse hepatitis virus-A59. J Virol. 1993;67:1–8. doi: 10.1128/jvi.67.1.1-8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dveksler G S, Gagneten S E, Scanga C A, Cardellichio C B, Holmes K V. Expression of the recombinant anchorless N-terminal domain of mouse hepatitis virus (MHV) receptor makes hamster or human cells susceptible to MHV infection. J Virol. 1996;70:4142–4145. doi: 10.1128/jvi.70.6.4142-4145.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dveksler G S, Pensiero M N, Cardellichio C B, Williams R K, Jiang G S, Holmes K V, Dieffenbach C W. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J Virol. 1991;65:6881–6891. doi: 10.1128/jvi.65.12.6881-6891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dveksler G S, Pensiero M N, Dieffenbach C W, Cardellichio C B, Basile A A, Elia P E, Holmes K V. Mouse hepatitis virus strain A59 and blocking antireceptor monoclonal antibody bind to the N-terminal domain of cellular receptor. Proc Natl Acad Sci USA. 1993;90:1716–1720. doi: 10.1073/pnas.90.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edlund M, Blikstad I, Obrink B. Calmodulin binds to specific sequences in the cytoplasmic domain of C-CAM and down-regulates C-CAM self-association. J Biol Chem. 1996;271:1393–1399. doi: 10.1074/jbc.271.3.1393. [DOI] [PubMed] [Google Scholar]
- 27.Frana M F, Behnke J N, Sturman L S, Holmes K V. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: host-dependent differences in proteolytic cleavage and cell fusion. J Virol. 1985;56:912–920. doi: 10.1128/jvi.56.3.912-920.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagneten S, Scanga C A, Dveksler G S, Beauchemin N, Percy D, Holmes K V. Attachment glycoproteins and receptor specificity of rat coronaviruses. Lab Anim Sci. 1996;46:159–166. [PubMed] [Google Scholar]
- 29.Gallagher T M. Overexpression of the MHV receptor. Effect on progeny virus secretion. Adv Exp Med Biol. 1995;380:331–336. [PubMed] [Google Scholar]
- 30.Gallagher T M. A role for naturally occurring variation of the murine coronavirus spike protein in stabilizing association with the cellular receptor. J Virol. 1997;71:3129–3137. doi: 10.1128/jvi.71.4.3129-3137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godfraind C, Langreth S G, Cardellichio C B, Knobler R, Coutelier J P, Dubois-Dalcq M, Holmes K V. Tissue and cellular distribution of an adhesion molecule in the carcinoembryonic antigen family that serves as a receptor for mouse hepatitis virus. Lab Invest. 1995;73:615–627. [PubMed] [Google Scholar]
- 32.Greve J M, Forte C P, Marlor C W, Meyer A M, Hoover-Litty H, Wunderlich D, McClelland A. Mechanisms of receptor-mediated rhinovirus neutralization defined by two soluble forms of ICAM-1. J Virol. 1991;65:6015–6023. doi: 10.1128/jvi.65.11.6015-6023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gromeier M, Lu H H, Bernhardt G, Harber J J, Bibb J A, Wimmer E. The human poliovirus receptor. Receptor-virus interaction and parameters of disease specificity. Ann N Y Acad Sci USA. 1995;753:19–36. doi: 10.1111/j.1749-6632.1995.tb27528.x. [DOI] [PubMed] [Google Scholar]
- 34.Hoover-Litty H, Greve J M. Formation of rhinovirus-soluble ICAM-1 complexes and conformational changes in the virion. J Virol. 1993;67:390–397. doi: 10.1128/jvi.67.1.390-397.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.James D C, Goldman M H, Hoare M, Jenkins N, Oliver R W, Green B N, Freedman R B. Posttranslational processing of recombinant human interferon-gamma in animal expression systems. Protein Sci. 1996;5:331–340. doi: 10.1002/pro.5560050217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarvis D L, Finn E E. Biochemical analysis of the N-glycosylation pathway in baculovirus-infected lepidopteran insect cells. Virology. 1995;212:500–511. doi: 10.1006/viro.1995.1508. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan G, Freistadt M S, Racaniello V R. Neutralization of poliovirus by cell receptors expressed in insect cells. J Virol. 1990;64:4697–4702. doi: 10.1128/jvi.64.10.4697-4702.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keck U, Nedéllec P, Beauchemin N, Thompson J A, Zimmerman W. The CEA10 gene encodes a secreted member of the murine carcinoembryonic antigen family and is expressed in the placenta, gastrointestinal tract and bone marrow. Eur J Biochem. 1995;229:455–464. doi: 10.1111/j.1432-1033.1995.0455k.x. [DOI] [PubMed] [Google Scholar]
- 39.Knobler R, Tunison L A, Oldstone M B. Host genetic control of mouse hepatitis virus type 4 (JHM strain) replication. I. Restriction of virus amplification and spread in macrophages from resistant mice. J Gen Virol. 1984;65:1543–1548. doi: 10.1099/0022-1317-65-9-1543. [DOI] [PubMed] [Google Scholar]
- 40.Lange G, Lewis S J, Murshudov G N, Dodson G G, Moody P C, Turkenburg J P, Barclay A N, Brady R L. Crystal structure of an extracellular fragment of the rat CD4 receptor containing domains 3 and 4. Structure. 1994;2:469–481. doi: 10.1016/s0969-2126(00)00048-4. [DOI] [PubMed] [Google Scholar]
- 41.Lin S, Luo W, Earley K, Cheung P, Hixson D C. Structure and function of C-CAM1: effects of the cytoplasmic domain on cell aggregation. Biochem J. 1995;311:239–245. doi: 10.1042/bj3110239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCuaig K, Rosenberg M, Nédellec P, Turbide C, Beauchemin N. Expression of the Bgp gene and characterization of mouse colon biliary glycoprotein isoforms. Gene. 1993;127:173–183. doi: 10.1016/0378-1119(93)90716-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore J P, McKeating J A, Norton W A, Sattentau Q J. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J Virol. 1991;65:1133–1140. doi: 10.1128/jvi.65.3.1133-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nédellec P, Dveksler G S, Daniels E, Turbide C, Chow B, Basile A A, Holmes K V, Beauchemin N. Bgp2, a new member of the carcinoembryonic antigen-related gene family, encodes an alternative receptor for mouse hepatitis viruses. J Virol. 1994;68:4525–4537. doi: 10.1128/jvi.68.7.4525-4537.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norkin L C. Virus receptors: implications for pathogenesis and the design of antiviral agents. Clin Microbiol Rev. 1995;8:293–315. doi: 10.1128/cmr.8.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohlin A, Hoover-Litty H, Sanderson G, Paessens A, Johnson S L, Holgate S T, Huguenel E, Greve J M. Spectrum of activity of soluble intercellular adhesion molecule-1 against rhinovirus reference strains and field isolates. Antimicrob Agents Chemother. 1997;38:1413–1415. doi: 10.1128/aac.38.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohtsuka N, Taguchi F. Mouse susceptibility to mouse hepatitis virus infection is linked to viral receptor genotype. J Virol. 1997;71:8860–8863. doi: 10.1128/jvi.71.11.8860-8863.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohtsuka N, Yamada Y K, Taguchi F. Difference in virus-binding activity of two distinct receptor proteins for mouse hepatitis virus. J Gen Virol. 1996;77:1683–1692. doi: 10.1099/0022-1317-77-8-1683. [DOI] [PubMed] [Google Scholar]
- 49.Pensiero M N, Dveksler G S, Cardellichio C B, Jiang G, Elia P E, Dieffenbach C W, Holmes K V. Binding of the coronavirus mouse hepatitis virus A59 to its receptor expressed from a recombinant vaccinia virus depends on posttranslational processing of the receptor glycoprotein. J Virol. 1992;66:4028–4039. doi: 10.1128/jvi.66.7.4028-4039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Racaniello V R. Virus-receptor interaction in poliovirus entry and pathogenesis. Harvey Lect. 1991;87:1–16. [PubMed] [Google Scholar]
- 51.Rao P V, Gallagher T M. Identification of a contiguous 6-residue determinant in the MHV receptor that controls the level of virion binding to cells. Virology. 1997;229:336–348. doi: 10.1006/viro.1997.8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rowe C L, Baker S C, Nathan M J, Fleming J O. Evolution of mouse hepatitis virus: detection and characterization of spike deletion variants during persistent infection. J Virol. 1997;71:2959–2969. doi: 10.1128/jvi.71.4.2959-2969.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rudert F, Saunders A M, Rebstock S, Thompson J A, Zimmerman W. Characterization of murine carcinoembryonic antigen gene family members. Mamm Genome. 1992;3:262–273. doi: 10.1007/BF00292154. [DOI] [PubMed] [Google Scholar]
- 54.Saeki K, Ohtsuka N, Taguchi F. Identification of spike protein residues of murine coronavirus responsible for receptor-binding activity by use of soluble receptor-resistant mutants. J Virol. 1997;71:9024–9031. doi: 10.1128/jvi.71.12.9024-9031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Signoret N, Poignard P, Blanc D, Sattentau Q J. Human and simian immunodeficiency viruses: virus-receptor interactions. Trends Microbiol. 1993;1:328–333. doi: 10.1016/0966-842x(93)90072-y. [DOI] [PubMed] [Google Scholar]
- 56.Smith A L, Cardellichio C B, Winograd D F, de Souza M S, Barthold S W, Holmes K V. Monoclonal antibody to the receptor for murine coronavirus MHV-A59 inhibits viral replication in vivo. J Infect Dis. 1991;163:879–882. doi: 10.1093/infdis/163.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith M S, Click R E, Plagemann P G. Control of mouse hepatitis virus replication in macrophages by a recessive gene on chromosome 7. J Immunol. 1984;133:428–432. [PubMed] [Google Scholar]
- 58.Sridhar P, Panda A K, Pal R, Talwar G P, Hasnain S E. Temporal nature of the promoter and not relative strength determines the expression of an extensively processed protein in a baculovirus system. FEBS Lett. 1993;315:282–286. doi: 10.1016/0014-5793(93)81179-4. [DOI] [PubMed] [Google Scholar]
- 59.Sturman L S, Holmes K V, Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J Virol. 1980;33:449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sturman L S, Ricard C S, Holmes K V. Conformational change of the coronavirus peplomer glycoprotein at pH 8.0 and 37°C correlates with virus aggregation and virus-induced cell fusion. J Virol. 1990;64:3042–3050. doi: 10.1128/jvi.64.6.3042-3050.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turbide C, Rojas M, Stanners C P, Beauchemin N. A mouse carcinoembryonic antigen gene family member is a calcium-dependent cell adhesion molecule. J Biol Chem. 1991;266:309–315. [PubMed] [Google Scholar]
- 62.Wege H, Siddell S, ter Meulan V. The biology and pathogenesis of coronaviruses. Curr Top Microbiol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- 63.Weiser W, Vellisto I, Bang F B. Congenic strains of mice susceptible and resistant to mouse hepatitis virus. Proc Soc Exp Biol Med. 1976;152:499–502. doi: 10.3181/00379727-152-39426. [DOI] [PubMed] [Google Scholar]
- 64.Wessner D R, Shick P C, Lu J-H, Cardellichio C B, Gagneten S E, Beauchemin N, Holmes K V, Dveksler G S. Mutational analysis of the virus and monoclonal antibody binding sites in MHVR, the cellular receptor of the murine coronavirus mouse hepatitis virus strain MHV-A59. J Virol. 1998;72:1941–1948. doi: 10.1128/jvi.72.3.1941-1948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams R K, Jiang G S, Holmes K V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci USA. 1991;88:5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams R K, Jiang G S, Snyder S W, Frana M F, Holmes K V. Purification of the 110-kilodalton glycoprotein receptor for mouse hepatitis virus (MHV)-A59 from mouse liver and identification of a nonfunctional, homologous protein in MHV-resistant SJL/J mice. J Virol. 1990;64:3817–3823. doi: 10.1128/jvi.64.8.3817-3823.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams R K, Snyder S W, Holmes K V. MHV-resistant SJL/J mice express a non-functional homolog to the MHV receptor glycoprotein. Adv Exp Med Biol. 1990;276:45–50. doi: 10.1007/978-1-4684-5823-7_7. [DOI] [PubMed] [Google Scholar]
- 68.Yafal A G, Kaplan G, Racaniello V R, Hogle J M. Characterization of poliovirus conformational alteration mediated by soluble cell receptors. Virology. 1993;197:501–505. doi: 10.1006/viro.1993.1621. [DOI] [PubMed] [Google Scholar]
- 69.Yokomori K, Asanaka M, Stohlman S A, Lai M M. A spike protein-dependent cellular factor other than the viral receptor is required for mouse hepatitis virus entry. Virology. 1993;196:45–56. doi: 10.1006/viro.1993.1453. [DOI] [PubMed] [Google Scholar]
- 70.Yokomori K, Lai M M. Mouse hepatitis virus utilizes two carcinoembryonic antigens as alternative receptors. J Virol. 1992;66:6194–6199. doi: 10.1128/jvi.66.10.6194-6199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yokomori K, Lai M M. The receptor for mouse hepatitis virus in the resistant mouse strain SJL is functional: implications for the requirement of a second factor for viral infection. J Virol. 1992;66:6931–6938. doi: 10.1128/jvi.66.12.6931-6938.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]